Introduction
Periodontal disease is a chronic inflammatory
disease that is the leading cause of tooth loss in adults (1). This disease is characterized by an
inflammatory response around the teeth, which is primarily caused
by oral microbial biofilms and maintained by an uncoordinated
immune inflammatory response, ultimately leading to progressive
destruction of the tissues supporting the teeth (2). This condition is related to many
systemic diseases, such as vascular disease, diabetes and heart
disease (3). To the best of our
knowledge, no suitable method for periodontal tissue regeneration
has yet been developed.
The periodontal ligament contains pluripotent
periodontal stem cells that express mesenchymal stem cell surface
markers and show self-renewal and pluripotency (4). Periodontal ligament stem cells
(PDLSCs) form cementum/periodontal ligament-like structures after
transplantation in vivo, which indicates that PDLSCs play an
important role in the reconstruction and regeneration of
periodontal tissue, providing a new prospect for periodontal tissue
regeneration (5). In addition,
animal experiments have shown that PDLSCs from different sources
(including human, canine, and porcine) can initiate the homing
effect and promote the regeneration of periodontal tissue after
implantation (6,7). Transplantation of PDLSCs effectively
regenerates alveolar bone in alveolar defects in minipigs, showing
encouraging results in preclinical trials (8,9).
Due to their proliferative and pluripotent differentiation
abilities, as well as their ability to form Sharpey fibers and
cementum-like structures, PDLSCs are considered optimal seeding
cells for periodontal engineering (10).
Researchers have increasingly examined new plant and
fruit bioactive compounds that can be used to combat chronic
disease and certain types of cancer (11,12). Mangiferin (MAG), a natural
polyphenolic compound commonly found in mango and papaya, exhibits
beneficial biological activities, including antioxidant, antitumor,
antiviral, antidiabetic and immunomodulatory activities (13–18). MAG has also been reported to have
anti-osteoclast activity for the treatment and prevention of bone
disease (19). To the best of our
knowledge, however, the effect of MAG on the osteogenic
differentiation of PDLSCs has not been reported.
The present study examined the effect of MAG on the
proliferation and osteogenic differentiation of human PDLSCs
(hPDLSCs) in vitro as well as the molecular pathways that
are involved. It was hypothesized that MAG can promote the
differentiation of hPDLSCs into osteoblasts and may be a potential
drug for periodontal tissue regeneration.
Materials and methods
Primary culture of hPDLSCs
The first or second premolars that were removed from
healthy individuals due to orthodontic needs were collected at the
Beijing Stomatological Hospital after obtaining verbal patient
consent, with approval of the Ethics Committee of Capital Medical
University in China. A total of 10 patients (4 male, 6 females)
aged 18–23 years were recruited from January 2021 to July 2021.
Periodontal tissue was scraped from the middle third of the root of
a healthy premolar extracted by orthodontic treatment. Tissues were
minced and digested in equal volumes with collagenase type I (3
mg/ml) and neutral protease (4 mg/ml) for 1 h at 37°C. The isolated
cells were then cultured in α-MEM with 10% fetal bovine serum and
1% penicillin/streptomycin (all Gibco) and placed in a 37°C and 5%
CO2 cell incubator. Cells at third and fourth passage
were used for the subsequent experiments.
Cell viability assay
The effect of MAG on viability of hPDLSCs was
determined by Cell Counting Kit-8 (CCK-8) assays. Briefly, hPDLSCs
were cultured in 96-well plates (1×104 cells/well) and
cultured with 0, 50, 100, 200 or 500 µmol/l MAG (labeled MAG0,
MAG50, MAG100, MAG200 or MAG500) for 7 days. Then, 10 µl CCK-8
reagent (Dojindo Laboratories, Inc.) was added dropwise to each
well for 2 h. The optical density values in each well were measured
at a wavelength of 490 nm under a microplate reader (Omega Bio-Tek,
Inc.).
Osteogenic induction
The osteogenic medium consisted of 15% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, 100 U/ml
streptomycin, 1% glutamine (Gibco; Thermo Fisher Scientific, Inc.),
10 nmol/l dexamethasone (MilliporeSigma), 0.2 mmol/l ascorbic acid
(MilliporeSigma) and 10 mmol/l sodium β-glycerophosphate
(MilliporeSigma) in α-MEM (Gibco). MAG at 0, 200 and 500 µmol/l
(labeled as MAG0, MAG200, MAG500) were selected for hPDLSC
treatment in subsequent cell experiments.
Alkaline phosphatase (ALP) activity
assay
ALP activity in hPDLSCs was assessed using an ALP
activity assay kit (MilliporeSigma) and absorbance was measured at
a wavelength of 520 nm on a microplate reader. ALP staining was
performed using an ALP staining kit (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
protocol.
Alizarin red staining
Alizarin red staining was used to observe mineral
deposition in each group. Cultured cells were fixed in 95% ethanol
for 30 min and then stained with 0.1% Alizarin red staining
solution (pH 4.2) for 20 min at room temperature. After cells were
washed with PBS, 100 mmol/l cetylpyridinium chloride
(MilliporeSigma) was added to each well and incubated at room
temperature for 30 min. The absorbance was measured at a wavelength
of 562 nm on a microplate reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) detection of
osteogenesis-related genes
Total cellular RNA was extracted from hPDLSCs using
TRIzol (Invitrogen, Thermo Fisher Scientific, Inc.) and a total of
2 µg RNA/sample was reverse transcribed to complementary DNA (cDNA)
using a cDNA RT kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The expression of the
osteogenesis-related genes ALP, biomineralization associated
(ALPL), collagen type 1 (COL1) and runt-related transcription
factor 2 (RUNX2) was determined by RT-qPCR using TaqMan Gene
Expression Assay (Invitrogen, Thermo Fisher Scientific, Inc.), then
qPCR was performed with denaturation at 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 15
sec. The expression of the housekeeping gene GAPDH was used as an
internal reference. Data were analyzed using the 2−∆∆Cq
relative expression method (20).
The primer sequences are listed in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers used. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers used.
| Gene name | Sequence,
5′→3′ |
|---|
| GAPDH |
|
|
Forward |
CGGACCAATACGACCAAATCCG |
|
Reverse |
AGCCACATCGCTCAGACACC |
| ALPL |
|
|
Forward |
GACCTCCTCGGAAGACACTC |
|
Reverse |
TGAAGGGCTTCTTGTCTGTG |
| COL1 |
|
|
Forward |
GCTGATGATGCCAATGTGGTT |
|
Reverse |
CCAGTCAGAGTGGCACATCTTG |
| RUNX2 |
|
|
Forward |
GGAATGCCTCTGCTGTTATGAA |
|
Reverse |
GCTTCTGTCTGTGCCTTCTG |
Western blot analysis
Cells were harvested, washed and lysed in
immunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). The protein content was determined using the BCA
method. Proteins were separated by 12% (w/v) sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (20 µg protein per lane)
and blotted onto polyvinylidene fluoride membranes. After blocking
with Quick Blot Buffer (Applygen Technologies Inc.) at room
temperature for 30 min, the proteins were detected by overnight
incubation with rabbit polyclonal primary antibodies against RUNX2
(1:1,000, ab23981; Abcam), ALP (1:1,000, ab83295; Abcam), COL1
(1:1,000, ab233080, Abcam), HSP90 (1:1,000, ab13495; Abcam), TGF-β1
(1:1,000, ab92486; Abcam), p-SMAD2 (1:1,000, 3108T; Cell Signaling
Technology), SMAD2 (1:1,000, 5339T; Cell Signaling Technology),
SMAD3 (1:1,000, 9523T; Cell Signaling Technology) and β-actin
(1:1,000, ab8227; Abcam) at 4°C. After washing, the membranes were
incubated with horseradish peroxidase-conjugated goat-anti-rabbit
IgG secondary antibody (1:2,000, cat. no. ab6721; Abcam) for 1 h at
room temperature. Specific complexes were visualized using
SuperEnhanced chemiluminescence detection kit (Applygen
Technologies, Inc.). Band intensities were quantified using ImageJ
1.53 software (National Institutes of Health). The background was
subtracted, and the signal of each target band was normalized to
that of HSP90 or β-actin.
Galunisertib treatment of hPDLSCs
hPDLSCs were cultured for 5 days at 37°C in 4 groups
as follows: untreated control, 200 µM MAG, 100 nM galunisertib or
100 nM galunisertib + 200 µM MAG. After multiple washes with PBS,
hPDLSCs were harvested and subjected to western blot analysis and
alizarin red staining analysis.
Statistical analysis
Statistical analysis was performed using SPSS 10.2
(SPSS, Inc.). One-way ANOVA and Tukey's post hoc test was used to
assess differences between groups and P-values were calculated
using unpaired Student's t test. All data are presented as the mean
± standard deviation (SD). A 95% confidence level (P<0.05) was
considered to indicate a statistically significant difference. The
number of replicates in each experiment is indicated in the figure
legends.
Results
MAG has no effect on hPDLSC
viability
Five concentrations of MAG (0, 50, 100, 200 and 500
µM) were selected for the experiments. The effect of different
amounts of MAG on the viability of hPDLSCs was assessed using CCK-8
assays. The cell viability of hPDLSCs gradually increased from day
1 to 7, and different concentrations of MAG had no effect on the
cell viability of hPDLSCs on days 1, 3, 5 and 7 compared with that
of the MAG0 group (Fig. 1A).
However, MAG500 significantly reduced cell viability compared with
MAG50 and MAG10 at 7 days. Therefore, 0, 200 and 500 µM MAG were
chosen for subsequent experiments.
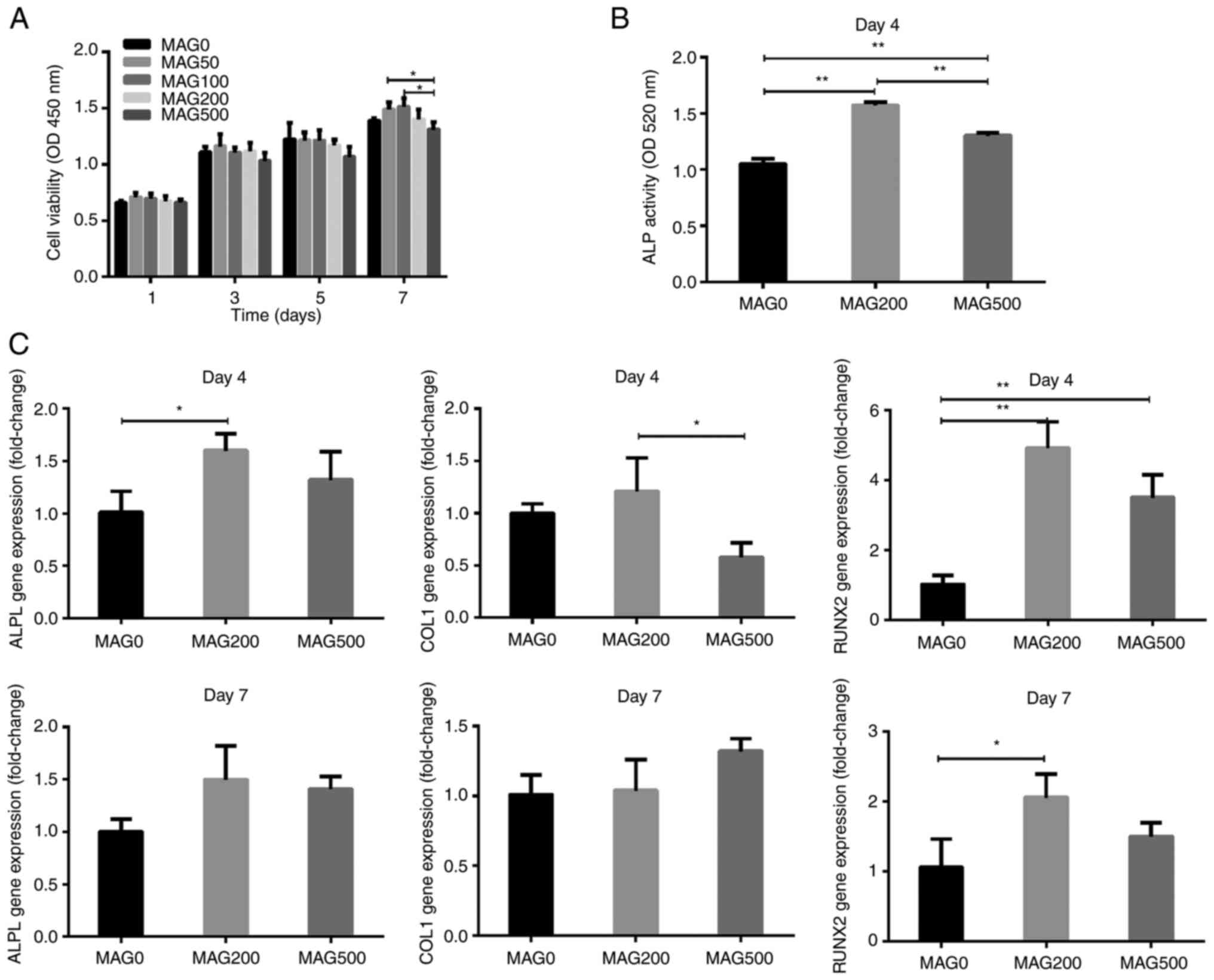 | Figure 1.Proliferation and osteogenic
differentiation of hPDLSCs following MAG treatment. (A)
Proliferation of hPDLSCs following MAG stimulation at different
concentrations (0, 50, 100, 200, 500 µmol/ml) was evaluated by a
Cell Counting Kit-8 assay on days 1, 3, 5 and 7. (B) ALP activity
of hPDLSCs treated with 0, 200, and 500 µmol/ml MAG on day 4. (C)
Effects of MAG on mRNA expression of osteogenic differentiation
markers. hPDLSCs were treated with MAG at concentrations of 0, 200
and 500 µmol/ml, and the mRNA levels of COL1, ALPL and RUNX2 were
determined via reverse transcription-quantitative PCR on days 4 and
7 post-MAG treatment. Data are presented as the mean ± SD (n=3).
*P<0.05, **P<0.01. ALPL, alkaline phosphate,
biomineralization associated; COL1, collagen type 1; RUNX2,
runt-related transcription factor 2; hPDLSC, human periodontal
ligament stem cells; OD, optical density; MAG, mangiferin. |
MAG promotes osteogenic
differentiation of hPDLSCs
ALP activity is important for bone mineralization
and is a useful biochemical marker of bone formation (21). Both MAG200 and MAG500
significantly increased the ALP activity of hPDLSCs on day 4 of
osteogenic induction (Fig. 1B).
The ALP activity of the MAG200 group was the highest and was ~1.5
times that of the MAG0 group. The ALP activity of the MAG500 group
was ~1.2 times that of the MAG0 group.
Next, the expression of osteogenic genes, including
ALPL, COL1, and RUNX2, at 4 and 7 days was analyzed. MAG200
significantly increased the expression of ALPL on the 4th day
(Fig. 1C). On both the 4 and 7th
days, the expression of RUNX2 was significantly different from that
of the MAG0 group. On day 7, neither MAG200 nor MAG500 had a
significant effect on the expression of ALPL and COL1.
The protein levels of the osteogenesis-related genes
COL1, RUNX2, SMAD5, ALP and bone morphogenetic protein 2 (BMP2)
were semiquantified using western blotting (Fig. 2A and B). The SMAD5, ALP, and BMP2
protein levels in hPDLSCs in the MAG200 group were significantly
upregulated after 7 days compared with the MAG0 group.
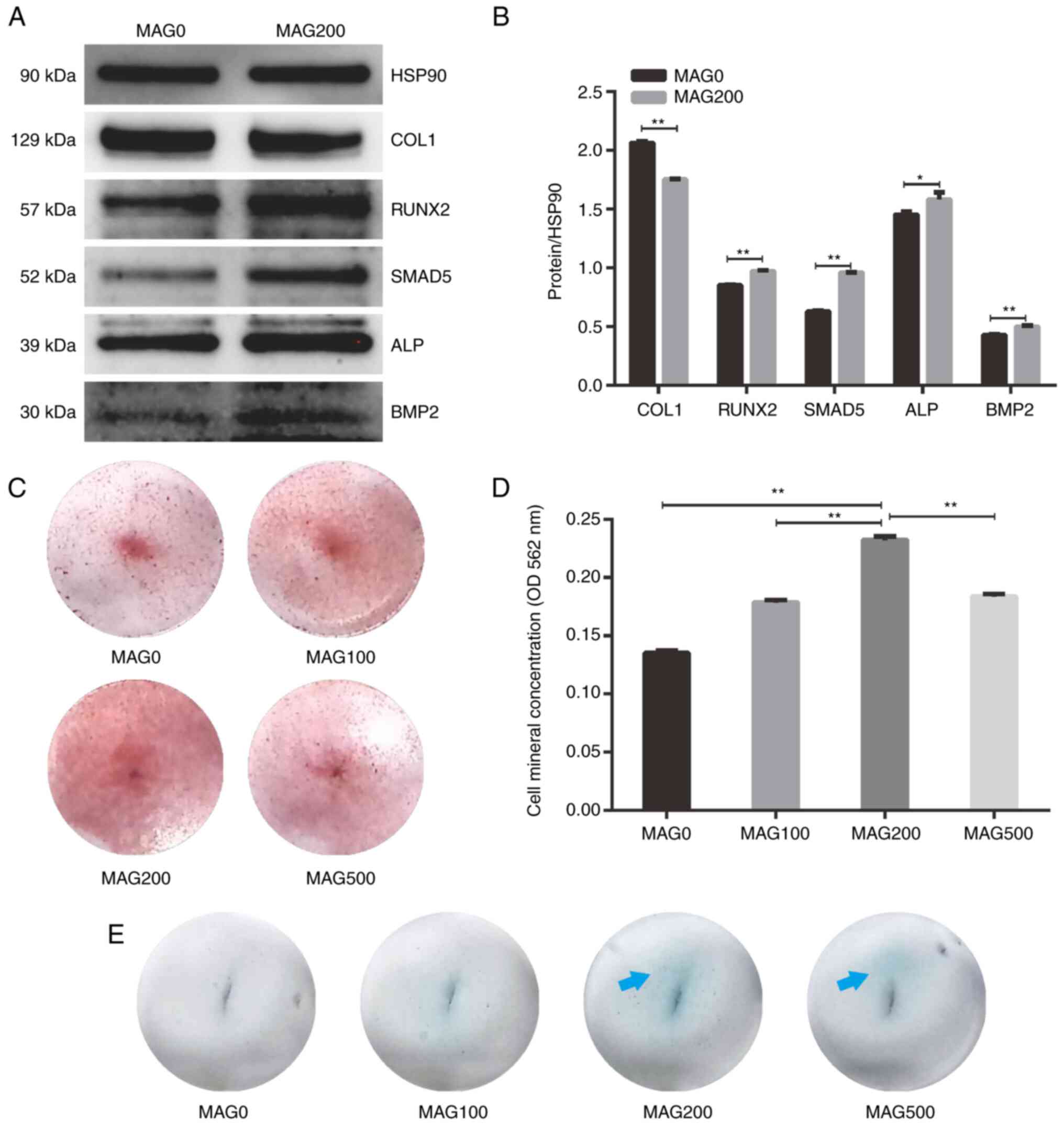 | Figure 2.Western blot analysis and Alizarin
red staining of hPDLSCs following MAG treatment. (A) Western blot
analysis of hPDLSCs treated with 0 and 200 µM MAG on day 7. (B)
Semi-quantitative analysis of protein expression. (C)
Representative Alizarin red staining images following MAG
stimulation at different concentrations (0, 100, 200, 500 µmol/ml)
in hPDLSCs at 1× magnification. (D) Concentration of
Alizarin-stained mineral deposits. (E) Representative alkaline
phosphatase staining images following MAG stimulation at different
concentrations (0, 100, 200, 500 µmol/ml) in hPDLSCs at
1×magnification. Blue arrows indicate positive staining for ALP.
Data are presented as the mean ± SD (n=4). *P<0.05, **P<0.01.
hPDLSCs, human periodontal ligament stem cells; OD, optical
density; MAG, mangiferin; ALP, alkaline phosphate; COL1, collagen
type 1; RUNX2, runt-related transcription factor 2; BMP2, bone
morphogenetic protein 2; HSP90, heat shock protein 90. |
In addition to proteins associated with bone
formation, mineralization is another marker for assessing
osteogenesis (22). Calcium
deposition was assessed to study the mineralization of cultured
hPDLSCs in different groups. MAG treatment increased calcium
nodules compared with the MAG0 group (Fig. 2C and D). However, the formation of
mineralized nodules was not proportional to the concentration of
MAG, with MAG200 resulting in the most mineralized nodules. As a
confirmation of osteogenic induction, ALP staining performed after
4 days of culture showed positive staining for all of the
MAG-containing groups (Fig. 2E).
In particular, ALP activity was significantly upregulated in the
MAG200 group compared with that in the MAG0 group. This was in
accordance with the aforementioned ALP activity results. These
results suggest that MAG served an important role in promoting
mineralization and has a strong ability to induce osteogenic
differentiation of hPDLSCs.
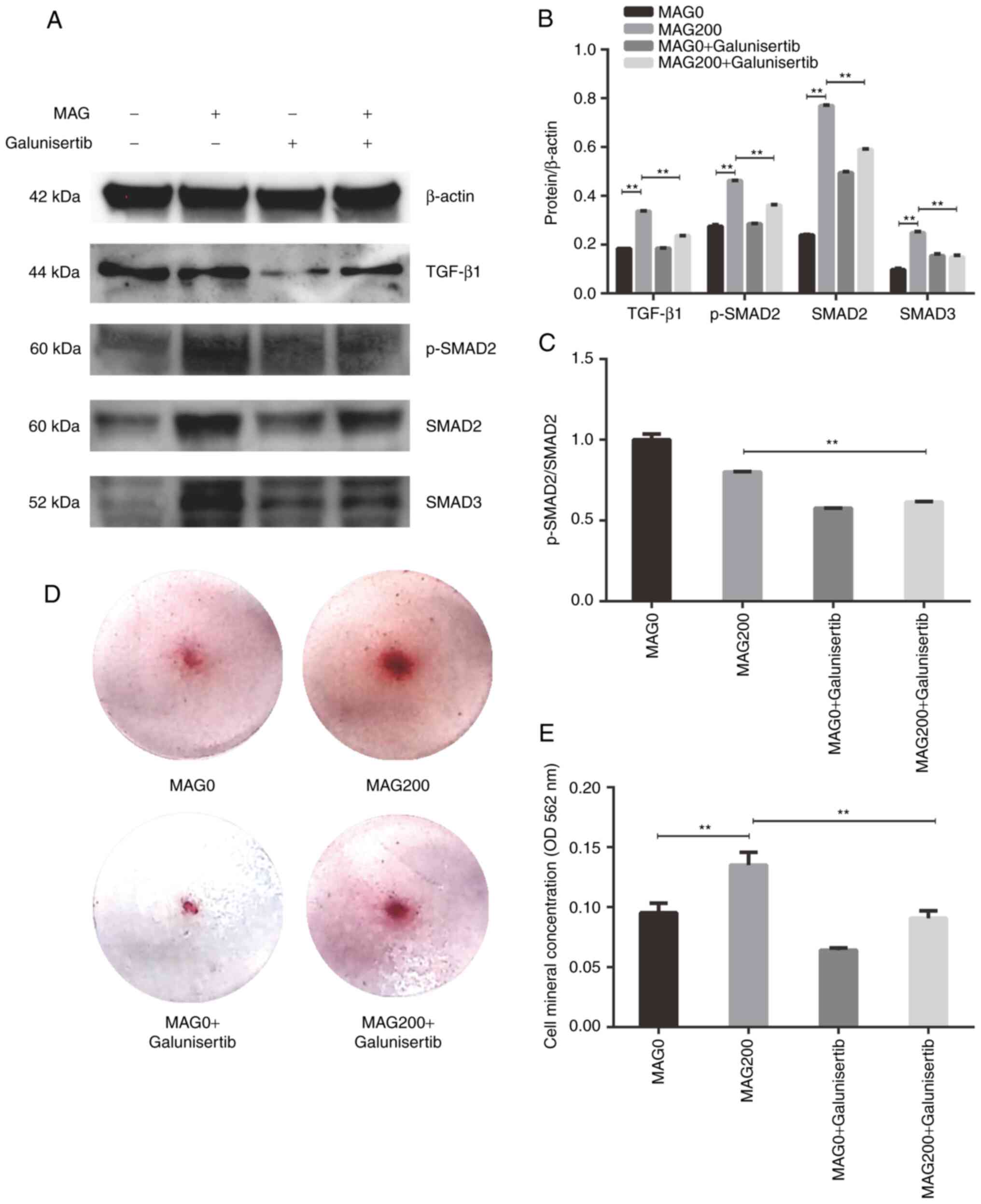
MAG promotes osteogenesis via the
TGF-β/SMAD2 signaling pathway
To analyze the mechanism by which MAG promotes
osteogenic differentiation, four key proteins of the TGF-β/SMAD2
signaling pathway, TGF-β, p-SMAD2, SMAD2 and SMAD3 were analyzed by
western blotting. Galunisertib is a small molecule inhibitor of
TGF-β receptor I kinase (23).
The expression of TGF-β1 in the MAG200 group in the presence of
galunisertib was significantly lower compared with the MAG200
group, indicating that galunisertib successfully inhibited
expression of TGF-β1 (Fig. 3A and
B). Western blotting showed that the expression levels of
p-SMAD2, SMAD2 and SMAD3 were significantly upregulated in the
MAG200 group after hPDLSCs were cultured for 7 days. Addition of
the pathway inhibitor galunisertib partially reversed the
upregulation of protein expression caused by MAG (Fig. 3A and B). The ratio of
p-SMAD2/SMAD2 in the MAG200 + galunisertib group was significantly
lower than the MAG200 group, indicating that galunisertib decreases
SMAD2 phosphorylation (Fig. 3C).
Alizarin red staining and calcium deposits quantification also
demonstrated that the addition of galunisertib could partially
inhibit the effect of MAG in promoting the osteogenic
differentiation of hPDLSCs (Fig. 3D
and E). Collectively, these data indicated that MAG activates
the TGF-β/SMAD2 signaling pathway during osteogenic
differentiation.
Discussion
Periodontal tissue is a complex group of tissues
consisting of gingiva, periodontal ligament, cementum, and alveolar
bone (24,25). Given this complexity, regeneration
of lost or damage periodontal tissue due to periodontitis remains a
challenge for current treatments (26–28). PDLSCs can generate structures
similar to the cementum/periodontal ligament in vivo,
suggesting the important role of PDLSCs in periodontal regeneration
(29). PDLSCs exhibit pluripotent
differentiation and are good candidates for tissue engineering due
to their ability to promote regeneration of dental and nondental
tissues (30).
A number of previous studies have shown that MAG
inhibits the inflammatory response (31–33). Studies have shown that MAG has a
notable inhibitory effect on intracellular adhesion molecule and
endothelial leukocyte adhesion molecule expression, which are
required for the transportation of leukocytes during inflammation
(34–36). Orally administered MAG (50 mg/kg
body weight, once daily) was used to treat periodontitis in mice
for 8 weeks; MAG was found to markedly inhibit alveolar bone loss,
TNF-α production and NF-κB in gingival epithelial cells and
phosphorylation of the JAK1-STAT1/3 pathway (37). Therefore, MAG has good therapeutic
potential for prevention and treatment of periodontitis.
Researchers have found that MAG promotes bone tissue
regeneration (38–42). Sekiguchi et al (39) showed that MAG can promote
osteoblastic bone formation by promoting cell proliferation and
inducing cell differentiation through RUNX2 in pre-osteoblast
MC3T3-E1 cells. Demeyer et al (40) prepared MAG-loaded chitosan-silica
hybrid nanocomposite scaffolds using sol gel synthesis and
freeze-drying processes; investigation of biomineralization and
cell viability showed that the addition of bioactive MAG further
promoted the effects of hybrid nanocomposite scaffolds in guided
bone regeneration applications. Li et al (41) used a freeze-drying technique to
prepare MAG-loaded poly(D, L-lactide-co-glycolide) scaffolds. The
scaffolds were then implanted into the alveolar bone defect of
diabetic rats and bone repair was examined using hematoxylin and
eosin staining. Under diabetic conditions in vitro, the
MAG-loaded scaffolds increased the histological score of bone
regeneration and improved delayed alveolar bone defect healing in
diabetic rats. Huh et al (42) isolated mesenchymal stem cells
(MSCs) from the subchondral bone of rabbits and treated them with
MAG and/or IL-1β. MAG induced chondrogenic differentiation of MSCs
by upregulating the expression of several key chondrogenic markers,
including TGF-β, BMP-2, and BMP-4. The experimental results of the
present study are similar to those of the aforementioned
experiments. In the present study, hPDLSCs were used as the
research object, and MAG was found to promote the osteogenic
differentiation of hPDLSCs. This result indicated that MAG may
promote the regeneration of periodontal tissue by promoting the
osteogenic differentiation of stem cells and may be a potential
drug for periodontal treatment.
Osteoblast differentiation is critical for bone
formation and involves the TGF-β/SMAD signaling pathway in bone
morphogenesis (43). SMAD
proteins are intermediate molecules that transmit the signal
generated by the binding of TGF-β to its receptor from the
cytoplasm to the nucleus, thereby playing an important role in
signal transmission and regulation of transcription of downstream
target genes (44). During signal
transduction in the TGF-β/SMAD pathway, TGF-β first binds to its
type II receptor and activates its type I receptor. Activated type
I receptors promote the phosphorylation of SMAD2 or SMAD3 at the
C-terminus, and these molecules bind with SMAD4 and translocate
into the nucleus, thereby affecting osteoblast proliferation and
differentiation (45–47). Therefore, compounds or drugs that
activate the SMAD signaling pathway through the TGF-β or BMP
pathways can modulate osteoporosis (48).
Previous studies have shown that SMAD2/3 serves an
important role in the regulation of bone formation (49) and the expression of SMAD2/3 and
phosphorylated (p)-SMAD2/3 was found to be elevated during
cementoblast differentiation and mineralization (50). Typically, activation of the
TGF-β/SMAD signaling pathway mediates the phosphorylation of
SMAD2/3, which dimerizes with SMAD4 and translocates to the
nucleus, leading to transcription of downstream genes to direct
cell differentiation (51). In
the present study, western blot analysis showed that the addition
of MAG could upregulate the expression of p-SMAD2, SMAD2, and SMAD3
in hPDLSCs, which revealed that MAG could activate the TGF-β/SMAD2
signaling pathway.
In the TGF-β/SMAD2 signaling pathway, serine in
SMAD2 is directly phosphorylated by the TGF-β1 receptor, resulting
in SMAD2 activation. Galunisertib (LY2157299 monohydrate) is a
small molecule inhibitor of TGF-β receptor I kinase that
specifically decreases SMAD2 phosphorylation and eliminates
activation of the classic pathway (52). In the present study, addition of
galunisertib partially reversed the MAG-mediated upregulation of
the protein expression of p-SMAD2 and SMAD2. Alizarin red staining
also indicated that galunisertib treatment could partially inhibit
the effect of MAG in promoting the osteogenic differentiation of
hPDLSCs. Collectively, these data indicated that MAG promotes the
osteogenic differentiation of hPLDSCs by activating the TGF-β/SMAD2
signaling pathway.
In conclusion, MAG can promote osteogenic
differentiation of hPDLSCs, and the TGF-β/SMAD2 signaling pathway
was involved in this process. In addition, given the potential of
MAG in antibacterial treatment and treatment of inflammation,
studying the regulatory effect of MAG on bone regeneration has
implications for the clinical treatment of periodontal disease. MAG
may be an effective drug for preventing periodontitis and promoting
periodontal bone regeneration.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82071144), National Key R&D
Program of China (grant no. YFC1104304) and Young Scientist Program
of Beijing Stomatological Hospital Capital Medical University
(grant no. YSP202001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and YB conceived the original idea and performed
experiments. YG and LZ confirm the authenticity of all the raw
data. LZ analyzed the data. YG, LZ and YB wrote the manuscript. YB
supervised the project and give final approval of the version to be
published. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The first or second premolars that were removed from
healthy individuals due to orthodontic needs were collected at the
Beijing Stomatological Hospital (Beijing, China) after obtaining
patient verbal consent with approval of the Ethics Committee of
Capital Medical University (approval no. KJ-2021-016-C-01-CS).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Highfield J: Diagnosis and classification
of periodontal disease. Aust Dent J. 54 (Suppl 1):S11–S26. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Dyke TE: The management of
inflammation in periodontal disease. J Periodontol. 79:1601–1608.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
John V, Alqallaf H and De Bedout T:
Periodontal disease and systemic diseases: An update for the
clinician. J Indiana Dent Assoc. 95:16–23. 2016.PubMed/NCBI
|
|
4
|
Liu N, Gu B, Liu N, Nie X, Zhang B, Zhou X
and Deng M: Wnt/β-catenin pathway regulates cementogenic
differentiation of adipose tissue-deprived stem cells in dental
follicle cell-conditioned medium. PLoS One. 9:e933642014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu L, Liu Y and Wang S: Stem cell-based
tooth and periodontal regeneration. Oral Dis. 24:696–705. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouchi T and Nakagawa T: Mesenchymal stem
cell-based tissue regeneration therapies for periodontitis. Regen
Ther. 14:72–78. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Zheng Y, Ding G, Fang D, Zhang C,
Bartold PM, Gronthos S, Shi S and Wang S: Periodontal ligament stem
cell-mediated treatment for periodontitis in miniature swine. Stem
Cells. 26:1065–1073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding G, Liu Y, Wang W, Wei F, Liu D, Fan
Z, An Y, Zhang C and Wang S: Allogeneic periodontal ligament stem
cell therapy for periodontitis in swine. Stem Cells. 28:1829–1838.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Xing Y, Jia L, Ji Y, Zhao B, Wen
Y and Xu X: An in vitro comparative study of multisource derived
human mesenchymal stem cells for bone tissue engineering. Stem
Cells Dev. 27:1634–1645. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scalbert A, Johnson IT and Saltmarsh M:
Polyphenols: Antioxidants and beyond. Am J Clin Nutr. 81 (1
Suppl):215S–217S. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mann S, Sarkar A, Sharma A, Gupta RK and
Biswas S: Antitumor activity of choerospondias axillaris fruit
extract by regulating the expression of SNCAIP and SNCA on
MDA-MB-231 cells. Asian Pac J Cancer Prev. 23:1577–1586. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imran M, Arshad MS, Butt MS, Kwon JH,
Arshad MU and Sultan MT: Mangiferin: A natural miracle bioactive
compound against lifestyle related disorders. Lipids Health Dis.
16:842017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang M, Liang Y, Chen K, Wang M, Long X,
Liu H, Sun Y and He B: The management of diabetes mellitus by
mangiferin: Advances and prospects. Nanoscale. 14:2119–2135. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walia V, Chaudhary SK and Kumar Sethiya N:
Therapeutic potential of mangiferin in the treatment of various
neuropsychiatric and neurodegenerative disorders. Neurochem Int.
143:1049392021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morozkina SN, Nhung Vu TH, Generalova YE,
Snetkov PP and Uspenskaya MV: Mangiferin as new potential
anti-cancer agent and mangiferin-integrated polymer systems-a novel
research direction. Biomolecules. 11:792021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren K, Li H, Zhou HF, Liang Y, Tong M,
Chen L, Zheng XL and Zhao GJ: Mangiferin promotes macrophage
cholesterol efflux and protects against atherosclerosis by
augmenting the expression of ABCA1 and ABCG1. Aging (Albany NY).
11:10992–11009. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu P, Liu C, Li B, Zhao C, Zhou T, Xue X
and Zhang B: Mangiferin attenuates IL-1β-induced chondrocytes
apoptosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 46:25–31. 2021.(In
English, Chinese). PubMed/NCBI
|
|
19
|
Ang E, Liu Q, Qi M, Liu HG, Yang X, Chen
H, Zheng MH and Xu J: Mangiferin attenuates osteoclastogenesis,
bone resorption, and RANKL-induced activation of NF-κB and ERK. J
Cell Biochem. 112:89–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rastogi D, Gautam N, Ara Z, Waliullah S
and Srivastava RN: Prevalence of abnormal bone-specific alkaline
phosphatase in orthopaedic trauma patients: A cross-sectional study
from a tertiary trauma centre. Cureus. 14:e242642022.PubMed/NCBI
|
|
22
|
Kato K, Ozaki M, Nakai K, Nagasaki M,
Nakajima J, Koshi R, Tanaka H, Kawato T and Tonogi M: Effect of
azithromycin on mineralized nodule formation in MC3T3-E1 cells.
Curr Issues Mol Biol. 43:1451–1459. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hira SK, Rej A, Paladhi A, Singh R, Saha
J, Mondal I, Bhattacharyya S and Manna PP: Galunisertib drives treg
fragility and promotes dendritic cell-mediated immunity against
experimental lymphoma. iScience. 23:1016232020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hassell TM: Tissues and cells of the
periodontium. Periodontol. 2000.3:9–38. 1993. View Article : Google Scholar
|
|
25
|
Cho MI and Garant PR: Development and
general structure of the periodontium. Periodontol. 2000.24:9–27.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rasperini G, Tavelli L, Barootchi S,
McGuire MK, Zucchelli G, Pagni G, Stefanini M, Wang HL and
Giannobile WV: Interproximal attachment gain: The challenge of
periodontal regeneration. J Periodontol. 92:931–946. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Jong T, Bakker AD, Everts V and Smit
TH: The intricate anatomy of the periodontal ligament and its
development: Lessons for periodontal regeneration. J Periodontal
Res. 52:965–974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hughes FJ, Ghuman M and Talal A:
Periodontal regeneration: A challenge for the tissue engineer? Proc
Inst Mech Eng H. 224:1345–1358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomokiyo A, Wada N and Maeda H:
Periodontal ligament stem cells: Regenerative potency in
periodontium. Stem Cells Dev. 28:974–985. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Liu A, Zhang L, Wang Z, Hui N, Zhai
Q, Zhang L, Jin Z and Jin F: Epithelial cell rests of malassez
provide a favorable microenvironment for ameliorating the impaired
osteogenic potential of human periodontal ligament stem cells.
Front Physiol. 12:7352342021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Yuwen T and Yanqin T: Mangiferin
inhibits inflammation and cell proliferation, and activates
proapoptotic events via NF-κB inhibition in DMBA-induced mammary
carcinogenesis in rats. J Environ Pathol Toxicol Oncol. 40:1–9.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong M, Li L, Li G, Song J, Liu B, Liu X
and Wang M: Mangiferin protects against alcoholic liver injury via
suppression of inflammation-induced adipose hyperlipolysis. Food
Funct. 11:8837–8851. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bulugonda RK, Kumar KA, Gangappa D, Beeda
H, Philip GH, Muralidhara Rao D and Faisal SM: Mangiferin from
Pueraria tuberosa reduces inflammation via inactivation of NLRP3
inflammasome. Sci Rep. 7:426832017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beltrán Núñez AE, Naranjo NL, Penabad CR,
Sironi M, Rodríguez GQ, Garrido GG and Hernández RD:
VIMANG® y mangiferina inhiben la expresión de ICAM-1 en
células endoteliales estimuladas con citocinas proinflamatorias.
Rev Cubana Invest Biomed. 22:164–172. 2003.
|
|
35
|
Yang H, Bai W, Gao L, Jiang J, Tang Y, Niu
Y, Lin H and Li L: Mangiferin alleviates hypertension induced by
hyperuricemia via increasing nitric oxide releases. J Pharmacol
Sci. 137:154–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dou W, Zhang J, Ren G, Ding L, Sun A, Deng
C, Wu X, Wei X, Mani S and Wang Z: Mangiferin attenuates the
symptoms of dextran sulfate sodium-induced colitis in mice via
NF-κB and MAPK signaling inactivation. Int Immunopharmacol.
23:170–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Wang Q, Ding Y, Bao C and Li W:
Mangiferin ameliorates porphyromonas gingivalis-induced
experimental periodontitis by inhibiting phosphorylation of nuclear
factor-κB and Janus kinase 1-signal transducer and activator of
transcription signaling pathways. J Periodontal Res. 52:1–7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bai Y, Liu C, Fu L, Gong X, Dou C, Cao Z,
Quan H, Li J, Kang F, Dai J, et al: Mangiferin enhances
endochondral ossification-based bone repair in massive bone defect
by inducing autophagy through activating AMP-activated protein
kinase signaling pathway. FASEB J. 32:4573–4584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sekiguchi Y, Mano H, Nakatani S, Shimizu
J, Kataoka A, Ogura K, Kimira Y, Ebata M and Wada M: Mangiferin
positively regulates osteoblast differentiation and suppresses
osteoclast differentiation. Mol Med Rep. 16:1328–1332. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Demeyer S, Athipornchai A, Pabunrueang P
and Trakulsujaritchok T: Development of mangiferin loaded
chitosan-silica hybrid scaffolds: Physicochemical and bioactivity
characterization. Carbohydr Polym. 261:1179052021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Liao H, Bao C, Xiao Y and Wang Q:
Preparation and evaluations of mangiferin-loaded PLGA scaffolds for
alveolar bone repair treatment under the diabetic condition. AAPS
PharmSciTech. 18:529–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huh JE, Koh PS, Seo BK, Park YC, Baek YH,
Lee JD and Park DS: Mangiferin reduces the inhibition of
chondrogenic differentiation by IL-1β in mesenchymal stem cells
from subchondral bone and targets multiple aspects of the Smad and
SOX9 pathways. Int J Mol Sci. 15:16025–16042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu J, Xu L, Li K, Xie N, Xi Y, Wang Y,
Zheng X, Chen X, Wang M and Ye X: Zinc-modified calcium silicate
coatings promote osteogenic differentiation through TGF-β/Smad
pathway and osseointegration in osteopenic rabbits. Sci Rep.
7:34402017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luo K: Signaling CROSS TALK BETWeen
TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect
Biol. 9:a0221372017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Runyan CE, Liu Z and Schnaper HW:
Phosphatidylinositol 3-kinase and Rab5 GTPase inversely regulate
the Smad anchor for receptor activation (SARA) protein
independently of transforming growth factor-β1. J Biol Chem.
287:35815–35824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ota K, Quint P, Ruan M, Pederson L,
Westendorf JJ, Khosla S and Oursler MJ: TGF-β induces Wnt10b in
osteoclasts from female mice to enhance coupling to osteoblasts.
Endocrinology. 154:3745–3752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li XL, Liu YB, Ma EG, Shen WX, Li H and
Zhang YN: Synergistic effect of BMP9 and TGF-β in the proliferation
and differentiation of osteoblasts. Genet Mol Res. 14:7605–7615.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang W, Rigueur D and Lyons KM: TGFβ as a
gatekeeper of BMP action in the developing growth plate. Bone.
137:1154392020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Heo SY, Ko SC, Nam SY, Oh J, Kim YM, Kim
JI and Jung WK: Fish bone peptide promotes osteogenic
differentiation of MC3T3-E1 pre-osteoblasts through upregulation of
MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem
Funct. 36:137–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li L, Zhu Z, Xiao W and Li L: Multi-walled
carbon nanotubes promote cementoblast differentiation and
mineralization through the TGF-β/Smad signaling pathway. Int J Mol
Sci. 16:3188–3201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Whitman M: Smads and early developmental
signaling by the TGFbeta superfamily. Genes Dev. 12:2445–2462.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Herbertz S, Sawyer JS, Stauber AJ,
Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba
SC, Benhadji KA, et al: Clinical development of galunisertib
(LY2157299 monohydrate), a small molecule inhibitor of transforming
growth factor-beta signaling pathway. Drug Des Devel Ther.
9:4479–4499. 2015.PubMed/NCBI
|