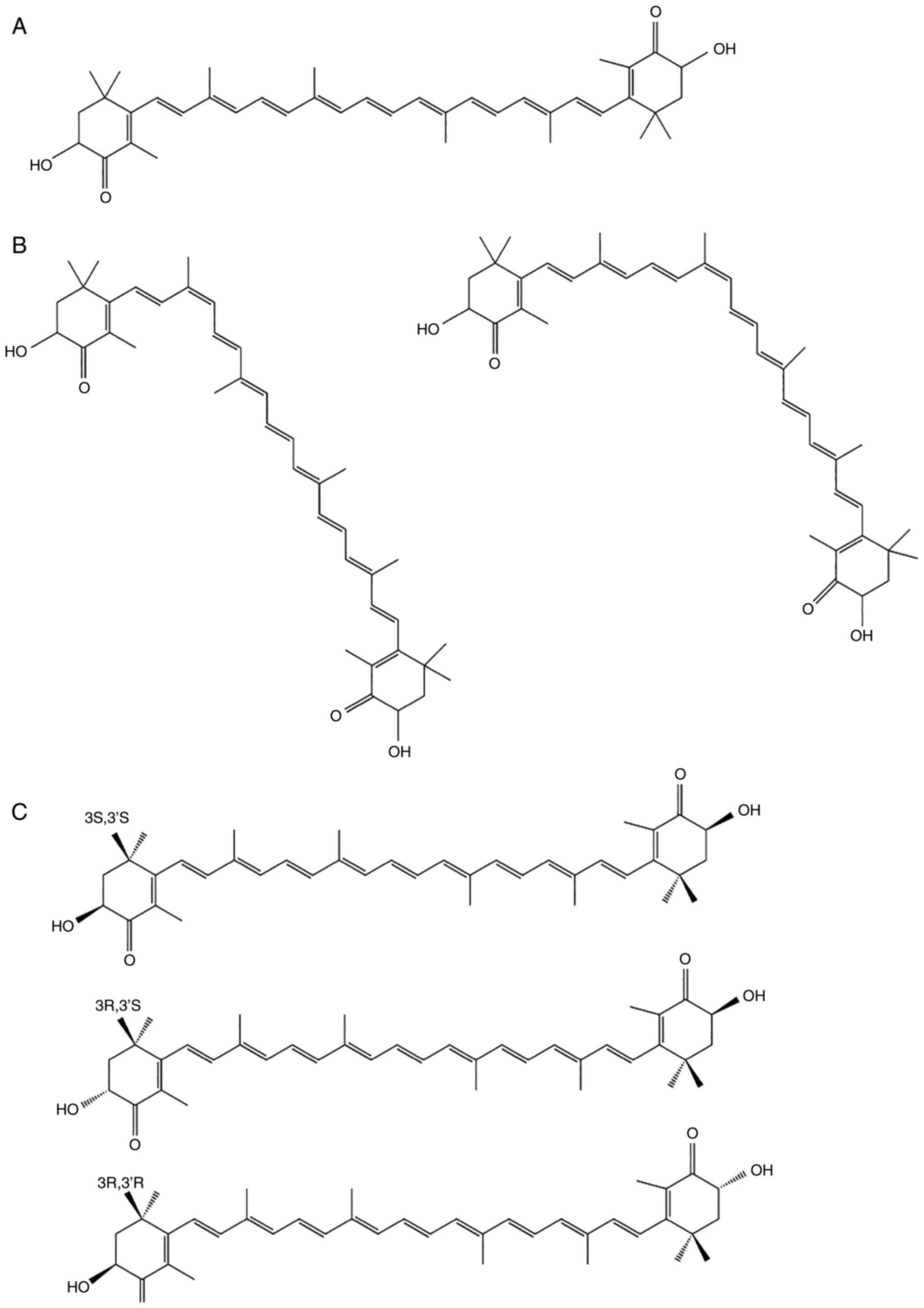
AST has a molecular structure similar to those of
β-carotene and other carotenoids (4). However, the oxygen groups in its
molecular structure distinguish AST from other carotenoid subtypes
(5). AST has a polar region at
each end of the molecule's ionone rings that neutralizes free
radicals. In contrast to the 11 carbon-carbon double
polyunsaturated bonds in β-carotene, the central nonpolar zone of
AST is made up of 13 bonds, which allow AST to remove high-energy
electrons (6). The hydroxyl and
ketone moieties on both rings increase the polarity of AST and
greatly enhance its capacity to cross the cell membrane (7,8).
These unique chemical properties bestow AST with some
bioactivity-related advantages, including a higher antioxidant
effect than other carotenoids (9). Owing to its carbon-carbon double
polyunsaturated bonds, AST has two isomeric forms; trans and cis
(Fig. 2A) (10). The cis isomer, cis-AST, includes
9-cis and 13-cis configurations (Fig.
2B). Due to the two stereogenic carbon atoms at the C-3 and
C-3′ positions, all-trans-AST has three stereoisomers: (3S, 3′S),
(3R, 3′R) and (3R, 3′S) (Fig.
2C). The structure of all-trans-AST is more stable than that of
cis-AST, indicating that all-trans-AST is the predominant form of
AST in nature (11). In addition,
3S, 3-S-AST is a more powerful antioxidant than the other
stereoisomers (12). Owing to the
stability of all-trans-AST, it has been used as an experimental
material in several studies. Therefore, the aim of the present
review was to explore the biological activities and neurological
functions of all-trans-AST.
AST is extracted from microorganisms; phytoplankton;
bacteria; yeast; and marine animals, such as shrimps, lobster,
asteroidean, algae, crustaceans, trout, krill, red sea bream and
salmon (13,14). In nature, AST is initially
synthesized by microalgae and phytoplankton, accumulating in
zooplankton and crustaceans and reaching higher level marine
animals through the food chain (14). Haematococcus pluvialis
(H. pluvialis) produces the largest quantity of natural AST
(15). However, large-scale
cultivation of H. pluvialis is considered costly (16); thus, synthetic production is
currently the predominant source of AST. However, synthetic AST
shows only 50% of the biological activity of natural AST (17).
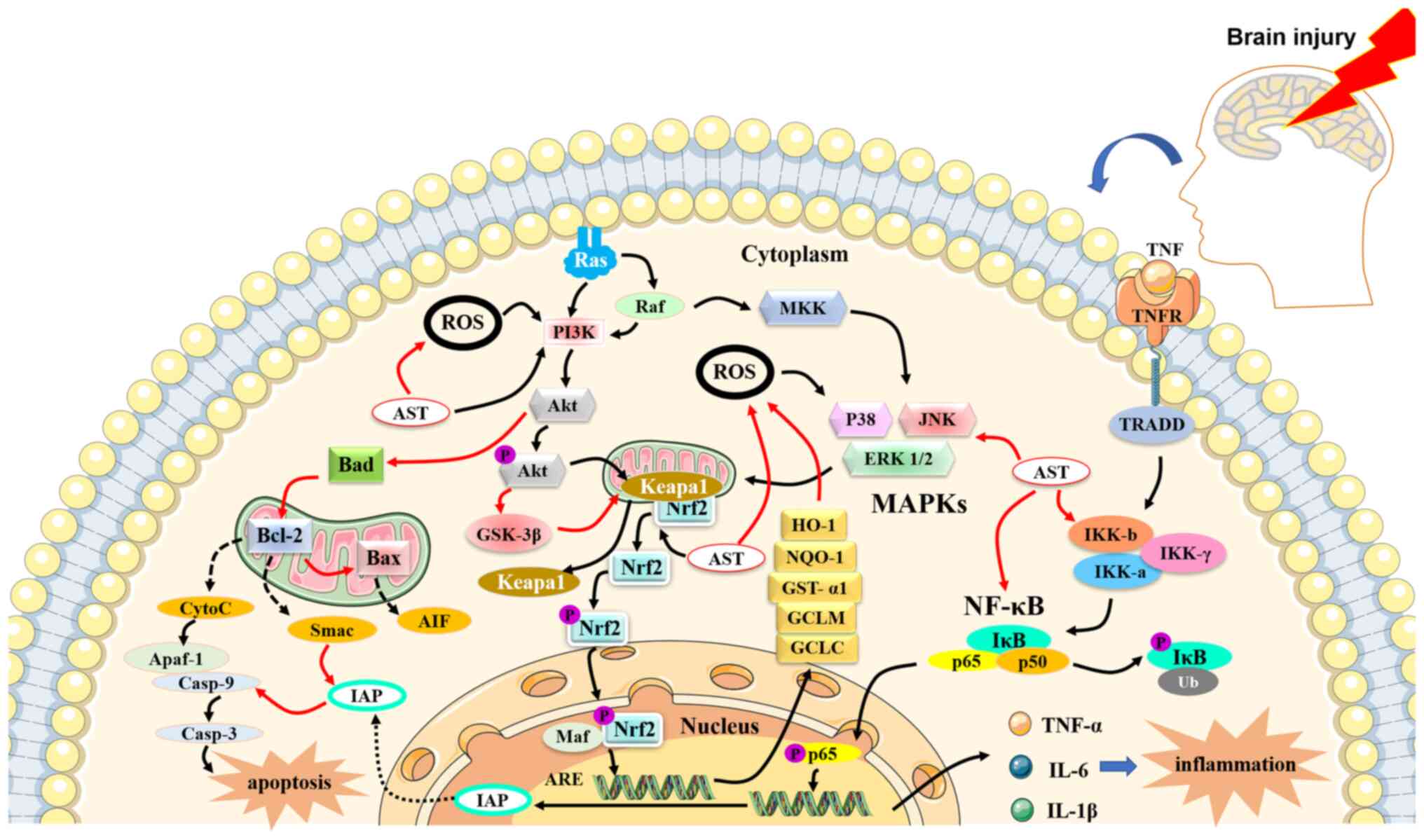
Previous studies have shown that AST can mediate the
processes of various diseases through antioxidant,
anti-inflammatory and anti-apoptotic activities (Fig. 3). In a study of a diabetic
retinopathy model, AST treatment increased the levels of the
antioxidant enzyme heme oxygenase-1 (HO-1) and maintained the
homeostasis of retinal ganglion cells (18). Yoshihisa et al (19) found that AST can protect
keratinocytes from ultraviolet-related damage by decreasing the
expression of oxidative factors [inducible nitric oxide, (iNOS)]
and the inflammatory factors IL-1β and TNF-α. In addition, a
clinical trial demonstrated that oral AST protects the skin from
ultraviolet injury (20).
Furthermore, AST maintains the homeostasis of lipid metabolism
(21) and controls the courses of
cardiovascular diseases and cancer by regulating apoptosis factors
and cell proliferation (22,23).
Previous studies have identified the health benefits
of AST against neurological disorders, including Alzheimer's
disease (AD), Parkinson's disease (PD), Huntington's disease (HD),
amyotrophic lateral sclerosis (ALS), cerebral ischemia/reperfusion
(IR), subarachnoid hemorrhage (SAH) and cognitive disorders
(24,25). Therefore, the present review
focused on the biological activities and neurological functions of
AST.
AST activates the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) pathway (33) and the mitogen-activated protein
kinase (MAPK)/extracellular signal-regulated protein kinase (ERK)
pathway. The two pathways facilitate the dissociation of nuclear
factor erythroid 2-related factor 2 (Nrf2) from Kelch-like
ECH-associated protein 1. Nrf2 is translocated to the nucleus and
activates the Nrf2 antioxidant response element (ARE) signaling
pathway (33). The PI3K/Akt
pathway upregulates the expression of HO-1, NAD(P)H quinone
oxidoreductase-1 (NQO-1), glutathione-S-transferase-α1, the
glutamate-cysteine ligase modifier subunit and the
glutamate-cysteine ligase catalytic subunit, which provide
protection against OS both in vitro and in vivo
(24,34–36). In addition, it has been reported
that rats fed AST show elevated levels of other antioxidant
enzymes, such as superoxide dismutase (SOD), catalase (CAT)
(37,38), thiobarbituric acid reactive
substances and peroxidase, in the liver and plasma (24,39).
Inflammation is a complex host defense response to
infection, injury, ischemia, toxins and radiation. Inflammation
also facilitates the tissue repair process through the actions of
immune cells and inflammatory mediators. However, excessive or
inappropriate inflammatory activity is deleterious to the host and
can cause or aggravate numerous diseases (40). The NF-κB signaling pathway is an
important and ubiquitous nuclear transcription pathway that serves
important roles in inflammatory and immune responses (41). Excessive activation of the NF-κB
signaling pathway is related to inflammatory changes in rheumatoid
arthritis and heart and brain diseases. In unstimulated conditions,
NF-κB (p50-p65) remains inactive in the cytoplasm and interacts
with the inhibitory (IκB) family (IκB-α) (42). Under stimulation by extracellular
agents, NF-κB is activated through dissociation of IκB, which is
phosphorylated by the IκB kinase complex (IKK, including IKKα and
IKKβ). Dissociated NF-κB enters the nucleus and binds to κB
regulatory elements that produce the pro-inflammatory cytokines
IL-1β, IL-6 and TNF-α (41,43). Therefore, blocking the NF-κB
signaling pathway is important for the mediation of inflammatory
diseases. A recent study showed that AST can block excessive NF-κB
signaling by downregulating the phosphorylation of IκB-α or
increasing the cellular expression of IκB-α mRNA and protein
(44). AST also exhibits
anti-inflammatory effects by inhibiting cyclooxygenase-1 and nitric
oxide in lipopolysaccharide-stimulated BV2 microglial cell
biogenesis through the regulation of multiple genes (39).
Apoptosis, a process of cell suicide, is a common
mechanism in living systems. This process is vital for tissue
development, maintenance of homeostasis and defense against a
variety of extracellular and intracellular insults and mutations
(45). However, excessive
apoptosis disrupts homeostasis and leads to numerous diseases. The
apoptotic pathway is regulated by the Bcl-2 family, including the
pro-apoptotic cytokines Bad and Bax and the anti-apoptotic
cytokines Bcl-2 and Bcl-xL (46,47). Under apoptotic stimulation, Bad
and Bax promote the release of cytochrome c (Cyt c) from the
mitochondria into the cytoplasm. A complex comprising Cyt c,
apoptotic protease activator-1 and caspase-9 then activates
caspase-3, which triggers apoptosis. Bcl-2 and Bcl-xL inhibit the
release of Cyt c and induce apoptosis (48). In addition, the PI3K/Akt pathway
inhibits Bad and Bax and the JAK/STAT pathway or the SCR/STAT
pathway promotes the expression of Bcl-2 and Bcl-xL, contributing
to anti-apoptosis.
The central nervous system (CNS) is one of the most
important systems in the human body and it contains billions of
neuronal and glial cells. The blood-brain barrier (BBB) is a
selectively permeable barrier between capillaries and the brain
that isolates the CNS from other systems of the body. This barrier
is crucial for maintaining brain homeostasis and protecting the
neuronal environment from harmful materials (54). However, the BBB occasionally
prevents the transportation of therapeutic agents to the CNS for
the treatment of neurological disorders. As mentioned previously,
AST is a lipid-soluble pigment that can cross the BBB, a feature
that is crucial for the treatment of neurological diseases. Manabe
et al (55) found that AST
accumulates in the hippocampi and cerebral cortexes of rat brains
after single and repeated dietary ingestion. The accumulation of
AST in the cerebral cortex may maintain and improve cognitive
function. Some studies have shown that treatment using AST can
promote nerve cell regeneration and increase gene expression of
proteins important for brain recovery, such as glial fibrillary
acidic protein (GFAP), microtubule associated protein 2 (MAP-2),
brain-derived neurotrophic factor (BDNF) and growth-associated
protein 43 (GAP-43) (56–59). GFAP serves significant roles in
the repair of CNS injury, promotion of cell communication and
alleviation of BBB damage (60).
MAP-2 can regulate microtubule growth and neuronal regeneration.
BDNF is responsible for neuronal survival and growth and the
differentiation of new neurons (61), whereas upregulation of GAP-43
stimulates the protein kinase pathway and promotes neurite
formation, regeneration and plasticity (61). The biological activities of AST in
the courses of neurological diseases are summarized in Table I.
Neurodegenerative disorders are difficult to prevent
and treat. In addition, improving their prognoses is quite
challenging. AD is the most common neurodegenerative disorder among
older individuals (62).
Extensive research has demonstrated that the number of people with
AD is steadily increasing. AD tends to have a long course, various
comorbidities and medical requirements for long-term care. These
data suggest that AD places a heavy socioeconomic burden on the
families of patients and the society at large (63,64).
Patients with AD show a significant degree of
oxidative damage in the brain. This oxidate damage is associated
with the accumulation of amyloid-β peptide (Aβ) (65). Aβ is the main component of senile
plaques, neurofibrillary tangles and neutrophil threads in the
brain (66). In addition to Aβ,
mitochondrial abnormalities and hyperphosphorylated tau also induce
oxidative and inflammatory reactions that contribute to the
pathology of AD (67).
Furthermore, metal ions (68,69), LPO (66) and DNA abnormalities (70) are implicated in the oxidative
process of AD.
AST, with its antioxidant and anti-inflammatory
effects, is recommended for the prevention or reduction of the
progression of AD and the improvement of its prognosis (37). Indeed, two double-blind
placebo-controlled studies conducted in Japan demonstrate that AST
supplementation could effectively improve cognitive ability, which
enables individuals to accomplish tasks more precisely and rapidly
(71,72). In their study, Taksima et
al (73) found that Wistar
rats treated with AST decreased their escape latency time and
increased the time spent in the target quadrant in the Morris water
maze test. AST intake has been found to reduce brain oxidative
indices, such as the LPO product malondialdehyde (MDA) and the
percentage of superoxide anion and increase glutathione peroxidase
activity. A previous study demonstrated that neurodegenerative
disorders may be related to insulin resistance, which could lead to
the accumulation of Aβ, mitochondrial dysfunction and increased
levels of inflammatory cytokines (74). In a previous animal study, Rahman
et al (75) found that AST
not only improved the cognitive assessment results of rats, but
also attenuated central insulin resistance indicators, Aβ level and
TNF-α level in the hippocampi of Wistar rats (76).
In a study of a PM2.5-induced neuroinflammation
model, AST treatment decreased the expression of M1
pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) and increased
the expression of M2 anti-inflammatory cytokines (IL-10 and
arginase-1) (77). Similar
anti-inflammatory activities of AST have been reported in other
studies. For example, a previous study showed that AST (50 µM)
significantly reduces the release of inflammatory mediators in
activated microglial cells through the modulation of factors
involved in the NF-κB cascade (e.g., IKKα/β, IκBα, NF-κB p65, IL-6
and MAPK) (78). HT12 cells and
PC12 cells are rat-derived neuronal cells that are used to imitate
the nervous system in vivo for studying neurodegeneration.
Another study demonstrated that after treatment using 1.25-5 µM
AST, these neuronal cells are protected from neurotoxicity
stimulated by glutamate-induced cytotoxicity and reduced lactate
dehydrogenase (LDH) release. This protective effect is attributable
to decreased caspase-3/8/9 expression, poly (ADP-ribose) polymerase
(PARP), suppressed ROS accumulation, increased nuclear Nrf2 and
HO-1 expression and modulated Akt/glycogen synthase kinase 3β
(GSK-3β) signaling (79). It has
been reported that AST improves the behavioral scores of rats in
hippocampal-dependent tasks; however, the underlying molecular
process is not fully understood. These results show that
administration of AST can serve as an augmentative treatment for
AD.
PD is the second most common neurodegenerative
disease globally. As with AD, the proportion of the global
population with PD is increasing (80). James Parkinson first described
this disease as a ‘shaking palsy’ 200 years ago (81). It is now recognized as a complex
and heterogeneous disorder characterized by classic motor symptoms
(bradykinesia, rigidity and tremor) induced by the loss of
dopaminergic neurons and non-motor manifestations (altered posture,
balance and gait) (81). Numerous
studies have shown that neuronal loss and formation of Lewy bodies
are the pathological hallmarks of PD. These pathological features,
which have been identified in the basal forebrain, anterior
thalamus, hypothalamus, amygdala and cerebral cortex, disrupt the
normal function of the brain and interrupt the actions of important
chemical messengers, such as acetylcholine and dopamine (82). Although the pathogenesis of PD is
not completely understood, increasing evidence from human and
animal studies suggest that OS and mitochondrial and calcium
dysfunction are important mediators in its pathogenesis (83). The antioxidant and
anti-inflammatory properties of AST make it a promising therapeutic
agent for PD. In a previous study of a PD model, AST was found to
increase PC12 cell viability, decrease mRNA production and decrease
the expression of proteins linked to neurodegenerative disorders,
such as activated transcription factor Sp1 and NR1 (32). AST also suppresses NADPH oxidase 2
levels, generates ROS and considerably increases Nrf2 and HO-1
levels (84). Studies have
demonstrated that AST induces mitochondrial protection and reduces
oxidative injury through the ERK1/2 and PI3K/Akt/Nrf2/HO-1 pathways
(36,85). In a study of
MPP+/1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced
apoptosis in SH-SY5Y cells and a PD model, Lee et al
(86) found that AST shows
anti-apoptotic and neuroprotective effects through the upregulation
of Bcl-2 protein expression and the inhibition of Bax and
α-synuclein expression and caspase-3 activation. However, there are
currently no clinical trials on AST for the treatment of PD.
Considering the findings of previous trials on PD and carotene
(87), it is possible that AST
could serve an important role in improving the progression of PD in
the future.
Ischemic stroke (IS) has a high incidence rate and
is one of the most common causes of serious morbidity and mortality
worldwide (88). Blockage of
cerebral blood flow results in pathophysiological responses,
including excitotoxicity, mitochondrial disorders, ROS release,
inflammatory changes, apoptosis, calcium imbalance and DNA damage
(88,89). In a study of a rat model, IR was
found to activate the redox-sensitive transcription factors NF-κB,
AP-1, MAPKs, JNK and p38 under minimal activation of ERK (90). Superoxide dismutase (SOD) and
glutathione (GSH), which are free radical scavengers found in the
brain tissue after cerebral IR, can suppress the harmful effects of
IS (91,92). Previous studies have shown the
neuroprotective effect of AST in reducing adverse reactions related
to cerebral IR injury during brain recovery (93–97).
SAH is a severe disease with high morbidity and
mortality rates worldwide. The clinical symptoms of SAH include
coma and varying degrees of neurological disorders, such as
aphasia, hemiplegia, hemianopia, paresthesia, headache and
dysphrenia (98). The
pathological course of SAH within the first 72 h is defined as
early brain injury, which includes destruction of the BBB, cerebral
edema, inflammation, increased intracranial pressure and neuronal
apoptosis (99–101). These changes suggest an
unfavorable prognosis and create significant individual and social
burden. A series of studies conducted by Zhang et al
(102) showed that AST
ameliorates inflammation and OS and improves neuronal survival in
SAH by modifying the Nrf2-ARE and Akt/Bad pathways and the
toll-like receptor 4 signaling pathway (34). As mentioned previously, these
pathways can inhibit the expression of inflammatory cytokines
(IL-1β, TNF-α and NF-кB p65) and apoptotic cytokines (Bax, Cyt c
and caspase-3) while rescuing mitochondrial function and BBB
integrity (34,52,102,103). Wang et al (104) show that AST inhibits
mitochondria-associated neuronal apoptosis after SAH by stabilizing
the mitochondrial membrane potential, decreasing the Bax/Bcl-2
ratio, inhibiting Cyt c and suppressing caspase-3 enzyme activity.
In addition, AST has been found to restore the expression of
synapsin-1, postsynaptic density-95, GAP-4, BDNF and purine-rich
binding protein-α associated with nerve growth and neuronal
differentiation.
ALS is a progressive and lethal neurological disease
characterized by irreversible loss of the upper and lower spinal or
bulbar motor neurons (105).
Most patients with ALS experience muscle paralysis until death,
which is caused by respiratory failure within 3–5 years of the
onset of symptoms. The number of patients with ALS has been rapidly
increasing as a result of increasing aging of the global
population. It has been reported that approximately 400,000 people
worldwide will have ALS by 2040 (106). Although the underlying
mechanisms of ALS are not fully understood, the most common cause
may be related to a mutation in the gene encoding Cu/Zn SOD1. SOD1
is a significant cytosolic metalloenzyme that catalyzes the
dismutation of the superoxide anion radical
(O2−) into H2O2 and
O2. In addition, mitochondrial dysfunction,
neuroinflammation and calcium flux-related excitotoxicity serve
important roles in the progression of ALS (105,107). The unstable structure of mutant
SOD1 leads to the accumulation free radicals from OS. OS causes
oxidative damage to lipids, proteins and nucleic acids, resulting
in neuronal death. Free radicals can be produced by antioxidants,
such as vitamin C, vitamin E and AST. Bond et al (108) showed that antioxidants are
promising as therapeutic agents for increasing the quality of life
of patients with ALS. Moreover, Isonaka et al (109) demonstrate that spinal motor
neurons treated with antioxidants and the SOD1 inhibitor
diethyldithiocarbamate (DDC) have longer neurite lengths than
neurons treated with DDC alone, indicating that antioxidants may
improve pathological changes in ALS. In addition, clinical dietary
studies have shown that carotenoid intake improves respiratory
function in patients with ALS and reduces the risk for the disease
(110,111).
AST, with its antioxidant, anti-inflammatory and
anti-apoptotic properties, has various health benefits for humans.
The present review highlighted the mechanisms of action and
benefits of AST in neurological diseases. In addition, owing to its
lipid-soluble characteristics, AST may serve an important role in
improving neurological diseases. However, previous studies on AST
are mainly focused on animal models. Thus, further in vivo
and in vitro studies on AST are warranted to clarify the
specific signaling pathways involved in its effects and to
elucidate its benefits for effective therapy. More research is
needed to explore the potential applications of AST in the
prevention, management and treatment of neurological diseases.
Not applicable.
Funding: No funding was received.
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
PS was a major contributor to writing the manuscript
and prepared the figures. CZ performed the literature search and
selection and was responsible for editing the references. The two
authors read and approved the final version of the manuscript, were
responsible for all aspects of the work and approved the submission
in its current form. Data authentication is not applicable.
No applicable.
No applicable.
The authors declare that they have no competing
interests.
|
1
|
Hu J, Nagarajan D, Zhang Q, Chang JS and
Lee DJ: Heterotrophic cultivation of microalgae for pigment
production: A review. Biotechnol Adv. 36:54–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng YF, Bae SH, Kwon MJ, Park JB, Choi
HD, Shin WG and Bae SK: Inhibitory effects of astaxanthin,
β-cryptoxanthin, canthaxanthin, lutein, and zeaxanthin on
cytochrome P450 enzyme activities. Food Chem Toxicol. 59:78–85.
2013. View Article : Google Scholar
|
|
3
|
Guerin M, Huntley ME and Olaizola M:
Haematococcus astaxanthin: Applications for human health and
nutrition. Trends Biotechnol. 21:210–216. 2003. View Article : Google Scholar
|
|
4
|
Boussiba S: Carotenogenesis in the green
alga Haematococcus pluvialis: Cellular physiology and stress
response. Physiol Plant. 108:111–117. 2010. View Article : Google Scholar
|
|
5
|
Higuera-Ciapara I, Félix-Valenzuela L and
Goycoolea FM: Astaxanthin: A review of its chemistry and
applications. Crit Rev Food Sci Nutr. 46:185–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tume RK, Sikes AL, Tabrett S and Smith DM:
Effect of background colour on the distribution of astaxanthin in
black tiger prawn (Penaeus monodon): Effective method for
improvement of cooked colour. Aquaculture. 296:129–135. 2009.
View Article : Google Scholar
|
|
7
|
Mosaad YO, Gobba NA and Hussein MA:
Astaxanthin; a promising protector against gentamicin-induced
nephrotoxicity in rats. Curr Pharm Biotechnol. 17:1189–1197. 2016.
View Article : Google Scholar
|
|
8
|
Curek GD, Cort A, Yucel G, Demir N, Ozturk
S, Elpek GO, Savas B and Aslan M: Effect of astaxanthin on
hepatocellular injury following ischemia/reperfusion. Toxicology.
267:147–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishimoto Y, Yoshida H and Kondo K:
Potential anti-atherosclerotic properties of astaxanthin. Mar
Drugs. 14:352016. View Article : Google Scholar
|
|
10
|
Zajac G, Machalska E, Kaczor A, Kessler J,
Bouř P and Baranska M: Structure of supramolecular astaxanthin
aggregates revealed by molecular dynamics and electronic circular
dichroism spectroscopy. Phys Chem Chem Phys. 20:18038–18046. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Armstrong DW and Chang CD: Rapid
baseline separation of enantiomers and a mesoform of
all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and
adonixanthin in standards and commercial supplements. J Chromatogr
A. 1194:172–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Luo Q, Cao Y, Goulette T, Liu X and
Xiao H: Mechanism of different stereoisomeric astaxanthin in
resistance to oxidative stress in caenorhabditis elegans. J Food
Sci. 81:H2280–H2287. 2016. View Article : Google Scholar
|
|
13
|
Yuan JP, Peng J, Yin K and Wang JH:
Potential health-promoting effects of astaxanthin: A high-value
carotenoid mostly from microalgae. Mol Nutr Food Res. 55:150–165.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambati RR, Phang SM, Ravi S and
Aswathanarayana RG: Astaxanthin: Sources, extraction, stability,
biological activities and its commercial applications-a review. Mar
Drugs. 12:128–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fakhri S, Abbaszadeh F, Dargahi L and
Jorjani M: Astaxanthin: A mechanistic review on its biological
activities and health benefits. Pharmacol Res. 136:1–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raja R, Hemaiswarya S, Kumar NA, Sridhar S
and Rengasamy R: A perspective on the biotechnological potential of
microalgae. Crit Rev Microbiol. 34:77–88. 2008. View Article : Google Scholar
|
|
17
|
Capelli B, Bagchi D and Cysewski GR:
Synthetic astaxanthin is significantly inferior to algal-based
astaxanthin as an antioxidant and may not be suitable as a human
nutraceutical supplement. Nutrafoods. 12:145–152. 2013. View Article : Google Scholar
|
|
18
|
Baccouche B, Benlarbi M, Barber AJ and Ben
Chaouacha-Chekir R: Short-term administration of astaxanthin
attenuates retinal changes in diet-induced diabetic psammomys
obesus. Curr Eye Res. 43:1177–1189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshihisa Y, Rehman MU and Shimizu T:
Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced
apoptosis in keratinocytes. Exp Dermatol. 23:178–183. 2014.
View Article : Google Scholar
|
|
20
|
Ito N, Seki S and Ueda F: The protective
role of astaxanthin for UV-induced skin deterioration in healthy
people-a randomized, double-blind, placebo-controlled trial.
Nutrients. 10:8172018. View Article : Google Scholar
|
|
21
|
Bhuvaneswari S, Arunkumar E, Viswanathan P
and Anuradha CV: Astaxanthin restricts weight gain, promotes
insulin sensitivity and curtails fatty liver disease in mice fed a
obesity-promoting diet. Process Biochem. 45:1406–1414. 2010.
View Article : Google Scholar
|
|
22
|
Fan CD, Sun JY, Fu XT, Hou YJ, Li Y, Yang
MF, Fu XY and Sun BL: Astaxanthin attenuates homocysteine-induced
cardiotoxicity in vitro and in vivo by inhibiting mitochondrial
dysfunction and oxidative damage. Front Physiol. 8:10412017.
View Article : Google Scholar
|
|
23
|
Kim JH, Park JJ, Lee BJ, Joo MK, Chun HJ,
Lee SW and Bak YT: Astaxanthin inhibits proliferation of human
gastric cancer cell lines by Interrupting cell cycle progression.
Gut Liver. 10:369–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Niu H, Shao A, Wu C, Dixon BJ, Zhang
J, Yang S and Wang Y: Astaxanthin as a potential neuroprotective
agent for neurological diseases. Mar Drugs. 13:5750–5766. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grimmig B, Kim SH, Nash K, Bickford PC and
Douglas Shytle R: Neuroprotective mechanisms of astaxanthin: A
potential therapeutic role in preserving cognitive function in age
and neurodegeneration. Geroscience. 39:19–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khademian M and Imlay JA: How microbes
evolved to tolerate oxygen. Trends Microbiol. 29:428–440. 2021.
View Article : Google Scholar
|
|
27
|
Hammarlund EU, Flashman E, Mohlin S and
Licausi F: Oxygen-sensing mechanisms across eukaryotic kingdoms and
their roles in complex multicellularity. Science. 370:eaba35122020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamath BS, Srikanta BM, Dharmesh SM,
Sarada R and Ravishankar GA: Ulcer preventive and antioxidative
properties of astaxanthin from Haematococcus pluvialis. Eur
J Pharmacol. 590:387–395. 2008. View Article : Google Scholar
|
|
29
|
Rao AR, Sindhuja HN, Dharmesh SM, Sankar
KU, Sarada R and Ravishankar GA: Effective inhibition of skin
cancer, tyrosinase, and antioxidative properties by astaxanthin and
astaxanthin esters from the green alga Haematococcus
pluvialis. J Agric Food Chem. 61:3842–3851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Naguib YM: Antioxidant activities of
astaxanthin and related carotenoids. J Agric Food Chem.
48:1150–1154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakajima Y, Inokuchi Y, Shimazawa M,
Otsubo K, Ishibashi T and Hara H: Astaxanthin, a dietary
carotenoid, protects retinal cells against oxidative stress
in-vitro and in mice in-vivo. J Pharm Pharmacol. 60:1365–1374.
2008. View Article : Google Scholar
|
|
32
|
Ye Q, Zhang X, Huang B, Zhu Y and Chen X:
Astaxanthin suppresses MPP(+)-induced oxidative damage in PC12
cells through a Sp1/NR1 signaling pathway. Mar Drugs. 11:1019–1034.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zarneshan SN, Fakhri S, Farzaei MH, Khan H
and Saso L: Astaxanthin targets PI3K/Akt signaling pathway toward
potential therapeutic applications. Food Chem Toxicol.
145:1117142020. View Article : Google Scholar
|
|
34
|
Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li
W, Zhou ML and Wang XL: Astaxanthin activates nuclear factor
erythroid-related factor 2 and the antioxidant responsive element
(Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in
rats and attenuates early brain injury. Mar Drugs. 12:6125–6141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y,
Hou D and Zhang X: Astaxanthin protects ARPE-19 cells from
oxidative stress via upregulation of Nrf2-regulated phase II
enzymes through activation of PI3K/Akt. Mol Vis. 19:1656–1666.
2013.PubMed/NCBI
|
|
36
|
Wang HQ, Sun XB, Xu YX, Zhao H, Zhu QY and
Zhu CQ: Astaxanthin upregulates heme oxygenase-1 expression through
ERK1/2 pathway and its protective effect against
beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res.
1360:159–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Al-Amin MM, Mahmud W, Pervin MS, Ridwanul
Islam SM, Ashikur Rahman M and Zinchenko A: Astaxanthin ameliorates
scopolamine-induced spatial memory deficit via reduced
cortical-striato-hippocampal oxidative stress. Brain Res.
1710:74–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SH, Lim JW and Kim H: Astaxanthin
inhibits mitochondrial dysfunction and interleukin-8 expression in
helicobacter pylori-infected gastric epithelial cells. Nutrients.
10:13202018. View Article : Google Scholar
|
|
39
|
Ranga Rao A, Raghunath Reddy RL, Baskaran
V, Sarada R and Ravishankar GA: Characterization of microalgal
carotenoids by mass spectrometry and their bioavailability and
antioxidant properties elucidated in rat model. J Agric Food Chem.
58:8553–8559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Netea MG, Balkwill F, Chonchol M,
Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, Golenbock D,
Gresnigt MS, Heneka MT, Hoffman HM, et al: A guiding map for
inflammation. Nat Immunol. 18:826–831. 2017. View Article : Google Scholar
|
|
41
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar
|
|
42
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar
|
|
43
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang C, Hassan YI, Liu R, Zhang H, Chen Y,
Zhang L and Tsao R: Anti-inflammatory effects of different
astaxanthin isomers and the roles of lipid transporters in the
cellular transport of astaxanthin isomers in Caco-2 cell
monolayers. J Agric Food Chem. 67:6222–6231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grilo AL and Mantalaris A: Apoptosis: A
mammalian cell bioprocessing perspective. Biotechnol Adv.
37:459–475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Adams JM and Cory S: The BCL-2 arbiters of
apoptosis and their growing role as cancer targets. Cell Death
Differ. 25:27–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar
|
|
49
|
Zhang L and Wang H: Multiple mechanisms of
anti-cancer effects exerted by astaxanthin. Mar Drugs.
13:4310–4330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dong LY, Jin J, Lu G and Kang XL:
Astaxanthin attenuates the apoptosis of retinal ganglion cells in
db/db mice by inhibition of oxidative stress. Mar Drugs.
11:960–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guo SX, Zhou HL, Huang CL, You CG, Fang Q,
Wu P, Wang XG and Han CM: Astaxanthin attenuates early acute kidney
injury following severe burns in rats by ameliorating oxidative
stress and mitochondrial-related apoptosis. Mar Drugs.
13:2105–2123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang XS, Zhang X, Wu Q, Li W, Zhang QR,
Wang CX, Zhou XM, Li H, Shi JX and Zhou ML: Astaxanthin alleviates
early brain injury following subarachnoid hemorrhage in rats:
Possible involvement of Akt/bad signaling. Mar Drugs. 12:4291–4310.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li S, Takahara T, Fujino M, Fukuhara Y,
Sugiyama T, Li XK and Takahara S: Astaxanthin prevents
ischemia-reperfusion injury of the steatotic liver in mice. PLoS
One. 12:e01878102017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Klein RS and Hunter CA: Protective and
pathological immunity during central nervous system infections.
Immunity. 46:891–909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Manabe Y, Komatsu T, Seki S and Sugawara
T: Dietary astaxanthin can accumulate in the brain of rats. Biosci
Biotechnol Biochem. 82:1433–1436. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
El-Agamy SE, Abdel-Aziz AK, Wahdan S,
Esmat A and Azab SS: Astaxanthin ameliorates doxorubicin-induced
cognitive impairment (Chemobrain) in experimental rat model: Impact
on oxidative, inflammatory, and apoptotic machineries. Mol
Neurobiol. 55:5727–5740. 2018. View Article : Google Scholar
|
|
57
|
Lee H, Lim JW and Kim H: Effect of
astaxanthin on activation of autophagy and inhibition of apoptosis
in helicobacter pylori-infected gastric epithelial cell line AGS.
Nutrients. 12:17502020. View Article : Google Scholar
|
|
58
|
Damodara Gowda KM, Suchetha Kumari N and
Ullal H: Role of astaxanthin in the modulation of brain-derived
neurotrophic factor and spatial learning behavior in perinatally
undernourished Wistar rats. Nutr Neurosci. 23:422–431. 2020.
View Article : Google Scholar
|
|
59
|
Wang YL, Zhu XL, Sun MH and Dang YK:
Effects of astaxanthin onaxonal regeneration via cAMP/PKA signaling
pathway in mice with focal cerebral infarction. Eur Rev Med
Pharmacol Sci. 23 (3 Suppl):S135–S143. 2019.
|
|
60
|
Cullen DK, Simon CM and LaPlaca MC: Strain
rate-dependent induction of reactive astrogliosis and cell death in
three-dimensional neuronal-astrocytic co-cultures. Brain Res.
1158:103–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ahmed S, Reynolds BA and Weiss S: BDNF
enhances the differentiation but not the survival of CNS stem
cell-derived neuronal precursors. J Neurosci. 15:5765–5778. 1995.
View Article : Google Scholar
|
|
62
|
Tublin JM, Adelstein JM, Del Monte F,
Combs CK and Wold LE: Getting to the heart of Alzheimer disease.
Circ Res. 124:142–149. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Alzheimer's Association: 2016 Alzheimer's
disease facts and figures. Alzheimers Dement. 12:459–509. 2016.
View Article : Google Scholar
|
|
64
|
Jia J, Wang F, Wei C, Zhou A, Jia X, Li F,
Tang M, Chu L, Zhou Y, Zhou C, et al: The prevalence of dementia in
urban and rural areas of China. Alzheimers Dement. 10:1–9. 2014.
View Article : Google Scholar
|
|
65
|
Nakamura A, Kaneko N, Villemagne VL, Kato
T, Doecke J, Doré V, Fowler C, Li QX, Martins R, Rowe C, et al:
High performance plasma amyloid-β biomarkers for Alzheimer's
disease. Nature. 554:249–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Butterfield DA, Castegna A, Lauderback CM
and Drake J: Evidence that amyloid beta-peptide-induced lipid
peroxidation and its sequelae in Alzheimer's disease brain
contribute to neuronal death. Neurobiol Aging. 23:655–664. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pradeepkiran JA and Reddy PH: Defective
mitophagy in Alzheimer's disease. Ageing Res Rev. 64:1011912020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Squitti R, Mendez A, Ricordi C, Siotto M
and Goldberg R: Copper in glucose intolerance, cognitive decline,
and Alzheimer disease. Alzheimer Dis Assoc Disord. 33:77–85. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bjørklund G, Dadar M, Peana M, Rahaman MS
and Aaseth J: Interactions between iron and manganese in
neurotoxicity. Arch Toxicol. 94:725–734. 2020. View Article : Google Scholar
|
|
70
|
Khan MM, Xiao J, Patel D and LeDoux MS:
DNA damage and neurodegenerative phenotypes in aged Ciz1 null mice.
Neurobiol Aging. 62:180–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ito N, Saito H, Seki S, Ueda F and Asada
T: Effects of composite supplement containing astaxanthin and
sesamin on cognitive functions in people with mild cognitive
impairment: A randomized, double-blind, placebo-controlled trial:
Erratum. J Alzheimers Dis. 68:8392019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sekikawa T, Kizawa Y, Li Y and Takara T:
Cognitive function improvement with astaxanthin and tocotrienol
intake: A randomized, double-blind, placebo-controlled study. J
Clin Biochem Nutr. 67:307–316. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Taksima T, Chonpathompikunlert P, Sroyraya
M, Hutamekalin P, Limpawattana M and Klaypradit W: Effects of
astaxanthin from shrimp shell on oxidative stress and behavior in
animal model of Alzheimer's disease. Mar Drugs. 17:6282019.
View Article : Google Scholar
|
|
74
|
Kellar D and Craft S: Brain insulin
resistance in Alzheimer's disease and related disorders: Mechanisms
and therapeutic approaches. Lancet Neurol. 19:758–766. 2020.
View Article : Google Scholar
|
|
75
|
Rahman SO, Panda BP, Parvez S, Kaundal M,
Hussain S, Akhtar M and Najmi AK: Neuroprotective role of
astaxanthin in hippocampal insulin resistance induced by Aβ
peptides in animal model of Alzheimer's disease. Biomed
Pharmacother. 110:47–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Craft S and Watson GS: Insulin and
neurodegenerative disease: Shared and specific mechanisms. Lancet
Neurol. 3:169–178. 2004. View Article : Google Scholar
|
|
77
|
Kim RE, Shin CY, Han SH and Kwon KJ:
Astaxanthin suppresses PM2.5-induced neuroinflammation by
regulating Akt phosphorylation in BV-2 microglial cells. Int J Mol
Sci. 21:72272020. View Article : Google Scholar
|
|
78
|
Kim YH, Koh HK and Kim DS: Down-regulation
of IL-6 production by astaxanthin via ERK-, MSK-, and
NF-κB-mediated signals in activated microglia. Int Immunopharmacol.
10:1560–1572. 2010. View Article : Google Scholar
|
|
79
|
Wen X, Huang A, Hu J, Zhong Z, Liu Y, Li
Z, Pan X and Liu Z: Neuroprotective effect of astaxanthin against
glutamate-induced cytotoxicity in HT22 cells: Involvement of the
Akt/GSK-3β pathway. Neuroscience. 303:558–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ascherio A and Schwarzschild MA: The
epidemiology of Parkinson's disease: Risk factors and prevention.
Lancet Neurol. 15:1257–1272. 2016. View Article : Google Scholar
|
|
81
|
Samii A, Nutt JG and Ransom BR:
Parkinson's disease. Lancet. 363:1783–1793. 2004. View Article : Google Scholar
|
|
82
|
Sayre LM, Smith MA and Perry G: Chemistry
and biochemistry of oxidative stress in neurodegenerative disease.
Curr Med Chem. 8:721–738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Issa AR, Sun J, Petitgas C, Mesquita A,
Dulac A, Robin M, Mollereau B, Jenny A, Chérif-Zahar B and Birman
S: The lysosomal membrane protein LAMP2A promotes autophagic flux
and prevents SNCA-induced Parkinson disease-like symptoms in the
Drosophila brain. Autophagy. 14:1898–1910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ye Q, Huang B, Zhang X, Zhu Y and Chen X:
Astaxanthin protects against MPP(+)-induced oxidative stress in
PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 13:1562012.
View Article : Google Scholar
|
|
85
|
Brasil FB, Bertolini Gobbo RC, Souza de
Almeida FJ, Luckachaki MD, Dall'Oglio EL and de Oliveira MR: The
signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the
mitochondrial protection promoted by astaxanthin in the SH-SY5Y
cells exposed to hydrogen peroxide. Neurochem Int. 146:1050242021.
View Article : Google Scholar
|
|
86
|
Lee DH, Kim CS and Lee YJ: Astaxanthin
protects against MPTP/MPP+-induced mitochondrial dysfunction and
ROS production in vivo and in vitro. Food Chem Toxicol. 49:271–280.
2011. View Article : Google Scholar
|
|
87
|
Kim JH, Hwang J, Shim E, Chung EJ, Jang SH
and Koh SB: Association of serum carotenoid, retinol, and
tocopherol concentrations with the progression of Parkinson's
disease. Nutr Res Pract. 11:114–120. 2017. View Article : Google Scholar
|
|
88
|
Campbell BCV, De Silva DA, Macleod MR,
Coutts SB, Schwamm LH, Davis SM and Donnan GA: Ischaemic stroke.
Nat Rev Dis Primers. 5:702019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
George PM and Steinberg GK: Novel stroke
therapeutics: Unraveling stroke pathophysiology and its impact on
clinical treatments. Neuron. 87:297–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lazou A, Bogoyevitch MA, Clerk A, Fuller
SJ, Marshall CJ and Sugden PH: Regulation of mitogen-activated
protein kinase cascade in adult rat heart preparations in vitro.
Circ Res. 75:932–941. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang R, Liu C, Liu X and Guo Y:
Protective effect of spatholobus suberectus on brain tissues in
cerebral ischemia. Am J Transl Res. 8:3963–3969. 2016.PubMed/NCBI
|
|
92
|
Vani JR, Mohammadi MT, Foroshani MS and
Jafari M: Polyhydroxylated fullerene nanoparticles attenuate brain
infarction and oxidative stress in rat model of ischemic stroke.
EXCLI J. 15:378–390. 2016.PubMed/NCBI
|
|
93
|
Xue Y, Qu Z, Fu J, Zhen J and Wang W, Cai
Y and Wang W: The protective effect of astaxanthin on learning and
memory deficits and oxidative stress in a mouse model of repeated
cerebral ischemia/reperfusion. Brain Res Bull. 131:221–228. 2017.
View Article : Google Scholar
|
|
94
|
Pan L, Zhou Y, Li XF, Wan QJ and Yu LH:
Preventive treatment of astaxanthin provides neuroprotection
through suppression of reactive oxygen species and activation of
antioxidant defense pathway after stroke in rats. Brain Res Bull.
130:211–220. 2017. View Article : Google Scholar
|
|
95
|
Lee DH, Lee YJ and Kwon KH:
Neuroprotective effects of astaxanthin in oxygen-glucose
deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J
Clin Biochem Nutr. 47:121–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lu YP, Liu SY, Sun H, Wu XM, Li JJ and Zhu
L: Neuroprotective effect of astaxanthin on H(2)O(2)-induced
neurotoxicity in vitro and on focal cerebral ischemia in vivo.
Brain Res. 1360:40–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang BB, Zou M, Zhao L and Zhang YK:
Astaxanthin attenuates acute cerebral infarction via Nrf-2/HO-1
pathway in rats. Curr Res Transl Med. 69:1032712021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Budohoski KP, Guilfoyle M, Helmy A,
Huuskonen T, Czosnyka M, Kirollos R, Menon DK, Pickard JD and
Kirkpatrick PJ: The pathophysiology and treatment of delayed
cerebral ischaemia following subarachnoid haemorrhage. J Neurol
Neurosurg Psychiatry. 85:1343–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Vergouwen MD, Ilodigwe D and Macdonald RL:
Cerebral infarction after subarachnoid hemorrhage contributes to
poor outcome by vasospasm-dependent and -independent effects.
Stroke. 42:924–929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen S, Feng H, Sherchan P, Klebe D, Zhao
G, Sun X, Zhang J, Tang J and Zhang JH: Controversies and evolving
new mechanisms in subarachnoid hemorrhage. Prog Neurobiol.
115:64–91. 2014. View Article : Google Scholar
|
|
101
|
Serrone JC, Maekawa H, Tjahjadi M and
Hernesniemi J: Aneurysmal subarachnoid hemorrhage: Pathobiology,
current treatment and future directions. Expert Rev Neurother.
15:367–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang X, Lu Y, Wu Q, Dai H, Li W, Lv S,
Zhou X, Zhang X, Hang C and Wang J: Astaxanthin mitigates
subarachnoid hemorrhage injury primarily by increasing sirtuin 1
and inhibiting the Toll-like receptor 4 signaling pathway. FASEB J.
33:722–737. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang XS, Zhang X, Zhou ML, Zhou XM, Li N,
Li W, Cong ZX, Sun Q, Zhuang Z, Wang CX and Shi JX: Amelioration of
oxidative stress and protection against early brain injury by
astaxanthin after experimental subarachnoid hemorrhage. J
Neurosurg. 121:42–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang Y, Liu Y, Li Y, Liu B, Wu P, Xu S and
Shi H: Protective effects of astaxanthin on subarachnoid
hemorrhage-induced early brain injury: Reduction of cerebral
vasospasm and improvement of neuron survival and mitochondrial
function. Acta Histochem. 121:56–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hardiman O, Al-Chalabi A, Chio A, Corr EM,
Logroscino G, Robberecht W, Shaw PJ, Simmons Z and van den Berg LH:
Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 3:170712017.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Arthur KC, Calvo A, Price TR, Geiger JT,
Chiò A and Traynor BJ: Projected increase in amyotrophic lateral
sclerosis from 2015 to 2040. Nat Commun. 7:124082016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nuevo Ordoñez Y, Montes-Bayón M,
Blanco-González E and Sanz-Medel A: Quantitative analysis and
simultaneous activity measurements of Cu, Zn-superoxide dismutase
in red blood cells by HPLC-ICPMS. Anal Chem. 82:2387–2394. 2010.
View Article : Google Scholar
|
|
108
|
Bond L, Bernhardt K, Madria P, Sorrentino
K, Scelsi H and Mitchell CS: A metadata analysis of oxidative
stress etiology in preclinical amyotrophic lateral sclerosis:
Benefits of antioxidant therapy. Front Neurosci. 12:102018.
View Article : Google Scholar
|
|
109
|
Isonaka R, Hiruma H, Katakura T and
Kawakami T: Inhibition of superoxide dismutase selectively
suppresses growth of rat spinal motor neurons: Comparison with
phosphorylated neurofilament-containing spinal neurons. Brain Res.
1425:13–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Fitzgerald KC, O'Reilly ÉJ, Fondell E,
Falcone GJ, McCullough ML, Park Y, Kolonel LN and Ascherio A:
Intakes of vitamin C and carotenoids and risk of amyotrophic
lateral sclerosis: Pooled results from 5 cohort studies. Ann
Neurol. 73:236–245. 2013. View Article : Google Scholar
|
|
111
|
Nieves JW, Gennings C, Factor-Litvak P,
Hupf J, Singleton J, Sharf V, Oskarsson B, Fernandes Filho JA,
Sorenson EJ, D'Amico E, et al: Association between dietary intake
and function in amyotrophic lateral sclerosis. JAMA Neurol.
73:1425–1432. 2016. View Article : Google Scholar
|