Introduction
Ischemic stroke represents the most common type of
stroke threatening human health, which is characterized by the
sudden loss of blood circulation to an area of the brain (1). Currently, re-perfusing the ischemic
area via drugs or early thrombolysis has been considered to be
effective for the treatment of this disease, but restoration of
blood reperfusion may aggravate brain injury and dysfunction, a
condition termed cerebral ischemia/reperfusion (I/R) injury
(2,3). Accumulating studies have
demonstrated that apoptosis and inflammation are the main factors
in I/R-induced nerve cell injury (4–7).
Hence, exploring the molecular biology underlying I/R injury may be
a remedy target to moderate the deterioration of ischemic
stroke.
MicroRNAs (miRNAs/miRs) are a subtype of highly
conserved endogenous single-stranded, non-coding small RNAs with a
length of 22–25 nucleotides that can negatively regulate the
expression of their target genes via binding to the 3′-untranslated
regions (3′-UTRs) of target mRNAs (8). It is well known that miRNAs
participate in diverse biological processes, including
proliferation, apoptosis, survival and inflammation (9,10),
which are implicated in the adjustment of neurodegenerative
diseases (11). With the
increasing studies on ischemic stroke, related investigation on
miRNAs regulating cerebral I/R injury has been increased. For
instance, Wang et al (12)
elucidated that miR-186-5p was upregulated and induced apoptosis in
an oxygen and glucose deprivation/reperfusion (OGD/R) model by
targeting insulin-like growth factor (IGF)-1. Conversely, Ren et
al (13) reported that
overexpression of miR-195-5p efficiently enhanced cell viability,
while it reduced lactate dehydrogenase (LDH) release and the
apoptotic rate in OGD-treated endothelial cells by targeting PTEN.
In addition, miR-124-5p could reduce ROS production and improve the
inflammatory microenvironment to attenuate cerebral I/R injury by
targeting NOX2 (14). The
attention of the authors was aroused by studies by Sohrabji and
Selvamani (15,16) which revealed miR-363-3p as a
target for ischemic stroke by improving ischemic stroke outcomes in
female rats. Moreover, miR-363-3p has been reported to be
intensively involved in cell proliferation (17), apoptosis (18) and inflammation (19) in different diseases. Nevertheless,
the expression level and regulatory role of miR-363-3p in cerebral
I/R injury remain unclear.
Programmed cell death 6-interacting protein
(PDCD6IP), known as apoptosis-linked gene-2-interacting protein 1
(ALIX), is one of the most intensely studied multifunctional
cytosolic and multi-domain scaffold proteins (20), which usually binds to the
pro-apoptotic protein PDCD6 (21). In addition, it was revealed that
ALIX was correlated with inflammation reaction in response to
different stimuli, including cytokines (22,23) and glutaminase 1 (GLS1) (24) during neuroinflammation. This
evidence indicated that PDCD6IP may play an important role in
cerebral I/R injury by regulating apoptosis and inflammation
status.
Based on the online prediction that PDCD6IP is a
target of miR-363-3p, the present study hypothesized that
miR-363-3p regulated apoptosis and inflammation in cerebral I/R
injury by targeting PDCD6IP. To validate this hypothesis, an OGD/R
cell injury model was first established and this model was assessed
by analyzing cell apoptosis, inflammation and cell viability.
Furthermore, the effects of miR-363-3p on OGD/R-induced injury were
analyzed and it was explored whether PDCD6IP was the downstream
regulator involved in the miR-363-3p-mediated effects.
Materials and methods
Cell culture and transfection
Human neuronal cell line SH-SY5Y (CRL-2266) was
obtained from the American Type Culture Collection (ATCC) and
cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone;
Cytiva) containing 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere with 5%
CO2. The miR-363-3p mimics
(5′-AAUUGCACGGUAUCCAUCUGUA-3′), small interference RNA targeting
PDCD6IP (si-PDCD6IP, 5′-GCTCCTGAGATATTATGATCA-3′) and their
corresponding negative controls, miR-NC
(5′-CAGUACUUUUGUGUAGUACAA-3′) and si-NC
(5′-GTGTTACGTTACCAACTAGAT-3′), respectively, were purchased from
Shanghai GenePharma Co., Ltd. To overexpress PDCD6IP, the full
length of PDCD6IP was cloned into the pcDNA3.1 vector (Sangon
Biotech Co., Ltd.) to generate the overexpression plasmid PDCD6IP,
and the empty vector was used as the negative control. All of miRNA
mimics, siRNA and NCs were diluted into 50 nM for use and 0.5 µg
recombinant plasmid was transfected into SH-SY5Y cells seeded in
six-well plates (3×105 cells/well) for 48 h at 37°C
before OGD/R treatment in accordance with the instructions of
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.).
OGD/R establishment
After 48 h of transfection, SH-SY5Y cells were
exposed to glucose-free DMEM and cultured for 4 h with
N2/CO2/O2 (94/5/1%) at 37°C, as
previously reported (25,26). Thereafter, the glucose-free DMEM
was replaced by standard culture medium and the cells were
transferred to normal conditions to receive re-oxygenation. Cells
in the control group were still cultured in normal DMEM medium in a
normoxic atmosphere. Subsequently, the cells received 4 h of OGD
followed by 6, 12, 24 and 48 h of re-oxygenation.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from SH-SY5Y cells using the
TRIzol reagent (Thermo Fisher Scientific, Inc.) in accordance with
the manufacturer's instructions. For detection of miR-363-3p, cDNA
was synthesized using the TaqMan™ MicroRNA Reverse
Transcription Kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) at 37°C for 5 min. Next, RT-qPCR was performed with Express
SYBR GreenER miRNA PCR kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). For detection of PDCD6IP, reverse transcription
was conducted with EasyScript First-Strand cDNA Synthesis SuperMix
(TransGen Biotech Co., Ltd.) and RT-qPCR was performed using SYBR
Premix Ex Taq (Takara Bio, Inc.). The expression levels of
miR-363-3p and PDCD6IP were normalized against U6 and GAPDH,
respectively. Relative quantification of target genes was conducted
with the 2−ΔΔCq method (27). The primers used in the present
study were as follows: miR-363-3p forward,
5′-GCCGAGAATTGCACGGTAT-3′ and reverse, 5′-CTCAACTGGTGTCGTGGA-3′
(28); PDCD6IP forward,
5′-CTGCCTTAAGTCGAGAGCCG-3′ and reverse,
5′-CAGGGAACACCTCCTGGAAATA-3′; GAPDH forward,
5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse, 5′-GCATCGCCCCACTTGATTTT-3′;
and U6 forward, 5′-TCTTCGTCATCACATATACTAAAAT-3′ and reverse,
5′-CTCTTCACGAATTTTCGTGTCAT-3′.
Cell viability assay
Cells at a density of 5×103 cells/well
were seeded into 96-well plates and cultured overnight at 37°C. The
following day, 10 µl of Cell Counting Kit-8 (CCK-8) solution
(Dojindo Molecular Technologies, Inc.) was added to each well.
Following incubation for 2 h at 37°C, the optical density at 450 nm
was measured by a microplate reader. Subsequently, cell viability
was calculated as the percentage of the OD value in the
experimental group/control group cells. The experiment was
performed in triplicate.
Detection of LDH release
Cell cytotoxicity was detected using the LDH
Cytotoxicity Assay Kit (cat. no. A020-1; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's protocol
(29). In brief, cells at a
density of 5×103 cells/well were plated into 96-well
plates and incubated with 150 µl of LDH release reagent for 1 h at
37°C. According to the absorbance at 490 nm in each well, the LDH
release was expressed as concentration units per liter. The
experiment was performed in triplicate.
Cell apoptosis
Cell apoptosis was assessed by an Annexin
V-FITC/propidium (PI) Apoptosis Detection Kit (Nanjing Keygen
Biotech Co. Ltd.) according to the manufacturer's instructions.
Briefly, cells were digested and obtained cell lysates were
incubated with 5 µl Annexin V/PI in 200 µl binding buffer for 15
min in the dark at room temperature. Subsequently, the proportion
of apoptotic cells was determined by FACSCalibur flow cytometer and
BD Accuri C6 Plus software (both BD Biosciences).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA assay (30)
was performed to investigate the alteration of inflammatory status
in cell-free supernatants in response to the indicated treatments.
In brief, the concentration levels of pro-inflammatory cytokines,
including interleukin (IL)-1β (ab214025), IL-6 (ab178013) and tumor
necrosis factor (TNF)-α (ab181421) in the supernatants of SH-SY5Y
cells were measured using commercially available ELISA kits (Abcam)
in accordance with the standard methods. The experiment was
performed in triplicate.
Luciferase reporter assay
The putative binding site between miR-363-3p and the
3′-UTR of PDCD6IP was predicted by TargetScan v.7.1 (http://www.targetscan.org/vert_71/). For the
luciferase reporter assay, the wild-type (WT) and mutant (MUT) of
PDCD6IP, containing the putative binding site with miR-363-3p, were
inserted into the pmirGLO luciferase reporter vector (Promega
Corporation) to generate recombinant reporter constructs termed as
WT PDCD6IP or MUT PDCD6IP, respectively. SH-SY5Y cells were
incubated in 24-well plates and co-transfected with 200 ng of the
luciferase reporter plasmid and 50 nM of miR-363-3p mimics or
miR-NC using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h of transfection, firefly and
Renilla luciferase activities were determined and relative
luciferase activity was calculated with Renilla luciferase
used for normalization (Promega Corporation).
Western blot analysis
Total protein sample was extracted from cells with
ice-cold RIPA lysis buffer (Beyotime Institute of Biotechnology)
and quantified by BCA assay (Beyotime Institute of Biotechnology)
according to the standard protocols. Equal amounts of protein
sample (30 µg) were separated by 10% SDS-PAGE gels and then
transferred onto PVDF membranes (EMD Millipore). After being
blocked with 5% skim milk containing 0.1% TBST for 2 h, the
membranes were incubated with primary antibodies against PDCD6IP
(cat. no. ab225555, 1:500; Abcam) and GAPDH (cat. no. ab245355,
1:5,000; Abcam) at 4°C overnight. Subsequently, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. ab97051; Abcam) for 2 h at room temperature,
followed by protein visualization using ECL detection reagent
(Bio-Rad Laboratories, Inc.). The band intensities were quantified
using ImageJ v1.8.0 (National Institutes of Health).
Statistical analysis
Statistical analyses were performed by GraphPad
Prism 6.0 (GraphPad Software, Inc.). The data are expressed as the
means ± standard deviation (SD) of three independent experiments.
Differences between two groups were analyzed by Student's t-test,
while differences between multiple groups was determined by one-way
analysis of variance (ANOVA) followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MiR-363-3p is downregulated following
OGD/R-induced neuronal injury in vitro
To explore the potential role of miR-363-3p in
cerebral I/R injury, SH-SY5Y cells were used to establish the in
vitro I/R injury model by exposing the cells to 4 h of OGD
followed by 6, 12, 24 and 48 h of re-oxygenation. The results of
RT-qPCR revealed that the expression level of miR-363-3p was
significantly lower in the OGD-4 h/R model than that of the
normoxic control group and gradually reached the lowest expression
level at 24 h reoxygenation compared with the normoxic control
(Fig. 1A). Subsequently, the
constructed in vitro cerebral I/R model was evaluated
through evaluation of cell survival. As revealed in Fig. 1B, cell viability was significantly
impaired in the OGD-4 h/R model compared with the normoxic
condition and reduced cell viability reached the lowest level at 24
h reoxygenation. Consistently, the release of LDH in the culture
supernatant was significantly increased when the cells were under
OGD-4 h/R conditions, which reached its peak at 24 h reoxygenation
compared with the normoxic condition (Fig. 1C). Thus, 4-h OGD/24-h
reoxygenation was selected as the optimal time-point for subsequent
experiments. Flow cytometric analysis further demonstrated that
OGD/R treatment significantly induced apoptosis of SH-SY5Y cells
compared with that in the control group (Fig. 1D). In addition, the inflammatory
status of SH-SY5Y cells under OGD/R treatment was examined by ELISA
assay. As expected, the levels of pro-inflammatory cytokines,
including IL-1β (Fig. 1E), IL-6
(Fig. 1F) and TNF-α (Fig. 1G) were significantly increased
when the cells were under OGD/R conditions. These results suggested
that miR-363-3p may play an important role in OGD/R induced
neuronal injury.
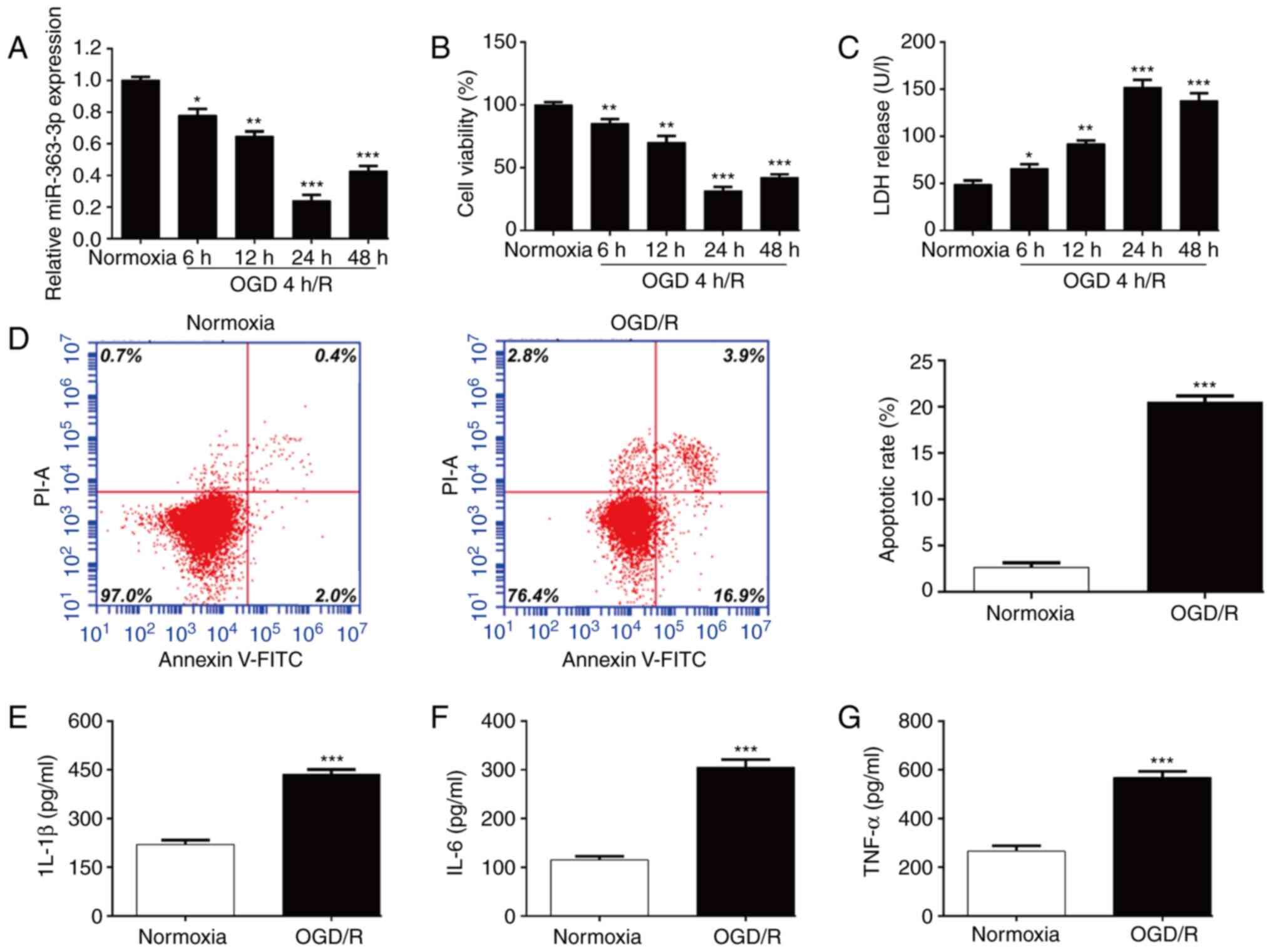 | Figure 1.MiR-363-3p expression levels in an
OGD/R model. SH-SY5Y cells were exposed to 4 h OGD, followed by 6,
12, 24 and 48 h of re-oxygenation. (A) The expression of miR-363-3p
was determined by RT-qPCR. (B) Cell viability and (C) cytotoxicity
were analyzed using CCK-8 and LDH assays, respectively. (D) Cell
apoptosis was analyzed in the 4-h OGD/24-h R model and in the
normoxic control group of SH-SY5Y cells by flow cytometry. ELISA
assays were applied to determine the levels of (E) IL-1β, (F) IL-6
and (G) TNF-α in the 4-h OGD/24-h R model and in the normoxic
control group of SH-SY5Y cells. Data are presented as the mean ±
SD. *P<0.05, **P<0.01 and ***P<0.001 compared with
normoxia. MiR, microRNA; OGD/R, oxygen and glucose
deprivation/re-oxygenation; RT-qPCR, reverse
transcription-quantitative PCR; CCK-8, Cell Counting Kit-8; LDH,
lactate dehydrogenase; IL, interleukin; TNF, tumor necrosis
factor. |
Overexpression of miR-363-3p
attenuates OGD/R-induced apoptosis and inflammation in SH-SY5Y
cells
To further determine the functional role of
miR-363-3p in the constructed OGD/R model, miR-363-3p mimics were
transfected into SH-SY5Y cells, followed by OGD/R treatment. The
results from the RT-qPCR assay showed that the expression of
miR-363-3p in SH-SY5Y cells after exposure to OGD/R was
significantly increased after transfection with miR-363-3p mimics
compared with that after miR-NC transfection (Fig. 2A). It was then observed that
miR-363-3p overexpression significantly increased cellular
viability (Fig. 2B) and reduced
LDH release (Fig. 2C) induced by
OGD/R exposure in SH-SY5Y cells. Moreover, the percentage of
apoptotic cells was significantly lower in the miR-363-3p
mimic-transfected OGD/R SH-SY5Y cells than in the
miR-NC-transfected group (Fig.
2D). ELISA assay further confirmed that transfection of
miR-363-3p mimics significantly suppressed the levels of IL-1β
(Fig. 2E), IL-6 (Fig. 2F) and TNF-α (Fig. 2G) in OGD/R-treated SH-SY5Y
cells.
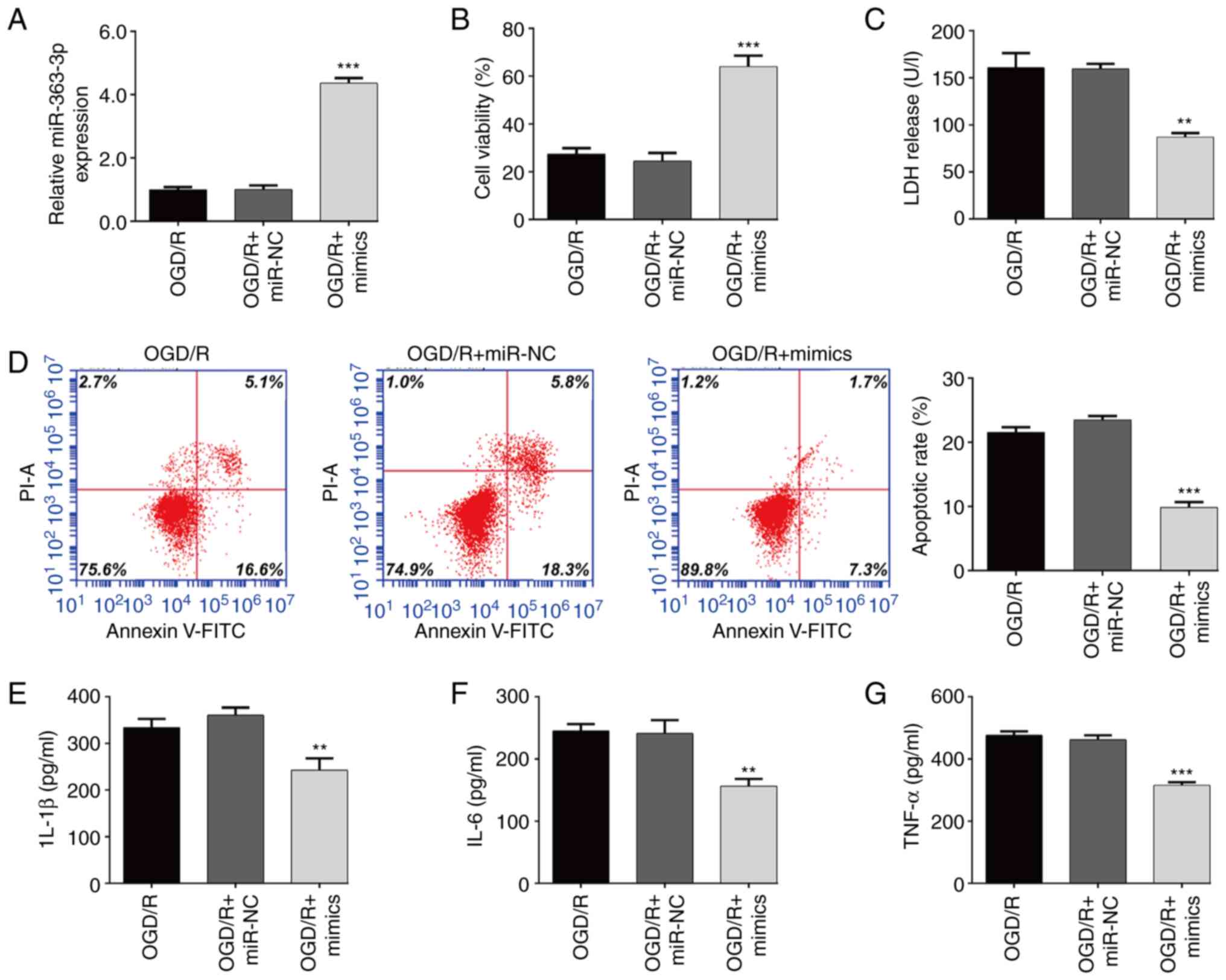 | Figure 2.Overexpression of miR-363-3p
attenuates OGD/R-induced apoptosis and inflammation in SH-SY5Y
cells. SH-SY5Y cells were transfected with miR-363-3p mimics and
miR-NC, and then received 4 h of OGD followed by 24 h of
re-oxygenation. (A) The transfection efficiency was determined by
RT-qPCR. (B) Cell viability was evaluated by CCK-8 assay. (C) The
release of LDH was detected by specific cytotoxicity assay kit. (D)
Cell apoptosis was evaluated by flow cytometer. ELISA assay was
applied to determine the levels of (E) IL-1β, (F) IL-6 and (G)
TNF-α. Data are presented as the mean ± SD. **P<0.01 and
***P<0.001 compared with OGD/R + miR-NC. miR, microRNA; OGD/R,
oxygen and glucose deprivation/re-oxygenation; NC, negative
control; RT-qPCR, reverse transcription-quantitative PCR; CCK-8,
Cell Counting Kit-8; LDH, lactate dehydrogenase; IL, interleukin;
TNF, tumor necrosis factor. |
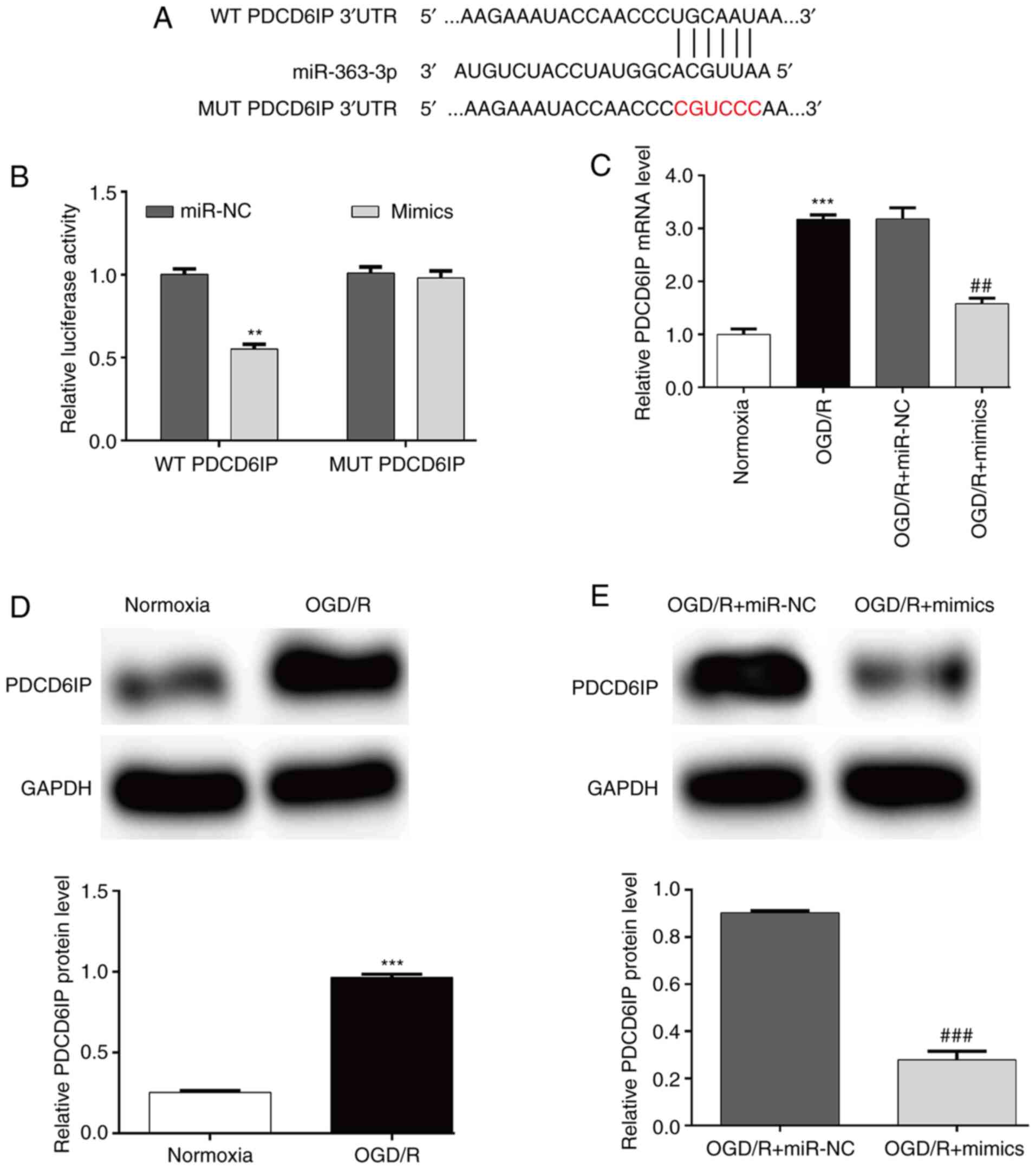
MiR-363-3p directly targets the 3′-UTR
of PDCD6IP
The mechanisms of how miR-363-3p attenuated
OGD/R-induced injury were further explored by searching its
possible targets. Among the predicted target genes, PDCD6IP was
screened as a candidate target for being reported to be involved in
apoptosis (31). As shown in
Fig. 3A, there was a putative
binding site between miR-363-3p and the 3′-UTR of PDCD6IP.
Luciferase reporter assay further demonstrated that transfection
with miR-363-3p mimics significantly reduced the relative
luciferase activity of WT PDCD6IP compared with that of the control
in SH-SY5Y cells, while the vector containing the site-mutated
sequence was not influenced by miR-363-3p mimics (Fig. 3B). Additionally, RT-qPCR analysis
indicated that the increased PDCD6IP mRNA expression induced by
OGD/R exposure could be significantly reversed by transfection with
miR-363-3p mimics in SH-SY5Y cells (Fig. 3C). Western blot analysis further
confirmed that the protein level of PDCD6IP was upregulated in the
OGD/R treatment group (Fig. 3D)
and this increased protein level of PDCD6IP was attenuated after
miR-363-3p overexpression (Fig.
3E). These results indicated that miR-363-3p may directly
target the expression of PDCD6IP by binding to its 3′UTR in
OGD/R-induced SH-SY5Y cells.
PDCD6IP knockdown suppresses
OGD/R-induced apoptosis and inflammation
Since increased PDCD6IP expression induced by OGD/R
treatment was reversed by miR-363-3p overexpression, it was thus
theorized that PDCD6IP could promote OGD/R-induced cell injury. To
confirm this, loss-of-function assays were performed in
OGD/R-treated SH-SY5Y cells. First, western blot analysis confirmed
that si-PDCD6IP transfection caused a significant decrease in
PDCD6IPO expression in OGD/R-induced SH-SY5Y cells (Fig. 4A). Next, the effects of PDCD6IP
knockdown on cell viability, apoptosis and inflammation were
examined. As revealed in Fig. 4B,
knockdown of PDCD6IP could increase cell viability in OGD/R-induced
cells. In line with miR-363-3p overexpression, knockdown of PDCD6IP
significantly alleviated OGD/R-induced LDH release (Fig. 4C) and apoptosis (Fig. 4D), and suppressed proinflammatory
cytokines, including the levels of IL-1β (Fig. 4E), IL-6 (Fig. 4F) and TNF-α (Fig. 4G).
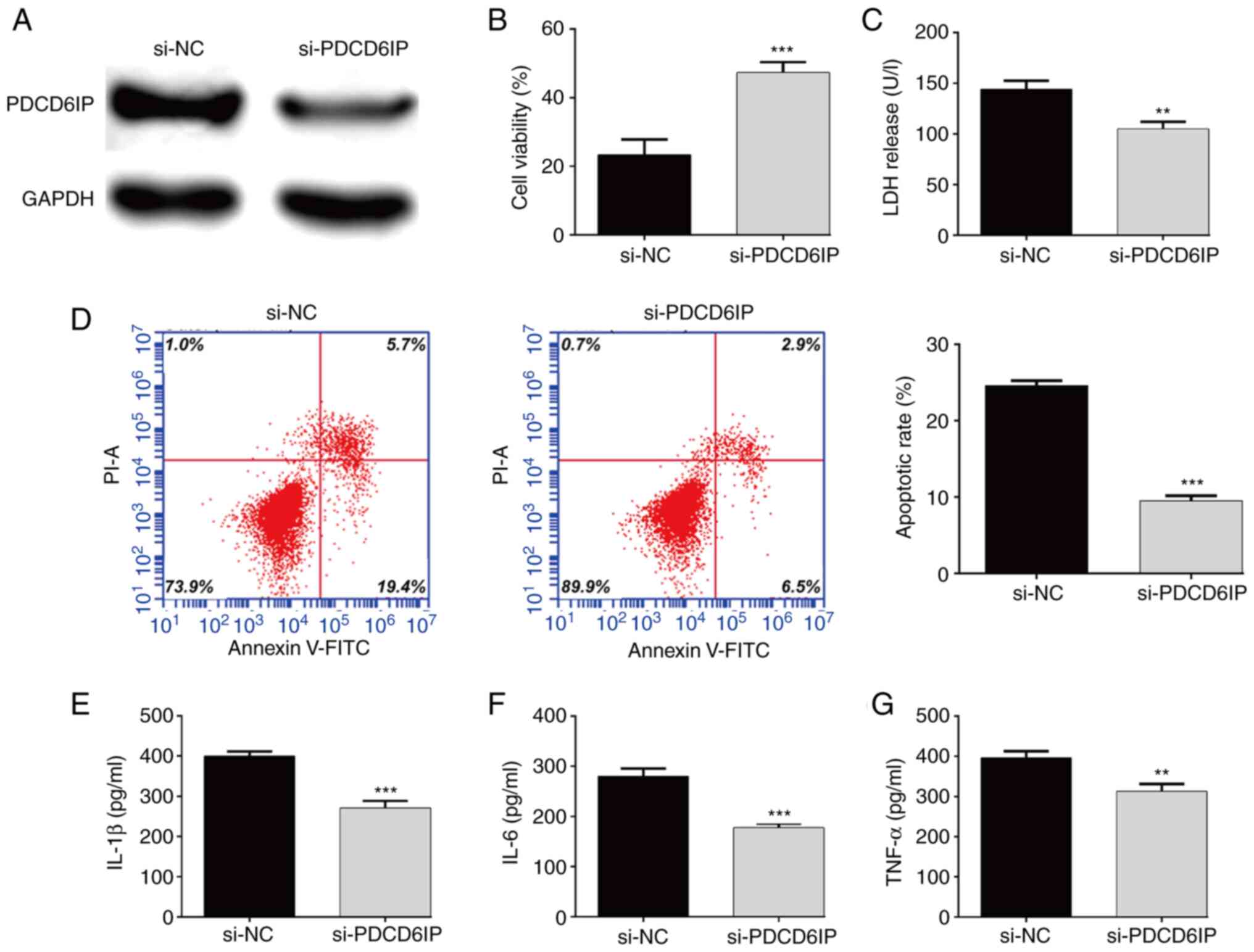 | Figure 4.PDCD6IP knockdown suppresses
OGD/R-induced apoptosis and inflammation. SH-SY5Y cells were
transfected with si-PDCD6IP, followed by OGD/R treatment. (A) The
protein expression level of PDCD6IP in SH-SY5Y cells was detected.
(B) The viability of SH-SY5Y cells was evaluated using the CCK-8
assay. (C) Cell cytotoxicity was analyzed using LDH assay. (D) Flow
cytometric analysis was conducted to detect apoptosis of SH-SY5Y
cells. ELISA assay was applied to determine the levels of (E)
IL-1β, (F) IL-6 and (G) TNF-α. Data are presented as the mean ± SD.
**P<0.01 and ***P<0.001 compared with si-NC. PDCD6IP,
programmed cell death 6-interacting protein; OGD/R, oxygen and
glucose deprivation/re-oxygenation; si-, small interfering; CCK-8,
Cell Counting Kit-8; LDH, lactate dehydrogenase; IL, interleukin;
TNF, tumor necrosis factor; NC, negative control. |
Overexpression of PDCD6IP counteracts
miR-363-3p-mediated inhibitory effects against OGD/R-induced
injury
To determine whether PDCD6IP was the downstream
regulator involved in miR-363-3p exerting protection against
OGD/R-induced injury, the overexpression of PDCD6IP in SH-SY5Y
cells after transfection with pcDNA3.1-PDCD6IP was first confirmed
(Fig. 5A). Rescue experiments
were then performed in SH-SY5Y cells by co-transfection with
miR-363-3p mimics and PDCD6IP overexpression plasmid, followed by
OGD/R treatment. It was first confirmed that the downregulation of
PDCD6IP induced by transfection with miR-363-3p mimics was markedly
reversed after PDCD6IP transfection (Fig. 5B). It was then observed that
overexpression of PDCD6IP counteracted the promoting effect of
miR-363-3p overexpression on the viability of OGD/R-induced SH-SY5Y
cells (Fig. 5C). PDCD6IP
overexpression reversed the miR-363-3p mimics-induced decrease in
LDH release (Fig. 5D) and
apoptosis (Fig. 5E) of
OGD/R-induced SH-SY5Y cells. Furthermore, the decrease in the
levels of IL-1β (Fig. 5F), IL-6
(Fig. 5G) and TNF-α (Fig. 5H) induced by miR-363-3p
overexpression were rescued after PDCD6IP overexpression. These
findings ascertained that the suppressive effects of miR-363-3p on
OGD/R-induced injury may be mediated via PDCD6IP inhibition.
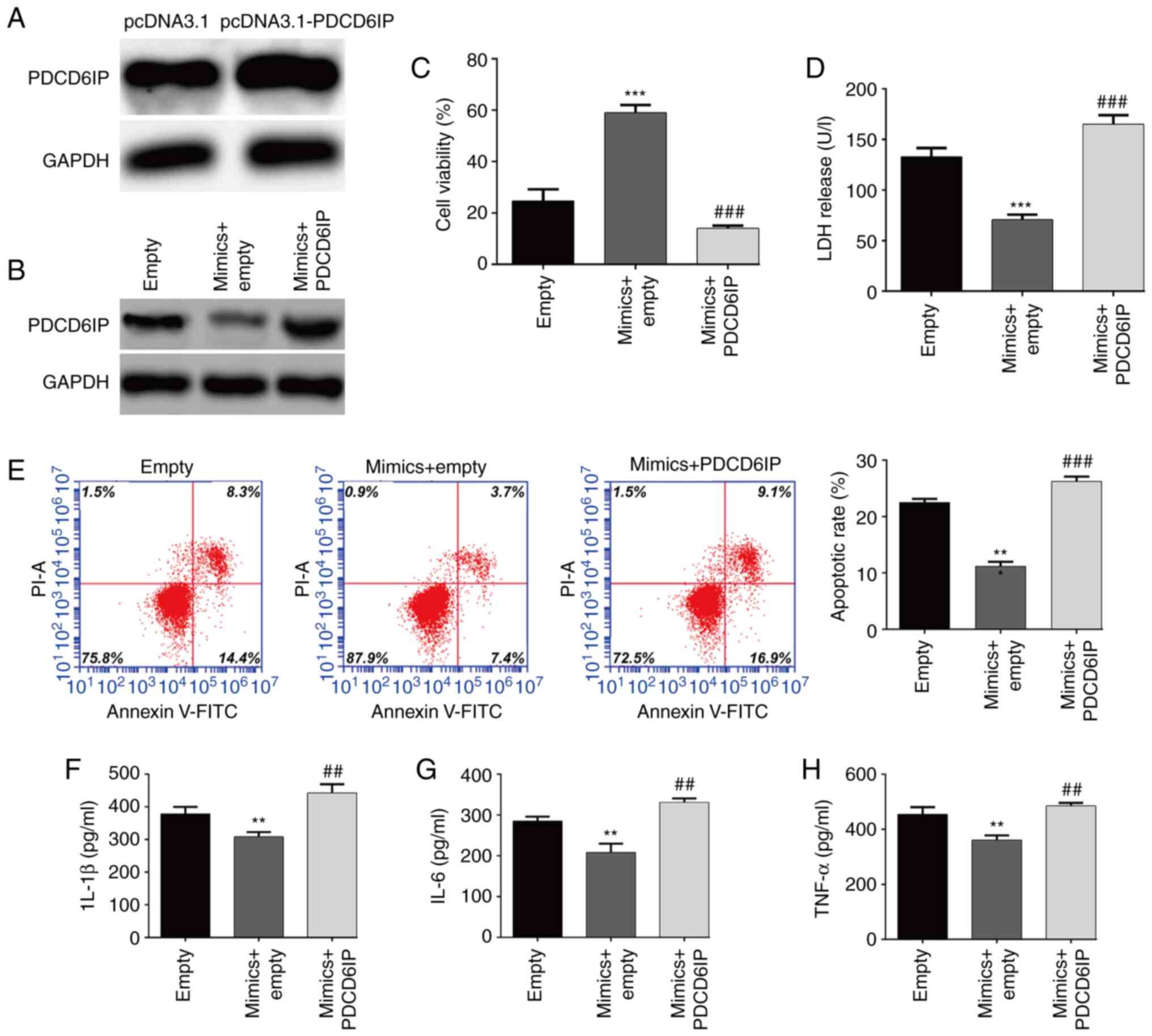 | Figure 5.Overexpression of PDCD6IP counteracts
the miR-363-3p-mediated protective effects against OGD/R-induced
injury. SH-SY5Y cells were divided into the following groups
according to different transfections: pcDNA3.1, pcDNA3.1-PDCD6IP,
empty vector, empty + miR-363-3p mimics, and miR-363-3p mimics +
PDCD6IP, followed by OGD/R treatment. (A and B) The protein
expression level of PDCD6IP was detected in SH-SY5Y cells from
different groups. (C) Cell viability, (D) LDH release and (E)
apoptosis were evaluated by CCK-8 assay, LDH assay and flow
cytometric analysis, respectively. (F-H) ELISA assays were applied
to determine the levels of IL-1β, IL-6 and TNF-α. Data are
presented as the mean ± SD. **P<0.01 and ***P<0.001 compared
with empty; and ##P<0.01 and ###P<0.001 compared with mimics
+ empty. PDCD6IP, programmed cell death 6-interacting protein; miR,
microRNA; OGD/R, oxygen and glucose deprivation/re-oxygenation;
LDH, lactate dehydrogenase; CCK-8, Cell Counting Kit-8; IL,
interleukin; TNF, tumor necrosis factor. |
Discussion
Cerebral I/R injury, a common event that occurs in
patients with acute ischemic stroke, is a complex systemic process
that involves inflammation, protein synthesis inhibition and
impaired mitochondrial function, causing irreversible dysfunctions
and structural damage (32). In
the last decades, miRNAs have become promising therapeutic targets
in ischemic stroke (33). When
studying the role of certain miRNAs in the pathogenesis of ischemic
stroke, the in vitro OGD/R procedure has usually been
applied to mimic cerebral I/R injury (34,35). Moreover, SH-SY5Y cells have been
frequently selected as the most commonly used cell line in five
models of ischemia-related injury, including OGD,
H2O2-induced oxidative stress, oxygen
deprivation, glucose deprivation and glutamate excitotoxicity
because of its human origin, catecholaminergic neuronal properties,
and ease of maintenance (36). In
the present study, an OGD/R injury model was successfully
constructed by exposing SH-SY5Y cells to OGD for 4 h, followed by
24 h of re-oxygenation. Before establishing 24 h of re-oxygenation,
it was determined that re-oxygenation for 48 h had different
effects compared with 24 h, including cell viability and LDH
release. In fact, OGD/R is an in vitro model that mimics the
in vivo process of a series of pathological reactions
initiated by I/R, which is a dynamic process previously described
(12,25,37). Similar with the aforementioned
studies (15,16), the expression of miR-363-3p was
decreased gradually and reached the lowest expression at 24 h
reoxygenation. The difference of cell viability and LDH release
between 24 and 48 h may be associated with the corresponding
altered miR-363-3p expression level, although these differences
were not significant. To sum up, the significantly decreased
miR-363-3p expression at OGD 4 h, followed by 24 h of
re-oxygenation may be associated with the pathogenesis of I/R
injury (15).
To the best of our knowledge, miR-363-3p has been
revealed to play an important role in the pathogenic processes of
malignant tumors, including proliferation, apoptosis, and
epithelial-mesenchymal transition in colorectal cancer (38), lung cancer (39), retinoblastoma (40), oral squamous cell carcinoma
(41), and acute myeloid leukemia
(42). Recent studies have
reported that miR-363-3p as a stroke neuroprotectant could improve
ischemic stroke outcomes in female rats (15,16). However, the regulatory role of
miR-363-3p in cerebral I/R-induced injury remains largely unclear.
In agreement with a previous study which reported that miR-363-3p
plays a protective role in renal fibrosis (RF) in vitro by
regulating the TGF-β2/Smad3 signaling pathway (43), our results revealed that
overexpression of miR-363-3p could significantly promote cell
viability, and suppress the production of pro-inflammatory
cytokines (TNF-α, IL-1β and IL-6) and cell apoptosis in
OGD/R-induced SH-SY5Y cells. Considering that excitotoxicity,
apoptosis and inflammation are the primary pathological causes of
neuronal loss following cerebral I/R (44,45), it was thus inferred that
miR-363-3p may have neuroprotective effects on simulated cerebral
ischemia in vitro. Moreover, miR-363-3p was revealed to
reduce inflammatory response and cell apoptosis in coronary
arterial endothelial cells (CAECs) but increase their viability,
which acts as a promising therapeutic target for coronary heart
disease (CHD) (19). By contrast,
miR-363-3p was significantly upregulated in osteoarthritis (OA)
model rats and in LPS-induced chondrocytes, which could promote
chondrocyte injury and apoptosis (46). In addition, downregulation of
miR-363-3p improved acute myocardial infarction-associated
endothelial injury by targeting KLF2 (47). These opposite regulatory roles of
miR-363-3p on cell apoptosis and inflammation may be ascribed to
different disease factors.
Mechanistically, our findings revealed that
miR-363-3p bound to the 3′-UTR region of PDCD6IP mRNA to inhibit
its expression, subsequently resulting in the suppression of
apoptosis and inflammation in OGD/R-induced SH-SY5Y cells. PDCD6IP
encodes for a protein that is known to bind to the products of the
PDCD6 gene, which is involved in the apoptosis pathway and was
demonstrated to decrease the risk of breast cancer in a sample of
Iranian women (21). PDCD6IP,
also named ALIX (20), was
previously reported to promote neuronal death (48,49). On the other hand, PDCD6IP has been
shown to be associated with inflammation reaction in response to
different stimuli, including cytokines (22,23) and glutaminase 1 (GLS1) during
neuroinflammation (24). Hence,
targeting PDCD6IP may be a potential therapeutic aim for preventing
apoptosis and inflammation causing neuronal cell injury. Our study
provided a new regulatory axis of miR-363-3p and PDCD6IP in
cerebral I/R injury, which may contribute to understanding of the
pathogenesis of ischemic stroke. However, there were some
limitations in the present study including i) lack of further
experiments to confirm how PDCD6IP can completely counteract the
effects of miR-363-3p; ii) lack of an in vivo I/R injury rat
model to further confirm the role of the miR-363-3p/PDCD6IP axis in
cerebral I/R injury; and iii) more targets of miR-363-3p need to be
identified in cerebral 1/R injury.
In summary, our study revealed that miR-363-3p was
significantly downregulated in OGD/R-induced neurons, and
overexpression of miR-363-3p could attenuate OGD/R-induced neuron
apoptosis and inflammation in vitro, which may be mediated,
at least in part, via inhibition of PDCD6IP (Fig. S1). Our findings suggest that
miR-363-3p may be a potential therapeutic target for cerebral I/R
injury.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC made substantial contributions to the conception
of the present study. YW and JJ were involved in the acquisition,
analysis and interpretation of data for the work. ZX dperformed the
statistical analysis and wrote the manuscript. HC and YW confirm
the authenticity of all the raw data. All authors agreed the final
version of manuscript to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chugh C: Acute ischemic stroke: Management
approach. Indian J Crit Care Med. 23 (Suppl 2):S140–S146. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu F, Ma R, Zhang G, Wang S, Yin J, Wang
E, Xiong E, Zhang Q and Li Y: Estrogen and propofol combination
therapy inhibits endoplasmic reticulum stress and remarkably
attenuates cerebral ischemia-reperfusion injury and OGD injury in
hippocampus. Biomed Pharmacother. 108:1596–1606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan RY, Wang SJ, Yao GT, Liu ZG and Xiao
N: The protective effect and its mechanism of 3-n-butylphthalide
pretreatment on cerebral ischemia reperfusion injury in rats. Eur
Rev Med Pharmacol Sci. 21:5275–5282. 2017.PubMed/NCBI
|
|
4
|
Tuttolomondo A, Di Sciacca R, Di Raimondo
D, Renda C, Pinto A and Licata G: Inflammation as a therapeutic
target in acute ischemic stroke treatment. Curr Top Med Chem.
9:1240–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ornellas FM, Ornellas DS, Martini SV,
Castiglione RC, Ventura GM, Rocco PR, Gutfilen B, de Souza SA,
Takiya CM and Morales MM: Bone marrow-derived mononuclear cell
therapy accelerates renal ischemia-reperfusion injury recovery by
modulating inflammatory, antioxidant and apoptotic related
molecules. Cell Physiol Biochem. 41:1736–1752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yingjie K, Haihong Y, Lingwei C, Sen Z,
Yuanting D, Shasha C, Liutong P, Ying W and Min Z: Apoptosis
repressor with caspase recruitment domain deficiency accelerates
ischemia/reperfusion (I/R)-induced acute kidney injury by
suppressing inflammation and apoptosis: The role of AKT/mTOR
signaling. Biomed Pharmacother. 112:1086812019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv Z, Liu C, Zhai M, Zhang Q, Li J, Zheng
F and Peng M: LPS pretreatment attenuates cerebral
ischaemia/reperfusion injury by inhibiting inflammation and
apoptosis. Cell Physiol Biochem. 45:2246–2256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rippe C, Blimline M, Magerko KA, Lawson
BR, LaRocca TJ, Donato AJ and Seals DR: MicroRNA changes in human
arterial endothelial cells with senescence: Relation to apoptosis,
eNOS and inflammation. Exp Gerontol. 47:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh RP, Massachi I, Manickavel S, Singh
S, Rao NP, Hasan S, Mc Curdy DK, Sharma S, Wong D, Hahn BH and
Rehimi H: The role of miRNA in inflammation and autoimmunity.
Autoimmun Rev. 12:1160–1165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrante M and Conti GO: Environment and
neurodegenerative diseases: An update on miRNA role. Microrna.
6:157–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang R, Bao H, Zhang S, Li R, Chen L and
Zhu Y: miR-186-5p promotes apoptosis by targeting IGF-1 in SH-SY5Y
OGD/R model. Int J Biol Sci. 14:1791–1799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren X, Wang Z and Guo C: MiR-195-5p
ameliorates cerebral ischemia-reperfusion injury by regulating the
PTEN-AKT signaling pathway. Neuropsychiatr Dis Treat. 17:1231–1242.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Yao J and Feng K: miR-124-5p/NOX2
axis modulates the ROS production and the inflammatory
microenvironment to protect against the cerebral I/R injury.
Neurochem Res. 45:404–417. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sohrabji F and Selvamani A: Sex
differences in miRNA as therapies for ischemic stroke. Neurochem
Int. 127:56–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Selvamani A and Sohrabji F: Mir363-3p
improves ischemic stroke outcomes in female but not male rats.
Neurochem Int. 107:168–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang K, Yan L and Lu F: miR-363-3p
inhibits osteosarcoma cell proliferation and invasion via targeting
SOX4. Oncol Res. 27:157–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Lai W, Deng Y, Liu M, Dong M, Liu
Z, Wang T, Li X, Zhao Z, Yin X, et al: MicroRNA-363-3p promotes
apoptosis in response to cadmium-induced renal injury by
down-regulating phosphoinositide 3-kinase expression. Toxicol Lett.
345:12–23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou T, Li S, Yang L and Xiang D:
microRNA-363-3p reduces endothelial cell inflammatory responses in
coronary heart disease via inactivation of the NOX4-dependent p38
MAPK axis. Aging (Albany NY). 13:11061–11082. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Missotten M, Nichols A, Rieger K and
Sadoul R: Alix, a novel mouse protein undergoing calcium-dependent
interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell
Death Differ. 6:124–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashemi M, Yousefi J, Hashemi SM, Amininia
S, Ebrahimi M, Taheri M and Ghavami S: Association between
programmed cell death 6 interacting protein insertion/deletion
polymorphism and the risk of breast cancer in a sample of Iranian
population. Dis Markers. 2015:8546212015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ajasin DO, Rao VR, Wu X, Ramasamy S,
Pujato M, Ruiz AP, Fiser A, Bresnick AR, Kalpana GV and Prasad VR:
CCL2 mobilizes ALIX to facilitate Gag-p6 mediated HIV-1 virion
release. Elife. 8:e355462019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Sun G, Yang J, Sun Q and Tong Z:
Interleukin-13 promotes expression of Alix to compromise renal
tubular epithelial barrier function. Cell Biol Int. 39:548–553.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu B, Liu J, Zhao R, Li Y, Peer J, Braun
AL, Zhao L, Wang Y, Tong Z, Huang Y and Zheng JC: Glutaminase 1
regulates the release of extracellular vesicles during
neuroinflammation through key metabolic intermediate
alpha-ketoglutarate. J Neuroinflammation. 15:792018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Yang C and Wang Y: miR-126
overexpression attenuates oxygen-glucose deprivation/reperfusion
injury by inhibiting oxidative stress and inflammatory response via
the activation of SIRT1/Nrf2 signaling pathway in human umbilical
vein endothelial cells. Mol Med Rep. 23:1652021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Cai Q, Lin S, Chen B, Jia B, Ye
R, Weygant N, Chu J and Peng J: Qingda granule exerts
neuroprotective effects against ischemia/reperfusion-induced
cerebral injury via lncRNA GAS5/miR-137 signaling pathway. Int J
Med Sci. 18:1687–1698. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rong H, Chen B, Wei X, Peng J, Ma K, Duan
S and He J: Long non-coding RNA XIST expedites lung adenocarcinoma
progression through upregulating MDM2 expression via binding to
miR-363-3p. Thorac Cancer. 11:659–671. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JF, Zhang L, Shi LL, Zhao ZH, Xu H,
Liang F, Li HB, Zhao Y, Xu X, Yang K and Tian YF: Parthenolide
attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β
pathway in PC12 cells. Biomed Pharmacother. 89:1159–1165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian M, Yang M, Li Z, Wang Y, Chen W, Yang
L, Li Y and Yuan H: Fluoxetine suppresses inflammatory reaction in
microglia under OGD/R challenge via modulation of NF-κB signaling.
Biosci Rep. 39:BSR201815842019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatellard-Causse C, Blot B, Cristina N,
Torch S, Missotten M and Sadoul R: Alix (ALG-2-interacting protein
X), a protein involved in apoptosis, binds to endophilins and
induces cytoplasmic vacuolization. J Biol Chem. 277:29108–29115.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaller B and Graf R: Cerebral ischemia
and reperfusion: the pathophysiologic concept as a basis for
clinical therapy. J Cereb Blood Flow Metab. 24:351–371. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vasudeva K and Munshi A: miRNA
dysregulation in ischaemic stroke: Focus on diagnosis, prognosis,
therapeutic and protective biomarkers. Eur J Neurosci.
52:3610–3627. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu S, Li Y, Chen JP, Li DZ, Jiang Q, Wu T
and Zhou XZ: Oxygen glucose deprivation/re-oxygenation-induced
neuronal cell death is associated with Lnc-D63785 m6A methylation
and miR-422a accumulation. Cell Death Dis. 11:8162020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chai Z, Gong J, Zheng P and Zheng J:
Inhibition of miR-19a-3p decreases cerebral ischemia/reperfusion
injury by targeting IGFBP3 in vivo and in vitro. Biol Res.
53:172020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Eaton ED, Wills TE, McCann SK,
Antonic A and Howells DW: Human ischaemic cascade studies using
SH-SY5Y cells: A systematic review and meta-analysis. Transl Stroke
Res. 9:564–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiang Y, Zhang Y, Xia Y, Zhao H, Liu A and
Chen Y: LncRNA MEG3 targeting miR-424-5p via MAPK signaling pathway
mediates neuronal apoptosis in ischemic stroke. Aging (Albany NY).
12:3156–3174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong J, Geng J and Tan W: MiR-363-3p
suppresses tumor growth and metastasis of colorectal cancer via
targeting SphK2. Biomed Pharmacother. 105:922–931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang J, Gao F, Chu H, Lou L, Wang H and
Chen Y: miR-363-3p inhibits migration, invasion, and
epithelial-mesenchymal transition by targeting NEDD9 and SOX4 in
non-small-cell lung cancer. J Cell Physiol. 235:1808–1820. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma X, Jin L, Lei X, Tong J and Wang R:
MicroRNA-363-3p inhibits cell proliferation and induces apoptosis
in retinoblastoma cells via the Akt/mTOR signaling pathway by
targeting PIK3CA. Oncol Rep. 43:1365–1374. 2020.PubMed/NCBI
|
|
41
|
Zhu L, Zhang L, Tang Y, Zhang F, Wan C, Xu
L and Guo P: MicroRNA-363-3p inhibits tumor cell proliferation and
invasion in oral squamous cell carcinoma cell lines by targeting
SSFA2. Exp Ther Med. 21:5492021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Chen S, Lu J, Yuan D, He L, Qin P,
Tan H and Xu L: MicroRNA-363-3p promote the development of acute
myeloid leukemia with RUNX1 mutation by targeting SPRYD4 and
FNDC3B. Medicine (Baltimore). 100:e258072021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong X, Li Y, Cao R and Xu H:
MicroRNA-363-3p inhibits the expression of renal fibrosis markers
in TGF-β1-treated HK-2 cells by targeting TGF-β2. Biochem Genet.
59:1033–1048. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dirnagl U, Iadecola C and Moskowitz MA:
Pathobiology of ischaemic stroke: An integrated view. Trends
Neurosci. 22:391–397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chamorro Á, Dirnagl U, Urra X and Planas
AM: Neuroprotection in acute stroke: Targeting excitotoxicity,
oxidative and nitrosative stress, and inflammation. Lancet Neurol.
15:869–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang M, Wang Z, Li B, Sun F, Chen A and
Gong M: Identification of microRNA-363-3p as an essential regulator
of chondrocyte apoptosis in osteoarthritis by targeting NRF1
through the p53-signaling pathway. Mol Med Rep. 21:1077–1088.
2020.PubMed/NCBI
|
|
47
|
Gao C, Qian H, Shi Q and Zhang H:
MicroRNA-363-3p serves as a diagnostic biomarker of acute
myocardial infarction and regulates vascular endothelial injury by
targeting KLF2. Cardiovasc Diagn Ther. 10:421–430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hemming FJ, Fraboulet S, Blot B and Sadoul
R: Early increase of apoptosis-linked gene-2 interacting protein X
in areas of kainate-induced neurodegeneration. Neuroscience.
123:887–895. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Blum D, Hemming FJ, Galas MC, Torch S,
Cuvelier L, Schiffmann SN and Sadoul R: Increased Alix
(apoptosis-linked gene-2 interacting protein X) immunoreactivity in
the degenerating striatum of rats chronically treated by
3-nitropropionic acid. Neurosci Lett. 368:309–313. 2004. View Article : Google Scholar : PubMed/NCBI
|