Introduction
Hepatocellular carcinoma (HCC) was the sixth most
commonly reported cancer and the third most common cause of cancer
death in the world in 2020 (1).
With research of HCC, the methods of treatment have developed
rapidly. Following traditional surgery, radiotherapy and
chemotherapy, novel methods (such as interventional, molecular
targeted and cell therapy and immunotherapy) have been widely used
in the treatment of HCC (2–4).
However, the prognosis of most patients is still poor, which is
related to the poor response of regimens for treatment (5), numerous side effects (including
gastrointestinal reaction, cutaneous pruritus) (6) and multidrug resistance (7). Therefore, exploration of effective
and safe drugs for the treatment of HCC is an urgent clinical
problem.
Rhodiola rosea L, also known as ‘golden
root’, is a perennial herbaceous plant of the crassulaceae family
and is widely distributed in zones with severe cold, dry and
hypoxic conditions at high altitude (3,500-5,000 meters) (8). In Asia, Rhodiola rosea L is
used as a herbal medicine to relieve certain symptoms, such as
headache and hernias (9). In
Europe and North America, Rhodiola rosea L is used as a
dietary supplement (10,11). According to modern pharmacology
reports, the key metabolites of Rhodiola rosea L include
phenethyl alcohol derivatives, monoterpenes, triterpenes,
flavonoids and phenolic acids (12,13). Salidroside (Sal), a primary
component, is extracted from the rhizome of Rhodiola rosea
L. An increasing number of studies have reported that Sal not only
demonstrates anti-hypoxia and anti-aging effects (14,15), but also demonstrates anti-fibrotic
and immune regulatory effects (16,17). Therefore, development of Sal for
treatment of disease is a promising field.
Induction of cancer cell apoptosis is one of the
goals of anti-cancer treatment. Apoptosis is an active
physiological response and the primary form of type I programmed
cell death, which is characterized by eversion of the membrane,
condensation of nuclear chromatin, splitting of the nucleus and
formation of apoptotic bodies (18). Autophagy is a pathway of type II
programmed cell death, which is different from apoptosis. Autophagy
primarily refers to the action of autophagic vesicles, which wrap
senescent or damaged organelles in a double-layered membrane to
form autophagosomes when cells receive certain stimuli (such as
starvation or hypoxia). Autophagosomes combine with lysosomes to
form autolysosomes, which degrade the wrapped contents to maintain
the stability of internal environment and the renewal of organelles
(19). Autophagy has become a hot
topic in anti-cancer therapy (20,21). At present, the association between
autophagy and apoptosis is still controversial (22). In tumorigenesis and development,
autophagy may serve two opposite roles; autophagy can act as a
protective mechanism to resist stress response and inhibit
apoptosis. For example, Wang et al (23) reported that pseudolaric acid B
induces autophagy and inhibits apoptosis in human lung fibroblast
MRC5 cells via the response to DNA damage. Furthermore, autophagy
produces certain products (such as amino acids and fatty acids),
which provide nutrients for the growth of tumor (24,25). However, autophagy synergistically
induces apoptosis. For example, resveratrol synergistically treats
ovarian cancer by inducing autophagy in human ovarian cancer cells
(26). Combined treatment with
matrine and 5-fluorouracil upregulates expression of
autophagy-associated genes Atg5 and Beclin-1 to enhance the
chemosensitivity of liver cancer cells (27). Thus, autophagy exerts a dual
effect in the therapy of cancer.
Increasing studies have reported that glycosides,
which are derived from traditional Chinese medicine, treat cancer
through the autophagy pathway (28,29). For example, ginsenoside F2 induces
autophagy by upregulating expression levels of Atg7, Beclin-1 and
microtubule-associated protein light chain 3 (LC3)B proteins in
human breast cancer stem cells; moreover, combined treatment with
the autophagy inhibitor chloroquine enhances ginsenoside F2-induced
apoptosis (30). Pulsatilla
saponin D induces autophagy by activating phosphorylation of ERK
and inhibiting phosphorylation of mTOR and p70S6K, which enhances
the level of human cervical cancer HeLa cell apoptosis (31).
There are numerous reports for the use of Sal in the
treatment of cancer (32,33), but to the best of our knowledge,
there are few studies (34,35) on Sal-induced apoptosis and
autophagy of liver cancer cells in Chinese and international
literature. In particular, there are no reports on the mechanism of
Sal-induced apoptosis and autophagy in liver cancer cells.
Therefore, in the present study, the mechanisms of Sal-induced
apoptosis and autophagy in highly metastatic human hepatoma cells
(MHCC97-H, 97H) were evaluated. Moreover, to assess the association
between autophagy and apoptosis, chloroquine diphosphate (CQ), a
commonly used inhibitor of the autophagic pathway, was used in the
present study.
Materials and methods
Reagents and antibodies
Sal (purity, 99.56%), CQ (purity, 99.65%) and
rapamycin (Rap; purity, 99.30%) were purchased from Selleck
Chemicals. DMEM, RPMI-1640 medium and phosphate-buffered saline
(PBS) were purchased from Cytiva. Trypsin-EDTA solution, 1% crystal
violet stain solution, 1% osmium tetroxide, saturated uranyl
acetate, lead citrate, Hoechst33342 dye, dansylcadaverine (MDC,
cat. no. G0170) staining kit, radioimmunoprecipitation assay (RIPA,
cat. no. R0010), phenylmethylsulfonyl fluoride (PMSF, cat. no.
P0100), SDS-PAGE gel preparation kit (cat. no. P1200), Tween-20,
10% SDS, 20X Tris-HCl buffered saline, 10X transfer buffer, BCA
protein assay kit and 4X bromophenol blue buffer were purchased
from Beijing Solarbio Science & Technology Co., Ltd.
Penicillin-streptomycin (100X) was purchased from
Shanghai Basal Media Technologies Co., Ltd. Fetal bovine serum
(FBS) was purchased from Sartorius AG. 4.0 and 2.5%
paraformaldehyde and extra-enhanced chemiluminescence substrate kit
(cat. no. BL520A) were purchased from Biosharp Life Sciences. Cell
Counting Kit-8 (CCK-8) and three-color pre-stained protein marker
were purchased from Yeasen Biotechnology (Shanghai) Co., Ltd. 95%
absolute ethanol was purchased from Tianjin Damao Chemical Reagent
Factory Difco™ skimmed milk and glycine were purchased from Beijing
Biotopped Technology Co., Ltd. Tris was purchased from Shanghai
Scigrace Biotech Co., Ltd. Anti-β-actin (cat. no. GTX109639),
anti-GAPDH (cat. no. GTX627408), anti-β-tubulin (cat. no.
GTX101279) and anti-p62 (cat. no. GTX100685) antibodies were
purchased from GeneTex, Inc. Anti-C-myc (cat. no. 67447-1-Ig),
anti-Bax (cat. no. 60267-1-Ig), anti-Bcl-2 (cat. no. 12789-1-AP),
anti-Caspase-3 (cat. no. 19677-1-AP), anti-Caspase-9 (cat. no.
10380-1-AP) and anti-Beclin-1 (cat. no. 1C10C4) antibodies were
purchased from ProteinTech Group, Inc. Anti-LC3-I/II (cat. no.
ab192890) antibodies were purchased from Abcam. Anti-PI3K (cat. no.
YM3503), anti-p-PI3K (cat. no. YP0765), anti-Akt (cat. no. YT0185),
anti-p-Akt (cat. no. YP0006), anti-mTOR (YT2913) and anti-p-mTOR
(YP0176) antibodies were purchased from ImmunoWay Biotechnology
Company. Horseradish peroxidase-conjugated goat anti-rabbit IgG
antibodies (cat. no. TA130023) were purchased from OriGene
Technologies, Inc.
Cell culture and treatment
The hepatocellular carcinoma 97H and epithelial
THLE-2 and Hs578Bst cell lines were purchased from the Cell Bank of
The Shanghai Institute for Biological Sciences. 97H cells were
cultured in DMEM with 10% FBS. THLE-2 and Hs578Bst cells were
cultured in RPMI-1640 medium with 10% FBS. All cells were incubated
at 37°C in a humidified atmosphere with 5% CO2. Cells
were treated with or without Sal (5, 10, 20, 40 and 80 µM) and/or
CQ (5, 10 and 20 µM) for 24, 48 and 72 h at 37°C. Cells were also
treated with or without Sal (80 µM) and/or Rap (400 nM) for 48 h at
37°C.
Cell growth curve
The 97H cells (80–90% confluence) were seeded in
12-well plates (3,000 cells/well) at 37°C for 24 h. Following
incubation, the number of untreated 97H cells was assessed using a
multifunctional cell analyzer (ACEABioscience, Inc.).
Cell viability assay
97H, THLE-2 and Hs578Bst cells (5×103
cells/well) were seeded in 96-well plates and incubated at 37°C
with 5% CO2 in a humidified environment for 24 h. The
cells were treated with Sal (0, 5, 10, 20, 40 and 80 µM) and/or CQ
(5, 10 and 20 µM), as aforementioned. A total of 10 µl CCK-8
reagent was added into each well and the cells were incubated at
37°C in the dark for 1 h. The cell viability assay was performed
using a microplate reader (Bio-Rad Laboratories, Inc.; excitation
wavelength, 450 nm).
Colony formation assay
97H cells (80–90% confluence) were seeded in 60-mm
dishes (500 cells/ml) and incubated at 37°C for 24 h. Following
incubation, the untreated cells in the blank group and treated 97H
cells were washed twice using PBS, cultured with complete medium
(10% FBS + 90% DMEM) and incubated in the incubator (5%
CO2, 37°C) for 14 days. The colonies (>50 cells) were
fixed using 4% paraformaldehyde for 30 min at room temperature and
stained using 1% crystal violet for 30 min at room temperature.
Stained cells were observed and photographed using an inverted
microscope (Olympus Corporation, magnification, ×40, ×200), and the
colonies of each group were counted manually.
Transmission electron microscopy
(TEM)
To evaluate the ultrastructure, cells in the blank
group and treated 97H cells were washed twice with PBS. Cells were
fixed using 2.5% paraformaldehyde for 90 min and 1% osmium
tetroxide for 30 min at 37°C. Following gradient dehydration with
95% absolute ethanol, cells were embedded in the resin, sectioned
into 60 nm thin slices, stained using saturated uranyl acetate for
30 min at 37°C, and stained using lead citrate for 10 min at 37°C.
Images were captured using a transmission electron microscope
(Hitachi, Ltd.; magnification, ×5,000 and ×8,000).
Hoechst33342 staining
97H cells (80–90% confluence) were incubated on a
glass slide in 12-well plates at 37°C for 24 h. Following
incubation, the glass slides were washed twice with PBS and cells
were fixed using 4% paraformaldehyde for 30 min at 37°C. Glass
slides were stained using 10 µg/ml Hoechst33342 dye for 30 min in
the dark at 37°C and washed twice with PBS. Stained cells were
observed using a fluorescence microscope (Olympus Corporation;
excitation wavelength, 488 nm; magnification, ×100 and ×200).
MDC staining
97H cells (80–90% confluence) were plated on glass
slides in 12-well plates at 37°C for 24 h. Following incubation,
cells in blank group and treated 97H cells were washed twice with
1X wash buffer, stained with MDC dye for 30 min in the dark at 37°C
and washed again with 1X wash buffer three times. Stained cells
were observed under a fluorescence microscope (excitation
wavelength, 355 nm; magnification, ×200).
Western blotting
Cells in the blank group and treated 97H cells were
lysed using pre-cooled RIPA lysis buffer (500 µl RIPA; 5 µl PMSF)
for 30 min at 4°C. Protein concentration was assessed using BCA
protein assay kit. Protein (30 µg/lane) was separated using 6, 10
and 15% SDS-PAGE. According to the different molecular weight of
the target protein, the corresponding concentration of SDS-PAGE was
selected. Subsequently, the SDS-PAGE was transferred to
polyvinylidene fluoride membranes. Membranes were blocked using 5%
skimmed milk for 2 h at room temperature and washed using
Tris-buffered saline with 0.1% Tween-20 for 15 min at room
temperature. Membranes were probed at 4°C overnight with antibodies
as follows: Anti-β-actin (1:2,000), anti-GAPDH (1:5,000),
anti-β-tubulin (1:2,000), anti-C-myc (1:1,000), anti-Bax (1:1,000),
anti-Bcl-2 (1:1,000), anti-Caspase-3 (1:1,000), anti-caspase-9
(1:1,000), anti-Beclin-1 (1:2,000), anti-p62 (1:1,000),
anti-LC3-I/II (1:1,000), anti-PI3K (1:1,000), anti-p-PI3K (1:500),
anti-Akt (1:1,000), anti-p-Akt (1:1,000), anti-mTOR (1:1,000) and
anti-p-mTOR (1:500). Membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG antibody (1:5,000) for 2
h at room temperature. Protein expression was assessed using an
extra-enhanced chemiluminescence substrate kit and gel imaging
system (Bio-Rad Laboratories, Inc.). Protein expression levels were
semi-quantified using Image Lab 5.1.0 software (Bio-Rad
Laboratories, Inc.) and processed using GraphPad Prism 8.0.2
software (GraphPad Software, Inc.).
Statistical analysis
All experiments were performed at least three times.
The data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS 22.0 statistical
software (IBM Corp.). Differences between multiple groups were
compared using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sal suppresses proliferation of 97H
cells in vitro
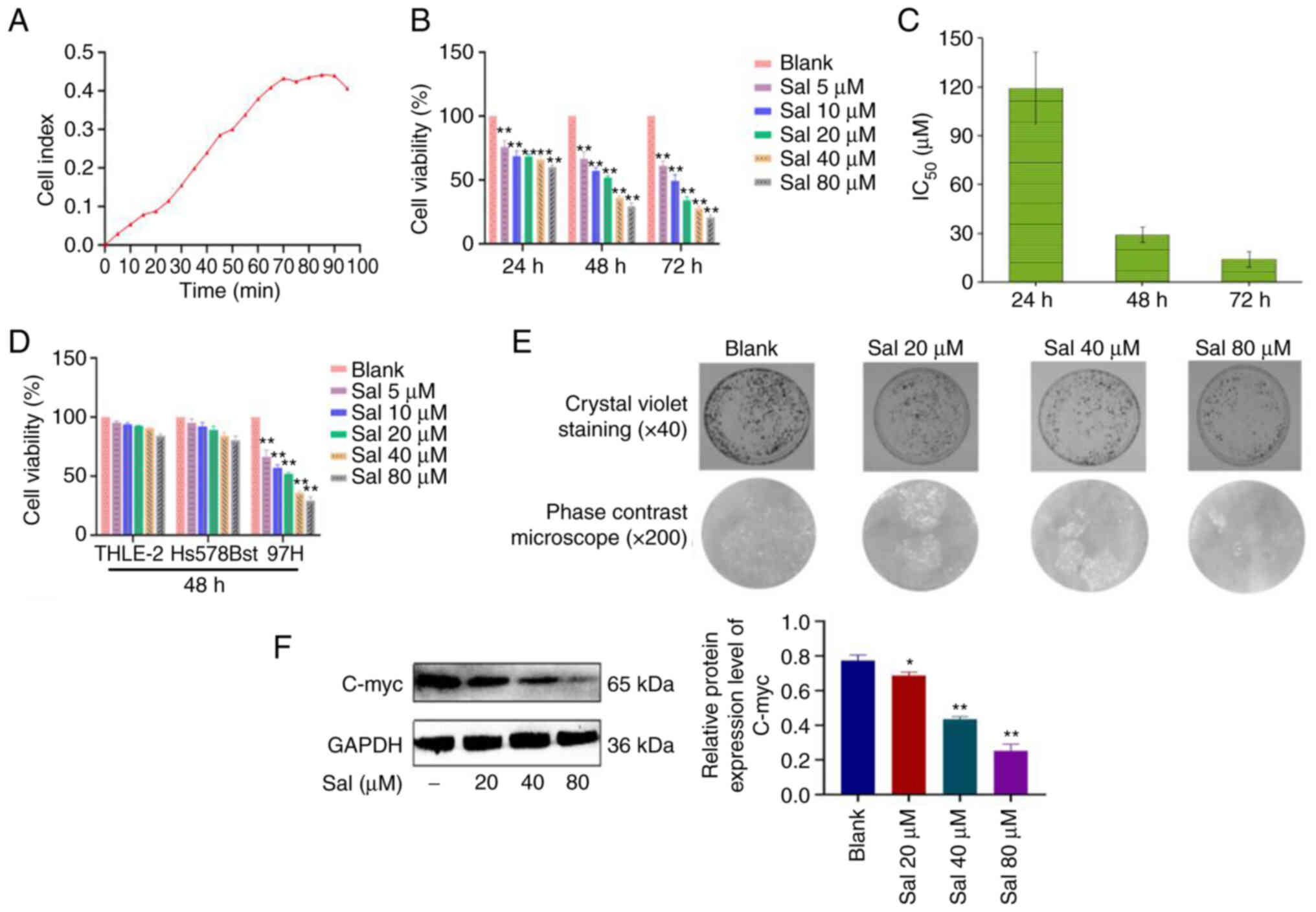
The growth curves of 97H cells were assessed using a
multifunctional cell analyzer without any drug intervention. The
growth curve of untreated cells can be divided into slow growth
latency (0–25 h), exponential growth phase with a larger slope
(25–70 h), plateau-shaped flat-top phase (70–90 h) and degenerate
decay phase (90–95 h) (Fig. 1A).
These data provided a basis for the processing time of subsequent
experiments. 97H cells were treated with Sal (5, 10, 20, 40 and 80
µM) for 24, 48 and 72 h. Compared with the blank group, Sal
demonstrated significant inhibitory effects on viability of 97H
cells in a dose-dependent manner at different times (P<0.01;
Fig. 1B). The values of half
maximal inhibitory concentration (IC50) at 24, 48 and 72
h were 119.2, 29.1 and 14.1 µM respectively (Fig. 1C). To evaluate the safety of Sal,
normal human liver THLE-2 cells and normal human breast Hs578Bst
cells were treated with Sal (5, 10, 20, 40 and 80 µM) for 48 h. Sal
had no significant effect on the viability of THLE-2 and Hs578Bst
cells (P>0.05; Fig. 1D). Based
on these results, it was decided to treat 97H cells with 20, 40 and
80 µM Sal for 48 h in subsequent experiments. Following treatment
with Sal (20, 40 and 80 µM) for 48 h, compared with the blank
group, the Sal group markedly decreased the number of 97H colonies
in a dose-dependent manner (Fig.
1E). Results of western blotting demonstrated that compared
with the blank group, expression levels of C-myc protein in the Sal
group were significantly down-regulated in a dose-dependent manner
(P<0.05; Fig. 1F).
Sal induces organelle impairment and
autophagy
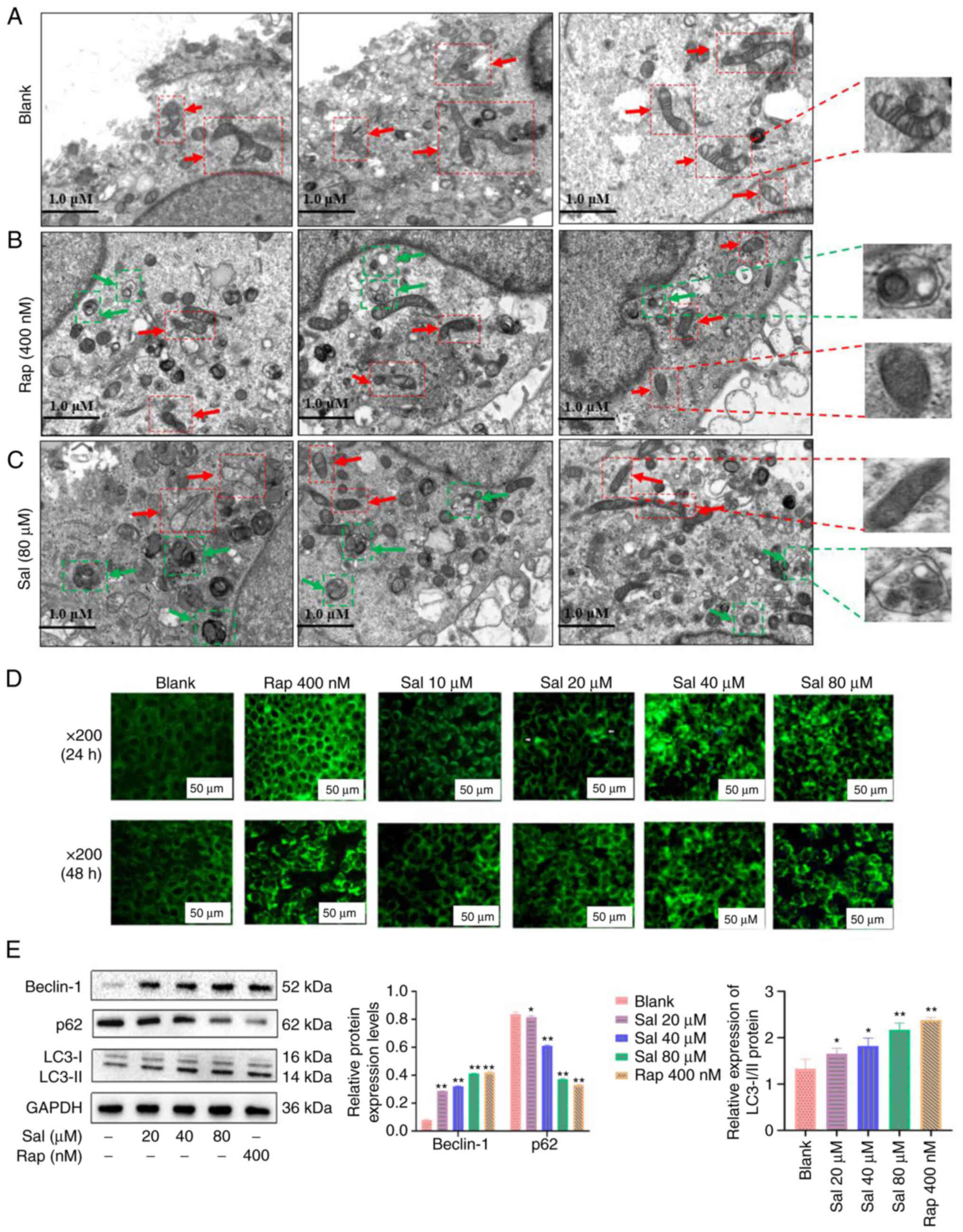
In the present study, TEM was used to evaluate the
organelle of 97H cells following Sal or autophagic inducer (Rap)
treatment. The mitochondria of blank cells were rich in content,
uniform in size, clear in mitochondrial crista, and complete in
membranous structure (Fig. 2A),
numerous damaged mitochondria were observed in the Sal- or
Rap-treated 97H cells at 48 h (Fig.
2B and C, respectively). Furthermore, numerous autophagosomes
with double membranes were observed in the Sal or Rap-treated 97H
cells at 48 h. Cytoplasmic material and/or membrane vesicles were
encapsulated within the autophagosomes. To evaluate the phenomenon
of autophagy, MDC staining was used to assess the autophagy product
(autophagosomes) following Sal or Rap treatment in 97H cells. The
green fluorescence associated with autophagosomes was not
demonstrated in the blank group; green fluorescence was gradually
increased in a dose-dependent manner in the Sal or Rap-treated 97H
cells (Fig. 2D). Western blotting
demonstrated that compared with the blank and Rap group, the ratio
of LC3-II to LC3-I protein expression levels was markedly
upregulated in the Sal group with a dose-dependent manner
(P<0.05); meanwhile, the Beclin-1 protein expression levels in
the Sal group were significantly upregulated in a dose-dependent
manner (P<0.05); furthermore, expression levels of p62 protein
in the Sal group were significantly downregulated in a
dose-dependent manner (P<0.05; Fig. 2E).
These results indicated that Sal or Rap treatment
resulted in organelle impairment and induced autophagy.
Sal induces apoptosis in 97H
cells
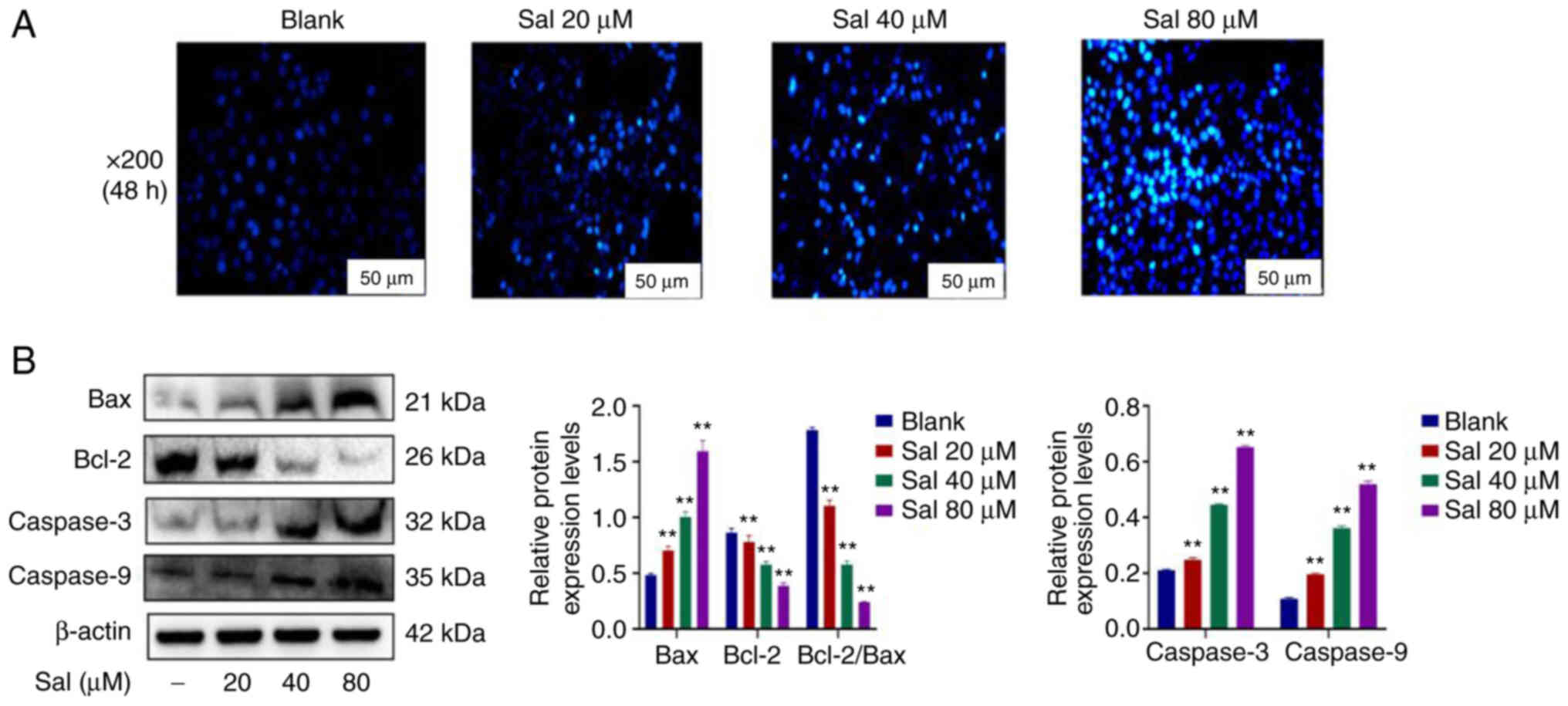
Hoechst33342 staining demonstrated markedly
increased numbers of apoptotic cells in a dose-dependent manner in
Sal-treated groups (Fig. 3A). To
evaluate the apoptotic effect induced by Sal, expression levels of
mitochondrial apoptosis-related proteins were assessed using
western blotting (Fig. 3B). The
results demonstrated that compared with the blank group, the ratio
of the Bcl-2 to Bax protein expression levels was significantly
down-regulated. Furthermore, expression levels of Caspase-3 and
Caspase-9 proteins were significantly up-regulated in a
dose-dependent manner at 48 h after treatment with Sal (P<0.01).
These results suggested that Sal could induce 97H cells
apoptosis.
Inhibiting autophagy increases
mitochondrial damage in 97H cells
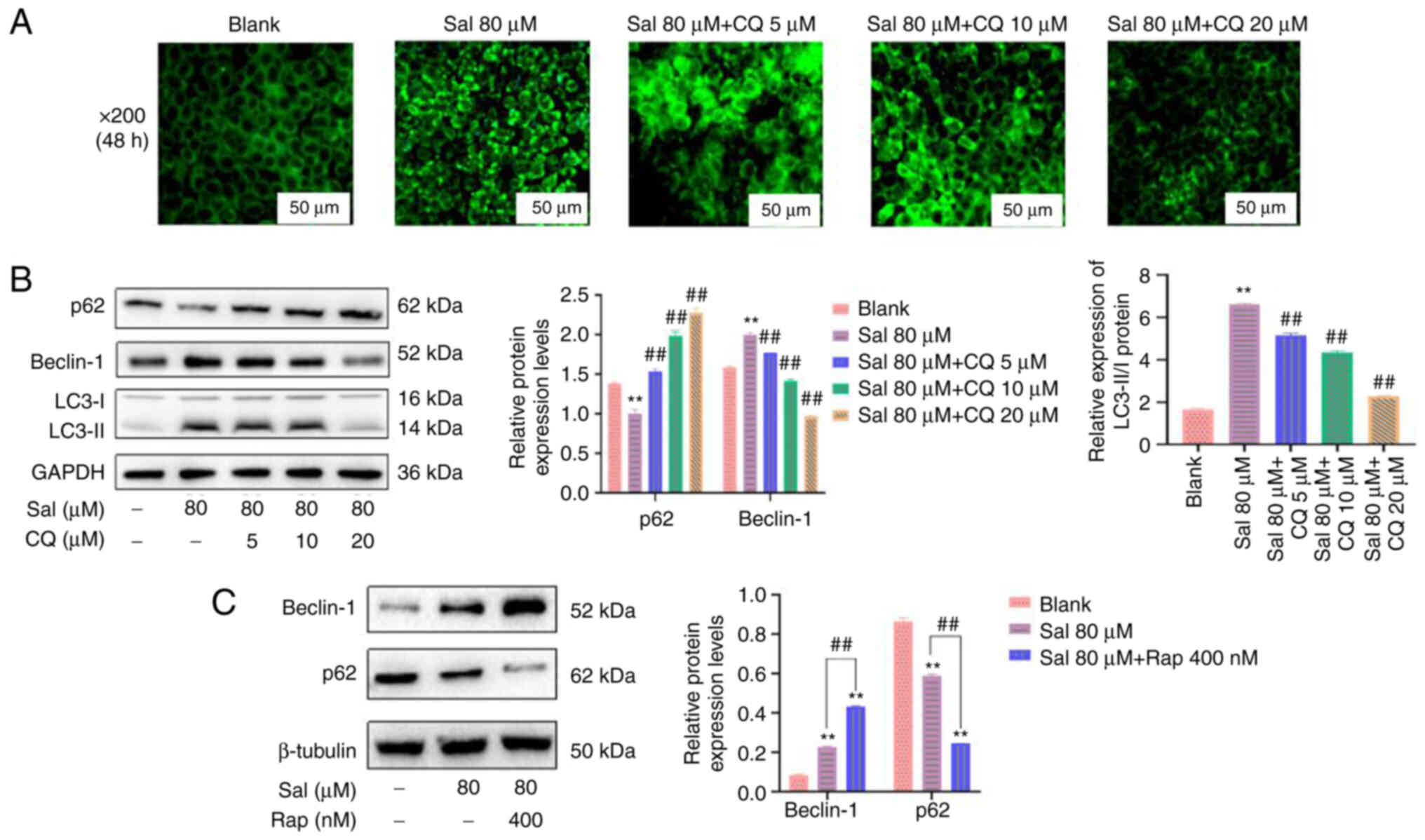
CQ is commonly used as an autophagy inhibitor. It
has been reported to inhibit autophagy by preventing the
combination of autophagosomes and lysosomes (36). Therefore, in the present study, CQ
was used to inhibit autophagy. Compared with the blank and Sal
groups, the amount and brightness of green fluorescence was
markedly decreased in a dose-dependent manner in the Sal + CQ group
(Fig. 4A). Furthermore, p62
protein expression levels were significantly upregulated and
Beclin-1 and LC3-II/I protein expression levels were significantly
downregulated following treatment with CQ compared with the Sal
group (P<0.01; Fig. 4B).
Moreover, Beclin-1 protein expression levels were significantly
upregulated and p62 protein expression levels were significantly
downregulated following treatment with the autophagy inducer Rap
compared with the Sal group (P<0.01; Fig. 4C). These data indicated that CQ
effectively inhibited Sal-induced autophagy in the present
study.
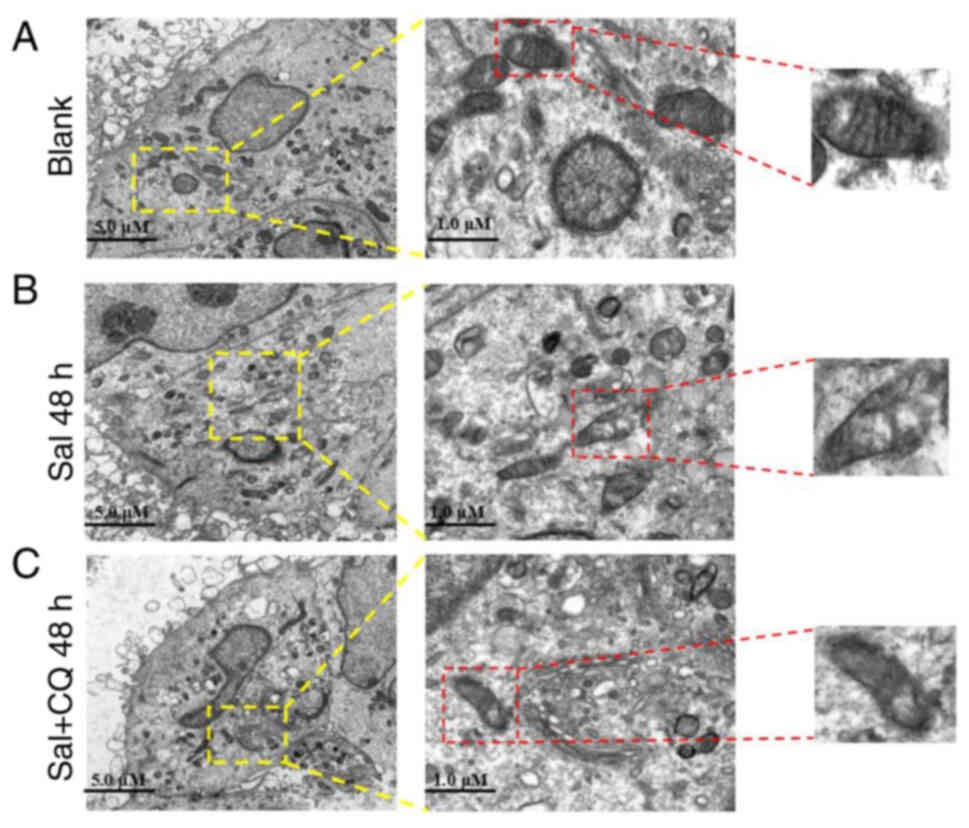
Mitochondria are not only key organelles for the
production of energy, but also regulate cellular redox signaling
pathways and programmed cell death (37). To evaluate the effect of CQ on
Sal-treated mitochondria, TEM was used to assess the ultrastructure
of mitochondria (Fig. 5A-C).
Swollen mitochondria were demonstrated following Sal treatment,
compared with the blank group. Furthermore, more serious
mitochondrial damage was observed in the Sal + CQ group (Fig. 5C), including swollen mitochondria,
double-membrane destruction and loss of normal morphology. These
results demonstrated that inhibiting autophagy increased the extent
of mitochondrial damage in Sal-treated cells.
Taken together, these results suggested that
inhibition of autophagy increased the degree of mitochondrial
damage.
Inhibiting autophagy enhances
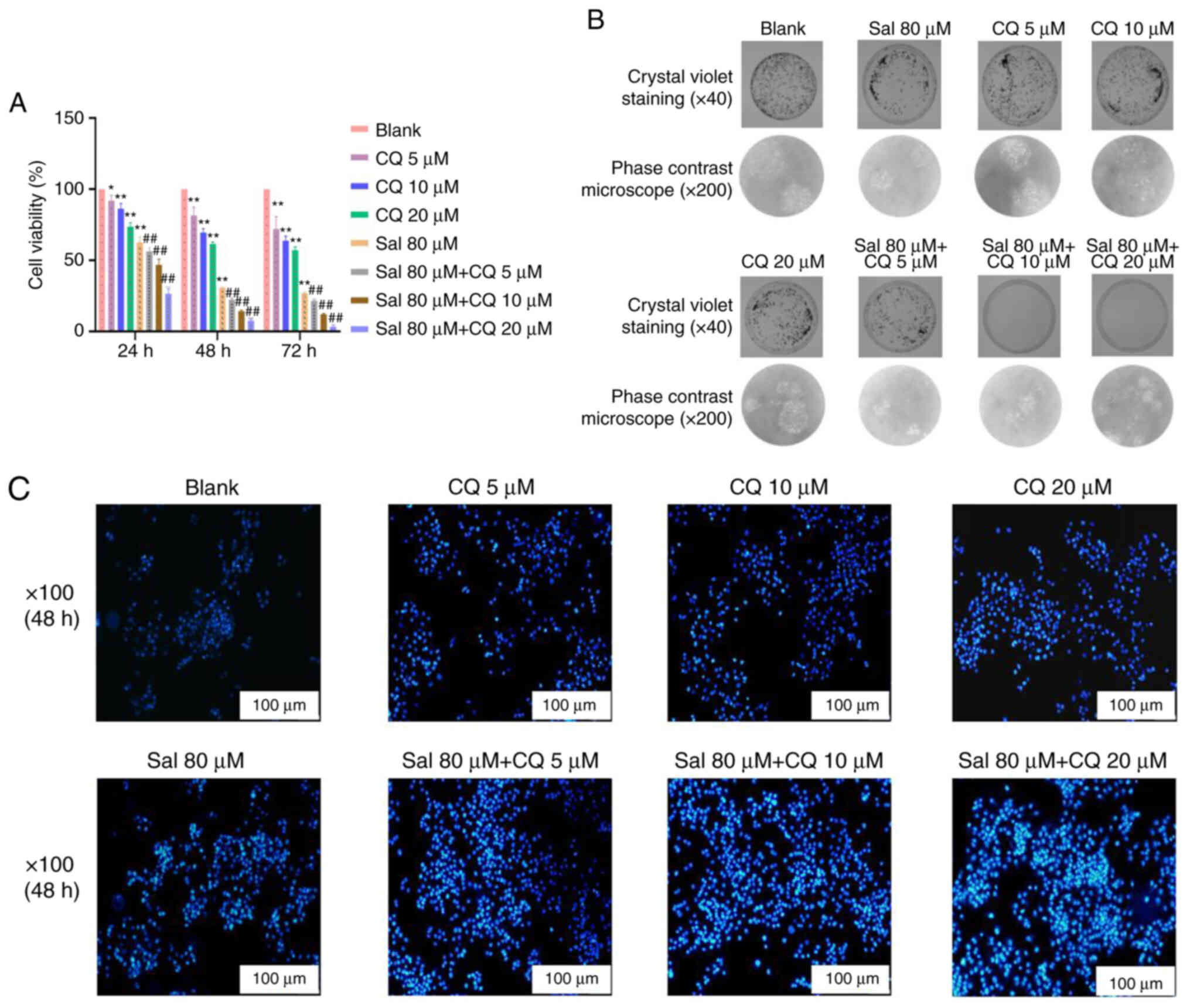
Sal-induced apoptosis
To evaluate the apoptotic effect of CQ in
Sal-treated 97H cells, CCK-8 method, plate colony formation assay
and Hochest33342 staining were used to assess apoptosis. The
results demonstrated that compared with the blank group, 80 µM Sal
group both demonstrated inhibitory effects on viability of 97H
cells at different times (P<0.01). Moreover, compared with the
Sal group, the viability of 97H cells was all decreased
significantly with a dose-dependent manner in the Sal + CQ groups
at different times (P<0.01; Fig.
6A). As seen in Fig. 6B,
compared with the blank group, the number of 97H cells' colonies
was decreased at 48 h after treatment with 80 µM Sal, and fewer
colonies appeared in the Sal + CQ groups at 48 h after treatment.
The result of Hoechst33342 staining demonstrated the number of
apoptotic cells was markedly increased at 48 h after treatment with
Sal. More apoptotic cells were demonstrated in the Sal + CQ groups
at 48 h after treatment compared with the blank (Fig. 6C).
Taken together, these results suggested that
inhibition of autophagy increased apoptosis.
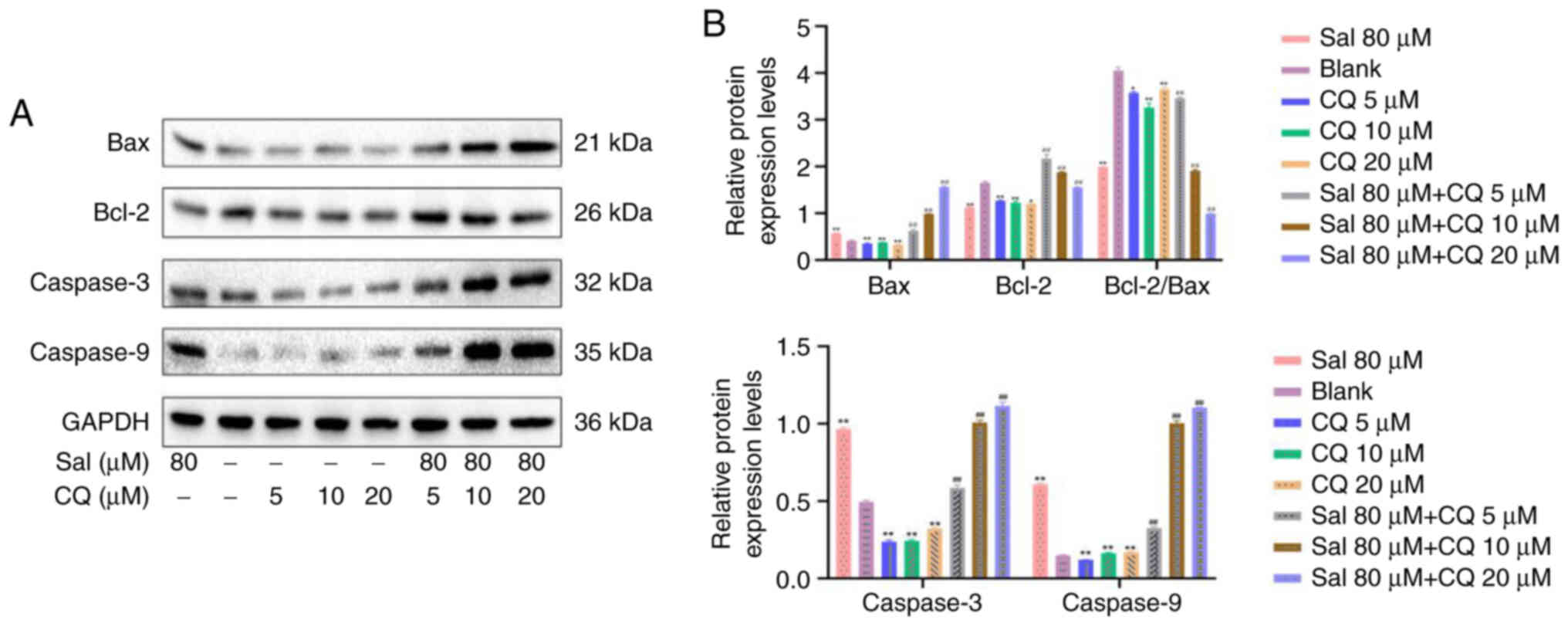
CQ regulates expression of Caspases in
97H cells
The caspase family serves an important role in
mitochondrially-regulated programmed death (38). Following Sal treatment, Caspase-3,
Caspase-9 and Bax/Bcl-2 protein expression levels all demonstrated
significant increases compared with the blank (P<0.01; Fig. 7A and B). Following combination
with CQ treatment, compared with the blank and Sal groups, protein
expression levels of Caspase-3, Caspase-9, Bax and Bcl-2 were
significantly increased (P<0.01). These results indicated that
inhibition of autophagy increased apoptosis and this may be
associated with the Caspase-mediated intrinsic apoptosis pathway in
97H cells.
Sal and CQ regulate the PI3K/Akt/mTOR
signaling pathway
Western blotting demonstrated that compared with the
blank group, the ratios of p-PI3K/PI3K, p-Akt/Akt and p-mTOR/mTOR
proteins were significantly decreased with a dose-dependent manner
in the Sal group (P<0.01; Fig. 8A
and B). Compared with the Sal group, combined treatment with
Sal and CQ promoted the ratios of p-PI3K/PI3K, p-Akt/Akt and
p-mTOR/mTOR proteins in a dose-dependent manner (P<0.01;
Fig. 8C and D). These data
demonstrated that Sal induced autophagy via inhibition of the
activation of the PI3K/Akt/mTOR signaling pathway. Inhibition of
autophagy may have increased apoptosis via the PI3K/Akt/mTOR
signaling pathway in 97H cells.
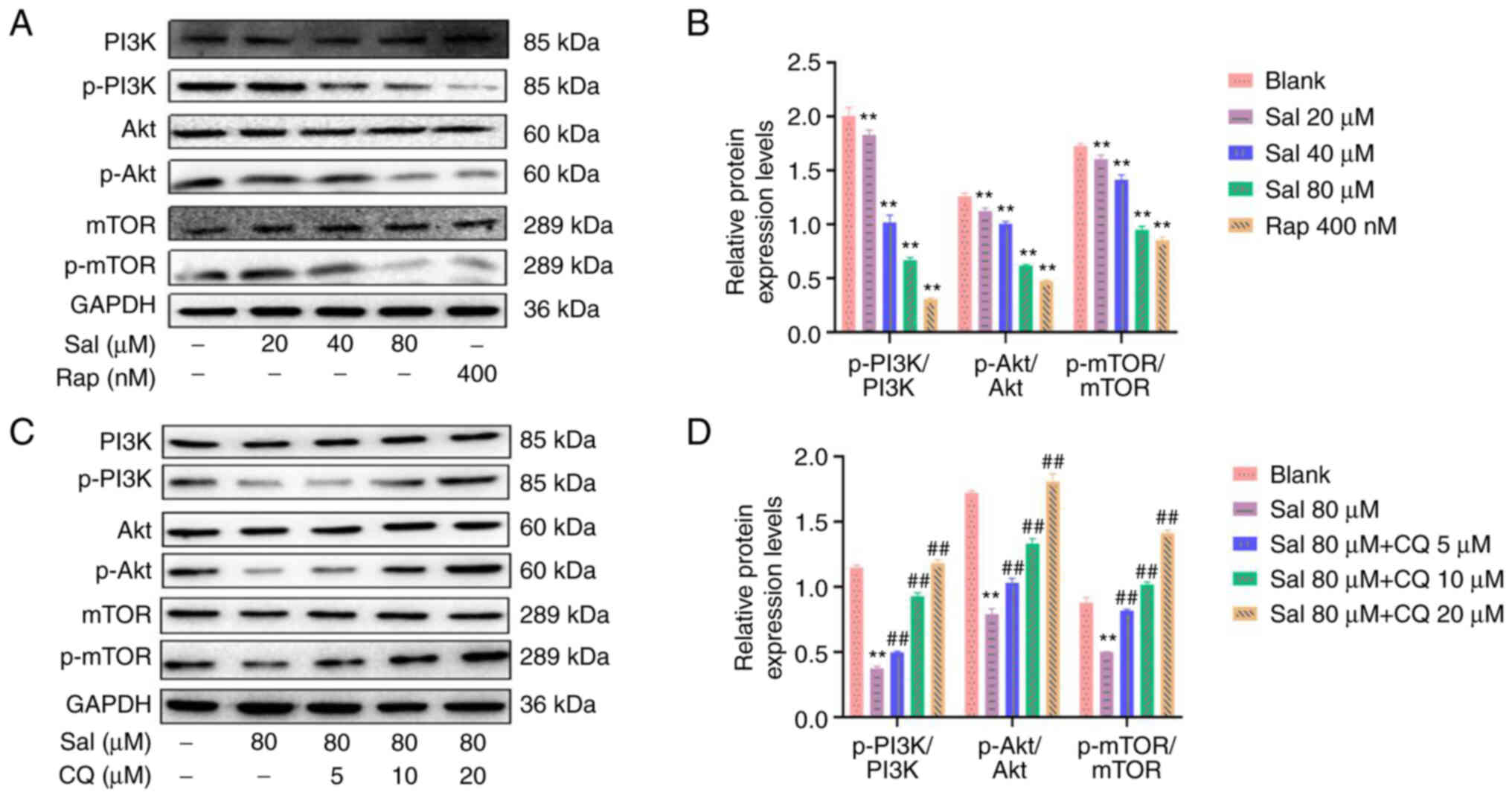 | Figure 8.Expression levels of proteins
associated with the PI3K/Akt/mTOR signaling pathway in 97H cells
treated with Sal and CQ. (A) Expression levels of PI3K, p-PI3K,
Akt, p-Akt, mTOR and p-mTOR proteins were assessed using western
blotting in 97H cells treated with Sal or Rap. (B)
Semi-quantification of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR
protein expression levels using densitometry. (C) Expression levels
of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR protein were assessed
using western blotting in 97H cells treated with Sal and CQ. (D)
Semi-quantification of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR
protein expression levels using densitometry. **P<0.01 vs.
blank. ##P<0.01 vs. Sal. CQ, chloroquine diphosphate;
Sal, salidroside; Rap, rapamycin; p-, phosphorylated. |
Discussion
In the present study, a Sal-treated 97H cell model
in vitro demonstrated that both apoptosis and autophagy were
increased following treatment with Sal. Further experiments
demonstrated that combination treatment with CQ and Sal inhibited
autophagy and promoted apoptosis, which indicated that inhibitors
of autophagy might accelerate the apoptotic process of 97H cells.
The results of the present study may aid in the elucidation of the
underlying molecular mechanisms of crosstalk between autophagy and
apoptosis in the stress-stimulated environment, which may
contribute to the development of novel therapy for liver cancer. In
cell biology, autophagy defines the catabolic process that
regulates the degradation of cell components through the lysosomal
machinery (39). Apoptosis is a
process of programmed cell death in normal physiological conditions
and is also an important part of maintaining the homeostasis of
internal environment (40). As
two distinct self-destructive processes, apoptosis (‘self-killing’)
and autophagy (‘self-eating’) are initiated and regulated by their
own molecular mechanism (40).
However, a complex relationship exists between autophagy and the
apoptotic cell death pathway, whereby regulators of apoptosis also
serve as regulators of autophagic activation (41).
Inhibition of autophagy by using specific inhibitors
(such as CQ) or suppression of autophagy regulatory pathways, may
increase the apoptotic efficiency of chemotherapeutic agents in
pancreatic (42), breast
(43), colon (44) and lung cancer (45) cells. These reports indicated that
autophagy may also be a therapeutic approach for cancer and that
inhibition of autophagy significantly promotes occurrence of
apoptosis in the development of cancer. Moreover, reports indicate
that CQ and interventional therapy combination may exert a
synergistic effect and induce apoptosis in Wistar liver cancer rats
(46,47). Similar to these results, in the
present study, Sal induced apoptosis and autophagy in the human 97H
liver cancer cell line. The viability of 97H cells incubated in
Sal-treated culture was evaluated. The viability of 97H cells after
Sal treatment was markedly lower compared with that of normally
cultured cells assessed using CCK-8 assay, Hoechst33342 staining
and western blotting. Cells were more dispersed and decreased in
number in the Sal + CQ group. Autophagosomes are key elements in
autophagy (48). Many methods can
be used to detected the formation of autophagosomes. For example,
Zhang et al (49) observed
changes of autophagosomes in hypoxia-induce myocardial cells injury
in mice using TEM technique. Cui et al (50) used MDC staining to detect the
number of autophagosomes in each group to evaluate the effect of
hydroxysafflor yellow A on the formation of autophagosomes in
vascular adventitial fibroblasts (VAFs) induced by angiotensinogen
II. Similar to their results, we also observed the formation of
autophagosomes in 97H cells treated with Sal or Rap by using the
TEM and MDC staining. Beclin-1, p62 and LC3B have been reported to
be key proteins in autophagy. The Beclin-1 gene, which encodes the
autophagy-induced protein, has been reported to regulate
localization of other autophagy proteins in the structure of
autophagy precursors by forming a complex with type III PI3K, which
regulates autophagy activity (51,52). p62 protein is reported to bind LC3
in the membrane of the autophagic vesicle to recruit the autophagic
degradation substrate into the autophagic vesicle for degradation
(53,54). LC3 is a soluble, cytosolic protein
that is cleaved during autophagic induction and is involved in the
formation of the autophagic vacuole membrane. When the autophagic
process starts, LC3-I (16 kDa) is converted to LC3-II (14 kDa)
(55,56). Therefore, western blotting was
used to assess protein expression levels of Beclin-1, p62, LC3-II
and LC3-I in Sal-treated 97H cells to evaluate the level of cell
autophagy. Following Sal treatment, the number of autophagosomes
gradually increased, Beclin-1 protein expression levels and
LC3-II/LC3-I ratio also increased, whereas p62 protein expression
levels decreased, which indicated that Sal increased cell
autophagy.
In the present study, Sal induced apoptosis and
autophagy in 97H cells. Based on these findings and related
literature (57,58), it could be hypothesized that
Sal-induced autophagy is a protective mechanism for hepatoma cells
and that inhibition of autophagy may promote apoptosis. To evaluate
this hypothesis, autophagy agonists were used as positive controls
for autophagic evaluation and autophagy inhibitors were used as
negative controls. CQ is a commonly used inhibitor of the
autophagic pathway and has been reported to inhibit autophagy by
prevention of the combination of autophagosomes and lysosomes
(36). Furthermore, under normal
conditions, autophagic flux of cells is low (59). If CQ is added first, autophagic
flux could be too low to be detected. Therefore, 97H cells were
pretreated with Sal and CQ was then used. CQ was used not only to
evaluate autophagic induction of Sal in liver cancer cells, but
also to evaluate the effect of the inhibition of autophagy on
Sal-induced apoptosis of liver cancer cells. In the present study,
in human 97H liver cancer cells, CQ and Sal in combination
increased cell death markedly compared with Sal alone. This
indicated that CQ enhanced Sal-induced liver cancer death and
autophagy increased the apoptotic effect in 97H cells. Inhibitors
of autophagy were used to evaluate the association between
apoptosis and autophagy. Inhibition of Sal-induced autophagy
promoting cell apoptosis; following the inhibition of autophagy in
Sal-treated cells, more apoptotic cells were observed.
The mitochondria, the energy metabolism center of
cell, and serve an important role in the regulation of cell death
and survival (60). Therefore, in
the present study, TEM was used to evaluate the ultrastructure of
mitochondria. The swollen mitochondria appeared in the Sal-treated
group; however, following combination treatment with CQ, the
morphological damage of mitochondria was more serious. The cascades
triggered by the Caspase family serve a key role in the regulation
of mitochondrial function (61).
Caspase-3 has been reported to be downstream of this cascade
reaction and causes cell death via degradation of intracellular
substrates (62). Caspase-9, as a
downstream signaling molecule of Caspase-3, damages the nuclear
pore, which helps Caspase-3 enter the nucleus and hydrolyze
deoxyribonuclease inhibitor (ICAD). ICAD is hydrolyzed and
separated by Caspase-3 to release CAD, which causes DNA
degradation, cytoskeleton separation and nuclear fragmentation,
which leads to apoptosis (63).
Therefore, the present study used western blotting to assess
expression levels of Bax, Bcl-2, Caspase-3 and Caspase-9 proteins
in Sal-treated 97H cells to evaluate the relationship between
mitochondrial dysfunction and Caspase. Compared with Sal alone,
using CQ and Sal in combination significantly increased protein
expression levels of Caspase-3 and Caspase-9 and Bax/Bcl-2 ratio,
which was associated with the damage to mitochondrial morphology.
These results indicated that following treatment with Sal,
inhibition of autophagy damaged the ultrastructure of cell
mitochondria, which led to increased levels of cell apoptosis.
The PI3K/Akt signaling pathway is a classical
pathway that regulates apoptosis. This signaling pathway affects
the activity of downstream apoptosis-associated molecules (Bax,
Bcl-2, Caspase-3 and Caspase-9) to regulate proliferation and
apoptosis of cancer cells (64,65). For example, Chen and Liu (66) treated human gastric cancer SGC7901
cells with quercetin, which is extracted from Dendrobium bark, and
reported that quercetin downregulated the protein expression levels
of p-PI3K and p-Akt and upregulated the protein expression levels
of Caspase-3, which indicated that quercetin induces apoptosis of
SGC7901 cells via inhibition of the PI3K/Akt signaling pathway.
Similarly, data from the present study demonstrated that Sal
decreased phosphorylation of PI3K and Akt proteins and upregulated
the protein expression levels of Bax/Bcl-2, Caspase-3 and
Caspase-9. mTOR is not only a downstream target of the PI3K/Akt
pathway but also serves an important role in the regulation of
autophagy (67,68). For example, Rong et al
(33) treated human gastric
cancer AGS cells with Sal in vitro and reported that Sal
treatment decreases protein expression levels of p-PI3K, p-Akt and
p-mTOR and that combined treatment with Sal and autophagy inhibitor
(insulin like growth factor-1, IGF-1) decreases expression levels
of Beclin-1 and LC3-II proteins, which indicated that Sal induces
autophagy in AGS cells via the PI3K/Akt/mTOR signaling pathway.
Similarly, data from the present study demonstrated that Sal
significantly decreased phosphorylation of PI3K, Akt and mTOR
proteins, whereas combined treatment with Sal and CQ significantly
increased expression levels of p-PI3K, p-Akt and p-mTOR proteins.
Furthermore, Sal significantly upregulated the protein expression
levels of Beclin-1 and LC3-II/LC3-I and significantly downregulated
expression levels of p62 protein. These results suggested that Sal
induced apoptosis and autophagy in human liver cancer cells via
inhibition of the PI3K/Akt/mTOR signaling pathway.
In summary, the present study demonstrated that Sal
induced 97H cell apoptosis via regulation of both mitochondrial
function and the autophagic response. This may have involved the
activation of both the PI3K/Akt/mTOR signaling pathway and directly
or indirectly regulate mitochondrial dysfunction and autophagy for
the survival of 97H cells. Following combination with autophagy
inhibitor CQ, this autophagy was inhibited and Sal-induced
apoptosis was increased. The present study demonstrated that
autophagy may serve a role as a defense mechanism in Sal-treated
human liver cancer cells and its inhibition may be a promising
strategy for the adjuvant chemotherapy of liver cancer. However,
the present study only evaluated the effects of one inhibitor in
one cell line. Therefore, further research is required to evaluate
the potential molecular mechanisms and develop targeted therapeutic
drugs.
Acknowledgements
Not applicable.
Funding
This present study was supported by the Program of Technology
Plan in Gansu Province (grant no. 18JR2FA010) and the Research
Project of Traditional Chinese Medicine in Gansu Province (grant
no. GZKP-2021-21).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
HS made substantial contributions to the conception
and design of the present study. BJ performed the experiments. LF
and TY acquired the data and confirm the authenticity of all the
raw data. WG, YL and CL analyzed the data. BJ drafted the
manuscript. HS and TW interpreted data and critically revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram L,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. Apr 5–2021.(Epub ahead of
print). View Article : Google Scholar
|
|
2
|
Keda M, Morizane C, Ueno M, Okusaka T,
Ishii H and Furuse J: Chemotherapy for hepatocellular carcinoma:
Current status and future perspectives. Jpn J Clin Oncol.
48:103–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kudo M: Targeted and immune therapies for
hepatocellular carcinoma: Predictions for 2019 and beyond. World J
Gastroenterol. 25:789–807. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin JW and Chung YH: Molecular targeted
therapy for hepatocellular carcinoma: Current and future. World J
Gastroenterol. 19:6144–6155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marin JJG, Briz O, Herraez E, Lozano E,
Asensio M, Di Giacomo S, Romero MR, Osorio-Padilla LM,
Santos-Llamas AI, Serrano MA, et al: Molecular bases of the poor
response of liver cancer to chemotherapy. Clin Res Hepatol
Gastroenterol. 42:182–192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian KJ: Observation and prevention of
side effects in interventional chemotherapy for liver cancer. J
Clin Med Pract. 4:135–136. 2000.
|
|
7
|
Sun AH, Chen J and Guan HM: Reversal
effect of paeonol on multidrug resistance of liver cancer cell line
HepG2/ADM. Shandong Med J. 56:1–4. 2016.
|
|
8
|
Li W and Huang QN: Advances in studies and
applications on Rhodiola rosea L. J Cap Norm Univ. 3:55–59.
2003.
|
|
9
|
Panossin A, Wikman G and Sarris J:
Rosenroot (Rhodiola rosea): Traditional use, chemical
composition, pharmacology and clinical efficacy. Phytomedicine.
17:481–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Booker A, Jalil B, Frommenwiler D, Reich
E, Zhai L, Kulic Z and Heinrich M: The authenticity and quality of
Rhodiola rosea products. Phytomedicine. 23:754–762. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruhsam M and Hollingsworth PM:
Authentication of Eleutherococcus and Rhodiola herbal supplement
products in the United Kingdom. J Pharm Biomed Anal. 149:403–409.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ming DS, Hillhouse BJ, Guns ES, Eberding
A, Xie S, Vimalanathan S and Towers GH: Bioactive compounds from
Rhodiola rosea (crassulaceae). Phytother Res. 19:740–743.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buchwald W, Mscisz A and Krajewska-Patan
A: Contents of biologically active compounds in Rhodiola
rosea roots during the vegetation period. Res Gate.
10:1413–1416. 2006.
|
|
14
|
Chen T, Ma Z, Zhu L, Jiang W, Wei T, Zhou
R, Luo F, Zhang K, Fu Q, Ma C and Yan T: Suppressing
receptor-interacting protein 140: A new sight for salidroside to
treat cerebral ischemia. Mol Neurobiol. 53:6240–6250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen JJ, Yuan LG and Li DD: Research on
the anti-aging role of salidroside in naturally aged mice. Chin Med
Bio. 7:412–417. 2012.
|
|
16
|
Wang S, He H, Chen L, Zhang W, Zhang X and
Chen J: Protective effects of salidroside in the
MPTP/MPP(+)-induced model of Parkinson's disease through
ROS-NO-related mitochondrion pathway. Mol Neurobiol. 51:718–728.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng T, Yang XY, Wu D, Xing S, Bian F, Li
W, Chi J, Bai X, Wu G, Chen X, et al: Salidroside ameliorates
insulin resistance through activation of a mitochondria-associated
AMPK/PI3K/Akt/GSK3β pathway. Br J Pharmacol. 172:3284–3301. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiang YC, Peng P, Liu XW, Jin X, Shen J,
Zhang T, Zhang L, Wan F, Ren YL, Yu QQ, et al: Paris saponin VII, a
Hippo pathway activator, induces autophagy and exhibits therapeutic
potential against human breast cancer cells. Acta Pharmacol Sin.
43:1568–1580. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji YC, Hu WW, Jin Y, Yu H and Fang J:
Liquiritigenin exerts the anti-cancer role in oral cancer via
inducing autophagy-related apoptosis through PI3K/AKT/mTOR pathway
inhibition in vitro and in vivo. Bioengineered. 12:6070–6082. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bata N and Cosford NDP: Cell survival and
cell death at the intersection of autophagy and apoptosis:
Implications for current and future cancer therapeutics. ACS
Pharmacol Transl Sci. 4:1728–1746. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Liu CY and Wang ZY: Pseudolaric
acid B induced autophagy through DNA damage response to inhibit
apoptosis. Jilin J Chin Med. 37:381–384. 2017.
|
|
24
|
Shi Y, Han JJ, Tennakoon JB, Mehta FF,
Merchant FA, Burns AR, Howe MK, McDonnell DP and Frigo DE:
Androgens promote prostate cancer cell growth through induction of
autophagy. Mol Endocrinol. 27:280–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang S, Wang X, Contino G, Liesa M, Sahin
E, Ying H, Bause A, Li Y, Stommel JM, Dell'antonio G, et al:
Pancreatic cancers require autophagy for tumor growth. Genes Dev.
25:717–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang P, Sun Y and Yao YY: Effects of
resveratrol on female reproductive system malignant tumors. J
Dalian Med Univ. 37:403–407. 2015.
|
|
27
|
Zhan XJ, Xie DZ and Hu YY: Experimental
study on anti-hepatocellular carcinoma and sensitizing effect of
matrine combined with 5-fluorouracil in vitro. Jiangxi J Trad Chin
Med. 47:42–45. 2016.
|
|
28
|
Chang MZ, Zhang SY and Hao YJ: Astragalus
polysaccharide inhibits the proliferation of esophageal cancer
EC109 cells by inducing cell autophagy. Central South Phar.
20:856–862. 2022.
|
|
29
|
Miao H, Yang JL, Zhang SQ and Yan N;
Gynecology Third Treatment Area Jilin Central Hospital, : Effects
of schisandra chinensis polysaccharides on proliferation, autophagy
and endoplasmic reticulum stress apoptosis of ovarian cancer SKOV3
cells. Systems Med. 6:135–137. 2021.
|
|
30
|
Mai TT, Moon J, Song Y, Viet PQ, Phuc PV,
Lee JM, Yi TH, Cho M and Cho SK: Ginsenoside F2 induces apoptosis
accompanied by protective autophagy in breast cancer stem cells.
Cancer Lett. 321:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Bao J, Wang K, Jia X, Zhang C,
Huang B, Chen M, Wan JB, Su H, Wang Y and He C: Pulsatilla saponin
D inhibits autophagic flux and synergistically enhances the
anticancer activity of chemotherapeutic agents against HeLa cells.
Am J Chin Med. 43:1657–1670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y
and Song F: Salidroside induces apoptosis and protective autophagy
in human gastric cancer AGS cells through the PI3K/Akt/mTOR
pathway. Biomed Pharmacother. 122:1097262020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang B, Yang T and Feng LF: Salidroside
induces the autophagy of 97H cells. Gansu Med J. 41:193–197.
2022.
|
|
35
|
Lu L, Liu S, Dong Q and Xin Y: Salidroside
suppresses the metastasis of hepatocellular carcinoma cells by
inhibiting the activation of the Notch1 signaling pathway. Mol Med
Rep. 19:4964–4972. 2019.PubMed/NCBI
|
|
36
|
Pan WY, Zhu XD, Zhao W, Qu S, Li L, Su F
and Li XY: The effects of chloroquine diphosphate and rapamycin at
different concentration on autophagy of CNE-2 cells. Chin J Oncol
Prev Treat. 3:280–283. 2011.
|
|
37
|
Zhang XY, Zhang YJ and Zhong Y: Research
progress of mitochondrial dynamics disorder in cancer. Nat Rev Mol
Cell Biol. 28:1219–1223. 2022.
|
|
38
|
DeVorkin L and Gorski SM: A
mitochondrial-associated link between an effector caspase and
autophagic flux. Autophagy. 10:1866–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kroemer G and Levine B: Autophagic cell
death: The story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fimia GM and Piacentini M: Regulation of
autophagy in mammals and its interplay with apoptosis. Cell Mol
Life Sci. 67:1581–1588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Subramani R, Gonzalez E, Arumugam A, Nandy
S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S, Narayan
M, et al: Nimbolide inhibits pancreatic cancer growth and
metastasis through ROS-mediated apoptosis and inhibition of
epithelial-to-mesenchymal transition. Sci Rep. 6:198192016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang PD, Zhao YL, Deng XQ, Mao YQ, Shi W,
Tang QQ, Li ZG, Zheng YZ, Yang SY and Wei YQ: Antitumor and
antimetastatic activities of chloroquine diphosphate in a murine
model of breast cancer. Biomed Phaemacother. 64:609–614. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sasaki K, Tsuno N, Sunami E, Tsurita G,
Okaji Y, Nishikawa T, Syuno Y, Hongo K, Kitayama J, Takahashi K and
Nagawa H: Abstract #383: Potentiation of pro-apoptotic effect of
5-fluorouracil on HT29 colon cancer cells by inhibition of
autophagy. Cancer Res. 69 (Suppl 9):S3832009.
|
|
45
|
Fan C, Wang W, Zhao B, Zhang S and Miao J:
Chloroquine inhibits cell growth and induces cell death in A549
lung cancer cells. Bioorg Med Chem. 14:3218–3222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hao X and Li W: Chloroquine diphosphate
suppresses liver cancer via inducing apoptosis in Wistar rats using
interventional therapy. Oncol Lett. 21:2332021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou KJ, Wang C and Xie MY: Effect of
chloroquine on tumor growth in mice with hepatic carcinoma and its
mechanism. Anhui Med J. 39:1167–1170. 2018.
|
|
48
|
Wang ZB, Wang J and Wang L:
Ultrastructural analysis of autophagosome. J Nanjing Med Uni (Nat
Sci). 36:426–429. 2016.
|
|
49
|
Zhang BN, Ye DY and Zhang DG: Application
of laser confocal scanning microscope in observing autophagy. J
Shantou Uni (Nat Sci Edi). 36:76–81. 2021.
|
|
50
|
Cui QZ, Liu BY and Li YY: Hydroxysafflor
yellow A represses Ang II-induced migration through activation of
autophagy in VAFs. Chin Phar Bull. 37:1680–1687. 2021.
|
|
51
|
Wang YW and Hou JS: Function of autophagy
gene Beclin 1 in tumor and its relationship with oral cancer. Chin
J Pra Stom. 4:374–376. 2011.
|
|
52
|
Li BX, Li CY and Peng R: Expression of
beclin-1, an autophagy-related protein, in stage IIIB colon cancer
and its relationship with prognosis. Chin J Clin Onc. 36:146–149.
2009.
|
|
53
|
Zhang Q, Su H, Ranek MJ and Wang X:
Autophagy and p62 in cardiac proteinopathy. Circ Res. 109:296–308.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li XY, Zhao WD and Zhou Y: Expression of
autophagy marker protein p62 in cervical squamous cell cancer and
its clinical significance. Chin J Clin Exp Path. 30:38–41.
2014.
|
|
55
|
Kabeya Y, Mizushima N, Yamamoto A,
Oshitani-Okamoto S, Ohsumi Y and Yoshimori T: LC3, GABARAP and
GATE16 localize to autophagosomal membrane depending on form-II
formation. J Cell Sci. 117:2805–2812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shen Y, Liang LZ, Hong MH, Xiong Y, Wei M
and Zhu XF: Expression and clinical significance of
microtubule-associated protein 1 light chain 3 (LC3) and Beclin1 in
epithelial ovarian cancer. Ai Zheng. 27:595–599. 2008.(In Chinese).
PubMed/NCBI
|
|
57
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ding SY, Wang MT, Dai DF, Peng JL and Wu
WL: Salidroside induces apoptosis and triggers endoplasmic
reticulum stress in human hepatocellular carcinoma. Biochem Biophys
Res Commun. 527:1057–1063. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Esteban-Martínez L and Boya P: Autophagic
flux determination in vivo and ex vivo. Methods. 75:79–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zamzami N and Kroemer G: The mitochondrion
in apoptosis: How Pandora's box opens? Nat Rev Mol Cell Biol.
2:67–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen AWG, Tseng YS, Lin CC, His YT, Lo YS,
Chuang YC, Lin SH, Yu CY, Hsieh MJ and Chen MK: Norcantharidin
induce apoptosis in human nasopharyngeal carcinoma through caspase
and mitochondrial pathway. Environ Toxicol. 33:343–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xu C, Sun G, Yuan G, Wang R and Sun X:
Effects of platycodin D on proliferation, apoptosis and PI3K/Akt
signal pathway of human glioma U251 cells. Molecules.
19:21411–21423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen LY and Liu Y: Quercitrin promotes
apoptosis of gastric cancer cell line SGC7901 by inhibiting
PI3K/AKT signaling pathway. Chin J Pathophysiol. 34:1976–1980.
2018.
|
|
67
|
Fu WW, Ou YY and Huang CY: Research
progress in the treatment of cardiovascular diseases based on mTOR
regulating autophagy. J Hainan Med Univ. 27:635–640. 2021.
|
|
68
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18:10882017. View Article : Google Scholar : PubMed/NCBI
|