Introduction
Allergic rhinitis (AR) and bronchial asthma (BA) are
inflammatory diseases of the upper and lower respiratory tracts,
respectively. They have similar pathological characteristics and
exhibit airway inflammation and hyperresponsiveness. A previous
study demonstrated close connections between AR and BA (1). In the absence of BA, subjects with AR
have significant lower airway dysfunction, including histological,
physiological and biochemical changes (2,3).
Studies have suggested that AR typically precedes the occurrence of
BA and could be an independent risk factor for the development of
BA (4,5). However, the pathogenesis of AR's
development into BA is complex and not entirely understood.
Nerve growth factor (NGF) reportedly participates in
regulating neuronal survival, differentiation, neurotransmission
and neurite growth (6). NGF also
exerts multidirectional effects on the mediation of several
pathophysiological processes, including immune responses,
inflammatory responses and catecholamine production (7–9).
Reportedly, the expressions of NGF, IL-6 and vasoactive intestinal
peptide (VIP) increase considerably in the nasal lavage fluids of
AR mice compared with control group mice (10). Previous evidence suggests that NGF
is persistently and highly expressed in nasal epithelium and
submucosal tissues of patients with AR and is mostly localized in
inflammatory cells (11,12). The level of serum NGF has equally
been shown to markedly increase in subjects with AR following
allergen stimulation (13,14). In addition, NGF is allegedly
involved in the pathophysiological processes of airway inflammation
and hyperresponsiveness in patients with AR (15). NGF mediates inflammation not only
in the upper airway but also in the lower airway (12,16).
NGF-mediated neurogenic inflammation is increasingly being linked
to the pathogenesis of BA (16).
The concentration of NGF has been shown to be significantly
enhanced in the serum of patients with BA (17). Increased NGF in the bronchial and
alveolar epithelia of asthmatic mice is likewise said to be
involved in the formation of airway hyperresponsiveness (18). These findings point to NGF being a
possible key inflammatory factor linking AR to BA. Previous studies
have shown that K252a is a potent protein kinase inhibitor that
blocked NGF-induced neurite outgrowth and protein phosphorylation
changes (19,20).
The synthesis and secretion of epinephrine (EPI)
have been shown to decrease in patients with BA, resulting in the
inability of epinephrine in vivo to effectively relieve
bronchospasm during an asthma attack (21). EPI is synthesized by adrenal
medulla chromaffin cells (AMCCs) and then released into the
circulatory system. In addition to their endocrine function, AMCCs
and nerve cells have the same origin and may also have the
potential to transform into nerve cells (22,23).
This feature of AMCCs is called redundancy in biology (24). There is evidence that asthmatic
pregnancy promotes the differentiation of AMCCs into sympathetic
nerve cells in offspring rats, inhibits the synthesis of EPI and
causes the dysfunction of bronchodilation (25). An in vitro study
demonstrated that primary cultured AMCCs transform into neural cell
phenotypes following stimulation by exogenous NGF. However,
glucocorticoids obstruct this NGF-induced phenotypic transformation
of AMCCs (26).
Previous studies reported the presence of an EPI
secretion disorder in asthmatic model rats, with a mechanism
closely associated with NGF-induced neuronal transformation and
endocrine dysfunction in AMCCs (21,27).
The present study hypothesized that NGF increased significantly in
an AR mouse model and assessed its ability to induce the
transformation of AMCCs from endocrine phenotypes to neuronal cell
phenotypes and then cause a decrease in EPI secretion. The present
study also sought to elucidate the potential underlying molecular
mechanisms of the evolution of AR into BA.
Materials and methods
Animals
A total of 40 eight-week-old male C57BL6 mice (22±2
g) was obtained from Hunan SJA Laboratory Animal Co., Ltd. and
acclimated to their novel environment for a week before
experimentation. The rodents were raised in a pathogen-free
environment and fed ad libitum. The study protocol used was
approved by the Animal Care Committee of Jiangxi Provincial
People's Hospital (protocol: 2022-008).
Cells, reagents and antibodies
AMCCs were obtained from the adrenal glands of mice.
The ovalbumin (OVA; cat. no. SLBK6455V) was purchased from
MilliporeSigma, Aluminum hydroxide [Al(OH)3; cat. no.
20150417] was from Tianjin Damao Chemical Reagent Factory, the
Opti-MEM I Reduced Serum Medium (cat. no. 31985070), Dulbecco's
Modified Eagle Medium (DMEM; cat. no. 10565016) and
Lipofectamine® 3000 (cat. no. L3000150) were from Thermo
Fisher Scientific, Inc. Giemsa's stain (cat. no. KGA228) and
hematoxylin stain (cat. no. KGA223) were from KeyGen Biotech Co.,
Ltd. Eosin stain (cat. no. AR1180-2) was from Boster Biological
Technology Co., Ltd. BCA Protein Quantitative kit (cat. no.
CW0014S), DAB kit (cat. no. CW0125M), TRIzon reagent (cat. no.
CW0580S), HiFiScript cDNA Synthesis kit (cat. no. CW2569M),
Ultrapure RNA kit (cat. no. CW0581M) and UltraSYBR Mixture (cat.
no. CW0957M) were obtained from CoWin Biotechnology Co., Ltd.
Polyvinylidene difluoride (PVDF) membranes (cat. no. IPVH00010)
were from MilliporeSigma, bovine serum albumin (BSA; cat. no.
A8020) and enhanced chemiluminescent solution (ECL; cat. no.
PE0010) were from Beijing Solarbio Science & Technology Co.,
Ltd. Non-fat milk powder (cat. no. P1622) was from Applygen
Technology Co., Ltd. The recombinant mouse NGF (cat. no.
50385-MNAC) was purchased from Sino Biological Co., Ltd, K252a
(cat. no. 11338) was from Neo Bioscience, Inc. and Mouse
Norepinephrine (NE; cat. no. ml063805) and Mouse Epinephrine (EPI)
ELISA kits (cat. no. ml002049) from Mlbio (Shanghai Enzyme-linked
Biotechnology Co., Ltd.). Primary antibodies against synaptophysin
(SYP; cat. no. ab 32127), phenylethanolamine N-methyl transferase
(PNMT; cat. no. ab167427) were procured from Abcam. NGF (cat. no.
bsm-10806m) and tropomyosin receptor kinase (Trk) A (cat. no.
bs-10210R) were purchased from BIOSS. GAPDH (cat. no. TA-08),
HRP-conjugated goat-anti-rabbit IgG secondary antibody (cat. no.
ZB-2301) and HRP-conjugated goat-anti-mouse IgG secondary antibody
(cat. no. ZB-2305) were from OriGene Technologies, Inc. and
Cy3-conjugated Goat Anti-Rabbit IgG secondary antibody (cat. no.
CW0159) was from CoWin Biotechnology Co., Ltd.
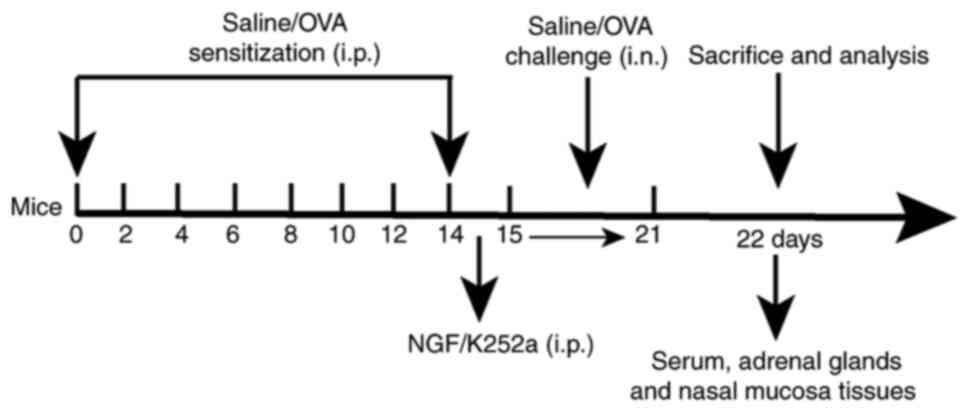
Animal model
During the present study, the mice were kept in a
12-h light/dark cycle room with a temperature of 22±2°C and
humidity of 40–70% and allowed food and water freely. They were
randomly divided into four groups after 7 days of adaptive feeding:
control group (n=10), Model group (OVA group, n=10), Model + NGF
group (n=10) and Model + K252a group (n=10).
To establish the AR model, 50 µg ovalbumin (OVA;
MilliporeSigma) and 5 mg Al(OH)3 were precipitated at a
1:1 ratio in 1 ml of saline. The mice were immunized by
intraperitoneally injecting them with 1 ml of
OVA-Al(OH)3 suspension every other day for eight
consecutive spells. On the 15th day, 20 µl of saline containing 5%
OVA was administered into both airways of the nasal cavity daily
for one week. Mice in the control group received the same treatment
but were given saline instead of the OVA solution. Prior to the
intranasal challenge, mice in the Model + NGF group were
intraperitoneally injected with 8 ng/kg of a 1 ng/ml NGF solution.
The Model + K252a group mice received 20 µg/kg of a 2 µg/ml K252a
solution (an inhibitor of NGF receptor) via intraperitoneal
injections prior to the intranasal challenge. During the study,
three mice were sacrificed due to reduced food intake and weight
loss. Finally, valid data was obtained from a total of 37 mice. On
day 22, blood samples were collected via cardiac puncture (2 ml per
mouse) and adrenal glands were immediately removed under aseptic
conditions. The mice were anesthetized with sodium pentobarbital
(70 mg/kg) via intraperitoneal injection and then sacrificed
through vertebral dislocation; death was confirmed when the animals
developed cardiac arrest, respiratory arrest and corneal reflex
arrest. All procedures were conducted strictly in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (28). The
experimental protocol is presented in Fig. 1.
Assessment of Frequencies of Nose
Scratching and Sneezing
As previously reported (29), the present study conducted
behavioral observations on mice by counting the number of sneezing
and nasal rubbing movements for 10 min immediately following the
final nasal challenge. Sneezing was characterized by an explosive
exhalation followed by a deep inhalation. Nasal rubbing is
characterized by scratching around the nose with the animal's
forelimbs.
Histopathological analysis of nasal
tissues
Nasal mucosa tissues from the mice were fixed in 10
formaldehyde solution at room temperature for 10 days and
dehydrated in a graded ethanol series (70, 80, 90 and 99.9%). The
transparent tissues were placed in melted paraffin and embedded
after the paraffin was completely incorporated into the tissues.
Subsequently, the paraffin-embedded nasal mucosa tissues were
sectioned into 5 µm and stained with hematoxylin and eosin for 5
min at room temperature. The remaining sections were then
deparaffinized in toluene and rehydrated in ethanol with increasing
concentrations of water. The endogenous peroxidase was blocked with
3% hydrogen peroxide for 10 min at room temperature. The sections
were incubated with polyclonal anti-NGF (1:200) and polyclonal
anti-TrkA (1:200) at 4°C overnight and then incubated with
horseradish peroxidase-conjugated secondary antibodies (1:100) at
room temperature for 30 min. The sections were developed with DAB
at 37°C for 3 min and then the nuclei were stained with hematoxylin
at 37°C for 3 min. Pathological changes in the nasal mucosa were
observed under a light microscope (CX41; Olympus Corporation).
Immunofluorescence staining
The slices of adrenal glands were dewaxed with
xylene for 10 min, dehydrated by graded ethanol (100, 100, 95 and
80%) and 0.03% hydrogen peroxide was used to eliminate endogenous
peroxidase activity. Antigen-repaired solution was added to repair
the antigen of tissues. Following washing with PBS three times for
5 min each time they were permeabilized in 0.5% Triton X-100 at
room temperature for 20 min. Non-specific binding was blocked by 5%
BSA at room temperature for 30 min and the cells were incubated
with anti-phenylethanolamine N-methyl transferase (PNMT; 1:100) at
4°C overnight, followed by incubation with the Cy3-conjugated Goat
Anti-Rabbit IgG (1:200) secondary antibody at room temperature for
30 min. Following washed with PBS three times, nuclei were stained
with DAPI for 5 min without light at room temperature and detected
with a fluorescence microscope (BX51; Olympus Corporation).
Separation and primary culture of
AMCCs
Adrenal glands were immediately collected and washed
three times in a cleansing solution containing three antibiotics
(100 µg/ml penicillin, 100 µg/ml streptomycin and 50 µg/ml
gentamicin) and cut in halves lengthwise and the outer cortex was
excised. The medullas were cut into small pieces using sterile
curved scissors. Collagenase (0.1%; 45 ml) was then added to the
cut pieces and the slices were placed in a 37°C water bath for 45
min to dissolve. A 5% BSA D-Hanks solution was introduced and the
mixture was centrifuged at 250 × g for 5 min at room temperature.
The supernatant was removed and a 1% D-Hanks solution was added to
the precipitate. A D-Hanks solution containing 5% BSA was poured
into another sterile centrifuge tube and the cell suspension was
put into the solution. Another centrifugation at 250 × g for 5 min
at room temperature yielded cell pellets at the bottom of the tube.
Cells were washed with a 1% BSA D-Hanks solution and a 10% FBS-DMEM
medium was added. The cells were divided into six groups: Control
group, Control + NGF group, Control + K252a group, Model group,
Model + NGF group and Model + K252a group. Control and Model group
cells were stained with Giemsa at 37°C for 1–2 min and the results
were observed using a light microscope (CX41; Olympus
Corporation).
Transmission electron microscopy
(TEM)
Cells were fixed with 2.5% glutaraldehyde at 37°C
for 3 h, washed with 0.01 M PBS, post-fixed with 1% osmium
tetroxide at 37°C for 1 h, washed with 0.01 M PBS, dehydrated in
graded acetone acid, soaked in an epoxy resin embedding medium and
100% acetone at a 1:1 ratio and drenched and embedded in a pure
embedding medium. Ultrathin sections of cells (50 nm) were stained
with uranyl acetate at 37°C for 15 min and then lead nitrate at
37°C for 10 min. Ultrastructural changes were observed using TEM
(JEM-1230; JEOL Ltd.).
Cell transfection
For transfection, short hairpin (sh)RNA against
STAT1 (sh-STAT1,
5′-GATCCAACATCTGCCTAGATCGGCTACTGTGAAGCCACAGATGGGTAGCCGATCTAGGCAGATGTTTTTTTTA-3′)
and shRNA negative control (sh-NC,
5′-AGCTTAAAAAAAAACATCTGCCTAGATCGGCTACCCATCTGTGGCTTCACAGTAGCCGATCTAGGCAGATGTTG-3′)
were designed and synthesized by Shanghai GenePharma Co., Ltd.
Following the manufacturer's instructions, the synthetics were
transfected into AMCCs using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). The mass of the
plasmids was 2.5 µg. The entire transfection process was carried
out in Opti-MEM, a reduced serum medium, at 37°C for 4 h in a
humidified environment containing 5% CO2. Following
transfection, the medium was replaced with a DMEM complete medium
containing 20% FBS. Transfection efficiency was detected using
reverse-transcription-quantitative polymerase chain reaction
(RT-q)PCR and western blotting 48 h post-transfection.
RT-qPCR
Total RNA was extracted from AMCCs using the TRIzon
reagent following the manufacturer's instructions. cDNA was
synthesized from total RNA using the HiFiScript cDNA Synthesis kit
and stored at −70°C until further use. qPCR was carried out using
UltraSYBR Mixture. The thermocycling conditions for PCR were:
Initial denaturation for 5 min at 95°C, followed by 40 cycles of
95°C for 15 sec and amplification at 60°C for 1 min. The
concentrations of the RT-qPCR products were normalized to GAPDH,
which was used as an internal control. The 2−∆∆Cq method
was used to calculate the relative mRNA expression (30). The primer sequences are listed in
Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequences
(5′-3′) |
|---|
| SYP | F:
CTACTCCTCCTCGGCTGAAT |
|
| R:
GCACATAGGCATCTCCTTGATA |
| JAK1 | F:
CCAGATTTGTAAGGGGATGGAC |
|
| R:
GACCAGACATCAGAGGCGAT |
| STAT1 | F:
GTCCCAGAACGGAGGTGAAC |
|
| R:
AGTTCGCTTAGGGTCGTCA |
| p38 | F:
CCCAGATGCCGAAGATGAACT |
|
| R:
TCATAGGTCAGGCTCTTCCACTC |
| ERK | F:
ATCACATCCTGGGTATTCTTGG |
|
| R:
TGGAGTCAGCATTTGGGAAC |
| GAPDH | F:
TCAACGGCACAGTCAAGG |
|
| R:
TGAGCCCTTCCACGATG |
Western blot analysis
The AMCCs from different groups were harvested in a
RIPA lysis buffer using 1 mM PMSF. Protein concentration was
determined using a BCA protein assay kit. Following extraction of
total proteins, 30 µg of protein was fractionated on 10% sodium
dodecyl sulfate-polyacrylamide gels, electrophoretically
transferred onto PVDF membranes, blocked with 5% non-fat milk for 2
h at room temperature, incubated with specific primary antibodies
(monoclonal anti-synaptophysin (SYP, 1:1,000), monoclonal anti-p38
(1:1,000), monoclonal anti-STAT1 (1:1,000), polyclonal anti-ERK
(1:1,000) and monoclonal anti-JAK1 (1:3,000) at 4°C overnight and
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:2,000) at room temperature for 1 h. Fluorescent
signals were developed using enhanced chemiluminescent solution.
Band densities were analyzed using ImageJ 6.0 software (National
Institutes of Health), with the GAPDH protein as an internal
reference.
Immunohistochemistry
AMCCs were put in Petri dishes and fixed with 4%
paraformaldehyde at room temperature for 15 min, following which 3%
hydrogen peroxide was added to remove endogenous peroxidase
blocking solution before incubation at room temperature for 10 min.
BSA (5%) was added to the Petri dishes dropwise and the content was
blocked at 37°C for 30 min. Next, the cells were exposed to an
anti-SYP antibody (Abcam; 1:400) at 4°C overnight, washed with PBS
and incubated with horseradish peroxidase-conjugated secondary
antibodies (1:2,000) for 30 min at room temperature. Following
another incubation at room temperature without light for 10 min,
the signals were detected using 3,3-diaminobenzidine. The cells
were then stained with hematoxylin at room temperature for 3 min
and examined under a light microscope (CX41; Olympus
Corporation).
ELISA
EPI and NE levels were quantified using a
commercially available ELISA kit per the manufacturer's
recommendations.
Statistical analysis
Each experiment was repeated at least three times.
Statistical analyses of all data were conducted using GraphPad
Prism 7.0 (GraphPad Software, Inc.). Values were expressed as the
mean ± standard deviation. One-way ANOVA, followed by the Tukey
post hoc test, was employed for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphological changes in the nasal
mucosa and adrenal gland in an OVA-induced allergic rhinitis mouse
model
Sensitization of OVA in AR mice resulted in
histopathological changes such as disordered arrangement of
epithelial cells and cilia, inflammatory cells infiltration
(lymphocytes and eosinophils) and interstitial swelling, compared
with control mice (Fig. 2A). The
expression of NGF and TrkA proteins in the AR mice nasal mucosa
tissues increased significantly compared with control mice
(Fig. 2B and C). As expected,
OVA-sensitized mice developed increased sneezing and nasal rubbing
compared with the control mice (Fig.
2D). TEM analyses revealed a regular arrangement of the
cortical and medulla cells of adrenal glands in the control group:
Medulla cells were characterized by clear mitochondrial structures,
uniform distributions of chromaffin particles, round nuclei and
smooth nuclear membranes. In the AR model group, there were changes
in the apoptosis levels of AMCCs, manifested as nuclear membrane
shrinkage, loose particle distribution and disintegration of cell
contents. The AMCCs in the NGF group were characterized by swelling
of cytoplasm, edema of mitochondria and protrusions. However,
widened intercellular spaces and decreased mitochondrial swelling
in AMCCs of K252a group were observed by TEM (Fig. 2E).
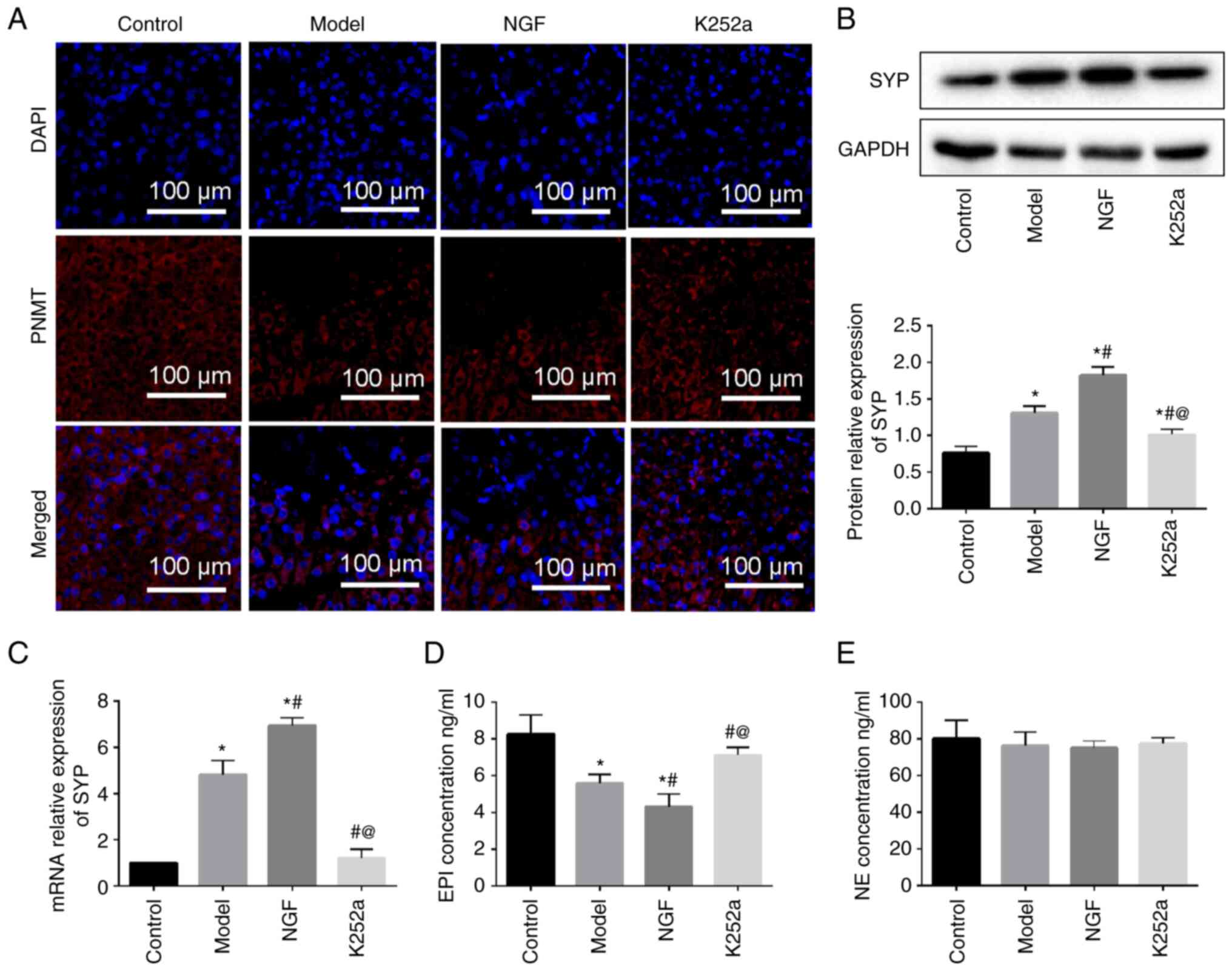
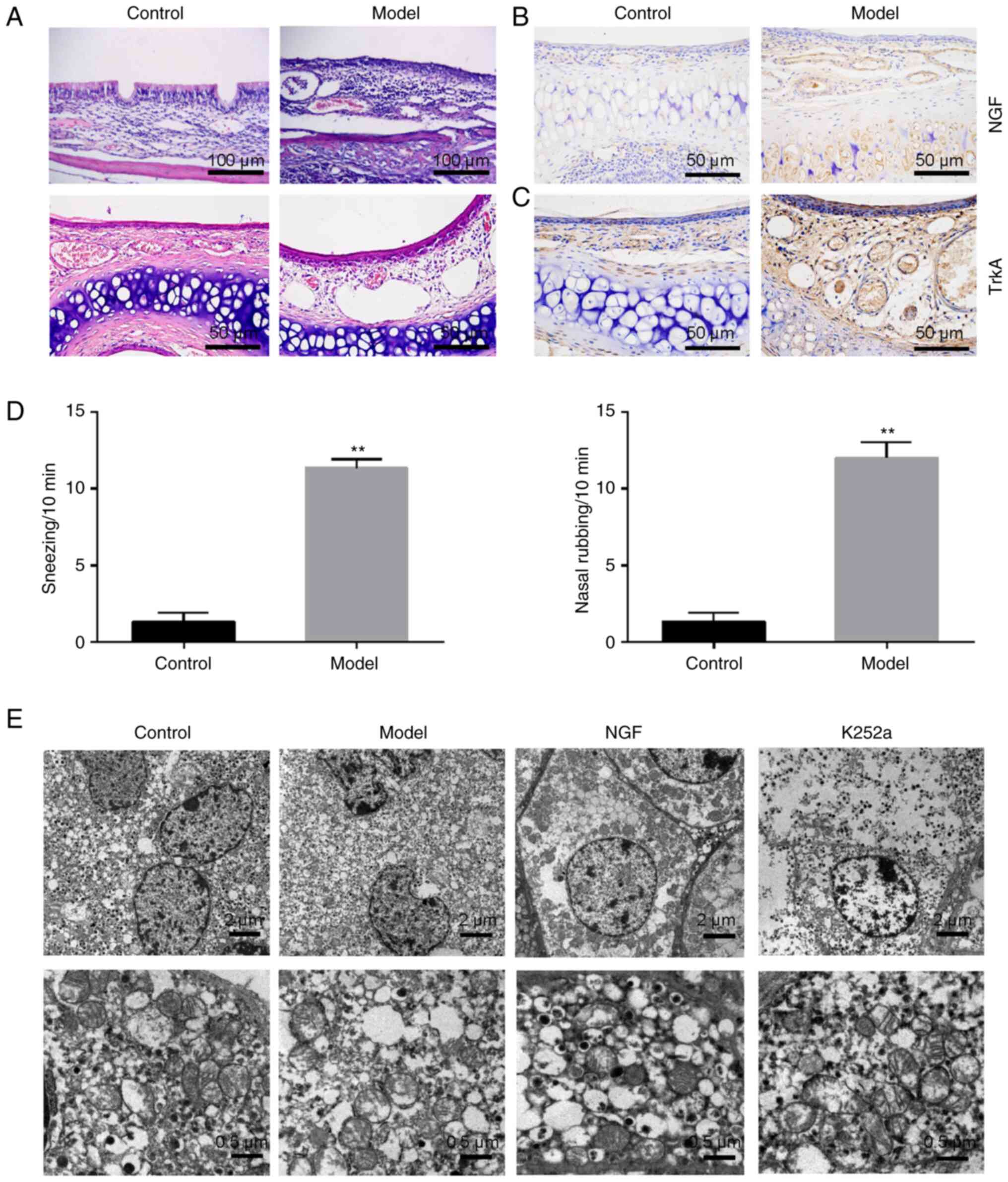 | Figure 2.Pathological changes in the nasal
mucosa and adrenal medulla tissues in the allergic rhinitis mouse
model. (A) Hematoxylin and eosin-stained nasal mucosa tissue
sections from mice. Representative micrographs of nasal mucosa
tissues from control and model groups. Scale bars, 50 µm (above),
100 µm (below). (B) The representative sample of NGF immunostaining
(DAB) was presented for the control and model groups. Scale bars,
50 µm. (C) A representative sample of TrkA immunostaining (DAB) was
presented for the control and model groups. Scale bars, 50 µm. (D)
Observation of the number of sneezing and nasal rubbing in mice
following intranasal challenge. (E) TEM-captured morphological
changes in the control group, model group, NGF group and K252a
group. Scale bars, 2 µm (above) and 0.5 µm (below). **P<0.01 vs.
control group. NGF, nerve growth factor; Trk, tropomyosin receptor
kinase; TEM, transmission electron microscope. |
Endocrine functional changes of
adrenal medulla from mice with the intervention of NGF
From Fig. 2, it was
found that immunostaining of PNMT was mainly expressed in the
adrenal cytoplasm. The expression levels of PNMT were much weaker
in model and NGF groups compared with the control group. However,
K252a treatment could promote the expression level of PNMT
(Fig. 3A). To explore whether SYP
is associated with the pathogenesis of AR, the expression levels of
SYP mRNA and protein were detected by RT-qPCR and western blotting,
respectively. Compared with the control group, significantly
increased SYP mRNA and protein were found in the model and NGF
groups. In addition, the NGF group had even stronger expressions of
both the SYP mRNA and protein than that in the model group, while
the mRNA and protein levels of SYP were inhibited using the K252a
(Fig. 3B and C).
The levels change of EPI and NE in
serum of each group
The results showed that EPI secretions from serum
were decreased in the model and NGF groups compared with control
group, but this trend was inhibited with treatment K252a (Fig. 3D). However, there was no
significant change in NE concentration in serum of mice in each
group following intervention. (Fig.
3E). These results indicated that the endocrine function of
adrenal medulla of mice changed following exogenous factors
treatment, leading to the disorder of EPI secretion.
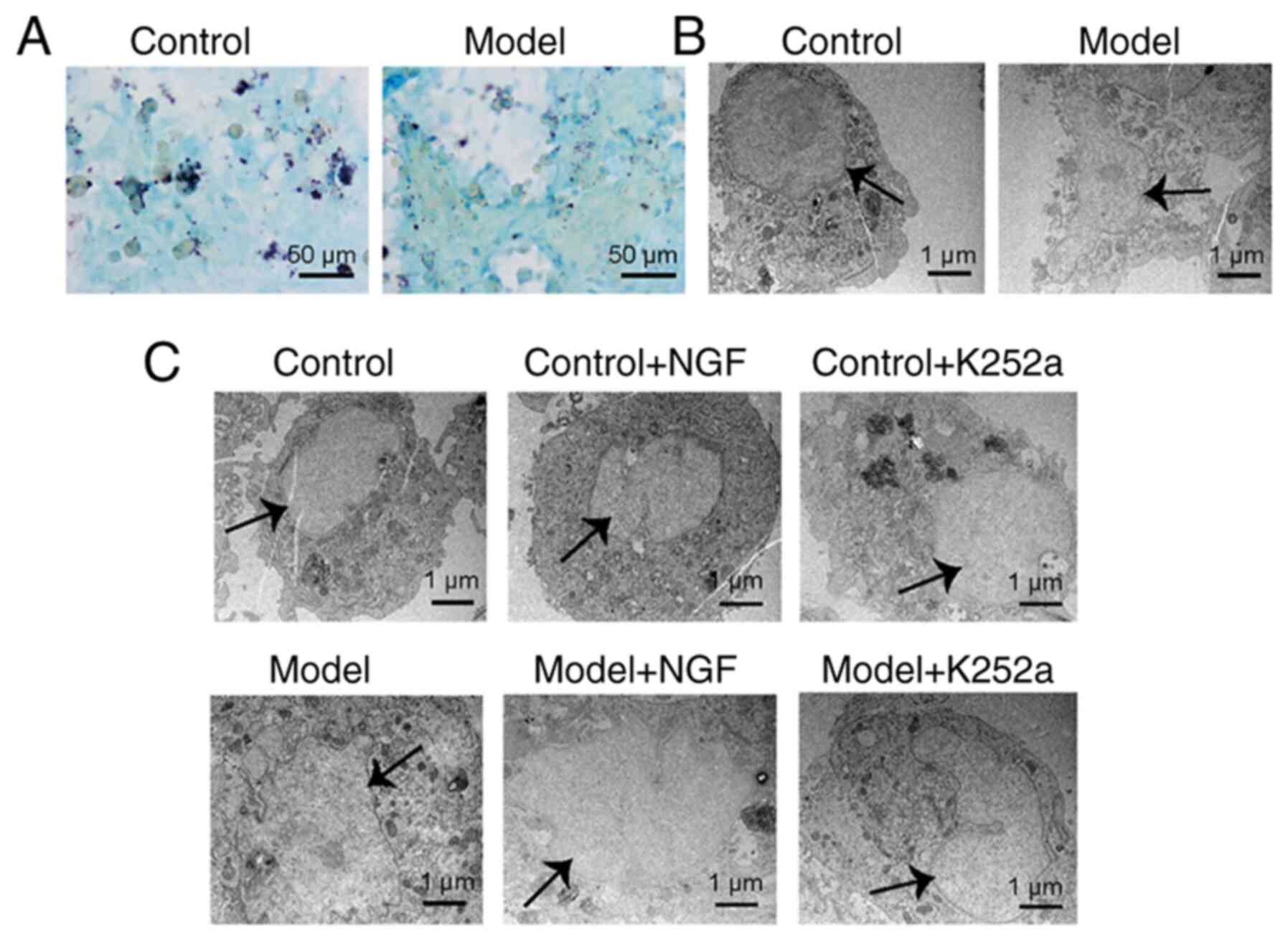
Identification of AMCCs and
NGF-mediated morphological changes in AMCCs
Giemsa staining revealed that primary AMCCs isolated
from the control and model groups had characteristically pale
yellow cytoplasmic chromaffin granules (Fig. 4A). In addition, adrenal medulla
cytoplasm harbored a uniform distribution of light black chromaffin
cell secretory granules under TEM (Fig. 4B). Therefore, the isolated primary
cells were possibly AMCCs. The AMCCs in the control group had
intact morphologies and normal mitochondrial structures; however,
AMCCs in the model group featured damaged nuclear structures,
enlarged mitochondria and disintegrated contents (Fig. 4C). The AMCCs in the NGF + model
group were more damaged compared with those in the model group,
showing shrinkage of the nuclear membranes and further
disintegration of contents. Treatment with K252a resulted in
abundant villiform protrusions on cell membrane surfaces, the
appearance of normal mitochondrial structures in the cytoplasm and
the formation of small vesicles near cell membranes (Fig. 4C).
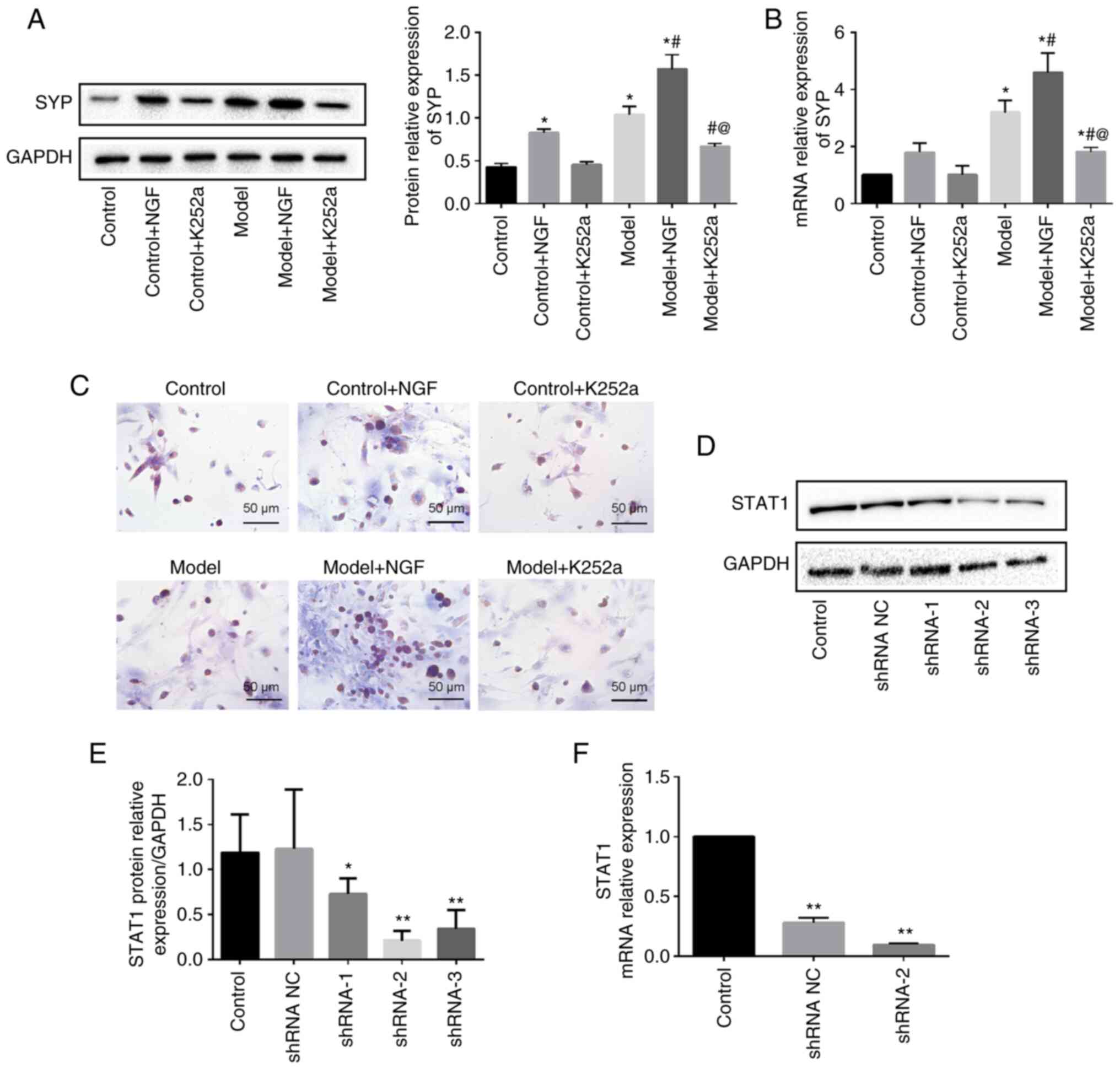
The effect of NGF and K252a on the
biological behavior of AMCCs
The assessment of whether NGF affected the
expression of SYP in AMCCs culminated in significantly increased
SYP mRNA and protein levels when AMCCs were treated with NGF
(Fig. 5A and B). In the presence
of K252a, the mRNA and protein levels of SYP markedly decreased in
AMCCs. Immunostaining localized SYP to the cytoplasm of AMCCs. The
expression of immunostained SYP was enhanced in the presence of
NGF, but K252a suppressed this expression (Fig. 5C). In summary, NGF participated in
the alterations in the biological behavior of AMCCs.
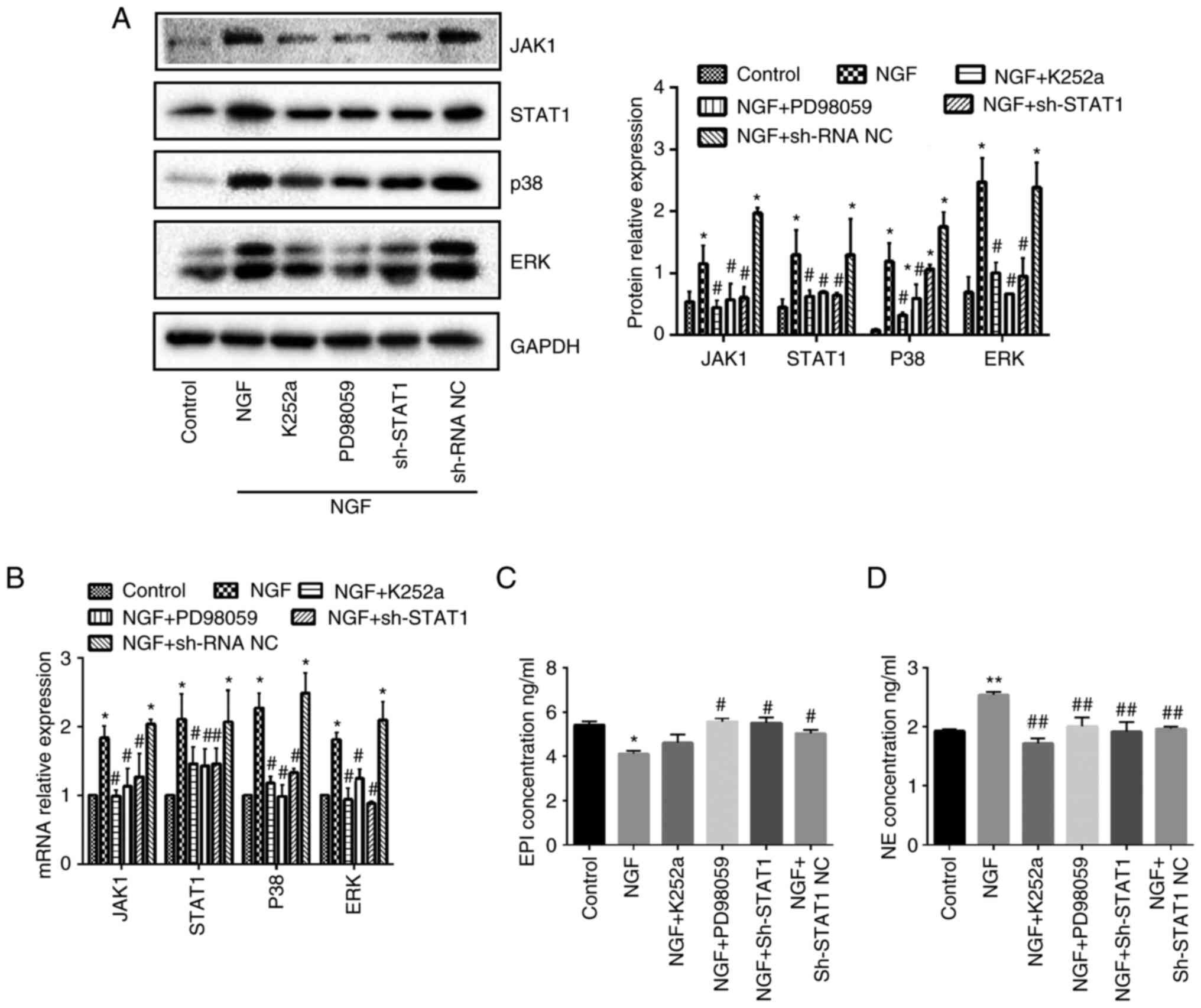
The JAK1/STAT1, p38 and ERK pathways
are substantially activated in AMCCs in the presence of NGF
STAT1 was knocked down with three pairs of designed
shRNAs and selected the shRNA2 with the highest interference
efficiency (Fig. 5D-F). NGF
treatment significantly promoted the protein and mRNA expression
levels of JAK1, STAT1, p38 and ERK in AMCCs compared with the
control group. However, in the presence of K252a, PD98059 (the
inhibitor of ERK) and STAT1 shRNA, the opposite observations were
made. (Fig. 6A and B). These
findings suggest that blocking NGF, ERK and STAT1 inhibited
NGF-triggered signaling events in AMCCs.
EPI and NE concentrations change in
cell supernatants treated with NGF
Compared with the control group, NGF presence
resulted in diminished EPI concentrations and amplified NE levels
in the supernatants of AMCCs. However, K252a, PD98059 and STAT1
shRNA treatments reversed this trend, increasing EPI expression and
decreasing the levels of NE (Fig. 6C
and D).
Discussion
The nasal mucosa, which is the first line of defense
against allergen exposure, has an important role in maintaining the
normal physiological function of the upper respiratory tract
(31,32). Past investigations have pointed out
that AR involves the infiltration of inflammatory cells, such as
lymphocytes, eosinophils and basophils, into the nasal mucosa
(33,34). The present study confirmed that
lymphocytes and eosinophils infiltrated the nasal mucosa of AR
mice, but not the control group. Additionally, the AR mice were
characterized by increased nasal scratching and sneezing. Thus, an
OVA-induced mouse model of allergic rhinitis was successfully
established.
Adrenal glands are crucial to maintaining the
pathophysiological processes of an organism via the control of the
endocrine system involved mainly in regulating hormone synthesis
and responding to stress (35).
The adrenal medulla is formed mostly by adrenergic and
noradrenergic chromaffin cells. Histologically, both the chromaffin
cells of the adrenal medulla and the sympathetic nerves of the
autonomic nervous system originate from the neural crest of the
ectoderm (36). Therefore, in
addition to endocrine functions, chromaffin cells can also
potentially transform into neuronal phenotypes. One previous
investigation suggested that both mature and immature adrenal
medulla cells possess varying degrees of pluripotent
differentiation capacity (37).
NGF, a member of the neurotrophin family, is critical in promoting
the differentiation of cells (7).
Stimulated by NGF, chromaffin cells can transform into neuronal
phenotypes, with their original endocrine function also occurring
with corresponding changes (27,37).
In the present study, in vivo, lesions of the adrenal
medulla were also accompanied by an AR onset and featured
nucleolemma shrinkage, loose particle distribution and
disintegration of cell contents under TEM. Notably, the present
study revealed that treatment with NGF further aggravated adrenal
medulla lesions in vitro; however, the degree of adrenal
medulla lesions was alleviated by K252a. As an inhibitor of NGF
receptor, K252a inhibits the activation of NGF-induced signaling
pathway and downstream signals. A previous study reported that NGF
could induce the transformation of microglial cells to a
neuroprotective and anti-inflammatory phenotype, while K252a
reversed this phenotypic transformation (38). K252a promotes the apoptosis of
endometrial cancer cells, but it has little effect on normal human
endometrial epithelial cells (39). As expected, K252a had no adverse
effect on AMCCs. By contrast, it could antagonize the adverse
effects of NGF on AMCCs. Together, when stimulated by NGF,
ultrastructural changes (including mitochondrial swelling and
nucleolemma shrinkage) occurred in the AMCCs of the AR mouse model
and are possible evidence of cell phenotype switching.
SYP, which is a transmembrane protein of synaptic
vesicles, is involved in the formation and circulation of synaptic
vesicles (40,41). Additionally, SYP is a specific
marker of neuroendocrine differentiation and is largely distributed
in multiple organs, including the neurons, spinal cord and adrenal
medulla (42). The present study
found that NGF mediation resulted in notable augmentations in the
SYP mRNA and protein levels in vitro; however, K252a
reversed these trends. The results of immunohistochemistry further
confirmed that the expression of SYP in the AMCCs of the AR model
group and NGF intervention group was higher compared with that in
the control group. Together, NGF presence induced an increase in
the number of synapses in AMCCs to promote neuronal
differentiation, while K252a inhibits this reaction.
PNMT is an enzyme biosynthesized by the adrenal
medulla, which catalyzes the biosynthesis of catecholamines and
methylates NE to form EPI (43).
Since the final step in catalyzing EPI synthesis is PNMT, the
changes in its expression levels indirectly reflect the number of
EPI secretion. The endocrine function of AMCCs could be indirectly
assessed using the level of PNMT (21). In the present study, it was
observed that the levels of PNMT in adrenal gland were
downregulated in NGF and model groups, especially the NGF group.
These results showed that reduced expression of PNMT by NGF
stimulation might result in decreased EPI production. Therefore,
the present study confirmed the changes in secretion of EPI in
vivo and in vitro by ELISA. Compared with the control
group, the serum levels of EPI were weakened in the model and NGF
groups. However, the opposite trend was observed following K252a
intervention. Decreased secretion of EPI in supernatants was
observed when AMCCs were co-cultured with NGF. Some AMCCs possibly
transform from endocrine phenotypes to neuronal cell phenotypes,
accompanied by a decline in endocrine function, resulting in
decreased synthesis and release of EPI in adrenal medulla cells.
Chromaffin cells can differentiate into sympathetic neurons under
NGF stimulation (26). Markedly,
sympathetic neurons cannot synthesize and secrete EPI, but they
produce and secrete NE (44).
Furthermore, it was hypothesized that the increased levels of NE in
cell supernatants arguably stemmed from the direct impact of NGF on
AMCCs in vitro, resulting in the transformation of most
chromaffin cells into sympathetic neuronal cells, widening the
production of NE.
However, the signaling pathway through which NGF
regulates the transdifferentiation of AMCCs remains to be
elucidated. The ERK signaling pathway possibly participates in
NGF-mediated neurite outgrowth in adrenal chromaffin cells
(45). Neurotrophins including NGF
and brain-derived neurotrophic factor (BDNF) regulate neuronal
differentiation, survival and growth by binding to members of the
Trk family, namely TrkA, TrkB and TrkC (46). A recent study showed that
activation of BDNF/TrkB signaling pathway can lead to
transactivation of several downstream signaling pathways, including
PI3K/Akt, JAK/STAT, PLCγ, Ras-Raf-MEK-ERK and NF-κB (47). Activation of TrkA by NGF triggered
phosphorylation of STAT3 at S727 and enhanced its DNA binding and
transcriptional activity. TrkA-induced STAT3 activation mediated
several downstream functions of NGF signaling, particularly
neuronal differentiation (48).
The present study showed that NGF stimulation was linked to
significantly enhanced protein and mRNA levels of JAK1, STAT1, p38
and ERK in AMCCs; however, K252a, PD98058 and STAT1 shRNA markedly
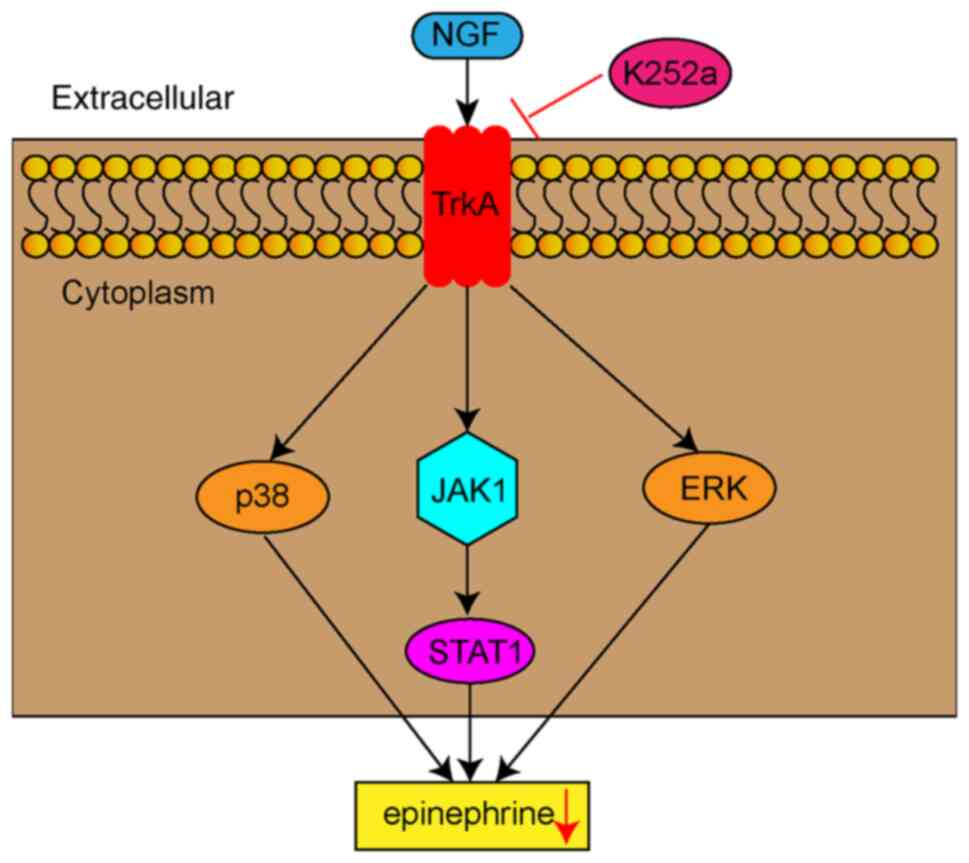
suppressed the activation of these pathways. Therefore, NGF induced
the conversion of AMCCs to a neuronal phenotype by activating
JAK1/STAT1, p38 and ERK signaling pathways, resulting in
epinephrine release dysfunction (Fig.
7). The present study was limited by the lack of analysis of
their downstream signaling pathways. Whether the activation of
these signaling pathways will be enhanced with the higher
concentration of NGF is one focus of future research.
In summary, the present study suggested that NGF
possibly affects the differentiation of AMCCs phenotypes in two
main ways. First, sufficient amounts of NGF directly act on
chromaffin cells to differentiate between endocrine phenotypes and
sympathetic neuronal phenotypes. Second, increased NGF levels
continuously act on chromaffin cells at different differentiation
stages, gradually reducing their redundancy threshold, paving the
way to eventually breaking through the anti-redundancy effect of
glucocorticoids and transforming into sympathetic neuronal
phenotypes. Hence, the mechanism of the transformation of AR into
BA in a mouse model could be as follows: the increased
concentration of NGF in AR mice directly affects the
differentiation of chromaffin cells and then transforms them from
an endocrine phenotype to a neuronal phenotype, thereby inhibiting
EPI synthesis and release them into AMCCs, making it difficult to
reach the concentration of diastolic airways to induce BA.
Overall, the present study demonstrated the effect
of NGF on AMCCs in vitro and vivo and elucidated the
pathogenesis of AR's transformation into BA and the correlation
between the two airway diseases. Therefore, it could provide new
theoretical support for the theory of ‘one airway, one disease’
(49).
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of China (grant nos. 81460004 and 82160006); the Jiangxi
Provincial Cultivation Program for Academic and Technical Leaders
of Major Subjects (grant no. 20172BCB22025); and the Jiangxi
Provincial Natural Science Foundation General Project (grant no.
20202BAB206003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and CL conceived and designed the present study.
XH, ZL, ZW, ND and AZ performed the experiments. JL and XH analyzed
the experimental data. CL and JL wrote the manuscript. CL and JW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Committee of Jiangxi Provincial People's Hospital (approval no.
2022-008; Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Testa D, DI Bari M, Nunziata M, Cristofaro
G, Massaro G, Marcuccio G and Motta G: Allergic rhinitis and asthma
assessment of risk factors in pediatric patients: A systematic
review. Int J Pediatr Otorhinolaryngol. 129:1097592020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corren J: The connection between allergic
rhinitis and bronchial asthma. Curr Opin Pulm Med. 13:13–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Lan F and Zhang L: Advances and
highlights in allergic rhinitis. Allergy. 76:3383–3389. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen M, Wu Y, Yuan S, Tang M, Zhang L,
Chen J, Li L, Wu J, Zhang J and Yin Y: Allergic rhinitis
improvement in asthmatic children after using acaricidal bait: A
randomized, double-blind, cross-placebo study. Front Pediatr.
9:7091392021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nappi E, Paoletti G, Malvezzi L, Ferri S,
Racca F, Messina MR, Puggioni F, Heffler E and Canonica GW:
Comorbid allergic rhinitis and asthma: Important clinical
considerations. Expert Rev Clin Immunol. 18:747–758. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ceci FM, Ferraguti G, Petrella C, Greco A,
Tirassa P, Iannitelli A, Ralli M, Vitali M, Ceccanti M, Chaldakov
GN, et al: Nerve growth factor, stress and diseases. Curr Med Chem.
28:2943–2959. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freund-Michel V and Frossard N: The nerve
growth factor and its receptors in airway inflammatory diseases.
Pharmacol Ther. 117:52–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rocco ML, Soligo M, Manni L and Aloe L:
Nerve growth factor: Early studies and recent clinical trials. Curr
Neuropharmacol. 16:1455–1465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang N, Xu J, Jiang C and Lu S:
Neuro-Immune regulation in inflammation and airway remodeling of
allergic asthma. Front Immunol. 13:8940472022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen PC, Hsieh MH, Kuo WS, Kao HF, Hsu CL
and Wang JY: Water-Soluble chitosan inhibits nerve growth factor
and attenuates allergic inflammation in mite allergen-induced
allergic rhinitis. J Allergy Clin Immunol. 140:1146–1149.e8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bresciani M, Lalibertè F, Lalibertè MF,
Gramiccioni C and Bonini S: Nerve growth factor localization in the
nasal mucosa of patients with persistent allergic rhinitis.
Allergy. 64:112–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu W, Chen X, Wu Q, Ying X, He R, Lou X,
Yang G, Zhou K and Jiang S: Acupoint application inhibits nerve
growth factor and attenuates allergic inflammation in allergic
rhinitis model rats. J Inflamm (Lond). 17:42020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raap U, Fokkens W, Bruder M, Hoogsteden H,
Kapp A and Braunstahl GJ: Modulation of neurotrophin and
neurotrophin receptor expression in nasal mucosa after nasal
allergen provocation in allergic rhinitis. Allergy. 63:468–475.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobkowiak P, Langwiński W, Nowakowska J,
Wojsyk-Banaszak I, Szczepankiewicz D, Jenerowicz D, Wasilewska E,
Bręborowicz A and Szczepankiewicz A: Neuroinflammatory gene
expression pattern is similar between allergic rhinitis and atopic
dermatitis but distinct from atopic asthma. Biomed Res Int.
2020:71969812020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coffey CS, Mulligan RM and Schlosser RJ:
Mucosal expression of nerve growth factor and brain-derived
neurotrophic factor in chronic rhinosinusitis. Am J Rhinol Allergy.
23:571–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu P, Li S and Tang L: Nerve growth
factor: A potential therapeutic target for lung diseases. Int J Mol
Sci. 22:91122021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szczepankiewicz A, Rachel M, Sobkowiak P,
Kycler Z, Wojsyk-Banaszak I, Schöneich N, Szczawińska-Popłonyk A
and Bręborowicz A: Neurotrophin serum concentrations and
polymorphisms of neurotrophins and their receptors in children with
asthma. Respir Med. 107:30–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogawa H, Azuma M, Uehara H, Takahashi T,
Nishioka Y, Sone S and Izumi K: Nerve growth factor derived from
bronchial epithelium after chronic mite antigen exposure
contributes to airway hyperresponsiveness by inducing
hyperinnervation, and is inhibited by in vivo siRNA. Clin Exp
Allergy. 42:460–470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashimoto S: K-252a, a Potent protein
kinase inhibitor, blocks nerve growth factor-induced neurite
outgrowth and changes in the phosphorylation of proteins in PC12h
cells. J Cell Biol. 107:1531–1539. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terad K, Matsushima Y, Matsunaga K, Takata
J, Karube Y, Ishige A and Chiba K: The kampo medicine yokukansan
(YKS) enhances nerve growth factor (NGF)-induced neurite outgrowth
in PC12 cells. Bosn J Basic Med Sci. 18:224–233. 2018.PubMed/NCBI
|
|
21
|
Hu CP, Zou YQ, Feng JT and Li XZ: The
effect of unilateral adrenalectomy on transformation of adrenal
medullary chromaffin cells in vivo: A potential mechanism of asthma
pathogenesis. PLoS One. 7:e445862012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furlan A, Dyachuk V, Kastriti ME,
Calvo-Enrique L, Abdo H, Hadjab S, Chontorotzea T, Akkuratova N,
Usoskin D, Kamenev D, et al: Multipotent peripheral glial cells
generate neuroendocrine cells of the adrenal medulla. Science.
357:eaal37532017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng JT and Hu CP: Dysfunction of
releasing adrenaline in asthma by nerve growth factor. Med
Hypotheses. 65:1043–1046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krakauer DC and Plotkin JB: Redundancy,
antiredundancy, and the robustness of genomes. Proc Natl Acad Sci
USA. 99:1405–1409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu XM, Hu CP, Li XZ, Zou YQ, Zou JT, Li YY
and Feng JT: Asthma pregnancy alters postnatal development of
chromaffin cells in the rat adrenal medulla. PLoS One.
6:e203372011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Unsicker K, Krisch B, Otten U and Thoenen
H: Nerve growth factor-induced fiber outgrowth from isolated rat
adrenal chromaffin cells: Impairment by glucocorticoids. Proc Natl
Acad Sci USA. 75:3498–3502. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li QG, Wu XR, Li XZ, Yu J, Xia Y, Wang AP
and Wang J: Neural-Endocrine mechanisms of respiratory syncytial
virus-associated asthma in a rat model. Genet Mol Res.
11:2780–2789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
National Research Council (US) Committee
for the Update of the Guide for the Care: Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
29
|
Li HT, Chen ZG, Lin YS, Liu H, Ye J, Zou
XL, Wang YH, Yang HL and Zhang TT: CpG-ODNs and budesonide act
synergistically to improve allergic responses in combined allergic
rhinitis and asthma syndrome induced by chronic exposure to
ovalbumin by modulating the TSLP-DC-OX40L Axis. Inflammation.
41:1304–1320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bousquet J, Anto JM, Bachert C, Baiardini
I, Bosnic-Anticevich S, Walter Canonica G, Melén E, Palomares O,
Scadding GK, Togias A and Toppila-Salmi S: Allergic Rhinitis. Nat
Rev Dis Primers. 6:952020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nur Husna SM, Tan HT, Md Shukri N, Mohd
Ashari NS and Wong KK: Nasal epithelial barrier integrity and tight
junctions disruption in allergic rhinitis: Overview and pathogenic
insights. Front Immunol. 12:6636262021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eifan AO and Durham SR: Pathogenesis of
Rhinitis. Clin Exp Allergy. 46:1139–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng Y, Wang C and Zhang L: Advances and
novel developments in allergic rhinitis. Allergy. 75:3069–3076.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haase M, Willenberg HS and Bornstein SR:
Update on the corticomedullary interaction in the adrenal gland.
Endocr Dev. 20:28–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Unsicker K, Huber K, Schütz G and Kalcheim
C: The chromaffin cell and its development. Neurochem Res.
30:921–925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barreto-Estrada JL, Medina-Ortíz WE and
García-Arrarás JE: The morphological and biochemical response of
avian embryonic sympathoadrenal cells to nerve growth factor is
developmentally regulated. Brain Res Dev Brain Res. 144:1–8. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rizzi C, Tiberi A, Giustizieri M, Marrone
MC, Gobbo F, Carucci NM, Meli G, Arisi I, D'Onofrio M, Marinelli S,
et al: NGF steers microglia toward a neuroprotective phenotype.
Glia. 66:1395–1416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: K252a is highly effective in suppressing the growth of
human endometrial cancer cells, but has little effect on normal
human endometrial epithelial cells. Oncol Rep. 19:749–753.
2008.PubMed/NCBI
|
|
40
|
Raja MK, Preobraschenski J, Del
Olmo-Cabrera S, Martinez-Turrillas R, Jahn R, Perez-Otano I and
Wesseling JF: Elevated synaptic vesicle release probability in
synaptophysin/gyrin family quadruple knockouts. Elife.
8:e407442019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang CW, Hsiao YT and Jackson MB:
Synaptophysin regulates fusion pores and exocytosis mode in
chromaffin cells. J Neurosci. 41:3563–3578. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kasprzak A, Zabel M and Biczysko W:
Selected markers (Chromogranin a, neuron-specific enolase,
synaptophysin, protein gene product 9.5) in diagnosis and prognosis
of neuroendocrine pulmonary tumours. Pol J Pathol. 58:23–33.
2007.PubMed/NCBI
|
|
43
|
Sørensen DB, Johnsen PF, Bibby BM, Böttner
A, Bornstein SR, Eisenhofer G, Pacak K and Hansen AK: PNMT
transgenic mice have an aggressive phenotype. Horm Metab Res.
37:159–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vega A, Luther JA, Birren SJ and Morales
MA: Segregation of the classical transmitters norepinephrine and
acetylcholine and the neuropeptide Y in sympathetic neurons:
Modulation by ciliary neurotrophic factor or prolonged growth in
culture. Dev Neurobiol. 70:913–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mullenbrock S, Shah J and Cooper GM:
Global expression analysis identified a preferentially nerve growth
factor-induced transcriptional program regulated by sustained
mitogen-activated protein kinase/extracellular signal-regulated
kinase (ERK) and AP-1 protein activation during PC12 cell
differentiation. J Biol Chem. 286:45131–45145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Uren RT and Turnley AM: Regulation of
Neurotrophin Receptor (Trk) signaling: Suppressor of cytokine
signaling 2 (SOCS2) is a new player. Front Mol Neurosci. 7:392014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Malekan M, Nezamabadi SS, Samami E,
Mohebalizadeh M, Saghazadeh A and Rezaei N: BDNF and its signaling
in cancer. J Cancer Res Clin Oncol. Sep 29–2022.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Miranda C, Fumagalli T, Anania MC, Vizioli
MG, Pagliardini S, Pierotti MA and Greco A: Role of STAT3 in in
vitro transformation triggered by TRK oncogenes. PLoS One.
5:e94462010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Klain A, Indolfi C, Dinardo G, Licari A,
Cardinale F, Caffarelli C, Manti S, Ricci G, Pingitore G, Tosca M,
et al: United airway disease. Acta Biomed.
92:e20215262021.PubMed/NCBI
|