Introduction
Secreted protein acidic and rich in cysteine
(SPARC), also known as basement-membrane protein 40 or osteonectin,
is a 43-kDa glycoprotein classified as a matricellular protein
(1,2). SPARC is the most abundant
non-collagenous protein in the bone matrix and demonstrates high
affinity for calcium, type I collagen and hydroxyapatite,
affinities associated with their mineralization (1). Furthermore, SPARC has been reported
to localize in non-mineralized tissues, which makes its
distribution ubiquitous (3,4).
SPARC not only binds to collagen fibrils (types I, II, III and V)
but also to basement collagen type IV (5,6) and
vascular endothelial growth factor A (7). Phenotypically, mice with Sparc
gene knock out (KO) are characterized by cataracts, decreases in
collagens and bone mineral density, and increases in levels of
adipose tissue (8–10). Bone marrow stromal-derived cells
from Sparc KO mice have been reported to demonstrate reduced
ability to form mineralized nodules and reduced osteocalcin
(OCN) mRNA expression levels, and enhanced adipocyte
development through the upregulation of adipsin and
CCAAT/enhancer-binding protein δ (C/EBPδ) expression levels
(11). Sparc KO cells have
also been reported to show reduced collagen type I and transforming
growth factor-β1 mRNA and protein expression levels (12). Taken together, these findings
indicated that SPARC may commit cells to the osteoblast lineage by
inhibiting their differentiation into adipocytes.
SPARC has also been reported to localize to nuclei
(13,14) and the endoplasmic reticulum (ER)
(15). Intracellularly, SPARC has
been reported to be associated with tubulin (16) and osteopontin (OPN) (17), and to accumulate in nasopharyngeal
angiofibromas (18). Moreover,
SPARC was not secreted into the culture medium but was reported to
have been detected in lysates of chronic myelogenous leukemia cells
(19). Bioinformatics analysis
studies have reported that SPARC binds to xeroderma pigmentosum
group C complex subunit and zinc finger protein 579 intracellularly
(20). Therefore, although SPARC
has been reported to be localized intracellularly, its
intracellular activity associated with
osteoblastogenesis/adipogenesis remains unclear.
Adipogenesis by mesenchymal stem cells is induced by
several transcription factors, such as peroxisome
proliferator-activated receptor-γ (PPARγ) and C/EBPα. C/EBPβ and
C/EBPδ are factors that induce PPARγ- and C/EBPα-expressing
preadipocytes, which suggests that PPARγ and C/EBPα serve roles
during the late stages of adipogenic differentiation (21). However, activator protein-1 (AP-1)
is an important transcription factor involved in early stage
commitment to the adipocyte lineage (21).
AP-1 is a homo- or hetero-dimeric transcription
factor consisting of basic region leucine zipper domain proteins,
such as Fos and Jun, that translocates into the nucleus. Although
the Fos proteins, such as c-Fos, Fra-1, Fra-2, FosB and the short
isoform of FosB (ΔFosB), heterodimerize only with their counterpart
Jun proteins, such as c-Jun, JunB and JunD, the Jun proteins
homodimerize or heterodimerize with Fos proteins. The Fos/Jun and
Jun/Jun dimers bind to TPA-response element (TRE; 5′-TGACTCA-3′)
(22). c-Fos has been reported to
induce the production of adipocyte P2/fatty acid-binding protein 4
(FABP4), which serves an important role in adipogenesis (23). Mutations in the c-FOS gene
have been reported to predict the phenotypic development of
congenital generalized lipodystrophy and Berardinelli-Seip
congenital lipodystrophy, rare genetic syndromes characterized by
the absence of adipose tissue (24), which suggests that c-Fos is
important for adipogenesis. The present study evaluated whether
SPARC interacted with c-Fos to commit to the osteoblastic lineage
by the inhibition of adipogenesis.
Materials and methods
Reagents
RPMI 1640 medium and simvastatin were purchased from
MilliporeSigma; α-minimum essential medium (α-MEM) was purchased
from MP Biomedicals; and ascorbic acid, β-glycerophosphate alizarin
red-S, Oil red O and ethidium bromide were purchased from FUJIFILM
Wako Pure Chemical Corporation. Fetal bovine serum (FBS) was
purchased from Hyclone (Cytiva). Guanidinium thiocyanate, phenol,
chloroform and Bacto Yeast Extract were purchased from Nacalai
Tesque, Inc. Prime STAR GXL DNA Polymerase and Xfect Transfection
Reagent, SYBR Premix Ex Taq II, In-Fusion® HD
Cloning Kit and Brevibacillus expression system II were
purchased from Takara Bio, Inc.; and Blocking Regent N102 was from
NOF Corporation. Immobilon-P polyvinylidene fluoride (PVDF)
membranes and the chemiluminescent reagent Luminata™
Forte Western HRP substrate were purchased from MilliporeSigma; and
Hybond-C nitrocellulose membranes and Hybond-N+ nylon membranes
were purchased from GE Healthcare. Radioimmunoprecipitation assay
(RIPA) Buffer, Dulbecco's modified Eagle medium (DMEM),
Bacto™ Soytone, SuperScript IV Reverse Transcriptase and
a High-Capacity cDNA Reverse Transcription Kit (cat. no. 4387406)
were purchased from Thermo Fisher Scientific, Inc. Prime STAR GXL
DNA Polymerase and Dual-Luciferase® Reporter Assay
System were purchased from Promega Corporation; and avidin-linked
HRP and DCÔ protein assay kits were from Bio-Rad
Laboratories, Inc.
Antibodies
Anti-SPARC polyclonal antibody (pAb) (1:1,000; cat.
no. ab55847) was purchased from Abcam, anti-c-Jun (G-4) monoclonal
antibody (mAb) (1:1,000; cat. no. sc-74543), and anti-c-Fos (H-125)
mAb (1:1,000; cat. no. sc-7202) were purchased from Santa Cruz
Biotechnology, Inc. Anti-β actin pAb (1:1,000; cat. no. GTX109639)
and anti-GAPDH pAb (1:1,000; cat. no. GTX100118) were purchased
from GeneTex, Inc.; anti-Lamin-A pAb (1:1,000; cat. no. 3267) was
purchased from BioVision, Inc. Anti-FLAG M2 mAb (1:1,000; cat. no.
F1804) was purchased from MilliporeSigma; and biotin-conjugated
anti-rabbit IgG pAb (1:10,000; cat. no. 111-065-144) and
biotin-conjugated anti-mouse IgG pAb (1:10,000; cat. no.
115-065-003) were purchased from Jackson ImmunoResearch
Laboratories.
Vectors
p3×FLAG-CMV-9 and p3×FLAG-CMV-10 vectors were
purchased from MilliporeSigma. The pAP1(1)-Luc vector was purchased
from Affymetrix, Inc.; pGL4.75[hRluc CMV] was from Promega
Corporation; and pNCMO2 was from Takara Bio, Inc.
Sequence datasets
Sequence data for cDNA and proteins were downloaded
from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov). Accession numbers for
the datasets used were as follows: Human SPARC (NM_003118.4 and
NP_003109.1); mouse SPARC (NM_009242.5); mouse c-Fos,
cDNA(NM_010234.2 and NP_034364); mouse c-Jun (NM_010591.2 and
NP_034721.1).
Cells and cell culture
The mouse bone marrow stromal ST2 cell line was
purchased from the RIKEN BioResource Center (25,26).
The human oral cancer Ca9-22 cell line was a gift from Dr Kimiharu
Hirose (Ohu University School of Dentistry, Koriyama, Japan) and
was originally obtained from Japanese Collection of Research
Bioresources Cell Bank (27). ST2
cells were cultured in RPMI 1640 supplemented with 10% FBS and used
for osteoblastic and adipocyte differentiation experiments
(28). Ca9-22 cells were cultured
in DMEM supplemented with 10% FBS and used for cloning of the
SPARC gene. All cultures were maintained at 37°C under a
humidified atmosphere containing 5% CO2 (unless
otherwise noted).
The ST2 cell clone with low SPARC production
(Fig. 1A) was selected using the
limiting dilution method. Briefly, ST2 cells were inoculated into a
96-well culture plate at a density of 1 cell/well in RPMI 1640
supplemented with 10% FBS; this culture medium was used throughout
cell cloning. Immediately after inoculation, an inverted
phase-contrast microscope was used to demonstrate that there was 1
cell/well. When the colony formed 2 weeks after seeding, cell
culture vessels were scaled up stepwise when they reached 70–80%
confluence after culturing for ~10 days: i.e., one well of a
96-well plate, one well of a 24-well culture plate, one well of a
6-well plate, and three wells of a 6-well plate. When cells reached
70–80% confluence in three wells in a 6-well plate, with cells in
one well used for RNA extraction, those in the other used for cell
lysate preparation and those remaining frozen at −80°C as the
stock. SPARC mRNA and protein expression levels were assessed using
reverse transcription-quantitative PCR (RT-qPCR) and western
blotting, respectively. Finally, the clone where SPARC mRNA was not
detected after 30 cycles of RT-qPCR was used for subsequent
experiments in the present study, these cells were subsequently
referred to as SPARC-low ST2 cells.
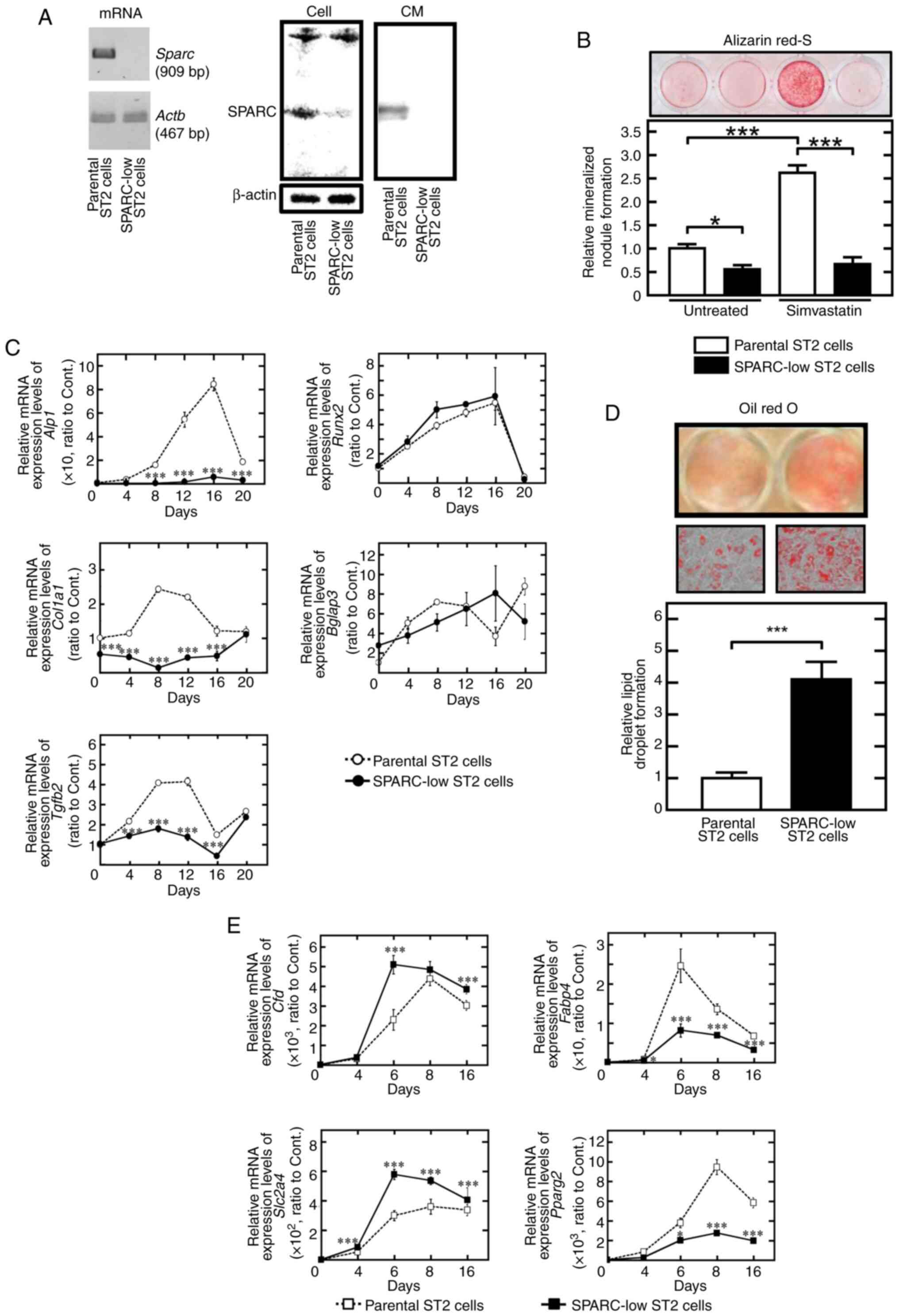 | Figure 1.Adipogenesis is higher but
osteoblastogenesis is lower in SPARC-low ST2 cells compared with in
parental ST2 cells. (A) SPARC expression was assessed at the mRNA
and protein levels in cells and CM using RT-PCR and western
blotting. (B) SPARC-low ST2 cells demonstrated low mineralized
nodule formation activity. Data were presented relative to those of
parental ST2 cells cultured in osteogenic medium without
simvastatin. (C) Relative mRNA expression levels of
osteoblastogenesis-associated genes, including Runx2, Tgfg2,
Alp1, Col1a1 and Bglap3 were assessed using RT-qPCR. The
mRNA expression levels were normalized against each control, which
was non-stimulated (time 0) parental ST2 cells. (D) SPARC-low ST2
cells demonstrated significantly higher lipid droplet formation
activity compared with parental ST2 cells. (High-power images,
objective magnification, ×20). The data were presented relative to
those of parental ST2 cells cultured in adipogenic medium. (E)
Relative mRNA expression levels of adipogenesis-associated genes,
including Cfd, Fabp4, Pparg2 and Slc2a4 were assessed
using RT-qPCR. The mRNA expression levels were normalized against
each control which was non-stimulated (time 0) parental ST2 cells.
*P<0.05 and ***P<0.001. CM, conditioned medium; RT, reverse
transcription; qPCR, quantitative PCR; Sparc/SPARC, secreted
protein acidic and rich in cysteine; Alp1, alkaline
phosphatase; Col1a1, type I collagen; Bglap3,
osteocalcin; Cfd, adipsin; Fabp4, fatty acid-binding
protein 4; Pparg2, proliferator-activated receptor-γ;
Slc2a4, glucose transporter type 4; Tgfb2,
transforming growth factor β2. |
For osteoblastic differentiation, 70% confluent ST2
cells were incubated with α-MEM supplemented with 10% FBS, 50 µg/ml
ascorbic acid and 10 mM β-glycerophosphate for 3 weeks. In some
cases, 10−6 M simvastatin was also added and cultured
for 3 weeks. Calcified deposits were assessed by staining with 40
mM alizarin red-S (pH, 4.2) at room temperature for 10 min. After
washing with distilled water five times, images of the culture
plates were captured using a digital camera and the red-stained
areas were quantified using ImageJ software version 1.5.3 (National
Institutes of Health) (26). For
adipocyte differentiation, 70% confluent ST2 cells were further
incubated with RPMI 1640 medium supplemented with 5 mg/ml insulin,
1 mM dexamethasone and 0.5 mM 1-methyl-3-isobutylxanthine (IBMX)
for 2 weeks. Triacylglycerol (TAG) deposits were assessed by
staining with Oil Red O at room temperature for 10 min. After
washing with 60% isopropanol three times, Oil Red O-stained images
of culture plates were captured using a digital camera and
high-power images were also captured by an inverted light
microscope (objective magnification, ×20), and Oil Red O was eluted
using 100% dimethyl sulfoxide at room temperature for 5 min and
quantified using spectrophotometry at 531 nm (25).
Construction of expression vectors for
SPARC and its mutant and transfection
Total RNA was extracted from Ca9-22 cells using the
standard acid guanidinium-phenol-chloroform (AGPC) extraction
method (29). RNA was reverse
transcribed using SuperScript IV Reverse Transcriptase at 55°C for
10 min followed by 80°C for 10 min for inactivation of the enzyme
and cDNA was amplified using Prime STAR GXL DNA Polymerase and
specific primers (Table I). The
qPCR thermocycling protocol consisted of denaturation at 98°C for
10 sec, followed by 30 cycles of annealing at 60°C for 15 sec and
extension at 68°C for 60 sec. The resulting fragments were cloned
into the vectors p3×FLAG-CMV-9 and p3×FLAG-CMV-10. The amino acid
sequence of human SPARC (Fig. 2B)
consisted of amino acid residues (AA) 1–17 as the signal peptide
and AA 18–303 as the mature protein. The expression vectors were
constructed using the In-Fusion® HD Cloning Kit as
follows: SPARC (AA 18–303) with wild-type signal peptide (AA 1–17),
SPARC (AA 18–303) with preprotrypsin signal peptide, and SPARC (AA
18–303) without signal peptide (Fig.
2C). To confirm the existence of the ectopically expressed
SPARC in the conditioned medium (CM), a SPARC-expression vector in
which the FLAG sequence was inserted between wild-type signal
peptide (AA 1–17) and mature SPARC (AA 18–303) was also
generated.
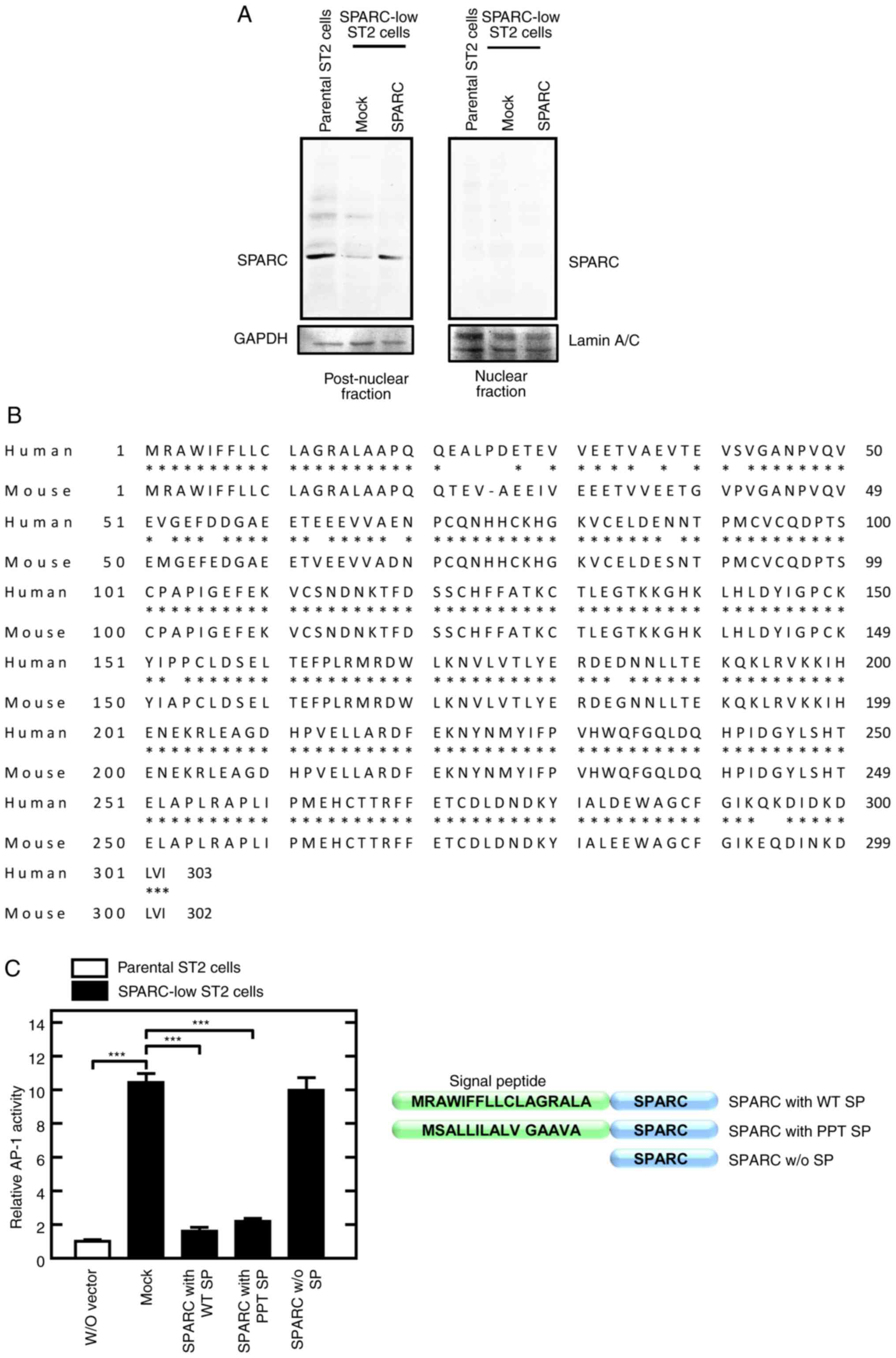 | Figure 2.Rescued secreted SPARC localizes to
the post-nuclear fraction and inhibits AP-1 activity. (A) SPARC
localized to the post-nuclear fraction. Cells were lysed and
fractionated into nuclear and post-nuclear fractions. SPARC protein
expression levels were assessed using western blotting. Lamin A/C
and GAPDH were used as internal controls for the nuclear fraction
and post-nuclear fraction, respectively. Mock, empty vector. (B)
Comparison of the amino acid sequences of human and mouse SPARC.
The amino acid sequences of SPARC were downloaded from NCBI (human,
NP_003109.1; mouse, NP_033268.1). Mouse SPARC was 92% homologous
with human SPARC including the signal peptide (AA 1 to 17). (C)
Secreted SPARC reduced AP-1 activity (left panel). Cells were
transfected with the AP1(1)-Luc vector, with or without SPARC
expression vectors, as follows: w/o vector; Mock, empty vector;
SPARC (AA 18 to 303) with WT SP; SPARC (AA 18 to 303) with PPT SP
(AA 1 to 17); SPARC (AA 18 to 303) w/o SP. Luciferase activity was
measured 24 h after transfection. N-terminal structure of the SPARC
expression vectors is illustrated (right panel). ***P<0.001.
AP-1, activator protein-1; AA, amino acid residue; SPARC, secreted
protein acidic and rich in cysteine; w/o, without; WT, wild type;
SP, signal peptide; PPT, preprotrypsin. |
 | Table I.Sequences of primers used for reverse
transcription-PCR. |
Table I.
Sequences of primers used for reverse
transcription-PCR.
| Gene | Sequence,
5′-3′ | Product, bp | Accession
number |
|---|
| Sparc | F:
ATGAGGGCCTGGATCTTCTTTCTCCTTT | 909 | NM_001290817.1 |
|
| R:
TTAGATCACCAGATCCTTGTTGATGTCCTG |
|
|
| Actb | F:
CGCAGCCACTGTCGAGTC | 467 | NM_007393.5 |
|
| R:
AAGGTCTCAAACATGATCTGGGT |
|
|
| SPARC | F:
ATGAGGGCCTGGATCTTCTTTCTCCTTT | 909 | NM_003118.4 |
|
| R:
GATCACAAGATCCTTGTCGATATCCTTCTG |
|
|
| c-Fos | F:
ATGATGTTCTCGGGTTTCAACGCCGACTAC | 1,155 | NM_010234.2 |
|
| R:
TTCTCTGACTGCTCACAGGGCCAGCA |
|
|
| c-Jun | F:
ATGACTGCAAAGATGGAAACGACCTTCTAC | 1,005 | NM_010591.2 |
|
| R:
TCAAAACGTTTGCAACTGCTGCGTTAG |
|
|
Transfection of vectors into SPARC-low ST2 cells was
proceeded using Xfect Transfection Reagent according to the
manufacturers' protocol. Briefly, 5 µg plasmids were mixed with 1.5
µl Xfect polymer and incubated at room temperature for 10 min,
followed by the mixture being added to the cultures in a 6-well
culture plate. After incubation of the cells in a CO2
incubator for 4 h, the culture medium was refreshed and the cells
were further incubated for 24 h for AP-1 measurements and 48 h for
preparation of CM.
Preparation of SPARC-present/absent
CM
SPARC-low ST2 cells were transfected with mock or
Flag-tagged SPARC expression vector as aforementioned.
Post-transfection, the medium was refreshed with RPMI 1640
supplemented with 10% FBS, and the cells were incubated for a
further 48 h. The CM was collected and clarified by centrifugation
at 1,000 × g for 10 min to obtain the following: SPARC (+) CM and
SPARC (−) CM.
Serum-free SPARC (+) or (−) CM was prepared from the
parallel cultures and subjected to western blot analysis. Briefly,
after the transfected cells were cultured for 24 h in RPMI 1640
supplemented with 10% FBS, they were cultured for a further 24 h in
serum-free RPMI 1640. The serum-free CM was collected and clarified
by centrifugation at 1,000 × g at room temperature for 10 min. The
supernatant was analyzed by western blotting.
RT-PCR and RT-qPCR
Total RNA was extracted from parental ST2 cells,
SPARC-low ST2 cells and transfected cells using the (AGPC)
extraction method. RNA was reverse transcribed at 55°C for 10 min,
followed by 80°C for 10 min for inactivation of the enzyme, using
SuperScript IV Reverse Transcriptase with Oligo dT for PCR and at
37°C for 60 min, followed by 95°C for 5 min for inactivation of the
enzyme, using a High-Capacity cDNA Reverse Transcription Kit for
qPCR.
For RT-PCR, the resultant cDNA was amplified using
Prime STAR GXL DNA Polymerase using specific primers (Table I). The thermocycling protocol
consisted of 98°C for 30 sec, followed by 30 cycles for
Sparc or 19 cycles for Actb of denaturation at 98°C
for 10 sec, annealing at 60°C for 15 sec and extension at 68°C for
60 sec. The PCR products were separated using 1% agarose gel
electrophoresis with 10 mM sodium borate medium and visualized
using 0.5 µg/ml ethidium bromide staining at room temperature for
20 min.
For quantification of mRNA expression, the resultant
cDNA was amplified using SYBR Premix Ex Taq II in a TP-870
Thermal Cycler Dice Real Time System (Takara Bio, Inc.) with
specific primers (Table II).
Two-step thermal cycling after an initial denaturation phase at
95°C for 30 sec was used in the present study as follows: 45 cycles
of denaturation at 95°C for 5 sec and, annealing and extension at
60°C for 30 sec. The mRNA expression levels of each target gene
were normalized relative to the mRNA expression levels of
Actb in the same samples. The data were assessed using the
2−ΔΔCq method (30).
 | Table II.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table II.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Genes | Sequence,
5′-3′ | Accession
number |
|---|
| Tgfb2 | F:
AGTTTACACTGCCCCTGCT | NM_009367.4 |
|
| R:
AGAGGTGCCATCAATACCTGC |
|
| Alp1 | F:
GCAGTATGAATTGAATCGGAACAA | NM_007431.3 |
|
| R:
ATGGCCTGGTCCATCTCCAC |
|
| Col1a1 | F:
GACATGTTCAGCTTTGTGGACCTC | NM_007742.4 |
|
| R:
GGGACCCTTAGGCCATTGTGTA |
|
| Bglap | F:
CAACAGGAGGGTGCAGAACAGA | NM_001305448.1 |
|
| R:
GCTTGGACATGAAGGCTTTGTC |
|
| Cfd | F:
GGATGGAGTGACGGATGACG | NM_013459.4 |
|
| R:
TGAGGCACTACACTCTGCAC |
|
| Fabp4 | F:
TGAAATCACCGCAGACGACA | NM_024406.4 |
|
| R:
ACACATTCCACCACCAGCTT |
|
| Pparg2 | F:
GCTTATTTATGATAGGTGTGATC | NM_001127330.2 |
|
| R:
GCATTGTGAGACATCCCCAC |
|
| Slc2a4 | F:
GCCCGGACCCTATACCCTAT | NM_009204.2 |
|
| R:
GGGTTCCCCATCGTCAGAG |
|
| Actb | F:
CATCCGTAAAGACCTCTATGCCAAC | NM_007393.5 |
|
| R:
ATGGAGCCACCGATCCACA |
|
AP-1 activity
AP-1 activity was assessed using a luciferase
reporter assay. Briefly, SPARC-low ST2 cells were seeded at a
density of 1×105 cells/well in 24-well culture plates,
incubated overnight in a CO2 incubator and
co-transfected at 37°C for 4 h with the AP1(1) Luc vector and the
pGL4.75 vector using Xfect Transfection Reagent. After removal of
the transfection reagent, the cells were incubated for an
additional 24 h at 37°C and luminescence intensity was assessed
using a TriStar2 LB942 plate reader (Titertek-Berthold) using a
dual reporter assay kit (31). The
data was normalized to co-transfected Renilla luciferase
activity.
Western blotting
Whole cell lysates of parental ST2 cells, SPARC-low
ST2 cells and transfected cells were prepared using RIPA buffer.
Nuclear fractions were extracted as described previously (32). Briefly, 1×106 cells were
lysed using 400 µl Buffer A [10 mM HEPES-NaOH, pH 7.9; 10 mM KCl;
0.1 mM EDTA; 1 mM DTT and 0.5 mM phenylmethylsulfonyl fluoride
(PMSF)] and incubated on ice for 20 min. The suspensions were
centrifuged at 21,000 × g for 10 sec, and the supernatants were
collected as post-nuclear fractions. The precipitates were
resuspended in 50 µl Buffer C [20 mM HEPES-NaOH, pH 7.9; 100 mM
KCl; 1 mM MgCl2; 0.2 mM CaCl2; 0.2 mM EDTA;
10% (v/v) glycerol; 1 mM DTT and 0.2 mM PMSF] and incubated on ice
for 20 min, followed by centrifugation of the suspensions at 21,000
× g for 2 min, with the resultant supernatants collected as nuclear
extracts. Following reduction with DTT, 10 µg protein aliquots were
separated by SDS-PAGE on 10% gels, followed by transfer of the
proteins to PVDF membranes. The membranes were blocked by
incubation with TBS-T (20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.05%
Tween-20) containing 20% blocking reagent N102 at room temperature
for 1 h, followed by overnight incubation with the aforementioned
primary antibodies at 4°C overnight and incubation with the
aforementioned biotinylated secondary antibodies for 1 h at room
temperature. The blots were subsequently incubated with
streptavidin-conjugated HRP for 20 min at room temperature, and the
signals were detected using Luminata™ Forte Western HRP
luminescent substrate (33).
Preparation of recombinant proteins
using Brevibacillus brevis
Total RNA, which was extracted from Ca9-22 cells for
SPARC and parental ST2 cells for c-Fos and
c-Jun, according to the AGPC extraction method, was reverse
transcribed at 55°C for 10 min using SuperScript IV Reverse
Transcriptase with Oligo dT. cDNA was amplified using the Prime
STAR GXL DNA Polymerase kit, as follows: Initial denaturation at
98°C for 30 sec, followed by 30 cycles of denaturation at 98°C for
10 sec, annealing at 60°C for 15 sec and extension at 68°C for 3.5
min (SPARC) or 60 sec (c-Fos and c-Jun). The
specific primers are shown in Table
I. The resultant cDNAs were cloned into the vector pNCMO2 with
4×His sequences using an In-Fusion® HD Cloning Kit
according to the manufacturer's protocol. The details of the cloned
genes were as follows: SPARC [human, NM_003118.4 (nt 116–973
corresponding to AA 18 to 303)]; c-Fos [mouse, NM_010234.2
(nt 140–1282 corresponding to AA 1 to 330)]; c-Jun [mouse,
NM_010591.2 (nt 952–1956 corresponding to AA 1 to 334)]. These
constructs were transfected into Brevibacillus brevis using
the Brevibacillus expression system II. Each
protein-expressing strain was cultured in 2SY medium (20.0 g/l
glucose, 40.0 g/l Bacto Soytone, 5.0 g/l Bacto Yeast Extract, 0.15
g/l CaCl2·2H2O) at 37°C overnight and the
culture supernatants were subjected to Ni Sepharose excel (cat. no.
17-3712-01; GE Healthcare) chromatography to purify these proteins
according to the manufacturer's protocol.
Electrophoretic mobility shift assay
(EMSA)
EMSA was performed using oligo DNA with a
biotinylated 5′-end. Briefly, the following sequences: DNA
oligonucleotide containing TRE sequence forward (F),
5′-TGAGTCAATGAGTCAGCTGACTCATTGACTCA-3′ and reverse (R),
5′-GGTGAGTCAATGAGTCAGCTGACTCATTGACTCA-3′; and DNA oligonucleotide
containing mutant TRE sequence F,
5′-TGTGACAATGTGACAGCTGTCACATTGTCACA-3′ and R,
5′-GGTGTGACAATGTGACAGCTGTCACATTGTCACA-3′, were annealed and used as
probes (underlined bases indicate mutation positions of the TRE
element). Recombinant proteins (2 µg), which were prepared using
the Brevibacillus expression system II as aforementioned,
were incubated with probes (2 fmol) in buffer containing 10 mM Tris
(pH 7.5), 50 mM KCl, 5 mM MgCl2, 1 mM DTT, 100 ng
Poly(dI/dC), 4% glycerol and 0.05% NP-40 for 15 min at room
temperature, followed by electrophoresis on undenatured 4% PAGE.
The nucleic acids were subsequently transferred to Hybond-N+
membranes and detected using avidin-conjugated HRP and HRP
luminescent substrate (34).
Far western blotting
To evaluate the direct binding of SPARC and AP-1
components, far western blotting was performed using recombinant
proteins: The subjected protein is blotted on the membrane and then
the candidate protein of the counterpart is overplayed. Finally,
the yielded complex on the membrane is detected. This assay is
considered suitable to assess the direct binding of proteins and is
comparable with other assays, such as the immunoprecipitation
assay, which are also able to detect indirect binding complex.
Briefly, recombinant proteins (0.5, 1.0 and 2.0 µg) from
Brevibacillus brevis were spotted onto Hybond-C membranes.
Membranes were blocked using 5% skim milk at room temperature for 1
h, then 1.2 µg/cm2 of each recombinant protein (c-Fos,
c-Jun and SPARC) was overlaid and incubated at room temperature for
1 h. They were blocked again using 10% N102 blocking solution in
TBST. After washing with TBST, the primary antibodies (anti-SPARC,
anti-c-Fos and anti-c-Jun) were used to detect their respective
proteins, according to the aforementioned method described for
western blotting.
Protein assay
Protein concentrations were determined prior to
western blotting, EMSA and far western blotting according to the
Lowry protein assay method using DC™ protein assay kits
with bovine serum albumin as the standard, according to the
manufacturer's protocol.
Statistical analysis
All experiments were performed in triplicate to
ensure that ≥2 independent experiments yielded similar results.
Results were presented as the mean ± standard error of the mean
(n=3). Comparisons between two independent groups were evaluated
using unpaired Student's t-test using Microsoft Excel version 2210
(Microsoft Corporation) and multiple comparisons of ≥3 groups were
evaluated using one-way ANOVA followed by Scheffé's F-test
(https://astatsa.com/OneWay_Anova_with_TukeyHSD/).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Phenotype of SPARC-low ST2 cells
A clone without obvious expression of SPARC was
selected from parental ST2 cells and was thereafter named SPARC-low
ST2 cells. The mRNA and protein expression levels of SPARC in the
parental and SPARC-low ST2 cells were shown in Fig. 1A. SPARC-low ST2 cells did not
express Sparc mRNA nor secrete SPARC into the culture
medium, although a non-specific signal showing the almost same
molecular weight as SPARC was detected from the lysate (Fig. 1A). Culture of these SPARC-low ST2
cells in osteogenic differentiation medium containing ascorbic acid
and β-glycerophosphate reduced calcification when compared with
parental ST2 cells (Fig. 1B),
which is in agreement with previous reports (11,35).
Simvastatin has been reported to be a strong promoter of
calcification in the presence of ascorbic acid and
β-glycerophosphate (36), an
effect which was significantly lower in SPARC-low ST2 cells than in
the parental cell line (Fig. 1B).
RT-qPCR demonstrated that the mRNA expression levels of
osteoblastic differentiation-associated genes, such as alkaline
phosphatase, transforming growth factor β2 and type I collagen were
significantly lower in SPARC-low ST2 cells compared with those in
parental ST2 cells. However, Runx2 and osteocalcin mRNA
expression levels were not significantly altered in SPARC-low ST2
cells compared with those in parental ST2 cells (Fig. 1C). Whether there were changes in
the rate of adipogenic differentiation in SPARC-low ST2 cells was
then assessed using adipogenic differentiation medium containing
insulin, IBMX and dexamethasone. This medium induced TAG deposition
in parental ST2 cells; however, accumulation was significantly
higher in SPARC-low ST2 cells compared with that in parental ST2
cells (Fig. 1D). The mRNA
expression levels of adipogenesis-related genes, such as adipsin
and glucose transporter 4 were significantly higher in SPARC-low
ST2 cells compared with those in the parental ST2 cells. In
addition, the mRNA expression levels of Pparg2 and
Fabp4 were induced by adipogenic differentiation medium;
however, the induced mRNA expression levels were, unexpectedly,
significantly lower in SPARC-low ST2 cells compared with those in
parental ST2 cells (Fig. 1E).
Fabp4 KO preadipocytes have previously been reported to
differentiate to adipogenic cells more than Fabp4 WT cells
(37), which suggested that the
contribution of FABP4 to adipogenesis was dependent on the
differentiation state of the cells (preadipocytes vs. bone marrow
stromal cells). Phenotypically, SPARC-low ST2 cells resemble the
cells derived from Sparc KO mice (8–10).
The 3T3-L1 cell line has been used for adipogenic differentiation
or as an adipocyte model (38);
however, it has not been reported to differentiate into
osteoblasts. The MC3T3-E1 cell line has been used to assess
osteoblastic differentiation (26), but has rarely been reported to
differentiate into adipogenic cells. However, ST2 cells have the
phenotypic ability to differentiate into either cell type depending
on the culturing conditions. These findings indicated that ST2
cells were a more appropriate model to test the effects of SPARC
than 3T3-L1 and MC3T3-E1 cells.
Extracellular SPARC incorporated into
cells reduces AP-1 activity
The intracellular function of SPARC was initially
evaluated by assessing its presence in the nuclear and post-nuclear
fractions of ST2 cell lysates, and in their CM. SPARC was not
detected in the nucleus but was demonstrated in the post-nuclear
fractions of both parental ST2 cells and SPARC-rescued SPARC-low
ST2 cells (Fig. 2A) and their CM
(Fig. 3). The amino acid sequence
of human SPARC is highly conserved among animal species (92%
homology with mouse origin) (Fig.
2B); therefore, the SPARC expression vector was constructed
using human cDNA.
The effects of Sparc expression on AP-1
activity were evaluated using the TRE-driven luciferase expression
vector. AP-1 activity was significantly higher in SPARC-low ST2
cells compared with its activity in parental ST2 cells. However,
the induction of SPARC expression using a signal peptide derived
from SPARC or preprotrypsin significantly reduced the activity
levels of AP-1 in SPARC-low ST2 cells compared with in the
SPARC-low ST2 cells (Fig. 2C);
however, this reduction was not demonstrated when SPARC was
expressed without the signal peptide. These data suggested that the
intracellular localization of SPARC depended on its signal peptide
sequence. Although it was initially hypothesized that the
intracellular localization of SPARC was due to the SPARC WT signal
peptide, the effect of replacement of the SPARC WT signal peptide
with preprotrypsin signal peptide was not significant and SPARC
lacking signal peptide demonstrated no AP-1 inhibitory activity,
which suggested that AP-1 inhibition occurred due to incorporation
of the secreted SPARC rather than intracellularly expressed
SPARC.
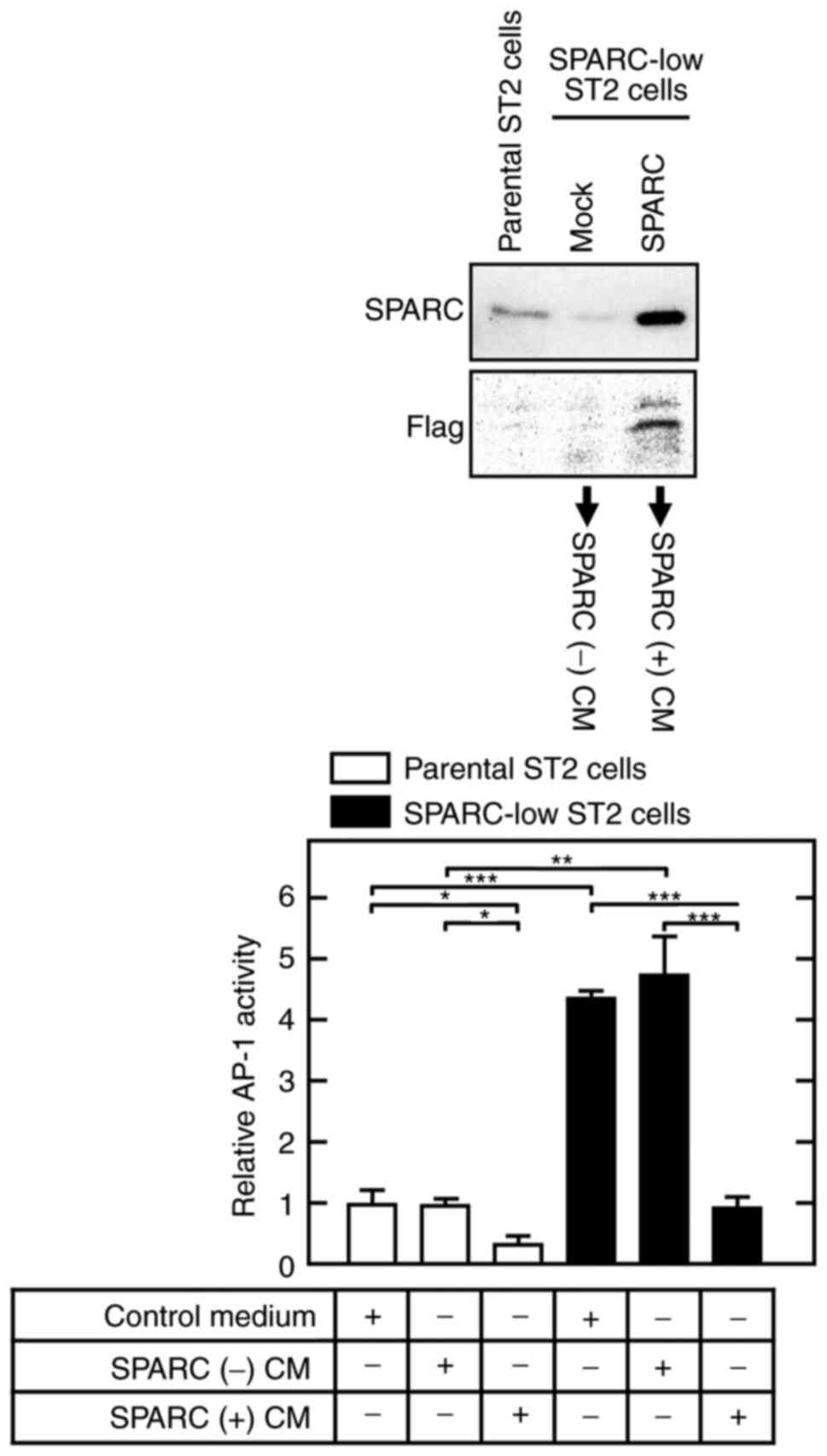
When SPARC-low ST2 cells and their parental cells
were cultured in CM from SPARC-rescued SPARC-low ST2 cells [SPARC
(+) CM], AP-1 activity was significantly lower compared with that
in cells cultured in CM from mock-transfected SPARC-low ST2 cells
[SPARC (−) CM] (Fig. 3).
Furthermore, the increased AP-1 activity in SPARC-low ST2 cells was
markedly reduced by SPARC (+) CM treatment to a similar level to
that shown in control/SPARC (−) CM-treated parental ST2 cells.
These results suggested that SPARC was secreted into the
extracellular space and incorporated into the cytosol, where it
interfered with the AP-1 complex/consensus sequence.
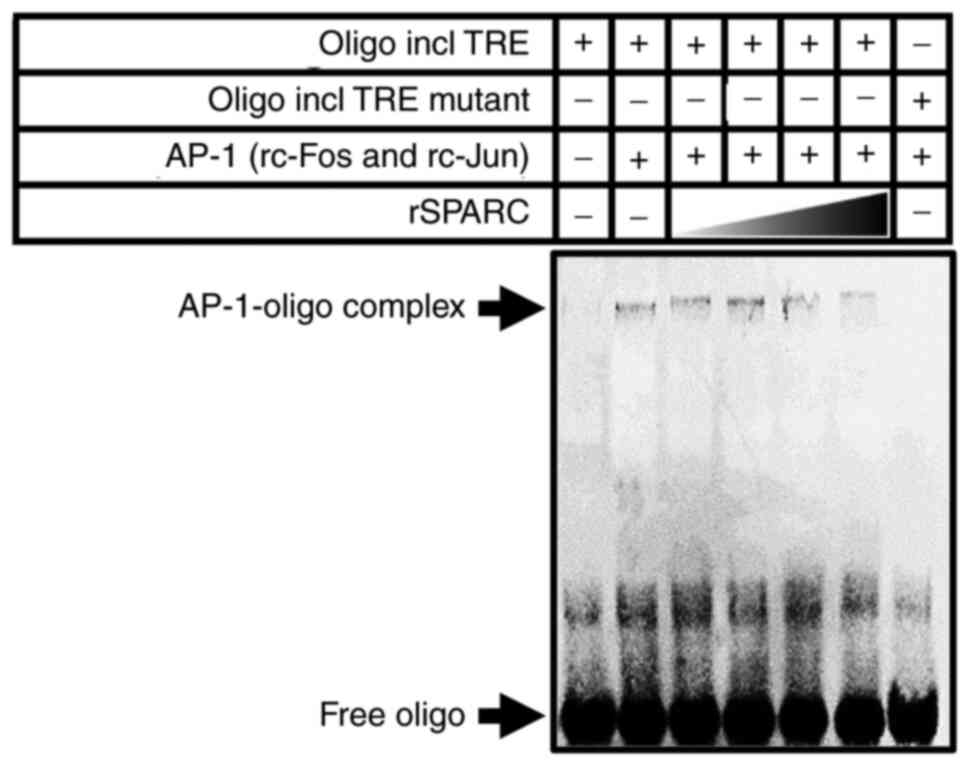
SPARC binds c-Fos
To evaluate whether SPARC reduced AP-1 transcription
activity by interfering with its dimerization, the recombinant
peptides c-Fos, c-Jun and SPARC were expressed in Brevibacillus
brevis, and EMSA was performed using these peptides and a
double-strand oligonucleotide containing the TRE sequence or
non-consensus sequence (mutant TRE) as the negative control
(Fig. 4). The complex
electrophoretically migrated slower than the free DNA oligo by the
addition of both c-Fos and c-Jun; however, the presence of SPARC
prevented those mobility shifts. Furthermore, mutation of the TRE
sequence markedly abrogated complex formation. As strong signals
were not detected using the immunoprecipitation assay in
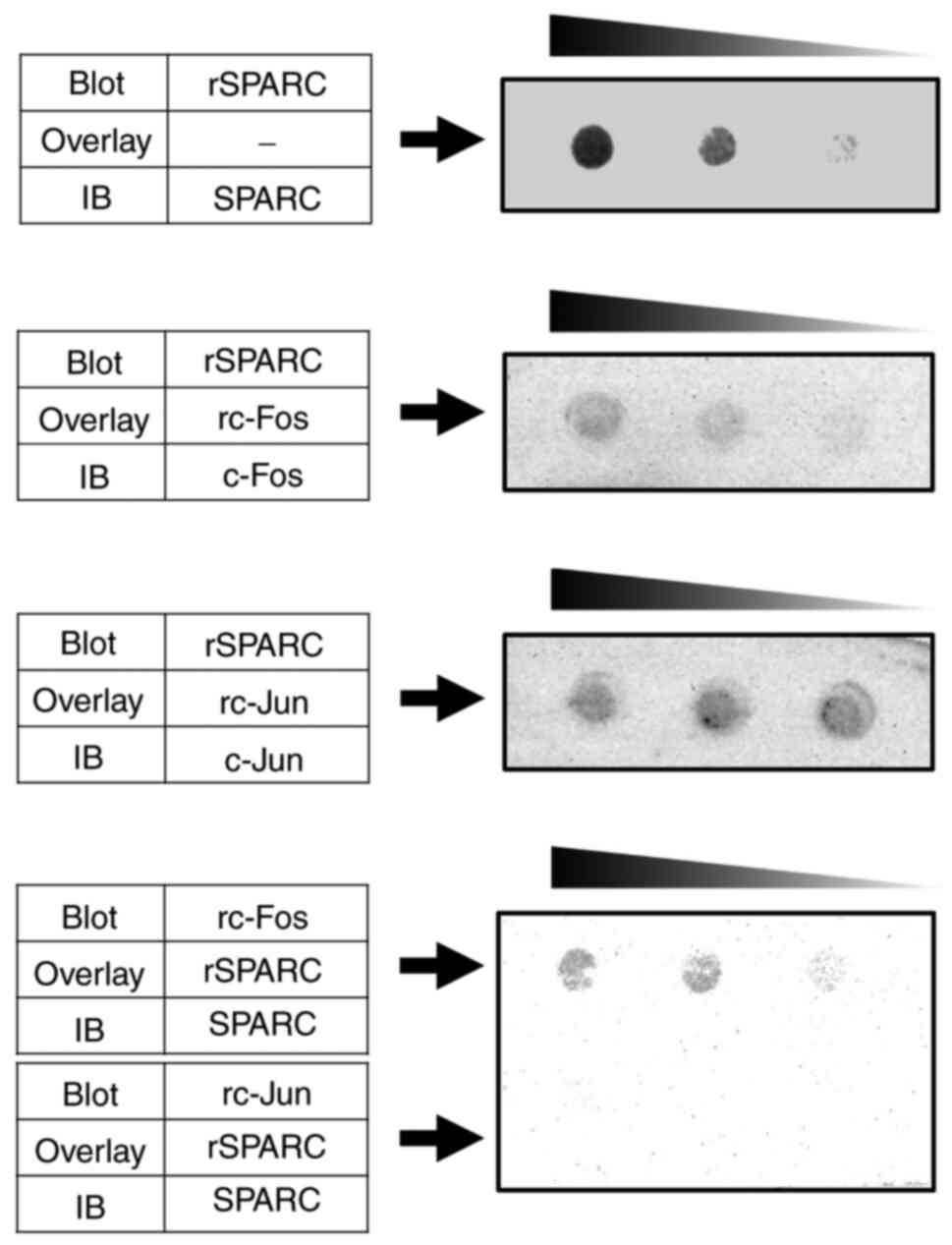
preliminary experiments to assess protein-protein interaction (data
not shown), far western blotting was performed to assess whether
SPARC directly bound c-Fos or c-Jun to prevent the dimerization of
AP-1 components. In the experiments in which the SPARC-blotted
membrane was overlaid with c-Fos and the experiments in which the
c-Fos-blotted membrane was overlaid with SPARC, specific and direct
binding of SPARC to c-Fos was detected (Fig. 5). However, both SPARC-blotted
membrane overlaid with c-Jun and c-Jun-blotted membrane overlaid
with SPARC did not show any specific binding.
Discussion
The matricellular proteins include SPARC,
thrombospondins, tenascins and CCNs, such as cysteine-rich 61 and
connective tissue growth factor (39). Also included are members of the
small integrin-binding ligand N-linked glycoprotein family,
such as OPN, periostin, bone sialoprotein, dentin
sialophosphoprotein, matrix extracellular phosphoglycoprotein and
dentin matrix protein-1. These proteins function at the cell
surface, interacting with cell surface receptors, growth factors,
proteinases and other extracellular matrix proteins. Some of these
proteins have been reported to have intracellular functions. For
example, OPN has been reported to interact with the intracellular
domain of CD44 (40) and
thrombospondin can bind to the ER luminal domain of activating
transcription factor 6α (41).
SPARC has been reported to be an intracellular protein (13–17,20);
however, its function is not completely understood. The present
study demonstrated that intracellular SPARC bound to c-Fos.
Phenotypically, Sparc KO mice have been
reported to be characterized by impaired bone formation and
enhanced adipose tissue formation (11). However, c-Fos KO mice have
been reported to be characterized by osteopetrosis, a condition in
which bone mass is abnormally high due to defective bone
resorption; however, bone-forming activity remains unclear
(42,43). As c-Fos has been reported to be
activated in osteoclast precursors and to be required for
osteoclast differentiation (44),
Thus, c-Fos is thought to serve an important role in bone
resorption through differentiation/activation of osteoclasts
directly to pre-osteoclasts and/or indirectly through osteoblasts.
However, to the best of our knowledge, the effects of c-Fos on
adipogenesis have not been previously reported. Transgenic mice
carrying Fra-1, a c-Fos-related protein and the counterpart of
c-Jun, have been reported to have increased bone formation
(45). Mouse adipose-derived
stromal cells with higher Fosl1 transgene expression have
been reported to have reduced adipogenesis (46). Fosl2-transgenic mice have
been reported to have an osteosclerotic phenotype due to excess
osteoblastogenesis, whereas Fosl2 KO in mice has been
reported to impair osteoblastogenesis and enhance an adipogenic
phenotype (47). Similarly, mice
transgenic for ΔFosB have been reported to have increased
osteosclerosis and reduced adipogenesis through two independent
cell-autonomous mechanisms (48).
Fra-1 and Fra-2 have been reported to inhibit PPARγ expression
levels, whereas c-Fos may enhance PPARγ expression in nonalcoholic
fatty liver disease (49). As Jun
family members have been hypothesized to contribute less to bone
formation than Fos proteins (50),
Fos proteins, with the exception of c-Fos, have been hypothesized
to serve an important role in the induction of cells of the
osteoblastic lineage concomitant with a decrease in adipogenesis.
The phenotype of Sparc KO mice is highly similar to that of
Fosl2 KO mice, including low bone density and an abundance
of adipose tissue (8,10,51).
These findings suggested that SPARC may be involved in the
activation of AP-1, via Fos proteins such as Fra-1, Fra-2 and
ΔFosB. That is, SPARC may bind to c-Fos and function as a decoy
counterpart of c-Fos to reduce dimerization, thus increasing the
ability of other Fos proteins to dimerize with Jun, inhibiting
adipogenesis and promoting osteoblastogenesis.
This hypothesis was supported by previous studies,
which reported that Fra-2 suppressed PPARγ2 in adipocytes (51) and increased bone formation
(45,52). The present study demonstrated that
unstimulated basal AP-1 activity was markedly higher in SPARC-low
ST2 cells compared with that in parental ST2 cells. Because most
basal AP-1 activity was attributed to c-Fos, AP-1 activity may be
higher in SPARC-low ST2 cells due to effects on c-Fos. That is,
SPARC may inhibit the apparent basal activity of AP-1 by binding to
c-Fos, suggesting that SPARC could inhibit c-Fos-containing AP-1
formation, enabling Jun proteins to dimerize with other Fos
proteins, thus promoting osteoblastogenesis. The DNA-binding
specificity of Fra-1/c-Jun heterodimers was previously reported to
be indistinguishable from that of c-Fos/c-Jun heterodimers
(53); however, a further study
reported that their binding specificities were clearly
distinguishable concerning PPARγ (49). The discrepant AP-1 activity changes
(AP-1 activity was higher, whereas the mRNA expression levels of
the AP-1 target gene PPARγ2 were lower in SPARC-low ST2 cells
compared with in parental ST2 cells) demonstrated in the present
study may also have been due to bio-physiological conditions that
limited response to the transiently introduced vector that
determined promoter activity and its inability to be integrated
into the genome (54).
In the present study, it was demonstrated that
secreted SPARC functioned intracellularly as a decoy counterpart of
c-Fos, after its incorporation from the extracellular space. The
un-secreted form (absence of signal peptide) demonstrated no
AP-1-inhibiting activity. Furthermore, the far western blotting
assay using recombinant proteins, which were not in their
glycosylated forms, demonstrated that SPARC bound c-Fos. Because
SPARC is a highly glycosylated protein, it is still possible that
glycosylation of SPARC may affect AP-1 activity. Further studies
are required to elucidate the inhibitory mechanism.
Sparc KO mice were initially established
using a Mf1/129Sv/Ev outbred mixed background by targeting exon 6
(8). Phenotypically, these mice
were characterized by obvious cataract formation and adipose tissue
accumulation, but their skeletal size was not changed. Other
Sparc KO mice were established with different backgrounds
and strategies. For example, 129SV/C57BL/6 Sparc KO mice
targeting exon 4 were characterized by cataract formation, adipose
tissue accumulation and osteopenia (9,10).
Osteopenia was also reported in Sparc KO 129Sv/Ev inbred
mice targeting exon 6, whereas symptoms of osteopenia were not
reported in Mf1/129Sv/Ev outbred mice with Sparc KO because
osteopenia was hidden by high degrees of individual variability
(55). Consequently, Sparc
deficiency has been reported to exert skeletal effects that are not
as severe as adipose tissue accumulation (9). Notably, the ability of SPARC to
inhibit adipogenesis was not hidden by high degrees of individual
variability, which suggested that SPARC strongly inhibited
adipogenesis but did not strongly induce osteoblastogenesis. This
inhibition of adipogenesis suggested that SPARC was involved in the
commitment of bone marrow stromal cells to differentiation into
osteoblasts.
SPARC levels (both mRNA and protein) have been
reported to be closely related to obesity and diabetes mellitus.
Weight loss resulting from a very low-calorie diet has been
reported to cause a concomitant 33% reduction of SPARC gene
expression in adipose tissue, whereas weight gain through
consumption of a fast-food diet increased SPARC gene
expression in adipose tissue by 30% (56). SPARC expression has also been
reported to be correlated with leptin-independent fat mass and
insulin resistance (56),
associations supported by the high SPARC expression in adipose
tissue of leptin receptor-deficient mice (57). SPARC mRNA expression levels
in both abdominal and visceral adipose tissue have been reported to
be correlated with body mass index, and serum SPARC concentration
has been shown to be associated with fasting insulin concentration
and the homeostasis model assessment of insulin resistance score
(58).
The aforementioned clinical observations seemingly
oppose the Sparc KO phenotype being the accumulation of
adipose tissue; however, SPARC no doubt serves an important role in
the regulation of adipogenesis, as well as in bone marrow stromal
cell differentiation, committing cells to the osteoblast lineage
through inhibition of adipogenesis as a decoy counterpart of c-Fos.
Our findings suggest providing novel insights into regenerative
medicine.
Acknowledgements
The present study was based on a PhD thesis (Tomoya
Hatori), which was published by Ohu University Press in 2020.
Funding
The present study was supported by JSPS KAKENHI (grant nos.
JP17K11885, JP18K09642 and JP19K10074.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH and YK wrote the manuscript. TH, TM and AS
performed the experiments. TH, KT and YK analyzed the data. TM, KT
and YK designed the experiments. TH, TM and YK confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Termine JD, Kleinman HK, Whitson SW, Conn
KM, McGarvey ML and Martin GR: Osteonectin, a bone-specific protein
linking mineral to collagen. Cell. 26:99–105. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan Q and Sage EH: SPARC, a matricellular
glycoprotein with important biological functions. J Histochem
Cytochem. 47:1495–1506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradshaw AD: The role of SPARC in
extracellular matrix assembly. J Cell Commun Signal. 3:239–246.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphy-Ullrich JE and Sage EH: Revisiting
the matricellular concept. Matrix Biol. 37:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giudici C, Raynal N, Wiedemann H, Cabral
WA, Marini JC, Timpl R, Bächinger HP, Farndale RW, Sasaki T and
Tenni R: Mapping of SPARC/BM-40/osteonectin-binding sites on
fibrillar collagens. J Biol Chem. 283:19551–19560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duncan S, Delage S, Chioran A, Sirbu O,
Brown TJ and Ringuette MJ: The predicted collagen-binding domains
of Drosophila SPARC are essential for survival and for collagen IV
distribution and assembly into basement membranes. Dev Biol.
461:197–209. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cydzik M, Abdul-Wahid A, Park S, Bourdeau
A, Bowden K, Prodeus A, Kollara A, Brown TJ, Ringuette MJ and
Gariépy J: Slow binding kinetics of secreted protein, acidic, rich
in cysteine-VEGF interaction limit VEGF activation of VEGF receptor
2 and attenuate angiogenesis. FASEB J. 29:3493–3505. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilmour DT, Lyon GJ, Carlton MB, Sanes JR,
Cunningham JM, Anderson JR, Hogan BL, Evans MJ and Colledge WH:
Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40
develop normally but show severe age-onset cataract formation and
disruption of the lens. EMBO J. 17:1860–1870. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delany AM, Amling M, Priemel M, Howe C,
Baron R and Canalis E: Osteopenia and decreased bone formation in
osteonectin-deficient mice. J Clin Invest. 105:915–923. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bradshaw AD, Graves DC, Motamed K and Sage
EH: SPARC-null mice exhibit increased adiposity without significant
differences in overall body weight. Proc Natl Acad Sci USA.
100:6045–6050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delany AM, Kalajzic I, Bradshaw AD, Sage
EH and Canalis E: Osteonectin-null mutation compromises osteoblast
formation, maturation, and survival. Endocrinology. 144:2588–2596.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Francki A, Bradshaw AD, Bassuk JA, Howe
CC, Couser WG and Sage EH: SPARC regulates the expression of
collagen type I and transforming growth factor-beta1 in mesangial
cells. J Biol Chem. 274:32145–32152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan Q, Weaver M, Perdue N and Sage EH:
Matricellular protein SPARC is translocated to the nuclei of
immortalized murine lens epithelial cells. J Cell Physiol.
203:286–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gooden MD, Vernon RB, Bassuk JA and Sage
EH: Cell cycle-dependent nuclear location of the matricellular
protein SPARC: Association with the nuclear matrix. J Cell Biochem.
74:152–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hecht JT and Sage EH: Retention of the
matricellular protein SPARC in the endoplasmic reticulum of
chondrocytes from patients with pseudoachondroplasia. J Histochem
Cytochem. 54:269–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huynh MH, Hong H, Delovitch S, Desser S
and Ringuette M: Association of SPARC (osteonectin, BM-40) with
extracellular and intracellular components of the ciliated surface
ectoderm of Xenopus embryos. Cell Motil Cytoskeleton. 47:154–162.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sodek J, Zhu B, Huynh MH, Brown TJ and
Ringuette M: Novel functions of the matricellular proteins
osteopontin and osteonectin/SPARC. Connect Tissue Res. 43:308–319.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krstulja M, Car A, Bonifacić D, Braut T
and Kujundzić M: Nasopharyngeal angiofibroma with intracellular
accumulation of SPARC-a hypothesis (SPARC in nasopharyngeal
angiofibroma). Med Hypotheses. 70:600–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fenouille N, Puissant A, Dufies M, Robert
G, Jacquel A, Ohanna M, Deckert M, Pasquet JM, Mahon FX, Cassuto
JP, et al: Persistent activation of the Fyn/ERK kinase signaling
axis mediates imatinib resistance in chronic myelogenous leukemia
cells through upregulation of intracellular SPARC. Cancer Res.
70:9659–9670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vinayagam A, Stelzl U, Foulle R, Plassmann
S, Zenkner M, Timm J, Assmus HE, Andrade-Navarro MA and Wanker EE:
A directed protein interaction network for investigating
intracellular signal transduction. Sci Signal. 4:rs82011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
White UA and Stephens JM: Transcriptional
factors that promote formation of white adipose tissue. Mol Cell
Endocrinol. 318:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karin M, Liu ZG and Zandi E: AP-1 function
and regulation. Curr Opin Cell Biol. 9:240–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Distel RJ, Ro HS, Rosen BS, Groves DL and
Spiegelman BM: Nucleoprotein complexes that regulate gene
expression in adipocyte differentiation: Direct participation of
c-fos. Cell. 49:835–844. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Knebel B, Kotzka J, Lehr S, Hartwig S,
Avci H, Jacob S, Nitzgen U, Schiller M, März W, Hoffmann MM, et al:
A mutation in the c-fos gene associated with congenital generalized
lipodystrophy. Orphanet J Rare Dis. 8:1192013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeda T and Horiuchi N: Simvastatin
suppresses leptin expression in 3T3-L1 adipocytes via activation of
the cyclic AMP-PKA pathway induced by inhibition of protein
prenylation. J Biochem. 145:771–781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maeda T, Suzuki A, Yuzawa S, Baba Y,
Kimura Y and Kato Y: Mineral trioxide aggregate induces
osteoblastogenesis via Atf6. Bone Rep. 2:36–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirose K, Isogai E, Mizugai H and Ueda I:
Adhesion of Porphyromonas gingivalis fimbriae to human gingival
cell line Ca9-22. Oral Microbiol Immunol. 11:402–406. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogawa M and Nishikawa S, Ikuta K, Yamamura
F, Naito M, Takahashi K and Nishikawa S: B cell ontogeny in murine
embryo studied by a culture system with the monolayer of a stromal
cell clone, ST2: B cell progenitor develops first in the embryonal
body rather than in the yolk sac. EMBO J. 7:1337–1343. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagaoka M, Maeda T, Chatani M, Handa K,
Yamakawa T, Kiyohara S, Negishi-Koga T, Kato Y, Takami M, Niida S,
et al: A delphinidin-enriched maqui berry extract improves bone
metabolism and protects against bone loss in osteopenic mouse
models. Antioxidants (Basel). 8:3862019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dignam JD, Lebovitz RM and Roeder RG:
Accurate transcription initiation by RNA polymerase II in a soluble
extract from isolated mammalian nuclei. Nucleic Acids Res.
11:1475–1489. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maeda T, Suzuki A, Koga K, Miyamoto C,
Maehata Y, Ozawa S, Hata RI, Nagashima Y, Nabeshima K, Miyazaki K
and Kato Y: TRPM5 mediates acidic extracellular pH signaling and
TRPM5 inhibition reduces spontaneous metastasis in mouse B16-BL6
melanoma cells. Oncotarget. 8:78312–78326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hellman LM and Fried MG: Electrophoretic
mobility shift assay (EMSA) for detecting protein-nucleic acid
interactions. Nat Protoc. 2:1849–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cornelius P, MacDougald OA and Lane MD:
Regulation of adipocyte development. Annu Rev Nutr. 14:99–129.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maeda T, Kawane T and Horiuchi N: Statins
augment vascular endothelial growth factor expression in
osteoblastic cells via inhibition of protein prenylation.
Endocrinology. 144:681–692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garin-Shkolnik T, Rudich A, Hotamisligil
GS and Rubinstein M: FABP4 attenuates PPARγ and adipogenesis and is
inversely correlated with PPARγ in adipose tissues. Diabetes.
63:900–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tseng C and Kolonin MG: Proteolytic
isoforms of SPARC induce adipose stromal cell mobilization in
obesity. Stem Cells. 34:174–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawakita F, Kanamaru H, Asada R and Suzuki
H: Potential roles of matricellular proteins in stroke. Exp Neurol.
322:1130572019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzuki K, Zhu B, Rittling SR, Denhardt DT,
Goldberg HA, McCulloch CA and Sodek J: Colocalization of
intracellular osteopontin with CD44 is associated with migration,
cell fusion, and resorption in osteoclasts. J Bone Miner Res.
17:1486–1497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lynch JM, Maillet M, Vanhoutte D,
Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ,
Osinska H, et al: A thrombospondin-dependent pathway for a
protective ER stress response. Cell. 149:1257–1268. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: A key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang ZQ, Ovitt C, Grigoriadis AE,
Möhle-Steinlein U, Rüther U and Wagner EF: Bone and haematopoietic
defects in mice lacking c-fos. Nature. 360:741–745. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boyce BF, Yamashita T, Yao Z, Zhang Q, Li
F and Xing L: Roles for NF-kappaB and c-Fos in osteoclasts. J Bone
Miner Metab. 23 (Suppl 1):S11–S15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jochum W, David JP, Elliott C, Wutz A,
Plenk H Jr, Matsuo K and Wagner EF: Increased bone formation and
osteosclerosis in mice overexpressing the transcription factor
Fra-1. Nat Med. 6:980–984. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schwabe K, Garcia M, Ubieta K, Hannemann
N, Herbort B, Luther J, Noël D, Jorgensen C, Casteilla L, David JP,
et al: Inhibition of osteoarthritis by adipose-derived stromal
cells overexpressing Fra-1 in mice. Arthritis Rheumatol.
68:138–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Eferl R, Hasselblatt P, Rath M, Popper H,
Zenz R, Komnenovic V, Idarraga MH, Kenner L and Wagner EF:
Development of pulmonary fibrosis through a pathway involving the
transcription factor Fra-2/AP-1. Proc Natl Acad Sci USA.
105:10525–10530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kveiborg M, Sabatakos G, Chiusaroli R, Wu
M, Philbrick WM, Horne WC and Baron R: DeltaFosB induces
osteosclerosis and decreases adipogenesis by two independent
cell-autonomous mechanisms. Mol Cell Biol. 24:2820–2830. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hasenfuss SC, Bakiri L, Thomsen MK,
Williams EG, Auwerx J and Wagner EF: Regulation of steatohepatitis
and PPARγ signaling by distinct AP-1 dimers. Cell Metab. 19:84–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wagner EF and Eferl R: Fos/AP-1 proteins
in bone and the immune system. Immunol Rev. 208:126–140. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Luther J, Ubieta K, Hannemann N, Jimenez
M, Garcia M, Zech C, Schett G, Wagner EF and Bozec A: Fra-2/AP-1
controls adipocyte differentiation and survival by regulating PPARγ
and hypoxia. Cell Death Differ. 21:655–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roschger P, Matsuo K, Misof BM, Tesch W,
Jochum W, Wagner EF, Fratzl P and Klaushofer K: Normal
mineralization and nanostructure of sclerotic bone in mice
overexpressing Fra-1. Bone. 34:776–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cohen DR, Ferreira PC, Gentz R, Franza BR
Jr and Curran T: The product of a fos-related gene, fra-1, binds
cooperatively to the AP-1 site with Jun: Transcription factor AP-1
is comprised of multiple protein complexes. Genes Dev. 3:173–184.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yan C, Wang H, Aggarwal B and Boyd DD: A
novel homologous recombination system to study 92 kDa type IV
collagenase transcription demonstrates that the NF-kappaB motif
drives the transition from a repressed to an activated state of
gene expression. FASEB J. 18:540–541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mansergh FC, Wells T, Elford C, Evans SL,
Perry MJ, Evans MJ and Evans BA: Osteopenia in Sparc
(osteonectin)-deficient mice: Characterization of phenotypic
determinants of femoral strength and changes in gene expression.
Physiol Genomics. 32:64–73. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kos K, Wong S, Tan B, Gummesson A, Jernas
M, Franck N, Kerrigan D, Nystrom FH, Carlsson LM, Randeva HS, et
al: Regulation of the fibrosis and angiogenesis promoter
SPARC/osteonectin in human adipose tissue by weight change, leptin,
insulin, and glucose. Diabetes. 58:1780–1788. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tartare-Deckert S, Chavey C, Monthouel MN,
Gautier N and Van Obberghen E: The matricellular protein
SPARC/osteonectin as a newly identified factor up-regulated in
obesity. J Biol Chem. 276:22231–22237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee SH, Lee JA, Park HS, Song YS, Jang YJ,
Kim JH, Lee YJ and Heo Y: Associations among SPARC mRNA expression
in adipose tissue, serum SPARC concentration and metabolic
parameters in Korean women. Obesity (Silver Spring). 21:2296–2302.
2013. View Article : Google Scholar : PubMed/NCBI
|