Introduction
Ischemic stroke accounts for ~80% of all types of
strokes and is one of the leading causes of disability and
mortality worldwide (1).
Sequential pathological lesions occur in the brain tissues
following ischemia due to the insufficient supply of oxygen and
glucose. As a result of reactive oxygen species (ROS) generation
during hypoxia, unexpected aggravation of brain damage will
typically reoccur after recanalization (2). This has been reported to be caused by
a combination of pathophysiological processes, including
inflammatory responses, oxidative stress and cell apoptosis
(3).
During cerebral ischemia-reperfusion, a number of
cell types in the brain can release inflammatory mediators that
upregulate the expression of inflammatory cytokines in cerebral
microvascular endothelial cells and microglia. These molecules can
either directly or indirectly regulate the function of components
in blood-brain barrier (BBB), leading to tight junction impairment
and BBB breakdown (4). The BBB
serves as a major interface between cells in central nervous system
(CNS) and those that are blood-derived in the periphery, which also
serves a fundamental role in maintaining CNS homeostasis and normal
neuronal function (5). When the
BBB is impaired, blood-derived water and cells can extravasate into
the brain parenchyma, resulting in severe complications, such as
cerebral edema, hemorrhagic transformation, intracranial
hypertension and even herniation. In addition, infiltrating
leukocytes can then exacerbate the inflammatory response and
aggravate the brain injury further (6). In particular, inflammatory signaling
is involved at all stages of the ischemic injury process (7,8). One
of the main manifestations of inflammation is the morphological and
functional transformation of inflammatory cells, such as the
microglia. Following ischemia onset, microglia become activated,
where their morphology and function are then changed and migrate to
the site of the lesions. The function of the microglia after
activation is mainly phagocytosis and cytokine production including
TNF, IL-1 and IL-6 (9). These
cytokines are important mediators of the ischemia-induced
inflammatory responses and are involved in the progression of
cerebral infarction, the disease severity and outcome (10). Therefore, preserving BBB integrity
and preventing neuroinflammation are considered to be the key
scientific aims in the therapeutic research area of cerebral
vascular diseases.
Apolipoprotein E (ApoE) is a 34 kDa protein that
comprises 299 amino acids and has been reported to confer
neuroprotection in various CNS disease models (11). In total, three isoforms of ApoE
exists, namely ApoE2, ApoE3 and ApoE4 and they differ by
cysteine-arginine bridges at specific sites. Although ApoE3 and
ApoE4 differ structurally at only position 112, their reported
roles in neurological diseases are completely distinct (12). ApoE3 has been shown to exert
protective effects on the neurovascular units against a number of
CNS diseases, whilst ApoE4 has been reported to induce deleterious
effects (13). Specifically,
during the process of neurovascular injuries in the CNS, ApoE3 has
been observed to exhibit potent anti-inflammatory, anti-apoptotic
and anti-oxidative effects (14).
ApoE is unfortunately not a good therapeutic
candidate for CNS damage owing to its high molecular weight and
inability to cross BBB. Therefore, a BBB-permeable ApoE-mimetic
peptide COG1410 was previously developed and synthesized to
preserve the receptor-binding region whilst also mimicking ApoE3
with aims of providing neuroprotection (15). COG1410 is a polypeptide that is
comprised of 12 amino acids with a molecular weight of 1,410 Da.
Wang et al (16) verified
that COG1410 can cross BBB and decreased to half of maximum content
within 60 min. COG1410 has been previously shown to protect neurons
against injuries induced by subarachnoid hemorrhage (SAH) (17–19)
and traumatic brain injury (TBI) (20–22),
relieving neuroinflammation while suppressing apoptosis in various
CNS disease models (23–25). In addition, COG1410 has been
demonstrated to reduce the volume and radiographic progression of
infarct zones, thereby improving functional outcomes in murine
models of focal brain ischemia (14,16).
However, the effects of COG1410 on BBB injury following cerebral
ischemia-reperfusion remains unclear and exploring the role of
COG1410 on the BBB may reveal novel therapeutic targets for
controlling complications associated with BBB disruption.
Previous studies suggest that the triggering
receptor expressed on myeloid cells 2 (TREM2) is a novel ApoE
receptor with high affinity (26,27).
It is predominantly located on the membrane surfaces of microglia
and macrophages (28). In CNS,
microglial TREM2 has been revealed to markedly attenuate
neuroinflammation and protect neurons against acute ischemic stroke
(29,30). Furthermore, it has even been
previously proposed as one of the markers of microglial M1
polarization (31). TREM2 also
serves a critical role in promoting the phagocytosis of apoptotic
debris in ischemic brains after a stroke, such that the elimination
of apoptotic debris can minimize the acute proinflammatory response
whilst simultaneously suppressing immune activation (32).
Considering this reported interaction between
COG1410 and TREM2, coupled with their possible neuroprotective and
anti-inflammatory roles, the present study hypothesized that the
ApoE mimetic peptide COG1410 can preserve BBB integrity and
alleviate neuroinflammation through TREM2. Therefore, the BBB
integrity and inflammatory state of COG1410-treated rats with
stroke were investigated in the present study. Furthermore, the
mechanism of COG1410 underlying its effects on BBB integrity was
explored using cultured microglial cell lines.
Materials and methods
Animals
A total of 90 8-week-old Male Sprague-Dawley rats
weighing 250–280 g were obtained from Changsha Tianqin Co., Ltd.
and transported to Hainan Provincial Hospital of Traditional
Chinese Medicine (Haikou, China) by air. All animals were housed in
a standard 12 h light-dark cycle with a temperature of 22–24°C and
a relative humidity of 50–60%. Food and water were provided ad
libitum. All animal experiments were performed according to the
National Research Council's Guide for the Care and Use of
Laboratory Animal (revised 1985; NIH publication no. 85–23,
http://olaw.nih.gov/policies-laws/phs-policy.htm)
and all procedures were approved by the Committee on Animal Care
and Use of Hainan Provincial Hospital of Traditional Chinese
Medicine (approval no. IACUC-HPHCM-2211001). Every effort was made
to minimize the number of animals used and their suffering. At the
end of the experiments, all animals were euthanized through the
inhalation of 5% isoflurane followed by cervical dislocation.
Transient focal brain
ischemia/reperfusion model
The transient focal cerebral ischemia/reperfusion
model was induced by using the middle cerebral artery occlusion
(MCAO) model as previously described (33). Briefly, rats were anesthetized by
4% isoflurane inhalation and then maintained at 1.5–2% isoflurane
during the MCAO operation. Rats were then placed in a supine
position before the left common carotid arteries, external carotid
artery (ECA) and internal carotid artery (ICA) were exposed and
ligated. Subsequently, a monofilament with silicon coating on the
tip and a diameter of 0.36 mm (cat. no. L3600; Jialing Co., Ltd.)
was inserted into the ICA from the ECA to occlude the middle
cerebral artery for 2 h. The suture was then removed to restore
blood flow for another 22 h to induce reperfusion. Sham control
rats were subjected to similar surgical operations without
occluding the middle cerebral artery. Rats were kept on a warm pat
until they woke up and recovered from surgery.
Experimental grouping and drug
treatment
Rats were randomly divided into the following three
groups: Sham operation group; MCAO with COG1410 group; and MCAO
with scrambled peptide group. Peptides were synthesized by
GenScript with a purity of 98% and they were both soluble in water
and DMSO. COG1410 (acetyl-AS-Aib-LRKL-Aib-KRLL-amide, 1 mg/kg in
saline) or scramble peptide (acetyl-ARLR-Aib-KLSA-Aib-KL-amide, 1
mg/kg in saline) was then intravenously administered through the
femoral vein immediately after the insertion of suture.
Sham-operated rats were administrated with an equivalent volume of
saline.
Extravasation of Evans blue
BBB integrity was assessed by measuring the
extravasation of Evans blue dye. Briefly, Evans blue (2% in saline;
4 ml/kg; MilliporeSigma) was administered through the femoral vein
at 4 h before perfusion. After 24 h of occlusion, the rats were
transcardially perfused with cold saline to remove the
intravascular dye. The hemispheres were weighed and incubated in
methanamide (Shanghai Yien Chemical Technology Co., Ltd.) in 65°C
for 24 h. Evans blue content was then determined in the
supernatants at 632 nm using a spectrophotometer (Genesys 180;
Thermo Fisher Scientific, Inc.). BBB leakage was represented as
µg/g brain. Gradient concentrations of Evans blue were used to
build the standard curve.
Cell culture
Mouse BV2 microglial cell lines were purchased from
the China Center for Type Culture Collection. Cells were cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere containing 95% air and 5% CO2. The
medium was replaced every 2 days.
Oxygen-glucose deprivation (OGD)
followed by reoxygenation (R) and drug treatment
BV2 cells were exposed to OGD/R conditions to mimic
cerebral ischemia-reperfusion injury in vitro as previously
described (34) with slight
modifications. The cells were incubated with DMEM without glucose
and placed in a humidified, air-tight chamber in which the
atmosphere was saturated with 95% N2 and 5%
CO2 at 37°C. For the control group, cells were incubated
with fresh DMEM with glucose at 37°C in a humidified incubator with
95% air and 5% CO2. After OGD treatment for 3 h, cells
were subjected to R and incubated with fresh DMEM with glucose for
3 h. During reoxygenation, cells were also treated with COG1410 (10
µM) or scramble peptide (10 µM). An equivalent volume of vehicle
was used as a control.
TREM2 short interfering (si)RNA
transfection
The TREM2 siRNA (TREM2 siRNA-1, GACTTCTGTTTCTGCTACT,
cat. no. siB151026023852; TREM2 siRNA-2, CTGTCAACTTCTGCACTTT, cat.
no. siB151026023922; TREM2 siRNA-3, GTACTTATGACGCCTTGAA, cat. no.
siG171219105940) or control siRNA (siNC, cat. no. siN0000001-1-5)
by Guangzhou RiboBio Co., Ltd. was transfected into BV2 cells, for
48 h using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the protocol provided by the
manufacturer. Normal culture medium was used, where cells were
exposed to ambient air until transfection. Following transfection,
the transfection efficiency and target expression were first
verified by western blot assay and a fitting TREM2 siRNA chosen for
subsequent experiments. The cells were then exposed to OGD/R with
either COG1410 or the scramble peptide. Protein samples were then
collected for western blotting.
Western blot analysis
Brain tissues or cultured cells were homogenized and
lysed by RIPA buffer (Nanjing KeyGen Biotech Co., Ltd.) containing
1% protease and phosphorylase inhibitor cocktail (Roche Diagnostics
(Shanghai) Co., Ltd.). After quantification and denaturation, an
equal amount of total protein was then loaded and separated with
SDS-PAGE in 8–10% gels, followed by transferal onto PVDF membranes.
After incubation of western blocking buffer (Nanjing KeyGen Biotech
Co., Ltd.), membranes were incubated overnight at 4°C with the
following primary antibodies: Anti-occludin (1:250; cat. no. A2601;
ABclonal Biotech Co., Ltd.), anti-MMP-9 (1:1,000; cat. no. 13667S;
Cell Signaling Technologies, Inc.), anti-TREM2 (1:1,000; cat. no.
ab209814; Abcam), anti-COX-2 (1:1,000; cat. no. 12282S; Cell
Signaling Technologies, Inc.) or anti-β-actin (1:1,000; cat. no.
3700S; Cell Signaling Technologies, Inc.). This was followed by
incubation with HRP-conjugated secondary antibodies (1:5,000; cat.
no. AS029; Cell Signaling Technologies, Inc.) for 2 h at room
temperature. Bands were detected by ECL advanced western blot
detection reagents (Nanjing KeyGen Biotech Co., Ltd.). The band
signals were quantified using ImageJ software (version 1.50i;
National Institutes of Health).
Immunofluorescence
Cryosections cut from rat brains (0–2.0 mm posterior
to the bregma) were immunolabelled by primary antibodies conjugated
with Alexa Fluor®555 against IgG (1:500; cat. no. 4417S;
Cell Signaling Technologies, Inc.) to evaluate the permeability of
BBB at 24 h. Subsequently, the sections were fixed with 4%
formaldehyde at room temperature for 20 min, permeabilized with
Triton X-100 and blocked with donkey serum (NeoBioscience
Technology Co., Ltd.) at room temperature for 1 h, followed by
incubation with anti-ionized calcium binding adaptor molecule 1
(Iba-1, 1:200; cat. no. ab178846; Abcam) or CD68 (1:200; cat. no.
ab201340; Abcam) antibodies overnight at 4°C, then with the goat
anti-mouse (1:250; cat. no. P1076; Beyotime Institute of
Biotechnology) secondary antibody in dark place at room temperature
for 2 h. Finally, the sections were counterstained with DAPI and
antifade Mounting Medium (Beyotime Institute of Biotechnology) at
room temperature for 5 min. Fluorescent images were captured using
Olympus Fluoview laser scanning confocal microscope (Olympus
Corporation).
In situ zymography
Gelatinolytic activities of MMP-2/9 in the brain
sections were analyzed by in situ zymography using EnzCheck
collagenase kit (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Frozen brain sections
were incubated with a reaction buffer containing 40 µg/ml
FITC-labeled DQ™ gelatin at 37°C for 2 h. Gelatin-FITC is cleaved
by gelatinases, which yields fluorescent peptides. Their
fluorescence intensity is was used as representatives of the net
gelatinolytic activity in the brain samples. Fluorescence intensity
was measured from images captured using the Olympus Fluoview laser
scanning confocal microscope (Olympus Corporation) and calculated
using Image-Pro Plus version 6.0 (Media Cybernetics, Inc.).
Olink proteomics study
Protein levels in the rat sera were measured using
the Olink® Mouse Exploratory panel (Olink Proteomics AB)
according to the manufacturer's protocols. The Proximity Extension
Assay technology used for the Olink protocol has been previously
described (35). It enables 92
analytes to be analyzed simultaneously, using 1 µl of each sample.
Briefly, pairs of oligonucleotide-labeled antibody probes are first
used to bind to their targeted proteins. If the two probes were
brought in close proximity with each other, then the
oligonucleotides would hybridize in a pair-wise manner. This
proximity-dependent DNA polymerization event was subsequently
detected and quantified using a microfluidic real-time PCR
instrument (Biomark HD; Fluidigm Corporation). Data were then
quality controlled and normalized using an internal extension
control and an inter-plate control to adjust for intra- and
inter-run variation. The final assay read-out was presented at
Normalized Protein eXpression values, with the arbitrary unit on a
log2-scale. High values were considered to indicate
higher protein expression levels. All assay validation data are
available on manufacturer's website (http://www.olink.com). In Gene Ontology (GO)
enrichment analysis, all DEPs were mapped to GO terms in the Gene
Ontology database (http://www.geneontology.org/), protein numbers were
calculated for every term, significantly enriched GO terms in DEPs
comparing to the genome background were defined by hypergeometric
test. Kyoto Encyclopedia of Genes and Genome (KEGG, http://www.kegg.jp/kegg/) is the major public
pathway-related database, the analysis was similar to GO enrichment
analysis.
Statistical analysis
Experimental data are presented as the means ±
standard deviation. Normality analysis was determined by skewness
coefficient, kurtosis coefficient and Shapiro-Wilk test. For the
data conforming to a normal distribution, comparisons were made by
one-way ANOVA followed by Tukey test for ≥2 group comparisons. For
the data not normally distributed, comparisons were determined
using Kruskal-Wallis test and followed by Nemenyi test for 2 group
comparisons. All statistical analyses were performed using SPSS
22.0 (IBM Corp.) or GraphPad Prism 6.01 (Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
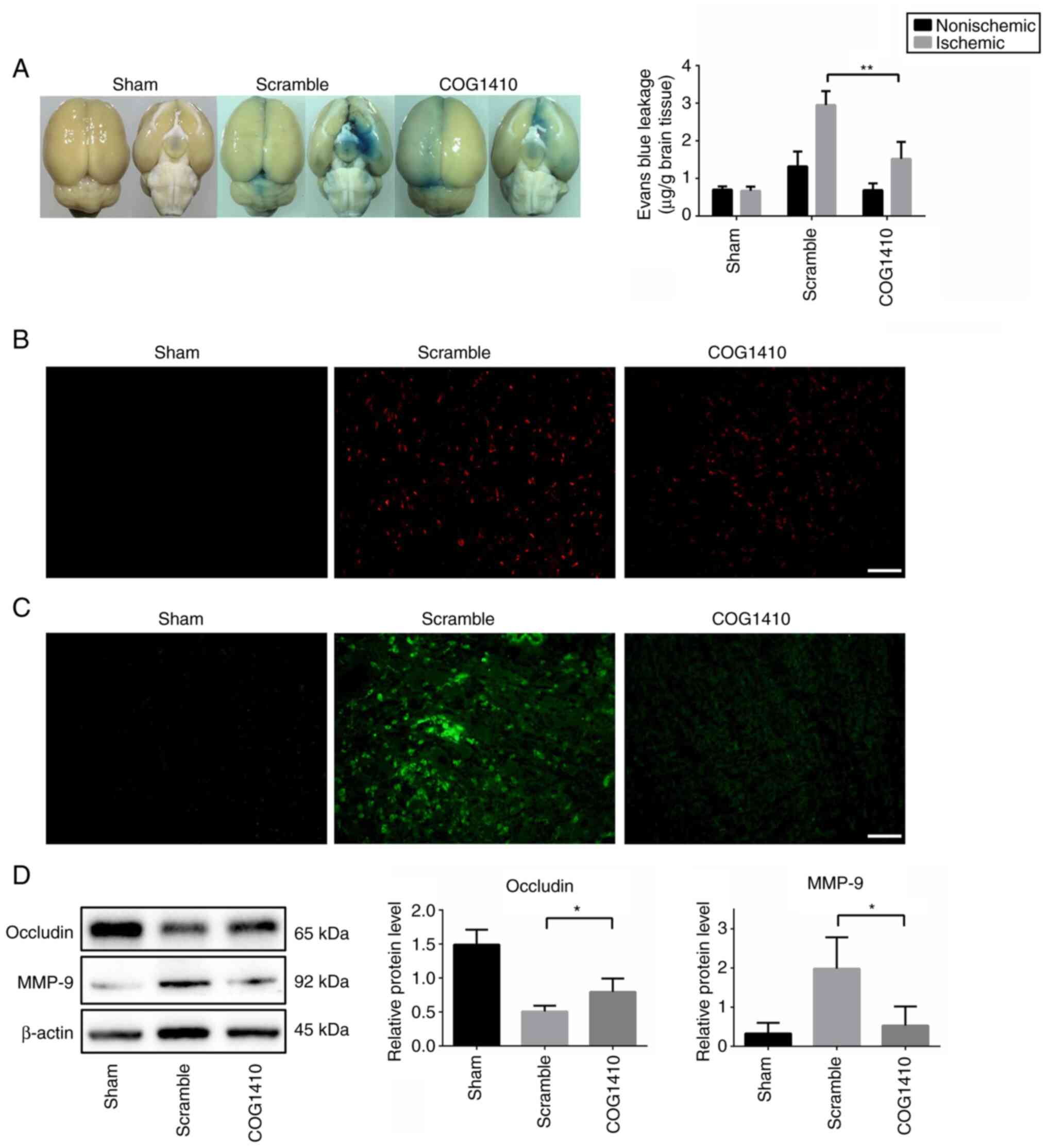
COG1410 alleviates BBB disruption in
MCAO rats
The extent of BBB permeability in rats following 2 h
of MCAO was first examined using Evans blue leakage and IgG
extravasation assays. Evans blue content in the ischemic
hemispheres was found to be significantly decreased in the COG1410
treatment group compared with that in the scramble peptide group
(Fig. 1A). In addition,
immunofluorescence staining assay revealed that IgG accumulated in
the cortex and striatum of the ischemic hemispheres 2 h after MCAO,
but COG1410 treatment markedly decreased the IgG content in these
cerebral areas (Fig. 1B).
To verify if COG1410 treatment can preserve BBB
integrity in MCAO rats, the expression levels of occludin, a tight
junction protein and MMP-9, a key enzyme that can digest
extracellular matrix to disrupt the BBB (36), were measured. The activity of MMPs
in the brain samples was also determined by in situ
zymography. According to Fig. 1C,
MCAO was found to increase MMP enzymatic activity in the brain
sections, which was in turn markedly reversed by COG1410
administration. Subsequent western blotting analysis revealed that
COG1410 can also reverse the MCAO-induced reduction of occludin
expression and MCAO-induced increments in MMP-9 expression
(Fig. 1D). These data, when taken
together, suggested that COG1410 can inhibit MMP-9 activity,
protect tight junctions and reduce BBB permeability during cerebral
ischemia-reperfusion injury.
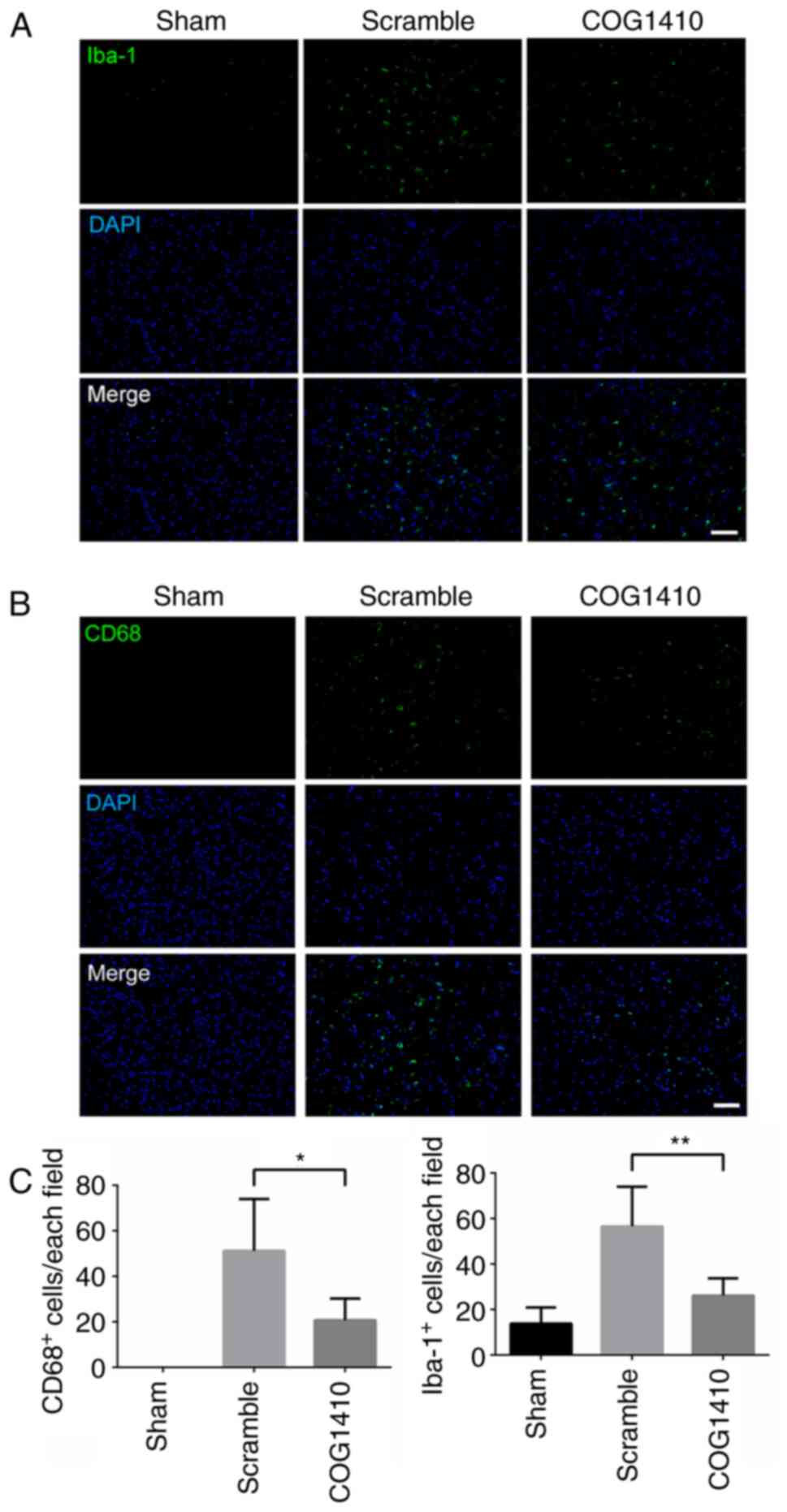
COG1410 suppresses microglial
activation and decreases inflammatory cytokine expression in the
brain tissues of MCAO rats
Since the inflammatory response is one of the key
events in ischemic brain injury and has been found to be involved
in BBB disruption (7), the
potential effect of COG1410 on inflammatory reactions in the rat
brain was then evaluated. Microglial activation was measured 24 h
after MCAO. MCAO was observed to activate the microglia and
increase their total levels, as evidenced by the increased number
of CD68- and Iba-1-positive cells in the peri-infarct area of the
brain tissues from rats in the MCAO group (Fig. 2). However, this aforementioned
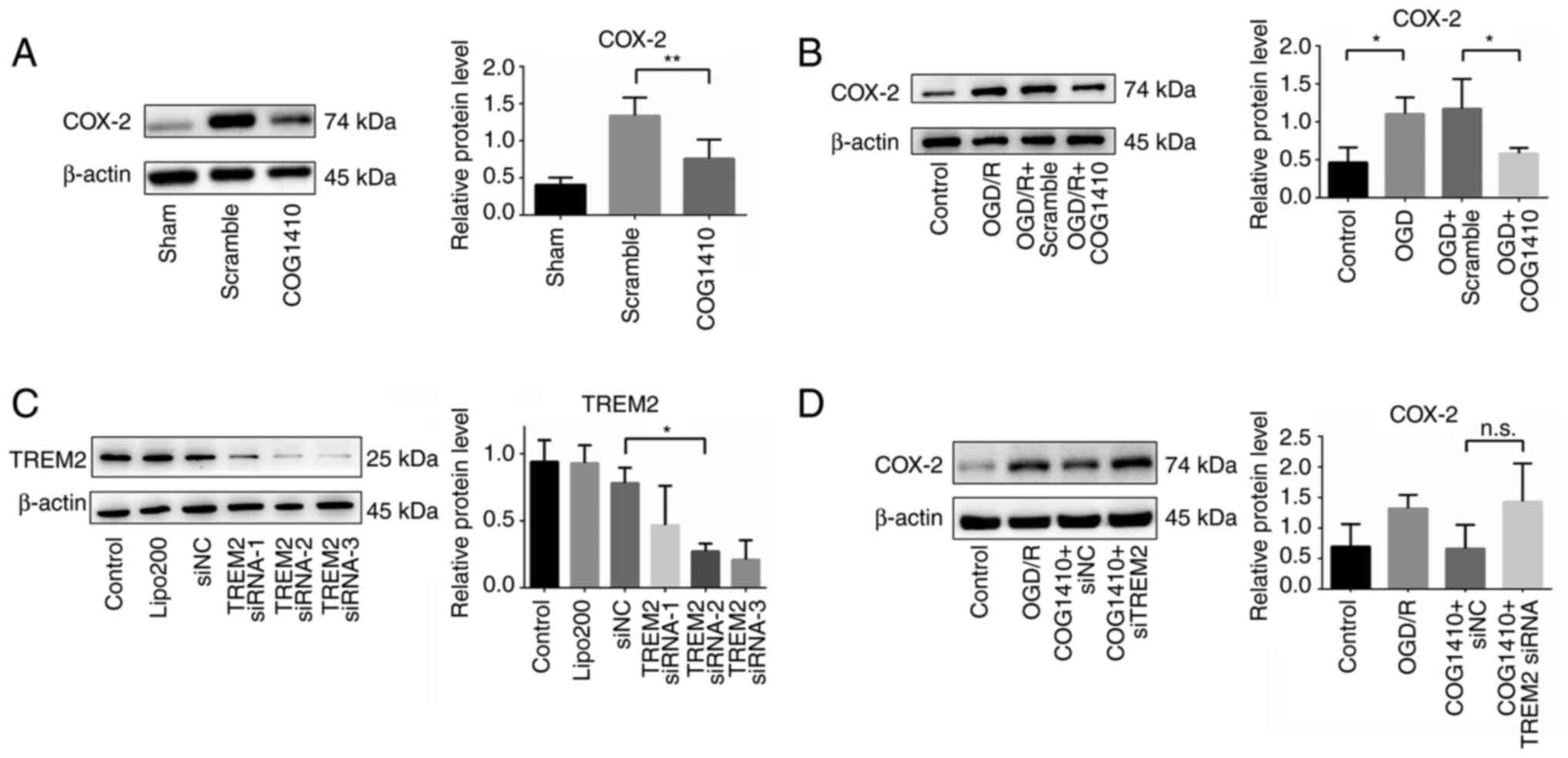
observation was markedly reversed by COG1410 treatment (Fig. 2). Subsequently, the protein
expression levels of cyclooxygenase (COX-2), a crucial mediator
contributing BBB damage because due to its ability to produce large
quantities of proinflammatory prostanoids (37), were measured by western blotting.
The results showed that COG1410 treatment markedly reduced the
protein expression of COX-2 in the ischemic hemisphere compared
with that in the scramble treatment group (Fig. 3A). Therefore, these data suggest
that COG1410 can reduce the inflammatory response in the ischemic
brain, which may contribute to BBB protection.
TREM2 siRNA abolishes the
downregulation of COX-2 in BV2 cells
To assess whether the TREM2, a receptor that can
bind to COG1410, is involved in the neuroprotective properties of
COG1410, the microglia cell line BV2 was used to explore the
potential molecular mechanism. The possible anti-inflammatory
properties of COG1410 in the BV2 cells were examined. As shown in
Fig. 3B, the results indicated
that COX-2 protein expression in OGD/R-treated BV2 cells was
markedly increased compared with that in the control group. By
contrast, COG1410 significantly decreased COX-2 protein expression
in the BV2 cells after OGD/R treatment compared with that in the
scramble-treated group.
To determine the relationship between COG1410 and
TREM2, siRNA was used to silence TREM2 expression prior to
investigating the COX-2 protein expression levels in BV2 cells. The
knockdown efficacy of TREM2 siRNA was verified by western blotting
and show in Fig. 3C. The results
indicated the protein expressions of TREM2 in TREM2 siRNA-2 and
TREM2 siRNA-3 group were significantly decreased compared with siNC
group, Lipofectamine® 2000 group and control group.
Based on the results, TREM2 siRNA-2 was chosen for subsequent
experiments. COG1410 was found to decrease COX-2 protein expression
OGD/R-treated BV2 cells. In addition, COX-2 expression tended to
increase after TREM2 knockdown in OGD/R-treated BV2 cells, but no
statistically significant differences could be found compared with
that in the control silencing group (Fig. 3D).
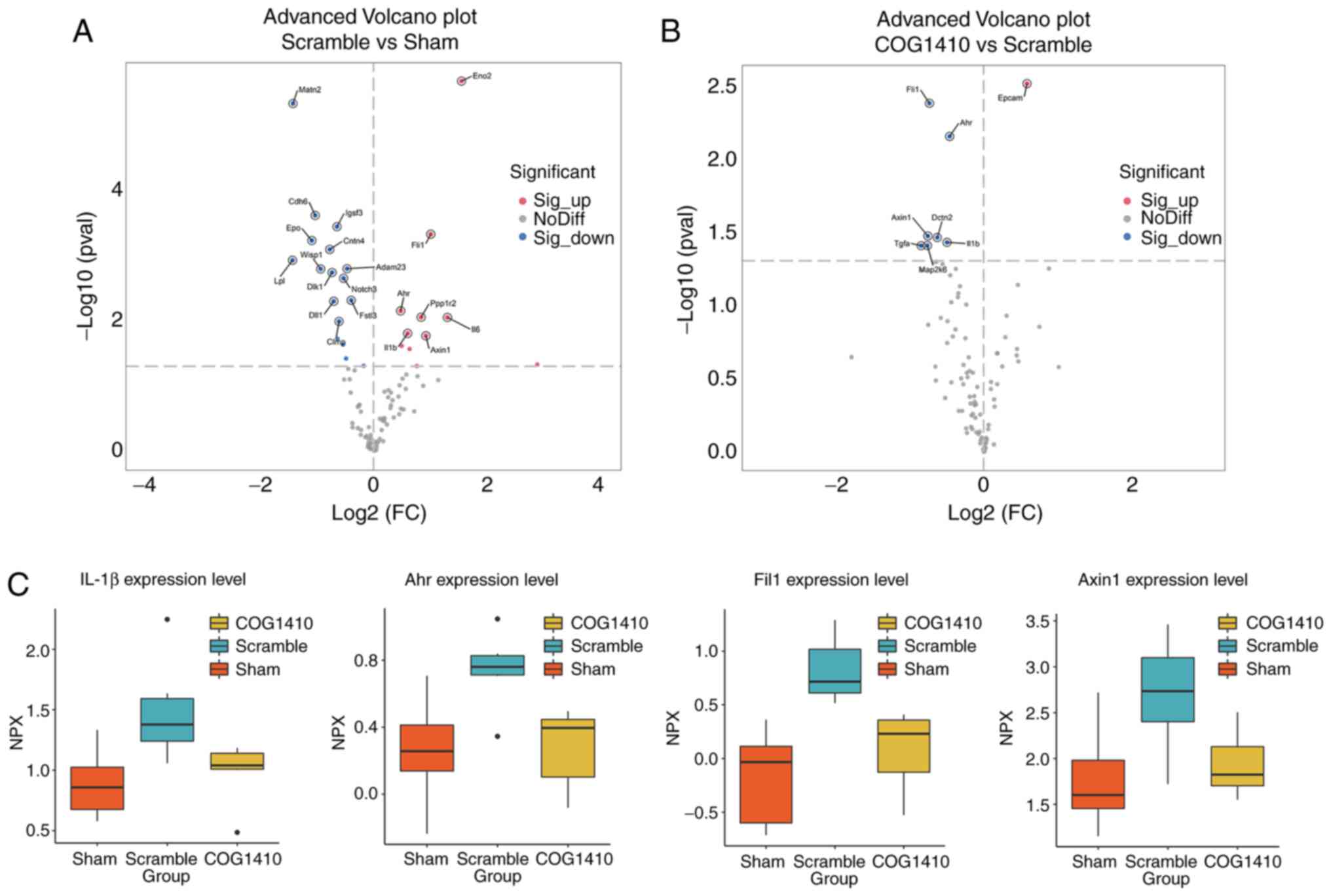
Olink proteomic study indicates that
COG1410 reduces peripheral inflammation in rats following MCAO
To examine the possible anti-inflammation properties
of COG1410, 92 proteins of the Mouse Exploratory Panel were then
further analyzed in the rat plasma samples using the Olink
proteomic method. The advanced volcano plot shown in Fig. 4A revealed that 17 proteins were
significantly upregulated whereas 11 were significantly
downregulated in MCAO rats compared with those in Sham control rats
(Scramble vs. Sham). In addition, one of the protein that was
previously elevated by MCAO, while seven of those previously found
to be reduced by MCAO, were in turn reversed by COG1410 treatment
(COG1410 vs. Scramble; Fig. 4B).
Gene Ontology and Kyoto Encyclopedia of Genes and Genome analysis
revealed no apparent enrichment in the specific terms. Comparison
of these three groups found four proteins to be increased in MCAO
rats but then significantly decreased by treatment with COG1410.
These were IL-1β, Aryl hydrocarbon receptor (AHR), Friend leukemia
integration 1 transcription factor and Axin-1 (Fig. 4C). In particular, all four of these
proteins are either inflammatory factors or important inflammation
regulators (6). These data
suggested that COG1410 could even reduce peripheral inflammation in
rats that undergo MCAO.
Discussion
The present study found that COG1410 can
significantly reduce BBB permeability and attenuate inflammatory
reactions in rats after brain ischemia-reperfusion. These findings
suggest that COG1410 can preserve BBB integrity by inhibiting MMP
activity and reducing tight junction protein degradation.
BBB disruption is central to poor outcomes and
increased mortality rates following ischemic stroke onset,
especially if thrombolytic therapy is delayed. Tissue plasminogen
activator treatment beyond the 4.5-h therapy window frequently
occurs as a result of the severe impairments in BBB, which is
mainly caused by the destruction of tight junction proteins and the
endothelial interface (6).
Therefore, preventing BBB injury after cerebral ischemia and
finding the optimal timing of thrombolytic therapy remain obstacles
that need to be overcome for ischemic stroke treatment. ApoE3 has
been proved to protect neurovascular unit. However, macromolecular
compounds, such as recombinant proteins, cannot cross BBB and,
therefore, are not usually used in treating neurological diseases.
In the perspective of translational medicine, exploring relatively
small and cell permeable peptide, instead of proteins, markedly
increases the bioavailability and efficacy in treating CNS
diseases. At present no clinical trial of COG1410 is registered or
being conducted. However, it is probable that COG1410 might be used
in clinical trial, especially for the neurological critical
disorders, due to the high efficacy in protecting BBB.
The present study demonstrated that COG1410 can
significantly reduce BBB permeability, according to Evans blue
leakage and immunofluorescence staining. In addition, COG1410 can
attenuate the impairment of the tight junction protein occludin.
Therefore, COG1410 may correspondingly improve stroke outcomes by
maintaining the integrity of BBB after ischemia stroke.
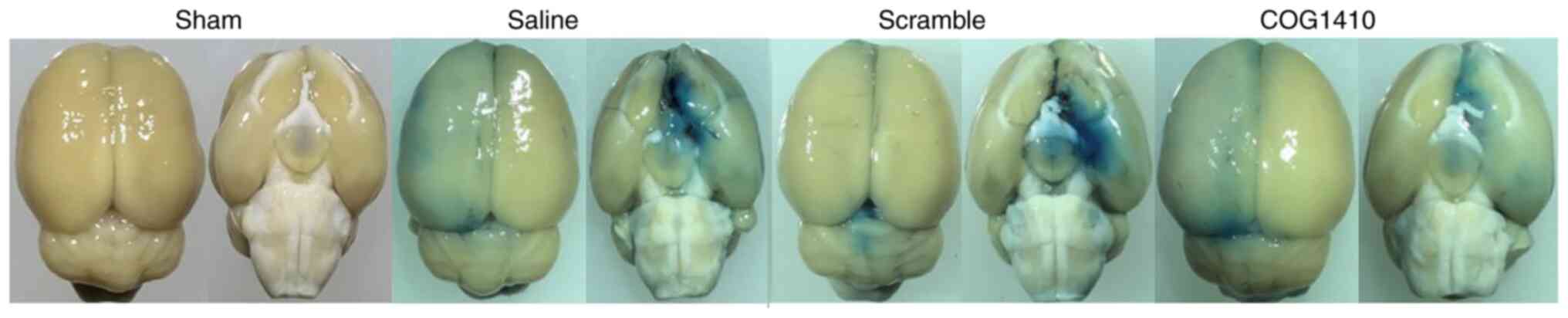
For the control in the present study, the best
efforts were exerted to guarantee its integrity during experimental
design. Usually, a scramble peptide with the same amino acids but
different sequence (with experimental peptide) was used as the
control. This can eliminate any potential efficacy/effect of
peptide or amino acid itself. The scramble peptide was also
compared with vehicle saline before conducting formal experiments.
In the Evans blue extravasation assay, the dye leakage was compared
between MCAO rats treated with saline or scramble peptide. The
result is represented as Fig 5, in
which Evans blue leakage to brain tissue of MCAO rats treated with
pseudopeptide and saline was similar, indicating that the scramble
peptide did not have any effect on its own.
In other CNS injury models, the protective effect of
COG1410 on the BBB is associated with the inhibition of MMPs
(14,17). Accumulating evidence has also
revealed that MMPs, particularly MMP-9, may mediate detrimental
effects on the BBB during the acute phase of ischemic stroke by
degrading the neurovascular matrix and disrupting tight junctions
(38). Furthermore, ApoE is
observed to suppress the cyclophillin A/NF-κB/MMP-9 pathway through
the low density lipoprotein receptor-related protein 1 receptor to
reverse BBB disruption (13). In
the present study, COG1410 reversed the increased MMP-9 protein
expression levels caused by cerebral ischemia-reperfusion injury,
in addition to inactivating the gelatinase activities of MMP in the
brain. In other words, COG1410 may mediate protective effects on
the BBB by suppressing activities of MMPs.
Neuroinflammation is one of the main causes of MMP-9
activation, leading to BBB breakdown (39). In addition, infiltrating
neutrophils and the microvascular endothelium are two key sources
of brain MMP-9 after cerebral ischemia (40). Several studies (18,23)
have demonstrated that COG1410 treatment can inhibit the activation
of microglia and macrophages, in addition to inhibiting the
infiltration of neutrophils in SAH and TBI models. Consistent with
these observations, the present data also revealed that COG1410
significantly reversed the activated microglia following MCAO.
COG1410 significantly reduced COX-2 protein expression after
ischemia-reperfusion injury both in vivo and in
vitro. Unlike COX-1, a constitutive isoform, COX-2 is rapidly
induced upon activation by inflammatory mediators during cerebral
ischemia-reperfusion. Recent studies have indicated that the
expression of COX-2 precedes the emergence of inflammatory factors
after brain injury and that the expression of inflammatory factors
induces an inflammatory cascade, leading to further cell death and
tissue injury (41–43). In addition, the catalytic products
of COX-2 are associated with the production of the free radical
superoxide and prostanoids (44,45).
The superoxide produced may react with NO to form the powerful
oxidant peroxynitrite. These reactive oxygen and nitrogen species
react with MMPs and increase their enzyme activities (46,47).
Furthermore, proinflammatory prostanoids, such as prostaglandin E2
produced by COX-2, induce a marked BBB breakdown in rats (48). Genetic deletion or pharmacological
inhibition of COX-2 dramatically reduces BBB damage by reducing
MMP-9 activity in a mouse model of ischemic stroke (37) and thus MMP-9 is an important
downstream effector contributing to COX-2-mediated neurovascular
damage in ischemic stroke (49).
In addition, in the present study, Olink proteomics
analysis showed that COG1410 could regulate the production of
IL-1β, which is a potent proinflammatory cytokine (39). Taken together, these findings
suggested that COG1410 can exert anti-inflammatory effects on the
cerebral ischemia-reperfusion model through the suppression of
microglial activation and inflammatory cytokine expression.
Therefore, this inhibition of neuroinflammation is proposed to
decrease the activity of MMPs, which is followed by reduced TJ
protein degradation, leading to the protection of BBB.
TREM2 is a high-affinity receptor for ApoE that is
expressed highly in the microglia compared with neurons and other
glial cells (50). TREM2 itself
has been reported to be mediate clear neuroprotective and
anti-inflammatory effects. TREM2 downregulates Toll-like receptor
4-mediated NF-κB activation and cytokine production (51,52).
By contrast, silencing TREM2 expression was previously found to
enhance lipopolysaccharide-induced proinflammatory cytokine
production in microglia (53). In
another study, knockdown of TREM2 increased PGE2 production by BV2
cells, where the overexpression of TREM2 was found to reduce
LPS-induced inflammation by inhibiting the PI3K/NF-κB signaling
pathway (28,54). According to previous researches,
the ApoE-TREM2 binding interface was found in amino acids 130–149
of ApoE (26,27,55–57),
while the amino acids of COG1410 are exactly in this area. ApoE has
been shown to exhibit an anti-inflammation role and protect neurons
in various CNS disease models. However, to the best of the authors'
knowledge, there is no direct evidence showing that TREM2 mediates
the anti-inflammation effects of ApoE. The biological functions of
ApoE-TREM2 mostly focus on the clearance of amyloid β and apoptotic
cells in the models of Alzheimer's disease (58,59).
Considering COG1410 contains the binding regions of
ApoE to TREM2, presumably it might also bind to TREM2. Other
supporting evidence includes: i) Radioligand
68Ga-NOTA-COG1410 was developed as specific PET image
probe targeting TREM2 for digestive tumor diagnosis (60); and ii) silencing endogenous TREM2
abolishes the neuroprotective and anti-inflammatory effects of
COG1410 in a mouse model of intracerebral hemorrhage (25). The binding surface and details
require more investigations using structural biology methods, such
as X-ray crystallography or nuclear magnetic resonance. TREM2 has
also been documented to contribute to the anti-inflammatory and
anti-apoptotic effects of COG1410 in an intracerebral hemorrhage
model through the PI3K/AKT signaling pathway (25). It is therefore hypothesized that
COG1410 can also activate TREM2 upstream of the neuroprotective
effects during cerebral ischemia-reperfusion injury. The mechanism
of how COG1410 works on microglia remains to be elucidated. Given
that TREM2 as a receptor predominantly locates on cell surface of
microglia and both COG1410 and TREM2 have anti-inflammatory effects
in CNS diseases, it was hypothesized that the binding of
COG1410-TREM2 should inhibit inflammation. In the present study, a
trend of increase in the COX-2 expression level was found following
TREM2 knockdown in OGD/R-treated BV2 cells. This partly supports
the aforementioned hypothesis. However, in-depth analysis of the
effects of TREM2 in additional mouse and cell models is necessary
to further explore its role in COG1410-mediated anti-inflammatory
and neuroprotective effects.
Olink proteomics assay was performed in the present
study to screen for any changes in the levels of a panel of
molecules before and after COG1410 treatment, to assess the role of
peripheral inflammation in this phenomenon This is a high-multiplex
immunoassay that can identify multiple proteins with high
reliability and data quality. Among the four identified proteins
that were reduced by COG1410, IL-1β is a well-characterized
cytokine that has been shown to induce BBB breakdown, neuron
apoptosis and brain edema (61).
By contrast, Axin1 is a scaffold protein in the β-catenin
destruction complex, functioning as an important regulator of the
Wnt/β-catenin pathway (62),
intestinal inflammation and cell apoptosis (63). A previous protein profiling study
of the rat ischemic stroke model revealed that Axin1 is amongst the
activated proteins in response to the penumbra tissue response
(64). AHR is a ligand-activated
transcription factor that is normally located in the cytoplasm. It
has been shown to be protective against hyperglycemia-induced BBB
dysfunction and brain injury after stroke (65,66),
in addition to regulating neuroinflammation and microglia activity
(67). Fil1 is expressed
predominantly in endothelial cells and immune cells, which mediates
important roles in regulating peripheral inflammation and
autoimmune diseases (68). These
four identified proteins are all, to varying extent, involved in
the regulation of inflammation or BBB injury. However, their
possible functions in the BBB-protective role of COG1410 remains
unclear and requires further study.
Taken together, the present study preliminarily
explored the potential neuroprotective effects of COG1410 on
ischemic stroke by regulating COX-2, which provides basic
theoretical support for developing COG1410 for clinical medicine.
TREM2 is a potential target for COG1410 on the microglia. The
molecular mechanisms of the physical binding of COG1410 and TREM2
and how COG1410/TREM2 complex regulates COX2 expression are
unclear. Further studies are required to verify the pharmacological
mechanism and evaluate its clinical applicability.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science Fund
of China (grant nos. 81960227, 82160247 and 82160899), Hainan
Province Science and Technology Special Fund (grant no.
ZDKJ2021034), Hainan Provincial Key Research and Development
Program (grant no. ZDYF2019196), and Hainan Provincial Clinical
Research Center Program (grant no. LCYX202104, LCYX202209).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and GW contributed to conception and design of
the study. YX, MG, CC, YY and YL performed the experiments and
analyzed the data. YX and YG wrote the manuscript. YX and YG
confirm the authenticity of all the raw data. All authors
contributed to manuscript revision, and all authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the committee on
Animal Care and Use of Hainan Provincial Hospital of Traditional
Chinese Medicine (approval no. IACUC-HPHCM-2211001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Suzuki K, Matsumaru Y, Takeuchi M,
Morimoto M, Kanazawa R, Takayama Y, Kamiya Y, Shigeta K, Okubo S,
Hayakawa M, et al: Effect of mechanical thrombectomy without vs
with intravenous thrombolysis on functional outcome among patients
with acute ischemic stroke: The SKIP randomized clinical trial.
JAMA. 325:244–253. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng T, Jiang Y, Farhan M, Lazarovici P,
Chen L and Zheng W: Anti-inflammatory effects of traditional
chinese medicines on preclinical in vivo models of brain
ischemia-reperfusion-injury: Prospects for neuroprotective drug
discovery and therapy. Front Pharmacol. 10:2042019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin R, Yang G and Li G: Molecular insights
and therapeutic targets for blood-brain barrier disruption in
ischemic stroke: Critical role of matrix metalloproteinases and
tissue-type plasminogen activator. Neurobiol Dis. 38:376–385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abbott NJ, Patabendige AA, Dolman DE,
Yusof SR and Begley DJ: Structure and function of the blood-brain
barrier. Neurobiol Dis. 37:13–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Andjelkovic AV, Zhu L, Yang T,
Bennett MVL, Chen J, Keep RF and Shi Y: Blood-brain barrier
dysfunction and recovery after ischemic stroke. Prog Neurobiol.
163–164. 144–171. 2018.PubMed/NCBI
|
|
7
|
Iadecola C and Anrather J: The immunology
of stroke: From mechanisms to translation. Nat Med. 17:796–808.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sincer I, Mansiroglu AK, Aktas G, Gunes Y
and Kocak MZ: Association between hemogram parameters and coronary
collateral development in subjects with Non-ST-Elevation myocardial
infarction. Rev Assoc Med Bras (1992). 66:160–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiong XY, Liu L and Yang QW: Functions and
mechanisms of microglia/macrophages in neuroinflammation and
neurogenesis after stroke. Prog Neurobiol. 142:23–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maida CD, Norrito RL, Daidone M,
Tuttolomondo A and Pinto A: Neuroinflammatory mechanisms in
ischemic stroke: Focus on cardioembolic stroke, background, and
therapeutic approaches. Int J Mol Sci. 21:64542020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mahley RW, Nathan BP and Pitas RE:
Apolipoprotein E. Structure, function, and possible roles in
Alzheimer's disease. Ann N Y Acad Sci. 777:139–145. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tai LM, Thomas R, Marottoli FM, Koster KP,
Kanekiyo T, Morris AW and Bu G: The role of APOE in cerebrovascular
dysfunction. Acta Neuropathol. 131:709–723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bell RD, Winkler EA, Singh I, Sagare AP,
Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et
al: Apolipoprotein E controls cerebrovascular integrity via
cyclophilin A. Nature. 485:512–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tukhovskaya EA, Yukin AY, Khokhlova ON,
Murashev AN and Vitek MP: COG1410, a novel apolipoprotein-E
mimetic, improves functional and morphological recovery in a rat
model of focal brain ischemia. J Neurosci Res. 87:677–682. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laskowitz DT, Fillit H, Yeung N, Toku K
and Vitek MP: Apolipoprotein E-derived peptides reduce CNS
inflammation: Implications for therapy of neurological disease.
Acta Neurol Scand Suppl. 185:15–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Anderson LG, Lascola CD, James ML,
Venkatraman TN, Bennett ER, Acheson SK, Vitek MP and Laskowitz DT:
ApolipoproteinE mimetic peptides improve outcome after focal
ischemia. Exp Neurol. 241:67–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pang J, Chen Y, Kuai L, Yang P, Peng J, Wu
Y, Chen Y, Vitek MP, Chen L, Sun X and Jiang Y: Inhibition of
blood-brain barrier disruption by an apolipoprotein E-Mimetic
peptide ameliorates early brain injury in experimental subarachnoid
hemorrhage. Transl Stroke Res. 8:257–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng J, Pang J, Huang L, Enkhjargal B,
Zhang T, Mo J, Wu P, Xu W, Zuo Y, Peng J, et al: LRP1 activation
attenuates white matter injury by modulating microglial
polarization through Shc1/PI3K/Akt pathway after subarachnoid
hemorrhage in rats. Redox Biol. 21:1011212019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Pang J, Peng J, Cao F, Vitek MP, Li
F, Jiang Y and Sun X: An apoE-derived mimic peptide, COG1410,
alleviates early brain injury via reducing apoptosis and
neuroinflammation in a mouse model of subarachnoid hemorrhage.
Neurosci Lett. 627:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao F, Jiang Y, Wu Y, Zhong J, Liu J, Qin
X, Chen L, Vitek MP, Li F, Xu L and Sun X: Apolipoprotein E-Mimetic
COG1410 reduces acute vasogenic edema following traumatic brain
injury. J Neurotrauma. 33:175–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laskowitz DT, McKenna SE, Song P, Wang H,
Durham L, Yeung N, Christensen D and Vitek MP: COG1410, a novel
apolipoprotein E-based peptide, improves functional recovery in a
murine model of traumatic brain injury. J Neurotrauma.
24:1093–1107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoane MR, Kaufman N, Vitek MP and McKenna
SE: COG1410 improves cognitive performance and reduces cortical
neuronal loss in the traumatically injured brain. J Neurotrauma.
26:121–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pang J, Peng J, Matei N, Yang P, Kuai L,
Wu Y, Chen L, Vitek MP, Li F, Sun X, et al: Apolipoprotein E exerts
a whole-brain protective property by promoting m1? microglia
quiescence after experimental subarachnoid hemorrhage in mice.
Transl Stroke Res. 9:654–668. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin X, You H, Cao F, Wu Y, Peng J, Pang J,
Xu H, Chen Y, Chen L, Vitek MP, et al: Apolipoprotein E mimetic
peptide increases cerebral glucose uptake by reducing blood-brain
barrier disruption after controlled cortical impact in mice: An
18F-Fluorodeoxyglucose PET/CT study. J Neurotrauma.
34:943–951. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Peng J, Sherchan P, Ma Y, Xiang S,
Yan F, Zhao H, Jiang Y, Wang N, Zhang JH and Zhang H: TREM2
activation attenuates neuroinflammation and neuronal apoptosis via
PI3K/Akt pathway after intracerebral hemorrhage in mice. J
Neuroinflammation. 17:1682020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krasemann S, Madore C, Cialic R, Baufeld
C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E, Xu Y, Fanek Z,
et al: The TREM2-APOE pathway drives the transcriptional phenotype
of dysfunctional microglia in neurodegenerative diseases. Immunity.
47:566–581.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Atagi Y, Liu CC, Painter MM, Chen XF,
Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, et al:
Apolipoprotein E is a ligand for triggering receptor expressed on
myeloid cells 2 (TREM2). J Biol Chem. 29:26043–26050. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Zhao B, Lin C, Gong Z and An X:
TREM2 inhibits inflammatory responses in mouse microglia by
suppressing the PI3K/NF-κB signaling. Cell Biol Int. 43:360–372.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu R, Li X, Xu P, Huang L, Cheng J, Huang
X, Jiang J, Wu LJ and Tang Y: TREM2 protects against cerebral
ischemia/reperfusion injury. Mol Brain. 10:202017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deczkowska A, Weiner A and Amit I: The
physiology, pathology, and potential therapeutic applications of
the TREM2 signaling pathway. Cell. 181:1207–1217. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhai Q, Li F, Chen X, Jia J, Sun S, Zhou
D, Ma L, Jiang T, Bai F, Xiong L and Wang Q: Triggering receptor
expressed on myeloid cells 2, a novel regulator of immunocyte
phenotypes, confers neuroprotection by relieving neuroinflammation.
Anesthesiology. 127:98–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawabori M, Kacimi R, Kauppinen T,
Calosing C, Kim JY, Hsieh CL, Nakamura MC and Yenari MA: Triggering
receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates
phagocytic activities of microglia and exacerbates ischemic damage
in experimental stroke. J Neurosci. 35:3384–3396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li M, Chen S, Shi X, Lyu C, Zhang Y, Tan
M, Wang C, Zang N, Liu X, Hu Y, et al: Cell permeable HMGB1-binding
heptamer peptide ameliorates neurovascular complications associated
with thrombolytic therapy in rats with transient ischemic stroke. J
Neuroinflammation. 15:2372018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsoi B, Wang S, Gao C, Luo Y, Li W, Yang
D, Yang D and Shen J: Realgar and cinnabar are essential components
contributing to neuroprotection of Angong Niuhuang Wan with no
hepatorenal toxicity in transient ischemic brain injury. Toxicol
Appl Pharmacol. 377:1146132019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Assarsson E, Lundberg M, Holmquist G,
Björkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E,
Ohlsson S, Edfeldt G, et al: Homogenous 96-plex PEA immunoassay
exhibiting high sensitivity, specificity, and excellent
scalability. PLoS One. 9:e951922014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sifat AE, Vaidya B and Abbruscato TJ:
Blood-brain barrier protection as a therapeutic strategy for acute
ischemic stroke. AAPS J. 19:957–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang C, Yang Y, DeMars KM, Rosenberg GA
and Candelario-Jalil E: Genetic deletion or pharmacological
inhibition of cyclooxygenase-2 reduces blood-brain barrier damage
in experimental ischemic stroke. Front Neurol. 11:8872020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Daneman R and Prat A: The blood-brain
barrier. Cold Spring Harb Perspect Biol. 7:a0204122015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lakhan SE, Kirchgessner A and Hofer M:
Inflammatory mechanisms in ischemic stroke: Therapeutic approaches.
J Transl Med. 7:972009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu X, Yao Y, Yang J, Zhengxie J, Li X, Hu
S, Zhang A, Dong J, Zhang C and Gan G: COX-2-PGE(2) signaling
pathway contributes to hippocampal neuronal injury and cognitive
impairment in PTZ-kindled epilepsy mice. Int Immunopharmacol.
87:1068012020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Luo W, Zhang J, Luo Y, Han W, Wang
H, Xia H, Chen Z, Yang Y, Chen Q, et al: Maternal inflammation
exaggerates offspring susceptibility to cerebral
ischemia-reperfusion injury via the COX-2/PGD2/DP2 pathway
activation. Oxid Med Cell Longev. 2022:15717052022.PubMed/NCBI
|
|
43
|
Yan W, Ren D, Feng X, Huang J, Wang D, Li
T and Zhang D: Neuroprotective and anti-inflammatory effect of
pterostilbene against cerebral ischemia/reperfusion injury via
suppression of COX-2. Front Pharmacol. 12:7703292021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang T, Zhang A, Pasumarthy A, Zhang L,
Warnock Z and Schnermann JB: Nitric oxide stimulates COX-2
expression in cultured collecting duct cells through MAP kinases
and superoxide but not cGMP. Am J Physiol Renal Physiol.
291:F891–F895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Manabe Y, Anrather J, Kawano T, Niwa K,
Zhou P, Ross ME and Iadecola C: Prostanoids, not reactive oxygen
species, mediate COX-2-dependent neurotoxicity. Ann Neurol.
55:668–675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sorokin A: Nitric oxide synthase and
cyclooxygenase pathways: A complex interplay in cellular signaling.
Curr Med Chem. 23:2559–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gu Y, Dee CM and Shen J: Interaction of
free radicals, matrix metalloproteinases and caveolin-1 impacts
blood-brain barrier permeability. Front Biosci (Schol Ed).
3:1216–1231. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schmidley JW, Dadson J, Iyer RS and
Salomon RG: Brain tissue injury and blood-brain barrier opening
induced by injection of LGE2 or PGE2. Prostaglandins Leukot Essent
Fatty Acids. 47:105–110. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Candelario-Jalil E, González-Falcón A,
García-Cabrera M, León OS and Fiebich BL: Post-ischaemic treatment
with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain
barrier disruption and leukocyte infiltration following transient
focal cerebral ischaemia in rats. J Neurochem. 100:1108–1120. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ford JW and McVicar DW: TREM and TREM-like
receptors in inflammation and disease. Curr Opin Immunol. 21:38–46.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X and
Fu J: Curcumin inhibits LPS-induced neuroinflammation by promoting
microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2
cells. Mol Immunol. 116:29–37. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sharif O, Gawish R, Warszawska JM, Martins
R, Lakovits K, Hladik A, Doninger B, Brunner J, Korosec A,
Schwarzenbacher RE, et al: The triggering receptor expressed on
myeloid cells 2 inhibits complement component 1q effector
mechanisms and exerts detrimental effects during pneumococcal
pneumonia. PLoS Pathog. 10:e10041672014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu K, Byers DE, Jin X, Agapov E,
Alexander-Brett J, Patel AC, Cella M, Gilfilan S, Colonna M, Kober
DL, et al: TREM-2 promotes macrophage survival and lung disease
after respiratory viral infection. J Exp Med. 212:681–697. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu R, He Y and Chen Z: Maprotiline
ameliorates isoflurane-induced microglial activation via regulating
triggering receptor expressed in myeloid cells 2 (TREM2).
Bioengineered. 12:12332–12344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bailey CC, DeVaux LB and Farzan M: The
triggering receptor expressed on myeloid cells 2 binds
apolipoprotein E. J Biol Chem. 290:26033–26042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yeh FL, Wang Y, Tom I, Gonzalez LC and
Sheng M: TREM2 binds to apolipoproteins, including APOE and
CLU/APOJ, and thereby facilitates uptake of amyloid-beta by
microglia. Neuron. 91:328–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jendresen C, Rskog V, Daws MR and Nilsson
LN: The Alzheimer's disease risk factors apolipoprotein E and TREM2
are linked in a receptor signaling pathway. J Neuroinflammation.
14:592017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nguyen AT, Wang K, Hu G, Wang X, Miao Z,
Azevedo JA, Suh E, Van Deerlin VM, Choi D, Roeder K, et al: APOE
and TREM2 regulate amyloid-responsive microglia in Alzheimer's
disease. Acta Neuropathol. 140:477–493. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wolfe CM, Fitz NF, Nam KN, Lefterov I and
Koldamova R: The Role of APOE and TREM2 in Alzheimer's
disease-current understanding and perspectives. Int J Mol Sci.
20:812018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shi D, Si Z, Xu Z, Cheng Y, Lin Q, Fu Z,
Fu W, Yang T, Shi H and Cheng D: Synthesis and Evaluation of
68Ga-NOTA-COG1410 Targeting to TREM2 of TAMs as a
Specific PET probe for digestive tumor diagnosis. Anal Chem.
94:3819–3830. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Voet S, Srinivasan S, Lamkanfi M and van
Loo G: Inflammasomes in neuroinflammatory and neurodegenerative
diseases. EMBO Mol Med. 11:e102482019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fiedler M, Mendoza-Topaz C, Rutherford TJ,
Mieszczanek J and Bienz M: Dishevelled interacts with the DIX
domain polymerization interface of Axin to interfere with its
function in down-regulating β-catenin. Proc Natl Acad Sci USA.
108:1937–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jin D, Zhang YG, Wu S, Lu R, Lin Z, Zheng
Y, Chen H, Cs-Szabo G and Sun J: Vitamin D receptor is a novel
transcriptional regulator for Axin1. J Steroid Biochem Mol Biol.
165:430–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Uzdensky A, Demyanenko S, Fedorenko G,
Lapteva T and Fedorenko A: Protein profile and morphological
alterations in penumbra after focal photothrombotic infarction in
the rat cerebral cortex. Mol Neurobiol. 54:4172–4188. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cuartero MI, Ballesteros I, de la Parra J,
Harkin AL, Abautret-Daly A, Sherwin E, Fernández-Salguero P, Corbí
AL, Lizasoain I and Moro MA: L-kynurenine/aryl hydrocarbon receptor
pathway mediates brain damage after experimental stroke.
Circulation. 130:2040–2051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ren R, Lu Q, Sherchan P, Fang Y, Lenahan
C, Tang L, Huang Y, Liu R, Zhang JH, Zhang J and Tang J: Inhibition
of aryl hydrocarbon receptor attenuates hyperglycemia-induced
hematoma expansion in an intracerebral hemorrhage mouse model. J Am
Heart Assoc. 10:e0227012021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Khan AS, Wolf A and Langmann T: The AhR
ligand 2, 2′-aminophenyl indole (2AI) regulates microglia
homeostasis and reduces pro-inflammatory signaling. Biochem Biophys
Res Commun. 579:15–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
He YS, Yang XK, Hu YQ, Xiang K and Pan HF:
Emerging role of Fli1 in autoimmune diseases. Int Immunopharmacol.
90:1071272021. View Article : Google Scholar : PubMed/NCBI
|