Introduction
The intestinal microbiota constitutes the most
important part of the human microbiota. It is considered that
~1,000 different bacteria are in each healthy individual's colon
mucosa and feces. The microbiota serves a role in a variety of
diseases, including metabolic disorders, inflammatory and
autoimmune diseases, allergies and even conditions where microbiome
involvement seems implausible (1–5).
Escherichia coli and Staphylococcus
aureus, intestine flora, cause adverse effects on intestinal
permeability by increasing their numbers in the intestinal
microbiota in dysbiosis. The alpha toxin of Staphylococcus
aureus alters intestinal integrity and impairs the barrier
function of intestinal cells in vitro (6). Staphylococcus aureus serves a
role in inflammatory bowel disease, as evidenced by the fact that
gut-derived Staphylococcus aureus superantigens can induce
inflammatory responses. A number of studies have shown that the
colon can be a reservoir of antibiotic resistance genes. For
example, vancomycin-resistant Staphylococcus aureus
colonizes the intestinal tract. In previous studies, it has been
observed that the intestinal transport of Staphylococcus
aureus is increased among hospitalized patients and infants
(7–13). Escherichia coli is
gram-negative and some E. coli strains provide vitamin K and
vitamin B12, which are beneficial for the host (14). However, colitis and other
intestinal diseases develop as a result of the increase in the
Enterobacteriaceae family and especially Escherichia coli as
a result of high-fat contents, diet, inactivity, and unnecessary
and incorrect antibiotic use (15). Especially in patients with
immunosuppressed colon cancer, changes in the flora cause bad
results in a whole spectrum from metabolic diseases to neurological
diseases.
An increase in the pathogenic bacteria population,
especially E. coli and S. aureus in the intestine
tract leads to an increase in the incidence of tumor appearance.
Mitochondria contain DNA called mtDNA. As with nuclear DNA, the
presence of endogenous reactive oxygen species (ROS) causes mtDNA
damage. ROS is one of the well-known parameters in cancer
formation. A number of studies show that chronic ROS formation
induces RNA and DNA break and leads to cancer formation. 8-OHdG and
H2AX are markers to evaluate the RNA and DNA breakout (16–18).
The capacity of Lactobacilli to adhere to epithelial
cells of the host intestinal tract is crucial in inhibiting
enteropathogenic infections. Species of Lactobacillus, such
as L. acidophilus, L. helveticus, L. rhamnosus, L. case, L.
paracasei, L. reuteri, and L. fermentum, compete with
enteric pathogens for binding sites, such as Enteropathogenic E.
coli (EPEC), Enterohaemorrhagic E. coli (EHEC),
Enterotoxigenic E. coli (ETEC), Enteroinvasive E.
coli (EIEC), Enteroaggregative E. coli (EAEC), diffusely
adherent E. coli (DAEC), and, a new pathotype,
adherent-invasive E. coli (AIEC) (19–24).
In addition to probiotics, vitamin E, which is stated to have a
significant effect on the colon, acts as an important antioxidant
(against lipid peroxidation) (25). The use of probiotics can improve
the intestinal microbial population, increase mucus secretion, and
reduce the number of lipopolysaccharides (LPSs) to prevent the
breakdown of tight junction proteins. When LPS binds endothelial
cells to toll-like receptors (TLR) 2 and 4, dendritic cells and
macrophage cells are activated and inflammatory markers increase.
Furthermore, a reduction in intestinal dysbiosis and intestinal
leakage after probiotic treatment can minimize the development of
inflammatory biomarkers and blunt unnecessary activation of the
immune system. By contrast, probiotics enhance the differentiation
of T cells towards Th2 and the development of Th2 cytokines such as
IL-4 and IL-10 (24–26). Vitamin K, especially synthetic
vitamin K3 (menadione), is twice as strong as natural vitamins K1
and K2. K3 is produced by bacteria in the intestinal microflora and
serves an important role in blood coagulation (26). HT-29 and Caco-2 cells derived from
human colorectal carcinoma are the most commonly used in numerous
in vitro cell culture absorption models.
To date, few studies have investigated the ability
of lactobacilli to inhibit the adhesion of Escherichia
coli and Staphylococcus aureus to intestinal epithelial
cells using methods evaluating the incorporation of vital dyes or
the inhibition of cell colony formation (27–30).
The present study determined the effect of the Lactobacillus
acidophilus ATCC 1356 strain and vitamins K3 and E on
Escherichia coli and methicillin-resistant Staphylococcus
aureus (MRSA) infection in the human colon adenocarcinoma cell
line grade one HT-29 and Caco-2.
Materials and methods
Chemicals and reagents
Vitamin K3, vitamin E, 9% isotonic sodium chloride
solution, tryptic soy broth, ethanol, blood agar, agar, crystal
violet solution, Dulbecco modified Eagles medium (DMEM), fetal calf
serum (FBS), phosphate buffer solution (PBS), antibiotic
antimitotic solution (100X), L glutamine, and trypsin-EDTA were
obtained from MilliporeSigma.
Bacterial strains
Lactobacillus acidophilus ATCC 4356 strains
(American Type Culture Collection) were purchased commercially and
isolated in the Department of Medical Microbiology, Faculty of
Medicine, Ataturk University. The Escherichia coli ATCC
25922, MRSA ATCC 29213 strain, and Lactobacillus acidophilus
ATCC 4356 strains were used in the present study. Lactobacillus
acidophilus ATCC 4356 was incubated in Mann, Rogosa, and Sharpe
broth (MRS Broth; MilliporeSigma) at 37°C and 5% CO2 for
48 h. L. acidophilus was grown in Lactobacillus MRS
Broth (Himedia) for 24 h at 37°C in a bacteriological incubator
under microaerophilic conditions. The number of cells in suspension
was determined with a spectrophotometer (B582; Micronal) at a
concentration of 107 cells/ml. The optical density and
wavelength used were 0.296 and 600 nm for L. acidophilus.
Cell densities of the inoculum were confirmed by CFU/ml counting
after plating in Rogosa agar for L. acidophilus. For the
preparation of the L. acidophilus culture filtrate, 1 ml of
the standard suspension was transferred to a Falcon tube containing
6 ml MRS broth and incubated for 24 h at 37°C in a bacteriological
incubator under microaerophilic conditions. Then the broth was
centrifuged (2,000 × g for 10 min) at room temperature and filtered
through a membrane with a 0.22-µm pore size (Advantec MFS, Inc.).
Escherichia coli ATCC 25922 strain was used. Bacteria were
inoculated into an EMB medium and incubated for 24 h at 37°C. Then,
108 CFU/ml suspension was prepared from the growing
colonies according to the McFarland 0.5 chart. The MRSA ATCC 29213
strain was incubated in modified Giolitti and Cantoni broth for 14
h at 37°C and Baird-Parker plates (supplemented with Giolitti and
Cantoni broth 1.5%) for 48 h at 37°C.
Kirby-Bauer disk diffusion method
Microorganism colonies taken from 24 h cultures were
adjusted in sterile saline to McFarland 0.5 turbidity and
inoculated into Mueller Hinton medium (meat infusion 2 g/l; casein
hydrolysate 17.5 g/l; starch 1.5 g/l; agar-agar 13,0 g/l) with a
swab stick. Then, 6 mm diameter Sterile Paper Discs (Oxoid
Antibacterial Susceptibility Blank Test Disc; Oxoid Ltd.) were
impregnated with 20 µg/ml and placed in Muller Hinton Medium. Zone
diameters were measured after 24 h of incubation. Vancomycin was
used as the control antibiotic.
Minimal inhibitory concentration
Vitamins E and K were dissolved with Tween 20 to a
final concentration of 5 mg/ml. Escherichia coli ATCC 25922,
MRSA ATCC 29213 and Lactobacillus acidophilus ATCC 4356 used
in the minimum inhibitory concentration determination method were
passed into eosin methylene blue and blood agar and a 24 h fresh
culture was prepared by CLSI criteria (31). From the colonies taken from the
prepared cultures, a bacterial suspension was prepared in sterile
0.9% saline with the turbidity of Lactobacillus acidophilus
ATCC 4356, 0.5 McFarland 109 CFU/ml, and Escherichia
coli ATCC 25922, MRSA ATCC 29213, 0.5 McFarland
5×105. Then, 100 µl of tryptic soy broth medium was
added to all wells 1 to 12 of the sterile 96-well microplate. At
first, 100 µl of bacterial suspension was added to the 1st well and
100 µl of a 1:1 dilution was added to the 10th well. Secondly, 100
µl of vitamin K, E, and K + E (5–0.007 mg/ml) were added to the 1st
well, and 100 µl dilution was made up to the 10th well in a 1:1
ratio (32). For bacteria control
only bacterial suspension was added to the well, and only vitamins
K and E were added to the 12th well. Then, the microplate was
covered with parafilm and incubated for 24 h at 37°C. Vancomycin
was used as the control antibiotic (33).
Cell culture infection model
Colon cancer cell lines
The human colon cancer cell lines HT-29 (ATCC no:
HTB-38) and Caco-2 (ATCC no: HTB-37) were used. Frozen cells were
rapidly thawed and centrifuged at 200 × g at room temperature for 5
min. Cells were collected in a 25 cm2 flask by adding
fresh DMEM high glucose, 10% FBS and 1% antibiotics (penicillin,
streptomycin, and amphotericin B). When 80% of the flasks were
covered with cells, they were centrifuged at 200 × g at room
temperature for 5 min (by removing
trypsin-ethylenediaminetetraacetic acid (EDTA; 0.25% trypsin-0.02%
EDTA)). The supernatant was discarded, and the cell suspension was
seeded at 104 cells/well in 96-well cell culture
plates.
Preparation of cell culture
samples
Vitamins E and K, an MRSA strain, and a
Lactobacillus acidophilus strain were used in the present
study. Groups were: Control group, the group containing DMSO
(dimethyl sulfoxide) was used as a positive control group; vitamin
E (5 mg/ml), vitamin K3 (5 mg/ml), Lactobacillus acidophilus
(109 CFU/ml), Escherichia coli ATCC 25922,
(108 CFU/ml) and MRSA (5×105 CFU/ml). The
experiment began when the cells in the plates reached a density of
85–90%. A total of 10 replicates were used for each dose
(n=10).
MTT analysis (cytotoxicity
analysis)
When the experiment had finished, 10 µl of MTT
solution was added to each well and incubated for 4 h at 37°C with
5% CO2. To dissolve the formed formazan crystals, 100 µl
of dimethyl sulfoxide (DMSO) solution was added to the wells. Cell
viability (%) was read using a Multiskan GO microplate
spectrophotometer (Thermo Fisher Scientific, Inc.) at 570 nm. The
viability rates were compared with those of the control group.
Oxidative stress markers
To measure the oxidative stress level, the total
antioxidant capacity (TAC) and the total oxidant status (TOS) were
determined with a commercial kit (Rel Assay Diagnostics) according
to the manufacturer's instructions. TAC levels were examined at 660
nm and TOS at 530 nm in a Multiskan GO microplate spectrophotometer
(Thermo Fisher Scientific, Inc.).
Immunohistochemistry
Cultured cells were incubated for 30 min in
paraformaldehyde (4% and room temperature) solution. The cells were
then incubated in 3% H2O2 for 5 min. A 0.1%
Triton-X solution was dripped onto the cells, washed with PBS, and
left for 15 min. After incubation, serum-free blocking buffer (cat.
no. X090930-2; Agilent Technologies, Inc.) was dripped onto the
cells and kept in the dark for 5 min at room temperature. Then, the
primary antibody (8-OHdG cat. no. sc-66036; Santa Cruz
Biotechnology, Inc.; 1:100) was added dropwise and incubated in
accordance with the manufacturer's protocols. An immunofluorescence
secondary antibody was used as a secondary marker (FITC; cat. no.
ab6785; Abcam) and incubated in the dark for 45 min at room
temperature. The cells were stained with Texas Red (cat. no.
ab6787; Abcam; 1:1,000) in the dark for 45 min. Then, DAPI with
mounting medium (cat. no. D1306; Thermo Fisher Scientific, Inc.;
1:200) was dropped onto the sections and they were kept in the dark
for 5 min. Then, the sections were closed with a coverslip. The
stained sections were examined under a fluorescence microscope
(Zeiss Axio; Zeiss AG).
Statistical analyses
Cell culture
The results are given as the mean ± standard error
of the mean, a visual representation of statistical quantities
estimated from the data with assumptions about the underlying
distribution of the data obtained in box plot charts. The
statistical comparison of the groups with each other was calculated
by one-way ANOVA and Tukey's HSD method. One-way ANOVA calculations
to be used in statistical analysis were performed with SPSS 20
software (IBM Corp.).
Immunohistochemistry
To determine the intensity of positive staining from
the images captured from the stained samples, five random areas
were selected from each image and evaluated using the Zen Imaging
Software program (Zeiss AG). Data was statistically defined as the
mean ± standard deviation for the percentage area. The Mann-Whitney
U test was performed to compare positive immunoreactive cells and
immunopositive stained areas with healthy controls. The data are
presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically
significant difference.
Results
Vitamins and probiotics are frequently consumed for
their alleged health benefits. However, it is not known whether
they can interact and influence the health effects of each other.
The present study documented the interactions between vitamins E
and K with Lactobacillus acidophilus against MRSA or
Escherichia coli.
Effects on bacterial growth
The present study first assessed if the different
combinations of vitamins exerted toxic effects on bacterial growth.
After the concentrations of bacteria and probiotics were prepared,
the doses of vitamins were adjusted and inoculations were made. The
minimal inhibitory concentration (MIC) value of vitamin E on MRSA
was 1.25 mg/ml, and Lactobacillus acidophilus + vitamin E
did not show any MIC value. By comparison, the MIC value of MRSA +
Lactobacillus acidophilus + vitamin E was 1.25 mg/ml. While
no effect was observed in vitamin K MRSA, MIC values of
Lactobacillus acidophilus + vitamin K were found to be 1.25
mg/ml, and MRSA + Lactobacillus acidophilus + vitamin K MIC
values were determined to be 2.5 mg/ml. The MIC values of
Lactobacillus acidophilus + vitamins E + K against MRSA or
Escherichia coli are shown in Table I. Altogether, the combination of
vitamin E and K had the most potent effects and strongly inhibited
bacterial growth when Lactobacillus acidophilus against MRSA
or Escherichia coli was grown in isolation. The MIC values
were larger when the bacteria were co-cultured.
 | Table I.Minimal inhibitory concentration
values of vitamins in bacteria. |
Table I.
Minimal inhibitory concentration
values of vitamins in bacteria.
|
| Strain mg/ml |
|---|
|
|
|
|---|
| Vitamin | E. coli | MRSA | Lactobacillus
acidophilus | E. coli +
Lactobacillus acidophilus | MRSA +
Lactobacillus acidophilus |
|---|
| Vitamin E | 0.03 | 1.25 | >5 | 0.03 | 1.25 |
| Vitamin K | >5 | >5 | 1.25 | 2.5 | 2.5 |
| Vitamin E + K | 0.02 | 0.007 | 0.009 | 2.5 | 0.15 |
Kirby Bauer disk diffusion
results
In the present study, an antibiogram was performed
by inoculating Escherichia coli and MRSA suspension into
Mueller Hinton medium. The inhibition zones of discs impregnated
with vitamins and probiotics were measured at 6, 12, 18, and 24 h.
The most effective after 24 h was the vitamin E + vitamin K +
Lactobacillus acidophilus combination against Escherichia
coli by opening a 23 mm zone against 17 mm MRSA. The inhibition
results against Escherichia coli and MRSA are shown in
Table II. The results show a
time-dependent change in zone diameter, which was the most
pronounced with vitamin E.
 | Table II.Escherichia coli and MRSA zone
diameters at 6, 12, 18, and 24 h (mm). |
Table II.
Escherichia coli and MRSA zone
diameters at 6, 12, 18, and 24 h (mm).
|
| Time |
|---|
|
|
|
|---|
| Agent | 6 h | 12 h | 18 h | 24 h |
|---|
| Vitamin E | No
zonea,b | 2a, 5b | 6a, 12b | 11a, 17b |
| Vitamin K | No
zonea,b | No
zonea,b | No
zonea,b | No
zonea,b |
| Vitamin E + vitamin
K + Lactobacillus acidophilus | No
zonea,b | 7a,11** | 15a, 17** | 17a, 23b |
Cell culture results
MTT assay
The present study then examined how probiotic and
vitamin supplementation in HT-29 and Caco-2 colon cancer cells
exposed to the bacteria would affect the viability of the cells.
The MTT results obtained in the Escherichia coli and MRSA on
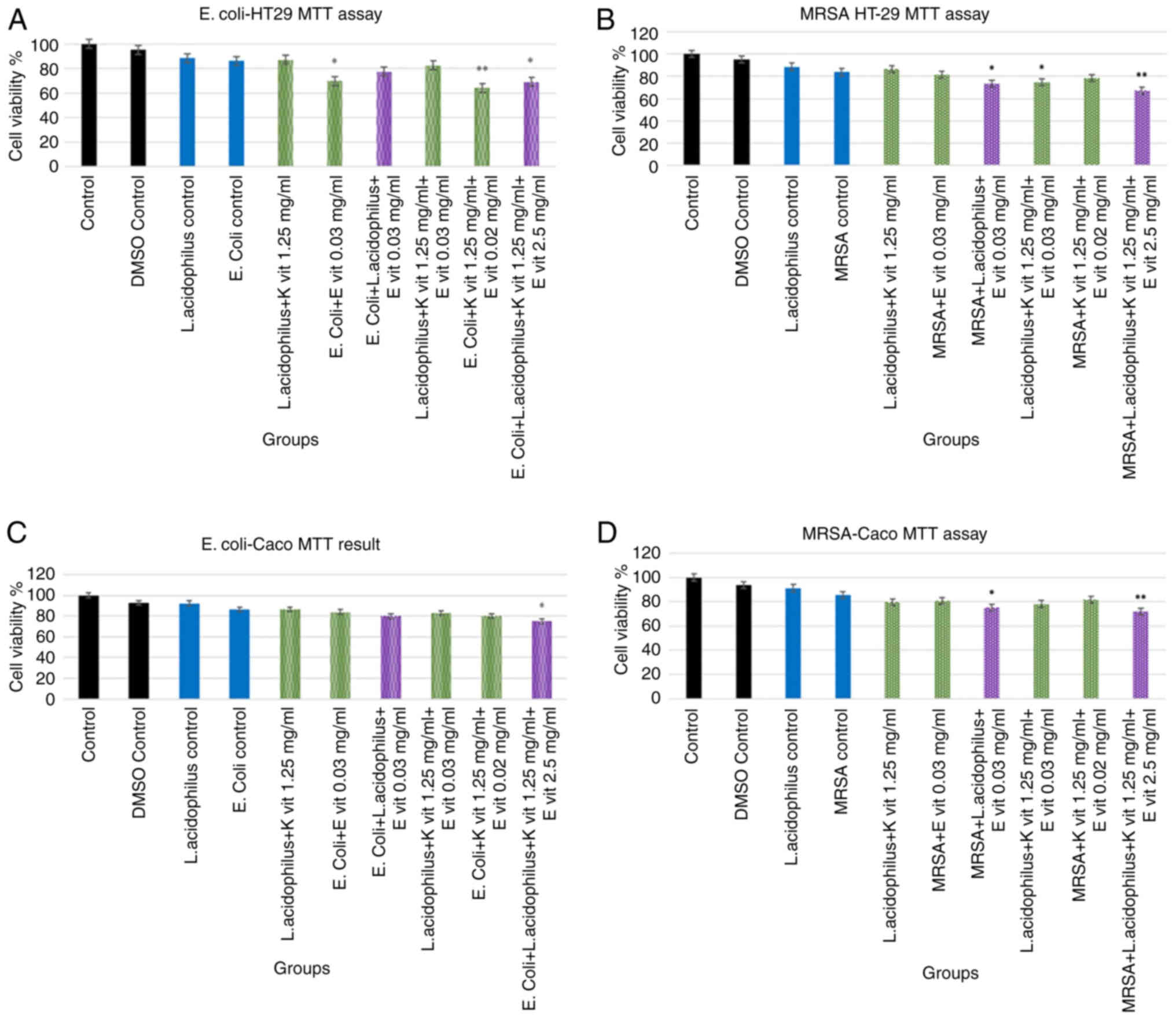
Caco and HT-29 cell lines are presented in Fig. 1. No significant difference was
found between the Lactobacillus acidophilus control group
and the control group. When Escherichia coli control and control
groups were compared, the viability rate was found to be 86.36%.
When Lactobacillus acidophilus + vitamin K 1.25 mg/ml,
vitamin E + Escherichia coli 0.03, and vitamin E + vitamin K
+ Escherichia coli 0.02 groups were compared with the
control group, the lowest viability rates were found with 64.4%
(**P<0.001), and 69.2% (*P<0.05), respectively. Regarding
MRSA, no significant difference was found between the control
groups and the positive control group. When the MRSA control and
control groups were compared, the viability rate was found to be
83.7%. When the Lactobacillus acidophilus + vitamin K 1.25
mg/ml + vitamin E 0.02 groups were compared with the control group,
the lowest viability rates were found at 67.4%, respectively, and
these values were statistically significant. (**P<0.001).
L. acidophilus did not decrease Caco cell
viability similar to the E. coli control group (P>0.05).
By contrast, the bacteria and vitamin combination, especially in
the vitamin E group, decreased the viability (P>0.05) but the
results were not statistically significant. E. coli + L.
acidophilus + vitamin E decreased cancer cell viability by
nearly 20% compared with the control group. The highest toxicity
was noticed in E. coli + L. acidophilus + vitamin E +
vitamin K near 25% (P<0.001). The MTT results are shown in
Fig. 1. L. acidophilus did
not decrease Caco cell viability, a result similar to MRSA
treatment. Bacteria and vitamin combination, especially in the
vitamin E group, decreased viability (P<0.05). MRSA + L.
acidophilus + vitamin E decreased cancer cell viability by
nearly 25% compared with the control group (P<0.05). The highest
toxicity was seen in MRSA + L. acidophilus + vitamin E +
vitamin K, by nearly 29% (P<0.001).
TAC and TOS measurement
The present study then evaluated whether antioxidant
capability or oxidative stress was changed by exposure to
probiotics, vitamins, or their combination. There was no
significant difference between TAC and TOS levels in the DMSO group
in HT-29 cells treated with E. coli compared with the
control group. TAC levels of the control group and Lactobacillus
acidophilus control group, Lactobacillus acidophilus +
vitamin K 1.25, Escherichia coli + vitamin E 0.03, and
Escherichia coli + vitamin K + vitamin E 0.02 were
significantly higher (**P<0.001). TOS values of these groups
were significantly lower compared with the control group
(**P<0.001, *P<0.05).
There was no significant difference between TAC and
TOS levels in the DMSO group in HT-29 cells given MRSA compared
with the control group. The TAC levels of the control group and the
L. acidophilus control, L. acidophilus + vitamin K 1.25, and
L. acidophilus + vitamin K + vitamin E + 1.25 groups were
significantly higher (**P<0.001). The TOS values of these groups
were significantly lower than those of the control group
(**P<0.001, *P<0.05). In addition, E. coli + L.
acidophilus + vitamin E affected antioxidant capacity compared
with the control group (P<0.05). The antioxidant capacity
decreased by adding vitamin E to both L. acidophilus (P<0.05)
and MRSA compared with the bacteria control group. while E and
vitamin K combination with bacteria lead to a decreased antioxidant
significantly in E. coli + vitamin E + K (P<0.05) and
E. coli + L. acidophilus + vitamin E + vitamin K
group 2 Trolox Equiv mmol/l−1 (P<0.001). E.
coli + vitamin E + K (P<0.05) and E. coli + L.
acidophilus + vitamin E + vitamin K group increased the
oxidative status significantly (P<0.05) compared with the
control group. The oxidative status results are shown in Fig. 2. The DMSO control did not affect
the antioxidant and oxidant status. In addition, the antioxidant
capacity decreased by adding vitamin E to both L.
acidophilus (P<0.05) and MRSA in comparison to the control
group. The combination of vitamin E and K with bacteria led to
decreased antioxidant capacity and increased oxidant status in the
Caco-2 culture. The antioxidant capacity primarily decreased in
MRSA + L. acidophilus + vitamin E + vitamin K group 2 Trolox
equiv mmol/l−1 (P<0.001). Oxidant status increased
slightly by adding vitamins to bacterial species (P>0.05).
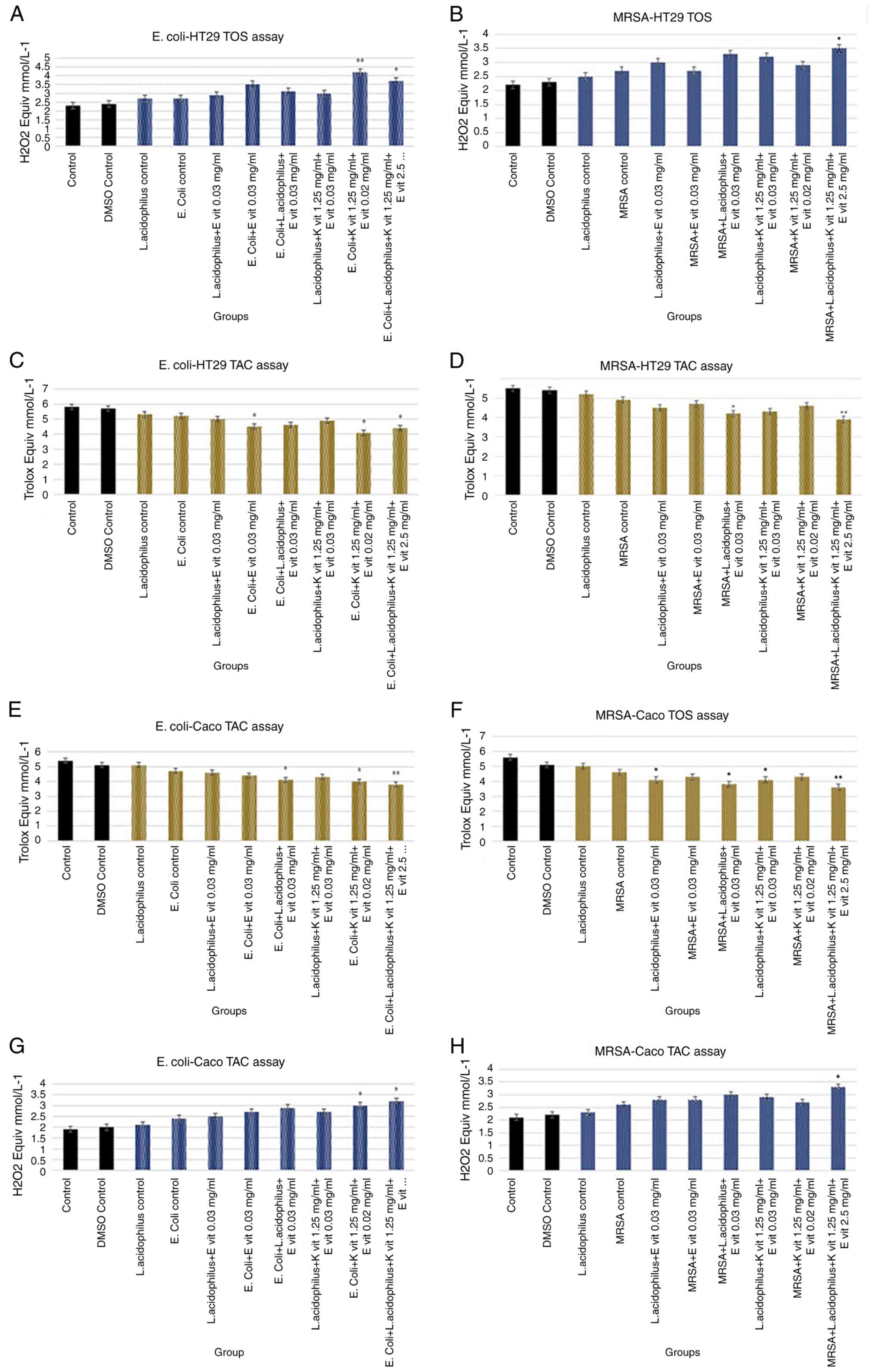 | Figure 2.Antioxidant and oxidant activity
measurement. TAC and TOS assay results of (A) Escherichia
coli-HT29 TOS, (B) MRSA-HT29 TOS, (C) E. coli-HT29 TAC,
(D) MRSA-HT29 TAC, (E) E. coli-Caco-2 TAC, (F) MRSA-Caco-2
TOS, (G) E. coli-Caco-2 TOS and (H) MRSA-Caco-2 TAC in cell
culture after 24 h. *P<0.05 and **P<0.001 vs. with the
control group. TAC, total antioxidant capacity; TOS, total oxidant
status; MRSA, methicillin-resistant Staphylococcus aureus; K
vit, vitamin K; E vit, vitamin E. |
Immunohistochemistry results
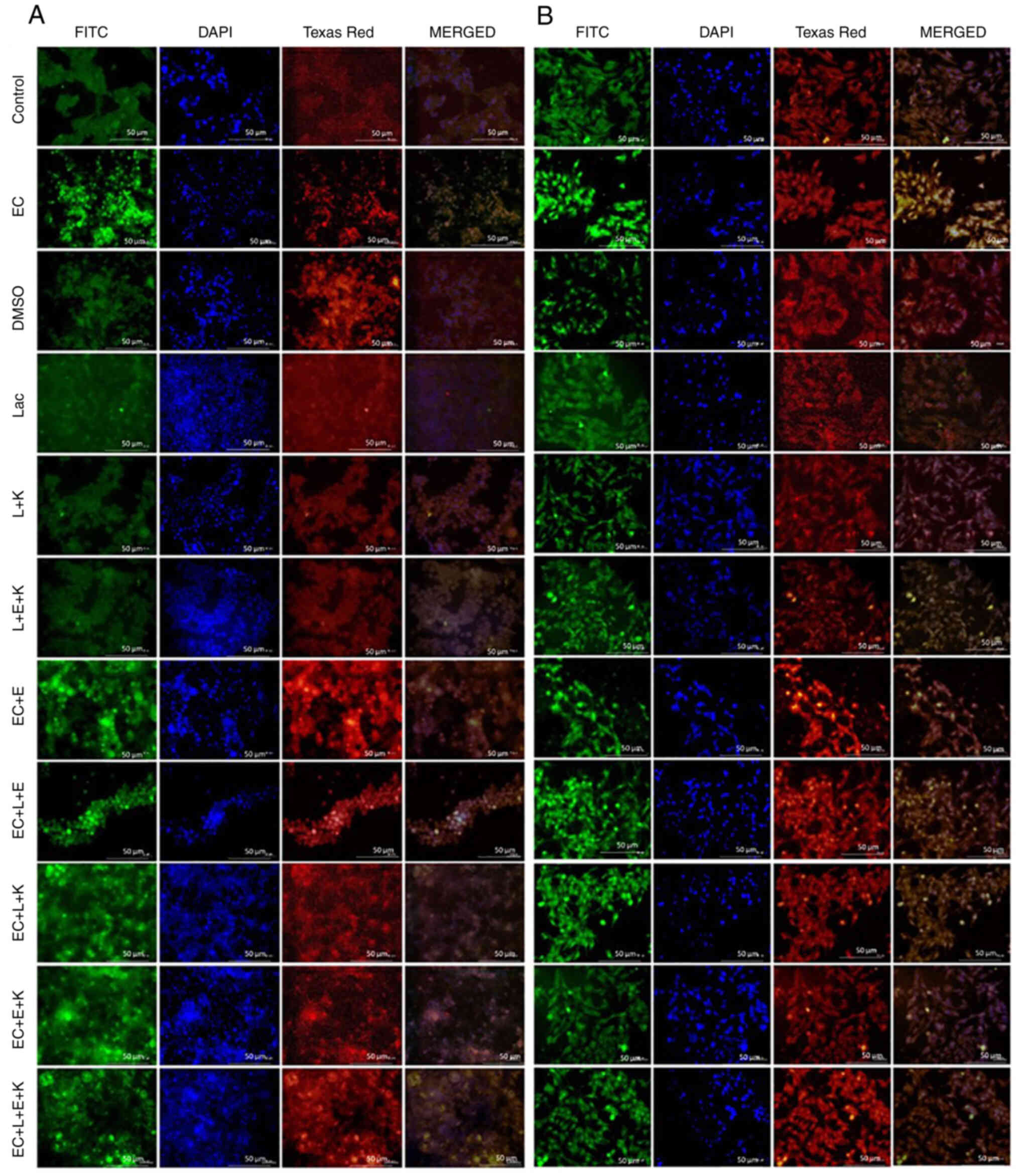
Whether the change in oxidative status could be
linked to the development of DNA damage was evaluated. For the
control group, in immunofluorescent staining, negative 8-OHdG, and
H2A.X expressions were established. For the E. coli control
group, the immunofluorescent staining showed strong 8-OHdG and
H2A.X expressions (Fig. 3). In the
DMSO control group, the immunofluorescent staining revealed
negative 8-OHdG and H2A.X expressions. The Lactobacillus
control group developed negative 8-OHdG and H2A.X expressions while
the L + vitamin K 1.25 mg/ml group was evaluated as negative 8-OHdG
and H2A.X expressions. The L + vitamin E + vitamin K 0.01 group
also showed negative 8-OHdG and H2A.X expressions. For the E.
coli + vitamin E 0.03 group, moderate expressions of 8-OHdG and
H2A.X were detected in immunofluorescent staining, while for the
E. coli + L + vitamin E 0.03 group, mild expressions of
8-OHdG and H2A.X were registered. E. coli + L + vitamin K
2.5 mg/ml group: moderate expressions of 8-OHdG and H2A.X detected.
For the E. coli + vitamin E + vitamin K 0.02 group, only
mild expressions of 8-OHdG and H2A.X were noted and for the E.
coli + L + vitamin E + vitamin K group, low expressions of
8-OHdG and H2A.X were registered in the immunofluorescent staining
(Table III).
 | Table III.Immunofluorescent result scores of
the study groups. |
Table III.
Immunofluorescent result scores of
the study groups.
|
| HT-29 8-OHdG | HT-29 H2A.X | Caco-2 8-OHdG | Caco-2 H2A.X |
|---|
| Control |
38.85±6.78a |
21.16±7.18a |
34.16±6.78a |
23.76±5.79a |
| E. coli
Control |
161.22±12.86b |
96.15±11.83b |
182.18±10.09b |
124.28±10.74b |
| DMSO Control |
38.15±5.76a |
23.84±5.12a |
37.18±5.42a |
25.18±6.18a |
|
Lactobacillus Control |
40.12±6.18a |
25.18±5.6a |
39.76±6.94a |
24.74±3.26a |
|
Lactobacillus + vitamin K 1.25 |
41.18±6.23a |
25.71±6.84a |
38.12±5.84a |
22.16±9.12a |
|
Lactobacillus + vitamin E + vitamin
K 0.01 |
40.16±6.84a |
24.6±5.76a |
36.15±4.12a |
22.18±5.74a |
| E. coli +
vitamin E 0.03 |
93.18±10.82c |
78.16±6.12c |
142.18±10.26c |
92.18±6.29c |
| E. coli +
Lactobacillus + vitamin E 0.03 |
66.85±10.63d |
50.24±11.38d |
94.18±14.13d |
60.12±9.26d |
| E. coli +
Lactobacillus + vitamin K 2.5 |
96.18±9.54c |
73.75±7.76c |
139.26±9.15c |
89.26±7.15c |
| E. coli +
vitamin E + vitamin K 0.02 |
68.12±13.79d |
47.16±9.91d |
99.16±16.85d |
61.74±12.7d |
| E. coli +
Lactobacillus + vitamin E + vitamin K |
57.96±11.12d |
39.97±11.9d |
85.26±16.28d |
52.42±10.28d |
For the control group, when the HT-29 cell line
samples were stained with the double immunofluorescence method,
8-OHdG, and H2A.X expression were evaluated as negative. For the
MRSA control group when HT-29 cell line samples were stained with
the double-immunofluorescence method, intense 8-OHdG, and H2A.X
expressions were observed. In the DMSO control group, negative
8-OHdG and H2A.X expression levels were determined., for the
Lactobacillus control group negative 8-OHdG and H2A.X
expressions were observed, for the L + vitamin K 1.25 group 8-OHdG,
and H2A.X were negative, while for L + vitamin E + vitamin K 0.01
group negative 8-OHdG and H2A.X expression levels were determined.
In the MRSA + vitamin E 1.25 mg/ml group, when HT-29 cell line
samples were stained with the double immunofluorescence method,
moderate levels of 8-OHdG and H2A.X was detected. The same results
were obtained for the MRSA + vitamin K 2.5 mg/ml group, namely
moderate 8-OHdG and H2A.X expressions. Mild 8-OHdG and H2A.X
expression levels were determined for the MRSA+L+ vitamin E 1.25
mg/ml and MRSA+ vitamin E + vitamin K 0.007 groups, while for the
MRSA + L + vitamin E + vitamin K 0.15 group low levels of 8-OHdG
and H2A.X was detected (Fig. 4).
The immunofluorescence results are shown in Table IV.
 | Table IV.Scoring of immunofluorescent findings
in cell cultures. |
Table IV.
Scoring of immunofluorescent findings
in cell cultures.
| Group | HT-29 8-OHdG | HT-29 H2A.X | Caco-2 8-OHdG | Caco-2 H2A.X |
|---|
| Control |
37.18±6.18a |
26.14±5.15a |
39.26±3.12a |
25.14±6.82a |
| MRSA control |
136.14±10.08b |
108.75±10.84b |
188.76±13.25b |
131.18±9.26b |
| DMSO control |
39.26±5.81a |
25.24±4.94a |
40.12±5.42a |
28.74±7.59a |
|
Lactobacillus control |
40.12±4.16a |
27.18±8.62a |
41.15±5.8a |
29.78±4.49a |
|
Lactobacillus + vitamin K 1.25 |
40.16±6.79a |
28.15±5.54a |
39.49±4.19a |
27.65±3a |
|
Lactobacillus + vitamin E + vitamin
K 0.01 |
40.74±5.92a |
27.99±4.84a |
40.33±6.74a |
29.48±5.74a |
| MRSA + vitamin E
1.25 |
91.18±9,74c |
74.12±6.18c |
141.16±7.2c |
102.12±8.18c |
| MRSA + vitamin K
2.5 |
88.75±8.4c |
80.16±10.21c |
137.75±6.74c |
96.12±9c |
| MRSA +
Lactobacillus + vitamin E 1.25 |
72.16±13.26d |
54.12±11.25d |
99.15±13.42d |
68.28±13.26d |
| MRSA + vitamin E +
vitamin K 0.007 |
71.84±10.3d |
52.28±8.12d |
96.84±14.35d |
68.15±12.64d |
| MRSA+
Lactobacillus + vitamin E + vitamin K 0.15 |
64.14±10.98d |
46.85±10.29d |
89.4±12.29d |
57.37±12.94d |
Discussion
There are intense scientific discussions on the
effects of probiotics and other supplements such as vitamins. The
present study examined how probiotic and vitamin supplementation in
HT-29 and Caco-2 colon cancer cells exposed to Escherichia
coli and MRSA infection would affect the toxicity and viability
of the cells. It compared two different colon cancer cells. While
HT-29 consists of first-degree cancer cells, Caco-2 cells consist
of second-degree cancer cells. By comparing the two levels, the
effect of probiotics and vitamin supplementation on the viability
of cells in an infection due to the progression of cancer prognosis
was examined. Although Escherichia coli normally settle in
the intestine as avirulent, they acquire virulent characteristics
that give them the ability to adapt to new niches and cause
intestinal and extra-intestinal diseases. This situation is closely
related to the immune system of the host. With these virulent
features, they can manage a process ranging from colitis to
diarrhea, cancer formation, and mortality (34). In the management of this process,
non-antibiotic treatments have gained popularity in recent years
with the emergence and spread of new antibiotic-resistant isolates.
Among these treatment approaches, the use of probiotics is a
promising alternative for the control of urinary tract infections
and infections caused by Escherichia coli.
Probiotics can adhere to uroepithelial cells and
inhibit the growth of pathogenic bacteria. In addition, oral
administration of Lactobacilli may colonize these microorganisms in
the urinary tract after intestinal colonization (35). Following a Staphylococcus
aureus infection, alpha-toxin reaches the basolateral
intestinal epithelium. The alpha-toxin then causes barrier
malfunction and, as a result, the intestinal lumen becomes
permeable to bacteria. As a result, bacteria and bacterial products
may pass into the lymph, lymph nodes and, finally, the blood,
aggravating the resulting septic condition (6). In the present study, the
antibacterial activity of Lactobacillus acidophilus and
vitamins E and K against Escherichia coli was evaluated
in vitro. The MIC value of vitamin E + vitamin K +
Lactobacillus acidophilus + MRSA was determined to be 0.15
mg/ml. Ghane et al (36) in
their study to determine the probiotic potential of
Lactobacillus strains isolated from kefir and to evaluate
their antimicrobial and antibiofilm activities against
uropathogenic Escherichia coli, screened 12
lactobacillus strains for their antimicrobial potentials
against uropathogenic Escherichia coli and seven of them
were isolated from Escherichia coli and Lactobacillus
strains showed high antagonistic activity. Different strains of
probiotics are known to produce compounds with antimicrobial
properties, including low molecular weight compounds, antimicrobial
peptides (bacteriocins) and organic acids (37–41).
The coaggregation between Lactobacilli and pathogenic bacteria
provides a barrier that prevents them from adhering to urinary and
intestinal epithelial cells. In parallel with the present study,
Ghane et al (36) isolated
probiotics in the study that showed antibacterial activity by
interacting with Escherichia coli.
The probiotic potential of three Pediococcus
spp. were investigated and 16S rRNA gene sequencing identified
Pediococcus acidilactici VKU2, P. acidilactici IAH-5,
and P. pentosaceus DHR005. All strains tolerated pH 3 and
0.3% oxalate and simulated gastric and intestinal juice for 3 h
(42). P. acidilactici
IAH-5 showed the highest cholesterol removal (67.52%), hydroxyl
radical scavenging activity (58.32%), hydrophobicity (40.3%), and
autoaggregation (48%). It was determined that it inhibited the
growth of the tested pathogens (Escherichia coli ATCC 25922,
Pseudomonas aeruginosa PTCC 1707, Salmonella
typhimurium PTCC 1609, and Staphylococcus aureus ATCC
25923) and the most susceptible strain was Staphylococcus
aureus (42). In the present
study, the Lactobacillus acidophilus strain showed a
positive effect against infection in HT-29 and Caco-2 cell
lines.
Probiotics are live microorganisms that, when
administered in adequate amounts, support health functions in the
human or animal host (36). In
addition, although various criteria have been proposed for
selecting probiotics, the most important feature is their ability
to bind to intestinal cells. Due to this adhesion ability,
probiotic strains can perform their functions by increasing their
persistence in the intestine (36). Due to the difficulty of studying
the in vivo adhesion of bacteria to the gastrointestinal
tract, in vitro evaluation using the adenocarcinoma cell
lines HT-29 and Caco-2 expresses the morphological and functional
features of normal enterocytes, is widely accepted (43). Although most studies on colon cell
lines show a correlation between hydrophobicity and adhesion,
Schillinger et al (44)
reported that Lactobacillus acidophilus BFE 719 showed good
adhesion to HT-29 cells while having a weak hydrophobicity of only
2%. In Lim and Ahn (2012) (45)
seven strains isolated from mustard leaf kimchi were screened for
their tolerance to simulated gastric and bile juices, their
adhesive properties to Caco-2 cells, and their ability to inhibit
Salmonella typhimurium ATCC 29631 adhesion. Lactobacillus
acidophilus GK20, Lactobacillus paracasei GK74 and
Lactobacillus plantarum GK81, which are resistant to bile
and gastric juices, have been found to have high bile salt
hydrolase activity against both sodium glycolate and sodium
taurocholate (45). One of the
most important features of probiotic bacteria is their ability to
survive in severe gastrointestinal conditions, including low pH
(i.e., stomach conditions) and high bile salt concentration (i.e.,
in the small intestine) until reaching their target (46,47).
The protection of the intestinal environment by Lactobacilli
strains is achieved by two mechanisms: the production of
antimicrobial compounds by Lactobacilli and binding to mucus and
coaggregation, which can form a barrier to prevent pathogenic
biofilm (48). The gut microbiome
is associated with the development of colorectal cancer (CRC).
Intestinal microbiota and bacterial mass population can trigger the
development of CRC by providing the formation of oncometabolite. In
a healthy colon, the main part of microbial metabolism is the
saccharolytic fermentation pathways (49). It has been suggested that oncogenic
bacteria such as Enterotoxigenic Bacteroides fragilis induce
the development of CRC through direct interactions with colon
epithelial cells and changes in microbiota composition in the
colorectal region. Escherichia coli, E. faecalis, Fusobacterium
nucleatum, and Streptococcus gallolyticus have been
identified as flora with higher populations in CRC patients.
However, it has been determined that there is a decrease in the
population of Bifidobacterium, Clostridium,
Faecalibacterium, Lactobacillus, and Roseburia
(50). Probiotics such as
Lactobacillus inhibit the growth of CRC by inhibiting
inflammation and angiogenesis and enhancing intestinal barrier
function through the secretion of short-chain fatty acids (51). Direct interactions with bacteria
and cancer cell combinations and vitamin combinations have not been
investigated. From the data of the present study, the toxicity of
E. coli is higher than that of lactobacillus bacteria
(52). This is due to the toxins
produced by E. coli. Cellular death occurs due to the
oxidative stress produced by the toxins within the cell. Vitamin E
is known to have anti-cancer effects (53). Vitamin E is known to allow
potentially beneficial Lactococcus and Bacteroides to
multiply in the gut (53). In a
colitis-associated cancer model, vitamin E suppresses
proinflammatory cytokines and modulates the gut microbiota
(54). The combination of two
bacteria and two vitamins in the present study did not cause damage
to the bacterial mass by reducing the neoplastic cell population
(55).
In recent years, microbiome methods have been used
in fecal collection using next-generation sequencing technology and
DNA analysis of all bacteria. The present study performed no
experiments on humans Future studies could experimentally
administer the probiotics, vitamin K and vitamin E. used here for
healthy subjects. and attempting to analyze their gut microbiota.
Furthermore, epidemiological studies linking the consumption of
these products in the human population would be another
strategy.
Probiotics, an alternative treatment option for
controlling infections caused by Escherichia coli and MRSA,
prevent the growth of pathogenic bacteria and this can be helped by
additional vitamin support. In this context, increasing additional
support studies for probiotic treatment are vital for the course of
treatment.
Acknowledgements
Not applicable.
Funding
The present study was partly funded by the European Union's
Horizon Europe framework program project ‘European Partnership for
the Assessment of Risks from Chemicals (PARC)’ and supported by
Decree no. 220 of the Russian Federation (grant no.
220-2961-3099).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
DC and AlT conceived the present study. ArT, OC,
DAS and DC were responsible for the methodology. ALA, RM and KG
were responsible for the formal analysis. AlT and RM performed
investigations. ArT, DAS, and AlT were responsible for resources.
ALA, KG and AlT wrote the original draft. ArT, OC, DC, RM and ALA
reviewed and edited the manuscript. KG and ALA were responsible for
visualization. ArT and AlT were responsible for supervision. DC and
AlT confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests. DS is the Editor-in-Chief for the journal, but had no
personal involvement in the reviewing process, or any influence in
terms of adjudicating on the final decision, for this article.
References
|
1
|
Caputi V and Giron MC:
Microbiome-gut-brain axis and toll-like receptors in Parkinson's
disease. Int J Mol Sci. 19:16892018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rea D, Coppola G, Palma G, Barbieri A,
Luciano A, Del Prete P, Rossetti S, Berretta M, Facchini G, Perdonà
S, et al: Microbiota effects on cancer: From risks to therapies.
Oncotarget. 9:17915–17927. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selber-Hnatiw S, Rukundo B, Ahmadi M,
Akoubi H, Al-Bizri H, Aliu AF, Ambeaghen TU, Avetisyan L, Bahar I,
Baird A, et al: Human gut microbiota: Toward an ecology of disease.
Front Microbiol. 8:12652017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajagopala SV, Vashee S, Oldfield LM,
Suzuki Y, Venter JC, Telenti A and Nelson KE: The human microbiome
and cancer. Cancer Prev Res (Phila). 10:226–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saud Hussein A, Ibraheem Salih N and
Hashim Saadoon I: Effect of Microbiota in the development of breast
cancer. Arch Razi Inst. 76:761–768. 2021.PubMed/NCBI
|
|
6
|
Kwak YK, Vikström E, Magnusson KE, Vécsey
Semjén B, Colque Navarro P and Möllby R: The Staphylococcus aureus
alpha-toxin perturbs the barrier function in Caco-2 epithelial cell
monolayers by altering junctional integrity. Infect. Immun.
80:1670–1680. 2012.PubMed/NCBI
|
|
7
|
Boyce JM and Havill NL: Nosocomial
antibiotic-associated diarrhea associated with
enterotoxin-producing strains of methicillin-resistant
Staphylococcus aureus. Am J Gastroenterol. 100:1828–1834. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Wang A, Ansari S, Hershberg RM and
McKay DM: Colonic bacterial superantigens evoke an inflammatory
response and exaggerate disease in mice recovering from colitis.
Gastroenterology. 125:1785–1795. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salyers AA, Gupta A and Wang Y: Human
intestinal bacteria as reservoirs for antibiotic resistance genes.
Trends Microbiol. 12:412–416. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sommer MOA, Dantas G and Church GM:
Functional characterization of the antibiotic resistance reservoir
in the human microflora. Science. 325:1128–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rimland D and Roberson B: Gastrointestinal
carriage of methicillin-resistant Staphylococcus aureus. J Clin
Microbiol. 24:137–138. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donskey CJ: the role of the intestinal
tract as a reservoir and source for transmission of nosocomial
pathogens. Clin Infect Dis. 39:219–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ray AJ, Pultz NJ, Bhalla A, Aron DC and
Donskey CJ: Coexistence of vancomycin-resistant enterococci and
Staphylococcus aureus in the intestinal tracts of hospitalized
patients. Clin Infect Dis. 37:875–881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blount ZD: The unexhausted potential of
E. coli. Elife. 4:e058262015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang SE, Lim SM, Jeong JJ, Jang HM, Lee
HJ, Han MJ and Kim DH: Gastrointestinal inflammation by gut
microbiota disturbance induces memory impairment in mice. Mucosal
Immunol. 11:369–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu M, Liang S, Wu J, Hua Y, Chen H, Zhang
Z, Liu J, Li X, Zhang B, Zhao W and Wan C: An Escherichia coli
Effector Protein EspF May Induce Host DNA damage via interaction
with SMC1. Front Microbiol. 12:6820642021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan R, Mo Y, Zhang Z, Jiang M, Tang S and
Zhang Q: Cobalt nanoparticles induce lung injury, DNA damage and
mutations in mice. Part Fibre Toxicol. 14:382017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu MH, Chen IC, Lee CH, Wu CW, Lee YC,
Kung YC, Hung CY and Wu KLH: Anti-neuroinflammation ameliorates
systemic inflammation-induced mitochondrial DNA impairment in the
nucleus of the solitary tract and cardiovascular reflex
dysfunction. J Neuroinflammation. 16:2242019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Candela M, Perna F, Carnevali P, Vitali B,
Ciati R, Gionchetti P, Rizzello F, Campieri M and Brigidi P:
Interaction of probiotic Lactobacillus and Bifidobacterium strains
with human intestinal epithelial cells: Adhesion properties,
competition against enteropathogens and modulation of IL-8
production. Int J Food Microbiol. 125:286–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fayol-Messaoudi D, Berger CN,
Coconnier-Polter MH, Lievin Le Moal V and Servin AL: pH-, lactic
acid-, and nonlactic acid-dependent activities of probiotic
lactobacilli against Salmonella enterica Serovar Typhimurium. Appl
Environ Microbiol. 71:6008–6013. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim Y, Kim SH, Whang KY, Kim YJ and Oh S:
Inhibition of Escherichia coli O157:H7 attachment by interactions
between lactic acid bacteria and intestinal epithelial cells. J
Microbiol Biotechnol. 18:1278–1285. 2008.PubMed/NCBI
|
|
22
|
Mappley LJ, Tchorzewska MA, Cooley WA,
Woodward MJ and La Ragione RM: Lactobacilli antagonize the growth,
motility, and adherence of Brachyspira pilosicoli: A potential
intervention against avian intestinal spirochetosis. Appl Environ
Microbiol. 77:5402–5411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Varma P, Dinesh KR, Menon KK and Biswas R:
Lactobacillus fermentum isolated from human colonic mucosal biopsy
inhibits the growth and adhesion of enteric and foodborne
pathogens. J Food Sci. 75:M546–M551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang YC, Zhang LW, Tuo YF, Guo CF, Yi HX,
Li JY, Han X and Du M: Inhibition of Shigella sonnei adherence to
HT-29 cells by lactobacilli from Chinese fermented food and
preliminary characterization of S-layer protein involvement. Res
Microbiol. 161:667–672. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horwıtt MK: Interpretations of
requirements for thiamin, riboflavin, niacin tryptophan, and
vitamin E plus comments on balance studies and vitamin B-6. Am J
Clin Nutr. 44:973–985. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andrade JC, Morais Braga MF, Guedes GM,
Tintino SR, Freitas MA, Quintans LJ Jr, Menezes IR and Coutinho HD:
Menadione (vitamin K) enhances the antibiotic activity of drugs by
cell membrane permeabilization mechanism. Saudi J Biol Sci.
24:59–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balimane PV, Chong S and Morrison RA:
Current methodologies used for evaluation of intestinal
permeability and absorption. J Pharmacol. Toxicol Methods.
44:301–312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blanchfield JT, Dutton JL, Hogg RC,
Gallagher OP, Craik DJ, Jones A, Adams DJ, Lewis RJ, Alewood PF and
Toth I: Synthesis, structure elucidation, in vitro biological
activity, toxicity, and Caco-2 cell permeability of lipophilic
analogs of r-Conotoxin MII. J Med Chem. 46:1266–1272. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Genc S, Pennisi M, Yeni Y, Yildirim S,
Gattuso G, Altinoz MA, Taghizadehghalehjoughi A, Bolat I, Tsatsakis
A, Hacımüftüoğlu A and Falzone L: Potential neurotoxic effects of
glioblastoma-derived exosomes in primary cultures of cerebellar
neurons via oxidant stress and glutathione depletion. Antioxidants
(Basel). 11:12252022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rogero SO, Higa OZ, Saiki M, Correa OV and
Costa I: Cytotoxicity due to corrosion of ear piercing studs.
Toxicol In Vitro. 14:497–504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Committee for Clinical Laboratory
Standards (NCCLS), Performance standards for antimicrobial disk
susceptibility tests, . Approved standard. NCCLS document M2-A5.
NCCLS; Villanova, PA: pp. 138–144. 1993
|
|
32
|
Ackermann G, Thomalla S, Ackermann F,
Schaumann R, Rodloff AC and Ruf BR: Prevalence and characteristics
of bacteria and host factors in an outbreak situation of
antibiotic-associated diarrhea. J Med Microbiol. 54:149–153. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Celebi D, Taghizadehghalehjoughi A, Baser
S, Genc S, Yilmaz A, Yeni Y, Yesilyurt F, Yildirim S, Bolat I,
Kordali S, et al: Effects of boric acid and potassium metaborate on
cytokine levels and redox stress parameters in a wound model
infected with methicillin-resistant Staphylococcus aureus. Mol Med
Rep. 26:2942022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le Gall T, Clermont O, Gouriou S, Picard
B, Nassif X, Denamur E and Tenaillon O: Extraintestinal virulence
is a coincidental by-product of commensalism in B2 phylogenetic
group Escherichia coli strains. Mol Biol Evol. 24:2373–2384. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zuccotti GV, Meneghin F, Raimondi C,
Dilillo D, Agostoni C, Riva E and Giovannini M: Probiotics in
clinical practice: An overview. J Int Med Res. 36 (Suppl 1):1A–53A.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghane M, Babaeekhou L and Ketabi SS:
Antibiofilm activity of kefir probiotic lactobacilli against
uropathogenic Escherichia coli (UPEC). Avicenna J Med Biotechnol.
12:221–229. 2020.PubMed/NCBI
|
|
37
|
Salminen S and Von Wright A: Lactic acid
bacteria: Microbiological and functional aspects. CRC Press; pp.
p6562004
|
|
38
|
Kumara SS, Bashisht A, Venkateswaran G,
Hariprasad P and Gayathri D: Characterization of novel
lactobacillus fermentum from curd samples of indigenous cows from
malnad region, Karnataka, for their aflatoxin B1 binding and
probiotic properties. Probiotics Antimicrob Proteins. 11:1100–1109.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vasudha M and Gayathri D: Kinetic and
modeling analyses of lactose-hydrolyzing β-galactosidase from
Lactiplantibacillus plantarum GV54. World Academy Sci J. 5:112023.
View Article : Google Scholar
|
|
40
|
Vasudha M, Prashantkumar CS, Bellurkar M,
Kaveeshwar V and Gayathri D: Probiotic potential of
β-galactosidase-producing lactic acid bacteria from fermented milk
and their molecular characterization. Biomed Rep. 18:232023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rashmi BS, Gayathri D, Vasudha M,
Prashantkumar CS, Swamy CT, Sunil KS, Somaraja PK and Prakash P:
Gluten hydrolyzing activity of Bacillus spp isolated from
sourdough. Microb Cell Fact. 19:1302020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vasiee A, Falah F, Behbahani BA and
Tabatabaee Yazdi F: Probiotic characterization of Pediococcus
strains isolated from Iranian cereal-dairy fermented product:
Interaction with pathogenic bacteria and the enteric cell line
Caco-2. J Biosci Bioeng. 130:471–479. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nantavisai K, Puttikamonkul S, Chotelersak
K and Taweechotipatr M: In vitro adhesion property and competition
against enteropathogens of Lactobacillus strains isolated from Thai
infants. Songklanakarin J Sci Technol. 40:69–74. 2018.
|
|
44
|
Schillinger U, Guigas C and Holzapfel WH:
In vitro adherence and other properties of lactobacilli used in
probiotic yoghurt-like products. Int Dairy J. 15:1289–1297. 2005.
View Article : Google Scholar
|
|
45
|
Lim SM and Ahn DH: Factors affecting
adhesion of lactic acid bacteria to Caco-2 cells and inhibitory
effect on infection of Salmonella typhimurium. J Microbiol
Biotechnol. 22:1731–1719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zommiti M, Bouffartigues E, Maillot O,
Barreau M, Szunerits S, Sebei K, Feuilloley M, Connil N and
Ferchichi M: In vitro assessment of the probiotic properties and
bacteriocinogenic potential of Pediococcus pentosaceus MZF16
isolated from artisanal Tunisian meat ‘Dried Ossban’. Front
Microbiol. 9:26072018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vasiee A, Behbahani BA, Tabatabaei Yazdi
F, Mortazavi SA and Noorbakhsh H: Diversity and probiotic potential
of lactic acid bacteria isolated from horreh, a traditional Iranian
fermented food. Probiotics Antimicrob. Proteins. 10:258–268.
2018.
|
|
48
|
Aslim B, Onal D and Beyatli Y: Factors
influencing autoaggregation and aggregation of Lactobacillus
delbrueckii subsp. bulgaricus isolated from handmade yoğurt. J Food
Prot. 70:223–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Khan AA and Cash P: E. coli and colon
cancer: Is mutY a culprit? Cancer Lett. 341:127–131. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chattopadhyay I, Dhar R, Pethusamy K,
Seethy A, Srivastava T, Sah R, Sharma J and Karmakar S: Exploring
the role of gut microbiome in colon cancer. Appl Biochem
Biotechnol. 193:1780–1799. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Licznerska K, Nejman-Faleńczyk B, Bloch S,
Dydecka A, Topka G, Gąsior T, Węgrzyn A and Węgrzyn G: Oxidative
stress in shiga toxin production by Enterohemorrhagic Escherichia
coli. Oxid Med Cell Longev. 2016:35783682016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xin J, Jiang X, Ben S, Yuan Q, Su L, Zhang
Z, Christiani DC, Du M and Wang M: Association between circulating
vitamin E and ten common cancers: Evidence from large-scale
Mendelian randomization analysis and a longitudinal cohort study.
BMC Med. 20:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Choi Y, Lee S, Kim S, Lee J, Ha J, Oh H,
Lee Y, Kim Y and Yoon Y: Vitamin E (α-tocopherol) consumption
influences gut microbiota composition. Int J Food Sci Nutr.
71:221–225. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang C, Zhao Y, Im S, Nakatsu C,
Jones-Hall Y and Jiang Q: Vitamin E delta-tocotrienol and
metabolite 13′-carboxychromanol inhibit colitis-associated colon
tumorigenesis and modulate gut microbiota in mice. J Nutr Biochem.
89:1085672021. View Article : Google Scholar : PubMed/NCBI
|