Introduction
Cardiovascular diseases (CVDs) are a series of heart
and vascular diseases, which cause a huge social and economic
burden worldwide. Acute myocardial infarction (AMI) is one
important type of CVD (1).
Mortality caused by CVD has been reported to account for 43.81 and
46.66% of total mortality in urban and rural areas respectively,
and the mortality rate of AMI in China has risen (2). AMI is one common type of coronary
heart disease, which is induced by acute and persistent ischemic
hypoxia and ultimately causes myocardial necrosis (3). Traditionally, AMI has been diagnosed
based on specific electrocardiogram (ECG) presentations and
elevated cardiac serum markers, including lactate dehydrogenase,
cardiac Troponin I (cTnI), cardiac muscle troponin T (cTnT) and
creatine kinase MB (CK-MB) (4,5).
However, the complexity of the condition of the patient, the onset
of acute pericarditis, myocarditis, heart failure, hypertension and
acute pulmonary embolism can also lead to elevated levels of
existing markers, which makes distinguishing AMI from these
conditions difficult (6,7). Only ST-segment elevation MI (STEMI)
can be detected by electrocardiography, while the infarcted vessels
cannot be predicted the first time. Although coronary angiography
is the most effective method to diagnose and treat AMI, it may also
be ineffective in certain cases where the infarcted vessels are
complex. Furthermore, some areas do not have sufficient access to
coronary angiography. This makes it difficult for a large
proportion of patients with AMI to receive the optimal treatment
(8). In summary, it is necessary
to develop more comprehensive clinical indicators to aid the
diagnosis and assessment of AMI as quickly as possible.
Exosomes (exos) are endogenously formed
extracellular vesicles that were first identified in the late 1960s
(9) and are considered
micro-vesicles with a diameter of 30–150 nm. Exos can be produced
by numerous types of cells and are widely present in the urine,
blood and saliva. Exos have a phospholipid bilayer structure and
the micro-vesicles are rich in biomolecules, such as proteins,
lipids, mRNA and non-coding RNA; therefore, exos can act as
messengers, which mediate intercellular communication and
participate in the regulation of cellular functions (10). Moreover, certain proteins have been
previously detected in exos, which demonstrate superiority in the
timeliness and specificity of different diseases compared with
traditional plasma markers, and these exos display great clinical
value by serving as novel biomarkers and therapeutic agents for
certain neoplastic diseases. For example, it has been shown that
ABCG1 and PTEN can be proteins in exosomes that delay the
progression of atherosclerosis (11,12).
In the study of cardiovascular system diseases, increasing
attention has been paid to the implications and functions of
exosomal microRNAs (miRNAs) (13).
miRNAs are a class of highly conserved small
non-coding RNAs, which widely exist in plasma or serum, either
bound to protein complexes or present in microvesicles or
lipoproteins (14–16). miRNAs mainly regulate genes by
binding to the 3′-UTR of mRNAs and interfering with subsequent
protein synthesis. Numerous miRNAs have been reported to have an
association with the pathogenesis of CVD (17,18).
It has been previously demonstrated that miRNA-133 (miR-133) widely
exists in cardiomyocytes, that the inhibition of miR-133 increases
cardiac hypertrophy and upregulation of miR-133 improves cardiac
function (19). miR-199a and
miR-590 have been reported to alleviate cardiac function after AMI
by inducing mitosis (20).
Furthermore, in terms of the therapeutic study of miRNAs,
inhibition of miR-92a reduces endothelial inflammation and promotes
angiogenesis and the functional recovery of ischemic myocardium
(21). It has been reported that
exosomal miRNA levels and corresponding disease scores, such as the
SYNTAX score, can be used to predict the complexity of coronary
lesions, which provides valuable references for the next step of
treatment (22,23).
The Wnt/β-catenin signaling pathway has been
reported to be reactivated after MI and is involved in the
regulation of pathophysiological processes, such as myocardial
apoptosis and myocardial fibrosis (24–26).
Secretory frizzled-related protein 1 (SFRP1) is a protein
structurally similar to the frizzled (FZ) receptor that inhibits
the binding of Wnt or FZ receptor/β-catenin signaling pathway
activity. It has been previously suggested that SFRP1 is closely
associated with myocardial fibrosis and cardiac remodeling, and it
is unclear whether its expression is affected by MI (27–33).
In conclusion, studies on AMI and exosomal miRNA are
rare; however, it is necessary to explore their association to
facilitate the clinical diagnosis and assessment of AMI. The aim of
the present study was to identify top differential miRNAs from
sequencing results, and to validate these miRNAs in AMI and healthy
control groups. The aim was also to identify new markers of AMI by
performing correlation analysis between exosomal miRNAs and
existing clinical indicators in AMI. Our previous study reported
the involvement of miRNAs carried by exosomes in the formation of
frontal atherosclerosis (34).
This study will investigate the value of miR-4516 and miR-203 in
aiding the diagnosis of acute myocardial infarction and predicting
the extent of vascular injury through sequencing analysis. These
will help in the future diagnosis and treatment in the clinic.
Materials and methods
Ethics approval
The present study was performed at The Central
Hospital Affiliated with Shandong First Medical University (Jinan,
China). Blood samples from patients with AMI and healthy controls
were collected from The Department of Cardiovascular Medicine and
The Emergency and Health Examination Center between October 2020
and May 2021. The present study was performed according to the
principles of the Declaration of Helsinki, and the study design was
approved by The Ethical Committee of The Central Hospital
Affiliated with Shandong First Medical University (approval no.
2018-039-01; Jinan, China).
Study participants
All participants in the present study were
volunteers and signed an informed consent form. Basic information
about the participants, including sex, age, disease situation and
other relevant personnel information were recorded. A total of 62
patients with AMI and 31 healthy controls were enrolled in the
present study. The diagnostic criteria for acute myocardial
infarction were defined by The Joint European Society of Cardiology
(35). The main AMI diagnostic
basis includes typical AMI symptoms and signs, abnormal ECG, the
level of serum biomarkers and coronary angiography results
(36).
Inclusion criteria
The inclusion criteria for selecting participants
were as follows: i) Aged 35–75 years old; ii) no history of smoking
or had quit smoking >3 months before the study; iii) chest pain
lasting >30 min with no relief. The time window was within 12 h
from the start of chest pain, including both acute ST elevation and
non-ST elevation MI, with samples collected from the AMI group
within 12 h of onset; iv) cTnT or cTnI >upper limit of a normal
level (99th percentile of the upper reference value) for at least
one-time point; v) patients with ≥70% stenosis of the pathogenic
vessel identified by coronary angiography, as this was required for
percutaneous coronary intervention (PCI); vi) no anticoagulants
administered; and vii) systolic blood pressure level was 90–140
mmHg, and diastolic blood pressure was <90 mmHg (patients with a
history of well-controlled hypertension were also enrolled). All
patients with acute myocardial infarction were given standard
treatment. The appropriate medical records were retrieved for all
participants.
Exclusion criteria
The exclusion criteria for selecting participants
for the present study were as follows: i) Presence of infection,
autoimmune disease, liver or kidney dysfunction, or history of
tumor; ii) aged <35 or >75 years old; iii) a blood glucose
level of >7 mmol/l; iv) systolic blood pressure <90 or
>140 mmHg and/or diastolic blood pressure >90 mmHg; and v)
psychiatric disorders (37).
SYNTAX score
SYNATX scores were assigned by assigning scores to
individual coronary vessels. The greater the sum of the scores, the
more severe the lesion. The complexity of coronary lesions was
quantitatively evaluated according to the integration system by two
or more experienced physicians according to anatomical
characteristics, such as lesion location, severity, bifurcation and
calcification of the left main coronary artery and/or three main
vessels (38).
Extraction of exos from blood
samples
A fasting blood sample (5 ml in a Becton, Dickinson
and Company EDTA anticoagulant tube) was collected from all members
of the healthy control group, and 5 ml of venous blood was
collected before emergency PCI from participants in the AMI group.
After centrifugation at 2,000 × g at 4°C for 10 min, plasma was
collected in 1.5 ml RNase-free tubes and stored at −80°C. After
16,260 × g centrifugation at 4°C for 45 min, the supernatant of the
plasma was collected and filtered using a 0.22 µm filter. The
filtered supernatant was transferred into an ultracentrifuge tube
and was centrifuged at 110,000 × g at 4°C for 120 min. At the end
of the first ultracentrifugation step, the supernatant was
aspirated, and the pellet was resuspended in 100 µl sterile PBS.
The aforementioned centrifugation parameters were repeated for
further centrifugation steps. After the second ultracentrifugation,
the supernatant was removed, and the pellet was resuspended in 100
µl PBS, transferred to a new RNase-free tube and stored in a −80°C
freezer.
Transmission electron microscopy (TEM)
and nanoparticle tracking analysis (NTA) examination
A total of 10 µl exo suspension solution was dropped
onto copper grids and subsequently transferred to 3% glutaraldehyde
solution for fixation for 30 min, followed by the addition of 4%
acetic acid oxygen dye solution and 1% methylcellulose solution,
for visualization under TEM (ht-7700; Hitachi, Ltd.). Dilute exos
obtained by ultracentrifugation were processed by NTA, to define
the particle size distribution and exo concentration of all
samples. Diluted exos obtained by ultracentrifugation were
processed by NTA, to define the particle size distribution and exo
concentration of all samples. The whole experiment was performed at
20–25°C.
Western blotting (WB)
RIPA lysis buffer (cat. no P0013B; Beyotime
Institute of Biotechnology) was used to extract proteins from the
exos previously isolated by ultracentrifugation. After ultrasonic
cell disruptor processing, the lysate was centrifuged at 16,260 × g
at 4°C for 5 min, and the supernatant was collected for
concentration quantification using a BCA assay kit. Loading buffer
was added to the samples, the samples were boiled for 10 min, and
then stored at −80°C. A total of 20 µg protein sample per well was
loaded on 10% sodium dodecyl sulfate-polyacrylamide gels. After
electrophoresis was completed, the separated proteins were
subsequently transferred onto a 0.22 µm PVDF membrane for 90 min at
70 V. The membranes were blocked with 5% non-fat milk powder
(diluted in TBST with 0.1% Tween 20) at room temperature. After
blocking, membranes were incubated with primary antibodies against
CD9 (1:1,000; cat. no. 20597-1-AP; Proteintech Group, Inc.), Alix
(1:1,000; cat. no. sc-53540; Santa Cruz Biotechnology, Inc.) and
calnexin (1:1,000; cat. no. 10427-2-AP; Proteintech Group, Inc.) as
a negative control, overnight at 4°C. The membranes were washed
three times using TBST and subsequently incubated with Anti-rabbit
IgG (1:20,000; cat. no. 7074; Cell Signaling Technology, Inc.) and
Anti-mouse IgG (1:20,000; cat. no. 7076; Cell Signaling Technology,
Inc.) secondary antibodies for 60 min at room temperature. The
chemiluminescence reagent was Immobilon Western (MilliporeSigma)
and Images were captured using a Tanon 5200 Multi Chemiluminescent
Imaging System.
Extraction of total RNA
Exo RNA was isolated using a column-based isolation
kit (cat. no. 217184; Qiagen China Co., Ltd.) according to the
manufacturer's instructions. Briefly, 1 ml of RNA lysate was added
to a 200 µl sample of exosomes, which was subsequently centrifuged
at 16,260 × g 4°C for 90 min, resuspended and precipitated,
centrifuged again and then isoacetone and anhydrous ethanol were
added in that order. Finally, the RNA was obtained by adding
enzyme-free water to resuspend the precipitate. The concentrations
were measured using a spectrophotometer(ND-ONE-WA30221; Thermo
Fisher Scientific, Inc.) at a wavelength of 260 nm.
Reverse transcription-quantitative
(q)PCR
Synthesis of complementary cDNA was performed using
an RT kit (cat. no. AG11717; Accurate Biology Inc.;) according to
the manufacturer's protocol. The total reaction volume was 20 µl,
which included 10 µl 2× miRNA RT Reaction Buffer, 2 µl miRNA RT
Enzyme Mix and 8 µl RNA. Thermocycling was performed as follows:
42°C for 60 min and 95°C for 3 min. Real-time fluorescent
quantitative PCR was performed on a QuanStudio 1 (Thermo Fish
Scientific, Inc.) with thermocycling conditions as follows: 95°C
for 30 sec for 1 cycle, and 35 cycles of denaturation at 95°C for 5
sec, annealing and extension at 60°C for 30 sec. This process was
repeated 3 times for each sample. qPCR was performed using the SYBR
Green PCR kit (cat. no. AG11702; Accurate Biology Inc.) according
to the manufacturer's protocol. The experiment was performed on a
Light Cycle 480 machine (Roche Diagnostics). Each experiment was
repeated three times and relative expression levels of miRNA were
analyzed using the 2−ΔΔCq method (11). U6 was used for normalization. For
RT-qPCR, a tailing method (addition of the poly(A) tail to the 3′
end of miRNA by a poly(A) polymerase to increase its length) was
used. A specific primer was used upstream and a universal primer
was used downstream. All miRNAs were purchased from Tiangen Biotech
Co., Ltd. The sequences of the primers used were as follows:
has-miR-203 forward (F), 5′-GUGAAAUGUUUAGGACCACUAG-3′; has-miR-4516
F, 5′-GGGAGAAGGGUCGGGGC-3′; and hsa-U6- F, 5′-CTCGCTTCGGCAGCACA-3′
and reverse 5′-AACGCTTCACGAATTTGCGT-3′. The miRNA reverse primer
was included in the RT-qPCR kit (cat. no. AG11717; Accurate Biology
Inc.).
Bioinformatics analysis
Target genes of differentially expressed miRNAs were
predicted using TargetScan 8.0 software (https://www.targetscan.org/vert_80/). Kyoto
Encyclopedia of Genes and Genomes (https://www.kegg.jp/) enrichment analyses were
performed using the David 6.8 online database (https://david.ncifcrf.gov). The ggplot2 package
(version 3.3.5) (https://cran.r-project.org/web/packages/ggplot2/)
in R (version 4.0.5) (https://www.r-project.org/) was used to plot bar and
Venn diagrams for visualization.
ELISA
Plasma was obtained by centrifuging blood samples at
2,000 × g at 4°C for 10 min and was then stored at −80°C. A Human
SFRP1 ELISA kit (cat. no. SEF880Hu; Cloud-Clone Corp.) was used to
assess the SFRP1 secretion level in each individual, according to
the manufacturer's protocols. The signal was measured at 450 nm
excitation light using a SpectraMax® i3 multifunctional
microplate reader. After the standard curve was established, the
plasma expression levels of SFRP1 were calculated based on the
absorbance values of each sample.
Statistical analysis
Mean ± standard deviation was used to present
relative clinical characteristics. Ordinal variables were compared
using one-way ANOVA. The statistical significance of normally
distributed data was assessed with the unpaired Student's t-test.
Pearson's correlation coefficient was used to validate the
relationships between continuous variables. Independent factor risk
analysis was performed using logistic regression. All experiments
were repeated ≥3 times. All statistical analyses were performed
using SPSS software (version 22.0; IBM Corp.) and GraphPad Prism
8.0 (GraphPad Software; Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics of the study
population
The baseline characteristics of the 31 healthy
control individuals and the 62 patients with AMI were summarized
(Table I). Unpaired Student's
t-test was used to compare the differences between the AMI and
control groups. In parallel, drug use data was collected from both
groups (Table II). There were no
statistically significant differences in these characteristics
between the two groups, except for total cholesterol).
 | Table I.Baseline data of the study
participants. |
Table I.
Baseline data of the study
participants.
| Baseline
characteristic | Control, mean ±
SD | AMI, mean ± SD | P-value |
|---|
| Age, years | 52.81±7.6 | 62.02±9.46 | 0.058 |
| HR, beats/min | 74.06±11.6 | 76.76±13.02 | 0.379 |
| SBP, mmHg | 105.03±9.83 | 125.21±19.58 | 0.086 |
| DBP, mmHg | 79.19±9.77 | 75.73±14.34 | 0.277 |
| Cr, µmol/l | 75.1±15.73 | 77.97±15.44 | 0.289 |
| Glu, mmol/l | 5.19±0.74 | 5.59±1.14 | 0.054 |
| TG, mmol/l | 1.49±0.63 | 1.49±0.73 | 0.917 |
| TC, mmol/l | 3.96±1.04 | 4.51±1.03 | 0.022 |
| LDL, mmol/l | 2.69±1.08 | 2.71±0.81 | 0.985 |
| HDL, mmol/l | 1.21±0.4 | 1.18±0.47 | 0.676 |
| Apolipoprotein a,
mmol/l | 231.83±291.8 | 330.56±304.14 | 0.144 |
| Apolipoprotein B,
mmol/l | 1.01±0.25 | 1±0.17 | 0.671 |
| Apolipoprotein E,
mmol/l | 46.63±12.61 | 51.59±16.35 | 0.185 |
 | Table II.Summary of medication use. |
Table II.
Summary of medication use.
| Drugs/drug groups
(Postoperative) | Control (n=31) | AMI (n=62) |
|---|
| Aspirin, n | 12 | 39 |
| Tegretol, n | NA | NA |
| Statins, n | 4 | 10 |
| Beta blockers,
n | 3 | 22 |
| Proton pump
inhibitors, n | NA | 20 |
| Isosorbide
mononitrate, n | NA | 2 |
| ACEI/ARB, n | 3 | 40 |
| Trimetazidine,
n | NA | NA |
| CCB, n | NA | 30 |
| Clopidogrel, n | NA | 25 |
| Diuretics, n | NA | 20 |
| Ivabradine, n | NA | 2 |
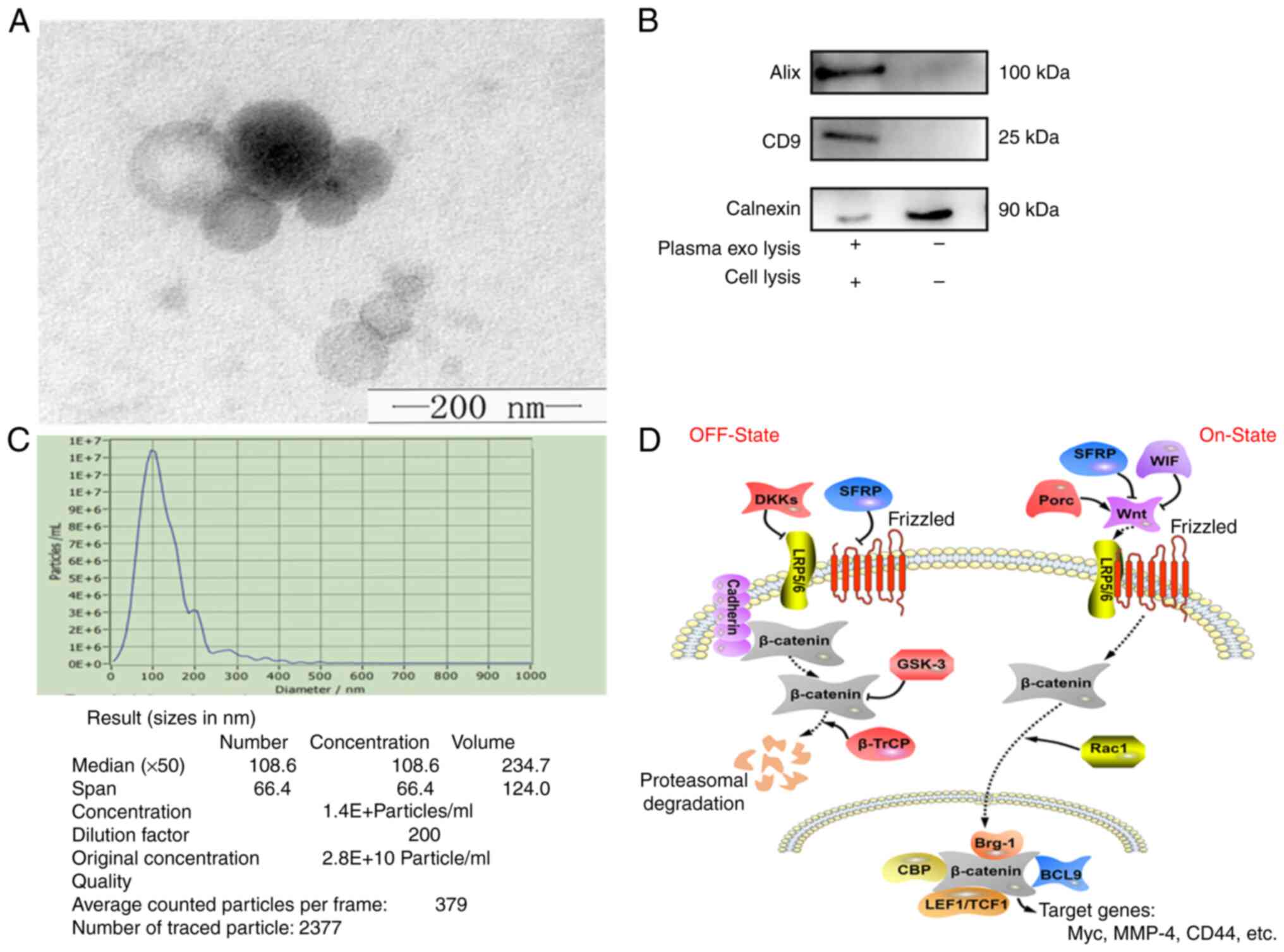
Characterization of exos
Previously published reports and the standards of
the International Society for Exosome Vesicles indicate that
verification of exosomes can be performed using transmission
electron microscopy, WB and particle size analysis, simultaneously
(34,39). The size and morphology of exosomes
can be objectively observed using transmission electron microscopy.
Particle size analysis allows the size, diameter and concentration
of exosomes to be assessed (40).
The typical disc-like structure of exos was observed using TEM,
which was a direct observation of plasma exosomes under TEM, whose
shape and size met the criteria for exosomes (Fig. 1A). WB allowed verification of
specific proteins on the surface of exosomes, such as CD9 and ALIX.
Most common cell membranes contain Calnexin proteins; however, exos
do not have Calnexin proteins on their membranes. Therefore, cell
lysis was chosen as a control group. The identity of the exos was
further confirmed by WB using antibodies against the exo-specific
markers, Alix and CD9, and the negative control, calnexin (Fig. 1B). NTA results demonstrated that
the average diameter of the isolated granule was 127.5 nm, with a
median diameter of 117.7 nm (Fig.
1C), which was in-line with the standard size of exos. These
results confirmed that exos were successfully extracted at a high
quality and purity. The regulatory relationship between SFRP1 and
the Wnt/β-Catenin signaling pathways, which serve a vital role in
atherosclerosis or AMI was illustrated (Fig. 1D) (41,42).
Expression levels of exosomal
miR-4516, miR-203 and plasma SFRP1 in each study group
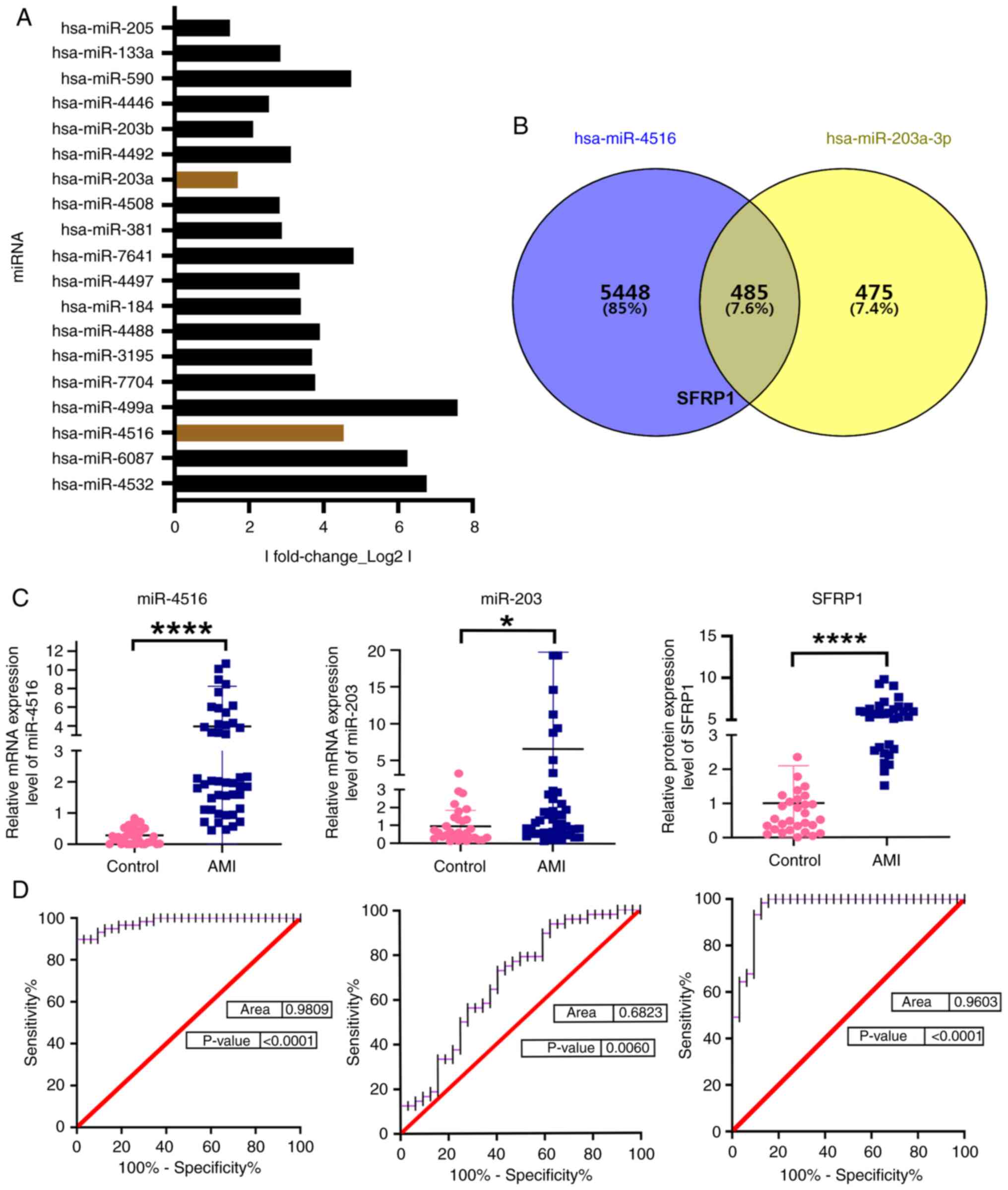
Previous sequencing results were analyzed and the
top 10 miRNAs with high variance ranking were selected for
validation (Fig. 2A) (34). SFRP1 was predicted as the target
protein for both miR-4516 and miR-203 using TargetScan software
(Fig. 2B). Statistical analysis
was performed using an unpaired Student's t-test. The levels of
miR-4516 and miR-203 in plasma exos from the AMI group were
significantly higher compared with those in the healthy control
group. In addition, the SFRP1 protein expression level was
significantly increased in the plasma of patients with AMI compared
with the control group (Fig. 2C
and Table III, Table IV, Table V). These data indicated that
exosomal miR-4516, miR-203 and plasma SFRP1 were associated with
AMI.
 | Table III.SFRP1 expression levels in AMI and
control groups. |
Table III.
SFRP1 expression levels in AMI and
control groups.
|
|
|
|
| 95% Confidence
interval |
|---|
|
|
|
|
|
|
|---|
| Group | Standard error | Mean deviation | P-value | Lower | Upper |
|---|
| Control
(miR-4516) | 4.284 | 0.272 | 0.001 | 0.176 | 0.368 |
| AMI (miR-4516) | 0.270 | 3.871 | 0.001 | 2.640 | 5.102 |
| Control
(miR-203) | 3.578 | 2.387 | 0.001 | 1.359 | 3.415 |
| AMI (miR-203) | 1.171 | 1.050 | 0.001 | 0.659 | 1.440 |
| Control
(SFRP1) | 1.802 | 4.657 | 0.001 | 4.196 | 5.119 |
| AMI (SFRP1) | 1.111 | 1.017 | 0.001 | 0.610 | 1.425 |
 | Table IV.MiR-203 expression levels in AMI and
control groups. |
Table IV.
MiR-203 expression levels in AMI and
control groups.
|
|
|
|
| 95% Confidence
interval |
|---|
|
|
|
|
|
|
|---|
| Group | Standard error | Mean deviation | P-value | Lower | Upper |
|---|
| Control | 3.578 | 2.387 | 0.001 | 1.359 | 3.415 |
| AMI | 1.171 | 1.050 | 0.001 | 0.659 | 1.440 |
 | Table V.Secretory frizzled-related protein
1expression levels in AMI and control groups. |
Table V.
Secretory frizzled-related protein
1expression levels in AMI and control groups.
|
|
|
|
| 95% Confidence
interval |
|---|
|
|
|
|
|
|
|---|
| Group | Standard error | Mean deviation | P-value | Lower | Upper |
|---|
| Control | 1.802 | 4.657 | 0.001 | 4.196 | 5.119 |
| AMI | 1.110 | 1.017 | 0.001 | 0.610 | 1.425 |
Exosomal miR-4516, miR-203 and plasma
SFRP1 levels as diagnostic biomarkers for AMI
The area under the curve (AUC) of exosomal miR-4516
and miR-203 were 0.9809 (P<0.0001) and 0.6823 (P=0.006),
respectively, and the AUC of plasma SFRP1 was 0.9603 (P<0.0001)
in the AMI group, which indicated that exosomal miR-4516, miR-203
and plasma SFRP1 may be candidate diagnostic biomarkers for AMI
(Fig. 2D).
Positive correlation between plasma
exosomal miR-4516 and SYNTAX score of patients with AMI
After collecting coronary angiography images from
each participant, the images were assessed using the SYNTAX scoring
system (Fig. 3A). Plasma exosomal
miR-4516 levels were significantly positively correlated with the
SYNTAX scores of patients with AMI (r2=0.7875;
P<0.0001) using Pearson's correlation analysis (Fig. 3B). However, there was no
significant correlation between either the exosomal miR-203 or
plasma SFRP1 levels and the SYNTAX score in the AMI group
(r2=−0.0145, P=0.9461 and r2=−0.229,
P=0.6572, respectively).
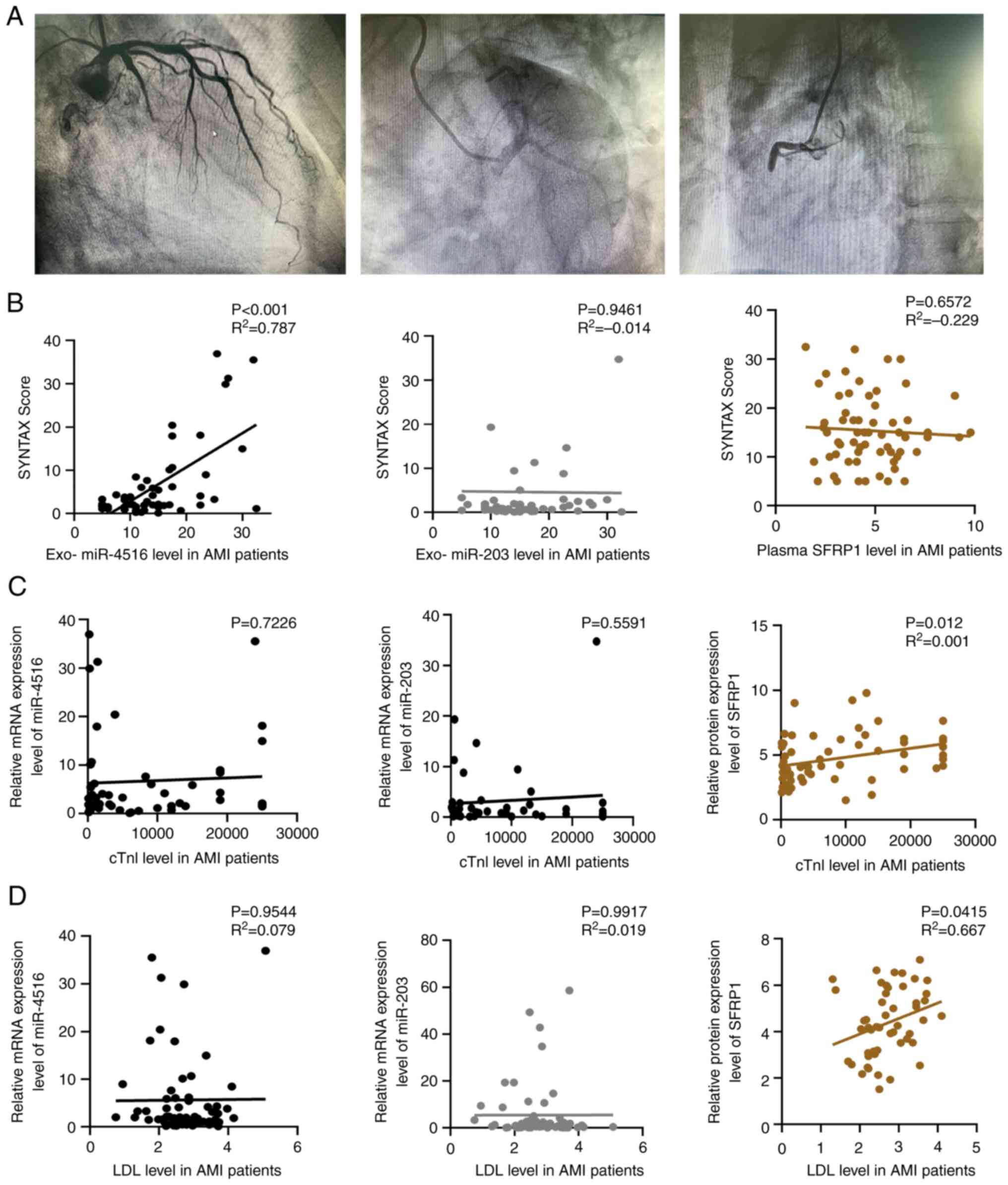 | Figure 3.Correlation of plasma exosomal
miR-4516, miR-203 and SFRP1 levels with SYNATX scores. (A)
Representative images from a coronary angiography procedure. The
three images show (left to right) occlusion of the gyral, anterior
descending and right coronary branches of the left coronary artery,
respectively. The degree of damage to the vessel was predicted
based on the blockage and was assessed using the SYNATX score. (B)
Correlation analysis of plasma exosomal miR-4516, miR-203 and SFRP1
levels with the SYNATX score of patients with AMI. Correlation of
plasma exosomal miR-4516, miR-203 and SFRP1 levels with (C) cTnI
and (D) LDL in patients with AMI. AMI, acute myocardial infarction;
cTnI, cardiac troponin I; exo, exosome; LDL, low-density
lipoprotein; miR, microRNA; SFRP1, secretory frizzled-related
protein 1. |
Plasma SFRP1 is positively correlated
with plasma low-density lipoprotein (LDL) levels in patients with
AMI
DBP and LDL of the participants correlated with
plasma exosomal miR-4516, miR-203 and SFRP1 levels (Table VI). There was no correlation
between the classical marker, cTnI, and plasma exosomal miR-4516,
miR-203 and SFRP1 levels (Fig.
3C). Plasma SFRP1 was positively correlated with serum LDL
levels (r2=0.667; P=0.0415); however, there was no
correlation between plasma exosomal miR-4516 or miR-203 and serum
LDL levels (Fig. 3D). A logistic
regression analysis of common risk factors for coronary artery
disease was performed.
 | Table VI.Correlations between serum exosomal
miR-4516, miR-203 and SRFP1 levels and traditional risk factors of
cardiovascular diseases in AMI patients. |
Table VI.
Correlations between serum exosomal
miR-4516, miR-203 and SRFP1 levels and traditional risk factors of
cardiovascular diseases in AMI patients.
|
|
| Exosomal
miR-4516 | Exosomal
miR-203 | SRFP1 |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Value | Control | AMI | Control | AMI | Control | AMI |
|---|
| Age, years | P | 0.802 | 0.918 | 0.746 | 0.994 | 0.691 | 0.175 |
|
| R2 | −0.051 | −0.015 | 0.160 | 0.007 | 0.573 | −0.025 |
| HR, beats/min | P | 0.492 | 0.572 | 0.374 | 0.434 | 0.361 | 0.775 |
|
| R2 | −0.189 | −0.109 | 0.5876 | −0.103 | 0.175 | 0.005 |
| SBP, mmHg | P | 0.590 | 0.222 | 0.081 | 0.076 | 0.003 | 0.984 |
|
| R2 | 0.206 | 0.342 | −1.567 | 0.359 | 0.737 | 0.001 |
| DBP, mmHg | P | 0.420 | 0.842 | 0.842 | 0.014 | 0.616 | 0.804 |
|
| R2 | 0.193 | 0.042 | 0.115 | 0.376 | −0.843 | 0.003 |
| Cr, µmol/l | P | 0.051 | 0.172 | 0.871 | 0.694 | 0.213 | 0.732 |
|
| R2 | −0.706 | 0.313 | −0.145 | 0.066 | 0.321 | −0.004 |
| Glu, mmol/l | P | 0.223 | 0.051 | 0.365 | 0.128 | 0.986 | 0.663 |
|
| R2 | 0.021 | −0.032 | 0.037 | 0.018 | −0.002 | 0.091 |
| TG, mmol/l | P | 0.867 | 0.085 | 0.835 | 0.933 | 0.374 | 0.731 |
|
| R2 | 0.002 | 0.018 | 0.007 | 0.001 | −0.091 | 0.118 |
| TC, mmol/l | P | 0.378 | 0.980 | 0.609 | 0.940 | 0.075 | 0.180 |
|
| R2 | 0.021 | 0.001 | −0.029 | 0.001 | 0.296 | 0.319 |
| LDL, mmol/l | P | 0.939 | 0.954 | 0.446 | 0.991 | 0.934 | 0.041 |
|
| R2 | −0.001 | 0.001 | −0.046 | 0.091 | −0.014 | 0.677 |
| HDL, mmol/l | P | 0.847 | 0.306 | 0.212 | 0.590 | 0.761 | 0.977 |
|
| R2 | −0.001 | −0.007 | −0.028 | 0.003 | −0.02 | 0.012 |
| Apolipoprotein
a | P | 0.592 | 0.223 | 0.476 | 0.193 | 0.554 | 0.092 |
|
| R2 | −3.757 | 0.005 | 0.119 | −0.042 | −0.29 | 0.001 |
| Apolipoprotein
B | P | 0.817 | 0.855 | 0.533 | 0.902 | 0.747 | 0.111 |
|
| R2 | 0.001 | −0.004 | −0.008 | 0.002 | −0.013 | 0.028 |
| Apolipoprotein
E | P | 0.259 | 0.489 | 0.828 | 0.994 | 0.311 | 0.952 |
|
| R2 | 0.336 | 0.17 | 0.156 | 0.001 | −0.211 | 0.001 |
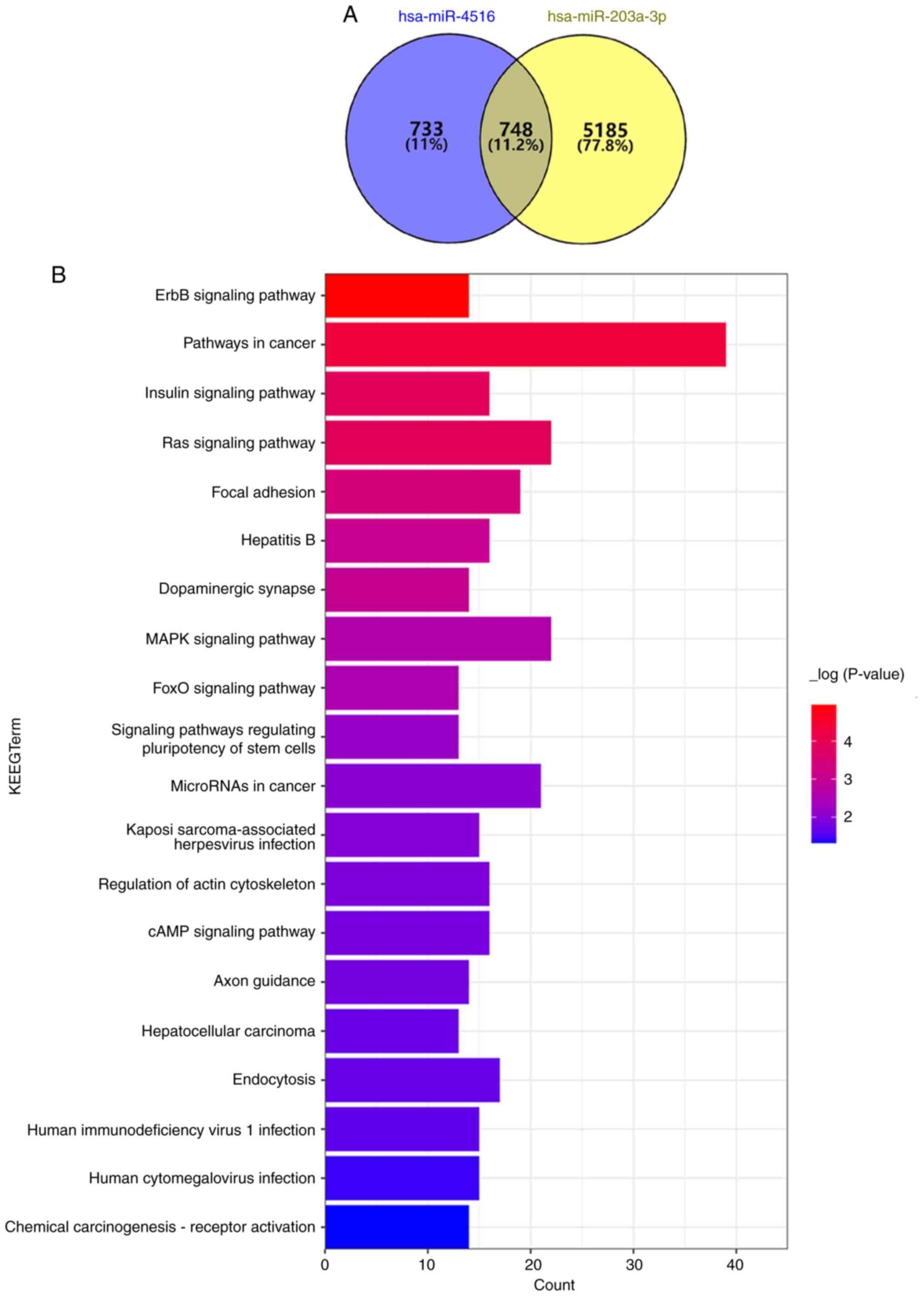
miR-4516 and miR-203 are associated
with adhesion
The KEGG enrichment analysis demonstrated that
miR-4516 and miR-203 shared 748 enrichment pathways, and that
miR-4516 and miR-203 were associated with adhesion function in the
enriched pathways (Fig. 4A and
B).
Discussion
CVD remains one of the leading causes of death
worldwide, and AMI is the most aggressive and problematic type of
CVD (1). With health education and
the continuous innovation of testing equipment, there is an
increased awareness of early diagnosis and early treatment of AMI
(43). Survival rates for AMI are
gradually improving in developed cities, However, the relative lack
of diagnostic capacity for AMI in less developed regions and the
poor access to treatment increase the mortality rate of patients
with AMI. Classical plasma markers of AMI, such as cTnI and CK-MB,
are becoming less specific and have certain drawbacks in clinical
diagnosis. This is due to multiple co-morbidities, such as
myocarditis, heart failure and pulmonary heart disease, which also
cause elevations in these markers (44). Exos are the products of cellular
secretion and are found in large numbers in various bodily fluids
and reflect intracellular status in real time. Previous studies
have reported that the miRNAs transported in exos are of
significant value in various CVDs. For example, miR-133, miR-146,
miR-499 and miR-26a in plasma can be used as potential diagnostic
markers for AMI (45).
In the present study, TargetScan predicted that
SFRP1 may be a target protein for miR-4516 and miR-203. The results
of the present study demonstrated that the expression levels of the
three indicators were significantly elevated in the AMI group
compared with the control, which prompted the hypothesis of a
relationship between the three indicators and AMI. Pearson's
correlation analysis further demonstrated that exosomal levels of
miR-4516 and miR-203 and plasma SFRP1 levels were significantly
correlated with AMI.
Coronary angiography can not only determine whether
a coronary artery is blocked and the extent of obstruction, but
also support the selection of a protocol for the next step of
treatment. SYNTAX scores can be used to quantitatively evaluate the
complexity of coronary lesions and provide a preliminary judgment,
which can inform the choice of the surgical procedure according to
a specific scoring system based on anatomical characteristics, such
as coronary lesion location, severity, bifurcation and
calcification. In addition, patients with MI often have multiple
co-morbidities, such as heart failure, anemia or even uremia at an
advanced age. Both ST-segment elevation myocardial infarction and
non-ST-segment elevation myocardial infarction have corresponding
blocked blood vessels, which can be identified on late coronary
angiography. Where this occurs, it would be advantageous if the
severity of the lesioned vessel could be determined as early as
possible in conjunction with the SYNTAX score. Early assessment of
the severity of the blockage in the vessel can predict the severity
of damage to the heart muscle. For example, if the blockage is
small, thrombolytic therapy may be an immediate option. However, if
the blockage is severe and combined with chronic occlusion of
multiple vessels, cardiac bypass surgery may be the immediate
treatment of choice (46,47). In the present study, the
relationship between the expression levels of exosomal miR-4516,
miR-203 and SFRP1 in the AMI group with their SYNTAX scores were
analyzed. Correlation analysis demonstrated that only miR-4516 was
significantly positively correlated with the SYNTAX score of
patients with AMI, and that neither miR-203 nor SFRP1 were
correlated with SYNTAX score (48,49).
These data indicated that exosomal miR-4516 may be used as a
non-invasive means of predicting the lesion complexity of coronary
vessels in patients with AMI, providing an initial basis for the
next treatment step (PCI or coronary artery bypass grafting). The
elevation of LDL in plasma is considered to be a major risk factor
for atherosclerosis, and the most direct cause of AMI is the
rupture of the atherosclerotic plate. Notably, in the present
study, Pearson's correlation analysis demonstrated that SFRP1
protein expression levels were significantly correlated with LDL.
After statistical analysis, no correlation was demonstrated between
SFRP1 mRNA expression levels and SYNTAX scores, but the AMI group
had significantly higher SFRP1 protein expression levels compared
with the control group. Statistical analysis demonstrated that
SFRP1 protein expression levels were significantly correlated with
cTNl. As SFRP1 is a key protein in the WNT signaling pathway, it
was hypothesized that SFRP1 may be positively correlated with the
severity of cardiomyocyte injury and angiogenesis (42). In addition to this, based on the
results of the present study, it was hypothesized that SFRP1 may be
involved in lipid metabolism. However, for reasons of time, further
validation was not performed.
Both miRNAs, miR-4516 and miR-203, were predicted by
bioinformatic analysis to be closely associated with adhesion and
endocytosis. The present study could not distinguish between STEMI
and non-STEMI as the subsequent treatment regimens for these two
types of AMI are different. Moreover, the detection of miRNA in
plasma exos was quite time-consuming. The time must be decreased to
facilitate use in a clinical setting. The main reason for this is
that the process of exo extraction takes about one hour. SYNTAX 2
scores combine the clinical variables of the patient, such as sex,
age, left ventricular ejection fraction, creatinine clearance and
other information, which allow accurate evaluation of the condition
of the patient (38). Therefore,
combination of the difference in exosomal miRNA and SYNTAX 2 scores
facilitates a more personalized treatment plan for patients.
The present study had limitations including that at
the time of diagnosis of AMI, all patients had the necessary tests
for cTnl and ECG. Obtaining the results of tests such as CK-MB and
BNP was slow compared with cTnl, and we did not consider these as a
necessary test considering that it would delay the patient's
treatment; therefore, the data for these two indicators are
relatively incomplete. The plasma samples collected were of human
origin and as such the composition of the plasma was complex and,
even if some of the more biased samples are excluded, the
dispersion of some of the samples was still large, this was also a
limitation of the present study. In addition to this, another
limitation of the present study was the lack of data and survival
information related to the later stages of recovery from myocardial
infarction in patients. Therefore, no relevant prognostic and
survival analysis were performed for this.
In conclusion, a combination of miR-4516, miR-203
and SFRP1 may help in the diagnosis of AMI and in the evaluation of
the degree of coronary stenosis. Furthermore, the present study
demonstrated a significant correlation between plasma SFRP1 protein
expression levels and LDL levels. These results may provide novel
diagnostic markers for AMI, which may contribute to the timely
diagnosis and treatment of AMI.
Acknowledgements
The authors would like to thank Dr Yan Zheng
(Research Center of Translational Medicine, Central Hospital
Affiliated to Shandong First Medical University, Jinan, Shandong,
China) for their technical assistance.
Funding
This work was supported by the Natural Science Foundation of
Shandong Province (grant no. ZR202103050087).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL, KL and SW designed the present study, searched
databases, extracted and assessed the literature and drafted the
manuscript. PL, JT, YY, LW and YM statistically analyzed the data.
YY and LW confirm the authenticity of all the raw data. PL, FD and
GS conceived and designed the present study, provided general
supervision and finalized the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Jinan Central Hospital (approval no. 2018-039-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao D, Liu J, Wang M, Zhang X and Zhou M:
Epidemiology of cardiovascular disease in China: Current features
and implications. Nat Rev Cardiol. 16:203–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma LY, Chen WW, Gao RL, Liu LS, Zhu ML,
Wang YJ, Wu ZS, Li HJ, Gu DF, Yang YJ, et al: China cardiovascular
diseases report 2018: An updated summary. J Geriatr Cardiol.
17:1–8. 2020.PubMed/NCBI
|
|
3
|
Bhatnagar P, Wickramasinghe K, Wilkins E
and Townsend N: Trends in the epidemiology of cardiovascular
disease in the UK. Heart. 102:1945–1952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rozenman Y and Gotsman MS: The earliest
diagnosis of acute myocardial infarction. Annu Rev Med. 45:31–44.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menown IB, Allen J, Anderson JM and Adgey
AA: ST depression only on the initial 12-lead ECG: Early diagnosis
of acute myocardial infarction. Eur Heart J. 22:218–227. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SJ, Kim MH, Lee KM, Kim TH, Choi SY,
Son MK, Park JW and Serebruany VL: Troponin I and D-dimer for
discriminating acute pulmonary thromboembolism from myocardial
infarction. Cardiology. 136:222–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roongsritong C, Warraich I and Bradley C:
Common causes of troponin elevations in the absence of acute
myocardial infarction: Incidence and clinical significance. Chest.
125:1877–1884. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Waters RE II, Singh KP, Roe MT, Lotfi M,
Sketch MH Jr, Mahaffey KW, Newby LK, Alexander JH, Harrington RA,
Califf RM and Granger CB: Rationale and strategies for implementing
community-based transfer protocols for primary percutaneous
coronary intervention for acute ST-segment elevation myocardial
infarction. J Am Coll Cardiol. 43:2153–2159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang YT, Huang YY, Zheng L, Qin SH, Xu XP,
An TX, Xu Y, Wu YS, Hu XM, Ping BH and Wang Q: Comparison of
isolation methods of exosomes and exosomal RNA from cell culture
medium and serum. Int J Mol Med. 40:834–844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Sun Y, Lin X, Zhang D, Hu C, Liu J,
Zhu Y, Gao A, Han H, Chai M, et al: Perivascular adipose-derived
exosomes reduce macrophage foam cell formation through miR-382-5p
and the BMP4-PPARγ-ABCA1/ABCG1 pathways. Vascul Pharmacol.
143:1069682022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang
L and Wang Y: Exosomes from nicotine-stimulated macrophages
accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC
migration and proliferation. Theranostics. 9:6901–6919. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turchinovich A, Weiz L, Langheinz A and
Burwinkel B: Characterization of extracellular circulating
microRNA. Nucleic Acids Res. 39:7223–7233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Ren S, Xia J, Wei Y and Xi Y:
EIF4A3-Induced circ-BNIP3 aggravated hypoxia-induced injury of H9c2
cells by targeting miR-27a-3p/BNIP3. Mol Ther Nucleic Acids.
19:533–545. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Muhtaresh HA, Salem AH and Al-Kafaji G:
Upregulation of circulating cardiomyocyte-enriched miR-1 and
miR-133 associate with the risk of coronary artery disease in type
2 diabetes patients and serve as potential biomarkers. J Cardiovasc
Transl Res. 12:347–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren J, Zhang J, Xu N, Han G, Geng Q, Song
J, Li S, Zhao J and Chen H: Signature of circulating microRNAs as
potential biomarkers in vulnerable coronary artery disease. PLoS
One. 8:e807382013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiese CB, Zhong J, Xu ZQ, Zhang Y, Ramirez
Solano MA, Zhu W, Linton MF, Sheng Q, Kon V and Vickers KC: Dual
inhibition of endothelial miR-92a-3p and miR-489-3p reduces renal
injury-associated atherosclerosis. Atherosclerosis. 282:121–131.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stone GW, Sabik JF, Serruys PW, Simonton
CA, Généreux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM
III, et al: Everolimus-eluting stents or bypass surgery for left
main coronary artery disease. N Engl J Med. 375:2223–2235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suh YJ, Hong YJ, Lee HJ, Hur J, Kim YJ,
Lee HS, Hong SR, Im DJ, Kim YJ, Park CH, et al: Prognostic value of
SYNTAX score based on coronary computed tomography angiography. Int
J Cardiol. 199:460–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Almeida M, Weinstein RS, O'Brien
CA, Manolagas SC and Jilka RL: Skeletal inflammation and
attenuation of Wnt signaling, Wnt ligand expression, and bone
formation in atherosclerotic ApoE-null mice. Am J Physiol
Endocrinol Metab. 310:E762–E773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Torres VI, Godoy JA and Inestrosa NC:
Modulating Wnt signaling at the root: Porcupine and Wnt acylation.
Pharmacol Ther. 198:34–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L and Wrana JL: The emerging role of
exosomes in Wnt secretion and transport. Curr Opin Genet Dev.
27:14–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y and Mlodzik M: Wnt-Frizzled/planar
cell polarity signaling: Cellular orientation by facing the wind
(Wnt). Annu Rev Cell Dev Biol. 31:623–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsutsumi N, Mukherjee S, Waghray D, Janda
CY, Jude KM, Miao Y, Burg JS, Aduri NG, Kossiakoff AA, Gati C and
Garcia KC: Structure of human Frizzled5 by fiducial-assisted
cryo-EM supports a heterodimeric mechanism of canonical Wnt
signaling. Elife. 9:e584642020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge
G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, et al:
Secreted Frizzled-related protein 2 is a procollagen C proteinase
enhancer with a role in fibrosis associated with myocardial
infarction. Nat Cell Biol. 11:46–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barandon L, Casassus F, Leroux L, Moreau
C, Allières C, Lamazière JM, Dufourcq P, Couffinhal T and Duplàa C:
Secreted frizzled-related protein-1 improves postinfarction scar
formation through a modulation of inflammatory response.
Arterioscler Thromb Vasc Biol. 31:e80–e87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sassi Y, Avramopoulos P, Ramanujam D,
Grüter L, Werfel S, Giosele S, Brunner AD, Esfandyari D,
Papadopoulou AS, De Strooper B, et al: Cardiac myocyte miR-29
promotes pathological remodeling of the heart by activating Wnt
signaling. Nat Commun. 8:16142017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Zheng X, Zhang C, Zhang C and Bu P:
Lcz696 alleviates myocardial fibrosis after myocardial infarction
through the sFRP-1/Wnt/β-catenin signaling pathway. Front
Pharmacol. 12:7241472021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu P, Wang S, Wang G, Zhao M, Du F, Li K,
Wang L, Wu H, Chen J, Yang Y and Su G: Macrophage-derived exosomal
miR-4532 promotes endothelial cells injury by targeting SP1 and
NF-κB P65 signalling activation. J Cell Mol Med. 26:5165–5180.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alpert JS, Thygesen K, Antman E and
Bassand JP: Myocardial infarction redefined-a consensus document of
the joint european society of cardiology/American college of
cardiology committee for the redefinition of myocardial infarction.
J Am Coll Cardiol. 36:959–969. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saraiva JFK and Franco D: Oral GLP-1
analogue: Perspectives and impact on atherosclerosis in type 2
diabetic patients. Cardiovasc Diabetol. 20:2352021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Biagini A, Testa R, Carpeggiani C,
Andreotti F, Mazzei MG, Emdin M and L'Abbate A: Detection of
spontaneous episodes in post-infarction angina. Comparison between
CCU and Holter monitoring. Eur Heart J. 7 (Suppl 3):S43–S46. 1986.
View Article : Google Scholar
|
|
38
|
Kundu A, Sardar P, O'Day K, Chatterjee S,
Owan T and Dawn Abbott J: SYNTAX score and outcomes of coronary
revascularization in diabetic patients. Curr Cardiol Rep.
20:282018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu X, Rong PZ, Song MS, Shi ZW, Feng G,
Chen XJ, Shi L, Wang CH and Pang QJ: lncRNA SNHG1 induced by SP1
regulates bone remodeling and angiogenesis via sponging miR-181c-5p
and modulating SFRP1/Wnt signaling pathway. Mol Med. 27:1412021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mafakher L, Rismani E, Rahimi H,
Enayatkhani M, Azadmanesh K and Teimoori-Toolabi L: Computational
design of antagonist peptides based on the structure of secreted
frizzled-related protein-1 (SFRP1) aiming to inhibit Wnt signaling
pathway. J Biomol Struct Dyn. 40:2169–2188. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu L, Liu F, Xie H and Feng J: Diagnostic
performance of microRNA-133a in acute myocardial infarction: A
meta-analysis. Cardiol J. 25:260–267. 2018.PubMed/NCBI
|
|
44
|
Xing X, Guo S, Zhang G, Liu Y, Bi S, Wang
X and Lu Q: miR-26a-5p protects against myocardial
ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT
signaling pathway. Braz J Med Biol Res. 53:e91062020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xin Y, Yang C and Han Z: Circulating
miR-499 as a potential biomarker for acute myocardial infarction.
Ann Transl Med. 4:1352016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duijvesz D, Luider T, Bangma CH and
Jenster G: Exosomes as biomarker treasure chests for prostate
cancer. Eur Urol. 59:823–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin B, Tian T, Lu Y, Liu D, Huang M, Zhu
L, Zhu Z, Song Y and Yang C: Tracing tumor-derived exosomal PD-L1
by dual-aptamer activated proximity-induced droplet digital PCR.
Angew Chem Int Ed Engl. 60:7582–7586. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Thuijs DJFM, Kappetein AP, Serruys PW,
Mohr FW, Morice MC, Mack MJ, Holmes DR Jr, Curzen N, Davierwala P,
Noack T, et al: Percutaneous coronary intervention versus coronary
artery bypass grafting in patients with three-vessel or left main
coronary artery disease: 10-Year follow-up of the multicentre
randomised controlled SYNTAX trial. Lancet. 394:1325–1334. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Duttagupta S, Thachathodiyl R, Rameshan A,
Venkatachalam A, Georgy S, Ts D and Menon J: Effectiveness of
Framingham and ASCVD risk scores in predicting coronary artery
disease-a comparative study with syntax score. J Assoc Physicians
India. 69:11–12. 2022.PubMed/NCBI
|