Introduction
Renal fibrosis is the final common pathological
feature of most forms of kidney disease, leading to irreversible
renal function impairment (1–4). Src
kinase belongs to the non-receptor tyrosine kinase family.
Activated Src has been implicated in the regulation of numerous
intracellular signaling cascades (e.g., MAPK, PI3K and protein
kinase C) and cellular processes such as cell proliferation,
differentiation, migration and invasion (5–8).
Among the numerous kinases that function as signaling hubs, Src has
a critical role in renal fibrosis through the integration of
multiple fibrogenic signal inputs (9,10).
In addition to its well-known ion pump function, Na/K-ATPase has
been identified as an important regulator of Src activation through
the formation of an Na/K-ATPase/Src complex, particularly in
epithelial cells (11,12). pNaKtide, a polypeptide derived and
constructed from the ND1 segment of the Na/K-ATPase α1 subunit, is
able to mimic Na/K-ATPase and bind to the kinase domain of Src,
preventing its activation without affecting the pump function of
Na/K-ATPase (13). A previous
study by our group indicated that the inhibition of Src and its
downstream signaling cascades by pNaKtide attenuated unilateral
ureter obstruction (UUO)-induced renal fibrosis, indicating that
Na/K-ATPase/Src complex-regulated signaling pathways are involved
in renal fibrosis (10).
Ouabain and other cardiotonic steroids are specific
Na/K-ATPase ligands. Oxidative stress, represented by reactive
oxygen species (ROS) production, has been observed upon activation
of the Na/K-ATPase/Src complex by cardiotonic steroids in various
cell types (14,15). In addition, there have been reports
that ROS can activate Na/K-ATPase/Src signaling cascades with
further generation of intracellular ROS (16–18).
This leads to a feed-forward oxidative amplification loop of
Na/K-ATPase/Src/ROS; ROS are generated by Na/K-ATPase/Src complex
activation and they enhance Na/K-ATPase/Src signaling cascade
activation (12,15). This oxidative amplification loop
has been observed in disease models such as uremic cardiomyopathy,
cognitive decline and neurodegeneration, salt-sensitive
hypertension and obesity (19–23).
In the above-mentioned study, increased ROS production was observed
in UUO mice, which was attenuated by pNaKtide (10); those findings provided support for
the hypothesis that an Na/K-ATPase/Src/ROS oxidant amplification
loop is involved in renal fibrosis.
The molecular mechanisms of ROS generation involved
in the oxidant amplification loop have not been fully elucidated
thus far, to the best of our knowledge. Nicotinamide adenine
dinucleotide phosphate oxidases (NOXs) have been regarded as a
major source of ROS in the kidney in various animal models
(24–27). A previous study by our group
observed increased expression levels of both NOX2 and NOX4 soon
after UUO induction, accompanied by increased expression levels of
oxidative stress markers (28).
The administration of apocynin, an inhibitor of NOXs, decreased the
expression levels of NOX2, NOX4 and oxidative stress markers,
indicating that NOXs are major contributors to oxidative stress in
UUO animals (29). The present
study investigated whether NOXs serve as a major source of ROS in
the Na/K-ATPase/Src/ROS oxidant amplification loop, which is
potentially involved in renal fibrosis.
Materials and methods
Reagents and antibodies
Monoclonal antibodies to phosphorylated
extracellular signal-related kinase (p-ERK; cat. no. sc-7383), ERK
(cat. no. sc-514302), c-Src (cat. no. sc-8056), α-smooth muscle
actin (α-SMA; cat. no. sc-32251), p47phox (cat. no. sc-17845),
p67phox (cat. no. sc-374510), 8-hydroxy-2′-deoxyguanosine (8-OHdG;
cat. no. sc-66036) and NOX2 (cat. no. sc-130548) were obtained from
Santa Cruz Biotechnology, Inc.; monoclonal antibodies to collagen I
(COL-I; cat. no. ab270993), Na/K-ATPase (cat. no. ab7671) and
3-nitrotyrosine (3-NT; cat. no. ab61392) were obtained from Abcam;
monoclonal antibodies to GAPDH (cat. no. 2118S), polyclonal
antibodies to p-Src (cat. no. 2101s) and horseradish
peroxidase-conjugated anti-rabbit (cat. no. 7074) and anti-mouse
antibodies (cat. no. 7076) were acquired from Cell Signaling
Technology, Inc.; a monoclonal antibody to 4-hydroxynonenal (4-HNE;
cat. no. MA5-27570) was purchased from Thermo Fisher Scientific,
Inc.; and monoclonal antibodies to nuclear factor E2-related factor
2 (Nrf2; cat. no. 16396-1-AP), heme-oxygenase 1 (HO-1; cat. no.
66743-1-Ig), NOX1 (cat. no. 17772-1-AP), NOX4 (cat. no. 14347-1-AP)
and NOX5 (cat. no. 25350-1-AP) were purchased from Proteintech
Group, Inc. An enhanced chemiluminescence kit was purchased from
Merck KGaA. Furthermore,
1-tert-butyl-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
(PP2) was purchased from Selleck Chemicals; and ouabain, apocynin
and N-acetylcysteine (NAC) were obtained from Sigma-Aldrich (Merck
KGaA). RIPA lysis buffer and a ROS Assay Kit (cat. no. S0033M) were
obtained from Beyotime Institute of Biotechnology. Medium199 and
fetal bovine serum were purchased from Thermo Fisher Scientific,
Inc.; a Sirius Red Staining Kit (cat. no. AG1470-2) was purchased
from Acmec Biochemical Co., Ltd. Small interfering RNAs (siRNAs)
specific for NOX2 were obtained from Hanbio Biotechnology Co., Ltd.
with the following sequences: 5′-GUGCACCAUGAUGAGGAGA-3′ (sense) and
5′-UCUCCUCAUCAUGGUGCAC-3′ (antisense). Scrambled siRNA sequences
were as follows: 5′-UUCUCCGAACGUGUCACGU-3′ (sense) and
5′-ACGUGACACGUUCGGAGAA-3′ (antisense).
Experimental animals and
treatment
All animal studies were approved by the Animal
Experimentation Ethics Committee of Peking University First
Hospital (Beijing, China; no. J2022005) and complied with the Guide
for the Care and Use of Laboratory Animals published by the
National Institutes of Health. Male C57BL/6J mice (age, 6–8 weeks;
weight, 18–23 g) were purchased from the Institute of Laboratory
Animal Science, Chinese Academy of Medical Sciences and housed at
25°C with 40% humidity and a 12-h light/dark cycle. They were
provided with free access to sterile water and food. The UUO model
was established as previously described (10,30).
All animals were anesthetized by intraperitoneal injection of 50
mg/kg pentobarbital sodium. The left ureter was exposed and ligated
with 4–0 silk sutures. In sham-operated mice, the ureter was
mobilized but not ligated. Animals were randomly divided into the
following six groups: Sham surgery, sham + PP2, sham + apocynin,
UUO, UUO + PP2 and UUO + apocynin. Apocynin (100 mg/kg) was
administered by gavage immediately after surgery and then daily for
7 days. PP2 (2 mg/kg) was intraperitoneally administered
immediately after ureteral ligation and then daily for 7 days. On
day 7 after surgery, the pre-specified experimental endpoint, the
mice were sacrificed through complete exsanguination under
anesthesia induced by intraperitoneal administration of 50 mg/kg
pentobarbital sodium and the left kidneys were harvested. Death was
confirmed when the animals' respiration and heartbeat ceased. Every
effort was made to minimize animal suffering during the procedure.
The harvested kidneys were decapsulated and then rapidly dissected.
Coronal sections (2–3 mm thick) through the middle portion of the
kidney were fixed in 10% neutral buffered formalin and embedded in
paraffin; the remaining kidney tissue was snap-frozen in liquid
nitrogen for further analysis.
Histological and immunohistochemical
staining of the kidney
Histological staining of collagen fibers was
performed on formalin-fixed and paraffin-embedded tissues using the
Sirius Red Staining kit in accordance with the manufacturer's
protocol. Immunohistochemical staining was conducted as described
in previous studies by our group (10,28).
In brief, deparaffinized serial sections were rehydrated in PBS and
subjected to antigen retrieval by microwave heating (1,400 W, 3
min, 30 sec). After natural cooling, sections were washed with PBS
and incubated with 0.3% hydrogen peroxide for 15 min at room
temperature to block endogenous peroxidase activity; they were
subsequently incubated with 3% bovine serum albumin for 30 min at
37°C in a humidified chamber. Sections were incubated overnight at
4°C with primary antibodies to COL-I (dilution, 1:200), α-SMA
(dilution, 1:1,000), NOX2 (dilution, 1:200), NOX4 (dilution,
1:200), 8-OHdG (dilution, 1:1,000) and Na/K-ATPase (dilution,
1:500). As a negative control, PBS was used instead of a primary
antibody. After tissues had been washed with PBS, they were
incubated with horseradish peroxidase-conjugated anti-mouse/rabbit
IgG polymer (cat. no. PV6000; ZSGB-BIO, Ltd.) for 1 h at room
temperature. Results were visualized using diaminobenzidine and
sections were observed under a microscope (Leica DM2500; Leica
Microsystems GmbH). Images were captured at a magnification of ×40
and then analyzed using ImageJ software [Version 1.53t; National
Institutes of Health (NIH)].
Cell culture and treatments
The pig renal proximal tubule cell line LLC-PK1 was
obtained from the American Type Culture Collection. The cells were
cultured in Medium199 containing 3% fetal bovine serum, 100
units/ml penicillin and 100 mg/ml streptomycin at 37°C in a
humidified incubator with 5% CO2. After the cells had
reached 80–90% confluence, they were serum-starved overnight and
then exposed to further treatments as indicated in the figure
legends. Certain cells were pre-treated with PP2 (5 µM) or NAC (3
mM) for 30 min prior to ouabain treatment (100 nM, 30 min).
siRNA transfection
LLC-PK1 cells were cultured to 50–60% confluence and
then transfected with siRNA oligonucleotides that specifically
targeted NOX2 (80 nM) using Lipofectamine 3000 (Thermo Fisher
Scientific, Inc.), in accordance with the manufacturer's
instructions. As a control, scrambled siRNA (80 nM) lacking
homology to any gene in the vertebrate transcriptome was
transfected into LLC-PK1 cells in a separate dish. After
transfection, cells were cultured for 48 h and then used for
experiments.
Measurement of intracellular ROS
Intracellular ROS levels were detected using a ROS
Assay Kit, in accordance with the manufacturer's instructions.
LLC-PK1 cells were seeded in black 96-well culture plates
(5×103 cells/well) and incubated with
diacetyldichlorofluorescein diacetate (10 µM) for 20 min at 37°C.
After cells had been washed with PBS, they were treated with
ouabain (100 nM, 30 min) with or without pretreatment with PP2 (5
µM) or NAC (3 mM) for 30 min. Dichlorofluorescein fluorescence
intensity was measured by a microplate reader (Bio Tek Synergy H1;
Agilent Technologies, Inc.) at an excitation wavelength of 488 nm
and an emission wavelength of 525 nm. Simultaneously, fluorescence
images were acquired by fluorescence microscopy.
Western blot analysis
Kidney tissue samples and LLC-PK1 cell homogenates
were prepared and analyzed as previously described (10,17).
In brief, tissue and cell samples were lysed with RIPA lysis buffer
containing a mixture of phosphatase and protease inhibitors
(Beyotime Institute of Biotechnology). After homogenization, the
lysates were centrifuged; supernatants were collected for western
blot analysis. Equal amounts of protein extracts were subjected to
SDS-PAGE (10%) and transferred onto polyvinylidene difluoride
membranes (Millipore; Merck KGaA). After membranes had been blocked
with 5% skimmed milk in Tris-buffered saline plus Tween-20 (TBS-T)
for 1 h at room temperature, they were incubated overnight at 4°C
with the indicated primary antibodies. Antibodies to E-cadherin,
p67phox, NOX1 and HO-1 were used at a dilution of 1:2,500; other
primary antibodies were used at a dilution of 1:1,000.
Subsequently, the membranes were washed with TBS-T and then
incubated with horseradish peroxidase-conjugated secondary
antibodies (dilution, 1:2,500) for 1 h at room temperature. Next,
western blots were developed with enhanced chemiluminescence
reagents and visualized using an Image Quant LAS 4000 mini
(Cytiva). ImageJ software (Version 1.53t, NIH) was used for
densitometric analysis of western blots. Band intensities were
calculated according to area and pixel value. For quantification,
the target protein content was normalized against the corresponding
GAPDH signal, for each experiment. The ratio was then expressed
relative to the content of the sham kidney in vivo
experiment or the control group, where applicable, which was
normalized to 1 in each experimental repeat.
Statistical analysis
Data shown in graphs represent the mean ± SEM for
each group. All experiments were performed at least three times.
Comparisons between two groups were performed using unpaired
Student's t-test. One-way analysis of variance was used for
multi-group comparisons, followed by Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PP2 and apocynin treatment attenuate
UUO-induced renal fibrosis and myofibroblast accumulation
UUO resulted in renal fibrosis onset, as determined
by Sirius Red staining (Fig. 1A),
and collagen-I expression, as determined by immunohistochemical
staining and homogenates analysis via western blotting (Fig. 1B and D; P<0.001 vs. sham alone).
Administration of either PP2 or apocynin significantly prevented
fibrosis in UUO mice (Fig. 1A, B and
D; both P<0.05 vs. UUO alone). Administration of either PP2
or apocynin to sham surgery mice did not significantly affect the
degree of renal fibrosis.
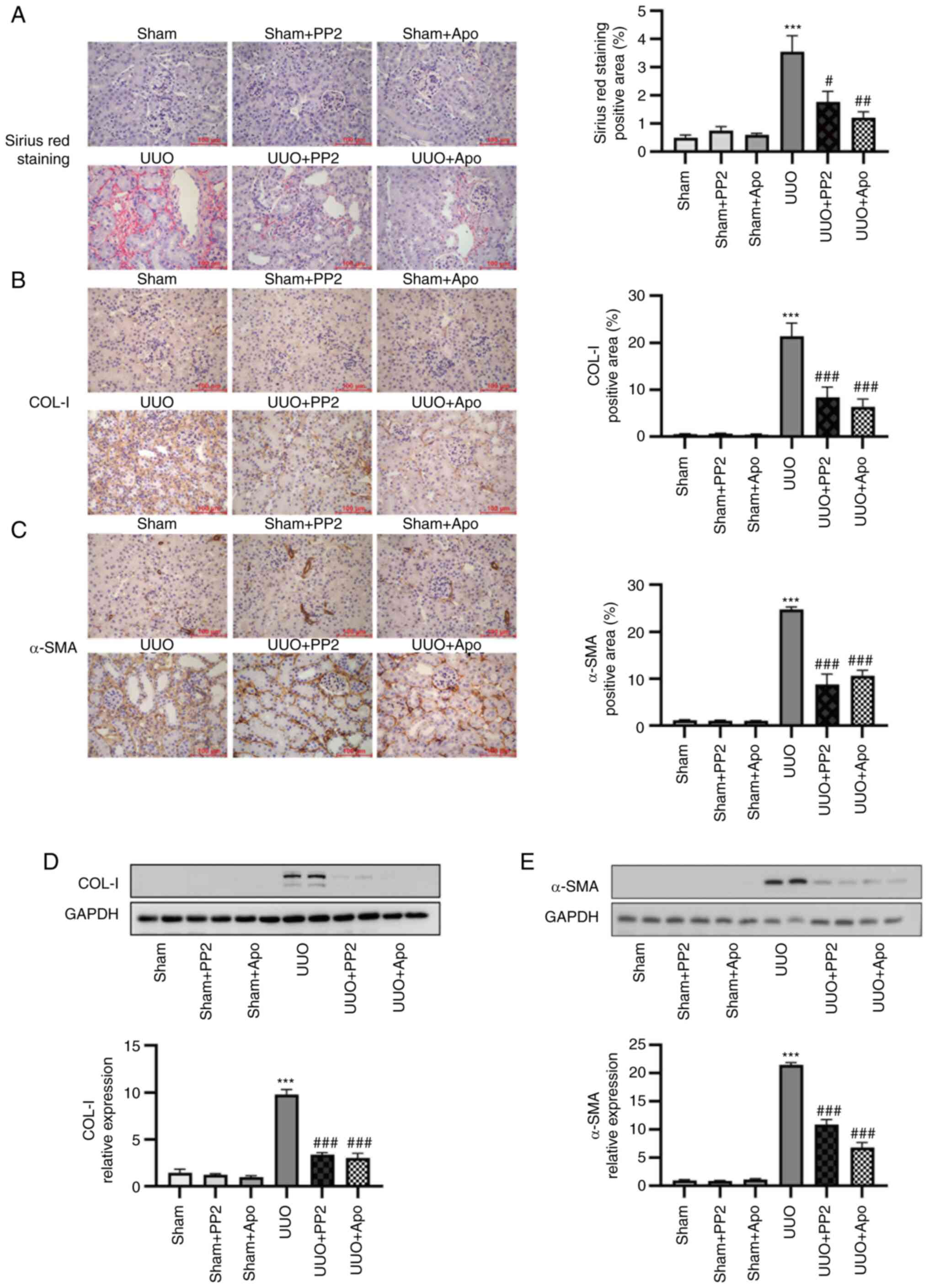 | Figure 1.UUO-induced renal fibrosis and
myofibroblast accumulation are attenuated by PP2 and apocynin
treatment. (A) Representative images and data analysis of Sirius
Red renal histology. (B) Representative images and data analysis of
immunohistochemical staining for COL-I. (C) Representative images
and data analysis of immunohistochemical staining for α-SMA
(magnification, ×400; scale bars, 100 µm). (D) Representative
western blot and data analysis of COL-I expression in kidney
homogenates. (E) Representative western blot and data analysis of
α-SMA expression in kidney homogenates. Values are expressed as
mean ± SEM (n=3-5). ***P<0.001 vs. sham alone;
#P<0.05, ##P<0.01,
###P<0.001 vs. UUO alone. COL-I, collagen I; α-SMA,
α-smooth muscle actin; PP2,
1-tert-butyl-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine;
Apo, apocynin; UUO, unilateral ureteral obstruction. |
α-SMA is regarded as a myofibroblast marker. In
addition to changes in extracellular matrix deposition, UUO induced
significant myofibroblast accumulation in the renal interstitium
(determined by immunohistochemical staining) and in kidney
homogenates (determined by western blot analysis) (Fig. 1C and E; P<0.001 vs. sham alone).
Administration of either PP2 or apocynin reduced myofibroblast
accumulation in UUO mice (Fig. 1C and
E; both P<0.001 vs. UUO alone). No significant effects of
PP2 or apocynin treatment were observed in sham surgery mice. These
findings indicated that the activation of Src and NOXs contributes
to myofibroblast accumulation in renal interstitium and renal
fibrosis onset after UUO injury.
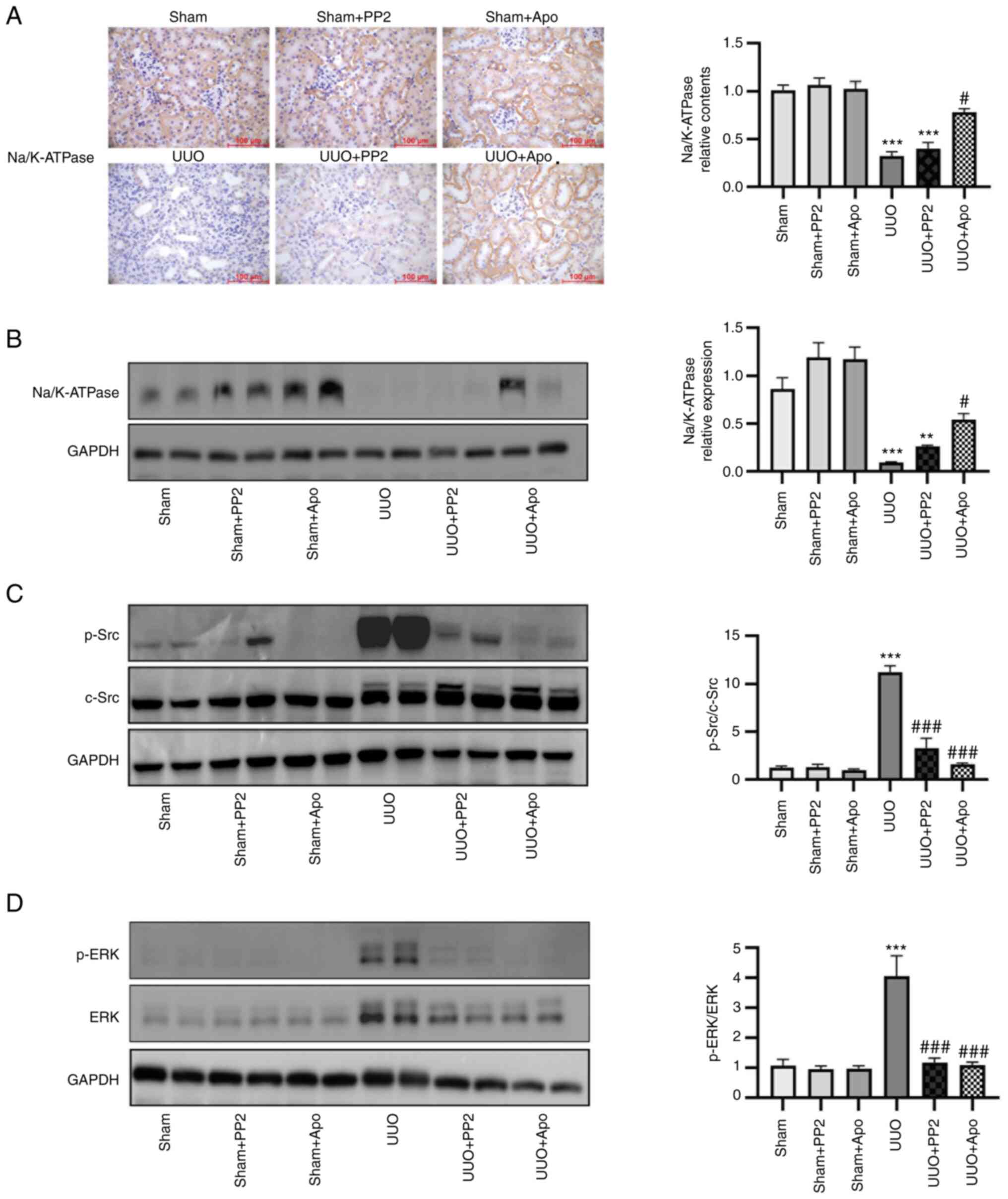
Effects of apocynin administration on
Na/K-ATPase expression and Src/ERK activation
The level of Na/K-ATPase expression is able to
regulate Src activation (17).
Therefore, changes in Na/K-ATPase expression in the kidney were
investigated. UUO led to a significant decrease in Na/K-ATPase
expression at 7 days postoperatively (Fig. 2A and B; P<0.001 vs. sham alone).
Significant activation of Src and ERK1/2 was also observed in
kidney homogenates from UUO mice (Fig.
2C and D; P<0.001 vs. sham alone). In UUO mice, apocynin
treatment partially but significantly reversed the decrease in
Na/K-ATPase expression and inhibited Src and ERK activation
(Fig. 2; both P<0.05 vs. UUO
alone). By contrast, PP2 treatment reduced Src and ERK1/2
activation in obstructed kidneys (Fig.
2C and D; both P<0.001 vs. UUO alone) but did not
significantly influence the UUO-induced decrease in Na/K-ATPase
expression.
Src inhibition attenuates UUO-induced
changes in oxidative stress profiles
Compared with the sham group, UUO significantly
increased activation of the Nrf2/HO-1 pathway and the levels of
oxidative stress biomarkers 3-NT and 4-HNE in kidney homogenates,
as determined by western blot analysis (Fig. 3A and B; all P<0.01 vs. sham
alone). Another oxidative stress biomarker, the DNA oxidation
product 8-OHdG, was also increased in the kidneys of UUO mice, as
determined by immunohistochemical staining (Fig. 3C; P<0.001 vs. sham alone).
Administration of PP2 significantly inhibited Nrf2/HO-1 axis
activation and 3-NT/4-HNE/8-OHdG expression in obstructed kidneys
with similar effects to apocynin (Fig.
3; all P<0.05 vs. UUO alone), suggesting that Src activation
contributed to the development of oxidative stress in UUO mice.
Collectively, these findings support the involvement of an
Na/K-ATPase/Src/ROS oxidative amplification loop in UUO-induced
renal fibrosis.
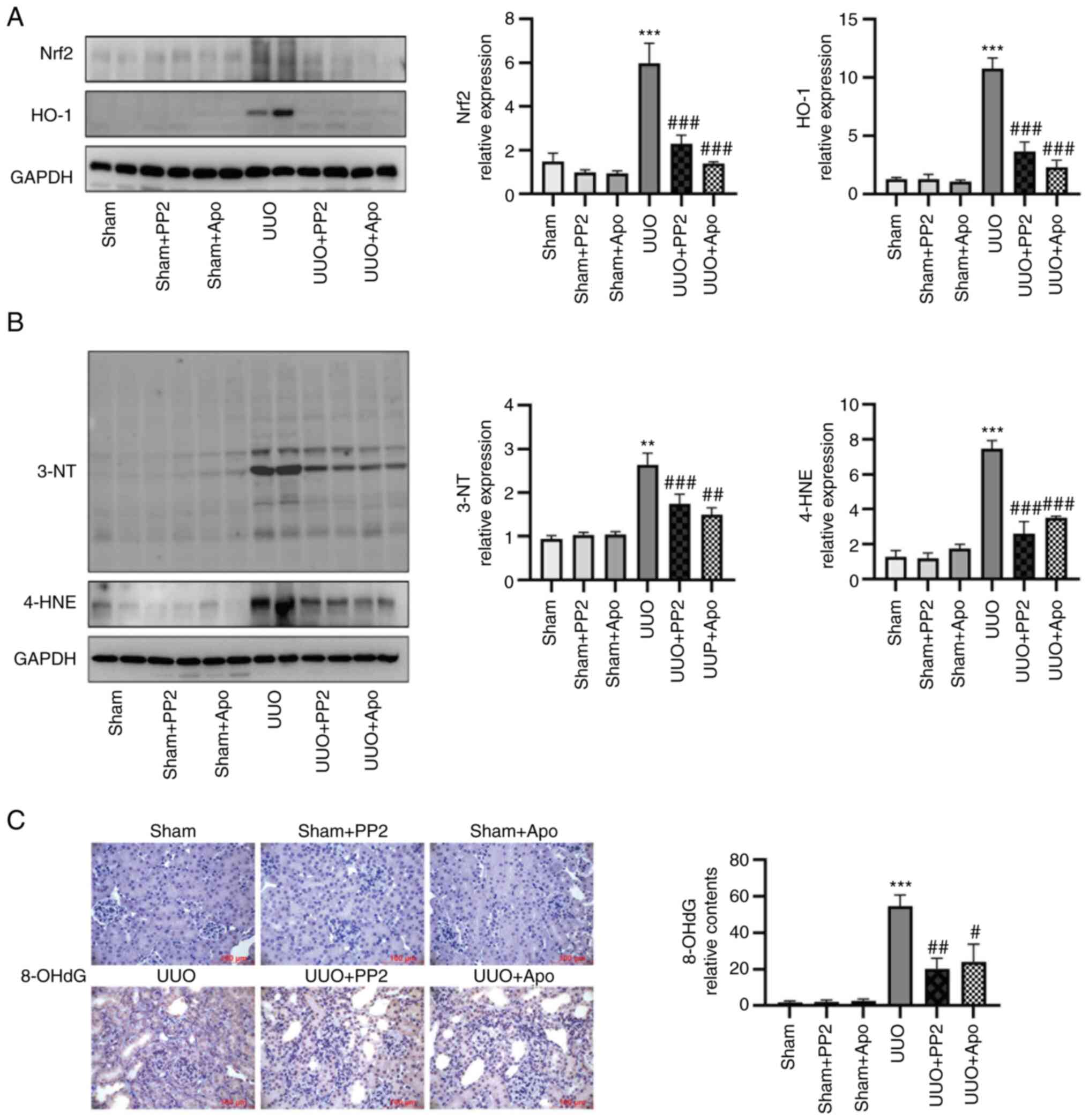 | Figure 3.Src inhibition decreases expression
levels of Nrf2, HO-1, 3-NT and 4-HNE. (A) Representative western
blot and data analysis of Nrf2 and HO-1 content in kidney
homogenates. (B) Representative western blot and data analysis of
3-NT and 4-HNE content in kidney homogenates. (C) Representative
images and data analysis of immunohistochemical staining of 8-OHdG
(magnification, ×400; scale bars, 100 µm). Values are expressed as
the mean ± SEM (n=3-5). **P<0.01, ***P<0.001 vs. sham alone;
#P<0.05, ##P<0.001,
###P<0.001 vs. UUO alone. 3-NT, 3-nitrotyrosine;
4-HNE, 4-hydroxynonenal; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; Apo,
apocynin; HO-1, heme-oxygenase 1; Nrf2, nuclear factor E2-related
factor 2; UUO, unilateral ureteral obstruction. |
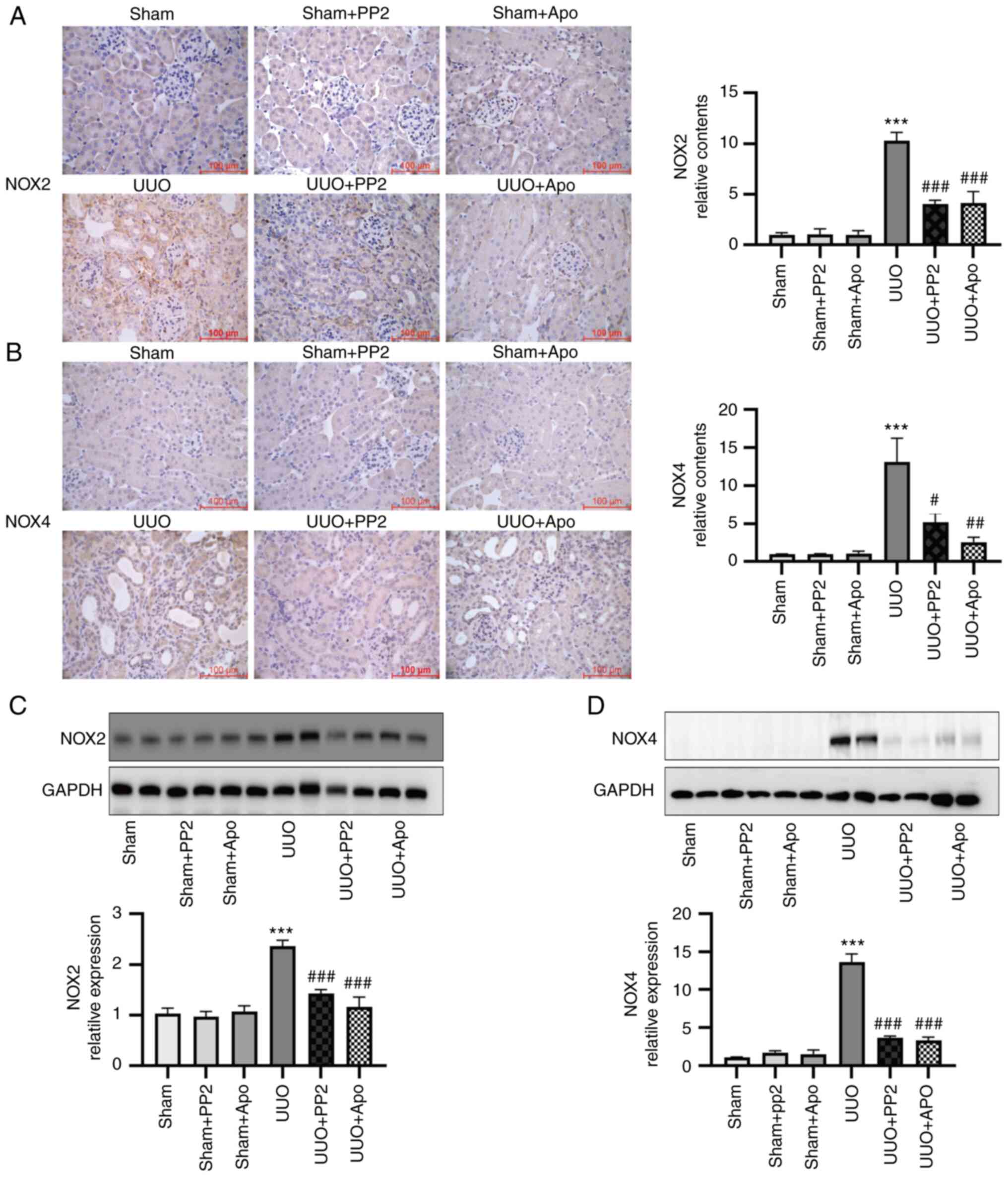
Involvement of NOXs in oxidative
stress generation in UUO-induced renal fibrosis
Considering that NOXs have key roles in the
production of ROS in various models (24–27),
the expression levels of NOX2 and NOX4 were examined. As presented
in Fig. 4, the expression levels
of both NOX2 and NOX4 were significantly increased (both P<0.001
vs. sham alone) in UUO mouse kidneys, as determined by
immunohistochemical staining and western blot analysis. PP2
treatment significantly attenuated the expression of NOX2 and NOX4
in UUO mice (Fig. 4; both
P<0.05 vs. UUO alone), consistent with the changes in oxidative
stress markers described above. In addition, administration of
apocynin significantly inhibited the activation of NOX2 and NOX4
(Fig. 4; both P<0.01 vs. UUO
alone). These results suggested that NOX activation contributes to
the Na/K-ATPase/Src/ROS oxidative amplification loop in UUO
mice.
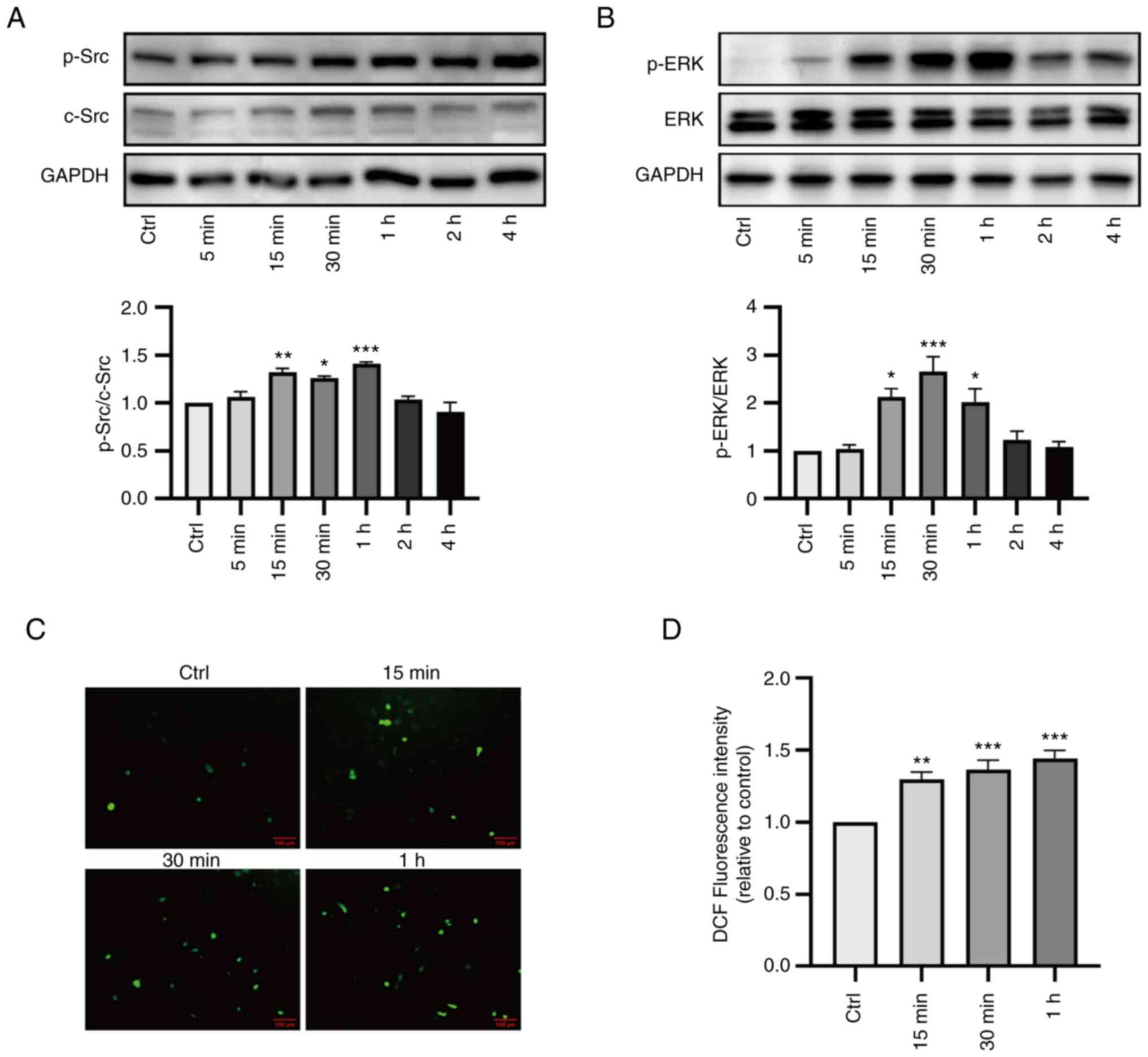
Effects of ouabain on ROS production
and Na/K-ATPase/Src signaling pathway in LLC-PK1 cells
Ouabain is a prototypic agonist of Na/K-ATPase. When
the Na/K-ATPase/Src complex is activated by ouabain, Src functions
as a master upstream regulator of intracellular signaling pathways
(17,18). As indicated in Fig. 5A and B, ouabain (100 nM) caused
significant activation of Src and ERK1/2 in LLC-PK1 cells between
15 min and 1 h post-treatment. A concomitant increase in
intracellular ROS levels was observed during ouabain treatment
(Fig. 5C and D). Based on these
results, 30 min was selected as the observation time-point in
subsequent cell experiments.
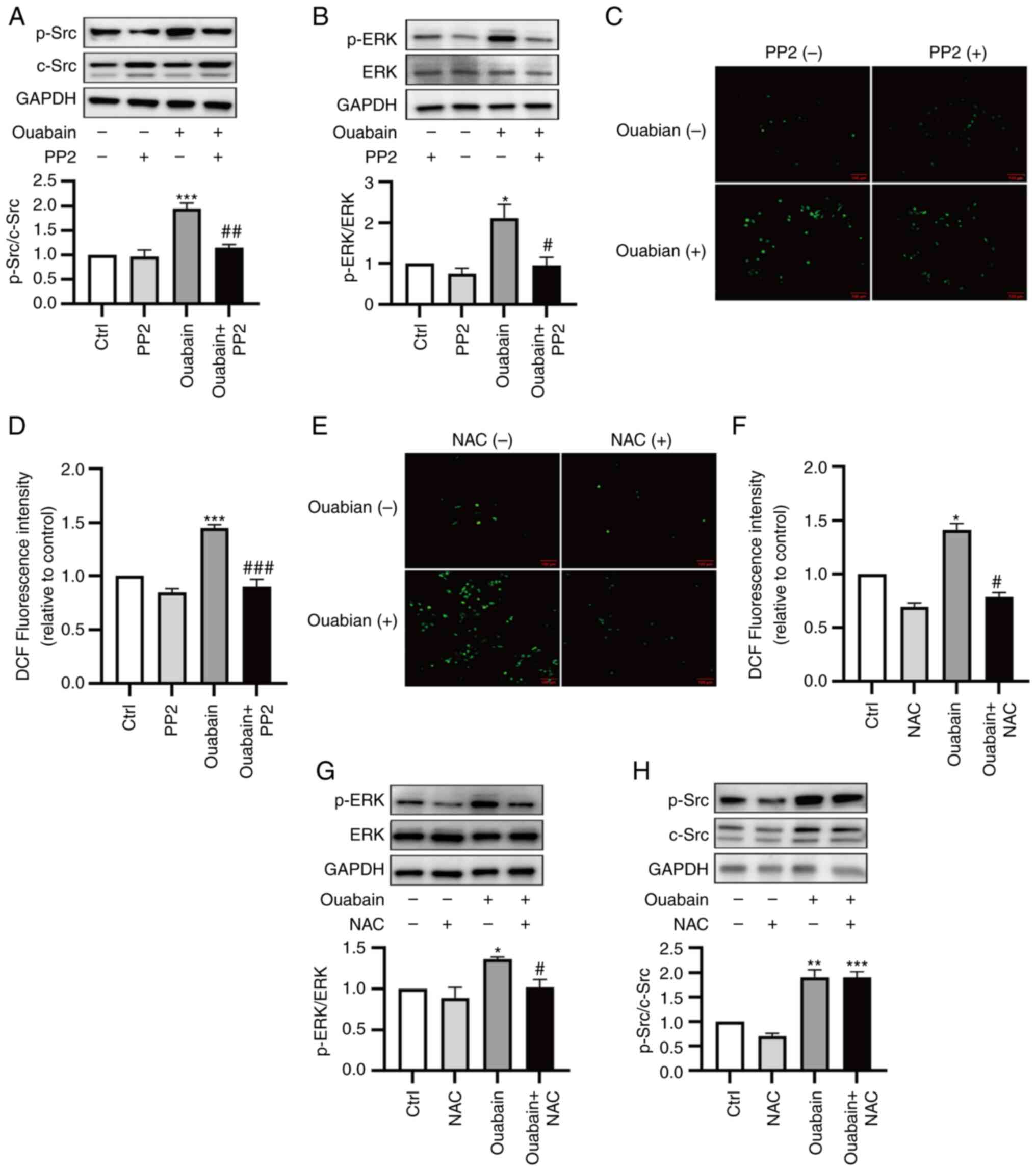
To investigate the roles of ROS in Src-regulated
signaling pathways, ouabain-induced signaling was assessed in the
presence and absence of the Src inhibitor PP2 and the antioxidant
NAC. Under basal conditions, PP2 and NAC treatments did not
influence Src/ERK1/2 activation or ROS production. Pretreatment
with PP2 (5 µM, 30 min) attenuated the ouabain-induced activation
of Src and ERK, as well as the ouabain-induced production of ROS
(Fig. 6A-D). Pretreatment of cells
with NAC (3 mM, 30 min) reduced ouabain-induced ROS production and
ouabain-induced activation of ERK1/2, but it had no effect on
ouabain-induced activation of Src (Fig. 6E-H). These results suggested that
Na/K-ATPase/Src activation is upstream of ouabain-induced ROS
production; this production is involved in regulating the
downstream activation of ERK1/2 in LLC-PK1 cells.
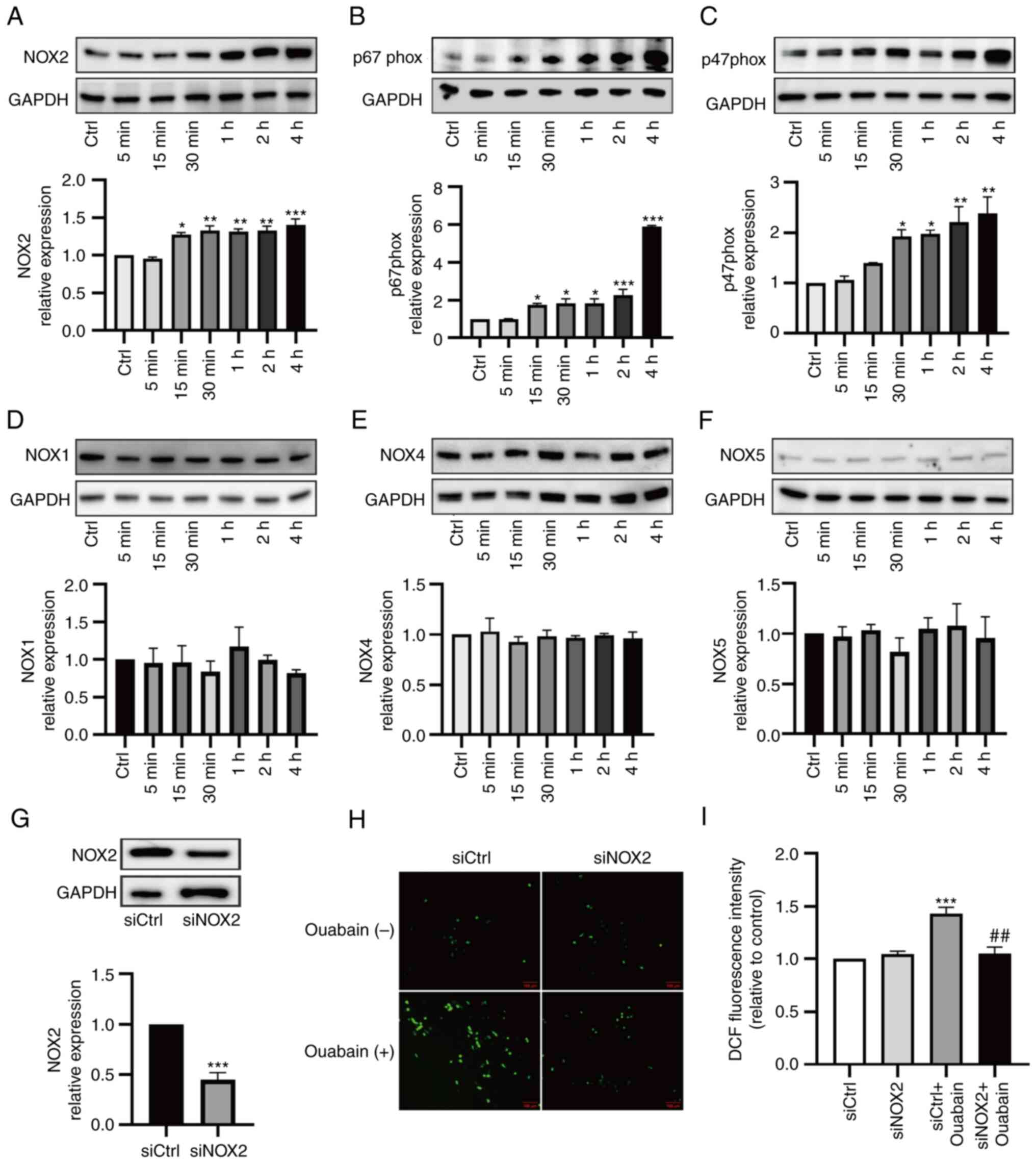
Increased expression of NOX2 as an
important ROS-generating NOX isoform in ouabain-treated LLC-PK1
cells
The NOX family is a major source of intracellular
ROS. To characterize NOX isoforms, the expression levels of NOX1,
NOX2, NOX4 and NOX5 were assessed in LLC-PK1 cells. Among the NOX
isoforms, the expression of NOX2 was significantly increased with
ouabain treatment, while there were no obvious differences in the
expression levels of NOX1, NOX4 or NOX5 (Fig. 7A and D-F). In addition, increased
expression levels of NOX2 components, such as p67phox and p47phox,
were observed, consistent with the changes in NOX2 expression
(Fig. 7B and C). As it was
demonstrated that NOX2 was upregulated in ouabain-treated LLC-PK1
cells, its role in oxidative stress generation was then examined in
a functional study with siRNA-mediated knockdown. Transfection with
NOX2 siRNA reduced basal expression of NOX2 by ~50% (Fig. 7G). NADPH-dependent ROS levels were
then measured in cells under basal conditions and after ouabain
stimulation. In cells transfected with control siRNA, ouabain
significantly increased ROS levels. However, ouabain did not
enhance ROS production in cells that had been transfected with NOX2
siRNA (Fig. 7H and I). Thus, NOX2
is likely to have an important role in ROS production in
ouabain-treated LLC-PK1 cells.
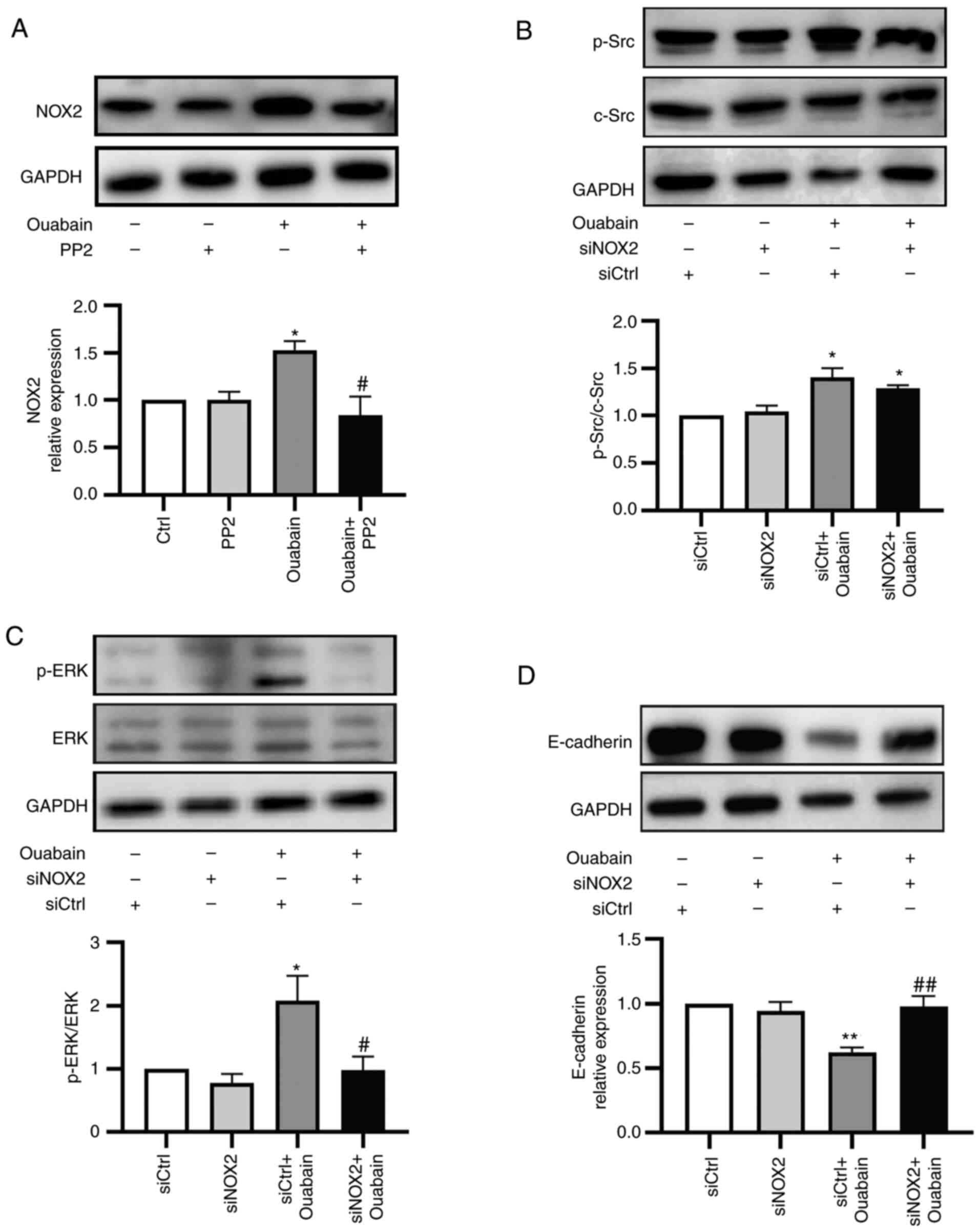
NOX2/ROS is involved in Src-regulated
signaling pathways and trans-differentiation in ouabain-stimulated
LLC-PK1 cells
Considering the upregulation of Src and NOX2/ROS in
ouabain-stimulated LLC-PK1 cells, their relationship was explored
in greater detail. Under basal conditions, PP2 did not influence
NOX2 expression. Pretreatment with PP2 (5 µM, 30 min) reduced the
ouabain-induced increase in NOX2 expression (Fig. 8A). NOX2 silencing did not affect
basal activation of Src or ERK1/2 in LLC-PK1 cells. Transfection
with NOX2 siRNA did not significantly reduce ouabain-induced Src
activation, although it did inhibit ERK1/2 activation in LLC-PK1
cells (Fig. 8B and C).
As a previous study by our group observed activation
of the Na/K-ATPase/Src/ERK1/2 cascade in LLC-PK1 cells to induce
phenotype transformation of LLC-PK1 cells via downregulation of
E-cadherin (17), the role of NOX2
in this process was then investigated. When NOX2 siRNA-transfected
cells were treated with ouabain for 24 h, E-cadherin expression was
not altered compared with the basal conditions. By contrast,
ouabain treatment led to decreased E-cadherin expression in control
siRNA-transfected cells. Thus, transfection with NOX2 siRNA
prevented the ouabain-induced downregulation of E-cadherin
expression (Fig. 8D).
Discussion
The involvement of the Na/K-ATPase/Src/ROS oxidative
amplification loop has been reported in various disease models,
including uremic cardiomyopathy, cognitive decline and
neurodegeneration, salt-sensitive hypertension and obesity
(19–23). However, the source of ROS in this
loop has remained to be fully elucidated. In the present study, the
role of the Na/K-ATPase/Src/ROS oxidative amplification loop and
the involvement of NOXs in renal fibrosis were examined. It was
demonstrated that NOXs-derived ROS mediated the Src-regulated
signaling cascade for fibrogenic processes, while regulating
Na/K-ATPase/Src complex activation, in UUO mice. Experiments with
LLC-PK1 cells further clarified the roles of specific NOXs in the
Na/K-ATPase/Src/ROS oxidative amplification loop via NOX2 silencing
with siRNA. It was found that NOX2-induced ROS production was
Na/K-ATPase/Src-dependent in ouabain-stimulated cells; it was
associated with downstream activation of the Src signaling pathway
and cellular transdifferentiation. Collectively, the present
results revealed that NOXs are key mediators of the
Na/K-ATPase/Src/ROS oxidant amplification loop involved in renal
fibrosis.
Renal fibrosis is the final common pathological
manifestation of chronic kidney disease resulting from various
etiologies. UUO is the most widely used model of interstitial
fibrosis. While the UUO model has certain limitations, such as the
rarity of absolute obstruction in humans, its lack of applicability
to certain renal fibrosis conditions and its inability to monitor
changes in renal function, the model also has several advantages.
These advantages include high reproducibility, ease of
implementation, a rapid time course and the capacity to replicate a
fibrotic sequence of events closely resembling the pathogenesis in
humans (31). Accordingly, the UUO
model was utilized as an example of renal interstitial fibrosis in
the present study.
Src kinase is a versatile non-receptor protein
tyrosine kinase that regulates numerous intracellular signaling
cascades upon activation by growth factors/cytokines from diverse
membrane receptors (5–7). There is evidence that Src activation
is involved in numerous renal lesions in animal models, including
the model of renal fibrosis (7,9,10).
In the present study, inhibition of Src activation by PP2
alleviated myofibroblast accumulation and extracellular matrix
deposition in UUO-induced renal fibrosis, supporting the central
role of Src in fibrosis onset.
In addition to its well-known pump function,
Na/K-ATPase has been identified as a regulator of Src kinase in
recent years. The α1 subunit of Na/K-ATPase binds to Src to form
the Na/K-ATPase/Src complex; this interaction maintains Src in an
inactive state (32,33). When the Na/K-ATPase conformation is
altered via ligand binding or the expression of Na/K-ATPase
decreases, Src is released and activated (34–36).
Increased ROS production has been observed upon activation of the
Na/K-ATPase/Src complex. Meanwhile, there is emerging evidence that
the Na/K-ATPase/Src complex may be activated by ROS in addition to
classic ligands for Na/K-ATPase (e.g., cardiotonic steroids)
(14–17,37).
Therefore, an oxidant amplification loop may exist between
oxidative stress and the Na/K-ATPase/Src complex. When the
Na/K-ATPase/Src complex is activated, a signaling cascade is
initiated; this results in ROS generation and subsequent activation
of the Na/K-ATPase/Src complex, thereby forming a reciprocal
regulatory process (12,14). Studies have indicated that this
Na/K-ATPase oxidant amplification loop is involved in diseases such
as uremic cardiomyopathy, obesity, aging, cognitive decline and
neurodegeneration, and cardiovascular disease (19–23).
A previous study by our group found that the UUO-induced increase
in 8-iso-prostaglandin F2α expression was reduced by pNaKtide
treatment, suggesting that oxidative stress in Na/K-ATPase-mediated
signaling is involved in UUO-induced renal fibrosis (10). ROS are difficult to directly
measure because of their short half-lives. ROS-induced oxidative
stress may cause oxidative damage in organelles (e.g. mitochondria)
and biomolecules such as proteins, lipids and DNA (38,39).
In general, macromolecules produced by ROS activity are used as
markers for estimation of oxidative stress (40). In the present study, levels of
3-NT, 4-HNE, 8-OHdG, Nrf2 and HO-1 were measured to determine ROS
production. It was found that oxidative stress was reduced in
PP2-treated UUO mice; Src-regulated signaling pathway inhibition in
apocynin-treated UUO mice was also observed. Furthermore, apocynin
administration partially reversed the downregulation of Na/K-ATPase
expression in UUO mice. Collectively, these in vivo findings
suggested that an Na/K-ATPase/Src/ROS oxidant amplification loop is
involved in UUO-induced renal fibrosis.
The source of ROS involved in the oxidant
amplification loop has not been elucidated thus far. The NOX family
consists of seven isoforms; NOX1, NOX2, NOX4 and NOX5 have the
highest expression in renal cells (26,41–43).
The levels of NOXs are elevated in several models of acute kidney
injury and chronic kidney disease, leading to excess ROS production
(37,44–46).
In a previous study by our group, it was observed that both NOX2
and NOX4 were upregulated and NOX activity was increased in UUO
model mice (28). Apocynin exerts
an antioxidant effect by inhibiting NOXs. The attenuation of NOXs
led to lower oxidative stress and a decrease in UUO-induced renal
fibrosis, suggesting that NOXs constitute an important source of
ROS in the UUO model (29). In the
present study, it was experimentally confirmed that NOX-derived ROS
generation was increased in UUO model mice; an interaction between
NOX regulation and the Src-regulated signaling cascade in renal
fibrosis was demonstrated. As expected, apocynin administration
significantly reduced the upregulation of NOX2 and NOX4, which led
to decreased levels of oxidative stress biomarkers in UUO mice.
Furthermore, apocynin treatment partially reversed the
downregulation of Na/K-ATPase expression and significantly
inhibited the activation of Src and downstream ERK. Furthermore,
PP2-induced inhibition of Src attenuated the upregulation of NOX2,
NOX4 and oxidative stress biomarkers to a level comparable with the
results of apocynin treatment in UUO mice. These findings suggest
that interactions between NOXs/ROS and Src activation are involved
in renal fibrosis.
Because the activation of Src and NOXs may be
simultaneously affected by convergent stimuli in vivo, the
specific roles of NOXs in the Na/K-ATPase/Src oxidant amplification
loop in LLC-PK1 cells were subsequently examined. Crosstalk between
Na/K-ATPase/Src and NOXs has been demonstrated in various cell
types. Liu et al (47)
found that angiotensin II inhibited the Na/K-ATPase pump in
vascular smooth muscle cells via NADPH oxidase-dependent
glutathionylation of the β1 subunit of Na/K-ATPase. Weaver et
al (48) reported that NOX1
expression was regulated by a ROS- and Src-mediated feed-forward
loop in beta cells. Camargo et al (49) demonstrated that NOX5 mediated the
Src oxidant amplification loop in human vascular cells. Li et
al (50) observed a reciprocal
relationship between NOX1/NOX2 and c-Src activation in fibroblasts
after hypoxia/reoxygenation. Furthermore, Yan et al
(51) reported that NOXs were the
source of ouabain-induced ROS in myocardial cells. In the present
study, a cellular model using ouabain, a classical ligand for
Na/K-ATPase, was generated to stimulate LLC-PK1 cells. LLC-PK1
cells were chosen due to their higher sensitivity to ouabain
compared to human or rodent cells, as well as the availability of
accumulated data from studies concerning the Na-K-ATPase/Src
(13,52). The present results revealed that
ouabain-induced Src/ERK activation and the simultaneous increases
in NOX2 expression and ROS generation in LLC-PK1 cells were
suppressed by PP2 pretreatment, supporting the notion that ROS
production in LLC-PK1 cells involves an Src-dependent process. The
interaction between P47phox and p67phox with NOX2 is critical for
the formation of an active NOX2 functional complex (41). In addition to the detection of
increased NOX2 expression, a paralleled increase in the expression
levels of p47phox and p67phox was also observed after ouabain
stimulation, supporting the role of NOX2 as the source of
ouabain-induced ROS production. NOX2 siRNA prevented
ouabain-induced ROS production and ERK activation without
influencing ouabain-induced Na/K-ATPase/Src activation, suggesting
that NOX2 has a role in mediating the Src downstream signaling
pathway via ROS production. To explore the functional implications
of the Na/K-ATPase/Src/NOXs/ROS axis, the phenotypic
differentiation of LLC-PK1 cells was examined. NOX2 silencing
partially reversed the ouabain-induced downregulation of E-cadherin
expression. In contrast to the in vivo findings, no
significant changes in NOX4 expression were detected in
ouabain-stimulated LLC-PK1 cells. This discrepancy may be related
to species differences or to the involvement of various NOX
isoforms in the Na/K-ATPase/Src/ROS amplification loop across a
diverse range of cell types that contribute to renal fibrosis. For
instance, NOX4 is continuously activated in vascular smooth muscle
cells for production of intracellular ROS under basal conditions
(53). Furthermore, no changes in
NOX1 or NOX5 expression were detected. Taken together, these
findings suggested that NOX2 was the major source of
ouabain-induced ROS in LLC-PK1 cells.
Mitochondria represent another major source of ROS
in the kidney. Liu et al (18) indicated that mitochondria were
involved in ouabain-induced ROS production in myocardial cells. In
the present study, the effect of mitochondria on ROS production was
not evaluated. Of note, interactions between mitochondria and NOXs
have been described. In models of acute kidney injury and chronic
kidney disease, the use of apocynin, a NOX inhibitor, decreased
mitochondrial ROS production (54,55);
this finding supports the notion that crosstalk between NOXs and
mitochondria is involved in establishing a pathological cycle of
ROS production.
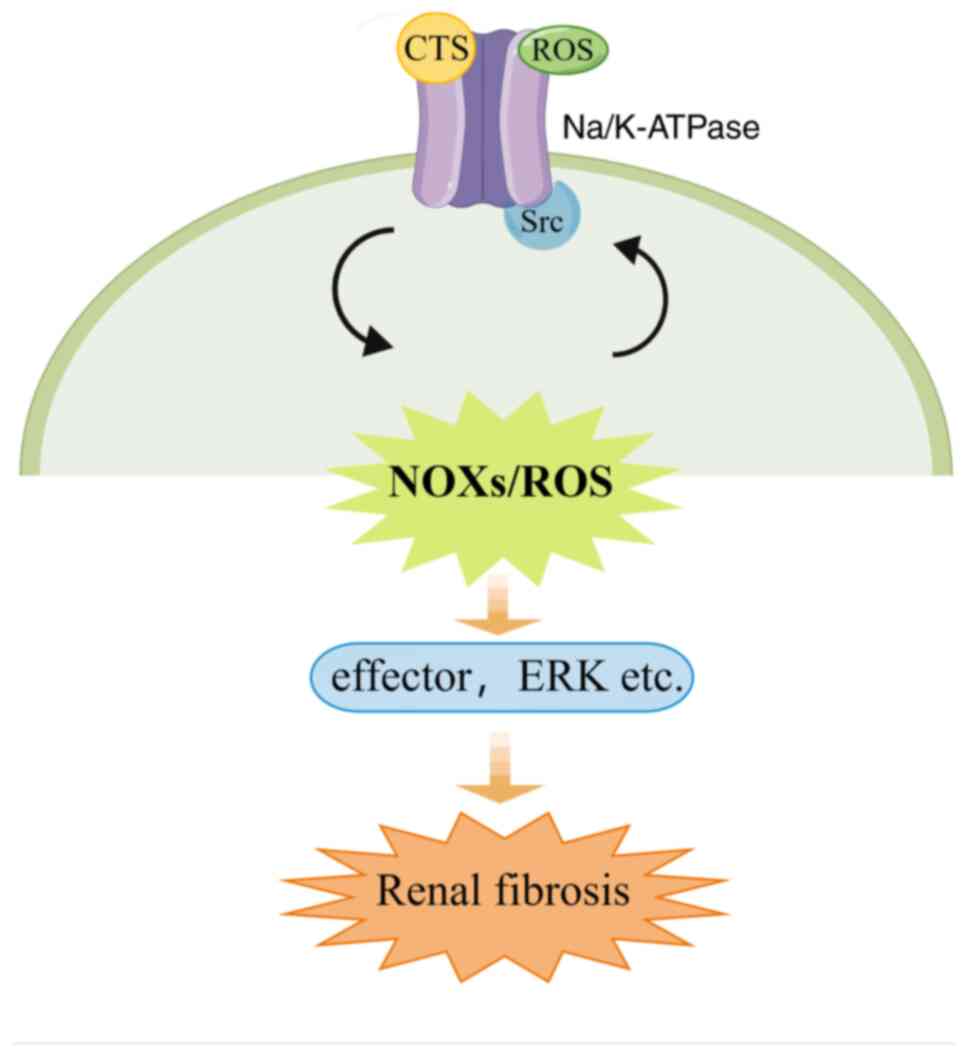
In conclusion, in the present study, NOXs were
identified as a major source of oxidative stress and a mediator of
the Na/K-ATPase/Src/ROS oxidant amplification loop in renal
fibrosis. ROS are produced via NOX activation after activation of
the Na/K-ATPase/Src complex, which in return activates the
Na/K-ATPase/Src complex and mediates Src-regulated downstream
signaling cascades and fibrogenic effects (Fig. 9). The disruption of this vicious
feed-forward loop between NOXs/ROS and redox-regulated
Na/K-ATPase/Src may have therapeutic applicability for renal
fibrosis disorders.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 81770671).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ, FL and XC performed the experiments and analyzed
the data. YW designed the experiments, interpreted the data and
wrote the manuscript. HZ and YW confirmed the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Experimentation Ethics Committee of Peking University First
Hospital (Beijing, China; approval no. J2022005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang T, Cui S, Liu X, Han L, Duan X, Feng
S, Zhang S and Li G: LncTUG1 ameliorates renal tubular fibrosis in
experimental diabetic nephropathy through the
miR-145-5p/dual-specificity phosphatase 6 axis. Ren Fail.
45:21739502023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang W, Sheng L, Chen Y, Li Z, Wu H, Ma J,
Zhang D, Chen X and Zhang S: Total coumarin derivates from
Hydrangea paniculata attenuate renal injuries in cationized-BSA
induced membranous nephropathy by inhibiting complement activation
and interleukin 10-mediated interstitial fibrosis. Phytomedicine.
96:1538862022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Webster AC, Nagler EV, Morton RL and
Masson P: Chronic kidney disease. Lancet. 389:1238–1252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Yang J, Li H, Li Y, Liu Y, Zhang
D, Zhang F, Zhou W and Chen X: Skimmin, a coumarin, suppresses the
streptozotocin-induced diabetic nephropathy in wistar rats. Eur J
Pharmacol. 692:78–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou D and Liu Y: Therapy for kidney
fibrosis: Is the Src kinase a potential target? Kidney Int.
89:12–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li N, Lin G, Zhang H, Sun J, Gui M, Liu Y,
Li W, Liu J and Tang J: Src family kinases: A potential therapeutic
target for acute kidney injury. Biomolecules. 12:9842022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J and Zhuang S: Src family kinases in
chronic kidney disease. Am J Physiol Renal Physiol. 313:F721–F728.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roskoski R Jr: Src protein-tyrosine kinase
structure, mechanism, and small molecule inhibitors. Pharmacol Res.
94:9–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan Y, Ma L, Zhou X, Ponnusamy M, Tang J,
Zhuang MA, Tolbert E, Bayliss G, Bai J and Zhuang S: Src inhibition
blocks renal interstitial fibroblast activation and ameliorates
renal fibrosis. Kidney Int. 89:68–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng X, Song Y and Wang Y: pNaKtide
ameliorates renal interstitial fibrosis through inhibition of
sodium-potassium adenosine triphosphatase-mediated signaling
pathways in unilateral ureteral obstruction mice. Nephrol Dial
Transplant. 34:242–252. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z and Xie Z: The Na/K-ATPase/Src
complex and cardiotonic steroid-activated protein kinase cascades.
Pflugers Arch. 457:635–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pratt RD, Brickman CR, Cottrill CL,
Shapiro JI and Liu J: The Na/K-ATPase signaling: From specific
ligands to general reactive oxygen species. Int J Mol Sci.
19:26002018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai F, Madan N, Ye Q, Duan Q, Li Z, Wang
S, Si S and Xie Z: Identification of a mutant α1 Na/K-ATPase that
pumps but is defective in signal transduction. J Biol Chem.
288:13295–13304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan Y, Shapiro AP, Haller S, Katragadda V,
Liu L, Tian J, Basrur V, Malhotra D, Xie ZJ, Abraham NG, et al:
Involvement of reactive oxygen species in a feed-forward mechanism
of Na/K-ATPase-mediated signaling transduction. J Biol Chem.
288:34249–34258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Z, Kometiani P, Liu J, Li J, Shapiro
JI and Askari A: Intracellular reactive oxygen species mediate the
linkage of Na+/K+-ATPase to hypertrophy and its marker genes in
cardiac myocytes. J Biol Chem. 274:19323–19328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu L, Li J, Liu J, Yuan Z, Pierre SV, Qu
W, Zhao X and Xie Z: Involvement of Na+/K+-ATPase in hydrogen
peroxide-induced hypertrophy in cardiac myocytes. Free Radic Biol
Med. 41:1548–1556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Ye Q, Liu C, Xie JX, Yan Y, Lai F,
Duan Q, Li X, Tian J and Xie Z: Involvement of Na/K-ATPase in
hydrogen peroxide-induced activation of the Src/ERK pathway in
LLC-PK1 cells. Free Radic Biol Med. 71:415–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Tian J, Haas M, Shapiro JI, Askari
A and Xie Z: Ouabain interaction with cardiac Na+/K+-ATPase
initiates signal cascades independent of changes in intracellular
Na+ and Ca2+ concentrations. J Biol Chem. 275:27838–27844. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartlett DE, Miller RB, Thiesfeldt S,
Lakhani HV, Shapiro JI and Sodhi K: The role of Na/K-atpase
signaling in oxidative stress related to aging: Implications in
obesity and cardiovascular disease. Int J Mol Sci. 19:21392018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shah PT, Martin R, Yan Y, Shapiro JI and
Liu J: Carbonylation modification regulates Na/K-ATPase signaling
and salt sensitivity: A review and a hypothesis. Front Physiol.
7:2562016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sodhi K, Pratt R, Wang X, Lakhani HV,
Pillai SS, Zehra M, Wang J, Grover L, Henderson B, Denvir J, et al:
Role of adipocyte Na,K-ATPase oxidant amplification loop in
cognitive decline and neurodegeneration. iScience. 24:1032622021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Yan Y, Liu L, Xie Z, Malhotra D,
Joe B and Shapiro JI: Impairment of Na/K-ATPase signaling in renal
proximal tubule contributes to Dahl salt-sensitive hypertension. J
Biol Chem. 286:22806–22813. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sodhi K, Wang X, Chaudhry MA, Lakhani HV,
Zehra M, Pratt R, Nawab A, Cottrill CL, Snoad B, Bai F, et al:
Central role for adipocyte Na,K-ATPase oxidant amplification loop
in the pathogenesis of experimental uremic cardiomyopathy. J Am Soc
Nephrol. 31:1746–1760. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jha JC, Dai A, Garzarella J, Charlton A,
Urner S, Østergaard JA, Okabe J, Holterman CE, Skene A, Power DA,
et al: Independent of Renox, NOX5 promotes renal inflammation and
fibrosis in diabetes by activating ROS-sensitive pathways.
Diabetes. 71:1282–1298. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alhasson F, Seth RK, Sarkar S, Kimono DA,
Albadrani MS, Dattaroy D, Chandrashekaran V, Scott GI, Raychoudhury
S, Nagarkatti M, et al: High circulatory leptin mediated
NOX-2-peroxynitrite-miR21 axis activate mesangial cells and
promotes renal inflammatory pathology in nonalcoholic fatty liver
disease. Redox Biol. 17:1–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holterman CE, Read NC and Kennedy CRJ: Nox
and renal disease. Clin Sci (Lond). 128:465–481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eid AA, Gorin Y, Fagg BM, Maalouf R,
Barnes JL, Block K and Abboud HE: Mechanisms of podocyte injury in
diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes.
58:1201–1211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu C, Song Y, Qu L, Tang J, Meng L and
Wang Y: Involvement of NOX in the regulation of renal tubular
expression of Na/K-ATPase in acute unilateral ureteral obstruction
rats. Nephron. 130:66–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng X, Zheng X, Song Y, Qu L, Tang J,
Meng L and Wang Y: Apocynin attenuates renal fibrosis via
inhibition of NOXs-ROS-ERK-myofibroblast accumulation in UUO rats.
Free Radic Res. 50:840–852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu M, Liu T, Shang P, Zhang Y, Liu L, Liu
T and Sun S: Acetyl-11-keto-β-boswellic acid ameliorates renal
interstitial fibrosis via Klotho/TGF-β/Smad signalling pathway. J
Cell Mol Med. 22:4997–5007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nogueira A, Pires MJ and Oliveira PA:
Pathophysiological mechanisms of renal fibrosis: A review of animal
models and therapeutic strategies. In Vivo. 31:1–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas
M, Maksimova E, Huang XY and Xie ZJ: Binding of Src to
Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell.
17:317–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui X and Xie Z: Protein interaction and
Na/K-ATPase-mediated signal transduction. Molecules. 22:9902017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shinoda T, Ogawa H, Cornelius F and
Toyoshima C: Crystal structure of the sodium-potassium pump at 2.4
A resolution. Nature. 459:446–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laursen M, Yatime L, Nissen P and Fedosova
NU: Crystal structure of the high-affinity Na+K+-ATPase-ouabain
complex with Mg2+ bound in the cation binding site. Proc Natl Acad
Sci USA. 110:10958–10963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie JX, Li X and Xie Z: Regulation of
renal function and structure by the signaling Na/K-ATPase. IUBMB
Life. 65:991–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan Y, Shapiro AP, Mopidevi BR, Chaudhry
MA, Maxwell K, Haller ST, Drummond CA, Kennedy DJ, Tian J, Malhotra
D, et al: Protein carbonylation of an amino acid residue of the
Na/K-ATPase α1 subunit determines Na/K-ATPase signaling and sodium
transport in renal proximal tubular cells. J Am Heart Assoc.
5:e0036752016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv W, Booz GW, Fan F, Wang Y and Roman RJ:
Oxidative stress and renal fibrosis: Recent insights for the
development of novel therapeutic strategies. Front Physiol.
9:1052018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Li Z, Wu H, Wang J and Zhang S:
Esculetin alleviates murine lupus nephritis by inhibiting
complement activation and enhancing Nrf2 signaling pathway. J
Ethnopharmacol. 288:1150042022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Demirci-Çekiç S, Özkan G, Avan AN, Uzunboy
S, Çapanoğlu E and Apak R: Biomarkers of oxidative stress and
antioxidant defense. J Pharm Biomed Anal. 209:1144772022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gill PS and Wilcox CS: NADPH oxidases in
the kidney. Antioxid Redox Signal. 8:1597–1607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aranda-Rivera AK, Cruz-Gregorio A,
Aparicio-Trejo OE, Ortega-Lozano AJ and Pedraza-Chaverri J: Redox
signaling pathways in unilateral ureteral obstruction (UUO)-induced
renal fibrosis. Free Radic Biol Med. 172:65–81. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee SR, An EJ, Kim J and Bae YS: Function
of NADPH oxidases in diabetic nephropathy and development of Nox
inhibitors. Biomol Ther (Seoul). 28:25–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shin HS, Yu M, Kim M, Choi HS and Kang DH:
Renoprotective effect of red ginseng in gentamicin-induced acute
kidney injury. Lab Invest. 94:1147–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoo JY, Cha DR, Kim B, An EJ, Lee SR, Cha
JJ, Kang YS, Ghee JY, Han JY and Bae YS: LPS-induced acute kidney
injury is mediated by Nox4-SH3YL1. Cell Rep. 33:1082452020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu CC, Karimi Galougahi K, Weisbrod RM,
Hansen T, Ravaie R, Nunez A, Liu YB, Fry N, Garcia A, Hamilton EJ,
et al: Oxidative inhibition of the vascular Na+-K+ pump via NADPH
oxidase-dependent β1-subunit glutathionylation: implications for
angiotensin II-induced vascular dysfunction. Free Radic Biol Med.
65:563–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weaver JR and Taylor-Fishwick DA:
Regulation of NOX-1 expression in beta cells: A positive feedback
loop involving the Src-kinase signaling pathway. Mol Cell
Endocrinol. 369:35–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Camargo LL, Montezano AC, Hussain M, Wang
Y, Zou Z, Rios FJ, Neves KB, Alves-Lopes R, Awan FR, Guzik TJ, et
al: Central role of c-Src in NOX5-mediated redox signalling in
vascular smooth muscle cells in human hypertension. Cardiovasc Res.
118:1359–1373. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Q, Zhang Y, Marden JJ, Banfi B and
Engelhardt JF: Endosomal NADPH oxidase regulates c-Src activation
following hypoxia/reoxygenation injury. Biochem J. 411:531–541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yan X, Xun M, Dou X, Wu L, Han Y and Zheng
J: Regulation of Na+-K+-ATPase effected high
glucose-induced myocardial cell injury through c-Src dependent
NADPH oxidase/ROS pathway. Exp Cell Res. 357:243–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yan Y, Haller S, Shapiro A, Malhotra N,
Tian J, Xie Z, Malhotra D, Shapiro JI and Liu J: Ouabain-stimulated
trafficking regulation of the Na/K-ATPase and NHE3 in renal
proximal tubule cells. Mol Cell Biochem. 367:175–183. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Clempus RE, Sorescu D, Dikalova AE,
Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassègue B and Griendling
KK: Nox4 is required for maintenance of the differentiated vascular
smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol.
27:42–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kozieł R, Pircher H, Kratochwil M, Lener
B, Hermann M, Dencher NA and Jansen-Dürr P: Mitochondrial
respiratory chain complex I is inactivated by NADPH oxidase Nox4.
Biochem J. 452:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fukai T and Ushio-Fukai M: Cross-talk
between NADPH oxidase and mitochondria: Role in ROS signaling and
angiogenesis. Cells. 9:18492020. View Article : Google Scholar : PubMed/NCBI
|