Erectile dysfunction (ED) is a common disease that
prevents males from achieving or maintaining a penile erection that
is sufficient for satisfactory sexual intercourse (1–3). ED
exerts notable effects on the quality of life of patients and their
sexual partners. Several risk factors, including diabetes,
metabolic syndrome and systemic disease may increase the prevalence
of ED (4–6). Approximately 52% of males worldwide
have been reported to experience ED, which affects ~150 million men
worldwide (7). A previous study
predicted that the number of patients with diabetes will reach 592
million by 2035, and reported that 75% of male patients with
diabetes experience differing degrees of ED (8). ED may also arise as a discrete
idiopathic condition, as a consequence of an infection or in
conjunction with systemic conditions, such as autoimmune disorders.
Notably, imbalances in regulatory inflammatory cytokines lead to
epithelial cell dysfunction, which induces the release of
endotoxins into the peripheral blood circulation, thereby causing a
systemic inflammatory response (9). Numerous therapeutic options are
currently available for the treatment of ED, including oral
phosphodiesterase type 5 (PDE5) inhibitors, alprostadil
intraurethral suppositories, intracavernosal injection therapy,
vacuum devices and penile prostheses or implants. PDE5 inhibitors
are the most commonly used treatment options, which exert
anti-inflammatory effects; however, 30% of patients are not
sensitive to them (10).
Furthermore, a previous review reported that PDE5 inhibitors induce
headache, inflammation, digestive issues and other adverse effects,
which limits their widespread usage in clinical practice (11).
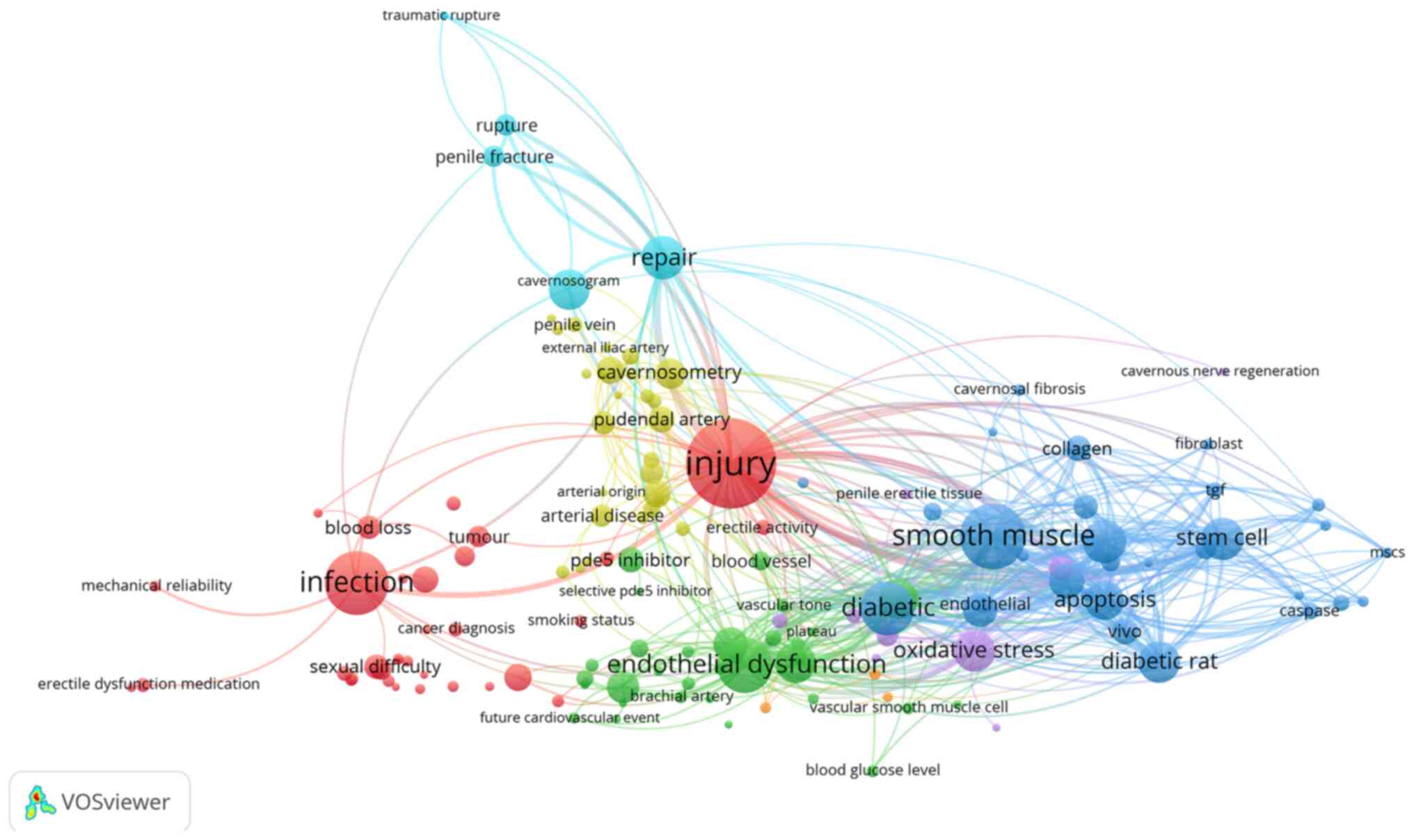
The present review analyzed the literature on ED and
created a graph that included 36,447 articles obtained from the Web
of Science, with publication dates ranging from 1945 to 2020. Key
words were used to provide a high-level description of the topic in
each article, and high-frequency key words were considered to
reflect the research hotspots in ED. Following the screening and
analysis of all ED-associated key words, 141 high-frequency key
words were collected. In addition, a symbiotic map of core key
words was created to summarize the research hotspots of ED.
Notably, ED was found to be associated with injury, endothelial
dysfunction, smooth muscle, infection, and oxidative stress
(Fig. 1) (12–20).
Numerous previous studies have demonstrated the effects of
pyroptosis in cardiovascular disease, including atherosclerosis,
myocardial infarction, diabetic cardiomyopathy and cardiac
hypertrophy, and in cancer (21–27).
However, studies focused on the role of pyroptosis in ED, and the
associated molecular mechanisms and pathogenic pathways are
lacking.
The results of a previous study demonstrated that a
poly ADP-ribose polymerase inhibitor decreased apoptosis in the
corpus cavernosum of diabetic rats; however, this inhibitor
improved erectile function, but not fully restore the erectile
response, indicating that types of cell death other than apopotosis
may be involved in ED (28). Types
of cell death include pyroptosis, apoptosis and necrosis, and these
three types have multiple similarities and differences. Notably,
these types of cell death play key roles in tissue homeostasis,
basic biological functions and disease occurrence (Table I) (14,24).
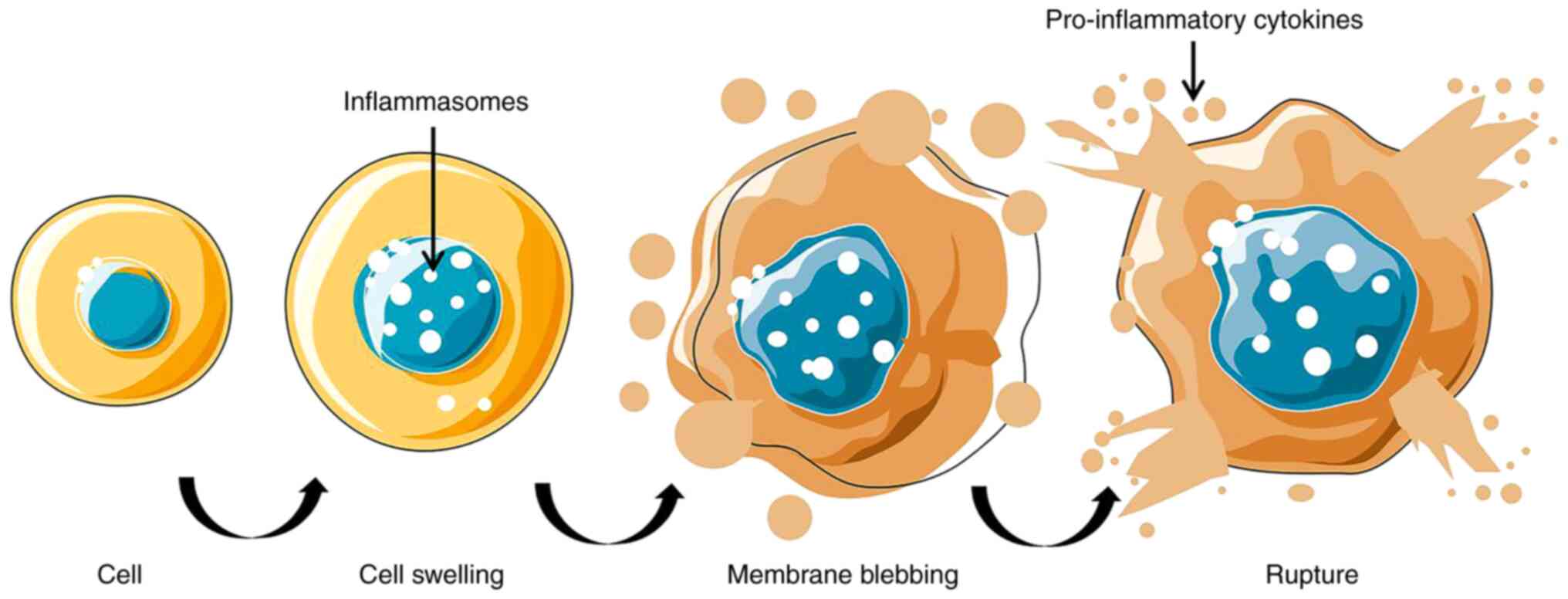
The inflammasome-induced activation of the pyroptosis pathway
accelerates cell swelling and causes a large number of
pro-inflammatory cytokines to be released from the cell, leading to
cell death (Fig. 2). Pyroptosis is
inflammatory programmed necrosis, which was initially reported in
Salmonella-infected macrophages (29). Notably, pyroptosis occurs in
response to a variety of pathogens and non-infectious factors, and
is dependent on caspase-1, −4, −5 and −11, which are associated
with the activation of downstream inflammatory factors. Cell death
induced by caspase-1 activation is known as canonical pyroptosis,
while cell death induced by caspase-4, −5 and −11 activation is
known as noncanonical pyroptosis (30). Inflammasome activation in the
canonical signaling pathway may induce the release of functional
caspase-1 in vivo, the activation of immune cells, and the
secretion of chemokines, inflammatory factors and adhesion
molecules. This exacerbates the inflammatory response, leading to
severe inflammation (31). A
previous study demonstrated that activation of the pyroptosis
signaling pathway is associated with the development of ED
(32). Increases in
proinflammatory cytokines in ED indicate that inflammatory factors
play a significant role in the progression of this disease
(33,34). The present study aimed to review
the mechanisms and the biological importance of pyroptosis in
inflammation-mediated ED.
The activity of inflammasomes in the corpora
cavernosa may modulate the function of this tissue at a
physiological level. In addition, inflammasome activation may
contribute to functional changes occurring in pathophysiological
states (40). Notably,
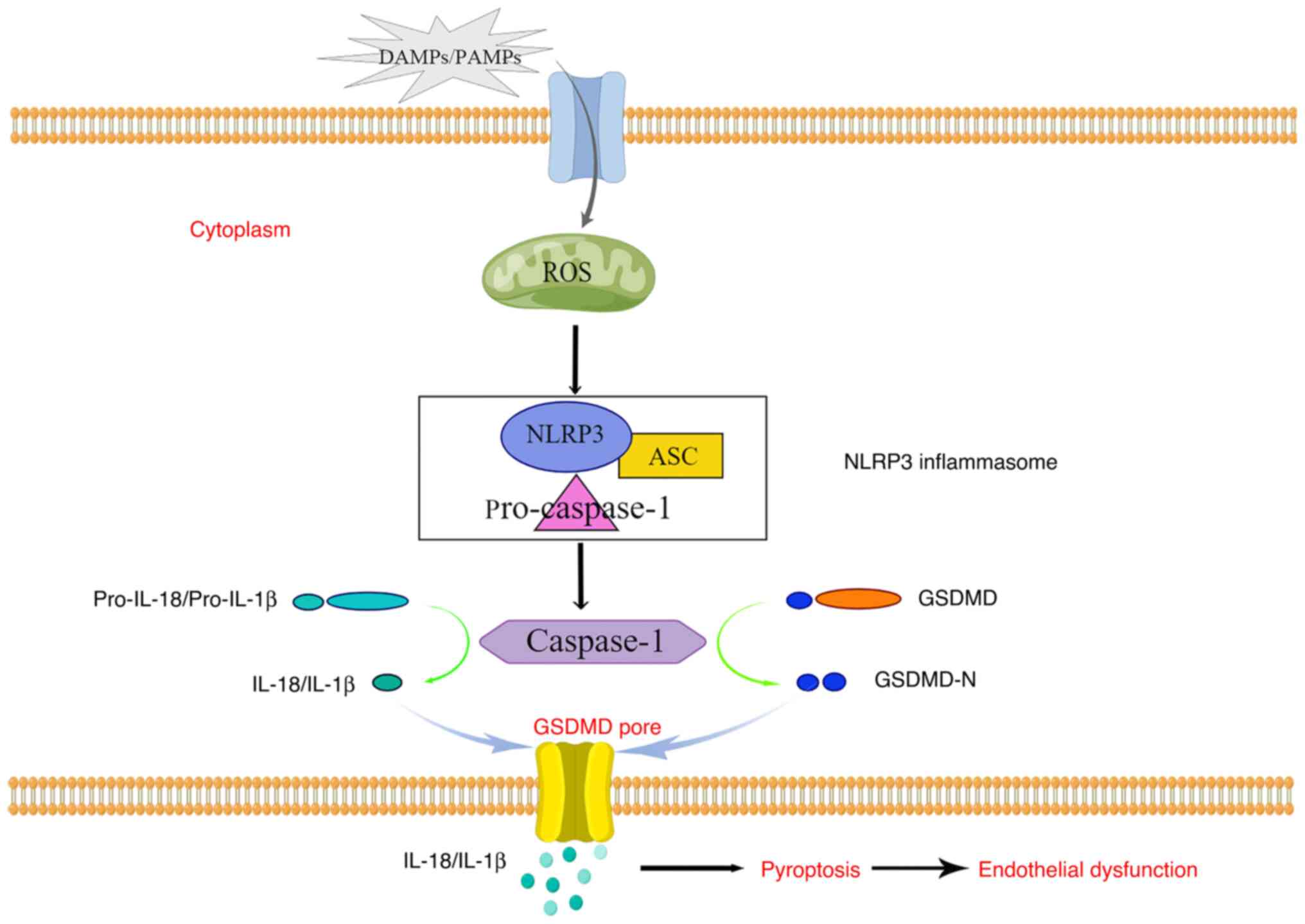
inflammasomes are important components of the pyroptosis pathway
that participate in immune regulation. NLRP3 is a member of the
NOD-like receptor family of pattern recognition receptors, which
also includes NLRP1, NOD-like receptor family caspase recruitment
domain containing 4, absent in melanoma 2 receptor and pyrin. Among
this family, the most commonly studied inflammasome is NLRP3
(42). Following the specific
stimulation of NLRP3, apoptosis-associated speck-like proteins
containing a caspase-1 precursor recruitment domain are activated.
These apoptosis-associated speck-like proteins are bridging
adaptors in inflammatory complexes that interact with cell death
activators and are essential for inflammasome integrity. They form
a multi-protein complex known as the NLRP3 inflammasome, which is
associated with various immune and inflammatory diseases, including
chronic obstructive pulmonary disease, bronchial asthma and hepatic
fibrosis. The NLRP3 inflammasome is crucial for innate immunity;
however, its aberrant activation promotes various inflammatory
disorders, including atherosclerosis and ED (43). The findings of a previous study
suggested that NLRP3 inflammasomes mediate innate immune responses
to induce ED. Moreover, the study indicated that NLRP3 increases
the accumulation of inflammatory factors in the blood vessel wall,
activates caspase-1, increases the thickness of the vessel wall and
reduces blood circulation, thereby leading to ED (44). Another study demonstrated that the
NLRP3 inflammasome participates in the mediation of
lipopolysaccharide-induced pyroptosis, and is positively associated
with vascular inflammation (45).
Activation of the NLRP3 inflammasome decreases the sensitivity of
the corpora cavernosa to NO and endothelium-dependent relaxation;
these are processes that may be associated with NLRP3-mediated
vascular functional and structural damage (46,47).
The inhibition of NLRP3 has been demonstrated to prevent the
endothelin-1-induced impairment of endothelial relaxation in the
corpora cavernosa in mice, which exhibits a positive effect on
erectile function (48). Thus, the
NLPR3 inflammasome may play a role in the pathogenesis of ED.
Notably, inflammasomes stimulate proinflammatory cytokines and
promote the expression of inflammatory factors, which induces
systemic inflammation (45).
Therefore, NLRP3 activation may serve as a novel target for the
modulation of ED. Further investigations are required to determine
whether inhibition of the inflammasome alleviates
inflammation-mediated ED.
The results of a transcriptomic study demonstrated
that IL-1β and IL-18 mediate multiple pathways in ED induced by a
high-fat diet (63). The IL-1
family has numerous members, including IL-1α, IL-1β and IL-18,
which play a key role in the regulation of innate immunity and the
adaptive immune response. IL-1β and IL-18 are cellular inflammatory
factors that exist on the surface of cell membranes, and are
produced by a variety of cells, including monocytes, macrophages
and neutrophils. These factors induce the inflammatory immune
response and lead to inflammation. Mature IL-1β and IL-18
contribute to numerous biological processes, including the
occurrence and development of ED. Song et al (64) demonstrated that IL-1β and IL-18
reduce the production of NO via downregulation of the expression
and activity of eNOS, which suggests that IL-1β and IL-18 may be
involved in the development of ED. In addition, another study
demonstrated that the local levels of the inflammatory factors
IL-1β and IL-18 were significantly increased in patients with ED,
and the elevation of these levels was sustained for a prolonged
period (65). Activation of the
inflammasome induces the maturation of inactive IL-1β and IL-18,
and increases the synthesis, expression and release of these
cytokines. The release of IL-1β and IL-18 through GSDMD pores
recruits immune cells to the site of inflammation, stimulates
secondary cytokine production and triggers acute-phase immunity
responses. Studies have revealed that the IL-18 receptor is
associated with susceptibility to vascular damage, and its
expression increases the risk of disease (66,67).
Moreover, the pyroptosis-mediated release of IL-1β and IL-18
initiates systemic inflammatory cascades and the development of ED
(10). However, the association
between pyroptosis and inflammation-mediated ED requires further
investigation.
Numerous factors influence the development and
progression of ED, including immunological and inflammatory
microenvironments. Notably, inflammatory cytokines are closely
associated with ED. Ferlin et al (68) observed that the volume of
inflammatory components in the blood of patients with ED was
increased compared with that in healthy individuals, and was
inversely associated with sexual performance. The occurrence of
oxidative stress in penile tissues in response to inflammatory
factors promotes the accumulation of reactive oxygen species and
triggers endothelial dysfunction, leading to ED. Matos et al
(69) demonstrated that
inflammatory factors can be used as predictors of ED, which further
verifies the role of inflammatory factors in the occurrence of ED.
Pyroptosis is associated with the pathogenesis of numerous chronic
inflammatory diseases due to being a pro-inflammatory process.
Oxidized low-density lipoproteins activate the NF-κB/NLRP3
signaling pathway and cause endothelial cell dysfunction, thereby
increasing the occurrence of pyroptosis in vascular smooth muscle
cells (48). A study in
atherosclerotic mice demonstrated that the numbers of
atherosclerotic plaques and pyroptotic factors were significantly
reduced, and oxidative low-density lipoprotein-induced pyroptosis
was inhibited following treatment with the caspase-1 inhibitor
VX-765 (70). It has also been
reported that hypercholesterolemia promotes activation of the NLRP3
inflammasome, downregulates the expression of eNOS and NO in
coronary arteries, induces endothelial dysfunction and accelerates
the pyroptosis of endothelial cells (71). Moreover, inflammation induces
endothelial dysfunction in human umbilical vein endothelial cells
through the increased expression of IL-1β, IL-18, caspase-1 and
NLRP3 induced via activation of the PI3K/AKT pathway (72). A combination of lipopolysaccharides
and adenosine triphosphate has been demonstrated to inhibit the
erectile function of mice via activation of the NLRP3 inflammasome
and upregulation of the expression of caspase-1 and IL-1β (73). It has also been demonstrated that
upregulation of the expression of yes-associated protein, a
component of the hippo signaling pathway, is associated with
inhibition of the proliferation of corpus cavernosum smooth muscle
cells. In addition, activated caspase-1 cleaves GSDMD into its
N-terminal form, thereby inducing an inflammatory response in cells
and pyroptosis, which leads to a decline in erectile function
(63). Luo et al (74) revealed that adipose-derived stem
cells improve erectile function in rats via the suppression of
NLRP3 inflammasome-mediated pyroptosis and inflammation in the
corpus cavernosum tissue. Notably, IL-1β and IL-18 exert
inflammatory effects (53), with
IL-1β playing a key role in the inflammatory response (75). The release of IL-1β from pyroptotic
cells promotes collagen production and downregulates the expression
of eNOS, leading to penile fibrosis, reduced NO synthesis (64,76)
and inflammation-mediated ED. Furthermore, serum IL-1β levels have
been demonstrated to be elevated in patients with ED (77). IL-18 plays a key role in
inflammatory cells and is a chemotactic cytokine that attracts
leukocytes to the site of inflammation (78). It has been demonstrated that NLRP3,
caspase-1 and IL-1β expression levels are increased in rats with
ED, indicating that pyroptosis may play a role in the development
of ED. Therefore, the inflammatory microenvironment created by the
release of a high number of inflammatory factors during pyroptosis
may induce the initiation and progression of ED (34).
Present research is focused on pyroptosis as a form
of pro-inflammatory programmed cell death. Notably, pyroptosis
plays an important role in the inflammatory response and
accelerates the progression of ED. The pathogenesis of ED is
complex, and cell death is important in the occurrence and
development of inflammatory-mediated ED. The present review
demonstrates that data surrounding the mechanisms underlying ED and
associated treatment options are lacking, despite the high
incidence of ED. As pyroptosis plays a key role in inflammation, it
may exhibit potential as a target in the treatment of
inflammation-mediated ED. However, the specific cell death pathways
and key upstream factors activated in inflammatory ED remain to be
fully elucidated. Thus, further investigations into the
pathogenesis of ED and cell pyroptosis are required. In addition,
the development of novel pyroptosis inhibitors and strategies for
the prevention and treatment of inflammatory-mediated ED is
necessary.
Not applicable.
This study was supported by the National Natural Science
Foundation of China (grant no. 81860781).
Not applicable.
XJ collected the data. YN edited the manuscript. BZ
and FL performed data management and wrote the manuscript. HG was
responsible for protocol/project development and edited the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
Bingbing Zhu, ORCID: 0000-0003-3713-7468.
The authors declare that they have no competing
interests.
|
1
|
Azmi S, Ferdousi M, Alam U, Petropoulos
IN, Ponirakis G, Marshall A, Asghar O, Fadavi H, Jones W, Tavakoli
M, et al: Small-fibre neuropathy in men with type 1 diabetes and
erectile dysfunction: A cross-sectional study. Diabetologia.
60:1094–1101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yafi FA, Jenkins L, Albersen M, Corona G,
Isidori AM, Goldfarb S, Maggi M, Nelson CJ, Parish S, Salonia A, et
al: Erectile dysfunction. Nat Rev Dis Primers. 2:160032016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sangiorgi G, Cereda A, Benedetto D,
Bonanni M, Chiricolo G, Cota L, Martuscelli E and Greco F: Anatomy,
pathophysiology, molecular mechanisms, and clinical management of
erectile dysfunction in patients affected by coronary artery
disease: A review. Biomedicines. 9:4322021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cantone E, Massanova M, Crocetto F, Barone
B, Esposito F, Arcaniolo D, Corlianò F, Romano L, Motta G and Celia
A: The relationship between obstructive sleep apnoea and erectile
dysfunction: An underdiagnosed link? A prospective cross-sectional
study. Andrologia. 54:e145042022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Romano L, Pellegrino R, Sciorio C, Barone
B, Gravina AG, Santonastaso A, Mucherino C, Astretto S, Napolitano
L, Aveta A, et al: Erectile and sexual dysfunction in male and
female patients with celiac disease: A cross-sectional
observational study. Andrology. 10:910–918. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romano L, Zagari RM, Arcaniolo D, Crocetto
F, Spirito L, Sciorio C, Gravina AG, Dajti E, Barone B, La Rocca R,
et al: Sexual dysfunction in gastroenterological patients: Do
gastroenterologists care enough? A nationwide survey from the
italian society of gastroenterology (SIGE). Dig Liver Dis.
54:1494–1501. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ayta IA, McKinlay JB and Krane RJ: The
likely worldwide increase in erectile dysfunction between 1995 and
2025 and some possible policy consequences. BJU Int. 84:50–56.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Defeudis G, Mazzilli R, Tenuta M, Rossini
G, Zamponi V, Olana S, Faggiano A, Pozzilli P, Isidori AM and
Gianfrilli D: Erectile dysfunction and diabetes: A melting pot of
circumstances and treatments. Diabetes Metab Res Rev. 38:e34942022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeshi K, Ruscher R, Hunter L, Daly NL,
Loukas A and Wangchuk P: Revisiting inflammatory bowel disease:
Pathology, treatments, challenges and emerging therapeutics
including drug leads from natural products. J Clin Med. 9:12732020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersson KE: PDE5 inhibitors-pharmacology
and clinical applications 20 years after sildenafil discovery. Br J
Pharmacol. 175:2554–2565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pyrgidis N, Mykoniatis I, Haidich AB,
Tirta M, Talimtzi P, Kalyvianakis D, Ouranidis A and Hatzichristou
D: Effect of phosphodiesterase-type 5 inhibitors on erectile
function: An overview of systematic reviews and meta-analyses. BMJ
Open. 11:e0473962021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
MacDonald SM and Burnett AL: Physiology of
erection and pathophysiology of erectile dysfunction. Urol Clin
North Am. 48:513–525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Defeudis G, Mazzilli R, Di Tommaso AM,
Zamponi V, Carlomagno F, Tuccinardi D, Watanabe M, Faggiano A and
Gianfrilli D: Effects of diet and antihyperglycemic drugs on
erectile dysfunction: A systematic review. Andrology. 11:282–294.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lane-Cordova AD, Kershaw K, Liu K,
Herrington D and Lloyd-Jones DM: Association between cardiovascular
health and endothelial function with future erectile dysfunction:
The multi-ethnic study of atherosclerosis. Am J Hypertens.
30:815–821. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu MC, Chang ML, Wang YC, Chen WH, Wu CC
and Yeh SD: Revisiting the regenerative therapeutic advances
towards erectile dysfunction. Cells. 9:12502020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song J, Sun T, Tang Z, Ruan Y, Liu K, Rao
K, Lan R, Wang S, Wang T and Liu J: Exosomes derived from smooth
muscle cells ameliorate diabetes-induced erectile dysfunction by
inhibiting fibrosis and modulating the NO/cGMP pathway. J Cell Mol
Med. 24:13289–13302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Souza ILL, Ferreira EDS, Vasconcelos
LHC, Cavalcante FA and da Silva BA: Erectile dysfunction: Key role
of cavernous smooth muscle cells. Front Pharmacol. 13:8950442022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng H, Liu Q, Deng Z, Li H, Zhang H, Song
J, Liu X, Liu J, Wen B and Wang T: Human umbilical cord mesenchymal
stem cells ameliorate erectile dysfunction in rats with diabetes
mellitus through the attenuation of ferroptosis. Stem Cell Res
Ther. 13:4502022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyata Y, Matsuo T, Nakamura Y, Mitsunari
K, Ohba K and Sakai H: Pathological significance of macrophages in
erectile dysfunction including peyronie's disease. Biomedicines.
9:16582021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji N, Qi Z, Wang Y, Yang X, Yan Z, Li M,
Ge Q and Zhang J: Pyroptosis: A new regulating mechanism in
cardiovascular disease. J Inflamm Res. 14:2647–2666. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai Y, Zhou Y, Li Z, Xia P, ChenFu X, Shi
A, Zhang J and Yu P: Non-coding rnas in necroptosis, pyroptosis,
and ferroptosis in cardiovascular diseases. Front Cardiovasc Med.
9:9097162022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian K, Yang Y, Zhou K, Deng N, Tian Z, Wu
Z, Liu X, Zhang F and Jiang Z: The role of ROS-induced pyroptosis
in CVD. Front Cardiovasc Med. 10:11165092023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhaolin Z, Guohua L, Shiyuan W and Zuo W:
Role of pyroptosis in cardiovascular disease. Cell Prolif.
52:e125632019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in
inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu L, Lu H, Pan Y, Liu C, Wang J, Chen B
and Wang Y: The role of pyroptosis and its crosstalk with immune
therapy in breast cancer. Front Immunol. 13:9739352022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu A, Shen L, Li N, Shen L and Li Z:
Pan-cancer analyses of pyroptosis with functional implications for
prognosis and immunotherapy in cancer. J Transl Med. 20:1092022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li WJ, Peng Y, Zhou J, Li B, Wang H, Zhang
J and Wang Z: Poly(ADP-ribose) polymerase inhibition improves
erectile function by activation of nitric oxide/cyclic guanosine
monophosphate pathway in diabetic rats. J Sex Med. 9:1319–1327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perez-Lopez A, Rosales-Reyes R,
Alpuche-Aranda CM and Ortiz-Navarrete V: Salmonella downregulates
Nod-like receptor family CARD domain containing protein 4
expression to promote its survival in B cells by preventing
inflammasome activation and cell death. J Immunol. 190:1201–1209.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL,
Cheng K, Sun RY, Zhou D, Han J and Wu Q: Tom20 senses
iron-activated ros signaling to promote melanoma cell pyroptosis.
Cell Res. 28:1171–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Zhao B, Gao WW, Song W, Hou J,
Zhang L and Xia Z: Inhibition of PINK1-Mediated mitophagy
contributes to postoperative cognitive dysfunction through
activation of caspase-3/GSDME-Dependent pyroptosis. ACS Chem
Neurosci. 14:1249–1260. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen ZB, Li G, Lin H, Jiang J and Jiang R:
Low androgen status inhibits erectile function by increasing
pyroptosis in rat corpus cavernosum. Andrology. 9:1264–1274. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Demirtaş Şahin T, Yazir Y, Utkan T, Gacar
G, Halbutoğulları ZS and Gocmez SS: Depression induced by chronic
stress leads to penile cavernosal dysfunction: Protective effect of
anti-TNF-α treatment. Can J Physiol Pharmacol. 96:933–942. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yazir Y, Demirtaş Şahin T, Furat Rençber
S, Gacar G, Halbutoğulları ZS, Utkan T and Aricioglu F: Restorative
effect of resveratrol on expression of endothelial and neuronal
nitric oxide synthase in cavernous tissues of chronic unpredictable
mild stress-exposed rats: An impact of inflammation. Int J Impot
Res. 30:318–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo M, Meng J, Yan J, Shang F, Zhang T, Lv
D, Li C, Yang X and Luo S: Role of the nucleotide-binding
domain-like receptor protein 3 inflammasome in the endothelial
dysfunction of early sepsis. Inflammation. 43:1561–1571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li T, Zheng G, Li B and Tang L:
Pyroptosis: A promising therapeutic target for noninfectious
diseases. Cell Prolif. 54:e131372021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T,
Huang J, Wang F, Zhou F and Zhang L: Role of pyroptosis in
inflammation and cancer. Cell Mol Immunol. 19:971–992. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Wang Z, Zheng Y, Yu Q, Zeng M,
Bai L, Yang L, Guo M, Jiang X and Gan J: Inhibitors of the NLRP3
inflammasome pathway as promising therapeutic candidates for
inflammatory diseases (review). Int J Mol Med. 51:352023.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen C and Xu P: Activation and
pharmacological regulation of inflammasomes. Biomolecules.
12:10052022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu P, Zhang X, Liu N, Tang L, Peng C and
Chen X: Pyroptosis: Mechanisms and diseases. Signal Transduct
Target Ther. 6:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kesavardhana S, Malireddi RKS and
Kanneganti TD: Caspases in cell death, inflammation, and
pyroptosis. Annu Rev Immunol. 38:567–595. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W
and Tang Q: STING-IRF3 contributes to lipopolysaccharide-induced
cardiac dysfunction, inflammation, apoptosis and pyroptosis by
activating NLRP3. Redox Biol. 24:1012152019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ren M, Chen JH, Xu HW, Li W, Wang T, Chi
Z, Lin Y, Zhang A, Chen G, Wang X, et al: Ergolide covalently binds
NLRP3 and inhibits NLRP3 inflammasome-mediated pyroptosis. Int
Immunopharmacol. 120:1102922023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zeng C, Wang R and Tan H: Role of
pyroptosis in cardiovascular diseases and its therapeutic
implications. Int J Biol Sci. 15:1345–1357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao LR, Xing RL, Wang PM, Zhang NS, Yin
SJ, Li XC and Zhang L: NLRP1 and NLRP3 inflammasomes mediate
LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol Med Rep.
17:5463–5469. 2018.PubMed/NCBI
|
|
46
|
Bruder-Nascimento T, Ferreira NS, Zanotto
CZ, Ramalho F, Pequeno IO, Olivon VC, Neves KB, Alves-Lopes R,
Campos E, Silva CA, et al: NLRP3 inflammasome mediates
aldosterone-induced vascular damage. Circulation. 134:1866–1880.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yin Y, Pastrana JL, Li X, Huang X,
Mallilankaraman K, Choi ET, Madesh M, Wang H and Yang XF:
Inflammasomes: Sensors of metabolic stresses for vascular
inflammation. Front Biosci (Landmark Ed). 18:638–649. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sobrano Fais R, Menezes da Costa R,
Carvalho Mendes A, Mestriner F, Comerma-Steffensen SG, Tostes RC,
Simonsen U and Silva Carneiro F: NLRP3 activation contributes to
endothelin-1-induced erectile dysfunction. J Cell Mol Med. 27:1–14.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matsui H, Musicki B, Sopko NA, Liu X,
Hurley PJ, Burnett AL, Bivalacqua TJ and Hannan JL: Early-stage
type 2 diabetes mellitus impairs erectile function and neurite
outgrowth from the major pelvic ganglion and downregulates the gene
expression of neurotrophic factors. Urology. 99:287.e1–287.e7.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yan CS, Ma Y, Li H, Cui J, Guo X, Wang G
and Ji L: Endoplasmic reticulum stress promotes caspase-1-dependent
acinar cell pyroptosis through the PERK pathway to aggravate acute
pancreatitis. Int Immunopharmacol. 120:1102932023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fan ZH, Xu L, Tian Y, Cao YL, Zhang XY,
Duan ZP and Ren F: The study of a key molecule Caspase-1 of
inflammasome in hepatitis B virus-related diseases. Zhonghua Gan
Zang Bing Za Zhi. 30:1158–1162. 2022.(In Chinese). PubMed/NCBI
|
|
52
|
Wang SH, Sun MJ, Ding SY, Liu CL, Wang JM,
Han SN, Lin X and Li Q: Ticagrelor reduces doxorubicin-induced
pyroptosis of rat cardiomyocytes by targeting GSK-3β/caspase-1.
Front Cardiovasc Med. 9:10906012023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li YF, Nanayakkara G, Sun Y, Li X, Wang L,
Cueto R, Shao Y, Fu H, Johnson C, Cheng J, et al: Analyses of
caspase-1-regulated transcriptomes in various tissues lead to
identification of novel IL-1β-, IL-18- and sirtuin-1-independent
pathways. J Hematol Oncol. 10:402017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yuan YY, Xie KX, Wang SL and Yuan LW:
Inflammatory caspase-related pyroptosis: Mechanism, regulation and
therapeutic potential for inflammatory bowel disease. Gastroenterol
Rep (Oxf). 6:167–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Di LJ, Zha CJ and Liu YH: Platelet-derived
microparticles stimulated by anti-β2GPI/β2GPI
complexes induce pyroptosis of endothelial cells in
antiphospholipid syndrome. Platelets. 34:21564922023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Barnett KC and Ting JP: Mitochondrial
GSDMD pores DAMpen pyroptosis. Immunity. 52:424–426. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fink SL and Cookson BT: Apoptosis,
pyroptosis, and necrosis: Mechanistic description of dead and dying
eukaryotic cells. Infect Immun. 73:1907–1916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Burdette BE, Esparza AN, Zhu H and Wang S:
Gasdermin D in pyroptosis. Acta Pharm Sin B. 11:2768–2782. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kumar V: Inflammation research sails
through the sea of immunology to reach immunometabolism. Int
Immunopharmacol. 73:128–145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gou X, Xu W, Liu Y, Peng Y, Xu W, Yin Y
and Zhang X: IL-6 prevents lung macrophage death and lung
inflammation injury by inhibiting GSDME- and GSDMD-mediated
pyroptosis during pneumococcal pneumosepsis. Microbiol Spectr.
10:e02049212022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ye B, Chen X, Dai S, Han J, Liang X, Lin
S, Cai X, Huang Z and Huang W: Emodin alleviates myocardial
ischemia/reperfusion injury by inhibiting gasdermin D-mediated
pyroptosis in cardiomyocytes. Drug Des Devel Ther. 13:975–990.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lei Q, Yi T and Chen C:
Nf-κB-gasdermin D (GSDMD) axis couples oxidative stress and
NACHT, LRR and PYD domains-containing protein 3 (NLRP3)
inflammasome-mediated cardiomyocyte pyroptosis following myocardial
infarction. Med Sci Monit. 24:6044–6052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen Y, Wang L, Huang ZS, Feng JX, Li SX,
Du ZJ, Zhang ZB, Liu J, Yang J, Hu ZM, et al: Cytoskeletal protein
SPTA1 mediating the decrease in erectile function induced by
high-fat diet via hippo signaling pathway. Andrology. 11:591–610.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song Y, Tian X, Wang X and Feng H:
Vascular protection of salicin on Il-1β-induced endothelial
inflammatory response and damages in retinal endothelial cells.
Artif Cells Nanomed Biotechnol. 47:1995–2002. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Maiorino MI, Bellastella G and Esposito K:
Lifestyle modifications and erectile dysfunction: What can be
expected? Asian J Androl. 17:5–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yasuda K, Nakanishi K and Tsutsui H:
Interleukin-18 in health and disease. Int J Mol Sci. 20:6492019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yoshimoto T and Nakanishi K: Roles of
il-18 in basophils and mast cells. Allergol Int. 55:105–113. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ferlin A, Arredi B, Speltra E, Cazzadore
C, Selice R, Garolla A, Lenzi A and Foresta C: Molecular and
clinical characterization of y chromosome microdeletions in
infertile men: A 10-year experience in italy. J Clin Endocrinol
Metab. 92:762–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Matos G, Hirotsu C, Alvarenga TA, Cintra
F, Bittencourt L, Tufik S and Andersen ML: The association between
TNF-α and erectile dysfunction complaints. Andrology. 1:872–878.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Y, Niu X, Xu H, Li Q, Meng L, He M,
Zhang J and Zhang Z and Zhang Z: VX-765 attenuates atherosclerosis
in ApoE deficient mice by modulating VSMCs pyroptosis. Exp Cell
Res. 389:1118472020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang Y, Li X, Pitzer AL, Chen Y, Wang L
and Li PL: Coronary endothelial dysfunction induced by nucleotide
oligomerization domain-like receptor protein with pyrin domain
containing 3 inflammasome activation during hypercholesterolemia:
Beyond inflammation. Antioxid Redox Signal. 22:1084–1096. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang F, Qin Y, Lv J, Wang Y, Che H, Chen
X, Jiang Y, Li A, Sun X, Yue E, et al: Silencing long non-coding
RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic
cardiomyopathy. Cell Death Dis. 9:10002018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fais RS, Rodrigues FL, Pereira CA, Mendes
AC, Mestriner F, Tostes RC and Carneiro FS: The inflammasome nlrp3
plays a dual role on mouse corpora cavernosa relaxation. Sci Rep.
9:162242019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Luo C, Peng Y, Zhou X, Fan J, Chen W,
Zhang H and Wei A: NLRP3 downregulation enhances engraftment and
functionality of adipose-derived stem cells to alleviate erectile
dysfunction in diabetic rats. Front Endocrinol (Lausanne).
13:9132962022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
He X, Qian Y, Li Z, Fan EK, Li Y, Wu L,
Billiar TR, Wilson MA, Shi X and Fan J: TLR4-upregulated IL-1β and
IL-1RI promote alveolar macrophage pyroptosis and lung inflammation
through an autocrine mechanism. Sci Rep. 6:316632016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu Y, Niu X, Wang G, Huang J, Liu M and
Peng B: Chronic prostatitis/chronic pelvic pain syndrome impairs
erectile function through increased endothelial dysfunction,
oxidative stress, apoptosis, and corporal fibrosis in a rat model.
Andrology. 4:1209–1216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vlachopoulos C, Aznaouridis K, Ioakeimidis
N, Rokkas K, Vasiliadou C, Alexopoulos N, Stefanadi E, Askitis A
and Stefanadis C: Unfavourable endothelial and inflammatory state
in erectile dysfunction patients with or without coronary artery
disease. Eur Heart J. 27:2640–2648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu W, Li M, Zhang X, Zhou Z, Shen Z and
Shen X: Association of polymorphisms in Th1/Th2-related cytokines
(IFN-γ, TGFβ1, IL-1β, IL-2, IL-4, IL-18) with oral lichen planus: A
pooled analysis of case-control studies. J Dent Sci. 18:560–566.
2023. View Article : Google Scholar : PubMed/NCBI
|