Introduction
Despite improvements having been made to standard
treatments, including surgery, radiotherapy and adjuvant
chemotherapy, the prognosis of patients with malignant glioma
remains poor (1). Therefore, the
development of novel treatment strategies for glioma is crucial.
Angiogenesis is a process through which new blood vessels are
generated from pre-existing vessels and this process plays a
critical role in the development and progression of human
malignancies such as glioma (2).
Malignant glioma is considered one of the most extensively
vascularized types of tumor (3).
Therefore, anti-angiogenesis therapy appears to be a promising
treatment strategy for malignant glioma (4).
Previous research has found that fibrinogen E
fragment, formed by plasmin cleavage of human fibrinogen, has an
antiangiogenic effect, which is mainly associated the first 24
amino acids of the α chain of human fibrinogen contained within it.
This 24-amino acid peptide is called alphastatin (5). A previous study confirmed that
alphastatin could inhibit both the migration and tube formation of
endothelial cells (ECs) induced by VEGF or basic fibroblast growth
factor (bFGF) in vitro and inhibit glioma growth by
inhibiting angiogenesis in vivo (6). The mechanism of the anti-angiogenic
effect of alphastatin involves blocking the JNK and ERK
phosphorylation pathways at the initial stage of angiogenesis and
subsequent inhibition of EC migration and differentiation (6). In addition, alphastatin exhibits
selective cytotoxicity on activated ECs in tumor blood vessels,
which could cause extensive thrombosis of tumor blood vessels and
lead to tumor necrosis (5).
Notably, alphastatin selectively disrupts activated ECs in tumor
vessels but has no detectable effect on vessels in normal tissues
such as liver, lungs and kidneys, indicating its potential
therapeutic effect for specifically targeting tumor angiogenesis
(5). Therefore, alphastatin
appears to be a safe and effective anti-angiogenic agent. However,
to maximize the efficacy of alphastatin, it is essential to
identify the appropriate method of administration of this
molecule.
In recent years, the drug delivery system (DDS) has
exhibited tremendous promise for enhancing the therapeutic effects
of drugs. DDS can significantly enhance the pharmaceutical effects
of drugs and can reduce the side effects of therapeutics in the
treatment of various disease conditions (7). Compared with traditional DDS,
cell-mediated DDS has numerous advantages, such as circulating in
the bloodstream for a period of time, abundant surface ligands,
targeting tumor cells and flexible morphology through biological
barriers due to its unique cellular properties (8). Among all cell-mediated DDSs, the
unique biological characteristics of mesenchymal stem cells (MSCs)
make them valuable cytoreagents for tumor gene therapy. MSCs can
relatively easily introduce and persistently express markers and/or
therapeutic genes. MSCs can be directly obtained from patients and
cultured and expanded in vitro, thus avoiding the immune
rejection and ethical concerns caused by the use of allogeneic stem
cells (9). Notably, their ability
to migrate to tumor tissue makes them an ideal delivery vehicle for
tumor treatment (8).
As a type of MSCs, adipose-derived stem cells
(ADSCs) are isolated from human adipose tissue and are
characterized by their morphology, surface markers and their
potential to differentiate into mesenchymal and neuronal lineages
(10). ADSCs have been
demonstrated to be attractive cellular vehicles for gene therapy
against malignancies due to their targeted tropism for cancer and
the intrinsic attribute of autologous transplantation (11). In addition to the above advantages
of MSCs, the success rate of ADSC isolation can reach 100% and the
yield of ADSCs is ~40-fold higher than that of bone marrow-derived
MSCs (11). Therefore, ADSCs can
be easily prepared and genetically modified as targeting DDS for
glioma therapy (12). In summary,
ADSCs have advantages such as tumor tropism, wide availability,
easy accessibility and low immunogenicity, making it an ideal tumor
targeted drug delivery system.
The present study constructed an ADSC-mediated
alphastatin targeted delivery system and investigated its effects
on angiogenesis and tumor growth in glioma.
Materials and methods
Materials
The U87 MG American Type Culture Collection (ATCC)
human glioblastoma cell line of unknown origin (cat no. CL-0238),
SHG-44 human glioma cell line (cat no. CL-0207), 293T cell line
(cat no. CL-0005) and human umbilical vein endothelial cell line
(HUVEC; cat no. CL-0122) were purchased from Procell Life Science
& Technology Co., Ltd. The SVG p12 human astroglia cell line
(cat no. CRL-8621) was purchased from the ATCC. The HUVECs used in
the present study were not primary cells. Suppliers have
authenticated all the aforementioned cell lines by short tandem
repeat profiling. ADSCs (cat no. HUXMD-01001) were purchased from
Cyagen Biosciences, Inc. A total of 15 male, 6-week-old, specific
pathogen-free, BALB/C nude mice, weighing 16–18 g, were purchased
from Changzhou Cavens Laboratory Animal Co., Ltd., and were housed
in a full-barrier rodent facility with a filtered air supply at
constant temperature (25°C) and humidity (50%) under a 12-h
light/dark cycle. Water and food for animals were provided ad
libitum. The housing environment for animals, water and animal
feeds were sterilized. All animal procedures were performed in
accordance with the guidance of the Research Ethics Committee of
The First Affiliated Hospital of Xi'an Jiaotong University
(approval number: 2020-G-263).
Cell culture
The U87, SHG44, 293T and SVG p12 cells were cultured
in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.). HUVECs were cultured in F12K
medium (Procell Life Science & Technology Co., Ltd.)
supplemented with 10% fetal bovine serum. ADSCs were cultured in
human adipose-derived stem cell growth medium (cat. no.
HUXMD-90011; Cyagen Biosciences, Inc.). All cells were cultured in
an atmosphere of 5% CO2 at 37°C.
Construction and transfection of
alphastatin lentivirus
The lentiviral vector plasmid
pLVX-mCMV-ZsGreen-IRES-Puro was purchased from Wuhan Viraltherapy
Technologies Co., Ltd. The pUC57-NT4-alphastatin plasmid containing
the sequence encoding the human neurotrophin-4 (NT4) signal peptide
and alphastatin fusion gene fragment (NT4-Al) and FLAG tag was
synthesized and purchased from GenScript. The NT4-Al-FLAG sequence
was cloned into pLVX-mCMV-ZsGreen-IRES-Puro and identified by
restriction enzyme digestion and sequence analysis. Lentiviral
particles were produced by the transfection of 293T cells with a
lentivirus packaging kit (cat. no. R003; Wuhan Viraltherapy
Technologies Co., Ltd.) according to the manufacturer's
instructions and lentiviral supernatants were collected after 48 h.
The construction of negative control (NC) lentivirus was performed
as described above. ADSCs were seeded in 24-well plates at a
concentration of 5×104 cells/well and infected with
concentrated alphastatin lentiviral particles (Al-ADSCs) or NC
lentiviral particles (NC-ADSCs; multiplicity of infection=20) in an
atmosphere of 5% CO2 at 37°C for 24 h. Successful
transfection of cells was verified by expression of green
fluorescent protein.
Detection of MSC surface markers in
transfected ADSCs
Flow cytometric detection of MSC surface markers was
used to determine whether lentivirus transfection affected the
biological characteristics of ADSCs. Al-ADSCs, NC-ADSCs and ADSCs
were seeded in six-well plates at a concentration of
5×105 cells/well and cultured in an atmosphere of 5%
CO2 at 37°C for 48 h. The cells were then quantified
using a cell counting board and resuspended with 0.1 ml
phosphate-buffered saline (PBS) containing 0.5% bovine serum
albumin (BSA; Thermo Fisher Scientific, Inc.). Allophycocyanin
(APC)-conjugated anti-human CD13 antibody (cat. no. 301705;
BioLegend, Inc.), APC-conjugated anti-mouse/human CD44 antibody
(cat. no. 103011; BioLegend, Inc.), phycoerythrin
(PE)/Cyanine7-conjugated anti-human CD90 (Thy1) antibody (cat. no.
328123; BioLegend, Inc.) and PE-conjugated anti-human CD105
(endoglin) antibody (cat. no. 12-1057-41; eBioscience; Thermo
Fisher Scientific, Inc.) were added according to the manufacturer's
instructions. Following incubation at 4°C for 30 min, the cells
were washed twice with PBS containing 0.5% BSA. A single cell
suspension was prepared by the addition of 0.2 ml PBS and stem cell
surface markers were then detected using flow cytometry (CytoFLEX;
Beckman Coulter, Inc.) and CytExpert 2.4 software (Beckman Coulter,
Inc.).
Western blot analysis
The expression level of alphastatin was detected
indirectly by detecting the expression of the FLAG tag. Extraction
of total cellular proteins and western blot analysis were performed
as previously described (13).
Briefly, cells were washed twice with PBS and lysed on ice
following the addition of 300 µl RIPA buffer (Beyotime Institute of
Biotechnology) with 1 mmol/l phenylmethylsulfonyl fluoride. Protein
concentration was determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Protein samples
(40 µg) were boiled in 1X SDS-PAGE sample loading buffer, resolved
using 10% SDS-PAGE and transferred onto polyvinylidene fluoride
membranes (MilliporeSigma). The membranes were then blocked with
TBS-0.1% Tween-20 (TBST) containing 5% non-fat dry milk at room
temperature for 2 h and probed with primary antibodies overnight at
4°C, followed by incubation with secondary antibodies conjugated to
horseradish peroxidase at 37°C for 2 h. Membranes were developed
using SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo
Fisher Scientific, Inc.). The primary antibodies employed in
western blotting were as follows: Goat anti-FLAG tag antibody
(1:1,000; cat. no. ab1257; Abcam) and rabbit anti-GAPDH antibody
(1:5,000; cat. no. ap0063, Bioworld Technology, Inc.). The
secondary antibodies employed in western blotting were as follows:
HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody
(1:5,000; cat. no. BA1054) for GAPDH detection and HRP-conjugated
rabbit anti-goat IgG antibody (1:5,000; cat. no. BA1060) for FLAG
tag detection (both from Wuhan Boster Biological Technology, Ltd.).
ImageJ 1.8 software (National Institutes of Health) was used for
densitometry.
Measurement of alphastatin secretion
from ADSCs
The secretion levels of alphastatin from alphastatin
lentivirus-transfected ADSCs (Al-ADSCs) were assessed using a FLAG
tag protein ELISA kit (cat. no. E4700-100; BioVision, Inc.).
Briefly, the supernatant of ADSCs in each group was collected at 48
h following infection with lentivirus. The samples were centrifuged
at room temperature for 20 min at 850 × g to remove particulates
and were immediately assayed using the aforementioned kit according
to the manufacturer's instructions.
Isolation of CD133+ glioma
stem cells (GSCs)
U87 and SHG44 cells were resuspended in 1 ml PBS
containing 0.5% BSA in an Eppendorf tube containing with
1×106 cells per tube. Following centrifugation (200 × g
for 3 min at 4°C), the cells were washed with PBS containing 0.5%
BSA once and then incubated with 5 µl anti-CD133 antibody (cat. no.
17-1338-42; Invitrogen; Thermo Fisher Scientific, Inc.) at 4°C for
30 min. Subsequently, the cells were washed twice with PBS
containing 0.5% BSA and resuspended for cell sorting using a flow
cytometer (FACSAria III; BD Biosciences).
In vitro analysis of tropism of
transfected ADSCs to GSCs
Cell migration assay was used to analyze the tropism
of Al-ADSCs to GSCs in vitro. Transwell chambers (cat. no.
353097; BD Biosciences) with 8-µm pore size polyethylene
terephthalate (PET) membranes were placed in 24-well plates
(Corning, Inc.). GSCs, CD133− glioma cells or SVG p12
cells were seeded in the lower chambers (2.5×105
cell/well) and cultured overnight in an atmosphere of 5%
CO2 at 37°C. Cell suspension of Al-ADSCs, NC-ADSCs or
ADSCs (3×105 cells/ml) was then added to the upper
chambers (200 µl/well) and co-cultured with cells in the lower
chamber in an atmosphere of 5% CO2 at 37°C overnight.
The migrated cells adhering to the bottom surface of the membranes
were fixed with a 70% ethanol solution at 4°C for 1 h and stained
with 0.5% crystal violet (cat. no. C0121; Beyotime Institute of
Biotechnology) at room temperature for 10 min. The migrated cells
were then counted under an optical microscope (IX51; Olympus
Corporation; magnification, ×200).
In vitro tube formation assay
The effect of Al-ADSCs on the angiogenesis of ECs
was analyzed using a tube formation assay in vitro. Matrigel
(cat. no. 356234; Corning, Inc.) was incubated at 4°C for 24 h and
then 0.1 ml Matrigel was evenly plated onto 24-well plates
(Corning, Inc.), precooled at −20°C for ≥10 min and then incubated
for 30 min at 37°C. Subsequently, HUVECs (1.5×105
cells/well) were seeded onto the Matrigel-coated plates and
incubated in an atmosphere of 5% CO2 at 37°C for 2.5 h.
Transwell chambers (cat. no. 353095; BD Biosciences) with 0.4-µm
pore size PET membranes were then placed in the 24-well plates and
a cell suspension of Al-ADSCs, NC-ADSCs or ADSCs (3×105
cells/ml) was added to the Transwell chambers (200 µl/well). The
cells in the upper and lower chambers were co-cultured in an
atmosphere of 5% CO2 at 37°C for 8 h. The EC-derived
tube-like structures in each well were visualized and analyzed
directly under an optical microscope (IX51; Olympus Corporation;
magnification, ×100).
EC scratch wound healing assay
A scratch wound healing assay was used to examine
the effects of Al-ADSCs on the migration of ECs. The HUVECs were
seeded at a density of 5×105 cells/well in six-well
plates. After 24 h, the cell monolayer was scraped in a straight
line using a 20-µl pipette tip and the cells were washed three
times with PBS. Transwell chambers (cat. no. 353090; BD
Biosciences) with 0.4-µm pore size PET membranes were then placed
in the six-well plates and a cell suspension of Al-ADSCs, NC-ADSCs
or ADSCs (5×105 cells/well) was added to the Transwell
chambers. The cells in the upper and low chambers were co-cultured
in serum-free medium in an atmosphere of 5% CO2 at 37°C
for 24 h. Images of the plates were captured under an optical
microscope (IX51; Olympus Corporation; magnification, ×100) at 0
and 24 h, respectively. ImageJ 1.8 software (National Institutes of
Health) was used to measure the migration area and the percentage
of wound healing was calculated. The calculation method was (Area
0 h-Area 24 h)/Area 0 h.
Cell Counting Kit-8 (CCK-8) cell
proliferation assay
A CCK-8 kit (cat. no. E-CK-A362; Elabscience
Biotechnology, Inc.) was used to examine the effect of Al-ADSCs on
the proliferation of ECs. HUVECs were seeded at a density of
5×103 cells/well in 24-well plates. After 24 h,
Transwell chambers (cat. no. 353095; BD Biosciences) with 0.4-µm
pore size PET membranes were placed in the 24-well plates and a
cell suspension of Al-ADSCs, NC-ADSCs or ADSCs (5×103
cells/well) was added to the Transwell chambers. The cells in the
upper and low chambers were co-cultured in an atmosphere of 5%
CO2 at 37°C. The HUVECs in low chambers were then
analyzed by CCK-8 assay at 24 and 48 h following co-culture.
Briefly, 50 µl CCK-8 solution was added to each well. Following
incubation at 37°C for 4 h, the absorbance at 450 nm was measured
with a microplate reader (Flexstation 3; Molecular Devices,
LLC).
Animal experiments
The establishment of intracranial xenograft models
was conducted as previously described (14). The 15 BALB/C nude mice were
randomly divided into three groups. To prevent accidental animal
death, one additional mouse was added to each group. Each group of
six mice was randomly numbered 1 to 6. The animals were
anaesthetized with an intraperitoneal injection of 30 mg/kg
pentobarbital sodium. U87 glioma cells transfected with
LV-mCherry-Puromycin Lentivirus [cat. no. FLV050; Fubio (Suzhou)
Biomedical Technology Co., Ltd.] and able to stably express a red
fluorescent protein mCherry were injected intracranially using a
stereotaxic apparatus (2.5 µl/mouse; cell concentration,
2×105 cells/10 µl). At day 3 after model establishment,
a suspension of NC-ADSCs or Al-ADSCs (2.5 µl/mouse; cell
concentration, 2×105 cells/10 µl) was injected into the
periphery of the tumor modeling area. The number of intracranial
injection cells followed the guidelines for the welfare and use of
animals in cancer research (15).
Animal health and behavior were monitored twice a week. After 28
days of tumor cell implantation, due to no accidental animal death,
5 mice numbered 1 to 5 in each group underwent subsequent
experiments, while mice numbered 6 continued to be raised until the
humane endpoint. The 15 tumor-bearing mice (5 mice/group) were
sacrificed after observing intracranial tumorigenesis using an
in vivo imaging instrument (IVIS Lumina III; PerkinElmer,
Inc.) at 28 days following tumor cell implantation by an
intraperitoneal injection of 200 mg/kg pentobarbital sodium
solution. Cardiac arrest for 2 min was used to identify mortality.
The humane endpoints included labored breathing, inability to
remain upright, impaired mobility, a hunched posture for >48 h
and no response to external stimuli. The mice were perfused with 4%
paraformaldehyde for 1 h at 4°C immediately following sacrifice,
before the brain and tumor samples were obtained and fixed
overnight with 4% paraformaldehyde at 4°C. Paraffin-embedded
sections were prepared of a thickness of 4 µm. Briefly, the tissues
were dehydrated by serial incubations in 75% alcohol (4 h), 85%
alcohol (2 h), 90% alcohol (1.5 h), 95% alcohol and 100% alcohol (2
times, 0.5 h each). The tissues were then washed in a 1:1 mixture
of 100% alcohol and xylene (10 min) and xylene (2 times, 10 min
each). After washing, the tissues were immersed in paraffin (3
times, 1 h each) and then embedded in paraffin blocks. The
distribution of NC-ADSCs or Al-ADSCs was observed under a
fluorescence microscope (BX53; Olympus Corporation).
Detection of CD133+ GSCs in
the xenograft model
CD133 immunofluorescence staining was used to
detected CD133+ GSCs in the xenograft model. Sections
were incubated overnight with an anti-CD133 antibody (cat. no.
66666-1-IG; Proteintech Group, Inc.) diluted 1:100 at 4°C. The NC
sections were incubated with PBS instead of the antibody. After
washing, the sections were incubated with cyanine 3-conjugated goat
anti-mouse IgG (cat. no. BA1031; Wuhan Boster Biological
Technology, Ltd.) diluted 1:100 at 37°C for 1 h. The
CD133+ GSCs were observed under a fluorescence
microscope (BX53; Olympus Corporation).
Microvessel density (MVD) detection in
xenograft models
CD34 staining was performed to detect MVD. All
procedures were performed according to the manufacturer's
protocols. The sections were incubated overnight at 4°C with a
rabbit anti-CD34 antibody (1:200; cat. no. ab81289; Abcam). The NC
sections were incubated with PBS instead of the antibody. Antibody
localization was determined using a 3,3′-diaminobenzidine substrate
kit (Wuhan Boster Biological Technology, Ltd.). MVD was evaluated
by detecting the cluster of CD34+ cells. Briefly, the
tumor sections were scanned at low magnifications (×40 or ×100) to
determine the areas of most intense tumor angiogenesis, termed ‘hot
spots’. Following ‘hot spot’ identification, the MVD was calculated
by averaging the number of individual microvessels in five fields
under an optical microscope (IX51; Olympus Corporation) at high
magnification (×400).
Statistical analysis
Data are presented as the mean ± standard deviation
of three replicates and analyzed using SPSS 17.0 software (SPSS,
Inc.). One-way analysis of variance was used to compare the groups
and the least significant difference post hoc test (≤3 groups) or
Tukey's test (>3 groups) were performed to further determine
inter-group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction of an ADSC-mediated
alphastatin targeted delivery system
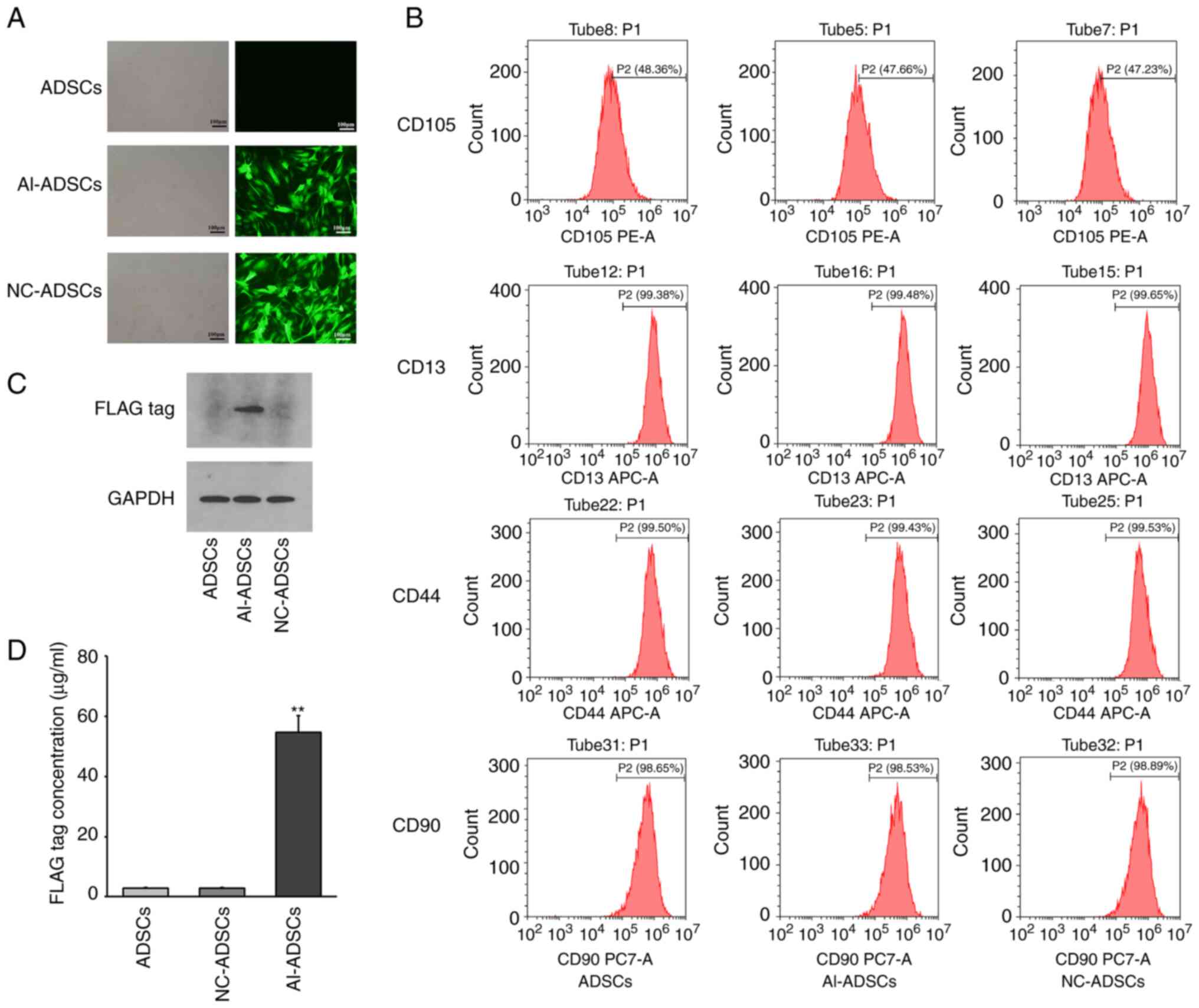
As presented in Fig.
1, following transfection with alphastatin lentivirus (Fig. 1A), the FLAG tag protein could be
detected in Al-ADSCs, which indicated that the Al-ADSCs expressed
alphastatin. No alphastatin expression was detected in the control
or NC groups (Fig. 1C). In
addition, FLAG tag protein could be detected in the supernatant of
Al-ADSCs using ELISA, which demonstrated that Al-ADSCs had the
function of exocrine alphastatin secretion (Fig. 1D). Compared with the ADSCs, there
was no significant difference in the expression of MSC surface
markers, including CD13, CD44, CD90 and CD105, in Al-ADSCs, which
indicated that alphastatin lentiviral transfection had no
significant effect on the stem cell characteristics of ADSCs
(Fig. 1B).
Isolation of GSCs and tropism of ADSCs
to GSCs
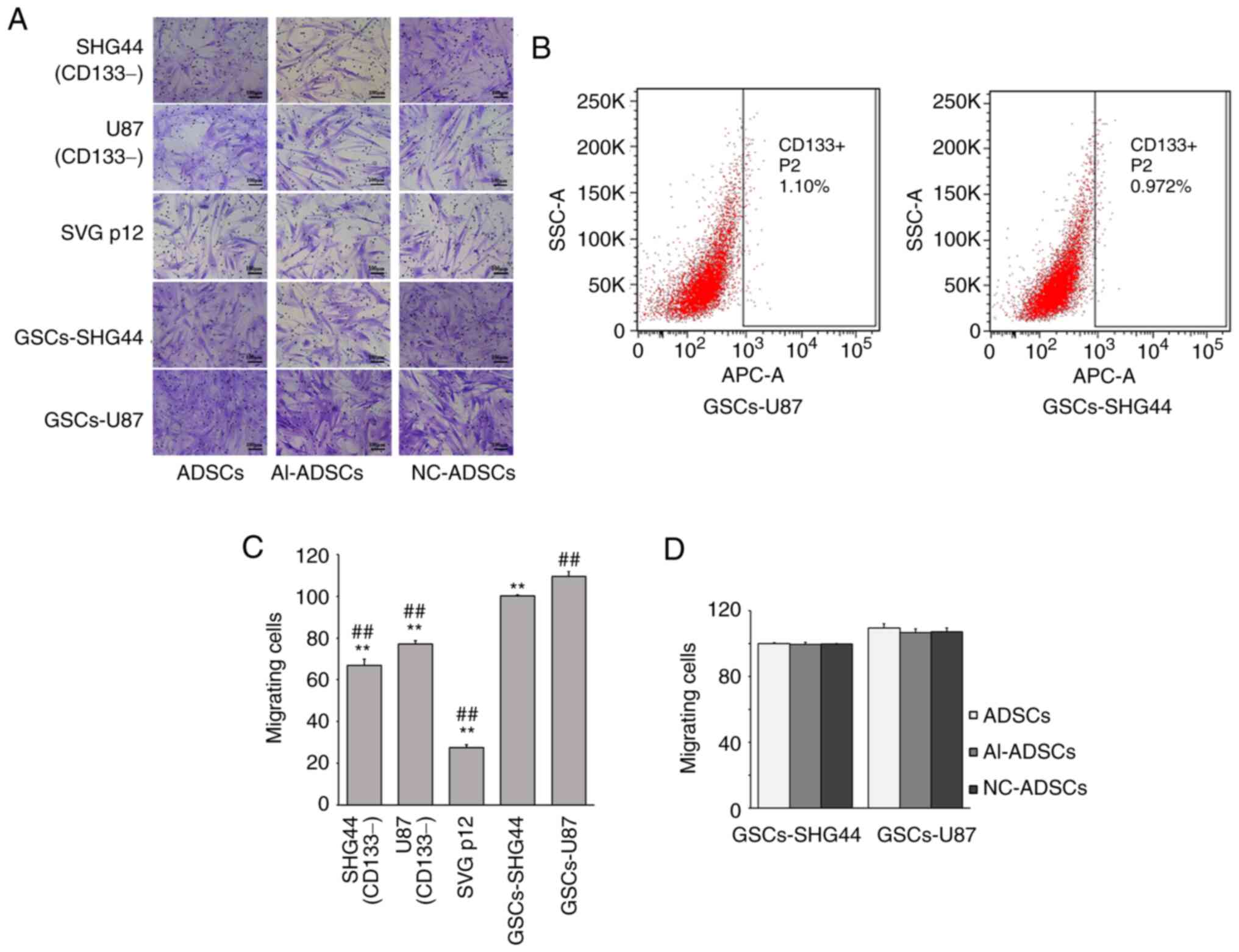
As presented in Fig.
2, GSCs were isolated from U87 and SHG44 glioma cell lines
using flow cytometry (Fig. 2B).
Cell migration assay revealed that ADSCs exhibited more obvious
tropism to GSCs than to CD133− glioma or SVG p12 cells
(F=708.439; P=0.001; Fig. 2A and
C). There was no significant difference between ADSCs, NC-ADSCs
or Al-ADSCs regarding tropism to GSCs (F=0.484; P=0.639 for
GSCs-SHG44; and F=1.122; P=0.386 for GSCs-U87; Fig. 2D), which indicated that alphastatin
lentiviral transfection had no significant effect on the tropism of
ADSCs to GSCs.
Effects of ADSC-mediated alphastatin
targeted delivery system on ECs in vitro
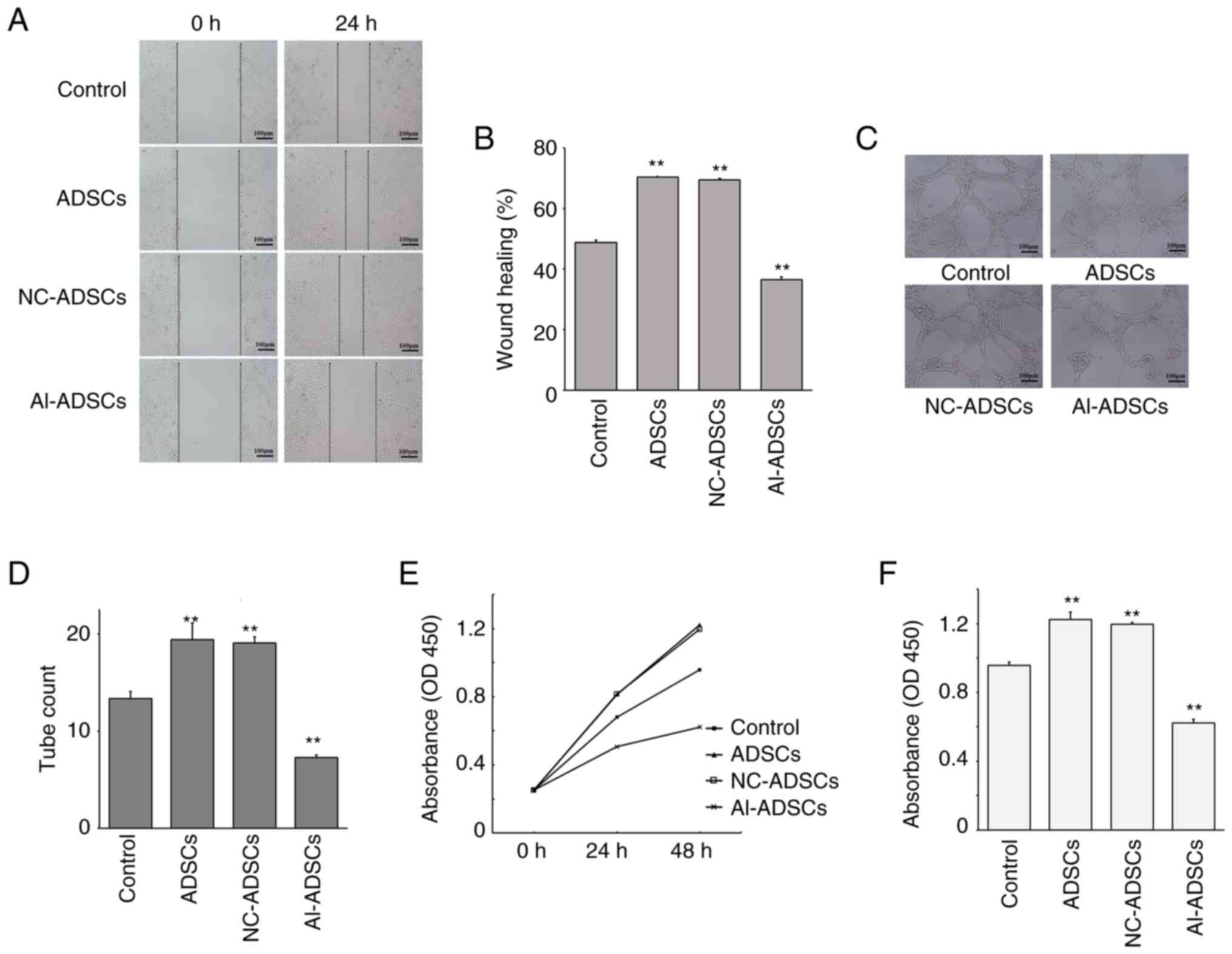
As presented in Fig.
3, tube formation assay revealed that Al-ADSCs significantly
inhibited the angiogenesis of HUVECs in vitro (F=98.262;
P=0.001; Fig. 3C and D). Scratch
wound healing assay revealed that the wound healing ability of the
HUVECs decreased following coculture with Al-ADSCs, suggesting that
the Al-ADSCs inhibited HUVEC migration (F=554.852; P=0.001;
Fig. 3A and B). In addition, cell
proliferation assay revealed that the proliferation of HUVECs was
inhibited by Al-ADSCs. At 48 h following co-culture with Al-ADSCs,
the absorbance of HUVECs at 450 nm was lower than that of the
control group, indicating that Al-ADSCs significantly inhibited the
proliferation of HUVECs (F=310.614; P=0.001; Fig. 3E and F). Notably, the ADSCs and
NC-ADSCs exerted promoting effects on the proliferation, migration
and tube formation abilities of HUVECs in vitro.
Effects of ADSC-mediated alphastatin
targeted delivery system on the glioma xenograft model
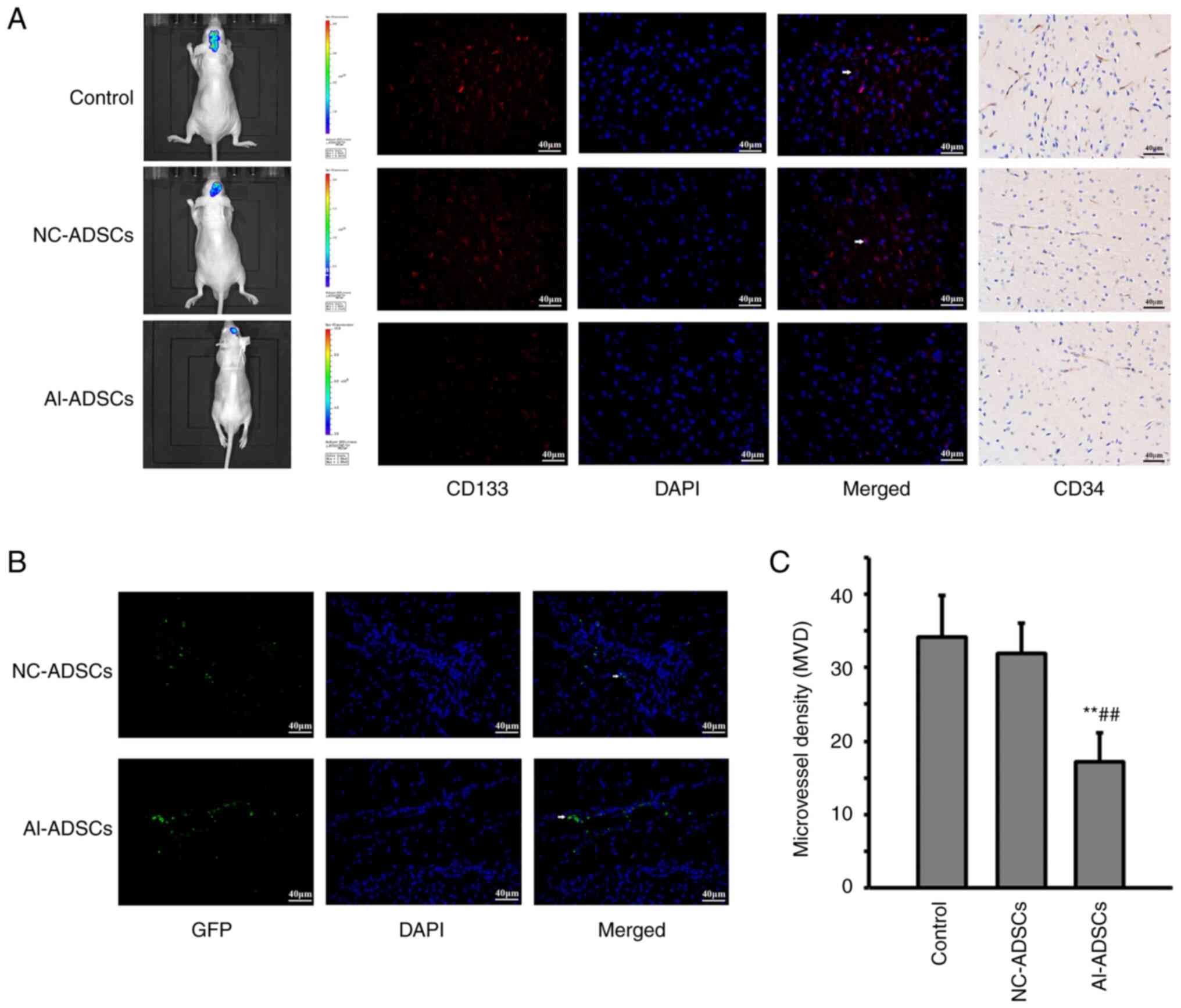
As presented in Fig.
4, both NC-ADSCs and Al-ADSCs were detected in glioma xenograft
tissue using a fluorescence microscope (Fig. 4B), which indicated that Al-ADSCs
could migrate into tumor tissue. In vivo imaging revealed
that the Al-ADSCs significantly inhibited tumor growth in the
glioma xenograft model. Furthermore, the number of
CD133+ cells in the Al-ADSC group was significantly
reduced (Fig. 4A).
Immunohistochemical assay revealed that Al-ADSCs significantly
reduced the MVD in glioma xenograft tissue (F=16.236; P=0.001;
Fig. 4C).
Discussion
Alphastatin has been confirmed to exert an antitumor
effect in several tumor models, such as gastric cancer (16), mammary carcinoma (17) and glioma (6,18).
The antitumor effect of alphastatin is mainly due to its
anti-angiogenic effect. Alphastatin can not only inhibit new vessel
growth, but can also significantly lead to the regression of
existing tumor vessels (18).
Although alphastatin has been shown to exert an effective antitumor
effect in previous studies (6,18),
it is necessary to identify an appropriate method of administration
in order to maximize its effect. In previous studies,
lentivirus-mediated gene transfer was used to express alphastatin
in HUVECs and human glioma cell lines (6,18).
Since the alphastatin sequence alone is not sufficient for
efficient expression and secretion in mammalian cells, the
alphastatin coding sequence should be fused to a signal peptide
sequence to allow sufficient secretion of expressed alphastatin. At
present, the NT4 signal peptide is one of the commonly used signal
peptides for the construction of secreted and expressed peptide
fusion genes. We successfully fused the NT4 signal peptide and
pro-region sequence to the alphastatin coding sequence (NT4-Al) in
our previous research and verified its expression effect (19). Therefore, the present study
continued to use this fusion gene as the expression sequence of
alphastatin. It has been demonstrated that the recombinant
lentivirus-mediated NT4-Al gene delivery system of alphastatin can
significantly suppress tumor growth and tumor angiogenesis in a
xenograft glioma model (6,18). In the present study, a lentiviral
vector carrying the NT4-Al fusion gene was used to infect ADSCs to
construct an ADSC-mediated alphastatin targeted delivery system.
The results revealed that NT4-Al lentiviral transfection did not
affect the stem cell characteristics of ADSCs or the tropism to
GSCs. At the same time, the ADSCs transfected with NT4-Al
lentivirus significantly expressed and secreted alphastatin. These
results indicated that an ADSC-mediated alphastatin targeted
delivery system was successfully constructed.
Subsequently, the present study examined the effects
of the ADSC-mediated alphastatin targeted delivery system on EC
proliferation, migration and angiogenesis in vitro. The
results revealed that the ADSC-mediated alphastatin targeted
delivery system significantly inhibited the angiogenesis, migration
and proliferation of ECs in vitro. Previous studies have
shown that alphastatin inhibits the migration and tubule formation
of ECs mediated by VEGF or bFGF (5,6,18).
As VEGF and bFGF do not bind to alphastatin directly (5), it is hypothesized that
antiangiogenetic activity of alphastatin probably operates at a
post-receptor locus common to the VEGF and bFGF signaling pathways.
MAPK signals have been reported to be involved in VEGF and bFGF
mediated EC proliferation, migration, differentiation and lumen
formation. Among them, activation of the ERK-MAPK pathway can
promote endothelial cell proliferation, while the JNK-MAPK pathway
plays an important role in both EC proliferation and migration
(20). Our previous study
confirmed that alphastatin attenuated the VEGF or bFGF induced
phosphorylation of JNK and ERK at the initial stage of
angiogenesis, thus inhibiting the proliferation and migration of
ECs and ultimately inhibiting angiogenesis (6). A further study suggested that
alphastatin inhibited the downregulation of vascular endothelial
cadherin (VE-cadherin) on the surface of EC induced by VEGF or
bFGF, which led to the turnover of VE-cadherin on the EC membrane
(18). VE-cadherin is an adhesion
molecule crucial for vascular integrity and endothelial cell
survival (21). VE-cadherin
turnover on the EC membrane can suppress angiogenesis and is
closely related to the MAPK pathways (22). Therefore, our previous data suggest
that the anti-angiogenic mechanism of alphastatin is partially
achieved by inhibiting the activation of JNK and ERK kinases and
blocking the VEGF and bFGF signaling pathways, leading to VE
cadherin turnover in ECs and then inhibiting EC migration and
differentiation, thus inhibiting angiogenesis. In addition, the
inhibition of angiogenesis by alphastatin also involves the
sphingosine kinase/sphingosine-1-phospate (SPK/S1P) signaling
pathway. It has been confirmed that the SKP/S1P signaling pathway
is involved in the regulation of hepatocyte growth factor-induced
EC migration (23) and also
mediates the activation of the MAPK signaling pathway induced by
VEGF (24), thus playing an
important role in EC angiogenesis. Another study indicated that
alphastatin decreases EC SPK activity and reduced S1P production,
which inhibits the process of angiogenesis (16). However, the mechanism by which
alphastatin inhibits endothelial cell angiogenesis may go beyond
this. A previous study has shown that after alphastatin treatment,
29 proteins are significantly differentially expressed in ECs
(25). Among them are nucleoside
diphosphate kinase B (NDPKB) and profilin-2 (PFN2), which are
involved in EC angiogenesis. NDPKB is essential for VEGF-induced
angiogenesis and contributes to the correct localization of VEGF
receptor type 2 and VE-cadherin at the endothelial adherens
junctions (26). PFN2 promotes EC
proliferation, migration and tube formation through the
phosphoinositide 3-kinase-PFN2-ERK axis (27). Alphastatin significantly inhibits
the expression of NDPKB and PFN2 in EC (25), which may also be one of the
possible mechanisms by which alphastatin inhibits EC angiogenesis.
The above data indicate that the mechanism by which alphastatin
inhibits angiogenesis is very complex and further research is
needed.
In addition, the present study found that ADSCs
promoted the proliferation, migration and tube formation abilities
of ECs, which was consistent with previous findings (28,29).
Of note, the present study demonstrated that alphastatin
significantly inhibited these promoting effects of ADSCs on ECs.
The promoting effect of ADSCs on ECs is mainly mediated through a
variety of angiogenic factors contained in extracellular vesicles,
such as VEGF (29), and
alphastatin can inhibit the migration and differentiation of ECs in
the presence of VEGF or bFGF (6).
This may be one of the reasons why it can inhibit ADSC-mediated
angiogenesis. However, the mechanisms involved warrant further
investigation.
In animal experiments, the tropism of the
ADSC-mediated alphastatin targeted delivery system to glioma in
vivo was confirmed. ADSC can be chemotactically recruited into
glioma (30). The stromal-derived
factor-1/C-XC chemokine receptor type 4 axis plays a critical role
in this innate tumor-homing ability of ADSCs (12). Previous studies have shown that
ADSCs can be used as an effective drug targeted delivery system for
glioma (31,32). In addition, the safety of the
ADSC-mediated drug delivery system has also been preliminarily
verified (31,33). The present study found that the
ADSC-mediated alphastatin targeted delivery system could migrate
into glioma tissue, which inhibited angiogenesis and tumor growth.
Notably, the number of GSCs decreased significantly in tumors with
the ADSC-mediated alphastatin targeted delivery system. GSCs reside
in specialized niche where interaction with the microenvironment
regulates their stem cell behavior (34). ECs in the GSC niche
microenvironment play a key role in the maintenance and
self-renewal of GSCs (35).
Currently, there is no evidence to suggest that alphastatin can
directly affect GSC. However, a previous study showed that
disrupting the niche microenvironment by inhibiting angiogenesis
can hinder the self-renewal of GSCs (36). Therefore, considering the
inhibitory effect of alphastatin on EC proliferation, migration and
angiogenesis, the reduction of GSCs may be associated with the
interference of alphastatin of the interaction between ECs and GSCs
in the GSC niche. The decreased number of GSCs can also affect
tumor growth (37). Therefore, the
mechanisms of the ADSC-mediated alphastatin targeted delivery
system regarding the inhibition of glioma growth may be mediated
via the suppression of tumor angiogenesis and GSCs. However, these
mechanisms require further investigation.
As aforementioned, the inhibitory effect of
alphastatin on tumor angiogenesis has been confirmed in previous
studies. The present study innovatively established an
ADSC-mediated alphastatin targeting delivery system. Through this
system, the specific expression of alphastatin and its
anti-angiogenesis effect in glioma tissue have been achieved, which
has not been previously reported. In addition, the present study
found that targeted anti-angiogenic strategy using ADSCs as
carriers appeared to reduce the number of GSCs in glioma tissue,
which has rarely been reported in previous studies. This appears to
provide some new ideas for glioma treatment strategies targeting
GSCs. In summary, the present study may provide a new strategy for
the treatment of glioma.
As the ADSC-mediated alphastatin targeted delivery
system is a new concept, there are currently no other studies that
use this targeted delivery system to treat glioma or other types of
tumors. Therefore, more research is needed to analyze its
advantages and limitations in the treatment of tumors. However,
there are still some noteworthy issues in this delivery system.
First, exogenous therapeutic genes may affect the biological
characteristics of ADSCs. Therefore, after introducing exogenous
genes, it is necessary to evaluate the tumor tropism and stem cell
characteristics of ADSCs. Second, before clinical application, the
safety of this delivery system needs to undergo a more
comprehensive and rigorous evaluation. Third, since the present
study only observed the anti-glioma effect of the targeted delivery
system for a relatively short period of time, its long-term
efficacy needs further observation.
In conclusion, in the present study, an
ADSC-mediated alphastatin targeted delivery system was constructed
and its tumor tropism to glioma was confirmed in vitro and
in vivo. Furthermore, this targeted delivery system was
demonstrated to be able to express and secrete alphastatin, which
inhibited angiogenesis and reduced the number of GSCs and inhibited
tumor growth. These results indicated that the ADSC-mediated
alphastatin targeted delivery system is a promising strategy for
glioma treatment. However, since it is a preliminary study, the
present study had certain limitations. First, it did not evaluate
the effect of the ADSC-mediated alphastatin targeted delivery
system on blood vessels in normal tissues throughout the body.
Second, the underlying mechanisms by which this delivery system
inhibits glioma angiogenesis, GSCs and tumor growth warrant further
investigation. Furthermore, the present study did not directly
detect alphastatin secreted by this targeted delivery system using
methods such as time-of-flight mass spectrometry. These will be the
focus of future research.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from Key Research and
Development Plan of Shaanxi Province, China (grant no.
2023-YBSF-242), Natural Science Basic Research Program of Shaanxi
(grant nos. 2019JQ-247 and 2022JQ-792) and National Natural Science
Foundation of China (grant no. 82102014).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL was responsible for writing the main manuscript
and data analysis. CL and CN contributed to the study design. TW
and TZ contributed to the molecular biology experiment and animal
experiment. CL and CN confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study has been approved by the Research
Ethics Committee of The First Affiliated Hospital of Xi'an Jiaotong
University (approval no. 2020-G-263) and complies with the
International Society for Stem Cell Research guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Lukas RV and Hegi ME: Improving
survival in molecularly selected glioblastoma. Lancet. 393:615–617.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahir BK, Engelhard HH and Lakka SS: Tumor
development and angiogenesis in adult brain tumor: Glioblastoma.
Mol Neurobiol. 57:2461–2478. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
García-Romero N, Palacín-Aliana I, Madurga
R, Carrión-Navarro J, Esteban-Rubio S, Jiménez B, Collazo A,
Pérez-Rodríguez F, Ortiz de Mendivil A, Fernández-Carballal C, et
al: Bevacizumab dose adjustment to improve clinical outcomes of
glioblastoma. BMC Med. 18:1422020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schulte JD, Aghi MK and Taylor JW:
Anti-angiogenic therapies in the management of glioblastoma. Chin
Clin Oncol. 10:372021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Staton CA, Brown NJ, Rodgers GR, Corke KP,
Tazzyman S, Underwood JC and Lewis CE: Alphastatin, a 24-amino acid
fragment of human fibrinogen, is a potent new inhibitor of
activated endothelial cells in vitro and in vivo. Blood.
103:601–606. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo SW, Che HM and Li WZ: Anti-tumor
effect of lentivirus-mediated gene transfer of alphastatin on human
glioma. Cancer Sci. 102:1038–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quiñones JP, Peniche H and Peniche C:
Chitosan based self-assembled nanoparticles in drug delivery.
Polymers (Basel). 10:2352018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu H, Yang Z, Li F, Xu L and Sun Y:
Cell-mediated targeting drugs delivery systems. Drug Deliv.
27:1425–1437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Porada CD and Almeida-Porada G:
Mesenchymal stem cells as therapeutics and vehicles for gene and
drug delivery. Adv Drug Deliv Rev. 62:1156–1166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Liu Y, Chen Y, Yuan L, Liu H,
Wang J, Liu Q and Zhang Y: Adipose-derived stem cells: Current
applications and future directions in the regeneration of multiple
tissues. Stem Cells Int. 2020:88108132020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kucerova L, Altanerova V, Matuskova M,
Tyciakova S and Altaner C: Adipose tissue-derived human mesenchymal
stem cells mediated prodrug cancer gene therapy. Cancer Res.
67:6304–6313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SA, Lee JY, Kwon SE, Wang KC, Phi JH,
Choi JW, Jin X, Lim JY, Kim H and Kim SK: Human adipose
tissue-derived mesenchymal stem cells target brain tumor-initiating
cells. PLoS One. 10:e01292922015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang C, Shangguan J, Yang L and Guo S:
Downregulation of astrocyte elevated gene-1 expression inhibits the
development of vasculogenic mimicry in gliomas. Exp Ther Med.
21:222021.PubMed/NCBI
|
|
14
|
Liang C, Guo S and Yang L: Effects of
all-trans retinoic acid on VEGF and HIF-1α expression in glioma
cells under normoxia and hypoxia and its anti-angiogenic effect in
an intracerebral glioma model. Mol Med Rep. 10:2713–2719. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, et al: Guidelines for the welfare and use of animals in
cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Li T, Li R, Wei B and Peng Z:
Alphastatin downregulates vascular endothelial cells sphingosine
kinase activity and suppresses tumor growth in nude mice bearing
human gastric cancer xenografts. World J Gastroenterol.
12:4130–4136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Staton CA, Stribbling SM,
Garcia-Echeverria C, Bury JP, Tazzyman S, Lewis CE and Brown NJ:
Identification of key residues involved in mediating the in vivo
anti-tumor/anti-endothelial activity of alphastatin. J Thromb
Haemost. 5:846–854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Che H, Song J, Guo S, Wang W and Gao G:
Inhibition of xenograft human glioma tumor growth by
lentivirus-mediated gene transfer of alphastatin. Oncol Rep.
29:1101–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo SW, Che HM and Li WZ: Construction of
recombinant lentivirus vector for tumor vasoinhibitory peptide
alphastatin gene delivery. Mol Med Rep. 3:923–928. 2010.PubMed/NCBI
|
|
20
|
Meadows KN, Bryant P, Vincent PA and
Pumiglia KM: Activated Ras induces a proangiogenic phenotype in
primary endothelial cells. Oncogene. 23:192–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carmeliet P and Collen D: Molecular basis
of angiogenesis. Role of VEGF and VE-cadherin. Ann N Y Acad Sci.
902:249–264. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chrifi I, Louzao-Martinez L, Brandt M, van
Dijk CGM, Burgisser P, Zhu C, Kros JM, Duncker DJ and Cheng C:
CMTM3 (CKLF-Like marvel transmembrane domain 3) mediates
angiogenesis by regulating cell surface availability of VE-cadherin
in endothelial adherens junctions. Arterioscler Thromb Vasc Biol.
37:1098–1114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan HF, Wu CT, Lu Y, Wang H, Liu HJ,
Zhang QW, Jia XX, Lu ZZ and Wang LS: Sphingosine kinase activation
regulates hepatocyte growth factor induced migration of endothelial
cells. Exp Cell Res. 298:593–601. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu X, Wu W, Mosteller RD and Broek D:
Sphingosine kinase mediates vascular endothelial growth
factor-induced activation of ras and mitogen-activated protein
kinases. Mol Cell Biol. 22:7758–7768. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang XX, Sun RJ, Wu M, Li T, Zhang Y and
Chen L: Differential protein expression in EC304 gastric cancer
cells induced by alphastatin. Asian Pac J Cancer Prev.
13:1667–1674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng Y, Gross S, Wolf NM, Butenschön VM,
Qiu Y, Devraj K, Liebner S, Kroll J, Skolnik EY, Hammes HP and
Wieland T: Nucleoside diphosphate kinase B regulates angiogenesis
through modulation of vascular endothelial growth factor receptor
type 2 and endothelial adherens junction proteins. Arterioscler
Thromb Vasc Biol. 34:2292–2300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Huo X, Chen K, Yang F, Tan W, Zhang
Q, Yu H, Li C, Zhou D, Chen H, et al: Profilin 2 and endothelial
exosomal profilin 2 promote angiogenesis and myocardial infarction
repair in mice. Front Cardiovasc Med. 9:7817532022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang B, Huang LF, Zhao L, Zeng Z, Wang X,
Cao D, Yang L, Ye Z, Chen X, Liu B, et al: Microvesicles (MIVs)
secreted from adipose-derived stem cells (ADSCs) contain multiple
microRNAs and promote the migration and invasion of endothelial
cells. Genes Dis. 7:225–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gangadaran P, Rajendran RL, Oh JM, Oh EJ,
Hong CM, Chung HY, Lee J and Ahn BC: Identification of angiogenic
cargo in extracellular vesicles secreted from human adipose
tissue-derived stem cells and induction of angiogenesis in vitro
and in vivo. Pharmaceutics. 13:4952021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pendleton C, Li Q, Chesler DA, Yuan K,
Guerrero-Cazares H and Quinones-Hinojosa A: Mesenchymal stem cells
derived from adipose tissue vs bone marrow: In vitro comparison of
their tropism towards gliomas. PLoS One. 8:e581982013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chuang CC, Chen YN, Wang YY, Huang YC, Lin
SY, Huang RY, Jang YY, Yang CC, Huang YF and Chang CW: Stem
cell-based delivery of gold/chlorin e6 nanocomplexes for combined
photothermal and photodynamic therapy. ACS Appl Mater Interfaces.
12:30021–30030. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang WC, Lu IL, Chiang WH, Lin YW, Tsai
YC, Chen HH, Chang CW, Chiang CS and Chiu HC: Tumortropic
adipose-derived stem cells carrying smart nanotherapeutics for
targeted delivery and dual-modality therapy of orthotopic
glioblastoma. J Control Release. 254:119–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng Y, Zhu M, Dangelmajer S, Lee YM,
Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q,
Zhang H, et al: Hypoxia-cultured human adipose-derived mesenchymal
stem cells are non-oncogenic and have enhanced viability, motility,
and tropism to brain cancer. Cell Death Dis. 5:e15672014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heddleston JM, Hitomi M, Venere M,
Flavahan WA, Yang K, Kim Y, Minhas S, Rich JN and Hjelmeland AB:
Glioma stem cell maintenance: The role of the microenvironment.
Curr Pharm Des. 17:2386–2401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schiffer D, Annovazzi L, Casalone C,
Corona C and Mellai M: Glioblastoma: Microenvironment and niche
concept. Cancers (Basel). 11:52018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Calabrese C, Poppleton H, Kocak M, Hogg
TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et
al: A perivascular niche for brain tumor stem cells. Cancer Cell.
11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng L, Ming Y, Zhang L, Zhou J, Xiang W,
Zeng S, He H and Chen L: MicroRNA-30a suppresses self-renewal and
tumorigenicity of glioma stem cells by blocking the NT5E-dependent
Akt signaling pathway. FASEB J. 34:5128–5143. 2020. View Article : Google Scholar : PubMed/NCBI
|