Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer and ranks as the sixth most common
malignancy in the world (1). Early
stages of HCC lack noticeable symptoms, resulting in ~80% of
diagnoses occurring at an advanced stage when curative liver
resection is not feasible (2).
Consequently, there is an urgent need to develop novel and
effective therapeutic targets for the prevention and treatment of
HCC. Furthermore, HCC is typically linked with chronic liver
diseases and primarily associated with hepatitis B and C virus
infections, excessive alcohol consumption and metabolic syndrome
(3–6). However, the molecular mechanism
underling HCC is yet to be fully elucidated. Understanding the
mechanisms involved in HCC carcinogenesis and progression is
necessary for identifying new and more effective treatment
strategies against this disease.
Hypoxia serves a crucial role in malignancy and is
often observed in tumors as a result of poorly organized blood
vessels and rapid tumor growth that outpaces vascularization
(7). Hypoxic tumor cells are
typically located away from blood vessels, making it difficult for
conventional drugs to reach the hypoxic tissues (8). Heightened glycolysis in hypoxic
tumors results in increased acidity, which is closely linked to
tumor progression and drug resistance (9). Additionally, tumor hypoxia can induce
genomic instability which results in the development of more
aggressive tumors. Tumor metastasis is responsible for >90% of
cancer-related deaths (10).
Hypoxia-inducible factor-1α (HIF-1α) is the primary
transcription factor involved in cellular adaptation to hypoxic
conditions. HIF-1α contributes to tumor progression by modulating
processes such as angiogenesis, energy metabolism, migration,
invasion, proliferation and apoptosis (11,12).
Consequently, inhibition of the expression of the HIF-1α gene in a
hypoxic microenvironment may offer a novel approach for therapeutic
interventions in malignant tumors such as HCC.
Rac GTPase activating protein 1 (RACGAP1), also
known as MgcRacGAP and CYK4, is a type of GTPase activating protein
that stimulates the intrinsic activity of Rho GTPases and enhances
GTP hydrolysis (13). Abnormal
expression of RACGAP1 has been implicated in the pathogenesis and
progression of numerous malignant tumors (14,15).
For example, recent studies have reported that upregulation of
RACGAP1 can be an indicator of a worse overall survival rate in
patients with HCC (16). The
activation of RACGAP1 and demethylation of its promoter region,
specifically at the H3K4me2 site, have been reported to promote
early recurrence, metastasis and microvascular invasion in HCC
(17,18). However, the specific role of
RACGAP1 in HCC within a hypoxic microenvironment has not been
extensively evaluated.
The objective of the present study was to assess the
impact of hypoxia on gene expression and its role in HCC
development and progression. Specifically, the gene expression
levels of HIF-1 α and RACGAP1 were manipulated to assess their
effects on numerous cellular processes, including proliferation,
apoptosis, migration and invasion of HCC cells. These
investigations are crucial to gain insight into the underlying
mechanisms involved in the pathogenesis of HCC. Furthermore, such
knowledge could aid in the identification of novel and effective
therapeutic targets for the prevention and treatment of HCC.
Materials and methods
Cell culture
Human liver cancer Hep3B and Huh7 cell lines were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences and cultured in DMEM medium (cat. no.
12430054; Gibco; Thermo Fisher Scientific, Inc.) which contained
10% FBS (cat. no. 10100147; Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin (cat. no. 15070063; Gibco; Thermo
Fisher Scientific, Inc.). Cells were grown in a 37°C incubator with
1% O2, 5% CO2 and 94% N2 for 48
h.
Reverse transcriptase
(RT)-quantitative polymerase chain reaction (qPCR)
TRIzol (1 ml; cat. no. R1030; Applygen Technologies,
Inc.) was added to the Hep3B and Huh7 cells pellets to extract the
total RNA. The concentration and quality of extracted RNA were then
assessed using a Nano Drop 2000 Spectrophotometer (Thermo Fisher
Scientific, Inc.). The extracted RNA was reverse-transcribed into
cDNA using the Promega M-MLV kit (cat. no. M1705; Promega
Corporation) according to the manufacturer's protocol. qPCR was
performed with the KAPA SYBR FAST qPCR kit (Kapa Biosystems; Roche
Diagnostics) using SimpliAmp™ PCR System (Thermo Fisher Scientific,
Inc.). Amplifications were performed using a two-step method as
follows: 95°C for 30 sec; 95°C for 10 sec followed by 40 cycles of
95°C for 10 sec, 60°C for 30 sec. The relative mRNA expression of
each sample was calculated using the 2−ΔΔCq method
(19). The sequences of the
primers used were as follows: HIF-1α upstream:
5′-CAGCCAGATCTCGGCGAAG-3′, downstream:
5′-CAGCATCCAGAAGTTTCCTCACA-3′; RACGAP1 upstream:
5′-CTGATGAATCACTGGATTGGGACTC-3′, downstream:
5′-GGTCTACTGCAGAGCCAATGG-3′; and GAPDH upstream:
5′-CCAGGTGGTCTCCTCTGA-3′, downstream:
5′-GCGCCCAATACGACCAAATC-3′.
Western blotting
Total proteins were extracted from Hep3B and Huh7
cells using RIPA lysis buffer (cat. no. P0013B, Beyotime Institute
of Biotechnology) were semi-quantified by the BCA method (cat. no.
23225; Thermo Fisher Scientific, Inc.). Proteins were mixed with 5X
SDS-PAGE protein loading buffer (cat. no. 20315ES05, Shanghai
Yeasen Biotechnology Co., Ltd.). The mass of protein loaded per
lane was 20 µg. Proteins were separated on 12% SDS-acrylamide gels
and transferred onto PVDF membranes, which were incubated with 5%
non-fat milk at room temperature for 2 h, followed by incubation
with rabbit anti-HIF-1α (1:200; cat. no. 20960-1-AP; Proteintech
Group, Inc.), rabbit anti-RACGAP1 (1:500; cat. no. 13739-1-AP; Cell
Signaling Technology, Inc.) and mouse anti-GAPDH (1:5,000; cat. no.
66004-1-Ig; Proteintech Group, Inc.) primary antibodies overnight
at 4°C. Membranes were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit/mouse IgG (1:5,000; cat.
nos. BA1070 and BM2002; Boster Biological Technology) secondary
antibodies for 1.5 h at room temperature. Immunoreactive protein
bands were assessed using an ECL hypersensitive chemiluminescence
kit (cat. no. P0018M; Beyotime Institute of Biotechnology) with an
Odyssey Scanning System (version 3.0, LI-COR Biosciences).
Immunofluorescence
Hep3B and Huh7 cells were plated in a 35 mm confocal
dish and cultured for 48 h followed by fixing with 4%
paraformaldehyde for 20 min at room temperature. After rinsing with
PBS, cells were permeabilized with 0.5% Triton X100 in PBS and
blocked with 3% BSA (cat. no. AR1006; Wuhan Boster Biological
Technology, Ltd.) at 37°C for 1 h. Cells were then incubated
overnight with anti-HIF-1α (1:50; cat. no. 20960-1-AP; Proteintech
Group, Inc.) or anti-RACGAP1 (1:50; cat. no. 13739-1-AP; Cell
Signaling Technology, Inc.) antibodies at 4°C, followed by 2 h
incubation with goat anti-mouse Alexa Fluor® 488 (1:200,
cat. no. A28175, Thermo Fisher Scientific, Inc.) and goat
anti-rabbit Alexa Fluor® 555 (1:200, cat. no. A27039;
Thermo Fisher Scientific, Inc.) at room temperature. After three
TBST (0.05% Tween-20) washing steps, cells were incubated with DAPI
(cat. no. D1306; Thermo Fisher Scientific, Inc.) at 37°C for 10
min. Finally, cells were observed using an LSM 510 META confocal
microscope with a Plan Apochromat 63X oil/1.4 DIC objective (Zeiss
GmbH).
Vector construction and lentiviral
transfection
Overexpressed and knocked down HIF-1α or RACGAP1
lentiviral vectors were constructed based on human HIF-1α and
RACGAP1 sequences from the Ensembl Release 110 (July 2023) (gene
nos. ENSG00000100644 and ENSG00000161800) and synthesized by the
Shanghai GeneChem Co., Ltd. HIF-1α/RACGAP1 overexpressing sequences
were cloned into GV358 vectors to produce HIF-1α- and
RACGAP1-overexpressing vectors. HIF-1α-specific short-hairpin RNA
(shRNA)-targeting coding sequences (sense (S):
5′-AAUGUGAGUUCGCAUCUUGAU-3′; antisense (AS):
5′-AUCAAGAUGCGAACUCACAUU-3′), RACGAP1-specific shRNA-targeting
coding sequences (S: 5′-CUAGGACGACAAGGCAACUUU-3′; AS:
5′-AAAGUUGCCUUGUCGUCCUAG-3′) and non-targeting negative control
sequences (S: 5′-UUCUCCGAACGAGUCACGU-3′; AS:
5′-ACGUGACUCGUUCGGAGAA-3′) (Shanghai GeneChem Co., Ltd) were cloned
into GV248 vectors to produce HIF-1α- and RACGAP1-knockdown
vectors.
A 3rd generation system was used to package the
lentivirus. For lentiviral production, 293T human embryonic kidney
cells (The Cell Bank of Type Culture Collection of The Chinese
Academy of Medical Science) were used to generate lentiviral
packaging and the lentivirus supernatant was collected and filtered
using a 0.45 µm filter at 48 h after transfection. Hep3B and Huh7
cells were seeded in six-well plates at a density of 1
×105 cells/well and cultured in DMEM with 10% FBS at 5%
CO2 at 37°C. The cells were transfected with 10 µg
lentiviral plasmids, 7.5 µg packaging plasmid and 5 µg envelope
plasmid at a multiplicity of infection (MOI) of 10 using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) the following day when the cells were ~70%
confluent. Hep3B and Huh7 cells were cultured at 37°C for 6 h
followed by replacement of the medium. The cells were grown at 37°C
for 48 h and subsequently treated with puromycin (1 µg/ml;
Invitrogen; Thermo Fisher Scientifics, Inc.) for 72 h to select
transfected clones. Then the transfected cells were collected to
assess the transfected efficiency using qPCR, western blotting and
immunofluorescence according to the aforementioned methods.
Structure determination and
refinement
The 3D protein structures of HIF-1α and RACGAP1 were
modeled using AlphaFold 2 (www.hpc.caltech.edu/documentation/software-and-modules/alphafold-2),
a protein structure prediction tool. Protein structures were
prepared using AutoDockTools (version 1.5.7; http://autodock.scripps.edu/) to ensure the accuracy
of the docking results (20).
Water molecules were manually removed from the protein structures
and polar hydrogen atoms were added. Protein-protein docking was
performed using the Global RAnge Molecular Matching: Docking Web
Server (release 306; Virtua Drug Ltd, http://www.dockingserver.com) (21). Finally, protein-protein
interactions were predicted and visualized using PyMOL software
(Version 2.5, Schrödinger, Inc.).
Cell Counting Kit-8 (CCK-8) assay
Hep3B and Huh7 were seeded at a density of 1,000
cells/well in 96-well plates and were cultured in an incubator at
37°C with 5% CO2 for four consecutive days. CCK-8
solution (10 µl; cat. no. 96992; MilliporeSigma) was added to each
well and incubated for 2 h. Optical density at 450 nm was then
measured with a Spectrafluor microreader plate (Molecular Devices,
LLC). These experiments were repeated three times.
Apoptosis analysis
Apoptotic Hep3B and Huh7 cell frequencies were
determined via staining with annexin V (cat. no. V13241; Thermo
Fisher Scientific, Inc.) and propidium iodide (PI). After rinsing
twice with PBS, Hep3B and Huh7 cells (1 ×106 cells per
sample) were centrifuged at 300 × g for 5 min at room temperature
and resuspended using 195 µl annexin V-FITC/PI binding buffer,
followed by incubation at room temperature with annexin V-FITC (5
µl) and PI (10 µl) for 40 min in the dark. Apoptosis levels were
analyzed using a BD LSR II flow cytometer (BD Biosciences). Ten
thousand events were collected per sample and data were processed
using CXP analysis software (version 2.1; Beckman Coulter,
Inc.).
Cell migration assay
The migration ability of Hep3B and Huh7 cells was
assessed using a Transwell kit. A cell suspension prepared in DMEM
(5×104 cells; 300 µl) was added to the top Transwell
chamber (cat. no. 3422; Corning, Inc.) and 500 µl 30% FBS medium
was added to the bottom. After incubating in a 37°C incubator for
48 h, the cells that had transferred on to the surface of the
bottom chamber were stained with 0.5% crystal violet for 20 min at
room temperature, then the chamber was rinsed with PBS and air
dried at room temperature. Images were obtained using a
fluorescence microscope and the number of migrated cells were
manually counted in six randomly selected fields per insert.
Wound healing assay
The motility of Hep3B and Huh7 cells were assessed
using wound healing assays. Hep3B and Huh7 cells (1×105
cells/well) were seeded in 24-well culture plates and cultured
until the confluence was ~90%. Then the culture medium was changed
to serum-free medium for 12 h (22). A 96-Wounding Replicator (cat. no.
VP408FH; V&P Scientific, Inc.) was subsequently used to scratch
wounds across each cell layer. Each well was then rinsed three
times with PBS and images were obtained at 0, 24 and 48 h using a
fluorescence microscope. The width of the gap was quantified using
ImageJ software (version 1.8.0, National Institutes of Health).
Healing rate=(wound area at 0 h-wound area at 24/48 h)/wound area
at 0 h.
Gene expression analysis using
publicly available datasets
Gene expression levels of HIF-1α and RACGAP1 in
normal and HCC tumor samples were obtained from The Cancer Genome
Atlas Program (TCGA)-HCC dataset (accession no. 202208; HCC tissue
samples, n=371 and normal tissue samples, n=226). Gene Expression
Profiling Interactive Analysis (GEPIA; version 2.0,
gepia.cancer-pku.cn/) was used for gene expression analysis. Median
mRNA expression levels of the HIF-1α and RACGAP1 for all patients
with HCC were calculated. Patients with expression levels in the
fourth quartile were classified as high expression, whereas those
with expression levels in the first quartile were classified as low
expression. Gene expressions were compared using the Wilcoxon
rank-sum test. Kaplan-Meier curves were created using GraphPad
Prism software (version 8.0; Dotmatics). Spearman's rank
correlation coefficient was used for correlation analysis and the
log-rank test was used to assess the significance of between-group
differences.
Co-immunoprecipitation (Co-IP)
experiments
A co-Immunoprecipitation kit (cat. No. 26149, Thermo
Scientific) was used to perform co-immunoprecipitation according to
the manufacturer's instructions. Briefly, 293T cells
(1×107) were collected in 1 ml RIPA buffer supplemented
with 1% protease inhibitor cocktail, and subsequently lysed by
sonication on ice for 3 min (10 sec pulse at 50% amplitude with 10
s rest times). The cellular debris was pelleted by centrifugation
at 15,000 × g for 15 min at 4°C. The cleared protein lysates were
then incubated with 80 µl Control Agarose Resin and anti-HIF-1α
(1:1 dilution by coupling Resin; cat. no. 20960-1-AP; Proteintech
Group, Inc.) antibodies with rotation overnight at 4°C. The
mixtures were then incubated with immobilized protein A/G beads
(Thermo Fisher Scientific, Inc.) with rotation at 4°C for 2 h. The
beads were collected by centrifugation at 3,000 × g for 2 min at
room temperature, and then washed five times with 0.5 ml IP wash
buffer. SDS loading buffer was added to the beads, and the samples
were denatured at 95°C for 8–10 min. Finally, the supernatants were
collected and stored at −80°C or immediately analyzed by western
blotting according to the aforementioned method.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 8.0; GraphPad Software; Dotmatics). Each
experiment was repeated three times. Data are presented as the mean
± standard deviation. Unpaired Student's t-test was used for
two-group comparisons and one-way ANOVA, followed by Tukey's test,
was used for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
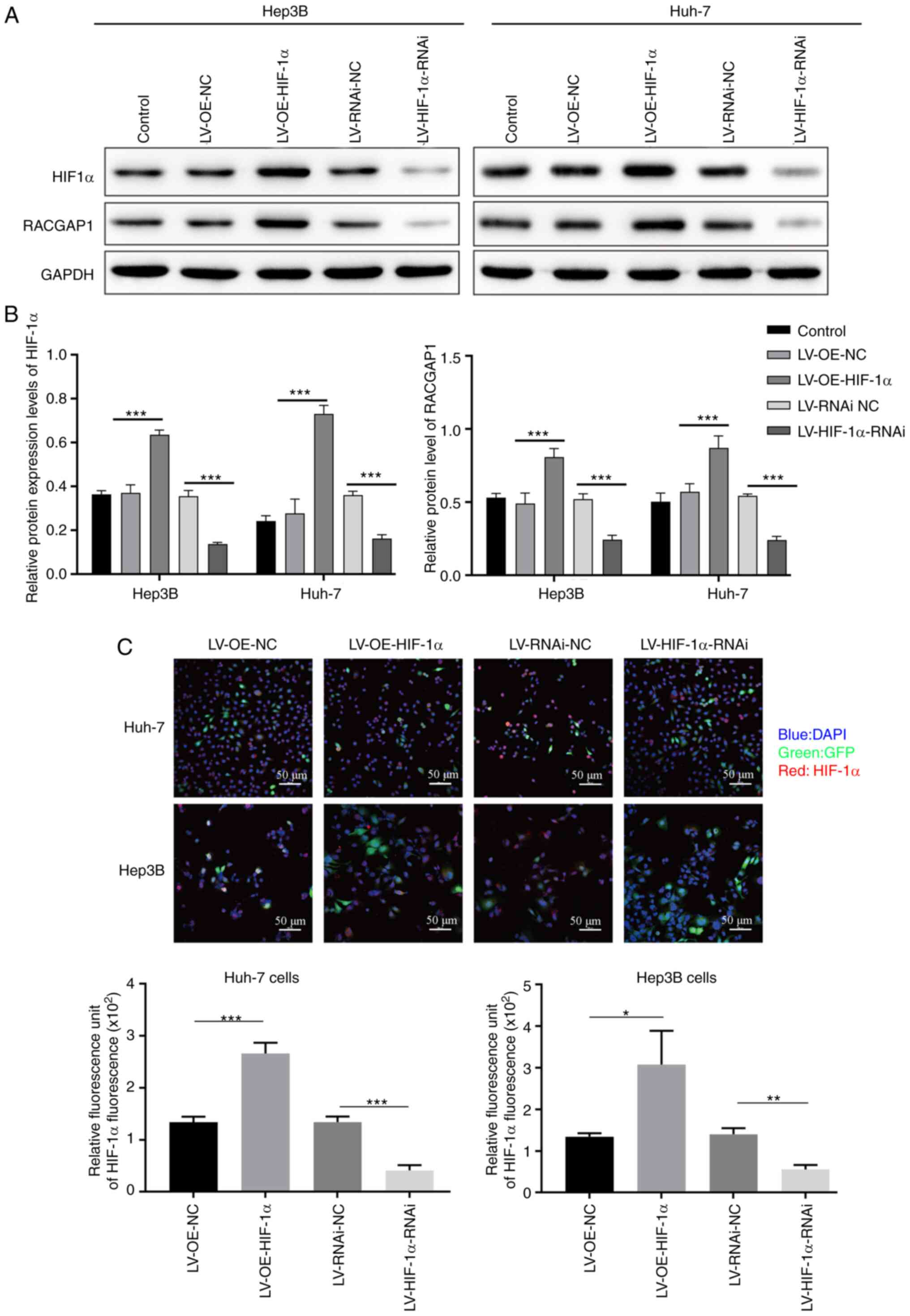
HIF-1α positively regulates RACGAP1
expression
HIF-1α overexpression and knockdown lentivirus
vectors were used to transfect Hep3B and Huh7 cells to assess the
potential role of HIF-1α in HCC. The results from qPCR, western
blotting and immunofluorescence staining demonstrated that HIF-1α
mRNA and protein expression levels were significantly increased in
the HIF-1α-overexpression group, compared with the empty vector
control group, whereas the expression significantly decreased in
the HIF-1α-knockdown group, compared with the non-targeting
negative control group (Figs. 1
and 2A). These findings indicated
that both overexpression and knockdown of HIF-1α in Hep3B and Huh7
cells was achieved. Additionally, it was demonstrated that RACGAP1
protein expression levels were significantly increased in the
HIF-1α-overexpression group, compared with the empty vector control
group, whereas the protein expression levels were significantly
decreased in the HIF-1α-knockdown group, compared with the
non-targeting negative control group (Figs. 1A, B and 2B). This suggested that HIF-1α positively
regulated RACGAP1 expression in the HCC cells.
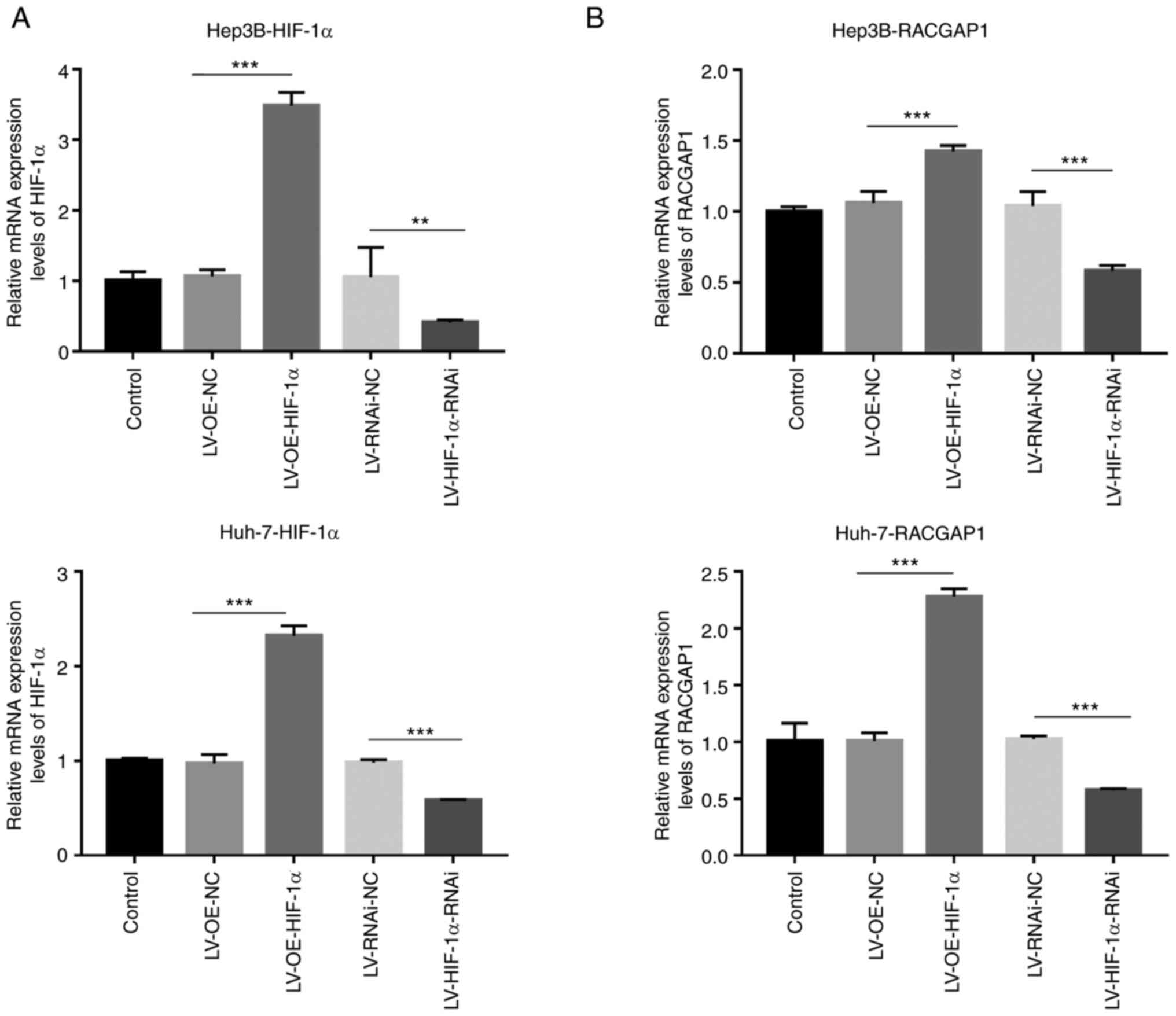
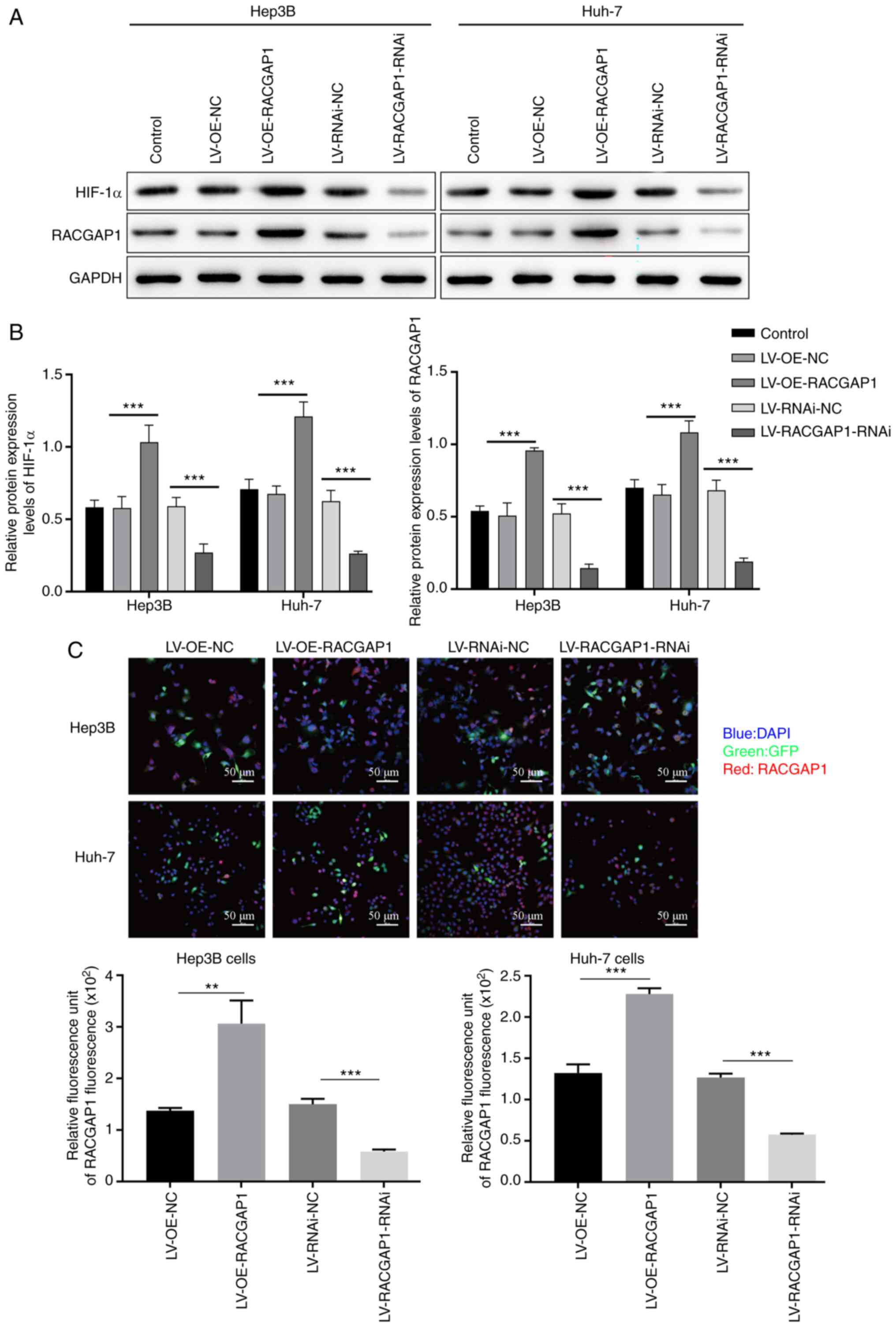
RACGAP1 positively regulates HIF-1α
expression
Hep3B and Huh7 cells were transfected with RACGAP1
knockdown and overexpression lentivirus vectors. The results from
qPCR, western blotting and immunofluorescence indicated that there
was successful knockdown and overexpression of RACGAP1 in Hep3B and
Huh7 cells. RACGAP1 mRNA and protein expression levels were
significantly increased in the RACGAP1-overexpression group,
compared with the empty vector control group, whereas the mRNA and
protein expression levels were significantly reduced in the
RACGAP1-knockdown group, compared with the non-targeting negative
control group (Figs. 3A-C and
4A). Additionally, it was
demonstrated that HIF-1α protein expression levels were
significantly increased in the RACGAP1-overexpression group,
compared with the empty vector control group, whereas the protein
expression levels were significantly decreased in the
RACGAP1-knockdown group, compared with the non-targeting negative
control group (Figs. 3A, B and
4B). The 3D protein structures
revealed interacting residues and nucleotides between HIF-1α and
RACGAP1, suggesting the presence of binding sites between the two
genes (Fig. 4C). Using CoIP with
an anti-HIF-1α antibody in 293T cells, it was demonstrated that
RACGAP1 was pulled down with HIF-1α, which indicated that HIF-1α
and RACGAP1 interacted directly within a complex (Fig. 4D). These results indicated the
existence of a mutual regulatory relationship between HIF-1α and
RACGAP1.
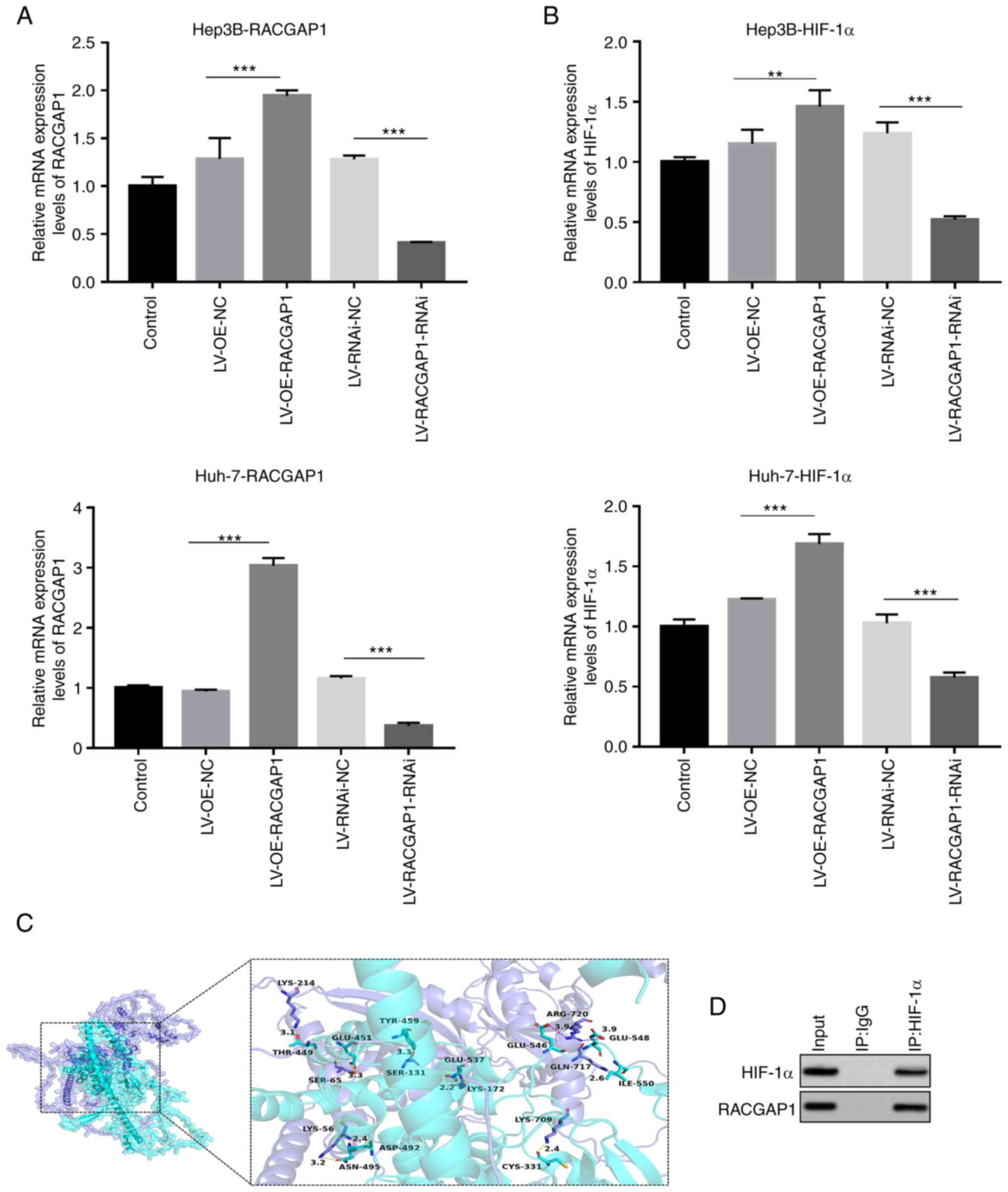 | Figure 4.Transfection efficiency of RACGAP1
knockdown and overexpression lentivirus vectors. mRNA levels of (A)
RACGAP1 and (B) HIF-1α in transfected Hep3B and Huh-7 cells,
determined by quantitative PCR. n=3. **P<0.01 and ***P<0.001.
(C) Predicted structures of HIF-1α and RACGAP1, visualized using
PyMOL software. HIF-1α protein is represented as a slate-colored
cartoon model, whilst RACGAP1 protein is shown as a cyan-colored
cartoon model. The binding sites of each protein are depicted as
corresponding colored stick structures. The binding site is
highlighted and presented in the context of the respective protein
within the binding region. (D) Co-immunoprecipitation analysis of
HIF-1α and RACGAP1. RACGAP1, Rac GTPase activating protein 1;
HIF-1α, hypoxia-inducible factor-1α; LV, lentivirus vector; OE,
overexpression; NC, negative control; RNAi, RNA interference; IP,
immunoprecipitation. |
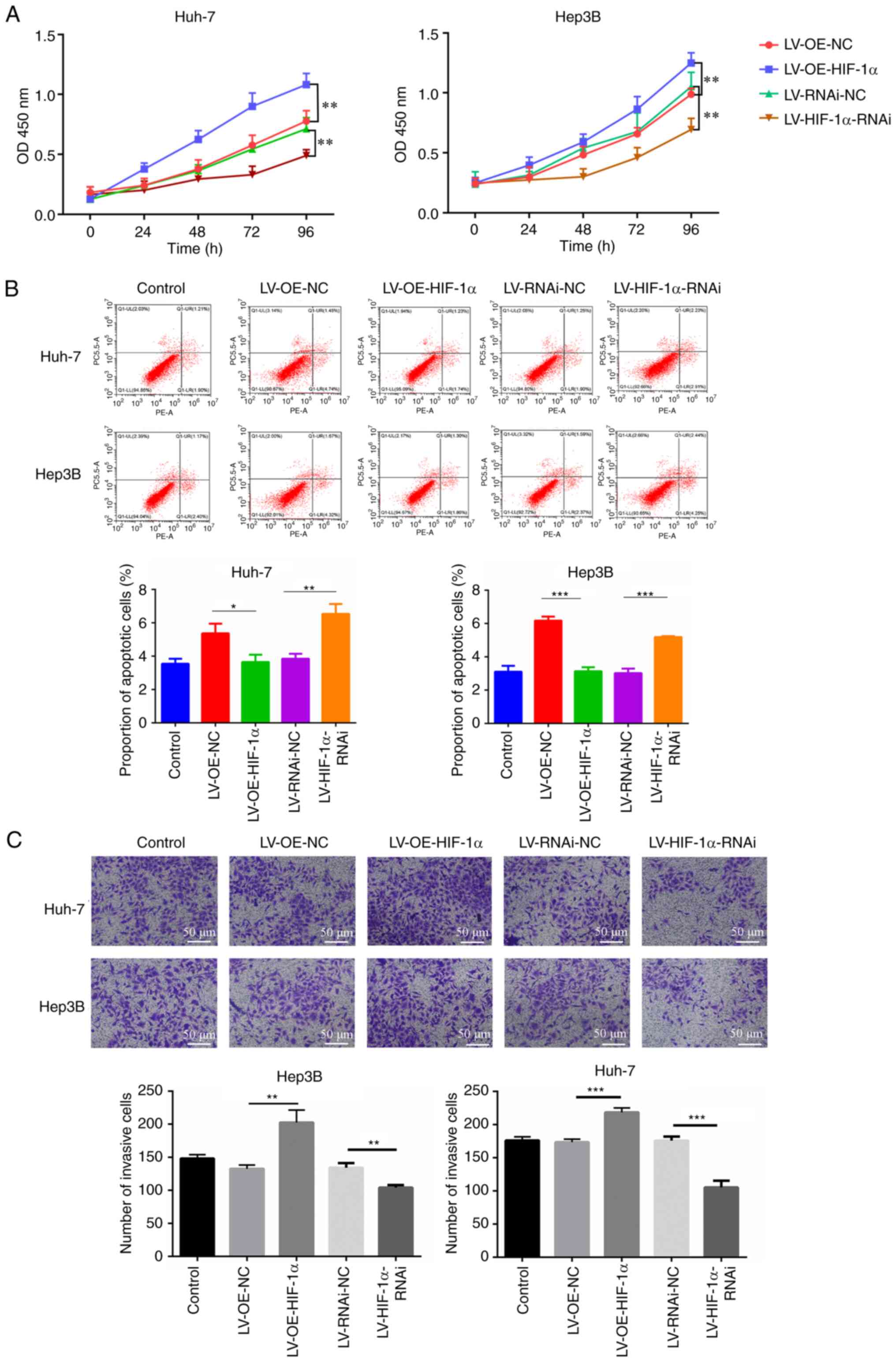
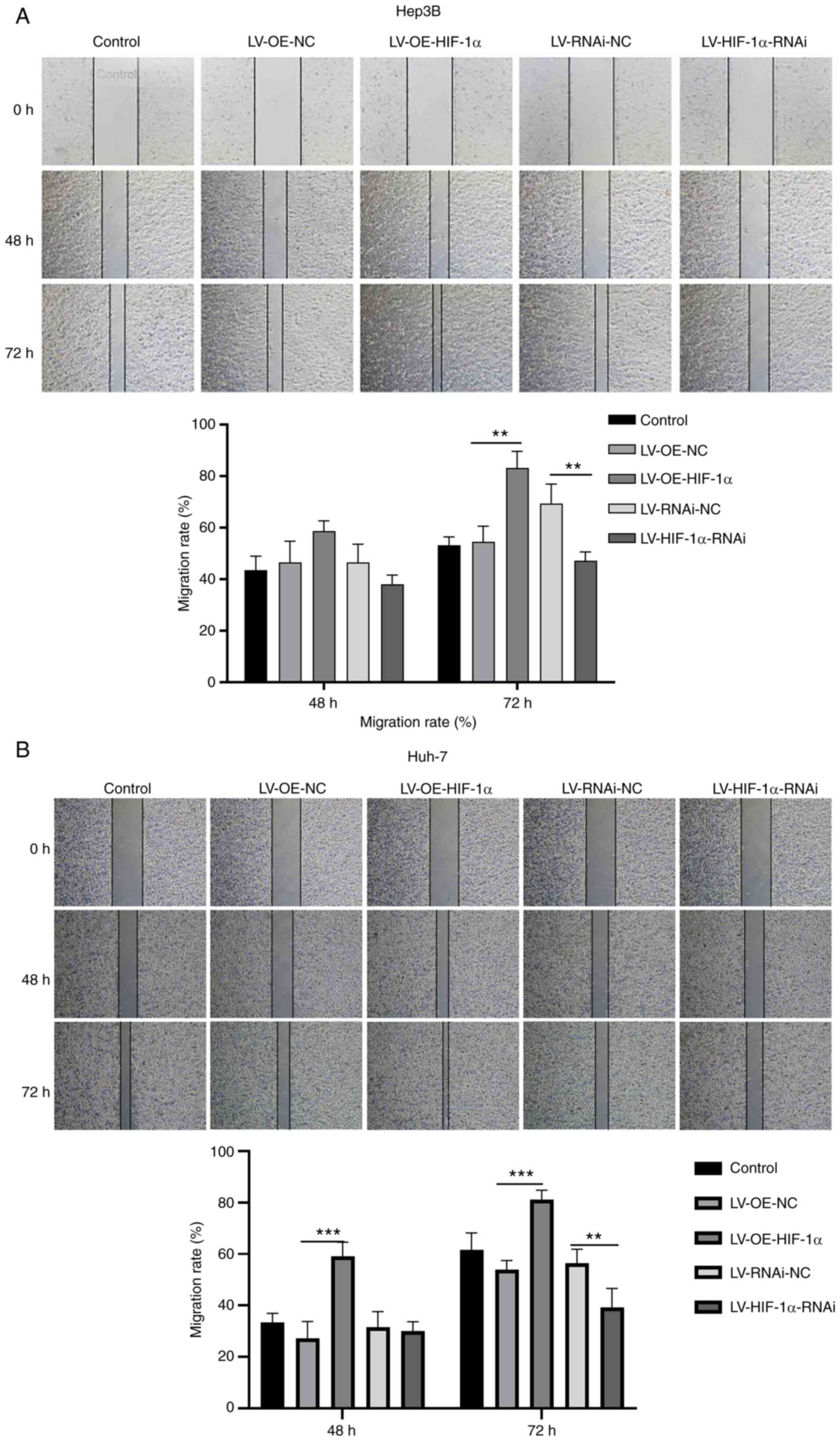
HIF-1α promotes the progression of HCC
cells
The CCK-8 assay was utilized to evaluate the impact
of HIF-1α on the viability of HCC cells. Overexpression of HIF-1α
significantly increased the viability of Hep3B and Huh7 cells,
compared with the empty vector control, whereas knockdown of HIF-1α
significantly reduced Hep3B and Huh7 cell viability, compared with
the non-targeting negative control (Fig. 5A). Overexpression of HIF-1α
significantly decreased the proportion of apoptotic Hep3B and Huh7
cells, compared with the empty vector control, whilst knockdown of
HIF-1α expression significantly increased the proportion of
apoptotic cells for both cell types, compared with the
non-targeting negative control (Fig.
5B). In addition, cell migration assays demonstrated that
overexpression of HIF-1α in Hep3B and Huh7 cells significantly
increased cell migration, compared with the empty vector control,
whereas knockdown of HIF-1α significantly decreased migration in
both cell types, compared with the non-targeting negative control
(Fig. 5C). Moreover,
overexpression of HIF-1α resulted in a reduced scratch width and
increased healing rate, indicating a significant increase in the
rate of cell migration in Hep3B and Hih7 cells, compared with the
empty vector control. Conversely, knockdown of HIF-1α resulted in a
greater scratch width and decreased healing rate, indicating a
significant decrease in the rate of cell migration in Hep3B cells
and Huh7 cells, compared with the non-targeting negative control
(Fig. 6A and B). Conversely,
knockdown of HIF-1α led to a greater scratch width and decreased
healing rate, which indicated a significant decrease in the rate of
cell migration in Hep3B and Huh7 cells compared with non-targeting
negative control.
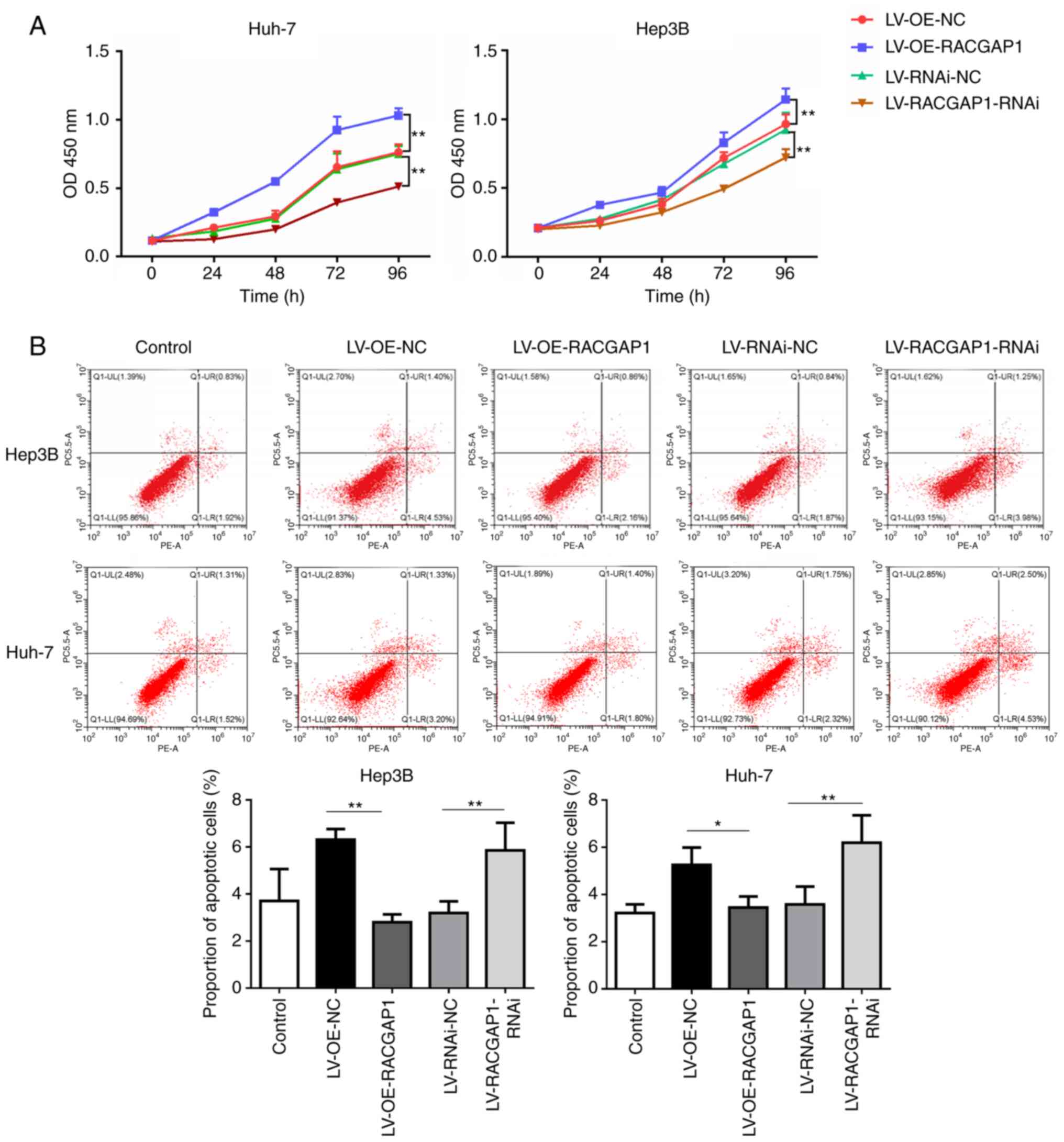
RACGAP1 promotes the progression of
HCC cells
The effect of RACGAP1 on cell viability and
apoptosis was assessed to further evaluate the potential regulatory
role of RACGAP1 in HCC progression. RACGAP1 overexpression
significantly increased the viability of Hep3B and Huh7 cells,
compared with the empty vector control, whilst knockdown of RACGAP1
significantly decreased cell viability in Hep3B and Huh7 cells,
compared with the non-targeting negative control (Fig. 7A). Conversely, overexpression of
RACGAP1 significantly decreased the proportion of apoptotic Hep3B
and Huh7 cells, compared with the empty vector control, whereas
knockdown of RACGAP1 expression significantly increased the
proportion of apoptotic cells for both cell types, compared with
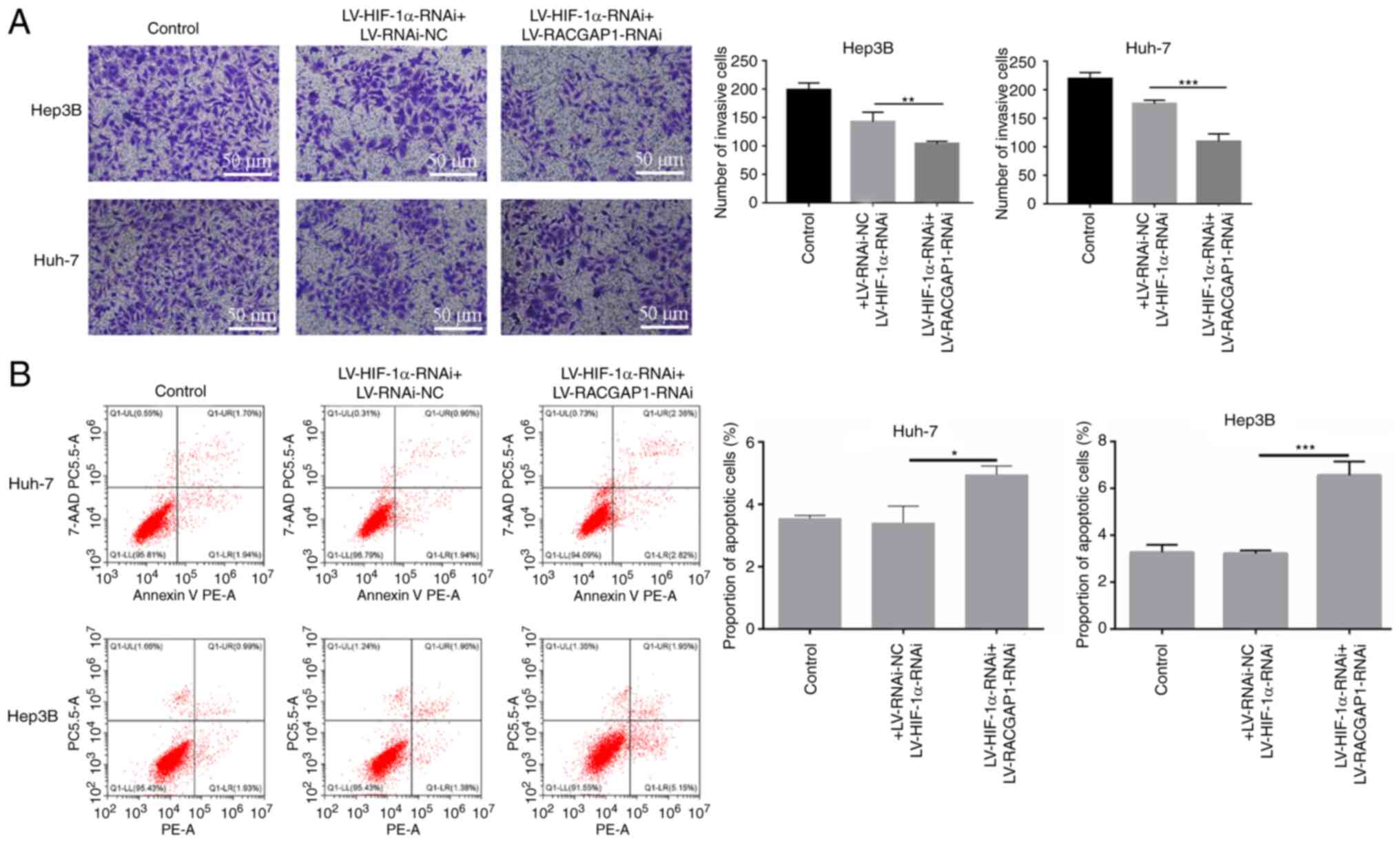
the non-targeting negative control (Fig. 7B). Furthermore, the interaction
between HIF-1α and RACGAP1 was evaluated by assessing their
combined effects on cell migration and apoptosis. After knockdown
of HIF-1α, transfection with RACGAP1 knockdown lentivirus vectors
had a superposition effect, resulting in a reduced cell migration
ability (Fig. 8A) and increased
proportion of apoptotic cell (Fig.
8B). These findings suggested the existence of a co-regulation
network between HIF-1α and RACGAP1 in the HCC cells.
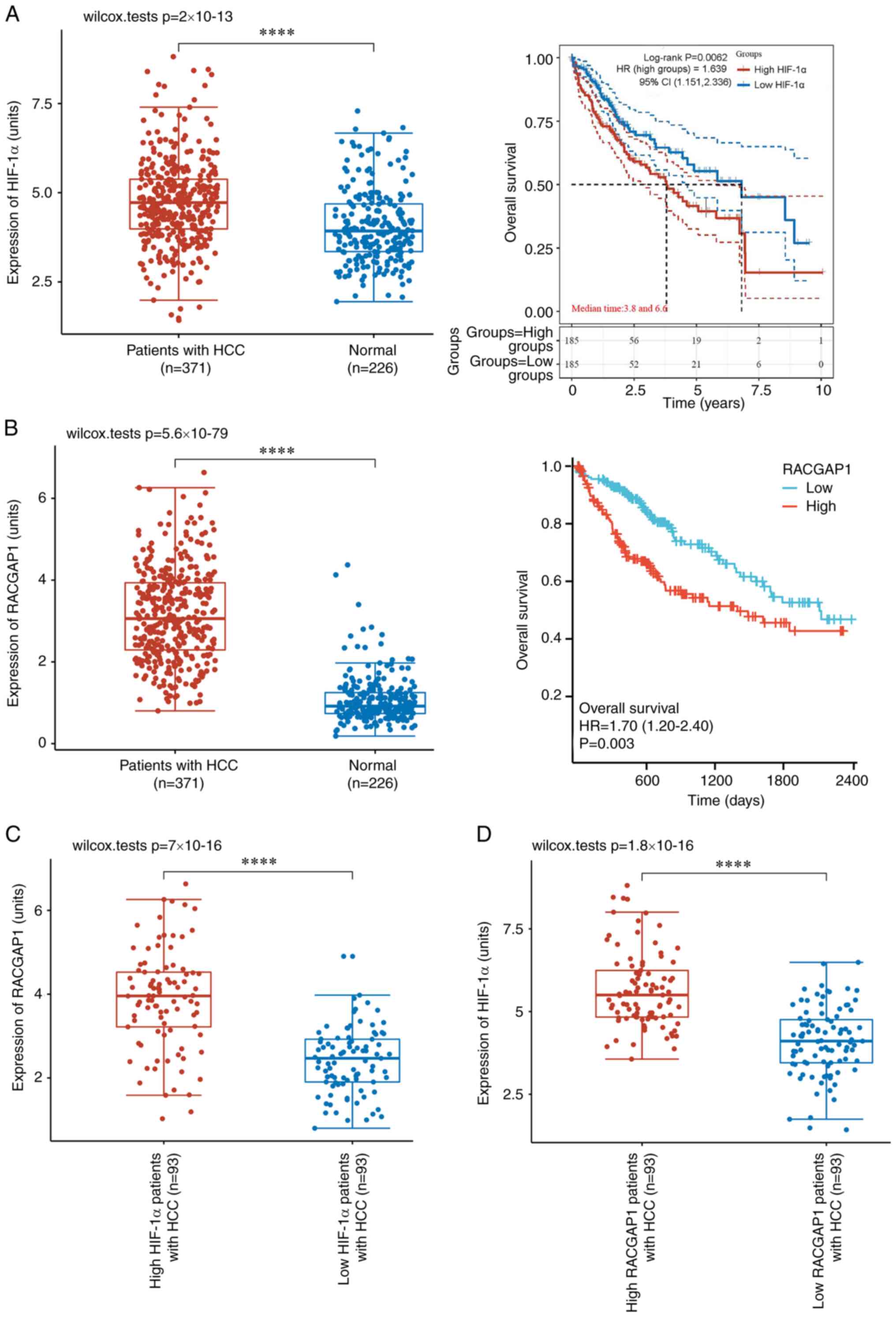
HIF-1α and RACGAP1 have a reciprocal
regulatory relationship in HCC tissue
Clinical data from the TCGA database was utilized to
further assess the potential regulatory relationship between HIF-1α
and RACGAP1. Analysis of such data demonstrated a significant
upregulation of HIF-1α expression in human HCC tissues that was
significantly associated with a lower overall survival probability
in patients with HCC (Fig. 9A).
Additionally, significantly increased RACGAP1 expression was
demonstrated in HCC tissues and this was also significantly
associated with a decreased overall survival probability in
patients with HCC (Fig. 9B).
Significant upregulation of RACGAP1 was demonstrated in patients
with HCC and high HIF-1α expression levels, compared with those
with low HIF-1α expression levels (Fig. 9C). Moreover, there was
significantly higher HIF-1α expression in patients with HCC and
high RACGAP1 expression levels, compared with those with low
RACGAP1 expression levels (Fig.
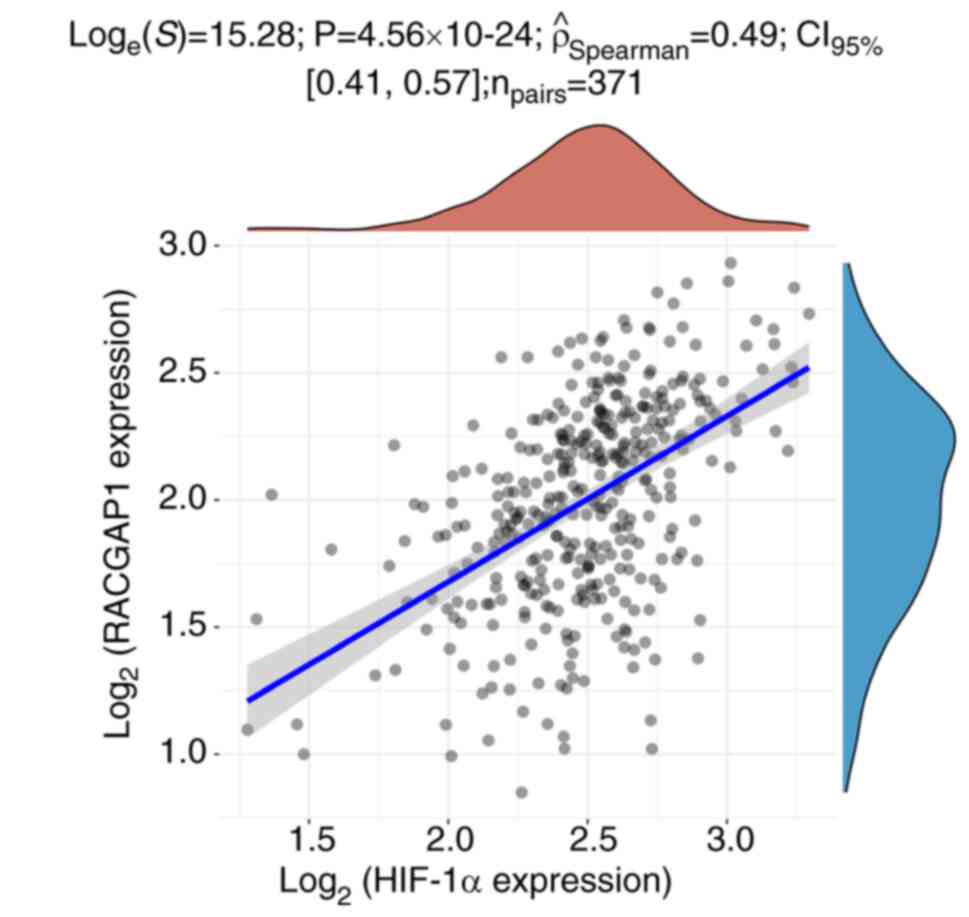
9D). Furthermore, Spearman's rank correlation analysis
demonstrated a significant positive correlation between the
expression levels of HIF-1α and RACGAP1 in HCC tissues (Fig. 10). These findings further
suggested the involvement of HIF-1α and RACGAP1 in HCC progression
through intricate reciprocal regulation.
Discussion
Hepatocellular carcinoma (HCC) is the sixth most
common cancer worldwide and ranks as the second leading cause of
cancer-related deaths (23,24).
Although several molecular and signaling pathways contributing to
HCC have been elucidated (25,26),
there is a need for increased early diagnoses and a lack of
effective therapies targeting HCC in clinic (27).
HIF-1α is a major regulator of hypoxia-responsive
genes in the tumor microenvironment and is a characteristic marker
of solid tumors (28). HIF-1α
overexpression has been reported to be a negative prognostic
indicator for patients with HCC (29). It serves a crucial role in
facilitating tumor migration, migration, metastasis, angiogenesis,
as well as regulating glycolysis, epithelial-mesenchymal transition
and lipid metabolism (30–35). RACGAP1, a member of the
GTPase-activating protein family, is a modulator of cytokinesis,
migration and differentiation (14). RACGAP1 is frequently upregulated in
HCC and is associated with shorter survival times in patients
(36). Overexpression of RACGAP1
promotes HCC cell proliferation by inhibiting the activation of the
Hippo and yes-associated protein pathways (32). Additionally, RACGAP1 acts as an
oncogenic competing endogenous RNA by sequestering microRNA-15-5p,
thereby promoting HCC recurrence (37). Therefore, HIF-1α and RACGAP1
exhibit similar functions in the progression of HCC under normal
oxygen levels (28–36). In the present study, HIF-1α and
RACGAP1 were demonstrated to promote HCC progression in a mutually
regulatory manner under hypoxic conditions, providing evidence for
their potential use in the clinical diagnosis and treatment of
HCC.
Angiogenesis is tightly regulated in normal tissues,
resulting in a well-organized vasculature that adequately supplies
oxygen. By contrast, malignant tumors, including HCC, exhibit
abnormal angiogenesis and an irregular vasculature, resulting in
insufficient oxygen concentrations within solid tumors (38). This hypoxic microenvironment
contributes to the metabolic reprogramming of cancer cells and
triggers the production of numerous bioactive molecules that
increase the resistance of cancer cells to radiation and
chemotherapy (39–41). For example, in HCC cells, the
activation of the FABP5/HIF-1α axis has been reported to lead to
increased lipid uptake and storage, which results in lipid
accumulation within the cells (35). Hypoxia has been reported to have
significantly enhanced the resistance of human malignant
mesothelioma cells to cisplatin by promoting epithelial to
mesenchymal transition (41).
Under hypoxic conditions, the degradation pathway of
oxygen-dependent HIF-1α is inhibited, resulting in increased levels
of HIF-1α within the tumor cells. HIF-1α serves a crucial role in
promoting tumor progression by inducing sustained growth factor
signaling, angiogenesis, epithelial-mesenchymal transition and
replicative immortality (42,43).
Hypoxia also leads to the selection of cancer cells that can evade
growth suppressors or apoptotic triggers and disrupt cellular
energetics (44,45). Additionally, HIF-1α is associated
with genetic instability, tumor-promoting inflammation and evasion
of the immune response (46,47).
Therefore, HIF-1α represents an important therapeutic target, given
its role in cancer development and progression. In the present
study, overexpression of HIF-1α significantly promoted the
proliferation, migration and metastasis of Huh-7 and Hep3B cells,
whilst inhibiting their apoptosis under hypoxic conditions.
Conversely, knockdown of HIF-1α effectively suppressed the
proliferation, migration and metastasis of Huh-7 and Hep3B cells,
whilst promoting apoptosis. These findings further support the role
of HIF-1α as a promoter of cancer progression in HCC.
A previous study reported that RACGAP1 serves a
crucial role in cell division and proliferation (37). RACGAP1 was initially identified in
the testis and in germ cells and its significance has been reported
in numerous malignant tumors, including colorectal cancer,
epithelial ovarian cancer, HCC, pancreatic and stomach cancer and
meningiomas (37,48,49).
Upregulation of RACGAP1 has been associated with doxorubicin
resistance in squamous cell carcinoma and is associated with worse
overall survival in patients (9).
In esophageal carcinoma, high RACGAP1 expression has been reported
to be significantly associated with worse overall survival,
disease-free survival, lymphatic migration, vessel migration and
advanced tumor, node, metastasis stage (50). Similarly, high nuclear RACGAP1
expression is associated with poor survival in patients with
colorectal cancer (51). In HCC,
RACGAP1 overexpression promotes disease progression and has been
reported to be significantly associated with worse overall survival
(16,17). In the present study, knockdown of
RACGAP1 significantly suppressed cell proliferation and induced
apoptosis, whilst overexpression of RACGAP1 significantly promoted
cell proliferation and suppressed apoptosis in Huh-7 and Hep3B
cells under hypoxic conditions. These findings demonstrated the
oncogenic role of RACGAP1 in HCC progression and suggested that
targeting the RACGAP1-mediated oncogenic pathway may hold
therapeutic value for HCC.
However, the interaction between HIF-1α and RACGAP1
has not been previously reported in the literature and to the best
of our knowledge, this is the first study that has demonstrated the
interaction between HIF-1α and RACGAP1. In the present study,
knockdown of HIF-1α resulted in a decrease in RACGAP1 expression,
whereas overexpression of HIF-1α promoted RACGAP1 expression.
Additionally, modulation of RACGAP1 expression had a reciprocal
effect on HIF-1α expression. These findings suggested that HIF-1α
and RACGAP1 mutually regulate each other and promote HCC
progression under hypoxic conditions. Furthermore, simultaneous
knockdown of HIF-1α and RACGAP1 had a more pronounced inhibitory
effect on HCC cell migration compared with knockdown of HIF-1α
alone. Similarly, simultaneous overexpression of HIF-1α and RACGAP1
had a more significant facilitation effect on HCC cell apoptosis
than HIF-1α knockdown alone. Clinical data analysis in the present
study also demonstrated RACGAP1 expression was significantly
increased in HCC tissues with high HIF-1α expression levels and
HIF-1α expression was significantly increased in HCC tissue with
high RACGAP1 expression levels. Spearman's rank correlation
analysis further demonstrated a significant positive correlation
between the expression levels of HIF-1α and RACGAP1 in HCC tissues,
emphasizing the reciprocal regulatory relationship between HIF-1α
and RACGAP1.
The regulatory mechanism between HIF-1α and RACGAP1
may involve a feedback loop. HIF-1α, a transcription factor, can
directly bind to specific DNA sequences, called hypoxia response
elements (HREs), present in the promoter region of the RACGAP1 gene
(52). Binding of HIF-1α to HREs
promotes the transcription of RACGAP1, leading to increased
expression of RACGAP1 (53).
RACGAP1 may regulate HIF-1α through multiple mechanisms, including
post-translational modifications and protein-protein interactions.
RACGAP1 can modulate the stability of HIF-1α protein by inhibiting
the ubiquitin-proteasome pathway (54,55).
Additionally, RACGAP1 can inhibit the activity of prolyl
hydroxylases, enzymes that regulate HIF-1α stability under normoxic
conditions, leading to increased stabilization of HIF-1α (56). In the present study, AlphaFold 2
and PyMOL were utilized to predict the interactions between HIF-1α
and RACGAP1 and the direct interaction between HIF-1α and RACGAP1
was further assessed using CoIP experiments. These reciprocal
regulatory mechanisms between HIF-1α and RACGAP1 contribute to HCC
progression.
The present study provided valuable insights into
the roles of HIF-1α and RACGAP1 in HCC, however there are certain
limitations to consider. Rescue experiments were not performed
during the investigation into the reciprocal relationship between
HIF-1α and RACGAP1 expression levels. The expression of HIF-1α and
RACGAP1 were instead manipulated to assess their specific impact on
the expression of the other. However, the inclusion of rescue
experiments could have provided further support for the findings of
the present study. Moreover, the absence of animal models limits
the generalizability of the results. The present study utilized
liver cancer clinical samples and cell information from the TCGA
database to assess the regulatory effects of HIF-1α and RACGAP1.
Future studies should utilize mouse and rat liver cancer models to
evaluate the roles of HIF-1α and RACGAP1 in liver cancer
progression.
In conclusion, HIF-1α and RACGAP1 cooperatively
contribute to the pathogenesis of HCC, which may provide a valuable
therapeutic target for HCC.
Acknowledgements
Not applicable.
Funding
This work was supported by Guangxi Clinic Medicine Research
Center of Hepatobiliary Diseases (grant. no. AD17129025) and the
Science Foundation of Youjiang Medical College for Nationalities
(grant. no. yy2020ky022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, ZX and JP conceived and designed the study. XW
and YL performed experiments. WL performed the analyses and also
participated in the study design. YL and JP wrote the manuscript.
XW and JP confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Halegoua-De Marzio D and Hann HW:
Prevention of hepatocellular carcinoma and its recurrence with
anti-hepatitis B viral therapy. Minerva Gastroenterol Dietol.
60:191–200. 2014.PubMed/NCBI
|
|
2
|
Brown ZJ, Hewitt DB and Pawlik TM:
Experimental drug treatments for hepatocellular carcinoma: Clinical
trial failures 2015 to 2021. Expert Opin Investig Drugs.
31:693–706. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan W and Ye G: Microarray analysis for
the identification of specific proteins and functional modules
involved in the process of hepatocellular carcinoma originating
from cirrhotic liver. Mol Med Rep. 17:5619–5626. 2018.PubMed/NCBI
|
|
4
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maucort-Boulch D, de Martel C, Franceschi
S and Plummer M: Fraction and incidence of liver cancer
attributable to hepatitis B and C viruses worldwide. Int J Cancer.
142:2471–2477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Martel C, Maucort-Boulch D, Plummer M
and Franceschi S: World-wide relative contribution of hepatitis B
and C viruses in hepatocellular carcinoma. Hepatology.
62:1190–1200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh IJ, Liu KT, Shen JH, Wu YH, Liu YH,
Yen MC and Kuo PL: Identification of the potential prognostic
markers from the miRNA-lncRNA-mRNA interactions for metastatic
renal cancer via next-generation sequencing and bioinformatics.
Diagnostics (Basel). 10:2282020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piasentin N, Milotti E and Chignola R: The
control of acidity in tumor cells: A biophysical model. Sci Rep.
10:136132020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung C, Mader CC, Schmitz JC, Atladottir
J, Fitchev P, Cornwell ML, Koleske AJ, Crawford SE and Gorelick F:
The vacuolar-ATPase modulates matrix metalloproteinase isoforms in
human pancreatic cancer. Lab Invest. 91:732–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spennati G, Horowitz LF, McGarry DJ,
Rudzka DA, Armstrong G, Olson MF, Folch A and Yin H: Organotypic
platform for studying cancer cell metastasis. Exp Cell Res.
401:1125272021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarbell J, Mahmoud M, Corti A, Cardoso L
and Caro C: The role of oxygen transport in atherosclerosis and
vascular disease. J R Soc Interface. 17:201907322020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drenckhan A, Freytag M, Supuran CT, Sauter
G, Izbicki JR and Gros SJ: CAIX furthers tumour progression in the
hypoxic tumour microenvironment of esophageal carcinoma and is a
possible therapeutic target. J Enzyme Inhib Med Chem. 33:1024–1033.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hodge RG and Ridley AJ: Regulating Rho
GTPases and their regulators. Nat Rev Mol Cell Biol. 17:496–510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bian R, Dang W, Song X, Liu L, Jiang C,
Yang Y, Li Y, Li L, Li X, Hu Y, et al: Rac GTPase activating
protein 1 promotes gallbladder cancer via binding DNA ligase 3 to
reduce apoptosis. Int J Biol Sci. 17:2167–2180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imaoka H, Toiyama Y, Saigusa S, Kawamura
M, Kawamoto A, Okugawa Y, Hiro J, Tanaka K, Inoue Y, Mohri Y and
Kusunoki M: RacGAP1 expression, increasing tumor malignant
potential, as a predictive biomarker for lymph node metastasis and
poor prognosis in colorectal cancer. Carcinogenesis. 36:346–354.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu Y, Chen B, Guo D, Pan L, Luo X, Tang J,
Yang W, Zhang Y, Zhang L, Huang J, et al: Up-Regulation of RACGAP1
promotes progressions of hepatocellular carcinoma regulated by
GABPA via PI3K/AKT pathway. Oxid Med Cell Longev. 2022:30341502022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao S, Wang K, Zhang L, Shi G, Wang Z,
Chen Z, Zhu P and He Q: PRC1 and RACGAP1 are diagnostic biomarkers
of early HCC and PRC1 Drives Self-Renewal of liver cancer stem
cells. Front Cell Dev Biol. 10:8640512022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pu J, Wang J, Wei H, Lu T, Wu X, Wu Y,
Shao Z, Luo C and Lu Y: lncRNA MAGI2-AS3 Prevents the Development
of HCC via Recruiting KDM1A and Promoting H3K4me2 Demethylation of
the RACGAP1 Promoter. Mol Ther Nucleic Acids. 18:351–362. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morris GM, Huey R and Olson AJ: Using
AutoDock for ligand-receptor docking. Curr Protoc Bioinformatics
Chapter 8: Unit 8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vakser IA: Long-distance potentials: An
approach to the multiple-minima problem in ligand-receptor
interaction. Protein Eng. 9:37–41. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng D, Liu M, Liu Y, Zhao X, Sun H, Zheng
X, Zhu J and Shang F: Micheliolide suppresses the viability,
migration and invasion of U251MG cells via the NF-κB signaling
pathway. Oncol Lett. 20:672020.PubMed/NCBI
|
|
23
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rebouissou S and Nault JC: Advances in
molecular classification and precision oncology in hepatocellular
carcinoma. J Hepatol. 72:215–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zucman-Rossi J, Villanueva A, Nault JC and
Llovet JM: Genetic landscape and biomarkers of hepatocellular
carcinoma. Gastroenterology. 149:1226–1239.e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dimri M and Satyanarayana A: Molecular
signaling pathways and therapeutic targets in hepatocellular
carcinoma. Cancers (Basel). 12:4912020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wegiel B, Vuerich M, Daneshmandi S and
Seth P: Metabolic Switch in the tumor microenvironment determines
immune responses to Anti-Cancer therapy. Front Oncol. 8:2842018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SL, Liu LP, Jiang JX, Xiong ZF, He QJ
and Wu C: The correlation of expression levels of HIF-1α and HIF-2α
in hepatocellular carcinoma with capsular migration, portal vein
tumor thrombi and patients' clinical outcome. Jpn J Clin Oncol.
44:159–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng W, Xue T, Huang S, Shi Q, Tang C, Cui
G, Yang G, Gong H and Guo H: HIF-1α promotes the migration and
migration of hepatocellular carcinoma cells via the IL-8-NF-κB
axis. Cell Mol Biol Lett. 23:262018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng B, Zhu Y, Su Z, Tang L, Sun C, Li C
and Zheng G: Basil polysaccharide attenuates hepatocellular
carcinoma metastasis in rat by suppressing H3K9me2 histone
methylation under hepatic artery ligation-induced hypoxia. Int J
Biol Macromol. 107:2171–2179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu
F, Zhang Y, Dong X and Sun B: HIF-1α promoted vasculogenic mimicry
formation in hepatocellular carcinoma through LOXL2 up-regulation
in hypoxic tumor microenvironment. J Exp Clin Cancer Res.
36:602017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng J, Dai W, Mao Y, Wu L, Li J, Chen K,
Yu Q, Kong R, Li S, Zhang J, et al: Simvastatin re-sensitizes
hepatocellular carcinoma cells to sorafenib by inhibiting
HIF-1α/PPAR-gamma/PKM2-mediated glycolysis. J Exp Clin Cancer Res.
39:242020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng B, Zhu Y, Sun C, Su Z, Tang L, Li C
and Zheng G: Basil polysaccharide inhibits hypoxia-induced
hepatocellular carcinoma metastasis and progression through
suppression of HIF-1α-mediated epithelial-mesenchymal transition.
Int J Biol Macromol. 137:32–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seo J, Jeong DW, Park JW, Lee KW, Fukuda J
and Chun YS: Fatty-acid-induced FABP5/HIF-1 reprograms lipid
metabolism and enhances the proliferation of liver cancer cells.
Commun Biol. 3:6382020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang XM, Cao XY, He P, Li J, Feng MX,
Zhang YL, Zhang XL, Wang YH, Yang Q, Zhu L, et al: Overexpression
of Rac GTPase activating protein 1 contributes to proliferation of
cancer cells by reducing hippo signaling to promote cytokinesis.
Gastroenterology. 155:1233–1249.e22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang MY, Chen DP, Qi B, Li MY, Zhu YY, Yin
WJ, He L, Yu Y, Li ZY, Lin L, et al: Pseudogene RACGAP1P activates
RACGAP1/Rho/ERK signalling axis as a competing endogenous RNA to
promote hepatocellular carcinoma early recurrence. Cell Death Dis.
10:4262019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang WH, Forde D and Lai AG: Dual
prognostic role of 2-oxoglutarate-dependent oxygenases in ten
cancer types: Implications for cell cycle regulation and cell
adhesion maintenance. Cancer Commun (Lond). 39:232019.PubMed/NCBI
|
|
39
|
Ozensoy Guler O, Supuran CT and Capasso C:
Carbonic anhydrase IX as a novel candidate in liquid biopsy. J
Enzyme Inhib Med Chem. 35:255–260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mast JM and Kuppusamy P: Hyperoxygenation
as a therapeutic supplement for treatment of triple negative breast
cancer. Front Oncol. 8:5272018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim MC, Hwang SH, Kim NY, Lee HS, Ji S,
Yang Y and Kim Y: Hypoxia promotes acquisition of aggressive
phenotypes in human malignant mesothelioma. BMC Cancer. 18:8192018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li HS, Zhou YN, Li L, Li SF, Long D, Chen
XL, Zhang JB, Feng L and Li YP: HIF-1α protects against oxidative
stress by directly targeting mitochondria. Redox Biol.
25:1011092019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, de Carvalho Ribeiro M,
Iracheta-Vellve A, Lowe P, Ambade A, Satishchandran A, Bukong T,
Catalano D, Kodys K and Szabo G: Macrophage-Specific
hypoxia-inducible factor-1α contributes to impaired autophagic flux
in nonalcoholic steatohepatitis. Hepatology. 69:545–563. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia YJ, Jiang XT, Jiang SB, He XJ, Luo JG,
Liu ZC, Wang L, Tao HQ and Chen JZ: PHD3 affects gastric cancer
progression by negatively regulating HIF1A. Mol Med Rep.
16:6882–6889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang J, Xu J, Dong Y and Huang B:
Down-regulation of HIF-1alpha inhibits the proliferation,
migration, and migration of gastric cancer by inhibiting PI3K/AKT
pathway and VEGF expression. Biosci Rep. 38:BSR201807412018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh R, Mishra MK and Aggarwal H:
Inflammation, immunity, and cancer. Mediators Inflamm.
2017:60273052017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lawson CD and Der CJ: Filling GAPs in our
knowledge: ARHGAP11A and RACGAP1 act as oncogenes in basal-like
breast cancers. Small GTPases. 9:290–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang C, Wang W, Liu Y, Yong M, Yang Y and
Zhou H: Rac GTPase activating protein 1 promotes oncogenic
progression of epithelial ovarian cancer. Cancer Sci. 109:84–93.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yin C, Toiyama Y, Okugawa Y, Shigemori T,
Yamamoto A, Ide S, Kitajima T, Fujikawa H, Yasuda H, Okita Y, et
al: Rac GTPase-Activating Protein 1 (RACGAP1) as an oncogenic
enhancer in esophageal carcinoma. Oncology. 97:155–163. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yeh CM, Sung WW, Lai HW, Hsieh MJ, Yen HH,
Su TC, Chang WH, Chen CY, Ko JL, Chen CJ, et al: Opposing
prognostic roles of nuclear and cytoplasmic RACGAP1 expression in
colorectal cancer patients. Hum Pathol. 47:45–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang Y, Lu H, Chen C, Lyu Y, Cole RN and
Semenza GL: HIF-1 Interacts with TRIM28 and DNA-PK to release
paused RNA polymerase II and activate target gene transcription in
response to hypoxia. Nat Commun. 13:3162022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Smythies JA, Sun M, Masson N, Salama R,
Simpson PD, Murray E, Neumann V, Cockman ME, Choudhry H, Ratcliffe
PJ and Mole DR: Inherent DNA-binding specificities of the HIF-1α
and HIF-2α transcription factors in chromatin. EMBO Rep.
20:e464012019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Song Z, Cao Q, Guo B, Zhao Y, Li X, Lou N,
Zhu C, Luo G, Peng S, Li G, et al: Overexpression of RACGAP1 by
E2F1 promotes neuroendocrine differentiation of prostate cancer by
stabilizing EZH2 expression. Aging Dis. 2023.doi:
10.14336/AD.2023.0202 (Epub ahead of print). View Article : Google Scholar
|
|
55
|
Rashid M, Zadeh LR, Baradaran B, Molavi O,
Ghesmati Z, Sabzichi M and Ramezani F: Up-down regulation of HIF-1α
in cancer progression. Gene. 798:1457962021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jaskiewicz M, Moszynska A, Kroliczewski J,
Cabaj A, Bartoszewska S, Charzyńska A, Gebert M, Dąbrowski M,
Collawn JF and Bartoszewski R: The transition from HIF-1 to HIF-2
during prolonged hypoxia results from reactivation of PHDs and
HIF1A mRNA instability. Cell Mol Biol Lett. 27:1092022. View Article : Google Scholar : PubMed/NCBI
|