Introduction
Anti-N-methyl-D-aspartate receptor (anti-NMDAR)
encephalitis, originally described as limbic encephalitis, is
characterized by seizures, dyskinesia, behavioral changes, mood
disturbances, cognitive impairment, autonomic dysfunction and
altered levels of consciousness (1). Anti-NMDAR antibodies in serum react
with the N-terminal domain of N-methyl-D-aspartate receptor subunit
1 (NMDAR1; also known as NR1, GRIN1 or GluN1) subunit of NMDAR.
This interaction results in the internalization of neuronal cell
surface receptors, reduced cell surface and synaptic NMDAR levels
and symptoms such as memory and behavioral changes (2,3).
However, the pathogenic mechanisms underlying anti-NMDAR
encephalitis are poorly understood.
To comprehensively evaluate anti-NMDAR encephalitis,
an animal model of the disease is necessary. A previous study
performed by Planagumà et al (3) continuously perfused cerebrospinal
fluid from patients with anti-NMDAR encephalitis into the
ventricles of mice, which resulted in progressive memory deficits,
anhedonia and depression-like behaviors. This was the first proof
of concept study that demonstrated a mouse model could be generated
using autoantibodies from patients with autoimmune encephalitis.
Subsequently, Jones et al (4) reported for the first time in 2019 the
immunization of C57BL/6 mice with purified GluN1/GluN2B NMDA fully
assembled tetrameric receptors embedded in NMDA receptor
proteoliposomes. Linnoila et al (5) inoculated six mice intranasally with
the herpes simplex virus and reported that four of these mice
developed serum NMDAR antibodies. A decrease in the NMDAR protein
expression level in the hippocampal postsynaptic membrane of the
mice was reported. Pan et al (6) actively immunized ApoE−/−
mice with NMDAR peptides to produce a large amount of serum NMDAR
antibodies. Ding et al (7)
successfully constructed an anti-NMDAR encephalitis model by
actively immunizing female C57BL/6 mice with the amino-terminal
domain (ATD) peptide (GluN1356-385) of the GluN1 protein subunit.
At present, there are few studies on the construction of anti-NMDAR
encephalitis mouse models and there is scope for exploring how such
models could be built to better replicate the clinical
manifestations of the disease.
In the present study, C57 ApoE−/− mice
were immunized with prokaryote-expressed human NMDA1 protein, and
an anti-NMDAR encephalitis mouse model was successfully
constructed, which was confirmed using ELISA, western blotting,
behavioral experiments and immunofluorescence.
Materials and methods
Protein expression and
purification
The NMDA1 (19–559 aa) gene was inserted into the
expression vector pET30a by whole gene synthesis using the
restriction enzyme digestion sites NdeI and HindIII, and the
accuracy of the final expression vector was confirmed by enzyme
digestion and Sanger sequencing (Sangon Biotech Co., Ltd.).
Finally, it was introduced into the bacterial host Escherichia
coli Rosetta blue (DE3). The expression of NMDA1 (19–559 aa)
protein was induced using 0.2 mmol/l
Isopropyl-β-D-1-thiogalactoside (IPTG) and 50 ug/ml kanamycin at
15°C for 16 h, and then NMDA1 (19–559 aa) protein was expressed as
a form of inclusion body. Subsequently, the fraction with the
inclusion bodies was dissolved in the solubilization buffer (10 mM
Tris-HCl, 100 mM sodium phosphate, 6 M guanidine-HCl, 10 mM
imidazole, 2 mM 2-mercaptoethanol, pH 8.0) and the clarified
supernatant was run through a 45–165 µm Ni-IDA column (cat. no.
C600292, Sangon Biotech Co., Ltd.) for purification of His-tagged
protein. Elution buffers including different concentrations of
imidazole (50 mM Tris; 300 mM NaCl; 50, 100 or 300 mM Imidazole; pH
8.0) were used to elute the His-tagged protein. The purity of NMDA1
(19–559 aa) in the eluted solutions was assessed using 10% SDS-PAGE
and the gels were stained using Coomassie Brilliant Blue R-250
(cat. no. 1610436; Bio-Rad Laboratories) according to the
manufacturer's protocols. Consequently, we chose the concentration
of 300 mM imidazole as the elution condition for the subsequent
protein purification (8,9).
Mouse immunization
C57BL/6 mice (age, 12 weeks; female;
ApoE−/−; n=10) were immunized with a mixture of NMDA1
(19–559 aa; Nanjing MerryBio Co., Ltd.) and OVA peptide (cat. no.
HY-P1489A; MedChemExpress) emulsified in an equal volume of
complete Freund's adjuvant [Mycobacterium tuberculosis H37RA
(cat. no. 231141; Becton, Dickinson and Company) plus incomplete
Freund's] at a final concentration of 1 mg/ml. The right groin of
each of the animals in the NMDA1 treatment group (n=5) was
subcutaneously injected with 100 µg of NMDA1 peptide and 20 µg of
OVA. The control mice (n=5) received an emulsion mixture of OVA and
CFA (cat. no. F5881; MilliporeSigma) and an equal volume of 0.9%
NaCl. All mice were injected intraperitoneally with 200 ng
pertussis toxin (cat. no. B7273; APExBIO Technology LLC) on the day
of the last immunization and again 48 h later. All mice were housed
in a 12 h light/dark cycle at 24°C with 60% humidity and ad
libitum access to food and water The animal study was reviewed
and approved by the Laboratory Animal Ethical and Welfare Committee
of Laboratory Animal Center, Ningxia Medical University (Yinchuan,
China; approval no. IACUC-NYLAC-2019-072).
Euthanasia of mice
Mice were placed in a euthanasia box, which was then
infused with CO2 at a rate of 30% vol/min. Animals were
observed for 3 min to confirm death. The heartbeat was observed,
respiration was monitored and pupil dilation was assessed to ensure
successful animal euthanasia. Heart blood and brain tissue were
then collected for further experimental use and the rest of the
remains of the mice were disposed of by the Experimental Animal
Center of Ningxia Medical University within 1 week.
Neuronal cell culture
HT-22 immortalized mouse hippocampal cells were
purchased from Procell Life Science & Technology Co., Ltd.
HT-22 cells were cultured and maintained in Dulbecco's modified
Eagle's medium supplemented with 10% FBS, 100 units/ml penicillin
and 100 µg/ml streptomycin at 37°C in humidified conditions under
5% CO2. The medium was changed thrice weekly and
cultures were split in a ratio of 1:5 weekly.
Behavioral assessments
Mice were transferred to the behavioral analysis
room a day before observational experiments to allow them to adapt
to the environment. All devices were cleaned before the
experiments. For the open field test (OFT), the experimental area
was classified into a central area (25% of the total area) and a
peripheral area (75% of the total area). The mice were placed in
the center of the open field and the behavioral parameters were
recorded for 5 min using a video tracking system (Smart 3.0;
Panlab). Next, the new object experiment (NOR) was performed. The
mice were placed into a box for 5 min with two identical objects
and taken out to rest for 1 h. Then, one of the objects was
replaced with an object of the same material but different shapes
and colors and the experiment continued for 5 min. Exploratory
behavior was classed as the mouse nose tip being within 2 cm of the
object. The video tracking system was used to record the time
during which the mouse explored the new and old objects separately.
In addition, the NOR index was calculated as follows: Percentage of
time spent on new objects/total time spent on both objects. After
each mouse experiment, the box was cleaned and ethanol was used to
remove residual odors.
ELISA
Plasma collected from mouse hearts after the
euthanasia of animals was stored at −80°C. ELISA plates (96-wells)
were coated with 0.5 µg of NMDA1 protein in 100 µl PBS/well
overnight at 4°C and blocked with 10% FBS/PBS with 0.1% Tween20
(cat. no. 04-001-1A; Biological Industries; Sartorius AG). Then,
the mouse plasma (1:1,000; 100 µl/well) was added to the wells for
2 h at 37°C. The signal was amplified with HRP-linked goat
anti-mouse IgG antibodies (1:5,000; 100 µl/well; cat. no. A21010;
Abbkine Scientific Co., Ltd.). Absorbance was measured at 450 nm
using a microplate reader.
Immunoblotting analyses
Hippocampi were dissected from the thawed brain and
lysed in RIPA buffer (cat. no. KGP250; Nanjing KeyGen Biotech Co.,
Ltd.). Protein concentration in the lysates was determined by the
using the BCA method according to the manufacturer's protocols
(cat. no. KGP902; Nanjing KeyGen Biotech Co., Ltd.). Then, 80 µg of
whole protein was denatured at 100°C for 5 min. Protein (80 µg) was
separated on 10% Mini-Protean TGX gels and subsequently transferred
onto a PVDF membrane. After blocking with 5% BSA (cat. no. A6010A;
Biotopped Life Sciences) for 30 min at 25°C, the membranes were
incubated with NMDA1 (1:1,000; cat. no. ab134308; Abcam),
postsynaptic density protein 95 (PSD-95; 1:1,000; cat. no. ab18258;
Abcam) and GAPDH antibodies (1:1,000; cat. no. TA802519; OriGene
Technologies, Inc.) overnight at 4°C. The membrane was washed
thrice with TBST (0.1% Tween-20 in TBS) and incubated with Dylight
680 goat anti-rabbit IgG (1:1,000; cat. no. A23720; Abbkine) and
IRDye 800CW goat anti-mouse IgG antibodies (1:2,000; cat. no.
926-32210; LI-COR Biosciences) for 1 h at 37°C. Protein bands were
visualized using the Odyssey CLx imager (LI-COR Biosciences). The
grayscale value of the protein was semi-quantified using ImageJ
(version 1.53c; National Institutes of Health).
Immunofluorescence
For the determination of antibodies bound to brain
tissues by immunofluorescence, 20 µm coronal sections were blocked
using 10% normal goat serum (Wuhan Boster Biological Technology
Ltd.) for 60 min at room temperature, incubated overnight at 4°C
with NMDA1 antibodies (1:100) and visualized after staining with
DyLight 488 goat anti-mouse IgG (1:500; cat. no. A23210; Abbkine)
for 30 min at 37°C. Then, the tissue sections were first incubated
overnight at 4°C with PSD-95 antibodies (1:100; cat. no. ab18258;
Abcam) and then incubated with the DyLight 680 goat anti-rabbit IgG
antibodies (1:500; cat. no. A23720; Abbkine) for 30 min at 37°C.
The slides were mounted and scanned using a DM6 fluorescence
microscope (Leica Microsystems GmbH). HT-22 cells (1×105
cells/ml) were incubated in 24 well plates with NMDA1 antibodies
(20 ng/µl) or mouse plasma (0.1 µl/µl) at 37°C for 24 h. The cells
were then fixed with 100% methanol at −20°C for 5 min, blocked with
10% normal goat serum for 30 min at room temperature, incubated
overnight at 4°C with NMDA1 antibodies (1:100) and visualized after
staining with the DyLight 488 goat anti-mouse IgG (1:500; cat. no.
A23210; Abbkine) for 30 min at 37°C. Then, the slides were
incubated overnight at 4°C with PSD-95 antibodies (1:100) and
incubated with the DyLight 680 goat anti-rabbit IgG antibodies
(1:500) for 30 min at 37°C. The slides were then mounted and imaged
using a fluorescence microscope (Zeiss). The protein cluster
density was analyzed using the ‘colocalization’ and ‘spot’
functions of the Imaris software (version 9.0.1; Oxford
Instruments).
Statistical analysis
The data of each group were tested for normality and
homogeneity of variance. An unpaired Student's t-test, one-sample
t-test and Welch's t-test was performed for comparison between two
groups. A two-way ANOVA followed by Sidak's post hoc test was
performed for comparisons between multiple groups. P<0.05 was
considered to indicate statistical significance. Data are presented
as mean ± standard error of the mean, all experiments were
performed three times. Statistical analyses were performed using
GraphPad Prism software (version 8; Dotmatics).
Results
NMDA1 caused behavioral changes in
mice
To study the behavioral alterations in mice treated
with NMDA1, OFTs were performed on days 7, 16 and 25 after
immunization and NORs were performed on days 9, 18 and 27 after
immunization (Fig. 1A). The
open-field loci of the mice in the control (CON) and NMDA1 groups
were measured (Fig. 1B and C).
Compared with the CON group, the total horizontal movement distance
of the NMDA1 group significantly decreased on day 16 after
immunization (P<0.01) (Fig.
1D). Moreover, the time in the central area was significantly
reduced in NMDA1-treated mice compared with CON mice on days 16 and
25 after immunization (P<0.05 and P<0.01, respectively)
(Fig. 1E). The aforementioned
results demonstrated that the mice in the NMDA1 group exhibited
greater anxiety and depression compared with those in the CON
group.
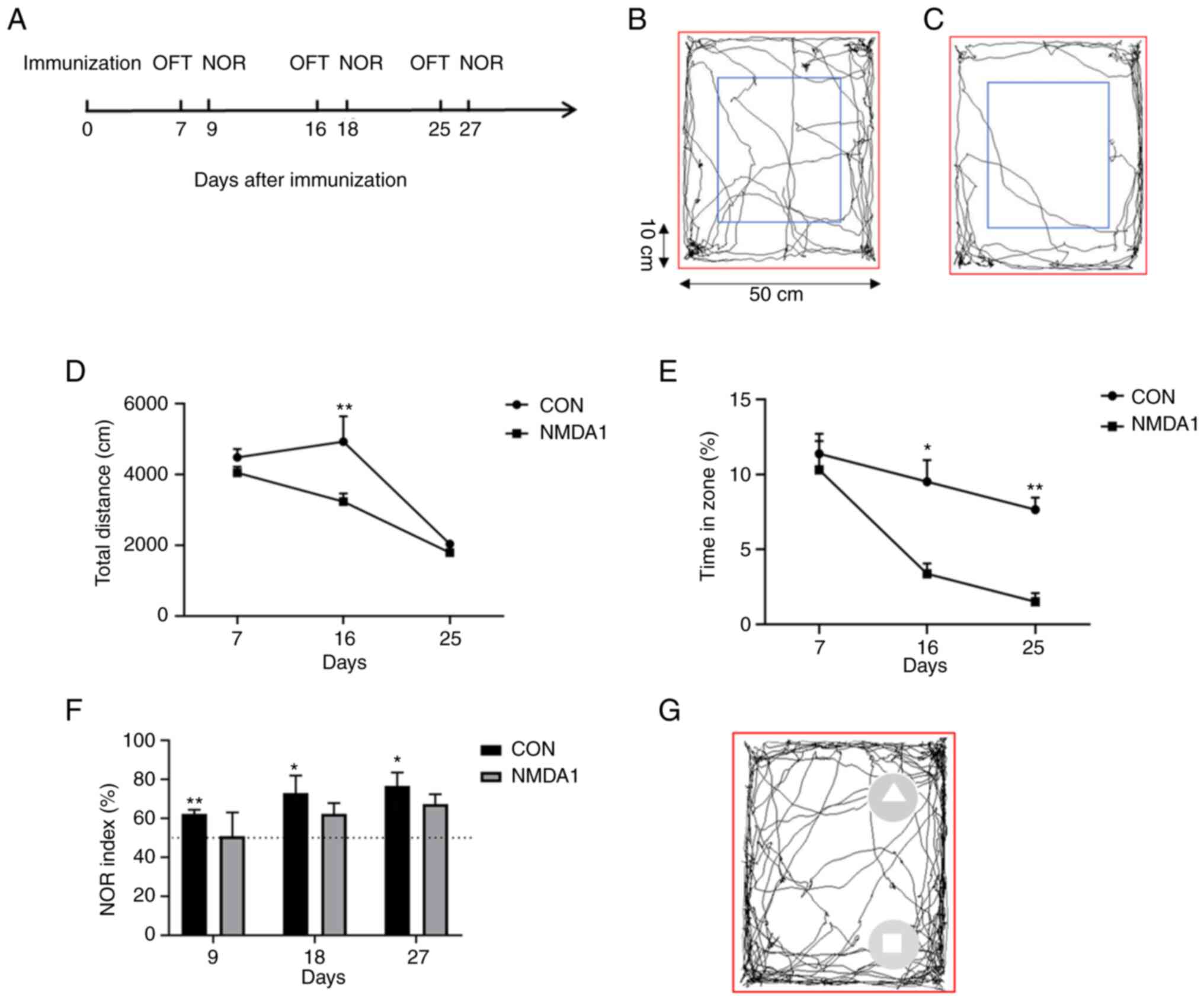 | Figure 1.Behavioral changes were observed in
mice after immunization with NMDA1. (A) OFT were performed on days
7, 16 and 25 after immunization, while NOR experiments were
performed on days 9, 18 and 27 after NMDA1 immunization. Movement
trajectories of the mice were measured in a 50×50 cm area (red
box), an area 10 cm from the edge of the area (blue box, central
area) and the area between the red and blue boxes (peripheral area)
in (B) CON mice and (C) NMDA1-treated mice. (D) The total distance
moved by mice in the NMDA1 and CON groups. *P<0.05, **P<0.01
vs. CON. (E) Time spent in the central region of the NMDA1 and CON
groups. *P<0.05, **P<0.01 vs. CON. (F) Recognition index of
mice in the NMDA1 and CON groups. *P<0.05, **P<0.01 vs. 50%.
(G) Mouse movement trajectory map, square represents an old object
and a triangle represents a new object. Data are presented as mean
± standard error of the mean. n=5. OFT, open field experiments;
NOR, novel object recognition experiments; NMDA1,
N-methyl-D-aspartate receptor subunit 1; CON, control. |
Healthy mice tend to explore new objects, hence, the
recognition index exhibited by these mice would be >50%.
However, mice with impaired memory cannot recognize new or old
objects, therefore, the recognition index of these mice would be
~50% (10). On days 9, 18 and 27
after immunization, the NOR index in the CON group was >50% and
was significantly greater than the expected 50% (P<0.01,
P<0.05 and P<0.05, respectively); however, there was no
significant difference between the NOR and the expected 50% in the
NMDA1 group over the three days (Fig.
1F). The movement trajectory diagram of the mouse NOR was
recorded, the mouse in the CON group spent longer exploring a new
object than an old object (Fig.
1G). The aforementioned results demonstrated that compared with
the mice in the CON group, those in the NMDA1 group demonstrated
memory impairment.
Production of plasma anti-NMDA1
antibodies reduced the protein expression levels of the
hippocampal, NMDA1 protein
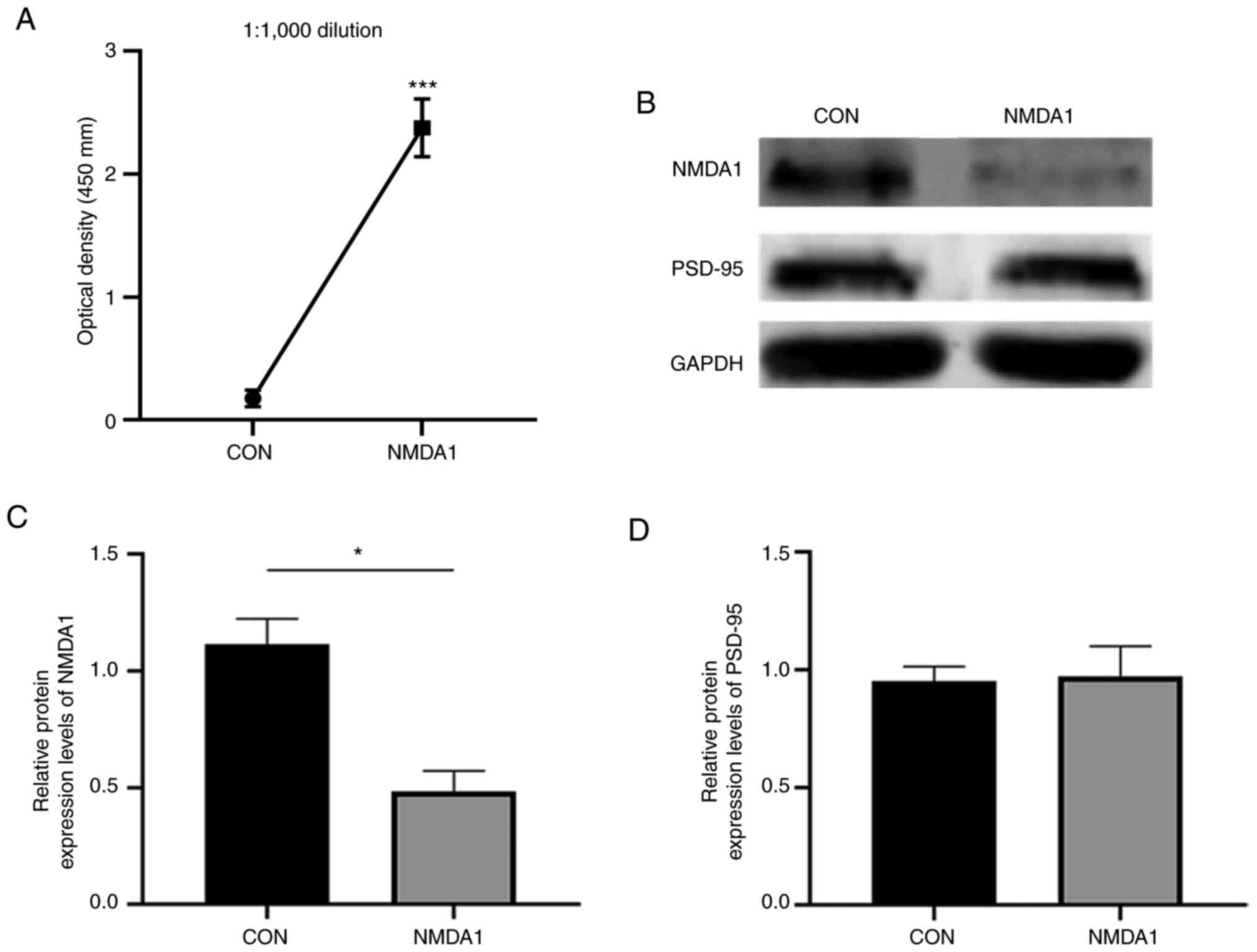
Anti-NMDA1 antibody levels in mouse plasma were
assessed using ELISA (Fig. 2A).
Compared with the CON group, the level of anti-NMDA1 antibodies in
the plasma of the mice in the NMDA1 group was significantly
increased (P<0.001).
Western blotting was used to assess the protein
expression levels of NMDA1 and PSD-95 proteins in the mouse
hippocampus (Fig. 2B-D). These
results demonstrated that compared with the CON group, the protein
expression levels of the NMDA1 protein were significantly decreased
in the NMDA1 group (P<0.05). However, there was no significant
difference in the expression levels of the PSD-95 protein between
the two groups.
Production of anti-NMDA1 antibodies
reduced the protein expression levels of NMDA1 in the mouse
hippocampal postsynaptic membrane
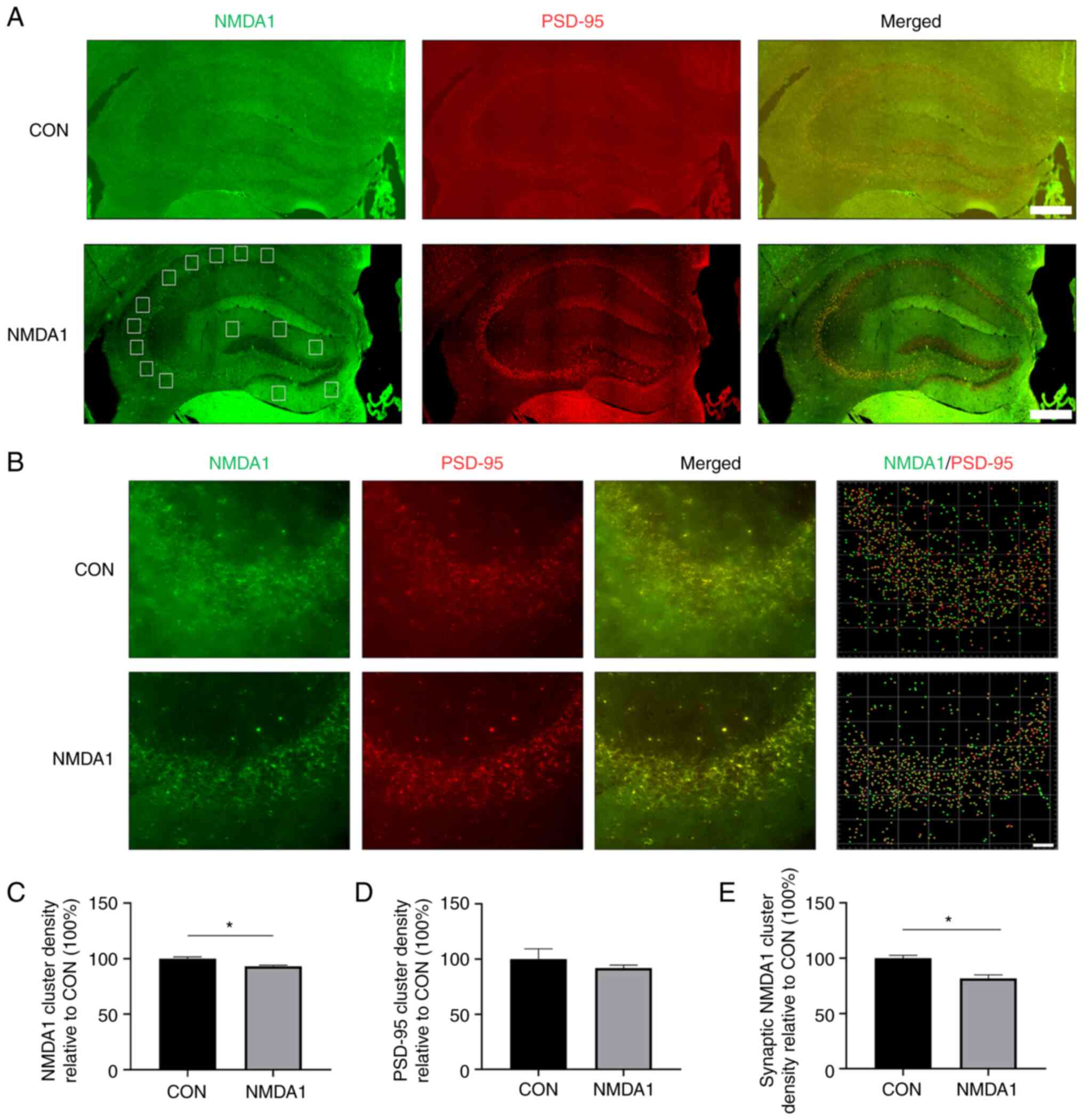
To evaluate the effect of anti-NMDA1 antibodies in
mouse plasma on the expression of the NMDA1 protein in the
hippocampus, frozen tissue section immunofluorescence imaging was
used to detect the protein expression levels of NMDA1 and PSD-95 in
the mouse hippocampus. Immunolabeling of NMDA1 and PSD-95 proteins
in the hippocampus of mice in the CON and NMDA1 groups was
performed (Fig. 3A) and a
representative 2D image of the CA3 region and the density analysis
of protein clusters was obtained (Fig.
3B). The total cluster density of NMDA1 protein in the
hippocampus of the mice in the CON group was significantly higher
compared with the mice in the NMDA1 group (P<0.05; Fig. 3C). However, the total cluster
density of PSD-95 protein in the hippocampus of the mice in the CON
group was not significantly different compared with the NMDA1 group
(Fig. 3D). When the NMDA1 protein
in the hippocampal synapse of the mice was examined, the cluster
density in the CON group was significantly higher compared with the
NMDA1 group (P<0.05; Fig.
3E).
Reduction in the expression of the
NMDA1 protein in the HT-22 cellular postsynaptic membrane by the
production of anti-NMDA1 antibodies
To determine the effect of anti-NMDA1 antibodies on
the expression levels of NMDA1 and PSD-95 proteins in neuronal
synapses, immunofluorescence was used to evaluate the protein
expression levels of NMDA1 and PSD-95 in HT-22 cells (Fig. 4A). The number of protein clusters
of NMDA1 and PSD-95 and the number of synaptic clusters of NMDA1
immunolabeled were quantified (Fig.
4B-D). The number of protein clusters of NMDA1 in the PBS group
was significantly higher compared with the NMDA1 Ab group
(P<0.05). The total PSD-95 number of protein clusters in the PBS
group was not significantly different compared with the NMDA1 Ab
group. The number of synaptic NMDA1 clusters in the PBS group was
significantly higher compared with the NMDA1 Ab group (P<0.05).
After adding mouse plasma to the cultured HT-22 cells, the number
of clusters of NMDA1 and PSD-95 proteins and the number of synaptic
clusters of NMDA1 were immunolabeled (Fig. 4E). The number of clusters of NMDA1
in the CON group was significantly higher compared with the NMDA1
group (P<0.05) (Fig. 4F). The
number of clusters of PSD-95 in the CON group was not significantly
different compared with the NMDA1 group (Fig. 4G). The synaptic cluster of NMDA1
protein in the CON group was significantly higher compared with the
NMDA1 group (P<0.05) (Fig.
4H).
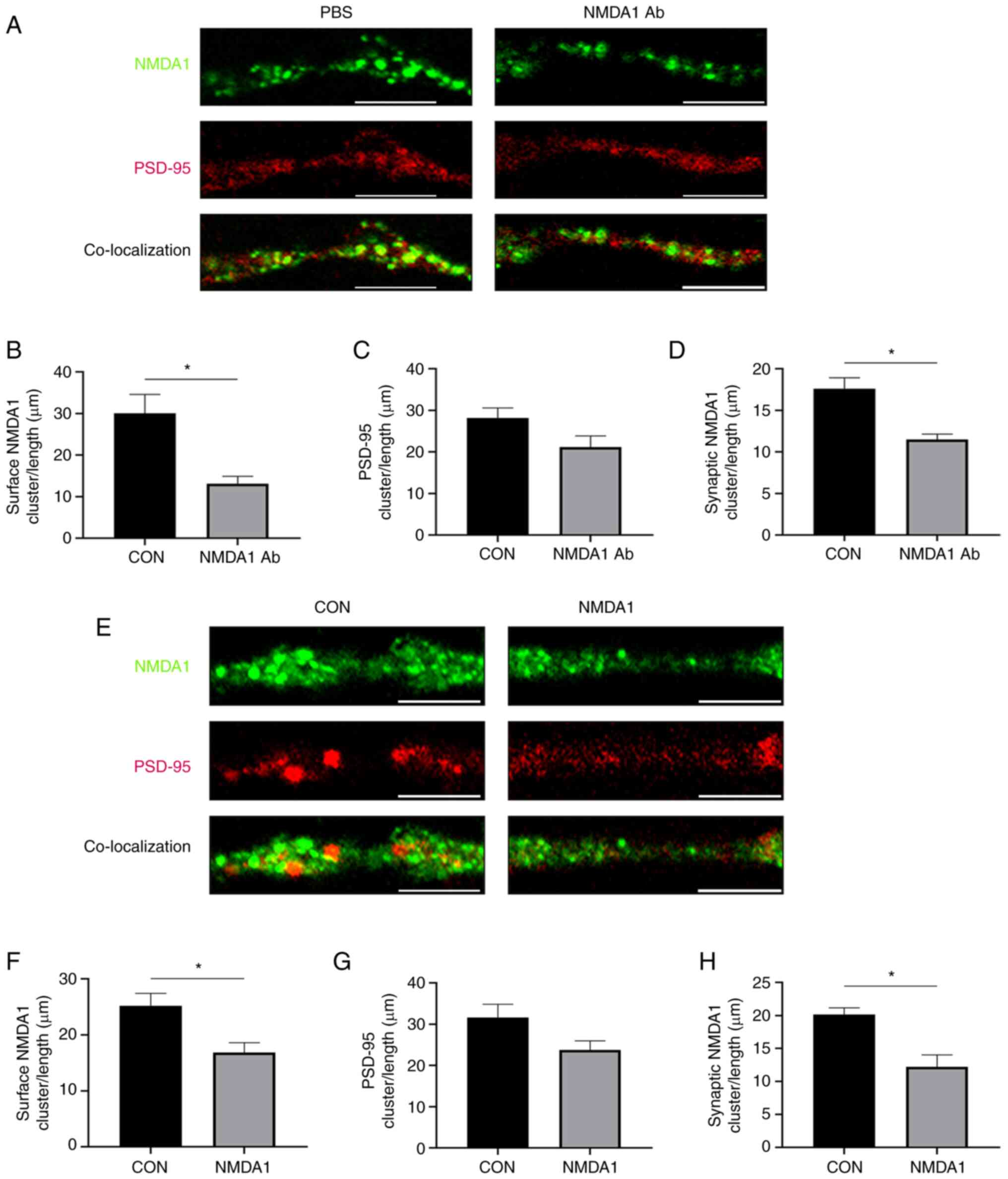 | Figure 4.NMDA1 antibodies reduce NMDA1 protein
expression in the HT-22 cell postsynaptic membrane. (A)
Representative synapses of HT-22 cells in the PBS and the NMDA1 Ab
group, demonstrating the total cluster of NMDA1 protein (green),
the total cluster of PSD-95 protein (red) and the synaptic cluster
of NMDA1 protein (green and red co-localized). Scale bar, 5 µm.
Quantitative analysis of (B) the total cluster of NMDA1 protein in
the PBS and NMDA1 Ab groups, (C) PSD-95 protein in the PBS and
NMDA1 Ab groups and (D) synaptic clusters of NMDA1 protein in the
PBS and NMDA1 Ab groups. (E) Representative HT-22 cell synapses in
CON and NMDA1 groups. NMDA1 protein total cluster (green), PSD-95
protein total cluster (red) and NMDA1 protein synaptic cluster
(green and red co-localized). Cluster density was defined as:
Number of spots/the synapse length (µm). Scale bar, 5 µm. Multiple,
10×100. (F) Quantitative analysis of (F) total clusters of NMDA1
protein in the CON and NMDA1 groups, (G) total clusters of PSD-95
protein in the CON and NMDA1 groups and (H) NMDA1 protein synaptic
clusters in the CON and NMDA1 groups. *P<0.05 vs. CON. Data are
presented as mean ± standard error of the mean. n=3. CON, control;
NMDA1, N-methyl-D-aspartate receptor subunit 1; PSD-95,
postsynaptic density protein 95; Ab, antibodies; NMDAR,
N-methyl-D-aspartate receptor. |
Discussion
The results of the present study demonstrated that
active immunization of mice with the NMDA1 (19–559 aa) protein
induced a disease state that exhibited the major features of human
anti-NMDAR encephalitis, such as abnormal behavior, cognitive
dysfunction or memory deficit (11). The establishment of animal models
via active immunization has previously served a key role in the
research of neurological diseases (12). For example, peptide fragments
extracted from myelin sheaths have previously been used to generate
experimental autoimmune encephalomyelitis (13). Similarly, active immunization with
neuromuscular junction proteins has been reported to result in
myasthenia-like features in mice (14). Earlier studies have reported that
active immunization of ApoE−/− mice with NMDA1 peptide
fragments resulted in high levels of circulating anti-NMDA1
antibodies and induced psychotic-like symptoms, such as
hyperactivity, upon MK-801 challenge (6,15).
The present study demonstrated that active immunization with the
NMDA1 protein induced high titers of pathogenic anti-NMDA1
autoantibodies. Furthermore, this immunization reproduced many of
the typical symptoms of anti-NMDA encephalitis in mice, such as
anxiety, depression and memory loss. The model of nascent
autoimmune anti-NMDA receptor encephalitis constructed in the
present study may potentially provide a novel way to investigate
the pathophysiology of human diseases and develop appropriate
treatment methods in the future.
The NMDA1 (19–559 aa) protein was expressed and
purified and it was found to exhibit good antigenicity.
Subsequently, mice were immunized with NMDA1 to produce a large
number of peripheral blood anti-NMDA1 antibodies. However, the
triggering mechanism of the autoimmune response to the NMDA1
protein remains unclear. The epitopes of patient-derived anti-NMDA1
antibodies have previously been reported to be to be extracellular
domains of the NMDA1 protein subunit (2,16–18).
The use of whole protein immunogens with intact extracellular
domains may have served a role in the pathogenesis of mice in the
present study.
Anti-NMDA1 antibodies did not directly bind to the
mouse hippocampus in the present study, nonetheless, mice with high
levels of plasma anti-NMDA1 antibodies exhibited reduced numbers of
postsynaptic NMDA1 protein clusters in the hippocampus and reduced
total hippocampal NMDA1 protein expression levels. The number of
PSD-95 protein clusters in the hippocampus remained unaltered and
the same finding was demonstrated in the cellular model of NMDA1
immunization. PSD-95 is a postsynaptic scaffolding protein that
serves a critical role in synaptogenesis and synaptic plasticity by
providing a platform for the postsynaptic clustering of crucial
synaptic proteins (19,20). PSD-95 interacts with the
cytoplasmic tail of NMDA receptor subunits and shaker-type
potassium channels and is required for synaptic plasticity
associated with NMDA receptor signaling (19). In the present study, the reduction
in the expression levels of the hippocampal NMDA1 protein suggested
that these mouse antibodies were similar to patient-derived
anti-NMDA1 antibodies.
Previous studies have reported that autoantibodies
targeting the NMDA1 protein are involved in disease pathogenesis,
such as NMDAR encephalitis (15,21).
In the present study, mice demonstrated memory deficits on days 9,
18 and 27 after immunization with NMDA1. This observation was
consistent with previously reported memory impairments in passive
and active immunization models (3,4).
Moreover, previous studies reported that treatment with anti-NMDA1
antibodies significantly reduced the density of the NMDA1 protein
in the membrane of hippocampal neurons, thus impairing its function
(3). Functional studies based on
drug inhibition of NMDAR or hippocampal CA1-specific ablation of
the GluN1 subunit have reported that hippocampal NMDAR was involved
in object recognition memory (22,23).
The NMDAR protein is essential for synaptic plasticity and memory
formation. Anatomically, extensive synapses and interconnections
are present between the limbic cortex and the hippocampus,
particularly the CA1 subregion and the inferior colliculus
(24) but not the dentate gyrus
(25). Furthermore, several
studies have reported that NMDARs located within the limbic cortex
serve a pertinent role in recognition memory (26–28).
Mice exhibit thigmotaxis as they are afraid of open,
unknown and potentially dangerous places. Hence, they show the
characteristic of ‘sticking to the wall’. It is generally believed
that the more time spent in the central area of the enclosure, the
lower the thigmotaxis and anxiety of the mice. Decreased total
horizontal movement distance indicates depression in mice (29,30).
The present study demonstrated that mice exhibited significantly
reduced central area motor time and anxiety-related behaviors on
days 16 and 25 after immunization with NMDA1. This finding was in
accordance with the key symptoms, such as anxiety and depression,
reported in patients with anti-NMDAR encephalitis (11). A previous study by Li et al
(31) reported a shorter time
spent in the center of the open area in NMDA-CSF-treated mice, but
this effect was not statistically significant. However, a previous
study reported a significant increase in tropism in
NMDA-CSF-treated animals in the Morris water maze (23). Based on these reports, Kersten
et al (10) hypothesized
that anxiety-related behaviors might be enhanced in
NMDA-CSF-treated animals. The present study demonstrated that the
total distance of horizontal movement was significantly decreased
in the NMDA1 group on day 16 after immunization compared with the
CON group. This observation is less consistent with previous
reports that the total distance during the observation period did
not differ between the experimental groups, which suggested that
spontaneous motor activity was completely normal (23,31).
The spontaneous activity was derived by calculating the total
movement distance of mice from 20–60 min in the mining experiment
(32). The present study
calculated the total horizontal movement distance of mice within 5
min and these results demonstrated an increase in avoidance in the
NMDA1 group or an association with depressive behavior. This was
consistent with the progressive memory deficits and depression-like
behaviors exhibited by the anti-NMDAR encephalitis mouse model
previously constructed by Planagumà et al (3).
The triggers of the autoimmune response of NMDAR
proteins are presently unknown. A previous study reported the use
of tetrameric xenopus holoprotein immunogens, NMDA1/GluN2B
receptors or rat NMDA1/GluN2A receptors to generate NMDAR
antibodies to produce fulminant encephalitis (4). Using the GluN1356-385 peptide that
targets the ATD of GluN1, an anti-NMDAR encephalitis model was
previously established via active immunization (7). Intranasal infection with herpes
simplex virus has also been reported to induce circulating
anti-NMDAR antibodies in mice, which may explain the pathogenesis
of secondary anti-NMDAR encephalitis in patients with herpes
simplex virus encephalitis (5). In
the present study, the thin outer segment from the NMDA1 subunit
was used as the immunogen, which was adequate to induce high titers
of pathogenic anti-NMDA1 autoantibodies. Actively immunized mice
developed memory deficits and anxiety-related symptoms typical of
mouse anti-NMDAR encephalitis. This active immunization model may
be useful for studying NMDAR encephalitis and could aid in further
investigations on the pathogenesis of the disease, thus
contributing to the development of potential new therapies in the
future.
Nonetheless, the present study has certain
limitations. Although NMDAR density in the hippocampus of mice
immunized with the NMDA1 protein was reduced, the mice did not
develop spontaneous seizures. In addition, the role of B and T
cells in disease induction is yet to be determined and further
studies are warranted.
The present study successfully constructed a mouse
model of anti-NMDAR encephalitis. The model mice exhibited symptoms
of anxiety, depression and memory impairment and a significant
increase in anti-NMDA1 antibodies in the peripheral blood of the
mice. Synaptic and total NMDA1 protein expressions were
significantly reduced in mouse hippocampus tissue and cultured
hippocampal cells. However, the expression of the PSD-95 protein
was not significantly different between the control and NMDA1
immunization models.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation
of China (grant no. 81960233) and the Key Research and Development
Project of Ningxia (grant nos. 2018BFG02017 and 2019BCG01003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY and YW confirm the authenticity of all the raw
data. LY contributed to the study planning, data collection, data
analysis, statistical analysis, data interpretation and manuscript
writing. YW, JY, GW, NZ, XG and JG performed the experimental
procedures. ZW contributed to the study conception and design,
supervision of data collection and analysis, data interpretation
and writing and revising the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures involved in this study were approved
by the Laboratory Animal Ethical and Welfare Committee of
Laboratory Animal Center, Ningxia Medical University (Yinchuan,
China; approval no. IACUC-NYLAC-2019-072).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NMDAR
|
N-methyl-D-aspartate receptor
|
|
NMDA1
|
N-methyl-D-aspartate receptor subunit
1
|
|
PSD-95
|
postsynaptic density protein 95
|
|
NR1
|
N-methyl-D-aspartate receptor subunit
1
|
|
GluN2B
|
N-methyl-D-aspartate receptor subunit
2B
|
|
GluN1
|
N-methyl-D-aspartate receptor subunit
1
|
|
GRIN1
|
N-methyl-D-aspartate receptor subunit
1
|
|
ATD
|
amino-terminal domain
|
|
CFA
|
complete freund's adjuvant
|
|
NOR
|
new object experiment
|
|
OFT
|
open field test
|
References
|
1
|
Yang J and Liu X: Immunotherapy for
refractory autoimmune encephalitis. Front Immunol. 12:7909622021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mikasova L, De Rossi P, Bouchet D, Georges
F, Rogemond V, Didelot A, Meissirel C, Honnorat J and Groc L:
Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors
in anti-NMDA encephalitis. Brain. 135:1606–1621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Planagumà J, Leypoldt F, Mannara F,
Gutiérrez-Cuesta J, Martín-García E, Aguilar E, Titulaer MJ,
Petit-Pedrol M, Jain A, Balice-Gordon R, et al: Human N-methyl
D-aspartate receptor antibodies alter memory and behaviour in mice.
Brain. 138:94–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones BE, Tovar KR, Goehring A,
Jalali-Yazdi F, Okada NJ, Gouaux E and Westbrook GL: Autoimmune
receptor encephalitis in mice induced by active immunization with
conformationally stabilized holoreceptors. Sci Transl Med.
11:eaaw00442019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linnoila J, Pulli B, Armangué T, Planagumà
J, Narsimhan R, Schob S, Zeller MWG, Dalmau J and Chen J: Mouse
model of anti-NMDA receptor post-herpes simplex encephalitis.
Neurol Neuroimmunol Neuroinflamm. 6:e5292018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan H, Oliveira B, Saher G, Dere E, Tapken
D, Mitjans M, Seidel J, Wesolowski J, Wakhloo D, Klein-Schmidt C,
et al: Uncoupling the widespread occurrence of anti-NMDAR1
autoantibodies from neuropsychiatric disease in a novel autoimmune
model. Mol Psychiatry. 24:1489–1501. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding Y, Zhou Z, Chen J, Peng Y and Wang H,
Qiu W, Xie W, Zhang J and Wang H: Anti-NMDAR encephalitis induced
in mice by active immunization with a peptide from the
amino-terminal domain of the GluN1 subunit. J Neuroinflammation.
18:532021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin Y, Liu Z, Jiang J, Jiang Z, Ji Y and
Sun B: Expression of intracellular domain of epidermal growth
factor receptor and generation of its monoclonal antibody. Cell Mol
Immunol. 1:137–141. 2004.PubMed/NCBI
|
|
9
|
Liu B, Kong Q, Zhang D and Yan L: Codon
optimization significantly enhanced the expression of human 37-kDa
iLRP in Escherichia coli. 3 Biotech. 8:2102018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kersten M, Rabbe T, Blome R, Porath K,
Sellmann T, Bien CG, Köhling R and Kirschstein T: Novel object
recognition in rats with NMDAR dysfunction in CA1 after
stereotactic injection of anti-NMDAR encephalitis cerebrospinal
fluid. Front Neurol. 10:5862019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Graus F, Titulaer MJ, Balu R, Benseler S,
Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M,
et al: A clinical approach to diagnosis of autoimmune encephalitis.
Lancet Neurol. 15:391–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hemmer B, Kerschensteiner M and Korn T:
Role of the innate and adaptive immune responses in the course of
multiple sclerosis. Lancet Neurol. 14:406–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bjelobaba I, Begovic-Kupresanin V, Pekovic
S and Lavrnja I: Animal models of multiple sclerosis: Focus on
experimental autoimmune encephalomyelitis. J Neurosci Res.
96:1021–1042. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Viegas S, Jacobson L, Waters P, Cossins J,
Jacob S, Leite MI, Webster R and Vincent A: Passive and active
immunization models of MuSK-Ab positive myasthenia:
Electrophysiological evidence for pre and postsynaptic defects. Exp
Neurol. 234:506–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Castillo-Gómez E, Oliveira B, Tapken D,
Bertrand S, Klein-Schmidt C, Pan H, Zafeiriou P, Steiner J, Jurek
B, Trippe R, et al: All naturally occurring autoantibodies against
the NMDA receptor subunit NR1 have pathogenic potential
irrespective of epitope and immunoglobulin class. Mol Psychiatry.
22:1776–1784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dalmau J, Gleichman AJ, Hughes EG, Rossi
JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R and
Lynch DR: Anti-NMDA-receptor encephalitis: Case series and analysis
of the effects of antibodies. Lancet Neurol. 7:1091–1098. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hughes EG, Peng X, Gleichman AJ, Lai M,
Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J and Balice-Gordon
RJ: Cellular and synaptic mechanisms of anti-NMDA receptor
encephalitis. J Neurosci. 30:5866–5875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gleichman AJ, Spruce LA, Dalmau J,
Seeholzer SH and Lynch DR: Anti-NMDA receptor encephalitis antibody
binding is dependent on amino acid identity of a small region
within the GluN1 amino terminal domain. J Neurosci. 32:11082–11094.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Migaud M, Charlesworth P, Dempster M,
Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG,
Morrison JH, et al: Enhanced long-term potentiation and impaired
learning in mice with mutant postsynaptic density-95 protein.
Nature. 396:433–439. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
He J, Bellini M, Xu J, Castleberry AM and
Hall RA: Interaction with cystic fibrosis transmembrane conductance
regulator-associated ligand (CAL) inhibits beta1-adrenergic
receptor surface expression. J Biol Chem. 279:50190–50196. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malviya M, Barman S, Golombeck KS,
Planagumà J, Mannara F, Strutz-Seebohm N, Wrzos C, Demir F,
Baksmeier C, Steckel J, et al: NMDAR encephalitis: Passive transfer
from man to mouse by a recombinant antibody. Ann Clin Transl
Neurol. 4:768–783. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Planagumà J, Haselmann H, Mannara F,
Petit-Pedrol M, Grünewald B, Aguilar E, Röpke L, Martín-García E,
Titulaer MJ, Jercog P, et al: Ephrin-B2 prevents
N-methyl-D-aspartate receptor antibody effects on memory and
neuroplasticity. Ann Neurol. 80:388–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Q, Tanaka K, Sun P, Nakata M,
Yamamoto R, Sakimura K, Matsui M and Kato N: Suppression of
synaptic plasticity by cerebrospinal fluid from anti-NMDA receptor
encephalitis patients. Neurobiol Dis. 45:610–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naber PA, Witter MP and Lopez da Silva FH:
Perirhinal cortex input to the hippocampus in the rat: Evidence for
parallel pathways, both direct and indirect. A combined
physiological and anatomical study. Eur J Neurosci. 11:4119–4133.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Witter MP, Naber PA and Lopes da Silva F:
Perirhinal cortex does not project to the dentate gyrus.
Hippocampus. 9:605–606. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abe H, Ishida Y and Iwasaki T: Perirhinal
N-methyl-D-aspartate and muscarinic systems participate in object
recognition in rats. Neurosci Lett. 356:191–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Winters BD and Bussey TJ: Glutamate
receptors in perirhinal cortex mediate encoding, retrieval, and
consolidation of object recognition memory. J Neurosci.
25:4243–4251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barker GRI, Warburton EC, Koder T, Dolman
NP, More JC, Aggleton JP, Bashir ZI, Auberson YP, Jane DE and Brown
MW: The different effects on recognition memory of perirhinal
kainate and NMDA glutamate receptor antagonism: Implications for
underlying plasticity mechanisms. J Neurosci. 26:3561–3566. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simon P, Dupuis R and Costentin J:
Thigmotaxis as an index of anxiety in mice. Influence of
dopaminergic transmissions. Behav Brain Res. 61:59–64. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu GX, Cai GQ, Cai YQ, Sheng ZJ, Jiang J,
Mei Z, Wang ZG, Guo L and Fei J: Reduced anxiety and
depression-like behaviors in mice lacking GABA transporter subtype
1. Neuropsychopharmacology. 32:1531–1539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Tanaka K, Wang L, Ishigaki Y and
Kato N: Induction of memory deficit in mice with chronic exposure
to cerebrospinal fluid from patients with anti-N-Methyl-D-aspartate
receptor encephalitis. Tohoku J Exp Med. 237:329–338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu YP, Liu T, Bi H, Zhang L and Dang YH:
Effects of strain, sex and circadian rhythm on open field test on
mice. Xi'an Jiaotong Daxue Xuebao Yixue Ban. 35:634–638. 2014.
|