Introduction
Depending on its nature, duration and intensity,
stress has a distinctive effect on pain perception. Notably, acute
or robust stress can induce analgesia; however, chronic or repeated
exposure to various stressors can induce hyperalgesia or exacerbate
existing pain, known as stress-induced hyperalgesia (SIH) (1). Various neural pathways participate in
the development of SIH, including the cortex, amygdala,
periaqueductal grey, rostral ventromedial medulla and spinal cord,
involving diverse neurotransmitters and neuromodulatory systems
(2). For example, glutamate
content is increased at the spinal and supraspinal levels during
stress to facilitate hyperalgesia if connected with ionotropic, but
not metabotropic, glutamate receptors (3). Traumatic stress can promote
hyperalgesia in rats by activating the corticotropin-releasing
factor (CRF)/CRF receptor 1 pathway in the central amygdala
(4), or via the interaction
between microglia and neurons in the spinal dorsal horn (5). However, the molecular mechanisms
underlying SIH remain unclear.
The heat shock cognate 71 kDa protein (Hsc70) is an
ATPase that protects cells against various harmful stimuli. In
particular, it is enriched in the nervous system and present at a
high level in neuronal cell bodies (6). Hsc70 and its ATPase activity regulate
transient receptor potential vanilloid 1 (TRPV1) expression and
function via inhibiting ROCK phosphorylation at the TRPV1-S502 site
in an Hsc70-dependent manner (7).
Notably, TRPV1 is a well-documented nociceptor expressed in primary
afferent dorsal root ganglion (DRG) neurons that participates in
nociceptive perception (8).
Increased TRPV1 expression is a characteristic of channel
sensitization that results in hyperalgesia (9).
In addition, according to the dual-luciferase
reporter assay and miRanda v1.0b prediction performed by Scott
et al (10), microRNA
(miRNA/miR)-3120 is an endogenous regulator of Hsc70. miRNAs are
small non-coding RNA molecules that are comprised of 18–25
nucleotides, which recognize the 3′ untranslated regions of target
mRNA to modulate gene and protein expression, and are thus involved
in a variety of biological processes (11). The etiological role of miRNAs in
diverse painful conditions, such as migraine, fibromyalgia,
visceral pain, and inflammatory or neuropathic pain, has been
demonstrated (12). Although
miRNAs have attracted great attention as diagnostic biomarkers and
therapeutic targets, to the best of our knowledge, their
participation in SIH has not been reported.
In the present study, complete Freund's adjuvant
(CFA) hind paw injection was performed in rats to induce
inflammatory pain (CFA rats). Furthermore, forced swim (FS) stress
was performed for 3 days before CFA injection to evoke mechanical
hyperalgesia (FS + CFA rats). Using this SIH rat model, the
participation of Hsc70 and its regulator miR-3120 were investigated
in FS stress-induced mechanical hyperalgesia in rats in an
inflammatory state.
Materials and methods
Animals and ethics approval
The present study was approved by the Institutional
Ethics Committee of Nanjing Medical University (approval no.
1706017; Nanjing, China). The present study was conducted using
adult male Sprague-Dawley rats (age, 6–8 weeks; weight, 180–240 g;
Qinglongshan Animal Center). Rats were housed individually with
free access to food and water, and were acclimated to the
environment with a temperature of 23–25°C, a humidity of 40–60% and
a 12-h light/dark cycle for at least 1 week before the experiment.
After the experiment, all rats were euthanized by decapitation
under deep anesthesia with 4–5% sevoflurane.
Animal models establishment
FS was induced in rats according to a previously
described method (13). Briefly,
in a cylinder (diameter, 30 cm; height, 50 cm) containing water at
24–26°C to a height of 20 cm, the rats were forced to swim for 3
consecutive days, for 10 min on the first day and then for 20 min
on the subsequent 2 days. After each FS session, the rats were
carefully dried and rewarmed. Inflammatory pain was induced via a
single injection of CFA (50 µl; MilliporeSigma) into the right hind
paw. FS stress has previously been reported to increase pain-like
behaviors in the hind paw (14).
In the present study, FS stress-induced mechanical hyperalgesia in
rats in an inflammatory state was established using FS stress for 3
days, followed by a single CFA injection. Control rats were
injected with 50 µl deionized water. To verify the efficacy of FS
stress-induced mechanical hyperalgesia in rats in an inflammatory
state, 6 rats were used in each group.
ELISA detection for serum
corticosterone
For serum corticosterone detection, 300 µl blood was
collected from the tail vein under 2–3% sevoflurane anesthesia 2 h
after FS stress, and the plasma was separated and stored at −80°C
until the ELISA was performed. A commercially available ELISA kit
(cat. no. ab108821; Abcam) was used to measure serum corticosterone
levels according to the manufacturer's instructions. The OD value
was acquired at 450 nm using a multi-function microplate reader (MD
Spectramac M3; Molecular Devices, LLC). Different rat groups were
measured across days 1–3, with three rats assessed at each time
point. For control rats, the blood was collected at the
approximately same time as for rats in the other groups.
Pain behavioral test
Mechanical pain was examined using von-Frey
filaments. Briefly, the rats were individually placed in a
transparent Plexiglass chamber for 30 min. Thereafter, a series of
von Frey filaments (0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0 and 15 g;
Danmic Global, LLC) were vertically applied to the central plantar
of the hind paw to evoke a flinch response using an up-down method
(15). The paw withdrawal
threshold was detected five times with an interval of 5 min, and
the mean value of the last three results was calculated as the
mechanical withdrawal threshold.
Western blot analysis
Hsc70 and TRPV1 expression levels in the DRG were
examined by western blot, as previously described (16). After the pain behavioral test, the
rats were sacrificed by decapitation under 4–5% sevoflurane
anesthesia and L4-5 DRG was collected for western blot analysis.
Briefly, the DRG was separated and homogenized in RIPA lysis buffer
(cat. no. bl504a; Biosharp Life Sciences), and the resulting
supernatant was collected. After protein quantification using the
Bradford assay, 20 µg proteins were separated by SDS-PAGE 8–12% on
gels and transferred onto polyvinylidene difluoride membranes. The
membranes were blocked with 5% non-fat milk for 1 h at room
temperature, and then incubated with primary antibodies against
Hsc70 (1:500; cat. no. ab51052), TRPV1 (1:200; cat. no. ab305299)
overnight at 4°C, and GAPDH (1:1,000; cat. no. ab8245) (all from
Abcam) was used as an internal control. After washing in
Tris-buffered saline-0.05% Tween three times, the membranes were
incubated with horseradish peroxidase-labeled immunoglobulin G
(1:5,000; cat. no. bl003A; Biosharp Life Sciences) for 1 h at room
temperature. The bands were detected using a luminescent imaging
system (G:BOX Chemi XR5; Syngene) and the blots were analyzed using
ImageJ software (v. 1.53a; National Institutes of Health).
Immunofluorescence (IF) analysis
In the DRG, Hsc70 and TRPV1 expression levels were
also detected by IF analysis. After sacrifice, the rats were
transcardially perfused with 4% paraformaldehyde. The L4-5 DRG was
removed, fixed in the same fixative at 4°C for 6 h, and thereafter
embedded in paraffin. For IF staining, the DRG tissue (3 µm) was
mounted on glass slides. After sequential dewaxing, hydration and
microwave antigen retrieval in sodium citrate for 20 min, the
sections were blocked with 5% bovine serum albumin (cat. no.
4240GR500; Biofroxx; neoFroxx GmbH) at 37°C for 1 h. After
overnight incubation with a rabbit antibody against Hsc70 (1:200;
cat. no. ab51052) or a mouse antibody against TRPV1 (1:200; cat.
no. ab203103) (both from Abcam) at 4°C, the sections were incubated
with fluorescein isothiocyanate-conjugated (1:200; cat. no. BL033A;
Biosharp Life Sciences) or tetramethylrhodamine-conjugated (1:100;
cat. no. 115-025-003; Jackson ImmunoResearch Europe Ltd.) secondary
antibodies for 1 h at 37°C. Nuclear DNA was labelled with DAPI. The
obtained IF sections were scanned and stored using an OlyVIA system
(Olympus VS200; Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for miR-3120 and Hsc70
RT-qPCR detection of miR-3120 and Hsc70 expression
in the DRG was performed. Briefly, total RNA was extracted from the
DRG with ice-cold TRIzol® reagent (cat. no. 15596-026;
Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, RNA
concentration and purity were determined using an ultraviolet
photometer (Shimadzu UV-2450; Shimadzu Corporation) at 260 and 280
nm; a result of 1.8–2.1 was considered an appropriate purity. Using
2 µg total RNA as a template, cDNA was synthesized using a reverse
transcriptase reagent kit (cat. no. RR036B; Takara Bio, Inc.)
according to the manufacturer's instructions. qPCR amplification
was performed using the One Step TB Green PrimeScript RT-PCR Kit II
(cat. no. RR086B; Takara Bio, Inc.). The cycling conditions were:
95°C for 5 min, followed by 40 cycles at 95°C for 10 sec and 60°C
for 30 sec. The 2−ΔΔCq method was used to calculate the
gene expression relative to the reference gene (17).
For detection of miR-3120, Bulge-loop™ miRNA qPCR
Primer Sets specific for miR-3120 were designed by Nanjing KeyGen
Biotech Co., Ltd. The primer sequences for miR-3120 and its
internal control U6, as well as for Hsc70 and its internal control
GAPDH, which were used for qPCR, are shown in Table I. To examine stress-induced changes
in miR-3120 expression, the same rats used for serum corticosterone
detection were assessed. Briefly, three rats were used to detect
serum corticosterone levels at each time point, after which they
were sacrificed, the DRG was dissected and miR-3120 expression was
detected.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| miR-3120 |
CGCGCACAGCAAGTGTAGA |
AGTGCAGGGTCCGAGGTATT |
| U6 |
CTCGCTTCGGCAGCACA |
TGGTGTCGTGGAGTCG |
| Hsc70 |
TACCCGTGCTCGATTTGAGG |
GAACCACCCACCAGGACAAT |
| GAPDH |
CGGCAAGTTCAACGGCACAGT |
CGCTCCTGGAAGATGGTGATGG |
miR-3120 agomir production and DRG
injection
Cy3-labeled miR-3120 agomir, which encodes the red
fluorescence protein Cy3 and miR-3120, as well as its negative
control (NC; Cy3-labeled agomir NC), were produced by Nanjing
KeyGen Biotech Co., Ltd. For the DRG injection, the paraspinal
muscles were carefully separated along the vertebrae under 2–3%
sevoflurane anesthesia and 4 mg/kg lidocaine was intraincisionally
injected. Part of the bone covering the right L5 DRG was then
removed to completely expose the ganglion. miR-3120 agomir or
agomir NC (0.5 nM dissolved in RNAase-free deionized water to 5 µl)
was slowly injected into the L5 DRG using a Hamilton microliter
syringe with a 30-gauge needle 3 days before CFA injection in rats
in the CFA + miR-3120 agomir or CFA + agomir NC groups.
Fluorescence in situ hybridization
(FISH) for miR-3120
Cellular distribution of miR-3120 in the DRG was
examined using FISH according to a previously described method
(18). Briefly, paraffin-embedded
DRG slides were prepared using the same method as that described
for IF analysis. After sequential dewaxing, hydration and
permeabilized with 0.4% Triton X-100 for 15 min at room
temperature, the slides were washed twice with 2X saline sodium
citrate (SSC). The oligonucleotide probe was denatured for 5 min at
75°C in a water bath, and the slides were incubated with the
pre-hybridization solution (cat. no. AR0152; Boster Biological
Technology) at 42°C for 30 min, followed by 40 nM probe at 42°C
overnight. After sequential washing with 2, 1, 0.5 and 0.1X SSC,
the slides were counterstained with Hoechst 33258 (cat. no. C1017;
Beyotime Institute of Biotechnology). The sections were scanned and
stored using the OlyVIA system (Olympus VS200) with the required
images exported when necessary. In addition, the cellular
distribution of miR-3120 and Hsc70 was examined in the DRG using
FISH and IF analysis, respectively. After FISH and IF staining, the
colocalization, as well as the size of miR-3120- and Hsc70-labeled
cells was analyzed using ImageJ software.
Lentiviral vector construction and
production
Full-length Hsc70 (Gene ID: 24468; Transcript:
NM_024351.2) or scramble oligonucleotides were subcloned into the
GV280 lentiviral vector (Nanjing KeyGen Biotech Co., Ltd.) to
construct a lentiviral vector overexpressing Hsc70 (LV-Hsc70;
1×108 TU/ml) and an LV-scramble, respectively. According
to a previously reported method (19), lentiviral vector (10 µl) was
intrathecally microinjected into the L5-6 intervertebral space of
rats through the skin using a 30-gauge needle attached to a
Hamilton microliter syringe under 2–3% sevoflurane anesthesia 1
week before CFA injection in the CFA + agomir + LV-Hsc70 and CFA +
agomir + LV-scramble groups.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 8.0 software (Dotmatics). Data are shown as the mean ± SEM.
One-way ANOVA was used to assess the changes in the mechanical
withdrawal threshold at each time point among the groups. In
addition, one-way ANOVA was used to assess differences in the
results of ELISA, western blotting, IF analysis and RT-qPCR among
the groups. If a significant difference was observed, the
Bonferroni post hoc test was applied. P<0.05 was considered to
indicate a statistically significant difference.
Results
FS stress increases serum
corticosterone and exacerbates CFA-induced mechanical pain in
rats
In the present study, CFA hind paw injection was
performed in rats to induce inflammatory pain (CFA rats). In
addition, FS stress was performed for 3 days before CFA injection
to evoke mechanical hyperalgesia to establish an SIH rat model (FS
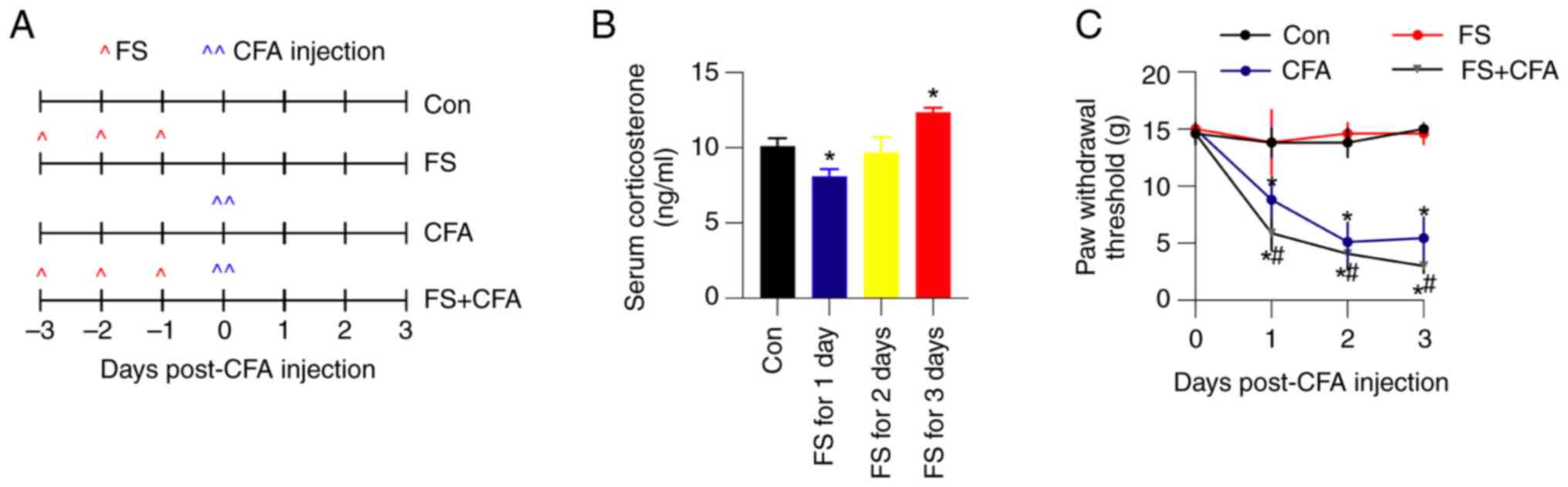
+ CFA rats). A schematic diagram of the model is presented in
Fig. 1A. To verify the efficiency
of FS stress in rats, serum corticosterone levels were examined
using ELISA. In control rats, the average serum corticosterone
level was 10.1±0.5 ng/ml. After 3 days of FS stress, the average
corticosterone concentration significantly increased to 12.3±0.3
ng/ml (Fig. 1B). Moreover, CFA
hind paw injection quickly decreased the paw withdrawal threshold,
which was further exacerbated by 3 days of FS stress (Fig. 1C). All of these observations
indicated the successful establishment of FS stress-induced
mechanical hyperalgesia in rats in an inflammatory state.
CFA upregulates Hsc70 and TRPV1
expression in the DRG, which is partially inhibited or further
enhanced by FS stress
Hsc70 participates in CFA-induced inflammatory pain
(6); however, its involvement in
FS stress-induced mechanical hyperalgesia remains largely unknown.
Therefore, the expression of Hsc70 in the DRG was detected using
western blot and IF analysis. The results showed that Hsc70 protein
expression was increased by ~1.9-fold in CFA; however, this
increase was partially inhibited in FS + CFA rats (Fig. 2A). Consistent with the results of
western blot, the results of IF analysis suggested a similar change
in Hsc70 expression (Fig. 2B).
However, FS stress alone did not significantly influence Hsc70
expression.
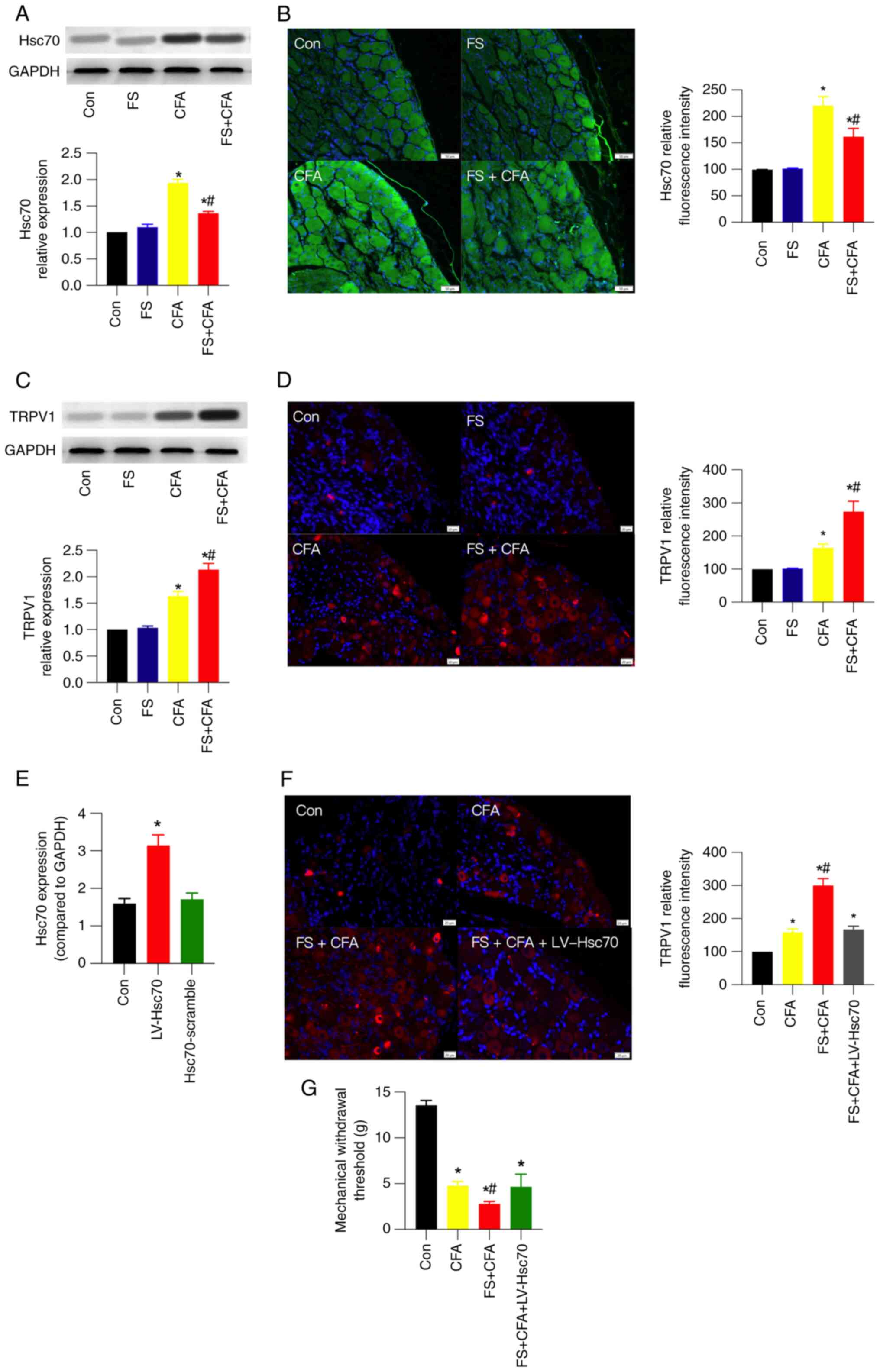 | Figure 2.CFA upregulates Hsc70 and TRPV1
expression in the DRG, which is partially inhibited or further
enhanced by FS stress, respectively. (A) Western blotting images
and their statistical analysis, and (B) IF images and their
statistical analysis for Hsc70 in control, FS, CFA and FS + CFA
rats (n=3 rats/group). Scale bar, 50 µm. (C) Western blotting
images and their statistical analysis, and (D) IF images and their
statistical analysis for TRPV1 expression in the four groups. (n=3
rats/group). Scale bar, 20 µm. To verify the etiological role of
Hsc70 in FS stress-induced mechanical hyperalgesia, LV-Hsc70 was
intrathecally injected into FS + CFA rats to increase its
expression the day before FS. (E) Reverse
transcription-quantitative PCR results showed that intrathecal
injection of LV-Hsc70, but not its scramble, successfully increased
Hsc70 expression in the DRG. (F) IF images and corresponding
statistical analysis of TRPV1 expression in the control, CFA, FS +
CFA and FS + CFA + LV-Hsc70 rats. Scale bar, 20 µm. (G) Mechanical
withdrawal threshold in rats in the four groups (n=6 rats/group).
For statistical analysis of IF images, 4–5 images from 2–3 rats
were analyzed in each group. Data are presented as the mean ± SEM.
*P<0.05 vs. Con; #P<0.05 vs. CFA. Con, control;
CFA, complete Freund's adjuvant; DRG, dorsal root ganglion; FS,
forced swim; Hsc70, heat shock cognate 71 kDa protein; LV,
lentivirus; TRPV1, transient receptor potential vanilloid 1. |
TRPV1 protein expression was increased by ~1.6-fold
in CFA rats, which was further increased to ~2-fold after 3 days of
FS stress (Fig. 2C). IF analysis
exhibited a similar expression change (Fig. 2D). Notably, TPRV1 is a well-known
nociceptor in pain perception. Moreover, to verify the etiological
role of Hsc70 in FS stress-induced mechanical hyperalgesia,
LV-Hsc70 was intrathecally injected into FS + CFA rats to increase
the expression of Hsc70 the day before FS. The efficiency of
intrathecal LV-Hsc70 was verified using RT-qPCR after 1 week
(Fig. 2E). In the presence of
intrathecal LV-Hsc70, TRPV1 expression in the DRG was partially
inhibited (Fig. 2F) and the
mechanical withdrawal threshold was increased (Fig. 2G) compared with that in the FS +
CFA group. These results suggested that Hsc70 and TRPV1 may have an
essential role in FS stress-induced mechanical hyperalgesia in rats
in an inflammatory state.
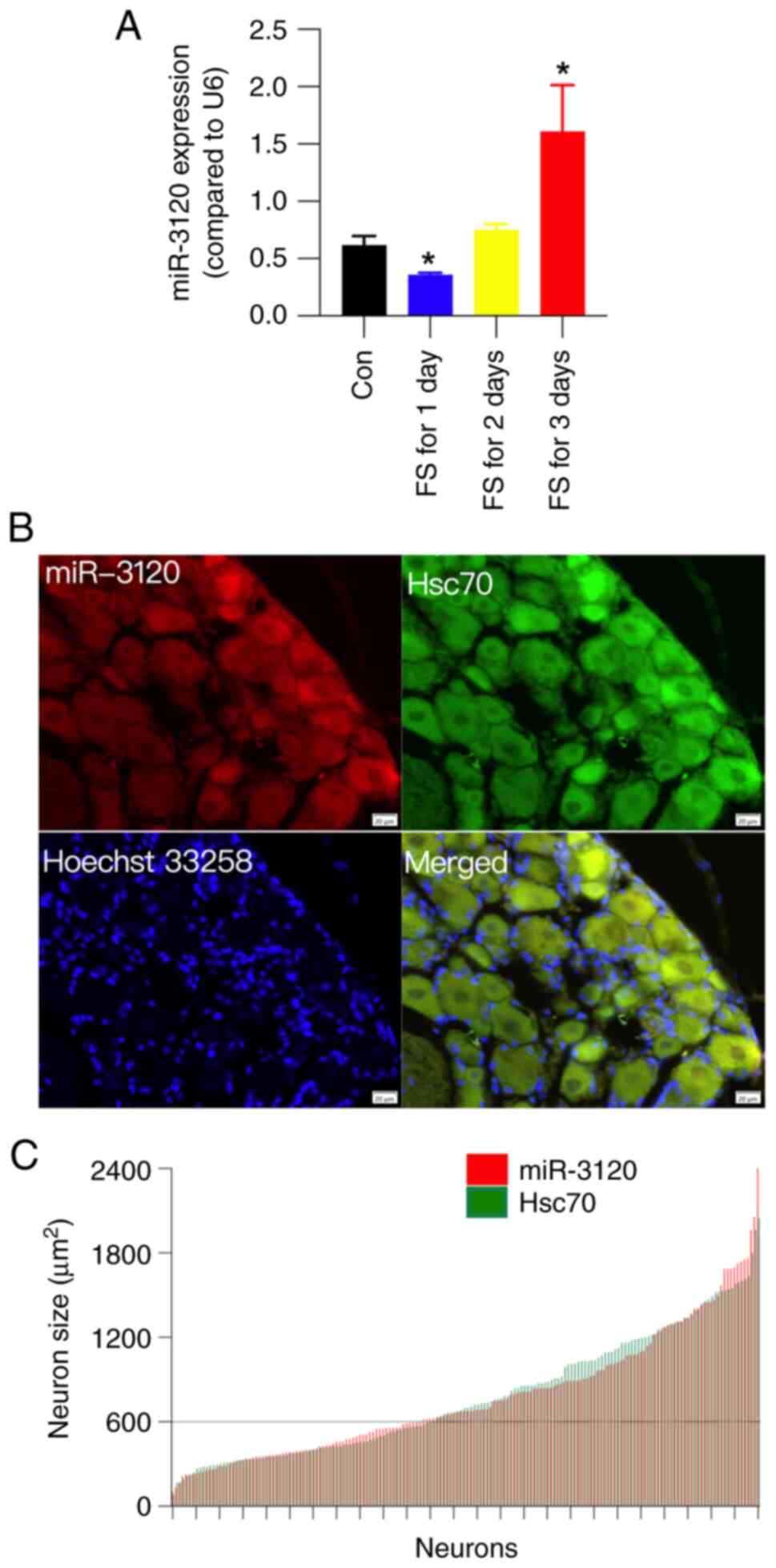
FS stress upregulates miR-3120
expression in the DRG
As an endogenous ligand of Hsc70, miR-3120 is
located in the neuronal cell body, and it targets and inhibits
Hsc70 (10). To explore the
potential regulatory role of miR-3120 for Hsc70, miR-3120
expression was examined by RT-qPCR in the DRG following FS stress.
The results showed that 3 days of FS stress significantly
upregulated the expression levels of miR-3120 by ~2.6-fold
(Fig. 3A). Moreover, FS stress for
1 day transiently downregulated miR-3120 expression. This
downregulation was consistent with that observed for serum
corticosterone; however, the underlying reason remains unclear.
The cellular distribution of miR-3120 and Hsc70 was
then examined in the DRG using FISH and IF analysis, respectively.
After double fluorescence staining, the colocalization of miR-3120
and Hsc70 was analyzed, as well as the diameter of the labeled
cells. miR-3120 and Hsc70 were both expressed on the cell surface
and in the cytoplasm, and a number of miR-3120 and Hsc70
double-labelled neurons were observed (Fig. 3B). Cell size distribution
histograms for miR-3120 (red) and Hsc70 (green) showed that more
than half of the miR-3120 and Hsc70 double-labeled neurons were
medium and large-sized neurons (cross-sectional area >600
µm2) (Fig. 3C). These
results indicated that miR-3120 and Hsc70 may act on the same
population of neurons.
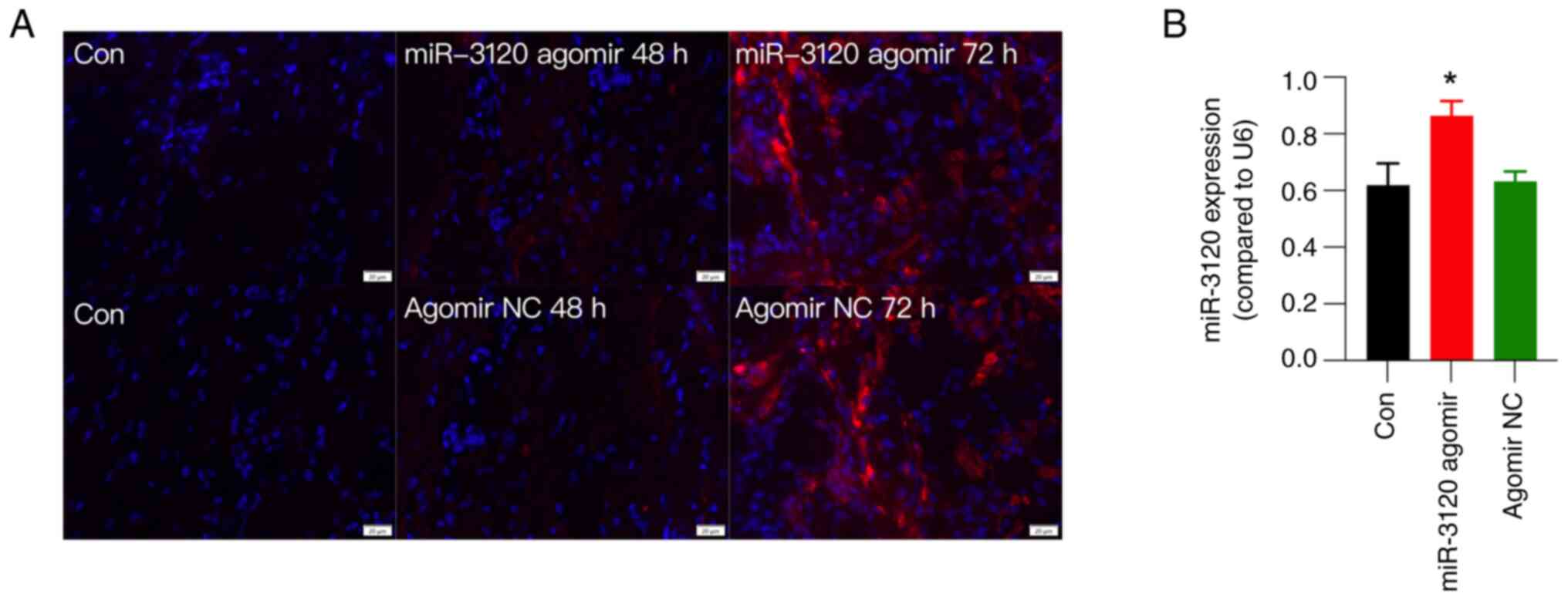
Fluorescence detection and RT-qPCR
verify the efficiency of miR-3120 agomir injection
To explore the participation of miR-3120 in FS
stress-induced mechanical hyperalgesia, miR-3120 agomir was
injected into the DRG in CFA rats. Agomirs are specially labeled
and chemically modified double-stranded small RNA molecules that
simulate endogenous miRNAs to regulate the biological functions of
target genes. Compared with miRNA mimics, agomirs can enrich in
target cells, presenting higher stability in vivo and
resulting in more effective interference (20).
The efficiency of the miR-3120 agomir was examined
using fluorescence detection and RT-qPCR. The results showed that
the injection of Cy3-labeled miR-3120 agomir encoding both the red
fluorescence protein Cy3 and miR-3120 into the L5 DRG induced red
fluorescence in all sizes of DRG neurons as early as 48 h after
injection (Fig. 4A). Although DRG
injection of agomir NC also presented red fluorescence (Fig. 4A), RT-qPCR showed that miR-3120
agomir, but not agomir NC, significantly increased the expression
levels of miR-3120 at 72 h after DRG injection compared with those
in the control group (Fig. 4B),
collectively verifying the efficiency of miR-3120 agomir after DRG
injection.
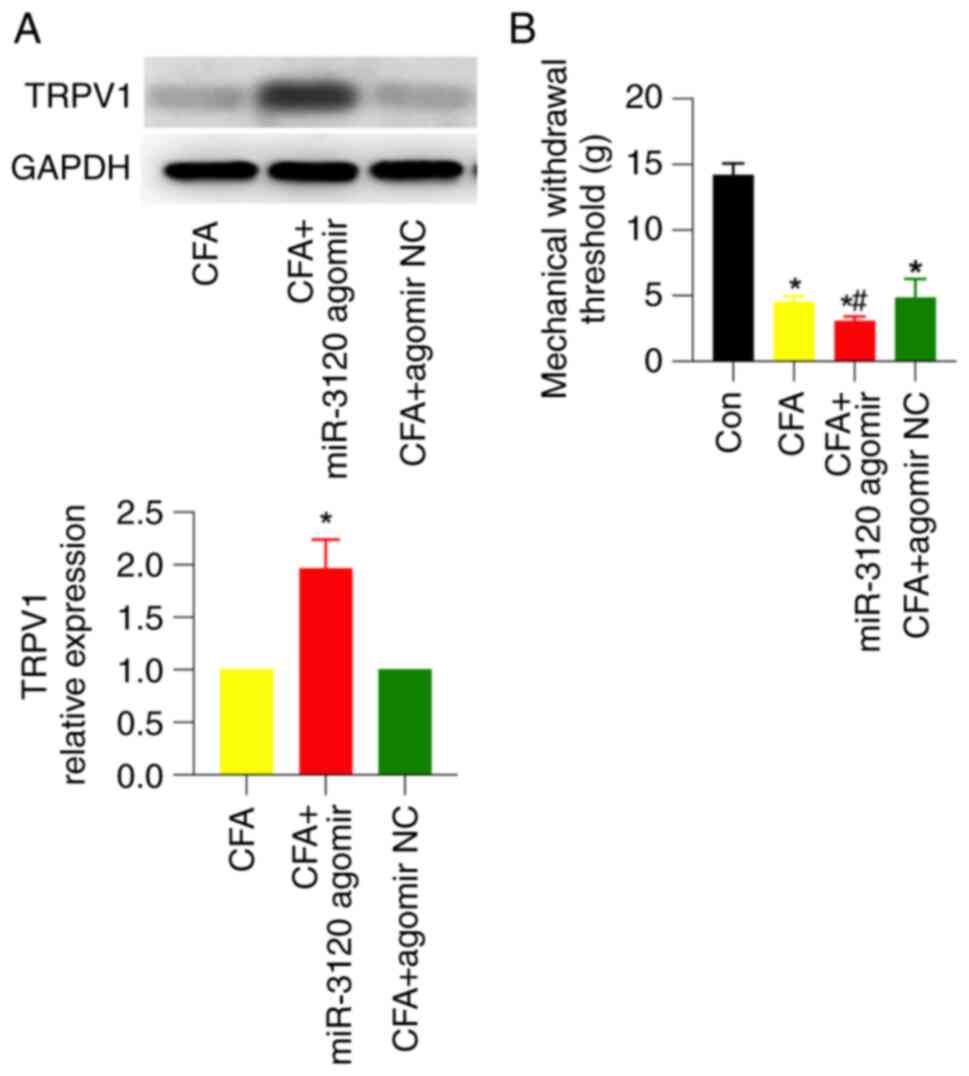
DRG injection of miR-3120 agomir
upregulates TRPV1 expression and induces mechanical hyperalgesia in
CFA rats
After verifying the efficiency of DRG injection of
miR-3120 agomir, the miR-3120 agomir or its NC was injected into
the DRG 3 days before CFA injection. Western blotting showed that
miR-3120 agomir, but not its NC, significantly upregulated TRPV1
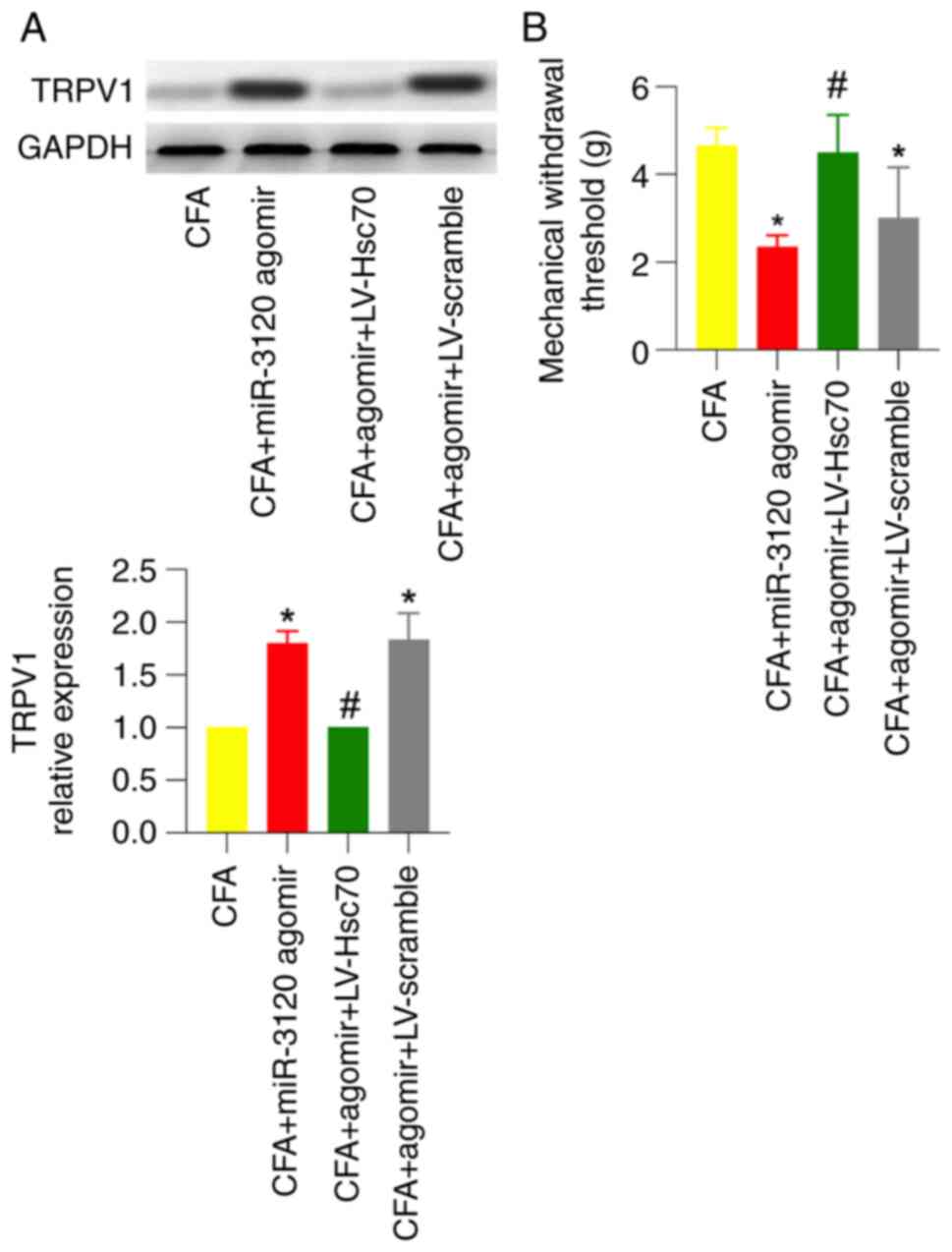
expression by ~2-fold in CFA rats (Fig. 5A). Meanwhile, miR-3120 agomir
significantly enhanced CFA-induced mechanical pain, which was
similar to FS stress-induced behavioral changes detected in CFA
rats (Fig. 5B). These results
suggested that miR-3120 may participate in FS stress-induced
mechanical hyperalgesia.
miR-3120 agomir-induced TRPV1
expression and mechanical hyperalgesia in CFA rats are abolished in
the presence of Hsc70 overexpression
To further investigate whether Hsc70 is involved in
miR-3120 agomir-induced TRPV1 expression and behavioral changes,
miR-3120 agomir and LV-Hsc70 were injected into the DRG 3 days and
intrathecally injected 1 week before CFA injection, respectively.
As shown by western blotting and behavioral testing, miR-3120
agomir-induced TRPV1 expression and mechanical hyperalgesia in CFA
rats were significantly abolished in the presence of intrathecal
injection of LV-Hsc70 but not LV-scramble (Fig. 6A and B).
Discussion
The results of the present study suggested that
miR-3120/Hsc70 may participate in FS stress-induced mechanical
hyperalgesia in rats in an inflammatory state, possibly via
disinhibiting TRPV1 expression in the DRG neurons. In CFA rats,
upregulated Hsc70 may inhibit TRPV1 expression and work as a
protective mechanism to control pain signals.
Hsc70 and TRPV1 expression was increased in the DRG
after CFA injection. As a member of the Hsp70 family, Hsc70 is a
type of constitutively expressed molecular cognate protein that is
essential for a number of cellular functions. Notably, Hsc70 acts
as a clathrin-uncoating ATPase during clathrin-mediated
endocytosis, maintaining protein homeostasis, antigen processing
and presentation, and importing proteins into organelles or
cellular compartments (21).
In the DRG, exogenous Hsc70 has been shown to
prevent injury-induced death of nearly all sensory neurons in
neonatal mice undergoing bilateral transection of the sciatic nerve
(22). Inhibition of Hsp90 to
upregulate Hsp70 may protect against glucose-induced embryonic DRG
neuron death, improve nerve conduction velocity and sensory
deficits, and reverse sensory hypoalgesia in diabetic mice
(23). However, whether Hsc70 in
the DRG has a protective role in inflammatory pain has seldom been
reported. Iftinca et al (7)
detected increased Hsc70 protein levels in the DRG 3 days post-CFA
hind paw injection. The increased Hsc70 was proposed to inhibit
TRPV1 expression via inhibiting ROCK phosphorylation of the
TRPV1-S502 site in an Hsc70-dependent manner, thus forming a
functional link between Hsc70 and TRPV1. The present also observed
an increase in TRPV1 expression in CFA rats in the present study,
which is consistent with previous reports (24,25).
The present study focused on the possible
contribution of Hsc70 and TRPV1 to FS stress-induced mechanical
hyperalgesia in CFA rats. As shown by western blotting and IF
analysis, FS stress inhibited CFA-induced upregulation of Hsc70
expression. Sato et al (26) reported that chronic stress-related
dexamethasone significantly decreased lysosomal Hsc70 expression in
human cell lines and primary cultured rat neurons, indicating that
chronic stress-related corticosteroid secretion may alter Hsc70
expression. In the present study, FS stress for 3 days markedly
increased serum corticosterone levels, indicating the successful
establishment of a chronic stress model. Such FS stress partially
inhibited the increase in Hsc70 expression in CFA rats, which was
accompanied by a further increase in TRPV1 expression and more
severe pain in CFA rats. As a type of capsaicin-sensitive
nociceptor, TRPV1 has been shown to protect against stress-induced
mechanical hyperalgesia, potentially by influencing neuronal
plasticity (27). Following
stress-induced visceral hyperalgesia, increased TRPV1 expression in
the DRG was observed, suggesting the stress sensitivity of TRPV1
nociceptors (28).
FS stress for 3 days increased the expression of
miR-3120, which has been reported to participate in synapse vesicle
function and neuronal plasticity (10,29).
Numerous miRNAs in the DRG have been suggested to participate in
CFA-related inflammatory pain. For example, decreased miR-485-5p
and miR-134 expression have been shown to contribute to CFA-induced
inflammatory pain by upregulating ASIC1 and MOR1 expression,
respectively (30,31). Moreover, CFA-related inflammation
can reduce miR-1, −16 and −206 expression in the DRG (32), indicating that miRNAs may
participate in the nociceptive process following noxious stimuli.
However, to the best of our knowledge, the role of miR-3120 in pain
regulation has not been reported. According to the dual-luciferase
reporter assay and miRanda v1.0b prediction performed by Scott
et al (10), miR-3120 may
target and inhibit Hsc70, playing an essential role in regulating
the constitutive level of Hsc70. In the present study, DRG
injection of miR-3120 agomir significantly increased miR-3120 and
TRPV1 expression, and exacerbated CFA-induced inflammatory pain,
replicating FS stress-induced inflammatory hyperalgesia in CFA
rats.
To explore the potential role of Hsc70 in
miR-3120-induced TRPV1 expression and behavioral changes, miR-3120
and Hsc70 were double stained in the DRG. Both miR-3120 and Hsc70
were found on the cell surface and in the cytoplasm. Cell size
distribution analysis for miR-3120 and Hsc70 showed that in normal
rats, more than half of the miR-3120 and Hsc70 double-labeled
neurons were large. Small-, medium- and large-sized neurons in the
DRG were assumed to be C-fiber-, Aδ- and Aβ-related sensory
neurons, respectively. Under physiological conditions, small- and
medium-sized neurons have been suggested to mediate nociceptive
behaviors in the DRG; however, Aβ primary afferent neurons have
recently been proposed to become hyperexcitable in response to
painful stressors (33,34). In the present study, more than half
of the miR-3120 and Hsc70 double-labeled neurons were observed to
be medium and large-sized neurons, suggesting that Aδ and Aβ
primary afferent neurons and their fibers may play an essential
role in FS stress-related mechanical hyperalgesia. However, the
present study did not examine the distribution of miR-3120- or
Hsc70-expressing neurons after CFA or FS stress. Further research
is required to assess this.
LV-Hsc70 was intrathecally injected 1 week before
CFA injection to induce the overexpression of Hsc70 in rats. As
expected, miR-3120 agomir-induced TRPV1 upregulation and mechanical
hyperalgesia in CFA rats were abolished in the presence of Hsc70
overexpression. This result suggested the involvement of Hsc70 in
miR-3120 agomir-induced TRPV1 upregulation. Increased Hsc70
expression may control TRPV1 expression and act as a protective
mechanism to control pain signals mediated by TRPV1 activation in
CFA rats. Numerous studies have suggested a protective role of
Hsc70 in various neurological diseases, including Parkinson's
disease, Huntington's disease, Alzheimer's disease, amyotrophic
lateral sclerosis and multiple system atrophy (35,36).
Hsc70 translocates to synapse-enriched areas in the cerebral cortex
to refold denatured proteins after thermal stress (6). In addition, Hsc70 directly
participates in cell survival during neurulation, and acts as an
intrinsic protector of neuroepithelial and neural precursor cells
(37). Exogenous Hsc70 incubation
prior to oxidative injury has been shown to protect motor neurons
from oxidative stress injury (38). Furthermore, intracerebroventricular
administration of Hsp70/Hsc70 can reduce the severity of chemically
induced seizures (39). To the
best of our knowledge, the present study is the first to suggest
the potential protective role of Hsc70 in CFA-induced inflammatory
pain.
In addition to the miR-3120/Hsc70 mechanism, the
underlying mechanisms for FS stress-induced inflammatory
hyperalgesia have not been elucidated. The role of the gut
microbiota, neuronal plasticity in the brain, dysfunction of the
descending pain regulatory system and excitatory-inhibitory
neurotransmission imbalance at the spinal level have been suggested
to participate in FS stress-related hyperalgesia (40,41).
Furthermore, the functional study of miR-3120, Hsc70 and TRPV1 in
SIH remains to be elucidated. One specific finding of the present
study was that serum corticosterone levels and miR-3120 expression
in the DRG were transiently decreased following FS stress for 1
day. Considering the etiological role of increased serum
corticosterone and miR-3120 in SIH, such a transient decrease may
contribute to another contrasting pain phenomenon, stress-induced
analgesia. However, this was not explored in the present study and
thus requires further verification.
In conclusion, the results of the present study
suggested that miR-3120/Hsc70 may participate in FS stress-induced
mechanical hyperalgesia in rats in an inflammatory state, possibly
via disinhibiting TRPV1 expression in the DRG neurons.
Acknowledgements
Not applicable.
Funding
This work is supported by grants from the National Natural
Science Foundation of China (grant nos. 81600960, 81971040 and
81971045) and the Jiangsu Provincial Key Research and Development
Program (grant no. BE2021615).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and SF designed the study and provided funding
support. SX and SL performed the behavioral test and were major
contributors in writing the manuscript. JY, RL and MM performed all
biological and histological examinations in this study, collected
original data and performed statistical analysis. XW and SF confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of Nanjing Medical University (approval no.
1706017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jennings EM, Okine BN, Roche M and Finn
DP: Stress-induced hyperalgesia. Prog Neurobiol. 121:1–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imbe H, Iwai-Liao Y and Senba E:
Stress-induced hyperalgesia: Animal models and putative mechanisms.
Front Biosci. 11:2179–2192. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lian YN, Lu Q, Chang JL and Zhang Y: The
role of glutamate and its receptors in central nervous system in
stress-induced hyperalgesia. Int J Neurosci. 128:283–290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itoga CA, Roltsch Hellard EA, Whitaker AM,
Lu YL, Schreiber AL, Baynes BB, Baiamonte BA, Richardson HN and
Gilpin NW: Traumatic stress promotes hyperalgesia via
corticotropin-releasing factor-1 receptor (CRFR1) signaling in
central amygdala. Neuropsychopharmacology. 41:2463–2472. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi J, Chen C, Meng QX, Wu Y, Wu H and Zhao
TB: Crosstalk between activated microglia and neurons in the spinal
dorsal horn contributes to stress-induced hyperalgesia. Sci Rep.
6:394422016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S and Brown IR: Translocation of
constitutively expressed heat shock protein Hsc70 to
synapse-enriched areas of the cerebral cortex after hyperthermic
stress. J Neurosci Res. 85:402–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iftinca M, Flynn R, Basso L, Melo H,
Aboushousha R, Taylor L and Altier C: The stress protein heat shock
cognate 70 (Hsc70) inhibits the transient receptor potential
vanilloid type 1 (TRPV1) channel. Mol Pain.
12:17448069166639452016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang YK, Lu YG, Zhao X, Zhang JB, Zhang
FM, Chen Y, Bi LB, Gu JH, Jiang ZJ, Wu XM, et al: Cytokine activin
C ameliorates chronic neuropathic pain in peripheral nerve injury
rodents by modulating the TRPV1 channel. Br J Pharmacol.
177:5642–5657. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bourinet E, Altier C, Hildebrand ME, Trang
T, Salter MW and Zamponi GW: Calcium-permeable ion channels in pain
signaling. Physiol Rev. 94:81–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scott H, Howarth J, Lee YB, Wong LF,
Bantounas I, Phylactou L, Verkade P and Uney JB: MiR-3120 is a
mirror microRNA that targets heat shock cognate protein 70 and
auxilin messenger RNAs and regulates clathrin vesicle uncoating. J
Biol Chem. 287:14726–14733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hammond SM: An overview of microRNAs. Adv
Drug Delivery Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakai A and Suzuki H: Emerging roles of
microRNAs in chronic pain. Neurochem Int. 77:58–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imbe H and Kimura A: Attenuation of pCREB
and Egr1 expression in the insular and anterior cingulate cortices
associated with enhancement of CFA-evoked mechanical
hypersensitivity after repeated forced swim stress. Brain Res Bull.
134:253–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piriyaprasath K, Kakihara Y, Kurahashi A,
Taiyoji M, Kodaira K, Aihara K, Hasegawa M, Yamamura K and Okamoto
K: kojipreventive roles of rice-extracts and ergothioneine on
anxiety- and pain-like responses under psychophysical stress
conditions in male mice. Nutrients. 15:39892023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang FM, Wang B, Hu H, Zhang YY, Chen HH,
Jiang ZJ, Zeng MX and Liu XJ: Transcriptional profiles of TGF-β
superfamily members in the lumbar DRGs and the effects of activins
A and C on inflammatory pain in rats. J Physiol Biochem.
79:313–325. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Zhou F, Zhang S, Mao M, Feng S and
Wang X: Participation of transient receptor potential vanilloid 1
in the analgesic effect of duloxetine for paclitaxel induced
peripheral neuropathic pain. Neurosci Lett. 773:1365122022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KL and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Q, Zhang S, Zhang BY, Zhang Y, Yao L,
Hu J and Zhang HH: microRNA-181a contributes to gastric
hypersensitivity in rats with diabetes by regulating TLR4
expression. Mol Pain. 19:174480692311593562023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang FM, Wang B, Hu H, Li QY, Chen HH,
Luo LT, Jiang ZJ, Zeng MX and Liu XJ: Transcriptional profiling of
TGF-β superfamily members in lumbar DRGs of rats following sciatic
nerve axotomy and activin C inhibits neuropathic pain. Endocr Metab
Immune Disord Drug Targets. 23:375–388. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang V and Wu W: MicroRNA-based
therapeutics for cancer. BioDrugs. 23:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stricher F, Macri C, Ruff M and Muller S:
HSPA8/HSC70 chaperone protein: Structure, function, and chemical
targeting. Autophagy. 9:1937–1954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Houenou LJ, Li L, Lei M, Kent CR and
Tytell M: Exogenous heat shock cognate protein Hsc 70 prevents
axotomy-induced death of spinal sensory neurons. Cell Stress
Chaperones. 1:161–166. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Urban MJ, Li C, Yu C, Lu Y, Krise JM,
Mcintosh MP, Rajewski RA, Blagg BS and Dobrowsky RT: Inhibiting
heat-shock protein 90 reverses sensory hypoalgesia in diabetic
mice. ASN Neuro. 2:e000402010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Ma S, Ke X, Yi Y, Yu H, Yu D, Li
Q, Shang Y, Lu Y and Pei L: The mechanism of Annexin A1 to modulate
TRPV1 and nociception in dorsal root ganglion neurons. Cell Biosci.
11:1672021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruan Y, Ling J, Ye F, Cheng N, Wu F, Tang
Z, Cheng X and Liu H: Paeoniflorin alleviates CFA-induced
inflammatory pain by inhibiting TRPV1 and
succinate/SUCNR1-HIF-1α/NLPR3 pathway. Int Immunopharmacol. 101((Pt
B)): 1083642021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato M, Ueda E, Konno A, Hirai H, Kurauchi
Y, Hisatsune A, Katsuki H and Seki T: Glucocorticoids negatively
regulates chaperone mediated autophagy and microautophagy. Biochem
Biophys Res Commun. 528:199–205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scheich B, Vincze P, Szőke É, Borbély É,
Hunyady Á, Szolcsányi J, Dénes Á, Környei Z, Gaszner B and Helyes
Z: Chronic stress-induced mechanical hyperalgesia is controlled by
capsaicin-sensitive neurones in the mouse. Eur J Pain.
21:1417–1431. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong S, Zheng G, Wu X, Snider NT, Owyang C
and Wiley JW: Corticosterone mediates reciprocal changes in CB 1
and TRPV1 receptors in primary sensory neurons in the chronically
stressed rat. Gastroenterology. 140:627–637.e4. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang C, Zhao Y, Wang Y, Wu H, Fang X and
Chen H: Deep RNA sequencing reveals that microRNAs play a key role
in lactation in rats. J Nutr. 144:1142–1149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu M, Wu R, Zhang L, Zhu HY, Xu GY, Qian W
and Zhang PA: Decreased miR-485-5p contributes to inflammatory pain
through post-transcriptional upregulation of ASIC1 in rat dorsal
root ganglion. J Pain Res. 13:3013–3022. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ni J, Gao Y, Gong S, Guo S, Hisamitsu T
and Jiang X: Regulation of µ-opioid type 1 receptors by microRNA134
in dorsal root ganglion neurons following peripheral inflammation.
Eur J Pain. 17:313–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kusuda R, Cadetti F, Ravanelli MI, Sousa
TA, Zanon S, De Lucca FL and Lucas G: Differential expression of
microRNAs in mouse pain models. Mol Pain. 7:172011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun W, Yang F, Wang Y, Fu H, Yang Y, Li
CL, Wang XL, Lin Q and Chen J: Contribution of large-sized primary
sensory neuronal sensitization to mechanical allodynia by
upregulation of hyperpolarization-activated cyclic nucleotide gated
channels via cyclooxygenase 1 cascade. Neuropharmacology. 113((Pt
A)): 217–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao WH and Bennett GJ: Persistent
low-frequency spontaneous discharge in A-fiber and C-fiber primary
afferent neurons during an inflammatory pain condition.
Anesthesiology. 107:813–821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Daniels CK and Cao S: Comprehensive
review on the HSC70 functions, interactions with related molecules
and involvement in clinical diseases and therapeutic potential.
Pharmacol Ther. 136:354–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang YT and Lu JH: Chaperone-mediated
autophagy in neurodegenerative diseases: Molecular mechanisms and
pharmacological opportunities. Cells. 11:22502022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rubio E, Valenciano AI, Segundo C, Sánchez
N, De Pablo F and De La Rosa EJ: Programmed cell death in the
neurulating embryo is prevented by the chaperone heat shock cognate
70. Eur J Neurosci. 15:1646–1654. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robinson MB, Taylor AR, Gifondorwa DJ,
Tytell M and Milligan CE: Exogenous Hsc70, but not thermal
preconditioning, confers protection to motoneurons subjected to
oxidative stress. Dev Neurobiol. 68:1–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ekimova IV, Nitsinskaya LE, Romanova IV,
Pastukhov YF, Margulis BA and Guzhova IV: Exogenous protein
Hsp70/Hsc70 can penetrate into brain structures and attenuate the
severity of chemically-induced seizures. J Neurochem.
115:1035–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu X, Zhan G, Chen R, Wang D, Guan S and
Xu H: Gut microbiota and its role in stress-induced hyperalgesia:
Gender-specific responses linked to different changes in serum
metabolites. Pharmacol Res. 177:1061292022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quintero L, Cardenas R and Suarez-Roca H:
Stress-induced hyperalgesia is associated with a reduced and
delayed GABA inhibitory control that enhances post-synaptic NMDA
receptor activation in the spinal cord. Pain. 152:1909–1922. 2011.
View Article : Google Scholar : PubMed/NCBI
|