Introduction
Major depressive disorder (MDD) is a mental illness
that is related to suicide and is characterized by cognitive
impairment, hopelessness, loss of pleasure, debilitation and low
self-esteem (1). The currently
available antidepressants for patients with depression include
selective serotonin and norepinephrine reuptake inhibitors
(2). However, poor treatment
adherence and high discontinuation rates result in treatment
failure (40 to 60%) (3,4), which indicates the need for a means
of preventing MDD. Numerous factors, such as parental depression,
chronic disease, sleeplessness, socioeconomic status, stress and
interpersonal dysfunction, influence the risk for depression
(5). Previous studies have
reported that the inflammatory response participates in the
pathogenesis of depression (6,7). The
protein expression of TNF-α and IL-6 are elevated in patients with
depression (8). Reduced cytokine
levels within the brain affect neurotrophic support and
neurotransmitter metabolism, ultimately leading to inhibition of
activated microglia cells (9).
SalB significantly decreases expression of pro-inflammatory
cytokines IL-1β and TNF-α, thereby inhibiting the activation of
microglia in the hippocampus and cortex (10). A previous study reported that
lipopolysaccharides (LPS) can induce inflammation-related
depression by altering brain-derived neurotrophic factor
(BDNF)-TrκB signaling (11).
Selanylimidazopyridine alleviates depressive-like behavior by
downregulating NFκB, promoting antioxidant activity, and increasing
BDNF expression in LPS-induced mice (12).
Cytokines cross the blood-brain barrier and impair
microglial function, which leads to depression (13). LPS activates the microglia and
induces inflammatory responses (14). Inducible nitric oxide synthase
(iNOS) and TNF-α are markers of pro-inflammatory M1 microglia
(15). Arginase 1 (Arg-1) and
IL-10 are markers of anti-inflammatory M2 microglia (16). Therefore, neuroinflammation may be
ameliorated by M2 microglial polarization that further alleviates
depression (17).
Monocyte chemotactic protein-1-induced protein 1
(MCPIP1), also termed ZC3H12A and Regnase-1, possesses
endoribonuclease and deubiquitinase activities (18). MCPIP1 ameliorates inflammatory
responses by destabilizing mRNAs encoding cytokines (19). MCPIP1 induces autophagy and
alleviates inflammatory responses in keratitis (20). MCPIP1 depletion aggravates
psoriasis-like inflammation in keratinocytes by IL-23/Th17 and
STAT3 pathways (21). MCPIP1
promotes TNF-α-induced apoptosis by decreasing NF-κB and cFLIP
protein expression (22). MCPIP1
inhibits IL-1β or LPS-induced ubiquitination of TNF receptor
associated factor 6 (TRAF6) (23).
The present study assessed if the role of MCPIP1 in reducing
inflammatory responses was associated with depression progression
and whether may represent an effective therapeutic target for
depression.
Materials and methods
Animals and treatments
Male C57BL/6J mice (weight, 18–20 g, n=20) were
purchased from the Jinan Pengyue Laboratory Animal Breeding Co.,
Ltd. Mice were housed 2 mice per cage under a 12 h light/dark cycle
at 18–22°C. The present study complied with the guidelines of the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (24) and was
approved by the Ethics Committee of Qingdao Mental Health Center
(Qingdao, China; approval no. 2022061). The mice were randomly
divided into four groups as follows: i) control group (PBS; n=5);
ii) LPS group (n=5); iii) LPS + NC group (n=5); and iv) LPS +
MCPIP1 group (n=5). An adenovirus-associated vector (AAV)
containing MCPIP1 (Shanghai GenePharma Co., Ltd.) was used. After
induction using 4% isoflurane and maintenance using 2% isoflurane,
2 µl of negative control (NC) AAV or MCPIP1 AAV were injected into
the hippocampus regions (−1.6 mm anteroposterior of bregma, ±1.8 mm
lateral and −1.6 mm dorsoventral of bregma) of mice (age, 6 weeks,
n=5) using a brain location microinjection pump (200 nl/min;
Stoelting Co.). The mice in control and LPS groups were subjected
to a sham procedure. At 4 weeks after injection, mice were
intraperitoneally injected with PBS or LPS (1 mg/kg) for 2 days
(25). If improper injection
operation caused the death of the mouse, the mouse was excluded and
replaced. All data derived from animal studies were analyzed by an
experimenter blind to experimental conditions. The order of the
animals was randomized prior to behavioral tests. Sucrose
preference test (SPT), open field test (OFT), tail suspension test
(TST) and forced swimming test (FST) were performed as described
previously (26). After testing
depression-like behavior, the mice were sacrificed by anesthesia
with isoflurane (4% for induction and 2% for maintenance) and
transcardial perfusion using ice-cold PBS and then 4%
paraformaldehyde in PBS overnight at 4°C. Following perfusion, the
brains were collected for subsequent analysis. The left hippocampus
was frozen and the right hippocampus was treated with paraffin.
SPT
During the adaptation phase, the mice were housed in
cages with two bottles of 1% sucrose solution for two days. On the
following day, one bottle of 1% sucrose solution and one bottle of
tap water were placed in each cage. On day 4 mice were deprived of
water and food for 24 h. Mice were allowed access to the 1% sucrose
solution and tap water bottles for 3 h, to avoid bottle side
preference, the positions of the two bottles were swapped. Sucrose
preference during the 3 h test was calculated as: Sucrose
consumption/(water consumption + sucrose consumption) ×100.
OFT
Briefly, mice were placed in a square wooden box
(40×40×40 cm) for 5 min. The mice were gently placed in the center
of the square facing in the same direction each time. Anilab
software (AVTAS version 5.0, Anilab Scientific Instruments Ltd.
Co.) was used to analyze the travel distance of the mice.
TST
Mice were individually suspended from a retort stand
with adhesive tape placed 1 cm from the tail tip and placed 50 cm
above the floor. The duration of immobility was recorded during a 6
min test and analyzed using Anilab software (ver 6.50, Anilab
Scientific Instruments Ltd. Co.).
FST
Mice were placed in a glass beaker (5,000 ml)
containing 4,000 ml of water (25±1°C) for 6 min, and the struggling
time was recorded.
Cells and treatments
Mouse BV2 transformed microglial cells (cat. no.
CL-0493; Procell Life Science & Technology Co., Ltd.) were
incubated at 37°C in MEM-non-essential amino acid solution
containing 10% fetal bovine serum (Gibco) and 1%
penicillin/streptomycin. Cells were transfected with 50 nM
pcDNA-MCPIP1, empty vector (Shanghai GenePharma Co., Ltd.), short
interfering RNA (si)-MCPIP1 or si-NC (Shanghai GenePharma Co.,
Ltd.) at 37°C for 24 h using Lipofectamine 3000. LPS (1 µg/ml; cat.
no. L8880; Beijing Solarbio Science & Technology Co., Ltd.) was
added to the transfected BV2 cells at 37°C for 12 h. LPS +
si-MCPIP1 + TAK-242 group cells were pretreated with TAK-242, a
selective TLR4 inhibitor (cat. no. HY-11109; MedChemExpress), at
37°C for 30 min. The sequences were as follows: si-MCPIP1 Sense:
5′-CCGAGAUCCUCUCCUACAAGU-3′, Anti-sense:
5′-UUGUAGGAGAGGAUCUCGGCA-3′. Si-NC Sense:
5′-UUCUCCGAACGUGUCACGUTT-3′; Anti-sense:
5′-ACGUGACACGUUCGGAGAATT-3′.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. RNA (1.0 µg) was reverse
transcribed using HiScript® II Q RT SuperMix for qPCR
(cat. no. R223-01; Vazyme Biotech Co., Ltd.) according to the
manufacturer's protocols. mRNA levels were quantified using a ChamQ
SYBR qPCR Master Mix kit (cat. no. Q311-02; Vazyme Biotech Co.,
Ltd.) according to the manufacturer's protocols. Thermocycling
conditions were as follows: Initial denaturation at 94°C for 30
sec, followed by 35 cycles of denaturation at 94°C for 5 sec and
extension at 60°C for 30 sec. GAPDH was used as the endogenous
control. Relative mRNA expression levels were assessed using the
2−ΔΔCq method (27).
Specific primer pairs are listed in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| MCPIP1 | F:
AACTGGTTTCTGGAGCGAGG |
|
| R:
CGAAGGATGTGCTGGTCTGT |
| CD16 | F:
CAGACAGGCAGAGTGCAGC |
|
| R:
ACGTGTAGCTGGATTGGACC |
| CD32 | F:
AAGCAGGTTCCAGACAATCCT |
|
| R:
TGGCTTGCTTTTCCCAATGC |
| iNOS | F:
GGTGAAGGGACTGAGCTGTT |
|
| R:
ACGTTCTCCGTTCTCTTGCAG |
| IL-4 | F:
TCACAGCAACGAAGAACACCA |
|
| R:
CAGGCATCGAAAAGCCCGAA |
| IL-10 | F:
GCATGGCCCAGAAATCAAGG |
|
| R:
ACACCTTGGTCTTGGAGCTTATTA |
| Arg-1 | F:
GTACATTGGCTTGCGAGACG |
|
| R:
ATCGGCCTTTTCTTCCTTCCC |
| CD206 | F:
TTCAGCTATTGGACGCGAGG |
|
| R:
GAATCTGACACCCAGCGGAA |
| GAPDH | F:
AGGTCGGTGTGAACGGATTT |
|
| R:
ACTGTGCCGTTGAATTTGCC |
Western blotting
Proteins were extracted from tissues and cells using
RIPA buffer (cat. no. P0013C; Beyotime Institute of Biotechnology).
The protein concentration was determined using a BCA protein assay
kit (P0012S; Beyotime Institute of Biotechnology). Proteins (30
µg/line) were separated using 10% SDS-PAGE (cat. no. P0012A;
Beyotime Institute of Biotechnology) and transferred onto a PVDF
membrane (cat. no. FFP24; Beyotime Institute of Biotechnology).
After blocking with 5% non-fat dry milk in Tris-buffers saline for
2 h at 25°C, the membrane was incubated with primary antibodies
against MCPIP1 (1:2,000; cat. no. 25009-1-AP; Proteintech Group
Inc.), Iba-1 (1:2,000; cat. no. ab178846; Abcam), TLR4 (1:1,000;
cat. no. A5258; Abclonal Biotech Co., Ltd.), MyD88 (1:1,000; cat.
no. A21905; Abclonal Biotech Co., Ltd.), TRAF6 (1:2,000 cat. no.
ab33915; Abcam), phosphorylated (p)-IκBα (1:1,000; cat. no.
ab133462; Abcam), IκBα (1:2,000; cat. no. 4814T; CST Biological
Reagents Co., Ltd.), p-p65 NF-κB (1:1,000; cat. no. 3033T; CST
Biological Reagents Co., Ltd.), p65 NF-κB (1:2,000; cat. no.
ab16502; Abcam), IL-6 (1:1,000; cat. no. 21865-1-AP; Proteintech
Group Inc.), TNF-α (1:2,000; cat. no. A0277; Abclonal Biotech Co.,
Ltd.), IL-1β (1:2,000; cat. no. 16806-1-AP; Proteintech Group
Inc.), iNOS (1:2,000; cat. no. ab178945; Abcam), Arg-1 (1:2,000;
cat. no. 16001-1-AP; Proteintech Group Inc.) and GAPDH (1:10,000,
cat. no. 10494-1-AP; Proteintech Group Inc.) at 4°C overnight.
GAPDH was used as an internal reference protein. Following washing
with TBS with 0.1% Tween20, membranes were then incubated with the
HRP-conjugated Goat Anti-Rabbit IgG (H+L) secondary antibodies
(1:10,000; SA00001-2; Proteintech Group Inc.) for 1 h at 25°C. ECL
solution (cat. no. E412-02; Vazyme Biotech Co., Ltd.) was used to
detect the bands. Analysis was performed using Image-Pro plus 6.0
software (Media Cybernetics).
Immunofluorescence staining
Frozen brain sections (thickness, 30 µm) were
blocked using 10% bovine serum albumin (Thermo Fisher Scientific
Inc.) for 1 h at 25°C. Sections were incubated with antibodies
against MCPIP1 (1:400; cat. no. 25009-1-AP; Proteintech Group Inc.)
at 4°C overnight, followed by incubation with Alexa
Fluor® 488-conjugated secondary antibody (1:1,000;
ab150077, Abcam) at room temperature in the dark for 1 h and DAPI
at 25°C for 5 min. Sections were imaged using a Leica DMI 4000 B
fluorescence microscope (Leica Microsystems GmbH). Images were
taken from each rat for analysis by Image-Pro plus 6.0 software
(Media Cybernetics).
Hematoxylin and eosin (H&E)
staining
Paraffin-embedded sections were deparaffinized in
xylene at 25°C for 5 min followed by rehydration using 95, 70 and
50% ethanol solution 3 min in each. The sections were stained with
H&E at 25°C for 8 min and imaged using a Leica DMI 4000 B
fluorescence microscope (Leica Microsystems GmbH). Proportion of
damaged neuronal cell was calculated by the number of cells
exhibiting shrinkage.
Enzyme-linked immunosorbent assay
(ELISA)
The serum levels of IL-6, TNF-α, and IL-1β in mice
were assessed using Mouse IL-6 ELISA Kit, Mouse TNF-α ELISA Kit,
and Mouse IL-1β ELISA Kit (cat. nos. KE10007, KE10002 and KE10003,
respectively; Proteintech Group Inc.).
Flow cytometry assay
BV2 cells were collected using trypsin, washed, and
suspended in PBS. The cells were then incubated with
FITC-conjugated CD16/CD32 antibodies (1:100; cat. no. 561728; BD
Biosciences) and PE-conjugated CD206 antibodies (1:100; cat. no.
568273; BD Biosciences) at 4°C for 20 min. The expression of
CD16/CD32 and CD206 was analyzed using an Accuri C6 Plus flow
cytometer (BD Biosciences) and FlowJo v10.9.0 software (Tree Star
Inc.).
Ubiquitination assay
pcDNA3.1-Flag-TRAF6, pcDNA3.1-His-MCPIP1, and
pcDNA3.1-HA-Ubiquitin plasmids were purchased from Beijing
Syngentech Co., Ltd. (Chian). BV2 cells were transfected with
Flag-TRAF6, His-MCPIP1 and HA-ubiquitin at 37°C for 48 h. Then,
cells (with the exception of control cells) were stimulated with 1
µg/ml LPS for 12 h and then treated with or without MG132 (20 µM)
for an additional 4 h to block proteasomal degradation. The cells
were lysed with buffer (50 mM Tris, 140 mM NaCl, 1% SDS), followed
by clearing of cell lysates through centrifugation at 10,000 × g
for 10 min. For IP assays, the anti-Flag (5 µg, cat. no.
20543-1-AP, Proteintech Group Inc.) were added at 4°C overnight.
After incubation, 50 µl protein A/G Plus-Agarose (Santa Cruz) was
added to the protein-antibody complexes and incubated at 4°C on a
rotating device overnight. Immunoprecipitates were washed five
times with immunoprecipitation buffer, centrifuged at 10,000 × g
for 1 min, and a 2× sample loading buffer was added to the beads
before boiling for 5 min. Pull-down samples were subjected to
immunoblotting with an HA-tagged antibody (1:5,000; cat. no.
51064-2-AP; Proteintech Group Inc.), Flag-tagged antibody
(1:20,000; cat. no. 20543-1-AP; Proteintech Group Inc.), His-tagged
antibody (1:2,000; cat. no. 10,001-0-AP; Proteintech Group Inc.),
and GAPDH (1:10,000; cat. no. 10494-1-AP; Proteintech Group Inc.).
Following washing with TBS with 0.1% Tween20, membranes were then
incubated with the HRP-conjugated Goat Anti-Rabbit IgG (H+L)
secondary antibodies (1:10,000; SA00001-2; Proteintech Group Inc.)
for 1 h at 25°C. ECL solution (cat. no. E412-02; Vazyme Biotech
Co., Ltd.) was used to detect the bands. Analysis was performed
using Image-Pro plus 6.0 software (Media Cybernetics).
Statistical analysis
Data are presented as mean ± SD. Statistical
significance was evaluated using unpaired Student's t-test and
one-way analysis of variance with Tukey's post hoc test using
GraphPad Prism version 8 (Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
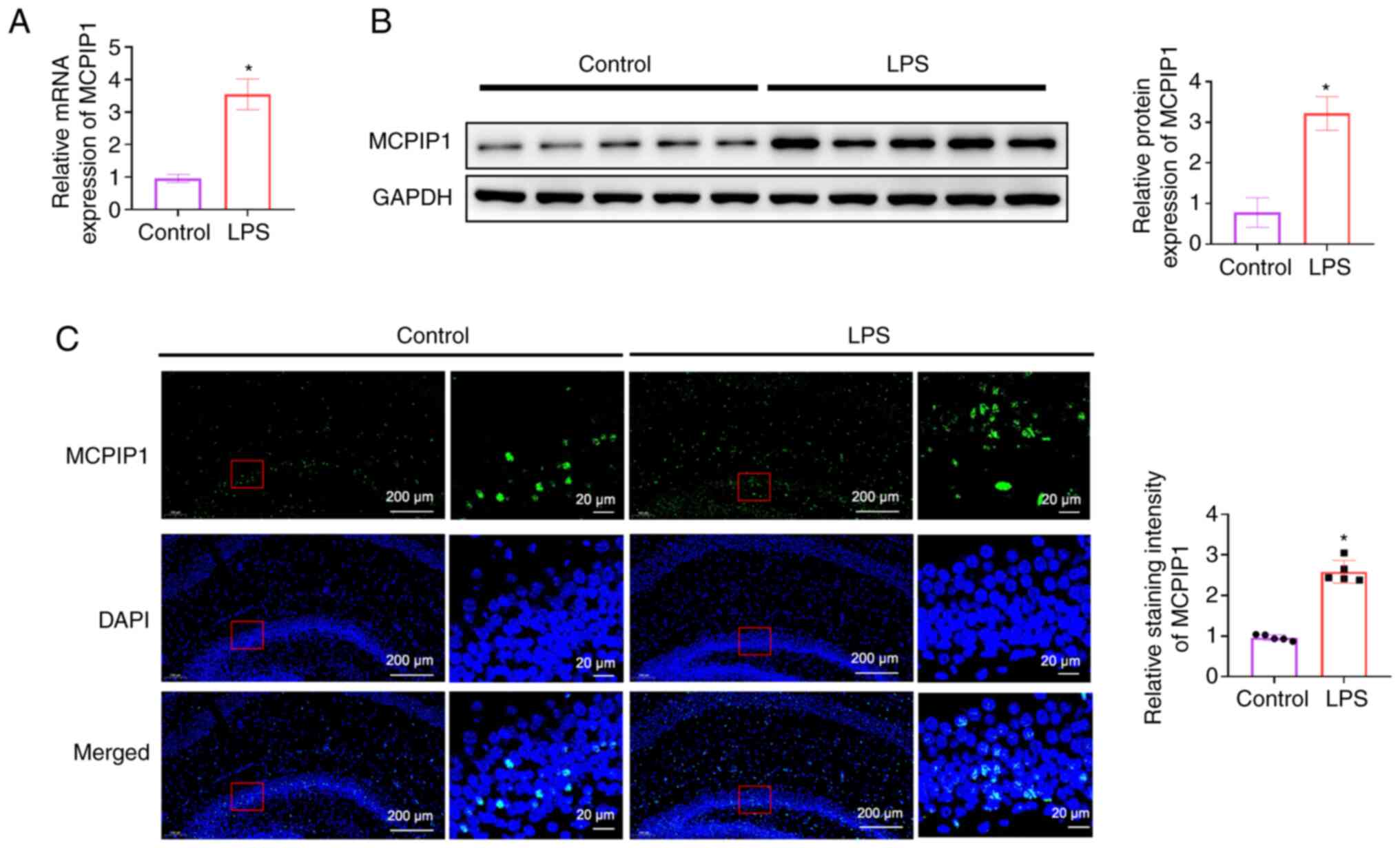
MCPIP1 is enhanced in LPS-treated
mice
A significant increase in MCPIP1 mRNA and protein
expression levels was demonstrated in the hippocampus of
LPS-treated mice compared with the control (Fig. 1A and B). Immunofluorescence results
also indicated that the intensity of MCPIP1 staining increased
significantly in LPS-treated mice compared with the control
(Fig. 1C).
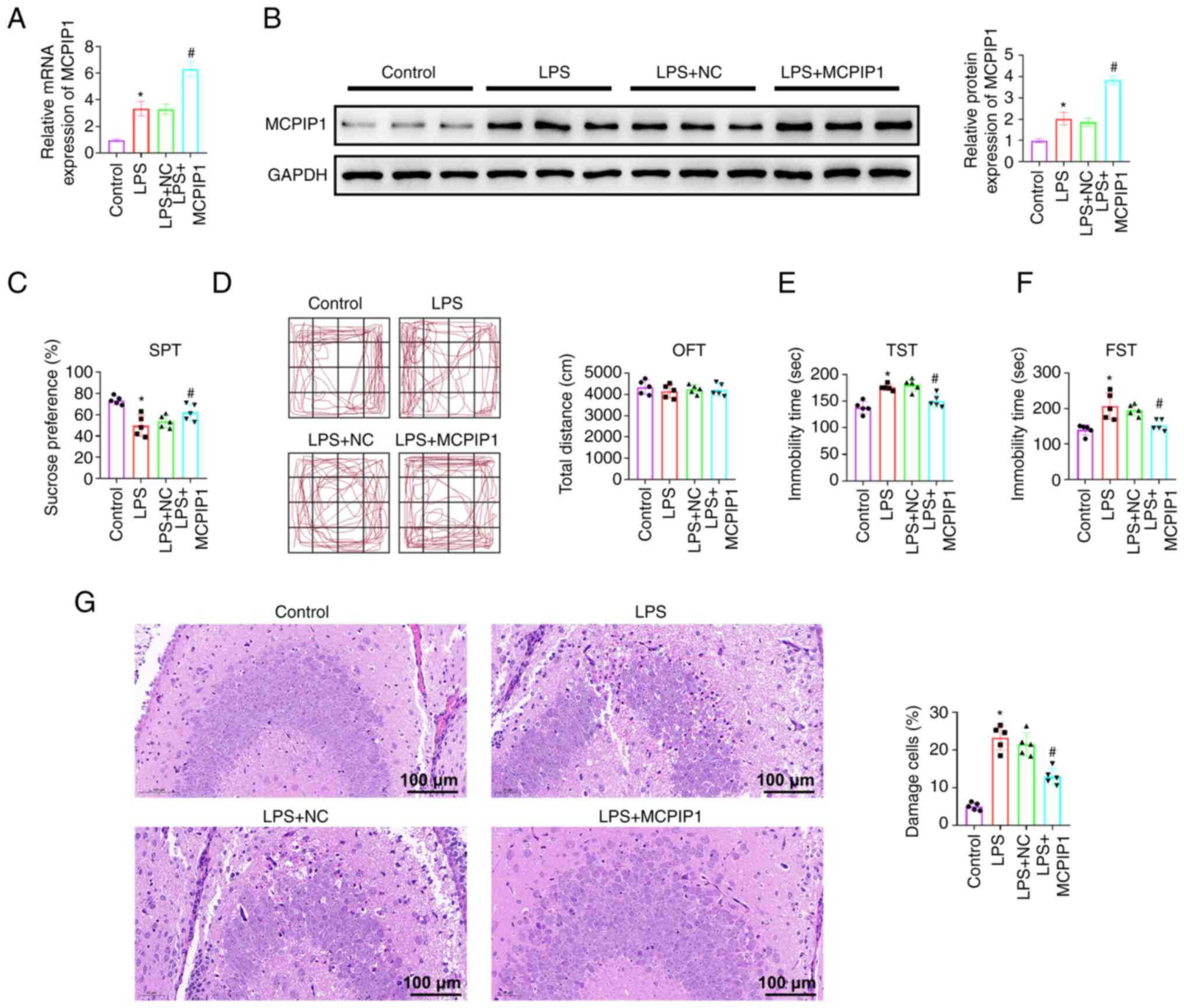
MCPIP1 overexpression mice exhibit
reduced depressive-like behaviors
To further evaluate the potential effect of MCPIP1
on depression, an AAV that overexpressed MCPIP1 was injected into
the hippocampus of mice. MCPIP1 mRNA and protein expression levels
increased significantly after AAV infusion compared with the AAV-NC
(Fig. 2A and B). The SPT provides
a measurement of anhedonia, lack of interest in reward stimuli, and
depression. The results indicated that LPS treated MCPIP1
overexpression mice exhibited a significantly higher preference for
sucrose solution compared with the LPS + NC mice (Fig. 2C), which indicated decreased
anhedonia behavior in MCPIP1 overexpression mice. There was no
significant difference in the distance moved between mice in the
LPS + NC and LPS + MCPIP1 groups in the OFT (Fig. 2D), which indicated that MCPIP1
overexpression did not significantly affect locomotor activity. The
TST was then performed as an acute stress assay to measure the
immobility time that was associated with the induction of
depression using LPS. MCPIP1 overexpression resulted in less
despair behavior, as indicated by the shorter time of immobility,
compared with that in the LPS + NC mice (Fig. 2E), and this was also confirmed by
the FST (Fig. 2F) that serves as
an alternative acute stress assay. MCPIP1 overexpression decreased
time of immobility, compared with the LPS + NC group (Fig. 2F). H&E staining demonstrated
that the hippocampal neurons were normal in the control group and
shrunken after LPS treatment. MCPIP1 overexpression also
significantly decreased the number of injured neurons compared with
the LPS + NC group (Fig. 2G).
MCPIP1 facilitates M2-polarization of
microglia and alleviates the inflammatory response via inhibition
of the TLR4/TRAF6/NF-κB signaling pathway in vivo
MCPIP1 significantly reduced Iba-1 protein
expression levels in LPS-treated mice compared with the LPS + NC
group (Fig. 3A). RT-qPCR analysis
demonstrated that MCPIP1 overexpression significantly inhibited the
LPS-induced elevation of the mRNA expression levels of CD16, CD32
and iNOS, compared with the LPS + NC group (Fig. 3B). MCPIP1 overexpression
significantly increased IL-4 and Arg-1 mRNA expression levels
compared with the LPS + NC; mRNA expression levels of IL-4 and
Arg-1 were significantly reduced by LPS treatment compared with the
control. IL-10 and CD206 mRNA expression levels were significantly
increased by MCPIP1 overexpression compared with the LPS + NC
group; however, LPS treatment did not demonstrate a significant
effect on their expression compared with the control (Fig. 3C). ELISA results demonstrated that
MCPIP1 overexpression significantly decreased IL-6, TNF-α and IL-1β
expression levels in LPS-treated mice compared with the LPS + NC
group (Fig. 3D-F). The
aforementioned results indicated that MCPIP1 served a role in the
M2-polarization of microglia. Western blotting was performed to
examine the TLR4/TRAF6/NF-κB signaling pathway. LPS-triggered
significantly increased TLR4, MyD88, TRAF6, p-IκBα and p-p65 NF-κB
protein expression levels compared with the control, which were
significantly reduced by MCPIP1 overexpression compared with the
LPS + NC group (Fig. 3G).
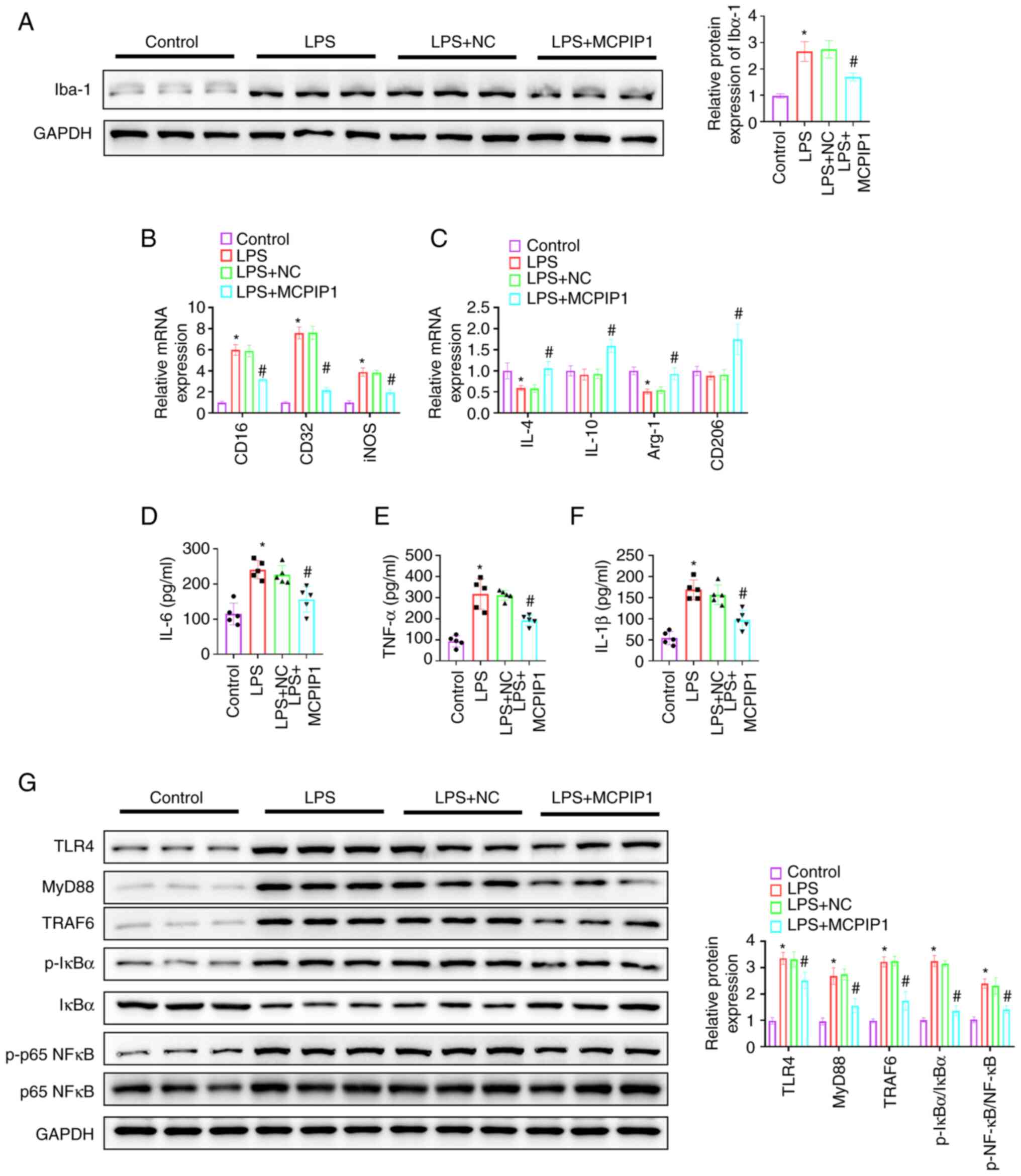 | Figure 3.MCPIP1 facilitates M2-polarization of
microglia and alleviates the inflammatory response via inhibition
of the TLR4/TRAF6/NF-κB signaling pathway in vivo. (A)
Western blotting was used to semi-quantify Iba-1 protein
expression. mRNA expression levels of (B) CD16, CD32 and iNOS, and
(C) IL-4, IL-10, Arg-1 and CD206 were quantified using Reverse
transcription-quantitative PCR. (D) IL-6, (E) TNF-α and (F) IL-1β
levels were assessed using ELISA. (G) Protein expression levels
were semi-quantified using western blotting. *P<0.05 vs.
control. #P<0.05 vs. LPS + NC group. MCPIP1, monocyte
chemotactic protein-1-induced protein 1; LPS, lipopolysaccharide;
NC, negative control; iNOS, inducible nitric oxide synthase; Arg-1,
arginase 1; TRAF6, TNF receptor associated factor 6; p,
phosphorylated. |
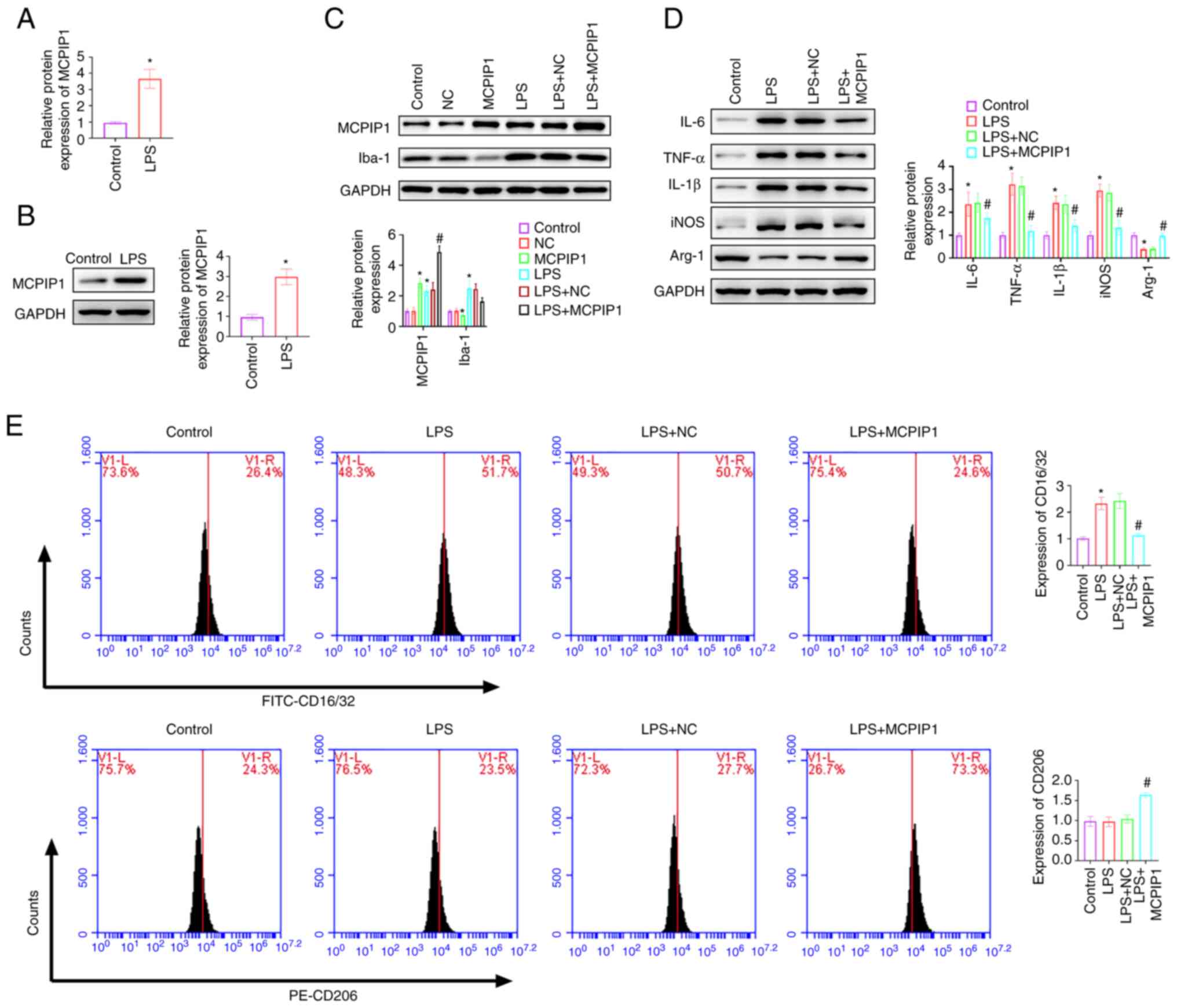
MCPIP1 accelerates M2-polarization of
microglia
LPS treatment significantly upregulated MCPIP1 mRNA
and protein expression levels in BV2 cells compared with the
control (Fig. 4A and B). MCPIP1
overexpression and knockdown plasmids were transfected into BV2
cells. MCPIP1 protein expression levels significantly increased and
Iba-1 protein expression levels significantly decreased after
transfection with the MCPIP1 overexpression vector compared with
the control (Fig. 4C). MCPIP1
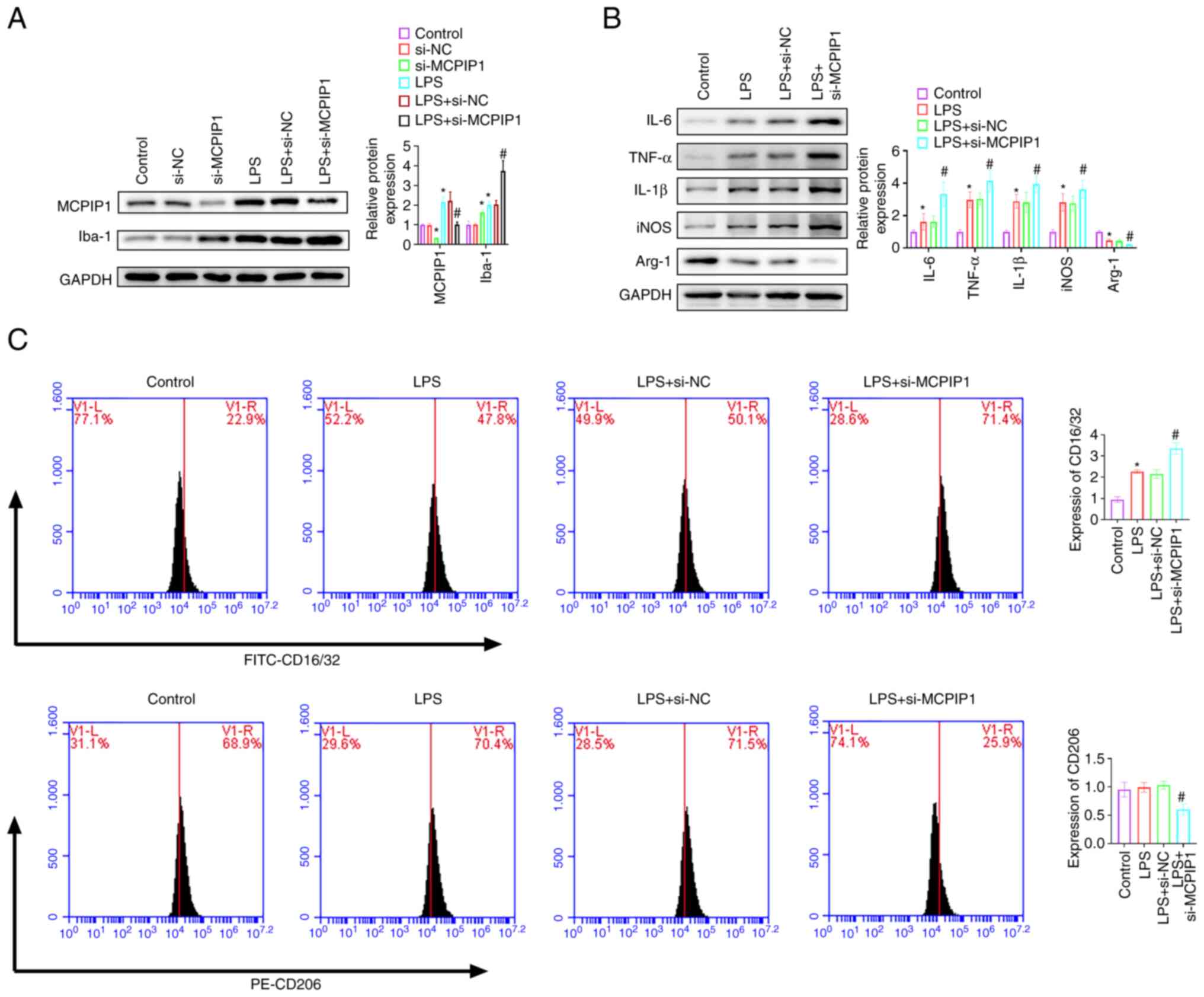
knockdown decreased MCPIP1 protein expression levels and
significantly increased Iba-1 protein expression levels (Fig. 5A). LPS treatment significantly
increased the protein expression levels of IL-6, TNF-α, IL-1β and
iNOS, and significantly decreased the protein expression levels of
Arg-1, all compared with the control. MCPIP1 overexpression
decreased protein expression levels of IL-6, TNF-α, IL-1β and iNOS,
and increased the protein expression levels of Arg-1, all compared
with the LPS + NC group (Fig. 4D).
MCPIP1 knockdown aggravated PS-induced the protein expression
levels of IL-6, TNF-α, IL-1β and iNOS, and further decreased arg-1
expression reduced by LPS (Fig.
5B). MCPIP1 overexpression significantly inhibited the
LPS-induced expression of CD16/32 compared with the LPS + NC group.
CD206 expression was significantly increased by MCPIP1
overexpression compared with the LPS + NC group; however, LPS
treatment did not significantly affect CD206 expression levels
compared with the control (Fig.
4E). MCPIP1 knockdown decreased CD206 expression levels
compared with the LPS + si-NC group (Fig. 5C).
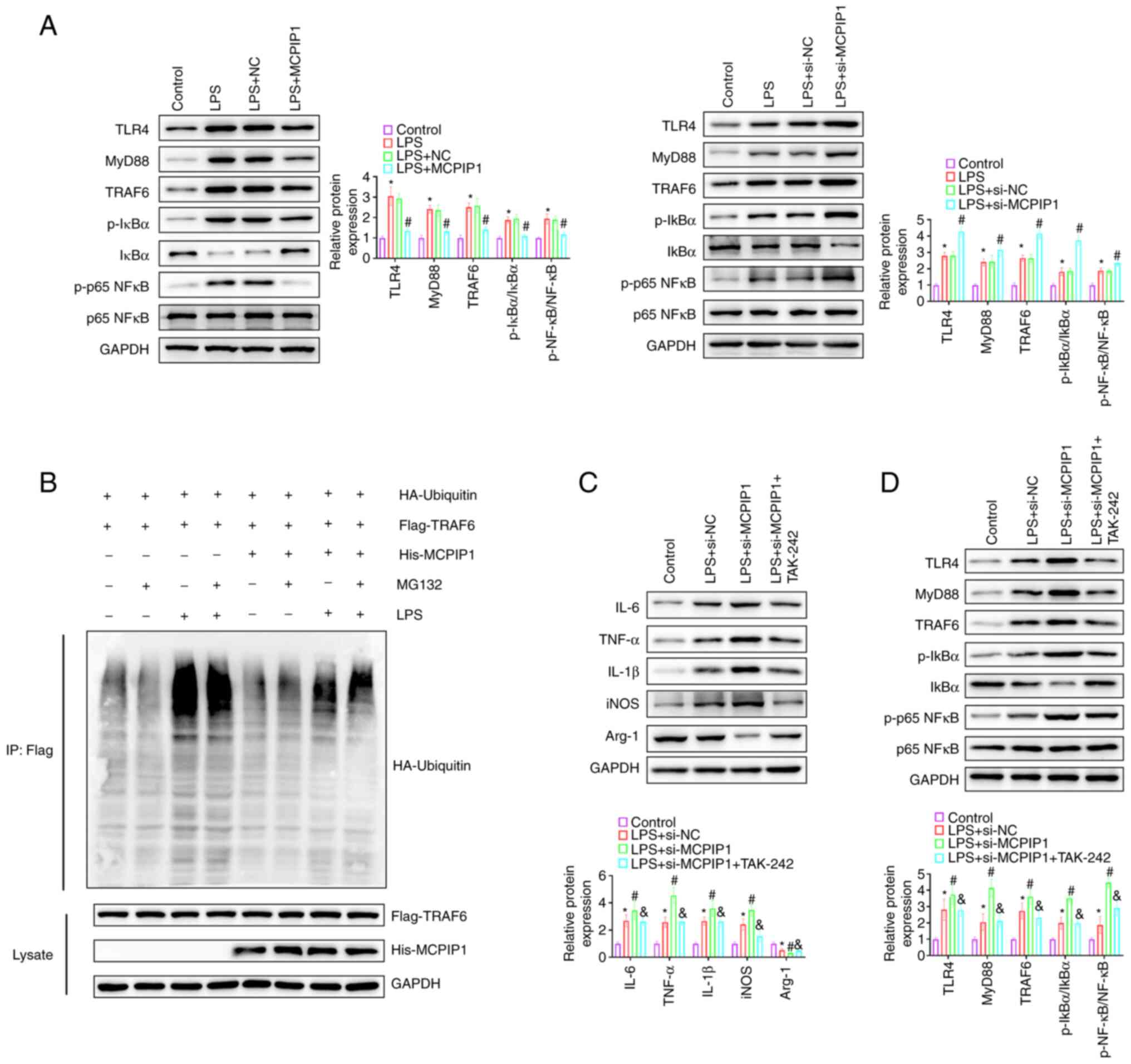
MCPIP1 alleviates the inflammatory
response by inhibiting the TLR4/TRAF6/NF-κB signaling pathway
MCPIP1 overexpression significantly reduced the
LPS-induced increase in the protein expression levels of TLR4,
MyD88, TRAF6, p-IκBα and p-p65 NF-κB compared with the LPS + NC
group. Moreover, MCPIP1 knockdown significantly increased the
protein expression levels of TLR4, MyD88, TRAF6, p-IκBα and p-p65
NF-κB compared with the LPS + NC group, indicating that LPS-induced
NF-κB activation was increased by MCPIP1 knockdown (Fig. 6A). LPS treatment markedly promoted
the ubiquitination of TRAF6 and MCPIP1 notably inhibited
LPS-induced TRAF6 ubiquitination in BV2 cells with or without MG132
treatment (Fig. 6B). TAK-242 was
used to evaluate the function of MCPIP1 in the LPS-induced
inflammatory response via the TLR4//TRAF6/NF-κB signaling pathway
in BV2 cells. Western blotting demonstrated that TAK-242 treatment
significantly reversed the upregulated protein expression levels of
IL-6, TNF-α, IL-1β and iNOS and the downregulated protein
expression levels of Arg-1 induced by MCPIP1 knockdown in BV2
cells, compared with the LPS + si-MCPIP1 group (Fig. 6C). Treatment with TAK-242
significantly reversed the upregulated protein expression levels of
TLR4, MyD88, TRAF6, p-IκBα and p-NF-κB p65 which were induced by
MCPIP1 knockdown (Fig. 6D). These
data indicated that MCPIP1 knockdown promoted the LPS-induced
inflammatory response via activation of the TLR4/TRAF6/NF-kB
signaling pathway in BV2 cells.
Discussion
MDD is a mental health condition associated with
numerous symptoms that include physical, cognitive, emotional and
social aspects (28). Although
advances have been made in the anti-depressant treatment of MDD, it
still presents a high mortality rate (29). Therefore, new therapeutic
biomarkers must be identified. previous studies have reported that
MCPIP1 serves important biological roles in lipid homeostasis
(30), insulin secretion (31) and the inflammatory response
(32), and also regulates the
development of certain diseases, such as hidradenitis suppurativa
(33), primary biliary cholangitis
(34), skin inflammation (35) and clear cell renal cell carcinoma
(36). Here, LPS could induce
MCPIP1 expression and overexpression of MCPIP1 decreased the
LPS-induced inflammatory response. Overexpression of MCPIP1 in
macrophages partially protects mice from LPS-induced septic shock
(37){Huang, 2013 #74}. Han et
al (38) reported that the
level of MCPIP1 increased and the level of SIRT1 decreased in LPS
induced Kupffer cells or RAW 264.7 macrophages. Overexpression of
MCPIP1 alleviated cytokine secretion and p65 nuclear translocation.
MCPIP1 overexpression induced by MG132 has been reported to
alleviate sepsis-induced pathologic changes, water content and
protein leakage in the lungs, the induction of systemic
inflammatory mediators and to improve the 7-day mortality rate in a
rat model (39). A previous study
reported that MCPIP1 expression was upregulated in LPS-treated
microglia and the mouse brain, and that levels of pro-inflammatory
cytokines were increased in MCPIP1 deficient mouse brains (40). Similarly, in the present study,
LPS-treated mice presented injured neurons, microglial activation
and depression-like behaviors. Overexpression of MCPIP1 in mice
alleviated the pathological symptoms, and this was consistent with
the observation that melatonin alleviated LPS-induced
depressive-like behaviors (41).
Collectively, these results indicated that MCPIP1 may represent a
novel therapeutic target for MDD and that MCPIP1 overexpression may
relieve depressive-like behaviors in LPS-induced mice.
Previous studies have reported that
neuroinflammation is a risk factor for depression (42–44).
LPS increases cytokine levels and strengthens depressive-like
behaviors in mice (45). It has
been reported that ibrutinib inhibits LPS-induced depressive-like
behaviors and pro-inflammatory cytokine levels by inhibiting NF-κB
activation (46). Wang et
al (47) reported that
palmatine relieved depressive like behaviors, inhibited
pro-inflammatory cytokines (TNF-α, IL-6, CD68 and iNOS) and
enhanced anti-inflammatory cytokines (IL-4, IL-10, CD206, Arg1 and
Ym1) in LPS-induced mice and BV2 cells. Zhang et al
(14) reported that curcumin
converted M1 phenotype to M2 phenotype by reducing iNOS, IL-1β,
IL-6 and CD16/32 expression and inducing Arg-1, IL-4, IL-10 and
CD206 expression in LPS-stimulated BV2 cells. Similarly, the
present study demonstrated that MCPIP1 overexpression alleviated
depressive-like behaviors in LPS-induced mice, significantly
inhibited the expression of pro-inflammatory cytokines and markers
of M1 microglia, such as IL-6, TNF-α, IL-1β, CD16/32 and iNOS, and
significantly increased the expression of anti-inflammatory
cytokines and markers of M2 microglia, such as IL-4, IL-10, Arg-1
and CD206. These results demonstrated that MCPIP1 inhibited
inflammation and depressive-like behaviors during LPS
treatment.
TLR4 is an important component of the inflammatory
response, and its upregulation is related to depression and the
activation of microglia (48). A
previous study reported that baicalin relieved LPS-induced
depressive-like behavior through a decrease in TLR4 expression and
activation of the PI3K/AKT/FOXO1 signaling pathway (49). TLR4 inhibition has been reported to
decrease MCPIP1 expression in LPS/ischemia-induced microglia
(50). In the present study,
MCPIP1 overexpression significantly decreased TLR4 expression in
LPS-stimulated mice and BV-2 cells. TLR4 elevated TRAF6 expression
through the MyD88-dependent pathway, thereby promoting cytokine
release (51). A previous study
reported that repression of TLR4/MyD88/TRAF6 alleviated
pro-inflammatory microglial polarization (52). In agreement with these studies, the
present study demonstrated that MCPIP1 reduced LPS-induced
neuroinflammation via deactivation of the TLR4/TRAF6/NF-κB
signaling pathway. NF-κB has been reported to be a crucial
downstream component of the TLR4 signaling pathway in the context
of LPS induced neuroinflammation (53). It has been previously reported that
hesperetin, a citrus Flavonoid, inhibited the inflammatory response
by inhibiting the TLR4/NF-kB signaling pathway in LPS-induced mice
and microglia (54). Similarly,
curcumin ameliorated LPS-induced neuroinflammation by inducing M2
polarization of microglia though the TREM2/TLR4/NF-κB signaling
pathway (14). In the present
study, MCPIP1 promoted M2-polarization of microglia and alleviated
neuroinflammation by suppressing the TLR4/TRAF6/NF-κB signaling
pathway. These results were consistent with those of a previous
study, which reported that loganin, an iridoid glycoside obtained
from traditional Chinese medicine Cornus officinalis, attenuated
Aβ-induced inflammatory response in BV-2 cells by suppressing the
TLR4/TRAF6/NF-κB signaling pathway (55). These results demonstrated that
MCPIP1 attenuated LPS-induced inflammation via inhibition of the
TLR4/TRAF6/NF-κB signaling pathway.
There are limitations of the present study. First,
the inflammatory mechanism mediated by MCPIP1 is complicated, and
we only targeted the TLR4/TRAF6/NF-κB pathway. Thus, other pathways
involved in the treatment of depressive-like behaviors need to be
further study. Second, we studied the role of MCPIP1 only in animal
models of LPS-induced depressive-like behaviors, and its role in
other animal models of depression needs to be further studied.
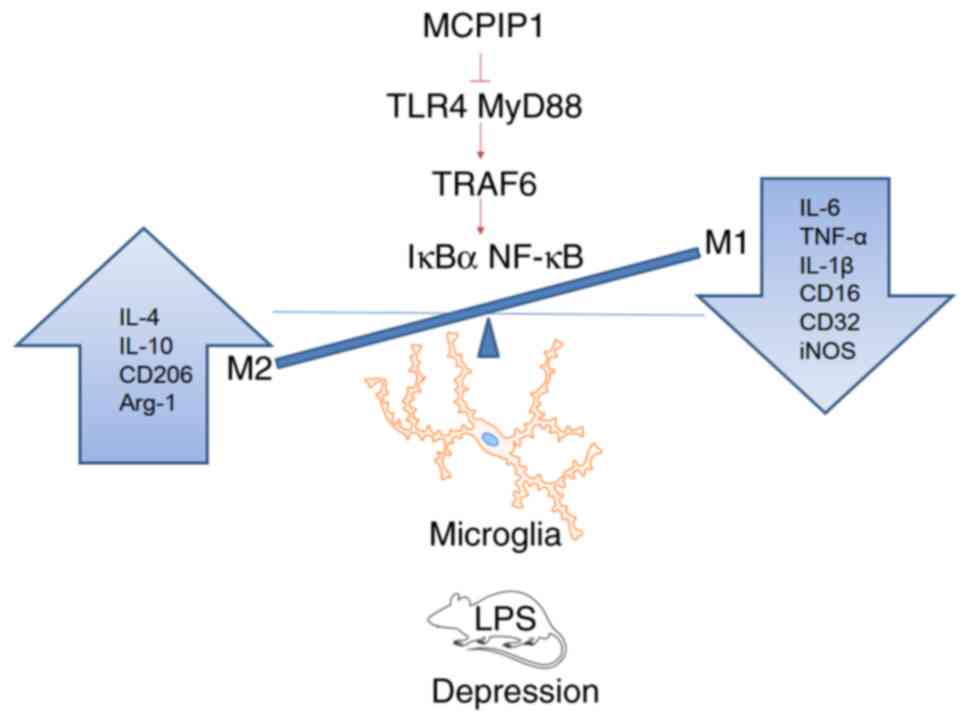
The aforementioned results indicated that MCPIP1
alleviated LPS-induced depressive-like behaviors and that MCPIP1
promoted the M2-polarization of microglia via the inhibition of the
TLR4/TRAF6/NF-κB signaling pathway (Fig. 7).
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (81872605).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QA was responsible for conception and design. QA and
JX performed the experiments. FP and SS performed data analysis and
interpretation. QA and JX confirm the authenticity of all the raw
data. QA and SS wrote the manuscript. All authors reviewed the
final manuscript.
Ethics approval and consent to
participate
The experimental protocol of our study was performed
in accordance with the guidelines of the National Institutes of
Health Guide for the Care and Use of Laboratory Animals and was
approved by the Ethics Committee of Qingdao Mental Health Center
(Qingdao, China; approval no. 2022061).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abdoli N, Salari N, Darvishi N, Jafarpour
S, Solaymani M, Mohammadi M and Shohaimi S: The global prevalence
of major depressive disorder (MDD) among the elderly: A systematic
review and meta-analysis. Neurosci Biobehav Rev. 132:1067–1073.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rink L, Adams A, Braun C, Bschor T, Kuhr K
and Baethge C: Dose-response relationship in selective serotonin
and norepinephrine reuptake inhibitors in the treatment of major
depressive disorder: A meta-analysis and network meta-analysis of
randomized controlled trials. Psychother Psychosom. 91:84–93. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chin T, Huyghebaert T, Svrcek C and
Oluboka O: Individualized antidepressant therapy in patients with
major depressive disorder: Novel evidence-informed decision support
tool. Can Fam Physician. 68:807–814. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masand PS: Tolerability and adherence
issues in antidepressant therapy. Clin Ther. 25:2289–2304. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammen C: Risk factors for depression: An
autobiographical review. Ann Rev Clin Psychol. 14:1–28. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Debnath M, Berk M and Maes M:
Translational evidence for the inflammatory response system
(IRS)/compensatory immune response system (CIRS) and
neuroprogression theory of major depression. Prog
Neuropsychopharmacol Biol Psychiatry. 111:1103432021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu JJ, Wei YB, Strawbridge R, Bao Y,
Chang S, Shi L, Que J, Gadad BS, Trivedi MH, Kelsoe JR and Lu L:
Peripheral cytokine levels and response to antidepressant treatment
in depression: A systematic review and meta-analysis. Mol
Psychiatry. 25:339–350. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dowlati Y, Herrmann N, Swardfager W, Liu
H, Sham L, Reim EK and Lanctôt K: A meta-analysis of cytokines in
major depression. Biol Psychiatry. 67:446–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller AH, Maletic V and Raison CL:
Inflammation and its discontents: The role of cytokines in the
pathophysiology of major depression. Biol Psychiatry. 65:732–741.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JQ, Wu XH, Feng Y, Xie XF, Fan YH,
Yan S, Zhao QY, Peng C and You ZL: Salvianolic acid B ameliorates
depressive-like behaviors in chronic mild stress-treated mice:
Involvement of the neuroinflammatory pathway. Acta Pharmacol Sin.
37:1141–1153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang JC, Yao W and Hashimoto K:
Brain-derived neurotrophic factor (BDNF)-TrkB signaling in
inflammation-related depression and potential therapeutic targets.
Curr Neuropharmacol. 14:721–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domingues M, Casaril AM, Birmann PT,
Lourenço DA, Vieira B, Begnini K, Lenardão EJ, Collares T, Seixas
FK and Savegnago L: Selanylimidazopyridine prevents
lipopolysaccharide-induced depressive-like behavior in mice by
targeting neurotrophins and inflammatory/oxidative mediators. Front
Neurosci. 12:4862018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yirmiya R, Rimmerman N and Reshef R:
Depression as a microglial disease. Trends Neurosci. 38:637–658.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X and
Fu J: Curcumin inhibits LPS-induced neuroinflammation by promoting
microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2
cells. Mol Immunol. 116:29–37. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim RE, Shin CY, Han SH and Kwon KJ:
Astaxanthin suppresses PM2.5-induced neuroinflammation by
regulating Akt phosphorylation in BV-2 microglial cells. Int J Mol
Sci. 21:72272020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XL, Chen F, Shi H, Zhang M, Yan L,
Pei XY and Peng XD: Oxymatrine inhibits neuroinflammation
byRegulating M1/M2 polarization in N9 microglia through the
TLR4/NF-κB pathway. Int Immunopharmacol. 100:1081392021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang K, Zhai Q, Wang S, Li Q, Liu J, Meng
F, Wang W, Zhang J, Wang D, Zhao D, et al: Cryptotanshinone
ameliorates CUS-induced depressive-like behaviors in mice. Transl
Neurosci. 12:469–481. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu R, Li Y, Liu Y, Qu J, Cao W, Zhang E,
He J and Cai Z: How are MCPIP1 and cytokines mutually regulated in
cancer-related immunity? Protein Cell. 11:881–893. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uehata T and Akira S: mRNA degradation by
the endoribonuclease Regnase-1/ZC3H12a/MCPIP-1. Biochim Biophys
Acta. 1829:708–713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han F, Shen L, Ma H, Wang L, Guo H and Wu
X: MCPIP1 alleviates inflammatory response through inducing
autophagy in Aspergillus fumigatus keratitis. Int Immunopharmacol.
113:1092792022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lichawska-Cieslar A, Konieczny P, Szukala
W, Declercq W, Fu M and Jura J: Loss of keratinocyte Mcpip1
abruptly activates the IL-23/Th17 and Stat3 pathways in skin
inflammation. Biochim Biophys Acta Mol Cell Res. 1868:1188662021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suk FM, Chang CC, Sun PC, Ke WT, Chung CC,
Lee KL, Chan TS and Liang YC: MCPIP1 enhances TNF-α-mediated
apoptosis through downregulation of the NF-κB/cFLIP axis. Biology
(Basel). 10:6552021.PubMed/NCBI
|
|
23
|
Wang W, Huang X, Xin HB, Fu M, Xue A and
Wu ZH: TRAF family member-associated NF-κB activator (TANK)
inhibits genotoxic nuclear factor κB activation by facilitating
deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase. J
Biol Chem. 290:13372–13385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39:208–211. 1996.PubMed/NCBI
|
|
25
|
Li W, Ali T, Zheng C, He K, Liu Z, Shah
FA, Li N, Yu ZJ and Li S: Anti-depressive-like behaviors of APN KO
mice involve Trkb/BDNF signaling related neuroinflammatory changes.
Mol Psychiatry. 27:1047–1058. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J,
Wang YL, Xu LZ and Wu WN: NLRP1 inflammasome contributes to chronic
stress-induced depressive-like behaviors in mice. J
Neuroinflammation. 17:1782020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hauenstein EJ: Depression in adolescence.
J Obstet Gynecol Neonatal Nurs. 32:239–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Touloumis C: The burden and the challenge
of treatment-resistant depression. Psychiatriki. 32:11–14. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moody J, Yang C, Sedinkin J and Chang Y:
Systemic MCPIP1 deficiency in mice impairs lipid homeostasis. Curr
Res Pharmacol Drug Discov. 1:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tyka K, Jörns A, Dunst A, Tang Y, Bryde
TH, Mehmeti I, Walentinsson A, Marselli L, Cnop M, Tyrberg B, et
al: MCPIP1 is a novel link between diabetogenic conditions and
impaired insulin secretory capacity. Biochim Biophys Acta Mol Basis
Dis. 1867:1661992021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bugara B, Konieczny P, Wolnicka-Glubisz A,
Eckhart L, Fischer H, Skalniak L, Borowczyk-Michalowska J, Drukala
J and Jura J: MCPIP1 contributes to the inflammatory response of
UVB-treated keratinocytes. J Dermatol Sci. 87:10–18. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krajewski PK, Szukała W, Lichawska-Cieślar
A, Matusiak Ł, Jura J and Szepietowski JC: MCPIP1/Regnase-1
expression in keratinocytes of patients with hidradenitis
suppurativa: Preliminary results. Int J Mol Sci. 22:72412021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kotlinowski J, Hutsch T, Czyzynska-Cichon
I, Wadowska M, Pydyn N, Jasztal A, Kij A, Dobosz E, Lech M, Miekus
K, et al: Deletion of Mcpip1 in Mcpip1(fl/fl)Alb(Cre) mice
recapitulates the phenotype of human primary biliary cholangitis.
Biochim Biophys Acta Mol Basis Dis. 1867:1660862021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Monin L, Gudjonsson JE, Childs EE, Amatya
N, Xing X, Verma AH, Coleman BM, Garg AV, Killeen M, Mathers A, et
al: MCPIP1/Regnase-1 restricts IL-17A- and IL-17C-dependent skin
inflammation. J Immunol. 198:767–775. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gorka J, Marona P, Kwapisz O, Rys J, Jura
J and Miekus K: The anti-inflammatory protein MCPIP1 inhibits the
development of ccRCC by maintaining high levels of tumour
suppressors. Eur J Pharmacol. 888:1735912020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang S, Miao R, Zhou Z, Wang T, Liu J,
Liu G, Chen YE, Xin HB, Zhang J and Fu M: MCPIP1 negatively
regulates toll-like receptor 4 signaling and protects mice from
LPS-induced septic shock. Cell Signal. 25:1228–1234. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han S, Li Z, Ji P, Jia Y, Bai X, Cai W, Li
X, Yang C, Yang Y, Yang K, et al: MCPIP1 alleviated
lipopolysaccharide-induced liver injury by regulating SIRT1 via
modulation of microRNA-9. J Cell Physiol. 234:22450–22462. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Huang T, Jiang L, Gao J, Yu D, Ge
Y and Lin S: MCP-induced protein 1 attenuates sepsis-induced acute
lung injury by modulating macrophage polarization via the JNK/c-Myc
pathway. Int Immunopharmacol. 75:162019. View Article : Google Scholar
|
|
40
|
Liang J, Wang J, Saad Y, Warble L, Becerra
E and Kolattukudy PE: Participation of MCP-induced protein 1 in
lipopolysaccharide preconditioning-induced ischemic stroke
tolerance by regulating the expression of proinflammatory
cytokines. J Neuroinflammation. 8:1822011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arioz BI, Tastan B, Tarakcioglu E, Tufekci
KU, Olcum M, Ersoy N, Bagriyanik A, Genc K and Genc S: Melatonin
attenuates LPS-induced acute depressive-like behaviors and
microglial NLRP3 inflammasome activation through the SIRT1/Nrf2
pathway. Front Immunol. 10:15112019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slavich GM and Sacher J: Stress, sex
hormones, inflammation, and major depressive disorder: Extending
social signal transduction theory of depression to account for sex
differences in mood disorders. Psychopharmacology (Berl).
236:3063–3079. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Troubat R, Barone P, Leman S, Desmidt T,
Cressant A, Atanasova B, Brizard B, El Hage W, Surget A, Belzung C
and Camus V: Neuroinflammation and depression: A review. Eur J
Neurosci. 53:151–171. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Carlessi AS, Borba LA, Zugno AI, Quevedo J
and Réus GZ: Gut microbiota-brain axis in depression: The role of
neuroinflammation. Eur J Neurosci. 53:222–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ali T, Rahman SU, Hao Q, Li W, Liu Z, Shah
FA, Murtaza I, Zhang Z, Yang X, Liu G and Li S: Melatonin prevents
neuroinflammation and relieves depression by attenuating autophagy
impairment through FOXO3a regulation. J Pineal Res. 69:e126672020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li W, Ali T, He K, Liu Z, Shah FA, Ren Q,
Liu Y, Jiang A and Li S: Ibrutinib alleviates LPS-induced
neuroinflammation and synaptic defects in a mouse model of
depression. Brain Behav Immun. 92:10–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Li M, Zhu C, Qin A, Wang J and Wei
X: The protective effect of Palmatine on depressive like behavior
by modulating microglia polarization in LPS-induced mice. Neurochem
Res. 47:3178–3191. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH,
Cui YX, Li J, Ma J, Piao HR, Jin X and Piao LX: Arctigenin protects
against depression by inhibiting microglial activation and
neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB
pathways. Br J Pharmacol. 177:5224–5245. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang
RY, Zhao QW, Ma ZQ, Deng XY and Ma SP: Baicalin ameliorates
neuroinflammation-induced depressive-like behavior through
inhibition of toll-like receptor 4 expression via the
PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 16:952019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen S, Lyu C, Zhou J, Huang S, Zhang Y,
Liu G, Liu K, Chen D, Hu Y, Zhou L and Gu Y: TLR4 signaling pathway
mediates the LPS/ischemia-induced expression of monocytechemotactic
protein-induced protein 1 in microglia. Neurosci Lett. 686:33–40.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
El-Shamarka ME, Eliwa HA and Ahmed MAE:
Inhibition of boldenone-induced aggression in rats by curcumin:
Targeting TLR4/MyD88/TRAF-6/NF-κB pathway. 51. El-Shamarka ME,
Eliwa HA and Ahmed MAE: Inhibition of boldenone-induced aggression
in rats by curcumin: Targeting TLR4/MyD88/TRAF-6/NF-κB pathway. J
Biochem Mol Toxicol. 36:e229362022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ran Y, Qie S, Gao F, Qie S, Gao F, Ding Z,
Yang S, Tian G, Liu Z and Xi J: Baicalein ameliorates ischemic
brain damage through suppressing proinflammatory microglia
polarization via inhibiting the TLR4/NF-κB and STAT1 pathway. Brain
Res. 1770:1476262021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jin X, Liu MY, Zhang DF, Zhong X, Du K,
Qian P, Yao WF, Gao H and Wei MJ: Baicalin mitigates cognitive
impairment and protects neurons from microglia-mediated
neuroinflammation via suppressing NLRP3 inflammasomes and
TLR4/NF-κB signaling pathway. CNS Neurosci Ther. 25:575–590. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Muhammad T, Ikram M, Ullah R, Rehman SU
and Kim MO: Hesperetin, a citrus flavonoid, attenuates LPS-induced
neuroinflammation, apoptosis and memory impairments by modulating
TLR4/NF-κB signaling. Nutrients. 11:6482019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cui Y, Wang Y, Zhao D, Feng X, Zhang L and
Liu C: Loganin prevents BV-2 microglia cells from Aβ(1–42)-induced
inflammation via regulating TLR4/TRAF6/NF-κB axis. Cell Biol Int.
42:1632–1642. 2018. View Article : Google Scholar : PubMed/NCBI
|