Introduction
Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) infection leads to transient hyperphosphatemia
(1), which is associated with a
higher risk of death from atherosclerosis in both the general
population and patients with chronic kidney disease (2–4).
Hyperphosphatemia on admission has been associated with late acute
kidney injury in patients with coronavirus disease 2019 (COVID-19)
(5). Likewise, an increased risk
of death from severe cases of COVID-19 has been observed in
patients with chronic kidney disease (6). Therefore, the present study aimed to
clarify the mechanism by which hyperphosphatemia promotes the
progression of SARS-CoV-2-related cardiovascular disease,
particularly the effect of excess inorganic phosphate (PI) on
SARS-CoV-2-induced cytokine storm.
Sterol regulatory element binding protein (SREBP)
cleavage-activating protein (SCAP) serves a central role in
regulating lipid homeostasis and inflammasome activation. Low serum
cholesterol concentration increases the activity of SREBP2 in
patients with COVID-19 (7,8). In addition, SARS-CoV-2 activates
SREBP2 and can lead to a cytokine storm in the peripheral blood
monocytes of patients with COVID-19 (9). Moreover, an inhibitor of SCAP-SREBP,
25-hydroxycholesterol, has been shown to suppress SARS-CoV-2
replication and excessive inflammatory responses in patients with
COVID-19 (10). Therefore, the
SCAP-SREBP signaling pathway has been suggested to serve a crucial
role in SARS-CoV-2-induced cytokine storm and organ damage.
Serum phosphate levels are positively correlated
with SCAP-SREBP2-mediated cholesterol synthesis (11). PI has been reported to accelerate
foam cell and atherosclerotic plaque formation in an apolipoprotein
E (ApoE) knockout (KO) mouse model (12) through enhanced SCAP-SREBP2
activation (13). In addition, it
has been demonstrated that SCAP overexpression in vascular smooth
muscle cells (VSMCs) induces NLRP3 inflammasome activation and
atherosclerosis (14). Recently,
Pan et al (15) found that
the SARS-CoV-2 N protein can induce NLRP3 inflammasome activation,
indicating that NLRP3 may be a coronavirus inflammatory target.
However, the underlying effects of PI on SARS-CoV-2 N
protein-induced cytokine storm remain unclear.
SARS-CoV-2 N protein is responsible for condensation
of the viral genome and promotes NLRP3 inflammasome activation to
induce hyperinflammation (15).
The present study aimed to investigate the effect of PI and
SARS-CoV-2 N protein on NLRP3 inflammasome activation. Furthermore,
this study investigated whether PI was able to amplify SARS-CoV-2 N
protein-induced NLRP3 inflammasome activation and lipogenesis
through SCAP-SREBP signaling pathways.
Materials and methods
Cell culture
Mouse VSMCs (MOVAS cell line; CRL-2797) were
purchased from the American Type Culture Collection, and were
cultured in DMEM/F-12 (HyClone; Cytiva) supplemented with 10% fetal
bovine serum (Shanghai ExCell Biology, Inc.) as described
previously (14). Thereafter, the
VSMCs were cultured at 2×106 cells/dish for 24 h at 37°C
and then starvation was induced with serum-free DMEM for 24 h at
37°C before the experimental treatment. In some experiments, the
VSMCs were pretreated with lycorine (20 µM; MedChemExpress) for 12
h before 3 mmol/l PI treatment for 24 h at 37°C. For some
experiments, SARS-CoV-2 N protein-overexpressing VSMCs were
pretreated with 1.0 mmol/l phosphonoformate sodium (PFA; CAS. no.
63585-09-1; Shanghai Aladdin Biochemical Technology Co., Ltd.) for
24 h before PI treatment for 24 h at 37°C. PFA is not only an
antiviral drug, but also a specific cell membrane sodium phosphate
transporter (pit1/2) inhibitor that prevents extracellular
phosphate from entering the cell (13). Phosphate stock solution contained
NaH2PO4 and Na2HPO4 (pH
7.40).
Lentivirus-mediated SARS-CoV-2 N
protein transduction
VSMCs were transduced with 50 µl SARS-CoV-2 N
protein lentivirus or negative control empty vector
(Lenti-EF1α-2019-nCOV-N-Flag/His-CMV-Puro; 1×108 IU/ml;
cat. no. C3021; Beyotime Institute of Biotechnology) for 24 h with
a multiplicity of infection of 50 at 37°C. A total of 48 h
post-transduction, the VSMCs were cultured for 4–7 days with 2
µg/ml puromycin (Beyotime Institute of Biotechnology) at 37°C.
Subsequently, the VSMCs were lysed and the specific protein
expression in VSMCs was confirmed by western blotting.
RNA interference
The VSMCs or SARS-CoV-2 N protein-overexpressing
VSMCs were transfected with 100 pmol SREBP2 small interfering RNA
(siRNA) or negative control (NC) siRNA using 5 µl
Lipo8000™ (cat. no. C0533; Beyotime Institute of
Biotechnology) for 24 h at 37°C, according to the manufacturer's
instructions. Subsequently, the SARS-CoV-2 N protein-overexpressing
VSMCs were further cultured for 12 h and then treated with or
without 0.2 mmol PI for 24 h at 37°C. The sequence of the
SREBP2-targeted siRNA was 5′-GCGGACAACACACAAUAUCAU-3′ and the
sequence of the scrambled siRNA control was
5′-UUCUCCGAACGUGUCACGUTT-3′. SREBP2 siRNA and the scrambled siRNA
were constructed by Sangon Biotech Co., Ltd.
Western blotting
Total protein was extracted from VSMCs using
ice-cold RIPA Lysis Buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology), containing a protease and phosphatase inhibitor
cocktail (cat. no. P1045; Beyotime Institute of Biotechnology).
Protein concentrations were measured using a BCA protein assay kit
(cat. no. P0010S; Beyotime Institute of Biotechnology) and 80 µg
protein/lane was separated by SDS-PAGE on a 10 or 12% gel. The
separated proteins were then transferred to a PVDF membrane and
blocked for 2 h. The PVDF membranes were incubated with the
following antibodies overnight at 37°C: SARS-CoV-2 N protein (cat.
no. AF0325; 1:500; Beyotime Institute of Biotechnology), SCAP (cat.
no. NBP2-04113; 1:1,000; Novus Biologicals, LLC), mature N-terminal
SREBP2 (N-SREBP2; 1:1,000; 70 Kd, cat. no. ab30682; Abcam), NLRP3
(cat. no. WL02635; 1:1,000; Wanleibio Co., Ltd.), procaspase-1
(cat. no. WL02996; 1:800; Wanleibio Co., Ltd.), cleaved caspase-1
(cat. no. WL03450; 1:800; Wanleibio Co., Ltd.), IL-1β (cat. no.
16806-1-AP; 1:600; Wuhan Sanying Biotechnology), IL-18 (cat. no.
10663-1-AP; 1:500; Wuhan Sanying Biotechnology) and β-actin (cat.
no. 81115-1-RR; 1:5,000; Wuhan Sanying Biotechnology). After
overnight incubation, the blots were incubated with goat
anti-rabbit IgG-HRP antibody (1:5,000; cat. no. A0208; Beyotime
Institute of Biotechnology) for 2 h at 37°C. Finally, the bands
were visualized using the ECL reagent kit (cat. no. WBKlS0100;
MilliporeSigma) and the densities of the bands were analyzed using
ImageJ 1.47i software (National Institutes of Health) for
semi-quantification.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The total RNA was extracted from the cells with
TRIzol® reagent (cat. no. 15596-026; Invitrogen; Thermo
Fisher Scientific, Inc.) and reverse-transcribed into cDNA using a
high-capacity cDNA synthesis kit (cat. no. BL696A; Biosharp Life
Sciences). The RT protocol was as follows: 25°C for 10 min, 55°C
for 15 min and 85°C for 5 min. qPCR analysis was performed using
the SYBR Green PCR Mix kit (cat. no. BL697A; Biosharp Life
Sciences) and the ABI7500 System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
Initial denaturation at 95°C for 2 min; followed by 40 cycles at
95°C for 15 sec and 60°C for 30 sec, with a final extension at 72°C
for 30 sec. The specific primer sequences are listed in Table I. The relative fold changes in gene
expression were normalized to the mRNA expression levels of β-actin
and were quantified using the 2−ΔΔCq method (16).
 | Table I.Reverse transcription-quantitative
PCR primer sequences. |
Table I.
Reverse transcription-quantitative
PCR primer sequences.
| Mouse gene
name | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| SREBP2 |
GCGTTCTGGAGACCATGGA |
ACAAAGTTGCTCTGAAAACAAATCA |
| HMGCoAR |
AGCTTGCCCGAATTGTATGTG |
TCTGTTGTGAACCATGTGACTTC |
| HMGCS1 |
GCCGTGAACTGGGTCGAA |
GCATATATAGCAATGTCTCCTGCAA |
| LDLR |
TGACTCAGACGAACAAGGCTG |
ATCTAGGCAATCTCGGTCTCC |
| PCSK9 |
CAGCGGCACCCTCATAGG |
CCCGAGGGCTGGATTAGC |
| SREBP1c |
ATCGGCGCGGAAGCTGTCGGGGTAGCGTG |
ACTGTCTTGGTTGTTGATGAGCTGGAGCCAT |
| FASN |
GCTGCGGAAACTTCAGGAAAT |
AGAGACGTGTCACTCCTGGACTT |
| SCD1 |
TTCTTGCGATACACTCTGGTGC |
CGGGATTGAATGTTCTTGTCGT |
| ACACA |
TGACAGACTGATCGCAGAGAAAG |
TGGAGAGCCCCACACACA |
| ACLY |
GCCAGCGGGAGCACATC |
CTTTGCAGGTGCCACTTCATC |
| NLRP3 |
TGTGAGAAGCAGGTTCTACTCT |
GACTGTTGAGGTCCACACTCT |
| IL-1β |
GAAATGCCACCTTTTGACAGTG |
TGGATGCTCTCATCAGGACAG |
| Β-actin |
GTGACGTTGACATCCGTAAAGA |
GCCGGACTCATCGTACTCC |
Confocal microscopy
VSMCs were fixed with 4% paraformaldehyde for 30 min
and permeabilized with 0.3% Triton X-100 for 5 min at 37°C.
Thereafter, the VSMCs were treated overnight with anti-SCAP (1:100;
cat. no. NBP2-04113; Novus Biologicals, LLC), anti-NLRP3 (1:200;
cat. no. NBP1-97601; Novus Biologicals, LLC) and anti-Golgin 97
(1:150; cat. no. PA5-30048; Thermo Fisher Scientific, Inc.) at
37°C. Then, the VSMCs were stained with goat anti-rabbit IgG
(TRITC; 1:100; cat. no. SA00007-2; Proteintech Group, Inc.) or goat
anti-mouse IgG (FITC; 1:100; cat. no. SA00003-1; Proteintech Group,
Inc.) for 45 min at 37°C. The nuclei were stained with DAPI for 8
min at 37°C and the images were viewed with a confocal laser
scanning microscope (Zeiss GmbH).
Statistical analysis
Data are presented as the mean ± SD. The
significance between two groups was assessed using unpaired
Student's t-test. Differences among multiple groups were analyzed
by one-way analysis of variance with Tukey's post hoc test. All
statistical analyses were performed using SPSS software (version
18, SPSS, Inc.). All of the experiments were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
PI amplifies SARS-CoV-2 N
protein-induced lipogenesis and NLRP3 inflammasome activation
through SCAP-SREBP2
SCAP transports SREBP2 to the Golgi apparatus, and
then SREBP2 becomes transcriptionally active when its N-terminal
DNA-binding fragment is proteolytically cleaved to its mature,
active form (mature N-terminal SREBP2, N-SREBP2) (17,18).
Subsequently, the cytoplasmic N-SREBP2 translocates to the nucleus
to bind to DNA and initiate gene transcription. This process can be
assessed by measuring the mature N-SREBP2 protein in the whole cell
lysate (17–19). Our previous study indicated that
SARS-CoV-2 N protein activates the dissociation of SCAP from the
endoplasmic reticulum (ER), resulting in SREBPs activation,
increased lipogenic gene expression and NLRP3 inflammasome
activation (20). To examine the
effect of PI on the activation of SCAP-SREBP2, SARS-CoV-2 N
protein-overexpressing VSMCs were incubated with PI (3 mmol/l) for
24 h. Firstly, SARS-CoV-2 N protein was significantly increased in
SARS-CoV-2 N protein-overexpressing VSMCs (Fig. 1A). Secondly, compared with those in
the SARS-CoV-2 N protein group, PI increased the mRNA expression
levels of SREBP2-targeted genes, i.e. HMG-CoA reductase (HMGCoAR),
low-density lipoprotein receptor (LDLR) and proprotein convertase
subtilisin/kexin type 9 (PCSK9) in SARS-CoV-2 N
protein-overexpressing VSMCs (Fig.
1B). In addition, the mRNA expression levels of
SREBP1c-targeted genes, i.e. recombinant fatty acid synthase
(FASN), stearyl coenzyme A desaturase 1 (SCD1), acetyl-CoA
carboxylase α (ACACA) and ATP-citrate lyase (ACLY) were
significantly increased in SARS-CoV-2 N protein-overexpressing
VSMCs (Fig. 1C). In addition, the
results indicated that PI significantly increased SCAP and SREBP2
protein expression levels in SARS-CoV-2 N protein-overexpressing
VSMCs compared with in the non-phosphate-treated control (Fig. 1D). Likewise, PI significantly
increased the relative mRNA expression levels of NLRP3 and IL-1β
(Fig. 1E), and the protein
expression levels of NLRP3, procaspase-1, cleaved caspase-1 and
IL-1β compared with those in the control group (Fig. 1F). These results indicated that PI
could amplify SARS-CoV-2 N protein-induced NLRP3 inflammasome
activation and lipogenesis, which may be mediated by
SCAP-SREBPs.
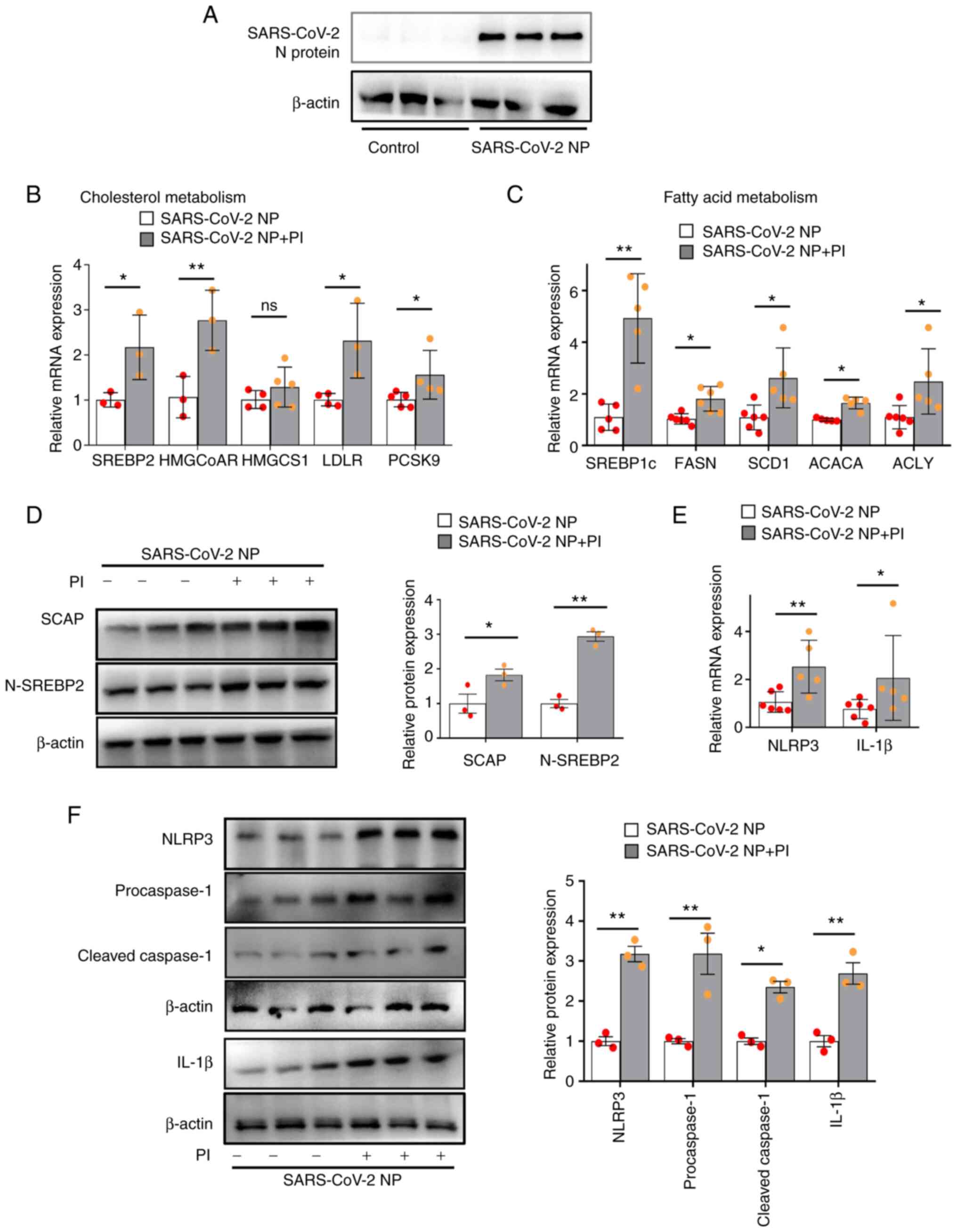 | Figure 1.PI promotes SCAP-SREBP-induced
lipogenesis and NLRP3 inflammasome activation in VSMCs
overexpressing the SARS-CoV-2 N protein. The SARS-CoV-2 N
protein-overexpressing VSMCs were treated with 3 mmol/l PI for 24
h. (A) Representative western blot images of SARS-CoV-2 N protein.
(B) Quantification of SREBP2, HMGCoAR, HMGCS1, LDLR and PCSK9 mRNA
expression levels. (C) Quantification of SREBP1c, FASN, SCD1, ACACA
and ACLY mRNA expression levels. (D) Western blot analysis of SCAP
and N-SREBP2. (E) Quantification of NLRP3 and IL-1β mRNA expression
levels. (F) Western blot analysis of NLRP3, procaspase-1, cleaved
caspase-1, and IL-1β expression (n≥3). *P˂0.05, **P˂0.01. PI,
inorganic phosphate; SARS-CoV-2, severe acute respiratory syndrome
coronavirus 2; SREBP, sterol regulatory element binding protein;
SCAP, SREBP cleavage-activating protein; N-SREBP2, mature
N-terminal SREBP2; NP, N protein; HMGCoAR, HMG-CoA reductase; LDLR,
low-density lipoprotein receptor; PCSK9, proprotein convertase
subtilisin/kexin type 9; FASN, recombinant fatty acid synthase;
SCD1, stearyl coenzyme A desaturase 1; ACACA, acetyl-CoA
carboxylase α; ACLY, ATP-citrate lyase; VSMCs, vascular smooth
muscle cells; ns, not significant.. |
PFA reverses the effect of PI and
SARS-CoV-2 N protein on SCAP-SREBP-induced lipogenesis and NLRP3
inflammasome activation
PFA is a specific inhibitor of intracellular
phosphate uptake (21). In VSMCs
overexpressing SARS-CoV-2 N protein, PFA significantly decreased
the relative protein expression levels of SCAP and N-SREBP2
compared with those in the PI-treated group, suggesting that PFA
reversed PI-induced SCAP-SREBP2 activation (Fig. 2A). Likewise, PFA significantly
decreased the mRNA expression levels of SREBP2-targeted genes, i.e.
HMGCoAR, LDLR and PCSK9 in PI-treated and SARS-CoV-2 N
protein-overexpressing VSMCs (Fig.
2B). In addition, PFA significantly decreased the relative mRNA
expression levels of NLRP3 and IL-1β (Fig. 2C), and the relative protein
expression levels of NLRP3, procaspase-1, cleaved caspase-1 and
IL-1β (Fig. 2D) compared with
those in the phosphate-treated controls. These data indicated that
PFA ameliorated the effect of PI and SARS-CoV-2 N protein on
SCAP-SREBP-induced lipogenesis and NLRP3 inflammasome
activation.
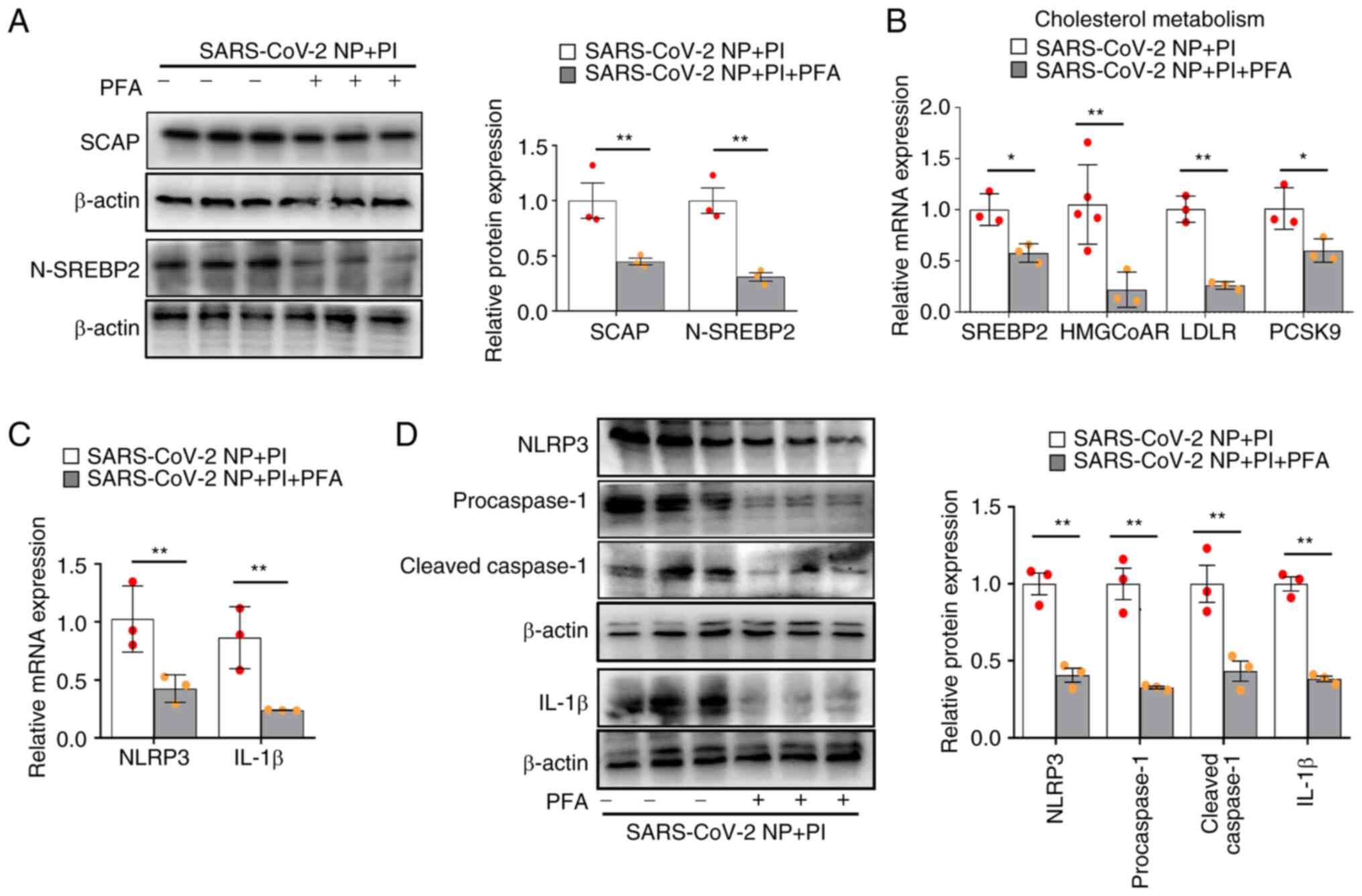 | Figure 2.PFA inhibits lipogenesis and NLRP3
inflammasome activation in VSMCs stimulated with PI and
overexpressing the SARS-CoV-2 N protein. The SARS-CoV-2 N
protein-overexpressing VSMCs were treated with PI and 1.0 mmol/l
PFA for 24 h. (A) Western blot analysis of SCAP and N-SREBP2. (B)
Quantification of SREBP2, HMGCoAR, LDLR and PCSK9 mRNA expression
levels. (C) Quantification of NLRP3 and IL-1β mRNA expression
levels. (D) Western blot analysis of NLRP3, procaspase-1, cleaved
caspase-1, and IL-1β expression (n≥3). *P˂0.05, **P˂0.01. PI,
inorganic phosphate; SARS-CoV-2, severe acute respiratory syndrome
coronavirus 2; SREBP, sterol regulatory element binding protein;
SCAP, SREBP cleavage-activating protein; N-SREBP2, mature
N-terminal SREBP2; NP, N protein; VSMCs, vascular smooth muscle
cells; HMGCoAR, HMG-CoA reductase; LDLR, low-density lipoprotein
receptor; PCSK9, proprotein convertase subtilisin/kexin type 9;
PFA, phosphonoformate sodium. |
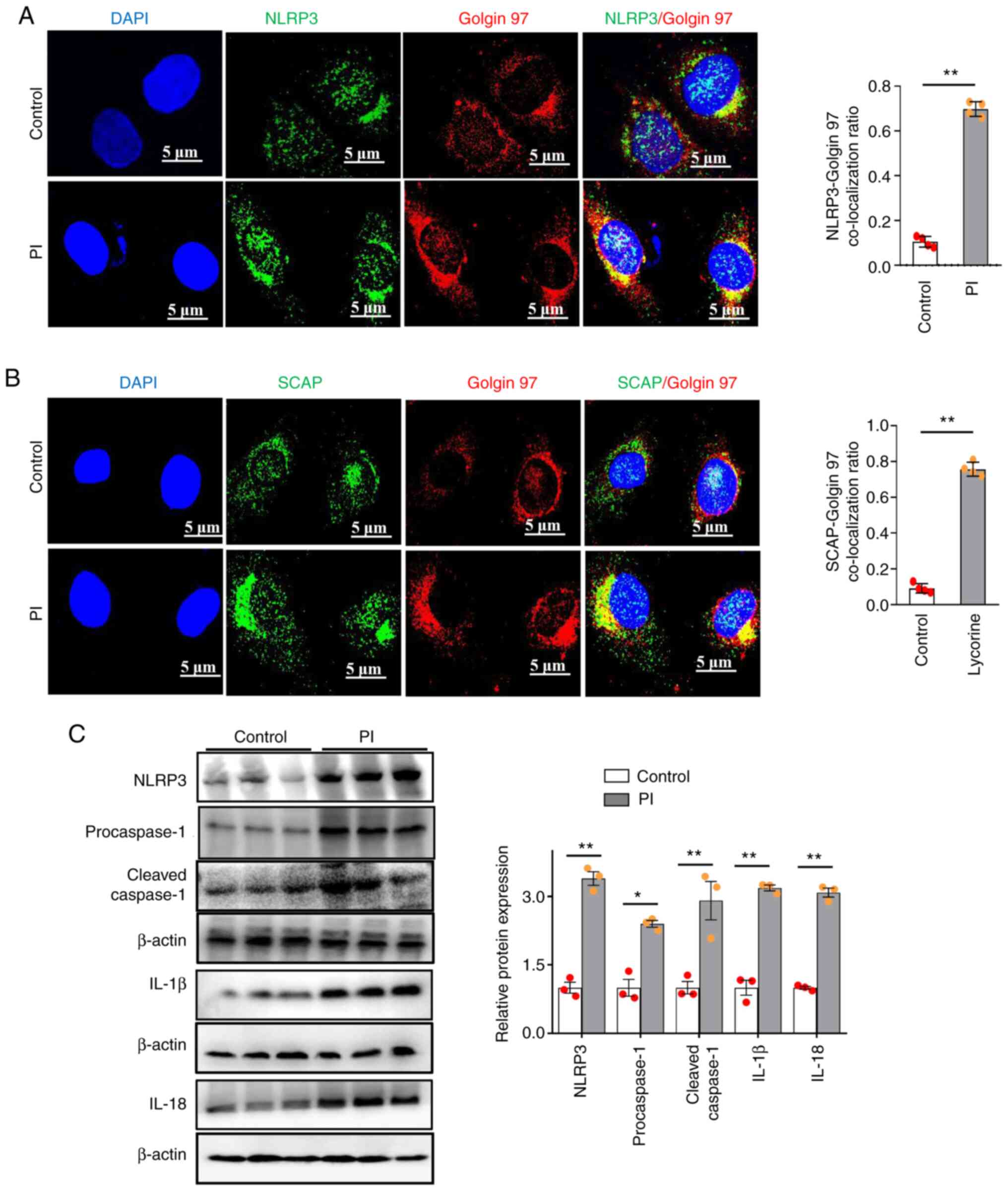
PI induces NLRP3 inflammasome
activation through increased SCAP and NLRP3 translocation to the
Golgi
It has previously been shown that SCAP-SREBP2 binds
with NLRP3 to form a ternary complex that promotes its
translocation to the Golgi, thereby facilitating NLRP3 inflammasome
activation (22). Consistent with
previous studies (14), we found
that NLRP3 (Fig. 3A) or SCAP
(Fig. 3B) proteins were separately
localized to Golgin 97 after PI stimulation, compared with that in
the non-phosphate-treated controls.
Furthermore, the relative protein expression levels
of NLRP3, procaspase-1, cleaved caspase-1, IL-1β and IL-18 were
significantly increased by PI compared with those in the
non-phosphate-treated control group (Fig. 3C). Therefore, these data
demonstrated that PI could induce NLRP3 inflammasome activation by
promoting SCAP and NLRP3 translocation to the Golgi.
PI promotes cholesterol metabolism and
fatty acid metabolism via the SCAP-SREBP pathway in VSMCs
It was demonstrated that the SCAP-SREBP inhibitor
lycorine decreased the protein expression levels of SCAP and
N-SREBP2 in VSMCs treated with PI (Fig. 4A). Compared with in the control
group, PI also increased SREBP2-mediated cholesterol biosynthesis
(i.e. SREBP2, HMGCoAR, LDLR and PCSK9 mRNA expression) (Fig. 4B) and SREBP 1c-mediated fatty acid
synthesis (i.e. SREBP1c, FASN, ACACA and SCD1 mRNA expression)
(Fig. 4C). Moreover, compared with
in the PI-treated VSMCs, lycorine significantly decreased
SEBP2-mediated cholesterol biosynthesis (i.e. SREBP2, HMGCoAR, LDLR
and PCSK9 mRNA expression) and SREBP 1c-mediated fatty acid
synthesis (i.e. SREBP1c, FASN, ACACA and SCD1 mRNA expression)
(Fig. 4B and C). These results
indicated that PI induced lipogenesis through SCAP-SREBPs.
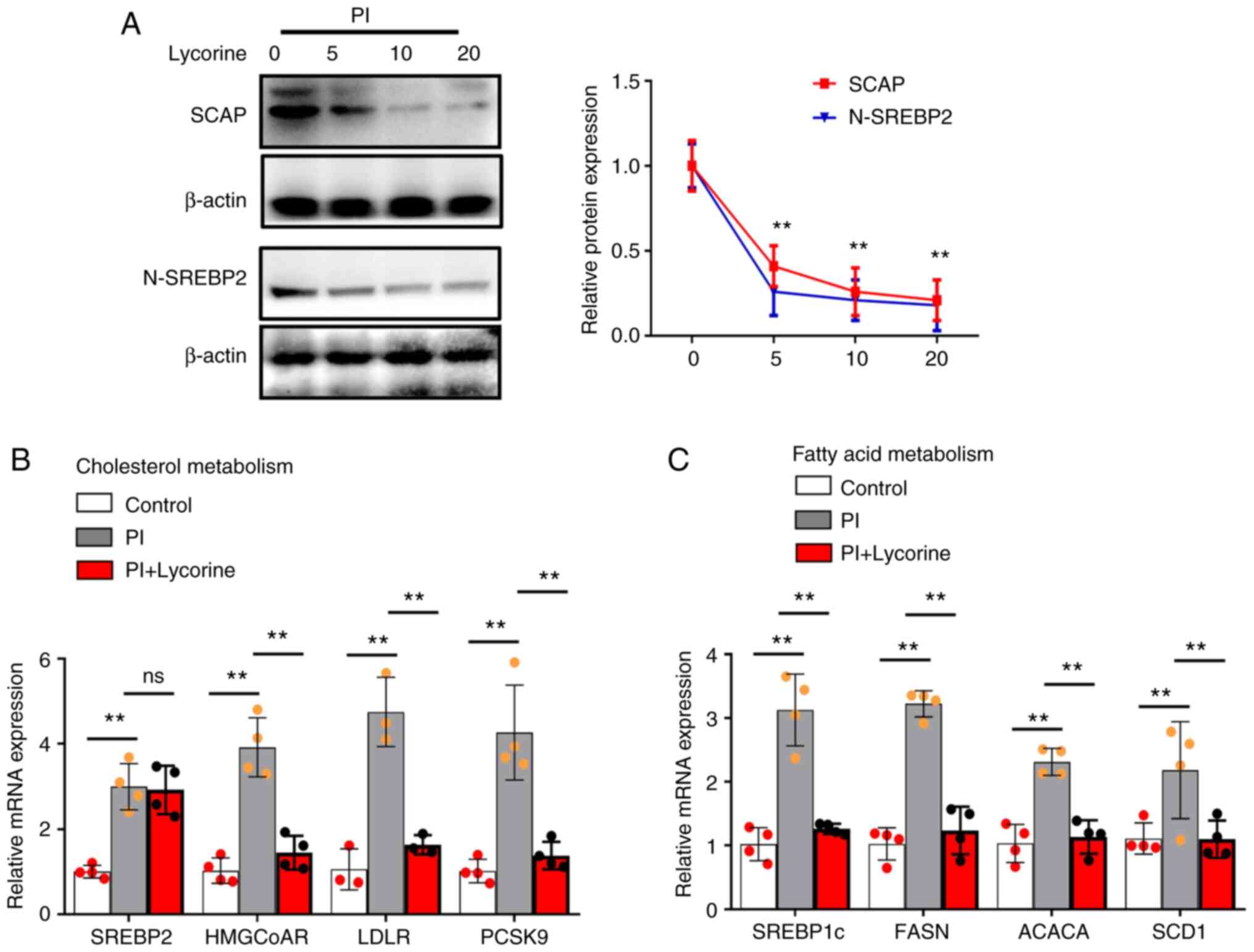 | Figure 4.PI induces cholesterol metabolism and
fatty acid metabolism via the SCAP-SREBP pathway in VSMCs. The
VSMCs were treated with 3 mmol/l PI for 24 h with or without
lycorine. (A) Western blot analysis of SCAP and N-SREBP2. (B)
Quantification of SREBP2, HMGCoAR, LDLR and PCSK9 mRNA expression
levels. (C) Quantification of SREBP1c, FASN, ACACA and SCD1 mRNA
expression levels (n≥3). **P˂0.01. PI, inorganic phosphate; SREBP,
sterol regulatory element binding protein; N-SREBP2, mature
N-terminal SREBP2; SCAP, SREBP cleavage-activating protein;
HMGCoAR, HMG-CoA reductase; LDLR, low-density lipoprotein receptor;
PCSK9, proprotein convertase subtilisin/kexin type 9; FASN,
recombinant fatty acid synthase; ACACA, acetyl-CoA carboxylase α;
SCD1, stearyl coenzyme A desaturase 1; VSMCs, vascular smooth
muscle cells; ns, not significant. |
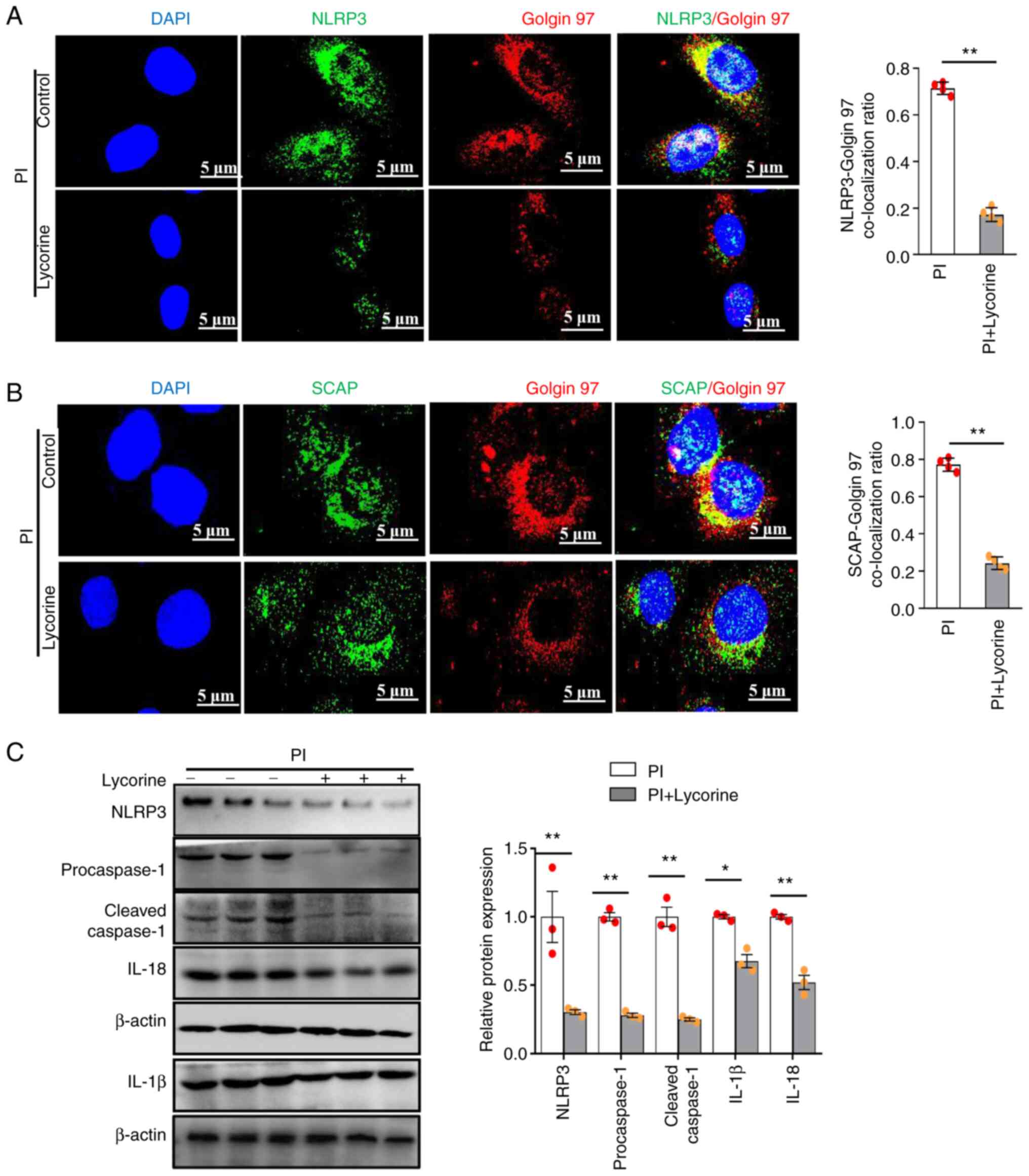
PI promotes NLRP3 inflammasome
activation via the SCAP-SREBP pathway in VSMCs
VSMCs were treated with lycorine to confirm whether
SCAP was involved in PI-induced NLRP3 inflammasome activation, it
was shown that in PI-treated VSMCs, lycorine significantly
decreased the NLRP3 (Fig. 5A) and
SCAP (Fig. 5B) Golgi
co-localization compared with the PI-only control.
Likewise, lycorine significantly decreased the
relative protein expression levels of NLRP3, procaspase-1, cleaved
caspase-1, IL-1β and IL-18 compared with those in the PI-only
control (Fig. 5C). Therefore, PI
was predicted to induce NLRP3 inflammasome activation by promoting
SCAP-SREBP ER-to-Golgi translocation.
Inhibitor of SCAP or knockdown of
SREBP2 suppresses PI and SARS-CoV-2 N protein-induced lipogenesis
synthesis and inflammasome formation in VSMCs
SCAP-SREBP2 has previously been identified as
serving a key role in inflammation and cholesterol metabolism
(22). The present results
demonstrated that lycorine significantly decreased SREBP2-mediated
cholesterol biosynthesis (i.e. SREBP2, HMGCoAR, LDLR and PCSK9 mRNA
expression) and SREBP1c-mediated fatty acid synthesis (i.e. FASN
and ACLY mRNA expression) (Fig. 6A and
B), compared with in PI-treated SARS-CoV-2 N-protein
overexpressing VSMCs. Furthermore, SREBP2 siRNA significantly
decreased the protein expression levels of SREBP2 in untreated
VSMCs compared with those in the control siRNA group (Fig. 6D). Likewise, it was demonstrated
that in PI-treated SARS-CoV-2 N-protein overexpressing VSMCs,
lycorine and SREBP2 siRNA markedly reduced NLRP3, procaspase-1,
cleaved caspase-1 and IL-1β expression compared with those in the
control groups (Fig. 6C and E).
These findings suggested that SCAP-SREBPs are necessary for
lipogenesis and NLRP3 inflammasome activation in PI and SARS-CoV-2
N protein-overexpressing VSMCs.
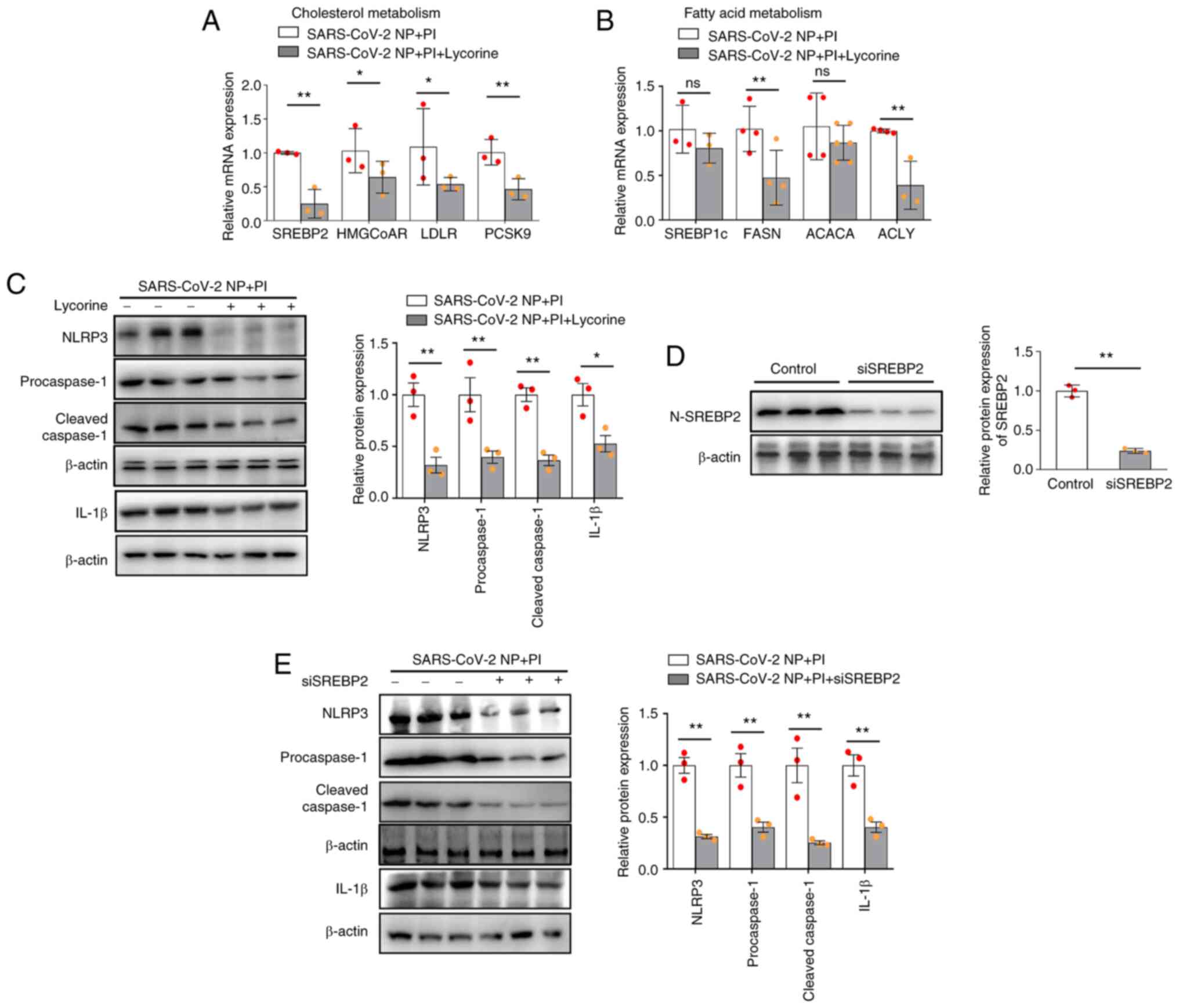 | Figure 6.Inhibition of SREBP
cleavage-activating protein or knockdown of SREBP2 alleviates
inflammasome formation in VSMCs stimulated with PI and SARS-CoV-2 N
protein. The SARS-CoV-2 N protein-overexpressing VSMCs were treated
with lycorine or transfected with siSREBP2. (A) Quantification of
SREBP2, HMGCoAR, LDLR and PCSK9 mRNA expression levels. (B)
Quantification of SREBP1c, FASN, ACACA and ACLY mRNA expression
levels. (C) NLRP3, procaspase-1, cleaved caspase-1 and IL-1β
protein expression levels in SARS-CoV-2 N protein-overexpressing
VSMCs treated with lycorine were examined via western blotting. (D)
Western blot analysis of N-SREBP2 protein expression in VSMCs
transfected with siSREBP2). (E) Western blot analysis of NLRP,
procaspase-1, cleaved caspase-1, and IL-1β level in SARS-CoV-2 N
protein-overexpressing VSMCs transfected with siSREBP2. *P˂0.05,
**P˂0.01. PI, inorganic phosphate; si, small interfering; OE,
overexpression; SARS-CoV-2, severe acute respiratory syndrome
coronavirus 2; NP, N protein; SREBP, sterol regulatory element
binding protein; N-SREBP2, mature N-terminal SREBP2; HMGCoAR,
HMG-CoA reductase; LDLR, low-density lipoprotein receptor; PCSK9,
proprotein convertase subtilisin/kexin type 9; FASN, recombinant
fatty acid synthase; SCD1, stearyl coenzyme A desaturase 1; ACACA,
acetyl-CoA carboxylase α; ACLY, ATP-citrate lyase; VSMCs, vascular
smooth muscle cells; ns, not significant. |
Discussion
The risk of mortality in patients hospitalized with
COVID-19 infection is strongly influenced by chronic kidney disease
(CKD) (23). COVID-19-related
mortality is ~10-times higher than that of patients with CKD and
without COVID-19 (24). These
studies indicated the incidence of COVID-19 in patients with CKD
was higher than that in the community (24,25),
and the risk of death from COVID-19 has been reported to be
increased by ~6-fold in patients with chronic kidney disease
(26). In addition, SARS-CoV-2
infection increases the risk of cardiovascular complications and
death in patients with acute kidney injury (27) and preexisting chronic kidney
disease (28). Therefore, COVID-19
may be linked to increased cardiovascular risk and mortality in
patients with CKD (29–31); however, few studies have examined
the nature or frequency of cardiovascular outcomes in patients with
COVID-19 and chronic kidney disease (27,32).
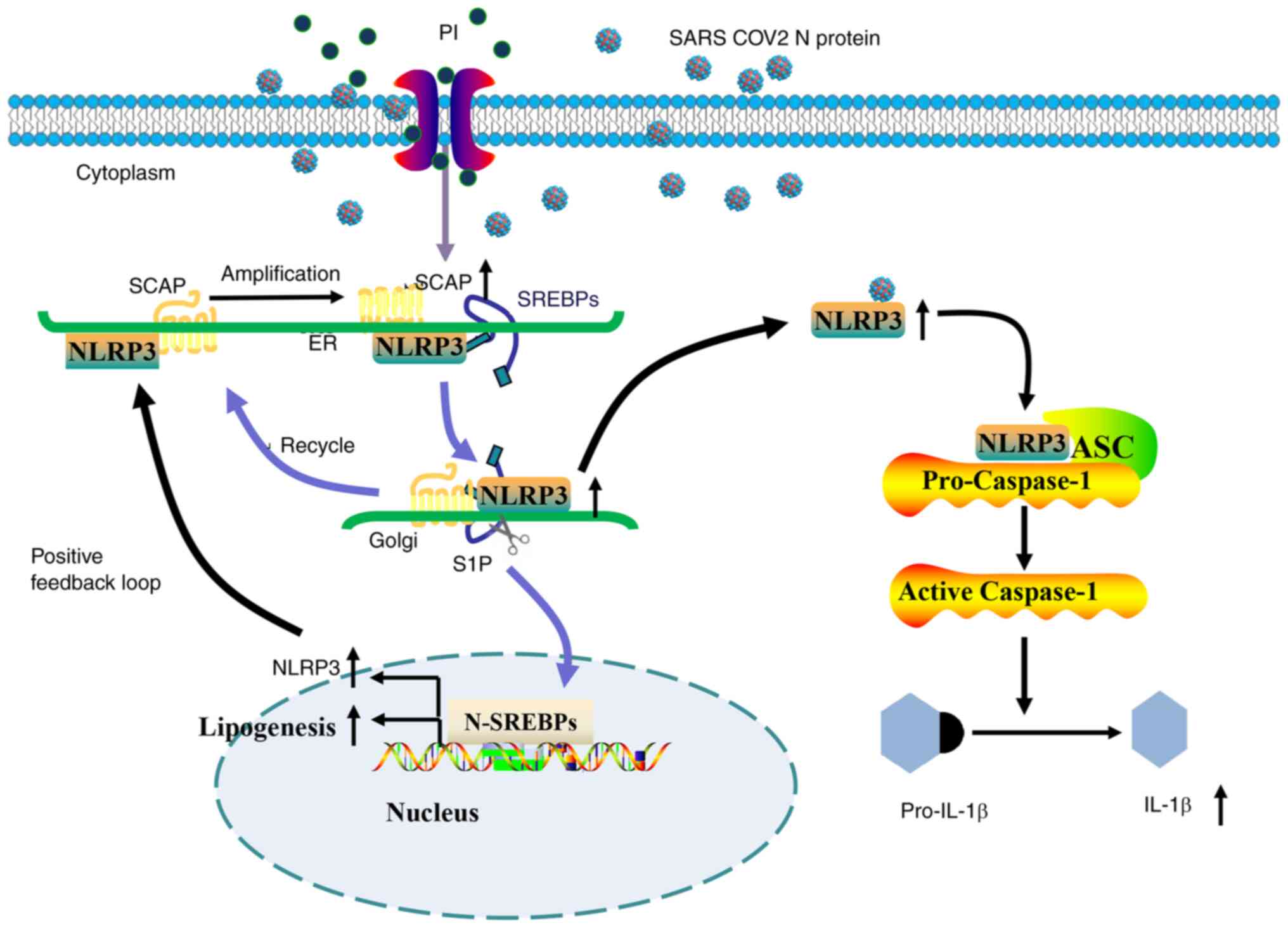
In our previous study, SARS-CoV-2 N protein promoted NLRP3
inflammasome activation by increased SREBP activation (20). However, the role of the SARS-CoV-2
N protein on NLRP3 inflammasome activation was not tested in this
study, The present study was the first to demonstrate that PI
amplified SARS-CoV-2 N protein-induced lipogenesis and NLRP3
inflammasome activation via the SCAP-SREBP signaling pathway
(Fig. 7), which suggested that PI
could potentially act alongside the SARS-CoV-2 N protein to enhance
the hyperinflammatory response. These results could provide novel
insights into the comorbidities in which PI may increase the risk
of mortality in patients with COVID-19 and chronic kidney disease,
and could provide new therapeutic targets for COVID-19-related
cardiovascular disease.
Hyperphosphatemia is highly prevalent and is
associated with increased mortality in patients with COVID-19
(33). Hyperphosphatemia
accelerates the occurrence and development of atherosclerosis
(12), which is associated with an
increased cardiovascular disease risk in the community (34). Until now, it has been widely
accepted that adequate control of hyperphosphatemia is important in
the clinical management of patients with CKD (35–37).
Treatment with the phosphate binders lanthanum carbonate and
sevelamer-HCl (35,36), or dietary phosphate restriction
(37), has been reported to
markedly ameliorate vascular calcification and atherosclerosis in
an apolipoprotein E-deficient mouse model. Notably, it has been
reported that specific inhibition of phosphate uptake reduces
SREBP2 mRNA levels and intracellular cholesterol accumulation
(13). Previous studies have
indicated that SCAP-SREBPs accelerate the accumulation of
cholesterol in cells by increasing intracellular cholesterol
synthesis (38). Moreover,
SREBP2-mediated cholesterol biosynthesis is a necessary step for
SARS-CoV-2 exocytosis and replication (39). SARS-CoV-2 also regulates host lipid
metabolism to facilitate viral replication (40). The present study demonstrated that
PI increased SCAP-SREBP and enhanced the expression of
lipogenic-associated mRNAs in SARS-CoV-2 N protein-overexpressing
VSMCs. The SCAP-SREBPs inhibitor AM580 has been shown to block
SREBP-mediated lipid biosynthesis and subsequent double-membrane
vesicle formation (41), thus
inhibiting SARS-CoV-2 replication (42). Therefore, PI could function
synergistically with the SARS-CoV-2 N protein to increase
intracellular cholesterol synthesis and SCAP-SREBPs could be used
as potential therapeutic targets to inhibit viral infection and
decrease the severity of COVID-19.
SCAP-SREBPs serve as a potential mediator of NLRP3
inflammasome activation in sterile inflammation and atherosclerosis
(43–45). Previous research (14,22)
has indicated that SCAP-SREBPs serve a role in NLRP3 inflammasome
activation. Thus, the present study aimed to evaluate the potential
effect of SCAP-SREBPs on SARS-CoV-2 N protein-induced NLRP3
inflammasome activation. It was demonstrated that PI in SARS-CoV-2
N protein-overexpressing VSMCs promoted SCAP or NLRP3 proteins
separately localizing to the Golgi, thus leading to robust
SCAP-SREBP-mediated NLRP3 inflammasome activation. SREBP2 has also
been reported to upregulate NLRP3 expression and to thus enhance
hemodynamic-induced endothelial inflammation and atherosclerosis
(46).
Consistent with a previous report (46), the present study demonstrated that
PI led to an increase in NLRP3 protein and mRNA expression levels,
thus potentially resulting in an inflammatory positive feedback
loop in NLRP3 inflammasome activation (20). Combined with our previous study, it
may be hypothesized that the SARS-CoV-2 N protein promotes NLRP3
inflammasome activation via the SCAP-SREBP signaling pathway
(20). Furthermore, the results
further verified that inhibition of SCAP with lycorine or
siRNA-induced silencing of SREBP2 almost completely abolished the
promoting effect of PI and SARS-CoV-2 N protein on the activation
of the NLRP3 inflammasome and the levels of IL-1β, further
supporting an amplified action of SCAP-SREBPs in SARS-CoV-2 N
protein-induced NLRP3 inflammasome activation. A recent report has
shown that COVID-19-activated SREBP2 disturbs cholesterol
biosynthesis and leads to cytokine storm (9). Moreover, the SARS-CoV2-N protein can
directly interact with the NLRP3 protein, promote the binding of
NLRP3 with ASC, and facilitate NLRP3 inflammasome assembly, thus
induces excessive inflammatory responses (22). Therefore, these results indicated
that PI may amplify SARS-CoV-2 N protein-induced NLRP3 inflammasome
activation via a SCAP-SREBP/NLRP3 positive feedback loop.
In summary, the present study provided new insight
into the effect of PI on SCAP-SREBP-mediated NLRP3 inflammasome
activation in SARS-CoV-2 N protein-induced cytokine storms.
Recently, it has been reported that the mortality of patients with
COVID-19 is linked to cytokine storm, which is triggered by the
overproduction of proinflammatory cytokines (47). Inhibition of the SREBP pathway
prevents SARS-CoV-2 replication (48) and cytokine storms (9). Therefore, SCAP-SREBPs may serve a
role in inducing lipogenesis and NLRP3 inflammasome activation in
COVID-19 (41) and targeting
SCAP-SREBPs may be a promising strategy for the treatment of
patients with COVID-19.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural
Foundation of China (grant no. 82360101), the Science and
Technology Plan Project of Jiangxi Provincial Health Commission
(grant no. 202210091), the Youth talent project of 2023
‘Technology+Medical’ Joint Plan Project/Ganzhou People's Hospital
Orientation Project (grant no. 2023NS127386), the Natural Science
Foundation Project of Jiangxi Province (grant no. 20224BAB206020),
the Science and Technology Plan of Jiangxi Provincial
Administration of Traditional Chinese Medicine (grant no. 2022A337)
and the Guiding Technology Plan of Quanzhou City (grant no.
GZ2022ZSF186/20222ZDX7577).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MHL collected and analyzed the data and wrote the
manuscript. MHL and LLX performed the experiments. XLL contributed
to the study design and data analyses and revised the manuscript.
MHL and LLX conducted the final verification and proofreading of
the article and confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tchidjou HK, Caron F, Ferec A, Braun K,
Hery L, Castelain S and Romeo B: Severe hyperphosphatasemia and
severe acute respiratory syndrome coronavirus 2 infection in
children. Blood Coagul Fibrinolysis. 31:575–577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin M, Valls J, Betriu A, Fernandez E
and Valdivielso JM: Association of serum phosphorus with
subclinical atherosclerosis in chronic kidney disease. Sex makes a
difference. Atherosclerosis. 241:264–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vervloet MG, Sezer S, Massy ZA, Johansson
L, Cozzolino M and Fouque D; ERA-EDTA Working Group on Chronic
Kidney Disease-Mineral and Bone Disorders and the European Renal
Nutrition Working Group, : The role of phosphate in kidney disease.
Nat Rev Nephrol. 13:27–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubio-Aliaga I and Krapf R: Phosphate
intake, hyperphosphatemia, and kidney function. Pflugers Arch.
474:935–947. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sabaghian T, Honarvar M, Safavi-Naini SAA,
Sadeghi Fadaki AS, Pourhoseingholi MA and Hatamabadi H: Effect of
electrolyte imbalance on mortality and late acute kidney injury in
hospitalized COVID-19 patients. Iran J Kidney Dis. 16:228–237.
2022.PubMed/NCBI
|
|
6
|
Rothan HA and Byrareddy SN: The
epidemiology and pathogenesis of coronavirus disease (COVID-19)
outbreak. J Autoimmun. 109:1024332020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu X, Chen D, Wu L, He G and Ye W:
Declined serum high density lipoprotein cholesterol is associated
with the severity of COVID-19 infection. Clin Chim Acta.
510:105–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei X, Zeng W, Su J, Wan H, Yu X, Cao X,
Tan W and Wang H: Hypolipidemia is associated with the severity of
COVID-19. J Clin Lipidol. 14:297–304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee W, Ahn JH, Park HH, Kim HN, Kim H, Yoo
Y, Shin H, Hong KS, Jang JG, Park CG, et al: COVID-19-activated
SREBP2 disturbs cholesterol biosynthesis and leads to cytokine
storm. Signal Transduct Target Ther. 5:1862020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim H, Lee HS, Ahn JH, Hong KS, Jang JG,
An J, Mun YH, Yoo SY, Choi YJ, Yun MY, et al: Lung-selective
25-hydroxycholesterol nanotherapeutics as a suppressor of
COVID-19-associated cytokine storm. Nano Today. 38:1011492021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okute Y, Shoji T, Shimomura N, Tsujimoto
Y, Nagata Y, Uedono H, Nakatani S, Morioka T, Mori K, Fukumoto S,
et al: Serum phosphate as an independent factor associated with
cholesterol metabolism in patients undergoing hemodialysis: A
cross-sectional analysis of the DREAM cohort. Nephrol Dial
Transplant. 38:1002–1008. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ellam T, Wilkie M, Chamberlain J, Crossman
D, Eastell R, Francis S and Chico TJ: Dietary phosphate modulates
atherogenesis and insulin resistance in apolipoprotein E knockout
mice-brief report. Arterioscler Thromb Vasc Biol. 31:1988–1990.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou C, He Q, Gan H, Zeng T, Liu Q,
Moorhead JF, Varghese Z, Ouyang N and Ruan XZ: Hyperphosphatemia in
chronic kidney disease exacerbates atherosclerosis via a
mannosidases-mediated complex-type conversion of SCAP N-glycans.
Kidney Int. 99:1342–1353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li D, Liu M, Li Z, Zheng G, Chen A, Zhao
L, Yang P, Wei L, Chen Y and Ruan XZ: Sterol-resistant SCAP
overexpression in vascular smooth muscle cells accelerates
atherosclerosis by increasing local vascular inflammation through
activation of the NLRP3 inflammasome in mice. Aging Dis.
12:747–763. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M,
Xiao F, Wang Z, Wang J, Jia Y, et al: SARS-CoV-2 N protein promotes
NLRP3 inflammasome activation to induce hyperinflammation. Nat
Commun. 12:46642021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ganji R, Paulo JA, Xi Y, Kline I, Zhu J,
Clemen CS, Weihl CC, Purdy JG, Gygi SP and Raman M: The p97-UBXD8
complex regulates ER-mitochondria contact sites by altering
membrane lipid saturation and composition. Nat Commun. 14:6382023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng H, Qin H, Liao M, Zheng E, Luo X,
Xiao A, Li Y, Chen L, Wei L, Zhao L, et al: CD36 promotes de novo
lipogenesis in hepatocytes through INSIG2-dependent SREBP1
processing. Mol Metab. 57:1014282022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong Y, Wu M, Wan X, Sun M, Zhang Y, Wu Z,
Li C, Liang X, Gao L, Ma C and Yue X: Lipophagy-mediated
cholesterol synthesis inhibition is required for the survival of
hepatocellular carcinoma under glutamine deprivation. Redox Biol.
63:1027322023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu MH, Lin XL and Xiao LL: SARS-CoV-2
nucleocapsid protein promotes TMAO-induced NLRP3 inflammasome
activation by SCAP-SREBP signaling pathway. Tissue Cell.
86:1022762023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jono S, McKee MD, Murry CE, Shioi A,
Nishizawa Y, Mori K, Morii H and Giachelli CM: Phosphate regulation
of vascular smooth muscle cell calcification. Circ Res. 87:E10–E17.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo C, Chi Z, Jiang D, Xu T, Yu W, Wang Z,
Chen S, Zhang L, Liu Q, Guo X, et al: Cholesterol homeostatic
regulator SCAP-SREBP2 integrates NLRP3 inflammasome activation and
cholesterol biosynthetic signaling in macrophages. Immunity.
49:842–856.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bepouka B, Mayasi N, Mandina M, Longokolo
M, Odio O, Mangala D, Mbula M, Kayembe JM and Situakibanza H: Risk
factors for mortality in COVID-19 patients in sub-Saharan Africa: A
systematic review and meta-analysis. PLoS One. 17:e02760082022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gibertoni D, Reno C, Rucci P, Fantini MP,
Buscaroli A, Mosconi G, Rigotti A, Giudicissi A, Mambelli E,
Righini M, et al: COVID-19 incidence and mortality in non-dialysis
chronic kidney disease patients. PLoS One. 16:e02545252021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung EYM, Palmer SC, Natale P, Krishnan
A, Cooper TE, Saglimbene VM, Ruospo M, Au E, Jayanti S, Liang A, et
al: Incidence and outcomes of COVID-19 in people with CKD: A
systematic review and meta-analysis. Am J Kidney Dis. 78:804–815.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai R, Zhang J, Zhu Y, Liu L, Liu Y and He
Q: Mortality in chronic kidney disease patients with COVID-19: A
systematic review and meta-analysis. Int Urol Nephrol.
53:1623–1629. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao A, Ranka S, Ayers C, Hendren N,
Rosenblatt A, Alger HM, Rutan C, Omar W, Khera R, Gupta K, et al:
Association of kidney disease with outcomes in COVID-19: Results
from the american heart association COVID-19 cardiovascular disease
registry. J Am Heart Assoc. 10:e0209102021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lambourg EJ, Gallacher PJ, Hunter RW,
Siddiqui M, Miller-Hodges E, Chalmers JD, Pugh D, Dhaun N and Bell
S: Cardiovascular outcomes in patients with chronic kidney disease
and COVID-19: A multi-regional data-linkage study. Eur Respir J.
60:21031682022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harrison SL, Buckley BJR, Rivera-Caravaca
JM, Zhang J and Lip GYH: Cardiovascular risk factors,
cardiovascular disease, and COVID-19: An umbrella review of
systematic reviews. Eur Heart J Qual Care Clin Outcomes. 7:330–339.
2021.PubMed/NCBI
|
|
30
|
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang
F, Gong W, Liu X, Liang J, Zhao Q, et al: Association of cardiac
injury with mortality in hospitalized patients with COVID-19 in
Wuhan, China. JAMA Cardiol. 5:802–810. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Linschoten M, Peters S, van Smeden M,
Jewbali LS, Schaap J, Siebelink HM, Smits PC, Tieleman RG, van der
Harst P, van Gilst WH, et al: Cardiac complications in patients
hospitalised with COVID-19. Eur Heart J Acute Cardiovasc Care.
9:817–823. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Podestà MA, Valli F, Galassi A, Cassia MA,
Ciceri P, Barbieri L, Carugo S and Cozzolino M: COVID-19 in chronic
kidney disease: The impact of old and novel cardiovascular risk
factors. Blood Purif. 50:740–749. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malinowska J, Malecka-Gieldowska M,
Bankowska D, Borecka K and Ciepiela O: Hypermagnesemia and
hyperphosphatemia are highly prevalent in patients with COVID-19
and increase the risk of death. Int J Infect Dis. 122:543–549.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dhingra R, Sullivan LM, Fox CS, Wang TJ,
D'Agostino RB Sr, Gaziano JM and Vasan RS: Relations of serum
phosphorus and calcium levels to the incidence of cardiovascular
disease in the community. Arch Intern Med. 167:879–885. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phan O, Ivanovski O, Nguyen-Khoa T, Mothu
N, Angulo J, Westenfeld R, Ketteler M, Meert N, Maizel J, Nikolov
IG, et al: Sevelamer prevents uremia-enhanced atherosclerosis
progression in apolipoprotein E-deficient mice. Circulation.
112:2875–2882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nikolov IG, Joki N, Nguyen-Khoa T,
Guerrera IC, Maizel J, Benchitrit J, Machado dos Reis L, Edelman A,
Lacour B, Jorgetti V, et al: Lanthanum carbonate, like
sevelamer-HCl, retards the progression of vascular calcification
and atherosclerosis in uremic apolipoprotein E-deficient mice.
Nephrol Dial Transplant. 27:505–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanaka S, Yamamoto H, Nakahashi O, Kagawa
T, Ishiguro M, Masuda M, Kozai M, Ikeda S, Taketani Y and Takeda E:
Dietary phosphate restriction induces hepatic lipid accumulation
through dysregulation of cholesterol metabolism in mice. Nutr Res.
33:586–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JH, Lee SH, Lee EH, Cho JY, Song DK,
Lee YJ, Kwon TK, Oh BC, Cho KW, Osborne TF, et al: SCAP deficiency
facilitates obesity and insulin resistance through shifting adipose
tissue macrophage polarization. J Adv Res. 45:1–13. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abu-Farha M, Thanaraj TA, Qaddoumi MG,
Hashem A, Abubaker J and Al-Mulla F: The role of lipid metabolism
in COVID-19 virus infection and as a drug target. Int J Mol Sci.
21:35442020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Al Heialy S, Hachim MY, Senok A, Gaudet M,
Abou Tayoun A, Hamoudi R, Alsheikh-Ali A and Hamid Q: Regulation of
angiotensin-converting enzyme 2 in obesity: Implications for
COVID-19. Front Physiol. 11:5550392020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan S, Chu H, Chan JF, Ye ZW, Wen L, Yan
B, Lai PM, Tee KM, Huang J, Chen D, et al: SREBP-dependent
lipidomic reprogramming as a broad-spectrum antiviral target. Nat
Commun. 10:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan S, Chan CC, Chik KK, Tsang JO, Liang
R, Cao J, Tang K, Cai JP, Ye ZW, Yin F, et al: Broad-spectrum
host-based antivirals targeting the interferon and lipogenesis
pathways as potential treatment options for the pandemic
coronavirus disease 2019 (COVID-19). Viruses. 12:6282020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Orr AW, Hastings NE, Blackman BR and
Wamhoff BR: Complex regulation and function of the inflammatory
smooth muscle cell phenotype in atherosclerosis. J Vasc Res.
47:168–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abbate A, Toldo S, Marchetti C, Kron J,
Van Tassell BW and Dinarello CA: Interleukin-1 and the inflammasome
as therapeutic targets in cardiovascular disease. Circ Res.
126:1260–1280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Burger F, Baptista D, Roth A, da Silva RF,
Montecucco F, Mach F, Brandt KJ and Miteva K: NLRP3 inflammasome
activation controls vascular smooth muscle cells phenotypic switch
in atherosclerosis. Int J Mol Sci. 23:3402021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang
WC, Marin T, Shentu TP, Wen L, Gongol B, et al: Sterol regulatory
element binding protein 2 activation of NLRP3 inflammasome in
endothelium mediates hemodynamic-induced atherosclerosis
susceptibility. Circulation. 128:632–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coperchini F, Chiovato L, Croce L, Magri F
and Rotondi M: The cytokine storm in COVID-19: An overview of the
involvement of the chemokine/chemokine-receptor system. Cytokine
Growth Factor Rev. 53:25–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Soares VC, Dias SG, Santos JC,
Azevedo-Quintanilha IG, Moreira IBG, Sacramento CQ,
Fintelman-Rodrigues N, Temerozo JR, da Silva MAN, Barreto-Vieira
DF, et al: Inhibition of the SREBP pathway prevents SARS-CoV-2
replication and inflammasome activation. Life Sci Alliance.
6:e2023020492023. View Article : Google Scholar : PubMed/NCBI
|