Introduction
Acute lung injury (ALI) is an acute inflammation
characterized by pulmonary hemorrhage, increased vascular
permeability and inflammatory cell infiltration (1). In severe cases, it may even lead to
acute respiratory failure and death (2). According to reports, the incidence of
ALI (~200,000 cases per year in the United States) and the overall
mortality rate remain high, with ALI also being a prevalent cause
of morbidity and mortality in critically ill patients (2,3).
Although the understanding of the pathogenesis of ALI has improved,
there is still no effective drug therapy to reduce the mortality
rates of patients with ALI (4).
Bacterial lipopolysaccharide (LPS) infection is one of the most
common causes of ALI (5), by
triggering inflammatory signal transduction, resulting in excessive
production of cytokines such as IL-1β and TNF-α, creating an
inflammatory storm (6). It has
been demonstrated that inhibiting the excessive inflammatory
cascade reaction of ALI is an effective strategy to reduce lung
injury (7). Therefore, controlling
the inflammatory response is a key measure for the prevention and
treatment of ALI.
Macrophages serve as the first line of defense
against the lung invasion of pathogens and are critical in
initiating and maintaining inflammatory responses, reducing
inflammation and restoring lung function (8). Previous studies have demonstrated
that glycolysis in macrophages increased during lung inflammation
and that inhibiting glycolysis could alleviate lung inflammation
(9,10). For instance, the glycolysis
inhibitor, 2-deoxy-D-glucose (2-DG) reduces triggering receptor
expressed on myeloid cells 1-triggered activation of the NLR family
pyrin domain containing 3 (NLRP3) inflammasome in lung macrophages
(11).
As the first rate-limiting enzyme of glycolysis,
hexokinase (HK) catalyzes the conversion of glucose into
glucose-6-phosphate (G-6P). There are four subtypes of HK in
mammals, namely HK1-4, and the distribution of the four HKs in the
mammalian body is also different, with tissue and cell specificity
(12). The HK subtypes distributed
in the lungs are dominated by HK1 and HK2, with HK1 tending to be
expressed in healthy tissues, whereas HK2 is more effective than
the other subtypes in promoting aerobic glycolysis (13,14),
supporting the involvement of HK2 in the regulation of pulmonary
disorders. Therefore, HK2 may be a more promising target. Previous
studies have suggested that HK2 is less extensively distributed and
expressed in healthy tissues but is specifically highly expressed
in various inflammation-related diseases and models, suggesting the
involvement of HK2 in regulating immune responses (12,15).
Therefore, it was hypothesized that selective inhibition of HK2 can
regulate the release of inflammatory factors in lung macrophages
with less impact on system stability and body metabolism. In
conclusion, reducing the inflammatory response by inhibiting HK2
activity may be a potential strategy for the treatment of ALI.
However, to the best of our knowledge, highly selective HK2
inhibitors have not been reported for the treatment of ALI.
The existing commonly used HK2 inhibitors have high
rates of adverse reactions due to their low specificity (16). Currently, the most commonly used
HK2 inhibitors in preclinical studies are lonidamine, 2-DG and
3-bromopyruvate, all of which are antitumor drugs that stem tumor
progression by suppressing glycolysis (17,18).
There is a high degree of homology between HK1 and HK2, and given
the wide distribution and important functions of HK1, these HK2
inhibitors are likely to inhibit HK1 activity while inhibiting HK2
activity (19,20).
To explore novel therapeutic strategies, the present
study attempted to reduce HK2 protein levels by chaperone-mediated
autophagy (CMA) (21). CMA is a
critical pathway in lysosomes for the specific degradation of
unnecessary proteins, particularly serving a pivotal role in
clearing misfolded proteins (22).
Therefore, using the lysosomal system to target the degradation of
pathogenic proteins may be an effective strategy to address
diseases. CMA-targeted chimeras are a class of molecules designed
using the CMA system, usually consisting of a cell-penetrating
peptide (TAT, transactivator of transcription protein of HIV-1),
target protein-binding domain (PBD) and CMA-targeting motif (CTM)
(23). Therefore, a targeting
peptide [TAT-ataxin 1 (ATXN1)-CTM] was synthesized based on the
knowledge that ATXN1 protein can bind HK2 protein. Mechanistically,
the peptide can interact with the endogenous HK2 protein and
effectively reduce HK2 protein levels through lysosomal degradation
(24).
In the present study, the targeting peptide
TAT-ATXN1-CTM was used to degrade HK2 in an LPS-induced THP-1 cell
model and murine ALI model. The results indicated that
TAT-ATXN1-CTM may serve an anti-inflammatory role by degrading the
HK2 protein. The present study provides a basis for novel treatment
strategies for ALI via selective inhibition of glycolysis.
Materials and methods
Experimental animals
Adult male Institute of Cancer Research mice (n=72
animals, 6–8 weeks old, 25±2 g) were purchased from Shanghai Slack
Experimental Animal Co., Ltd. [certificate no. SCXK (Hu)
2022–0004]. Mice were housed for 3 days to allow them to acclimate
to the environment prior to the experiment. Mice were bred in
specific pathogen-free conditions of 23±3°C with 55±15% humidity
and a 12 h light/dark cycle, and were allowed free access to
sterilized food and water were supplied. All handling procedures
used in the present study were approved by the Animal Care and Use
Committees at the Zhejiang University School of Medicine (Approved
No. ZJU20220294, Hangzhou, China) and were conducted in accordance
with the policies of institutional guidelines on the care and use
of laboratory animals. Euthanasia was performed with CO2
at a volume displacement rate of 30% vol/min.
Murine model of ALI and treatment
Animals were divided into six groups in a randomized
manner with 12 mice in each group. The groups were as follows: i)
Control group; ii) LPS group; iii) LPS + TAT-CTM (200 µg/kg) group;
iv) LPS + TAT-ATXN1-CTM (50 µg/kg) group; v) LPS + TAT-ATXN1-CTM
(200 µg/kg) group; and vi) LPS + TAT-ATXN1-CTM (1,000 µg/kg) group.
The mice were anesthetized by nasal inhalation of 4% isoflurane and
the depth of anesthesia was maintained with 1.5% isoflurane. Mice
in the control group were intratracheally injected with 0.9% sodium
chloride injection (2 mg/kg). The murine ALI model was established
by intratracheal injection of LPS (2 mg/kg; cat. no. L3129;
MilliporeSigma). Treatment groups received LPS (2 mg/kg) and
TAT-CTM (200 µg/kg) or TAT-ATXN1-CTM (50, 200, 1,000 µg/kg)
administered simultaneously. Mice were euthanized once they reached
humane endpoints or the study endpoint. The humane endpoints
included signs of loose fur, inactivity or reduced activity,
hunched posture, and respiratory distress, and these were not
observed in any of the animals. The study endpoint was 8 h after
LPS administration. The bronchoalveolar lavage fluid (BALF) was
prepared to measure the accumulation of inflammatory cells, lung
tissue was harvested for histopathological examination and the
degree of pathological injury was scored.
Collection of BALF and cell
counting
BALF preparation was described previously (25). Briefly, mice were sacrificed, and
the trachea of each mouse was surgically exposed and cannulated.
The left lung and accessory lobe were ligated to collect the BALF
of the right lung. The right lung was lavaged twice with a single
volume of warmed 0.5 ml of PBS containing 1% bovine serum albumin
(cat. no. A8020; Beijing Solarbio) and 5,000 IU/l heparin. White
blood cells were counted under a light microscope. The collected
BALF was centrifuged at 400 × g, 4°C for 10 min. The pelleted cells
were coated onto microscope slides after drying at room
temperature. Wright-Giemsa staining was performed, and the numbers
of neutrophils, macrophages and lymphocytes were counted as
cells/slide under a light microscope. Briefly, the slides were
stained in Wright's stain (cat. no. W100940; Shanghai Aladdin
Biochemical Technology Co., Ltd.) for 4 min and then in Giemsa's
stain (cat. no. G100959; Shanghai Aladdin Biochemical Technology
Co., Ltd.) for 2 min at 25°C. The cells were observed and counted
under the light microscope. Stained sections were scanned using the
software Multiscan (2.0.0.150) of a digital section scanner
(Convergence Technology Co., Ltd.) to obtain screenshots and
analyze the processing. Neutrophils have a dark blue multi-lobed
nucleus and pale pink cytoplasm; lymphocytes have purple nucleus
with sky blue cytoplasm; Macrophages have purple nucleus with blue
cytoplasm as lymphocytes but larger than other leukocytes.
Histological examination of the
lung
The left middle lobe of the lung was fixed in 10%
neutral formalin overnight at room temperature and embedded in
paraffin, and paraffin sections (5 µm) were prepared. The sections
were stained with hematoxylin staining for 3 min and eosin staining
for 30 sec at room temperature, and the lung injury and
inflammatory cell infiltration were observed under a light
microscope (26).
Inflammation score
The extent of histological lung damage was
quantified by a designated scoring system, which is made up of five
grades: 0 points, normal appearing lung; 1 point, mild inflammatory
cell infiltration, no tissue injury; 2 points, mild to moderate
inflammatory cell infiltration, mild tissue injury; 3 points,
moderate inflammatory cell infiltration, mild tissue injury; 4
points, moderate to severe inflammatory cell infiltration with
obvious tissue injury; and 5 points, severe inflammatory cell
infiltration with significant tissue injury and changes. Slides
were assessed by a pathologist blinded to the experimental
design.
Cell culture
THP-1 and 293T cell lines were obtained from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. The THP-1 human macrophages were cultured in RPMI-1640
growth medium (cat. no. CGM112.05; CellMax Technologies AB)
containing 10% Premium FBS (cat. no. SA101.02; CellMax Technologies
AB), 0.05 mM β-mercaptoethanol (cat. no. M6250; Shanghai Macklin
Biochemical Co., Ltd.) and 1% penicillin-streptomycin. The 293T
cells were cultured in DMEM (cat. no. CGM101.05; CellMax
Technologies AB) with 10% FBS (cat. no. 04-001-1ACS; Biological
Industries) and 1% penicillin-streptomycin. All cells were cultured
in a CO2 incubator at 37°C with 5% CO2. THP-1
cells (5×105 cells/ml) were treated with 100 ng/ml
phorbol-12-myristate acetate (PMA; cat. no. HY-18739;
MedChemExpress) for at 37°C 24 h to induce cell attachment. The
cells were further left to rest at 37°C for 24 h in complete RPMI
1640 medium before stimulation with LPS (1 µg/ml; cat. no. L3129;
MilliporeSigma) and 10 µM TAT-CTM or TAT-ATXN1-CTM treatments at
37°C for 4, 8 and 24 h.
Cell transfection
The human HK2 plasmid containing the HK2 sequences
and the pcDNA3.1 vector with three Flag tags were synthesized by
Shandong Weizhen Biotechnology Co., Ltd., and empty vector was used
as a control. The 293T cells were plated on a 6-well plate to ~80%
confluency 1 day before transfection, and then transfected with the
plasmid. For transfection, 2 µg plasmid was diluted in 100 µl basal
medium (DMEM) before adding to a 6-well plate. Polyethylenimine
(PEI; cat. no. 23966; Polysciences, Inc.) at a 1:3 (w/w) cDNA:PEI
ratio was added to the diluted plasmid solution and this was gently
mixed. The plasmid-PEI mixture was placed in a CO2
incubator for 20 min and then added to the cells, which were
cultured in an incubator with 5% CO2 at 37°C. After 6 h,
the solution in the 6-well plate was replaced with fresh complete
medium, and 10 µM TAT-CTM or TAT-ATXN1-CTM (GenScript) for testing
was added. Cells were harvested 48 h after transfection for
subsequent experiments.
Cell viability assay
For MTT assays, 5×103 THP-1 cells were
seeded into 96-well plates with complete RPMI 1640 medium and
treated with 100 ng/ml PMA at 37°C for 24 h. The cells were further
left to rest at 37°C for 24 h in complete RPMI 1640 medium only
before treatment with 0, 5, 10 and 20 µM of TAT-ATXN1-CTM or
TAT-CTM in RPMI 1640 medium in a total volume of 100 µl at 37°C for
24 h. Subsequently, 10 µl MTT/PBS (5 mg/ml; cat. no. M8180; Beijing
Solarbio Science & Technology Co., Ltd.) was added to the
medium and cells were incubated in a CO2 incubator at
37°C for 4 h. Subsequently, 100 µl dimethyl sulfoxide was added and
the 96-well plates were shaken for 10 min at room temperature.
Finally, optical density of the samples was measured at 490 nm
using a multipurpose microplate reader (SpectraMax iD3; Molecular
Devices, LLC).
Design, synthesis and testing of
targeting peptide
It has been reported that the amino acid sequence
594–610 of ATXN1 interacts with the HK2 protein (24). To increase the membrane
permeability of the peptide, a transmembrane domain (TAT) was added
to the ATXN1 (594–610) sequence. A CTM sequence was added to
degrade the endogenous targeted protein for lysosomal degradation
(Table I).
 | Table I.Amino acid sequences of ATXN1
(594–610), TAT, CTM, TAT-CTM and TAT-ATXN1-CTM. |
Table I.
Amino acid sequences of ATXN1
(594–610), TAT, CTM, TAT-CTM and TAT-ATXN1-CTM.
| Peptide | Amino acid
sequence |
|---|
| ATXN1
(594–610) |
KTEDFIQSAEISNDLKI |
| TAT | YGRKKRRQRR |
| CTM | KFERQKILDQRFFE |
| TAT-CTM |
YGRKKRRQRRKFERQKILDQRFFE |
| TAT-ATXN1-CTM |
YGRKKRRQRRKTEDFIQSAEISNDL |
|
|
KIKFERQKILDQRFFE |
TAT-ATXN1-CTM and TAT-CTM peptides, synthesized by
GenScript, were solid-phase synthesized as C-terminal amides and
purified using high-performance liquid chromatography (HPLC) using
a preparative column, and the final purity was ascertained as 98.1%
for TAT-ATXN1-CTM and 98.9% for TAT-CTM using analytical HPLC peak
area integration (Figs. S1 and
S2). Briefly, The TAT-ATXN1-CTM
or TAT-CTM crude product was purified using an HPLC system
(LC-20AB, Shimadzu) with the reversed-phase column (Inertsil ODS-3
4.6×250 mm, Shimadzu) at 25 °C. The samples were loaded onto the
column (12–15 µl loading volume). The flow rate was set to be 1
ml/min using 0.065% trifluoroacetic in 100% water (v/v) and 0.05%
trifluoroacetic in 100% acetonitrile (v/v) as the mobile phase
compositions of pump A and pump B, respectively. The wavelength of
the detector was set to 220 nm. The purified TAT-ATXN1-CTM or
TAT-CTM fractions were collected. The molecular masses of the
peptides were determined by electrospray ionization mass
spectrometry (ESI-MS, LCMS-2020, Shimadzu). MS parameters for the
peptides were: nitrogen gas temperature, 350°C; gas flow, 5 l/min;
nebulizer pressure, 15 psi; scan time, 500 msec. The observed mass
spectral values for TAT-ATXN1-CTM and TAT-CTM were 5,359.2 and
3,270.0, respectively, which was consistent with the theoretical
values (Figs. S3 and S4).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (cat. no. CW0580S; CoWin Biosciences)
according to the manufacturer's instructions. Total RNA was
quantified using a micro-spectrophotometer (NAno-300; cat. no.
MO00040009; Hangzhou Allsheng Instruments Co., Ltd.), and the
optical density 260/280 nm ratio of all samples was 1.8–2.0. Next,
cDNA was synthesized on a PCR instrument (SimpliAmp™; cat. no.
A24811; Thermo Fisher Scientific, Inc.) using the RNA reverse
transcription kit (cat. no. CW2569M; CoWin Biosciences). RT was
performed as follows: 42°C for 15 min, incubate at 85°C for 5 min,
and keep warm at 4°C. Primers and a fluorescent quantitative PCR
kit (cat. no. CW2601H; CoWin Biosciences) containing SYBR Green I
fluorescent dye were used to perform fluorescent qPCR on a PCR
system (cat. no. 4351106; Thermo Fisher Scientific, Inc.). Primer
sequences for human HK2, human GAPDH, mouse HK2 and mouse β-actin
(Sangon Biotech Co., Ltd.) are listed in Table II. The thermocycling conditions
were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 60 sec. The 2−ΔΔCq method was used
for analysis of results and gene levels were normalized to the
internal reference gene, GAPDH for human HK2 or β-actin for mouse
HK2 (27).
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| Human HK2 | Forward |
TTGACCAGGAGATTGACATGGG |
|
| Reverse |
CAACCGCATCAGGACCTCA |
| Human GAPDH | Forward |
GGAGCGAGATCCCTCCAAAAT |
|
| Reverse |
GGCTGTTGTCATACTTCTCATGG |
| Mouse HK2 | Forward |
GTGTGCTCCGAGTAAGGGTG |
|
| Reverse |
CAGGCATTCGGCAATGTGG |
| Mouse β-actin | Forward |
GGCTGTATTCCCCTCCATCG |
|
| Reverse |
CCAGTTGGTAACAATGCCATGT |
Western blotting
The cells were washed with pre-cooled 1X PBS (cat.
no. P1020; Beijing Solarbio Science & Technology Co., Ltd.) and
lysed with lysis buffer containing RIPA (cat. no. P0013B; Beyotime
Institute of Biotechnology) and PMSF (cat. no. P0100; Beijing
Solarbio Science & Technology Co., Ltd.) at a ratio of 1:100 of
PMSF:RIPA. The cell lysate was centrifuged at 13,800 × g for 5 min
at 4°C to obtain the supernatant. The protein content of the
supernatant was determined using a BCA Protein Assay Kit (cat. no.
BL521A; Biosharp Life Sciences) and then protein (20 µg/lane) were
loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis before being electrophoretically transferred onto
PVDF membranes (cat. no. 1214429; GVS). The membrane was blocked
with ready-to-use blocking solution (cat. no. P0252; Beyotime
Institute of Biotechnology) for 1 h at room temperature and then
incubated with the indicated primary antibodies overnight at 4°C.
The primary antibodies were as follows: Anti-β-actin (1:1,000; cat.
no. 3700; Cell Signaling Technology, Inc.), anti-HK1 (1:1,000; cat.
no. 2024S; Cell Signaling Technology, Inc.) and anti-HK2 (1:1,000;
cat. no. ab209874; Abcam). Subsequently, the following secondary
antibodies were added for 1 h at room temperature: Goat anti-rabbit
IgG HRP-conjugated (1:12,000; cat. no. 31460; Thermo Fisher
Scientific, Inc.) and goat anti-mouse IgG HRP-conjugated (1:12,000;
cat. no. 31430; Thermo Fisher Scientific, Inc.). Detection of
immunoreactive bands was performed using ECL Western Blotting
Detection kit (cat. no. SW2040; Beijing Solarbio Science &
Technology Co., Ltd.) on a chemiluminescence gel imaging system
(Peiqing JS-1070P; Shanghai Peiqing Science & Technology Co.,
Ltd.) and data were semi-quantified using ImageJ2× 2.1.4.7 (Rawak
Software Development) with β-actin as the loading control.
ELISA
The lung tissues from mice and supernatants from
THP-1 cells were collected. For lung tissues, the protein
concentration was assayed using a BCA Protein Assay Kit (cat. no.
BL521A; Biosharp Life Sciences). Levels of TNF-α, IL-1β and HK2
were analyzed using commercial ELISA kits according to the
manufacturer's instructions. Human TNF-α ELISA Kit (cat. no.
CHE0019; Beijing 4A Biotech Co., Ltd.), Human IL-1β ELISA Kit (cat.
no. CHE0001; Beijing 4A Biotech Co., Ltd.), Mouse TNF-α ELISA Kit
(cat. no. CME0004; Beijing 4A Biotech Co., Ltd.), Mouse IL-1β (cat.
no. CME0015; Beijing 4A Biotech Co., Ltd.) and Mouse HK2 ELISA kit
(cat. no. ml058727; Mlbio) were used. The absorbance of each well
was measured at 450 nm with a multifunctional microplate reader
(SpectraMax iD3; Molecular Devices, LLC), and the concentration of
TNF-α, IL-1β and HK2 was calculated according to the standard
curve.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 7.0 (Dotmatics). All quantitative results are presented as
the mean ± standard error of the mean (SEM) from at least three
independent experiments. Statistical analysis was performed using
one-way ANOVA followed by Dunnett's post hoc test, two-way ANOVA
followed by Sidak's post hoc test, Kruskal-Wallis test followed by
Dunn's post hoc test or an unpaired two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
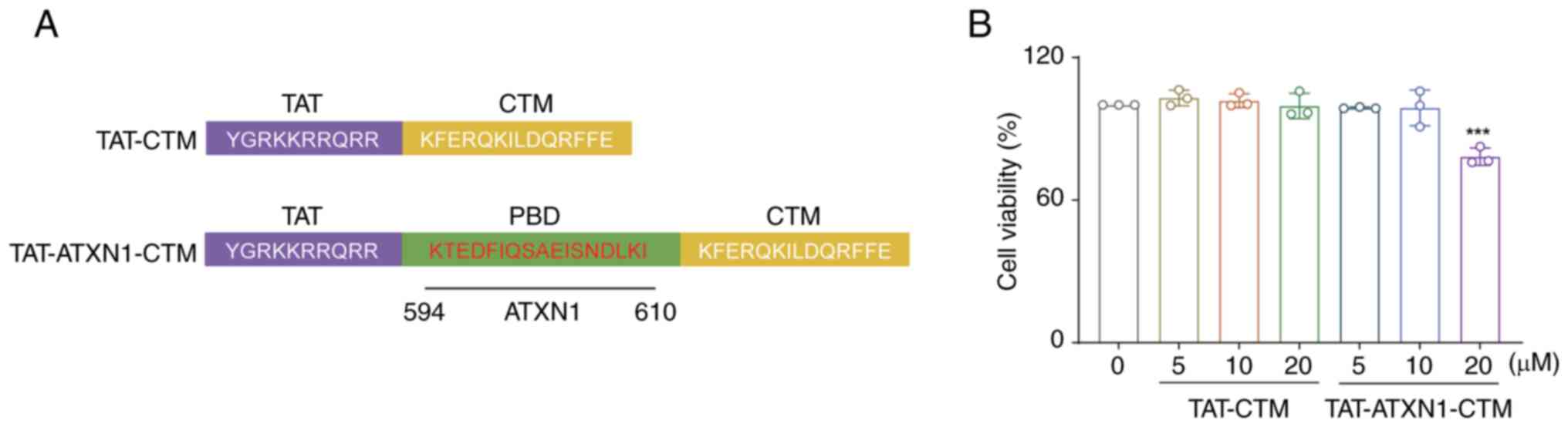
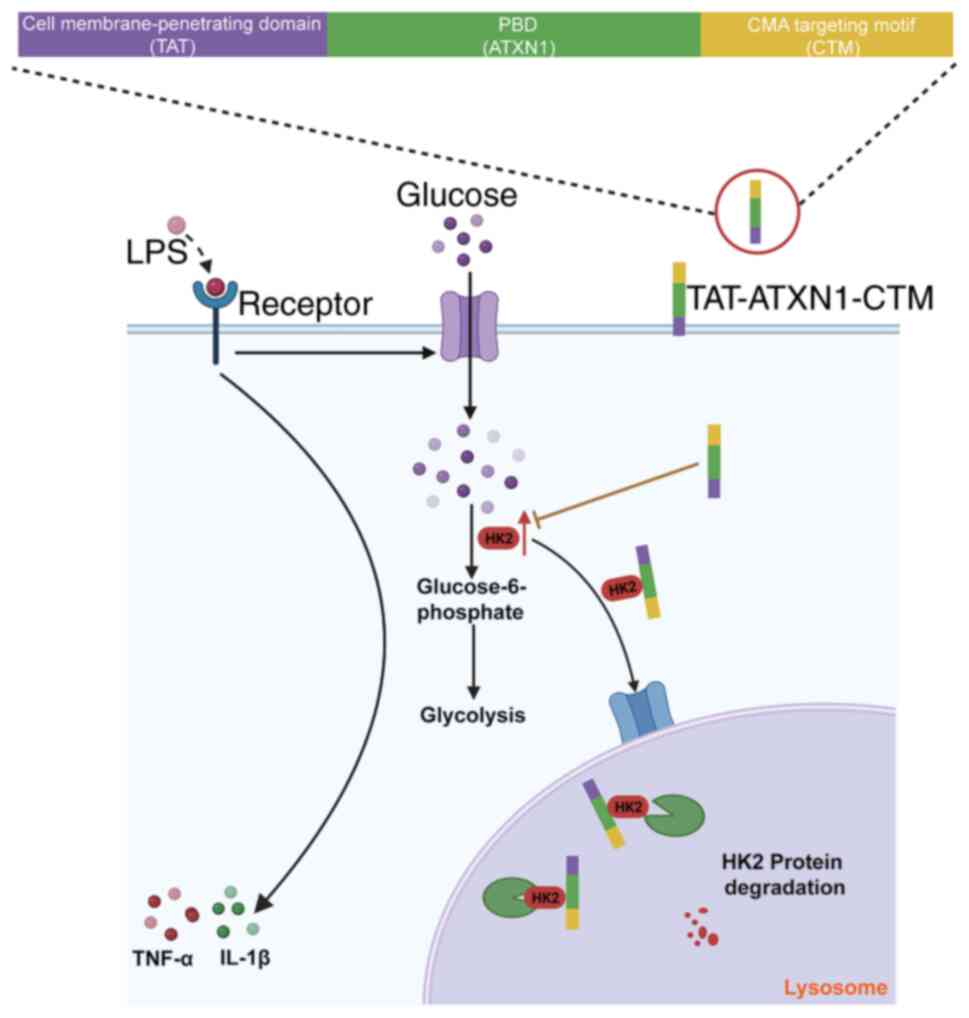
Composition and safety assessment of
TAT-ATXN1-CTM in vitro
TAT-ATXN1-CTM was designed to cross the cell
membrane and selectively degrade the HK2 protein. The peptide
consists of three functional domains. The first domain (TAT) can
induce the peptide to cross the cell membrane. The second domain
(PBD) is composed of ATXN1 protein, which can selectively recognize
and bind to the HK2 protein. The third domain, CTM, targets
lysosomes to degrade proteins (23,24).
In the present study, TAT-CTM was used as a control (Fig. 1A).
The effects of TAT-ATXN1-CTM at different
concentrations on cell viability were assessed using an MTT assay.
The results demonstrated that TAT-ATXN1-CTM treatment for 24 h at 5
and 10 µM had no significant effects on the viability of THP-1
cells, while 20 µM treatment significantly decreased the cell
viability compared with the 0 µM treated group [Fig. 1B; 20 µM (TAT-ATXN1-CTM) vs. 0 µM,
78.290±2.099 vs. 100.000±0.000; P<0.001]. Therefore, 10 µM
TAT-ATXN1-CTM was used for subsequent experiments to avoid
cytotoxicity (Fig. 1B).
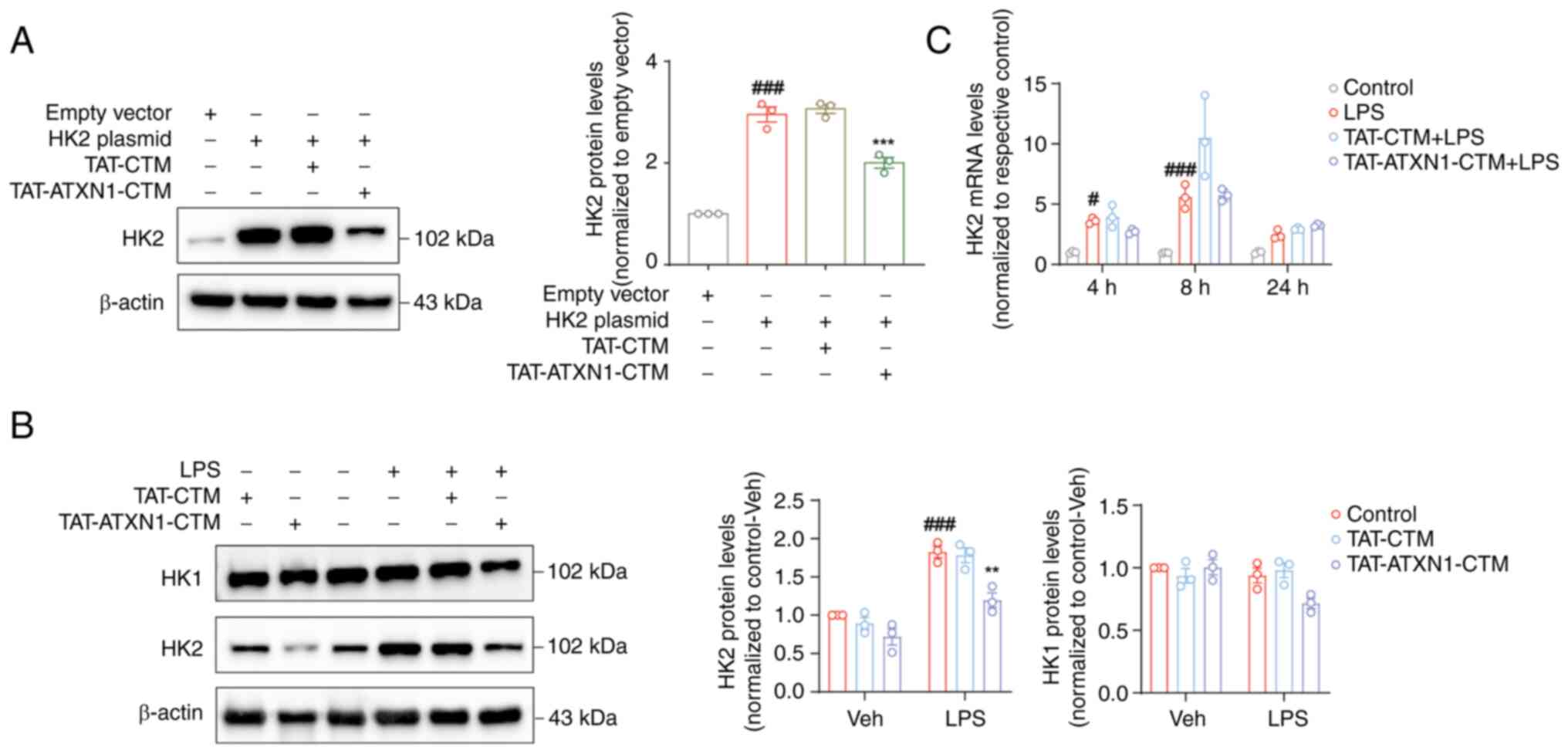
TAT-ATXN1-CTM selectively degrades HK2
protein without changing HK2 mRNA levels in vitro
Firstly, HK2 was overexpressed and it was confirmed
by both western blotting and RT-qPCR that HK2 expression was
increased following transfection with the HK2 plasmid compared with
the empty vector group (Fig. S5,
HK2 plasmid vs. empty vector, for mRNA, 8.257±0.905 vs.
1.016±0.121, P<0.01; Fig. 2A,
for protein, 2.955±0.148 vs. 1.000, P<0.001). Subsequently, the
degradation of the HK2 protein by TAT-ATXN1-CTM was verified.
TAT-ATXN1-CTM treatment significantly reduced HK2 expression
following HK2 plasmid-mediated overexpression in 293T cells
(Fig. 2A; HK2
plasmid+/TAT-ATXN1-CTM+ vs. HK2
plasmid+, 2.003±0.106 vs. 2.955±0.148; P<0.001). In
LPS-induced THP-1 cells, TAT-ATXN1-CTM treatment also markedly
reduced HK2 protein expression [Fig.
2B; LPS+/control+ vs. vehicle
(Veh)+/control+, 1.826±0.076 vs. 1.000;
P<0.001; LPS+/TAT-ATXN1-CTM+ vs.
LPS+/control+, 1.196±0.096 vs. 1.826±0.076;
P<0.01]. By contrast, HK2 mRNA levels were significantly
increased at 4 and 8 h after LPS treatment, but were not affected
at different time points (4, 8 and 24 h) after TAT-ATXN1-CTM
administration (Fig. 2C; LPS vs.
control, 4 h: 3.611±0.1444 vs. 1.003±0.0561, P<0.05, 8 h:
5.601±0.5804 vs. 0.973±0.0214, P<0.001). In addition,
TAT-ATXN1-CTM treatment tended to decrease HK1 protein expression
in LPS-induced THP-1 cells (Fig.
2B; LPS+/TAT-ATXN1-CTM+ vs.
LPS+/control+, 0.717±0.0417 vs. 0.939±0.060;
P=0.141), possibly through indirect mechanisms related to
inflammation suppression. These results suggested that
TAT-ATXN1-CTM was effective in selectively reducing HK2
expression.
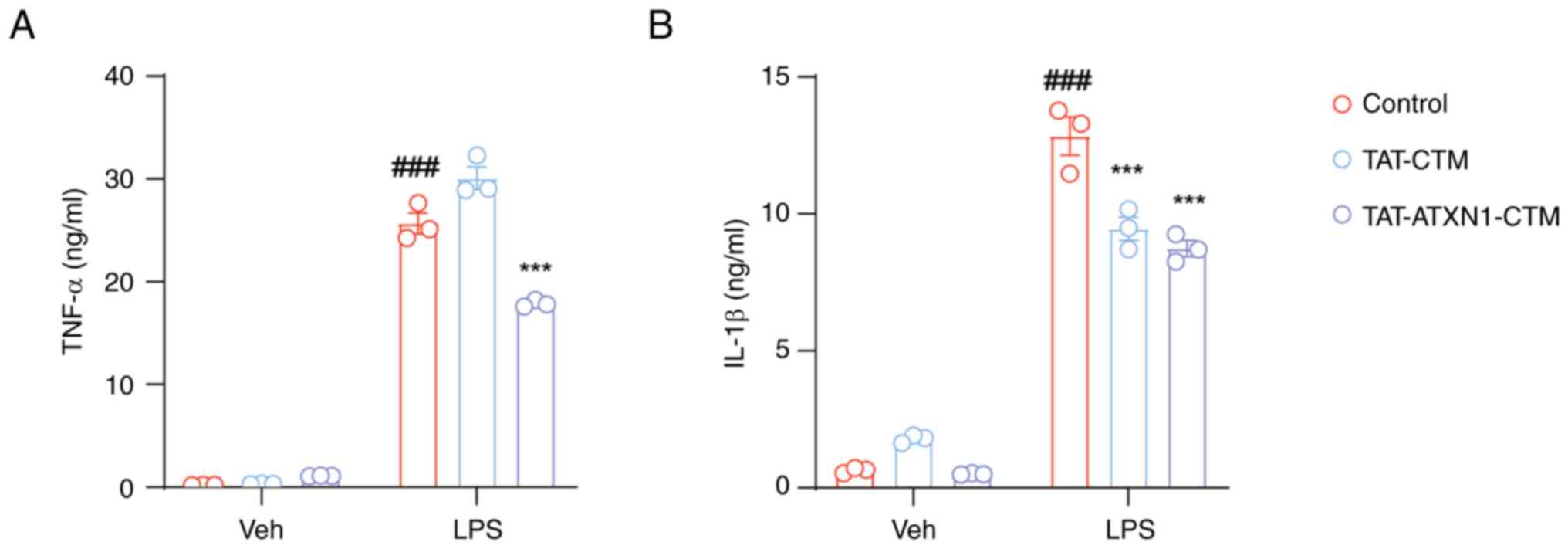
TAT-ATXN1-CTM reduces the production
of proinflammatory factors in LPS-induced THP-1 cells
HK2 expression is increased during inflammatory
processes (28) and whether
TAT-ATXN1-CTM exerted anti-inflammatory effects by degrading the
HK2 protein was next investigated. Treatment with 10 µM
TAT-ATXN1-CTM significantly reduced the levels of TNF-α and IL-1β
in the supernatant of THP-1 cells induced by LPS (Fig. 3;
LPS+/control+ vs.
Veh+/control+, TNF-α: 25.670±1.015 vs.
0.194±0.009, P<0.001, IL-1β: 12.840±0.699 vs. 0.637±0.0554,
P<0.001; LPS+/TAT-ATXN1-CTM+ vs.
LPS+/control+, TNF-α: 17.860±0.184 vs.
25.670±1.015, P<0.001, IL-1β: 8.739±0.291 vs. 12.840±0.699,
P<0.001). Notably, TAT-CTM, as a control treatment, also
decreased IL-1β protein expression (Fig. 3B;
LPS+/TAT-CTM+ vs.
LPS+/control+, IL-1β: 9.458±0.425 vs.
12.840±0.699; P<0.001). These data imply that TAT-ATXN1-CTM
could suppress proinflammatory cytokines in LPS-induced THP-1
cells.
TAT-ATXN1-CTM attenuates inflammatory
cell infiltration in the lung in an LPS-induced model of ALI
The anti-inflammatory effect of TAT-ATXN1-CTM was
investigated in a murine model of ALI (Fig. 4A). TAT-ATXN1-CTM (1,000 µg/kg)
treatment significantly reduced the number of total cells,
neutrophils, macrophages and lymphocytes in the BALF compared to
the control group [Fig. 4B-F;
LPS+ vs. control, total cells: 47.050±3.004 vs.
9.545±0.796, P<0.001, neutrophils: 15.900±2.424 vs. 0.05±0.017,
P<0.001, macrophages: 5.330±1.396 vs. 0.036±0.010, P<0.001
and lymphocytes: 25.820±2.746 vs. 9.460±0.784, P<0.001;
LPS+/TAT-ATXN1-CTM (1,000 µg/kg)+ vs.
LPS+, total cells: 20.000±1.676 vs. 47.050±3.004,
P<0.001, neutrophils: 5.985±1.044 vs. 15.900±2.424, P<0.01,
macrophages: 1.570±0.227 vs. 5.330±1.396, P<0.001 and
lymphocytes: 12.450±1.303 vs. 25.820±2.746, P<0.001]. Notably,
TAT-CTM also slightly decreased the number of total cells (Fig. 4C; total cells: 36.130±2.010 vs.
47.050±3.004; P<0.01), macrophages (Fig. 4E; 2.560±0.392 vs. 5.330±1.396;
P<0.05) and lymphocytes (Fig.
4F; 18.220±1.655 vs. 25.820±2.746; P<0.01) in BALF. In
conclusion, these data suggested that TAT-ATXN1-CTM treatment
inhibited the accumulation of inflammatory cells in the lungs of
LPS-induced mice.
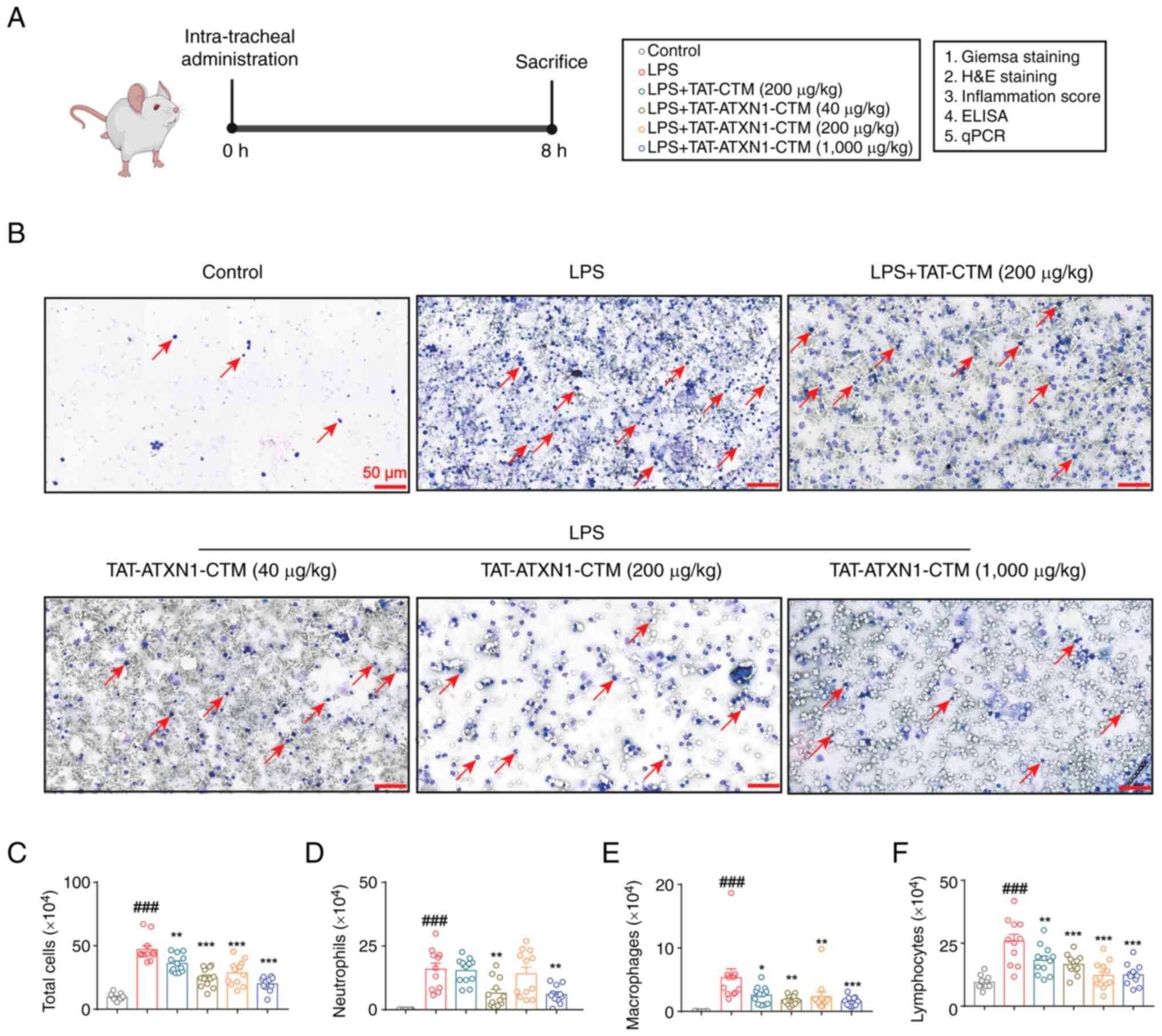 | Figure 4.TAT-ATXN1-CTM treatment reduces the
accumulation of inflammatory cells in LPS-induced mice. (A)
Schematic representation of the protocol used for the mouse
experiments. (B) Representative images of Wright-Giemsa staining of
the BALF cells are shown. Scale bar, 50 µm. The arrows point to
white blood cells. (C) Total cells, (D) neutrophils, (E)
macrophages and (F) lymphocytes in the BALF were counted. Data are
presented as the mean ± SEM (n=11-12). One-way ANOVA followed by
Dunnett's post hoc test was used to assess significance.
###P<0.001 vs. control; *P<0.05, **P<0.01,
***P<0.001 vs. LPS+. TAT, transactivator of
transcription protein of HIV-1; ATXN1, ataxin 1; BALF,
bronchoalveolar lavage fluid; CTM, chaperone-mediated
autophagy-targeting motif; LPS, lipopolysaccharide; qPCR,
quantitative PCR. |
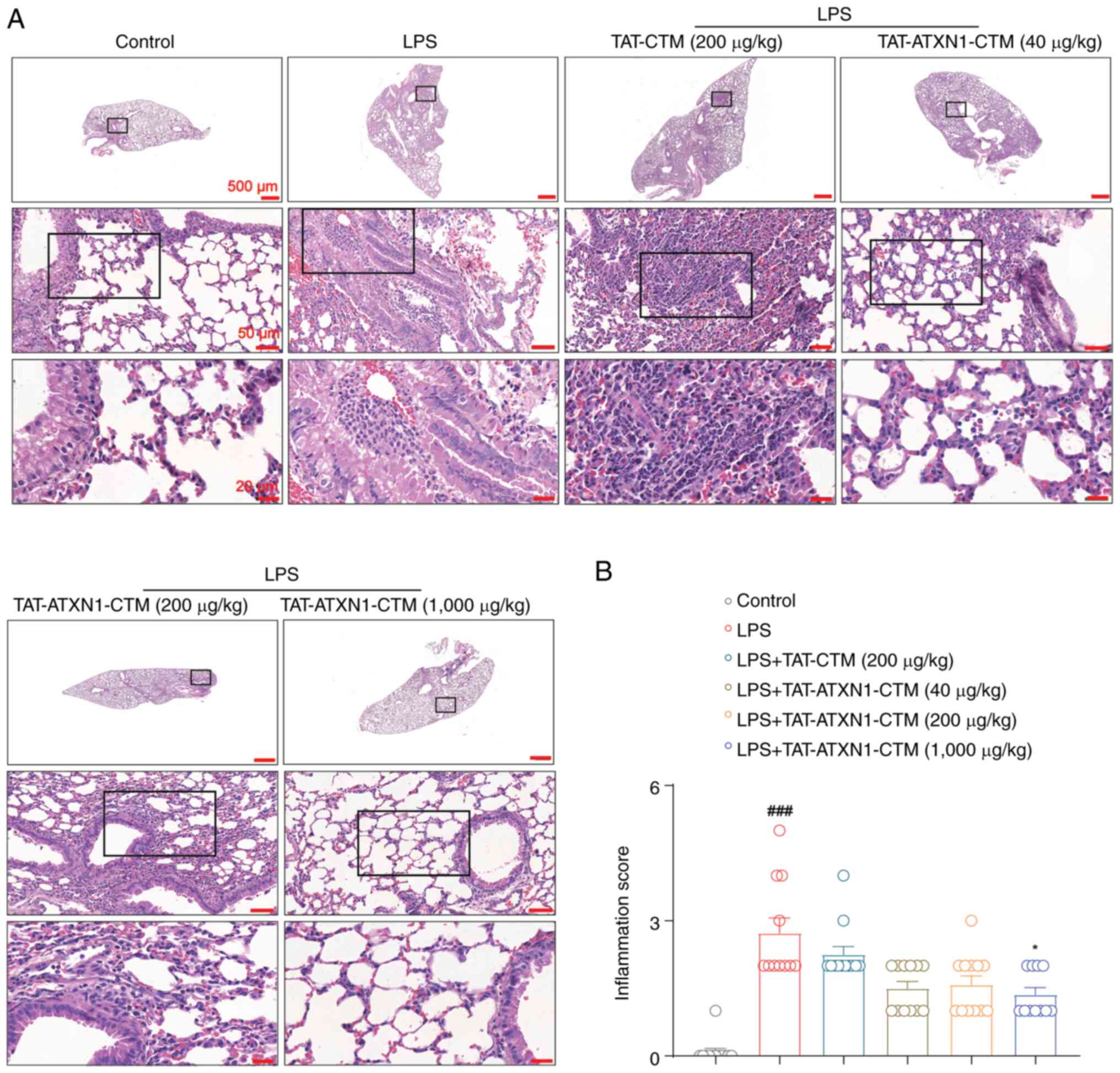
TAT-ATXN1-CTM attenuates pathological
injury in the lungs in an LPS-induced model of ALI
Based on lung histological examination, LPS induced
a pulmonary inflammatory response, including notable alveolar wall
thickening, congestion with inflammatory cell infiltration around
the alveoli or airways, which was significantly attenuated by 1,000
µg/kg TAT-ATXN1-CTM treatment [Fig.
5; LPS+ vs. control, 2.727±0.333 vs. 0.083±0.083,
P<0.001; LPS+/TAT-ATXN1-CTM (1,000 µg/kg)+
vs. LPS+, 1.364±0.152 vs. 2.727±0.333, P<0.05]. These
results indicated that inhibition of HK2 attenuated lung
histopathological damage in LPS-induced ALI in mice.
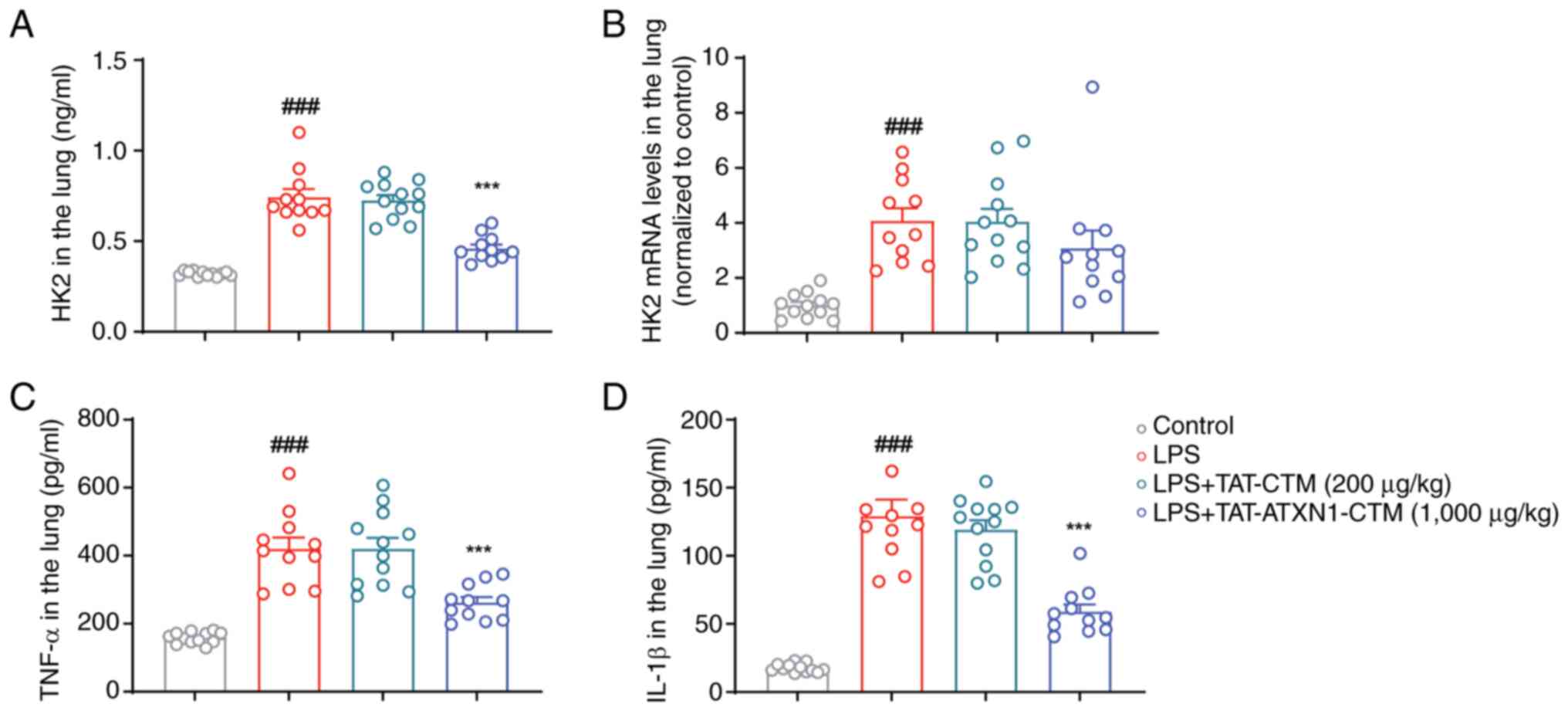
TAT-ATXN1-CTM treatment suppresses HK2
protein expression and proinflammatory factors in the lungs of
LPS-treated mice
Based on the aforementioned findings, TAT-ATXN1-CTM
(1,000 µg/kg) ameliorated ALI in vivo, whereas other low
concentrations of TAT-ATXN1-CTM had limited effect in treating ALI.
Consequently, the effects of TAT-ATXN1-CTM (1,000 µg/kg) on the
expression of HK2 and proinflammatory factors in LPS-challenged
mice only were investigated. TAT-ATXN1-CTM (1,000 µg/kg) treatment
reduced the protein expression levels of HK2, TNF-α and IL-1β
without affecting HK2 mRNA levels in the lung tissues of ALI mice
[Fig. 6; LPS+ vs.
control, HK2: 0.744±0.045 vs. 0.320±0.004, P<0.001, TNF-α:
420.600±32.250 vs. 158.800±4.788, P<0.001 and IL-1β:
129.300±12.040 vs. 17.630±0.921, P<0.001;
LPS+/TAT-ATXN1-CTM+ (1,000 µg/kg) vs.
LPS+, HK2: 0.461±0.021 vs. 0.744±0.045, P<0.001,
TNF-α: 262.500±15.750 vs. 420.600±32.250, P<0.001 and IL-1β:
59.040±5.229 vs. 129.300±12.040, P<0.001]. Collectively, these
data suggested that 1,000 µg/kg TAT-ATXN1-CTM reduced the protein
expression levels of HK2 and proinflammatory factors in ALI mice
induced by LPS.
Discussion
ALI is a devastating intrapulmonary inflammatory
disease and there is a lack of effective drug treatments (9). In the present study, a peptide,
TAT-ATXN1-CTM, was designed, and this could enter cells using TAT,
bind to the HK2 protein via the PBD and transport the peptide-HK2
protein complex to the lysosome for degradation via CTM, which
alleviates LPS-induced ALI (Fig.
7). The present study provided experimental evidence that
TAT-ATXN1-CTM exerted anti-inflammatory effects through HK2
degradation in the treatment of ALI. However, the THP-1 and 293T
cells used in the experiments were immortalized tumor cells, and
validation using primary macrophages from humans is needed. In
addition, while TAT-ATXN1-CTM exerted potent anti-inflammatory
activity in vitro, it had relatively minor efficacy in
vivo and higher drug concentrations are required to protect
mice from LPS-induced ALI, which may be related to factors such as
the shorter half-life and lower stability of the peptide (29). Therefore, TAT-ATXN1-CTM needs to be
further optimized or modified to improve its stability and potency
via the introduction of stabilizing α-helixes, salt bridge
formations, stapling or clipping of peptide sequences, or other
chemical modifications in future studies (29). Furthermore, an optimal dose needs
to be determined based on numerous factors, including the
bioavailability, pharmacokinetics and toxicity of the peptide,
which requires further characterization.
To date, there are two main ways to silence
proteins. RNA interference (RNAi) technology is the more commonly
used approach for protein expression knockdown due to its relative
maturity in terms of precision and efficacy (30). However, this technology presents
some experimental side effects, such as prolonged duration of
action, suppression of genes other than the desired gene target,
resulting in impeding other non-target protein expression and
disrupting the natural regulatory mechanism of normal cells
(31). Additionally, the
introduction of exogenous RNA can stimulate the production of
inflammatory factors and cause cell damage (32). Furthermore, the clustered regularly
interspaced short palindromic repeat-associated protein 9 (Cas9)
system is a highly efficient genome editing method that allows for
precise modification of genomic DNA to interfere with the
expression of specific proteins (33). Compared with RNAi technology, gene
knockout has demonstrated superior consistency (34). However, it still faces challenges
in terms of delivery, specificity, toxicity and immune responses.
For example, Cas9 can be delivered in the forms of DNA, mRNA, or
protein. Plasmid DNA poses a risk of insertional mutagenesis
(35); delivering the Cas9
protein, which is of bacterial origin, into cells may induce
carryover of bacterial endotoxin and trigger serious immunologic
responses (36); the
cell-targeting specificity of Cas9 delivery requires novel
biomaterials to address (37).
The method used in the present study has some
superiority over other methods for targeting peptides. The peptide
is easy and inexpensive to prepare, with specificity being its most
crucial characteristic. The specificity and effectiveness of
targeting peptides to degrade specific proteins largely depend on
the affinity and high selectivity of the interaction between target
proteins and PBDs (23). Common
methods for the identification of high-affinity and specific PBDs
include peptide arrays, phage display and computational modeling
(23,38). A sequence based on the binding site
between the HK2 and ATXN1 proteins was designed. We hypothesized
that ATXN1 has a specific motif for binding to HK2, and the binding
relationship between ATXN1 and HK2 was simulated. It was found that
ATXN1 (594–610) interacted with HK2, and thus, the ATXN1 (594–610)
sequence was used for the PBD.
Emerging evidence has indicated reciprocal
regulation between glucose metabolism and immunity (39). Increased glycolysis facilitated by
pyruvate kinase M2 contributes substrates for the biosynthesis of
proteins and nucleic acids needed for LPS-induced inflammatory
activation of immune cells (39,40).
Furthermore, previous studies have demonstrated that HK2 protein
was an important regulatory factor in the regulation of
inflammation-related diseases, and HK2 protein silencing could
inhibit the production of inflammatory factors (41,42).
In the present study, it was verified that TAT-ATXN1-CTM degraded
HK2 protein without affecting its mRNA levels both in vitro
and in vivo, which was consistent with the results of a
previous study (23). Under normal
conditions, TAT-ATXN1-CTM had no effect on the HK1 protein;
however, in THP-1 cell inflammatory models, it tended to
downregulate HK1 protein expression. This may be related to the
reduced inflammatory response resulting from HK2 degradation, which
can result in decreased HK1 expression (12). Furthermore, the degradation of HK2
may induce increased G-6P levels, and then upregulated cellular
glucose uptake, which may in turn inhibit HK1 (12,43).
In addition, the TAT-CTM control reduced levels of the inflammatory
factor IL-1β and decreased inflammatory leukocyte production. It
has been reported that increased expression and activation of NLRP3
inflammasome protein components promoted IL-1β production and
recruitment of immune cells to the site of injury, and NLRP3
proteins are considered to be substrates for CMA (44,45).
Therefore, we hypothesized that TAT-CTM containing the CMA
structure may reduce IL-1β production and inflammatory cell
infiltration by promoting NLRP3 protein degradation.
In conclusion, the present study demonstrated that
the TAT-ATXN1-CTM targeting peptide could effectively degrade the
endogenous HK2 protein and act as an anti-inflammatory agent in
vitro and in vivo. Therefore, the outcome of the
experiments provides novel perspectives for TAT-ATXN1-CTM
application in the development of ALI treatment, and provides novel
insights for drug discovery. Finally, it is hypothesized that
targeting peptides hold great potential as an effective therapeutic
approach in the future, as shown by a successful phase 2B clinical
trial, which elucidated the efficacy and safety of TAT-mediated
peptides (46).
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The National
Natural Science Foundation of China (grant no. 82173819) and
Natural Science Foundation of Zhejiang Province (grant no.
LTGY23H010006).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YX and HG conceived and designed the study. JY, LD
and YW performed the experiments and analyzed the results. JY and
YX wrote and revised the manuscript. LD, LG and YW participated in
the data analysis and interpretation. YX and JY confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All handling procedures used in the present study
were approved by the Animal Care and Use Committees at the Zhejiang
University School of Medicine (Approved No. ZJU20220294, Hangzhou,
China) and were conducted in accordance with the policies of
institutional guidelines on the care and use of laboratory
animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang R, Xu J, Zhang Y, Zhu X, Liu J and
Tan Y: Ligustrazine alleviate acute lung injury through suppressing
pyroptosis and apoptosis of alveolar macrophages. Front Pharmacol.
12:6805122021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mowery NT, Terzian WTH and Nelson AC:
Acute lung injury. Curr Probl Surg. 57:1007772020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dutta S, Zhu Y, Han Y, Almuntashiri S,
Wang X and Zhang D: Long Noncoding RNA: A novel insight into the
pathogenesis of acute lung injury. J Clin Med. 12:6042023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He YQ, Zhou CC, Yu LY, Wang L, Deng JL,
Tao YL, Zhang F and Chen WS: Natural product derived phytochemicals
in managing acute lung injury by multiple mechanisms. Pharmacol
Res. 163:1052242021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian J, Chen X, Shu S, Zhang W, Fang B,
Chen X, Zhao Y, Liu Z and Liang G: Design and synthesis novel
di-carbonyl analogs of curcumin (DACs) act as potent
anti-inflammatory agents against LPS-induced acute lung injury
(ALI). Eur J Med Chem. 167:414–425. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan R, Li Y, Han S, Chen X, Chen J, He J,
Gao H and Yang Y, Yang S and Yang Y: Fe-curcumin nanozyme-mediated
reactive oxygen species scavenging and Anti-Inflammation for acute
lung injury. ACS Cent Sci. 8:10–21. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnston LK, Rims CR, Gill SE, McGuire JK
and Manicone AM: Pulmonary macrophage subpopulations in the
induction and resolution of acute lung injury. Am J Respir Cell Mol
Biol. 47:417–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong WJ, Yang HH, Guan XX, Xiong JB, Sun
CC, Zhang CY, Luo XQ, Zhang YF, Zhang J, Duan JX, et al: Inhibition
of glycolysis alleviates lipopolysaccharide-induced acute lung
injury in a mouse model. J Cell Physiol. 234:4641–4654. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez-Prados JC, Traves PG, Cuenca J,
Rico D, Aragonés J, Martín-Sanz P, Cascante M and Boscá L:
Substrate fate in activated macrophages: A comparison between
innate, classic, and alternative activation. J Immunol.
185:605–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong WJ, Liu T, Yang HH, Duan JX, Yang
JT, Guan XX, Xiong JB, Zhang YF, Zhang CY, Zhou Y and Guan CX:
TREM-1 governs NLRP3 inflammasome activation of macrophages by
firing up glycolysis in acute lung injury. Int J Biol Sci.
19:242–257. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts DJ and Miyamoto S: Hexokinase II
integrates energy metabolism and cellular protection: Akting on
mitochondria and TORCing to autophagy. Cell Death Differ.
22:248–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeWaal D, Nogueira V, Terry AR, Patra KC,
Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR and Hay N:
Hexokinase-2 depletion inhibits glycolysis and induces oxidative
phosphorylation in hepatocellular carcinoma and sensitizes to
metformin. Nat Commun. 9:4462018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong L, Cui Z, Chen P, Han H, Peng J and
Leng X: Reduced survival of patients with hepatocellular carcinoma
expressing hexokinase II. Med Oncol. 29:909–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, Cao K, Wang F, Wu W, Mai W, Qiu L,
Luo Y, Ge WP, Sun B, Shi L, et al: Dual roles of hexokinase 2 in
shaping microglial function by gating glycolytic flux and
mitochondrial activity. Nat Metab. 4:1756–1774. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Varghese E, Samuel SM, Líšková A, Samec M,
Kubatka P and Büsselberg D: Targeting glucose metabolism to
overcome resistance to anticancer chemotherapy in breast cancer.
Cancers (Basel). 12:22522020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CH, Wang BW, Hsiao YC, Wu CY, Cheng
FJ, Hsia TC, Chen CY, Wang Y, Weihua Z, Chou RHT, et al:
PKCdelta-mediated SGLT1 upregulation confers the acquired
resistance of NSCLC to EGFR TKIs. Oncogene. 40:4796–4808. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ros S and Schulze A: Glycolysis back in
the limelight: Systemic targeting of HK2 blocks tumor growth.
Cancer Discov. 3:1105–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo D, Tong Y, Jiang X, Meng Y, Jiang H,
Du L, Wu Q, Li S, Luo S, Li M, et al: Aerobic glycolysis promotes
tumor immune evasion by hexokinase2-mediated phosphorylation of
IκBα. Cell Metab. 34:1312–1324.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu S and Herschman HR: A tumor agnostic
therapeutic strategy for hexokinase 1-Null/Hexokinase 2-Positive
cancers. Cancer Res. 79:5907–5914. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou QQ, Xiao HT, Yang F, Wang YD, Li P
and Zheng ZG: Advancing targeted protein degradation for metabolic
diseases therapy. Pharmacol Res. 188:1066272023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho PW, Leung CT, Liu H, Pang SY, Lam CS,
Xian J, Li L, Kung MH, Ramsden DB and Ho SL: Age-dependent
accumulation of oligomeric SNCA/α-synuclein from impaired
degradation in mutant LRRK2 knockin mouse model of Parkinson
disease: Role for therapeutic activation of chaperone-mediated
autophagy (CMA). Autophagy. 16:347–370. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan X, Jin WY, Lu J, Wang J and Wang YT:
Rapid and reversible knockdown of endogenous proteins by
peptide-directed lysosomal degradation. Nat Neurosci. 17:471–480.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Williamson NA and Bogoyevitch MA:
Complementary proteomics strategies capture an ataxin-1 interactome
in Neuro-2a cells. Sci Data. 5:1802622018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie YC, Dong XW, Wu XM, Yan XF and Xie QM:
Inhibitory effects of flavonoids extracted from licorice on
lipopolysaccharide-induced acute pulmonary inflammation in mice.
Int Immunopharmacol. 9:194–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Lei W, Zhang S and Yao L: MCC950,
a NLRP3 inhibitor, ameliorates lipopolysaccharide-induced lung
inflammation in mice. Bioorg Med Chem. 30:1159542021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang W, Zheng F, Lin C and Zhang A:
Changes in energy metabolism and macrophage polarization: Potential
mechanisms of arsenic-induced lung injury. Ecotoxicol Environ Saf.
204:1109482020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fosgerau K and Hoffmann T: Peptide
therapeutics: Current status and future directions. Drug Discov
Today. 20:122–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leung RK and Whittaker PA: RNA
interference: From gene silencing to gene-specific therapeutics.
Pharmacol Ther. 107:222–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin X, Ruan X, Anderson MG, McDowell JA,
Kroeger PE, Fesik SW and Shen Y: siRNA-mediated off-target gene
silencing triggered by a 7 nt complementation. Nucleic Acids Res.
33:4527–4535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Castanotto D and Rossi JJ: The promises
and pitfalls of RNA-interference-based therapeutics. Nature.
457:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yip BH: Recent advances in CRISPR/Cas9
delivery strategies. Biomolecules. 10:8392020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuscu C, Arslan S, Singh R, Thorpe J and
Adli M: Genome-wide analysis reveals characteristics of off-target
sites bound by the Cas9 endonuclease. Nat Biotechnol. 32:677–683.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen F, Alphonse M and Liu Q: Strategies
for nonviral nanoparticle-based delivery of CRISPR/Cas9
therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol.
12:e16092020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
You L, Tong R, Li M, Liu Y, Xue J and Lu
Y: Advancements and obstacles of CRISPR-Cas9 technology in
translational research. Mol Ther Methods Clin Dev. 13:359–370.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Behr M, Zhou J, Xu B and Zhang H: In vivo
delivery of CRISPR-Cas9 therapeutics: Progress and challenges. Acta
Pharm Sin B. 11:2150–2171. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou YF, Wang J, Deng MF, Chi B, Wei N,
Chen JG, Liu D, Yin X, Lu Y and Zhu LQ: The Peptide-Directed
lysosomal degradation of CDK5 exerts therapeutic effects against
stroke. Aging Dis. 10:1140–1145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O'Neill LA and Pearce EJ: Immunometabolism
governs dendritic cell and macrophage function. J Exp Med.
213:15–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Lu B, Sheng L, Zhu Z, Sun H, Zhou Y,
Yang Y, Xue D, Chen W, Tian X, et al: Hexokinase 2-dependent
hyperglycolysis driving microglial activation contributes to
ischemic brain injury. J Neurochem. 144:186–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bao C, Zhu S, Song K and He C: HK2: A
potential regulator of osteoarthritis via glycolytic and
non-glycolytic pathways. Cell Commun Signal. 20:1322022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan Y, Fan G, Liu Y, Liu L, Zhang T, Liu
P, Tu Q, Zhang X, Luo S, Yao L, et al: The transcription factor
KLF14 regulates macrophage glycolysis and immune function by
inhibiting HK2 in sepsis. Cell Mol Immunol. 19:504–515. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qian Y, Chen D, Zhu Y, Wu J, Wang Y and
Yang W: Targeting hexokinase 1 alleviates NLRP3-mediated
inflammation in apical periodontitis: A laboratory investigation.
Int Endod J. 56:734–747. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qiao L, Ma J, Zhang Z, Sui W, Zhai C, Xu
D, Wang Z, Lu H, Zhang M, Zhang C, et al: Deficient
Chaperone-Mediated autophagy promotes inflammation and
atherosclerosis. Circ Res. 129:1141–1157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hettwer J, Hinterdobler J, Miritsch B,
Deutsch MA, Li X, Mauersberger C, Moggio A, Braster Q, Gram H,
Robertson AAB, et al: Interleukin-1beta suppression dampens
inflammatory leucocyte production and uptake in atherosclerosis.
Cardiovasc Res. 118:2778–2791. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hill MD, Martin RH, Mikulis D, Wong JH,
Silver FL, Terbrugge KG, Milot G, Clark WM, Macdonald RL, Kelly ME,
et al: Safety and efficacy of NA-1 in patients with iatrogenic
stroke after endovascular aneurysm repair (ENACT): A phase 2,
randomised, double-blind, placebo-controlled trial. Lancet Neurol.
11:942–950. 2012. View Article : Google Scholar : PubMed/NCBI
|