Introduction
Periodontal disease is an infectious bacterial
disease occurring in the periodontal support tissue, which can lead
to continuous and irreversible destruction of the periodontal
support tissue, leading to tooth loss and seriously affecting
quality of life (1). Also,
periodontitis can lead to destruction of alveolar bone and
periodontal ligaments, and is associated with numerous types of
systemic disease (2), such as
diabetes, heart disease and osteoporosis. However, the treatment of
periodontitis is still primarily based on mechanical removal of
periodontal pathogenic factors such as plaque and dental calculus,
and lifelong periodontal maintenance therapy is required. Treating
periodontitis and restoring absorbed periodontal tissue remains a
major challenge (3).
Drugs are commonly used as adjuvant therapy for
periodontitis (4) to supplement
non-surgical treatment of periodontitis. The most commonly used
antibiotics, such as metronidazole, minocycline and doxycycline,
can enter the mucosa locally (5).
These drugs are used in periodontal pockets to inhibit or eliminate
periodontal disease-causing microorganisms and regulate
inflammatory response of tissue. However, the use of antibiotics
can cause immune disorders, or induce bacteria to develop drug
resistance, and these adverse effects restrict the use of
antibiotics in the treatment of periodontitis (6). Traditional Chinese medicine has shown
potential in the treatment of periodontitis (7). Oroxylin A is a flavonoid compound
isolated from the root of Scutellaria baicalensis. A number
of studies have confirmed that Oroxylin A has antioxidant,
anti-inflammatory and anti-tumor activity (8–10) In
terms of anti-inflammatory effect, Oroxylin A has been shown to
inhibit secretion of inflammatory cytokines (11), but the molecular mechanism is
unclear.
Heme oxygenase-1 (HO-1) is a key cell protective
enzyme. A number of studies have confirmed that HO-1 serves a
regulatory role in inflammation subsidence and is considered a
potential therapeutic target for numerous drugs to exert
anti-inflammatory effects (12–14).
Studies have confirmed that HO-1 serves an important role in the
occurrence and development of periodontitis and can regulate
certain cytokines, such as cyclooxygenase (COX)-2 and TNF-α, to
affect the process of periodontitis (15,16).
To the best of our knowledge, there has been no report that
Oroxylin A affects inflammation through HO-1. Therefore, it was
hypothesized that Oroxylin A may decrease the expression of COX-2
and TNF-α by regulating HO-1, thus alleviating periodontal local
inflammation, and at the same time downregulating the
RANKL:osteoprotegerin (OPG) ratio, thus inhibiting the absorption
of alveolar bone.
Materials and methods
Materials
Penicillin/streptomycin, Minimum Essential Medium-α,
trypsin and fetal bovine serum were provided by Biological
Industries. Lipopolysaccharide (LPS) and Oroxylin A were purchased
from Beijing Solarbio Science & Technology Co., Ltd. RNAiso
Plus, TB Green™ Premix Ex Taq™ II and PrimeScript™ RT reagent kit
with gDNA Eraser were provided by Takara Bio, Inc. PCR primers were
purchased from Sangon Biotech Co., Ltd.
Animals and treatments
The 8-week-old male Wistar rats weighing 250–300 g
were purchased from Charles River Laboratories, Inc. A total of 30
rats were housed in the Experimental Animal Center of the
Affiliated Hospital of Qingdao University and maintained in a
specific-pathogen-free environment at 25°C, 40% humidity, and a
12-h light/dark cycle. The animals had free access to food and
water. Periodontitis was induced by tying silk ligatures around the
maxillary second molars and high sugar diet, as previously
described (17).
Rats were randomly divided into the following groups
(n=10/group): Sham operation (PBS, no periodontitis, no silk
ligation), periodontitis (PBS+silk ligation) and treatment group
(silk ligation+Oroxylin A). General anesthesia was induced by
intraperitoneal injection of pentobarbital sodium (40 mg/kg, 2%).
Periodontitis was induced by placing a silk thread between the
right maxillary first and second molars. Rats in the treatment
group were injected with 8 µl Oroxylin A solution with a final
concentration of 0.5 µg/µl at the gingival site every 48 h for 2
weeks, while the sham group and the periodontitis group were
injected with the same volume of PBS solution. A total of 4 weeks
later, the rats were sacrificed by intraperitoneal injection of
sodium pentobarbital (200 mg/kg) followed by rapid decapitation;
death was confirmed by loss of respiration and heartbeat. The
gingival tissue was collected to extract total RNA and total
protein for detecting the expression of COX −2, TNF-α, RANKL and
OPG and paraffin sections were prepared. All animal studies were
approved by the Research Ethics Committee of the Affiliated
Hospital of Qingdao University (Qingdao, China, AHQU-MAL20210326).
The expression level of COX-2 was observed by immunohistochemistry.
The gingival tissue specimens were formalin-fixed and
paraffin-embedded at room temperature (fixative concentration, 4%;
duration 12 h). Sections of 3 µm thickness were examined with
immunohistochemistry. Antigen retrieval was performed by heating
the specimens at 97°C, followed by washing with xylene and gradual
rehydration with a descending ethanol series. Sections were blocked
with the bovine serum albumin(BSA,3%) for 30 min at room
temperature. Immunohistochemistry sections were incubated in 3%
H2O2 in methanol for 30 min to block
endogenous peroxidase activity. Primary antibody against COX-2
(1:1000, Abcam, ab151571) was incubated at 37°C for 1 h. Goat anti
mouse IgG conjugated with horseradish peroxidase (1:1000, Abcam,
ab6789) used as the secondary antibodies which was incubated at
37°C for 30 min. Chromogen detection was performed using DAB for 1
min at room temperature. Add sufficient amount of hematoxylin
solution to the tissue slices to completely cover and incubate for
1 min at room temperature. Images are acquired at 40× magnification
using a light microscope (Olympus, BX43). Image-Pro Plus version
6.0 software (Media Cybernetics, Inc., USA) was used for
analysis.
Isolation, culture and identification
of primary rat gingival fibroblasts (RGFs)
After anesthesia, rat gingival tissue obtained from
the attached gingiva of Wistar rats were cut into pieces and
cultured in complete Minimum Essential Medium-α containing 10%
fetal bovine serum and 1% penicillin/streptomycin, in a humidified
incubator of 5% CO2 at 37°C (18). When the cells grew out from the
edge of gingival tissue, and the fusion rate reached 80%, the cells
were digested, centrifuged, and passaged. Third-to-fifth passage
cells were used for subsequent analysis. RGFs were identified by
immunofluorescence staining with anti-vimentin, anti-fibronectin,
anti-cytokeratin antibodies and PBS for control group (19). Cells were divided into the
following groups: Control (PBS), LPS (10 pg/ml) and 50, 100, 200
and 400 µg/ml Oroxylin A+LPS (10 pg/ml). All treatments were
performed at 37°C for 24 h. Cells were formalin-fixed at 4°C
(fixative concentration, 4%; 20 min), and blocked with normal goat
serum(10%, Absin) for 1 h at 37°C. Cells were incubated with
primary antibodies as follows: Anti-vimentin (1:1000, Abcam,
ab8978); anti- fibronectin (1:1,000, Abcam, ab2413),
anti-cytokeratin (1:1000, Abcam, ab53280) at 37°C for 2 h. Goat
anti mouse IgG conjugated with horseradish peroxidase (1:1000,
Abcam, ab6789) used as the secondary antibodies which was incubated
at room temperature for 2h. Nucleus was stained with DAPI.
Images were captured with fluorescent microscopy (Keyence,
BZ-X800). Image-Pro Plus version 6.0 software (Media Cybernetics,
Inc.) was used for analysis.
Cell transfection
RGFs were transfected with small interfering (si)RNA
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C. The sequences used
were as follows: Rat HO-1 forward, 5′-ACAAGCAGAACCCAGUCUA-3′ and
reverse, 5′-UAGACUGGGUUCUGCUUGU-3′ and negative control (NC)
forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. RGFs (5×105 cells/well)
were transfected with either HO-1 (20 nM) or NC siRNA (20 nM) using
the RNAimax-transfection system (Invitrogen; Thermo Fisher
Scientific, Inc.). Following transfection for 36 h, cells were
treated with complete medium in the presence of baicalein (50 µM;
37°C, 5%CO2) for 12 h (for PCR) or 24 h (for western
blotting) (20).
Cell Counting Kit-8 (CCK8) assay
Cell viability was analyzed by CCK8 (Abcam)
according to the manufacturer's protocol Cells were seeded and
cultured for 3 h at a density of 5×103/well in 100 µl
medium into 96-well microplates(37°C). Then, the cells were treated
with Oroxylin A at 37°C for 24 h. 10 µl CCK-8 reagent was added to
each well and then cultured at 37°C for 2 h. All experiments were
performed in triplicate. The absorbance was analyzed at 450 nm
using a microplate reader.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol (Thermo Fisher Scientific, Inc.)was used to
isolate total RNA from RGFs. RT-qPCR was then performed using
RT-qPCR kits (SYBR Premix Ex Taq II, DRR820A, Takara), the
temperature and duration were according to the manufacturers
protocol. RNA was reverse-transcribed into cDNA using a Light
Cycler LC480 (Roche Diagnostics). Cycling conditions consisted of
initial incubations, followed by 40 cycles of denaturation,
annealing, and extension. The thermocycling conditions of the qPCR
steps were as follows: activation (temperature: 50°C; duration: 2
min), Dual-Lock DNA polymerase (temperature: 95°C; duration: 2
min), denaturation (temperature: 95°C; duration: 15 sec), and
annealing/extension (temperature: 60°C; duration: 1 min). The mRNA
expression levels were normalized to the level of GAPDH, a
housekeeping gene. The 2-ΔΔCq method was used to determine relative
expression of target genes. The primers used were as follows: GAPDH
forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′; TNF-α (GenBank: NM_013091.2) forward,
5′-ATGGGCTCCCTCTCATCAGT-3′ and reverse, 5′-AAATGGCAAATCGGCTGACG-3′;
COX-2 (GenBank: NM_017232.4) forward, 5′-CTCAGCCATGCAGCAAATCC-3′
and reverse, 5′-GGGTGGGCTTCAGCAGTAAT-3′; RANKL (GenBank:
NM_057149.2) forward, 5′-CCTGTACTTTCGAGCGCAGA-3′ and reverse,
5′-AGTCGAGTCCTGCAAACCTG-3′; OPG (GenBank: NM_012870.2) forward, 5′-
GAATGTGAGGAAGGGCGCTA-3′ and reverse, 5′-CTTCGCACAGGGTGACATCT-3′ and
HO-1 (GenBank: NM_012580.2) forward, 5′-ATGCCCCACTCTACTTCCCT-3′ and
reverse, 5′-TGTGTGGCTGGTGTGTAAGG-3′.
Western blotting
TRIzol (Thermo Fisher Scientific, Inc.) was used to
isolate total protein from RGFs. Protein concentrations were
estimated via BCA assay. 30 µg total protein was loaded per lane.
12% separation gel and 5% stacking gel were used. Proteins were
transferred to PVDF membranes. Membranes were blocked with 5%
skimmed milk for 1.5 h at room temperature prior to western blot
analysis. Incubate the blot with the primary antibody at 4°C
overnight followed by secondary antibody for 2 h at room
temperature. Primary antibodies against COX −2 (ab15191, 1:1000),
TNF-α (ab9579,1:1000), OPG (ab73400, 1:1000), RANKL (ab93719,
1:1000) and HO-1 (ab13248, 1:1000) were all purchased from Abcam.
Secondary horseradish peroxidase-linked antibodies against rabbit
IgG (7074, 1:1,000) and anti-β-actin were from Cell Signaling
Technology. Finally, protein bands were observed by ECL (Abcam,
ab133406) and quantified using GraphPad Prism (version 8;
Dotmatics) and ImageJ 2 (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism (version 8; Dotmatics). Data are presented as mean ± standard
deviation of three independent experimental repeats. One-way
analysis of variance followed by Tukey's post hoc test was used to
compare groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Isolation, culture and identification
of RGFs
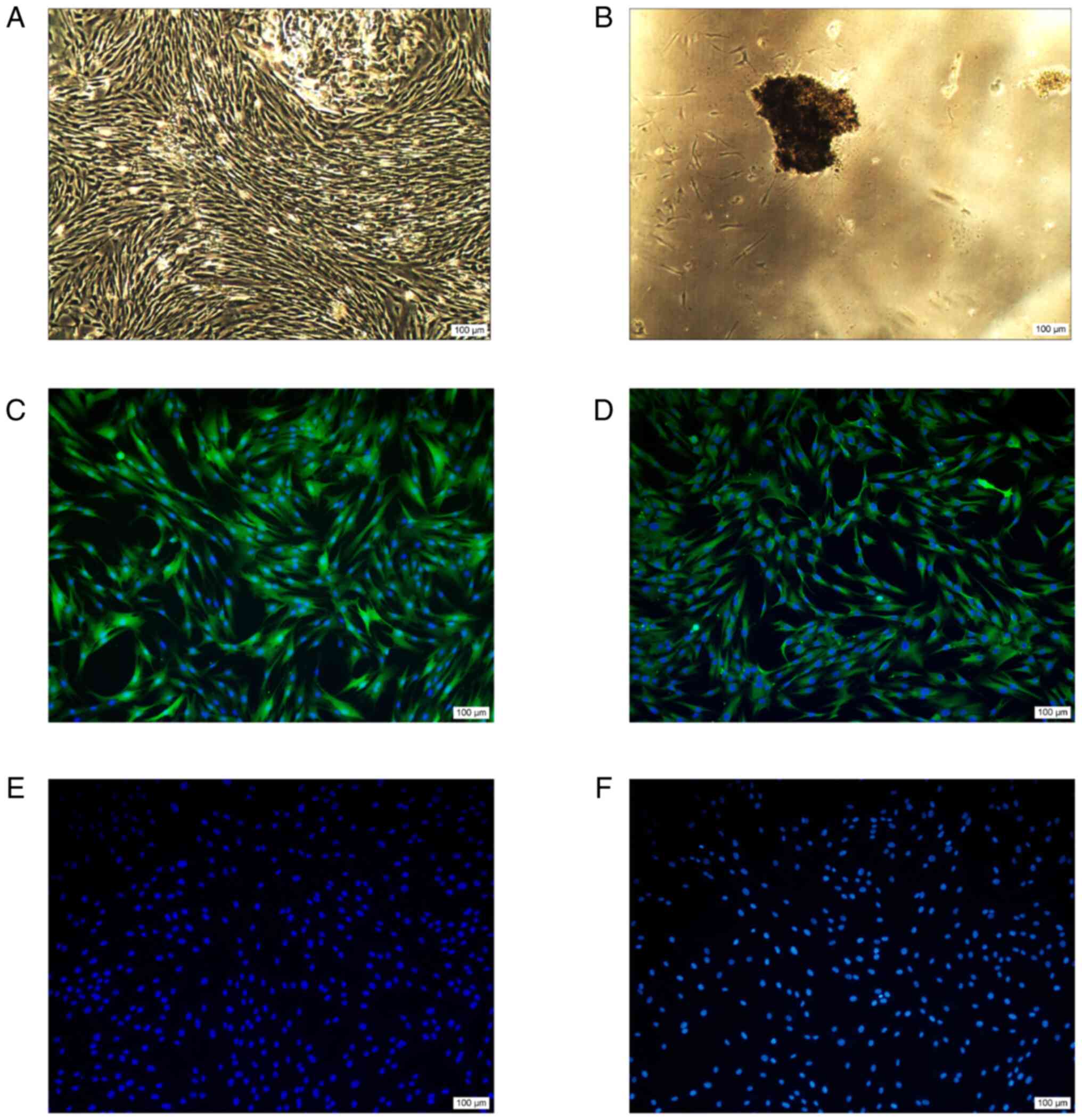
After being cultured for 3–7 days, the periodontal
tissue fragment adhered to the wall and some cells grew from the
edge of the tissue fragment (Fig.
1A). After 2–3 weeks of culture, Cells proliferated toward the
center of the tissue fragment where they form a swirl pattern. Cell
morphology was mostly fusiform, the nucleus was aggregated in the
center and the protrusion formed by the cytoplasm radiated
outwards. After 3–4 weeks, the fusion rate of the cells reached 80%
(Fig. 1B).
Immunofluorescence results showed positive
expression of fibronectin and vimentin antibodies (Fig. 1C and D), while negative expression
of keratin antibodies (Fig. 1E).
Fig. 1F shows a negative control,
and the primary antibody was replaced by PBS without any
expression. The outcome of positive expression of fibronectin and
vimentin antibodies, while negative expression of keratin
antibodies showed that periodontal membrane stem cells were derived
from mesenchyma.
Effect of Oroxylin A on expression of
inflammation and osteoclast factors of RGFs
Preliminary CCK-8 results showed that Oroxylin A
(50, 100, 200M, 400M) has no cytotoxicity (Fig. S1). RT-qPCR showed that compared
with the blank control, the mRNA expression levels of COX-2, TNF-α
and RANKL in the LPS-stimulated group decreased with the increase
of Oroxylin A concentration (Fig.
2A-C). LPS inflammatory stimulation did not significantly
increase the expression of OPG, however, preconditioning with
Oroxylin A increased the expression of OPG mRNA in LPS-stimulated
RGFs in Oroxylin A dose-dependent manner (Fig. 2D). The RANKL/OPG ratio was
significantly increased in the LPS group and showed a downward
trend with the increase of the dose of Oroxylin A (Fig. 2E).
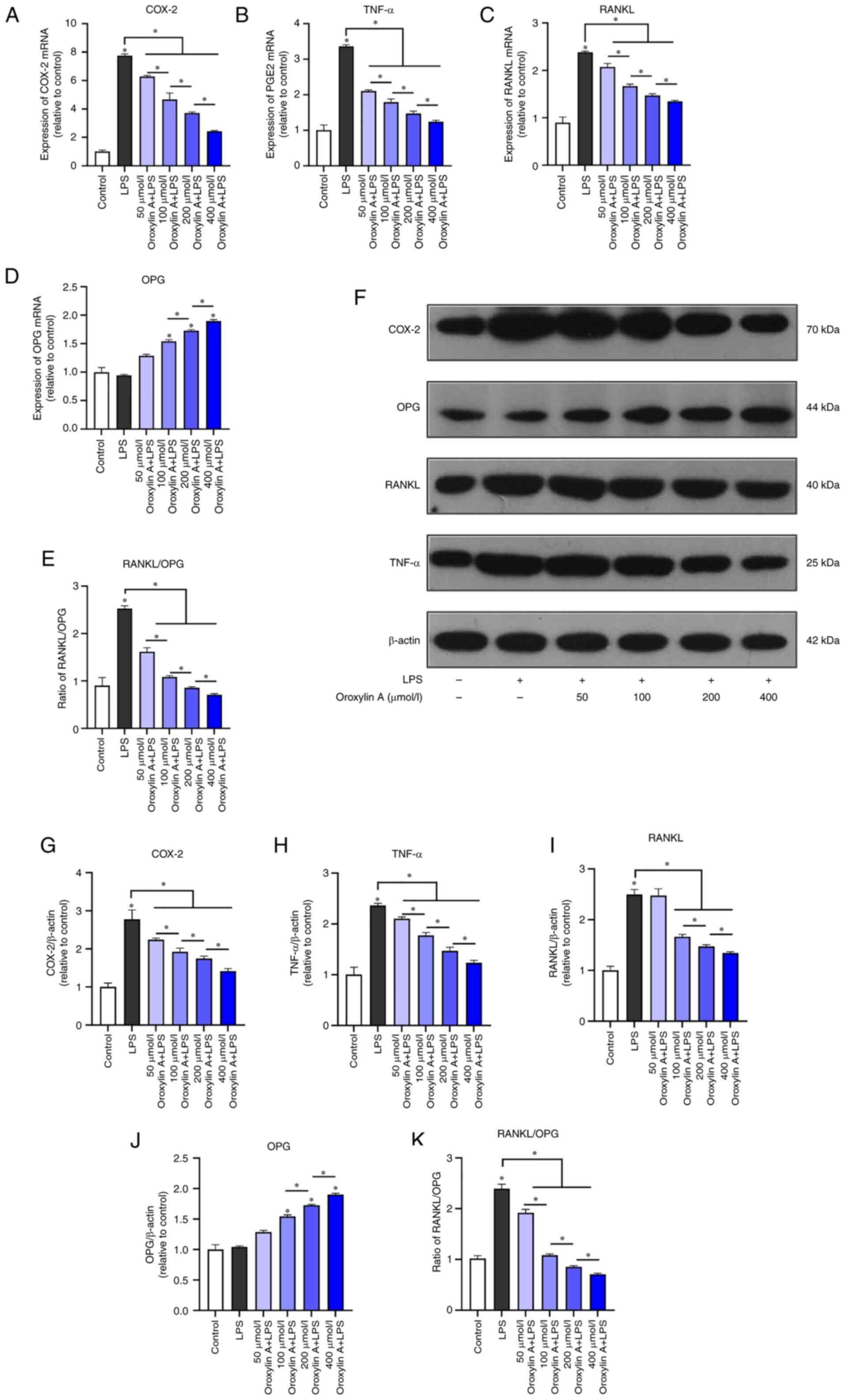 | Figure 2.RGFs were infected by LPS and treated
with Oroxylin A. Levels of mRNA of (A) COX-2, TNF-α, (B) RANKL, (C)
OPG and (D) RANKL/OPG (E) were analyzed by reverse
transcription-quantitative polymerase chain reaction. (F) Protein
levels of COX-2, TNF-α, RANKL and OPG were analyzed by western
blotting. Levels of COX-2, (G) TNF-α, (H) RANKL, (I) OPG and (J)
RANKL/OPG (K) between control group, LPS group and different
concentration of Oroxylin A groups. The mRNA levels of cytokines in
untreated controls were set as 1.0. The blots were stripped and
re-probed with β-actin as a loading control. Bars show the levels
of cytokines with mean ± SD (n=3). *P<0.05 vs. control. RGF, rat
gingival fibroblast; LPS, lipopolysaccharide; COX, cyclooxygenase;
OPG, osteoprotegerin. |
Western blotting showed that LPS could significantly
induce the protein expression levels of COX-2, TNF-α and RANKL and
Oroxylin A could downregulate their expression in a
concentration-dependent manner (Fig.
2F-I). Oroxylin A could induce OPG protein expression in a
concentration-dependent manner (Fig.
2J), while the RANKL/OPG ratio was increased by LPS stimulation
and decreased with the dose of Oroxylin A (Fig. 2K).
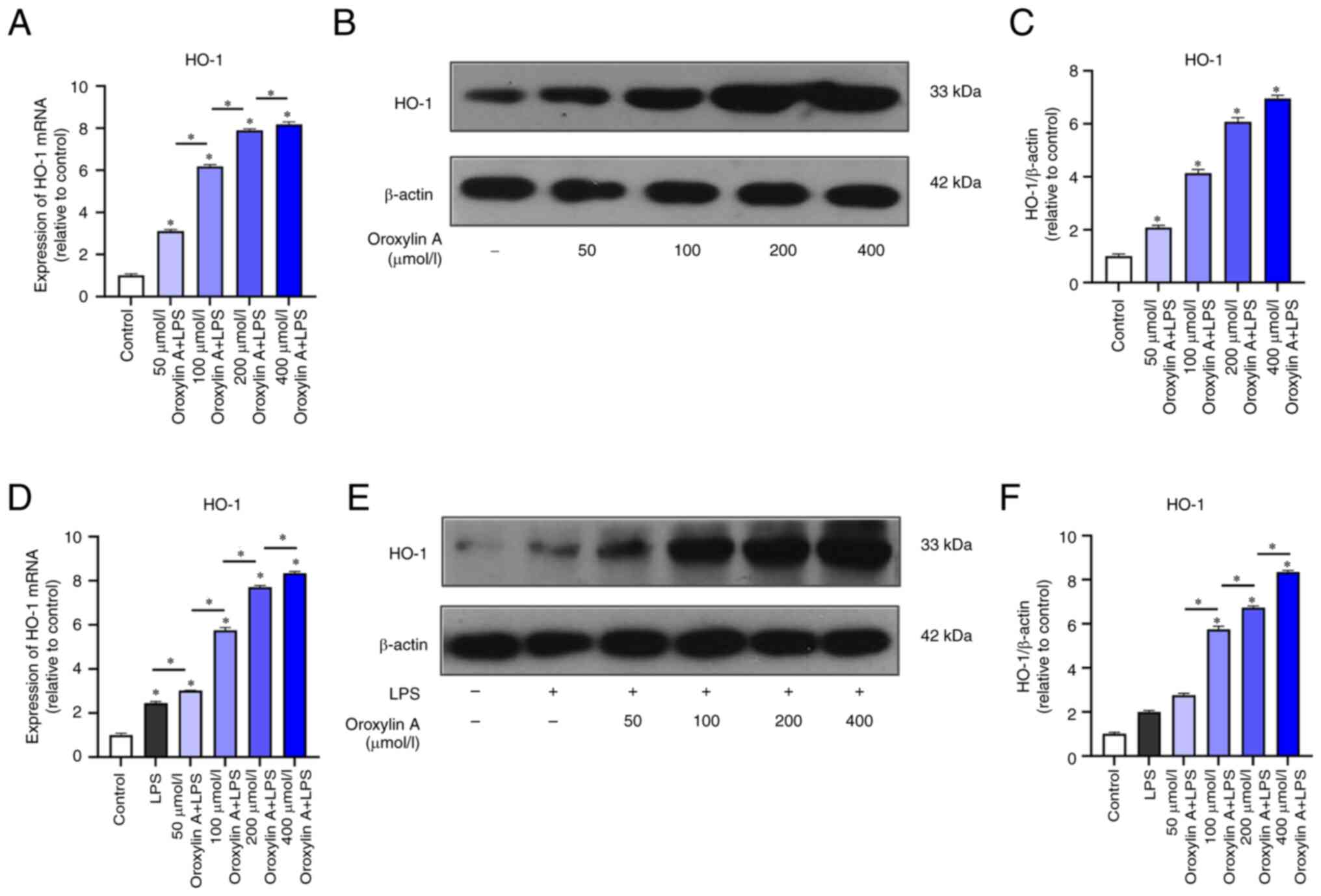
Regulation of HO-1 expression in
non-inflammatory and inflammatory RGFs by Oroxylin A
RT-qPCR results showed that the expression of HO-1
mRNA in normal RGFs was increased following Oroxylin A treatment
and the degree of increase was positively associated with the
concentration of Oroxylin A (Fig.
3A). The protein expression of HO-1 was increased following by
Oroxylin A treatment (Fig. 3B).
The expression of HO-1 increased following Oroxylin A treatment in
a dose-dependent manner (Fig.
3C)
Following LPS induction, the mRNA and protein
expression of HO-1 in RGFs were significantly higher than those in
blank control group without inflammation induction, and HO-1 was
upregulated with the increase of Oroxylin A concentration (Fig. 3D-F).
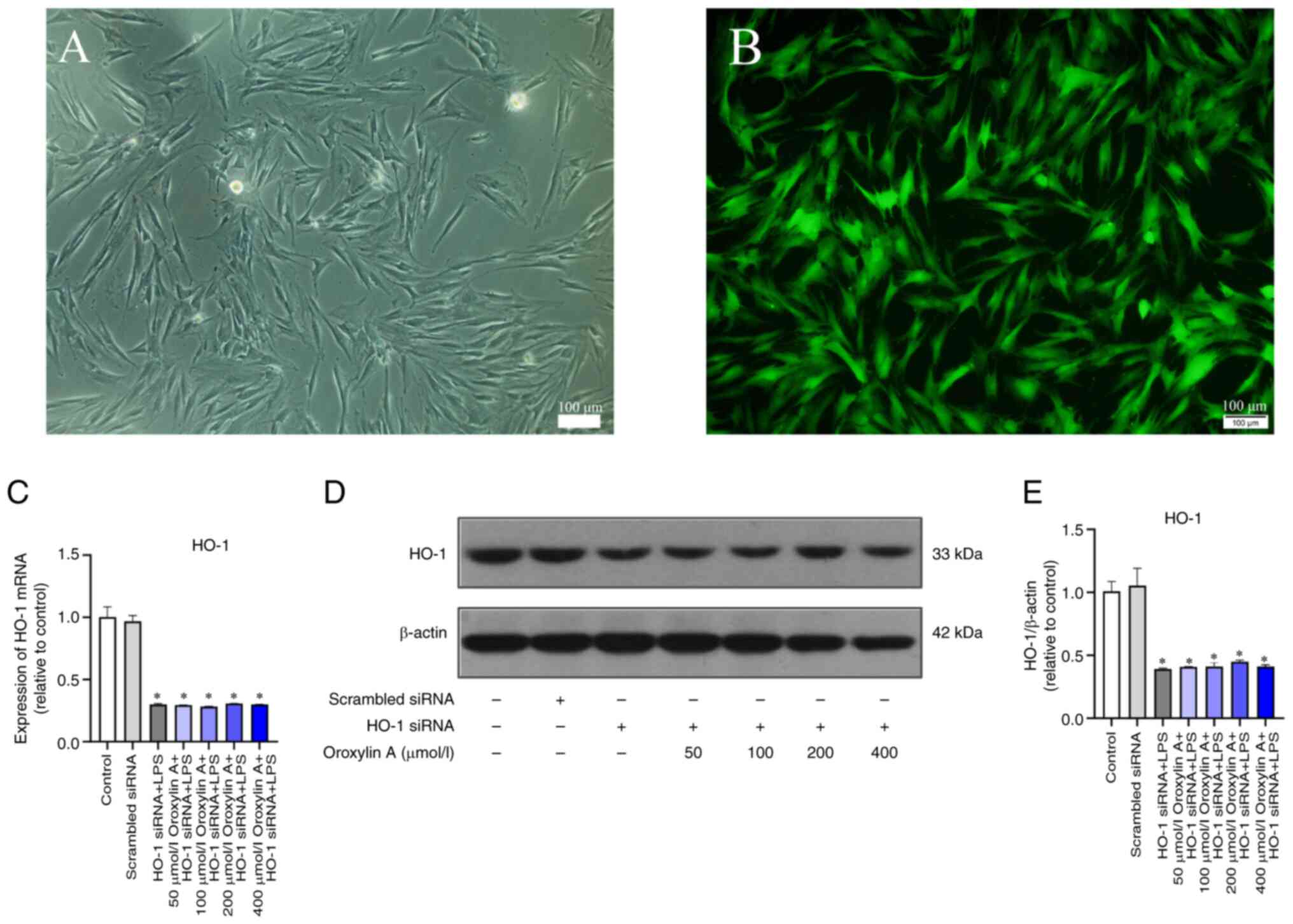
Transfection efficiency of siRNA and
effect of HO-1 knockdown in RGFs
Following the transfection of RGFs with
fluorescence-labeled siRNA, peripheral cells showed high brightness
under microscope (Fig. 4A). The
results of fluorescence microscopy showed that the fluorescence was
evenly distributed in the cells (Fig.
4B). RT-qPCR and western blotting showed no significant
differences in the HO-1 mRNA and protein expression levels of
scrambled group and the control group (Fig. 4C-E).
RGFs were induced by LPS inflammation following HO-1
gene silencing, and RT-qPCR and WB showed that the mRNA and protein
expression of COX-2, TNF-α and RANKL were significantly increased,
and there was no statistical difference in the OPG group (Fig. 5A-K). mRNA and protein expression
levels of COX-2, TNF-α, RANKL and OPG as well as the RANKL/OPG
ratio were not significantly decreased by different concentrations
of Oroxylin A (Fig. 5A-K).
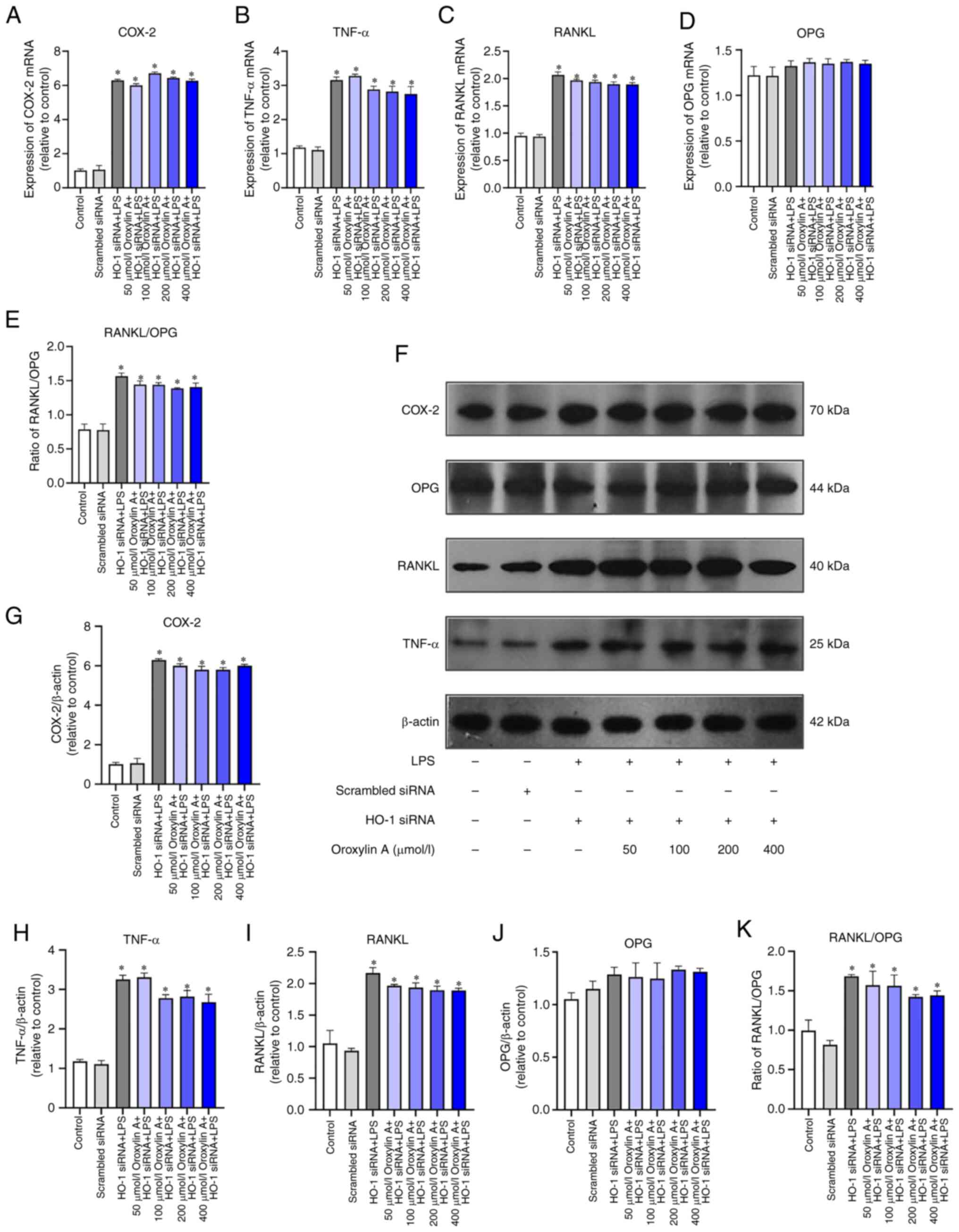 | Figure 5.RGFs (HO-1−) infected with
LPS and treated with Oroxylin A. Levels of mRNA of COX-2, (A)
TNF-α, (B) RANKL, (C) OPG (D) and RANKL/OPG (E) were analyzed by
reverse transcription-quantitative PCR. (F) Protein levels of
COX-2, TNF-α, RANKL and OPG were analyzed by western blotting.
Levels of COX-2, (G) TNF-α, (H) RANKL, (I) OPG and (J) RANKL/OPG
(K) between control group, scrambled siRNA group, LPS group and
different concentration of Oroxylin A groups. The mRNA levels of
cytokines in untreated controls were set as 1.0. The blots were
stripped and re-probed with β-actin as a loading control.
*P<0.05 vs. control. RGF, rat gingival fibroblast; HO, heme
oxygenase; COX, cyclooxygenase; OPG, osteoprotegerin; LPS,
lipopolysaccharide; si, small interfering. |
Effect of Oroxylin A in a rat model of
periodontitis
Immunohistochemical results showed that COX-2 was
expressed in the periodontal membrane of rats and the positive
cells were yellowish brown. Expression of COX-2+ cells
in every group was significantly different. The expression of COX-2
in the periodontal tissues of the control and the Oroxylin A
treatment group was significantly lower than that of the
periodontitis group (Fig. 6A-F)
and the expression of COX-2, TNF-α and RANKL/OPG ratio in the
periodontal tissue of the Oroxylin A treatment group was slightly
higher than that of the control, but the expression level decreased
with the increase of the dose (Fig.
6G-L).
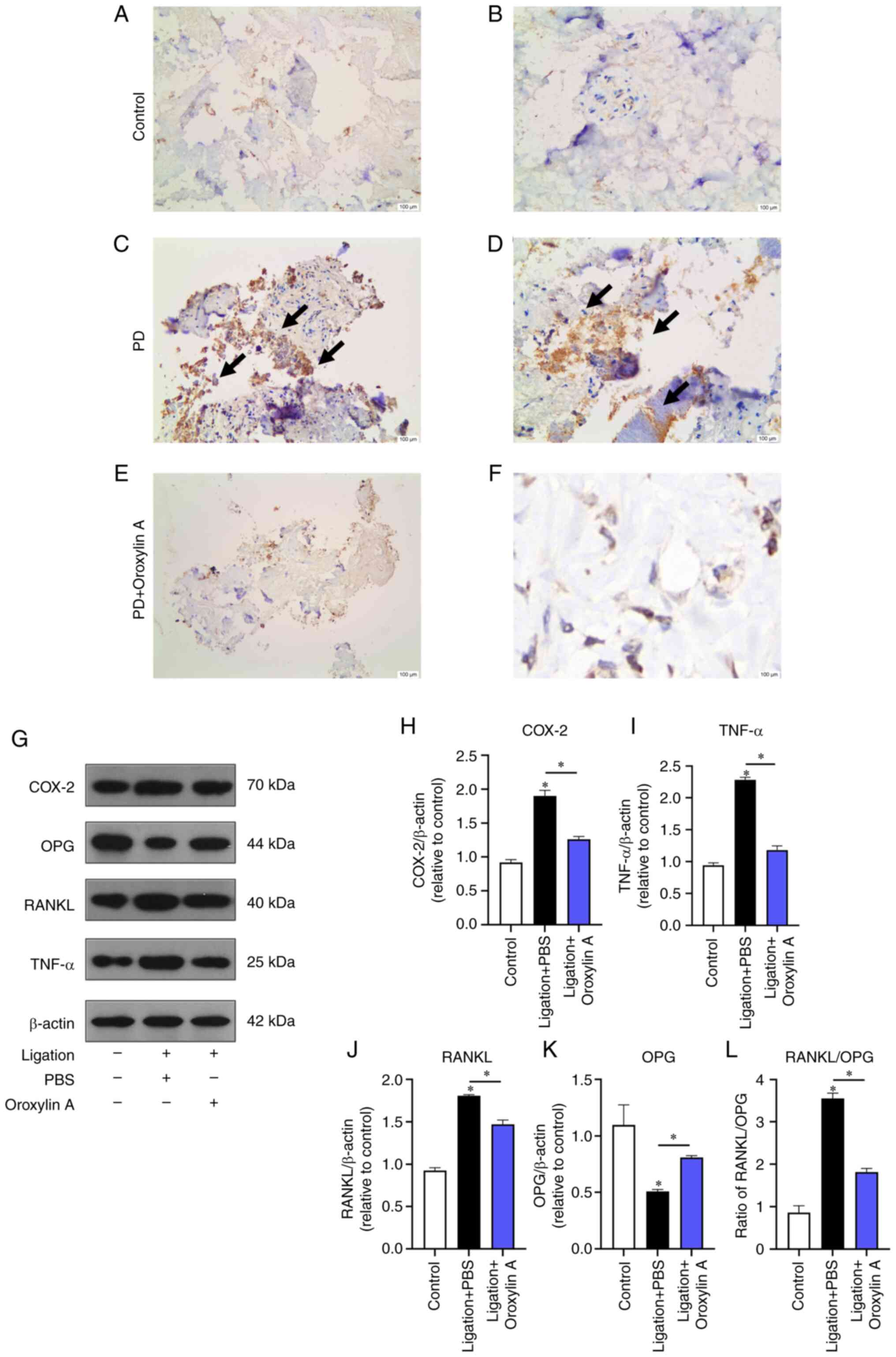 | Figure 6.Immunohistochemical expression of
COX-2 and the protein expression of COX-2, TNF-α and RANKL/OPG
ratio in the control group, the ligation group and the Oroxylin A
treatment group. Immunohistochemical results of the expression of
COX-2 in control group, (A) in control group, (B) in PD group, (C)
in PD group, (D) in PD +Oroxylin A group, (E) in PD+ Oroxylin A
group (F). (G) Protein levels of COX-2, OPG, RANKL and TNF-α in
gums. Levels of COX-2, (H) TNF-α, (I) RANKL(J), OPG and (K)
RANKL/OPG (L) between three groups of PBS, ligation +PBS, ligation
+PBS +Oroxylin A. COX, cyclooxygenase; OPG, osteoprotegerin.
*P<0.05 vs. control. |
Discussion
Traditional Chinese medicine and its extracts have
potential in treating periodontitis (21). Oroxylin A is the primary active
ingredient in S. baicalensis, with anti-inflammatory,
antitumor, antioxidant, vascular protection and other
pharmacological effects (22,23).
Because of these properties, Oroxylin A provides a promising
approach for treatment of periodontitis. The purpose of the current
study was to investigate the anti-inflammatory effect of Oroxylin A
on LPS-stimulated RGFs and the underlying molecular mechanism. In
preliminary experiments, in vitro CCK-8 assay was used to
detect the cytotoxic effect of Oroxylin A on RGFs; there was no
significant difference between fibroblasts treated with 0–400
µmol/l Oroxylin A and normal fibroblasts. These results indicate
that Oroxylin A has low toxicity on RGFs and has the potential to
be applied topically in periodontal tissue.
Techniques commonly employed to identify fibroblasts
include morphological observation, immunocytochemical staining,
biological characteristic assessment, and molecular biology assay
(24,25). Morphological observations via
microscopy provide a basic understanding of cell shape and
structure but lack specificity. Immunocytochemical staining using
antibodies against specific fibroblast markers such as collagen or
α-smooth muscle actin can offer higher specificity but may have
limitations related to antibody specificity and potential
cross-reactivity. Biological characteristic assessment, such as
analyzing cell dependency on culture media, proliferation rate and
cell cycle progression, could provide functional insights into
fibroblast behavior. Similarly, molecular biology techniques such
as RT-qPCR or western blotting can confirm the expression of
fibroblast-specific genes, offering molecular-level validation
(26).
Prostaglandins are synthesized from arachidonic acid
by COX (27). The most abundant
prostaglandin in the body is TNF-α; COX-2 appears to be the major
COX that controls TNF-α synthesis in the inflammatory response
(28). Numbers of studies have
proved that COX-2 and TNF-α are notably upregulated in the
occurrence and development of periodontitis and are involved in the
damage of the periodontal tissue, leading to gingival inflammation
and alveolar bone resorption (29–31).
Therefore, in the present study, COX-2 and TNF-α were selected to
detect the effects of Oroxylin A on these cytokines. According to
RT-qPCR and western blotting results, Oroxylin A can inhibit
production of COX-2 and TNF-α in LPS-induced RGFs suggesting that
Oroxylin A may serve a role in regulating the immune response
related to periodontal disease.
RANKL is the master regulator of osteoclast
differentiation and function, serving an integral role in
osteoclast formation (32,33). OPG, a decoy receptor of RANKL, is a
key bone protection factor that serves a core role in bone
homeostasis (34). A large number
of studies have confirmed that upregulation of RANKL, but also the
downregulation or degradation of OPG, are related to the loss of
periodontal bone, and the RANKL/OPG ratio reflects the level of
bone resorption (32,35,36).
The present study revealed that Oroxylin A regulated the RANKL/OPG
ratio on the mRNA and protein levels and participated in the
process of osteogenic repair in periodontitis. Oroxylin A can
affect the outcome of periodontitis in both decreasing inflammation
and promoting osteogenesis.
HO-1 expression is upregulated in the bone, which
may be an important antioxidant defense mechanism (37,38).
The present study showed that HO-1 increased with Oroxylin A dose.
Therefore, it was hypothesized that Oroxylin A exerted osteogenic
and anti-inflammatory properties by upregulating the expression of
HO-1. The expression of HO-1 was knocked down, and the mRNA and
protein levels of COX-2, TNF-α, RANKL and OPG were detected.
Oroxylin A did not decrease expression of these cytokines
upregulated by LPS stimulation. This indicates that Oroxylin A
relies on HO-1 to play its role and downregulates the expression of
inflammatory factors in LPS-stimulated RGFs.
Rankl regulates osteoclasts, serving a key role in
bone metabolism (39). Here, a rat
model of ligation-induced periodontitis was constructed and
Oroxylin A was used to intervene. Oroxylin A could protect the
periodontal tissues from damage of COX-2 and TNF-α and decrease the
RANKL/OPG ratio at the same time. Ligation promotes plaque
accumulation, leading to periodontitis with a pathogenesis similar
to human periodontitis (40).
Immunohistochemistry showed that Oroxylin A decreased the
expression of COX-2 in periodontitis rats. Western blotting showed
that COX-2, TNF-α and RANKL/OPG ratio were all downregulated in the
periodontal tissue of rats with periodontitis. A number of studies
have confirmed that Oroxylin A inhibits cellular inflammation, as
well as osteoarthritis, respiratory inflammation and skin tumors
(9,41,42).
Oroxylin A was here applied to the treatment of periodontitis,
providing novel potential options for the treatment of
periodontitis in the future.
The present study investigated the effect of
Oroxylin A on HO-1 expression to verify whether Oroxylin A inhibits
inflammatory cytokines in periodontitis via HO-1. Oroxylin A did
not induce expression of inflammatory cytokines in the case of HO-1
knockdown, demonstrating that Oroxylin A inhibiting inflammatory
cytokines in periodontitis via HO-1. In conclusion, Oroxylin A
decreased expression of inflammatory cytokines in LPS-induced RGFs
and has a good inhibitory effect on periodontitis in rats,
providing new drug options for the treatment of periodontitis.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Yuli Gao for
assistance with the experiments and Dr Congshan Li for valuable
discussion (Department of Orthodontics, The Affiliated Hospital of
Qingdao University) .
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 51703106) and the Natural Science
Foundation of Shandong Province of China (grant nos. ZR2016EMQ05
and ZR2022ME021).
Availability of data and materials
The data generated in the present study are included
in the figures and tables of this article.
Authors' contributions
TW, ZW and CJ performed experiments and wrote the
article. YZ and LT analyzed and interpreted data and wrote the
manuscript. JF and XX conceived and designed the study and revised
the manuscript. All authors have read and approved the final
manuscript. TW and JF confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee for the Welfare of Experimental Animals of the Affiliated
Hospital of Qingdao University (approval no. AHQU-MAL
20210326).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu S, Zhou Q, Jiang Z, Wang Y, Yang K, Qiu
X and Ji Q: The effect of doxycycline-containing
chitosan/carboxymethyl chitosan nanoparticles on NLRP3 inflammasome
in periodontal disease. Carbohydr Polym. 237:1161632020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slots J: Focal infection of periodontal
origin. Periodontol 2000. 79:233–235. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slots J: Periodontitis: Facts, fallacies
and the future. Periodontol 2000. 75:7–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herrera D, Alonso B, León R, Roldán S and
Sanz M: Antimicrobial therapy in periodontitis: The use of systemic
antimicrobials against the subgingival biofilm. J Clin Periodontol.
35 (Suppl):45–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graziani F, Karapetsa D, Alonso B and
Herrera D: Nonsurgical and surgical treatment of periodontitis: How
many options for one disease? Periodontology 2000. 75:152–188.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teughels W, Feres M, Oud V, Martín C,
Matesanz P and Herrera D: Adjunctive effect of systemic
antimicrobials in periodontitis therapy: A systematic review and
meta-analysis. J Clin Periodontol. 47 (Suppl 22):257–281. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu Y, Chen X, Wang R, Wang S, Wang X,
Zheng L, Zhang B, Chai Y, Zhu Z and Yuan Y: Comparative
two-dimensional HepG2 and L02/cell membrane
chromatography/C18/time-of-flight mass spectrometry for screening
selective anti-hepatoma components from Scutellariae Radix. J Pharm
Biomed Anal. 164:550–556. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin J, Chen S, Wang D, Chen Y, Wang Y, Guo
M, Zhou C and Dou J: Oroxylin A suppresses influenza A virus
replication correlating with neuraminidase inhibition and induction
of IFNs. Biomed Pharmacother. 97:385–394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Tong D, Liu J, Chen F and Shen Y:
Oroxylin A attenuates cigarette smoke-induced lung inflammation by
activating Nrf2. Int Immunopharmacol. 40:524–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han Q, Wang H, Xiao C, Fu BD and Du CT:
Oroxylin A inhibits H2O2-induced oxidative
stress in PC12 cells. Nat Prod Res. 31:1339–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao H, Ye J, Gao L and Liu Y: The main
bioactive compounds of Scutellaria baicalensis Georgi. for
alleviation of inflammatory cytokines: A comprehensive review.
Biomed Pharmacother. 133:1109172021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Y, Li C, Peng X, Jiang N, Hu L, Gu L,
Zhu G, Zhao G and Lin J: Perillaldehyde ameliorates aspergillus
fumigatus keratitis by activating the Nrf2/HO-1 signaling pathway
and inhibiting dectin-1-mediated inflammation. Invest Ophthalmol
Vis Sci. 61:512020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryter SW: Heme oxygenase-1: An
anti-inflammatory effector in cardiovascular, lung, and related
metabolic disorders. Antioxidants (Basel). 11:5552022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim EN, Kaygusuz O, Lee HS and Jeong GS:
Simultaneous quantitative analysis of ginsenosides isolated from
the fruit of Panax ginseng C.A. Meyer and regulation of HO-1
expression through EGFR signaling has anti-inflammatory and
osteogenic induction effects in HPDL cells. Molecules. 26:20922021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HY, Lee GH, Kim JH, Cheng J, Cho JH,
Suh JW and Chae HJ: Ixeris dentata and lactobacillus gasseri media
protect against periodontitis through Nrf2-HO-1 signalling pathway.
Sci Rep. 13:128612023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
França ALQ, Chaves HV, Freire JMO, de
Sousa LHT, Pimenta ATA, Lima MAS, de Oliveira BR, de Mattos MC,
Pinto VPT, Portela AMLR, et al: Molecular docking study and
antireabsorptive activity of a semi-synthetic coumarin derivative
from Platymiscium floribundum in the ligature-induced periodontitis
in rats: The involvement of heme oxygenase-1. Clin Oral Investig.
26:1701–1711. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pacios S, Kang J, Galicia J, Gluck K,
Patel H, Ovaydi-Mandel A, Petrov S, Alawi F and Graves DT: Diabetes
aggravates periodontitis by limiting repair through enhanced
inflammation. FASEB J. 26:1423–1430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu S, Zhou Q, Fan C, Zhao H, Wang Y, Qiu
X, Yang K and Ji Q: Doxycycline inhibits NAcht Leucine-rich repeat
protein 3 inflammasome activation and interleukin-1β production
induced by porphyromonas gingivalis-lipopolysaccharide and
adenosine triphosphate in human gingival fibroblasts. Arch Oral
Biol. 107:1045142019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park HJ, Salem M, Semlali A, Leung KP and
Rouabhia M: Antimicrobial peptide KSL-W promotes gingival
fibroblast healing properties in vitro. Peptides. 93:33–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwak HJ, Yang D, Hwang Y, Jun HS and Cheon
HG: Baicalein protects rat insulinoma INS-1 cells from
palmitate-induced lipotoxicity by inducing HO-1. PLoS One.
12:e01764322017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De G, Chen A, Zhao Q, Xie R, Wang C, Li M,
Zhao H, Gu X, McCarl LH, Zhang F, et al: A multi-herb-combined
remedy to overcome hyper-inflammatory response by reprogramming
transcription factor profile and shaping monocyte subsets.
Pharmacol Res. 169:1056172021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Jia Y, Li M, Wang L, Shao J, Guo
Q, Tan S, Ding H, Chen A, Zhang F and Zheng S: Blockade of
glycolysis-dependent contraction by Oroxylin A via inhibition of
lactate dehydrogenase-a in hepatic stellate cells. Cell Commun
Signal. 17:112019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin H, Lian N, Bian M, Zhang C, Chen X,
Shao J, Wu L, Chen A, Guo Q, Zhang F and Zheng S: Oroxylin A
prevents alcohol-induced hepatic steatosis through inhibition of
hypoxia inducible factor 1alpha. Chem Biol Interact. 285:14–20.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang W, Zhang Y, Qian C, Yuan Z and Du J:
Induction and mechanism of apoptosis by hydroxycamptothecin in
human Tenon's capsule fibroblasts. Invest Ophthalmol Vis Sci.
53:4874–4880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye J, Zhang B, Xu J, Chang Q, McNutt MA,
Korteweg C, Gong E and Gu J: Molecular pathology in the lungs of
severe acute respiratory syndrome patients. Am J Pathol.
170:538–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi T, Kim H, Liu X, Sugiura H,
Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, et al:
Matrix metalloproteinase-9 activates TGF-β and stimulates
fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol
Physiol. 306:L1006–L1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizuno R, Kawada K and Sakai Y:
Prostaglandin E2/EP signaling in the tumor microenvironment of
colorectal cancer. Int J Mol Sci. 20:62542019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JY, Pillinger MH and Abramson SB:
Prostaglandin E2 synthesis and secretion: The role of PGE2
synthases. Clin Immunol. 119:229–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sánchez GA, Miozza VA, Delgado A and Busch
L: Salivary IL-1β and PGE2 as biomarkers of periodontal status,
before and after periodontal treatment. J Clin Periodontol.
40:1112–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hienz SA, Paliwal S and Ivanovski S:
Mechanisms of bone resorption in periodontitis. J Immunol Res.
2015:6154862015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin LJ, Söder PO, Leung WK, Corbet EF,
Samaranayake LP, Söder B and Davies WI: Granulocyte elastase
activity and PGE2 levels in gingival crevicular fluid in relation
to the presence of subgingival periodontopathogens in subjects with
untreated adult periodontitis. J Clin Periodontol. 26:531–540.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsukasaki M and Takayanagi H:
Osteoimmunology: Evolving concepts in bone-immune interactions in
health and disease. Nat Rev Immunol. 19:626–642. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okamoto K, Nakashima T, Shinohara M,
Negishi-Koga T, Komatsu N, Terashima A, Sawa S, Nitta T and
Takayanagi H: Osteoimmunology: The conceptual framework unifying
the immune and skeletal systems. Physiol Rev. 97:1295–1349. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akiyama T, Miyamoto Y, Yoshimura K, Yamada
A, Takami M, Suzawa T, Hoshino M, Imamura T, Akiyama C, Yasuhara R,
et al: Porphyromonas gingivalis-derived lysine gingipain enhances
osteoclast differentiation induced by tumor necrosis factor-α and
interleukin-1β but suppresses that by interleukin-17A: Importance
of proteolytic degradation of osteoprotegerin by lysine gingipain.
J Biol Chem. 289:15621–15630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsukasaki M, Asano T, Muro R, Huynh NC,
Komatsu N, Okamoto K, Nakano K, Okamura T, Nitta T and Takayanagi
H: OPG production matters where it happened. Cell Rep.
32:1081242020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cawley KM, Bustamante-Gomez NC, Guha AG,
MacLeod RS, Xiong J, Gubrij I, Liu Y, Mulkey R, Palmieri M,
Thostenson JD, et al: Local production of osteoprotegerin by
osteoblasts suppresses bone resorption. Cell Rep. 32:1080522020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee D, Kook SH, Ji H, Lee SA, Choi KC, Lee
KY and Lee JC: N-acetyl cysteine inhibits H2O2-mediated reduction
in the mineralization of MC3T3-E1 cells by down-regulating
Nrf2/HO-1 pathway. BMB Rep. 48:636–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi EM, Suh KS, Kim YJ, Hong SM, Park SY
and Chon S: Glabridin alleviates the toxic effects of methylglyoxal
on osteoblastic MC3T3-E1 cells by increasing expression of the
glyoxalase system and Nrf2/HO-1 signaling and protecting
mitochondrial function. J Agric Food Chem. 64:226–235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nazar N, Mehmood MH, Siddique R and Faisal
MN: Assessment of antiarthritic potential of Asparagus dumosus
using formaldehyde and CFA-induced arthritic models in rats via
modulation of oxidative stress biomarkers and mRNA expression of
IL-1b, IL-6, RANKL, OPG, TNF-α and COX-2. Inflammopharmacology.
32:825–847. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu L, Li C, Cai X, Xiang J, Cao Z and
Dong W: The temporal expression and localization of extracellular
matrix metalloproteinase inducer (EMMPRIN) during the development
of periodontitis in an animal model. J Periodontal Res. 45:541–549.
2010.PubMed/NCBI
|
|
41
|
Chen DH, Zheng G, Zhong XY, Lin ZH, Yang
SW, Liu HX and Shang P: Oroxylin A attenuates osteoarthritis
progression by dual inhibition of cell inflammation and
hypertrophy. Food Funct. 12:328–339. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang H, Cai H, Zhang L, Hua Z, Shi J and
Wei Y: Oroxylin A inhibits carcinogen-induced skin tumorigenesis
through inhibition of inflammation by regulating SHCBP1 in mice.
Int Immunopharmacol. 80:1061232020. View Article : Google Scholar : PubMed/NCBI
|