Introduction
Primary osteoporosis is a metabolic disease in which
bone loss leads to increased bone fragility; notably, this
condition is more common in postmenopausal women (1). The rate of bone formation is lower
than the rate of bone resorption by osteoclasts, which ultimately
leads to osteoporosis (2). Further
investigations into potential indicators of osteoporosis are
required, to improve the timely diagnosis of osteoporosis and to
reduce the probability of fracture in clinical practice.
C1q/tumor necrosis factor-related protein-3 (CTRP3)
is a highly hydrophilic secreted protein that serves an important
role in a variety of metabolic and inflammatory diseases (3–5).
Serum CTRP3 levels have been shown to be decreased in patients with
osteoporosis, and a significant decrease in the incidence of
osteoporosis has been observed in the presence of CTRP3 (6). The results of a previous study
demonstrated that the serum levels of CTRP3 in patients with
primary hyperparathyroidism are lower than those observed in
healthy controls (7). In addition,
the serum levels of CTRP3 in patients with primary
hyperparathyroidism with osteoporosis were shown to be lower than
those in patients with primary hyperparathyroidism without
osteoporosis. The results of a logistic regression analysis further
revealed that CTRP3 levels may be used to independently determine
whether a patient has osteoporosis (7). However, the specific role of CTRP3 in
osteoporosis has yet to be fully elucidated. CTRP3 is highly
upregulated in fracture callus tissue, and delayed intrachondral
fracture healing can lead to abnormal mineral distribution in
CTRP3-knockout mice. By contrast, callus remodeling has been
reported to be accelerated in mice with CTRP3 overexpression
(8). The results of a previous
study also revealed that CTRP3 suppresses osteoclastogenic
factor-induced osteoclast differentiation and bone destruction
(9). Notably, CTRP3 significantly
accelerates the calcification of abdominal aorta and the arterial
ring, upregulates the expression of osteogenic marker genes, and
enhances the expression of osteogenic markers in
β-glycerophosphate-induced vascular smooth muscle cells (10). The results of these previous
studies highlighted that CTRP3 exhibits a specific regulatory
effect on the differentiation of osteoblasts and osteoclasts.
Activation of the AMP-activated protein kinase (AMPK)/sirtuin 1
(SIRT1) signaling pathway promotes the osteogenic differentiation
and antioxidant response of mouse bone mesenchymal stem cells
(11), and activation of
AMPK/nuclear factor estradiol (E2)-related factor 2 (Nrf2) inhibits
RANKL-induced osteoclast generation and oxidative stress (12). Therefore, it was hypothesized that
CTRP3 may inhibit oophorectomy (OVX)-induced bone loss in mice, and
this process may be associated with the AMPK/SIRT1/Nrf2 signaling
pathway.
Globular CTRP3 (gCTRP3) is a truncated form of
CTRP3, and is termed ‘globular’ because it comprises the C-terminal
globular domain of CTRP3 (13).
CTRPs all contain C1q globular domains and are jointly
characterized as the 1q/TNF superfamily, which comprises CTRP1,
CTRP3, CTRP4, CTRP5, CTRP6 and CTRP7 (14–16).
Notably, the globular structure of CTRP1 has been proven to be
consistent with the function of the full-length domain of CTRP1
(17). As previously described,
gCTRP3 is biologically active, and gCTRP3 has been demonstrated to
improve impaired vasodilatation of microvasculature in diabetes by
ameliorating endothelial cell function (13). Besides, supplementation with the
recombinant human gCTRP3 protects against high-fat diet-induced
spermatogenic deficiency in mice (18). gCTRP3 has been reported to promote
mitochondrial biogenesis in hypoxia/reoxygenation-induced rat
cardiomyocytes (19). A recent
study demonstrated that gCTRP3 treatment improves impaired
vasodilatation of the microvasculature in a murine model of type 2
diabetes mellitus by ameliorating endothelial cell function
(13). The present study aimed to
explore the effects of gCTRP3 on OVX-induced mice, and MC3T3-E1
cells were selected for in vitro experiments in this study.
The MC3T3-E1 mouse calvaria-derived preosteoblast cell line has
contributed to the investigation of the role of osteoblasts in bone
formation (20). In addition, the
role of AMPK/SIRT1/Nrf2 signaling was investigated using an AMPK
inhibitor (Compound C), SIRT1 inhibitor (EX527) and Nrf2 inhibitor
(ML385).
Materials and methods
Animal experiments
A total of 30 female C57BL/6J mice (age, 6 weeks;
weight, 21–25 g) purchased from Hangzhou Ziyuan Laboratory Animal
Technology Co., Ltd. were housed under the conditions of a 12-h
light/dark cycle at 21–23°C with a relative humidity of 60–65% with
free access to water and food. All experimental procedures were
carried out according to institutional guidelines and were approved
by the Animal Care and Ethics Committee of The Third Hospital of
Hebei Medical University (Shijiazhuang, China; approval no.
Z2023-020-1). All mice were anesthetized using an intraperitoneal
injection of 50 mg/kg sodium pentobarbital. OVX was achieved
through removal of the bilateral ovaries, and an equal amount of
adipose tissue was removed to create control-operated mice.
At 1 week post-surgery, the control-operated mice
were randomly divided into the following two groups (n=6/group): i)
Control group (control-operated mice treated with distilled water)
and ii) gCTRP3 group [control-operated mice treated with human
recombinant gCTRP3 (0.5 mg/kg)]. The OVX mice were randomly divided
into the following three groups (n=6/group): i) OVX group (OVX mice
treated with distilled water); ii) OVX + gCTRP3 group [OVX mice
treated with gCTRP3 (0.5 mg/kg)]; and iii) OVX + E2 group [OVX mice
treated with E2-valerate tablets (Bayer Healthcare Co., Ltd.; 1
mg/kg)]. Mice in the treatment groups were administered gCTRP3, E2
or an equal volume of distilled water once a day via gavage. gCTRP3
was purchased from Aviscera Bioscience. After 12 weeks of
treatment, all mice were fasted overnight and euthanized with an
intraperitoneal injection of 200 mg/kg sodium pentobarbital.
Subsequently, blood was collected from the femoral artery, and
serum was obtained following the centrifugation of blood at 1,000 ×
g for 10 min at 4°C. The left femurs of all mice were isolated for
micro-computed tomography (CT) and histological analysis.
Micro-CT scans and histological
analysis
For micro-CT scans, the left femurs were evaluated
using a Skyscan 1076 scanner (SkyScan NV) at 35 µm resolution.
Reconstructed images were segmented to quantify the trabecular bone
structure using CTAn software (v1.10.9.0; SkyScan NV), and 3D
images were visualized using Ant software (release 2.05; SkyScan
NV). The volume of interest (VOI) was 1–1.5 mm below the growth
plate. The bone mineral density (BMD), bone volume/tissue volume
(BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and
trabecular spacing (Tb.Sp) were calculated within the delimited VOI
(21). For histological analysis,
the left femurs were fixed in 4% paraformaldehyde for 24 h at room
temperature, decalcified in 12% EDTA (pH 7.4) for 21 days at room
temperature, and embedded in paraffin. The tissues were sliced into
5-µm sections, and these were subsequently used for hematoxylin and
eosin, and tartrate-resistant acid phosphatase (TRAP) staining. For
hematoxylin and eosin staining, the sections were stained with
hematoxylin for 5 min at room temperature and eosin for 5 min at
room temperature. TRAP staining was performed using a 387A kit
(Merck KGaA), Briefly, a mixed solution of citrate solution, Fast
Gamet GBC Base solution, naphthol AS-BI phosphonic acid solution
and sodium nitrite solution were preheated at 37°C before tartrate
solution was added to the mixture, the sections were then submerged
into the mixed solution for 1 h at 37°C. Stained sections were
photomicrographed using a light microscope (Olympus Corp.).
Immunofluorescence analysis
The paraffin-embedded sections (5 µm) were dewaxed
with xylene and rehydrated with a gradient ethanol series. The
sections were then permeabilized with 0.1% Triton X-100 (BioFroxx;
neoFroxx GmbH) for 3 min followed by incubation with 5% bovine
serum albumin (BSA; BioFroxx; neoFroxx GmbH) for 1 h at room
temperature. Subsequently, the sections were incubated with an
anti-Runt-related transcription factor-2 (RUNX2) antibody
(1:10,000; cat. no. 12556S; Cell Signaling Technology, Inc.)
overnight at 4°C. Subsequently, sections were washed with PBS and
incubated with goat anti-rabbit IgG secondary antibody conjugated
to Alexa Fluor® 488 (1:1,000; cat. no. 4412S; Cell
Signaling Technology, Inc.) at room temperature for 1 h. After
washing in PBS, the sections were counterstained with DAPI for 10
min at room temperature and were observed under a fluorescence
microscope (Olympus Corp.).
ELISA
The levels of C-telopeptide of type I collagen
(CTX-1), TRAP, osteocalcin (OCN) and procollagen type 1 N-terminal
propeptide (P1NP) in serum were detected using CTX-1 (cat. no.
E-EL-M3023; Elabscience Biotechnology, Inc.), TRAP (cat. no.
SP14794; SPBIO), OCN (cat. no. E-EL-M0864c; Elabscience
Biotechnology, Inc.) and P1NP (cat. no. E-EL-M0233c; Elabscience
Biotechnology, Inc.) ELISA kits according to the manufacturers'
instructions. Absorbance was measured using a microplate
reader.
Cell culture and treatment
MC3T3-E1 cells (cat. no. CRL-2593) were obtained
from the American Type Culture Collection, and were cultured in
α-MEM (HyClone; Cytiva) supplemented with 10% FBS (HyClone;
Cytiva), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C
with 5% CO2. Prior to osteogenic differentiation,
MC3T3-E1 cells were pretreated with different concentrations of
gCTRP3 (0.2, 1 and 2 µg/ml) for 24 h at 37°C. An AMPK inhibitor
(Compound C; 1 µM; MedChemExpress) (22), SIRT1 inhibitor (EX527; 10 µM;
MedChemExpress) (23) or Nrf2
inhibitor (ML385; 5 µM; MedChemExpress) (24) was used to treat MC3T3-E1 cells in
the presence of gCTRP3 for 24 h at 37°C. Subsequently, MC3T3-E1
cells were seeded into 6-well plates at a density of
1×104 cells/well and cultured in α-MEM supplemented with
10 mM β-glycerol phosphate, 50 µg/ml ascorbic acid and 100 nM
dexamethasone for up to 14 days at 37°C.
Cell Counting Kit-8 (CCK-8) assay
The viability of MC3T3-E1 cells was evaluated using
a CCK-8 assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.). Following pretreatment with gCTRP3, MC3T3-E1 cells were
incubated with CCK-8 solution (10 µl) for 2 h at 37°C. Absorbance
was detected using a microplate reader at a wavelength of 450
nm.
Alkaline phosphatase (ALP)
activity
The ALP activity of MC3T3-E1 cells was detected
using an ALP assay kit (cat. no. E-BC-K091-S; Elabscience
Biotechnology, Inc.) according to the manufacturer's instructions.
Absorbance was detected using a microplate reader at a wavelength
of 450 nm.
ALP staining and Alizarin red staining
(ARS)
For ALP staining, MC3T3-E1 cells were fixed with 4%
formaldehyde at room temperature for 30 min, rinsed with 0.05% TBS
supplemented with Tween-20 and stained with naphthol/Fast Red
Violet solution (Sigma-Aldrich; Merck KGaA) at room temperature for
15 min, according to the manufacturer's instructions. Then cells
were rinsed and imaged under a light microscope (Olympus
Corp.).
For ARS, MC3T3-E1 cells were fixed with 4%
formaldehyde at room temperature for 15 min and stained with 40 mM
ARS dye (pH 4.2; Beyotime Institute of Biotechnology) at room
temperature for 10 min. Calcium deposits were observed under a
light microscope (Olympus Corp.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Following treatment, total RNA was extracted from
MC3T3-E1 cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA was reverse transcribed into cDNA
using the PrimeScript RT reagent kit (Takara Bio, Inc.) according
to manufacturer's protocol, followed by qPCR using Kapa
SYBR® FAST qPCR Master Mix (Takara Bio, Inc.). The PCR
reaction conditions consisted of 95°C for 3 min, followed by 40
cycles of 95°C for 30 sec and 60°C for 30 sec. RUNX2, ALP
and OCN mRNA expression levels in MC3T3-E1 cells were
quantified using the 2−∆∆Cq method and normalized to the
internal reference gene GAPDH (25). The primer sequences were designed
by Sangon Biotech Co., Ltd. The following primer pairs were used
for qPCR: RUNX2, forward 5′-GCCAATCCCTAAGTGTGGCT-3′, reverse
5′-AACAGAGAGCGAGGGGGTAT-3′; ALP, forward
5′-TGGTCACAGCAGTTGGTAGC-3′, reverse 5′-CTGAGATTCGTCCCTCGCTG-3′;
OCN, forward 5′-ATGGCTTGAAGACCGCCTAC-3′, reverse
5′-GACAGGGAGGATCAAGTCCC-3′; and GAPDH, forward
5′-CCCTTAAGAGGGATGCTGCC-3′ and reverse
5′-TACGGCCAAATCCGTTCACA-3′.
Western blot analysis
Following treatment, MC3T3-E1 cells were lysed in
ice-cold RIPA lysis buffer (Beyotime Institute of Biotechnology).
The concentration of total proteins in MC3T3-E1 cells was detected
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts of protein (40 µg per lane) were
separated by SDS-PAGE on 10% gels and were transferred onto
polyvinylidene difluoride membranes. After blocking with 5% BSA for
1 h at room temperature, the membranes were incubated with primary
antibodies against phosphorylated (p)-AMPK (1:1,000 dilution; cat.
no. 2535T; Cell Signaling Technology, Inc.), AMPK (1:1,000; cat.
no. 2532S; Cell Signaling Technology, Inc.), SIRT1 (1:1,000; cat.
no. 9475T; Cell Signaling Technology, Inc.), Nrf2 (1:1,000; cat.
no. 80593-1-RR; Proteintech Group, Inc.) and GAPDH (1:1,000; cat.
no. 2118T; Cell Signaling Technology, Inc.) overnight at 4°C.
Subsequently, the membranes were incubated with HRP-conjugated goat
anti-rabbit IgG secondary antibody (1:10,000; cat. no. 7074P2; Cell
Signaling Technology, Inc.) for 1 h at room temperature. Protein
bands were visualized using ECL reagent (MilliporeSigma) and
protein expression was semi-quantified using ImageJ (version 1.8.0;
National Institutes of Health).
Statistical analysis
All experiments were independently repeated three
times and data are presented as the mean ± standard deviation.
Statistical analyses were performed using GraphPad Prism software
(version 8.0.1; Dotmatics). One-way ANOVA followed by Tukey's
post-hoc test was used to compare differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
gCTRP3 treatment alleviates bone loss
in OVX-induced mice
Results of the micro-CT scan demonstrated that loss
of the trabecular bone occurred in OVX-induced mice, and this loss
was improved in mice treated with gCTRP3 and E2 (the positive drug
group) (Fig. 1A). Morphometric
analysis of the left femurs of OVX-induced mice demonstrated that
BMD, BV/TV, Tb.N and Tb.Th were decreased in OVX-induced mice, and
these levels were markedly restored following treatment with gCTRP3
and E2 (Fig. 1B). By contrast,
Tb.Sp was increased in OVX-induced mice, and this was decreased
following treatment with gCTRP3 and E2 (Fig. 1B). Notably, the cortical bone
thickness and trabecular area were markedly decreased in
OVX-induced mice; however, the bone mass of OVX-induced mice was
increased in the gCTRP3 and E2 group (Fig. 2A). In addition, TRAP staining
indicated that OVX induced an increase in the number of
osteoclasts, and this number was decreased following treatment with
gCTRP3 and E2 (Fig. 2B). In
addition, the expression levels of RUNX2, a marker of bone
formation, were reduced in OVX-induced mice, and treatment with
gCTRP3 and E2 increased the expression levels (Fig. 2C). Simultaneously, the serum levels
of bone resorption markers (CTX-1 and TRAP) were increased, and
those of bone formation markers (OCN and P1NP) were decreased in
OVX-induced mice (Fig. 2D), which
was consistent with the results of previous studies (26–28).
By contrast, these levels were restored following treatment with
gCTRP3 and E2 (Fig. 2D).
Collectively, the results of the present study demonstrated that
gCTRP3 treatment alleviated bone loss in OVX-induced mice.
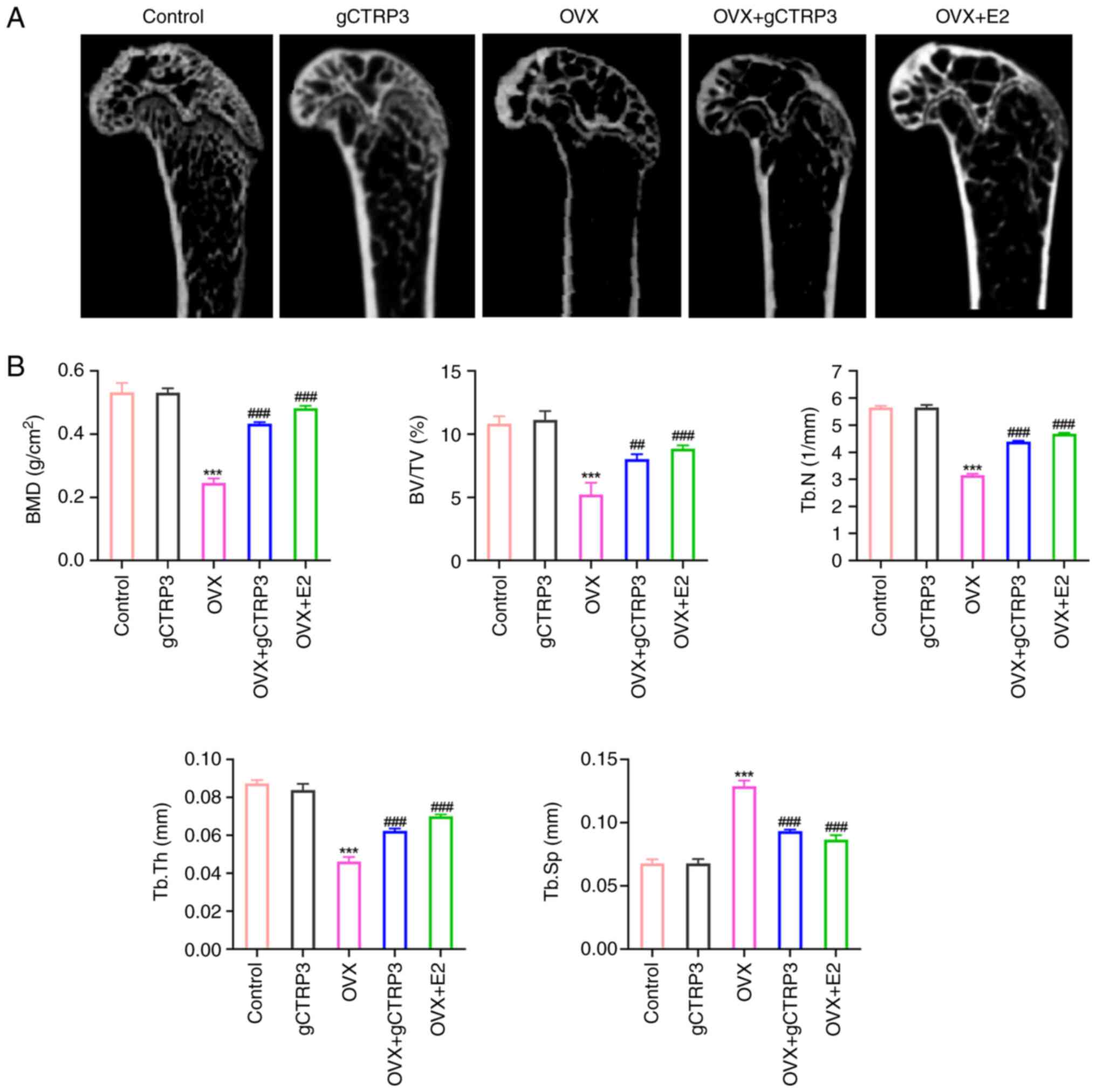 | Figure 1.gCTRP3 alleviates bone loss in
OVX-induced mice. (A) Left femurs of OVX-induced mice were examined
using a micro-computed tomography scan following gCTRP3 treatment.
(B) BMD, BV/TV, Tb.N, Tb.Th and Tb.Sp were quantified in the left
femurs of OVX-induced mice following treatment with gCTRP3.
***P<0.001 vs. Control group; ##P<0.01,
###P<0.001 vs. OVX group. gCTRP3, globular C1q/tumor
necrosis factor-related protein 3; OVX, oophorectomy; E2, estradiol
valerate; BMD, bone mineral density; BV/TV, bone volume/tissue
volume; Tb.N, trabecular number; Tb.Th, trabecular thickness;
Tb.Sp, trabecular separation. |
 | Figure 2.gCTRP3 inhibits bone resorption and
promotes bone formation. (A) Pathological changes of the left
femurs of OVX-induced mice were observed using hematoxylin and
eosin staining following treatment with gCTRP3 (scale bar, 25 µm).
(B) Number of osteoclasts in the left femurs of OVX-induced mice
were observed using TRAP staining following treatment with gCTRP3
(scale bar, 50 µm). (C) RUNX2 expression levels in the left femurs
of OVX-induced mice were detected using immunofluorescence analysis
following treatment with gCTRP3 (scale bar, 50 µm). (D) CTX-1,
TRAP, OCN and P1NP expression levels in the serum of OVX-induced
mice were detected using ELISA kits following treatment with
gCTRP3. ***P<0.001 vs. Control group; #P<0.05,
##P<0.01 and ###P<0.001 vs. OVX group.
gCTRP3, globular C1q/tumor necrosis factor-related protein 3; OVX,
oophorectomy; E2, estradiol valerate; TRAP, tartrate-resistant acid
phosphatase; RUNX2, Runt-related transcription factor-2; CTX-1,
C-telopeptide of type I collagen; OCN, osteocalcin; P1NP,
procollagen type 1 N-terminal propeptide. |
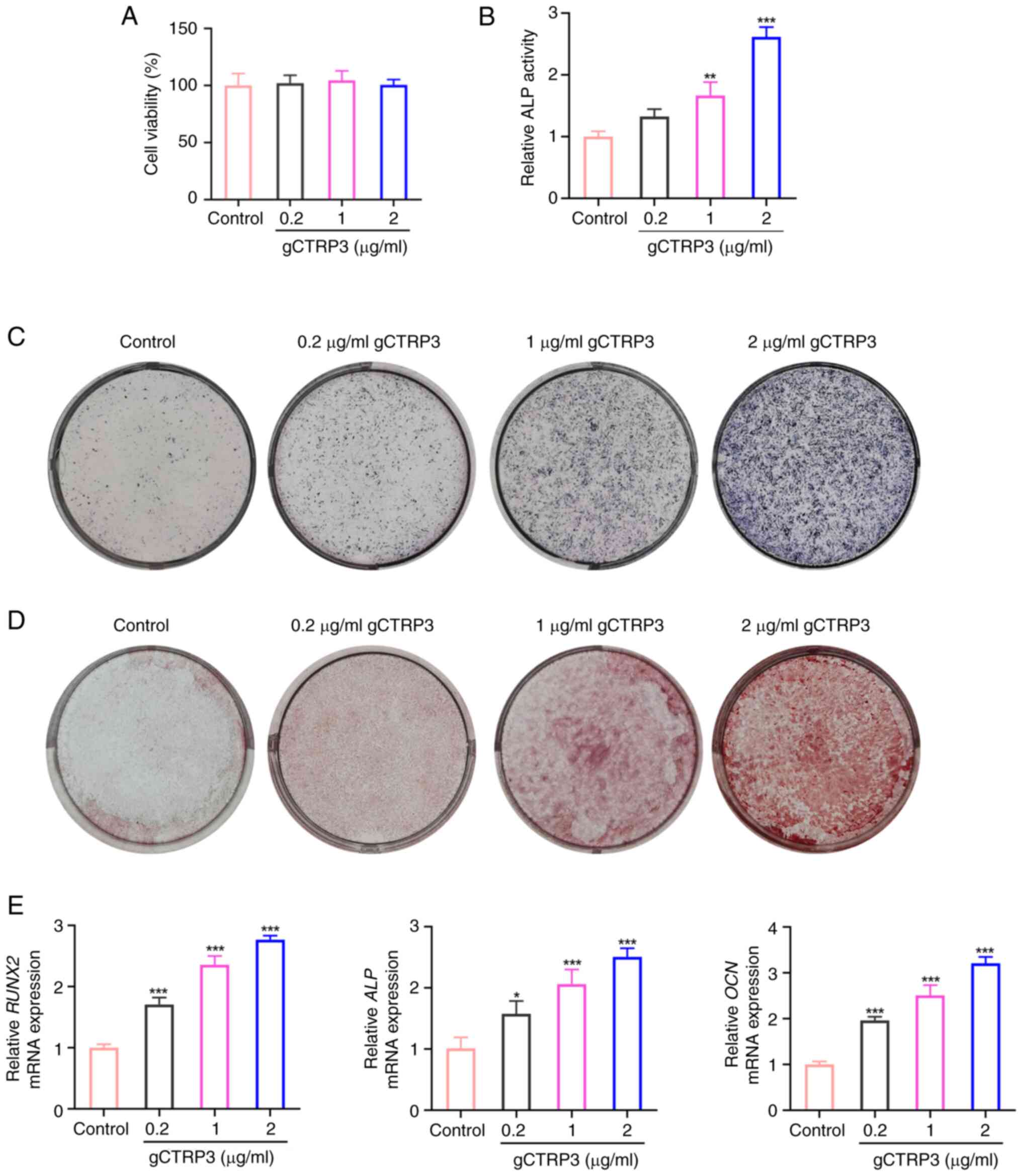
gCTRP3 promotes the osteogenic
differentiation of MC3T3-E1 cells
As shown in Fig.
3A, different concentrations of gCTRP3 did not affect the
viability of MC3T3-E1 cells, compared with that in the untreated
control group. Furthermore, ALP activity, osteoblast
differentiation and mineralized calcium nodules were all increased
following treatment with gCTRP3 from 0.2 to 2 µg/ml, when compared
with the control group (Fig.
3B-D). Consistently, RUNX2, ALP and OCN
expression levels were increased in MC3T3-E1 cells following
treatment with gCTRP3 from 0.2 to 2 µg/ml (Fig. 3E). Collectively, the results of the
present study suggested that gCTRP3 may promote the osteogenic
differentiation of MC3T3-E1 cells.
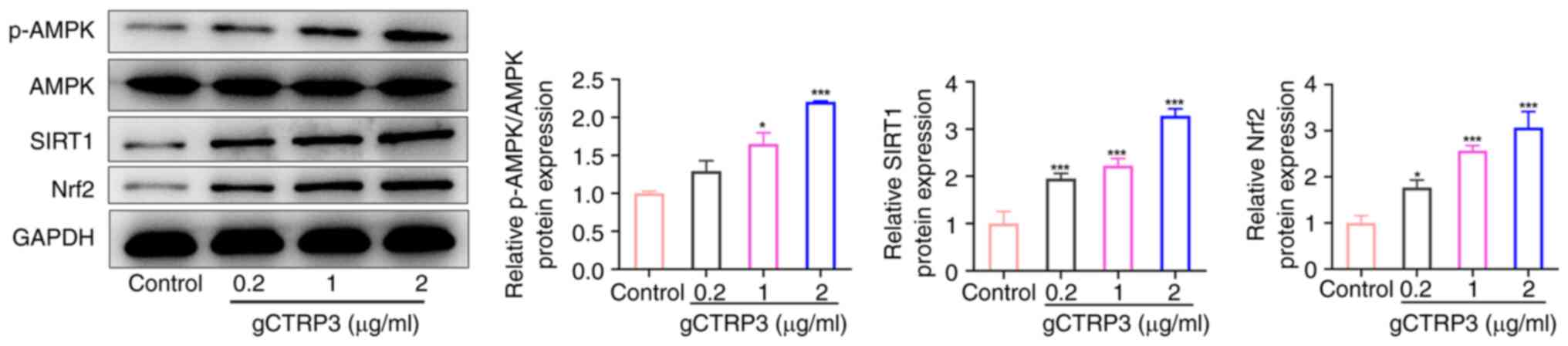
gCTRP3 activates the AMPK/SIRT1/Nrf2
signaling pathway in MC3T3-E1 cells
Western blot analysis was used to evaluate the
expression levels of proteins associated with AMPK/SIRT1/Nrf2
signaling in MC3T3-E1 cells, in the presence of gCTRP3. The results
of the present study demonstrated that p-AMPK, SIRT1 and Nrf2
expression levels were increased in MC3T3-E1 cells following
treatment with 1 and 2 µg/ml gCTRP3 (Fig. 4). By contrast, AMPK expression was
not altered in MC3T3-E1 cells following treatment with different
concentrations of gCTRP3 (Fig. 4).
Collectively, these data revealed that gCTRP3 activates the
AMPK/SIRT1/Nrf2 signaling pathway in MC3T3-E1 cells.
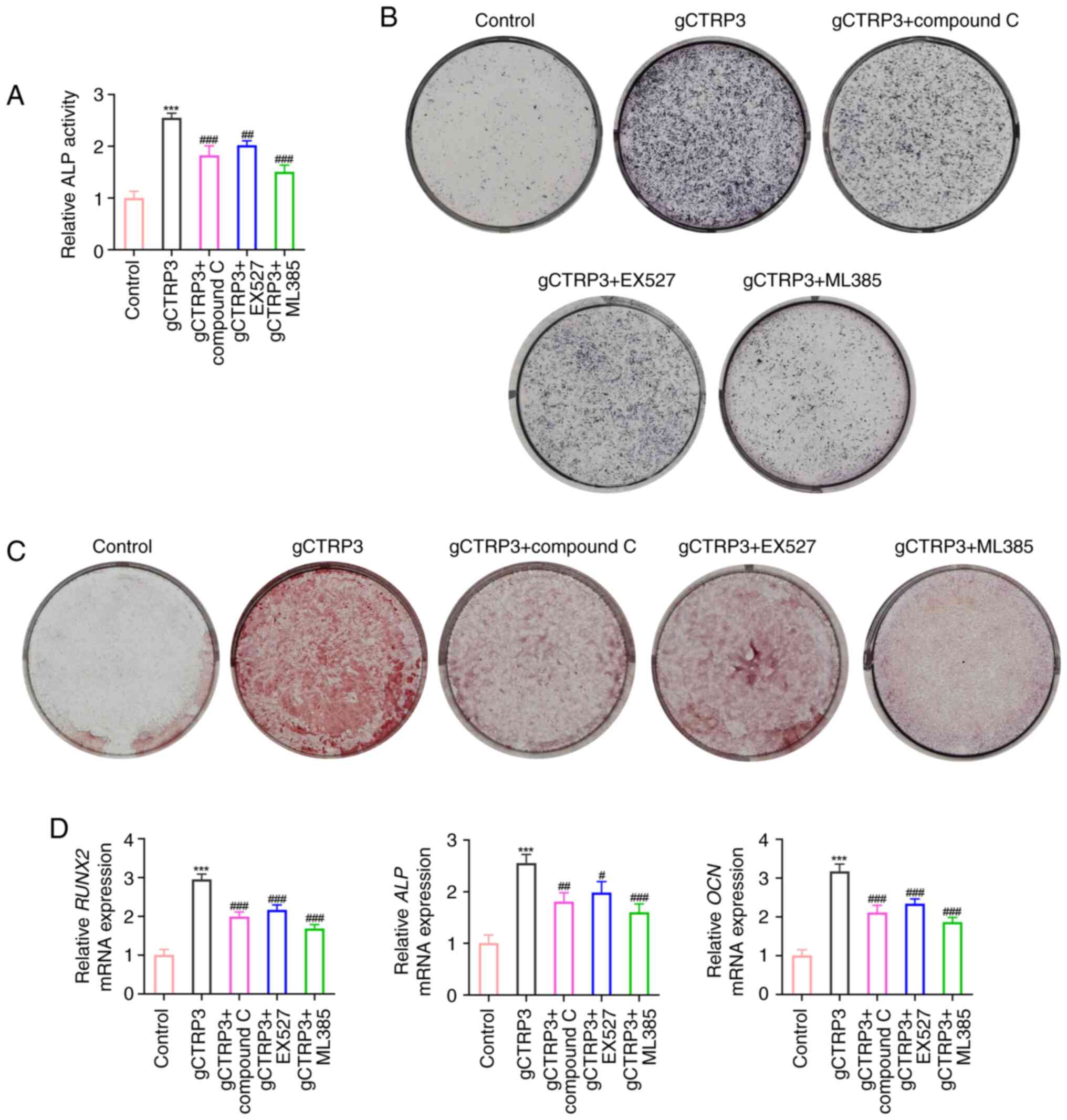
gCTRP3 promotes the osteogenic
differentiation of MC3T3-E1 cells through activating the
AMPK/SIRT1/Nrf2 signaling pathway
MC3T3-E1 cells were treated with an AMPK inhibitor
(Compound C), SIRT1 inhibitor (EX527) or Nrf2 inhibitor (ML385) to
assess whether gCTRP3 promoted osteogenic differentiation via the
AMPK/SIRT1/Nrf2 signaling pathway. As displayed in Fig. 5A-C, gCTRP3 treatment elevated ALP
activity, osteoblast differentiation and mineralized calcium
nodules, whereas these factors were reduced following treatment
with Compound C, EX527 and ML385. In addition, the gCTRP3-mediated
increases in the expression levels of RUNX2, ALP and
OCN were reduced following treatment with Compound C, EX527
and ML385 in MC3T3-E1 cells (Fig.
5D). Collectively, these data demonstrated that gCTRP3 promotes
the osteogenic differentiation of MC3T3-E1 cells through activating
the AMPK/SIRT1/Nrf2 signaling pathway.
Discussion
The results of the present study revealed that
gCTRP3 alleviated bone loss in OVX-induced mice. Moreover, gCTRP3
increased BMD, BV/TV, Tb.N and Tb.Th, decreased Tb.Sp, and improved
cortical bone thickness and trabecular area. The results of the
present study also demonstrated that treatment with gCTRP3
decreased the number of osteoclasts, promoted the levels of RUNX2,
OCN and P1NP, and inhibited the levels of CTX-1 and TRAP. In
vitro, gCTRP3 promoted ALP activity, osteoblast differentiation
and mineralized calcium nodules through activating the
AMPK/SIRT1/Nrf2 signaling pathway, and these factors were reversed
following treatment with Compound C, EX527 and ML385.
As a member of the CTRP family, CTRP3 is highly
expressed in cartilage and adipocytes. CTRP3 participates in the
proliferation and migration of chondrocytes, and regulates the
homeostasis of cartilage and bone metabolism in vivo
(7,8). In patients with reduced CTRP3
expression levels, the metabolism of calcium and phosphorus is
unbalanced, and the absorption capacity of calcium, phosphorus and
other substances is reduced, leading to osteoporosis (6). Maeda et al (29) reveled that CTRP3 may be involved in
chondrocyte development. Yokohama-Tamaki et al (30) demonstrated that CTRP3 may be
essential for mandible regulation via perichondrium maintenance and
neochondrogenesis. The results of a previous study revealed that
reduced CTRP3 expression inhibits the proliferation, migration and
invasion of osteosarcoma cells (31). Moreover, CTRP3 can inhibit the
hypoxia/serum deprivation-induced apoptosis of bone marrow-derived
mesenchymal stem cells (32). The
results of the present study demonstrated that gCTRP3 serves a key
role in bone formation in OVX-induced mice, and promoted the ALP
activity, osteoblast differentiation and mineralized calcium
nodules of MC3T3-E1 cells.
ALP hydrolyzes organophosphate, increases inorganic
phosphate local rates and facilitates mineralization as well as
reduces the concentration of extracellular pyrophosphate, an
inhibitor of mineral formation (33). In addition, ALP promotes bone
calcification and acts as a prerequisite for the formation of
calcium nodules (34). The
expression of RUNX2 indicates the onset of osteogenic
differentiation, which occurs at the earliest stage of bone
formation (35). The expression of
RUNX2 in bone tissue after OVX remains controversial, and may be
affected by different stages of osseointegration, mouse background
or various bone tissue (36–38).
OCN is a non-collagen protein that is present in bone tissue, and
levels are indicative of maturity during osteogenic differentiation
(39). The results of the present
study demonstrated that treatment with gCTRP3 significantly
increased the expression levels of ALP, RUNX2 and OCN
in MC3T3-E1 cells, suggesting that gCTRP3 may promote the
osteogenic differentiation of MC3T3-E1 cells.
The results of previous studies demonstrated that
AMPK is a therapeutic target for metabolic diseases, cancer and
atherosclerosis, which serves an important role in
anti-inflammatory, antitumor and anti-aging treatments (40–43).
At present, osteoporosis is considered a metabolic disease that is
comparable with diabetes and obesity, and AMPK promotes osteoblast
differentiation and bone formation (44). Through the AMPK signaling pathway,
Si-Zhi Wan, a traditional Chinese medicine used to treat
osteoporosis, inhibits osteoclast autophagy in osteoporosis to
attenuate osteoclastogenesis (45). Isovaleric acid stimulates AMPK
phosphorylation, and treatment with an AMPK inhibitor (Compound C)
blocks isovaleric acid-induced inhibition of osteoclast generation
(46). This suggests that the AMPK
signaling pathway serves a key role in bone metabolism in
osteoporosis. Compound C attenuates AMPK expression in different
types of cells (47), and inhibits
the osteogenic differentiation of cells (48,49).
AMPK activation directly inhibits the generation of osteoclasts
(50), stimulates the production
of osteoprotegerin in osteoblasts, reduces the expression of RANKL
and indirectly inhibits osteoclast differentiation (51). The results of the present study
revealed that AMPK inhibition reduced ALP activity, osteoblast
differentiation and mineralized calcium nodules of MC3T3-E1
cells.
SIRT1 is involved in intracellular energy metabolism
via the AMPK signaling pathway, and modulates bone metabolism in an
AMPK-dependent manner. Wang et al (52) revealed that AMPK promotes the
osteogenic differentiation of pre-osteoblast MC3T3-E1 cells through
the AMPK/GFI1/OPN pathway, and the osteogenic differentiation of
AMPKα2-overexpressed cell models has been shown to be markedly
increased. Notably, AMPK facilities bone metabolism via activation
of SIRT1, which deacetylates and activates the kinase upstream of
AMPK, LKB1. In addition, AMPK activation increases the
NAD+/NADH ratio and further activates SIRT1 (53). Notably, it has been reported that
cholesterol sulfate inhibits osteoclast differentiation and
survival by activating the AMPK/SIRT1 axis (54). El-Haj et al (55) revealed that SIRT1 expression levels
are markedly reduced in the bone tissue of female patients with
osteoporotic fractures. Moreover, treatment with the SIRT1 agonist,
SRT3025, significantly reduces the expression levels of osteoblast
proteins and maintains the osteogenic differentiation of bone
marrow mesenchymal stem cells. The results of the present study
indicated that gCTRP3 stimulated the expression of SIRT1, and
treatment with the SIRT1 inhibitor, EX527, suppressed ALP activity,
osteoblast differentiation and mineralized calcium nodules in
MC3T3-E1 cells.
Nrf2 has an important role in regulating bone
development and metabolism. During osteoclast formation, inhibition
of Nrf2 expression can increase the number of osteoclasts (56). Furthermore, azilsartan ameliorates
OVX-induced osteoporosis by activating Nrf2 signaling (46), and anemoside B4 attenuates
RANKL-induced osteoclastogenesis by upregulating Nrf2, and dampens
OVX-induced bone loss (57).
Moreover, Nrf2 overexpression reduces the number of osteoclasts and
downregulates RANKL expression (58). In the process of osteogenic
differentiation, osteoblasts cannot differentiate in the absence of
Nrf2, and osteogenic differentiation is inhibited in the presence
of high oxidative stress (59,60).
The present study demonstrated that gCTRP3 promoted Nrf2
expression, reduced the number of osteoclasts in OVX-induced mice
and promoted the osteogenic differentiation of MC3T3-E1 cells.
In conclusion, gCTRP3 inhibited OVX-induced
osteoporosis in mice, and promoted the osteogenic differentiation
of MC3T3-E1 cells through increasing ALP activity, osteoblast
differentiation and mineralized calcium nodules via activation of
the AMPK/SIRT1/Nrf2 signaling pathway. The results of the present
study provide a novel theoretical basis for the role of CTRP3 in
OVX-induced osteoporosis, and revealed that CTRP3 may exhibit
potential as a therapeutic target for the postoperative treatment
of OVX-induced osteoporosis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Government Funding for Clinical
Medical Excellence of Hebei Province (grant no. ZF2023087). The
funders had no role in the study design, data collection and
analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ and YZha contributed to the conception and design
of the study. DZ, JQ, HZ, HQ and FZ contributed to the experiments
and generated the figures. XJZ, YZho and YZha analyzed and
interpreted data. XZ drafted the manuscript and YZha revised and
edited it. All authors read and approved the final version of the
manuscript. XZ and YZha confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Third Hospital of Hebei Medical University
(approval no. Z2023-020-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ji MX and Yu Q: Primary osteoporosis in
postmenopausal women. Chronic Dis Transl Med. 1:9–13.
2015.PubMed/NCBI
|
|
2
|
Huang YF, Li LJ, Gao SQ, Chu Y, Niu J,
Geng FN, Shen YM and Peng LH: Evidence based anti-osteoporosis
effects of Periplaneta americana L on osteoblasts, osteoclasts,
vascular endothelial cells and bone marrow derived mesenchymal stem
cells. BMC Complement Altern Med. 17:4132017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peterson JM, Seldin MM, Wei Z, Aja S and
Wong GW: CTRP3 attenuates diet-induced hepatic steatosis by
regulating triglyceride metabolism. Am J Physiol Gastrointest Liver
Physiol. 305:G214–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murayama MA, Kakuta S, Maruhashi T,
Shimizu K, Seno A, Kubo S, Sato N, Saijo S, Hattori M and Iwakura
Y: CTRP3 plays an important role in the development of
collagen-induced arthritis in mice. Biochem Biophys Res Commun.
443:42–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim MJ, Park EJ, Lee W, Kim JE and Park
SY: Regulation of the transcriptional activation of CTRP3 in
chondrocytes by c-Jun. Mol Cell Biochem. 368:111–117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu ZH, Zhang X, Xie H, He J, Zhang WC,
Jing DF and Luo X: Serum CTRP3 Level is associated with
osteoporosis in postmenopausal women. Exp Clin Endocrinol Diabetes.
126:559–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demirtas D, Acıbucu F, Baylan FA, Gulumsek
E and Saler T: CTRP3 is significantly decreased in patients with
primary hyperparathyroidism and closely related with osteoporosis.
Exp Clin Endocrinol Diabetes. 128:152–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Youngstrom DW, Zondervan RL, Doucet NR,
Acevedo PK, Sexton HE, Gardner EA, Anderson JS, Kushwaha P, Little
HC, Rodriguez S, et al: CTRP3 regulates endochondral ossification
and bone remodeling during fracture healing. J Orthop Res.
38:996–1006. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JY, Min JY, Baek JM, Ahn SJ, Jun HY,
Yoon KH, Choi MK, Lee MS and Oh J: CTRP3 acts as a negative
regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling
in vitro and RANKL-induced calvarial bone destruction in vivo.
Bone. 79:242–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Wang JY, Feng H, Wang C, Li L, Wu
D, Lei H, Li H and Wu LL: Overexpression of c1q/tumor necrosis
factor-related protein-3 promotes phosphate-induced vascular smooth
muscle cell calcification both in vivo and in vitro. Arterioscler
Thromb Vasc Biol. 34:1002–1010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang N, Wang L, Yang J, Wang Z and Cheng
L: Quercetin promotes osteogenic differentiation and antioxidant
responses of mouse bone mesenchymal stem cells through activation
of the AMPK/SIRT1 signaling pathway. Phytother Res. 35:2639–2650.
2021. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Chen C, Zhu X, Li Y, Yu R and Xu W:
Glycyrrhizin Suppresses RANKL-Induced Osteoclastogenesis and
Oxidative Stress Through Inhibiting NF-κB and MAPK and Activating
AMPK/Nrf2. Calcif Tissue Int. 103:324–337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan Z, Cao X, Wang C, Liu S, Li Y, Lu G,
Yan W, Guo R, Zhao D, Cao J and Xu Y: C1q/tumor necrosis
factor-related protein-3 improves microvascular endothelial
function in diabetes through the AMPK/eNOS/NO signaling pathway.
Biochem Pharmacol. 195:1147452022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Wright GL and Peterson JM:
C1q/TNF-Related Protein 3 (CTRP3) function and regulation. Compr
Physiol. 7:863–878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirketerp-Møller N, Bayarri-Olmos R,
Krogfelt KA and Garred P: C1q/TNF-related protein 6 is a pattern
recognition molecule that recruits collectin-11 from the complement
system to ligands. J Immunol. 204:1598–1606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omeka WKM, Liyanage DS, Priyathilaka TT,
Kwon H, Lee S and Lee J: Characterization of four C1q/TNF-related
proteins (CTRPs) from red-lip mullet (Liza haematocheila) and their
transcriptional modulation in response to bacterial and
pathogen-associated molecular pattern stimuli. Fish Shellfish
Immunol. 84:158–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu L, Gao L, Zhang D, Yao R, Huang Z, Du
B, Wang Z, Xiao L, Li P, Li Y, et al: C1QTNF1 attenuates
angiotensin II-induced cardiac hypertrophy via activation of the
AMPKa pathway. Free Radic Biol Med. 121:215–230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mu Y, Yin TL, Yin L, Hu X and Yang J:
CTRP3 attenuates high-fat diet-induced male reproductive
dysfunction in mice. Clin Sci (Lond). 132:883–899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang CL, Feng H, Li L, Wang JY, Wu D, Hao
YT, Wang Z, Zhang Y and Wu LL: Globular CTRP3 promotes
mitochondrial biogenesis in cardiomyocytes through AMPK/PGC-1α
pathway. Biochim Biophys Acta Gen Subj. 1861:3085–3094. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang N, Zhang X, Li L, Xu T, Li M, Zhao Q,
Yu J, Wang J and Liu Z: Ginsenoside Rc promotes bone formation in
ovariectomy-induced osteoporosis in vivo and osteogenic
differentiation in vitro. Int J Mol Sci. 23:61872022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park CH, Lee B, Han M, Rhee WJ, Kwak MS,
Yoo TH and Shin JS: Canagliflozin protects against
cisplatin-induced acute kidney injury by AMPK-mediated autophagy in
renal proximal tubular cells. Cell Death Discov. 8:122022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin H, Zhang H, Zhang X, Zhang S, Zhu S
and Wang H: Resveratrol protects intestinal epithelial cells
against radiation-induced damage by promoting autophagy and
inhibiting apoptosis through SIRT1 activation. J Radiat Res.
62:574–581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Han N, Zhao K, Li Y, Chi Y and
Wang B: Protective effects of pyrroloquinoline quinine against
oxidative stress-induced cellular senescence and inflammation in
human renal tubular epithelial cells via Keap1/Nrf2 signaling
pathway. Int Immunopharmacol. 72:445–453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tripathi A, John AA, Kumar D, Kaushal SK,
Singh DP, Husain N, Sarkar J and Singh D: MiR-539-3p impairs
osteogenesis by suppressing Wnt interaction with LRP-6 co-receptor
and subsequent inhibition of Akap-3 signaling pathway. Front
Endocrinol (Lausanne). 13:9773472022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park OJ, Kwon Y, Kim J, Park C, Yun CH and
Han SH: Muramyl dipeptide alleviates estrogen deficiency-induced
osteoporosis through canonical Wnt signaling. J Pathol.
260:137–147. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu H, Xu J, Chen F, Liu T, Li J, Jiang L,
Jia Y, Hu C, Gao Z, Gan C, et al: Acanthopanax senticosus aqueous
extract ameliorates ovariectomy-induced bone loss in middle-aged
mice by inhibiting the receptor activator of nuclear factor-κB
ligand-induced osteoclastogenesis. Food Funct. 11:9696–9709. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maeda T, Jikko A, Abe M, Yokohama-Tamaki
T, Akiyama H, Furukawa S, Takigawa M and Wakisaka S: Cartducin, a
paralog of Acrp30/adiponectin, is induced during chondrogenic
differentiation and promotes proliferation of chondrogenic
precursors and chondrocytes. J Cell Physiol. 206:537–544. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yokohama-Tamaki T, Maeda T, Tanaka TS and
Shibata S: Functional analysis of CTRP3/cartducin in Meckel's
cartilage and developing condylar cartilage in the fetal mouse
mandible. J Anat. 218:517–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao G, Zhang L, Qian D, Sun Y and Liu W:
miR-495-3p inhibits the cell proliferation, invasion and migration
of osteosarcoma by targeting C1q/TNF-related protein 3. Onco
Targets Ther. 12:6133–6143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou M, Liu J, Liu F, Liu K and Yu B: C1q
tumor necrosis factor-related protein-3 protects mesenchymal stem
cells against hypoxia- and serum deprivation-induced apoptosis
through the phosphoinositide 3-kinase/Akt pathway. Int J Mol Med.
33:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vimalraj S: Alkaline phosphatase:
Structure, expression and its function in bone mineralization.
Gene. 754:1448552020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma U, Pal D and Prasad R: Alkaline
phosphatase: An overview. Indian J Clin Biochem. 29:269–278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Komori T: Molecular mechanism of
runx2-dependent bone development. Mol Cells. 43:168–175.
2020.PubMed/NCBI
|
|
36
|
Zhang K, Qiu W, Li H, Li J, Wang P, Chen
Z, Lin X and Qian A: MACF1 overexpression in BMSCs alleviates
senile osteoporosis in mice through TCF4/miR-335-5p signaling
pathway. J Orthop Translat. 39:177–190. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siqueira R, Ferreira JA, Rizzante FAP,
Moura GF, Mendonça DBS, de Magalhães D, Cimões R and Mendonça G:
Hydrophilic titanium surface modulates early stages of
osseointegration in osteoporosis. J Periodontal Res. 56:351–362.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin X, Jiang Q, Komori H, Sakane C,
Fukuyama R, Matsuo Y, Ito K, Miyazaki T and Komori T: Runt-related
transcription factor-2 (Runx2) is required for bone matrix protein
gene expression in committed osteoblasts in mice. J Bone Miner Res.
36:2081–2095. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu X, Liu S, Dong K and Liu Z: The effects
of p38MAPK inhibitor SB203580 on MC3T3-E1 cell proliferation and
differetiation under high glucose concentration. J Pract
Stomatology. 31:184–187. 2015.
|
|
40
|
Kim SG, Kim JR and Choi HC:
Quercetin-Induced AMP-Activated protein kinase activation
attenuates vasoconstriction through LKB1-AMPK signaling pathway. J
Med Food. 21:146–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Su Q, Peng M, Zhang Y, Xu W, Darko KO, Tao
T, Huang Y, Tao X and Yang X: Quercetin induces bladder cancer
cells apoptosis by activation of AMPK signaling pathway. Am J
Cancer Res. 6:498–508. 2016.PubMed/NCBI
|
|
42
|
Kim GT, Lee SH and Kim YM: Quercetin
Regulates Sestrin 2-AMPK-mTOR signaling pathway and induces
apoptosis via increased intracellular ROS in HCT116 colon cancer
cells. J Cancer Prev. 18:264–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Viollet B, Terkeltaub R and
Liu-Bryan R: AMP-activated protein kinase suppresses urate
crystal-induced inflammation and transduces colchicine effects in
macrophages. Ann Rheum Dis. 75:286–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Su J, Sun W, Cai L and Deng Z:
AMP-activated protein kinase stimulates osteoblast differentiation
and mineralization through autophagy induction. Int J Mol Med.
41:2535–2544. 2018.PubMed/NCBI
|
|
45
|
Huang Y, Yao H, Tjahjono AW, Xiang L, Li
K, Tang J and Gao Y: Si-Zhi Wan regulates osteoclast autophagy in
osteoporosis through the AMPK signaling pathway to attenuate
osteoclastogenesis. J Pharm Pharmacol. 76:236–244. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cho KM, Kim YS, Lee M, Lee HY and Bae YS:
Isovaleric acid ameliorates ovariectomy-induced osteoporosis by
inhibiting osteoclast differentiation. J Cell Mol Med.
25:4287–4297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dasgupta B and Seibel W: Compound
C/Dorsomorphin: Its use and misuse as an AMPK inhibitor. Methods
Mol Biol. 1732:195–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Langelueddecke C, Jakab M, Ketterl N,
Lehner L, Hufnagl C, Schmidt S, Geibel JP, Fuerst J and Ritter M:
Effect of the AMP-kinase modulators AICAR, metformin and compound C
on insulin secretion of INS-1E rat insulinoma cells under standard
cell culture conditions. Cell Physiol Biochem. 29:75–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su CW, Chang YC, Chien MH, Hsieh YH, Chen
MK, Lin CW and Yang SF: Loss of TIMP3 by promoter methylation of
Sp1 binding site promotes oral cancer metastasis. Cell Death Dis.
10:7932019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang H, Viollet B and Wu D: Genetic
deletion of catalytic subunits of AMP-activated protein kinase
increases osteoclasts and reduces bone mass in young adult mice. J
Biol Chem. 288:12187–12196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mai QG, Zhang ZM, Xu S, Lu M, Zhou RP,
Zhao L, Jia CH, Wen ZH, Jin DD and Bai XC: Metformin stimulates
osteoprotegerin and reduces RANKL expression in osteoblasts and
ovariectomized rats. J Cell Biochem. 112:2902–2909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang YG, Han XG, Yang Y, Qiao H, Dai KR,
Fan QM and Tang TT: Functional differences between AMPK α1 and α2
subunits in osteogenesis, osteoblast-associated induction of
osteoclastogenesis, and adipogenesis. Sci Rep. 6:327712016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cetrullo S, D'Adamo S, Tantini B, Borzi RM
and Flamigni F: mTOR, AMPK, and Sirt1: Key players in metabolic
stress management. Crit Rev Eukaryot Gene Expr. 25:59–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park JH, Lee J, Lee GR, Kwon M, Lee HI,
Kim N, Kim HJ, Lee MO and Jeong W: Cholesterol sulfate inhibits
osteoclast differentiation and survival by regulating the
AMPK-Sirt1-NF-κB pathway. J Cell Physiol. 238:2063–2075. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
El-Haj M, Gurt I, Cohen-Kfir E, Dixit V,
Artsi H, Kandel L, Yakubovsky O, Safran O and Dresner-Pollak R:
Reduced Sirtuin1 expression at the femoral neck in women who
sustained an osteoporotic hip fracture. Osteoporos Int.
27:2373–2378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Han J, Yang K, An J, Jiang N, Fu S and
Tang X: The Role of NRF2 in Bone Metabolism-Friend or Foe? Front
Endocrinol (Lausanne). 13:8130572022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cao Z, Niu X, Wang M, Yu S, Wang M, Mu S,
Liu C and Wang Y: Anemoside B4 attenuates RANKL-induced
osteoclastogenesis by upregulating Nrf2 and dampens
ovariectomy-induced bone loss. Biomed Pharmacother. 167:1154542023.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kanzaki H, Shinohara F, Kajiya M and
Kodama T: The Keap1/Nrf2 protein axis plays a role in osteoclast
differentiation by regulating intracellular reactive oxygen species
signaling. J Biol Chem. 288:23009–23020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun YX, Li L, Corry KA, Zhang P, Yang Y,
Himes E, Mihuti CL, Nelson C, Dai G and Li J: Deletion of Nrf2
reduces skeletal mechanical properties and decreases load-driven
bone formation. Bone. 74:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pitoniak A and Bohmann D: Mechanisms and
functions of Nrf2 signaling in Drosophila. Free Radic Biol Med.
88:302–313. 2015. View Article : Google Scholar : PubMed/NCBI
|