Introduction
Heterotopic ossification (HO) is a pathological
process characterized by formation of bone within muscle, ligaments
or other soft tissues (1,2). HO occurs after deep burn, fracture,
total hip replacement, brain or medullary injury (3–5).
Muscle HO is a form of HO characterized by abnormal formation of
bone tissue within skeletal muscle, typically in response to
trauma, surgery or other types of injury (6,7).
This can result in joint dysfunction, as the presence of
heterotopic bone within muscle tissue can cause pain, decreased
range of motion and other complications (8).
The morbidity of HO following central neurological
injury is 10 to 53% and the prevalence of genetic HO is extremely
rare, affecting 1 in 2,000,000 people (9). While the condition can occur at all
ages, it occurs most commonly in young adults, with the highest
occurrence observed during 20–40 years old. Muscle HO does not
appear to have a significant sex or racial or ethnic predilection
(10). Muscle HO is hypothesized
to involve a complex interplay of signaling pathways and cellular
processes (11,12). The mechanisms underlying this
process are not fully understood, but they may involve intricate
interactions between inflammatory molecules, growth factors and
other signaling pathways. Thus, better understanding of the
pathogenesis of muscle HO could have implications for the
development of novel treatments and therapies (13,14).
Peripheral denervation occurs when the nerve
innervating a muscle is disrupted or severed, leading to loss of
motor function and muscle atrophy. The peripheral nerves innervate
muscles via neuromuscular junctions, which are synaptic connections
established between motor neurons and muscles, as well as muscle
sensory organs (15). While
effects of denervation on muscle have been extensively studied
(16–18), research has also suggested a
potential link between peripheral denervation and development of
muscle HO (19). However, the
mechanisms underlying the association between peripheral
denervation and muscle HO are not fully understood. Better
understanding of the effects of denervation on muscle HO could
facilitate development of novel treatments and therapies for this
condition and could help to elucidate the underlying
pathophysiology.
Cytokines serve crucial functions in the regulation
of inflammation and immune responses (20–22).
The release of cytokines serves a role in the process of muscle
degeneration and regeneration following denervation (23). Following denervation, the affected
muscle tissue increases susceptibility of satellite cells to
apoptosis, contributing to the inability of muscle to regenerate
after prolonged periods without neural input. Loss of muscle mass
is frequently associated with an increase in pro-inflammatory
cytokines, as these are considered critical mediators of catabolic
responses, such as muscle-specific protein break down (17). Moreover, inflammatory cells are
involved in this process with a surrounding infiltration of
cytokines, such as substance P and calcitonin gene related peptide
(CGRP), from peripheral, sensory neurons. These cytokines exert
direct effects on muscle cells, including promoting the degradation
of muscle tissue and activation of muscle stem cells for both
degenerative and regenerative processes (24).
Neuregulin 3 (NRG3) is a member of the neuregulin
family of signaling molecules and serves a role in cellular
processes such as cell proliferation, differentiation and
migration. NRG3 is primarily expressed in the central nervous
system, where it serves a role in development and function of the
brain (25). NRG3 may also serve a
role in the mechanisms of bone formation and ossification (26). Achilleos and Trainor (27) reported that NRG3 is expressed in
osteoblasts that are responsible for bone formation and that the
expression of NRG3 is increased during bone healing. Lazard et
al (28) reported that NRG3
can promote the differentiation of mesenchymal stem cells into
osteoblasts, which are responsible for formation of bone
tissue.
Non-myogenic cells serve a role in skeletal muscle
degeneration (29,30). In particular, fibro-adipogenic
progenitors (FAPs) are regulators of muscle stem cell function and
skeletal muscle degeneration (31,32).
Previous studies have suggested that FAPs have the potential to
differentiate into osteogenic or chondrogenic cells when exposed to
specific conditions, such as bone morphogenetic protein-2 (BMP-2)
(33–35). These findings suggest that FAPs are
a cellular source of muscle HO. However, the association between
denervation-induced changes in cytokine expression and muscle HO
requires further assessment.
In the present study, a mouse model of gastrocnemius
muscle HO was established and the sciatic nerve was cut to simulate
peripheral nerve injury. The effect of peripheral nerve injury on
muscle HO and underlying mechanism were assessed by detecting the
changes in cytokine expression in muscle tissue and analyzing
transcriptome-wide differential expression, along with in
vitro experiments with FAPs.
Materials and methods
Experimental animals
A total of 90 C57BL/6 6–8 week-old male mice
(weight, 20–25 g) were obtained from Army Medical University,
Chongqing, China. All mice were housed in a pathogen-free and 60%
humidity environment with free access to food and water. The
temperature was maintained at 22–26°C with a 12-h light/12-h dark
cycle. All animal experimental procedures and euthanasia were
approved by The Laboratory Animal Welfare and Ethics Committee of
Third Military Medical University (Chongqing, China; approval no.
AMUWEC20210782). At the end of the experiment, mice were euthanized
by injection with 30 mg/kg pentobarbital sodium anesthetic
injection followed by cervical dislocation. Heartbeat and breathing
were checked to ensure successful euthanasia. Throughout the
experiment, mice were assessed once/day for general indicators and
once/week for specific indicators. When any mice demonstrated any
of the humane end points, the animal experiment was terminated
(Table SI). No mice died prior to
the end of the study.
Study design
Left hindlimb intramuscular injection of cardiotoxin
(CTX; 50 µl 10 µmol/l) was used to establish a muscle HO model. To
assess the role of peripheral denervation in muscle HO, mice were
randomly assigned to the following groups: i) CTX injection (CTX
group) and ii) CTX injection with left hindlimb peripheral
denervation group (CTX-DeN group). To assess the role of NRG3/ErbB4
signaling in muscle HO, mice were randomly assigned to the
following groups: i) CTX-DeN and ii) CTX-DeN + 10 mg/kg
dacomitinib. Finally, micro-computed tomography (micro-CT), PCR and
cytokine chip analyses were performed to assess the degree of
HO.
Surgical procedure
The peripheral denervation model was established
according to Akhter et al (36) as follows: Mice were administered 30
mg/kg pentobarbital sodium anesthetic injection in the right lower
abdomen. The mice were placed on a sterilized area for preparation
of the hindlimb, which was secured in an extended position with
surgical tape. The skin was palpated to identify the femur and a
small incision (<2 cm) was made 2 mm medially in the skin with a
fine scalpel. The biceps femoris was blunt dissected at mid-thigh
level using scissors, exposing the sciatic nerve, which was
carefully isolated from connective tissue and cut with
microscissors. After ensuring there was no excessive bleeding, the
wound was closed (CTX-DeN group). Mice in the CTX group underwent
the same procedure except for the cutting of sciatic nerve. After
peripheral denervation surgery, 50 µl 10 µmol/l CTX (cat. no.
C9759; MilliporeSigma) was injected into both calf muscles of each
mouse. A gelatin sponge (cat. no. 20153141505; JinLing
Pharmaceutical) was sectioned 1 mm thick discs and 5 µl aliquots
containing 0.5 µg of recombinant BMP-2 (cat. no. Z02913;
GenScript.) was adsorbed onto each disc. The medial surface of the
calf muscles in the leg was incised longitudinally. A single disc
containing BMP-2/gelatin was inserted intramuscularly. The disc was
implanted between the gastrocnemius and soleus muscles around the
CTX injection site. Finally, the skin was sutured closed. In the
CTX-DeN + dacomitinib group, 1 day after the animal model was
established, a single perimuscular injection of 10 mg/kg
dacomitinib (20 nM, dissolved in DMSO; 20 µl) was administered
around the gelatin implantation site (37).
Cell isolation and
fluorescence-activated cell sorting
The isolation of FAPs was conducted as described in
a previous study (38). Muscles
were obtained from both hind limbs of mice. Non-muscle tissue was
excised and finely minced. Subsequently, enzymatic digestion was
performed using 0.2% type II collagenase (cat. no. LS004176;
Worthington Biochemical Corporation) for 1 h at 37°C. The resulting
muscle plasma was filtered through cell filters with pore sizes of
100 and 40 µm (cat. nos. 431752 and 431750, respectively; BD
Biosciences). Red blood cell lysis buffer (cat. No. C3702, BD
Bioscience) was utilized to eliminate red blood cells, and the
cells were resuspended in PBS wash buffer containing 2% FBS (Cat.
No. SH30406.05, HyClone) and stained with antibodies for 30 min at
4°C in the absence of light. The gating strategy for flow cytometry
involved CD31−, CD45−, Integrin
α7−, stem cell antigen-1+ (Sca-1+)
and Platelet Derived Growth Factor Receptor Alpha+
(PDGFRα+) cells. The antibodies used included Alexa
Fluor 488-CD31 (1:100, Cat. No. 160,208, Biolegend), Alexa Fluor
488-CD45 (1:100, Cat. No. 160,306, Biolegend), APC-Integrinα7
(1:100, Cat. No. FAB3518A, R&D), APC-Cy7-Sca-1 (1:100, Cat. No.
108,126, Biolegend) and biotin-PDGFRα (1:100, Cat. No. AF1062,
R&D). The stained cells were analyzed using FACSAria III (BD
Biosciences, NJ, USA). The data was analysed by using FlowJo v10
(Flowjo, LLC., OR, USA).
Cell culture
Primary FAPs were cultured on Matrigel-coated tissue
culture plates (Cat. No. CLS430639, Corning, Inc.) in DMEM (Cat.
No. SH30243.01B, HyClone) supplemented with 20% fetal bovine serum
(Cat. No. SH30406.05, HyClone), 1% penicillin-streptomycin and 2.5
ng/ml basic fibroblast growth factor (cat. no. PHG0021; Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C. The medium was replaced
with fresh medium after 3 days. To induce osteogenic
differentiation, cells were treated with osteogenesis induction
medium (cat. no. HUXMX-90021; Cyagen Biosciences, Inc.) at 37°C in
a saturated humidity incubator containing 5% CO2 for 14
days; medium was replaced every 3 days. To verify osteogenic
differentiation of FAPs via the PI3K/Akt signaling pathway, FAPs
were cultured in vitro and divided into four groups as follows: i)
normal medium; ii) osteogenic-inducible medium (OIM); iii) OIM +
NRG3 (2.0 µg/ml, cat. no. ab276716, Abcam) and iv) OIM + NRG3 (2.0
µg/ml) + 1 µM dacomitinib. After 14 days of culture, Alizarin Red S
staining was performed.
Micro-CT
Following euthanasia, mouse hindlimbs were imaged by
micro-CT (vivaCT, Skyscan 1276, Bruker) to detect HO formation at 4
weeks post-surgery. The scanner was configured with a voltage of 60
kV and pixel resolution of 10 µm. Once the scans were complete, the
images were reconstructed, which allowed visualization and analysis
of the 3D microstructure of the hindlimbs. The results were
analyzed using Scanner software (3D.Suite, SKYSCAN 1276, Bruker)
for Micro-CT. Bone mineral density (BMD) were measured.
Histochemical staining
The fresh frozen muscle tissue was sectioned into
8-µm slices using a cryostat and fixed with 4% PFA at 4°C for 5
min. Muscle histology was assessed by hematoxylin-eosin (H&E)
staining (Beijing Solarbio Technology Science & Technology Co.,
Ltd.). Slices were stained for 5 min in hematoxylin and 2 min eosin
solution at 24°C before being dried and sealed with neutral gum.
The cells were fixed with 4% PFA for 10 min at 4°C and subsequently
rinsed in water and 60% isopropanol, prior to Alizarin Red S
staining. After staining the cells with Alizarin Red S for 30 min
at 24°C, they were again rinsed in water. The positive-stanined
cells of Alizarin Red S were counted manually. All the stained
sections and cells were viewed and photographed under a Zeiss
(Oberkochen) fluorescence Axiovert microscope equipped with a Zeiss
AxioCam digital colour camera connected to the Zeiss AxioVision 3.0
system (magnification, 40).
RNA-sequencing (seq)
For RNA-seq analysis of mouse hindlimbs. total RNA
was extracted from tissue samples using TRIzol (Cat. No. 15596026,
Invitrogen). Subsequently, total RNA was qualified and quantified
using a Fragment Analyzer or Agilent 2100 Bioanalyzer (Agilent, CA,
USA). Following addition of a single ‘A’ base and ligation of the
adapter to cDNA fragments, PCR was employed for the final cDNA
library creation using Optimal Dual-mode mRNA Library Prep kit(cat.
no. LR00R96, BGI). RNA was processed to generate a cDNA library,
which was subjected to high-throughput sequencing using a
new-generation sequencing platform (input 1 pM for DNA sequencing)
using MGISEQ-2000RS High-throughput Sequencing Set (cat. no. FCL
SE50)(60 cycles; Cat. No. 1000006138, MGI). The DNBs are loaded
into the patterned nanoarray and SE 50 (Single-end for direction of
sequencing, and 50 bp for length) bases reads are generated on
BGISEQ platform (BGI-Shenzhen, China). Subsequently, Dr. Tom
multi-omics data mining system (https://biosys.bgi.com) was used for data analysis,
mapping and mining. HISAT2 (v2.1.0) software was used to align
clean data to the reference genome for differential gene analysis.
We used the DESeq2 (version 1.4.5) for differential gene detection
with a condition of Q value ≤ 0.05 or FDR ≤ 0.001. Hypergeometric
test based on the Phyper (https://en.wikipedia.org/wiki/Hypergeometric_distribution)
function to perform GO (geneontology.org/) and KEGG (kegg.jp/)
enrichment analysis on the differential genes, with Qvalue ≤ 0.05
as the threshold.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from mouse hindlimbs using
TRIzol reagent (cat. no. 15596026, Invitrogen; Thermo Fisher
Scientific, Inc.). RNA was reverse-transcribed into cDNA using the
RevertAid First Strand cDNA Synthesis kit (cat. no. K1622, Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. A reaction system containing primers, dNTP and buffer
was prepared using an ABI 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The amplification
reaction was performed in a thermal cycler using a SYBR kit (cat.
no. RR4420L, Takara Bio, Inc.). Thermocycling conditions included
incubation at 95°C for ten seconds, followed by 40 cycles of 95°C
for five sec and 60°C for 30 sec. Relative gene expression was
quantified by densitometry and normalized to the expression of
GAPDH). Amplification results were analyzed and quantified using
the 2−ΔΔCq method (39). The primer sequences used for PCR
are listed in Table SII.
Western blotting
Total protein from FAPs was extracted using RIPA
lysis buffer (cat. no. P0013P, Beyotime) and quantified using a BCA
kit (cat. no. P0010, Beyotime) and 50 µg protein/lane was separated
by 10% SDS-PAGE (cat. no. 1620177; Bio-Rad Laboratories, Inc.), and
the separated proteins were transferred to polyvinylidene
difluoride membranes. The separated proteins were transferred onto
a membrane. Membranes were incubated overnight at 4°C in PBS
containing 5% BSA (cat. no. BS114, Biosharp) with primary
antibodies anti-PI3K (1:2,000; cat. no. bs-5570R, BIOSS),
anti-phosphorylated (p)-PI3K (1:2,000; cat. no. bs-5538P, BIOSS),
anti-AKT (1:2,000; cat. no. 10176-2-AP, Proteintech), anti-p-AKT
(1:2,000; cat. no. 66444-1-Ig, Proteintech) and GAPDH (1:5,000;
cat. no. 60004-1-Ig, Proteintech). Following primary incubation,
membranes were incubated with goat anti-rabbit IgG secondary
antibody (1:2,000; cat. no. SA00001-2,Proteintech) for 1 h at
ambient temperature. Protein bands were stimulated with ECL kit
(cat. no. WBULS0500, Millipore) and detected with a Bio-Rad
ChemiDoc MP System (170–8280; Bio-Rad Laboratories, Inc.). ImageJ
software (version 1.46r, National Institutes of Health) was used to
analyse the results.
Muscle tissue chip array
Muscles were obtained from hind limbs of mice at 4
weeks after modeling. Non-muscle tissue was carefully excised and
finely minced. The experiment was performed using the Raybiotech
reagent kit (Wayen Biomedical Technology) and was conducted
according to the standard operating procedures of Raybiotech chip.
Fresh muscle tissue culture supernatant was harvested and stored at
−80°C. Muscle tissue chips (AAM-BLG-308, Raybiotech) were obtained
from Shanghai Wayen Biomedical Technology Co. Ltd. Agilent SureScan
Dx Microarray Scanner chip scanner to scan the chip at 532 nm,
Power (100%) conditions. The fluorescence signal intensity, which
is then normalised after the data is read and generally analysed
using the FI of the fluorescence signal with the background removed
(F532 Median-B532 Median).
STRING database analysis of NRG3
By means of the STRING database (version
12.0, www.string-db.org/), we constructed
the protein-protein interaction (PPI) network for NRG3. A network
type of full STRING network with high confidence (minimum required
interaction score=0.7) was selected.
Statistical analysis
Data are presented as the mean ± SD of ≥3
experimental repeats. Unpaired t test was used for comparisons
between two groups. One-way analysis of variance with Tukey's post
hoc test was used for comparisons between multiple groups. Model
assumptions were checked using the Shapiro-Wilk normality test and
Levene's test for homogeneity of variance and by visual inspection
of residual and fitted value plots. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using SPSS 26.0 software (IBM Corp.).
Pearson correlation coefficients of gene expressions between each
two samples were calculated.
Results
Denervation leads to more severe HO of
muscle
To assess the effect of denervation on muscle HO, a
model of muscle HO was established by CTX-DeN, with a sham surgical
treatment performed in the control group. Micro-CT was performed 4
weeks post-surgery (Fig. 1A). The
BMD in the CTX-DeN group (0.780±0.020 g/cm3) was
significantly increased compared with CTX group (0.479±0.032
g/cm3) (Fig. 1B),
indicating a higher degree of HO in the denervated group compared
with the control. H&E staining demonstrated that the CTX-DeN
group had a significantly higher HO ratio compared with the CTX
group (Fig. 1C and D). RT-qPCR was
performed on muscle tissue to assess osteogenesis-related gene
expression. mRNA levels of osteopontin (OPN), RUNX2, osterix/SP7
(encoded by the SP7 gene, SP7), alkaline phosphatase (ALPL) and
bone morphogenetic protein-2 (BMP2) were significantly higher in
the CTX-DeN group compared with the CTX group (Fig. 1E). These results suggested that
denervation led to increased HO of muscles.
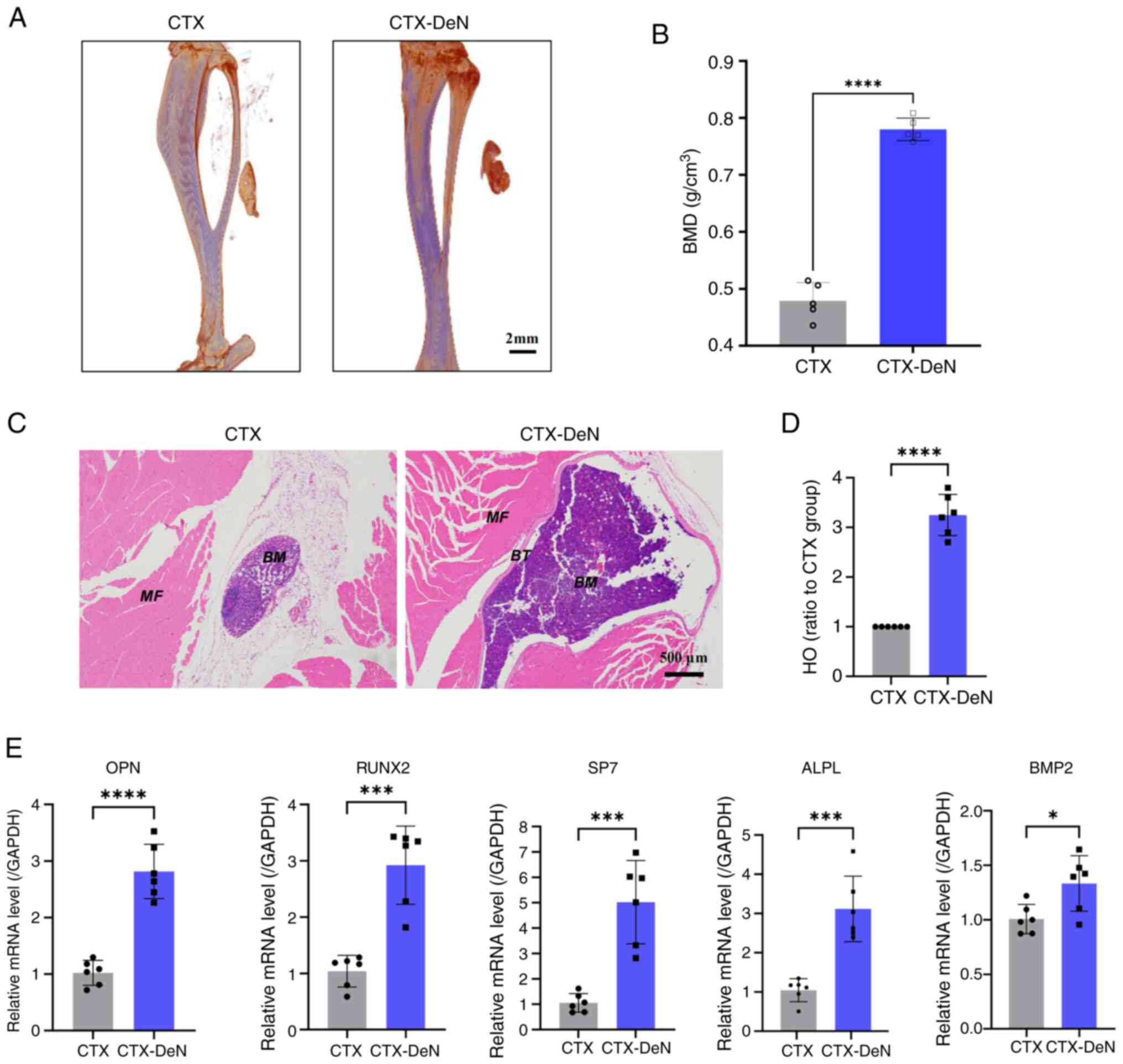 | Figure 1.Denervation leads to increased HO of
calf muscles. (A) Morphology of muscle HO was scanned by
micro-computed tomography. (B) BMD was subjected to statistical
analysis. (C) Calf muscle was subjected to hematoxylin-eosin
staining. (D) HO ratio to CTX. (E) RT-qPCR analysis of gene
expression levels of OPN, RUNX2, SP7, ALPL and BMP2. *P<0.05,
***P<0.005 and ****P<0.001. CTX, cardiotoxin; DeN,
denervation; MF, Muscle fiber; BT, Bone tissue; BM, Bone mineral;
OPN, Osteopontin; SP7, Osterix/SP7 (encoded by the SP7 gene); ALPL,
Alkaline phosphatase; BMP2, Bone morphogenetic protein-2; HO,
heterotopic ossification; BMD, bone mineral density; RT-qPCR,
reverse transcription-quantitative PCR. |
Denervation increases secretion of
NRG3 and NRG3 is most closely associated cell membrane surface
receptor was ErbB4
To assess the underlying factors contributing to
exacerbation of muscle HO resulting from denervation, fresh muscle
tissue was collected and the levels of 15 cytokines were
quantified. The protein level of NRG3 was increased in the CTX-DeN
group compared with the CTX group (Fig. 2A; Table SIII), suggesting that denervation
led to an increase in the secretion of the cytokine NRG3 in muscle
tissue. To determine the mechanism of how increased NRG3 secretion
affects HO, we constructed the protein-protein interaction (PPI)
network for NRG3 by means of the STRING database (version 12.0). A
network type of full STRING network with high confidence (minimum
required interaction score=0.7) was selected. The STRING database
was used for protein interaction network visualization and
predicted the molecular interactions of NRG3. This predicted that
the most closely associated cell membrane surface receptor was
ErbB4 (Fig. 2B). RT-qPCR was used
to measure the levels of ErbB4 mRNA in muscle tissue and
demonstrated that the expression of ErbB4 was significantly higher
in the denervated group compared with the control (Fig. 2C). This suggested that denervation
may exert its effects through upregulating the release of NRG3 in
muscle tissue and binding to ErbB4 receptors, thereby contributing
to the exacerbation of HO.
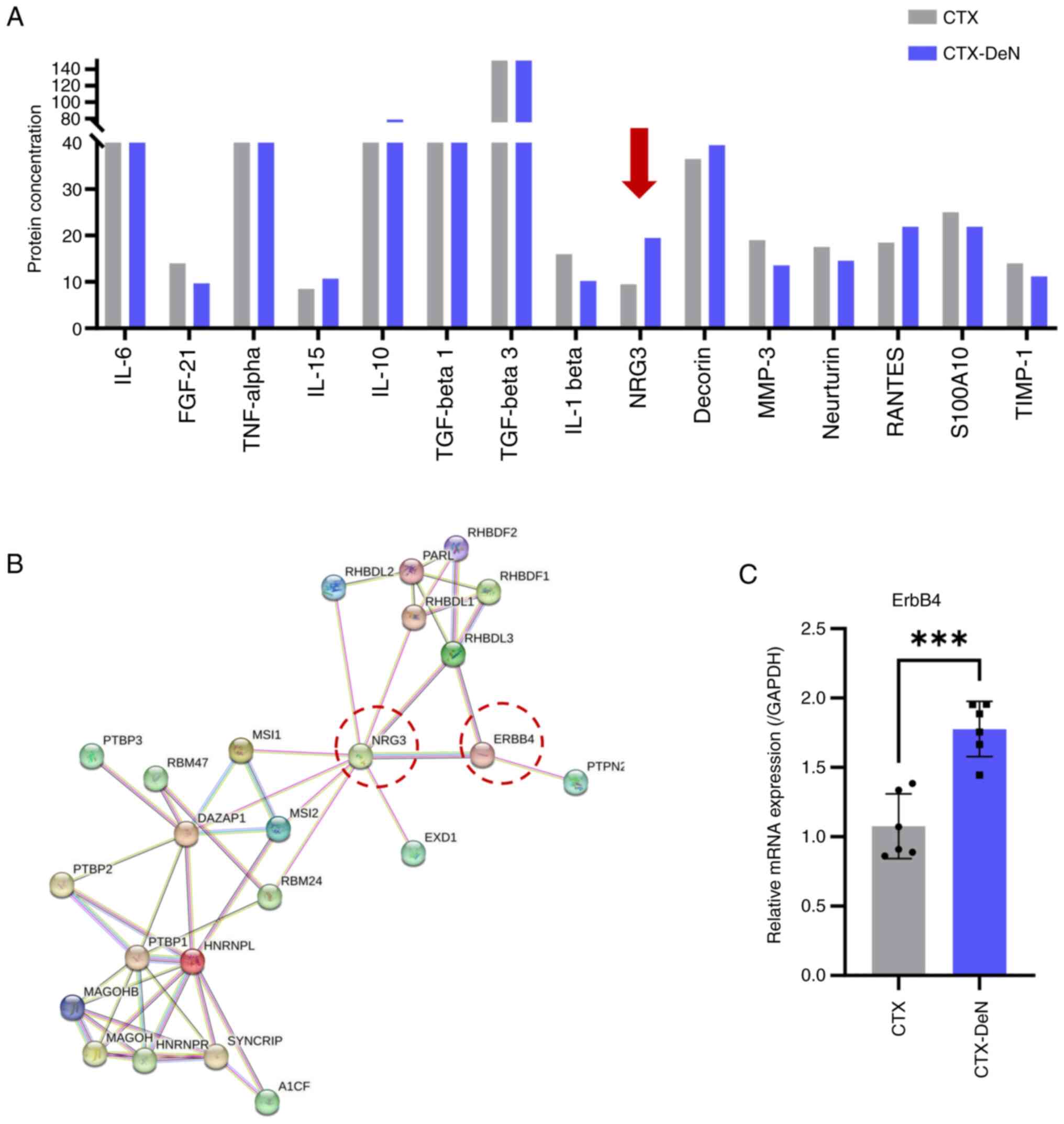 | Figure 2.DeN increases secretion of NRG3. (A)
Cytokine microarray analysis was used to quantify the levels of
cytokine secretion in muscle heterotopic ossification. Arrow
indicates the protein level of NRG3 was increased most in the
CTX-DeN group compared with the CTX group. (B) Molecular
interactions of NRG3. (C) Reverse transcription-quantitative PCR
analysis of ErbB4 mRNA levels. n=6/group. ***P<0.005. NRG3,
Neuregulin 3; CTX, Cardiotoxin; FGF, Fibroblast growth factor; TGF,
Transforming growth factor; RANTES, Regulated upon activation
normal T cell expressed and presumably secreted; S100A10, p11,
annexin II light chain, calpactin light chain; TIMP, The tissue
inhibitors of matrix metalloproteinases; DeN, Denervation. |
Ossification and cartilage
development-associated genes are enriched in denervation-aggravated
muscle HO
To assess how denervation causes exacerbation of
muscle HO, transcriptome sequencing was performed on muscle tissue
(Fig. S1). The results
demonstrated a total of 1,066 differentially expressed genes, of
which 752 were upregulated and 314 genes were downregulated in the
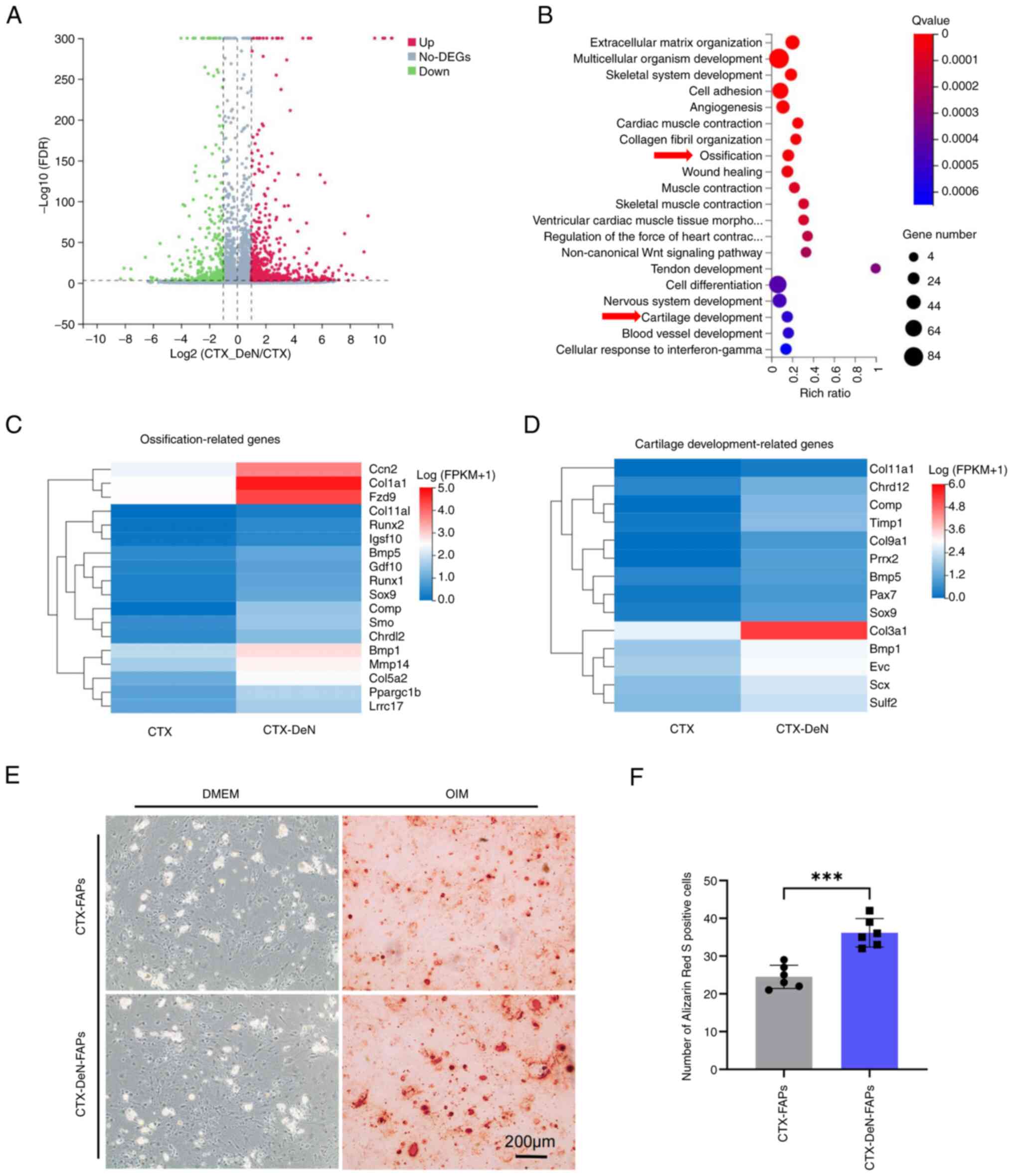
CTX-DeN group compared with the CTX group (Fig. 3A and Fig. S2). Gene Ontology functional
enrichment analysis of differentially expressed genes reported that
biological processes, including ‘ossification’ and ‘cartilage
development’ were significantly enriched (Fig. 3B-D). Freshly sorted FAPs were
cultured in osteogenic induction medium for 14 days. Alizarin Red S
staining was performed. The number of positively stained cells from
the CTX-DeN group was significantly increased compared with the CTX
group (Fig. 3E and F).
NRG3 affects osteogenic
differentiation of FAPs via the PI3K/Akt signaling pathway
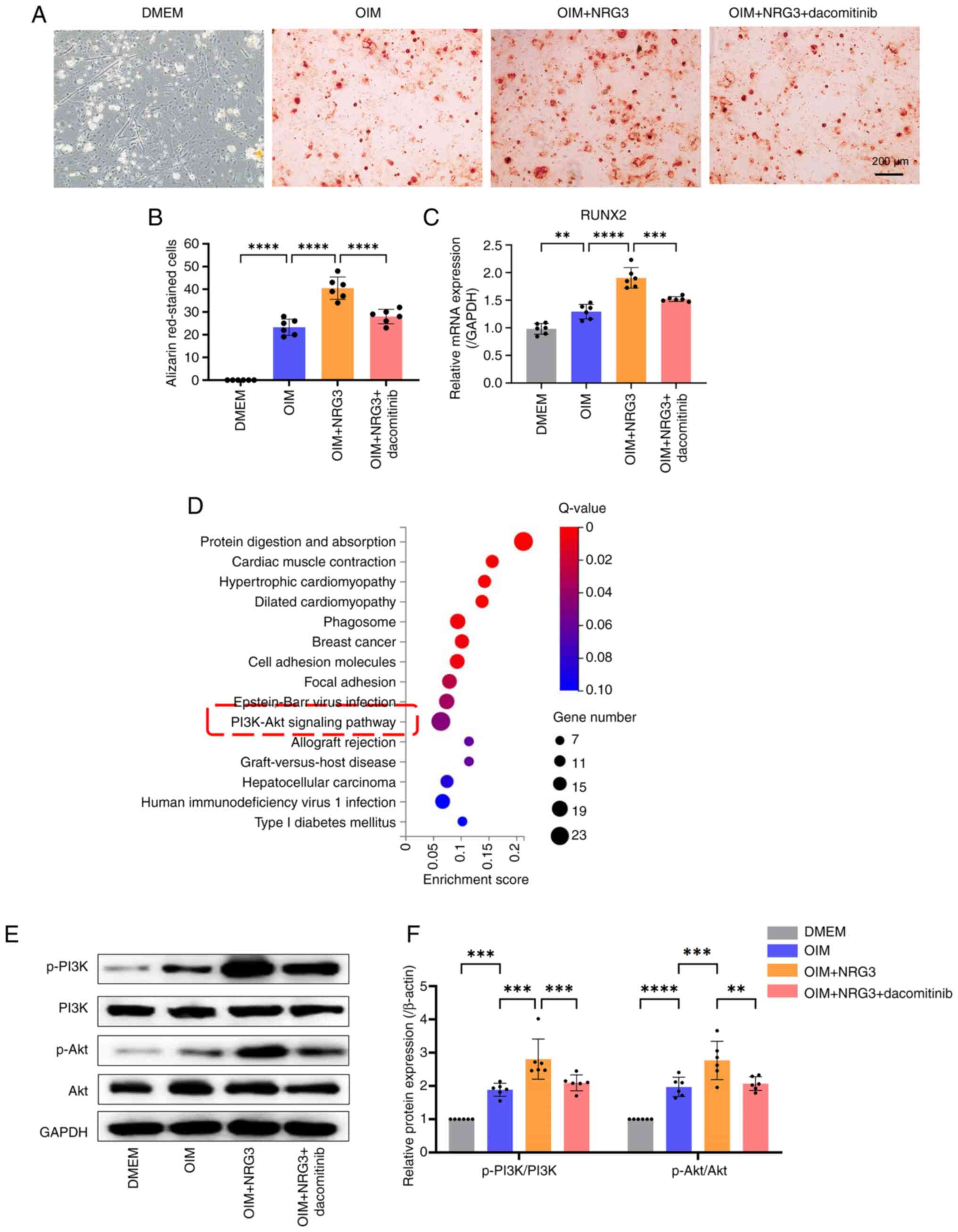
To assess the role of NRG3/ErbB4 signaling on the
PI3K/Akt signaling pathway in HO exacerbation, dacomitinib, an
inhibitor of ErbB4 was used. Osteogenic induction medium increased
the number of Alizarin Red S stained cells in OIM group was more
than DMEM group; this was enhanced by addition of NRG3. Following
addition of dacomitinib, osteogenic differentiation of FAPs was
inhibited, and the number of Alizarin Red S stained cells in
OIM+NRG3+dacomitinib group was less than OIM+NRG3 group (Fig. 4A and B). RT-qPCR analysis of
osteogenesis-associated genes was consistent with results of
alizarin Red S staining. The mRNA level of RUNX2 in OIM group was
more than DMEM group, and the mRNA level of RUNX2 in OIM+NRG3 group
was more than OIM group. Following addition of dacomitinib, the
mRNA level of RUNX2 in OIM+NRG3+dacomitinib group was less than
OIM+NRG3 group (Fig. 4C). KEGG
pathway enrichment analysis was performed on differentially
expressed genes; a number of pathways, including ‘protein digestion
and absorption’ were enriched. Based on previous literature
(40,41) and the results of KEGG, the PI3K/Akt
signaling pathway associated with osteogenesis was screened
(Fig. 4D). Western blotting of
PI3K/Akt pathway-associated proteins demonstrated that the protein
expression levels of p-PI3K and p-Akt were significantly increased
in the OIM group compared with DMEM group and OIM + NRG3 group
compared with the OIM group. Moreover, in the OIM + NRG3 +
dacomitinib group, protein expression levels of p-PI3K and p-Akt
were decreased compared with the OIM + NRG3 group (Fig. 4E and F).
Dacomitinib attenuates
denervation-aggravated HO of muscle
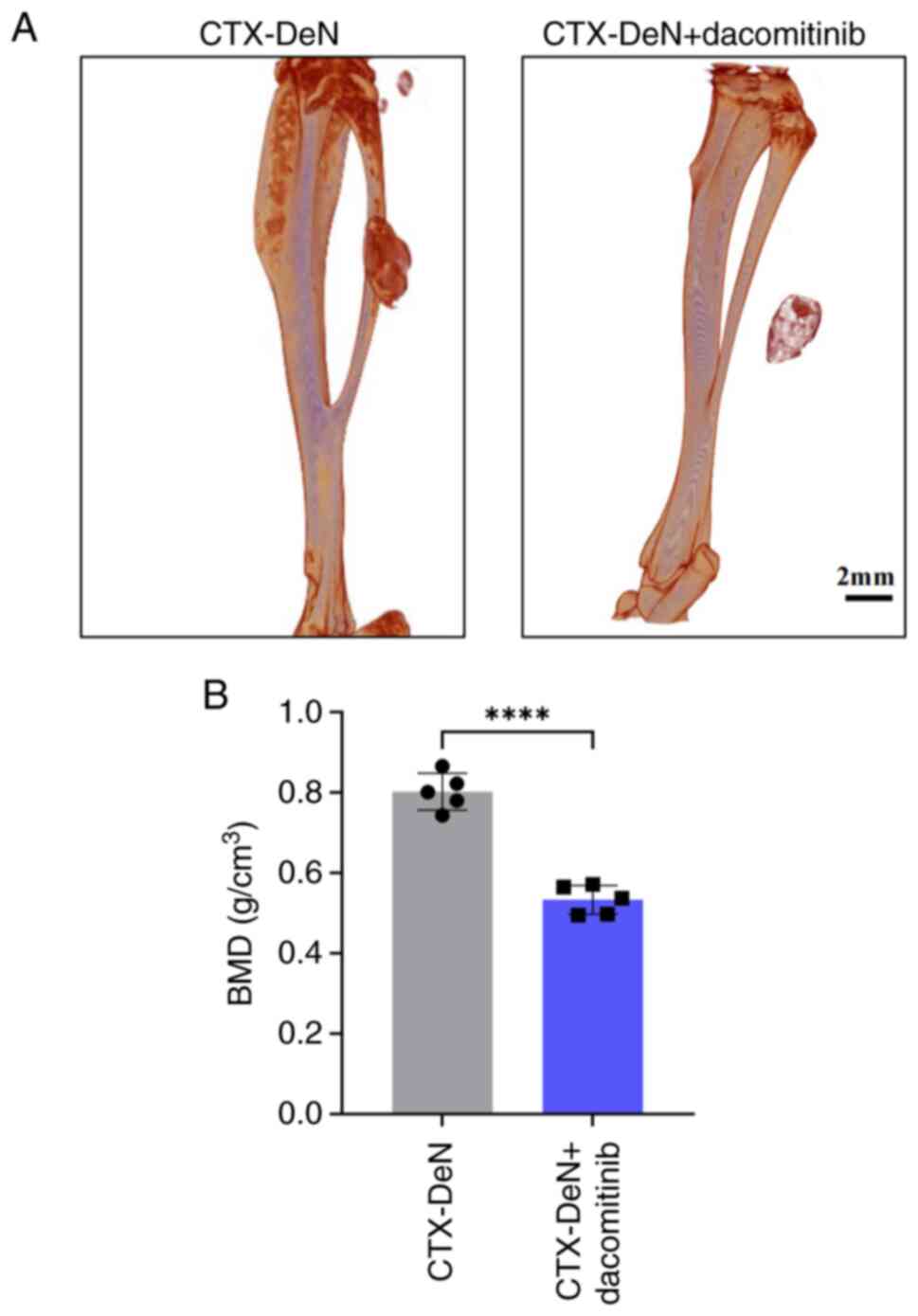
Finally, to confirm the mechanistic findings at the
cellular level, a muscle HO model was re-established in the mouse
gastrocnemius muscle. Micro-CT demonstrated that the BMD of the
CTX-DeN + dacomitinib group (0.533±0.036 g/cm3) was
significantly decreased compared with the CTX-DeN group(0.802±0.046
g/cm3) (Fig. 5A and
B).
Discussion
The association between peripheral nerves and
development of muscle HO has been demonstrated (42) but the relationship with the
progression of muscle HO is unclear. The understanding of the
association between the peripheral nerves and the progression of
muscle HO may serve as a diagnostic guide and aid in selecting an
appropriate treatment modality. The current treatment for extensive
injury-induced HO is surgical excision (43). However, certain patients may not be
suitable candidates for complete excision due to the nature or
location of ossification. Furthermore, the possibility of HO
recurrence after surgery exists (44), accompanied by associated procedural
risks such as infection and neurovascular injury. Improving
understanding of the underlying mechanisms of pathological bone
formation may facilitate the development of strategies aimed at
preventing unwanted ossification while using its regenerative
potential for applications in tissue engineering systems (45). In the present study, a mouse model
of gastrocnemius muscle HO was established by injury to the sciatic
nerve; imaging and histological observation demonstrated that
peripheral nerve injury promoted progression of muscle HO. This is
not only consistent with the findings of Qureshi et al
(46) and Olmsted-Davis et
al (47) but also increases
the knowledge of the association between peripheral nerve injury
and development of muscle HO and clarifies the relationship between
peripheral nerve injury and progression of muscle HO.
Increased expression of BMP-2 caused by damage to
the neurovascular barrier due to neuroinflammation is hypothesized
to be the mechanism by which peripheral nerve damage leads to
muscle HO (48–50). In the present study, a mouse model
of gastrocnemius muscle HO was established by CTX injection, based
on previous literature reports (51–53),
and locally applied gelatin solution saturated with BMP-2 to
increase the formation of ectopic bone. Denervation leads to more
severe HO of muscle and increases the formation of HO (51). The levels of cytokines in HO muscle
tissue were measured to determine their involvement in the
progression of HO. The expression levels of multiple cytokines,
including NRG3, were altered after peripheral nerve injury. Based
on analysis of absolute and differential expression, NRG3 may serve
a role in progression of muscle HO due to peripheral nerve
injury.
The activation of FAPs cells occurs following muscle
trauma. Previous research primarily focused on understanding
intracellular signaling pathways involved in wound repair (54). To the best of our knowledge,
however, there is a lack of research regarding upstream mediators
and mechanisms regulated by muscle innervation that govern FAP
recruitment, expansion and differentiation (54). Gallardo et al (55) developed a denervation mouse model
and reported activation of Yes-associated protein
1/(transcriptional co-activator with PDZ-binding motif) TAZ
signaling in FAPs, suggesting their involvement in FAP activation,
proliferation and differentiation. In the present study, using FAPs
as a model of muscle tissue, FAPs underwent significant osteogenic
differentiation upon induction of NRG3. This suggested that FAPs
may a cellular source of muscle HO. As non-myogenic cells, FAPs
serve a role in muscle injury repair, especially in skeletal muscle
degeneration and regeneration (38). Previous studies (34,56)
have reported that FAPs exhibit osteogenic or chondrogenic
differentiation under specific culture conditions in the presence
of BMP-2. Intramuscular transplantation of FAPs combined with
Matrigel and BMP-2 has demonstrated that these cells form cartilage
and calcium deposits in vivo (33). Lineage tracing using
PDGFRα− mice reported that the majority of osteoblasts
in BMP-2-induced in vivo HO are derived from FAPs (35). These findings suggest that FAPs
have multilineage differentiation potential and serve a role in
muscle HO.
The present study demonstrated through in
vitro and in vivo experiments that NRG3 modulated the
PI3K/Akt pathway, which induced osteogenic differentiation of FAPs
via binding to ErbB4 through protein-protein interaction network by
STRING database. NRG3 activates ErbB receptors, including ErbB2,
ErbB3 and ErbB4. Upon binding to its receptor, NRG3 initiates
downstream signaling pathways, such as the PI3K/Akt and
mitogen-activated protein kinase pathways, which serve roles in
cellular processes including survival, proliferation and
differentiation (57). This was
supported by results of KEGG pathway enrichment analysis of the
transcriptome sequencing data. These results suggested that the
cytological cause of the exacerbation of denervation-induced muscle
HO may be increased secretion of NRG3 and binding to ErbB4, thus
activating the PI3K/Akt pathway, leading to increased expression of
genes related to osteogenic differentiation (Fig. 6). Fisher et al (58) suggested that ErbB signaling serves
a role in bone formation and ossification. ErbB2 and ErbB3 are
expressed in osteoblasts, which are bone-forming cells involved in
regulation of differentiation and proliferation of periosteal
cells. Furthermore, Linder et al (59) reported that activation of the ErbB
receptor family by specific ligands can promote bone formation and
mineralization. In the present study, C57BL/6 mice were used as
animal models due to genetic and physiological similarity to
humans. The age of 8–12 weeks in mice corresponds to ~20 years of
age in humans, as they have fully developed but have not yet been
affected by aging (51–53).
Dacomitinib is a reversible pan-epidermal growth
factor receptor (EGFR) inhibitor (60), which selectively targets EGFR, and
is an irreversible inhibitor of three ErbB family kinase members
(ErbB1, ErbB2, and ErbB4) (61).
Dacomitinib decreased osteogenic differentiation of FAPs was
reduced. Western blotting of PI3K/Akt pathway-associated proteins
demonstrated expression levels of p-PI3K and p-Akt significantly
increased in the OIM + NRG3 group compared with the OIM group.
Moreover, in the OIM + NRG3 + dacomitinib group, protein expression
levels of p-PI3K and p-Akt were decreased compared with the OIM +
NRG3 group. These results suggested that denervation led to
osteogenesis-related biological processes in muscle tissue and the
PI3K/Akt signaling pathway may mediate this process. The
aforementioned results suggested that increased secretion of NRG3,
through binding to ErbB4, activates the PI3K/Akt pathway, leading
to increased expression of genes related to osteogenic
differentiation and exacerbation of denervation-induced muscle HO.
Dacomitinib may serve as a potential treatment option for muscle
HO.
A limitation of the present study is that FAPs were
not confirmed as a cellular source of muscle HO. ErbB4 inhibitor
dacomitinib was used to suppress the signaling of NRG3/ErbB4,
however, there may be other potential effects of this inhibitor. To
the best of our knowledge, the impact on proliferation of FAPs has
not been investigated. Future research should address this
knowledge gap. Cell tracing should be used to confirm that FAPs are
the cellular source of heterotopic calcification and gene knockout
mice should be used.
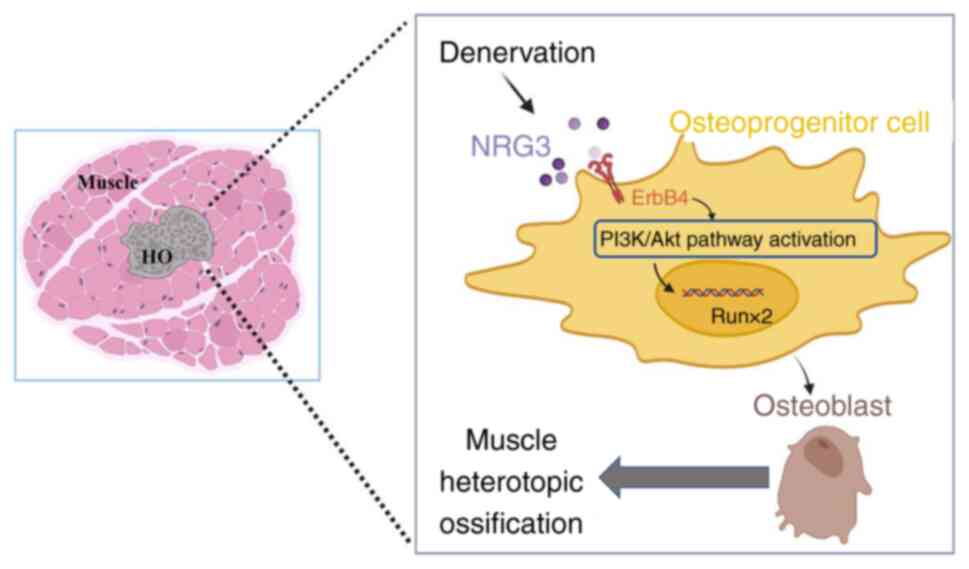
In summary, the present study demonstrated that
peripheral nerve injury exacerbated progression of muscle HO,
potentially via increased expression of BMP-2 caused by the release
of cytokine NRG3, which induced osteogenic differentiation of FAPs
via the ErbB4/PI3K/Akt signaling pathway.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Projects of the
Natural Science Foundation of China (grant no. 82130071).
Availability of data and materials
The data generated in the present study may be found
in The National Center for Biotechnology Information database under
accession number PRJNA1136075 or at the following URL:
ncbi.nlm.nih.gov/sra/PRJNA1136075.
Authors' contributions
LM, XK, KT, XL, YW, LG and XB conceived the study.
LM and JT performed experiments. LM, LG, HT and XK designed the
methodology. LM, YW, XL and HT collected data. JT, LM, YW, XL, KT
and XB analyzed data. LM, JT, YW, XL and HT wrote the manuscript.
LG, KT, XK and XB revised the manuscript. LM, KT and XB confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
The Laboratory Animal Welfare and Ethics Committee of Third
Military Medical University (Chongqing, China; approval no.
AMUWEC20210782).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Edwards DS and Clasper JC: Heterotopic
ossification: A systematic review. J R Army Med Corps. 161:315–321.
2015. View Article : Google Scholar
|
|
2
|
Ranganathan K, Loder S, Agarwal S, Wong
VW, Forsberg J, Davis TA, Wang S, James AW and Levi B: Heterotopic
Ossification: Basic-Science principles and clinical correlates. J
Bone Joint Surg Am. 97:1101–1111. 2015. View Article : Google Scholar
|
|
3
|
Sturbois-Nachef N, Gatin L, Salga M,
Geffrier A, Fontaine C and Allart E: Neurogenic heterotopic
ossification in the upper limb. Hand Surg Rehabil. 41S:S167–S174.
2022. View Article : Google Scholar
|
|
4
|
Reichel LM, Salisbury E, Moustoukas MJ,
Davis AR and Olmsted-Davis E: Molecular mechanisms of heterotopic
ossification. J Hand Surg Am. 39:563–566. 2014. View Article : Google Scholar
|
|
5
|
Nauth A, Giles E, Potter BK, Nesti LJ,
O'brien FP, Bosse MJ, Anglen JO, Mehta S, Ahn J, Miclau T and
Schemitsch EH: Heterotopic ossification in orthopaedic trauma. J
Orthop Trauma. 26:684–688. 2012. View Article : Google Scholar
|
|
6
|
Moore-Lotridge SN, Li Q, Gibson BHY,
Martin JT, Hawley GD, Arnold TH, Saito M, Tannouri S, Schwartz HS,
Gumina RJ, et al: Trauma-Induced nanohydroxyapatite deposition in
skeletal muscle is sufficient to drive heterotopic ossification.
Calcif Tissue Int. 104:411–425. 2019. View Article : Google Scholar
|
|
7
|
Ji Y, Christopherson GT, Kluk MW, Amrani
O, Jackson WM and Nesti LJ: Heterotopic ossification following
musculoskeletal trauma: Modeling stem and progenitor cells in their
microenvironment. Adv Exp Med Biol. 720:39–50. 2011. View Article : Google Scholar
|
|
8
|
Sanders BS, Wilcox RB III and Higgins LD:
Heterotopic ossification of the deltoid muscle after arthroscopic
rotator cuff repair. Am J Orthop (Belle Mead NJ). 39:E67–E71.
2010.
|
|
9
|
Xu Y, Huang M, He W, He C, Chen K, Hou J,
Huang M, Jiao Y, Liu R, Zou N, et al: Heterotopic Ossification:
Clinical features, basic researches, and mechanical stimulations.
Front Cell Dev Biol. 10:7709312022. View Article : Google Scholar
|
|
10
|
Edwards DS, Kuhn KM, Potter BK and
Forsberg JA: Heterotopic Ossification: A review of current
understanding, treatment, and future. J Orthop Trauma. 30 (Suppl
3):S27–S30. 2016. View Article : Google Scholar
|
|
11
|
Peake JM, Della Gatta P, Suzuki K and
Nieman DC: Cytokine expression and secretion by skeletal muscle
cells: Regulatory mechanisms and exercise effects. Exerc Immunol
Rev. 21:8–25. 2015.
|
|
12
|
Zhang S, Sun S, He J and Shen L: NT-3
promotes osteogenic differentiation of mouse bone marrow
mesenchymal stem cells by regulating the Akt pathway. J
Musculoskelet Neuronal Interact. 20:591–599. 2020.
|
|
13
|
Feng H, Xing W, Han Y, Sun J, Kong M, Gao
B, Yang Y, Yin Z, Chen X, Zhao Y, et al: Tendon-derived cathepsin
K-expressing progenitor cells activate Hedgehog signaling to drive
heterotopic ossification. J Clin Invest. 130:6354–6365. 2020.
View Article : Google Scholar
|
|
14
|
Xu R, Hu J, Zhou X and Yang Y: Heterotopic
ossification: Mechanistic insights and clinical challenges. Bone.
109:134–142. 2018. View Article : Google Scholar
|
|
15
|
Doherty C, Lodyga M, Correa J, Di
Ciano-Oliveira C, Plant PJ, Bain JR and Batt J: Utilization of the
rat tibial nerve transection model to evaluate cellular and
molecular mechanisms underpinning denervation-mediated muscle
injury. Int J Mol Sci. 25:18472024. View Article : Google Scholar
|
|
16
|
Bertin JSF, Marques MJ, Macedo AB, de
Carvalho SC and Neto HS: Effect of photobiomodulation on
denervation-induced skeletal muscle atrophy and autophagy: A study
in mice. J Manipulative Physiol Ther. 45:97–103. 2022. View Article : Google Scholar
|
|
17
|
Rodríguez MP and Cabello-Verrugio C:
Soluble factors associated with denervation-induced skeletal muscle
atrophy. Curr Protein Pept Sci. 25:189–199. 2024. View Article : Google Scholar
|
|
18
|
Komatsu M, Nakada T, Kawagishi H, Kato H
and Yamada M: Increase in phospholamban content in mouse skeletal
muscle after denervation. J Muscle Res Cell Motil. 39:163–173.
2018. View Article : Google Scholar
|
|
19
|
Lee J, Jang SH, Lee SJ and Lee O:
Synchrotron radiation imaging analysis of neural damage in mouse
soleus muscle. Sci Rep. 10:45552020. View Article : Google Scholar
|
|
20
|
Yoshimura A, Ito M, Chikuma S, Akanuma T
and Nakatsukasa H: Negative regulation of cytokine signaling in
immunity. Cold Spring Harb Perspect Biol. 10:a0285712018.
View Article : Google Scholar
|
|
21
|
Zhou P, Zheng T and Zhao B:
Cytokine-mediated immunomodulation of osteoclastogenesis. Bone.
164:1165402022. View Article : Google Scholar
|
|
22
|
Mansurov A, Lauterbach A, Budina E, Alpar
AT, Hubbell JA and Ishihara J: Immunoengineering approaches for
cytokine therapy. Am J Physiol Cell Physiol. 321:C369–C383. 2021.
View Article : Google Scholar
|
|
23
|
Schaible HG, Del Rosso A and
Matucci-Cerinic M: Neurogenic aspects of inflammation. Rheum Dis
Clin North Am. 3177–101. (ix)2005. View Article : Google Scholar
|
|
24
|
Salisbury E, Rodenberg E, Sonnet C, Hipp
J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA and Davis
AR: Sensory nerve induced inflammation contributes to heterotopic
ossification. J Cell Biochem. 112:2748–2758. 2011. View Article : Google Scholar
|
|
25
|
Zhang D, Sliwkowski MX, Mark M, Frantz G,
Akita R, Sun Y, Hillan K, Crowley C, Brush J and Godowski PJ:
Neuregulin-3 (NRG3): A novel neural tissue-enriched protein that
binds and activates ErbB4. Proc Natl Acad Sci USA. 94:9562–9567.
1997. View Article : Google Scholar
|
|
26
|
Jullien N, Maudinet A, Leloutre B, Ringe
J, Haupl T and Marie PJ: Downregulation of ErbB3 by Wnt3a
contributes to wnt-induced osteoblast differentiation in
mesenchymal cells. J Cell Biochem. 113:2047–2056. 2012. View Article : Google Scholar
|
|
27
|
Achilleos A and Trainor PA: Neural crest
stem cells: Discovery, properties and potential for therapy. Cell
Res. 22:288–304. 2012. View Article : Google Scholar
|
|
28
|
Lazard ZW, Olmsted-Davis EA, Salisbury EA,
Gugala Z, Sonnet C, Davis EL, Beal E II, Ubogu EE and Davis AR:
Osteoblasts Have a Neural Origin in Heterotopic Ossification. Clin
Orthop Relat Res. 473:2790–2806. 2015. View Article : Google Scholar
|
|
29
|
Joe AW, Yi L, Natarajan A, Le Grand F, So
L, Wang J, Rudnicki MA and Rossi FM: Muscle injury activates
resident fibro/adipogenic progenitors that facilitate myogenesis.
Nat Cell Biol. 12:153–163. 2010. View Article : Google Scholar
|
|
30
|
Helmbacher F and Stricker S: Tissue cross
talks governing limb muscle development and regeneration. Semin
Cell Dev Biol. 104:14–30. 2020. View Article : Google Scholar
|
|
31
|
Contreras O, Rossi FMV and Theret M:
Origins, potency, and heterogeneity of skeletal muscle
fibro-adipogenic progenitors-time for new definitions. Skelet
Muscle. 11:162021. View Article : Google Scholar
|
|
32
|
Vallecillo-Garcia P, Orgeur M, Vom
Hofe-Schneider S, Stumm J, Kappert V, Ibrahim DM, Börno ST, Hayashi
S, Relaix F, Hildebrandt K, et al: Odd skipped-related 1 identifies
a population of embryonic fibro-adipogenic progenitors regulating
myogenesis during limb development. Nat Commun. 8:12182017.
View Article : Google Scholar
|
|
33
|
Wosczyna MN, Biswas AA, Cogswell CA and
Goldhamer DJ: Multipotent progenitors resident in the skeletal
muscle interstitium exhibit robust BMP-dependent osteogenic
activity and mediate heterotopic ossification. J Bone Miner Res.
27:1004–1017. 2012. View Article : Google Scholar
|
|
34
|
Lees-Shepard JB, Yamamoto M, Biswas AA,
Stoessel SJ, Nicholas SE, Cogswell CA, Devarakonda PM, Schneider MJ
Jr, Cummins SM, Legendre NP, et al: Activin-dependent signaling in
fibro/adipogenic progenitors causes fibrodysplasia ossificans
progressiva. Nat Commun. 9:4712018. View Article : Google Scholar
|
|
35
|
Eisner C, Cummings M, Johnston G, Tung LW,
Groppa E, Chang C and Rossi FM: Murine tissue-resident PDGFRα+
fibro-adipogenic progenitors spontaneously acquire osteogenic
phenotype in an altered inflammatory environment. J Bone Miner Res.
35:1525–1534. 2020. View Article : Google Scholar
|
|
36
|
Akhter ET, Rotterman TM, English AW and
Alvarez FJ: Sciatic nerve cut and repair using fibrin glue in adult
mice. Bio Protoc. 9:e33632019. View Article : Google Scholar
|
|
37
|
Engelman JA, Zejnullahu K, Gale CM,
Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov
GN, Bradner JE, et al: PF00299804, an irreversible pan-ERBB
inhibitor, is effective in lung cancer models with EGFR and ERBB2
mutations that are resistant to gefitinib. Cancer Res.
67:11924–11932. 2007. View Article : Google Scholar
|
|
38
|
Kang X, Yang MY, Shi YX, Xie MM, Zhu M,
Zheng XL, Zhang CK, Ge ZL, Bian XT, Lv JT, et al: Interleukin-15
facilitates muscle regeneration through modulation of
fibro/adipogenic progenitors. Cell Commun Signal. 16:422018.
View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
40
|
Zhao SJ, Kong FQ, Jie J, Li Q, Liu H, Xu
AD, Yang YQ, Jiang B, Wang DD, Zhou ZQ, et al: Macrophage MSR1
promotes BMSC osteogenic differentiation and M2-like polarization
by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics.
10:17–35. 2020. View Article : Google Scholar
|
|
41
|
Pan JM, Wu LG, Cai JW, Wu LT and Liang M:
Dexamethasone suppresses osteogenesis of osteoblast via the
PI3K/Akt signaling pathway in vitro and in vivo. J Recept Signal
Transduct Res. 39:80–86. 2019. View Article : Google Scholar
|
|
42
|
Davis EL, Davis AR, Gugala Z and
Olmsted-Davis EA: Is heterotopic ossification getting nervous?: The
role of the peripheral nervous system in heterotopic ossification.
Bone. 109:22–27. 2018. View Article : Google Scholar
|
|
43
|
Hwang CD, Pagani CA, Nunez JH, Cherief M,
Qin Q, Gomez-Salazar M, Kadaikal B, Kang H, Chowdary AR, Patel N,
et al: Contemporary perspectives on heterotopic ossification. JCI
insight. 7:e1589962022. View Article : Google Scholar
|
|
44
|
Yuasa M, Mignemi NA, Nyman JS, Duvall CL,
Schwartz HS, Okawa A, Yoshii T, Bhattacharjee G, Zhao C, Bible JE,
et al: Fibrinolysis is essential for fracture repair and prevention
of heterotopic ossification. J Clin Invest. 125:3117–3131. 2015.
View Article : Google Scholar
|
|
45
|
Wan QQ, Qin WP, Ma YX, Shen MJ, Li J,
Zhang ZB, Chen JH, Tay FR, Niu LN and Jiao K: Crosstalk between
bone and nerves within bone. Adv Sci (Weinh). 8:20033902021.
View Article : Google Scholar
|
|
46
|
Qureshi AT, Crump EK, Pavey GJ, Hope DN,
Forsberg JA and Davis TA: Early characterization of blast-related
heterotopic ossification in a rat model. Clin Orthop Relat Res.
473:2831–2839. 2015. View Article : Google Scholar
|
|
47
|
Olmsted-Davis EA, Salisbury EA, Hoang D,
Davis EL, Lazard Z, Sonnet C, Davis TA, Forsberg JA and Davis AR:
Progenitors in peripheral nerves launch heterotopic ossification.
Stem Cells Transl Med. 6:1109–1119. 2017. View Article : Google Scholar
|
|
48
|
Alfieri KA, Forsberg JA and Potter BK:
Blast injuries and heterotopic ossification. Bone Joint Res.
1:192–197. 2012. View Article : Google Scholar
|
|
49
|
Smith JK, Miller ME, Carroll CG, Faillace
WJ, Nesti LJ, Cawley CM and Landau ME: High-resolution ultrasound
in combat-related peripheral nerve injuries. Muscle Nerve.
54:1139–1144. 2016. View Article : Google Scholar
|
|
50
|
Edwards DS, Clasper JC and Patel HD:
Heterotopic ossification in victims of the London 7/7 bombings. J R
Army Med Corps. 161:345–347. 2015. View Article : Google Scholar
|
|
51
|
Wang X, Li F, Xie L, Crane J, Zhen G,
Mishina Y, Deng R, Gao B, Chen H, Liu S, et al: Inhibition of
overactive TGF-β attenuates progression of heterotopic ossification
in mice. Nat Commun. 9:5512018. View Article : Google Scholar
|
|
52
|
Li L, Jiang Y, Lin H, Shen H, Sohn J,
Alexander PG and Tuan RS: Muscle injury promotes heterotopic
ossification by stimulating local bone morphogenetic protein-7
production. J Orthop Translat. 18:142–153. 2019. View Article : Google Scholar
|
|
53
|
O'Brien EJ, Frank CB, Shrive NG,
Hallgrímsson B and Hart DA: Heterotopic mineralization
(ossification or calcification) in tendinopathy or following
surgical tendon trauma. Int J Exp Pathol. 93:319–331. 2012.
View Article : Google Scholar
|
|
54
|
Wei X, Nicoletti C and Puri PL:
Fibro-Adipogenic Progenitors: Versatile keepers of skeletal muscle
homeostasis, beyond the response to myotrauma. Semin Cell Dev Biol.
119:23–31. 2021. View Article : Google Scholar
|
|
55
|
Gallardo FS, Cordova-Casanova A,
Bock-Pereda A, Rebolledo DL, Ravasio A, Casar JC and Brandan E:
Denervation Drives YAP/TAZ activation in muscular fibro/adipogenic
progenitors. Int J Mol Sci. 24:55852023. View Article : Google Scholar
|
|
56
|
Mejias Rivera L, Shore EM and Mourkioti F:
Cellular and molecular mechanisms of heterotopic ossification in
fibrodysplasia ossificans progressiva. Biomedicines. 12:7792024.
View Article : Google Scholar
|
|
57
|
Tumolo MR, Panico A, De Donno A, Mincarone
P, Leo CG, Guarino R, Bagordo F, Serio F, Idolo A, Grassi T and
Sabina S: The expression of microRNAs and exposure to environmental
contaminants related to human health: A review. Int J Environ
Health Res. 32:332–354. 2022. View Article : Google Scholar
|
|
58
|
Fisher MC, Clinton GM, Maihle NJ and Dealy
CN: Requirement for ErbB2/ErbB signaling in developing cartilage
and bone. Dev Growth Differ. 49:503–513. 2007. View Article : Google Scholar
|
|
59
|
Linder M, Hecking M, Glitzner E, Zwerina
K, Holcmann M, Bakiri L, Ruocco MG, Tuckermann J, Schett G, Wagner
EF and Sibilia M: EGFR controls bone development by negatively
regulating mTOR-signaling during osteoblast differentiation. Cell
Death Differ. 25:1094–1106. 2018. View Article : Google Scholar
|
|
60
|
Shirley M: Dacomitinib: First global
approval. Drugs. 78:1947–1953. 2018. View Article : Google Scholar
|
|
61
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964. 2017.
View Article : Google Scholar
|