Lens opacification, also termed cataract, is among
the leading causes of vision loss worldwide, and has resulted in
blindness in at least 53 million individuals globally at present
(1,2). Cataract phacoemulsification followed
by intraocular lens implantation is the current sole treatment for
this ailment (3). However,
cataract surgery is a great burden on global healthcare and
individuals. Firstly, as the global population ages, the number of
cataracts are expected to increase, which will place an even
heavier social and economic burden on healthcare (4). Unfortunately, expenses and the
overall medical condition limit surgery for numerous individuals.
For example, in China, the ophthalmologists are concentrated in
eastern urban areas (5) and the
current cost of cataract surgery is too expensive for numerous
individuals (6). Second, patients
may undergo complications after cataract surgery, such as
inflammation, xerophthalmia, macular edema or even posterior
capsular opacification (PCO) (4),
which greatly affects wellbeing. PCO is the most common
complication and when it occurs, it can lead to secondary vision
loss or blindness in 30–50% of adults and in 100% of children
(7). Therefore, elucidating the
changes that cause cataracts and developing pharmacological
preventative and therapeutic strategies is crucial.
Eye lenses are optically clear structures behind the
iris and in front of the vitreous body that focus light on the
retina (8). Lenses are formed from
ectodermal tissue and are comprised of lens epithelium and lens
fibers (9). The lens epithelium is
one single layer of anterior epithelial cells and, during lens
development, the lens epithelial cells gradually migrate towards
the lens equator, where they invert and elongate to differentiate
into fiber cells (10). Meanwhile,
differentiating cells synthesize large amounts of soluble
lenticular proteins, including crystallins, while degrading their
organelles and nuclei to increase lens transparency (10). Any disturbances in the lens
epithelium or lens fibers will result in a loss of lens
transparency (11).
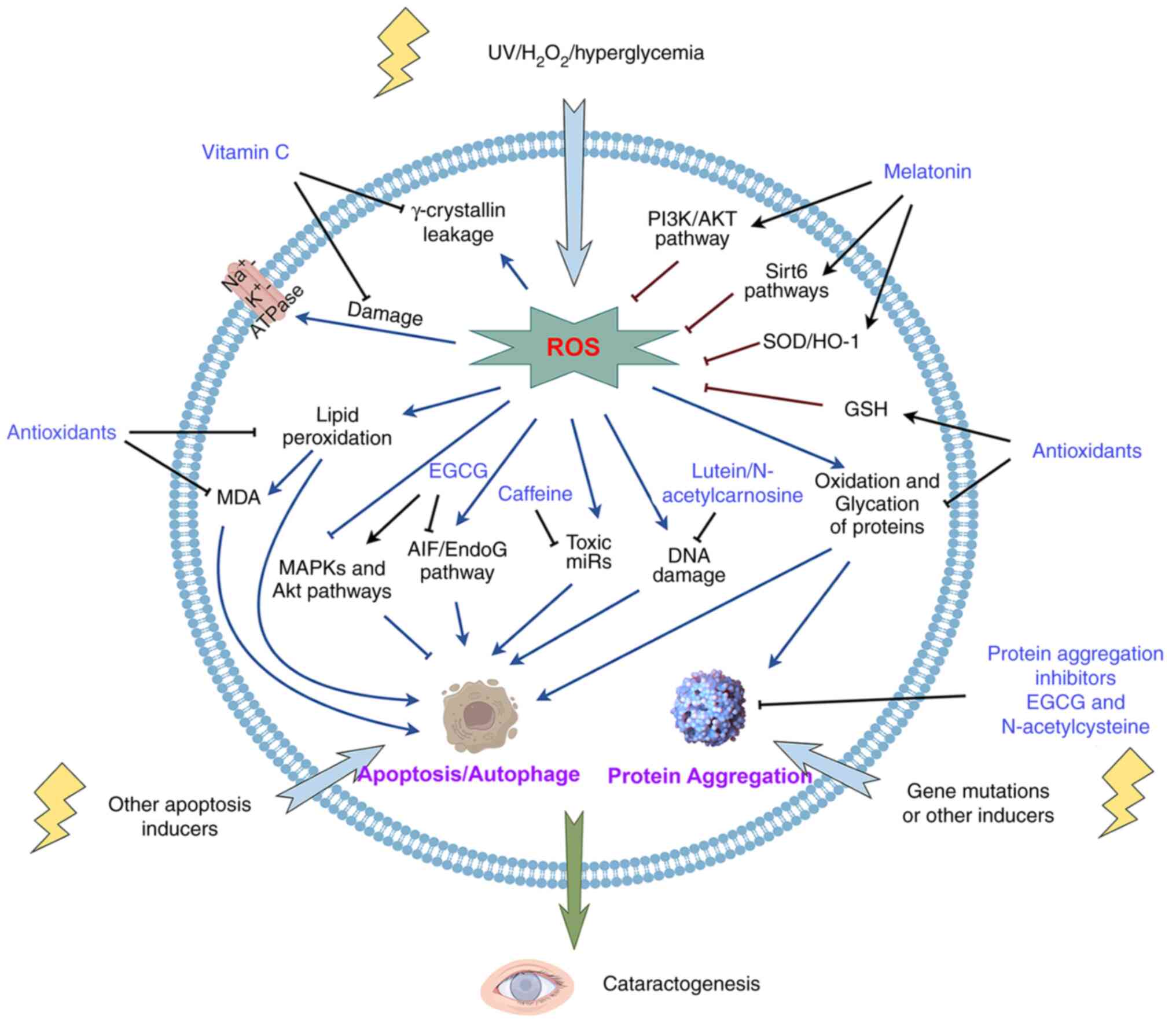
Vitamin C is essential for humans and is abundant in
the human lens, with the human ocular humors containing 50-fold
more vitamin C than plasma (34,35),
which protects the lens from UV light and other damage by reacting
with free radicals (36). In aged
human lenses, vitamin C levels are greatly decreased and thus may
fail to protect the lenses against oxidative stress-induced
cataracts (37). Vitamin C
supplementation has been revealed to help replenish and restore
endogenous vitamin C against cataract formation (33,38).
Under oxidative stress conditions, vitamin C prevents membrane LPO
(39) and
Na+K+-ATPase pump damage in the lens
(40).
Na+K+-ATPase-mediated ion transport is
crucial for maintaining the correct concentration of sodium in the
lens, and an abnormal elevation of lens sodium has been implicated
in the development of senile cataracts (41). In vitro, the physiological
concentration of vitamin C protects lens cells and dissected lenses
against H2O2 (42), UVB exposure (43) and other ROS-inducing factors such
as hyperglycemia (44), and thus
against induced oxidative damage. An in vivo study by
Devamanoharan et al (45)
found that a 0.3 mM per rat pup/day intraperitoneal vitamin C
injection maintained ATP and GSH levels and decreased MDA (the end
product of LPO) levels to prevent nuclear cataract development.
ATP, the intracellular energy currency molecule, has been shown to
act as a biological hydrotrope to prevent pathological protein
aggregation and maintain protein solubility (46), while elevated MDA levels are
associated with cataract formation (47). Additionally, a 1% (w/w) dietary
intake of vitamin C has been shown to reduce cataractogenesis in
streptozotocin (STZ)-induced diabetic rat models by decreasing
γ-crystallin leakage (48,49) and relieving oxidative stress by
increasing GSH peroxidase (GSH-Px) activity and reducing
peroxidation levels (50).
However, under pathological or overdose conditions,
vitamin C can switch from being an antioxidant to a pro-oxidant,
suggesting a role in stimulating the progression of cataracts.
First, a high concentration of vitamin C (1 M) has been reported to
promote the Fenton reaction, thus contributing to the formation of
hydroxy radicals as well as dehydroascorbic acid (DHA) and
H2O2, which are toxic to the lens (51). DHA is a reactive electrophile and
the primary oxidation product of vitamin C (51). DHA levels increase in response to
oxidative stress and are hypothesized to be associated with various
ROS and protein glycation-related diseases, including senile
cataracts (52). Similar to
oxidation, glycation is a deleterious form of post-translational
modification that is linked to age-related diseases, particularly
cataracts (53). The accumulated
glycation of proteins in the lens may induce protein conformational
changes that stimulate further glycation and oxidation as well as
trigger protein aggregation leading to a cataract (54). 2,3-diketo-L-gulonic acid (2,3-DKG)
is the further degradation product of DHA, and a heightened level
of 2,3-DKG has been shown to be related to increased
cataractogenesis in vivo (55). Vitamin C or its breakdown products
react with their substrate proteins to accelerate cataract
development. Fan et al (56) reported that vitamin C acts as a
chaperone of methylglyoxal hydroimidazolones, enhancing oxoaldehyde
stress, which promotes senile cataract progression. Additionally,
incubation of vitamin C and individual crystallins results in the
glycation and cross-linking of isolated lens crystallins (57). Furthermore, L-erythrulose, which
induces protein glycation and cross-linking, has been identified as
the major non-oxidative degradation product of vitamin C and
participates in diabetic and age-onset cataract formation (51). Overall, the existing experimental
data suggest that an appropriate level of vitamin C is essential to
protect the lens from oxidative damage. By contrast, boosting
vitamin C levels could be toxic to the lens and result in cataract
formation.
Similar to vitamin C, vitamin E has also been
identified as an antioxidant that protects against oxidative
stress-associated eye diseases such as cataracts and glaucoma
(58). It has been shown that
higher levels of vitamin E are associated with lower cataract risk
(59), while a reduced vitamin E
concentration is relevant to the development of senile cataracts
(60). Lens organ culture studies
have shown that the lipid solubility and antioxidant capabilities
of vitamin E shield membranes and scavenge free radicals to reduce
cataractogenesis (61–63). Animal studies have also confirmed
the protective effects of vitamin E on the lens. First, vitamin E
has been reported to prevent hyperglycemia-induced oxidative stress
and cataractogenesis by restoring GSH and reducing the formation of
MDA in the lenses of diabetic transgenic mice (64). Vitamin E has also been shown to
prevent cataracts induced by ionizing radiation (65), steroids (66), UV radiation (67) or selenite (68). Furthermore, a randomized human lens
sample study involving 50 patients with unilateral/bilateral
idiopathic immature senile cataracts showed that patients receiving
vitamin E had higher levels of reduced GSH and GSH-Px as well as
lower levels of MDA and lens opacity in the cortical cataractous
lenses compared with the placebo group (69), directly confirming the protective
effect of vitamin E in the human lens.
Carotenoids, a naturally occurring group of
lipo-soluble pigments, are potent antioxidants that neutralize and
scavenge free radicals (70). This
group comprises >600 natural compounds, among which lutein and
its stereoisomer, zeaxanthin, were revealed to assist in preventing
and mitigating oxidative-induced cataracts (71,72).
Additionally, lutein/zeaxanthin has been found to neutralize or
reduce free radicals in the human lens and filter against
high-energy and harmful blue light (73,74).
Oxidized proteins, LPO and DNA damage increase in human lens
epithelial cells in response to oxidative stress (75). However, pre-culture with 5 mM
lutein/zeaxanthin has been shown to notably prevent such
alterations (71), suggesting it
may lessen the incidence of senile cataracts by reducing oxidative
stress. Chitchumroonchokchai et al (76) found that 0.25 µM lutein protected
human lens epithelial cells from UV-induced oxidative stress by
inhibiting JNK and p38 activation. Both of which are implicated in
oxidative stress inhibition and lens cell protection (77). Furthermore, experimental evidence
shows that by filtering the high-energy and harmful blue light,
lutein attenuates photo-induced oxidation of lens proteins, thereby
protecting against age-related eye diseases, including cataracts
(74). More notably, in
vivo studies have demonstrated that lutein could counteract
certain types of cataracts. Specifically, Kinoshita et al
(78) found that 10 mg/kg/day
lutein administered orally for 29 days ameliorated cataracts in
type 1 diabetic rats by inhibiting the accumulation of
Nɛ-(carboxymethyl) lysine and
Nɛ-(carboxyethyl) lysine in the serum.
Nɛ-(carboxymethyl) lysine and
Nɛ-(carboxyethyl) lysine are glycoxidation products and
are significantly increased by diabetes, with the typical
complication being cataracts in both rats and humans (78). Combined with insulin, oral
administration of 0.5 mg/kg lutein has been demonstrated to prevent
the development of cataracts in STZ-induced diabetic rats by
preventing the diabetes-induced reduction of GSH levels (79). A clinical trial observation also
suggested that a higher dietary intake or higher blood levels of
lutein/zeaxanthin are associated with a lower incidence and a
slower progression of cataracts (80).
Besides its antioxidant properties, lutein inhibits
bovine lens epithelial cell growth and migration in vitro,
protecting the post-operative lens following phacoemulsification
(81). Considering that fibrotic
responses after surgery could result in blindness, this study
demonstrates the prospects of lutein in preventing PCO.
Polyphenols are the biggest group of phytochemicals,
which comprise >1,000 different compounds (82). Numerous polyphenols are linked to
health benefits such as antioxidant, anti-inflammatory or antiviral
activities (82). Moreover, recent
studies have described new findings regarding polyphenols, such as
(−)-epigallocatechin-3-gallate (EGCG) and resveratrol, in lens
protection.
EGCG is a primary component of green tea and has a
polyphenolic structure as well as a strong antioxidant capacity to
inhibit ROS generation by scavenging free radicals and chelating
metal ions (83). An in
vitro study has shown that 50 µM EGCG protects lens epithelial
cells against oxidative stress-induced apoptosis by activating the
MAPK and Akt pathways (84) as
well as UVB irradiation-induced apoptosis through the
apoptosis-inducing factor/Endonuclease G signaling pathway
(85). Crystallin is a major
structural protein present in the lens and its aggregation results
in an augmentation of lens opacity (86). EGCG also inhibits crystallin
aggregation, particularly αA (66–80),
a major fragment of αA-crystallin (87) and γB-crystallin (88), which protects the lens in a
concentration-dependent manner from 0 to 50 mM. In response to
hyperglycemia, EGCG suppresses the high glucose-induced expression
of apoptotic genes, c-Fos, c-Myc and p53 to protect human lens
epithelial cells, suggesting a protective role of EGCG in diabetic
cataract formation (89).
Furthermore, an in vivo study confirmed that oral
administration of 1 mg/kg EGCG prevented lens opacity and
αB-crystallin aggregation in diabetic rat models (90). Although EGCG is a redox-active
molecule, it auto-oxidizes to produce superoxide radicals and
H2O2 (91).
Contradictory data demonstrate that a high level of EGCG (200 mM)
inhibits lens epithelial cell growth and induces apoptosis
(92,93), indicating its use in PCO
prevention.
Resveratrol, another natural polyphenol, is a
radical-scavenging antioxidant and anti-aging agent (94). Accumulating evidence has
demonstrated that resveratrol has a therapeutic and preventive
effect on the eye, specifically the lens (95). In vitro research has
revealed that resveratrol protects human lens epithelial cells
against oxidative stress in a concentration-dependent manner by
enhancing catalase, SOD-1 and heme oxygenase-1 (HO-1) expression
(96), and activates autophagy to
protect cells against high glucose-induced oxidative stress
(97). Both HO-1 and its upstream
regulator, nuclear factor erythroid 2-related factor 2 (Nrf2), are
oxidative stress inhibitors (98–100). Autophagy refers to the
physiological and pathological processes of cellular lysosomal
degradation, which are not only essential for cell survival and
development but are also associated with various human diseases
including diabetes (101–103). The activation of autophagy has
been reported to protect against oxidative stress and apoptosis
under specific conditions (104,105). Resveratrol has also displayed a
protective role in animal models. First, in STZ-induced diabetic
rats, Singh et al (106)
and Higashi et al (107)
found that oral administration of 40 mg/kg/day resveratrol is
beneficial in the pharmacotherapy of diabetes and its secondary
complications, such as cataracts, through the attenuation of
oxidative damage to lens proteins. Second, Chen et al
(108) designed a nanosystem of
gold nanoparticles containing resveratrol (RGNPs). In the
selenite-induced cataract model, subcutaneous injection of RGNPs
improved lens opacity and decreased the mRNA and protein levels of
proteins associated with the lens (γA-crystallin and
βA1-crystallin) senescence markers (p16 and p21) and the activated
Sirtuin (Sirt) 1/Nrf2 pathway. These findings demonstrated the
anti-aging and anti-cataract effects of resveratrol (108). Resveratrol also has been shown to
be a candidate agent in preventing PCO. In FHL124 cells and human
lens capsular bags, 30 µM resveratrol significantly inhibited cell
growth, migration and epithelial-mesenchymal transition (EMT),
which are pivotal events for PCO development (109).
Melatonin, an amphiphilic tryptophan-derived
indolamine, is primarily secreted by the pineal gland and regulates
circadian rhythm (110,111). This hormone also acts a highly
potent antioxidant by activating GSH synthesis and scavenging free
radicals as well as an anti-inflammatory factor by functioning as
an immune modulator (112). It
has been demonstrated that melatonin is synthesized within the eye
to counteract age-related ocular diseases including glaucoma,
age-related macular degeneration, diabetic retinopathy and cataract
(113). In human lens epithelial
cells, 50–250 µM melatonin decreases
H2O2-induced intracellular ROS generation by
activating the PI3K/Akt signaling pathway (114) and inhibits UVB-promoted
ferroptosis by regulating two Sirt6 (Nrf2 or nuclear receptor
coactivator 4) pathways (115,116). PI3K/Akt signaling has a critical
role in lens protection by mediating apoptosis (117), while Sirt6 is a chromatin
regulatory protein that also plays a role in combating oxidative
stress (115). An in vivo
study confirmed that melatonin delayed the development of senile
cataract by activating Sirt6 (115). In an STZ-induced diabetic rat
model, intraperitoneal injection of 5 mg/kg/day melatonin reduced
cataract formation by increasing the GSH levels and decreasing the
activity of aldose reductase (AR) and the MDA level (118). AR is the crucial enzyme in the
polyol pathway and mediates the conversion of glucose to sorbitol
(119,120). Accumulation of sorbitol in the
lens results in osmotic trauma and eventually lens opacification
(121). A study by Karslioğlu
et al (122) revealed that
melatonin protects against radiation-induced cataract by
significantly increasing the activity of SOD enzymes and decreasing
the MDA level. As demonstrated by the aforementioned studies,
melatonin may be a promising candidate in cataract management.
Caffeine, a widely used drug as well as a dietary
constituent, has been identified as a ROS scavenger against
cataract formation. Firstly, in 2008, Varma et al (123) evaluated the effect of caffeine on
cultured and UV radiation-exposed mice lenses and revealed that
caffeine significantly maintained the active transport activity,
GSH levels and transparency of lenses. Following this study, the
same group then demonstrated that 5.15 µM intraperitoneally
injected or a 1% dietary intake of caffeine also had a positive
effect on preventing selenite-induced (124) and high sugar-induced (125) cataracts in animal models. A
further study in humans revealed that a higher level of coffee
consumption was co-related to a lower incidence of cataract
blindness (125,126). Mechanistically, the caffeine
effect could be multifactorial. First, as an antioxidant, caffeine
is an effective inhibitor of LPO and against all three reactive
species that cause membrane damage in vivo, including OH•,
peroxyl radical (ROO•) and singlet oxygen
(1O2), at certain concentrations (127). Caffeine also retains lens GSH and
ascorbic acid levels which were significantly lower in high-fat
diet-induced mice (128).
Moreover, caffeine suppresses the high-galactose diet-induced
elevation of toxic microRNAs particularly miR-16, miR-32, miR-218
that are known to induce apoptosis and cell death by gene silencing
to prevent the formation of cataracts (125,129). Overall, caffeine is a promising
candidate molecule for cataract prevention and treatment. However,
excessive maternal caffeine exposure (100 mg/kg/day,
intraperitoneally) during pregnancy has been indicated in inducing
cataracts (130), suggesting that
caution when consuming a high quantity caffeine is necessary for
pregnant women.
N-acetylcarnosine, a natural histidine-containing
dipeptide, has been applied as an eye drop to prevent or reverse
the progression of cataracts. N-acetylcarnosine, a prodrug, is
metabolized into L-carnosine in the front chamber of the eye
(131). L-carnosine is an in
vivo universal antioxidant and has a potent protective effect
against oxidative stress but cannot penetrate the cornea (131). Clinical trials have revealed that
an N-acetylcarnosine lubricant eye drop treatment
significantly improves visual function. First, an observation by
Babizhayev et al (30)
revealed that a short-period administration of
N-acetylcarnosine lubricant eye drops rejuvenated the visual
functions of older adult drivers and drivers with cataracts.
Second, a clinical experiment with >50,000 participants showed
that N-acetylcarnosine eye drops improved senile cataracts
and visual acuity in patients with diabetic ocular complications
(53,132,133). Mechanistically, the effect of
N-acetylcarnosine/L-carnosine on preventing or delaying
cataract formation may be through the anti-glycation of proteins,
antioxidative impairment, protecting proteins against cross-linking
and DNA damage (53,132,133). Protein glycation is also one of
the main factors contributing to diseases such as diabetes
mellitus, carcinoma and cataracts (53,134). It induces lens protein structural
changes that result in protein crosslinks, aggregation and high
molecular weight protein formation (135). Another study found that
N-acetylcarnosine decreased lens cell telomere shortening to
protect against oxidative stress (75,136) and the harmful effects of lipid
peroxides on the crystalline lens in vivo (137). Taken together,
N-acetylcarnosine/L-carnosine prevents and treats senile
cataracts and is a potentially effective and non-surgical
anti-cataract therapy.
The human lens is primarily comprised of
crystallins whose native tertiary structures and solubility ensure
lens transparency (147). The
crystallin superfamily includes α-, β- and γ-crystallins (148). During lens differentiation,
crystallin levels are highly upregulated, while degradation of
organelles such as nuclei, mitochondria, endoplasmic reticulum, and
ribosomes occurs (148). Both
gene mutation, which is considered to be related to congenital
cataract, or age-related protein damage induced by UV radiation,
oxidative stress and other factors such as hyperglycemia, may lead
to the generation of light-scattering protein particles and
cataract formation (18).
Furthermore, the mature fiber without organelles lacks the protein
synthesis and degradation machinery necessary for removing and
replacing damaged proteins (148). Therefore, the native
conformations of crystallins must have superior solubility and
long-term stability (147). If
not, preventing or reversing protein aggregation is an important
and novel strategy for cataract prevention and treatment.
The analog of lanosterol, 25-hydroxycholesterol,
has also been demonstrated to have a similar effect but a different
mechanism in cataract prevention and therapy (156). Lanosterol can release all
crystallin members by possibly binding with and destabilizing the
intramolecular β-sheet structures of the crystallin aggregates
(156). Specifically, Kang et
al (157) showed that
lanosterol binds to the hydrophobic dimerization interface to
disrupt the aggregation of human γD-crystallin. However,
25-hydroxycholesterol distinctly dissociates α-crystallin via a
certain binding site such as the dimer interface (158,159). Although 25-hydroxycholesterol is
specific to α-crystallin, it is able to improve the transparency a
solution composed of various crystallins. This may be due to the
release of α-crystallin, which weakens the intermolecular
interactions in the aggregates (156).
Although, lanosterol and 25-hydroxycholesterol have
shown promising results in preventing and treating cataracts by
dissolving lens crystallin proteins, certain researchers have
doubted these effect. First, Daszynski et al (160) found that 0.2 mM lanosterol and
0.25 mM or 0.5 mM 25-hydroxycholesterol did not raise the soluble
lens protein levels and restore cataract lens clarity. Second, the
therapeutic effect of lanosterol has not been observed in some
in vivo cases. For instance, it was reported that 25 mM
lanosterol failed to reverse opacification of human senile cataract
nuclei (161) and had little
effect on the nuclear cataracts of cynomolgus monkeys (150). These findings indicate that the
therapeutic potential of lanosterol may be restricted by its
capacity to dissolve protein aggregates or by its concentration and
the cataract type and severity. Therefore, further studies to
elucidate the pharmacological mechanisms of lanosterol and
25-hydroxycholesterol are necessary to promote utilization in the
clinical treatment of cataracts.
In the vertebrate lens, crystallins (α-, β- and γ-)
are typically considered structural proteins that constitute nearly
90% of the total lens protein (162). However, α-crystallins, which are
composed of the two subunits αA- and αB-crystallin, but not β- or
γ-crystallins, are also small heat-shock proteins that act as
molecular chaperones and anti-apoptotic proteins to help maintain
lens clarity (163).
α-crystallins contribute to the protection from numerous eye
diseases including cataracts, retinitis pigmentosa and macular
degeneration (164–166). In eye lenses, α-crystallins form
short-range contacts with other crystallin proteins to avoid
protein misfolding and aggregation-induced light scattering
(164). Mutations as well as
aging related modification of α-crystallins that affect the
structure, oligomerization and chaperone function, lead to
decreased solubility and increased protein aggregation, making the
lens prone to the development of congenital or senile cataracts
(163). Thus, modulating
chaperone activity by increasing the chaperone concentration in the
lens is one important strategy to interfere with protein
aggregation in the lens. Since the penetration to the eye is
limited by the size, stability and post-modification of chaperones
(162), the mini-chaperone
peptide is a potential candidate molecule for therapeutic use in
diseases associated with protein aggregation such as cataracts.
Previously, investigators established that both the
mini-αA70-88 (KFVIFLDVKHFSPEDLTVK) and mini-αB73-92
(DRFSVNLDVKHFSPEELKVK) peptide chaperones have a similar effect on
preventing protein aggregation of the native α-crystallin subunits
(167,168). These mini-chaperones had already
been demonstrated to inhibit selenite-induced cataract formation in
rats by intraperitoneal injection at concentrations of 2.5–10 µg
per animal (169). The prevention
of cataract development by these mini-chaperones is achieved by
inhibiting stress-induced apoptosis as well as protein aggregation
(169). It has been suggested
that these mini-chaperones provided Bax and procaspase-3 binding
sites to inhibit their activities and inhibited cytochrome c
release (169). However, the
mechanism by which these mini-chaperones interact with their
substrate proteins to inhibit protein aggregation has not yet been
elucidated (169). Furthermore,
the αA-mini-chaperone has also been shown to stabilize the cataract
causing αA-crystallin mutant, αAG98R, and rescue its chaperone
activity (170). The covalent
interactions of the αA-mini-chaperone with the αAG98R subunits has
been detected (170).
γD-crystallin is the natural substrate of αA-crystallin (171). A study by Banerjee et al
(172) revealed that the
αA-mini-chaperone binds to Phe56, Val132, and Val164 to Leu167 of
γD-crystallin to protect it from aggregation and oxidation. In
summary, mini-chaperones that exhibit a specific binding affinity
for crystallin and anti-apoptotic properties serve as promising
drug candidates for cataract prevention and treatment.
Besides sterols, a phenolic compound, rosmarinic
acid has also been identified as a lenticular protein aggregation
inhibitor (173), as well as an
antioxidant (174,175). In 2018, Chemerovski-Glikman et
al (173) reported that they
had developed an ex vivo screening platform in which human
lens particles removed from patients during cataract surgery were
treated with different protein aggregation modulator candidates.
The study confirmed the efficacy of 25-hydroxycholesterol in
reducing the cataract protein load. Moreover, it was revealed that
rosmarinic acid was potent cataract modulators and exhibited
improved optical clearance abilities compared with sterols.
Furthermore, an in vivo study in which model rats were
subcutaneously injected with rosmarinic acid confirmed that it
ameliorated cataract formation by modulating protein aggregation
(173). Mechanistically,
rosmarinic acid reduces cataract microparticle size and modifies
their amyloid-like features (173). Additionally, as an antioxidant,
intraperitoneally injected rosmarinic acid reduces estrogen
deficiency- and selenite-induced cataract development by inhibiting
oxidative stress (174,175).
Cataract is a major ophthalmic disease causing
severe visual impairment and even blindness in patients (1,2). To
date, cataract surgery is still the only effective treatment method
(3). However, cataract surgery has
a number of limitations. Surgery has a great economic burden on
public health and patients, and some patients may not even be able
to have surgery due to a lack of access and resources (4–6).
Additionally, surgery may cause PCO and further vision loss
(7). Therefore, researchers are
continuously seeking an available and effective non-surgical method
to prevent and treat cataracts.
Cataract development has several unknown causes;
however, oxidative stress is known to cause and develop cataracts
(20,106,130,143,174). The disturbance of pro- and
antioxidant systems leads to hyper-levels of free radicals, which
attack other molecules, thus resulting in aging-related diseases
such as glaucoma and cataracts (29). Therefore, inhibiting pro-oxidants
or enhancing the levels of antioxidants are primary strategies to
prevent cataracts. Vitamins, carotenoids, polyphenols, melatonin,
caffeine, N-acetylcarnosine and N-acetylcysteine are
strong antioxidants that target oxidative stress in the
pathogenesis of cataracts (19,28,107,118,132,145,176). These antioxidants have been
demonstrated to prevent or slow the progression of cataracts in
vitro, ex vivo and in vivo (31,106,116,130,143,144). Carotenoids and polyphenols
inhibit cell fibrosis and EMT, suggesting secondary cataract PCO
prevention potential (81,109). Besides their antioxidative and
anti-cataract properties, certain antioxidants (such as vitamins)
also display lens toxicity, which may be related to hyper-dosage or
oxidizing metabolites (56).
Moreover, some antioxidants (such as EGCG and
N-acetylcysteine) inhibit protein aggregation, thus enhanced
their application prospects in cataract prevention and treatment
(87,138) (Table
I). However, only N-acetylcarnosine has experimentally
demonstrated a partial efficacy in the restoration of vision
(30). Consequently, further
research is warranted to devise a comprehensive strategy that
enhances both the prophylactic efficacy of antioxidants and
combines the therapeutic efficacy of other pharmacological
treatments in cataract.
Crystallins are the vital structural and functional
proteins that are responsible for the refractive index in the lens
(177). The structural
conformational changes caused by post-translational modifications
or mutations produce a disorder of crystallin-crystallin
interactions and lenticular opacity (18,163). Inhibiting or reversing crystallin
aggregation is another major effective strategy for cataract
prevention and treatment (90,156). Zhao et al (149) first revealed that lanosterol
reverses protein aggregation in cataracts. Lanosterol releases all
crystallin family members, while its analog, 25-hydroxycholesterol,
specifically dissociates α-crystallin (156). With different mechanisms, these
drugs display similar crystallin aggregation inhibition effects
(149,156,173). Although they are promising
anti-cataract drugs, these drugs have little effect on nuclear
cataracts, suggesting multiple variables restrict their therapeutic
effect (150,161), such as the lens nuclear barrier
(150).
In the lens, high levels of chaperone protein are
vital for transparency maintenance (158,164). Due to gene mutation, oxidative
stress or environmental factors, these proteins may lose their
chaperone activity and become part of aggregates forming the
cataract (163). Thus, increasing
the activity or concentration of these chaperones in the lens would
be an effective strategy for cataract treatment. Recently,
mini-chaperones have been found to act like the native proteins,
inhibiting oxidative stress and protein aggregation (162,169). Further study is imperative to
promote its translation from bench to the clinic. Recently, the
phenolic compound, rosmarinic acid, has also been proposed to be an
anti-cataract candidate as it has lenticular protein aggregation
and antioxidative properties (173) (Table II).
Not applicable.
This work supported by the National Natural Science Foundation
of China (grant no. 82302277), The Science and Technology
Innovation Program of Hunan Province (grant no. 2022RC1232),
Research Foundation of Education Bureau of Hunan Province (grant
no. 22A0658), and Essential Science Indicators Discipline Special
Project of Changsha Medical University (grant nos. 2022CYY029 and
2022CYY010).
Not applicable.
LW, XLi, JL wrote and revised the main manuscript,
XM and XLiu collected and analyzed the data. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bernhisel A and Pettey J: Manual small
incision cataract surgery. Curr Opin Ophthalmol. 31:74–79. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flaxman SR, Bourne R, Resnikoff S, Ackland
P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen
JH, et al: Global causes of blindness and distance vision
impairment 1990–2020: A systematic review and meta-analysis. Lancet
Glob Health. 5:e1221–e1234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohnen T, Baumeister M, Kook D, Klaproth
OK and Ohrloff C: Cataract surgery with implantation of an
artificial lens. Dtsch Arztebl Int. 106:695–702. 2009.PubMed/NCBI
|
|
4
|
Marques AP, Ramke J, Cairns J, Butt T,
Zhang JH, Jones I, Jovic M, Nandakumar A, Faal H, Taylor H, et al:
The economics of vision impairment and its leading causes: A
systematic review. EClinicalMedicine. 46:1013542022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An L, Jan CL, Feng J, Wang Z, Zhan L and
Xu X: Inequity in access: Cataract surgery throughput of chinese
ophthalmologists from the china national eye care capacity and
resource survey. Ophthalmic Epidemiol. 27:29–38. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang XJ, Li EY, Leung CK, Musch DC, Zheng
CR, He MG, Chang DF and Lam DS: Willingness to pay for cataract
surgery in baiyin district, northwestern China. Ophthalmic
Epidemiol. 28:205–212. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rusin-Kaczorowska K and Jurowski P:
Qualification and methods of laser capsulotomy in pseudophakic eye.
Klin Oczna. 114:143–146. 2012.(In Polish). PubMed/NCBI
|
|
8
|
Kaplan HJ: Anatomy and function of the
eye. Chem Immunol Allergy. 92:4–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miesfeld JB and Brown NL: Eye
organogenesis: A hierarchical view of ocular development. Curr Top
Dev Biol. 132:351–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cvekl A and Ashery-Padan R: The cellular
and molecular mechanisms of vertebrate lens development.
Development. 141:4432–4447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmitt C and Hockwin O: The mechanisms of
cataract formation. J Inherit Metab Dis. 13:501–508. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thompson J and Lakhani N: Cataracts. Prim
Care. 42:409–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
West SK and Valmadrid CT: Epidemiology of
risk factors for age-related cataract. Surv Ophthalmol. 39:323–334.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Straatsma BR, Foos RY, Horwitz J, Gardner
KM and Pettit TH: Aging-related cataract: Laboratory investigation
and clinical management. Ann Intern Med. 102:82–92. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pichi F, Lembo A, Serafino M and Nucci P:
Genetics of congenital cataract. Dev Ophthalmol. 57:1–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan WH, Biswas S, Ashworth JL and Lloyd
IC: Congenital and infantile cataract: Aetiology and management.
Eur J Pediatr. 171:625–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Chen X, Yan Y and Yao K: Molecular
genetics of congenital cataracts. Exp Eye Res. 191:1078722020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiels A and Hejtmancik JF: Mutations and
mechanisms in congenital and age-related cataracts. Exp Eye Res.
156:95–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Babizhayev MA and Yegorov YE: Reactive
oxygen species and the aging eye: Specific role of metabolically
active mitochondria in maintaining lens function and in the
initiation of the Oxidation-induced maturity onset Cataract-A novel
platform of Mitochondria-targeted antioxidants with broad
therapeutic potential for redox regulation and detoxification of
oxidants in eye diseases. Am J Ther. 23:e98–e117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bohm EW, Buonfiglio F, Voigt AM, Bachmann
P, Safi T, Pfeiffer N and Gericke A: Oxidative stress in the eye
and its role in the pathophysiology of ocular diseases. Redox Biol.
68:1029672023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandrasekaran A, Idelchik M and Melendez
JA: Redox control of senescence and age-related disease. Redox
Biol. 11:91–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Nie Q, Gao M, Yang L, Xiang JW,
Xiao Y, Liu FY, Gong XD, Fu JL, Wang Y, et al: The transcription
factor CREB acts as an important regulator mediating oxidative
stress-induced apoptosis by suppressing αB-crystallin expression.
Aging (Albany NY). 12:13594–13617. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Babizhayev MA and Costa EB: Lipid peroxide
and reactive oxygen species generating systems of the crystalline
lens. Biochim Biophys Acta. 1225:326–337. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klos-Rola J and Zagorski Z: Peroxidation
of lipids in patients with senile cataract. Klin Oczna.
106:416–418. 2004.(In Polish). PubMed/NCBI
|
|
26
|
Li X, Luo JQ, Liao XQ, Zhang S, Yang LF,
Wu T, Wang L, Xu Q, He BS and Guo Z: Allicin inhibits the growth of
HONE-1 and HNE1 human nasopharyngeal carcinoma cells by inducing
ferroptosis. Neoplasma. 3:243–254. 2024. View Article : Google Scholar
|
|
27
|
Babizhayev MA, Deyev AI, Yermakova VN,
Brikman IV and Bours J: Lipid peroxidation and cataracts:
N-acetylcarnosine as a therapeutic tool to manage age-related
cataracts in human and in canine eyes. Drugs R D. 5:125–139. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thiagarajan R and Manikandan R:
Antioxidants and cataract. Free Radic Res. 47:337–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brennan LA, McGreal RS and Kantorow M:
Oxidative stress defense and repair systems of the ocular lens.
Front Biosci (Elite Ed). 4:141–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Babizhayev MA: Rejuvenation of visual
functions in older adult drivers and drivers with cataract during a
short-term administration of N-acetylcarnosine lubricant eye drops.
Rejuvenation Res. 7:186–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higgins MR, Izadi A and Kaviani M:
Antioxidants and exercise performance: With a focus on vitamin E
and C supplementation. Int J Environ Res Public Health.
17:84522020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang H, Yin Y, Wu CR, Liu Y, Guo F, Li M
and Ma L: Dietary vitamin and carotenoid intake and risk of
age-related cataract. Am J Clin Nutr. 109:43–54. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sella R and Afshari NA: Nutritional effect
on age-related cataract formation and progression. Curr Opin
Ophthalmol. 30:63–69. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lim JC, Caballero AM, Braakhuis AJ and
Donaldson PJL: Vitamin C and the lens: New insights into delaying
the onset of cataract. Nutrients. 12:31422020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Senthilkumari S, Talwar B, Dharmalingam K,
Ravindran RD, Jayanthi R, Sundaresan P, Saravanan C, Young IS,
Dangour AD and Fletcher AE: Polymorphisms in sodium-dependent
vitamin C transporter genes and plasma, aqueous humor and lens
nucleus ascorbate concentrations in an ascorbate depleted setting.
Exp Eye Res. 124:24–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reddy GB and Bhat KS: Protection against
UVB inactivation (in vitro) of rat lens enzymes by natural
antioxidants. Mol Cell Biochem. 194:41–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bron AJ and Brown NA: Perinuclear lens
retrodots: A role for ascorbate in cataractogenesis. Br J
Ophthalmol. 71:86–95. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taylor A, Jacques PF, Chylack LJ,
Hankinson SE, Khu PM, Rogers G, Friend J, Tung W, Wolfe JK, Padhye
N and Willett WC: Long-term intake of vitamins and carotenoids and
odds of early age-related cortical and posterior subcapsular lens
opacities. Am J Clin Nutr. 75:540–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garland DL: Ascorbic acid and the eye. Am
J Clin Nutr. 54 (6 Suppl):1198S–1202S. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Varma SD, Kumar S and Richards RD:
Light-induced damage to ocular lens cation pump: Prevention by
vitamin C. Proc Natl Acad Sci USA. 76:3504–3506. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Delamere NA and Tamiya S: Expression,
regulation and function of Na,K-ATPase in the lens. Prog Retin Eye
Res. 23:593–615. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shang F, Lu M, Dudek E, Reddan J and
Taylor A: Vitamin C and vitamin E restore the resistance of
GSH-depleted lens cells to H2O2. Free Radic Biol Med. 34:521–530.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu K, Kojima M, Shui YB, Sasaki H and
Sasaki K: Ultraviolet B-induced corneal and lens damage in guinea
pigs on low-ascorbic acid diet. Ophthalmic Res. 36:277–283. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hegde KR and Varma SD: Protective effect
of ascorbate against oxidative stress in the mouse lens. Biochim
Biophys Acta. 1670:12–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Devamanoharan PS, Henein M, Morris S,
Ramachandran S, Richards RD and Varma SD: Prevention of selenite
cataract by vitamin C. Exp Eye Res. 52:563–568. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Greiner JV and Glonek T: Adenosine
triphosphate (ATP) and protein aggregation in Age-Related
Vision-Threatening ocular diseases. Metabolites. 13:11002023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao W, Devamanoharan PS, Henein M, Ali AH
and Varma SD: Diabetes-induced biochemical changes in rat lens:
Attenuation of cataractogenesis by pyruvate. Diabetes Obes Metab.
2:165–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Linklater HA, Dzialoszynski T, McLeod HL,
Sanford SE and Trevithick JR: Modelling cortical cataractogenesis.
XI. Vitamin C reduces gamma-crystallin leakage from lenses in
diabetic rats. Exp Eye Res. 51:241–247. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang YY, Shi LX, Li JH, Yao LY and Xiang
DX: Piperazine ferulate ameliorates the development of diabetic
nephropathy by regulating endothelial nitric oxide synthase. Mol
Med Rep. 19:2245–2253. 2019.PubMed/NCBI
|
|
50
|
Özkaya D, Naziroğlu M, Armağan A, Demirel
A, Köroglu BK, Çolakoğlu N, Kükner A and Sönmez TT: Dietary vitamin
C and E modulates oxidative stress induced-kidney and lens injury
in diabetic aged male rats through modulating glucose homeostasis
and antioxidant systems. Cell Biochem Funct. 29:287–293. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Simpson GL and Ortwerth BJ: The
non-oxidative degradation of ascorbic acid at physiological
conditions. Biochim Biophys Acta. 1501:12–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bensch KG, Fleming JE and Lohmann W: The
role of ascorbic acid in senile cataract. Proc Natl Acad Sci USA.
82:7193–7196. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sadowska-Bartosz I and Bartosz G: Effect
of glycation inhibitors on aging and age-related diseases. Mech
Ageing Dev. 160:1–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ahmed N, Thornalley PJ, Dawczynski J,
Franke S, Strobel J, Stein G and Haik GM: Methylglyoxal-derived
hydroimidazolone advanced glycation end-products of human lens
proteins. Invest Ophthalmol Vis Sci. 44:5287–5292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Koshiishi I, Mamura Y, Liu J and Imanari
T: Degradation of dehydroascorbate to 2,3-diketogulonate in blood
circulation. Biochim Biophys Acta. 1425:209–214. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fan X, Sell DR, Hao C, Liu S, Wang B,
Wesson DW, Siedlak S, Zhu X, Kavanagh TJ, Harrison FE and Monnier
VM: Vitamin C is a source of oxoaldehyde and glycative stress in
age-related cataract and neurodegenerative diseases. Aging Cell.
19:e131762020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Prabhakaram M and Ortwerth BJ: The
glycation and cross-linking of isolated lens crystallins by
ascorbic acid. Exp Eye Res. 55:451–459. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tanito M: Reported evidence of vitamin E
protection against cataract and glaucoma. Free Radic Biol Med.
177:100–119. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jacques PF, Chylack LJ, McGandy RB and
Hartz SC: Antioxidant status in persons with and without senile
cataract. Arch Ophthalmol. 106:337–340. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Knekt P, Heliovaara M, Rissanen A, Aromaa
A and Aaran RK: Serum antioxidant vitamins and risk of cataract.
BMJ. 305:1392–1394. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zigler JJ, Bodaness RS, Gery I and
Kinoshita JH: Effects of lipid peroxidation products on the rat
lens in organ culture: A possible mechanism of cataract initiation
in retinal degenerative disease. Arch Biochem Biophys. 225:149–156.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Creighton MO, Sanwal M, Stewart-DeHaan PJ
and Trevithick JR: Modeling cortical cataractogenesis. V. Steroid
cataracts induced by solumedrol partially prevented by vitamin E in
vitro. Exp Eye Res. 37:65–76. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ross WM, Creighton MO, Inch WR and
Trevithick JR: Radiation cataract formation diminished by vitamin E
in rat lenses in vitro. Exp Eye Res. 36:645–653. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee AY and Chung SS: Contributions of
polyol pathway to oxidative stress in diabetic cataract. Faseb J.
13:23–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Karslioglu I, Ertekin MV, Kocer I, Taysi
S, Sezen O, Gepdiremen A and Balci E: Protective role of
intramuscularly administered vitamin E on the levels of lipid
peroxidation and the activities of antioxidant enzymes in the lens
of rats made cataractous with gamma-irradiation. Eur J Ophthalmol.
14:478–485. 2004. View Article : Google Scholar
|
|
66
|
Costagliola C, Iuliano G, Menzione M,
Apponi-Battini G and Auricchio G: Effect of topical glucocorticoid
administration on the protein and nonprotein sulfhydryl groups of
the rabbit lens. Ophthalmic Res. 19:351–356. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang J, Lofgren S, Dong X, Galichanin K
and Soderberg PG: Dose-response relationship for α-tocopherol
prevention of ultraviolet radiation induced cataract in rat. Exp
Eye Res. 93:91–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ayala MN and Soderberg PG: Vitamin E can
protect against ultraviolet radiation-induced cataract in albino
rats. Ophthalmic Res. 36:264–269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Seth RK and Kharb S: Protective function
of alpha-tocopherol against the process of cataractogenesis in
humans. Ann Nutr Metab. 43:286–289. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Stahl W, Nicolai S, Briviba K, Hanusch M,
Broszeit G, Peters M, Martin HD and Sies H: Biological activities
of natural and synthetic carotenoids: Induction of gap junctional
communication and singlet oxygen quenching. Carcinogenesis.
18:89–92. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gao S, Qin T, Liu Z, Caceres MA, Ronchi
CF, Chen CY, Yeum KJ, Taylor A, Blumberg JB, Liu Y and Shang F:
Lutein and zeaxanthin supplementation reduces H2O2-induced
oxidative damage in human lens epithelial cells. Mol Vis.
17:3180–3190. 2011.PubMed/NCBI
|
|
72
|
Ma L, Hao ZX, Liu RR, Yu RB, Shi Q and Pan
JP: A dose-response meta-analysis of dietary lutein and zeaxanthin
intake in relation to risk of age-related cataract. Graefes Arch
Clin Exp Ophthalmol. 252:63–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Landrum JT and Bone RA: Lutein,
zeaxanthin, and the macular pigment. Arch Biochem Biophys.
385:28–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Krinsky NI, Landrum JT and Bone RA:
Biologic mechanisms of the protective role of lutein and zeaxanthin
in the eye. Annu Rev Nutr. 23:171–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Babizhayev MA, Vishnyakova KS and Yegorov
YE: Telomere-dependent senescent phenotype of lens epithelial cells
as a biological marker of aging and cataractogenesis: The role of
oxidative stress intensity and specific mechanism of phospholipid
hydroperoxide toxicity in lens and aqueous. Fundam Clin Pharmacol.
25:139–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chitchumroonchokchai C, Bomser JA, Glamm
JE and Failla ML: Xanthophylls and alpha-tocopherol decrease
UVB-induced lipid peroxidation and stress signaling in human lens
epithelial cells. J Nutr. 134:3225–3232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Peng J, Zheng TT, Liang Y, Duan LF, Zhang
YD, Wang LJ, He GM and Xiao HT: P-Coumaric acid protects human lens
epithelial cells against oxidative Stress-Induced apoptosis by MAPK
signaling. Oxid Med Cell Longev. 2018:85490522018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kinoshita S, Sugawa H, Nanri T, Ohno RI,
Shirakawa JI, Sato H, Katsuta N, Sakake S and Nagai R: Trapa
bispinosa Roxb. And lutein ameliorate cataract in type 1 diabetic
rats. J Clin Biochem Nutr. 66:8–14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Arnal E, Miranda M, Almansa I, Muriach M,
Barcia JM, Romero FJ, Diaz-Llopis M and Bosch-Morell F: Lutein
prevents cataract development and progression in diabetic rats.
Graefes Arch Clin Exp Ophthalmol. 247:115–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Age-Related Eye Disease Study 2 (AREDS2)
Research Group, . Chew EY, SanGiovanni JP, Ferris FL, Wong WT,
Agron E, Clemons TE, Sperduto R, Danis R, Chandra SR, et al:
Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2
randomized trial report no. 4. JAMA Ophthalmol. 131:843–850. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hu Y and Xu Z: Effects of lutein on the
growth and migration of bovine lens epithelial cells in vitro. J
Huazhong Univ Sci Technolog Med Sci. 28:360–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li AN, Li S, Zhang YJ, Xu XR, Chen YM and
Li HB: Resources and biological activities of natural polyphenols.
Nutrients. 6:6020–6047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Higuchi A, Yonemitsu K, Koreeda A and
Tsunenari S: Inhibitory activity of epigallocatechin gallate (EGCg)
in paraquat-induced microsomal lipid peroxidation-a mechanism of
protective effects of EGCg against paraquat toxicity. Toxicology.
183:143–149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yao K, Ye P, Zhang L, Tan J, Tang X and
Zhang Y: Epigallocatechin gallate protects against oxidative
stress-induced mitochondria-dependent apoptosis in human lens
epithelial cells. Mol Vis. 14:217–223. 2008.PubMed/NCBI
|
|
85
|
Wu Q, Li Z, Lu X, Song J, Wang H, Liu D
and Bi H: Epigallocatechin gallate protects the human lens
epithelial cell survival against UVB irradiation through AIF/endo G
signalling pathways in vitro. Cutan Ocul Toxicol. 40:187–197. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ghosh D, Agarwal M and Radhakrishna M:
Molecular insights into the inhibitory role of α-Crystallin against
γD-Crystallin aggregation. J Chem Theory Comput. 20:1740–1752.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kumar V, Gour S, Peter OS, Gandhi S, Goyal
P, Pandey J, Harsolia RS and Yadav JK: Effect of green tea
polyphenol epigallocatechin-3-gallate on the aggregation of
αA(66–80) peptide, a major fragment of αA-crystallin involved in
cataract development. Curr Eye Res. 42:1368–1377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chaudhury S, Bag S, Bose M, Das AK, Ghosh
AK and Dasgupta S: Protection of human gammaB-crystallin from
UV-induced damage by epigallocatechin gallate: Spectroscopic and
docking studies. Mol Biosyst. 12:2901–2909. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ye P, Lin K, Li Z, Liu J, Yao K and Xu W:
(−)-Epigallocatechin gallate regulates expression of apoptotic
genes and protects cultured human lens epithelial cells under
hyperglycemia. Mol Biol (Mosk). 47:251–257. 2013.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Caesary AG, Handayani N and Sujuti H:
Effect of epigallocatechin gallate in green tea on preventing lens
opacity and αB-crystallin aggregation in rat model of diabetes. Int
J Ophthalmol. 16:342–347. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang Z, Zhang X, Bi K, He Y, Yan W, Yang
C and Zhang J: Potential protective mechanisms of green tea
polyphenol EGCG against COVID-19. Trends Food Sci Technol.
114:11–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang W, Li S, Zeng J, Liu Y, Wu M and
Zhang M: Growth inhibition, induction of apoptosis by green tea
constituent (−)-epigallocatechin-3-gallate in cultured rabbit lens
epithelial cells. Yan Ke Xue Bao. 16:194–198. 2000.PubMed/NCBI
|
|
93
|
Huang W, Liu Y, Zeng J and Wu M: Role of
p38MAPKs pathway in the growth inhibition of rabbit lens epithelial
cells induced by EGCG. Yan Ke Xue Bao. 19:236–238. 2472003.(In
Chinese). PubMed/NCBI
|
|
94
|
Zhou DD, Luo M, Huang SY, Saimaiti A,
Shang A, Gan RY and Li HB: Effects and mechanisms of resveratrol on
aging and Age-related diseases. Oxid Med Cell Longev.
2021:99322182021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bryl A, Falkowski M, Zorena K and Mrugacz
M: The role of resveratrol in eye Diseases-A review of the
literature. Nutrients. 14:29742022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z
and Liu P: Resveratrol protects human lens epithelial cells against
H2O2-induced oxidative stress by increasing catalase, SOD-1, and
HO-1 expression. Mol Vis. 16:1467–1474. 2010.PubMed/NCBI
|
|
97
|
Chen P, Yao Z and He Z: Resveratrol
protects against high glucose-induced oxidative damage in human
lens epithelial cells by activating autophagy. Exp Ther Med.
21:4402021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ran H, Liu H and Wu P: Echinatin mitigates
H2O2-induced oxidative damage and apoptosis in lens epithelial
cells via the Nrf2/HO-1 pathway. Adv Clin Exp Med. 30:1195–1203.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li J, Huang Y, Ma T, Liu Y, Luo Y, Gao L,
Li Z and Ye Z: Carbon monoxide releasing molecule-3 alleviates
oxidative stress and apoptosis in Selenite-induced cataract in rats
via activating Nrf2/HO-1 pathway. Curr Eye Res. 48:919–929. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen J, Li X, Liu H, Zhong D, Yin K, Li Y,
Zhu L, Xu C, Li M and Wang C: Bone marrow stromal cell-derived
exosomal circular RNA improves diabetic foot ulcer wound healing by
activating the nuclear factor erythroid 2-related factor 2 pathway
and inhibiting ferroptosis. Diabet Med. 40:e150312023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dai Z, Zhu B, Yu H, Jian X, Peng J, Fang C
and Wu Y: Role of autophagy induced by arecoline in angiogenesis of
oral submucous fibrosis. Arch Oral Biol. 102:7–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li F, Li D, Liu H, Cao BB, Jiang F, Chen
DN and Li JD: RNF216 regulates the migration of immortalized GnRH
neurons by suppressing Beclin1-mediated autophagy. Front Endocrinol
(Lausanne). 10:122019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Luo G, Zhou Z, Huang C, Zhang P, Sun N,
Chen W, Deng C, Li X, Wu P, Tang J and Qing L: Itaconic acid
induces angiogenesis and suppresses apoptosis via Nrf2/autophagy to
prolong the survival of multi-territory perforator flaps. Heliyon.
9:e179092023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jegal KH, Ko HL, Park SM, Byun SH, Kang
KW, Cho IJ and Kim SC: Eupatilin induces Sestrin2-dependent
autophagy to prevent oxidative stress. Apoptosis. 21:642–656. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Barlow AD and Thomas DC: Autophagy in
diabetes: β-cell dysfunction, insulin resistance, and
complications. Dna Cell Biol. 34:252–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Singh A and Bodakhe SH: Biochemical
evidence indicates the preventive effect of resveratrol and
nicotinamide in the treatment of STZ-induced diabetic cataract.
Curr Eye Res. 46:52–63. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Higashi Y, Higashi K, Mori A, Sakamoto K,
Ishii K and Nakahara T: Anti-cataract effect of resveratrol in
High-Glucose-Treated Streptozotocin-Induced diabetic rats. Biol
Pharm Bull. 41:1586–1592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen Q, Gu P, Liu X, Hu S, Zheng H, Liu T
and Li C: Gold nanoparticles encapsulated resveratrol as an
Anti-Aging agent to delay cataract development. Pharmaceuticals
(Basel). 16:262022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Smith A, Eldred JA and Wormstone IM:
Resveratrol inhibits wound healing and lens fibrosis: A putative
candidate for posterior capsule opacification prevention. Invest
Ophthalmol Vis Sci. 60:3863–3877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Vasey C, McBride J and Penta K: Circadian
rhythm dysregulation and restoration: The role of melatonin.
Nutrients. 13:34802021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang L, Xu S, Chen R, Ding Y, Liu M, Hou
C, Wu Z, Men X, Bao M, He B and Li S: Exploring the causal
association between epigenetic clocks and menopause age: Insights
from a bidirectional Mendelian randomization study. Front
Endocrinol (Lausanne). 15:14295142024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Abe M, Reiter RJ, Orhii PB, Hara M and
Poeggeler B: Inhibitory effect of melatonin on cataract formation
in newborn rats: Evidence for an antioxidative role for melatonin.
J Pineal Res. 17:94–100. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Crooke A, Huete-Toral F, Colligris B and
Pintor J: The role and therapeutic potential of melatonin in
age-related ocular diseases. J Pineal Res. 632017.doi:
10.1111/jpi.12430. PubMed/NCBI
|
|
114
|
Bai J, Dong L, Song Z, Ge H, Cai X, Wang G
and Liu P: The role of melatonin as an antioxidant in human lens
epithelial cells. Free Radic Res. 47:635–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Mi Y, Wei C, Sun L, Liu H, Zhang J, Luo J,
Yu X, He J, Ge H and Liu P: Melatonin inhibits ferroptosis and
delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and
SIRT6/NCOA4/FTH1 pathways. Biomed Pharmacother. 157:1140482023.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sun Z, Zou X, Bao M, Huang Z, Lou Y, Zhang
Y and Huang P: Role of ferroptosis in fibrosis diseases. Am J Med
Sci. 366:87–95. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu Y, Li H and Liu Y: MicroRNA-378a
regulates the reactive oxygen species (ROS)/Phosphatidylinositol
3-Kinases (PI3K)/AKT signaling pathway in human lens epithelial
cells and cataract. Med Sci Monit. 25:4314–4321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Khorsand M, Akmali M, Sharzad S and
Beheshtitabar M: Melatonin reduces cataract formation and aldose
reductase activity in lenses of streptozotocin-induced diabetic
rat. Iran J Med Sci. 41:305–313. 2016.PubMed/NCBI
|
|
119
|
Jedziniak JA, Chylack LJ, Cheng HM, Gillis
MK, Kalustian AA and Tung WH: The sorbitol pathway in the human
lens: Aldose reductase and polyol dehydrogenase. Invest Ophthalmol
Vis Sci. 20:314–326. 1981.PubMed/NCBI
|
|
120
|
Mi W, Xia Y and Bian Y: Meta-analysis of
the association between aldose reductase gene (CA)n microsatellite
variants and risk of diabetic retinopathy. Exp Ther Med.
18:4499–4509. 2019.PubMed/NCBI
|
|
121
|
Kiziltoprak H, Tekin K, Inanc M and Goker
YS: Cataract in diabetes mellitus. World J Diabetes. 10:140–153.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Karslioglu I, Ertekin MV, Taysi S, Kocer
I, Sezen O, Gepdiremen A, Koç M and Bakan N: Radioprotective
effects of melatonin on radiation-induced cataract. J Radiat Res.
46:277–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Varma SD, Hegde KR and Kovtun S:
UV-B-induced damage to the lens in vitro: Prevention by caffeine. J
Ocul Pharmacol Ther. 24:439–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Varma SD, Hegde KR and Kovtun S:
Inhibition of selenite-induced cataract by caffeine. Acta
Ophthalmol. 88:e245–e249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Varma SD and Kovtun S: Protective effect
of caffeine against high sugar-induced transcription of microRNAs
and consequent gene silencing: A study using lenses of galactosemic
mice. Mol Vis. 19:493–500. 2013.PubMed/NCBI
|
|
126
|
Kronschlager M, Ruiss M, Dechat T and
Findl O: Single high-dose peroral caffeine intake inhibits
ultraviolet radiation-induced apoptosis in human lens epithelial
cells in vitro. Acta Ophthalmol. 99:e587–e593. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Devasagayam TP, Kamat JP, Mohan H and
Kesavan PC: Caffeine as an antioxidant: Inhibition of lipid
peroxidation induced by reactive oxygen species. Biochim Biophys
Acta. 1282:63–70. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Nakazawa Y, Ishimori N, Oguchi J, Nagai N,
Kimura M, Funakoshi-Tago M and Tamura H: Coffee brew intake can
prevent the reduction of lens glutathione and ascorbic acid levels
in HFD-fed animals. Exp Ther Med. 17:1420–1425. 2019.PubMed/NCBI
|
|
129
|
Luo J, Wang L, Cui C, Chen H, Zeng W and
Li X: MicroRNA-19a-3p inhibits endothelial dysfunction in
atherosclerosis by targeting JCAD. BMC Cardiovasc Disor.
24:3942024. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Evereklioglu C, Guldur E, Alasehirli B,
Cengiz B, Sari I and Pirbudak L: Excessive maternal caffeine
exposure during pregnancy is cataractogenic for neonatal
crystalline lenses in rats: A biomicroscopic and histopathologic
study. Acta Ophthalmol Scand. 82:552–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Babizhayev MA, Yermakova VN, Sakina NL,
Evstigneeva RP, Rozhkova EA and Zheltukhina GA: N
alpha-acetylcarnosine is a prodrug of L-carnosine in ophthalmic
application as antioxidant. Clin Chim Acta. 254:1–21. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Babizhayev MA, Micans P, Guiotto A and
Kasus-Jacobi A: N-acetylcarnosine lubricant eyedrops possess
all-in-one universal antioxidant protective effects of L-carnosine
in aqueous and lipid membrane environments, aldehyde scavenging,
and transglycation activities inherent to cataracts: A clinical
study of the new vision-saving drug N-acetylcarnosine eyedrop
therapy in a database population of over 50,500 patients. Am J
Ther. 16:517–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Babizhayev MA, Guiotto A and Kasus-Jacobi
A: N-Acetylcarnosine and histidyl-hydrazide are potent agents for
multitargeted ophthalmic therapy of senile cataracts and diabetic
ocular complications. J Drug Target. 17:36–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Xiong T, Li Z, Huang X, Lu K, Xie W, Zhou
Z and Tu J: TO901317 inhibits the development of hepatocellular
carcinoma by LXRalpha/Glut1 decreasing glycometabolism. Am J
Physiol Gastrointest Liver Physiol. 316:G598–G607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Fan X and Monnier VM: Protein
posttranslational modification (PTM) by glycation: Role in lens
aging and age-related cataractogenesis. Exp Eye Res.
210:1087052021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Babizhayev MA and Yegorov YE: Telomere
attrition in lens epithelial cells-a target for N-acetylcarnosine
therapy. Front Biosci (Landmark Ed). 15:934–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Babizhayev MA: Analysis of lipid
peroxidation and electron microscopic survey of maturation stages
during human cataractogenesis: Pharmacokinetic assay of Can-C
N-acetylcarnosine prodrug lubricant eye drops for cataract
prevention. Drugs R D. 6:345–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Jain AK, Lim G, Langford M and Jain SK:
Effect of high-glucose levels on protein oxidation in cultured lens
cells, and in crystalline and albumin solution and its inhibition
by vitamin B6 and N-acetylcysteine: Its possible relevance to
cataract formation in diabetes. Free Radic Biol Med. 33:1615–1621.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhang S, Chai FY, Yan H, Guo Y and Harding
JJ: Effects of N-acetylcysteine and glutathione ethyl ester drops
on streptozotocin-induced diabetic cataract in rats. Mol Vis.
14:862–870. 2008.PubMed/NCBI
|
|
140
|
Tuzcu EA, Tuzcu K, Basarslan F, Motor S,
Coskun M, Keskin U, Ayintap E, Ilhan O and Oksuz H: Protective
effects of N-acetylcysteine on triamcinolone acetonide-induced lens
damage in rats. Cutan Ocul Toxicol. 33:294–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Aydin B, Yagci R, Yilmaz FM, Erdurmus M,
Karadag R, Keskin U, Durmus M and Yigitoglu R: Prevention of
selenite-induced cataractogenesis by N-acetylcysteine in rats. Curr
Eye Res. 34:196–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wang P, Liu XC, Yan H and Li MY:
Hyperoxia-induced lens damage in rabbit: Protective effects of
N-acetylcysteine. Mol Vis. 15:2945–2952. 2009.PubMed/NCBI
|
|
143
|
Erol G, Kartal H, Comu FM, Cetin E,
Demirdas E, Sicim H, Unal CS, Gunay C, Oz BS and Bolcal C: Effects
of N-Acetylcysteine and N-Acetylcysteine amide on erythrocyte
deformability and oxidative stress in a rat model of lower
extremity Ischemia-Reperfusion injury. Cardiol Res Pract.
2020:68418352020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Martis RM, Grey AC, Wu H, Wall GM,
Donaldson PJ and Lim JC: N-Acetylcysteine amide (NACA) and diNACA
inhibit H2O2-induced cataract formation ex
vivo in pig and rat lenses. Exp Eye Res. 234:1096102023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Maddirala Y, Tobwala S, Karacal H and
Ercal N: Prevention and reversal of selenite-induced cataracts by
N-acetylcysteine amide in Wistar rats. BMC Ophthalmol. 17:542017.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Carey JW, Pinarci EY, Penugonda S, Karacal
H and Ercal N: In vivo inhibition of
l-buthionine-(S,R)-sulfoximine-induced cataracts by a novel
antioxidant, N-acetylcysteine amide. Free Radic Biol Med.
50:722–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Moreau KL and King JA: Protein misfolding
and aggregation in cataract disease and prospects for prevention.
Trends Mol Med. 18:273–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Cvekl A, McGreal R and Liu W: Lens
development and crystallin gene expression. Prog Mol Biol Transl
Sci. 134:129–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, Hu
LD, Ouyang H, Patel SH, Jin X, Lin D, et al: Lanosterol reverses
protein aggregation in cataracts. Nature. 523:607–611. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang K, He W, Du Y, Zhou Y, Wu X, Zhu J,
Zhu X, Zhang K and Lu Y: Inhibitory effect of lanosterol on
cataractous lens of cynomolgus monkeys using a subconjunctival drug
release system. Precis Clin Med. 5:pbac0212022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Deguchi S, Kadowaki R, Otake H, Taga A,
Nakazawa Y, Misra M, Yamamoto N, Sasaki H and Nagai N: Combination
of lanosterol and nilvadipine nanosuspensions rescues lens
opacification in Selenite-Induced cataractic rats. Pharmaceutics.
14:15202022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Sweeney MH and Truscott RJ: An impediment
to glutathione diffusion in older normal human lenses: A possible
precondition for nuclear cataract. Exp Eye Res. 67:587–595. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Nagai N, Fukuoka Y, Sato K, Otake H, Taga
A, Oka M, Hiramatsu N and Yamamoto N: The intravitreal injection of
lanosterol nanoparticles rescues lens structure collapse at an
early stage in shumiya cataract rats. Int J Mol Sci. 21:10482020.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hua H, Yang T, Huang L, Chen R, Li M, Zou
Z, Wang N, Yang D and Liu Y: Protective effects of lanosterol
synthase Up-regulation in UV-B-induced oxidative stress. Front
Pharmacol. 10:9472019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Shen X, Zhu M, Kang L, Tu Y, Li L, Zhang
R, Qin B, Yang M and Guan H: Lanosterol synthase pathway alleviates
lens opacity in Age-related cortical cataract. J Ophthalmol.
2018:41258932018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Chen XJ, Hu LD, Yao K and Yan YB:
Lanosterol and 25-hydroxycholesterol dissociate crystallin
aggregates isolated from cataractous human lens via different
mechanisms. Biochem Biophys Res Commun. 506:868–873. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kang H, Yang Z and Zhou R: Lanosterol
disrupts aggregation of human gammaD-Crystallin by binding to the
hydrophobic dimerization interface. J Am Chem Soc. 140:8479–8486.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Makley LN, McMenimen KA, DeVree BT,
Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ,
Thompson AD, Sunahara R, et al: Pharmacological chaperone for
α-crystallin partially restores transparency in cataract models.
Science. 350:674–677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Molnar KS, Dunyak BM, Su B, Izrayelit Y,
McGlasson-Naumann B, Hamilton PD, Qian M, Covey DF, Gestwicki JE,
Makley LN and Andley UP: Mechanism of action of VP1-001 in
cryAB(R120G)-Associated and Age-Related cataracts. Invest
Ophthalmol Vis Sci. 60:3320–3331. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Daszynski DM, Santhoshkumar P, Phadte AS,
Sharma KK, Zhong HA, Lou MF and Kador PF: Failure of oxysterols
such as lanosterol to restore lens clarity from cataracts. Sci Rep.
9:84592019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Shanmugam PM, Barigali A, Kadaskar J,
Borgohain S, Mishra DK, Ramanjulu R and Minija CK: Effect of
lanosterol on human cataract nucleus. Indian J Ophthalmol.
63:888–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Raju M, Santhoshkumar P and Krishna SK:
Alpha-crystallin-derived peptides as therapeutic chaperones.
Biochim Biophys Acta. 1860:246–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Budnar P, Tangirala R, Bakthisaran R and
Rao CM: Protein aggregation and cataract: Role of Age-related
modifications and mutations in α-Crystallins. Biochemistry (Mosc).
87:225–241. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Sprague-Piercy MA, Rocha MA, Kwok AO and
Martin RW: α-Crystallins in the vertebrate eye lens: Complex
oligomers and molecular chaperones. Annu Rev Phys Chem. 72:143–163.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wang T, Yao J, Jia L, Fort PE and Zacks
DN: Loss of alphaA or alphaB-Crystallin accelerates photoreceptor
cell death in a mouse model of P23H autosomal dominant retinitis
pigmentosa. Int J Mol Sci. 23:702021. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Joachim SC, Bruns K, Lackner KJ, Pfeiffer
N and Grus FH: Analysis of IgG antibody patterns against retinal
antigens and antibodies to alpha-crystallin, GFAP, and
alpha-enolase in sera of patients with ‘wet’ age-related macular
degeneration. Graefes Arch Clin Exp Ophthalmol. 245:619–626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Sharma KK, Kumar RS, Kumar GS and Quinn
PT: Synthesis and characterization of a peptide identified as a
functional element in alphaA-crystallin. J Biol Chem.
275:3767–3771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Bhattacharyya J, Padmanabha UE, Wang J and
Sharma KK: Mini-alphaB-crystallin: A functional element of
alphaB-crystallin with chaperone-like activity. Biochemistry.
45:3069–3076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Nahomi RB, Wang B, Raghavan CT, Voss O,
Doseff AI, Santhoshkumar P and Nagaraj RH: Chaperone peptides of
α-crystallin inhibit epithelial cell apoptosis, protein
insolubilization, and opacification in experimental cataracts. J
Biol Chem. 288:13022–13035. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Raju M, Santhoshkumar P and Sharma KK:
AlphaA-Crystallin-derived mini-chaperone modulates stability and
function of cataract causing alphaAG98R-crystallin. PLoS One.
7:e440772012. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Ghosh KS, Pande A and Pande J: Binding of
γ-crystallin substrate prevents the binding of copper and zinc ions
to the molecular chaperone α-crystallin. Biochemistry.
50:3279–3281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Banerjee PR, Pande A, Shekhtman A and
Pande J: Molecular mechanism of the chaperone function of
mini-α-crystallin, a 19-residue peptide of human α-crystallin.
Biochemistry. 54:505–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Chemerovski-Glikman M, Mimouni M, Dagan Y,
Haj E, Vainer I, Allon R, Blumenthal EZ, Adler-Abramovich L, Segal
D, Gazit E and Zayit-Soudry S: Rosmarinic acid restores complete
transparency of sonicated human cataract ex vivo and delays
cataract formation in vivo. Sci Rep. 8:93412018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zych M, Wojnar W, Dudek S and
Kaczmarczyk-Sedlak I: Rosmarinic and sinapic acids may increase the
content of reduced glutathione in the lenses of Estrogen-Deficient
rats. Nutrients. 11:8032019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Tsai CF, Wu JY and Hsu YW: Protective
effects of rosmarinic acid against Selenite-induced cataract and
oxidative damage in rats. Int J Med Sci. 16:729–740. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Babizhayev MA and Yegorov YE: Telomere
attrition in human lens epithelial cells associated with oxidative
stress provide a new therapeutic target for the treatment,
dissolving and prevention of cataract with N-Acetylcarnosine
lubricant eye drops. Kinetic, pharmacological and
Activity-dependent separation of therapeutic targeting:
Transcorneal penetration and delivery of L-Carnosine in the aqueous
humor and Hormone-Like hypothalamic antiaging effects of the
instilled ophthalmic drug through a safe eye medication technique.
Recent Pat Drug Deliv Formul. 10:82–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Kumar B and Reilly MA: The development,
growth, and regeneration of the crystalline lens: A review. Curr
Eye Res. 45:313–326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Cetinel S and Montemagno C: Nanotechnology
for the prevention and treatment of cataract. Asia Pac J Ophthalmol
(Phila). 4:381–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Lee BJ and Afshari NA: Advances in drug
therapy and delivery for cataract treatment. Curr Opin Ophthalmol.
34:3–8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Marco-Benedi V, Laclaustra M,
Sanchez-Hernandez RM, Ortega-Martinez DVE, Pedro-Botet J, Puzo J
and Civeira F: Cataract surgery in elderly subjects with
heterozygous familial hypercholesterolemia in prolonged treatment
with statins. J Clin Med. 10:34942021. View Article : Google Scholar : PubMed/NCBI
|