Introduction
Total joint arthroplasty, including total hip and
total knee arthroplasties, is the most effective surgical treatment
for advanced symptomatic osteoarthritis. However, the inflammatory
response triggered by the byproducts of joint replacement
prostheses can lead to pain, bone defects and movement disorders.
In severe cases, these complications may result in aseptic
loosening and periprosthetic osteolysis, which are among the most
common complications limiting the lifespan of prostheses (1). This poses a challenging clinical
problem because significant symptoms often do not manifest until
the late stage of failure, at which point the pathological changes
are irreversible, necessitating further surgical intervention
(2).
A previous study identified particulate wear debris
from in vivo prosthesis degradation as a crucial factor
stimulating the release of proinflammatory substances, suppressing
bone formation, inducing osteoclastic bone resorption, and
contributing to prosthesis-related chronic inflammation (3). Therefore, a deeper understanding of
the impact of prosthesis material design and particulate wear
debris on the inflammatory response after joint replacement is
essential for preventing aseptic loosening and periprosthetic
osteolysis (4).
Among various biomaterials, titanium (Ti)-based
implants are the most widely used in the medical field, including
dental prostheses, hearing aids, pacemakers and joint replacements
(5). It is well established that
oral peri-implant and periodontal bone loss, primarily caused by
peri-implantitis and periodontitis, and wear debris, particularly
Ti particles near the implant site, are crucial triggers of
inflammation and bone resorption (6). Among the cells involved in Ti
particle-induced inflammation, macrophages are of particular
interest (7). Qu et al
(8) reported that the activation
of mononuclear macrophages and giant cells is the primary cause of
chronic inflammation induced by Ti-wear debris. These findings
suggested that macrophages play a critical role in aseptic
loosening and inflammation after Ti-based joint replacement.
However, the underlying mechanisms, including cytokine,
inflammatory expression patterns and regulatory pathways, remain
poorly understood. The present study aimed to explore the influence
of Ti release on macrophage-like RAW 264.7 cells in aseptic
loosening and inflammation pathogenesis.
Within the integrin family, β2 integrins (CD11/CD18)
are specifically expressed on the surfaces of macrophages and
leukocytes and are composed of a common β2 subunit (CD18) and four
different α subunits (CD11a-d) (9). Previous studies have shown that these
β2 integrins, acting as adhesion receptors, are crucial in the
activation of inflammatory diseases such as rheumatoid arthritis
(10) and systemic lupus
erythematosus (11) by mediating
inflammatory cell recruitment, cell-cell contact and downstream
cellular signaling (12). Han
et al (13) demonstrated
that β2 integrins expressed in macrophages contribute to cell
activation, cytotoxicity, chemotaxis and phagocytosis in a mouse
model of endotoxin shock. Lv et al (14) demonstrated that β2 integrins
promote the expression of inflammatory cytokines, resulting in a
pro-inflammatory transformation in macrophages in multi-organ
fibrosis. Among the inside-out and outside-in signaling, Toll-like
receptor (TLR) signaling has been shown to regulate β2
integrin-stimulated inflammatory responses, though the interaction
between these pathways remains controversial (15,16).
To further elucidate the signaling pathways involved and the role
of integrin-mediated inflammation in Ti-induced osteolysis, the
present study focused on TLR signaling.
The present study aimed to investigate the
expression patterns of integrins and inflammatory cytokines in RAW
264.7 cells treated with Ti particles to understand the role of
integrins in Ti particle-mediated inflammatory osteolysis and
identify potential signaling pathways involved.
Materials and methods
Cell treatment
Commercially pure Ti particles (mean diameter, 3.0
µm; 93% <20 µm) were purchased from Alfa Aesar. To sterilize the
particles, they were mixed with 95% ethanol and stirred
magnetically for 24 h. The particles were then resuspended in
sterile phosphate-buffered saline containing 6% rat serum,
penicillin (100 U/ml) and streptomycin (100 U/ml) at a
concentration of 1.2×108/ml, stored at 4°C.
Murine macrophage-like RAW 264.7 cells were
purchased from the Chinese Academy of Sciences and the Shanghai
Institute of Cell Biology. Cells were cultured at 37°C in a
humidified incubator with 5% CO2 in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.),
supplemented with 10% heat-inactivated fetal bovine serum and
antibiotics (100 units/ml of penicillin-G and 100 pg/ml of
streptomycin). Then, RAW 264.7 cells (3×105 cells/ml)
were plated in 96-well plates and treated with or without Ti
particles (0.1 mg/ml). Cell samples were collected at various time
points (6, 12, 24, 48 and 72 h) and frozen for further
analysis.
Cell viability
The viability of RAW 264.7 cells was assessed using
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay. At different time points (6, 12, 24, 48 and 72 h),
cell samples (seeded at 3×105 cells/ml) were incubated
with MTT (0.5 mg/ml) at 37°C for 2 h. The culture medium was then
replaced with an equal volume of dimethyl sulfoxide to dissolve
formazan crystals. After centrifugation, the supernatant from each
sample was transferred to a 96-well plate. The corresponding
absorbance was measured at a wavelength of 570 nm using a
microplate reader (Bio-Tek Instruments Inc.). Cell viability was
presented as the mean ± standard deviation (SD) of the optical
density values. All experiments were performed in triplicates.
Gene expression analysis: Reverse
transcription-quantitative PCR (RT-qPCR)
mRNA expression levels in RAW 264.7 were quantified
by RT-qPCR. RAW 264.7 samples treated with or without 0.1 mg/ml Ti
particles collected at 48 and 72 h were homogenized in
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) using a glass/Teflon homogenizer. Total RNA was isolated
according to the manufacturer's protocol. First-strand
complementary DNA (cDNA) was synthesized using M-MLV reverse
transcriptase (cDNA synthesis kit; Takara Bio, Inc.) according to
the manufacturer's protocol. qPCR was performed using 1.0 µl cDNA
from each sample with a commercial kit and a Thermal Cycler Dice
TP800 (Takara Bio, Inc.). Cycling conditions began with an initial
3-min denaturation step at 95°C followed by 40 cycles of 10 sec at
95°C for denaturation and 45 sec at 60°C for annealing and
extension. Primer sequences for murine integrin β1, integrin β2
(CD18), integrin β3, integrin α1, integrin α2, integrin α3,
integrin α4, integrin α5, integrin α6, integrin L (CD11a), integrin
M (CD11b), integrin X (CD11c), integrin D (CD11d), TLR1, TLR2,
TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR11, interleukins (ILs; IL
1β, IL-6, IL-8, IL-10 and IL-12), tumor necrosis factor (TNF),
interferons, matrix metalloproteinases (MMPs; MMP3, MMP8 and
MMP11), MyD88, TIPAR and the housekeeping gene
β-actin are listed in Table
I. Relative expression values were normalized to β-actin
and analyzed using the 2−ΔΔCq method (17). All samples were assayed in
triplicates.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR analysis of gene expression. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR analysis of gene expression.
| Gene name | Primer sequence
(5′-3′) |
|---|
| Integrin β1 | F:
GGACGCTGCGAAAAGATGAA |
| (CD29) | R:
CCACAATTTGGCCCTGCTTG |
| Integrin β2 | F:
CCCTAGCTGGACTGTTCTTCC |
| (CD18) | R:
GTGAAGTTCAGCTTCTGGCAC |
| Integrin β3 | F:
CAGTGGCCGGGACAACTCT |
| (CD61) | R:
CAGGTTACATCGGGGTGAGC |
| Integrin α1 | F:
ATGACGCTCTGCCAAACTCA |
| (CD49a) | R:
TGTTGTACGCACTGTCTCCC |
| Integrin α2 | F:
ACCCACGGAGAAAGCAGAAG |
| (CD49b) | R:
CGCCGATGGTTTAGCTGTTG |
| Integrin α3 | F:
CCGACGACTACTGAGGGGT |
| (CD49c) | R:
AGCGGGTCGGCTTAAAGAAG |
| Integrin α4 | F:
GAAGCATCCCTGGCCACTAC |
| (CD49d) | R:
CCACTGACCAGAGTTGCACA |
| Integrin α5 | F:
CTTCCGACAGGGAGGGACTA |
| (CD49e) | R:
AGGGCATGTGTAAACGAGGG |
| Integrin α6 | F:
ACAGTGAGCGTGAGCCG |
| (CD49f) | R:
GACAGGTAGAGCAGGCACAA |
| TLR1 | F:
CGGACGGGTAAGGTTGTCTT |
|
| R:
CCAACACGTGGGCTCTTAGT |
| TLR2 | F:
CAGTCTTCCTAGGCTGGTGC |
|
| R:
AAGGAAACAGTCCGCACCTC |
| TLR3 | F:
TCCTGTGTCCGAGATGTCCT |
|
| R:
CAGCCTGAAAGTGAAACTCGC |
| TLR4 | F:
GTGGCCCTACCAAGTCTCAG |
|
| R:
GCTGCAGCTCTTCTAGACCC |
| TLR5 | F:
CTGCAGGGCAAAACACTGTC |
|
| R:
TTGTGATGTCCACCGTCCAG |
| TLR6 | F:
GGTACCGTCAGTGCTGGAAA |
|
| R:
TATTAAGGCCAGGGCGCAAA |
| TLR7 | F:
TGCGAGTCTCGGTTTTCTGT |
|
| R:
TGAGAAGGGAGCCAAGGACA |
| TLR8 | F:
TGCACATTCCCTGGAGACAC |
|
| R:
GGAGAGGAAGCCAGAGGGTA |
| TLR11 | F:
CTGCCAGGGACTTTGGGATT |
|
| R:
TTGCTAAGGCCTGTCCTGTG |
| Integrin L | F:
TGTTCCCAGATGGAAGCCAC |
| CD11aLFA-1 | R:
CCCTTTTGGTCCCTTGGTGT |
| Integrin M | F:
CCACACTAGCATCAAGGGCA |
| CD11b | R:
AAGAGCTTCACACTGCCACC |
| Integrin X | F:
TCTTCTGCTGTTGGGGTTTGT |
| CD11c | R:
TGCCTGTGTGATAGCCACATT |
| Integrin D | F:
GGTAGACAGCAACAGGCTCC |
| CD11d | R:
AGGAGAACCGTACCCTCTGC |
| IL-1β | F:
TGCCACCTTTTGACAGTGATG |
|
| R:
TGATGTGCTGCTGCGAGATT |
| IL-6 | F:
TCCAGTTGCCTTCTTGGGAC |
|
| R:
AGTCTCCTCTCCGGACTTGT |
| IL-8 | F:
CTGGGATTCACCTCAAGAACATC |
|
| R:
CAGGGTCAAGGCAAGCCTC |
| IL-10 | F:
TAAGGCTGGCCACACTTGAG |
|
| R:
TGAGCTGCTGCAGGAATGAT |
| IL-12 | F:
TCTTCTCACCGTGCACATCC |
|
| R:
TGGCCAAACTGAGGTGGTTT |
| TNF | F:
GATCGGTCCCCAAAGGGATG |
|
| R:
CCTCCACTTGGTGGTTTGTG |
| IFN | F:
AGCAAGGCGAAAAAGGATGC |
|
| R:
TCATTGAATGCTTGGCGCTG |
| MMP-3 | F:
GGAGGCAGCAGAGAACCTAC |
|
| R:
AGGACCGGAAGACCCTTCAT |
| MMP-8 | F:
CTTCTGGAGACGGCATCCTC |
|
| R:
GCCCAGTACTGTCTGCCTTT |
| MMP-11 | F:
TCCGCTGACAACACTTTGGA |
|
| R:
TCCCCTGAGGAGACATAGGC |
| MyD88 | F:
GATGACTGGCCTGAGCAACT |
|
| R:
GCTATCCTTGGGAGCTGTCC |
| TIRAP | F:
CTCATTTTCCCCAACCGTGC |
|
| R:
CTCGGGATCTGTGTTTCGGT |
| β-actin | F:
CCTGGCTGGCCGGGACCTGAC |
|
| R:
ACCGCTCGTTGCCAATAGTGATGA |
Protein expression analysis by western
blot assay
RAW 264.7 macrophages were challenged with indicated
concentrations of Ti particles for 6, 12, 24, 48 and 72 h, and then
lysed in a buffer containing 1% Triton X-100, protease inhibitors
(MilliporeSigma), and 1 mM sodium orthovanadate (MilliporeSigma).
The protein concentration in the supernatant was measured using a
BCA protein assay kit. Equal amounts of protein (30 µg/lane) were
separated using Tris-bis SDS-PAGE gels (8 or 10% gel concentration
for different molecular weight proteins; Invitrogen; Thermo Fisher
Scientific, Inc.) and transferred onto polyvinylidene fluoride
membranes (MilliporeSigma). After incubation in 5% skimmed milk in
Tris-buffered saline with 0.05% Tween-20 (TBST) for 1 h at room
temperature (to block non-specific binding), the membranes were
incubated overnight at 4°C with the appropriate primary antibody
(Table II). A horseradish
peroxidase-conjugated donkey anti-rabbit IgG was used as a
secondary antibody (1:1,000; cat. no. NA934; Cytiva). Antibody
binding was detected using the Immobilon Chemiluminescence system
(MilliporeSigma). Quantitative densitometry analysis was conducted
and the relative densities of the protein bands were analyzed using
ImageJ software (version 1.53b; National Institutes of Health). The
relative expression levels of target proteins were normalized to
the corresponding intensities of tubulin.
 | Table II.Antibody suppliers and concentrations
used in western blotting. |
Table II.
Antibody suppliers and concentrations
used in western blotting.
| Antibody | Species | Supplier | Cat. no. | Dilution |
|---|
| CD18 | Rabbit mAb | Cell Signaling
Technology, Inc. | 72607 | 1:1,000 |
| CD11a | Rabbit | Abcam | ab228964 | 1:1,000 |
| CD11b | Rabbit mAb | Cell Signaling
Technology, Inc. | 93169 | 1:1,000 |
| CD11c | Rabbit mAb | Cell Signaling
Technology, Inc. | 97585 | 1:1,000 |
| MyD88 | Rabbit mAb | Cell Signaling
Technology, Inc. | 4283 | 1:1,000 |
| TIRAP | Rabbit mAb | Cell Signaling
Technology, Inc. | 13077 | 1:1,000 |
| Tubulin | Rabbit | Abcam | ab6046 | 1:500 |
Confocal immunofluorescence
RAW 264.7 cells at a density of 1×105
cells/ml were seeded on coverslips using six-well plates and
stained for immunofluorescence detection under confocal
fluorescence microscopy. Filamentous actin was used as a
housekeeping marker and stained with rhodamine-phalloidin
(Molecular Probes; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature in the dark. Samples were stained with the following
primary antibodies overnight at 4°C: CD11a (1:100; cat. no.
ab186873; Abcam), CD11b (1:500; cat. no. ab184308; Abcam), CD11c
(1:100; cat. no. 97585; Cell Signaling Technology, Inc.), CD18
(1:500; cat. no. 102259; SinoBiological), MyD88 (1:50; cat. no.
PA5-19919; Thermo Fisher Scientific, Inc.) and TIRAP (1:100; cat.
no. ab17218; Abcam). A secondary antibody of Alexa Fluor
488-conjugated goat anti-rabbit IgG (1:1,000; cat. no. A-11034;
Invitrogen; Thermo Fisher Scientific, Inc.) was then added and
incubated for 1 h at room temperature in the dark. Nuclei were
visualized using Hoechst 33342 (cat. no. H1399; Thermo Fisher
Scientific, Inc.) staining for 1 h at room temperature. The images
were captured with a 633 or 320 objective lens using appropriate
laser excitation (wavelengths of 540, 488 and 360 nm) on a
laser-scanning confocal microscope (Carl Zeiss AG) or an Olympus
IX81-FV1000 (Olympus Corporation) laser microscope system. Detector
gain was initially optimized by sampling several regions of the
coverslip and then fixed for each corresponding channel. Once set,
the detector gain value was kept stable throughout the image
acquisition process. Images were analyzed using Zeiss LSM Image
Examiner Software and FV10-ASW 3.0 Viewer. Immunofluorescence was
used to quantitatively analyze CD18, CD11b, CD11c, myeloid
differentiation primary response protein 88 (MyD88) and
TIR-domain-containing adapter protein (TIRAP) expression levels.
Specific antibodies were used and detected using green
fluorescence. Simultaneously, the cytoskeletal protein actin was
labeled with red fluorescence as an internal reference standard.
Relative fluorescence intensity was calculated as the ratio of the
average green fluorescence intensity (target proteins) to the red
fluorescence intensity (actin), yielding the fluorescence ratio.
Each experiment was repeated three times to ensure reliability.
Multiple group statistical analyses were performed to evaluate the
significance of differences between groups. All images were
captured under identical magnification and microscopy
conditions.
Flow cytometry and intracellular
cytokine staining
RAW 264.7, macrophages were treated with Ti
particles for 6, 12, 24, 48 and 72 h. For the final 2 h of
stimulation, macrophage Fc receptors were blocked with 2.4G2 for 10
min at 4°C, followed by surface staining with anti-F4/80 (1:100;
cat. no. 14-4801-85; BM8/eBioscience; Thermo Fisher Scientific,
Inc.). Peritoneal cells were surface stained with anti-Siglec F
(1:100; cat. no. 564514; E50-2440/BD Biosciences) and anti-Gr1
(1:200; cat. no. MA1-10402; eBioscience; Thermo Fisher Scientific,
Inc.). Macrophages were then fixed and permeabilized using the BD
Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences) for
20 min at 4°C. Flow cytometric analysis was conducted on an LSR2
(BD Biosciences) using FACS Diva software (BD Biosciences). The
gating strategy involved using Forward Scatter (FSC) and Side
Scatter (SSC) to isolate viable, intact cells while excluding
debris or dead cells. Fluorescence marker gating was applied to
quantify integrin expression with negative controls setting
baseline fluorescence and positive controls verifying accuracy. The
scale bars on histograms were adjusted to ensure clear separation
between marker-positive and marker-negative populations. All flow
cytometry analyses and procedures were conducted using FlowJo
software (version 10.8.1; Tree Star, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
RAW 264.7 cells were cultured and treated with Ti
particles to induce an inflammatory response. After 6, 24, 48 and
72 h of treatment, the cells were harvested and lysed to obtain
cellular extracts. Surface adhesion molecules and signaling protein
levels were quantitatively assessed using specific ELISA kits
according to the manufacturer's protocol. Briefly, cell lysates
were added to microtiter plates pre-coated with antibodies specific
to each target protein. Following incubation, the plates were
washed to remove unbound proteins, and a secondary enzyme-linked
antibody specific to the target protein was added. The
enzyme-substrate reaction produced a colorimetric signal, measured
at 450nm using a microplate reader. The concentrations of CD11a
(cat. no. SEB572Mu; Cloud-Clone Corp.), CD11b (cat. no. JM-12253M2;
Jiangsu Jingmei Biological Technology Co., Ltd.), CD11c (cat. no.
SEB159Mu; Cloud-Clone Corp.), CD18 (cat. no. JM-13347M2; Jiangsu
Jingmei Biological Technology Co., Ltd.), MyD88 (cat. no. SEB707Mu;
Cloud-Clone Corp.) and TIRAP (cat. no. JM-13352M2; Jiangsu Jingmei
Biological Technology Co., Ltd.) were subsequently calculated based
on the appropriate standard curve.
Wound healing assay
Wound healing assays were performed to assess
collective cell migration. After reaching 90% cell confluence, RAW
264.7 cells were scratched with a sterile 100-µl pipette tip and
then treated with serum-free medium. The distance between the wound
edges was measured using an IX71 inverted light microscope (Olympus
Corporation). Images were captured at 0-, 6-, 12-, 24- and 36-h
time points. The experiments were performed three times for each
group, and three measurements were taken for each sample using
ImageJ software.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Statistical analyses were performed using SPSS 28 software
(IBM Corp.). Differences between unpaired data groups were analyzed
using the unpaired Student's two-tail t-test after normality was
confirmed with the Kolmogorov-Smirnov test. A non-parametric
Mann-Whitney test was conducted for data that did not follow a
normal distribution. P<0.05 was considered to indicate a
statistically significant difference. Data analysis was conducted
by Prism 8.0 (GraphPad Software; Dotmatics) software.
Results
Ti particles effect on RAW 264.7 cell
viability
To evaluate the impact of Ti particles on cell
viability, RAW 264.7 cells were cultured with micron-sized pure Ti
particles at a concentration of 0.1 mg/ml. The viability of RAW
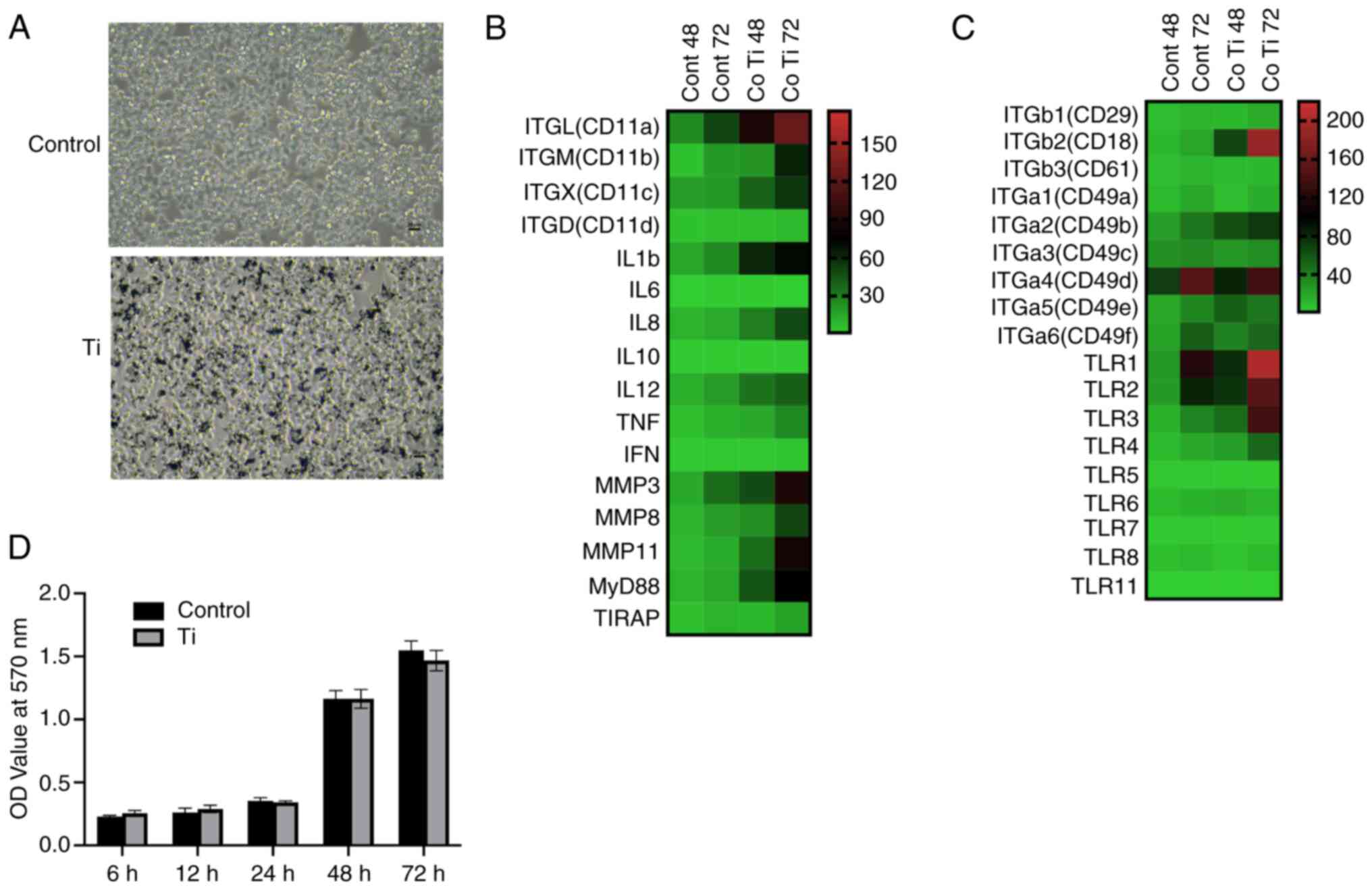
264.7 cells was not negatively affected by Ti particles (Fig. 1A and D). Compared with the control
group, no significant decrease in cell viability was observed at
any of the examined time points, including 6, 12, 24, 48 and 72 h
post-treatment.
Expression of integrin genes and
inflammatory cytokines heatmap by RT-qPCR
The expression levels of integrin family members,
TLRs, and inflammation response gene classes in RAW 264.7 cells,
with and without Ti particle treatment, were detected by RT-qPCR
(detailed results are presented in Fig. S1) and are presented as heat maps
in Fig. 1B and C.
The expression levels of integrin subunits CD18,
CD11a, CD11b and CD11c significantly increased following Ti
particle treatment (Fig. 1B).
Additionally, there was a notable upregulation in the gene
expression of TLRs (1–4) at 48 and 72 h after Ti treatment. The
relative expression folds of MyD88 and TIRAP, downstream molecules
in the TLR signaling pathway, were also elevated simultaneously
(P<0.05).
The expression patterns of inflammatory cytokines in
RAW 264.7 cells treated with Ti particles are shown in Fig. 1C. There was a significant increase
in the expression of TNF-α, IL 1β, IL-8 and IL-12 in cells cultured
with Ti particles compared with the blank control group at 72 h
(P<0.05). Furthermore, the matrix metalloproteinase-3 (MMP-3),
MMP-8 and MMP-11 levels significantly increased following Ti
treatment (P<0.05). Notably, the expression of IL 1β and IL 12
was upregulated earlier than that of the other inflammatory
cytokines.
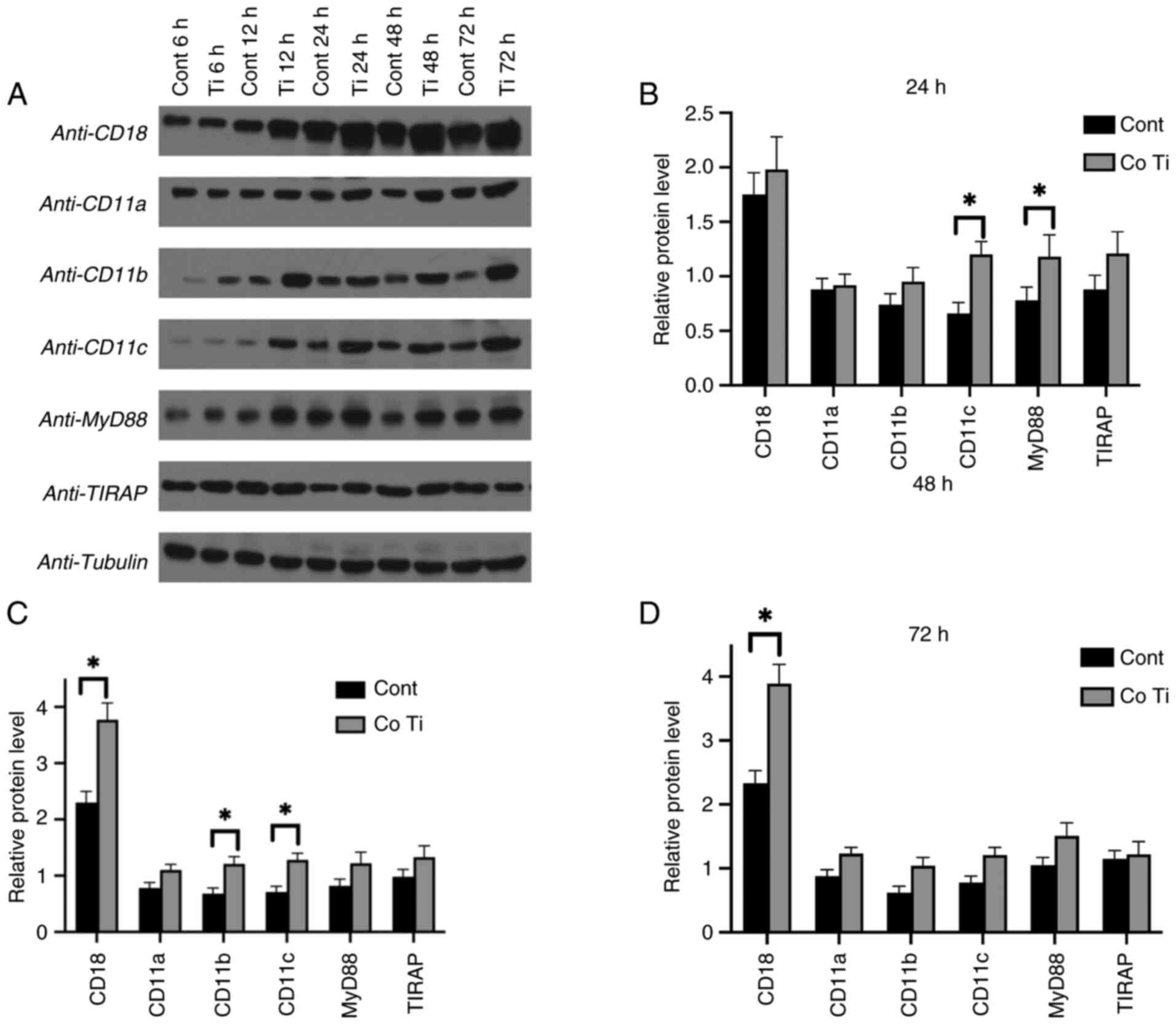
Promoted protein expression levels
with Ti stimulation by western blot assay
Protein expression levels in the Ti co-culture group
vs. the blank control group were analyzed by western blotting at
the 24-, 48- and 72-h time points (Fig. 2A). Higher protein expression levels
were observed in the Ti particle co-culture group, consistent with
the mRNA levels of the corresponding genes, CD18, CD11b,
CD11c and MyD88. However, discrepancies were noted where
protein levels did not align with mRNA expression profiles, such as
CD11a and TIRAP.
Quantitative analysis of western blot protein levels
was normalized to the housekeeping protein tubulin. The results
showed markedly increased protein levels of CD18 (at 48 and 72 h),
CD11b (at 48h) and CD11c (at 24 and 48 h) in RAW 264.7 cells
cultured with Ti particles compared with the control group
(P<0.05) (Fig. 2B-D). The
protein level of MyD88 was also significantly elevated in the
Ti-treated group compared with the control group at the 24-h
checking point (P<0.05).
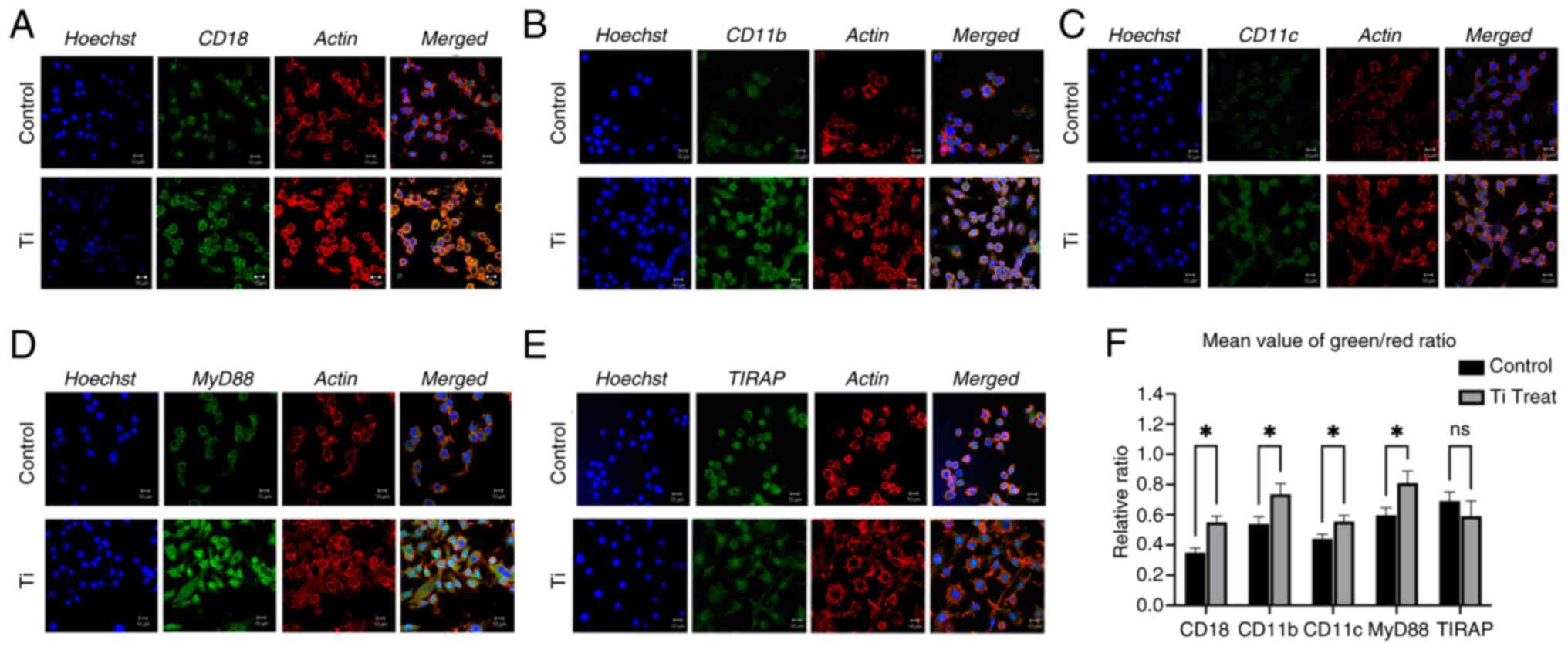
Ti-induced upregulation of integrin
and inflammatory response by confocal immunofluorescence assay
Immunofluorescent staining of RAW 264.7 cells was
conducted with and without Ti treatment and observed under a
confocal microscope (Fig. 3).
Consistent with the immunoblotting results, the confocal
immunofluorescent images demonstrated upregulation of CD18, CD11b
and CD11c in response to Ti particle co-culture (Fig. 3A-C). Additionally, MyD88 and TIRAP
exhibited significant changes in immunofluorescent intensity
following Ti co-culture (Fig. 3D and
E). Statistical analysis was performed in term of fluorescence
ratio (Fig. 3F). The observed
increase in the intensity of MyD88 and TIRAP aligned with the
protein and gene expression levels depicted in Figs. 1 and 2.
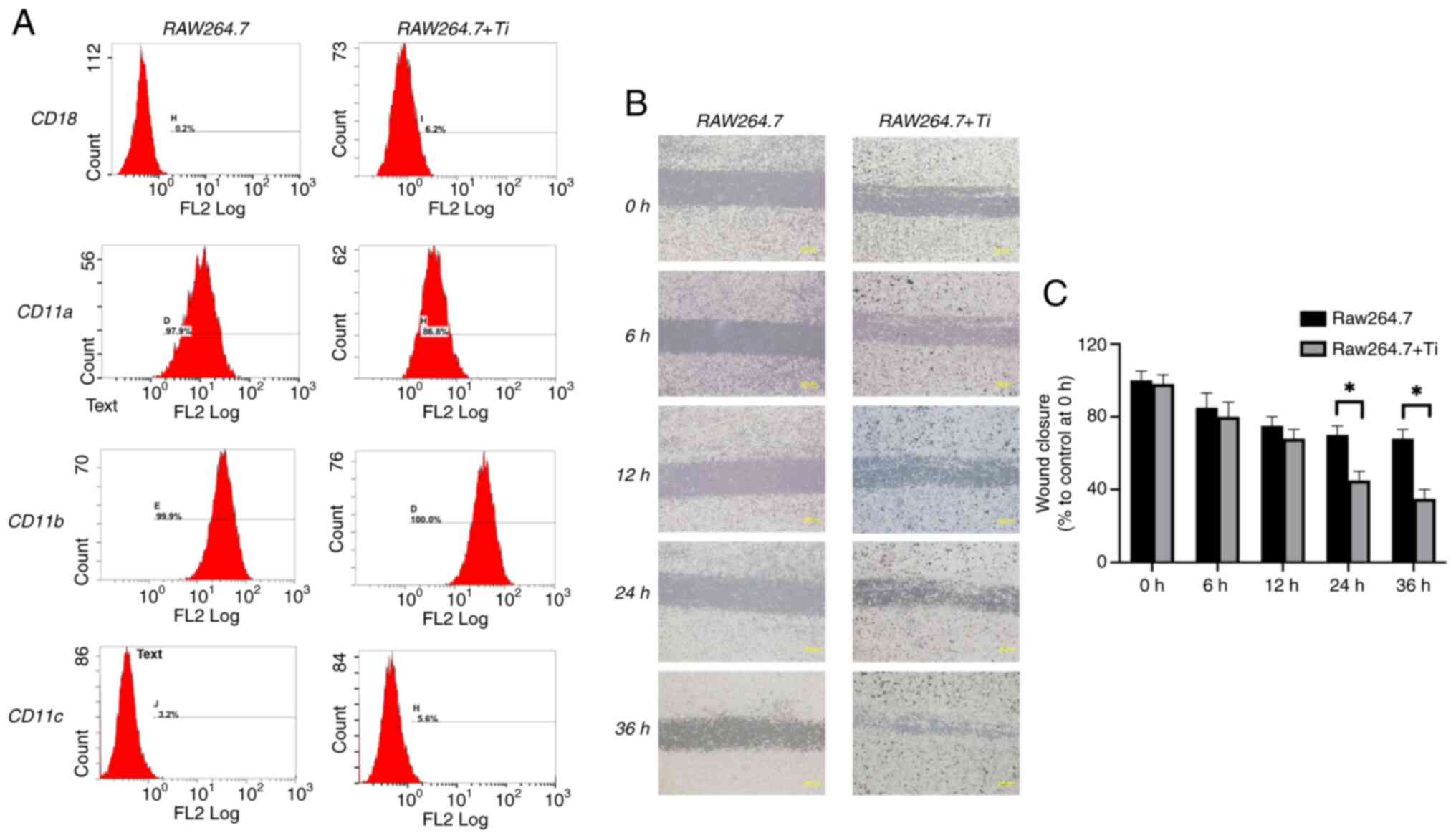
Integrin characteristics by flow
cytometry assessment
Flow cytometry was used to characterize and quantify
the impact of Ti particles on integrin protein expression on the
surface of RAW 264.7 cells (Fig.
4A). The analysis revealed enhanced CD18, CD11b and CD11c
expression in the Ti co-culture group compared with the control
group. The most notable increase was observed for CD18 (from
0.2–6.2%), while CD11b and CD11c showed slight increases. However,
CD11a expression decreased by over 10% (Fig. 4A).
Ti-induced changes in RAW 264.7 cell
migration features by scratch-wound healing assay
A wound healing assay was performed to assess the
migratory characteristics of RAW 264.7 cells co-cultured with Ti
particles. Representative images of cell migration at different
time points are revealed in Fig.
4B, with quantification presented in Fig. 4C. The results indicated that Ti
particles significantly enhanced the migration of RAW 264.7
compared with the control group at 24 and 36 h (P<0.05).
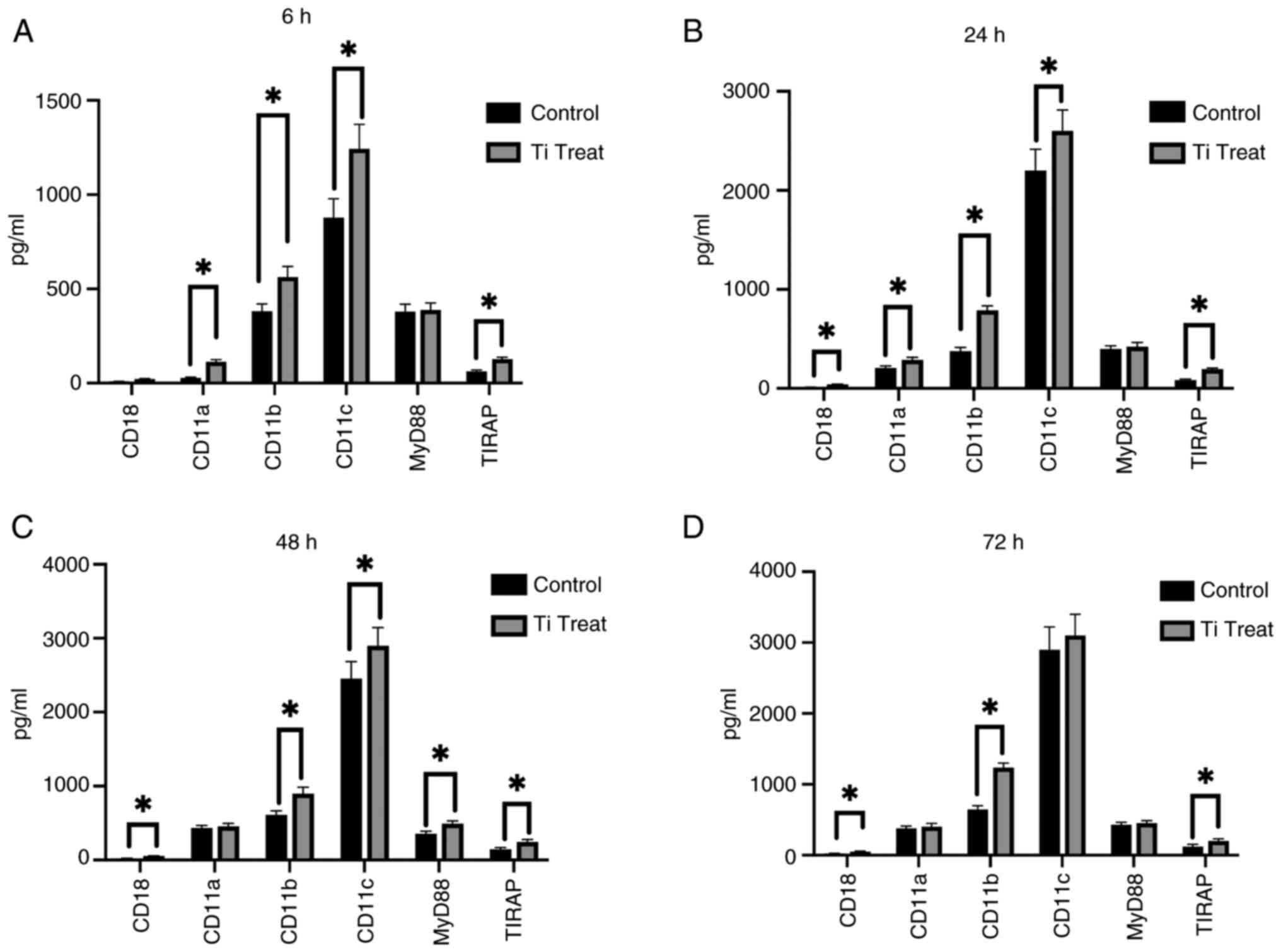
Ti-induced changes in RAW 264.7 cell
protein secretion by ELISA
The expression levels of CD18, CD11a, CD11b, CD11c,
MyD88 and TIRAP were analyzed in RAW 264.7 cells following
treatment with Ti particles at 6, 24, 48 and 72 h (Fig. 5A-D). At the 6-h mark, a significant
increase was observed in the expression of CD11a, CD11b and CD11c
but not CD18. However, at 24 and 48 h, there was significant
elevation in mostly all target molecules detected, including CD18,
CD11a, CD11b, CD11c and TIRAP, indicating a pronounced response. At
the 72-h time point, CD18, CD11b and TIRAP continued to show
significant increases in expression. These results suggested a
time-dependent activation of these markers in response to Ti
particle exposure.
Discussion
Previous studies have shown that macrophage-like
cells are involved in the chronic inflammation induced by Ti
particles. Vallés et al (18) found that primary macrophages
released increased amounts of TNF-α, IL-6 and IL-1β after
incubation with Ti particles. Pettersson et al (19) demonstrated that Ti particles
activate lipopolysaccharide-primed macrophages and induce the
release of interleukin-1β. Pajarinen et al (20) showed that Ti particles influence
the local cytokine microenvironment through foreign body reactions,
thereby modulating macrophage polarization. Lee et al
(21) demonstrated that
conditioned media of macrophages challenged with Ti particles
(Ti-CM) suppresses early and late differentiation markers of
osteoprogenitors. Bi et al (22) proved that conditioned media of
murine marrow cells challenged with Ti particles stimulate in
vitro bone resorption primarily by inducing osteoclast
differentiation with minor effects on osteoclast activity or
survival. In the present study, the impact of Ti particles on RAW
264.7 macrophage cells was investigated, focusing on cell
viability, inflammatory response, migratory behavior, and
particularly the underlying regulatory mechanisms of integrins.
Extensive research has focused on reducing the
inflammatory response and toxicity of implant wear particles in
local tissues. Studies indicate that the cytotoxic effects of
chemical components depend on factors such as dose, duration,
particle size, temperature and cell type (23). For Ti-based implants, macrophage
cell viability is primarily influenced by the concentration of Ti
particles and the surface characteristics of the implant (24). The present results demonstrated
that Ti particles did not compromise the viability of RAW 264.7
cells at any of the examined time points, suggesting that Ti
particles do not exert cytotoxic effects under the conditions
tested. This is consistent with previous studies reporting minimal
cytotoxicity of Ti particles on macrophages at ~0.1 mg/ml (25). Therefore, the Ti particles used in
the present study were considered non-toxic and suitable for
further experiments. The present study also provided insight into
the safe application of Ti-based implants under well-controlled
concentrations and surface modifications.
β2 integrins are crucial in macrophage functions,
including cell adhesion, migration, phagocytosis and immune
responses (26). The current
integrin expression analysis revealed that Ti particles
significantly upregulated the expression of CD18, CD11b and CD11c
at both the mRNA and protein levels, as shown by RT-qPCR, western
blotting and confocal immunofluorescence assays. In macrophages,
CD18, which mediates cell binding to the extracellular matrix
(27), was upregulated in response
to Ti particles, indicating increased cell adhesion and
proliferation, as depicted in Fig.
1. Previous studies have reported that CD11/CD18 is essential
for polymorphonuclear neutrophils recruitment and activation,
particularly later stages of immune cell activation through β2
integrin-mediated Syk activation (28). The delayed but significant increase
in CD18 expression at 48 and 72 h depicted in Fig. 2 was likely due to the time required
for its upregulation as part of the immune response, including cell
activation, migration and adhesion, which becomes more prominent at
later stages. For example, Ebnet et al (29) found that CD11/CD18 were involved in
the regulation of leukocyte-endothelial cell interaction. Through
their cytoplasmic domains, junctional adhesion molecules directly
associate with various tight junction-associated proteins including
ZO-1, AF-6, MUPP1 and PAR-3. CD11b and CD11c have close physical
and functional relationships (30)
but exhibit different expression levels and functions in various
macrophages and show cell-type-specific regulation under
inflammatory conditions (31).
CD11c expression increased as early as 24 h after Ti treatment in
RAW 264.7 cells, indicating its potential role in macrophage
activation. By contrast, CD11b, critical for macrophage recruitment
during the inflammatory response (32), showed significant increases at 48
h, reflecting the need for additional signaling events to mediate
macrophage migration and phagocytosis. The delayed expression
aligns with the role of macrophages in clearing debris and
promoting tissue remodeling, processes that typically follow the
initial inflammatory response. Sándor et al (33) reported that blocking CD11b
significantly enhanced the attachment of MDDCs and MDMs to
fibrinogen, demonstrating a competition between CD11b and CD11c for
this ligand.
Notably, differences in cell morphology revealed by
confocal immunofluorescence may be attributed to macrophage
activation induced by Ti particles. Flow cytometry showed that CD18
expression increased significantly on the cell surface, while CD11b
and CD11c exhibited moderate increases following Ti-particle
treatment. These results align with findings from western blotting
and immunofluorescence. However, while CD11a mRNA levels were
elevated, its surface expression decreased, indicating potential
post-transcriptional regulation or protein degradation mechanisms.
Further ELISA results depicted in Fig.
5 indicated that CD11a, CD11b and CD11c are involved in early
recognition with Ti particles. The delayed but significant increase
in CD18 at 24 and 48 h may indicate that CD18 plays a critical role
in sustaining and amplifying the immune response once
upregulated.
TLRs are pivotal in mediating inflammatory responses
in macrophages, primarily through the MyD88-dependent and
MyD88-independent pathways (34).
Previous research investigated the crosstalk between β2 integrins
(CD11/CD18) and TLRs in macrophages and other immune cells
(12), suggesting β2 integrin
regulates TLR signaling positively or negatively depending on cell
types and inflammatory status (35). Bai et al (36) demonstrated that CD11b enhances the
TIRAP enrichment in the plasma membrane, exerting a positive
regulatory effect via the MyD88-dependent pathway. Saitoh et
al (37) also found that MyD88
is required for the interplay between TLR7 and integrin
CD11a/CD18.
In the present study, increased expressions of TLR1,
TLR2, TLR3 and TLR4 were observed at 48 and 72 h, along with
elevated MyD88 and TIRAP expression levels following Ti particle
treatment, indicating the involvement of TLRs downstream
MyD88-dependent signaling in RAW 264.7 cell activation and
pro-inflammatory cytokine production. MyD88 expression may peak at
24 h owing to early activation. It was reported that MyD88 can be
regulated by upstream Src kinase activated by Rac1-induced F-actin
formation in macrophages (38).
MyD88 expression may not show significance as the signaling cascade
progresses or due to negative feedback mechanisms. Avbelj et
al (39) found a negative
feedback regulation of MyD88 by inflammasome-activated caspase-1.
Zhang and Ghosh (40) also found
that the negative regulation of MyD88-mediated signaling through
the suppression of IRAK1 and TRAF6 contributes to the attenuation
of the inflammatory response over time. Additionally, the
significant upregulation of pro-inflammatory cytokines, including
TNF-α, IL-1β, IL-8 and IL-12, along with MMP-3, MMP-8 and MMP-11,
indicated that Ti particles induce a robust inflammatory response
in RAW 267.4 cells.
As a well-known pro-inflammatory factor, LPS-induced
biomarker pattern in RAW 264.7 cells has been well studied
(41). As early as 1–4 h period,
LPS caused TLR4 and MyD88 levels to increase to more than twice
those of the control group (42),
while CD14 and TNF-alpha levels increased nearly 10-fold (43,44).
Additionally, IL-6, MMP-9 and IL-1β were significantly elevated
more than 3-fold compared with the untreated group (43). Among the integrin family,
LPS-induced RAW 264.7 cells were characterized by an increase in
CD11b/CD18, several-fold compared with the untreated group after 24
h (44). By contrast, Ti particles
induced a slower and more moderate inflammatory response in RAW
264.7 cells, which was markedly different from that caused by LPS,
both in terms of pattern and magnitude.
The role of β2 integrins in macrophage migration is
complex, depending on the specific context and cellular environment
(45). A previous study suggested
that β2 integrins inhibit macrophage migration by increasing
adhesion, leading to their retention at inflammation sites
(46). However, other research
suggested that the migration of human monocytes in vitro is
inhibited when integrin β2 function is blocked (47). The activation of TLR signaling can
upregulate integrin expression and other molecules, further
facilitating macrophage migration toward inflammation sites
(48). In the present study, Ti
particles significantly promoted RAW 264.7 cell migration at all
examined time points, as indicated by the wound healing assay,
accompanied by increased levels of pro-inflammatory cytokines and
MMPs, indicating that Ti particles enhance the inflammatory
response in macrophages. This effect is likely associated with the
activation of β2 integrins and the TLR signaling pathway.
The current study explored the role of integrin
mediated TLR signaling in Ti particle-induced inflammation in RAW
264.7 macrophage cells, and provided valuable insights for
developing strategies to mitigate aseptic loosening and
periprosthetic osteolysis in patients with Ti-based implants. The
limitations of the current study include the incomplete elucidation
of the crosstalk between integrins and TLRs in the response of RAW
264.7 macrophages induced by Ti particles. Additionally, the
effects of conditioned media from macrophages were not further
investigated on osteogenic and osteoclastic cells, which would
clarify the indirect effect of Ti particles.
In summary, Ti particles induced an inflammatory
response in RAW 264.7 macrophages, characterized by enhanced
integrin expression, TLR signaling activation, pro-inflammatory
cytokine production and increased cell migration, as illustrated in
Fig. S2. These findings
elucidated the molecular mechanisms underlying Ti particle-induced
inflammation and provided valuable insights for developing
strategies to mitigate aseptic loosening and periprosthetic
osteolysis in patients with Ti-based implants. Future studies
should identify specific regulatory pathways and potential
therapeutic targets to modulate macrophage activity and improve
implant longevity. Further study should also investigate the
mechanisms underlying the differences in inflammatory responses to
Ti particles and LPS, with the goal of identifying more targeted
therapeutic strategies.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Dai
Xuesong (Department of Orthopedic Surgery, The Second Affiliated
Hospital, School of Medicine, Zhejiang University, Hangzhou, China)
and Professor Ye Zhaoming (Department of Orthopedic Surgery, The
Second Affiliated Hospital, School of Medicine, Zhejiang
University, Hangzhou, China) for their assistance with statistical
advice and suggestions on the web interface.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81800782 and 81902279), the Medical
and Health Research Project of Zhejiang Association (grant no.
2022KY808) and the Zhejiang Traditional Chinese Medicine
Administration Science and Technology Project (grant no.
2024ZL574).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS and WGW conceptualized the study. YS and HN
acquired the data. HN and JFZ analyzed the data. YS wrote the
original draft. HN, JFZ and GWW wrote, reviewed and edited the
manuscript. All authors read and approved the final version of the
manuscript. YS, JFZ and WGW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsutsumi R, Xie C, Wei X, Zhang M, Zhang
X, Flick LM, Schwarz EM and O'Keefe RJ: PGE2 Signaling Through the
EP4 Receptor on Fibroblasts Upregulates RANKL and Stimulates
Osteolysis. J Bone Miner Res. 24:1753–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodman SB and Gallo J: Periprosthetic
Osteolysis: Mechanisms, prevention and treatment. J Clin Med.
8:20912019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Liu N, Liu K, Zhou G, Gan J, Wang
Z, Shi T, He W, Wang L, Guo T, et al: Autophagy mediated CoCrMo
particle-induced peri-implant osteolysis by promoting osteoblast
apoptosis. Autophagy. 11:2358–2369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stephens M, Liao S and von der Weid PY:
Ultra-purification of Lipopolysaccharides reveals species-specific
signalling bias of TLR4: Importance in macrophage function. Sci
Rep. 11:13352021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KT, Eo MY, Nguyen TTH and Kim SM:
General review of titanium toxicity. Int J Implant Dent. 5:102019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prestat M and Thierry D: Corrosion of
titanium under simulated inflammation conditions: Clinical context
and in vitro investigations. Acta Biomater. 136:72–87. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mombelli A, Hashim D and Cionca N: What is
the impact of titanium particles and biocorrosion on implant
survival and complications? A critical review. Clin Oral Implants
Res. 29 (Suppl 1):S37–S53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu R, Chen X, Yuan Y, Wang W, Qiu C, Liu
L, Li P, Zhang Z, Vasilev K, Liu L, et al: Ghrelin fights against
titanium particle-induced inflammatory osteolysis through
activation of beta-catenin signaling pathway. Inflammation.
42:1652–1665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blythe EN, Weaver LC, Brown A and Dekaban
GA: β2 Integrin CD11d/CD18: From expression to an emerging role in
staged leukocyte migration. Front Immunol. 12:7754472021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burt RK, Loh Y, Pearce W, Beohar N, Barr
WG, Craig R, Wen Y, Rapp JA and Kessler J: Clinical applications of
blood-derived and marrow-derived stem cells for nonmalignant
diseases. JAMA. 299:925–936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palomino-Morales RJ, Rojas-Villarraga A,
Gonzalez CI, Ramirez G, Anaya JM and Martin J: STAT4 but not
TRAF1/C5 variants influence the risk of developing rheumatoid
arthritis and systemic lupus erythematosus in Colombians. Genes
Immun. 9:379–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schittenhelm L, Hilkens CM and Morrison
VL: β2 integrins as regulators of dendritic cell, monocyte, and
macrophage function. Front Immunol. 8:18662017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han C, Jin J, Xu S, Liu H, Li N and Cao X:
Integrin CD11b negatively regulates TLR-triggered inflammatory
responses by activating Syk and promoting degradation of MyD88 and
TRIF via Cbl-b. Nat. Immunol. 11:734–742. 2010.
|
|
14
|
Lv L, Xie Y, Li K, Hu T, Lu X, Cao Y and
Zheng X: Unveiling the mechanism of surface
hydrophilicity-modulated macrophage polarization. Adv Healthc
Mater. 7:e18006752018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alépée N, Bahinski A, Daneshian M, De
Wever B, Fritsche E, Goldberg A, Hansmann J, Hartung T, Haycock J,
Hogberg H, et al: State-of-the-art of 3D cultures
(organs-on-a-chip) in safety testing and pathophysiology. ALTEX.
31:441–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yee NK and Hamerman JA: β(2) integrins
inhibit TLR responses by regulating NF-κB pathway and p38 MAPK
activation. Eur J Immunol. 43:779–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vallés G, González-Melendi P,
González-Carrasco JL, Saldaña L, Sánchez-Sabaté E, Munuera L and
Vilaboa N: Differential inflammatory macrophage response to rutile
and titanium particles. Biomaterials. 27:5199–5211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pettersson M, Kelk P, Belibasakis GN,
Bylund D, Molin Thorén M and Johansson A: Titanium ions form
particles that activate and execute interleukin-1β release from
lipopolysaccharide-primed macrophages. J Periodontal Res. 52:21–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pajarinen J, Kouri VP, Jämsen E, Li TF,
Mandelin J and Konttinen YT: The response of macrophages to
titanium particles is determined by macrophage polarization. Acta
Biomater. 9:9229–9240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SS, Sharma AR, Choi BS, Jung JS, Chang
JD, Park S, Salvati EA, Purdue EP, Song DK and Nam JS: The effect
of TNFα secreted from macrophages activated by titanium particles
on osteogenic activity regulated by WNT/BMP signaling in
osteoprogenitor cells. Biomaterials. 33:4251–4263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bi Y, VanDeMotter RR, Ragab AA, Goldberg
VM, Anderson JM and Greenfield EM: Titanium particles stimulate
bone resorption by inducing differentiation of murine osteoclasts.
J Bone Joint Surg Am. 83:501–508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dias Corpa Tardelli J, Lima da Costa
Valente M, Theodoro de Oliveira T and Cândido dos Reis A: Influence
of chemical composition on cell viability on titanium surfaces: A
systematic review. J Prosthet Dent. 125:421–425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao S, Feng X, Li W, Wang LN and Wang X:
Regulation of RAW 264.7 macrophages behavior on anodic TiO2
nanotubular arrays. Front Mater Sci. 11:318–327. 2017. View Article : Google Scholar
|
|
25
|
Messous R, Henriques B, Bousbaa H, Silva
FS, Teughels W and Souza JCM: Cytotoxic effects of submicron- and
nano-scale titanium debris released from dental implants: An
integrative review. Clin Oral Investig. 25:1627–1640. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weller S, Bonnet M, Delagreverie H, Israel
L, Chrabieh M, Maródi L, Rodriguez-Gallego C, Garty BZ, Roifman C,
Issekutz AC, et al: IgM+IgD+CD27+ B cells are markedly reduced in
IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients.
Blood. 120:4992–5001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Podolnikova NP, Kushchayeva YS, Wu Y,
Faust J and Ugarova TP: The Role of Integrins αMβ2 (Mac-1,
CD11b/CD18) and αDβ2 (CD11d/CD18) in Macrophage Fusion. Am J
Pathol. 186:2105–2116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schymeinsky J, Mócsai A and Walzog B:
Neutrophil activation via beta2 integrins (CD11/CD18): Molecular
mechanisms and clinical implications. Thromb Haemost. 98:262–273.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ebnet K, Suzuki A, Ohno S and Vestweber D:
Junctional adhesion molecules (JAMs): More molecules with dual
functions? J Cell Sci. 117((Pt 1)): 19–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ross GD and Větvička V: CR3 (CD11b, CD18):
A phagocyte and NK cell membrane receptor with multiple ligand
specificities and functions. Clin Exp Immunol. 92:181–184. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lukácsi S, Gerecsei T, Balázs K, Francz B,
Szabó B, Erdei A and Bajtay Z: The differential role of CR3
(CD11b/CD18) and CR4 (CD11c/CD18) in the adherence, migration and
podosome formation of human macrophages and dendritic cells under
inflammatory conditions. PLoS One. 15:e02324322020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kirby AC, Raynes JG and Kaye PM: CD11b
regulates recruitment of alveolar macrophages but not pulmonary
dendritic cells after pneumococcal challenge. J Infect Dis.
193:205–213. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sándor N, Lukácsi S, Ungai-Salánki R,
Orgován N, Szabó B, Horváth R, Erdei A and Bajtay Z: CD11c/CD18
dominates adhesion of human monocytes, macrophages and dendritic
cells over CD11b/CD18. PLoS One. 11:e01631202016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duan T, Du Y, Xing C, Wang HY and Wang RF:
Toll-Like receptor signaling and its role in cell-mediated
immunity. Front. Immunol. 13:8127742022.PubMed/NCBI
|
|
35
|
Alhamdan F, Bayarsaikhan G and Yuki K:
Toll-like receptors and integrins crosstalk. Front Immunol.
15:14037642024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai Y, Qian C, Qian L, Ma F, Hou J, Chen
Y, Wang Q and Cao X: Integrin CD11b Negatively Regulates
TLR9-Triggered dendritic cell cross-priming by upregulating
microRNA-146a. J Immunol. 188:5293–5302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saitoh SI, Abe F, Kanno A, Tanimura N,
Mori Saitoh Y, Fukui R, Shibata T, Sato K, Ichinohe T, Hayashi M,
et al: TLR7 mediated viral recognition results in focal type I
interferon secretion by dendritic cells. Nat Commun. 8:15922017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yi YS, Kim HG, Kim JH, Yang WS, Kim E,
Jeong D, Park JG, Aziz N, Kim S, Parameswaran N and Cho JY:
Syk-MyD88 axis is a critical determinant of inflammatory-response
in activated macrophages. Front Immunol. 12:7673662021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Avbelj M, Hafner-Bratkovič I, Lainšček D,
Manček-Keber M, Peternelj TT, Panter G, Treon SP, Gole B, Potočnik
U and Jerala R: Cleavage-Mediated Regulation of Myd88 signaling by
inflammasome-activated caspase-1. Front Immunol. 12:7902582022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang G and Ghosh S: Negative regulation
of toll-like receptor-mediated signaling by Tollip. J Biol Chem.
277:7059–7065. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Facchin BM, dos Reis GO, Vieira GN, Mohr
ETB, da Rosa JS, Kretzer IF, Demarchi IG and Dalmarco EM:
Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A
systematic review and meta-analysis. Inflamm Res. 71:741–758. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tian C, Liu X, Chang Y, Wang R, Yang M and
Liu M: Rutin prevents inflammation induced by lipopolysaccharide in
RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB
signalling pathway. J Pharm Pharmacol. 73:110–117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu R, Ma L, Chen T and Wang J:
Sophorolipid Suppresses LPS-Induced Inflammation in RAW264.7 Cells
through the NF-κB signaling pathway. Molecules. 27:50372022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barbour SE, Wong C, Rabah D, Kapur A and
Carter AD: Mature macrophage cell lines exhibit variable responses
to LPS. Mol Immunol. 35:977–987. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun H, Zhi K, Hu L and Fan Z: The
Activation and Regulation of beta2 integrins in phagocytes and
phagocytosis. Front Immunol. 12:6336392021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yakubenko VP, Belevych N, Mishchuk D,
Schurin A, Lam SC and Ugarova TP: The role of integrin alpha D
beta2 (CD11d/CD18) in monocyte/macrophage migration. Exp Cell Res.
314:2569–2578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chuluyan HE and Issekutz AC: VLA-4
integrin can mediate CD11/CD18-independent transendothelial
migration of human monocytes. J Clin Invest. 92:2768–2777. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gruber EJ and Leifer CA: Molecular
regulation of TLR signaling in health and disease:
Mechano-regulation of macrophages and TLR signaling. Innate Immun.
26:15–25. 2020. View Article : Google Scholar : PubMed/NCBI
|