Introduction
Lung adenocarcinoma (LUAD) is a major histological
subtype of non-small cell lung cancer, accounting for 35–40% of all
patients with lung cancer worldwide (1,2).
Despite advances in treatment, LUAD continues to pose significant
health challenges due to its high rates of tumor invasion,
metastasis and recurrence after treatment (3). The etiology of LUAD is
multifactorial, involving genetic susceptibility, environmental
factors (such as smoking and air pollution) and various gene
mutations (4). Current treatment
modalities for LUAD include surgical resection, radiotherapy,
chemotherapy and targeted therapy (5). Studies using single-cell
RNA-sequencing technology have shown that RAC1 serves a critical
role in promoting the brain metastasis of LUAD (6). Additionally, circulating tumor DNA
methods can detect and analyze the early dissemination of
metastasis in LUAD (7). However,
despite significant progress, there remain challenges in fully
understanding the molecular mechanisms driving LUAD metastasis.
Particularly, the role of specific genes and pathways in this
process remains poorly understood, which hampers the development of
more effective therapeutic strategies.
The phospholipid phosphatase 2 (PPAP2C) gene, also
known as PLPP2, serves a critical role in phospholipid metabolism
by catalyzing the conversion of phosphatidic acid (PA) to
diacylglycerol, a key step in cellular signaling (8,9).
Research has indicated that PPAP2C can promote the proliferation of
LUAD cells by regulating the synthesis of lipid rafts (10). Epithelial-mesenchymal transition
(EMT) is a process of cellular phenotypic transformation where
epithelial cells lose cell-cell adhesion and acquire mesenchymal
characteristics, resulting in enhanced cell migration and invasion
(11). Furthermore, overexpression
of PPAP2C has been shown to promote the development of breast
cancer by affecting the expression of CDC34, LSM7 and SGTA in
EMT-related pathways (8). Despite
these insights, the specific role of PPAP2C in LUAD cell metastasis
remains unclear.
The ERK and JNK pathways, which are MAPK signaling
pathways, regulate gene expression and cellular behavior by
activating specific protein kinases, leading to the phosphorylation
of downstream target proteins (12). The ERK pathway is typically
activated by the Ras-Raf-MEK-ERK signaling cascade, whereas the JNK
pathway is activated by MAP3Ks, MKK4/7 and JNK (13). The ERK/JNK pathways have critical
roles in various cellular processes, such as proliferation,
differentiation, apoptosis and stress responses (14). Research has shown that
overexpression of Notch4 may enhance the activities of ERK, JNK and
p38, thereby promoting the proliferation, anti-apoptosis and
migratory abilities of LUAD cells (15). Currently, the association between
PPAP2C and the ERK/JNK pathways in LUAD is unexplored, creating a
significant gap in the understanding of LUAD metastasis.
Given these challenges, the present study aimed to
systematically investigate the function of PPAP2C in LUAD, focusing
on its impact on the ERK/JNK signaling pathways and the EMT
process, thereby identifying novel potential therapeutic targets
for LUAD. By silencing the PPAP2C gene, its specific effects on
LUAD cell migration, invasion and related signaling pathways were
explored, providing new potential targets and a theoretical basis
for the diagnosis and treatment of LUAD.
Materials and methods
Database analysis
Data analysis was performed using R software
(version 4.2.1; http://www.r-project.org) with packages including
ggplot2 (version 3.3.6; http://ggplot2.tidyverse.org), stats (version 4.2.1)
and car (version 3.1–0; http://cran.r-project.org/web/packages/car/index.html).
Statistical analysis was carried out using the stats and car
packages specifically tailored to accommodate the structured and
quantitative nature of the data type. RNA-sequencing data from The
Cancer Genome Atlas (TCGA)-LUAD project were downloaded and
processed from TCGA database (https://portal.gdc.cancer.gov), by extracting
TPM-formatted data following the STAR pipeline (16). The data were log2
(value+1)-transformed and the expression differences of PPAP2C
(ENSG00000141934.10) between tumor and normal tissue samples were
evaluated using the Wilcoxon rank-sum test. Visualization was
performed using the ggplot2 package. Additionally, patients were
divided based on the median expression level of PPAP2C. Those with
expression levels above the median were classified as ‘high
expression,’ and those below as ‘low expression.’ This method
ensures a balanced distribution of patients across both groups,
facilitating more robust statistical comparisons. Co-expression
heatmaps were generated through Pearson's correlation coefficient
analysis to assess the correlation of PPAP2C with other genes
(MAPK1, MAPK3, MAPK8, CDH1, CDH2 and SNAI1).
Cell culture
A549 (cat. no. CL-0016; Wuhan Pricella Biotechnology
Co., Ltd.) and H1299 (cat. no. CL-0165; Wuhan Pricella
Biotechnology Co., Ltd.) cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; cat. no. 11965-092) containing 10% fetal
bovine serum (FBS; cat. no. 10270-106) and 1%
penicillin-streptomycin solution (cat. no. 15140-122) (all from
Gibco; Thermo Fisher Scientific, Inc.). Cells were maintained at
37°C in a humidified atmosphere containing 5% CO2 (Forma
3110; Thermo Fisher Scientific, Inc.). Upon reaching 80–90%
confluence, the cells were trypsinized with 0.25% trypsin-EDTA
(cat. no. 25200-072; Gibco; Thermo Fisher Scientific, Inc.) and
subcultured at a 1:3 ratio. Cell counting was performed using a
TC20 automated cell counter (Bio-Rad Laboratories, Inc.). All
experiments were conducted under sterile conditions in a biosafety
cabinet (1300 Series A2; Thermo Fisher Scientific, Inc.).
siRNA synthesis and cell
transfection
The siRNA targeting the PPAP2C gene (Gene ID: 8612)
was synthesized by Guangzhou Anerno Biotechnology Co., Ltd. A
scrambled negative control (NC) sequence was also synthesized
(Guangzhou Anerno Biotechnology Co., Ltd.).
Lipofectamine® 2000 (cat. no. 11668027; Invitrogen;
Thermo Fisher Scientific, Inc.) was used as the transfection
reagent. Briefly, A549 (cat. no. CL-0016; Wuhan Pricella
Biotechnology Co., Ltd.) and H1299 (cat. no. CL-0165; Wuhan
Pricella Biotechnology Co., Ltd.) cells were seeded into 6-well
plates at a density of 5×105 cells/well and were
cultured for 24 h to reach 80–90% confluence. For transfection, 4
µg of the constructed silencing vector DNA was incubated with 10 µl
Lipofectamine 2000 in 250 µl serum-free medium Opti-MEM (cat. no.
31985062; Gibco; Thermo Fisher Scientific, Inc.) at room
temperature for 20 min and was then added to each well containing 2
ml serum-free medium. After incubation at 37°C 6 h of incubation,
the medium was replaced with complete medium containing 10% FBS.
PPAP2C gene expression levels were detected at 48 h
post-transfection by reverse transcription-quantitative PCR
(RT-qPCR), and the relative expression levels were calculated using
the 2−ΔΔCq method (17). Each experiment was repeated three
times, with three technical replicates per experiment, to ensure
data reliability and accuracy. The siRNA sequences are presented in
Table I.
 | Table I.Sequences of siRNAs and primers. |
Table I.
Sequences of siRNAs and primers.
| ID | Sequence
(5′-3′) |
|---|
| si-PPAP2C #1 | Sense:
CCCCGUACAAGCGAGGAUUUU |
|
| Antisense:
AAAAUCCUCGCUUGUACGGGG |
| si-PPAP2C #2 | Sense:
CCCGUACAAGCGAGGAUUUUA |
|
| Antisense:
UAAAAUCCUCGCUUGUACGGG |
| si-PPAP2C #3 | Sense:
CCCCAAAUAUCCCCUUCUUUU |
|
| Antisense:
AAAAGAAGGGGAUAUUUGGGG |
| si-NC | Sense:
UUCUCCGAACGAGUCACGUUU |
|
| Antisense:
AAACGUGACUCGUUCGGAGAA |
| PPAP2C | F:
CTGCCCTTCGCTATCCTGAC |
|
| R:
CCGTGGGTGATGGTATCTGG |
| Fos | F:
CAAGCGGAGACAGACCAACT |
|
| R:
GTGAGCTGCCAGGATGAACT |
| Jun | F:
GAGACAAGTGGCAGAGTCCC |
|
| R:
TCTTCTCTTGCGTGGCTCTC |
| GAPDH | F:
GACCACAGTCCATGCCATCA |
|
| R:
CCGTTCAGCTCAGGGATGAC |
RT-qPCR
A549 and H1299 cells were ground thoroughly in
liquid nitrogen, followed by the addition of 1 ml RNAiso Plus (cat.
no. 9109; Takara Bio, Inc.), and were mixed thoroughly by
vortexing, then placed on ice for 5 min. Subsequently, 0.2 ml
chloroform (cat. no. C805077; Shanghai Macklin Biochemical Co.,
Ltd.) was added, shaken vigorously for 15 sec and incubated at 4°C
for 3 min. After centrifugation (D-1524R; Zhuhai Hema Medical
Instrument Co., Ltd.) at 1,969 × g for 15 min at 4°C, the aqueous
phase was transferred to a new tube. An equal volume of isopropanol
(cat. no. H920368; Shanghai Macklin Biochemical Co., Ltd.) was then
added, mixed well and incubated at −20°C for 20 min, followed by
centrifugation at 1,969 × g for 15 min at 4°C. The supernatant was
discarded, and the pellet was washed with 1 ml 75% DEPC-treated
ethanol (cat. no. H855322 Shanghai Macklin Biochemical Co., Ltd.)
and then centrifuged at 875 × g for 5 min at 4°C. The liquid was
discarded, and the pellet was air-dried at room temperature. RNA
was dissolved in 30 µl DEPC-treated ddH2O (cat. no.
W915679 Shanghai Macklin Biochemical Co., Ltd.) and stored at
−80°C. RNA concentration was measured using a Q6000UV UV
spectrophotometer (Quawell Technology). For cDNA synthesis, 2 µg
total RNA was used as a template, according to the instructions of
the Bestar™ qPCR RT Kit (cat. no. 2220; DBI), with a total reaction
volume of 10 µl. qPCR was performed using Bestar™ SybrGreen qPCR
MasterMix (cat. no. 2043; DBI) in a final reaction volume of 20 µl.
Fluorescence qPCR experiments were conducted on an Agilent
Stratagene fluorescence qPCR instrument (Mx3000P; Stratagene;
Agilent Technologies, Inc.). Thermocycling conditions for qPCR
included: 95°C for 2 min for initial denaturation, followed by 40
cycles of 95°C for 10 s for denaturation, and 60°C for 30 s for
annealing/extension. Results were processed using the
2−ΔΔCq method, with the data presented as the mean ±
standard deviation. Detailed primer sequences are provided in
Table I.
Western blot analysis
After washing the cells three times with PBS (cat.
no. 14190-144; Thermo Fisher Scientific, Inc.), the samples were
centrifuged at 22,000 × g for 10 min at 4°C using a refrigerated
high-speed centrifuge (Zhuhai Hema Medical Instrument Co., Ltd.).
The supernatant was collected and stored at −80°C. Proteins were
extracted from cells using RIPA lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology), containing 1% protease
inhibitor cocktail (cat. no. P1005; Beyotime Institute of
Biotechnology). Protein concentration was determined using the BCA
Protein Assay Kit (cat. no. P0012S; Beyotime Institute of
Biotechnology). Protein samples were separated by SDS-PAGE, where
the separating gel was prepared by mixing 30% acrylamide (cat. no.
1610156; Bio-Rad Laboratories, Inc.), bis-acrylamide (cat. no.
A3574; Sigma-Aldrich), 1.5 mol/l Tris-HCl (pH 8.8; cat. no. MA0053;
MeilunBio) and 10% SDS (cat. no. BL517A; Biosharp). A total of 20
µg of protein was loaded per lane. After polymerization, the
stacking gel was added. Following sample loading, electrophoresis
was performed using a constant voltage power supply (DYY-6C;
Beijing Liuyi Instrument Factory) at 100 V, and the voltage was
increased to 120 V after the samples entered the separating gel,
continuing until the bromophenol blue (cat. no. B0126;
Sigma-Aldrich) reached the bottom of the gel. After
electrophoresis, the proteins were transferred onto a PVDF membrane
(cat. no. IPVH00010; MilliporeSigma) using a constant current of
300 mA. The membrane was then washed with TBS buffer (cat. no.
T5030; MilliporeSigma) and blocked with 5% non-fat milk (cat. no.
A600669; Shanghai Sangon Biotechnology Co., Ltd.) at room
temperature for 1 h. The membrane was then incubated with primary
antibodies, including anti-PPAP2C (cat. no. PA5-98075; 1:1,200; 33
kDa), anti-phosphorylated (p)-ERK (cat. no. 44-680G; 1:1,000; 42/44
kDa), anti-ERK (cat. no. MA5-15134; 1:1,000; 42/44 kDa), anti-p-JNK
(cat. no. 44-682G; 1:1,000; 46/55 kDa), anti-JNK (cat. no. AHO1362;
1:1,000; 46/55 kDa) (all from Thermo Fisher Scientific, Inc.),
anti-GAPDH (cat. no. ab9485; 1;2,500; 37 kDa), anti-N-cadherin
(cat. no. ab76011; 1:10,000; 125 kDa), anti-E-cadherin (cat. no.
ab40772; 1:2,000; 130 kDa) and anti-Snail (cat. no. ab31787;
1:1,000; 68 kDa) (all from Abcam) for 1 h at room temperature.
Notably, the band intensity and clarity after 1 h of incubation
were comparable to those after overnight incubation. Subsequently,
the membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG secondary antibody (cat. no.
ab6721; 1:10,000; Abcam) or HRP-conjugated rabbit anti-rat IgG
secondary antibody (cat. no. ab6734; 1:2000; Abcam) for 40 min at
room temperature. Detection was performed using a chemiluminescence
substrate (cat. no. WBKLS0500; MilliporeSigma), and the results
were recorded on X-ray film (Guangxi Superstar Medical Device Co.,
Ltd.) and were analyzed using ImageJ software (version 1.53;
National Institutes of Health). The intensity of each band was
normalized to the loading control and background intensity was
subtracted to exclude non-specific signals; the final results were
expressed in relative intensity units.
Wound healing assay
Complete medium was prepared using high-glucose DMEM
(cat. no. E600003; Sangon Biotech Co., Ltd.), supplemented with 10%
premium FBS (cat. no. FBS-P01; Shanghai Excell Biological
Technology Co., Ltd.) and 1% penicillin-streptomycin (cat. no.
P1400; Beijing Solarbio Science & Technology Co., Ltd.) as per
the instructions provided by Sangon Biotech Co., Ltd. Horizontal
lines were evenly drawn on the back of a 6-well plate (TCP010006;
Guangzhou Jet Bio-Filtration Co., Ltd.) using a fine-tip marker
pen, with at least five lines crossing each well to standardize the
scratch position. Cells grown to 80% confluence were harvested, the
old culture medium was aspirated, and the cells were washed 1–2
times with PBS. Cells were treated with trypsin-EDTA (cat. no.
E607001; Shanghai Sangon Biotechnology Co., Ltd.) and gently
washed, the trypsin solution was aspirated, and the cells were
placed in a 37°C incubator (311; Thermo Fisher Scientific, Inc.)
for 2–3 min until the cells became round. Fresh complete medium was
added, and a single-cell suspension was prepared using a pipette
(cat. no. 7010101033; Dragonlab). The cells were counted, and the
cell density was adjusted to 5×105/ml. Subsequently, 1
ml cell suspension (5×105 cells/ml) was added to each
well of the 6-well plate, ensuring each well reached 80% confluence
prior to wounding. Fresh complete medium containing specific siRNA
treatments targeting PPAP2C gene (si-PPAP2C #1 and si-PPAP2C #2),
along with a scrambled siRNA as a NC (si-NC), was added to the
respective treatment groups was added. The cells were cultured for
48 h at 37°C and 5% CO2. A vertical scratch was made
using a pipette tip (PPT221010; Guangzhou Jet Bio-Filtration Co.,
Ltd.). The cells were washed three times with 2 ml PBS/well to
remove the detached cells and were then incubated with 2 ml
complete medium containing 10% serum (cat. no. A5256701; Thermo
Fisher Scientific, Inc.) at 37°C and 5% CO2 for 24 h.
The cells were observed and images were captured using a light
inverted microscope (CKX53; Olympus Corporation), and the wound
area was analyzed using Image-Pro Plus software (Ver. 6.0; Media
Cybernetics, Inc.). The relative migration rate was calculated by
measuring the initial wound size and the wound closure rate after
24 h of incubation, normalized to the control.
Transwell invasion assay
Matrigel (cat. no. 356234; Corning, Inc.) was thawed
overnight at 4°C and was mixed with pre-cooled serum-free medium at
a 1:15 ratio to prepare the gel solution. Subsequently, 100 µl gel
solution was added to the upper chamber of Transwell inserts (8 µm
pore size; 24-well format; CP012036; Guangzhou Jet Bio-Filtration
Co., Ltd.) and allowed to solidify at 37°C for 2 h. Excess liquid
was removed, and 50 µl sterile PBS was added to each well and
incubated at 37°C for 30 min to wash away the residual Matrigel.
Cells were serum-starved for 12 h before seeding. Cells were
trypsinized with 0.25% trypsin-EDTA for 2–3 min until they
detached, and the cell density was adjusted to 2×105/ml.
Subsequently, 100 µl cell suspension (2×104 cells/well)
was added to the upper chamber, and 700 µl complete medium
containing 10% FBS was added to the lower chamber. The Transwell
inserts were incubated at 37°C in an atmosphere containing 5%
CO2 and 100% humidity for 24 h. The inserts were then
removed, the non-invasive cells in the upper chamber were gently
wiped away with a cotton swab and the residual Matrigel was
removed. The inserts were washed three times with PBS, fixed with
4% paraformaldehyde at room temperature for 20 min, and washed
three more times with PBS. The inserts were then stained with 0.1%
crystal violet (cat. no. G1062; Beijing Solarbio Science &
Technology Co., Ltd.) solution at room temperature for 10 min,
washed with running water, and the membrane was removed from the
inserts using a scalpel, air-dried and mounted with neutral resin.
Slides were scanned, and three random fields were selected for cell
counting using a light microscope.
Molecular docking experiment
First, the X-ray crystal structure of ERK1 was
obtained from the Protein Data Bank (PDB) with the PDB ID: 3FHR
(https://www.rcsb.org/structure/3FHR.). The
three-dimensional structure of PPAP2C was constructed using
AlphaFold (version 2.1.1; http://alphafold.ebi.ac.uk/) with default settings. To
ensure the accuracy of the molecular docking results, the two
protein structures were preprocessed using AutoDockTools-1.5.7
(https://autodock.scripps.edu/),
including manual removal of water molecules and the addition of
polar hydrogens. Subsequently, protein-protein docking simulations
were performed using the GRAMM docking server (https://gramm.compbio.ku.edu/) with default
parameters. The resulting protein complex was further manually
optimized with AutoDockTools-1.5.7, where water molecules were
again removed, and polar hydrogens were added. Finally,
protein-protein interactions were visualized and analyzed using
PyMOL (https://pymol.org/), generating the corresponding
interaction diagrams.
Co-immunoprecipitation experiment
Total proteins were extracted from H1299 and A549
cell lines. The cells were washed three times with PBS and lysed
using RIPA lysis buffer containing protease inhibitors (cat. no.
P0013B; Beyotime Institute of Biotechnology). The lysates were
centrifuged at 22,000 × g for 10 min at 4°C using a refrigerated
high-speed centrifuge (TGL-16M; Zhuhai Hema Medical Instrument Co.,
Ltd.) and the supernatant was collected. PPAP2C antibody (cat. no.
ab168371; 1:10,000; 36 kDa; Abcam) was added to the cell lysates
and incubated overnight at 4°C to ensure sufficient binding. As a
control, IgG (cat. no. ab18443; 1:10,000; Abcam) was used in a
parallel experiment under the same conditions. A total of 50 µl
protein A/G magnetic beads (cat. no. 10002D; Thermo Fisher
Scientific, Inc.) were then added, and the mixture was incubated
for an additional 2 h at 4°C to capture the antibody-antigen
complexes. After incubation, the immune complexes were collected by
centrifugation at 22,000 × g for 10 min at 4°C and washed three
times with lysis buffer. The washed beads were suspended in SDS
loading buffer (cat. no. P0015F; Beyotime Institute of
Biotechnology) and heated to 95°C to elute the proteins from the
beads. The resulting protein samples underwent western blotting, as
aforementioned, using a 12% SDS-PAGE gel. The membrane was probed
with an anti-ERK1 antibody (cat. no. ab32537; 1:1,000; 43 kDa;
Abcam) followed by an HRP-conjugated secondary antibody (cat. no.
ab6721; 1:10,000; Abcam) at room temperature for 1 h each. Finally,
detection was performed using a chemiluminescence substrate and the
results were recorded using medical X-ray film.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 20 min
at room temperature, washed three times with 1 ml PBS, and
permeabilized with 0.1% Triton X-100 for 10 min at room
temperature, followed by three washes with 1 ml PBS. The cells were
then incubated overnight at 4°C with primary antibodies against
E-cadherin (cat. no. ab40772; 1:1,000; Abcam), followed by
incubation with Alexa Fluor 488-conjugated secondary antibodies
(cat. no. A-21206; 1:1,000; Thermo Fisher Scientific, Inc.) for 1 h
at room temperature. Nuclei were stained with DAPI (cat. no. D1306;
1:1,000; Thermo Fisher Scientific, Inc.) for 5 min at room
temperature, followed by three washes with 1 ml PBS, and the cells
were observed and images were captured using an inverted
fluorescence microscope (CKX53; Olympus Corporation).
Statistical analysis
Statistical analysis and data visualization were
performed using GraphPad Prism 9.0 software (Dotmatics). The
normality of the data distribution was assessed using the
Shapiro-Wilk test. For data that followed a normal distribution,
comparisons between two groups were performed using Student's
t-test. For comparisons between two groups not following a normal
distribution, the Wilcoxon rank-sum test was used. For comparisons
involving multiple groups, one-way ANOVA was used, followed by
Tukey's post hoc test to account for multiple comparisons. Data
following a normal distribution are presented as the mean ±
standard deviation, while data not following a normal distribution
are expressed as median and IQR. P<0.05 was considered to
indicate a statistically significant difference.
Results
PPAP2C is highly expressed in LUAD and
is correlated with the ERK/JNK pathway and EMT-related genes
The expression levels of PPAP2C were lower in normal
lung tissues, from healthy individuals (n=59), and were markedly
elevated in LUAD tissues (n=539; P<0.001; Fig. 1A). Further analysis demonstrated
associations between the expression levels of PPAP2C and the genes
MAPK1, MAPK3, MAPK8, CDH1, CDH2 and SNAI1. Specifically, high
expression of PPAP2C was associated with MAPK3, MAPK8, CDH1 and
SNAI1 (P<0.05, P<0.001; Fig.
1B).
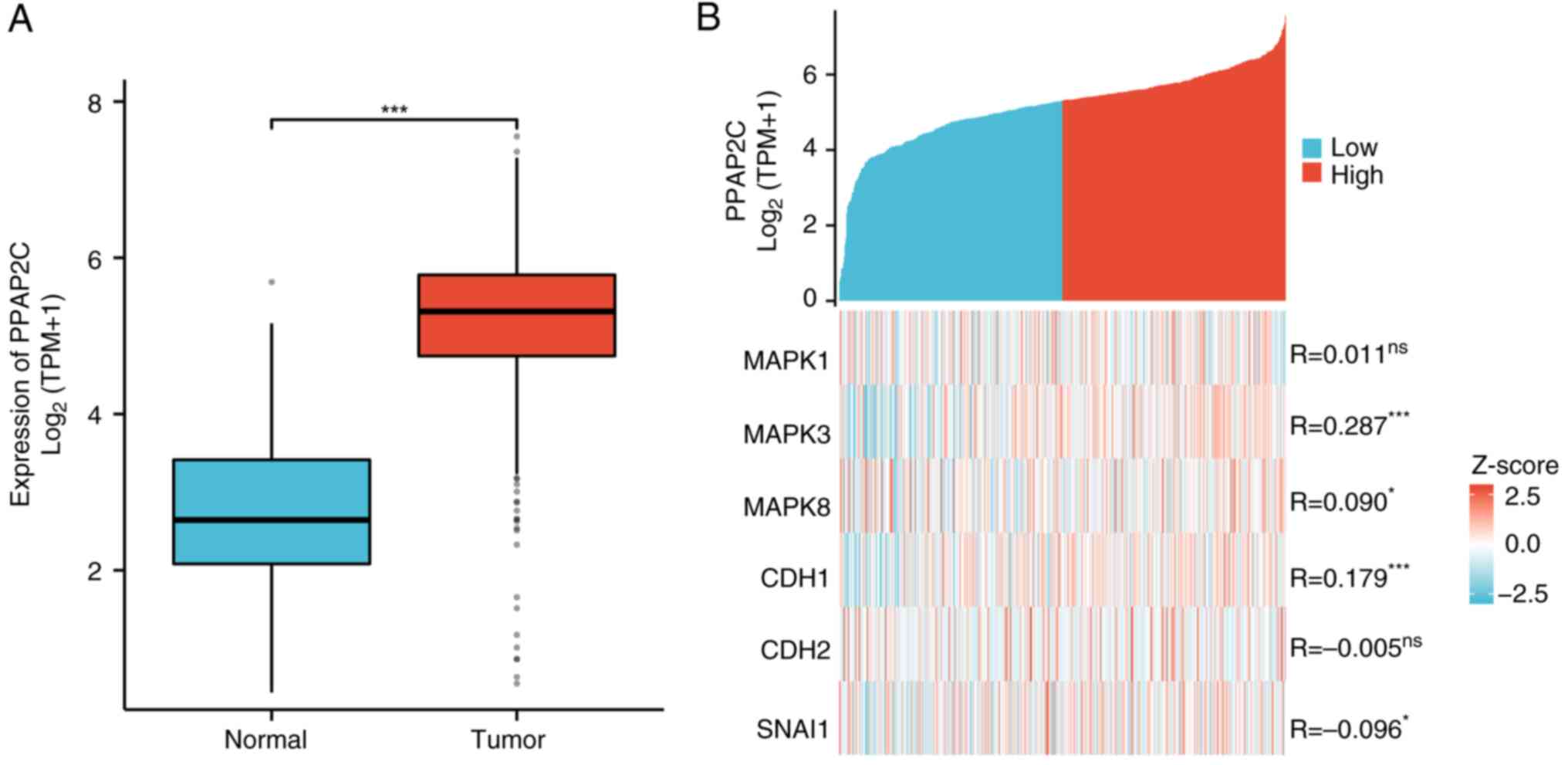 | Figure 1.High expression of PPAP2C in LUAD,
and its correlation with the ERK/JNK pathway and EMT-related genes.
(A) Expression levels of PPAP2C were compared between normal
tissues (n=59) and LUAD tissues (n=539). (B) Correlation between
PPAP2C expression levels and the expression of genes, including
MAPK1, MAPK3, MAPK8, CDH1, CDH2 and SNAI1, in groups with high and
low PPAP2C expression. The comparison of PPAP2C expression levels
between normal and LUAD tissues was conducted using the Wilcoxon
rank-sum test. *P<0.05, ***P<0.001. LUAD, lung
adenocarcinoma; ns, not significant; PPAP2C, phospholipid
phosphatase 2. |
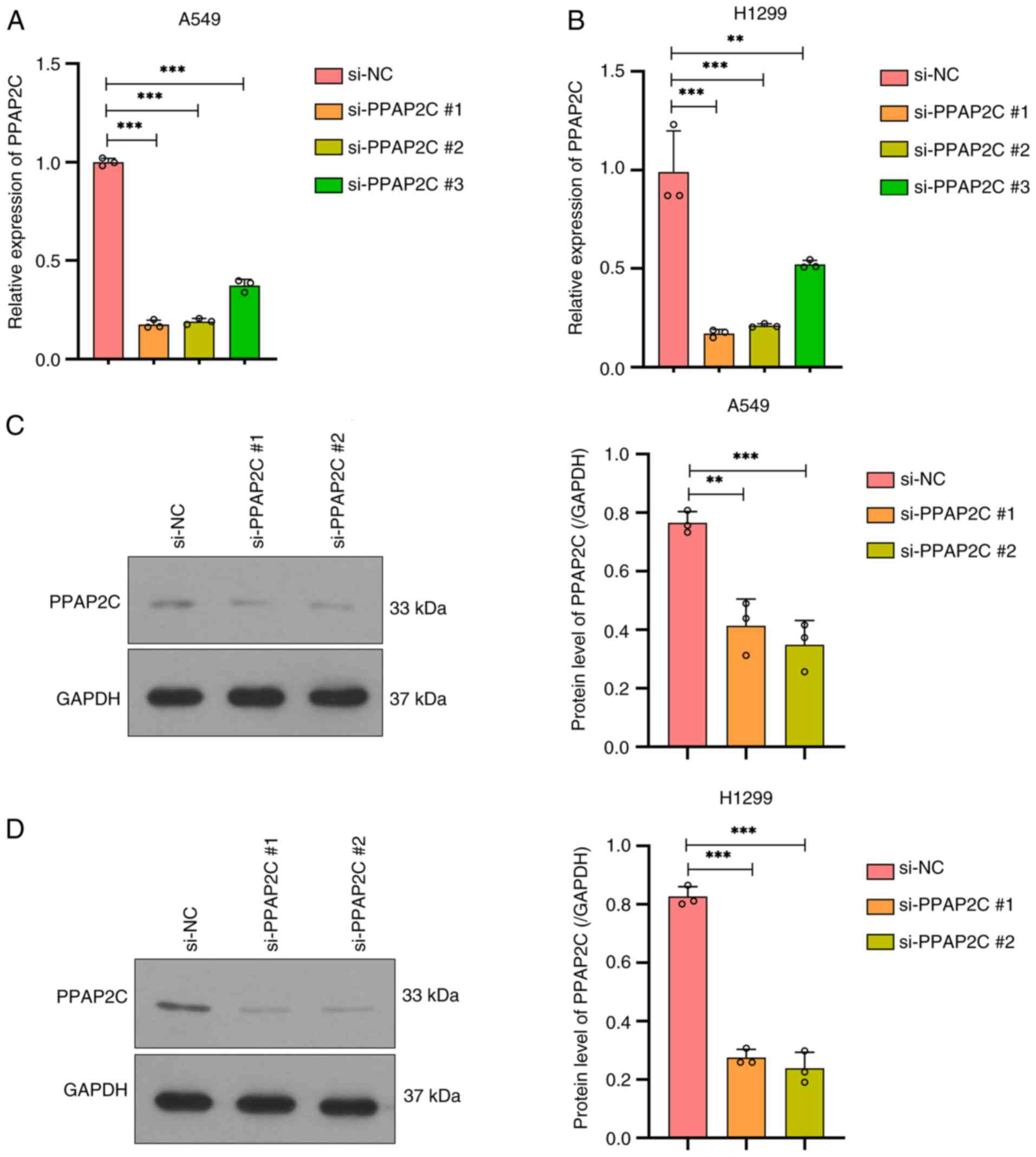
Successful construction of a PPAP2C
gene silencing model in LUAD cells
In A549 cells, RT-qPCR was used to detect the mRNA
expression levels of the PPAP2C gene after silencing. The results
showed that compared with the si-NC group, all three silencing
targets (small interfering RNA: si-PPAP2C#1, si-PPAP2C#2 and
si-PPAP2C#3) significantly reduced the mRNA expression levels of
PPAP2C, with si-PPAP2C#1 showing the greatest reduction
(P<0.001; Fig. 2A). The RT-qPCR
results in H1299 cells were similar to those in A549 cells, showing
significant downregulation of PPAP2C across all three silencing
targets, with si-PPAP2C#1 showing the most significant silencing
effect (P<0.001; Fig. 2B). At
the protein level, western blot analysis in A549 cells revealed
that PPAP2C protein expression was significantly reduced after
silencing, with both the si-PPAP2C#2 and si-PPAP2C#1 groups showing
marked downregulation compared with that in the si-NC group
(P<0.01 and P<0.001; Fig.
2C). The results of western blotting in H1299 cells
demonstrated a similar trend to that in A549 cells, with a
significant reduction in PPAP2C protein expression after silencing,
with si-PPAP2C#2 showing the most pronounced downregulatory effect
(P<0.001; Fig. 2D). As
si-PPAP2C #1 and si-PPAP2C #2 showed the most effective silencing,
they were selected for subsequent experiments.
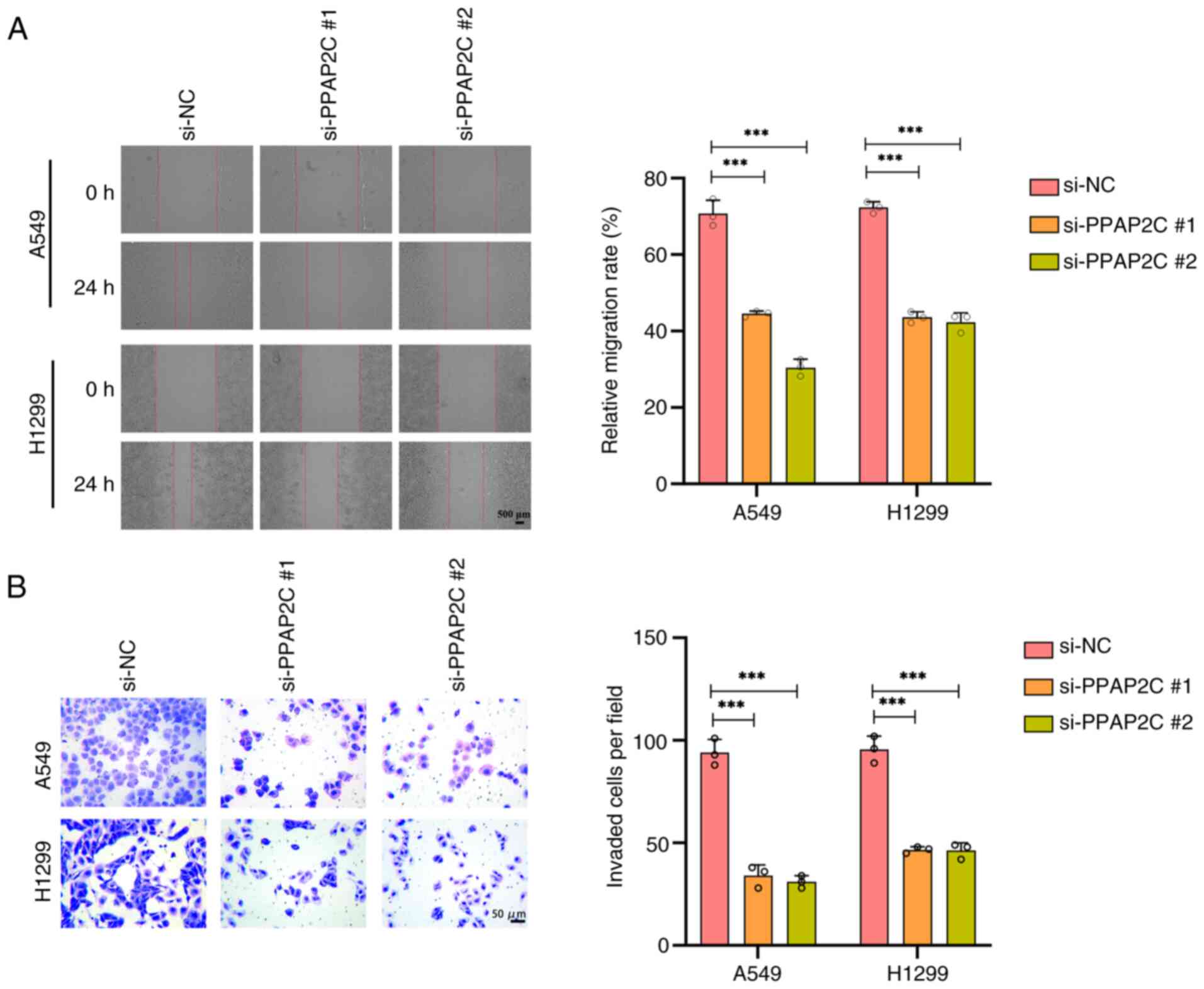
Silencing of the PPAP2C gene inhibits
the migration and invasion of LUAD cells
After PPAP2C silencing, the migratory capability of
A549 and H1299 cells significantly decreased within 24 h, and the
relative migration rate was significantly lower than that of the
si-NC group (P<0.001; Fig. 3A).
Additionally, the invasive ability of A549 and H1299 cells was
markedly reduced after PPAP2C silencing, as evidenced by a
significant decrease in the number of invasive cells per field
compared with that in the si-NC group (P<0.001; Fig. 3B).
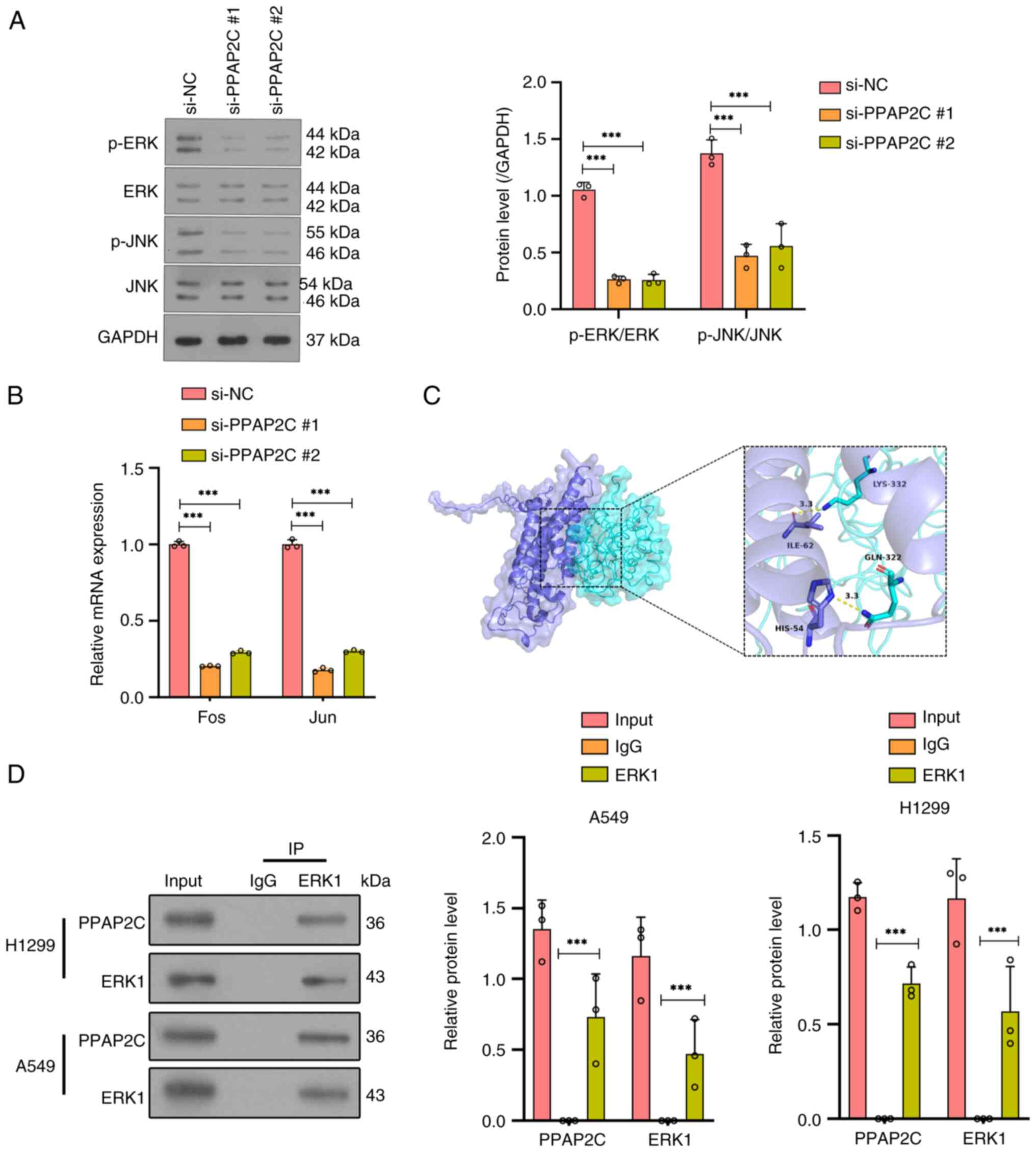
Silencing of PPAP2C significantly
inhibits the activity of the ERK/JNK signaling pathway
Western blot analysis detected the protein
expression levels of ERK and JNK, as well as their phosphorylated
forms (p-ERK and p-JNK), showing a significant reduction in
phosphorylation levels of both ERK and JNK in the si-PPAP2C#1 and
si-PPAP2C#2 groups compared with those in the si-NC group
(P<0.001; Fig. 4A). The RT-qPCR
results indicated that, compared with those in the si-NC group, the
mRNA expression levels of the ERK/JNK downstream genes Fos and Jun
were significantly reduced in the si-PPAP2C#1 and si-PPAP2C#2
groups (P<0.001; Fig. 4B).
Molecular docking results demonstrated that there were clear
binding sites between PPAP2C and ERK1, involving specific amino
acid residues such as LYS-332, ILE-62, GLN-322 and HIS-54 (Fig. 4C). Furthermore,
co-immunoprecipitation experiments confirmed the interaction
between PPAP2C and ERK1 in both H1299 and A549 cell lines compared
with the control IgG (P<0.001; Fig.
4D).
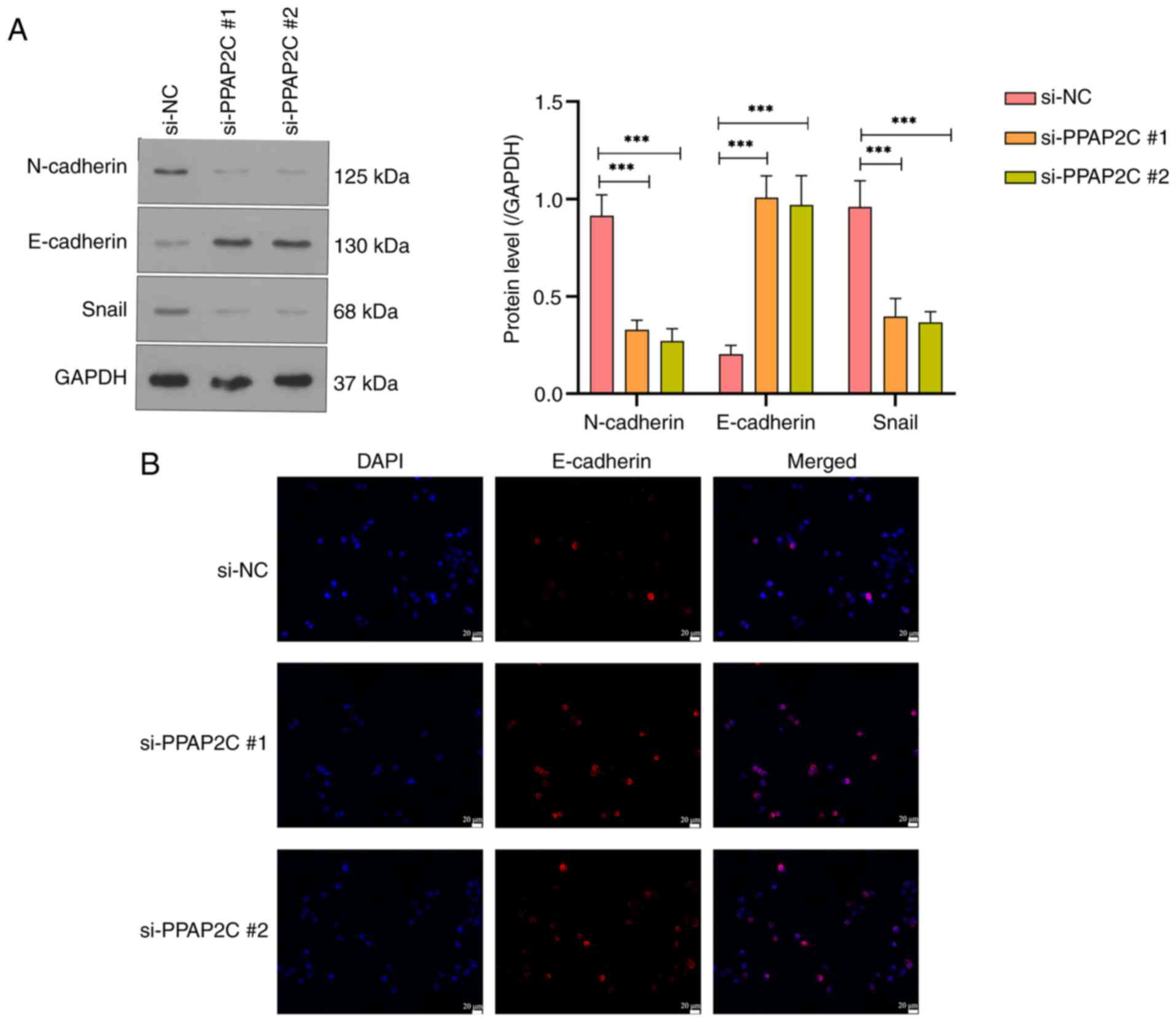
PPAP2C gene silencing inhibits EMT in
LUAD cells
Western blot analysis of N-cadherin, E-cadherin and
Snail revealed that the protein levels of N-cadherin and Snail were
significantly reduced, whereas E-cadherin protein levels were
significantly increased in the si-PPAP2C#1 and si-PPAP2C#2 groups
compared with those in the si-NC group (P<0.001; Fig. 5A). Immunofluorescence staining
further confirmed the elevated expression of E-cadherin in the
si-PPAP2C#1 and si-PPAP2C#2 groups, as evidenced by the markedly
enhanced fluorescence signal (Fig.
5B).
Discussion
LUAD is the most common subtype of lung cancer that
is characterized by high invasiveness and metastatic potential,
which contributes to its high mortality rates despite advancements
in diagnosis and treatment (4,18).
PPAP2C is a PA phosphatase that exhibits aberrant expression in
various tumors, such as LUAD (10)
and endometrial cancer (19), and
is closely associated with tumor progression and prognosis
(10,20). The ERK/JNK pathway serves a
critical role in cell proliferation, differentiation, migration and
apoptosis, and has been shown to be closely linked to tumorigenesis
and development in various types of cancer, such as LUAD and breast
cancer (15,21). EMT is a crucial process by which
tumor cells acquire invasive and migratory capabilities (22,23).
The A549 cell line is derived from human LUAD and harbors a KRAS
mutation, making it an ideal model for studying the RAS-ERK
pathway. This is because the KRAS mutation activates the ERK
signaling pathway, affecting cell proliferation and migration. By
contrast, the H1299 cell line is also derived from LUAD but lacks
the p53 gene, an important tumor suppressor gene. The absence of
p53 simulates common genetic defects found in LUAD (24,25).
Therefore, A549 and H1299 cells were used for the present
experiments. This study investigated the expression of PPAP2C in
LUAD, and its association with the ERK/JNK signaling pathway and
EMT-related genes, aiming to elucidate the role of PPAP2C in the
progression of LUAD.
Previous studies have demonstrated that PPAP2C is
highly expressed in various tumors, and its elevated levels are
closely associated with increased tumor invasiveness and poor
prognosis (8,19). For example, in breast cancer, high
expression of PPAP2C can promote cancer cell proliferation and
migration (8). In the present
study, it was revealed that PPAP2C was significantly upregulated in
LUAD tissues compared with that in normal lung tissues. This
finding aligns with previous studies on other types of cancer
(20,26), further suggesting that PPAP2C may
serve a promotive role in LUAD progression (10), and providing a potential target for
a more precise diagnosis and the treatment of LUAD.
The ERK/JNK signaling pathway, as a critical branch
of the MAPK signaling pathway, has been reported to be aberrantly
activated in various types of cancer, such as intrahepatic
cholangiocarcinoma (27) and colon
cancer (28), promoting tumor cell
proliferation and metastasis (14,29).
The MAPK1, MAPK3 and MAPK8 genes encode the ERK2, ERK1 and JNK1
proteins, respectively (30,31).
Research has shown that inhibiting EMT can significantly reduce the
invasiveness of cancer cells (32,33).
CDH1, CDH2 and SNAI1 are important genes closely related to EMT.
CDH1 encodes E-cadherin, which is primarily expressed in epithelial
cells, maintaining cell-cell adhesion and epithelial morphology,
while CDH2 encodes N-cadherin, which is mainly expressed in
mesenchymal cells, promoting cell migration and invasion (34). SNAI1 encodes the Snail
transcription factor, which drives EMT by inhibiting E-cadherin
expression and promoting N-cadherin expression (35). It has been demonstrated that PCSK9
promotes EMT and migration of tumor cells in colorectal cancer by
upregulating Snail and downregulating E-cadherin (36). The present study revealed that high
PPAP2C expression was associated with genes such as MAPK3, MAPK8,
CDH1 and SNAI1, suggesting that PPAP2C may interact with the
ERK/JNK pathway and EMT-related genes to promote LUAD
progression.
It has been reported that inhibiting the ERK/JNK
pathway can significantly reduce the invasiveness and migratory
capabilities of tumor cells (37).
The present results showed that silencing PPAP2C significantly
reduced the migration and invasion of LUAD cells, indicating that
PPAP2C may be critical in enhancing the migration and invasion of
LUAD cells by modulating key signaling pathways. Although the
current study identified the roles of PPAP2C in inhibiting LUAD
cell metastasis by silencing the gene, the lack of a
pharmacological inhibitor treatment group prevents a comprehensive
evaluation of its potential application in clinical therapy. Future
research should consider establishing a treatment group using known
chemotherapeutic agents as a positive control to confirm the
therapeutic efficacy and further clarify the clinical significance
of PPAP2C as a potential therapeutic target. Additionally, the
absence of a Transwell migration assay may lead to an incomplete
understanding of the specific role of PPAP2C in cell migration;
therefore, future studies should consider incorporating this assay
to further validate and strengthen the conclusions of this
research.
Fos and Jun are key downstream transcription factors
of the ERK/JNK signaling pathway, regulated by the activation of
ERK and JNK, respectively (38).
ERK1/2 can promote cell proliferation by phosphorylating
transcription factors, such as c-Fos and Elk-1. Furthermore, JNK1
can regulate gene expression by phosphorylating c-Jun and ATF2,
thereby participating in apoptosis and stress responses (39,40).
The present study demonstrated that the phosphorylation levels of
ERK and JNK were significantly decreased following PPAP2C
silencing. PPAP2C silencing also led to a significant reduction in
the mRNA expression levels of Fos and Jun genes, further
demonstrating that PPAP2C may influence the expression of
downstream transcription factors through regulation of the ERK/JNK
signaling pathway. Additionally, a clear binding site was
identified between PPAP2C and ERK1, and the co-immunoprecipitation
assay confirmed their physical interaction in both H1299 and A549
cell lines, further corroborating the interaction between PPAP2C
and ERK1. Based on the aforementioned experimental data, it may
reasonably be inferred that there is a direct interaction between
PPAP2C and ERK1, and this interaction may promote the activation of
ERK1. However, these findings are primarily based on in
vitro cell experiments. Although the results indicated a
significant interaction between PPAP2C and ERK1, further in
vivo studies are needed to validate this mechanism. As there
are currently no reports on the specific mechanism by which PPAP2C
affects ERK1, to the best of our knowledge, there is no literature
to support this finding at present.
The expression of Fra1 is associated with
mesenchymal characteristics in epithelial tumors, and it has been
shown that dephosphorylated JNK2 can increase Fra1 expression by
promoting the expression of c-Jun and Jun-B (41). Additionally, it has been reported
that haploinsufficiency of Gata3, a cell cycle inhibitor, in
p18Ink4c-deficient mice can upregulate Fra1 and downregulate c-Fos,
leading to EMT activation and promoting the initiation and
metastasis of breast tumors (42).
The present study suggested that PPAP2C may influence EMT through
the ERK/JNK signaling pathway. While the current study provides
strong evidence that PPAP2C influences EMT markers by modulating
the ERK/JNK signaling pathway and its downstream transcription
factors Jun and Fos, the exact molecular mechanisms remain unclear.
Future research should focus on elucidating the direct target genes
regulated by Jun and Fos during EMT, and how PPAP2C modulates these
pathways. A deeper understanding of these mechanisms may provide
novel therapeutic targets for diseases involving EMT, such as
cancer metastasis.
EMT is a critical process by which tumor cells
acquire migratory and invasive capabilities, characterized by a
decrease in the epithelial marker E-cadherin, and an increase in
the mesenchymal markers N-cadherin and Snail (32,43).
Studies have shown that modulating the expression of E-cadherin and
N-cadherin can significantly impact the invasiveness of tumor cells
(44,45). The current study demonstrated that
silencing the PPAP2C gene in LUAD cells significantly reduced the
protein levels of N-cadherin and Snail, while significantly
increasing the protein levels of E-cadherin. This indicated that
PPAP2C may enhance the invasiveness and migratory abilities of LUAD
cells by promoting the EMT process. Immunofluorescence staining
further confirmed this finding, showing that E-cadherin expression
and localization were notably enhanced after PPAP2C silencing.
These findings suggested that silencing the PPAP2C gene may reverse
the EMT process by restoring E-cadherin expression, and reducing
the levels of N-cadherin and Snail, thereby decreasing the
invasiveness and migratory capabilities of LUAD cells. Therefore,
targeting PPAP2C could be a promising strategy to inhibit EMT and
tumor metastasis in LUAD. However, the exact mechanism by which
PPAP2C influences EMT has not been fully elucidated in the current
study.
Despite the important insights provided by the
present study into the critical role of PPAP2C in LUAD, several
limitations should be acknowledged. Firstly, the study primarily
validated the role of PPAP2C in LUAD through in vitro cell
experiments, which limits the ability to fully understand the in
vivo mechanisms of PPAP2C in LUAD progression. Secondly,
immunohistochemistry and qPCR experiments were not performed on
actual human LUAD tissue samples. Due to the lack of sufficient
clinical samples, the expression levels of PPAP2C could not be
verified in LUAD tissues and the consistency with the in
vitro findings could not be assessed. In future studies, we aim
to collect and analyze clinical data from patients with LUAD, using
survival analysis, multivariate regression analysis and metastasis
risk assessment methods to evaluate whether PPAP2C can serve as an
independent prognostic marker and a predictor of metastatic risk.
Additionally, we aim to explore its relationship with other
clinical characteristics, such as tumor staging and treatment
response. These studies will help clarify the role of PPAP2C in
LUAD progression and provide new insights for personalized
treatment. Therefore, future research should consider using animal
models and clinical data to more comprehensively validate the key
role of PPAP2C in LUAD and to assess its potential as a therapeutic
target.
In conclusion, the present study revealed that
PPAP2C was highly expressed in LUAD, and could enhance the
migration and invasion of LUAD cells by activating the ERK/JNK
signaling pathway and promoting the EMT process, thus highlighting
its potential as a critical target for therapeutic intervention in
LUAD.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Xian Jiaotong University
Second Affiliated Hospital Fund for Free Exploration Project [grant
no. 2020YJ(ZYTS)086].
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL analyzed TCGA data, and conducted the Pearson
correlation coefficient analysis, molecular docking and
co-immunoprecipitation experiments. WD constructed the PPAP2C gene
silencing models, and performed RT-qPCR and western blot analysis.
TJ evaluated cell migration and invasion through wound healing and
Transwell invasion assays. MZ examined the effects of PPAP2C
silencing on the ERK/JNK signaling pathway and EMT using western
blotting and immunofluorescence staining. WL supervised the
project, contributed to study design and revised the manuscript. YL
and WL confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Su L, Zhao J, Su H, Wang Y, Huang W, Jiang
X and Gao S: CircRNAs in Lung Adenocarcinoma: Diagnosis and
Therapy. Curr Gene Ther. 22:15–22. 2022.PubMed/NCBI
|
|
2
|
Wei X, Li X, Hu S, Cheng J and Cai R:
Regulation of ferroptosis in lung adenocarcinoma. Int J Mol Sci.
24:146142023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song J, Liu W, Wang J, Hao J, Wang Y, You
X, Du X, Zhou Y, Ben J, Zhang X, et al: GALNT6 promotes invasion
and metastasis of human lung adenocarcinoma cells through
O-glycosylating chaperone protein GRP78. Cell Death Dis.
11:3522020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song Y, Kelava L and Kiss I: MiRNAs in
lung adenocarcinoma: Role, diagnosis, prognosis, and therapy. Int J
Mol Sci. 24:133022023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujikawa R, Muraoka Y, Kashima J, Yoshida
Y, Ito K, Watanabe H, Kusumoto M, Watanabe SI and Yatabe Y:
Clinicopathologic and genotypic features of lung adenocarcinoma
characterized by the international association for the study of
lung cancer grading system. J Thorac Oncol. 17:700–707. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen M, Li H, Xu X, Bao X, Xue L, Ai X, Xu
J, Xu M, Shi Y, Zhen T, et al: Identification of RAC1 in promoting
brain metastasis of lung adenocarcinoma using single-cell
transcriptome sequencing. Cell Death Dis. 14:3302023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abbosh C, Frankell AM, Harrison T,
Kisistok J, Garnett A, Johnson L, Veeriah S, Moreau M, Chesh A,
Chaunzwa TL, et al: Tracking early lung cancer metastatic
dissemination in TRACERx using ctDNA. Nature. 616:553–562. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Qi H, Zhang Y, Sun H, Dong J and
Wang H: PLPP2: Potential therapeutic target of breast cancer in
PLPP family. Immunobiology. 227:1522982022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun L, Gao M, Qian Q, Guo Z, Zhu P, Wang X
and Wang H: Triclosan-induced abnormal expression of miR-30b
regulates fto-mediated m(6)A methylation level to cause lipid
metabolism disorder in zebrafish. Sci Total Environ.
770:1452852021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Miao Z, Qin X, Yang Y, Wu S, Miao
Q and Li B, Zhang M, Wu P, Han Y and Li B: Transcriptomic landscape
based on annotated clinical features reveals PLPP2 involvement in
lipid raft-mediated proliferation signature of early-stage lung
adenocarcinoma. J Exp Clin Cancer Res. 42:3152023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, van der Zon G, Ma J, Mei H,
Cabukusta B, Agaser CC, Madunić K, Wuhrer M, Zhang T and Ten Dijke
P: ST3GAL5-catalyzed gangliosides inhibit TGF-β-induced
epithelial-mesenchymal transition via TβRI degradation. EMBO J.
42:e1105532023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang N, Zhang X, Liu K, Qian Y, Dai Y,
Song J, Zheng Y and Ye M: Roles of ERK/JNK in carbon black induced
AP-1 cell signaling pathway changes. Wei Sheng Yan Jiu. 50:533–538.
2021.(In Chinese). PubMed/NCBI
|
|
13
|
Qiu Q, Yu X, Chen Q and He X: Sema3A
inactivates the ERK/JNK signalling pathways to alleviate
inflammation and oxidative stress in lipopolysaccharide-stimulated
rat endothelial cells and lung tissues. Autoimmunity.
56:22009082023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Yang Z, Wang S, Wang X and Mao J:
Targeting MAPK-ERK/JNK pathway: A potential intervention mechanism
of myocardial fibrosis in heart failure. Biomed Pharmacother.
173:1164132024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Cao X, Zhao H, Guo M, Fang X, Li K,
Qin L, He Y and Liu X: Hypoxia Activates Notch4 via ERK/JNK/P38
MAPK signaling pathways to promote lung adenocarcinoma progression
and metastasis. Front Cell Dev Biol. 9:7801212021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du X, Xue Z, Lv J and Wang H: Expression
of the Topoisomerase II Alpha (TOP2A) gene in lung adenocarcinoma
cells and the association with patient outcomes. Med Sci Monit.
26:e9291202020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Cai X and Lin J, Wu Q, Zhang K,
Lin Y, Liu B and Lin J: A novel five-gene metabolism-related risk
signature for predicting prognosis and immune infiltration in
endometrial cancer: A TCGA data mining. Comput Biol Med.
155:1066322023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Tao Y, Chen Y, Wu Y, He Y, Yin S, Xu
S and Yu Y: Development of a metabolism-related signature for
predicting prognosis, immune infiltration and immunotherapy
response in breast cancer. Am J Cancer Res. 12:5440–5461.
2022.PubMed/NCBI
|
|
21
|
Liu K, Lu R, Zhao Q, Du J, Li Y, Zheng M
and Zhang S: Association and clinicopathologic significance of
p38MAPK-ERK-JNK-CDC25C with polyploid giant cancer cell formation.
Med Oncol. 37:62019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xin W, Zhang J, Zhang H, Ma X, Zhang Y, Li
Y and Wang F: CLCA2 overexpression suppresses
epithelial-to-mesenchymal transition in cervical cancer cells
through inactivation of ERK/JNK/p38-MAPK signaling pathways. BMC
Mol Cell Biol. 23:442022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng Y, Shen Y, Fang Q, Duan S, Wang Y,
Dai X and Chen Y: Identification of epithelial-mesenchymal
transition-related biomarkers in lung adenocarcinoma using
bioinformatics and lab experiments. Aging (Albany NY).
15:11970–11984. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du R, Shen W, Liu Y, Gao W, Zhou W, Li J,
Zhao S, Chen C, Chen Y, Liu Y, et al: TGIF2 promotes the
progression of lung adenocarcinoma by bridging EGFR/RAS/ERK
signaling to cancer cell stemness. Signal Transduct Target Ther.
4:602019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Chen X and Yi X: The effects of A549
and H1299 cell-derived exosomes on the proliferation and apoptosis
of BEAS-2B cells. Pharmazie. 76:379–387. 2021.PubMed/NCBI
|
|
26
|
Xu Y, Jin Y, Gao S, Wang Y, Qu C, Wu Y,
Ding N, Dai Y, Jiang L and Liu S: Prognostic signature and
therapeutic value based on membrane lipid biosynthesis-related
genes in breast cancer. J Oncol. 2022:72044152022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang J, Liao Y, He S, Shi J, Peng L, Xu X,
Xie F, Diao N, Huang J, Xie Q, et al: Autocrine parathyroid
hormone-like hormone promotes intrahepatic cholangiocarcinoma cell
proliferation via increased ERK/JNK-ATF2-cyclinD1 signaling. J
Transl Med. 15:2382017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee YS, Kim SY, Song SJ, Hong HK, Lee Y,
Oh BY, Lee WY and Cho YB: Crosstalk between CCL7 and CCR3 promotes
metastasis of colon cancer cells via ERK-JNK signaling pathways.
Oncotarget. 7:36842–36853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo M, Zhang M, Cao X, Fang X, Li K, Qin
L, He Y, Zhao J, Xu Y, Liu X and Li X: Notch4 mediates vascular
remodeling via ERK/JNK/P38 MAPK signaling pathways in hypoxic
pulmonary hypertension. Respir Res. 23:62022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kciuk M, Gielecińska A, Budzinska A,
Mojzych M and Kontek R: Metastasis and MAPK Pathways. Int J Mol
Sci. 23:38472022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allam EA, Ibrahim HF, Abdulmalek SA,
Abdelmeniem IM and Basta M: Coenzyme Q(10) alleviates testicular
endocrine and spermatogenic dysfunction induced by high-fat diet in
male Wistar rats: Role of adipokines, oxidative stress and
MAPK/ERK/JNK pathway. Andrologia. 54:e145442022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Yuan X, Wang B and Gao F: Icariin
alleviates transforming growth factor-β1-induced
epithelial-mesenchymal transition by targeting Smad and MAPK
signaling pathways. Am J Transl Res. 12:343–360. 2020.PubMed/NCBI
|
|
33
|
Gao M, Lai K, Deng Y, Lu Z, Song C, Wang
W, Xu C, Li N and Geng Q: Eriocitrin inhibits
epithelial-mesenchymal transformation (EMT) in lung adenocarcinoma
cells via triggering ferroptosis. Aging (Albany NY).
15:10089–10104. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
García-Cuellar CM, Santibáñez-Andrade M,
Chirino YI, Quintana-Belmares R, Morales-Bárcenas R,
Quezada-Maldonado EM and Sánchez-Pérez Y: Particulate Matter
(PM(10)) Promotes Cell Invasion through Epithelial-Mesenchymal
Transition (EMT) by TGF-β Activation in A549 Lung Cells. Int J Mol
Sci. 22:126322021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang C, Jing X, Wu Q and Ding K: Novel
pectin-like polysaccharide from Panax notoginseng attenuates renal
tubular cells fibrogenesis induced by TGF-β. Carbohydr Polym.
276:1187722022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Li S, Luo H, Lu Q and Yu S: PCSK9
promotes the progression and metastasis of colon cancer cells
through regulation of EMT and PI3K/AKT signaling in tumor cells and
phenotypic polarization of macrophages. J Exp Clin Cancer Res.
41:3032022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batzorig U, Wei PL, Wang W, Huang CY and
Chang YJ: Glucose-Regulated protein 94 mediates the proliferation
and metastasis through the regulation of ETV1 and MAPK pathway in
colorectal cancer. Int J Med Sci. 18:2251–2261. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin X, Zhou L, Han F, Han J, Zhang Y, Sun
Z, Zhao W, Wang Z and Zheng L: Beta-adrenoceptor Activation by
Norepinephrine Enhances Lipopolysaccharide-induced Matrix
Metalloproteinase-9 Expression Through the ERK/JNK-c-Fos Pathway in
Human THP-1 Cells. J Atheroscler Thromb. 24:55–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manios K, Tsiambas E, Stavrakis I,
Stamatelopoulos A, Kavantzas N, Agrogiannis G and C Lazaris A:
c-Fos/c-Jun transcription factors in non-small cell lung carcinoma.
J BUON. 25:2141–2143. 2020.PubMed/NCBI
|
|
40
|
Wang Q, Li Z, Wang D, Yang S and Feng Y:
Myocardial protection properties of parishins from the roots of
Gastrodia elata Bl. Biomed Pharmacother. 121:1096452020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu S, Dong X, Gao W, Stupack D, Liu Y,
Xiang R and Li N: Alternative promotion and suppression of
metastasis by JNK2 governed by its phosphorylation. Oncotarget.
8:56569–56581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Bai F, Wang Y, Wang C, Chan HL,
Zheng C, Fang J, Zhu WG and Pei XH: Loss of function of GATA3
regulates FRA1 and c-FOS to activate EMT and promote mammary
tumorigenesis and metastasis. Cell Death Dis. 14:3702023.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu H, Xu WH, Ren F, Wang J, Wang HK, Cao
DL, Shi GH, Qu YY, Zhang HL and Ye DW: Prognostic value of
epithelial-mesenchymal transition markers in clear cell renal cell
carcinoma. Aging (Albany NY). 12:866–883. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Na TY, Schecterson L, Mendonsa AM and
Gumbiner BM: The functional activity of E-cadherin controls tumor
cell metastasis at multiple steps. Proc Natl Acad Sci USA.
117:5931–5937. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei J, Wu L, Yang S, Zhang C, Feng L, Wang
M, Li H and Wang F: E-cadherin to N-cadherin switching in the
TGF-β1 mediated retinal pigment epithelial to mesenchymal
transition. Exp Eye Res. 220:1090852022. View Article : Google Scholar : PubMed/NCBI
|