Introduction
Soft tissue sarcomas (STSs) are rare tumors
representing <1% of all adult malignancies and accounting for at
least 11,410 new diagnoses each year (1). The most common primary sites are the
extremities, followed by the trunk, internal trunk (such as the
retroperitonium), pelvic cavity, intra-abdominal region, and the
head and neck (1). Detection of STS
is easier in cases where the tumor is superficial. However, the
early diagnosis of a deep STS may be difficult, since a number of
these tumors present as a painless mass (2,3). As a
result, numerous STSs are diagnosed at a late stage and have
already reached a large size at the time of presentation.
High-grade STS resection with an adequate wide margin reportedly
inhibits local tumor recurrence and improves patients prognosis
compared with a marginal or intra-lesional margin (4). Patients with larger tumors are in
greater risk of requiring an amputation as the primary treatment
strategy and have a higher incidence of postoperative surgical
wound complications, as well as mortality and tumor recurrence
(2,3,5–11). In addition, patients with high-grade
STS may have a higher wound complication rate. This is due to a
larger incision, large defect of soft tissue, which is a result of
a wider margin of tissue requiring removal, prolonged surgery
duration and other factors (5–9). In
addition to tumor size, tumor depth and tumor histological grade
have also been demonstrated to be predictive factor for survival
(10,11). Therefore, patients with high-grade,
deep and large STS must be carefully treated and followed-up.
Only a limited number of studies exist that report
detailed surgical and clinical outcome in such patients (2,6).
Therefore, the aim of the present study was to elucidate the
incidence of surgical complications and the survival in patients
with large and deep high-grade STS. Furthermore, the
disease-specific and event-free survival rates were examined in
these patients.
Patients and methods
Patients
Between January 1999 and March 2013, a total of 30
adult patients suffering from primary high-grade deep STS with a
size of 10 cm or greater were treated at the Mie University
Hospital (Tsu, Japan). A deep tumor was defined as a tumor with any
of following characteristics: Located exclusively beneath the
superficial fascia; superficial to the fascia, with invasion of or
through the fascia; or both superficial to and beneath the fascia
(12). All 30 patients underwent
surgical resection of the tumor. Patients that presented with
metastases and/or local recurrence at the diagnosis, as well as
patients with retroperitoneal sarcomas, were excluded from the
present study. The pre-treatment work-up included brain, lung,
abdomen and pelvic computed tomography scans with and without the
use of a contrast medium. The histopathological diagnosis and tumor
grade were determined using the French Federation of Cancer Centers
Sarcoma Group (FNCLCC) grading system (13) for all the patients; the grading was
reviewed and confirmed by two independent pathologists. In
addition, the surgical margin was microscopically evaluated. The
study was approved by the Ethics Committee of Mie University
Hospital. Written informed consent was obtained from the
patients.
Statistical analysis
Statistical associations of the clinicopathological
factors were evaluated using the Mann-Whitney U-test for
quantitative data, and the χ2 test or Fisher's exact
test for qualitative data. The duration of disease-specific or
event-free survival was defined as the interval between the date of
the initial treatment of the primary tumor and the date of
mortality, local recurrence or metastasis. Survival curves were
constructed using the Kaplan-Meier method. The log-rank test was
used to compare the survival and event rates. Statistical analyses
were performed using the Stat View version 5.0 software (SAS
Institute, Cary, NC, USA). A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient, tumor and treatment
characteristics
Table I reports the
characteristics and treatments of patients included in the present
study. The mean age at diagnosis was 62 years (range, 24–86 years),
while 20 male and 10 female patients were included. The mean
interval between the onset of symptoms and the histological
diagnosis was 1–50 months, with a median period of 5.5 months. In
total, 11 patients experienced a delay in symptom onset of >6
months. The main symptoms resulting in the patients consulting the
Mie University Hospital were as follows: Increased size of the mass
(n=12); awareness of the mass (n=7); and pain (n=13). In addition,
2 patients experienced a combination of two clinical symptoms,
which prompted them to consult our hospital. The mean tumor size at
diagnosis was 15.1 cm (range, 10–30 cm). According to the FNCLCC
grading system, 19 patients had grade 3 sarcomas and 11 patients
had grade 2 tumors. Furthermore, the tumors were histologically
classified as 9 cases of malignant fibrous
histiocytoma/undifferentiated pleomorphic sarcoma, 5 cases of
myxoid liposarcoma, 4 cases of malignant peripheral nerve sheath
tumor, 3 cases of leiomyosarcoma, 2 cases of myxofibrosarcoma, 2
cases of malignant granular cell tumor, 2 cases of fibrosarcoma,
and 1 case each of extra-skeletal chondrosarcoma, dedifferentiated
liposarcoma and epithelioid sarcoma. The primary tumor sites
included the thighs (n=18), buttocks (n=7), chest wall (n=2) and
other sites (n=3). Finally, the mean follow-up period after the
date of the initial treatment was 40 months (range, 6–148
months).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Patients, n=30 |
|---|
| Age (years) |
|
| Mean | 62 |
|
Range | 24–86 |
| Gender (n) |
|
| Male | 20 |
|
Female | 10 |
| Tumor size (cm) |
|
| Mean | 15.1 |
|
Range | 10–30 |
| Surgical procedure
(n) |
|
|
Amputation | 2 |
| Limb
salvage surgery | 28 |
|
Resection | 20 |
|
Resection and
prosthesis | 5 |
|
Resection and
mesh | 1 |
|
Resection and
intramedullary nail | 3 |
| Surgical margin
(n) |
|
|
Positive | 7 |
|
Negative | 23 |
Surgical treatment and wound
complications
In total, 28 of the 30 patients underwent limb
salvage surgery and 2 patients underwent amputation of the primary
sarcoma (Table I). The surgical
complications experienced by the patients are listed in Table II, while Table III reports the association between
the complications and patient characteristics.
 | Table II.Type of surgical wound
complication. |
Table II.
Type of surgical wound
complication.
| Surgical
complication | Patients
(n)a |
|---|
| Infection | 5 |
| Hematoma | 3 |
| Wound dehiscence | 10 |
| Lymphorrhea | 1 |
| None | 14 |
 | Table III.Association between wound
complications and patient characteristics. |
Table III.
Association between wound
complications and patient characteristics.
|
| Complication |
|
|---|
|
|
|
|
|---|
| Variables | Yes | No | P-valuea |
|---|
| Age (years) |
|
|
|
| Mean
age | 65 | 58 | 0.15 |
| Gender (n) |
|
|
|
| Male | 11 | 9 | 0.80 |
|
Female | 5 | 5 |
|
| Intraoperative blood
loss (ml) |
|
|
|
| Mean | 1,310 | 659 | 0.07 |
| Surgery duration
(min) |
|
|
|
| Mean | 264 | 179 | 0.04 |
| Reconstruction after
surgery (n) |
|
|
|
| Yes | 3 | 4 | 0.67 |
| Tumor resection
(n) |
|
|
|
| No | 13 | 10 |
|
| Neo-adjuvant Cx
(n) |
|
|
|
| Yes | 4 | 6 | 0.44 |
| No | 12 | 8 |
|
| Radiation therapy
(n) |
|
|
|
| Yes | 4 | 12 |
|
| No | 2 | 12 | 0.66 |
| Tumor grade (n) |
|
|
|
| Grade
2 | 7 | 4 |
|
| Grade
3 | 9 | 10 | 0.39 |
Of the 30 patients included in the present study, 16
(53%) developed a total of 19 wound complications (Table II). There were 10 cases of wound
dehiscence, 5 cases of infections, 3 cases of hematomas and 1 case
of lymphorrhea. No patients succumbed to these surgical
complications. Additional surgical treatment was performed in 3
patients with postoperative infections, while 2 patients with
postoperative hematomas underwent puncture.
Reconstruction subsequent to tumor resection was
required in 6 patients (Table III),
whereas prosthesis following tumor resection, including a part of
the femur, was required in 5 patients due to suspected bone
invasion based on the pre-treatment examination. For skeletal
reconstruction after tumor resection of normal muscle and rib
attached to the tumor at the chest wall, a prosthetic mesh was used
in 1 case. The mean intraoperative blood loss was 1,006 ml (range,
68–3,170 ml) and intraoperative blood transfusion was required in
12 patients (40%). The mean operative time was 224 min (range,
78–689 min). Histologically, a positive surgical margin was
observed in 7 patients, while a negative surgical margin was
identified in 23 patients (Table
I).
To prevent a fracture following brachytherapy, an
intramedullary nail was inserted at the femur in 3 patients. In
addition, 10 of the 30 patients received neoadjuvant and/or
adjuvant chemotherapy, 4 underwent brachytherapy and 2 received
postoperative radiotherapy.
The results of the statistical analysis indicated
that longer surgery duration was associated with wound
complications (P=0.04, Mann-Whitney U test). However, blood loss
(P=0.07, Mann-Whitney U test) and administration of adjuvant
therapy (chemotherapy or radiotherapy; P=0.70, χ2 test)
was not associated with wound complications. By contrast, the 2
patients treated with amputation experienced no complications.
Clinical outcome in patients with
high-grade STS
Of the 30 patients included in the present study, 12
were alive and disease-free in March 2014 (last review), while 3
patients were alive with disease. However, 13 patients succumbed to
the disease and 2 due to other causes; the average survival time
after the treatment in these 13 patients was 22.8 months (range,
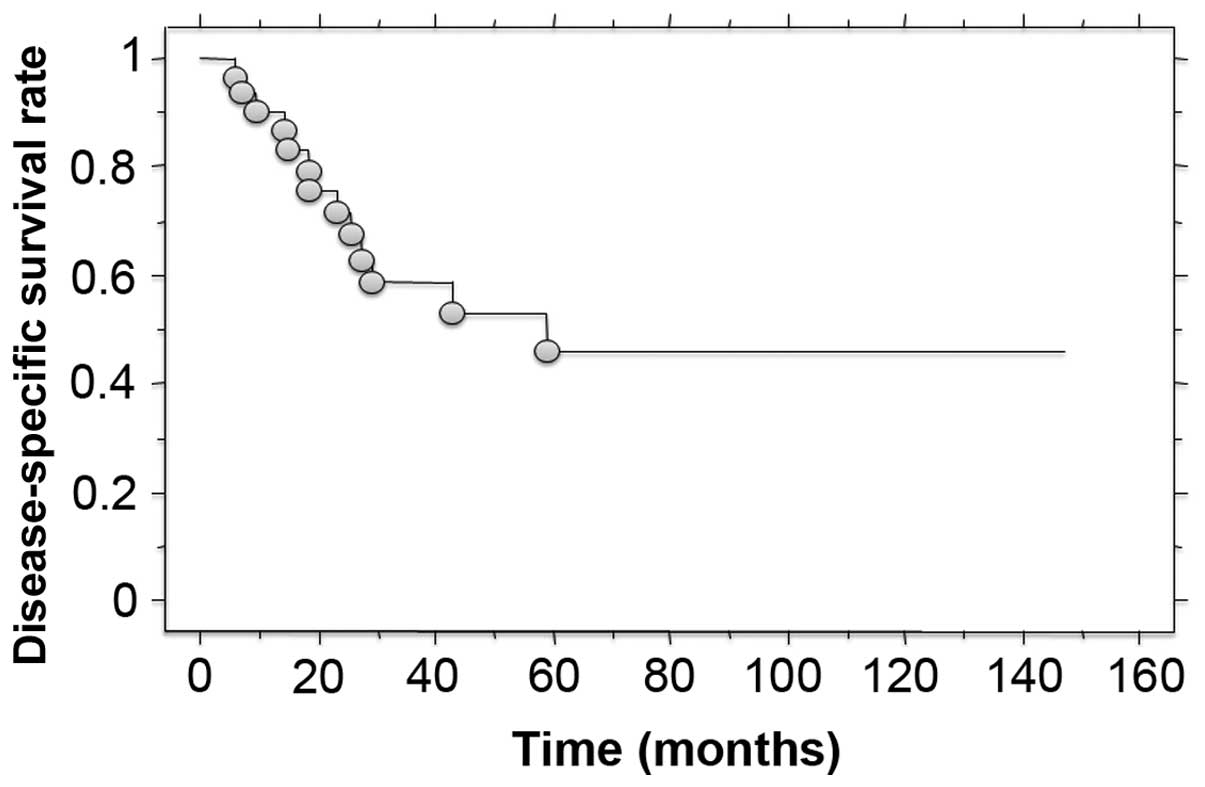
6–59 months) The disease-specific survival was 58.5% at 3 years and
46.1% at 5 years after treatment (Fig.
1). Local recurrence was detected in 13 patients, and distant
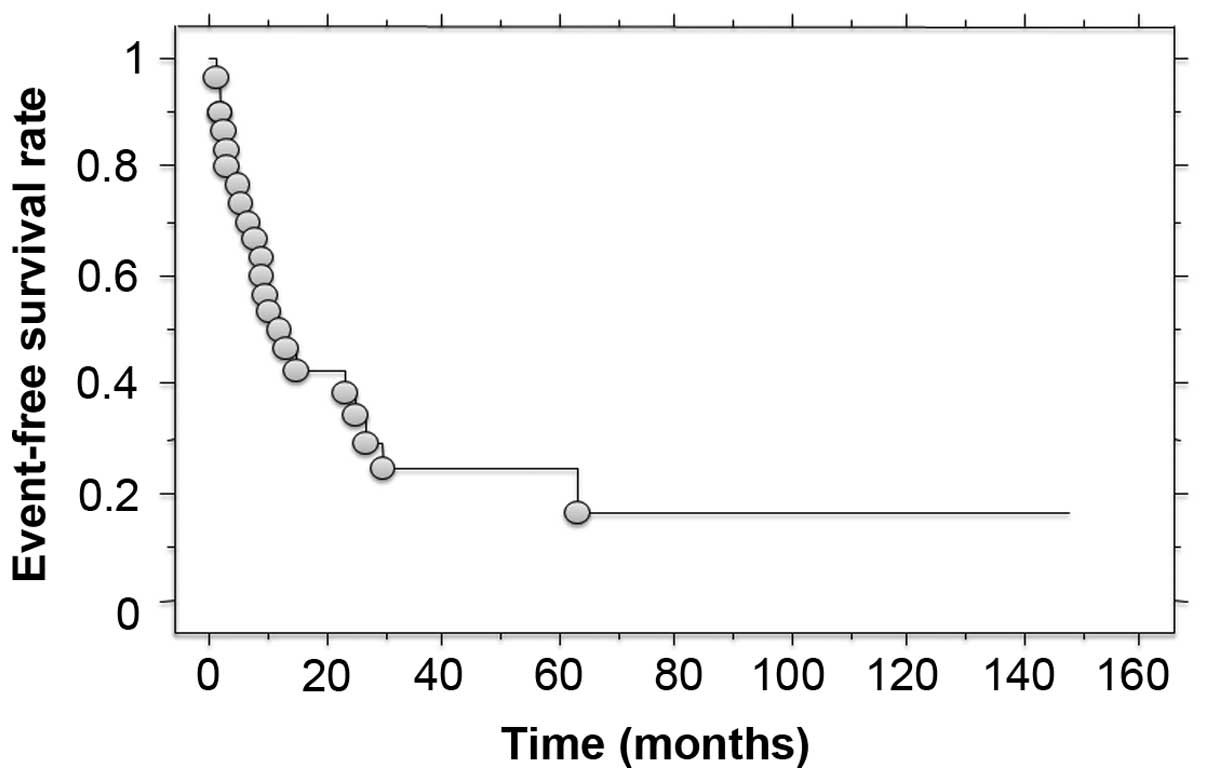
metastasis was identified in 17 patients as the first relapse. The
3- and 5-year event-free survival rates were 24.4 and 16.3%,
respectively (Fig. 2). Of the 28
patients treated with limb salvage surgery, 3 patients eventually
underwent amputation due to local recurrence. The log-rank test
revealed no statistically significant association between
disease-specific or event-free survival, and the following factors:
patient age and gender, tumor histological grade and administration
of adjuvant therapy.
Discussion
The early diagnosis of deep STS may be difficult,
particularly in tumors located at the thigh or buttock due to the
muscle volume of these areas (2,3).
Furthermore, numerous of these tumors present as a painless mass
(2,3).
Surgery continues to play an important role in the treatment of
soft tissue sarcoma, which can involve local resection and
subsequent reconstruction of the resultant deficit, or amputation.
However, particularly in large tumors, surgery for STS is
associated with several risk factors of surgical wound
complications, including a large defect following tumor resection,
blood loss, the administration of adjuvant therapy and the
requirement for prosthetic reconstruction following tumor
resection, such as bone reconstruction (5–9). In the
current study, 53% of patients with large and deep, high-grade STS
presented wound complications, and the surgery duration was found
to be significantly associated with the incidence of these
complications. Although a high incidence of wound complications was
observed, there were no fatal complications among the investigated
cases. Therefore, taking into account the risk of complications and
subsequent treatments, a large number of these patients were able
to recover.
While multiple factors affect the survival following
STS treatment, the tumor size, depth and histological grade are
certainly the most important factors (2,3,10,11). Weitz
et al (11) reported that the
5-year disease-specific survival rate was 51% for high-risk
patients (high-grade, deep tumors, with a size of >10 cm). In
the cases examined in the present study, the disease-specific
survival rate was 58.5% at 3 years and 46.1% at 5 years after
treatment. These results suggest that these tumors have a high
potential of local recurrence and metastases. In fact, in present
study, 43 and 57% of patients developed local recurrence and
metastases, respectively. Therefore, careful follow-up should be
performed to detect early relapse for these patients. Furthermore,
since limb salvage surgery results in several complications and
risk of recurrence, it must be considered whether amputation may be
a better strategy. No predictive factors for survival and event
incidence were detected in the current cases, possibly due to the
high risk of oncological event in the patient cohort.
However, the current study presents certain
limitations. First, the presence of systemic diseases may be
associated with a higher rate of wound complications. Clinical
factors, including medical comorbidities, obesity, smoking and the
patients' immunocompetence, were not considered in the study due to
the lack of information. Furthermore, the retrospective nature of
the study is another limitation.
In conclusion, surgical wound complications occurred
in 53% of patients with high-grade STS in the present study.
Therefore, careful wound care is required in these patients.
Furthermore, the fact that these patients are at a greater risk of
tumor-associated events and mortality should be considered during
treatment.
References
|
1
|
Kneisl JS, Coleman MM and Raut CP: Outcome
in the management of adult soft tissue sarcomas. J Surg Oncol.
110:527–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grimer RJ: Size matters for sarcomas! Ann
R Coll Surg Engl. 88:519–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura T, Matsumine A, Matsubara T, et
al: The symptom-to diagnosis delay in soft tissue sarcoma influence
the overall survival and the development of distant metastasis. J
Surg Oncol. 104:771–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsubara T, Kusuzaki K, Matsumine A,
Nakamura T and Sudo A: Can a less radical surgery using
photodynamic therapy with acridine orange be equal to a wide-margin
resection? Clin Orthop Relat Res. 471:792–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saddegh MK and Bauer HC: Wound
complication in surgery of soft tissue sarcoma. Analysis of 103
consecutive patients managed without adjuvant therapy. Clin Orthop
Relat Res. 289:247–253. 1993.PubMed/NCBI
|
|
6
|
Aded R and Younge D: Surgical management
of very large musculoskeletal sarcomas. Ann N Y Acad Sci.
1138:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geller DS, Hornicek FJ, Mankin HJ and
Raskin KA: Soft tissue sarcoma resection volume associated with
wound-healing complications. Clin Orthop Relat Res. 459:182–185.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwartz A, Rebecca A, Smith A, et al:
Risk factors for significant wound complications following wide
resection of extremity soft tissue sarcomas. Clin Orthop Relat Res.
471:3612–3617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peat BG, Bell RS, Davis A, et al:
Wound-healing complications after soft-tissue sarcoma surgery.
Plast Reconstr Surg. 93:980–987. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pisters PW, Leung DH, Woodruff J, Shi W
and Brennan MF: Analysis of prognostic factors in 1,041 patients
with localized soft tissue sarcomas of the extremities. J Clin
Oncol. 14:1679–1689. 1996.PubMed/NCBI
|
|
11
|
Weitz J, Antonescu CR and Brennan MF:
Localized extremity soft tissue sarcoma: Improved knowledge with
unchanged survival over time. J Clin Oncol. 21:2719–2725. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB, Byrd DR, Compton CC, et al: Soft
tissue sarcomaAJCC Cancer Staging Manual. 7th. Springer; New York,
NY: pp. 345–355. 2010
|
|
13
|
Trojani M, Contesso G, Coindre JM, et al:
Soft-tissue sarcomas of adults; study of pathological prognostic
variables and definition of a histopathological grading system. Int
J Cancer. 33:37–42. 1984. View Article : Google Scholar : PubMed/NCBI
|