Introduction
Osteosarcoma (OS) is the most common type of primary
malignant bone tumor diagnosed in children and adolescents
(1). Despite advancements in
multi-agent chemotherapies and surgical techniques, the prognosis
for patients with OS remains poor, owing to its high recurrence
rate and metastatic potential (2,3). The
precise mechanisms underlying OS carcinogenesis and progression
remain largely unknown. Therefore, the elucidation of the
mechanisms that mediate the initiation and progression of OS
carcinogenesis, and an exploration of potential therapeutic agents
are urgently required (4).
MicroRNAs (miRNAs) are a class of small non-coding
regulatory RNAs (19–25 nucleotides) that serve roles essential for
diverse biological processes, including cellular proliferation,
migration, invasion and apoptosis (5–8). miRNAs
usually bind imperfectly to the 3′-untranslated regions (3′-UTRs)
of target mRNAs. Previous studies have demonstrated that miR-544
serves pivotal roles in various types of cancer. miR-544 was
reported to suppress proliferation, invasion and migration, and to
induce cell apoptosis, in glioma by targeting
Parkinsonism-associated deglycase (9). miR-544 was demonstrated to act as an
oncogene in gastric cancer by repressing Iroquois homeobox 1
(10). However, to the best of our
knowledge, the role of miR-544 in OS has not yet been reported. In
the present study, we detected miR-544 expression in human OS
tissues and cell lines, the biological effects and potential
mechanisms of miR-544 in OS were investigated.
Materials and methods
Cell culture
Human OS cell lines, U2-OS, SAOS-2, MG-63 and
SOSP-9607, and the human osteoblast cell line h-FOB, were purchased
from the Cell Bank of Chinese Academy of Sciences (Shanghai, China)
and grown in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and 100 U/ml penicillin/streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.). Cell lines were cultured in a humidified
incubator at 37°C in an atmosphere containing 5%
CO2.
Clinical specimens
A total of 8 pairs of human OS tissues and
tumor-adjacent normal tissues (TATs) were obtained from 8 patients
(4 females and 4 males; age range, 31–55) with OS at the Department
of Orthopedics, Guangzhou First People's Hospital (Guangzhou,
China) from August 2014 to October 2015. The present study was
approved by the Ethics Committee of Guangzhou First People's
Hospital (Guangzhou, China). OS diagnosis was confirmed
pathologically by 2 pathologists independently. Informed consent
was obtained from all patients for tissue collection during
surgery. Tissues were immediately frozen in liquid nitrogen, and
stored at −80°C until use.
Plasmids, small interfering (si)RNA
and transfection
miR-544 mimics (HmiR0234), miR-544 inhibitor
(miR-544-in, HmiR-AN0623), and the negative control miRNAs
(CmiR0001 and CmiR-AN0001) were purchased from GeneCopoeia, Inc.,
(Rockville, MD, USA), and each miRNA (30 nM) was transfected into
OS cells using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The time interval between transfection and subsequent
experimentation was 48 h.
AXIN2-siRNA (5′-GCAGAGGGACAGGAATCAT-3′) and the
negative control siRNA (5′-GCAGGGACAAGGTAGACAT-3′) were purchased
from Qiagen, Inc., (Valencia, CA, USA). Transfection with 50 nM
siRNA was performed using Lipofectamine® 2000 reagent,
according to the manufacturer's protocol. The time interval between
transfection and subsequent experimentation was 48 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, was extracted from OS
cells and clinical tissues using TRIzol® (Life
Technologies; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. miRNA was converted to cDNA using a
TaqMan® miRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The expression levels
of miR-544 (HmiRQP0623, GeneCopoeia™, Guangzhou, China)
were quantified using primers within a miRNA-specific
TaqMan® miRNA assay kit (Applied Biosystems; Thermo
Fisher Scientific, Inc), according to the manufacturer's protocol.
PCR was performed to detect the expression of Cyclin D1 (CCND1,
HQP016204, GeneCopoeia™, Guangzhou, China) and c-Myc
(HQP011597, GeneCopoeia™, Guangzhou, China using the ABI
7500 Fast Real-Time PCR system (Thermo Fisher Scientific, Inc.)
with SYBR Green Mix Taq kit (Takara Bio, Inc., Otsu, Shiga, Japan).
The thermocycling conditions were as follows: At 95°C for 30 sec,
followed by 40 cycles of amplification at 95°C for 5 sec, at 59°C
for 30 sec and at 72°C for 30 sec. U6 (HmiRQP9001,
GeneCopoeia™, Guangzhou, China) and GAPDH (HQP064347,
GeneCopoeia™, Guangzhou, China) served as internal
controls for the miRNA and mRNA assays, respectively. Expression
was quantified using the 2−ΔΔCq method (11).
MTT assay
Transfected SOSP-9607 cells were seeded into 96-well
plates, in medium containing 10% FBS, at a density of
1×103 cells/well. The cells were stained with 20 µg MTT
dye (0.5 mg/ml; Sigma-Aldrich; Merck KGaA). The formazan crystals
formed were dissolved in 150 µl dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck KGaA) at 1, 2, 3, 4 or 5 days, and the
absorbance was recorded at 490 nm using a spectro-photometric plate
reader.
Colony formation assay
SOSP-9607 cells were seeded into 6-well plates
(1×103 cells/well) and incubated for 10 days in medium
containing 10% FBS. The colonies were stained with 0.5% crystal
violet at room temperature for 15 min following fixation in 4%
paraformaldehyde for 5 min at room temperature. The number of
colonies, each defined as a group of >50 cells, was counted per
plate.
Anchorage-independent growth
assay
SOSP-9607 cells were trypsinized, and
2×103 cells were suspended in complete medium
(Dulbecco's modified Eagle's medium supplemented with 10% FBS and
100 U/ml penicillin/streptomycin) containing 0.3% agar
(Sigma-Aldrich; Merck KGaA), and applied onto a layer of 1% agar in
complete medium in 6-well plates. Cells were incubated for 2 weeks
at 37°C prior to subjection to a colony formation assay, as
aforementioned, and cell colonies were imaged at magnification
×100. Only cell colonies >0.1 mm in diameter were counted.
MiRNA target prediction and
Dual-luciferase reporter assay
Based on the miR sequences, target genes were
predicted using TargetScan (version 3.1; http://www.targetscan.org/mamm_31/). AXIN2 was
amplified from SOSP-9607 cell cDNA and was sub-cloned into a
firefly luciferase reporter pGL3 plasmid (cat. no., GUR100013-P-2;
Guangzhou RiboBio Co., Ltd., Guangzhou, China). Cells were seeded
in triplicate in 24-well plates (5×104 cells/well) and
cultured for 24 h. pGL3-AXIN2-3L3-luciferase reporter pGL3 plasmids
were co-transfected into the cells with the pRL-TK Renilla
plasmids (Promega Corporation, Madison, WI, USA) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. After
48 h of transfection, the luciferase activities of the transformed
cells were assayed using a Dual-Luciferase Reporter assay system
(Promega Corporation), according to the manufacturer's protocol.
Firefly luciferase activity was normalized to Renilla
luciferase activity.
Western blotting
Total protein was extracted using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China), and protein concentrations were
determined with BCA Protein Assay Kit (Beyotime, Institute of
Biotechnology), accoding to the manufacturer's protocol. A total of
50 µg protein extracts were separated via 10% SDS-PAGE and
transferred onto PVDF membranes. The membranes were blocked with 5%
non-fat milk in TBS-T (20 mM Tris, pH 7.6, 137 mM NaCl, 0.05%
Tween-20) for 0.5 h at room temperature, and then incubated
overnight with anti-AXIN2 (catalog no., 2151), anti-CCND1 (catalog
no., 2978) and anti-c-Myc (catalog no., 13987) antibodies (all at a
dilution of 1:1,000; Cell Signaling Technology Inc., Danvers, MA,
USA) at 4°C overnight. α-tubulin (catalog no., 2144; 1:5,000;
Sigma-Aldrich; Merck KGaA) was used as the loading control. The
blots were then incubated for 2 h with a horseradish
peroxidase-conjugated anti-rabbit immunoglobulin secondary antibody
(cat no. P0023D; 1:5,000; Beyotime Institute of Biotechnology) at
37°C. Signals were visualized using enhanced chemiluminescence
(Thermo Fisher Scientific, Inc.) as a substrate, and images were
analyzed using an automated chemiluminescence system (LAS500; GE
Healthcare, USA) according to protocol of the manufacturer.
Statistical analysis
All data are presented as the mean ± standard
deviation, and all experiments were repeated independently at least
3 times. Statistical analyses, specifically a one-way analysis of
variance (ANOVA) or the Student's t test, were performed using SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-544 expression is upregulated in
OS cell lines and tissues
To investigate the role of miR-544 in OS
development, miR-544 expression was examined in OS cells and
clinical tissue samples. RT-qPCR analysis indicated that miR-544
expression was significantly increased in the OS cell lines, U2-OS,
SAO-2, MG-63 and SOSP-9607, compared with in the human osteoblast
h-FOB cells (Fig. 1A). Furthermore,
miR-544 expression was markedly upregulated in the OS tissues, as
compared with in the matched TATs (Fig.
1B). This suggests that miR-544 is upregulated in OS and that
it may serve a role in promoting OS development.
miR-544 promotes OS cell proliferation
and cell cycle progression
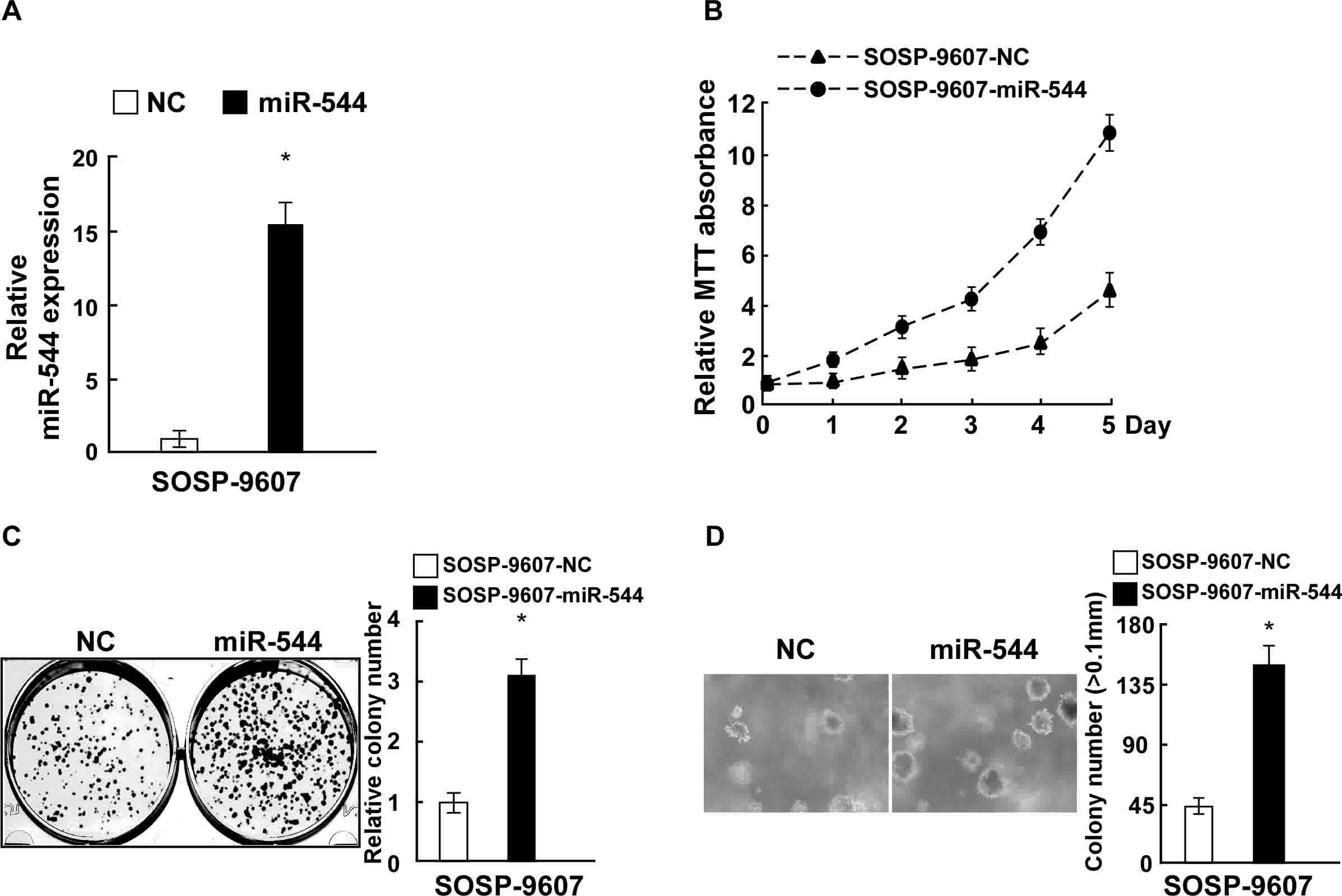
To explore the effect of miR-544 on OS cell
proliferation, SOSP-9607 cells were transfected with miR-544
mimics, a miR-544-in or the respective controls, and the expression
of miR-544 in the stably transfected SOSP-9607 cell line was
confirmed by RT-qPCR (Fig. 2A). MTT
and colony formation assays revealed that the overexpression of
miR-544 increased the proliferation of SOSP-9607 cells, as compared
with those cells transfected with negative control miRNA (Fig. 2B and C). Overexpression of miR-544 in
SOSP-9607 cells also significantly enhanced their
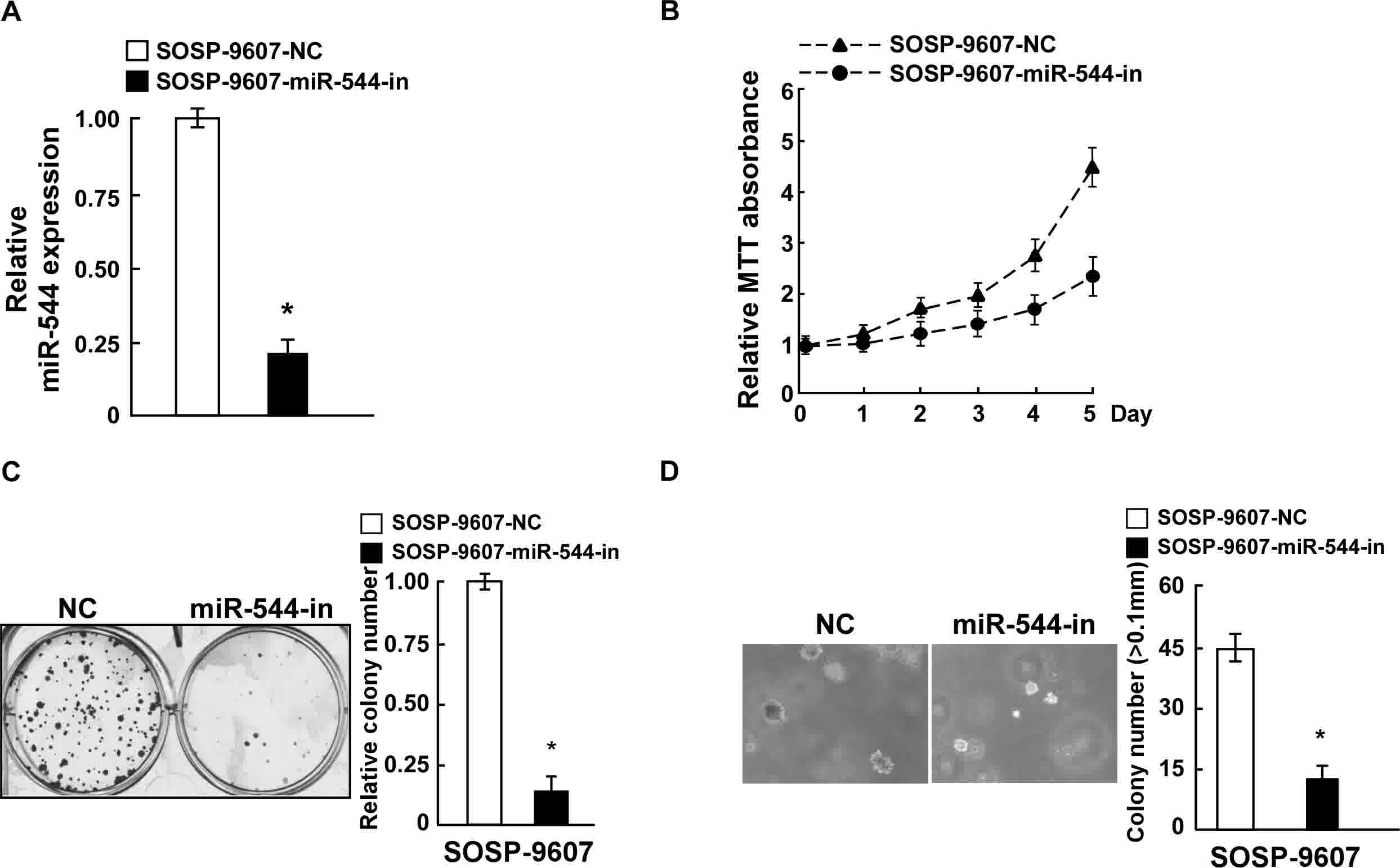
anchorage-independent growth ability (P<0.05; Fig. 2D). By contrast, SOSP-9607 cells were
transfected with miR-544-in or the respective control, and the
expression of miR-544 in the stably transfected SOSP-9607 cell line
was confirmed by RT-qPCR (Fig. 3A).
MiR544-in-transfected SOSP-9607 cells exhibited decreased
proliferation rates, colony formation ability, and
anchorage-independent cell growth ability, compared with in the
negative control cells (Fig. 3B-D).
Collectively, these data indicate that miR-544 acted as a tumor
promoter to endorse OS cell proliferation.
miR-544 directly targets AXIN2 by
binding its 3′-UTR in OS and alters the expression of
proliferation-associated proteins
According to bioinformatical predictions, AXIN2 is a
putative target gene of miR-544. To verify this prediction, WT
AXIN2 3′UTRs were generated (Fig.
4A). To determine whether miR-544 affected AXIN2 expression,
AXIN2 expression was analyzed by western blotting. Overexpression
of miR-544 inhibited the protein expression of AXIN2, while
SOSP-9607 cells transfected with miR-544-in exhibited enhanced
protein expression of AXIN2 (Fig.
4B). To investigate whether AXIN2 could be regulated by
miR-544, AXIN2 3′-UTR was co-transfected into SOSP-9607 cells with
miR-544 mimics, miR-544-in or miR-544-mut, followed by the
measurement of luciferase activity. As demonstrated in Fig. 4C, luciferase activity was markedly
reduced in cells that were co-transfected with the WT AXIN2 3′-UTR
and miR-544. By contrast, miR-544-in transfection increased
luciferase activity in cells transfected with WT AXIN2 3′-UTR.
miR-544-mut transfection did not alter the luciferase activity of
cells transfected with AXIN2 3′-UTR. These findings indicate that
miR-544 downregulated the protein expression of AXIN2 via direct
binding to the seed sequences in its 3′-UTR.
It has been reported that the Wnt signaling pathway
serves an essential role in the cancer cell cycle and proliferation
(12,13). C-Myc, CCND1, and AXIN2 are well-known
target genes of the Wnt signaling pathway (14–17). The
expression of these Wnt/β-catenin signaling pathway downstream
genes was determined in miR-544-transfected cells. Using RT-qPCR
and western blotting, it was demonstrated that the mRNA and protein
expression levels of CCND1 and c-Myc were upregulated in
miR-544-transfected SOSP-9607 cells, while transfection with
miR-544-in resulted in the opposite effect (Fig. 4D and E). This suggests that miR-544
modulated downstream genes of the Wnt signaling pathway (AXIN2,
CCND1 and c-Myc).
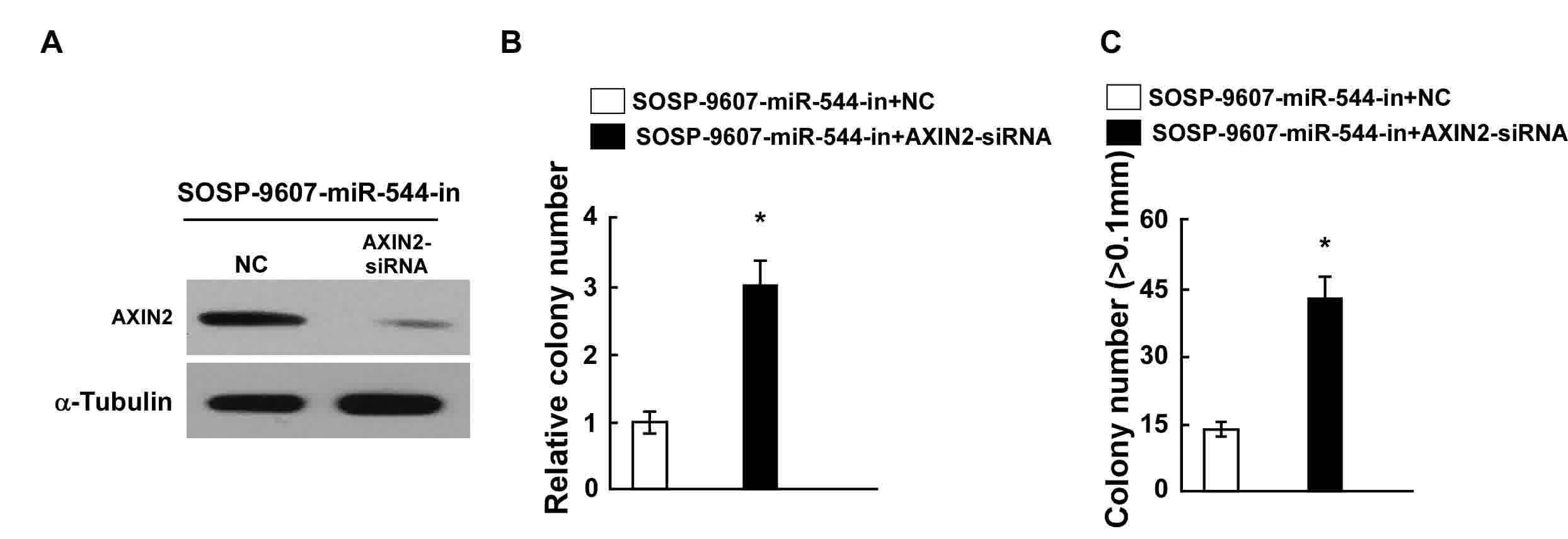
AXIN2 suppression counteracts the
proliferation arrest induced by miR-544-in
The effects of AXIN2 downregulation on proliferation
were examined in OS cells transfected with miR-544-in.
siRNA-mediated knockdown of AXIN2 was performed in
miR-544-in-transfected SOSP-9607 cells, and confirmed through
western blotting (Fig. 5A). Results
from the colony formation and anchorage-independent growth assays
indicated that the suppression of AXIN2 expression by AXIN2-siRNA
reversed the effects of miR-544-in in SOSP-9607 cells (Fig. 5B and C). Taken together, these results
demonstrated that the downregulation of AXIN2 counteracts the cell
proliferation arrest induced by miR-544-in.
Discussion
Prior studies have demonstrated that microRNAs
negatively regulate their target mRNAs in a sequence-specific
manner (18,19), to serve roles in the development of
human cancer types, including OS. miR-143 was reported to promote
the apoptosis of OS cells by targeting Bcl-2 (20). miR-99a was demonstrated to inhibit
cell proliferation by targeting TNF-α-induced protein 8 in OS cells
(21). Zhang et al (22) indicated that miR-30a regulates the
proliferation, migration and invasion of human OS by regulating
Runt-related transcription factor 2. However, to the best of our
knowledge, the role of miR-544 in OS has not been previously
investigated. miR-544 was demonstrated to suppress tumor growth in
human triple-negative breast cancer by downregulating both B-cell
CLL/lymphoma 6 and signal transducer and activator of transcription
3 (23). In the present study, it was
demonstrated that miR-544 serves a role in promoting OS cell
proliferation. miR-544 expression was increased in OS cell lines
and tissues, compared in with h-FOB cells and TATs. The
overexpression of miR-544 significantly increased OS cell
proliferation. By contrast, miR-544-in-transfection significantly
decreased cell proliferative ability, suggesting that miR-544 may
be a novel tumor promoter and serve a critical role in OS
carcinogenesis.
The results of the present study indicate that AXIN2
was a direct target gene of miR-544, and that it was implicated in
the functional effect of miR-544 on OS carcinogenesis. It has been
reported that AXIN2 is an important regulator of the Wnt/β-catenin
signaling pathway, and participates in various cellular functions
(24,25). Wei et al (26) suggested that AXIN2 expression was
downregulated in ameloblastoma, and was involved in its
tumorigenesis. Koinuma et al (27) indicated that epigenetic silencing of
AXIN2 was associated with colorectal cancer carcinogenesis. Growing
evidence suggests that AXIN2 can act as a tumor suppressor gene or
an oncogene, regulated by several miRNAs. miR-374a was reported to
promote esophageal cancer cell proliferation by suppressing AXIN2
expression (28). Additionally,
miR-107 was determined to promote hepatocellular carcinoma cell
proliferation by regulating AXIN2 (29). Similarly, Kim et al (30) indicated that miR-205 inhibited oral
carcinoma oncogenic activity by downregulating AXIN2 expression. In
the present study, the expression of CCND1 and c-Myc was
upregulated, AXIN2 expression was suppressed by miR-544, and cell
proliferation was elevated in OS. Furthermore, the knockdown of
AXIN2 in miR-544-in-transfected SOSP-9607 OS cells counteracted the
proliferation arrest induced by miR-544-in.
In conclusion, the results of the present study
demonstrated that miR-544 directly regulates AXIN2 expression and,
thus, contributes to OS tumorigenesis. This provides a novel
insight into the biology of OS, and suggests that miR-544 may be a
promising prognostic factor and therapeutic target for future OS
therapy.
Acknowledgements
Not applicable.
Funding
This work was supported by Guangdong Science and
Technology Project (2017ZC0320) and the Department of Orthopedics
of Guangzhou First People's Hospital (Guangzhou, China).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
GMZ and MC conceived and designed the experiments.
MC, YYL, MQZ and XLW performed the experiments. XHG and LC
collected the samples and analyzed the data. MC wrote the paper.
The final manuscript was also approved by all authors.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangzhou First People's Hospital (Guangzhou, China),
and informed consent was obtained from all patients for tissue
collection during surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu W, Zhu J, Wang Y, Wang J, Fang W, Xia
K, Shao J, Wu M, Liu B, Liang C, et al: A review and outlook in the
treatment of osteosarcoma and other deep tumors with photodynamic
therapy: From basic to deep. Oncotarget. 8:39833–39848.
2017.PubMed/NCBI
|
|
2
|
Nouri H, Ben Maitigue M, Abid L, Nouri N,
Abdelkader A, Bouaziz M and Mestiri M: Surface osteosarcoma:
Clinical features and therapeutic implications. J Bone Oncol.
4:115–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miao J, Wu S, Peng Z, Tania M and Zhang C:
MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects.
Tumour Biol. 34:2093–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang WL, Cao J, Xu B, Yang P, Shen F, Sun
Z, Li WL, Wang Q and Liu F: miR-892a regulated PPP2R2A expression
and promoted cell proliferation of human colorectal cancer cells.
Biomed Pharmacother. 72:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang C, Long F, Wan J, Hu Y and He H:
MicroRNA-205 acts as a tumor suppressor in osteosarcoma via
targeting RUNX2. Oncol Rep. 35:3275–3284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu ZF, Liang ZQ, Li L, Zhou YB, Wang ZB,
Gu WF, Tu LY and Zhao J: miR-335 functions as a tumor suppressor
and regulates survivin expression in osteosarcoma. Eur Rev Med
Pharmacol Sci. 20:1251–1257. 2016.PubMed/NCBI
|
|
8
|
Ma C, Zhan C, Yuan H, Cui Y and Zhang Z:
MicroRNA-603 functions as an oncogene by suppressing BRCC2 protein
translation in osteosarcoma. Oncol Rep. 35:3257–3264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin S, Dai Y, Li C, Fang X, Han H and Wang
D: MicroRNA-544 inhibits glioma proliferation, invasion and
migration but induces cell apoptosis by targeting PARK7. Am J
Transl Res. 8:1826–1837. 2016.PubMed/NCBI
|
|
10
|
Zhi Q, Guo X, Guo L, Zhang R, Jiang J, Ji
J, Zhang J, Zhang J, Chen X, Cai Q, et al: Oncogenic miR-544 is an
important molecular target in gastric cancer. Anticancer Agents Med
Chem. 13:270–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai G, Zheng D, Wang Q, Yang J, Liu G,
Song Q, Sun X, Tao C, Hu Q, Gao T, et al: Baicalein inhibits
progression of osteosarcoma cells through inactivation of the
Wnt/β-catenin signaling pathway. Oncotarget. 8:86098–86116. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng ZY, Xu XH, Cen DZ, Luo CY and Wu SB:
miR-590-3p promotes colon cancer cell proliferation via
Wnt/β-catenin signaling pathway by inhibiting WIF1 and DKK1. Eur
Rev Med Pharmacol Sci. 21:4844–4852. 2017.PubMed/NCBI
|
|
14
|
Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick
R, Hanash S, Cho KR and Fearon ER: Activation of AXIN2 expression
by beta-catenin-T cell factor. A feedback repressor pathway
regulating Wnt signaling. J Biol Chem. 277:21657–21665. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng Z, Wu T, Li Y, Xu Z, Zhang S, Liu B,
Chen Q and Tian D: MicroRNA-370-3p inhibits human glioma cell
proliferation and induces cell cycle arrest by directly targeting
β-catenin. Brain Res. 1644:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eo HJ, Park GH and Jeong JB: Inhibition of
Wnt signaling by silymarin in human colorectal cancer cells. Biomol
Ther (Seoul). 24:380–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Shen C, Li C, Yang G, Liu H, Chen
X, Zhu D, Zou H, Zhen Y, Zhang D and Zhao S: miR-577 inhibits
glioblastoma tumor growth via the Wnt signaling pathway. Mol
Carcinog. 55:575–585. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li WH, Wu HJ, Li YX, Pan HG, Meng T and
Wang X: MicroRNA-143 promotes apoptosis of osteosarcoma cells by
caspase-3 activation via targeting Bcl-2. Biomed Pharmacother.
80:8–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing B and Ren C: Tumor-suppressive
miR-99a inhibits cell proliferation via targeting of TNFAIP8 in
osteosarcoma cells. Am J Transl Res. 8:1082–1090. 2016.PubMed/NCBI
|
|
22
|
Zhang R, Yan S, Wang J, Deng F, Guo Y, Li
Y, Fan M, Song Q, Liu H, Weng Y and Shi Q: miR-30a regulates the
proliferation, migration, and invasion of human osteosarcoma by
targeting Runx2. Tumour Biol. 37:3479–3488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Z, Wang S, Zhu J, Yang Q, Dong H and
Huang J: MicroRNA-544 down-regulates both Bcl6 and Stat3 to inhibit
tumor growth of human triple negative breast cancer. Biol Chem.
397:1087–1095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Mastriani E, Yan ZQ, Yin SY, Zeng
Z, Wang H, Li QH, Liu HY, Wang X, Bao HX, et al: SOX7 co-regulates
Wnt/β-catenin signaling with Axin-2: Both expressed at low levels
in breast cancer. Sci Rep. 6:261362016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yanaka Y, Muramatsu T, Uetake H, Kozaki K
and Inazawa J: miR-544a induces epithelial-mesenchymal transition
through the activation of WNT signaling pathway in gastric cancer.
Carcinogenesis. 36:1363–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Z, Zhong M, Guo Y, Wang Y, Ren M and
Wang Z: Expression of β-catenin and AXIN2 in ameloblastomas.
Contemp Oncol (Pozn). 17:250–256. 2013.PubMed/NCBI
|
|
27
|
Koinuma K, Yamashita Y, Liu W, Hatanaka H,
Kurashina K, Wada T, Takada S, Kaneda R, Choi YL, Fujiwara SI, et
al: Epigenetic silencing of AXIN2 in colorectal carcinoma with
microsatellite instability. Oncogene. 25:139–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Xin H, Han Z, Sun H, Gao N and Yu
H: MicroRNA-374a promotes esophageal cancer cell proliferation via
Axin2 suppression. Oncol Rep. 34:1988–1994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JJ, Wang CY, Hua L, Yao KH, Chen JT
and Hu JH: miR-107 promotes hepatocellular carcinoma cell
proliferation by targeting Axin2. Int J Clin Exp Pathol.
8:5168–5174. 2015.PubMed/NCBI
|
|
30
|
Kim JS, Park SY, Lee SA, Park MG, Yu SK,
Lee MH, Park MR, Kim SG, Oh JS, Lee SY, et al: MicroRNA-205
suppresses the oral carcinoma oncogenic activity via
down-regulation of Axin-2 in KB human oral cancer cell. Mol Cell
Biochem. 387:71–79. 2014. View Article : Google Scholar : PubMed/NCBI
|