Introduction
Gastrointestinal stromal tumors (GIST) are the most
common mesenchymal tumors of the gastrointestinal tract. The major
genetic cause of GIST is the abnormal activation of c-KIT or
platelet-derived growth factor receptor α (PDGFRA) due to acquired
functional mutations (1). GIST
patients are usually not sensitive to traditional radiotherapy or
chemotherapy. Radical resection remains the treatment of choice,
but 40–80% of patients still develop recurrence or metastasis
(2). Imatinib is a targeted therapy
drug which inhibits tyrosine kinase (TK) activity. Imatinib has
been used to treat Philadelphia chromosome-positive chronic myeloid
leukemia (CML) and GIST patients who cannot undergo resection
and/or already have metastasis. However, it has been reported that
some patients did not respond to the drug, and some patients
developed drug resistance.
Mutations in GIST lead to aberrant activation of TK
in the absence of corresponding ligands. Signaling pathways such as
the phosphoinositide 3-kinase (PI3K)/AKT and mitogen-activated
protein kinase (MAPK) pathways are the most important signaling
pathways that activate TK abnormally (3). These pathways may be involved in the
mechanism contributing to the failure of Imatinib treatment
(4). The Sonic hedgehog (Shh)
pathway, on the other hand, is involved in the regulation of cell
differentiation and proliferation. Previously it was considered to
be highly conserved and plays a role only during embryonic
development and tissue differentiation, and becomes inhibited in
postnatal and adult tissues (5,6). Zhao
et al (7) first reported that
inhibitors of Shh signaling pathway not only reduces the spread of
wild-type BCR-ABL1 in CML, but also reduces the resistance to
Imatinib in CML tumor cells. Subsequently, several other studies
also suggested that the Shh pathway plays an important role in GIST
(8–10). Therefore, it is worth studying if Shh
pathway inhibitors may also be effective for Imatinib-resistant
GIST. In addition, we speculate that PI3K, and MAPK signaling
pathways may crosstalk with Shh for Shh inhibitor to act in
Imatinib-resistant GIST. The study objective is to investigate the
interaction among Shh, PI3K and MAPK pathways in GIST cells.
Materials and methods
Cells and major reagents
GIST-H1 cells were purchased from Shanghai Cell
Bank, Chinese Academy of Sciences. HEGF and hN-SHH were procured
from PeproTech and Sino Biological, respectively. Wortmannin and
cyclopamine (CPN)-KAAD were obtained from Gene Operation.
PD98059 is a MAPK signaling pathway inhibitor
commonly used in basic research. PD98059 can enter the cells
through facilitated diffusion and specifically inhibit the ERK
kinase MEK1, and ultimately inhibit the phosphorylation of ERK1/2
and block the MAPK signaling pathway. CPN is an isosteroid
alkaloids. In vertebrates, the SHH signaling pathway is mainly
composed of shh ligand, patched (PTC) receptor, smoothened (SMO)
transmembrane protein, and nuclear transcription factor Gli
(glioma-associated oncogene). Smo is an essential receptor for
activating hedgehog signal transduction. Studies have confirmed
that CPN can specifically antagonize Smo, so that Smo-dependent
intracellular signal transduction of tumor gene transcription and
expression was inhibited, thereby inhibiting the entire pathway
activity. P110β subunit, p85α subunit and the downstream molecules
are all important members of PI3K signaling pathway. Blocking any
one of these PI3K signaling pathways can be an option to block
PI3K. Wortmannin is considered to be a highly selective inhibitor
because it specifically binds only to the catalytic subunit P110β
in PI3K.
The following substances and antibodies were used:
MTT and mouse anti-tubulin antibody (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany); rabbit anti-p-ERK1/2 antibody and rabbit
anti-p-AKT antibody (Cell Signaling Technology, Inc., Danvers, MA,
USA); rabbit anti-AKT antibody and rabbit anti-ERK1/2 antibody
(BioWorld Technology, Inc., St. Louis Park, MN, USA); and PD 98059,
mouse anti-caspase-3 antibody, and rabbit anti-Gli-1 antibody
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Treatments
The GIST-H1 cells were induced by endothelial growth
factor (EGF) and recombinant Shh (N-shh) through the treatments of
blank control, CPN-KAAD, wortmannin, PD98059, CPN-KAAD+PD98059, and
CPN-KAAD+wortmannin. The cells were collected after 48 h, and the
expression levels of p-AKT, AKT, p-ERK1/2, ERK1/2, and Gli-1 were
detected via western blot.
Apoptosis test
One group was treated as follows: i) blank
treatment, ii) blank treatment after 48 h starvation, iii)
CPN-KAAD+wortmannin treatment after 48 h starvation, and iv)
CPN-KAAD+wortmannin treatment after 48 h starvation. The other
group was stimulated with 100 ng/ml EGF for 10 min with blank
treatment, blank treatment after 48 h starvation,
CPN-KAAD+wortmannin treatment, and CPN-KAAD+wortmannin treatment
after 48 h starvation. The caspase-3 expression was determined
through western blot.
Cell proliferation rate
The cells were divided into three groups: Group 1
was given blank treatment, blank treatment after 10 min CPN-KAAD,
EGF (or N-Shh) stimulation, or CPN-KAAD treatment after 10 min EGF
(or N-Shh) stimulation; group 2 was given PD98059 for 1 h with
blank treatment, blank treatment after 10 min CPN-KAAD, EGF (or
N-Shh) stimulation, or CPN-KAAD treatment after 10 min EGF (or
N-Shh) stimulation; and group 3 was provided wortmannin for 1 h
with blank treatment, blank treatment after 10 min CPN-KAAD, EGF
(or N-Shh) stimulation, or CPN-KAAD treatment after 10 min EGF
stimulation. Optical density was measured at 590 nm, and each
experiment was repeated twice.
Statistical analysis
SPSS 16.0 software was used for statistical
analysis. The analytical methods used were the Student's t-test and
chi-square test with P<0.05 as significance level. In
multi-group analysis, ANOVA followed by Tukey's test was used. In
the cell growth curve, time was listed in the horizontal axis and
light absorption value was assigned in the vertical axis.
Results
A number of MAPK, PI3K, and Shh signaling pathway
inhibitors were used in the study. Wortmannin can initiate specific
binding reactions with the catalytic subunit P110β in PI3K, which
can inhibit PI3K activity. PD98059 is a MAPK signaling pathway
inhibitor that inhibits the phosphorylation of ERK1/2 and blocks
the transduction of the MAPK signaling pathway. CPN is an
isosteroidal alkaloid that works specifically against Smo,
which inhibits the transcription and expression of tumor genes that
mediate the intracellular signal transduction of Smo and thus
blocks the activity of the entire pathway. CPN-KAAD, a derivative
of CPN, acts as a selective inhibitor of the Smo and hedgehog
signaling pathways and is 10–20 times more active than
CPN[2-3].
Effects of EGF
Fig. 1 shows the
relationship among PI3K, MAPK, and Shh signaling pathways under the
action of EGF p-AKT and p-ERK expression. For both p-AKT and p-ERK,
(1) The signal intensity of the
EGF-treated control group was significantly higher than that of the
non EGF-treated group (P<0.05) (2). The signal intensity between the
wortmannin-, CPN-KAAD-, and wortmannin and CPN-KAAD co-treated
groups, and the control group was significantly different
(P<0.05). The signal intensity of the CPN-KAAD-, wortmannin-,
and wortmannin+CPN-KAAD-treated groups was also lower than that of
the EGF-treated control group (P<0.05; Fig. 1A and B) (3). The signal intensity of the wortmannin-,
PD98059-, and PD98059+CPN-KAAD-treated group was significantly
lower than that of the control group (P<0.05). The signal
intensity of the PD98059-, CPN-KAAD-, and CPN-KAAD+PD98059-treated
groups was significantly lower than that of the EGF-treated control
group (P<0.05; Fig. 1C and D).
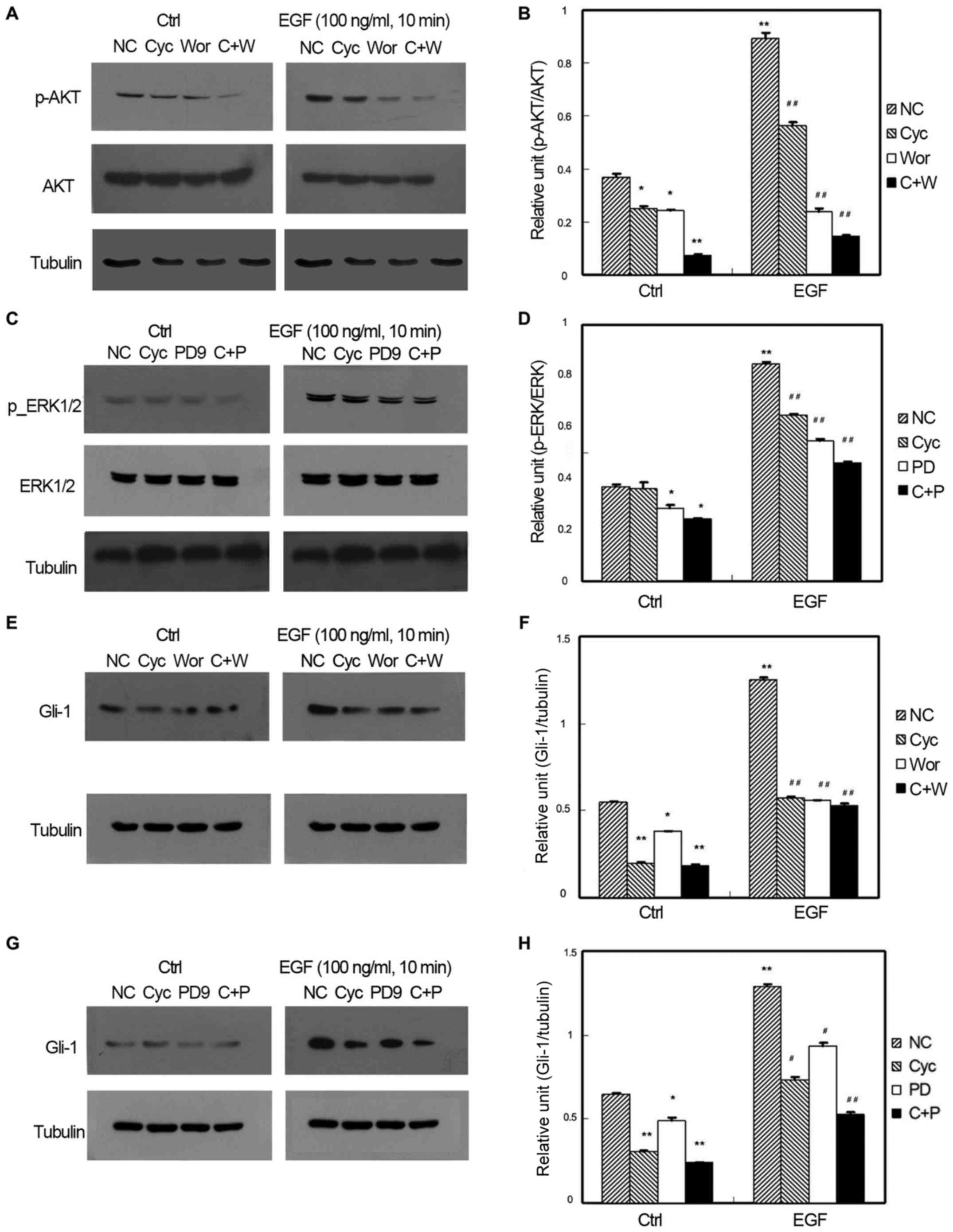 | Figure 1.Effect of N-Shh on GIST-H1 cell
signaling pathways. (A) Representative gel and quantification of
(B) the effect of N-Shh on GIST-H1 cell AKT activation and the
comparison of the effect from each treatment factor. (C)
Representative gel and quantification of (D) the effect of N-Shh on
GIST-H1 cell ERK activation and the comparison of the effect from
each treatment factor. (E) Representative gel and quantification of
(F) the effect of N-Shh on GIST-H1 cell Gli-1 expression and the
comparison of the effect from each treatment factor. (G)
Representative gel and quantification of (H) the effect of N-Shh on
GIST-H1 cell Gli-1 expression and the comparison of the effect from
each treatment factor (*P<0.05, **P<0.01 vs. ctrl-NC;
#P<0.05, ##P<0.05 vs. N-Shh-NC). N-Shh,
recombinant Sonic hedgehog; GIST, gastrointestinal stromal tumor;
EGF, endothelial growth factor; Gil-1, glioma-associated
oncogene-1; NC-normal control, Cyc-cyclopamine, PD-PD98059,
C+P-CPN-KAAD+PD98059, Wor-wortmannin, C+W- CPN-KAAD
+wortmannin. |
In Gli-1 expression, the signal intensity of the
wortmannin-treated group was slightly lower than that of the
control group (P<0.05). The signal intensity of the CPN-KAAD-
and wortmannin+CPN-KAAD-treated groups was significantly lower than
that of the control group (P<0.05). The signal intensity of the
EGF-treated group was obviously higher than that of the
non-EGF-treated group (P<0.05). In the EGF-treated group, the
signal intensity of the inhibitor-treated group was obviously lower
than that of the EGF-treated control group (P<0.05). Regardless
of EGF treatment, the signal intensity of the co-treatment group
was significantly lower than those of the wortmannin- and
CPN-KAAD-treated group (P<0.05; Fig.
1E and F). The signal intensity of the PD98059-treated group
was lower than that of the control group (P<0.05), and the
signal intensity of the CPN-KAAD- and PD98059+CPN-KAAD-treated
groups was lower than that of the control group. The signal
intensity of the EGF-treated group was significantly higher than
that of the non-EGF-treated group (P<0.05). In the EGF-treated
group, the signal intensity of the drug-treated group was
significantly lower than that of the EGF-treated group (P<0.05).
Regardless of the EGF treatment, the signal intensity of the
co-treatment, PD98059-, and CPN-KAAD-treated groups was
significantly lower than that of the control group (P<0.05;
Fig. 1G and H).
Effects of N-Shh
Fig. 2 shows the
relationship among PI3K, MAPK, and Shh signaling pathways under the
action of N-Shh. In p-AKT expression, the signal intensity of the
wortmannin-, CPN-KAAD-, and wortmannin+CPN-KAAD-treated groups was
significantly lower than that of the control group (P<0.05). The
signal intensity of the N-Shh-treated group was significantly
higher than that of the control group (P<0.05). In the
N-Shh-treated group, the signal intensity of the drug-treated group
was significantly lower than that of the N-Shh-treated control
group (P<0.05) (Fig. 2A and B). In
p-ERK expression, the signal intensity of the PD98059- and
CPN-KAAD-treated groups was lower than that of the control group
(P<0.05). The signal intensity of the PD98059+CPN-KAAD-treated
group was significantly higher than that of the control group
(P<0.05). The signal intensity of the N-Shh treatment group was
significantly higher than that of the control group (P<0.05). In
the treatment group, the signal intensity of the PD98059- and
PD98059+CPN-KAAD-treated groups was significantly lower than that
of the control group (P<0.05). The signal intensity of the
CPN-treated group was slightly lower than that of the control group
(P<0.05; Fig. 2C and D).
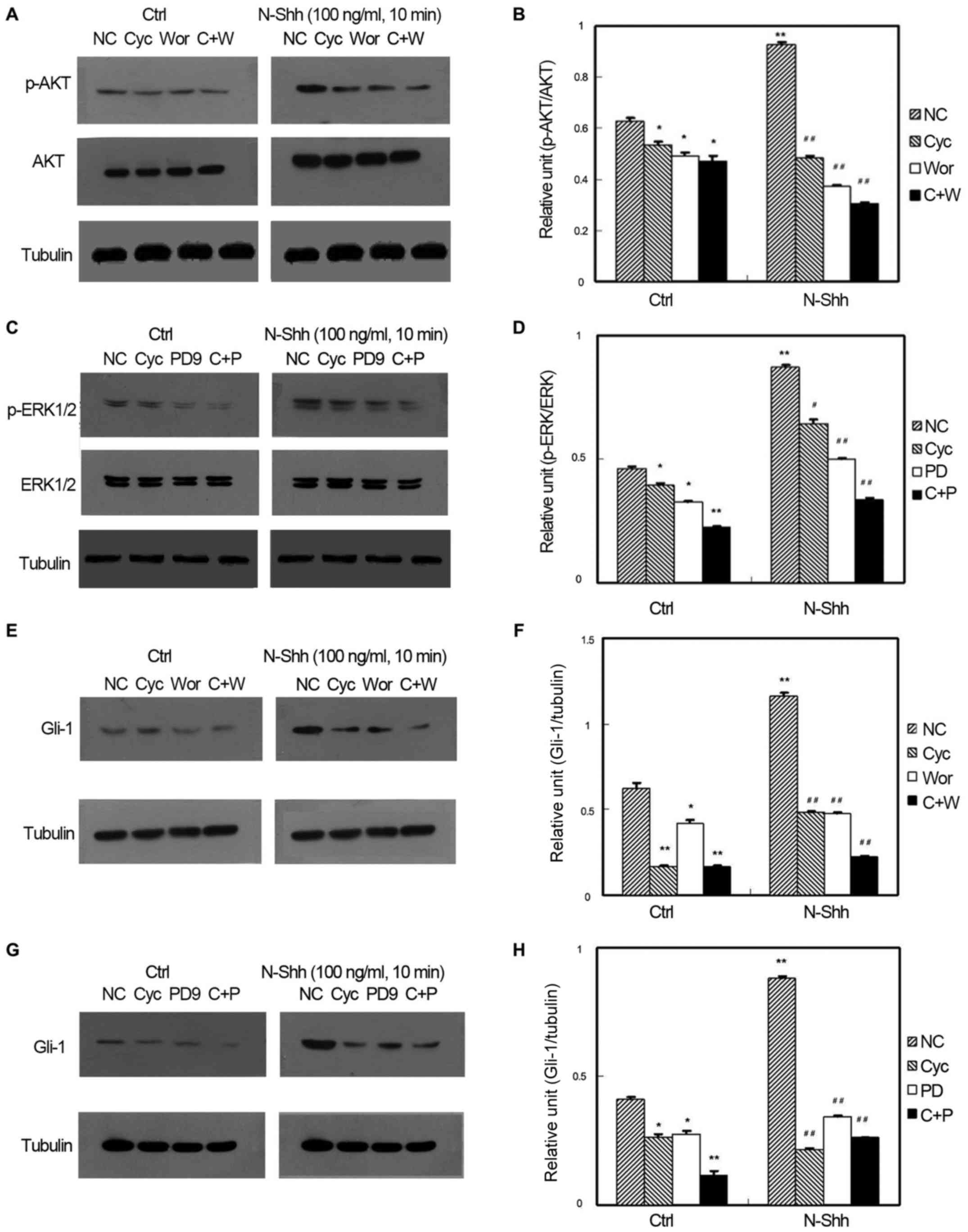 | Figure 2.Effect of N-Shh on GIST-H1 cell
signaling pathways. (A) Representative gel and quantification of
(B) the effect of N-Shh on GIST-H1 cell AKT activation and the
comparison of the effect from each treatment factor. (C)
Representative gel and quantification of (D) the effect of N-Shh on
GIST-H1 cell ERK activation and the comparison of the effect from
each treatment factor. (E) Representative gel and quantification of
(F) the effect of N-Shh on GIST-H1 cell Gli-1 expression and the
comparison of the effect from each treatment factor. (G)
Representative gel and quantification of (H) the effect of N-Shh on
GIST-H1 cell Gli-1 expression and the comparison of the effect from
each treatment factor (*P<0.05, **P<0.01 vs. ctrl-NC;
#P<0.05, ##P<0.05 vs. N-Shh-NC). N-Shh,
recombinant Sonic hedgehog; GIST, gastrointestinal stromal tumor;
EGF, endothelial growth factor; NC-normal control, Cyc-cyclopamine,
PD-PD98059, C+P-CPN-KAAD+PD98059, Wor-wortmannin, C+W-
CPN-KAAD+wortmannin. |
In Gli-1 expression induced by N-Shh and interfered
by the targeted drug of different signaling pathways, the signal
intensity of the wortmannin-treated group was lower than that of
the control group (P<0.05). The relative ratios of the
grayscales of the CPN-KAAD- and wortmannin+CPN-KAAD-treated groups
were lower than that of the control group (P<0.05). The signal
intensity of the N-Shh-treated group was significantly higher than
that of the non-N-Shh-treated group (P<0.05). In the
N-Shh-treated group, the signal intensity of the drug-treated group
was significantly lower than that of the N-Shh-induced control
group (P<0.05). Regardless of N-Shh treatment, the signal
intensity of the co-treatment group was about lower than those of
the wortmannin- and CPN-KAAD-treated groups (P<0.05) (Fig. 2E and F). In Fig. 2G and H, the signal intensity of the
PD98059-treated group was slightly lower than that of the control
group (P<0.05). The signal intensity of the CPN-KAAD- and
PD98059+CPN-KAAD-treated groups was lower than that of the control
group (P<0.05). The signal intensity of the N-Shh-treated group
was significantly higher than that of the non-N-Shh-treated control
group (P<0.05). In the N-Shh-treated group, the signal intensity
of the drug-treated group was lower than that of the N-Shh-treated
control group (P<0.05). Regardless of the N-Shh treatment, the
signal intensityof the co-treatment group was significantly lower
than those of the PD98059- and CPN-KAAD-treated groups
(P<0.05).
Effects of Shh, PI3K and MAPK
signaling inhibition on caspase-3 expression
Fig. 3 shows the
effect of inhibitory Shh, PI3K, and MAPK signaling pathways on
caspase-3 expression. With Shh and/or PI3K inhibition, after 12 h
of serum starvation of GIST-H1 cells, the relative ratios of the
grayscales of caspase-3 to tubulin were significantly higher than
that of the negative control group (P<0.05). After 12 h of serum
starvation of the GIST-H1 cells, the signal intensity of the
CPN-KAAD- and wortmannin-treated groups was slightly lower than
that of the starvation control group, but the difference was not
significant (P>0.05). The signal intensity of the EGF-treated
serum starvation group was slightly higher than that of the
EGF-treated control group, but the difference was not significant
(P>0.05). The signal intensity of the EGF-treated wortmannin
group was significantly higher than that of the control group
(P<0.01). The signal intensity of the EGF- and CPN-KAAD-treated
group was higher than that of the EGF-treated control group
(P<0.05; Fig. 3A and B).
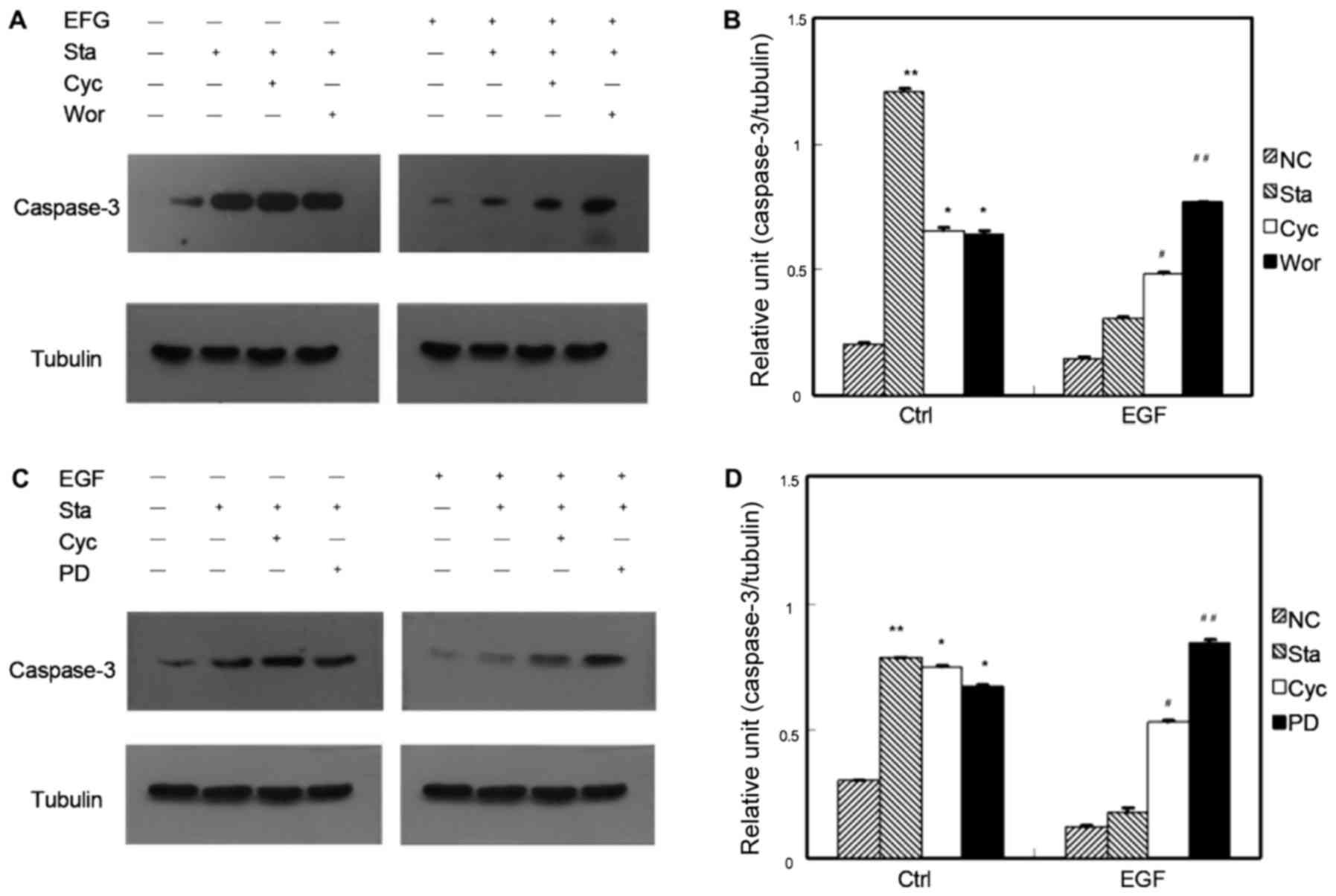 | Figure 3.Effect of inhibiting Shh, PI3K and
(or) MAPK signaling pathway on the expression of caspase-3 in GIST
cells. (A) The influence on the expression of caspase-3 by
inhibiting Shh and (or) PI3K signaling pathway in GIST cells. (B)
The caspase-3/Tubulin relative intensity ratios of each treatment
factor resulted from EGF-mediated cell apoptosis actions by
inhibiting Shh and (or) PI3K signaling pathway. (C) The influence
on the expression of caspase-3 by inhibiting Shh and (or) MAPK
signaling pathway in GIST cells. (D) The caspase-3/Tubulin relative
intensity ratios of each treatment factor resulted from
EGF-mediated cell apoptosis actions by inhibiting Shh and (or) MAPK
signaling pathway (*P<0.05, **P<0.05 vs. ctrl-NC;
#P<0.05, ##P<0.05 vs. EGF-NC). N-Shh,
recombinant Sonic hedgehog; GIST, gastrointestinal stromal tumor;
EGF, endothelial growth factor; NC-normal control, Sta-starvation,
Cyc-cyclopamine, PD-PD98059, Wor-wortmannin. |
In Fig. 3C and D, with
Shh and/or MAPK signaling pathway inhibition, after 12 h of serum
starvation of the GIST-H1 cells, the signal intensity of caspase-3
to tubulin was significantly higher than that of the negative
control group (P<0.05). After 12 h of serum starvation of the
GIST-H1 cells, the signal intensity of the CPN-KAAD- and PD-treated
groups was slightly higher than that of the starvation control
group (P<0.05). The signal intensity of the EGF-treated serum
starvation group was slightly higher than that of the EGF-treated
control group (P<0.05). The signal intensity of the EGF- and
PD98059-treated group was significantly higher than that of the
EGF-treated control group (P<0.01). The signal intensity of the
EGF- and CPN-KAAD-treated group was higher than that of the
EGF-treated control group (P<0.05; Fig. 3C and D).
Effects of Shh, PI3K, and MAPK
signaling inhibition on cell proliferation
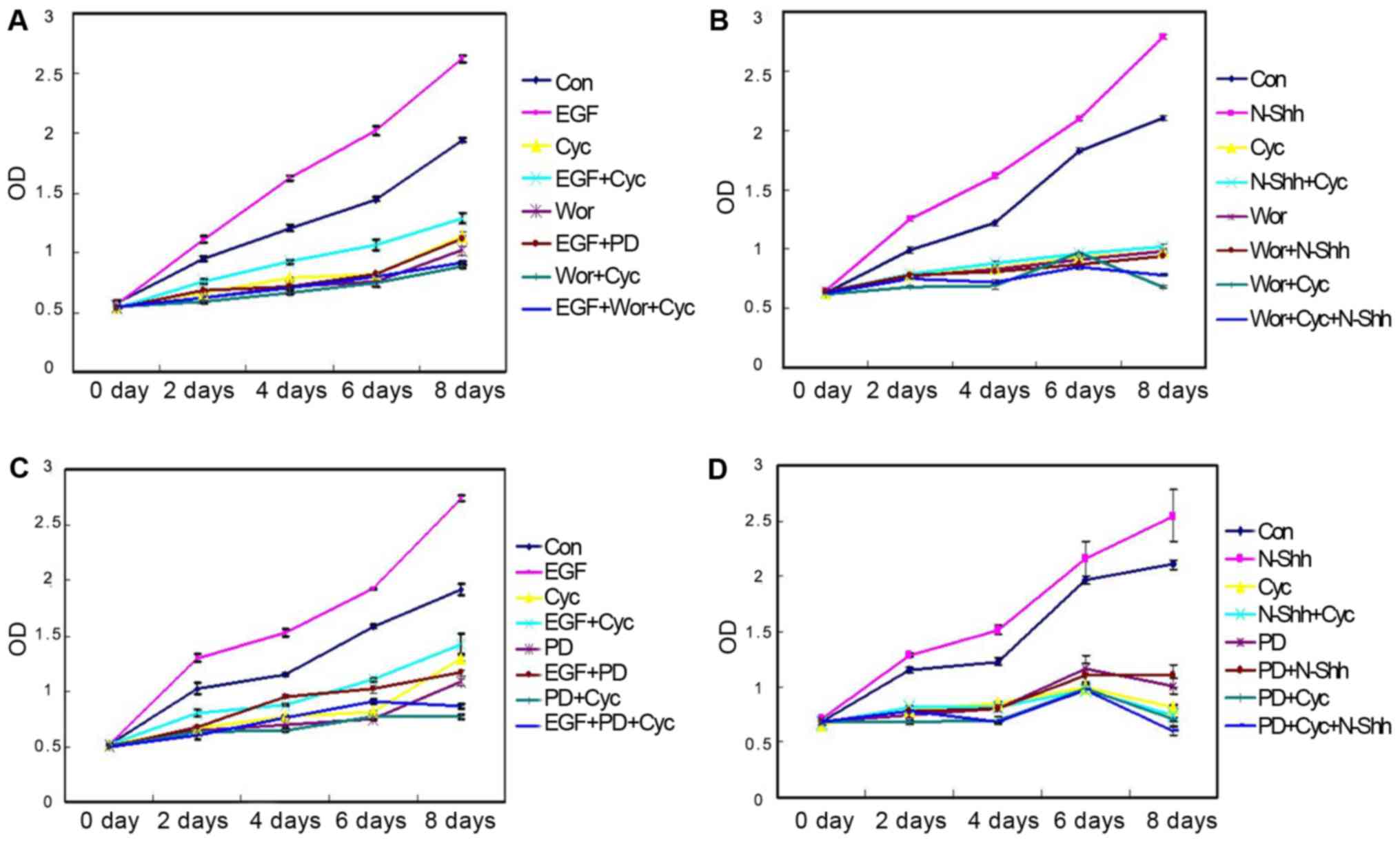
Fig. 4 shows the
effect of inhibitory Shh, PI3K, and MAPK signaling pathways on cell
proliferation. With Shh and PI3K signaling pathways inhibition,
compaered with that in the blank control treatment, the
proliferation rate of the GIST-H1 cells significantly increased
under EGF treatment (P<0.01), and the effect can be partially
blocked by wortmannin and CPN-KAAD pretreatment. Under N-shh
treatment (P<0.01), the proliferation rate of the GIST-H1 cells
also significantly increases (P<0.01), and the effect can be
prevented by wortmannin and CPN-KAAD pretreatment (Fig. 4A and B). With Shh and MAPK signaling
pathways inhibition, compared with that in the blank control
treatment, the proliferation rate of the GIST-H1 cells
significantly increased under EGF treatment, and the effect can be
partially blocked by wortmannin and CPN-KAAD pretreatment (Fig. 4C). Under N-shh treatment, the
proliferation rate of the GIST-H1 cells also significantly
increased, but the effect can be evidently inhibited by PD98059 and
CPN-KAAD pretreatment (Fig. 4D).
Discussion
Considering the important signal proteins of the
three signaling pathways as targets, we believe that the
development of targeted drugs will be one of the priorities in
tumor therapy in the future.
The Shh signaling pathway is expressed in GIST
tissues (10) and is associated with
the risk rating of GIST. Detecting in GIST-H1 cell lines via
western blot, we found that p-AKT, p-ERK, and Gli-1 are expressed
in the three signaling pathways. In GIST-H1 cells, the expression
of the PI3K/AKT and MAPK/ERK signaling pathways can be upregulated
by the stimulation of their self-specific stimulatory factor EGF
and by the stimulation of the stimulatory factor of the Shh
signaling pathway. Conversely, the expression of the PI3K/AKT and
MAPK/ERK signaling pathways can be inhibited by their self-specific
inhibitor and by the specific inhibitor of the Shh signaling
pathway. The combined use of the inhibitor (CPN-KAAD+wortmannin) of
the Shh and PI3K/AKT signaling pathways and the inhibitor of the
wortmannin+CPN-KAAD or PD98059+CPN-KAAD of the Shh signaling
pathway can further partially block or suppress the expression of
the two signaling pathways. Hence, the upregulation and inhibition
of PI3K/and MAPK signaling pathways in GIST-H1 cells are related to
the Shh signaling pathway.
In GIST-H1 cells, the expression of the Shh
signaling pathway can be upregulated by the stimulation of its
self-specific stimulatory factor N-Shh and by the stimulation of
the stimulatory factor EGF of the PI3K/AKT and MAPK/ERK signaling
pathways. The activity of the Shh signaling pathway can be
inhibited by its self-specific inhibitor and by the specific
inhibitor of the PI3K/AKT and MAPK/ERK signaling pathways. The
combined use of the inhibitor (CPN-KAAD+wortmannin) of the Shh and
PI3K/AKT signaling pathway or the inhibitor (CPN-KAAD+PD98059) of
the Shh and MAPK/ERK signaling pathways can further inhibit its
activity. Hence, the upregulation and inhibition of the Shh
signaling pathway in GIST-H1 cells are related to PI3K/and MAPK
signaling pathways.
Shh, PI, and MAPK signaling pathways can be
activated in GIST and can be inhibited by their corresponding
inhibitors. This result indicates that the three signaling pathways
exist in GIST and that cross regulation occurs among these
pathways.
The effect of inhibitory Shh, PI3K, and MAPK
signaling pathways on the caspase-3 expression in GIST-H1 cells can
be detected via western blot. Regardless of the stimulatory
factors, the relative ratios of the grayscales of these signaling
pathways are higher than that of the control group (P<0.05).
This result suggests that specific Shh, PI3K, and MAPK signaling
pathway inhibitors can accelerate GIST cell apoptosis.
Proliferation experiment demonstrates that the proliferation rate
of GIST-H1 cells significantly accelerates under EGF or N-shh
treatment and that it can be partially inhibited by PD98059,
CPN-KAAD, and wortmannin pretreatment. This result implies that the
inhibitory Shh, PI3K, and MAPK signaling pathways can block the
proliferation of GIST cells.
In summary, the data in GIST H1 cells confirmed that
the combination of the above three inhibitors of the signal pathway
can effectively inhibit the expression of GIST signaling and cell
proliferation, and therefore can be considered as targeted therapy
options in GIST.
Acknowledgements
Not applicable.
Funding
The present study received internal funding from the
First Affiliated Hospital of Anhui Medical University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ, WZ and XM contributed to the study design,
experimental design and manuscript writing. ZW, QX, and JC
contributed to the experimental design and manuscript writing.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Foo WC, Liegl-Atzwanger B and Lazar AJ:
Pathology of gastrointestinal stromal tumors. Clin Med Insights
Pathol. 5:23–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeMatteo RP, Lewis JJ, Leung D, Mudan SS,
Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal
tumors: Recurrence patterns and prognostic factors for survival.
Ann Surg. 231:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsutsui S, Matsuyama A, Yamamoto M,
Takeuchi H, Oshiro Y, Ishida T and Maehara Y: The Akt expression
correlates with the VEGF-A and -C expression as well as the
microvessel and lymphatic vessel density in breast cancer. Oncol
Rep. 23:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Fagan DH, Zeng X, Freeman KT,
Sachdev D and Yee D: Inhibition of cancer cell proliferation and
metastasis by insulin receptor downregulation. Oncogene.
29:2517–2527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daniels M, Lurkin I, Pauli R, Erbstösser
E, Hildebrandt U, Hellwig K, Zschille U, Lüders P, Krüger G, Knolle
J, et al: Spectrum of KIT/PDGFRA/BRAF mutations and
Phosphatidylinositol-3-Kinase pathway gene alterations in
gastrointestinal stromal tumors (GIST). Cancer Lett. 312:43–54.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wancket LM, Frazier WJ and Liu Y:
Mitogen-activated protein kinase phosphatase (MKP)-1 in immunology,
physiology and disease. Life Sci. 90:237–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao C, Chen A, Jamieson CH, Fereshteh M,
Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al:
Hedgehog signalling is essential for maintenance of cancer stem
cells in myeloid leukaemia. Nature. 458:776–779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshizaki A, Nakayama T, Naito S, Wen CY
and Sekine I: Expressions of sonic hedgehog, patched, smoothened
and Gli-1 in human intestinal stromal tumors and their correlation
with prognosis. World J Gastroenterol. 12:5687–5691. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Long B, Zhu H, Zhu C, Liu T and Meng W:
Activation of the Hedgehog pathway in chronic myelogeneous leukemia
patients. J Exp Clin Cancer Res. 30:82011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi Y, Zhao W, Wang Z and Meng X:
Expression of Shh signaling pathway factors in gastrointestinal
stromal tumor tissues and their associations with clinical
pathological factors. Int J Clin Exp Pathol. 9:2841–2848. 2016.
|