Neurotrophins are a family of proteins that regulate
neuron differentiation, survival, dendritic pruning, patterning of
innervation, synaptic function and plasticity in the central and
the peripheral nervous system (1,2). There are
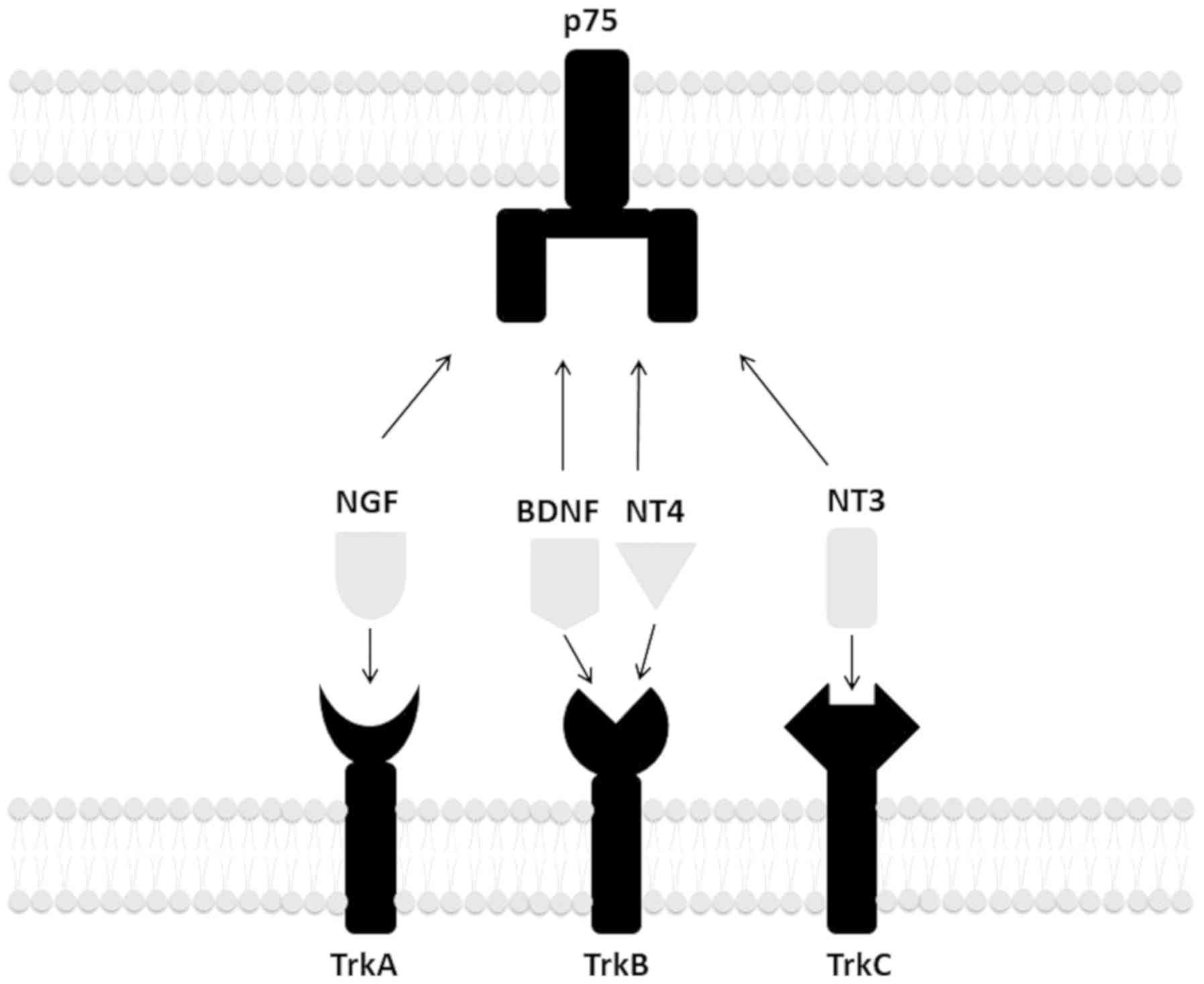
four neurotrophins: nerve growth factor (NGF), brain-derived
neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and NT-4. They
have two types of receptors: the p75 neurotrophin receptor and
tropomyosin receptor kinases (Trk) (2). P75 is the receptor for all four
neurotrophins. Regarding Trk receptors, NGF binds TrkA; BDNF and
NT-4 bind TrkB; and NT-3 mainly binds TrkC (2) (Fig.
1).
Initially, neurotrophins and their receptors were
thought to be expressed only in nervous system but further studies
showed they are also expressed by macrophages, endocrine cells,
immune cells, smooth and striated muscle fibers (3,4). Recently,
neurotrophins and their Trk receptors, especially BDNF and TrkB,
were found to be highly up-regulated and play a vital role in
various cancers, including breast, lung, colon-rectum, pancreas,
prostate, liver, myelomas and lymphoid tumors (5). Activation of these Trk receptors elicits
a series of downstream signalings, including PI3K/Akt,
Ras-Raf-MEK-ERK, PLCγ pathway, transactivation of EGFR, etc. As a
result, these pathways exhibit oncogenic effects by promoting
cancer cell's growth, proliferation, survival, migration,
epithelial to mesenchymal trasition, anoikis, relapse and
chemotherapeutic sensitivity (6–12). Drugs
targeting these Trk receptors have been put into clinical trials
for cancer therapy and promising results have been achieved with
moderate side effects (13).
This review will summarize all recent findings about
the role of BDNF/TrkB in tumor and its underlying downstream
pathways. We will also conclude and discuss clinical trials of
targeting Trk receptors for treatment of cancers and the potential
risk.
Among the 4 neurotrophins, BDNF is the most abundant
growth factor in the brain, which plays an important role in
sustaining physiological processes of the brain. For example, BDNF
regulates dendritic branching and dendritic spine morphology
(14,15), as well as synaptic plasticity and
long-term potentiation (LTP) (16).
BDNF also modulates hypothalamic metabolic function, further
reflecting the diversity of its role in the brain (17,18).
It has been revealed that BDNF is important in the
developmental and mature taste system, by supporting survival of
taste cells and geniculate ganglion neurons, and maintaining and
guiding taste nerve innervations (19–21). These
results demonstrated BDNF exhibits crucial effects in both of the
central and peripheral nervous system. Another study also showed
that BDNF/TrkB pathway may be involved in maintaining adult
hippocampal neurogenesis by promoting survival, proliferation, and
neural differentiation of neural stem cells (22,23). This
function and underlying mechanism is comparable to BDNF's role in
cancer (23–25).
Recently, lots of evidences showed BDNF and its
receptor TrkB play a vital role in tumor pathology (26–28). TrkB
is a type of receptor tyrosine kinases (RTKs) and some RTKs were
characterized as oncogenes (29).
Preclinical trials of target therapies on these RTKs showed
promising results (13). Recent
reports indicate that BDNF/TrkB pathway has an important function
in neural tumors, such as neuroblastoma (30). Further studies have shown that
BDNF/TrkB is oncogenic not only in neurogenic original tumors
(31), but also in other tumors
outside of the neural system (32,33).
In addition to TrkB, p75 is a receptor for a
precursor form of BDNF (pro-BDNF), which can be cleaved to form the
mature form by metalloproteinases (34). Unlike TrkB, which is the receptor for
the mature form of BDNF (35), the
role of p75 is not well established in tumors. It is found to be
overexpressed in glioblastoma (34,36),
melanoma (37,38) and breast cancer (6), implying it may exhibit an oncogenic
role. However, it also displays an oncolytic role by suppressing
tumor cell proliferation and migration in bladder (39), hepatocellular (40) and gastric cancers (41). Considering p75′s controversial role in
tumor, we will only summarize the oncogenic role of BDNF/TrkB
pathway for the following sections.
As said above, BDNF's oncogenic role in cancer has
initially been characterized in neuroblastoma, a type of cancer in
nervous tissue (30). It has ever
been demonstrated Tyro3, Axl and Mertk (TAM) receptor tyrosine
kinases promote neurogenesis by supporting neural stem cell
survival, proliferation and neuronal differentiation (22,42).
Removal of TAM receptors leads to a significantly-reduced level of
neurogenesis and BDNF expression, indicating TAM receptors support
neurogenesis by activating BDNF pathway (23). Some scientists also concluded BDNF
mediates development, migration, differentiation and survival of
newborn neurons (43). Further on,
high levels of BDNF were found in neuroblastoma cells and were
discovered to be linked with better prognosis of neuroblastoma
(44). An increase of BDNF and TrkB
signaling in neuroblastoma cells may represent an autocrine system
to support tumor growth, invasion and metastasis. Mover, BDNF/TrkB
pathway was implied to induce angiogenesis in neuroblastoma. It was
found that BDNF could stimulate neovascularization through
recruitment of TrkB-expressing endothelial progenitor cells
(45,46). Lastly, this signaling was also
demonstrated to promote resistance to chemotherapy in neuroblastoma
cells (47).
As evidence is accumulated, BDNF/TrkB signaling is
universally considered to have oncogenic consequences. It has been
found BDNF/TrkB are up-regulated in countless types of cancers,
such as breast cancer, carcinoid, cervical, colorectal, glioma,
liver, lung (6–12). Some studies even revealed BDNF might
be an important prognostic factor for cancers (12,48–50).
Recently, BDNF/TrkB pathway has been demonstrated to transactivate
EGFR, a growth factor receptor commonly up-regulated in many
cancers (43,51). This transactivation is important for
proliferation and migration of embryonic cortical neurons, lung
cancer cells and ovarian cancer cells (43,51,52).
Administration of BDNF prevents the oncolytic role of EFGR
inhibition in colon cancer. Besides, BDNF and EGFR seems to
compensate for each other so that dual inhibition of the two
pathways works effectively to suppress colon cancer cell
proliferation (53).
BDNF/TrkB can also decrease a cancer cell's
sensitivity to chemotherapy. It has been reported that BDNF
increases the survival of neuroblastoma cells from cisplatin,
etoposide and vinblastine in a dose-dependent manner (54,55).
Treatment with antibodies against BDNF made mice more susceptible
to chemotherapy in models of breast cancer (6), uterine sarcoma (56), and neuroblastoma (57,58). BDNF
administration was demonstrated to cause chemotherapeutic
resistance in head and neck squamous cell carcinoma (59). This protective role of BDNF from
chemotherapy was possibly due to its ability to support
proliferation and survival of cancer cells (55).
In addition, BDNF/TrkB pathway promotes resilience
against the programmed death of anchorage dependent cancer cells.
This programmed death of cancer cells, known as anoikis, is
important to fight against many types of cancers. As solid tumors
metastasize from the original sites and migrate to other regions
with plentiful nutrients, tumor cells may undergo anoikis (60). However, some tumor cells can have a
mesenchymal transition from an epithelial nature so that they
survive short travel through the blood to distant organs (61). Interestingly, BDNF/TrkB has been
reported to regulate the resistance to anoikis of several cancers
since up-regulation of BDNF/TrkB was found in metastatic tumor
cells. Nevertheless, no activation of BDNF/TrkB pathway was
observed in non-metastatic tumor cells or tumor cells that fail to
survive through metastasis (60,62,63). This
supports how BDNF/TrkB signaling may perhaps play a crucial role in
the progression and invasion of malignant tumors.
Finally, BDNF/TrkB pathway has been implied to
mediate cancer reformation after successful treatment of cancer.
Scientists overexpressed TrkB in a neural crest-derived cell line
and implanted them into mice. These cells formed tumors 10 days
after implantation and killed all mice within one week after tumor
formation (31). Cancer stem cells
are similar to neural crest-derived cells. They divide slowly but
can turn into cancer cells under some condition. Chemotherapy can
kill rapidly-dividing tumor cells but can't target these
slowly-dividing cells. It has been shown that TrkB-positive cancer
stem cells can cause tumor reformation after successful treatment
of mice with triple-negative breast cancers (32). These findings demonstrate the
importance of continuous treatment with TrkB inhibitor after
successful removal of tumor cells with chemotherapy.
As discussed above, it has been observed that BDNF
and TrkB levels increase in many types of cancers, conferring
aggressive phenotypes due to their resistance to chemotherapeutic
agents (64). BDNF binds its receptor
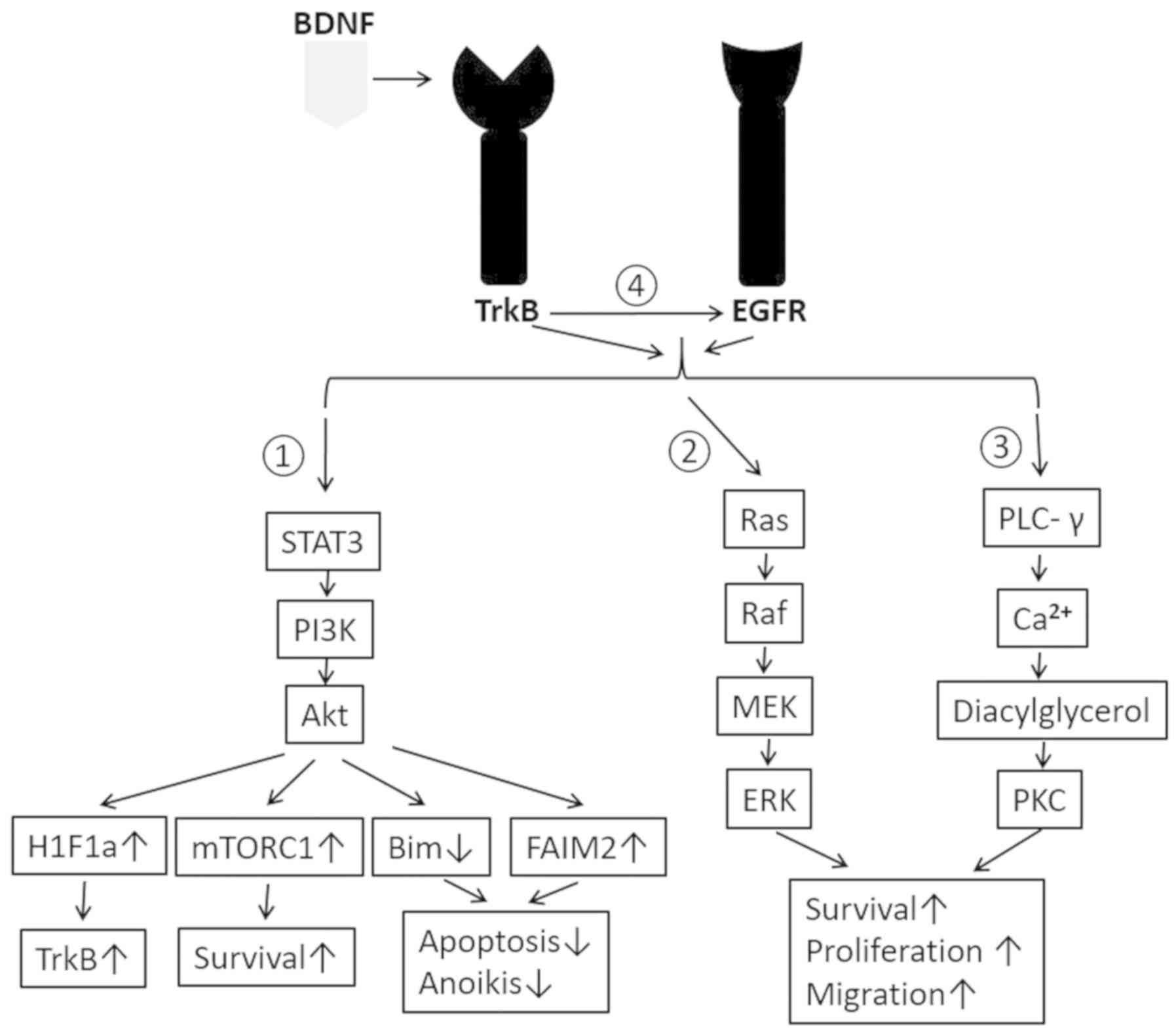
TrkB and triggers a cascade of signals, including PI3K/Akt,
Ras-Raf-MEK-ERK, PLCγ pathway, transactivation of EGFR, Jak/STAT,
nuclear factor kappa-light-chain-enhancer of activated B cells
(NF-kB), Urokinase-type plasminogen activator (UPAR)/UPA,
Wnt/β-catenin and Vascular endothelial growth factor (VEGF)
pathways, etc. Among these pathways, the first 4 are mostly studied
and therefore will be discussed in this review and summarized as
shown in Fig. 2.
PI3K/Akt can be activated by BDNF/TrkB and then
leads to production of pro-migratory, anti-apoptotic and
pro-survival proteins (65,66). Some recent studies have indicated that
TrkB receptor activation induces phosphorylation of tyrosine 705 of
STAT3, which then activates PI3K/Akt (67). This pathway will activate the
mammalian target of rapamycin complex 1 (mTORC1), resulting in
increased protein synthesis and cell survival by direct
phosphorylation of its effectors, such as the ribosomal S6 kinase1
(S6K1), and elF4E-binding proteins (4E-BPs) to terminate binding to
elF4E and relieve the block on translation (68,69).
Besides, PI3K/Akt pathway can transduce to amplify
hypoxia-inducible factor 1-alpha (H1F1a), which is a
transcriptional activator of TrkB expression. This positive
feedback loop aggravates and extends BDNF/TrkB's effect on tumor
(67,70).
As a universal attenuator of chemotherapeutic
efficacy, BDNF/TrkB exhibits this role by mediating PI3K/Akt
pathway, since inhibition of PI3K abrogated BDNF's ability to
protect cancer cells from etoposide (55). PI3K/Akt pathway promotes resistance to
extrinsic apoptosis through down-regulation of Bim, a pro-apoptotic
protein that facilitates mitochondrial-mediated or intrinsic
apoptosis (59,71). In addition, PI3K/Akt pathway is
involved in up-regulation of Fas apoptotic inhibitory molecule 2
(FAIM2), which works to inhibit Fas-mediated Caspase-8-dependent
apoptosis (72). The two possible
pathways also explain how BDNF promotes resistance to anoikis
through activation of PI3K/Akt.
BDNF/TrkB pathway has also been shown to
transactivate EGFR even without the endogenous EGF ligand (43) (Fig. 2).
In vitro study demonstrated administering BDNF leads to
expected TrkB phosphorylation and also EGFR phosphorylation no
matter if EGF is present in the culture media or not (43). Transactivation of EGFR stimulates
expression of PLC-γ, which then causes the release of calcium ions
from intracellular compartments and the generation of
diacylglycerol. Diacylglycerol can activate protein kinase C (PKC),
which is linked to carcinogenesis and maintenance of malignant
phenotype (73). Besides, EGFR can
result in the progression of cancer cells through G1 phase and into
S phase by regulating the cyclin dependent kinases (CDK) and the
cyclins (74). EGFR can also cause
Ras activation, which involves large number of protein factors,
including Raf, mitogen-activated protein kinase (MAPK), cytosolic
kinases and nuclear transcription factors (75,76).
Activation of Ras will in turn accelerate cell-cycle progression
and contribute to poor prognosis of patients with cancers (12,77).
Moreover, it is possible that transactivation of EGFR regulates
cancer through PI3K/Akt pathway, as shown in breast cancer
(78), head and neck cancer (79), and prostate cancer (80).
Finally, BDNF/TrkB is considered to be able to
directly activate Ras and PLC-γ pathways, both of which play a
vital role in a wide range of cancers (Fig. 2). In the central nervous system, it is
well documented that BDNF/TrkB activates the Ras-Raf-MEK-ERK
signaling and regulates the neuronal differentiation (81). BDNF/TrkB pathway is also demonstrated
to regulate synaptic plasticity by promoting the PLCγ-mediated
expression of protein kinase C (82).
Recent studies showed Ras and PLC-γ mediated oncogenic role may be
triggered by BDNF/TrkB. For example, BDNF/TrkB activates NF-κB
expression through stimulation of PLCγ and therefore enhances
ovarian cancer cell survival by suppressing anoikis (83). BDNF/TrkB is also demonstrated to
promote epithelia-mesenchymal transition, as well as the migration
and invasion of cervical cancer by activating Ras-Raf-MEK-ERK
pathway (84).
Scientists have developed two highly potent and
selective TrkB inhibitors, cyclotraxin-B (85) and antinuclear antibodies (ANA)-12
(86), which can inhibit TrkB and its
downstream processes. However, they are not applied to clinical
trials since targeting all Trk receptors seems more promising to
treat cancer. Like TrkB, TrkA and TrkC are up-regulated in many
types of cancers and demonstrated to be oncogenic as well (13). For example, Light et al
(87) reported TrkA up-regulation in
neuroblastomas was associated with poor prognosis, while activated
expression and signaling of TrkC corresponded to a more aggressive
and invasive neuroblastoma. Besides, the kinase domain of the three
receptors are remarkably conservative. TrkB and TrkC share 100%
identical residues in the ATP binding sites, and there is only a
2-residue difference between TrkA and TrkB (88). Considering kinase domain determines
their activity, it is reasonable and applicable to design
inhibitors targeting the conservative kinase domain. Based on the
fact that the three Trk receptors share similar kinase domain and
all have oncogenic role, drugs targeting all of them were designed
and applied to clinical trials.
Entrectinib is an ATP-competitive inhibitor of the
Trk proteins, c-ros oncogene 1 (ROS1), and anaplastic lymphoma
kinase (ALK). It is currently being investigated in multiple phase
II studies, including breast cancer, renal cancer, ovarian cancer,
non-small cell lung cancer (NSCLC), and sarcomas (89). Promising results have been reported,
including increased objective response rate, median
progression-free survival rate and overall survival rate.
Interestingly, Entrectinib has also shown the efficacy to treat
brain tumors, implying that it can penetrate blood-brain barrier
(BBB) (90). Similar to Entrectinib,
Larotrectinib is another pan-Trk inhibitor which is able to
penetrate BBB and shows positive results in multiple phase II
clinical trials, including glioblastoma, small cell lung cancer
(SCLC), colorectal cancers, melanoma pancreatic and ovarian
(91,92). A table was made to summarize all
clinical trials for the two Trk inhibitors (Table I).
Cabozantinib is an orally bioavailable small
molecule inhibitor of Trk receptors, c-Met, RET, ROS1, ALK, and
vascular endothelial growth factor 2 (VEGFR2) with approved
treatment for metastatic medullary thyroid cancer and prostate
cancer (13). Recently, Cabozantinib
was approved as an anti-angiogenic therapy for advanced renal cell
carcinoma, by eliciting significant improvements in response rates,
progression-free survival, and overall survival (93,94).
Currently, more clinical trials are underway to evaluate its role
in CNS tumors, like gliomas (95).
Considering its capacity of penetrating BBB, promising results are
expected.
Though the clinical trials received promising
results, we should not ignore the potential side effects of
targeting BDNF/TrkB for cancer treatment. As a neurotrophin factor
in the nervous system, BDNF regulates multiple processes including
neuron differentiation, survival, dendritic pruning, patterning of
innervation, synaptic function and plasticity (1,2). Our lab
has demonstrated BDNF plays a vital role in the central and
peripheral nervous system by regulating the developmental and
mature taste system (19–21), and maintaining adult hippocampal
neurogenesis (22,23). A recent clinical trial even showed
elevation of BDNF levels by using CX1846 can correct age-related
issues (96). Therefore, we should be
extremely careful when targeting BDNF/TrkB to treat cancer. Doses
of BDNF/TrkB inhibitors and side effects of nervous system should
be closely monitored. Dysfunction of central nervous system may be
expected, like memory loss, ataxia, anhedonia, lethargy and
depression (97). As a result, it is
necessary to specifically target tumors with administration of
BDNF/TrkB inhibitors. Gene delivery using viral vectors may be a
good option since it can specifically target tumor cells with
appropriate promoters.
Besides, recent studies suggest that BDNF
overexpression in the hypothalamus may have an oncolytic effect. It
is found that mice with enriched environmental (EE) housing had
high expression of BDNF in the hypothalamus and also got augmented
T-cell cytotoxicity. This increased anti-tumor immune response was
abrogated by hypothalamic knockdown of BDNF, implying BDNF mediates
the oncolytic effects of EE housing (98). Besides, tumors of EE mice had reduced
expression of several pro-survival proteins like VEGF, IGF-1 and
p-ERK, which normally confer resistance to chemotherapeutic agents.
These results were derived from mice transplanted with breast
cancer cells (99), melanoma cancer
cells (100), and even glioma cells
(101). It is suggested that
up-regulation of BDNF in the central nervous system may have an
effect of oncolysis rather than oncogenesis, even if the tumor is
within the central nervous system. The underlying mechanism may be
due to that BDNF supports survival and maturation of peripheral
T-cell (102). As a result, when we
target BDNF/TrkB for cancer treatment, a close monitoring of T-cell
activity is necessary so that the antitumor immune response won't
be attenuated.
BDNF plays an important role in a wide range of
cancers by binding its receptor TrkB (6–12).
Up-regulation of BDNF/TrkB results in a series of downstream
signalings, including PI3K/Akt, Ras-Raf-MEK-ERK, PLCγ pathway, and
transactivation of EGFR, etc (Fig.
2). Stimulation of these signalings exerts oncogenic effects by
mediating cancer cell's growth, proliferation, survival, migration,
epithelial to mesenchymal trasition, anoikis, relapse and
chemotherapeutic sensitivity (6–12).
Although BDNF/TrkB regulates several downstream signalings and
these pathways may correspond to each other, not all these
signalings will be stimulated in response to BDNF/TrkB. For
example, it was reported that BDNF can rescue neuroblastoma cells
from etoposide. Inhibition of PI3K but not MAPK can abrogate this
ability, indicating MAPK pathway may not be involved in this
oncogenic role (103).
Since BDNF plays a crucial role in oncogenesis, is
it still safe to activate BDNF/TrkB for treatment of some
neurodegenerative diseases? Like human hormones, too much or too
little of BDNF may be harmful. It is not suggested that healthy
people should take BDNF as a supplement due to the potential risk
of oncogenesis. Nevertheless, administration of BDNF may be
beneficial in some aging and neurodegenerative diseases, such as
amyotrophic lateral sclerosis (ALS), peripheral neuropathy,
Parkinson's disease and Alzheimer's disease (104–106).
Under these unhealthy conditions, BNDF/TrkB is down-regulated and
there is no evidence that administration of BDNF can cause cancer.
For clinical application, BDNF is not directly administrated since
it is a moderately-sized and charged protein, and can't easily
cross BBB. Therefore, scientists have spent decades trying to
establish small drugs that could penetrate BBB and safely augment
BDNF levels in the brain. Recently, Ampakines, a modulator of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptor, have been found to significantly elevate BDNF levels in
some brain regions and also successfully correct age-related memory
issues without severe side effects (96,107).
Not applicable.
The present review was supported by the National
Science Foundation of China (grant no. 81570344).
Not applicable.
LM, BL and RJ wrote the manuscript. XJ and XY
critically revised the manuscript for important intellectual
content. YX reviewed and edited the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bibel M and Barde YA: Neurotrophins: Key
regulators of cell fate and cell shape in the vertebrate nervous
system. Genes Dev. 14:2919–2937. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meldolesi J: Neurotrophin receptors in the
pathogenesis, diagnosis and therapy of neurodegenerative diseases.
Pharmacol Res. 121:129–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reichardt LF: Neurotrophin-regulated
signalling pathways. Rev Physiol Biochem Pharmacol. 361:1545–1564.
2006.
|
|
5
|
Meldolesi J: Neurotrophin Trk receptors:
New targets for cancer therapy. Rev Physiol Biochem Pharmacol.
174:67–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vanhecke E, Adriaenssens E, Verbeke S,
Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X and
Hondermarck H: Brain-derived neurotrophic factor and
neurotrophin-4/5 are expressed in breast cancer and can be targeted
to inhibit tumor cell survival. Clin Cancer Res. 17:1741–1752.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci A, Graziano P, Mariotta S, Cardillo
G, Sposato B, Terzano C and Bronzetti E: Neurotrophin system
expression in human pulmonary carcinoid tumors. Growth Factors.
23:303–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moon A, Won KY, Lee JY, Kang I, Lee SK and
Lee J: Expression of BDNF, TrkB, and p53 in early-stage squamous
cell carcinoma of the uterine cervix. Pathology. 43:453–458. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brunetto de Farias C, Rosemberg DB, Heinen
TE, Koehler-Santos P, Abujamra AL, Kapczinski F, Brunetto AL,
Ashton-Prolla P, Meurer L, Reis Bogo M, et al: BDNF/TrkB content
and interaction with gastrin-releasing peptide receptor blockade in
colorectal cancer. Oncology. 79:430–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilbertson RJ and Rich JN: Making a
tumour's bed: Glioblastoma stem cells and the vascular niche. Nat
Rev Cancer. 7:733–736. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka K, Okugawa Y, Toiyama Y, Inoue Y,
Saigusa S, Kawamura M, Araki T, Uchida K, Mohri Y and Kusunoki M:
Brain-derived neurotrophic factor (BDNF)-induced
tropomyosin-related kinase B (Trk B) signaling is a potential
therapeutic target for peritoneal carcinomatosis arising from
colorectal cancer. PLoS One. 9:e964102014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamura K, Harada T, Wang S, Ijichi K,
Furuyama K, Koga T, Okamoto T, Takayama K, Yano T and Nakanishi Y:
Expression of TrkB and BDNF is associated with poor prognosis in
non-small cell lung cancer. Lung Cancer. 78:100–106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lange AM and Lo HW: Inhibiting TRK
proteins in clinical cancer therapy. Cancers (Basel). 10(pii):
E1052018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka J, Horiike Y, Matsuzaki M, Miyazaki
T, Ellis-Davies GC and Kasai H: Protein synthesis and
neurotrophin-dependent structural plasticity of single dendritic
spines. Science. 319:1683–1687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horch HW and Katz LC: BDNF release from
single cells elicits local dendritic growth in nearby neurons. Nat
Neurosci. 5:1177–1184. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Figurov A, Pozzo-Miller LD, Olafsson P,
Wang T and Lu B: Regulation of synaptic responses to high-frequency
stimulation and LTP by neurotrophins in the hippocampus. Nature.
381:706–709. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu B, Goulding EH, Zang K, Cepoi D, Cone
RD, Jones KR, Tecott LH and Reichardt LF: Brain-derived
neurotrophic factor regulates energy balance downstream of
melanocortin-4 receptor. Nat Neurosci. 6:736–742. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao L, Lin EJ, Cahill MC, Wang C, Liu X
and During MJ: Molecular therapy of obesity and diabetes by a
physiological autoregulatory approach. Nat Med. 15:447–454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng L, Huang T, Sun C, Hill DL and Krimm
R: BDNF is required for taste axon regeneration following
unilateral chorda tympani nerve section. Exp Neurol. 293:27–42.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng L, Ohman-Gault L, Ma L and Krimm RF:
Taste bud-derived BDNF is required to maintain normal amounts of
innervation to adult taste buds. eNeuro. 2(pii):
ENEURO.0097-15.2015. 2015.PubMed/NCBI
|
|
21
|
Meng L, Jiang X and Ji R: Role of
neurotrophin in the taste system following gustatory nerve injury.
Metab Brain Dis. 30:605–613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji R, Meng L, Li Q and Lu Q: TAM receptor
deficiency affects adult hippocampal neurogenesis. Metab Brain Dis.
30:633–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji R, Meng L, Jiang X, Cvm NK, Ding J, Li
Q and Lu Q: TAM receptors support neural stem cell survival,
proliferation and neuronal differentiation. PLoS One.
9:e1151402014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Q, Ford MC, Lavik EB and Madri JA:
Modeling the neurovascular niche: VEGF- and BDNF-mediated
cross-talk between neural stem cells and endothelial cells: An in
vitro study. J Neurosci Res. 84:1656–1668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blurton-Jones M, Kitazawa M,
Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR,
Poon WW, Green KN and LaFerla FM: Neural stem cells improve
cognition via BDNF in a transgenic model of Alzheimer disease. Proc
Natl Acad Sci USA. 106:13594–13599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pearse RN, Swendeman SL, Li Y, Rafii D and
Hempstead BL: A neurotrophin axis in myeloma: TrkB and BDNF promote
tumor-cell survival. Blood. 105:4429–4436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakagawara A: Trk receptor tyrosine
kinases: A bridge between cancer and neural development. Cancer
Lett. 169:107–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brodeur GM, Minturn JE, Ho R, Simpson AM,
Iyer R, Varela CR, Light JE, Kolla V and Evans AE: Trk receptor
expression and inhibition in neuroblastomas. Clin Cancer Res.
15:3244–3250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Porter AC and Vaillancourt RR: Tyrosine
kinase receptor-activated signal transduction pathways which lead
to oncogenesis. Oncogene. 17:(11 Reviews). 1343–1352. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakagawara A, Azar CG, Scavarda NJ and
Brodeur GM: Expression and function of TRK-B and BDNF in human
neuroblastomas. Mol Cell Biol. 14:759–767. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin B, Ma ZY, Zhou ZW, Gao WC, Du ZG, Zhao
ZH and Li QQ: The TrkB+ cancer stem cells contribute to
post-chemotherapy recurrence of triple-negative breast cancers in
an orthotopic mouse model. Oncogene. 34:761–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang SY, Hui LP, Li CY, Gao J, Cui ZS and
Qiu XS: More expression of BDNF associates with lung squamous cell
carcinoma and is critical to the proliferation and invasion of lung
cancer cells. BMC Cancer. 16:1712016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Radin DP and Patel P: BDNF: An oncogene or
tumor suppressor? Anticancer Res. 37:3983–3990. 2017.PubMed/NCBI
|
|
34
|
Hwang JJ, Park MH, Choi SY and Koh JY:
Activation of the Trk signaling pathway by extracellular zinc. Role
of metalloproteinases. J Biol Chem. 280:11995–12001. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hempstead BL: Dissecting the diverse
actions of pro-and mature neurotrophins. Curr Alzheimer Res.
3:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnston AL, Lun X, Rahn JJ, Liacini A,
Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth
PA, et al: The p75 neurotrophin receptor is a central regulator of
glioma invasion. PLoS Biol. 5:e2122007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Herrmann JL, Menter DG, Hamada J,
Marchetti D, Nakajima M and Nicolson GL: Mediation of
NGF-stimulated extracellular matrix invasion by the human melanoma
low-affinity p75 neurotrophin receptor: melanoma p75 functions
independently of trkA. Mol Biol Cell. 4:1205–1216. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marchetti D, Aucoin R, Blust J, Murry B
and Greiter-Wilke A: p75 neurotrophin receptor functions as a
survival receptor in brain-metastatic melanoma cells. J Cell
Biochem. 91:206–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khwaja F and Djakiew D: Inhibition of
cell-cycle effectors of proliferation in bladder tumor epithelial
cells by the p75NTR tumor suppressor. Mol Carcinog. 36:153–160.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuanlong H, Haifeng J, Xiaoyin Z, Jialin
S, Jie L, Li Y, Huahong X, Jiugang S, Yanglin P, Kaichun W, et al:
The inhibitory effect of p75 neurotrophin receptor on growth of
human hepatocellular carcinoma cells. Cancer Lett. 268:110–119.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jin H, Pan Y, He L, Zhai H, Li X, Zhao L,
Sun L, Liu J, Hong L, Song J, et al: p75 neurotrophin receptor
inhibits invasion and metastasis of gastric cancer. Mol Cancer Res.
5:423–433. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji R, Tian S, Lu HJ and Lu Q, Zheng Y,
Wang X, Ding J, Li Q and Lu Q: TAM receptors affect adult brain
neurogenesis by negative regulation of microglial cell activation.
J Immunol. 191:6165–6177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Puehringer D, Orel N, Lüningschrör P,
Subramanian N, Herrmann T, Chao MV and Sendtner M: EGF
transactivation of Trk receptors regulates the migration of newborn
cortical neurons. Nat Neurosci. 16:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park JR, Eggert A and Caron H:
Neuroblastoma: Biology, prognosis, and treatment. Hematol Oncol
Clin North Am. 24:65–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kermani P and Hempstead B: Brain-derived
neurotrophic factor: A newly described mediator of angiogenesis.
Trends Cardiovasc Med. 17:140–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kermani P, Rafii D, Jin DK, Whitlock P,
Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR,
et al: Neurotrophins promote revascularization by local recruitment
of TrkB+ endothelial cells and systemic mobilization of
hematopoietic progenitors. J Clin Invest. 115:653–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ho R, Eggert A, Hishiki T, Minturn JE,
Ikegaki N, Foster P, Camoratto AM, Evans AE and Brodeur GM:
Resistance to chemotherapy mediated by TrkB in neuroblastomas.
Cancer Res. 62:6462–6466. 2002.PubMed/NCBI
|
|
48
|
Brierley GV, Priebe IK, Purins L, Fung KY,
Tabor B, Lockett T, Nice E, Gibbs P, Tie J, McMurrick P, et al:
Serum concentrations of brain-derived neurotrophic factor (BDNF)
are decreased in colorectal cancer patients. Cancer Biomark.
13:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiong L, Deng X, Wen Y, Yang Z and Miao X:
Association of BDNF and BMPR1A with clinicopathologic parameters in
benign and malignant gallbladder lesions. World J Surg Oncol.
11:802013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Choi B, Lee EJ, Shin MK, Park YS, Ryu MH,
Kim SM, Kim EY, Lee HK and Chang EJ: Upregulation of brain-derived
neurotrophic factor in advanced gastric cancer contributes to bone
metastatic osteolysis by inducing long pentraxin 3. Oncotarget.
7:55506–55517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Götz R and Sendtner M: Cooperation of
tyrosine kinase receptor TrkB and epidermal growth factor receptor
signaling enhances migration and dispersal of lung tumor cells.
PLoS One. 9:e1009442014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qiu L, Zhou C, Sun Y, Di W, Scheffler E,
Healey S, Kouttab N, Chu W and Wan Y: Crosstalk between EGFR and
TrkB enhances ovarian cancer cell migration and proliferation. Int
J Oncol. 29:1003–1011. 2006.PubMed/NCBI
|
|
53
|
de Farias CB, Heinen TE, dos Santos RP,
Abujamra AL, Schwartsmann G and Roesler R: BDNF/TrkB signaling
protects HT-29 human colon cancer cells from EGFR inhibition.
Biochem Biophys Res Commun. 425:328–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Heinen TE, dos Santos RP, da Rocha A, Dos
Santos MP, Lopez PL, Silva Filho MA, Souza BK, Rivero LF, Becker
RG, Gregianin LJ, et al: Trk inhibition reduces cell proliferation
and potentiates the effects of chemotherapeutic agents in Ewing
sarcoma. Oncotarget. 7:348602016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yee CL, Jones KR and Finger TE:
Brain-derived neurotrophic factor is present in adult mouse taste
cells with synapses. J Comp Neurol. 459:15–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Makino K, Kawamura K, Sato W, Kawamura N,
Fujimoto T and Terada Y: Inhibition of uterine sarcoma cell growth
through suppression of endogenous tyrosine kinase B signaling. PLoS
One. 7:e410492012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Iyer R, Evans AE, Qi X, Ho R, Minturn JE,
Zhao H, Balamuth N, Maris JM and Brodeur GM: Lestaurtinib enhances
the antitumor efficacy of chemotherapy in murine xenograft models
of neuroblastoma. Clin Cancer Res. 16:1478–1485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Iyer R, Wehrmann L, Golden RL, Naraparaju
K, Croucher JL, MacFarland SP, Guan P, Kolla V, Wei G, Cam N, et
al: Entrectinib is a potent inhibitor of Trk-driven neuroblastomas
in a xenograft mouse model. Cancer Lett. 372:179–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jaboin J, Kim CJ, Kaplan DR and Thiele CJ:
Brain-derived neurotrophic factor activation of TrkB protects
neuroblastoma cells from chemotherapy-induced apoptosis via
phosphatidylinositol 3′-kinase pathway. Cancer Res. 62:6756–6763.
2002.PubMed/NCBI
|
|
60
|
Bao W, Qiu H, Yang T, Luo X, Zhang H and
Wan X: Upregulation of TrkB promotes epithelial-mesenchymal
transition and anoikis resistance in endometrial carcinoma. PLoS
One. 8:e706162013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kupferman M, Jiffar T, El-Naggar A, Yilmaz
T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D and Myers JN:
TrkB induces EMT and has a key role in invasion of head and neck
squamous cell carcinoma. Oncogene. 29:2047–2059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ricci A, De Vitis C, Noto A, Fattore L,
Mariotta S, Cherubini E, Roscilli G, Liguori G, Scognamiglio G,
Rocco G, et al: TrkB is responsible for EMT transition in malignant
pleural effusions derived cultures from adenocarcinoma of the lung.
Cell Cycle. 12:1696–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Smit MA, Geiger TR, Song JY, Gitelman I
and Peeper DS: A Twist-Snail axis critical for TrkB-induced
epithelial-mesenchymal transition-like transformation, anoikis
resistance, and metastasis. Mol Cell Biol. 29:3722–3737. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee J, Jiffar T and Kupferman ME: A novel
role for BDNF-TrkB in the regulation of chemotherapy resistance in
head and neck squamous cell carcinoma. PLoS One. 7:e302462012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xia H, Li Y and Lv X: MicroRNA-107
inhibits tumor growth and metastasis by targeting the BDNF-mediated
PI3K/AKT pathway in human non-small lung cancer. Int J Oncol.
49:1325–1333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
DeWitt J, Ochoa V, Urschitz J, Elston M,
Moisyadi S and Nishi R: Constitutively active TrkB confers an
aggressive transformed phenotype to a neural crest-derived cell
line. Oncogene. 33:977–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen B, Liang Y, He Z, An Y, Zhao W and Wu
J: Autocrine activity of BDNF induced by the STAT3 signaling
pathway causes prolonged TrkB activation and promotes human
non-small-cell lung cancer proliferation. Sci Rep. 6:304042016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Navé BT, Ouwens M, Withers DJ, Alessi DR
and Shepherd PR: Mammalian target of rapamycin is a direct target
for protein kinase B: Identification of a convergence point for
opposing effects of insulin and amino-acid deficiency on protein
translation. Biochem J. 344:427–431. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Aoki M, Blazek E and Vogt PK: A role of
the kinase mTOR in cellular transformation induced by the
oncoproteins P3k and Akt. Proc Natl Acad Sci USA. 98:136–141. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Martens LK, Kirschner KM, Warnecke C and
Scholz H: Hypoxia-inducible factor-1 (HIF-1) is a transcriptional
activator of the TrkB neurotrophin receptor gene. J Biol Chem.
282:14379–14388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li Z, Zhang J, Liu Z, Woo CW and Thiele
CJ: Downregulation of Bim by brain-derived neurotrophic factor
activation of TrkB protects neuroblastoma cells from paclitaxel but
not etoposide or cisplatin-induced cell death. Cell Death Differ.
14:318–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Radin D, Lippa A, Patel P and Leonardi D:
Lifeguard inhibition of Fas-mediated apoptosis: A possible
mechanism for explaining the cisplatin resistance of
triple-negative breast cancer cells. Biomed Pharmacother.
77:161–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Voldborg BR, Damstrup L, Spang-Thomsen M
and Poulsen HS: Epidermal growth factor receptor (EGFR) and EGFR
mutations, function and possible role in clinical trials. Ann
Oncol. 8:1197–1206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hunter T and Pines J: Cyclins and cancer.
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bos JL: Ras oncogenes in human cancer: A
review. Cancer Res. 49:4682–4689. 1989.PubMed/NCBI
|
|
76
|
Davis RJ: The mitogen-activated protein
kinase signal transduction pathway. J Biol Chem. 268:14553–14556.
1993.PubMed/NCBI
|
|
77
|
Sinkevicius KW, Kriegel C, Bellaria KJ,
Lee J, Lau AN, Leeman KT, Zhou P, Beede AM, Fillmore CM, Caswell D,
et al: Neurotrophin receptor TrkB promotes lung adenocarcinoma
metastasis. Proc Natl Acad Sci USA. 111:10299–10304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Davis NM, Sokolosky M, Stadelman K, Abrams
SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro
A, et al: Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in
breast cancer: Possibilities for therapeutic intervention.
Oncotarget. 5:4603–4650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Morris LG, Taylor BS, Bivona TG, Gong Y,
Eng S, Brennan CW, Kaufman A, Kastenhuber ER, Banuchi VE, Singh B,
et al: Genomic dissection of the epidermal growth factor receptor
(EGFR)/PI3K pathway reveals frequent deletion of the EGFR
phosphatase PTPRS in head and neck cancers. Proc Natl Acad Sci USA.
108:19024–19029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lee DH, Szczepanski MJ and Lee YJ:
Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt
signaling pathway in human prostate cancer cells. J Cell Biochem.
106:1113–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cowley S, Paterson H, Kemp P and Marshall
CJ: Activation of MAP kinase kinase is necessary and sufficient for
PC12 differentiation and for transformation of NIH 3T3 cells. Cell.
77:841–852. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang EJ and Reichardt LF: Trk receptors:
Roles in neuronal signal transduction. Annu Rev Biochem.
72:609–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Siu MK, Wong OG and Cheung AN: TrkB as a
therapeutic target for ovarian cancer. Expert Opin Ther Targets.
13:1169–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yuan Y, Ye HQ and Ren QC: Upregulation of
the BDNF/TrKB pathway promotes epithelial-mesenchymal transition,
as well as the migration and invasion of cervical cancer. Int J
Oncol. 52:461–472. 2018.PubMed/NCBI
|
|
85
|
Cazorla M, Jouvenceau A, Rose C, Guilloux
JP, Pilon C, Dranovsky A and Prémont J: Cyclotraxin-B, the first
highly potent and selective TrkB inhibitor, has anxiolytic
properties in mice. PLoS One. 5:e97772010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cazorla M, Prémont J, Mann A, Girard N,
Kellendonk C and Rognan D: Identification of a low-molecular weight
TrkB antagonist with anxiolytic and antidepressant activity in
mice. J Clin Invest. 121:1846–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Light JE, Koyama H, Minturn JE, Ho R,
Simpson AM, Iyer R, Mangino JL, Kolla V, London WB and Brodeur GM:
Clinical significance of NTRK family gene expression in
neuroblastomas. Pediatr Blood Cancer. 59:226–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Bertrand T, Kothe M, Liu J, Dupuy A, Rak
A, Berne PF, Davis S, Gladysheva T, Valtre C, Crenne JY and Mathieu
M: The crystal structures of TrkA and TrkB suggest key regions for
achieving selective inhibition. J Mol Biol. 423:439–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Drilon A, Siena S, Ou SI, Patel M, Ahn MJ,
Lee J, Bauer TM, Farago AF, Wheler JJ, Liu SV, et al: Safety and
antitumor activity of the multitargeted pan-TRK, ROS1, and ALK
inhibitor entrectinib: Combined results from two phase I trials
(ALKA-372-001 and STARTRK-1). Cancer Discov. 7:400–409. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ardini E, Menichincheri M, Banfi P,
Bosotti R, De Ponti C, Pulci R, Ballinari D, Ciomei M, Texido G,
Degrassi A, et al: Entrectinib, a pan-TRK, ROS1, and ALK inhibitor
with activity in multiple molecularly defined cancer indications.
Mol Cancer Ther. 15:628–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Burris HA, Shaw AT, Bauer TM, Farago AF,
Doebele RC, Smith S, Nanda N, Cruickshank S, Low JA and Brose MS:
Pharmacokinetics (PK) of LOXO-101 during the first-in-human phase I
study in patients with advanced solid tumors: Interim update.
Cancer Res. 75:45292015. View Article : Google Scholar
|
|
92
|
Drilon A, Laetsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo
AS, et al: Efficacy of larotrectinib in TRK fusion-positive cancers
in adults and children. N Engl J Med. 378:731–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Al-Salama ZT and Keating GM: Cabozantinib:
A review in advanced renal cell carcinoma. Drugs. 76:1771–1778.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Abdelaziz A and Vaishampayan U:
Cabozantinib for renal cell carcinoma: Current and future
paradigms. Curr Treat Options Oncol. 18:182017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schiff D, Desjardins A, Cloughesy T,
Mikkelsen T, Glantz M, Chamberlain MC, Reardon DA and Wen PY: Phase
1 dose escalation trial of the safety and pharmacokinetics of
cabozantinib concurrent with temozolomide and radiotherapy or
temozolomide after radiotherapy in newly diagnosed patients with
high-grade gliomas. Cancer. 122:582–587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Radin DP, Zhong S, Purcell R and Lippa A:
Acute ampakine treatment ameliorates age-related deficits in
long-term potentiation. Biomed Pharmacother. 84:806–809. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Morgensztern D, Campo MJ, Dahlberg SE,
Doebele RC, Garon E, Gerber DE, Goldberg SB, Hammerman PS, Heist
RS, Hensing T, et al: Molecularly targeted therapies in
non-small-cell lung cancer annual update 2014. J Thorac Oncol.
10:(1 Suppl 1). S1–S63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cao L, Liu X, Lin EJ, Wang C, Choi EY,
Riban V, Lin B and During MJ: Environmental and genetic activation
of a brain-adipocyte BDNF/leptin axis causes cancer remission and
inhibition. Cell. 142:52–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu X, McMurphy T, Xiao R, Slater A, Huang
W and Cao L: Hypothalamic gene transfer of BDNF inhibits breast
cancer progression and metastasis in middle age obese mice. Mol
Ther. 22:1275–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Xiao R, Bergin SM, Huang W, Slater AM, Liu
X, Judd RT, Lin ED, Widstrom KJ, Scoville SD, Yu J, et al:
Environmental and genetic activation of hypothalamic BDNF modulates
T-cell immunity to exert an anticancer phenotype. Cancer Immunol
Res. 4:488–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Garofalo S, D'Alessandro G, Chece G, Brau
F, Maggi L, Rosa A, Porzia A, Mainiero F, Esposito V, Lauro C, et
al: Enriched environment reduces glioma growth through immune and
non-immune mechanisms in mice. Nat Commun. 6:66232015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Linker RA, Lee DH, Flach AC, Litke T, van
den Brandt J, Reichardt HM, Lingner T, Bommhardt U, Sendtner M,
Gold R, et al: Thymocyte-derived BDNF influences T-cell maturation
at the DN3/DN4 transition stage. Eur J Immunol. 45:1326–1338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jaboin J, Hong A, Kim CJ and Thiele CJ:
Cisplatin-induced cytotoxicity is blocked by brain-derived
neurotrophic factor activation of TrkB signal transduction path in
neuroblastoma. Cancer Lett. 193:109–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ochs G, Penn RD, York M, Giess R, Beck M,
Tonn J, Haigh J, Malta E, Traub M, Sendtner M and Toyka KV: A phase
I/II trial of recombinant methionyl human brain derived
neurotrophic factor administered by intrathecal infusion to
patients with amyotrophic lateral sclerosis. Amyotroph Lateral
Scler Other Motor Neuron Disord. 1:201–206. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Apfel SC, Kessler JA, Adornato BT, Litchy
WJ, Sanders C and Rask CA: Recombinant human nerve growth factor in
the treatment of diabetic polyneuropathy. NGF Study Group.
Neurology. 51:695–702. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kordower JH, Palfi S, Chen EY, Ma SY,
Sendera T, Cochran EJ, Cochran EJ, Mufson EJ, Penn R, Goetz CG and
Comella CD: Clinicopathological findings following intraventricular
glial-derived neurotrophic factor treatment in a patient with
Parkinson's disease. Ann Neurol. 46:419–424. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Radin DP, Purcell R and Lippa AS:
Oncolytic properties of ampakines in vitro. Anticancer Res.
38:265–269. 2018.PubMed/NCBI
|