Introduction
Colorectal cancer (CRC) is one of the most common
cancer types globally (1). In the
United States, New Zealand and France the incidence and mortality
rates of CRC have exhibited a downward trend, resulting from early
screening, improved therapy and preventive lifestyle choices
(2). The primary preventive measure
for CRC is a healthy lifestyle, including the maintenance of a
healthy weight, exercise, reduction of meat and alcohol
consumption, and avoidance of smoking (1). It is widely accepted that surgical
resection is the principal therapy option at the early stages of
CRC, which is defined as invasion and lymph node metastasis only
occurring locally (3). In addition,
adjuvant chemotherapy is an essential treatment for numerous
patients with resectable CRC (4).
Platelets are involved in multiple mechanisms of
cancer development and metastasis (5). Platelet-associated indicators assessed
by routine blood examination include platelet count (PLT), mean
platelet volume (MPV), platelet distribution width (PDW) and
plateletcrit (PCT). MPV reflects the average size, stimulation and
production rate of platelets. Pro-inflammatory and pro-thrombotic
conditions are closely associated with MPV (6,7). PCT is
calculated as the MPV multiplied by the PLT. It has been identified
that an elevated PCT is associated with coronary artery disease and
an increased risk of venous thrombosis (8,9). The PDW
is one of the platelet volume indicators, reflecting the average
change in platelet volume (10).
Therefore, platelet-associated indicators may be used as
inflammatory markers for CRC, in addition to their use in
inflammatory, thromboembolic and cardiovascular diseases.
In the present study, the hypothesis that
platelet-associated indices may provide useful prognostic
information was investigated in patients with resectable CRC.
Materials and methods
Subjects and inclusion criteria
The present study was conducted as a retrospective
investigation of patients with resectable CRC that had been
referred to the First Affiliated Hospital of Soochow University
(Jiangsu, China) between June 2006 and July 2016. The Medical
Ethics Committees of the First Affiliated Hospital of Soochow
University granted approval for the present study, and written
informed consent to participate was obtained from patients at the
beginning of the study. The clinical and pathological records of
all participating patients were reviewed periodically.
In total, 153 patients with resectable CRC were
recruited. All cases were confirmed by surgery and pathology. All
patients originally received six cycles of XELOX treatment, a
standard adjuvant chemotherapy including oxaliplatin administered
intravenously at a dose of 130 mg/m2 on day 1, and
capecitabine (850–1,250 mg/m2) twice a day for 2 weeks,
followed by a 1-week rest. Patient characteristics are detailed in
Table I. The median age of the
patients was 56 years (range, 27–85 years); 70 patients were male
and 83 were female. Cancer stage was determined using the
Tumor-Node-Metastasis (TNM) classification according to the
recommendations of the American Joint Committee on Cancer (AJCC)
(11). Prognostic analyses were
performed in regard to overall survival (OS).
 | Table I.Clinicopathological features of
patients with colorectal cancer. |
Table I.
Clinicopathological features of
patients with colorectal cancer.
|
|
| Platelet count | Plateletcrit | Mean platelet
volume | Platelet
distribution width |
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
features | Patients, n | Low, n | High, n | χ2 | P-value | Low, n | High, n | χ2 | P-value | Low, n | High, n | χ2 | P-value | Low, n | High, n | χ2 | P-value |
|---|
| Sex | 153 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Male | 70 | 45 | 25 | 10.058 | 0.002 | 42 | 28 | 3.616 | 0.057 | 35 | 35 | 0.006 | 0.941 | 36 | 34 | 0.038 | 0.845 |
|
Female | 83 | 32 | 51 |
|
| 37 | 46 |
|
| 42 | 41 |
|
| 44 | 39 |
|
|
| Age, years | 153 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≤56 | 81 | 38 | 43 | 0.802 | 0.370 | 39 | 42 | 0.837 | 0.360 | 34 | 47 | 4.802 | 0.028 | 46 | 35 | 1.399 | 0.237 |
|
>56 | 72 | 39 | 33 |
|
| 40 | 32 |
|
| 43 | 29 |
|
| 34 | 38 |
|
|
| Tumor size, cm | 153 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ≤5 | 86 | 46 | 40 | 0.785 | 0.376 | 45 | 41 | 0.038 | 0.846 | 44 | 42 | 0.055 | 0.815 | 42 | 44 | 0.937 | 0.333 |
|
>5 | 67 | 31 | 36 |
|
| 34 | 33 |
|
| 33 | 34 |
|
| 38 | 29 |
|
|
| Depth of
invasion | 153 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| T1,
T2 | 17 | 10 | 7 | 0.552 | 0.457 | 10 | 7 | 0.396 | 0.529 | 5 | 12 | 3.347 | 0.067 | 10 | 7 | 0.327 | 0.567 |
| T3,
T4 | 136 | 67 | 69 |
|
| 69 | 67 |
|
| 72 | 64 |
|
| 70 | 66 |
|
|
| Lymphonodus | 153 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| N0,
N1 | 111 | 54 | 57 | 0.456 | 0.500 | 53 | 58 | 2.445 | 0.118 | 55 | 56 | 0.098 | 0.755 | 62 | 49 | 2.064 | 0.151 |
| N2 | 42 | 23 | 19 |
|
| 26 | 16 |
|
| 22 | 20 |
|
| 18 | 24 |
|
|
| AJCC stage | 153 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| I,
II | 56 | 25 | 31 | 1.142 | 0.285 | 25 | 31 | 1.729 | 0.189 | 22 | 34 | 4.307 | 0.038 | 32 | 24 | 0.835 | 0.361 |
|
III | 97 | 52 | 45 |
|
| 54 | 43 |
|
| 55 | 42 |
|
| 48 | 49 |
|
|
Blood samples
Pre-surgery blood samples were collected up to 1
week before surgery. Post-surgery blood samples were regarded as
pre-chemotherapy samples and were collected 3 weeks after surgery.
Post-chemotherapy samples were collected after 3 months of standard
chemotherapy in order to eliminate the impact of treatment on
platelet-associated indicators. Peripheral venous blood (5–7 ml)
was collected into sterile EDTA tubes. All blood samples were
fasted and obtained between 6:30 and 7:30 a.m. in order to
standardize the known impact of circulating hormones (circadian
rhythm) on the number and subtype distribution of various white
blood cell indices (12).
Hematological parameters were analyzed up to 30 min after
collection, using a hematology analyzer (Sysmex XE-2100; Sysmex
Corporation). PLT, PCT, MPV and PDW blood levels are recorded in
Table I. The patients were divided
into two groups according to the median value of PLT, PCT, MPV or
PDW. The post-/pre-treatment ratios were defined as the rate of
pre- and post-treatment blood parameter values.
Computed tomography (CT)
evaluation
CT scans were performed every 2 months for response
assessment and evaluated according to the criteria of the Response
Evaluation Criteria in Solid Tumors 1.1 (13).
Follow-up
Patient survival time was recorded from the date of
chemotherapy until death or the last clinical evaluation.
Prognostic analyses were performed in relation to patient OS time.
OS was defined as the time from diagnosis to that of death from any
cause.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (IBM Corp.). For analysis of survival data,
Kaplan-Meier curves were constructed and statistical analysis was
performed using the log-rank test. The associations between
blood-parameter status and clinicopathological features were
determined using the χ2 test. The associations between
alterations in the status of platelet-associated indicators and
surgery or chemotherapy were assessed using a Student's t-test. The
multivariate logistic regression model was employed to identify the
independent risk factors associated with resectable CRC. P<0.05
was considered to indicate a statistically significant
difference.
Results
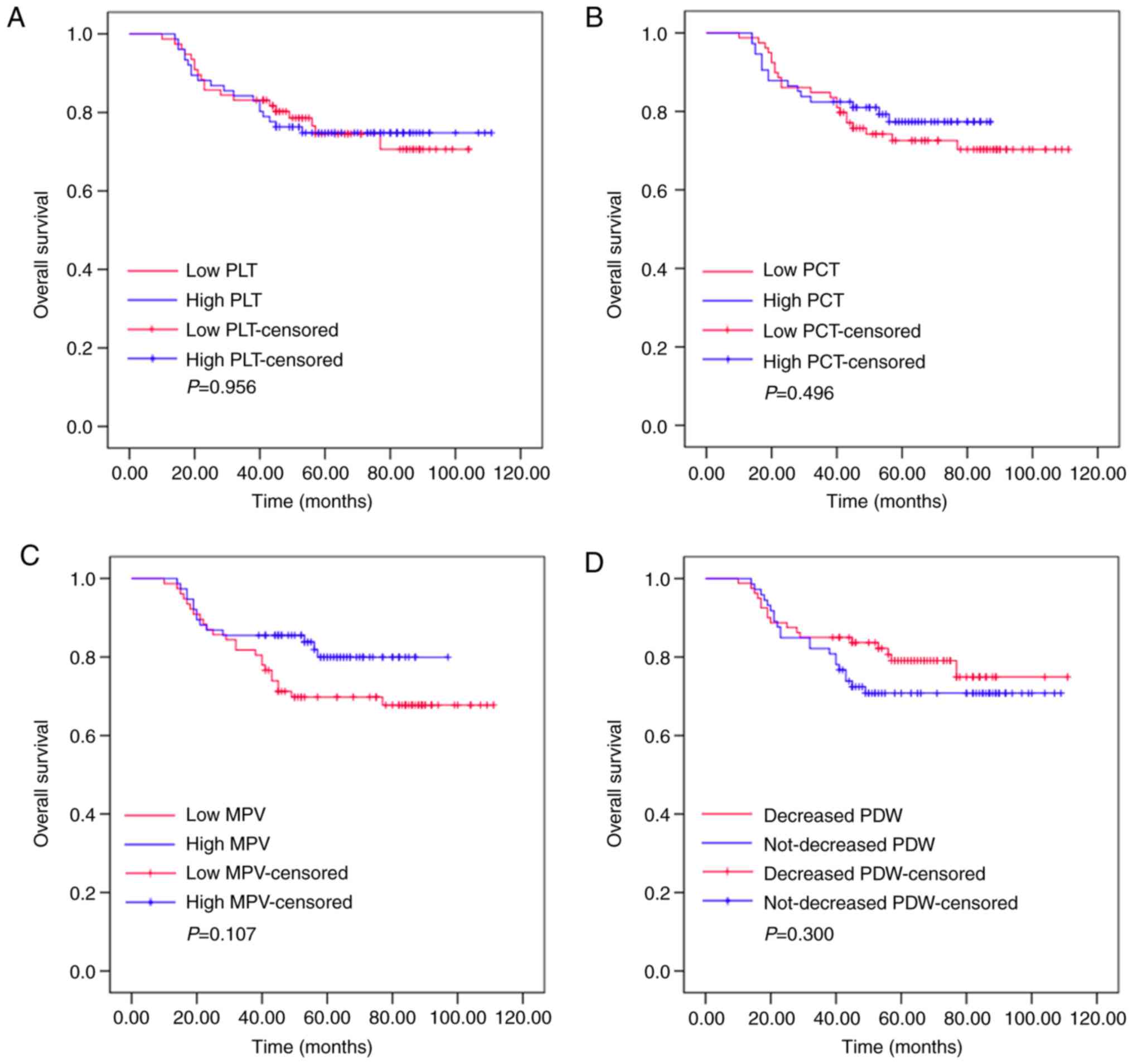
Pre-treatment PLT, PCT, MPV and PDW
values do not correlate with the outcome of patients with
resectable CRC
Kaplan-Meier plots were used to determine the
effects of pre-treatment PLT, PCT, MPV and PDW status on OS
(Fig. 1). The patients were divided
into two groups according to the median value of PLT (low PLT,
≤211.575×109/l, or high PLT,
>211.575×109/l), PCT (low PCT, ≤0.225 l/l, or high
PCT, >0.225 l/l), MPV (low MPV, ≤10.400 fl, or high MPV,
>10.400 fl) or PDW (low PDW, ≤15.485%, or high PDW,
>15.485%). The mean OS of the high PLT group was 63 months
(range, 53.392–72.608), while that of the low PLT group was 56
months (range, 49.312–62.688; P=0.956). The mean OS was 58 months
(range, 52.731–63.269) in the high PCT group and 64 months (range,
51.806–76.194) in the low PCT group (P=0.496). The mean OS of the
high MPV group was 58 months (range, 53.728–62.272), while that of
the low MPV group was 68 months (range, 46.025–89.975; P=0.107).
The mean OS was 52 months (range, 44.465–59.535) in the high PDW
group and 63 months (range, 58.623–67.377) in the low PDW group
(P=0.300). These results demonstrated that pre-treatment levels of
PLT, PCT, MPV or PDW had no effects on the OS of patients with
resectable CRC.
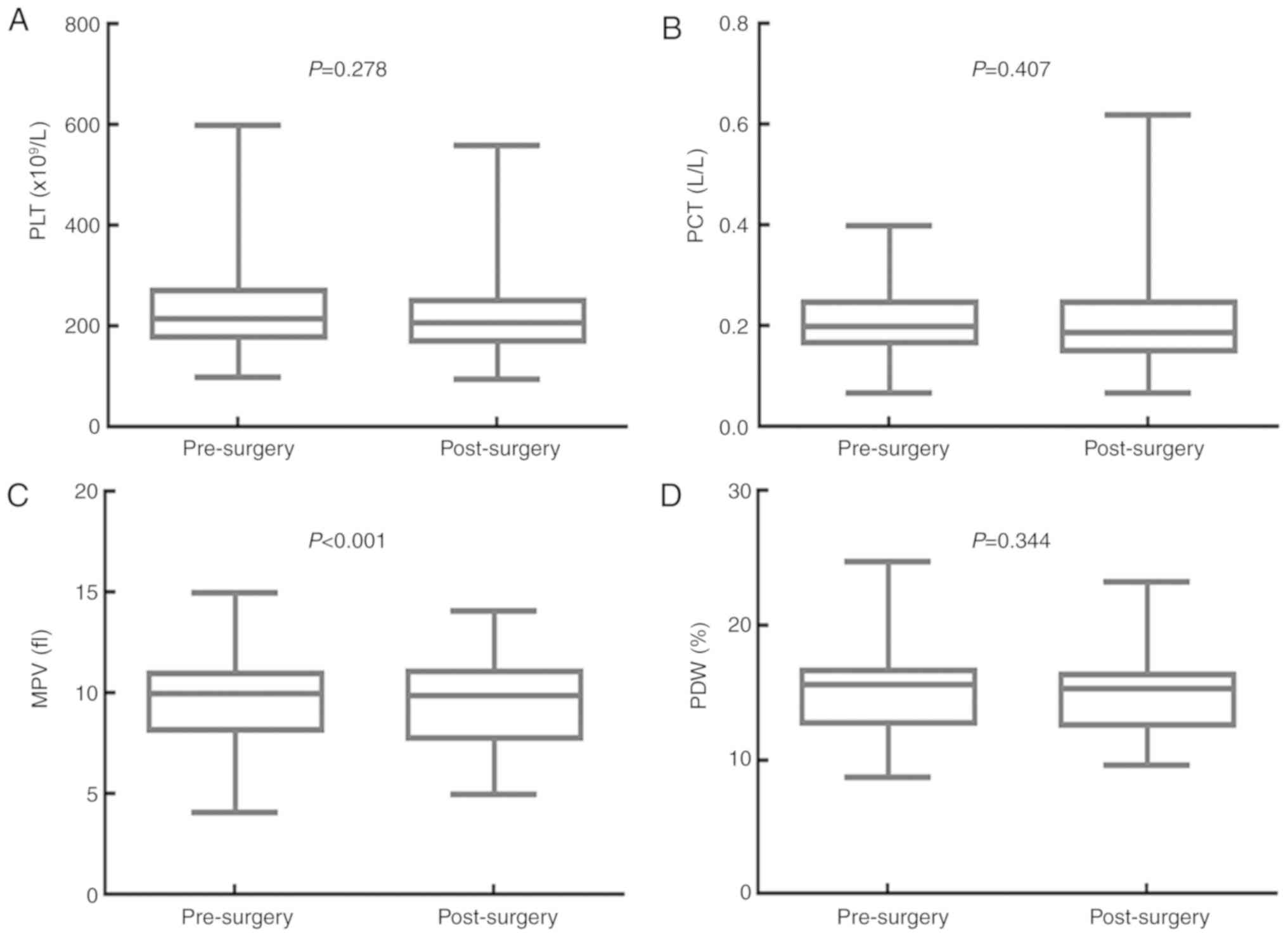
Surgery significantly decreases MPV, but has no
impact on PLT, PCT or PDW. The effects of surgery on the values for
PLT, PCT, MPV and PDW are presented in Fig. 2. The median PLT value was
216.000×109/l [95% confidence interval (CI),
201.030–230.970×109/l] before surgery, and
207.000×109/l (95% CI, 194.210–219.800×109/l)
after surgery (P=0.278). The median PCT value was 0.200 l/l (95%
CI, 0.190–0.220 l/l) before surgery, and 0.190 L/L (95% CI,
0.170–0.220 l/l) after surgery (P=0.407). The median MPV value was
10.000 fl (95% CI, 9.680–10.400 fl) before surgery, and 9.900 fl
(95% CI, 9.300–10.198 fl) after surgery (P<0.001). The median
PDW value was 15.600% (95% CI, 15.040–15.900%) before surgery, and
15.300% (95% CI, 14.760–15.700%) after surgery (P=0.344).
Therefore, surgery significantly decreased the MPV value, but had
no significant impact on the PLT, PCT and PDW values of patients
with resectable CRC.
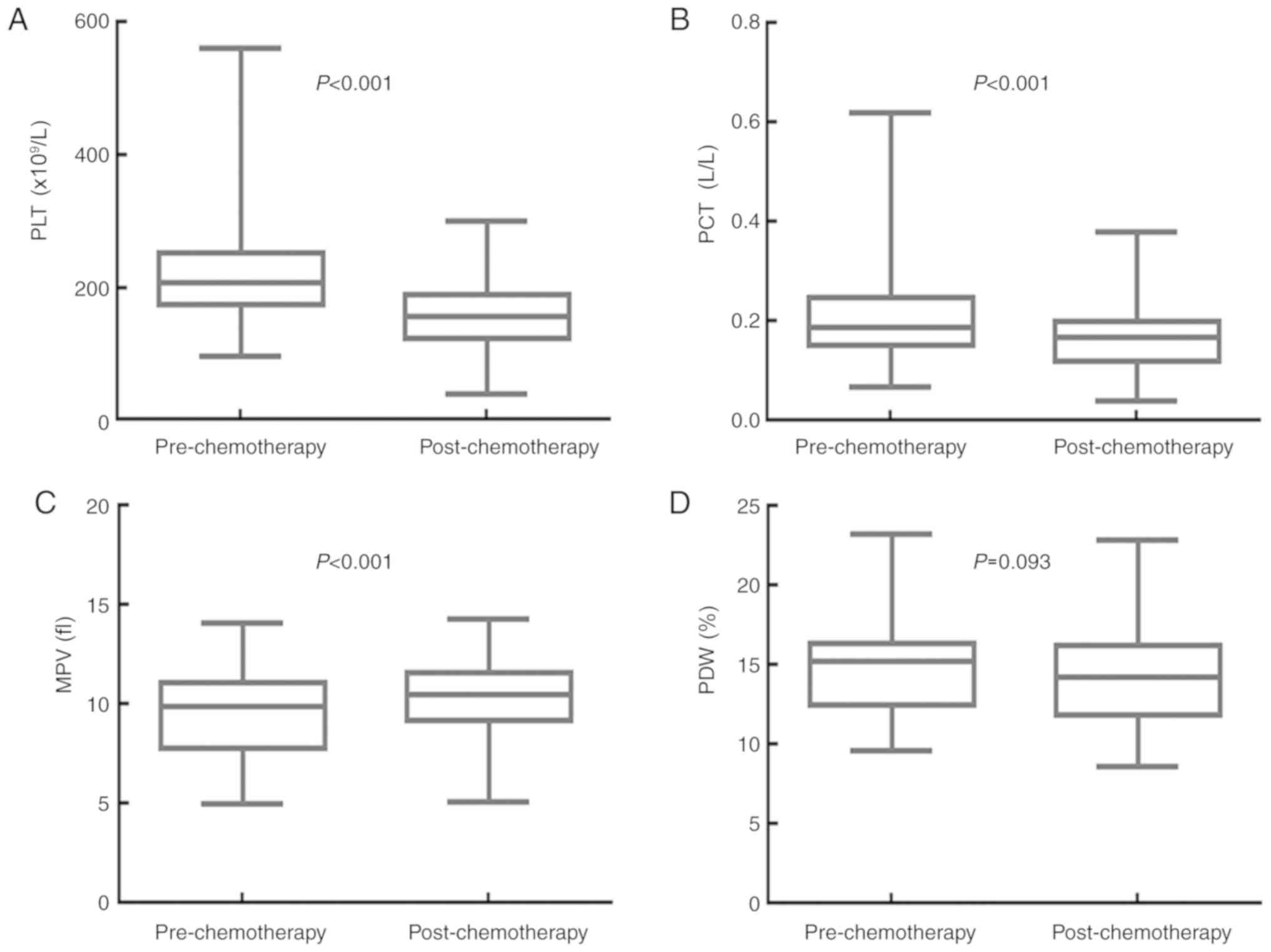
Adjuvant chemotherapy significantly
decreases PLT and PCT, and increases MPV, but has no effect on
PDW
The effects of adjuvant chemotherapy on the values
for PLT, PCT, MPV and PDW are shown in Fig. 3. The median PLT value was
207.000×109/l (95% CI, 194.210–219.800×109/l)
before, and 157.000×109/l (95% CI,
149.160–164.840×109/l) after adjuvant chemotherapy
(P<0.001). The median PCT value was 0.190 l/l (95% CI,
0.170–0.220 l/l) before, and 0.170 l/l (95% CI, 0.160–0.180 l/l)
following adjuvant chemotherapy (P<0.001). The median MPV value
was 9.900 fl (95% CI, 9.300–10.198 fl) before, and 10.500 fl (95%
CI, 10.200–10.800 fl) following adjuvant chemotherapy (P<0.001).
The median PDW value was 15.300% (95% CI, 14.760–15.700%) before
adjuvant chemotherapy, and 14.300% (95% CI, 13.200–15.000%) after
adjuvant chemotherapy (P=0.093). Adjuvant chemotherapy
significantly decreased the PLT and PCT values, but increased the
MPV value of patients with resectable CRC. There was no significant
impact on the PDW value.
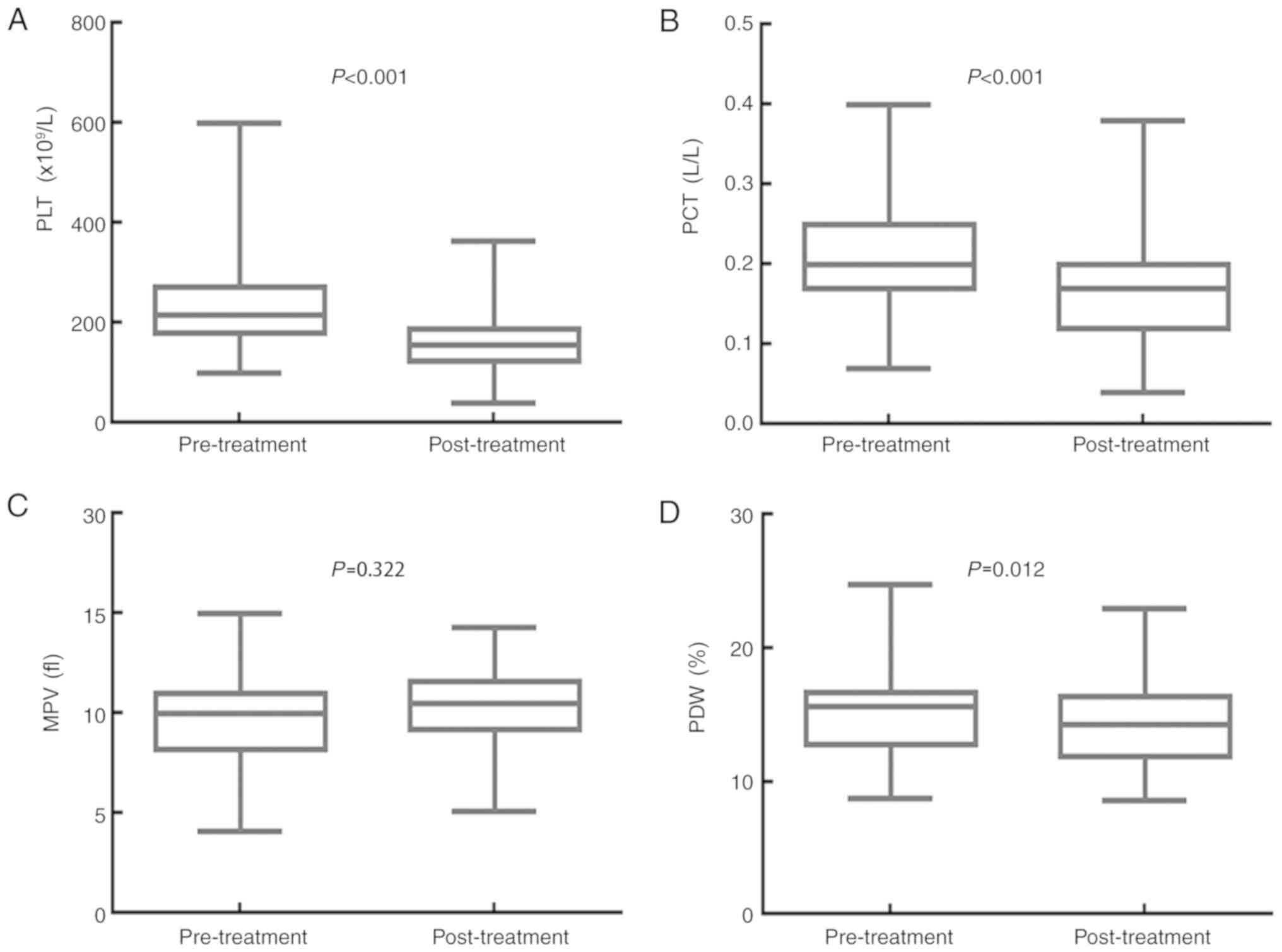
A whole course of treatment
significantly decreases PLT, PCT and PDW, but has no effect on
MPV
The impact of the whole course of treatment (surgery
and adjuvant chemotherapy) on the values for PLT, PCT, MPV and PDW
are displayed in Fig. 4. The median
PLT value was 216.000×109/l (95% CI,
201.030–230.970×109/l) before treatment and
157.000×109/l (95% CI, 149.160–164.840×109/l)
after treatment (P<0.001). The PCT median value was 0.200 l/l
(95% CI, 0.190–0.220 l/l) before treatment and 0.170 l/l (95% CI,
0.160–0.180 l/l) after treatment (P<0.001). The median MPV value
was 10.000 fl (95% CI, 9.680–10.400 fl) before treatment and 10.500
fl (95% CI, 10.200–10.800 fl) after treatment (P=0.322). The median
PDW value was 15.600% (95% CI, 15.040–15.900%) before treatment,
and 14.300% (95% CI, 13.200–15.000%) after treatment (P=0.012).
Treatment with surgery and adjuvant chemotherapy significantly
decreased the PLT, PCT and PDW values, but had no obvious effect on
the MPV value of patients with resectable CRC.
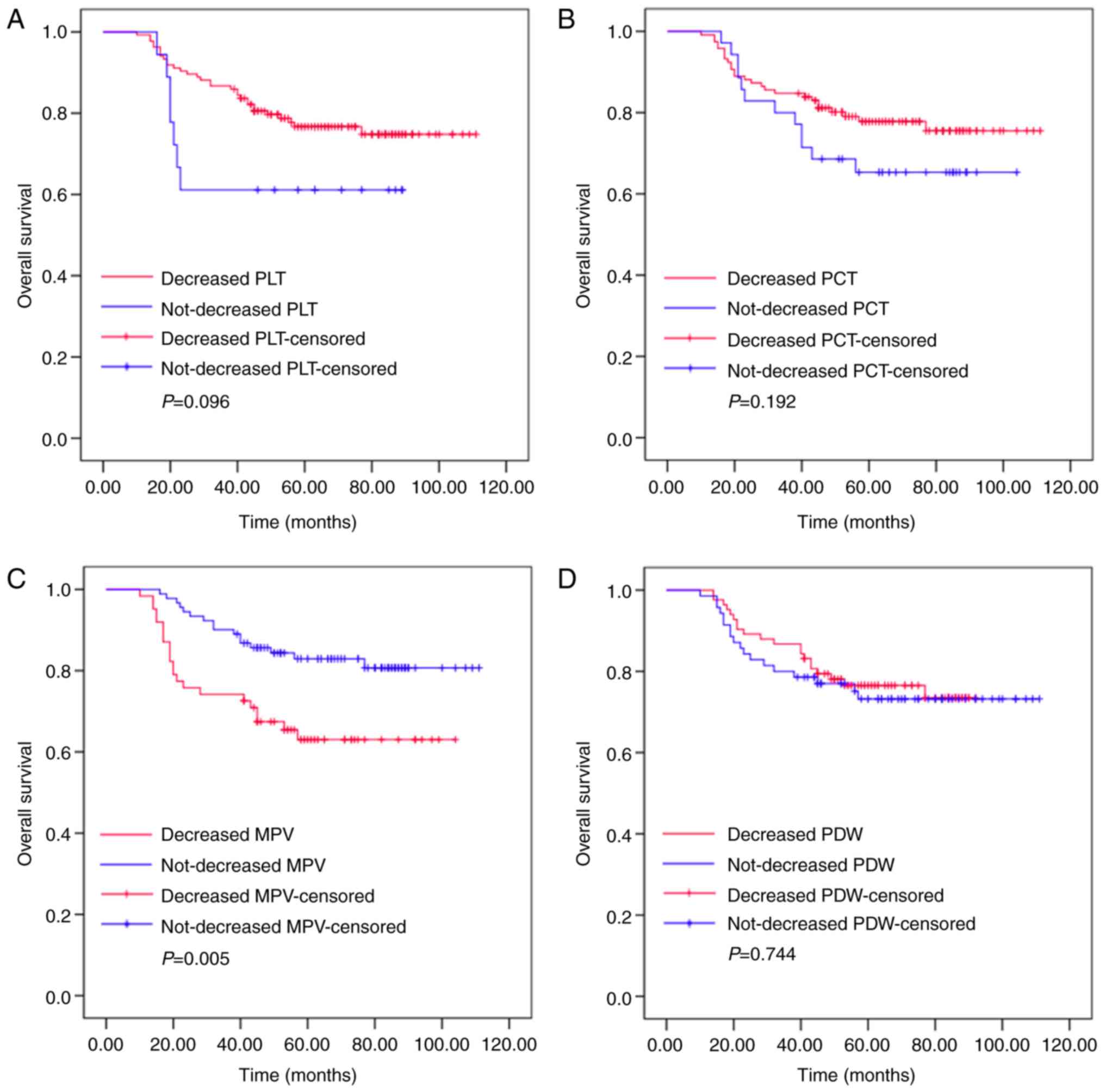
Changes in MPV following a whole
course of treatment predict the outcomes of patients with
resectable CRC
Kaplan-Meier plots were used to determine the effect
of changes in PLT, PCT, MPV and PDW status on OS (Fig. 5). The mean OS of patients whose MPV
decreased following a whole course of treatment (surgery and
adjuvant chemotherapy) was 75 months (range, 65.918–85.211), while
that of patients whose MPV did not decrease was 97 months (range,
90.599–103.210). Significant differences were identified for OS
between these two groups (P=0.005). The mean OS of patients whose
PLT level decreased following a whole course of treatment was 92
months (range, 85.875–97.804), while that of patients whose PLT did
not decrease was 62 months (range, 46.703–77.742; P=0.096). The
mean OS of patients whose PCT level decreased following a whole
course of treatment was 92 months (range, 85.409–98.433), while
that of the non-decreased group was 78 months (rang, 66.979–90.514;
P=0.192). The mean OS of patients whose PDW level decreased
following a whole course of treatment was 77 months (range,
71.466–83.015), while those where PDW did not decrease was 88
months (range, 79.668–97.597; P=0.744). Thus, the patients whose
MPV level increased following surgery and adjuvant chemotherapy had
increased survival ratios. However, changes in PLT, PCT and PDW had
no significant effect on OS.
Univariate and multivariate analyses
of prognostic factors in patients with resectable CRC
Univariate analyses demonstrated that sex [female;
hazard ratio (HR), 2.911; 95% confidence interval (CI),
1.413–5.998; P=0.004), tumor size (>5 cm; HR 2.613; 95% CI
1.351–5.054; P=0.004), lymphonodus metastasis (N2; HR 2.197; 95% CI
1.153–4.187; P=0.017), AJCC stage (III; HR 2.408; 95% CI
1.103–5.254; P=0.027) and decreased post/pre-treatment MPV ratio
(HR 2.430; 95% CI 1.274–4.636; P=0.007) were significant risk
factors for poor prognosis (Table
II). In multivariate analysis, sex (female; HR 3.236; 95% CI
1.553–6.745; P=0.002), tumor size (>5 cm; HR 2.058; 95% CI
1.007–4.203; P=0.048) and decreased post/pre-treatment MPV ratio
(HR 2.621; 95% CI 1.329–5.131; P=0.005) were found to be
independently associated with poor survival.
 | Table II.Univariate and multivariate logistic
regression analysis of risk factors for resectable colorectal
cancer. |
Table II.
Univariate and multivariate logistic
regression analysis of risk factors for resectable colorectal
cancer.
|
| Overall
survival |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Risk factors | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
| Female
or male | 2.911
(1.413–5.998) | 0.004 | 3.236
(1.553–6.745) | 0.002 |
| Age |
|
|
|
|
| >56
or ≤56 years | 1.003
(0.974–1.034) | 0.825 | – | – |
| Tumor size, cm |
|
|
|
|
| >5
or ≤5 | 2.613
(1.351–5.054) | 0.004 | 2.058
(1.007–4.203) | 0.048 |
| Depth of
invasion |
|
|
|
|
| T3-4 or
T1-2 | 2.591
(0.624–10.77) | 0.190 | – | – |
| Lymphonodus,
metastasis |
|
|
|
|
| N2 or
N0-1 | 2.197
(1.153–4.187) | 0.017 | 2.070
(0.967–4.429) | 0.061 |
| AJCC stage |
|
|
|
|
| III or
I–II | 2.408
(1.103–5.254) | 0.027 | 1.440
(0.588–3.529) | 0.425 |
| Pre-treatment
PLT |
|
|
|
|
|
≤211.575 or >
211.575×109/l | 1.018
(0.539–1.924) | 0.956 | – | – |
| Pre-treatment
PCT |
|
|
|
|
| ≤0.225
or >0.225 l/l | 1.250
(0.656–2.383) | 0.498 | – | – |
| Pre-treatment
MPV |
|
|
|
|
| ≤10.400
or >10.400 fl | 1.709
(0.883–3.309) | 0.112 | – | – |
| Pre-treatment
PDW |
|
|
|
|
| ≤15.485
or >15.485% | 0.714
(0.376–1.356) | 0.304 | – | – |
| Post-/pre-treatment
PLT ratio |
|
|
|
|
| <1
or ≥1 | 0.506
(0.222–1.150) | 0.104 | – | – |
| Post-/pre-treatment
PCT ratio |
|
|
|
|
| <1
or ≥1 | 0.638
(0.322–1.264) | 0.197 | – | – |
| Post-/pre-treatment
MPV ratio |
|
|
|
|
| <1
or ≥1 | 2.430
(1.274–4.636) | 0.007 | 2.612
(1.329–5.131) | 0.005 |
| Post-/pre-treatment
PDW ratio |
|
|
|
|
| <1
or ≥1 | 0.893
(0.472–1.688) | 0.727 | – | – |
Discussion
Platelets, derived from megakaryocytes, have a
crucial role in a variety of physiological and pathological
processes, including hemostasis, wound healing, inflammatory
responses and thrombosis. Previous studies have confirmed that a
high PLT, which is associated with tumorigenesis, metastasis and
angiogenesis, is a common phenomenon in patients with solid cancer
types (14,15). Meta-analyses have suggested that a
high PLT may have diagnostic and prognostic value in various types
of cancer, including CRC (16,17).
Platelet activation is involved in progressive tumor growth and
metastasis via the release of cytokines, chemokines and the
expression of numerous adhesion receptors (18,19).
Cancer cell-derived interleukin (IL-)6 is a trigger for high,
tumor-induced PLT in several types of cancer (20,21). By
protecting circulating tumor cells from immune-surveillance,
platelets contribute to metastasis (14). In addition, the obstructions formed
by platelets and tumor cells in the microcirculation facilitate the
extravasation of tumor cells to metastatic sites. In the present
study, surgery combined with adjuvant chemotherapy significantly
decreased the PLT of patients with CRC. Chemotherapy was likely the
primary cause of this downregulation, since surgery had no effect
on PLT status. However, the post/pre-treatment ratio of PLT, which
was based on changes in individual PLTs, had no apparent
association with OS.
Platelet-associated indicators are closely
associated with the activation and function of platelets. MPV
reflects early platelet activation (6–10,14–22)
and PCT, obtained by multiplying the PLT and the MPV, reflects the
percentage of the blood volume occupied by platelets. The PDW has
been one of the most commonly used markers to indicate platelet
activity. According to previous studies, there may be a correlation
between platelet-associated indicators and the prognosis of
patients with cancer (23,24). The PCT differs depending on the
stage, histological type and metastatic status of various types of
cancer. For instance, the PCT is higher in patients with epithelial
ovarian or endometrial cancer compared with that in healthy
controls (25,26). PCT was also reported to be positively
correlated with common causes of mortality, including venous
thrombosis and tumor metastasis, in patients with CRC (8,27,28).
Although surgery and adjuvant chemotherapy significantly reduced
PCT in the present study, the post/pre-treatment ratio of PCT was
not associated with OS.
The implication of the MPV is controversial at
present. Certain studies have indicated that MPV in patients with
cancer was higher compared with that in normal controls (29,30). In
addition, other studies have reported a positive correlation
between high MPV and advanced cancer stage (24,31).
However, several studies have indicated the opposite, stating that
a low MPV was associated with unfavorable prognoses in non-small
cell lung cancer (NSCLC) (32,33).
Kumagai et al (33)
determined that in patients with resectable NSCLC, an MPV of
<8.50 fl was an independent predictive indicator for shorter
disease-free survival and OS. In addition, Tuncel et al
(34) demonstrated that the MPV was
significantly higher in patients with metastatic CRC (mCRC)
compared with that in patients with non-mCRC, and that the benefits
of bevacizumab on progression-free survival were significantly
greater in patients with low MPV, compared with those with high MPV
status. Furthermore, in a study by Li et al (35), patients with a low MPV (≤8.6 fl) had
a significantly improved OS compared with those with a high MPV
(>8.6 fl). Multivariate analysis revealed that high MPV was an
independent risk factor affecting OS in patients with resectable
CRC. In the present study, surgery increased MPV, while
chemotherapy decreased it, leading to an MPV that was unchanged
(compared with baseline) following treatment with both surgery and
adjuvant chemotherapy. Furthermore, based on individual MPV values,
subjects whose MPV's were not decreased had better outcomes.
Univariate and multivariate analyses demonstrated that a decreased
post/pre-treatment MPV ratio was a significant and independent risk
factor associated with poor prognosis. Therefore, an unchanged MPV
following treatment may indicate a favorable prognostic index in
resectable CRC.
The association between decreased MPV and poor
prognosis may be due to a number of variables. The key to
explaining this decrease may be the association between blood
coagulation and cancer in general. A study has demonstrated that
tumor necrosis factor-α, IL-1β, vascular endothelial growth factor
and basic fibroblast growth factor released from various cells
stimulated the formation of vascular endothelial thrombi, and
enhanced the destruction of larger-sized platelets, resulting in a
decreased MPV in the circulating platelets (36). Another study suggested that increased
thrombotic activity in patients with metastatic colon cancer led to
a decrease in the MPV, which was in most cases considered to
correlate with poor survival (37).
PDW, another platelet index, has been reported to be
a diagnostic and prognostic factor for cancer (38). Oncel et al (10) suggested that the PDW was
significantly higher in patients with lung cancer compared with
that in the control group. Ma et al (25) identified that the PDW was higher in
the epithelial ovarian cancer group compared with that in the
benign tumor and healthy control groups. Hirahara et al
(39) focused on PDW and
cancer-specific survival in patients with esophageal cancer. Due to
a small proportion of patients with an elevated PDW, it was not
possible to assess the statistical association between PDW and
prognostic factors. In a recent retrospective study by Song et
al (40), univariate analysis
revealed that an elevated PDW was an independent risk factor for
recurrence-free survival and OS in patients with non-mCRC. In the
present study, it was identified that surgery combined with
adjuvant chemotherapy significantly decreased PDW. However, the
post/pre-treatment ratio of PDW had no significant impact on
OS.
In conclusion, the present study indicated that an
increased MPV following a whole course of treatment was associated
with improved OS in patients with resectable CRC, and that a low
post-/pre-treatment MPV ratio was an independent negative
prognostic factor for OS. Of note, the present study had certain
limitations. It was a retrospective study with a small sample size,
involving only 153 patients. Patients with systemic diseases,
including hypertension, diabetes, rheumatic disease and infection
were excluded in order to eliminate the impact of these conditions
on platelet-associated indicators. In spite of the small sample
size, the results of the present study were representative to a
certain extent. A multi-center study with a larger sample
population will be performed in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472296, 81602091,
81402176, 81402093, 81272542 and 81200369), the Six Major Talent
Peak Project of Jiangsu Province (grant no. 2015-WSN-022), the
Project of Invigorating Health Care through Science, Technology and
Education, Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016709), the Project of Jiangsu Provincial Commission of
Health and Family Planning (grant no. H201518), the Science and
Education for Health Foundation of Suzhou for Youth (grant no.
kjxw2015003), and the Science and Technology Project Foundation of
Suzhou (grant nos. SYS201464 and SYS201504).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT and WJW designed the study. WQ, JW, FRG and MYW
collected the data. MDX, LL and WL carried out the statistical
analysis. XXG interpreted and checked the data. WQ and XXG wrote
the paper. WQ and MDX were responsible for the revision of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Medical Ethics Committees of the First
Affiliated Hospital of Soochow University granted approval for the
present study. Written informed consent was obtained from all
patients.
Patient consent for publication
All patients provided consent for the publication of
their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Punt CJ, Koopman M and Vermeulen L: From
tumour heterogeneity to advances in precision treatment of
colorectal cancer. Nat Rev Clin Oncol. 14:235–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du C, Huang D, Peng Y, Yao Y, Zhao Y, Yang
Y, Wang H, Cao L, Zhu WG and Gu J: 5-Fluorouracil targets histone
acetyltransferases p300/CBP in the treatment of colorectal cancer.
Cancer Lett. 400:183–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho-Tin-Noé B, Goerge T, Cifuni SM,
Duerschmied D and Wagner DD: Platelet granule secretion
continuously prevents intratumor hemorrhage. Cancer Res.
68:6851–6858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colkesen Y and Muderrisoglu H: The role of
mean platelet volume in predicting thrombotic events. Clin Chem Lab
Med. 50:631–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lian L, Xia YY, Zhou C, Shen XM, Li XL,
Han SG, Zheng Y, Gong FR, Tao M and Li W: Mean platelet volume
predicts chemotherapy response and prognosis in patients with
unresectable gastric cancer. Oncol Lett. 10:3419–3424. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vázquez-Santiago M, Vilalta N, Ziyatdinov
A, Cuevas B, Macho R, Pujol-Moix N, Carrasco M, Mateo J,
Fontcuberta J, Soria JM and Souto JC: Platelet count and
plateletcrit are associated with an increased risk of venous
thrombosis in females. Results from the RETROVE study. Thromb Res.
157:162–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ifran A, Hasimi A, Kaptan K, Nevruz O,
Beyan C and Erbil K: Evaluation of platelet parameters in healthy
apheresis donors using the ADVIA 120. Transfus Apher Sci. 33:87–90.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oncel M, Kiyici A, Sunam GS, Sahin E and
Adam B: Evaluation of platelet indices in lung cancer patients.
Asian Pac J Cancer Prev. 16:7599–7602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim KH, Yang SS, Yoon YS, Lim SB, Yu CS
and Kim JC: Validation of the seventh edition of the American Joint
Committee on Cancer tumor-node-metastasis (AJCC TNM) staging in
patients with stage II and stage III colorectal carcinoma: Analysis
of 2,511 cases from a medical centre in Korea. Colorectal Dis.
13:e220–e226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dimitrov S, Benedict C, Heutling D,
Westermann J, Born J and Lange T: Cortisol and epinephrine control
opposing circadian rhythms in T cell subsets. Blood. 113:5134–5143.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keil S, Barabasch A, Dirrichs T, Bruners
P, Hansen NL, Bieling HB, Brümmendorf TH and Kuhl CK: Target lesion
selection: An important factor causing variability of response
classification in the response evaluation criteria for solid tumors
1.1. Invest Radiol. 49:509–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buergy D, Wenz F, Groden C and Brockmann
MA: Tumor-platelet interaction in solid tumors. Int J Cancer.
130:2747–2760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Lv Z, Yu H and Zhu J: The
clinicopathological and prognostic role of thrombocytosis in
patients with cancer: A meta-analysis. Oncol Lett. 13:5002–5008.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo T, Krzystanek M, Szallasi Z and
Szallasi A: Thrombocytosis portends adverse prognostic significance
in patients with stage II colorectal carcinoma. F1000Res.
3:1802014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M and Geng JG: P-selectin mediates
adhesion of leukocytes, platelets, and cancer cells in
inflammation, thrombosis, and cancer growth and metastasis. Arch
Immunol Ther Exp (Warsz). 54:75–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ho-Tin-Noé B, Demers M and Wagner DD: How
platelets safeguard vascular integrity. J Thromb Haemost. 9 (Suppl
1):S56–S65. 2011. View Article : Google Scholar
|
|
20
|
Nakashima J, Tachibana M, Horiguchi Y, Oya
M, Ohigashi T, Asakura H and Murai M: Serum interleukin 6 as a
prognostic factor in patients with prostate cancer. Clin Cancer
Res. 6:2702–2706. 2000.PubMed/NCBI
|
|
21
|
Paule B, Belot J, Rudant C, Coulombel C
and Abbou CC: The importance of IL-6 protein expression in primary
human renal cell carcinoma: An immunohistochemical study. J Clin
Pathol. 53:388–390. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen XM, Xia YY, Lian L, Zhou C, Li XL,
Han SG, Zheng Y, Gong FR, Tao M, Mao ZQ and Li W: Mean platelet
volume provides beneficial diagnostic and prognostic information
for patients with resectable gastric cancer. Oncol Lett.
12:2501–2506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wodarczyk M, Kasprzyk J,
Sobolewska-Wodarczyk A, Wƚodarczyk J, Tchórzewski M, Dziki A and
Dziki Ƚ: Mean platelet volume as a possible biomarker of tumor
progression in rectal cancer. Cancer Biomark. 17:411–417. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurtoglu E, Kokcu A, Celik H, Sari S and
Tosun M: Platelet indices may be useful in discrimination of benign
and malign endometrial lesions, and early and advanced stage
endometrial cancer. Asian Pac J Cancer Prev. 16:5397–5400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma X, Wang Y, Sheng H, Tian W, Qi Z, Teng
F and Xue F: Prognostic significance of thrombocytosis, platelet
parameters and aggregation rates in epithelial ovarian cancer. J
Obstet Gynaecol Res. 40:178–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karateke A, Kaplanoglu M and Baloglu A:
Relations of platelet indices with endometrial hyperplasia and
endometrial cancer. Asian Pac J Cancer Prev. 16:4905–4908. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borsig L: The role of platelet activation
in tumor metastasis. Expert Rev Anticancer Ther. 8:1247–1255. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ay C, Pabinger I and Cohen AT:
Cancer-associated venous thromboembolism: Burden, mechanisms, and
management. Thromb Haemost. 117:219–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurt M, Onal IK, Sayilir AY, Beyazit Y,
Oztas E, Kekilli M, Turhan N, Karaman K and Akdogan M: The role of
mean platelet volume in the diagnosis of hepatocellular carcinoma
in patients with chronic liver disease. Hepatogastroenterology.
59:1580–1582. 2012.PubMed/NCBI
|
|
30
|
Karaman K, Bostanci EB, Aksoy E, Kurt M,
Celep B, Ulas M, Dalgic T, Surmelioglu A, Hayran M and Akoglu M:
The predictive value of mean platelet volume in differential
diagnosis of non-functional pancreatic neuroendocrine tumors from
pancreatic adenocarcinomas. Eur J Intern Med. 22:e95–e98. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho SY, Yang JJ, You E, Kim BH, Shim J,
Lee HJ, Lee WI, Suh JT and Park TS: Mean platelet volume/platelet
count ratio in hepatocellular carcinoma. Platelets. 24:375–377.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inagaki N, Kibata K, Tamaki T, Shimizu T
and Nomura S: Prognostic impact of the mean platelet
volume/platelet count ratio in terms of survival in advanced
non-small cell lung cancer. Lung Cancer. 83:97–101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumagai S, Tokuno J, Ueda Y, Marumo S,
Shoji T, Nishimura T, Fukui M and Huang CL: Prognostic significance
of preoperative mean platelet volume in resected non-small-cell
lung cancer. Mol Clin Oncol. 3:197–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tuncel T, Ozgun A, Emirzeoglu L, Celik S,
Bilgi O and Karagoz B: Mean platelet volume as a prognostic marker
in metastatic colorectal cancer patients treated with
bevacizumab-combined chemotherapy. Asian Pac J Cancer Prev.
15:6421–6423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li N, Yu Z, Zhang X, Liu T, Sun YX, Wang
RT and Yu KJ: Elevated mean platelet volume predicts poor prognosis
in colorectal cancer. Sci Rep. 7:102612017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mutlu H, Berk V, Karaca H, Erden A, Aslan
T and Akca Z: Treatment regimen with bevacizumab decreases mean
platelet volume in patients with metastatic colon cancer. Clin Appl
Thromb Hemost. 18:546–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mutlu H, Artis TA, Erden A and Akca Z:
Alteration in mean platelet volume and platicrit values in patients
with cancer that developed thrombosis. Clin Appl Thromb Hemost.
19:331–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vagdatli E, Gounari E, Lazaridou E,
Katsibourlia E, Tsikopoulou F and Labrianou I: Platelet
distribution width: A simple, practical and specific marker of
activation of coagulation. Hippokratia. 14:28–32. 2010.PubMed/NCBI
|
|
39
|
Hirahara N, Matsubara T, Kawahara D,
Mizota Y, Ishibashi S and Tajima Y: Prognostic value of
hematological parameters in patients undergoing esophagectomy for
esophageal squamous cell carcinoma. Int J Clin Oncol. 21:909–919.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song X, Zhu H, Pei Q, Tan F, Li C, Zhou Z,
Zhou Y, Yu N, Li Y and Pei H: Significance of inflammation-based
indices in the prognosis of patients with non-metastatic colorectal
cancer. Oncotarget. 8:45178–45189. 2017.PubMed/NCBI
|