Introduction
Primary hepatocellular carcinoma (HCC) remains the
third leading cause of cancer-associated mortality in China.
Clinical treatment modalities include surgical resection,
transplantation, chemotherapy and radiotherapy. The 5-year survival
rate of patients with HCC was 5–30% between 2000 and 2014 (1). The main reason is that the initial
symptoms of HCC are occult, and the majority of patients are
diagnosed only at an advanced stage. Therefore, there is an urgent
need to identify early HCC biomarkers and novel treatment
targets.
In recent years, the reprogramming of metabolic
pathways in tumor cells is a field of cancer research that has
attracted attention (2,3). De novo lipogenesis increases in
liver cancer cells, and the endogenous lipid metabolism network
changes significantly. Endocannabinoids are a class of endogenous
lipids that target cannabinoid receptors 1 and 2
(CB1/2), the most extensively investigated of which are
anandamide (AEA) and 2-arachidonylglycerol (2-AG). Endocannabinoids
modulate multiple cell survival-associated signaling pathways,
including extracellular signal-regulated kinase (ERK) (4), p38 mitogen-activated protein kinase
(MAPK) (5) and the ceramide pathways
(6) in breast cancer (7), prostate cancer (8), rectal cancer (9) and glioma (10). Ceramides are widely distributed as
sphingolipid messengers involved in apoptosis and cell cycle
arrest. It was recently demonstrated that CB receptor
activation-dependent apoptosis signaling is associated with
ceramide accumulation (6).
Increased synthesis of endogenous mono-unsaturated
fatty acids (MUFA) is another biochemical hallmark of cancer
(11). Previous reports have shown
that high expression of stearoyl-CoA desaturase-1 (SCD1) catalyzes
the desaturation of saturated fatty acids (SFA), which provides
abundant MUFA substrates for membrane biosynthesis during tumor
cell proliferation. Inhibition of SCD1 suppressed the synthesis of
MUFA and induced de novo synthesis of long-chain ceramides,
which is a major mechanism mediating apoptosis in a variety of
tumor cells (12).
The endogenous lipid metabolism network is
associated with the occurrence and progression of malignancies. The
abundance of prostaglandin E2 in the tumor microenvironment further
impedes T cell infiltration and cancer immune evasion (13). However, the expression, metabolism
and regulation of these lipids in HCC tissues have yet to be
elucidated. In the present study, the pathological data from 67
patients with HCC who underwent surgical resection were
retrospectively analyzed, and the differences in the levels of
endogenous lipids and metabolic enzymes between tumor tissues and
their non-tumor counterparts were evaluated. Examination of
specific lipid metabolism profiles may provide targets and markers
for HCC treatment and early diagnosis.
Materials and methods
Human tissue samples
Samples from cancer tissues and their adjacent
normal counterparts were obtained from 67 patients who had
undergone surgical resection for HCC at the Fifth Hospital of
Xiamen (Xiamen, China) between January 2015 and December 2016. The
mean age of the patients was 52.6±11.3 years (range, 18–76 years).
In total, 83.6% of the patients were male, consistent with a prior
report that HCC is more prevalent in men compared with women
(14). The patients were diagnosed
with HCC via pathological examination (data not shown). All
patients were informed of the aims of this study, and they provided
written informed consent for the investigation in accordance with
the Ethics Committee in the Fifth Hospital of Xiamen, following the
clinical registration guidelines in China. This study was approved
by the Ethics Committee of the Fifth Hospital of Xiamen.
Tumor-node-metastasis (TNM) stage was determined according to the
World Health Organization TNM staging 7th edition and the
pathological analysis results. The samples were sectioned and some
were stored at −80°C for western blot and high-performance liquid
chromatography (HPLC)-mass spectrometry (MS) analysis, whereas
others were flash-frozen in liquid nitrogen for PCR analysis.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from tissue samples using
TRIzol reagent (Thermo Fisher Scientific, Inc.), and RNA
concentration was measured with a spectrophotometer (Beckman
Coulter, Inc.). Total RNA (1 µg) was reverse-transcribed to cDNA
with the ReverTra Ace® qPCR RT kit (Toyobo Life
Science), according to the manufacturer's protocols, and amplified
with Ex Taq DNA polymerase, according to the manufacturer's
protocols. qPCR was carried out on an Applied Biosystems 7500 Fast
Real-Time PCR System (Thermo Fisher Scientific, Inc.) with SYBR
Premix Ex Taq II (Takara Bio Inc., Otsu, Japan). The thermocycling
conditions were as follows: Denaturation at 95°C for 5 sec,
annealing/extension at 60°C for 31 sec (40 cycles). The
quantitative values of mRNA were analyzed using the
2–ΔΔCq method (15) and
normalized relative to the levels of 18S. Each sample was set up in
triplicate and the experiments were repeated three times.
The primers were as follows:
N-acylphosphatidylethanolamine-hydrolysingphospholipase D
(NAPE-PLD), forward primer (F): 5′-TGGCTGGGACACGCG-3′, reverse
primer (R): 5′-GGGATCCGTGAGGAGGATG-3′; fatty acid amide hydrolase
(FAAH), F: 5′-GCCTCAAGGAATGCTTCAGC-3′, R:
5′-TGCCCTCATTCAGGCTCAAG-3′; monoacylglycerol lipase (MAGL), F:
5′-CATGTGGATTCCATGCAGAAAG-3′, R: 5′-AGGATTGGCAAGAACCAGAGG-3′;
diacylglycerol lipase (DGL)-α, F: 5′-AGAATGTCACCCTCGGAATGG-3′, R:
5′-GTGGCTCTCAGCTTGACAAAGG-3′; CB1, F:
5′-AGCCTCTGGATAACAGCATGG-3′, R: 5′-AATCTTGACCGTGCTCTTGATG-3′;
CB2, F: 5′-CTCAGTGACCAGGTCAAGAAGG-3′, R:
5′-TTTTGCCTCTGACCCAAGG-3′; ceramide synthases (Cer)S1, F:
5′-TTTGGCTCCCGCACAATGT-3′, R: 5′-AAAAGCGAGATAGAGGTCCTCA-3′; CerS2,
F: 5′-GCTCTTCCTCATCGTTCGATAC-3′, R: 5′-GTGTAGCCACGTACAGCTCA-3′;
CerS3, F: 5′-CACCCAGCTGTCAAAGAGAAGG-3′, R:
5′-AGGACGATATCCGAAAGGTGG-3′; CerS4, F:
5′-CCGGATCCCGTCCAGTTTCAACGAG-3′, R:
5′-GGGAATTCGGCTATGTGGCTGTTGTG-3′; CerS5, F:
5′-GCTGCTCTTCGAGCGATTTAT-3′, R: 5′-CCTCCGATGGCGAAACCAG-3′; CerS6,
F: 5′-TTTGGCTCCCGCACAATGT-3′, R: 5′-AAAAGCGAGATAGAGGTCCTCA-3′; 18S,
F: 5′-CAGCCACCCGAGATTGAGCA-3′, R: 5′-TAGTAGCGACGGGCGGTGTG-3′.
Western blot analysis
Tumors and adjacent normal tissues were homogenized
and sonicated (50/60Hz) in ice-cold RIPA buffer (Beyotime
Biotechnology, Jiangsu, China) for 10 times, 5 sec each time. The
suspension was centrifuged for 15 min at 4°C and 15,294 × g. The
total protein content was determined using a bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocols, with bovine serum albumin (BSA;
Solarbio Science & Technology Co. Ltd., Beijing, China) as the
standard. Proteins (20 µg) were mixed with sample buffer, boiled,
and then separated by 15% SDS-PAGE. After transference to
polyvinylidene difluoride membranes (GE Healthcare Life Sciences),
the membrane was blocked in 5% milk in Tris-buffered saline with 1%
Tween-20 at room temperature for 1 h, and incubated at 4°C
overnight with primary antibodies against CB1 (Abcam;
1:1,000; cat. no. ab23703), CB2 (Abcam; 1:1,000; cat.
no., ab3561), SCD1 (Abcam; 1:500; cat. no. ab19862) and β-actin
(Sigma-Aldrich, Merck KGaA; 1:500; cat. no. A5316). The membranes
were subsequently incubated with an HRP-conjugated anti-rabbit IgG
antibody (Sigma-Aldrich, Merck KGaA; 1:10,000; cat. no. SAB5600127)
for 1 h at room temperature. Protein bands were visualized using an
Enhanced Chemiluminescence Plus kit (GE Healthcare Life Sciences).
Quantitative analysis was performed using the ImageJ software
(version 2.1.4.7; National Institute of Health) with β-actin as the
endogenous control.
Endogenous lipid extraction and
analysis
In total, 50 µg frozen tissue samples were
homogenized in 2 ml methanol/H2O mixture (50:50, v/v)
containing C17:1 FAE, and C17:0 ceramide as internal standards.
Endogenous lipids were extracted with 3 ml chloroform, and then
centrifuged for 10 min at 4°C and 3,000 × g. The organic phase was
collected and dried under nitrogen by a nitrogen evaporator
(Beijing TongTaiLian Technology Co., Ltd.). Lipids were
re-dissolved in 1 ml chloroform and transferred to small Silica Gel
G columns. Endogenous FAEs and ceramides were eluted with
methanol/chloroform (10:90, v/v), dried under nitrogen,
reconstituted in 100 µl methanol and detected using the 3200 Q Trap
HPLC-MS system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
coupling with the 1100-HPLC system (Agilent Technologies).
The parameters of isolation and elution condition,
ion monitor model and the molecular ion were all previously
described in detail (16). The
detailed information is as follows. The gradient elution of the
mobile phase was as follows: 85% methanol (containing 15%
H2O; pH 7.5) was kept for the first 3 min, followed by a
linear gradient from 85 to 100% methanol for 2 min, and then 100%
methanol was continued for another 15 min. Finally, the elution
condition went back to 85% methanol for 2 min at a flow rate of 0.7
ml/min. Column temperature was kept at 40°C. Ion detection was
monitored by APCI+-MRM mode. The molecular ions were
monitored at m/z 348.00/62.00 for AEA, m/z
379.10/287.10 for 2-AG, m/z 313.1/62.0 for C17:1 FAE,
m/z 464.4/264.2 for C12:0 ceramide, m/z 520.4/264.2
for C16:0 ceramide, m/z 534.3/264.2 for C17:0 ceramide,
m/z 548.4/264.2 for C18:0 ceramide, m/z 576.4/264.2
for C20:0 ceramide, m/z 604.5/264.2 for C22:0 ceramide,
m/z 632.4/264.2 for C24:0 ceramide, and m/z
630.4/264.2 for C24:1 ceramide.
Enzymatic assays
Tissue samples were cut into 200 µm thick sections
and were homogenized in ice-cold Tris-HCl buffer (50 mM, pH 7.4)
containing 0.32 M sucrose to obtain total proteins. To measure FAAH
and MAGL activity, tissue proteins were incubated at 37°C for 30
min in 50 mM Tris-HCl buffer (pH 8.0, containing 0.05% fatty
acid-free BSA), and 100 µg sample protein was incubated with 50 µM
AEA or 2-oleoylglycerol as substrates. The reaction was stopped by
adding 200 µl chloroform/methanol (1:1, v/v), containing C17:0
heptadecanoic acid as an internal standard. The reaction solution
was centrifuged at 1,500 × g at 4°C for 5 min and the organic
layers were subsequently collected and dried under nitrogen. The
residues were re-dissolved in 100 µl methanol, and analyzed by 3200
Q Trap HPLC-MS system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) coupled with the 1100-HPLC system (Agilent
Technologies, Inc.) in the negative-ion mode using 17:0
heptadecanoic acid as internal standard. A Hypersil Gold C18 column
(dimensions, 250 × 4.6 mm; particle size, 5 µm; Thermo Fisher
Scientific, Inc.) was used for analytical separation and the column
temperature was kept at 40°C. Fatty acids were eluted using a
linear gradient from 90% phase A (methanol containing 0.25% acetic
acid and 5 mM ammonium acetate) to 100% phase B (water containing
0.25% acetic acid and 5 mM ammonium acetate) in 2.5 min at a flow
rate of 1.0 ml/min. Capillary voltage was set at −4 kV and the
fragmentor voltage was 120V. Nitrogen was used as drying gas at a
flow rate of 13 liters/min and a temperature of 350°C. Nebulizer
pressure is set at 60 psi. [M-H]− ion was monitored in
the selected-ion monitoring (SIM) mode (m/z=303 for
arachidonic acid, m/z=281 for oleic acid, and m/z=269
for 17:0 heptadecanoic acid).
Statistical analysis
Data are expressed as the means ± standard error of
the mean. Experiments were performed in triplicate. Student's
t-test was performed using GraphPad Prism (version 5.01; GraphPad
Software, Inc.), and P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of 67 patients with
HCC
Tumor tissues and adjacent non-tumor tissues were
collected from patients with HCC who underwent surgical resection
between 2015 and 2016. The detailed clinical parameters of the 67
patients are listed in Table I. The
mean age of the patients was 52.6±11.3 years (range, 18–76 years).
In total, 83.6% of the patients were male, consistent with prior
reports that HCC is more prevalent in males compared to females. It
was reported that estrogen might repress HCC growth via inhibiting
alternative activation of tumor-associated macrophages (17). Of the 67 samples collected, 65 were
HBV infections, with a ratio of 92.5%. It was consistent with the
fact that >80% of patients with HCC were HBV carriers in Fujian
Province. As there was an insufficient number of HCC samples
without HBV infection in the current study, the effect of HBV on
the expression of these endogenous lipids and its role in promoting
HCC was not investigated. Approximately 10% of the patients were
recorded to have varying degrees of liver damage, and 23 patients
had cancerous thrombi. In the cohort, ~55% was diagnosed with
intermediate-grade cancer, while the percentage of high- grade was
25.4% and that of low-grade cases was 19.4% according to the
Standardization of diagnosis and treatment for hepatocellular
carcinoma (2017 edition, Bureau of Medical Administration, National
Health and Family Planning Commission of the PRC) (18).
 | Table I.Clinical pathological parameters of
67 patients with hepatocellular carcinoma. |
Table I.
Clinical pathological parameters of
67 patients with hepatocellular carcinoma.
| Parameter | Value, n (%) |
|---|
| Sex |
|
|
Male | 51 (76.1) |
|
Female | 16 (23.9) |
| HBsAg |
|
|
Positive | 62 (92.5) |
|
Negative | 5 (7.5) |
| AFP (ng/ml) before
surgery |
|
|
>25 | 42 (62.7) |
|
≤25 | 25 (37.3) |
| Tumor stage (TNM)
(36) |
|
| I or
II | 47 (70.1) |
|
III | 20 (29.9) |
| Grade |
|
|
High | 17 (25.4) |
|
Middle | 13 (19.4) |
|
Low | 37 (55.2) |
| Tumor diameter
(cm) |
|
|
>5 | 22 (32.8) |
| ≤5 | 45 (67.2) |
According to the current data, 38 patients (56%)
have a recorded pathological status of cirrhosis, and 14 patients
of them had Child-Pugh score. A total of 9 patients were recorded
as HBV-related decompensated liver cirrhosis, 7 of them had a
higher Child-Pugh score (10.29±0.68), while 2 of them had a lower
Child-Pugh score (5 and 6, respectively). A total of 4 patients
were recorded as decompensated alcoholic cirrhosis with Child-Pugh
scores of 5, 9, 12, and 13, respectively. And only one patient was
recorded as cardiogenic cirrhosis with a Child-Pugh score of 5.
The present study also included 7 patients who were
HCV RNA positive and 6 patients were HCV RNA negative. There are no
relevant records for the other patients. Some patients showed
dyslipidemia, 5 of them were diagnosed with fatty liver and
hypertriglyceridemia, 3 patients were diagnosed with
hypercholesterolemia, and only 1 patient was suspected as
nonalcoholic steatohepatitis.
Expression of endocannabinoids in
human HCC samples
The endogenous lipids in 67 tumor and matched
non-cancerous tissue pairs were detected and quantified by HPLC-MS.
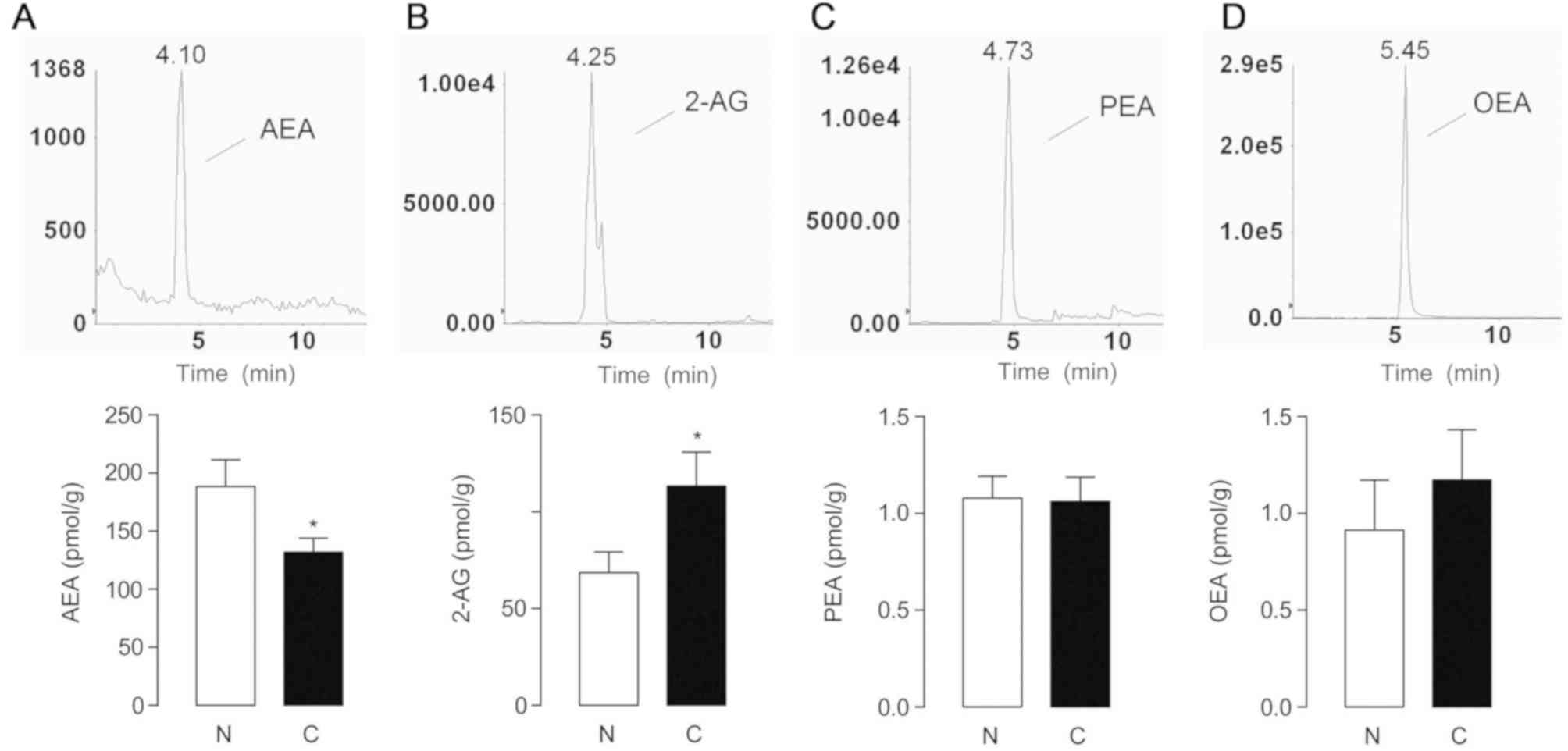
Compared with the non-tumor counterparts, AEA levels were
significantly decreased in tumor tissues (P=0.0329; 188.3±22.94
pmol/g in non-tumor tissues, and 131.7±12.04 pmol/g in HCC,
Fig. 1A), while the level of 2-AG,
another important endocannabinoid, was significantly increased in
tumor tissues (P=0.0278; 68.55±10.64 nmol/g in non-cancerous
controls, and 113.3±17.48 nmol/g in HCC, Fig. 1B). As congeners of AEA,
palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) belong to
the superfamily of N-acylethnolamines (NAEs), however PEA
and OEA interact with the PPAR-α receptor instead of cannabinoid
receptors (19). The expression of
PEA and OEA did not differ significantly between the two groups
(Fig. 1C and D).
Changes in endocannabinoid metabolism
in human HCC samples
The most notable endocannabinoid changes in the
present study were the decrease of AEA and increase of 2-AG. AEA is
generated by NAPE-PLD and metabolized by FAAH (20). The checkpoint in 2-AG synthesis is
considered to be DGL-α (21).
Furthermore, MAGL is mainly involved in 2-AG degradation (21). To further investigate the molecular
mechanisms underlying the aforementioned changes, the expression
and activity profiles of these enzymes were examined in tissue
samples.
There was no significant difference in the mRNA
expression of NAPE-PLD between tumors and non-cancerous controls
(Fig. 2A). The mRNA levels and
activity of FAAH increased in HCC samples relative to their
non-tumor counterparts (P=0.041; Fig. 2B
and C), which suggested increased degradation of AEA in tumor
tissues. A 5-fold higher level of DGL-α mRNA was detected in the
tumor group (1.05±0.09 in non-tumor tissues and 5.27±0.81 in HCC;
P<0.0001; Fig. 2D). The
expression of DGL-α exhibited a greater increase compared with MAGL
(Fig. 2E and F), indicating that
2-AG synthesis was markedly faster than its degradation. Whether
the elevated expression of 2-AG and DGL-α may be used as markers
for early HCC diagnosis warrants further investigation.
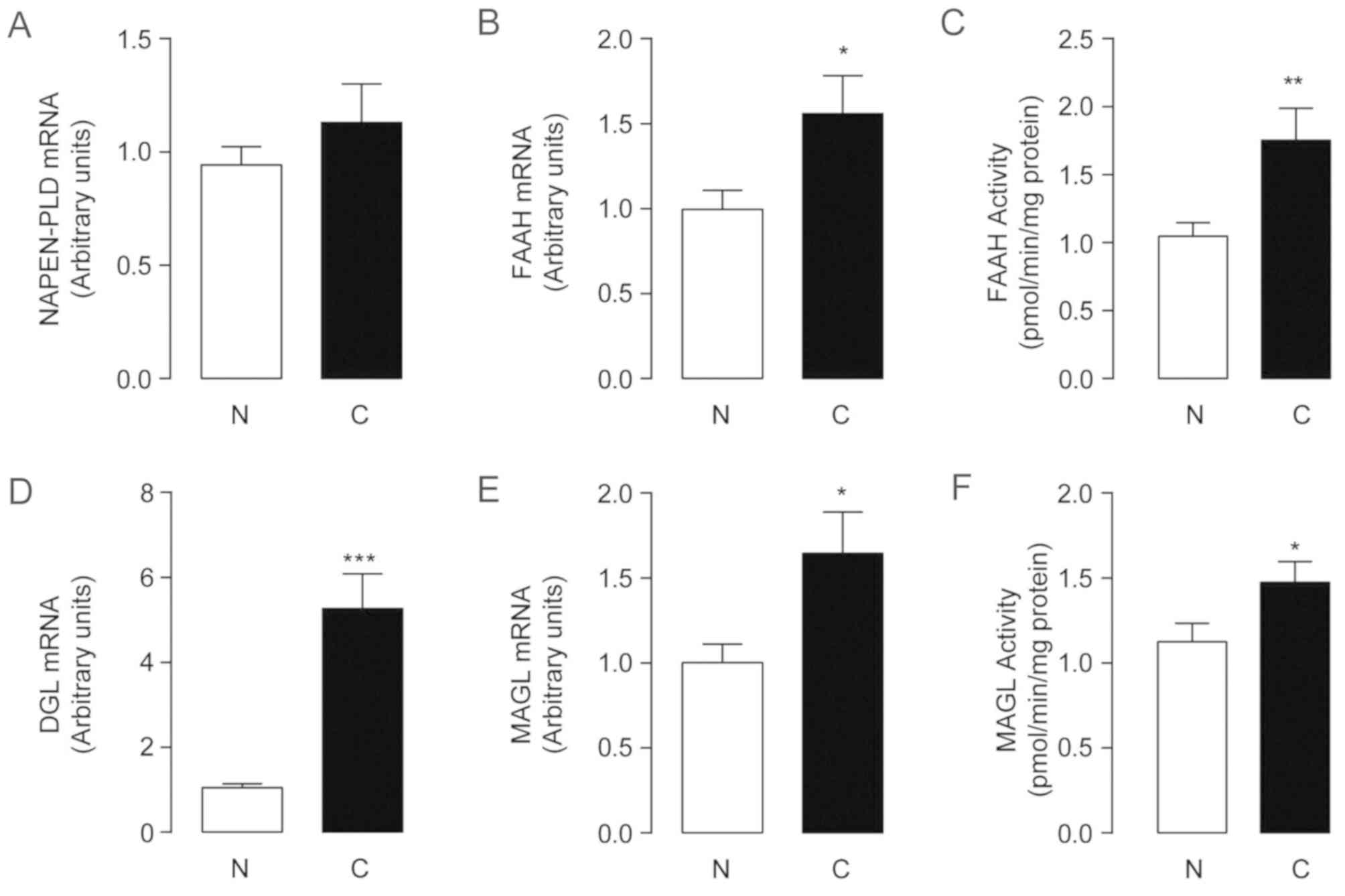 | Figure 2.Alteration of endocannabinoids
metabolic enzymes in human hepatocellular carcinoma. The mRNA
expression levels (A, B, D, E) and the enzyme activities (C and F)
of (A) NAPE-PLD, (B and C) FAAH, (D) DGL and (E and F) MAGL in
non-tumor tissue control and hepatocellular carcinoma tissues.
*P<0.05, **P<0.01 and ***P<0.001; n=67. N, non-tumor
tissue control; C, hepatocellular carcinoma tissues; NAPE-PLD,
N-acylphosphatidylethanolamine-hydrolysingphospholipase D;
FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase;
DGL, diacylglycerol lipase. |
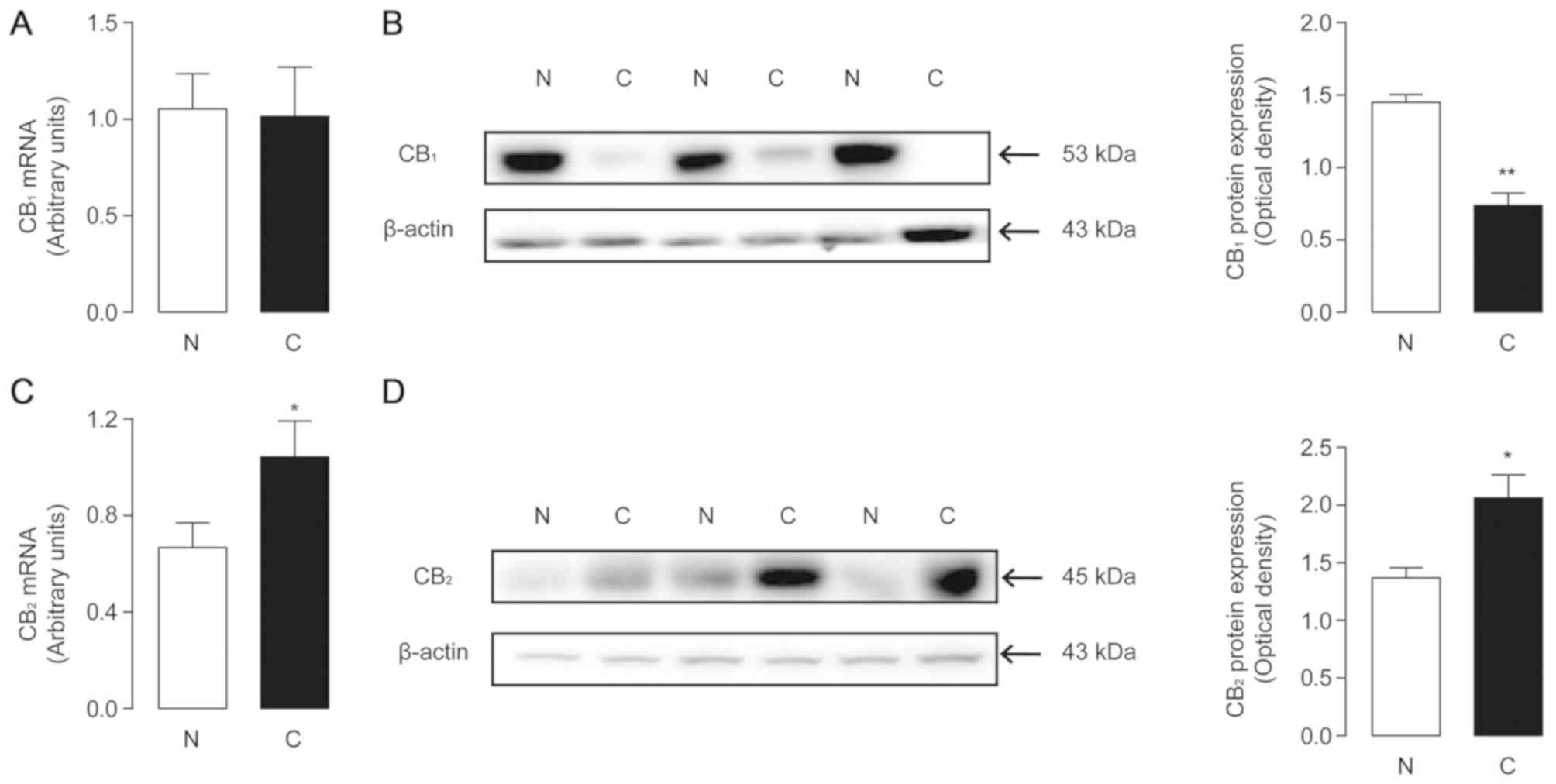
Expression of cannabinoid receptors in
human HCC samples
It has been reported that cannabinoid receptors 1
and 2 mediate functional responses to the AEA and 2-AG. As the
levels of these two endocannabinoids changed significantly in tumor
tissues, the present study investigated whether there were
corresponding changes in the receptors. The mRNA expression of
CB1 and CB2 was examined by quantitative PCR
in both tumor and non-tumor samples. There was no significant
difference between the two groups for CB1 mRNA levels
(Fig. 3A), while CB2
expression was increased in tumor tissues (Fig. 3C). The protein levels of the two
receptors were further investigated via western blot analysis.
ImageJ software was used to calculate the optical density of the
blots, using β-actin as the control. The results revealed that
CB1 protein levels in tumor tissues were decreased
significantly (1.45±0.054 in non-cancerous tissues and 0.74±0.086
in tumor tissues; P=0.0022; Fig.
3B), and those of CB2 were significantly increased
(1.37±0.088 in non-tumor tissues and 2.06±0.2 in tumor tissues;
P=0.0336, Fig. 3D). The trends in
receptor protein expression were consistent with the changes in
endogenous ligands.
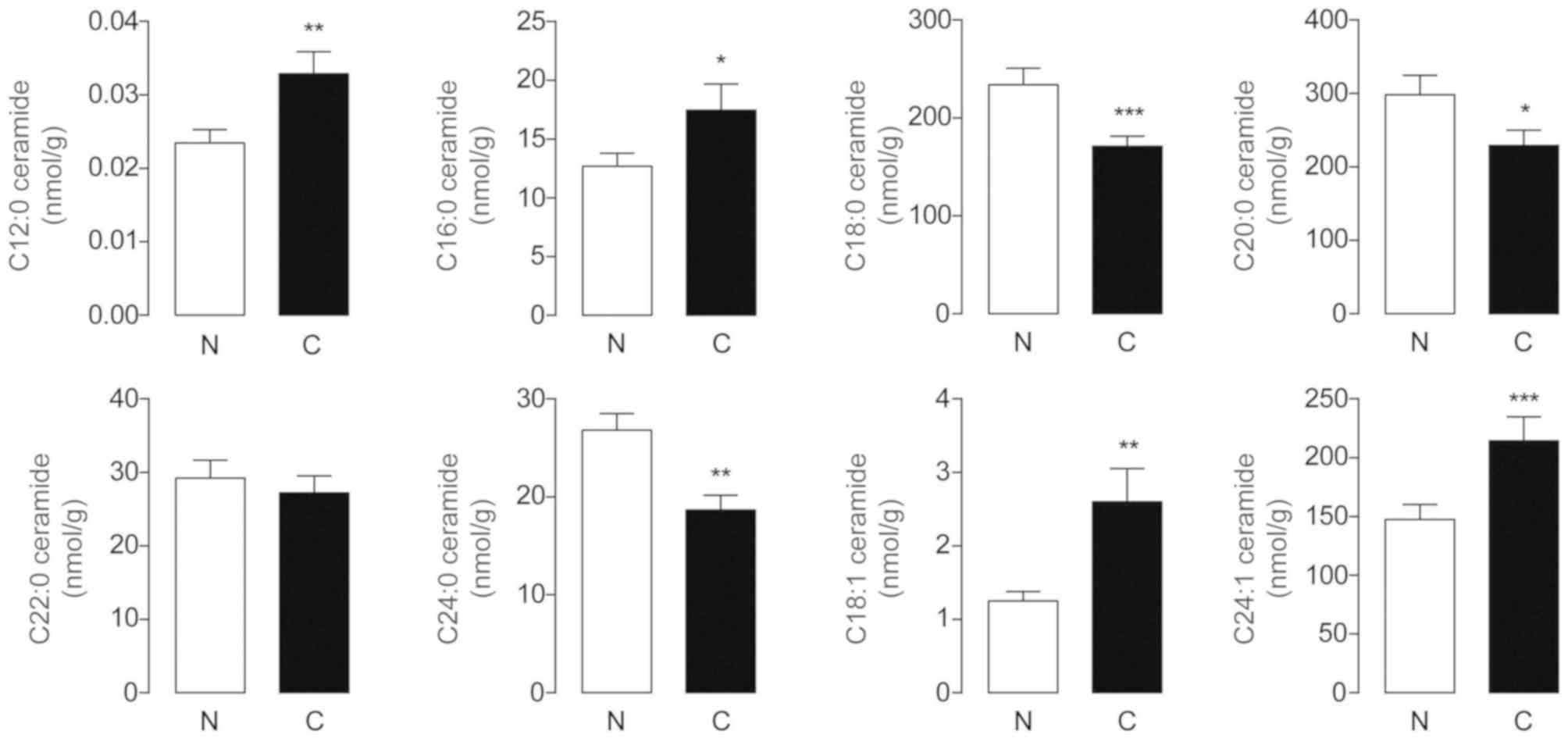
Expression of endogenous ceramides in
human HCC samples
Ceramides are a family of bioactive sphingolipids
with tumor-suppressive properties (22). In the present study, HPLC-MS
detection revealed that endogenous C12:0, C16:0, C18:1 and
C24:1-ceramides were markedly increased in HCC tissues, whereas the
levels of C18:0, C20:0 and C24:0-ceramides were significantly
decreased in these samples, each compared with their adjacent
normal counterparts (Fig. 4). The
differences in C22:0-ceramide levels between tumor and non-tumor
tissues were not significant. It was documented that C18:0-ceramide
exerted anti-proliferative effects on head and neck squamous cell
carcinomas (HNSCCs), while the roles of C18:0, C20:0 and
C24:0-ceramides in the pathogenesis and progression of HCC require
further investigation.
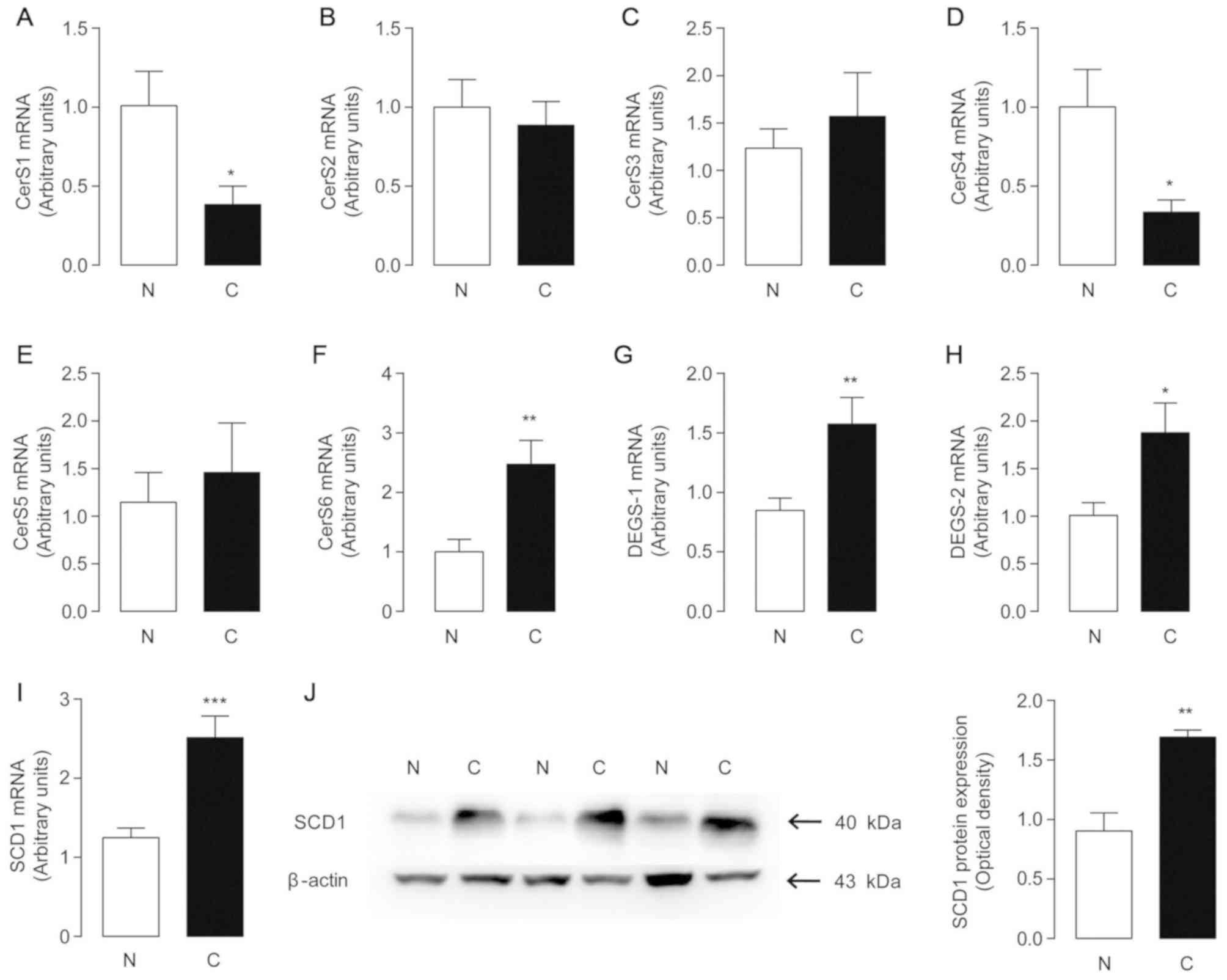
Changes in endogenous ceramide
metabolism in human HCC samples
To examine the mechanisms underlying the changes
observed with ceramides, the mRNA levels of CerS1-6 were examined
in 67 pairs of HCC tumor and adjacent non-tumor tissues (Fig. 5A-F). The results revealed that the
mRNA levels of CerS6 increased significantly in ~70% of the
patients, which were associated with increased tumor levels of
C16:0-ceramide. Furthermore, the decreased mRNA levels of CerS1
were in accordance with reduced C18:0-ceramide levels, which was
highly relevant to the lower levels of C18:0-ceramide observed in
HCC tissues. Decreases in C20:0 and C24:0-ceramides in tumor
tissues may be attributed to the downregulated expression of CerS4,
a synthase responsible for the synthesis of C18:0-24:0 ceramides
(22). Notably, the levels of
mono-unsaturated C18:1 and C24:1 ceramides were increased in tumor
samples, consistently with the elevated levels of dihydroceramide
desaturase 1 and 2 (DEGS-1/2) (Fig. 5G
and H). However, the main role of DEGS and mono-unsaturated
ceramides involved in HCC formation and progression warrants
further investigation.
SCD1 is a transmembrane protein that converts SFAs
to Δ-9 MUFAs to supply phospholipids for membrane biogenesis during
cancer cell proliferation (23). The
SCD1 mRNA and protein levels were significantly upregulated in HCC
tissues compared with their non-tumor counterparts (Fig. 5I). High expression of SCD1 may be
associated with the suppression of C18:0, 20:0 and 24:0-ceramide
de novo synthesis, while the crosstalk between the SCD1
pathway and the ceramide metabolism network has, to the best of our
knowledge, yet to be investigated.
Discussion
It has been reported that endocannabinoids are
generally upregulated in tumor tissues, including gliomas, colon
and breast cancer, compared with non-cancerous tissues. AEA is the
first discovered endogenous ligand for cannabinoid receptor 1.
Administration of AEA or inhibitors of FAAH, the main degradative
enzyme of AEA, has been shown to suppress the growth of different
types of tumor xenografts via CB receptor activation (24). Targeting endocannabinoid systems may
be a reasonable strategy for anticancer therapy. In previous
studies, endocannabinoids and the associated receptors were
detected in tissues and serum of patients with HCC by lipid
analysis, qPCR, and immunohistochemistry. However, a disadvantage
of the aforementioned is that there is no systematic detection of
the endocannabinoid system, as they cannot explain the reason for
these major endocannabinoids levels changes. The novelty of this
research is that a systematic detection including endocannabinoids,
ceramides, the associated synthetic and metabolic hydrolases and
the associated receptors is carried out. The changes of endogenous
lipids have been explained primarily to provide potential targets
and research directions for the diagnosis and treatment of HCC.
To date, to the best of our knowledge, little is
known on the physiological and pathological roles of AEA in HCC. In
a serum metabolic profiling analysis study of HCC, Zhou et
al (25) found that serum AEA
was significantly elevated in HCC groups compared with healthy
controls. In the present study, endogenous AEA levels were
decreased in liver cancer tissues, contrary to a previous report
(25). The downregulation of AEA in
HCC tissues reported herein was consistent with this study's
findings that the expression and activity of FAAH increased
significantly in HCC. In the study conducted by Xu et al
(26), serum was used instead of
liver tissues, recapitulating the systematic AEA metabolic profiles
of patients with HCC. This study only revealed the metabolic
characteristics of AEA in the local environment of the liver.
Therefore, this study suggests that a decrease in AEA levels in
tumor tissues shows that AEA may play an important role in
suppressing HCC cell proliferation. AEA is an endogenous agonist of
the CB1 receptor, and the activation of CB1
is beneficial for tumor cell apoptosis and autophagy. After
treatment with cannabinoids, the expression levels of Akt in breast
cancer cells decreased and the PI3K/Akt/mTOR pathway was inhibited,
which significantly suppressed tumor cell migration. Elevated serum
AEA levels may reflect antitumor auto-protection of the body;
however, the precise role of endogenous AEA in the occurrence and
progression of cancer remains unclear.
CB1 receptors are preferentially
expressed in the central nervous system. In the present study, a
notable decrease of CB1 receptor expression in HCC
tissues was observed by western blot analysis. However, it has been
reported that high expression of the CB1 receptor was
observed in 45% of the cases of HCC by immunohistochemical analysis
(26). These conflicting results may
be attributed to the selection of different differentiation
samples. The data demonstrated that high CB1 expression
was associated with high differentiation of HCC (26), however poorly differentiated HCCs
generally exhibited low CB1 expression. In the present
study, 74.6% of HCC samples were poorly to intermediately
differentiated, with lower CB1 levels, in accordance
with the aforementioned data, and CB1 receptor
expression in HCC was suggested to be associated with improved
prognosis (26).
It was recently reported that the CB1
receptor was upregulated in diethynitrosamine-induced HCC mouse
models (27). Peripheral blockade or
genetic ablation of the CB1 receptor suppressed its high
expression in the endocannabinoid system and the growth of HCC.
However, these conclusions were drawn from observations made with
an animal model; therefore, they cannot fully represent the true
conditions of the human body. Accordingly, further research is
required to validate that the pharmacological activation of
CB1 by enhancing AEA levels is effective for the
treatment of HCC.
The most distinct change to the endocannabinoid
system in the present study was the increase in 2-AG. The activity
and expression of MAGL, the enzyme responsible for 2-AG hydrolysis,
was increased in HCC compared with non-cancerous controls, which
was not consistent with the increase of 2-AG. However, the
expression of DGL-α, the 2-AG-biosynthesizing enzyme, increased in
HCC tissues, suggesting faster biosynthesis of 2-AGcompared with
its degradation. The upregulation of MAGL may be a result of the
negative feedback induced by the excessive production of DGL-α and
2-AG.
It has been reported that CB2 receptors
are more prevalent in peripheral tissues associated with immune
function. Hepatic CB2 protein levels are elevated in a
number of liver pathologies, including fatty liver, hepatic
fibrogenesis (28) and acute liver
injury (29). In 2006, by
immunohistochemical analysis, Xu et al (26) showed high expression of
CB1 and CB2 receptors in 45 and 52% cases of
HCC, respectively, which is consistent with this study's findings.
Overexpression of CB2 may have potential as prognostic
indicators for patients with HCC.
Studies have shown that the major endocannabinoid
2-AG and the associated receptor CB2 have a key role in
the pathogenesis of chronic liver injury. Activation of
CB2 receptor is known to inhibit tumor vascularization
(30), while, in another research,
elevated CB2 level was reported to facilitate the tumor
invasion through the suppression of the anti-tumor immune system
(31). The detailed role of the
CB2 receptor in HCC development and progression remains
unknown. To the best of our knowledge, there are currently no
reports on CB2 function in the treatment of HCC.
The present study provided new evidence confirming
the excessive expression of 2-AG and its associated receptor
CB2 in individuals with HCC, which may lead to the
identification of novel diagnostic and therapeutic targets against
HCC. However, there was no significant difference for 2-AG and
CB2 expression between stage I–II and III, or between
Grade high, middle, and low of HCC. Therefore, further studies
should delineate whether administration of CB2
agonist/antagonist may pave the way for anti-HCC treatment.
The expression levels of major endocannabinoids, AEA
and 2-AG, are modulated mainly through synthetic and metabolic
enzymes. However, changes in endocannabinoids levels cannot be
accurately explained only through the above enzymes. The
aforementioned suggests that by inhibiting highly expressed
metabolic enzymes, local endocannabinoid levels can be increased,
which seems likely to have some effects on tumor cells through
activating CB receptors. It was reported that the inhibitors of
FAAH and MGL, the metabolic enzymes of AEA and 2-AG, showed
anti-tumor effects in some types of malignancies (21), including colorectal cancer (9), non-small lung cancer (32)and prostate cancer (33). However, the precise role of
endocannabinoids system in the occurrence and progression of HCC is
still unclear. In the present research, of the 67 patients with
HCC, only 5 patients who were HBV negative were included. The
proportion of patients with hepatitis B positive reached 92.5%.
Future studies including a sufficient sample of patients with HCC
and hepatitis B negative should be conducted to provide the
metabolic profiling data for HBV infection.
Cannabinoid receptor activation was shown to induce
apoptosis via de novo ceramide synthesis in several types of
cancer cells, including glioma (5)
and colon cancer cells (34).
Ceramides are a class of pro-apoptotic sphingolipids with important
roles in the control of cancer cell fate. It has been reported that
the levels of specific ceramides, such as C18:0 and C20:0, may be
important in the inhibition of cell growth in human HNSCC (35). In the present study, notable
decreases in C18:0, 20:0 and 24:0-ceramides were found in HCC
tissues compared with non-tumor counterparts, which was in
accordance with previously reported data (35). In accordance with these findings in
the present study, the levels of C12:0, C16:0, C18:1 and
C24:1-ceramides were increased in HCC tumors compared with adjacent
normal tissues. The specific role of the changes in endogenous
ceramide expression in HCC has not been fully elucidated, and
further studies are required to determine the possible association
between altered ceramide levels and HCC progression. Increasing
ceramide levels is a potential strategy for the treatment of HCC,
and the inhibition of SCD1 in HCC may be a viable option (10).
Acknowledgements
The authors would like to thank Dr Xuan Zhu (School
of Pharmaceutical Sciences, Xiamen University) for their help in
revising the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81603145), the Fujian
Health-Education Research Grant (no. WKJ2016-2-03), Fujian
Provincial Natural Science Foundation (nos. 2015J01416 and
2017J01146), and the Xiamen Science and Technology Program Grant
(no. 3502Z20154069).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and FQ conceived and designed experiments. JY and
LL carried out the experiments. RZ analyzed data, prepared figures,
and finished the complementary experiments. JY, FQ and RZ wrote the
manuscript. YT, JY and FQ provided the clinical samples. All the
authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of the Fifth Hospital of Xiamen (Fuzhou, China) following
the clinical registration guidelines in China. All patients
provided written informed consent for the investigation.
Patient consent for publication
All patients consented to the publication of this
research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000–14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strickaert A, Corbet C, Spinette SA,
Craciun L, Dom G, Andry G, Larsimont D, Wattiez R, Dumont JE, Feron
O and Maenhaut C: Reprogramming of energetic metabolism: Increased
expression and roles of pyruvate carboxylase in papillary thyroid
cancer. Thyroid. 2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou X, Yang X, Sun X, Xu X, Li X, Guo Y,
Wang J, Li X, Yao L, Wang H and Shen L: Effect of PTEN loss on
metabolic reprogramming in prostate cancer cells. Oncol Lett.
17:2856–2866. 2019.PubMed/NCBI
|
|
4
|
Li X, Xu H, Lei T, Yang Y, Jing D, Dai S,
Luo P and Xu Q: A pulsed electromagnetic field protects against
glutamate-induced excitotoxicity by modulating the endocannabinoid
system in HT22 cells. Front Neurosci. 11:422017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dyall SC, Mandhair HK, Fincham REA, Kerr
DM, Roche M and Molina-Holgado F: Distinctive effects of
eicosapentaenoic and docosahexaenoic acids in regulating neural
stem cell fate are mediated via endocannabinoid signalling
pathways. Neuropharmacology. 107:387–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cinar R, Godlewski G, Liu J, Tam J,
Jourdan T, Mukhopadhyay B, Harvey-White J and Kunos G: Hepatic
cannabinoid-1 receptors mediate diet-induced insulin resistance by
increasingde novosynthesis of long-chain ceramides. Hepatology.
59:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sean M. Emery AHL and David A: Gewirtz:
Involvement of the endocannabinoid system in the development and
treatment of breast cancer. Virginia commonwealth univ richmond;
2014
|
|
8
|
Orellana-Serradell O, Poblete CE, Sanchez
C, Castellón EA, Gallegos I, Huidobro C, Llanos MN and Contreras
HR: Proapoptotic effect of endocannabinoids in prostate cancer
cells. Oncol Rep. 33:1599–1608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ligresti A, Bisogno T, Matias I, De
Petrocellis L, Cascio MG, Cosenza V, D'argenio G, Scaglione G,
Bifulco M, Sorrentini I and Di Marzo V: Possible endocannabinoid
control of colorectal cancer growth. Gastroenterology. 125:677–687.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galve-Roperh I, Sánchez C, Cortés ML,
Gómez del Pulgar T, Izquierdo M and Guzmán M: Anti-tumoral action
of cannabinoids: Involvement of sustained ceramide accumulation and
extracellular signal-regulated kinase activation. Nat Med.
6:313–319. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Igal RA: Stearoyl CoA desaturase-1: New
insights into a central regulator of cancer metabolism. Biochim
Biophys Acta. 12:1865–1880. 2016. View Article : Google Scholar
|
|
12
|
Chen L, Ren J, Yang L, Li Y, Fu J, Li Y,
Tian Y, Qiu F, Liu Z and Qiu Y: Stearoyl-CoA desaturase-1 mediated
cell apoptosis in colorectal cancer by promoting ceramide
synthesis. Sci Rep. 6:196652016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Wenes M, Romero P, Huang SC, Fendt
SM and Ho PC: Navigating metabolic pathways to enhance antitumour
immunity and immunotherapy. Nat Rev Clin Oncol. 2019.(Epub ahead of
print). View Article : Google Scholar
|
|
14
|
Mittal S and El-Serag HB: Epidemiology of
HCC: consider the population. J Clin Gastroenterol. 47:S2–S6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Chen H, Li Y, Li L, Qiu Y and Ren
J: Endocannabinoid and ceramide levels are altered in patients with
colorectal cancer. Oncol Rep. 34:447–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang W, Lu Y, Xu Y, Xu L, Zheng W, Wu Y,
Li L and Shen P: Estrogen represses hepatocellular carcinoma (HCC)
growth via inhibiting alternative activation of tumor-associated
macrophages (TAMs). J Biol Chem. 287:40140–40149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH,
Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, et al: Guidelines for
diagnosis and treatment of primary liver cancer in China (2017
Edition). Liver Cancer. 7:235–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu J, Gaetani S, Oveisi F, Lo Verme J,
Serrano A, Rodríguez De Fonseca F, Rosengarth A, Luecke H, Di
Giacomo B, Tarzia G and Piomelli D: Oleylethanolamide regulates
feeding and body weight through activation of the nuclear receptor
PPAR-alpha. Nature. 425:90–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bobrov MY, Shevchenko VP, Yudushkin IA,
Rogov SI, Remov MN, Fomina-Ageeva EV, Gretskaya NM, Nagaev IY,
Kuklev DV and Bezuglov VV: Hydrolysis of anandamide and
eicosapentaenoic acid ethanolamide in mouse splenocytes.
Biochemistry. 65:615–619. 2000.PubMed/NCBI
|
|
21
|
Nomura DK, Long JZ, Niessen S, Hoover HS,
Ng SW and Cravatt BF: Monoacylglycerol lipase regulates a fatty
acid network that promotes cancer pathogenesis. Cell. 140:49–61.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morad SA and Cabot MC:
Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer.
13:51–65. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsui H, Yokoyama T, Sekiguchi K, Iijima
D, Sunaga H, Maniwa M, Ueno M, Iso T, Arai M and Kurabayashi M:
Stearoyl-CoA desaturase-1 (SCD1) augments saturated fatty
acid-induced lipid accumulation and inhibits apoptosis in cardiac
myocytes. PLoS One. 7:e332832012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Massi P, Valenti M, Vaccani A, Gasperi V,
Perletti G, Marras E, Fezza F, Maccarrone M and Parolaro D:
5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the
antitumor activity of cannabidiol, a non-psychoactive cannabinoid.
J Neurochem. 104:1091–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Ding L, Yin P, Lu X, Wang X, Niu
J, Gao P and Xu G: Serum metabolic profiling study of
hepatocellular carcinoma infected with hepatitis B or hepatitis C
virus by using liquid chromatography-mass spectrometry. J Proteome
Res. 11:5433–5442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou
J, Fan W, Li Q, Wang Q, Zhong D and Miao X: Overexpression of
cannabinoid receptors CB1 and CB2 correlates with improved
prognosis of patients with hepatocellular carcinoma. Cancer Genet
Cytogenet. 171:31–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukhopadhyay B, Schuebel K, Mukhopadhyay
P, Cinar R, Godlewski G, Xiong K, Mackie K, Lizak M, Yuan Q,
Goldman D and Kunos G: Cannabinoid receptor 1 promotes
hepatocellular carcinoma initiation and progression through
multiple mechanisms. Hepatology. 61:1615–1626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Louvet A, Teixeira-Clerc F, Chobert MN,
Deveaux V, Pavoine C, Zimmer A, Pecker F, Mallat A and Lotersztajn
S: Cannabinoid CB2 receptors protect against alcoholic liver
disease by regulating Kupffer cell polarization in mice.
Hepatology. 54:1217–1226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teixeira-Clerc F, Belot MP, Manin S,
Deveaux V, Cadoudal T, Chobert MN, Louvet A, Zimmer A, Tordjmann T,
Mallat A and Lotersztajn S: Beneficial paracrine effects of
cannabinoid receptor 2 on liver injury and regeneration.
Hepatology. 52:1046–1059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blázquez C, Casanova ML, Planas A, Gómez
Del Pulgar T, Villanueva C, Fernández-Aceñero MJ, Aragonés J,
Huffman JW, Jorcano JL and Guzmán M: Inhibition of tumor
angiogenesis by cannabinoids. FASEB J. 17:529–531. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hermanson DJ and Marnett LJ: Cannabinoids,
endocannabinoids, and cancer. Cancer Metast Rev. 30:599–612. 2011.
View Article : Google Scholar
|
|
32
|
Ravi J, Sneh A, Shilo K, Nasser MW, Nasser
MW and Ganju RK: FAAH inhibition enhances anandamide mediated
anti-tumorigenic effects in non-small cell lung cancer by
downregulating the EGF/EGFR pathway. Oncotarget. 5:2475–2486. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nomura DK, Lombardi DP, Chang JW, Niessen
S, Ward AM, Long JZ, Hoover HH and Cravatt BF: Monoacylglycerol
lipase exerts dual control over endocannabinoid and fatty acid
pathways to support prostate cancer. Chem Biol. 18:846–856. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cianchi F, Papucci L, Schiavone N, Lulli
M, Magnelli L, Vinci MC, Messerini L, Manera C, Ronconi E,
Romagnani P, et al: Cannabinoid receptor activation induces
apoptosis through tumor necrosis factor α–mediated ceramide de novo
synthesis in colon cancer cells. Clin Cancer Res. 14:7691–7700.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karahatay S, Thomas K, Koybasi S, Senkal
CE, Elojeimy S, Liu X, Bielawski J, Day TA, Gillespie MB, Sinha D,
et al: Clinical relevance of ceramide metabolism in the
pathogenesis of human head and neck squamous cell carcinoma
(HNSCC): Attenuation of C(18)-ceramide in HNSCC tumors correlates
with lymphovascular invasion and nodal metastasis. Cancer Lett.
256:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Befeler AS and Di Bisceglie AM:
Hepatocellular carcinoma: Diagnosis and treatment.
Gastroenterology. 122:1609–1619. 2002. View Article : Google Scholar : PubMed/NCBI
|