Lung cancer is a disease with the highest morbidity
and mortality rates worldwide. It is reported that non-small cell
lung cancer (NSCLC) accounts for 85% of the total lung cancer cases
worldwide, of which squamous cell lung cancer (LUSC), often with
poor prognosis, accounted for 30% of NSCLC in 2017 (1,2). Data
has demonstrated that more than 1 in 3 patients with lung
adenocarcinoma (LUAD) benefit from molecular-targeted therapies
(3). Inhibitors of epidermal growth
factor receptor, v-ki-ras2 kirsten rat sarcoma viral oncogene
homologue and anaplastic lymphoma receptor tyrosine kinase are some
of the few molecules that are targeted in lung cancer therapy
(4). However, the application of
molecular-targeted therapies in the diagnosis and treatment of LUSC
in the clinical setting is very limited. Thus, the identification
of biomarkers that are associated with the diagnosis and treatment
of LUSC has become one of the main focus areas in research. An
increasing number of studies have revealed new genetic changes
associated with LUSC, including the oncogenes baculoviral IAP
repeat contain 5 (BIRC5) and GAPDH. BIRC5 is an important inhibitor
of apoptosis, which serves an important role in carcinogenesis and
progression of LUSC (5). Li et
al (6) reported a significantly
higher expression level of BIRC5 in LUSC tissues compared with
normal tissues, indicating the potential of BIRC5 as a target for
anti-tumor therapy. On the other hand, GAPDH has been reported to
serve a crucial role in regulating glycolysis in cancer cells.
GAPDH depletes ATP in cancer cells via the inhibition of
glycolysis, which eventually kills cancer cells (7,8). Hence,
GAPDH has become a therapeutic target of interest against cancer
cells. LUSC accounts for more than 400,000 deaths worldwide each
year (2); it is important to
highlight that the mortality rate of LUSC is inevitably high, even
at early stage, despite several discoveries of potential targets
such as BIRC5 and GAPDH. Thus, the investigation of other potential
molecular mechanisms associated with LUSC is important.
In recent years, gene microarray and gene chip
technologies have developed rapidly, which has provided a
theoretical basis for the detection of genetic alterations in
cancer cells (9,10). These technologies can be applied to
identify differentially expressed genes (DEGs), which can
potentially be associated with the carcinogenicity and progression
of LUSC. In the present study, in order to avoid false positive
results from a single microarray gene expression dataset, three
mRNA microarray datasets from the Gene Expression Omnibus (GEO)
database platform were downloaded. LUSC tissues and non-cancerous
tissues were analyzed in order to identify DEGs. Furthermore, Gene
Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analyses were conducted and a protein-protein
interaction (PPI) network was constructed in order to understand
the molecular mechanisms underlying the generation and progression
of LUSC. The associations between the hub genes and clinical tissue
samples were identified using the University of California Santa
Cruz (UCSC) Cancer Genomics Browser and ONCOMINE database. A total
of 67 DEGs and 17 hub genes were identified as potential diagnostic
and therapeutic biomarkers of LUSC. Five hub genes with the highest
node value were selected via CentiScaPe, in which the results from
the UCSC and ONCOMINE online clinical databases indicated all five
hub genes to be associated with unfavorable prognosis of LUSC.
Thymidylate synthetase (TYMS), cyclin B2 (CCNB2) and replication
factor C subunit 4 (RFC4) were suggested as potential and novel
target genes for the treatment of LUSC.
The Database for Annotation, Visualization and
Integrated Discovery database (DAVID; http://david.ncifcrf.gov; version 6.8) is an online
gene and pathway functional annotation database that contains
biological information and also provides analysis tools (14). Biological information can be
extracted from the comprehensive set of genes and proteins, which
provides functional annotations. The KEGG database can be used to
analyze genome information and gene function and study the gene
expression information as a whole network (15). GO is a type of bioinformatics tool
for annotating genes and analyzing their biological processes. GO
enrichment analyses contain three modules of molecular function,
cell composition and biological process (16). In order to analyze the function and
cell signaling pathways of DEGs, KEGG and GO enrichment analyses
were conducted using the DAVID database. KEGG and GO enrichment
bubble plots were drawn using online graphics tools Image GP
(http://www.ehbio.com/ImageGP/).
P<0.05 was considered as statistically significant.
The PPI network of DEGs was constructed by the
online analysis website Search Tool for the Retrieval of
Interacting Genes (STRING; http://string-db.org; version 11.0) (17) and the interaction of a combined score
>0.4 was considered as statistically significant. Analyzing the
function of PPI can provide insights into the mechanisms of disease
occurrence and development. Cytoscape (version 3.6.1) is an open
bioinformatics software platform that can be used to construct a
visual network of molecular interactions (18). The plug-in Molecular Complex
Detection (MCODE) (version 1.4.2) of Cytoscape is an APP for
detecting densely correlated regions in the PPI networks (19). The gene modules were visualized and
graphically displayed with the plug-in MCODE. The selection
criteria were as follows: MCODE score >5; node score cut-off,
0.2; degree cut-off, 2; k-score, 2; and Max depth, 100. CentiScaPe
(version 2.2), a Cytoscape APP specifically designed to calculate
centrality indexes for the selection of the most critical nodes in
a network (20). The plug-in
CentiScaPe 2.2 was used to identify hub genes for functional
analysis with interaction node degrees ≥10.
The hub genes with interaction node degrees ≥10 were
screened. The network of the genes and their co-expression genes
was constructed by the online platform cBioPortal (http://www.cbioportal.org) (21,22).
Hierarchical clustering of hub genes was constructed by online
analysis website UCSC Cancer Genomics Browser (http://genome-cancer.ucsc.edu) (23). Heat maps of hub genes expression in
three different studies of clinical LUSC samples vs. non-cancerous
tissue samples (24–26), and the associations between the
expression patterns and tumor stage, overall survival status (the
survival rate of patients from diagnosis to the end of the study)
and survival status at 5 years (the survival rate five years after
diagnosis) were analyzed using the Hou Lung dataset (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19188)
(27), which was obtained from the
Oncomine database (http://www.oncomine.com) (28,29).
The standard microarrays were obtained from the GEO
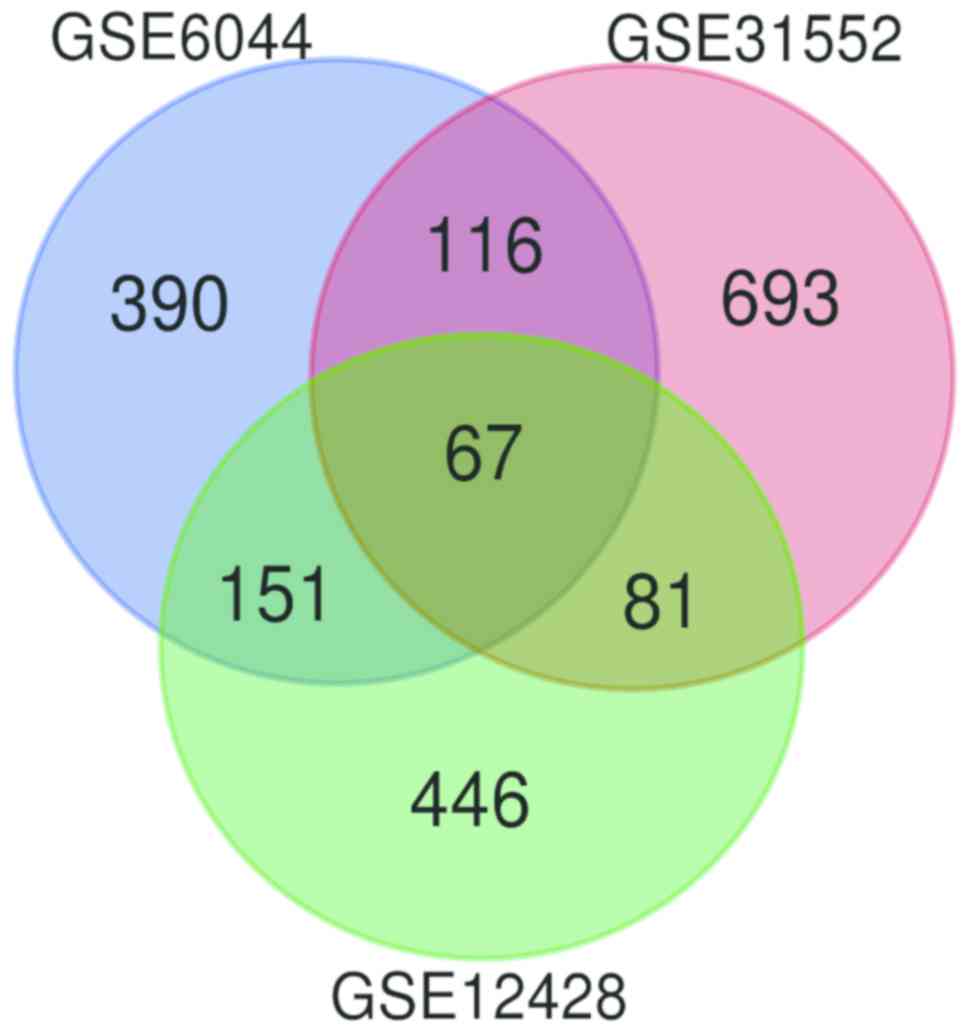
database platform. Following further analysis using GEO2R, DEGs
were identified from the GSE31552 (957), GSE6044 (724) and GSE12428
(745) datasets. The 67 DEGs between the three datasets are
presented in a Venn diagram (Fig.
1), consisting of 42 upregulated genes and 25 downregulated
genes between LUSC and non-cancerous tissues.
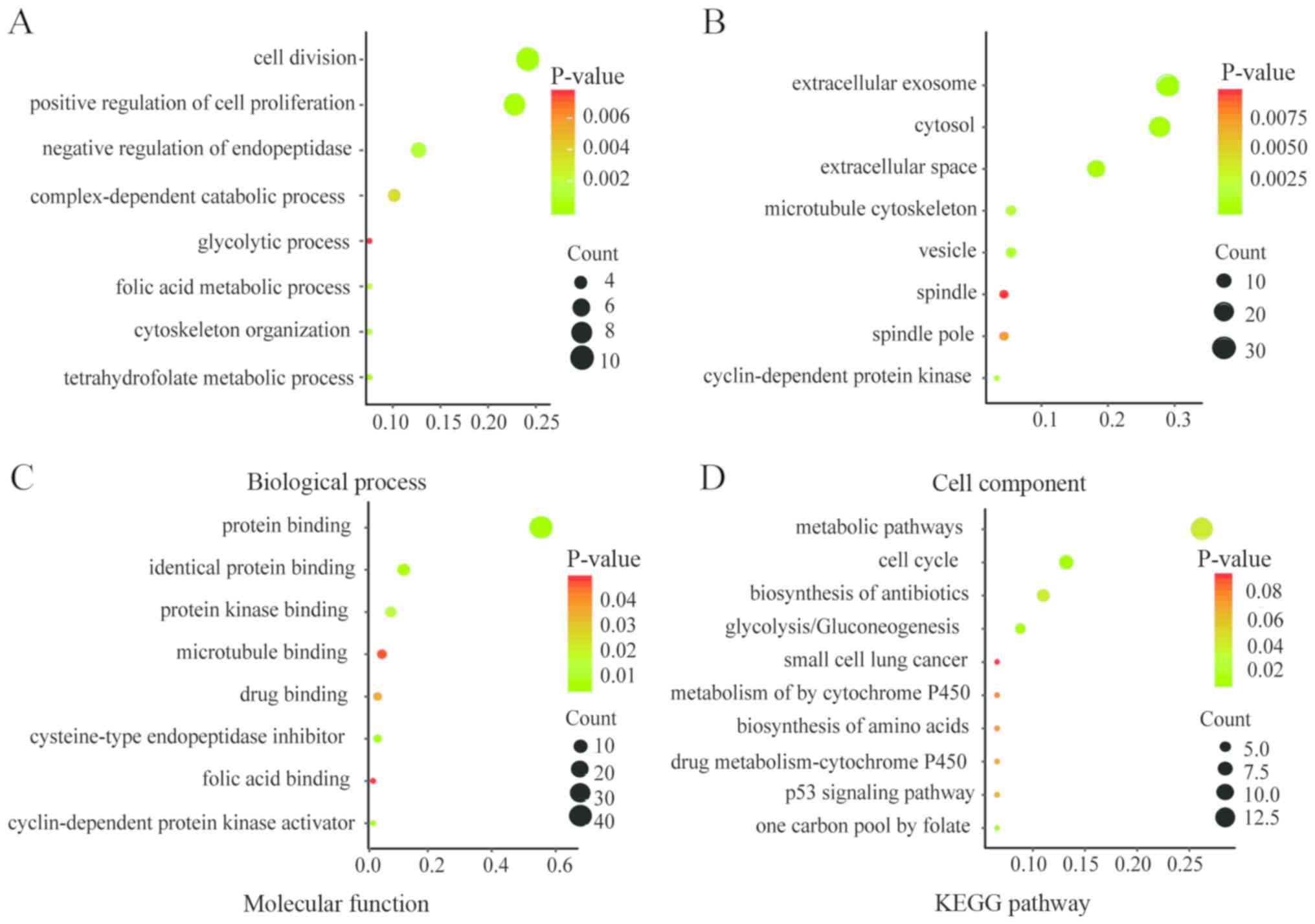
Functional and pathway enrichment analyses of DEGs
were conducted by DAVID to obtain the biological classification.
The GO enrichment analysis included biological processes (BP), cell
component (CC) and molecular function (MF) terms of the DEGs. The
results of the KEGG and GO enrichment analyses are presented as
bubble plots in Fig. 2. Changes in
BP were significantly enriched in ‘cell division’, ‘positive
regulation of cell proliferation’, ‘negative regulation of
endopeptidase activity’ and ‘tetrahydrofolate metabolic process’
(Fig. 2A). Changes in CC were
significantly enriched in ‘extracellular exosome’, ‘extracellular
space’, ‘cytosol’ and ‘vesicle’ (Fig.
2B). Changes in MF were mainly enriched in ‘protein binding’,
‘cysteine-type endopeptidase inhibitor activity’, ‘protein binding’
and ‘identical protein binding’ (Fig.
2C). The KEGG pathway analysis was mainly enriched in ‘cell
cycle’, ‘glycolysis or gluconeogenesis’, ‘metabolic pathways’ and
‘one carbon pool by folate’ (Fig.
2D).
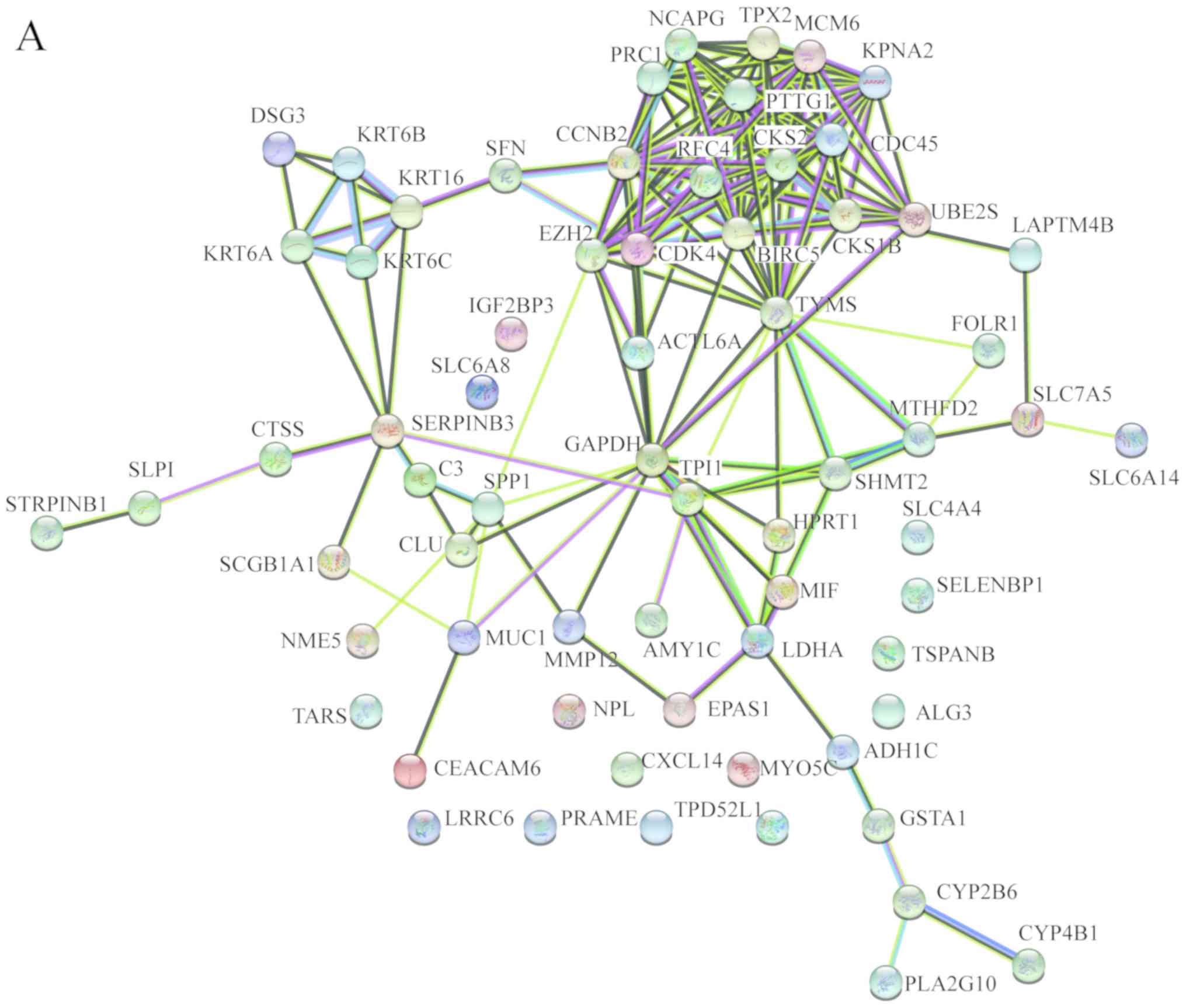
In order to identify the hub genes of LUSC, the PPI
network of DEGs was analyzed by STRING. The results revealed that
most genes interacted with each other and were located in the
center of the network, and were closely associated with the
surrounding proteins in the network (Fig. 3A). To enhance the accuracy of the
results, the PPI network was also analyzed by Cytoscape. The
obtained results were in correspondence with the results of STRING
(Fig. 3B), and the hub gene module
was obtained using MCODE (Fig.
3C).
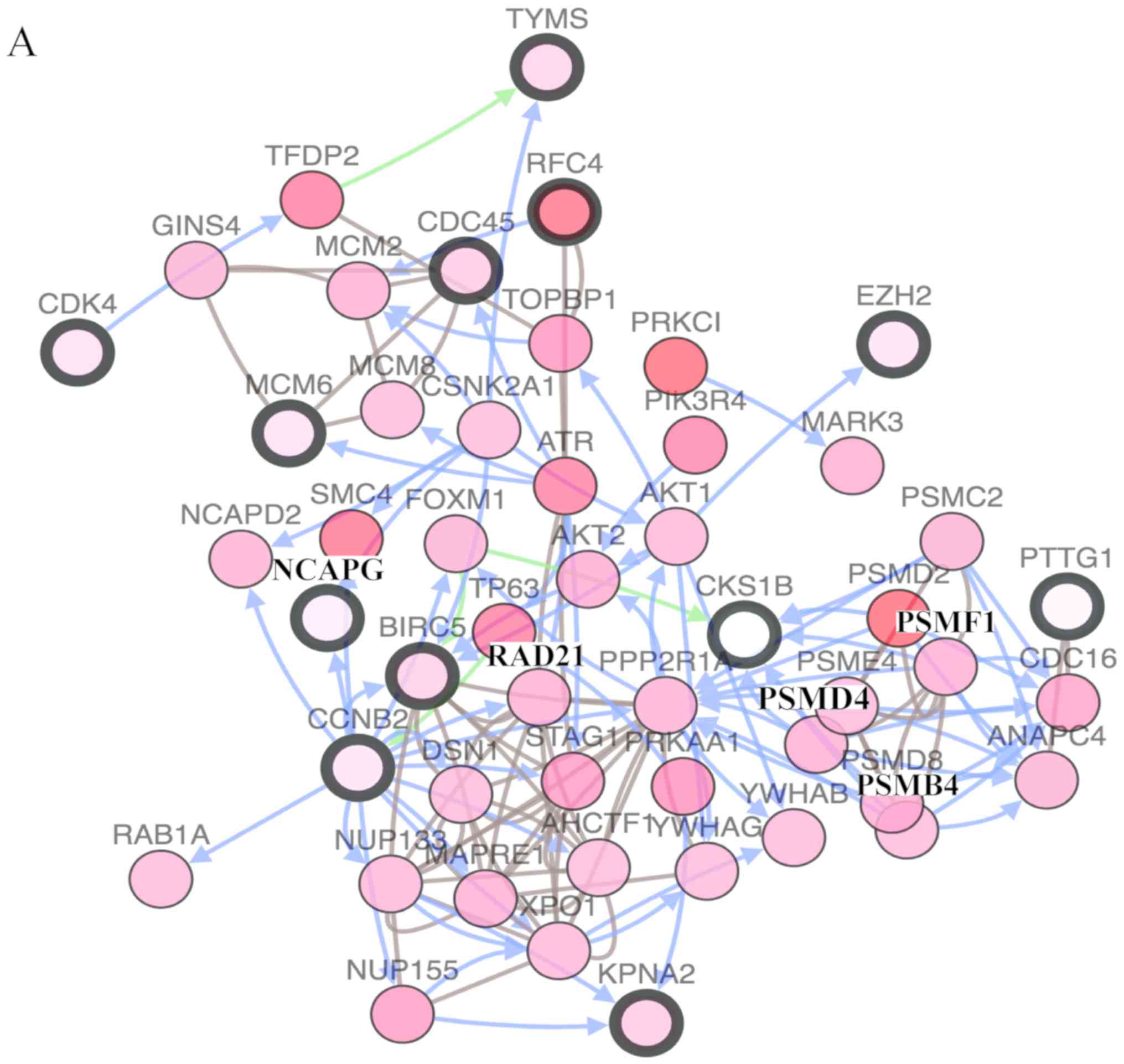
In total, 17 genes were regarded as hub genes with
degrees ≥10 using CentiScaPe. The names, abbreviations and
functions for each of these hub genes are summarized in Table I. The genes associated with the hub
genes and their co-expression network were obtained using the
cBioPortal online platform by performing interaction analysis
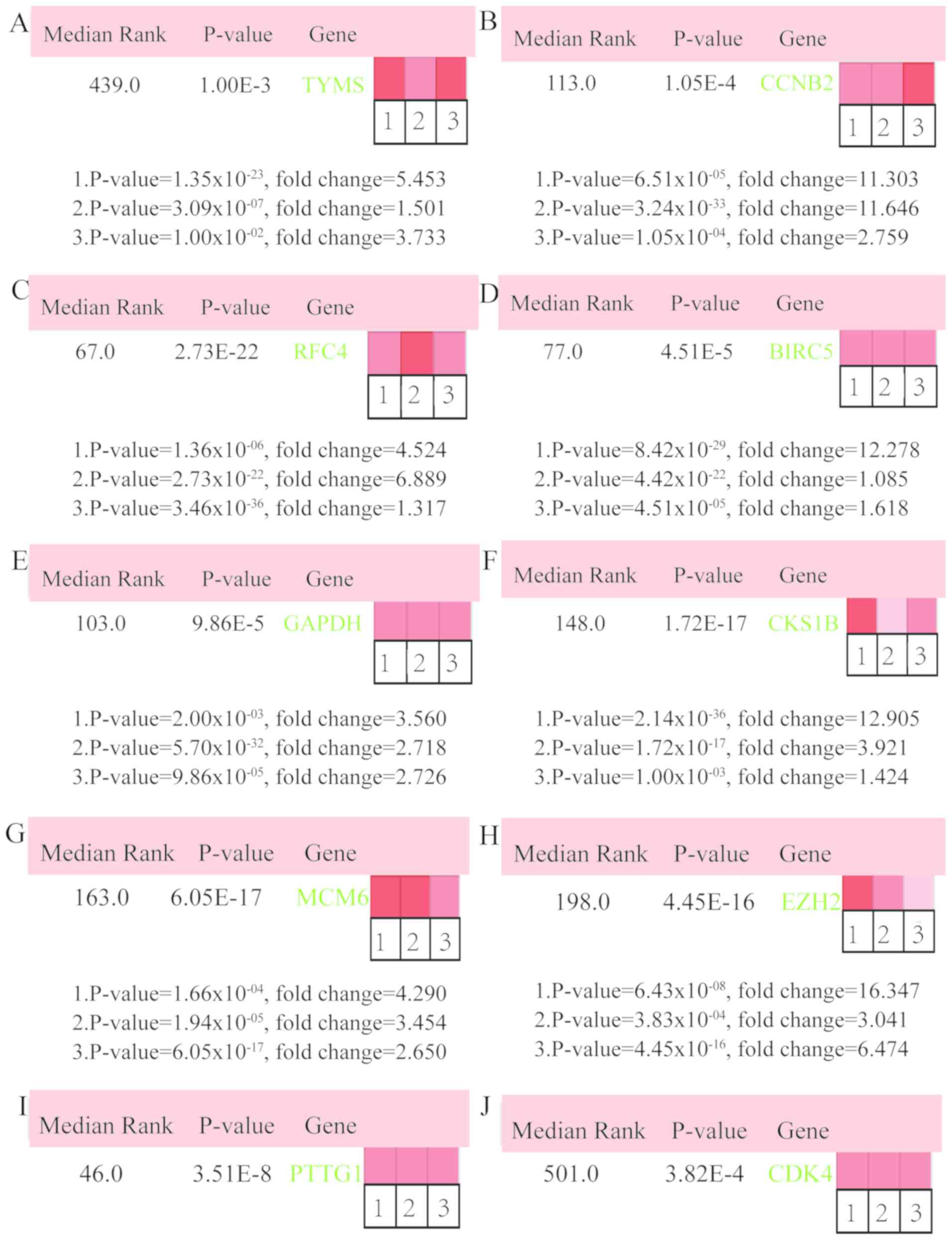
(Fig. 4A). The expression of 17 hub
genes in LUSC tissues and its association with the severity and
prognosis among LUSC patients were further explored using the UCSC
and ONCOMINE online databases. Furthermore, a heat map of
hierarchical clustering obtained using UCSC demonstrated that the
expression of hub genes in LUSC tissues was higher compared with
that of non-cancerous samples. However, the expression of hub genes
showed no differences with gender (Fig.
4B). The heat map of hub genes expression in clinical LUSC
tissue samples and normal tissue samples were analyzed using three
different datasets, using the ONCOMINE online platform. The results
revealed that most of the hub genes were significantly upregulated
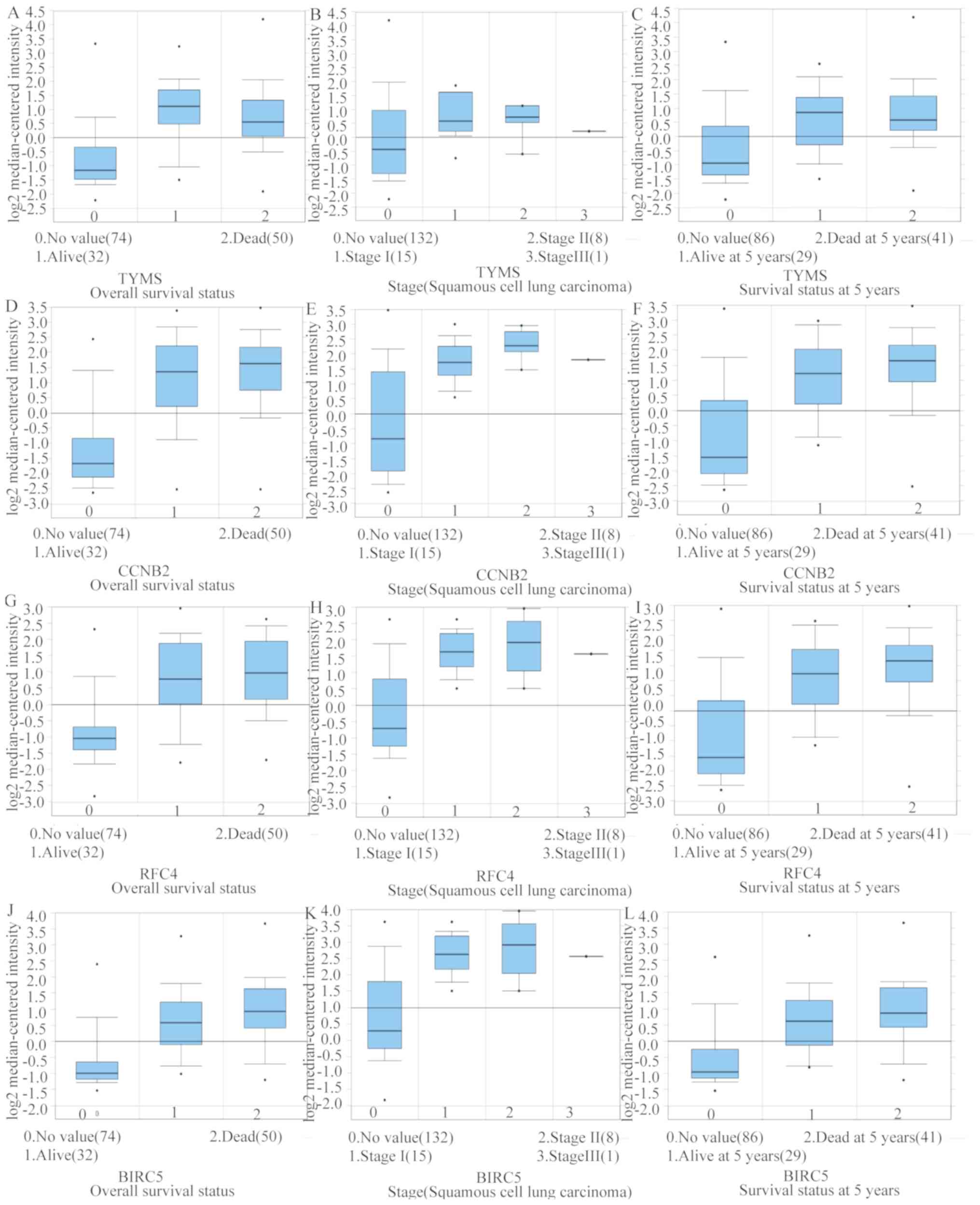
in clinical LUSC samples in all the datasets (Fig. 5). The hub genes whose interaction
node degree was among the top five were TYMS, CCNB2, RFC4, BIRC5
and GAPDH, indicating their potential role in the processes of
carcinogenesis, development and unfavorable prognosis of LUSC. The
associations between the upregulated hub genes and tumor stage,
overall survival status and survival status at 5 years were also
analyzed. The top five genes were associated with high tumor stage,
poor overall survival status and poor survival status at 5 years,
which suggests their upregulation to be involved in the promotion
of tumor progression and poor prognosis (Fig. 6).
In recent years, the incidence and mortality of lung
cancer has continued to increase rapidly worldwide (30). LUAD and LUSC are the two common types
of lung cancer. The incidence of LUSC was reported to be associated
with smoking, whereas the treatment of LUSC remains limited
compared with that of LUAD. The underlying pathological mechanisms
of LUSC at the molecular level are still at the exploration stage
(31). Mutations or amplifications
of phosphatidylinositol-3 kinases (PI3K), phosphatase and Tensin
homolog (PTEN), erythropoietin-producing hepatocellular A2 (EphA2)
and liver kinase B1 (LKB1) were reported to be associated with the
incidence, progression and prognosis of LUSC (32,33). A
study conducted using the Cancer Genome Atlas Research Network
demonstrated the dysfunction of NFE2L2, KEAP1, CDKN2A and RB1, and
the abnormal structures of their products are associated with the
occurrence and development of LUSC (34). The high mortality rate of LUSC is in
part due to the lack of early detection of LUSC biomarkers
(35). As a result, the
identification of key molecules involved in LUSC is required and
important for improving clinical efficacy. Microarray is a
high-throughput technology in obtaining novel biomarkers, which can
provide the basis for further studies on the mechanism of LUSC and
clinical targeted therapies at the molecular level.
In the present study, three mRNA microarray datasets
were analyzed to identify 67 common DEGs. The DEGs consisted of 42
upregulated and 25 downregulated genes between LUSC tissue samples
and normal tissue samples. GO terms and KEGG pathway enrichments
were analyzed in order to investigate interactions among the DEGs.
The results indicated that the 67 DEGs were significantly enriched
in cell cycle, cell proliferation, glycolysis or gluconeogenesis,
and tetrahydrofolate metabolic process. Previous studies have
illustrated that dysregulations of cell cycle and cell
proliferation serve roles in the carcinogenesis and malignant
change of LUSC (36–38). In addition, multiple studies have
also shown that glycolysis or gluconeogenesis serve important roles
in tumor initiation, progression and unfavorable prognosis in
cancer (39,40). Furthermore, gene polymorphism of
tetrahydrofolate induces a decreased activity of tetrahydrofolate
reductase, which affects the normal metabolism of folate in cells,
where tetrahydrofolate metabolic disorder is closely associated
with tumorigenesis (41,42). The findings of the present study were
in accordance with the conclusions of previous studies and showed
that GO and KEGG enrichment analyses were significantly enriched in
cell cycle, cell proliferation, glycolysis or gluconeogenesis, and
tetrahydrofolate metabolic process. A total of 17 DEGs were
identified as hub genes with an interaction node degree ≥10. The
hub genes whose degrees were among the top five were TYMS, CCNB2,
RFC4, BIRC5 and GAPDH, and the PPI network showed that they were
directly interacting with each other.
Several studies have suggested that TYMS is a
predictive biomarker to test for the effectiveness of pemetrexed
used in chemotherapy for treating NSCLC (43,44). Lu
et al (45) reported that the
expression of TYMS was significantly upregulated among patients
with lymph node metastasis. The expression of TYMS was also higher
among patients with 5-year recurrence rate. Besides, high
expression of TYMS was found in the case of breast cancer, which
resulted in increased susceptibility of an individual to the
progression of the disease. Gene polymorphisms of TYMS have been
reported to have potential in improving the diagnosis, prevention
and treatment of breast cancer (46). Hence, several studies are now
focusing on the association between gene polymorphic variations of
TYMS with various types of cancer (47–49).
BIRC5 was upregulated in 76% LUSC samples and the expression in
LUSC tissues was significantly higher compared with that in
non-cancerous tissues (50). BIRC5
is a potential biomarker or therapeutic target of
smoking-associated LUSC (51). The
expression of BIRC5 was higher among patients who are smokers
compared with non-smokers, and in squamous vs. non-squamous lung
tumor (P<0.001). The present study also demonstrated that BIRC5
expression level was negatively associated with the expression of
tumor suppressor gene Tp53 (52).
GAPDH serves a critical role in inhibiting the process of
glycolysis in tumor cells (53). The
expression of GAPDH was notably upregulated in LUSC tissues, and an
increased level of GAPDH significantly promotes the cell
proliferation and migration in LUSC (54). CCNB2 as a member of the cell cyclin
protein family, was significantly associated with different staging
and metastatic statuses of tumors (P<0.001). Thus, CCNB2 is a
potential biomarker for evaluating metastatic status and
therapeutic efficacy for cancer patients (55). Additionally, the upregulation of
CCNB2 was closely associated with the degree of differentiation,
progression, lymph node metastasis, invasion and adverse prognosis
in NSCLC (56). CCNB2 has also been
found to be upregulated in patients with bladder and colorectal
cancer (57). Thus, CCNB2 is a
potential diagnostic biomarker and a therapeutic target for LUSC.
RFC4 was involved in DNA replication and regulation of cell
proliferation and cell cycle. Studies reported an association of
RFC4 with cancer progression and worse survival outcome, and the
ability to predict response to radiotherapy and neoadjuvant
radiotherapy in rectal cancer (58,59).
RFC4 has been demonstrated to be associated with several types of
cancer, however the underlying carcinogenic mechanism needs to be
further explored. The top five hub genes reported in the present
study were associated with various types of cancer. Multiple
studies have demonstrated that GAPDH and BIRC5 are associated with
LUSC (50,54). However, to our knowledge, no previous
studies reported TYMS, CCNB2 and RFC4 to be directly associated
with LUSC. In the present study, ONCOMINE and UCSC analysis
confirmed that the top five hub genes from clinical LUSC samples
were significantly upregulated and were all associated with
different staging of cancer and survival rate compared to that of
other samples. Therefore, TYMS, CCNB2 and RFC4 are potential novel
biomarkers of LUSC for further investigation.
Among the other 12 hub genes identified in the
present study, MCM6, EZH2, CDK4, TPX2 and PRC1 were previously
reported to be associated with LUSC. Minichromosome maintenance
(MCM) proteins serve a critical role in cell proliferation and cell
cycle. Meanwhile, MCM6 is often associated with poor prognosis,
particularly among male patients with LUSC and with a history of
smoking (60). The presence of EZH2
was associated with the aggressiveness of cancer development.
Behrens et al (61) analyzed
221 LUSC samples and 320 lung adenocarcinomas samples, which
revealed significantly higher expression of EZH2 in LUSC compared
with that of LUAD (P<0.0001). Cell cycle protein CDK4 has an
established association with neoplasia and cancer progression.
Recent studies have found that pathways including that of CDK4/6
were frequently altered in LUSC via diverse mechanisms, suggesting
CDK4/6 inhibitors as potential target for the treatment of LUSC
(62,63). TPX2, which actively participates in
the formation of spindle microtubules during mitosis, was
significantly associated with cell differentiation and metastatic
status of LUSC cells, suggesting its potential as a prognostic
predictor of LUSC (64). PRC1, an
important protein involved in cytokinesis, plays an important role
in microtubule organization in eukaryotes (65). A recent study has implicated the
overexpression of PRC1 in LUSC to be associated with increased
susceptibility to lymph node metastasis and shorter survival time
in patients with LUSC (66).
Literature review revealed that the interaction
among LUSC and hub genes CKS1B, PTTG1, CKS2, CDC45, KPNA2, NCAPG
and UBE2S has not been widely reported. These genes are potential
novel biomarkers and therapeutic targets of LUSC. CKS1B, an adaptor
for cyclin-dependent kinases, was shown to be associated with
chemoresistance, low chemosensitivity and poor prognosis in cancer.
An elevated level of CKS1B has been reported to result in the
resistance of cancer cells to bortezomib, and activation of the
NEDD8 pathway, which in turn leads to further advancement of
cancer. Thus, CKS1B is a potential novel target in multiple myeloma
(67). In addition, CKS1B promotes
proteasomal degradation and ubiquitination of p27Kip1.
Overexpression of CKS1B contributes to an increased turnover rate
of p27Kip1 and promotion of cancer cell proliferation, resulting in
poor prognosis in many types of cancer (68). PTTG1 functions to regulate
transcription, the G-M phase of mitosis and the repair of DNA.
Overexpression of PTTG1 has been reported in multiple types of
cancer, in which PTTG1 is associated with metabolic processes, such
as carcinogenesis, migration, invasion and epithelial-mesenchymal
transition in squamous cell carcinomas (69). Recent studies demonstrated that
various non-coding RNAs lead to cancer cell growth and metastasis
via PTTG1 (70,71). Therefore, PTTG1 is a potential and
novel therapeutic target of LUSC tumor growth and metastasis. CKS2,
as a cyclin-dependent kinase-interacting protein, serves important
roles in regulating cell cycle, inducing apoptosis, as well as
regulating cancer cell invasion and metastasis. Upregulation of
CKS2 can lead to DNA damage in cells, which can increase the
proliferation of cancer cell (72).
Therefore, the molecular mechanism by which CKS2 regulates cell
cycle and induces cell apoptosis may be critical in investigating
diagnostic methods and treatment methods for LUSC. CDC45 is an
essential regulator of cell proliferation. An elevated expression
of CDC45 is correlated with DNA damage in the S-phase, in which the
anti-cancer effect of CDC45 suppressor is mediated by limiting DNA
damage during S phase (73). KPNA2,
as a member of the Karyopherin α family, actively participates in
the process of signal transduction from the extracellular space to
the nucleus. Furthermore, the upregulation of KPNA2 is correlated
with cancer cell proliferation and metastasis (74). Moreover, several studies reported
that KPNA2 is involved in the progression of cancer by regulating
nuclear translocation of cancer-associated proteins; which may
explain the significantly upregulated expression of KPNA2 in LUSC.
NCAPG is a novel mitotic gene and provides novel therapeutic
targets for cancer. Goto et al (75) reported that NCAPG, as a target of
miR-145-3p, could predict the survival rate of patients with
prostate cancer. UBE2S is a central protein in the process of
ubiquitination and is associated with the malignancy of various
types of cancer (76). Studies have
reported the upregulation of UBE2S to enhance the nuclear
translocation of β-catenin and induced expression of c-Myc and
cyclin D1, suggesting UBE2S as a potential prognostic factor and
oncogene in LUSC (77–78).
In conclusion, the present study was conducted in
order to identify potential DEGs that may be associated with
carcinogenesis or adverse progression of LUSC. A total of 67 DEGs
and 17 hub genes were identified, in which hub genes were regarded
as promising targets for the diagnosis and treatment of LUSC.
Meanwhile, TYMS, CCNB2 and RFC4 were identified as potential novel
biomarkers of LUSC. However, the present study was based on
bioinformatics methods and no experiments were performed to
validate the findings. Therefore, further studies are required to
explore the biological association between the genes identified in
the present study in LUSC, in order to improve treatments and
clinical outcomes of LUSC.
Not applicable.
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
81273696).
The datasets used and/or analyzed in the present
study are available from the corresponding authors on reasonable
request.
JM, XZ, HD and SL designed the study. JM and XZ
analyzed and interpreted the microarray datasets, and wrote the
manuscript. XY and LM made substantial contributions to acquisition
and interpretation of data. JM, XG, HY, JC and YL analyzed the
data. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Youlden DR, Cramb SM and Baade PD: The
International Epidemiology of Lung Cancer: Geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lazarus KA, Hadi F, Zambon E, Bach K,
Santolla MF, Watson JK, Correia LL, Das M, Ugur R, Pensa S, et al:
BCL11A interacts with SOX2 to control the expression of epigenetic
regulators in lung squamous carcinoma. Nat Commun. 9:33272018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kris MG, Johnson BE, Kwiatkowski DJ,
Iafrate AJ, Wistuba II, Aronson SL, Engelman JA, Shyr Y, Khuri FR,
Rudin CM, et al: Identification of driver mutations in tumor
specimens from 1,000 patients with lung adenocarcinoma: The NCI's
Lung Cancer Mutation Consortium (LCMC). J Clin Oncol.
29:CRA75062011. View Article : Google Scholar
|
|
4
|
Chan BA and Hughes BG: Targeted therapy
for non-small cell lung cancer: Current standards and the promise
of the future. Transl Lung Cancer Res. 4:36–54. 2015.PubMed/NCBI
|
|
5
|
Vayshlya NA, Zinovyeva MV, Sass AV,
Kopantzev EP, Vinogradova TV and Sverdlov ED: Increased expression
of BIRC5 in non-small cell lung cancer and esophageal
squamous cell carcinoma does not correlate with the expression of
its inhibitors SMAC/DIABLO and PML. Mol Biol. 42:579–587. 2008.
View Article : Google Scholar
|
|
6
|
Li S, Sun X, Miao S, Liu J and Jiao W:
Differential protein-coding gene and long noncoding RNA expression
in smoking-related lung squamous cell carcinoma. Thorac Cancer.
8:672–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ganapathy-Kanniappan S, Geschwind JF,
Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, Cole RN, Syed
LH, Rao PP, Ota S and Vali M: Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate
mediated cancer cell death. Anticancer Res. 29:4909–4918.
2009.PubMed/NCBI
|
|
8
|
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng
L, Bhalla KN, Keating MJ and Huang P: Inhibition of glycolysis in
cancer cells: A novel strategy to overcome drug resistance
associated with mitochondrial respiratory defect and hypoxia.
Cancer Res. 65:613–621. 2005.PubMed/NCBI
|
|
9
|
Dawany NB and Tozeren A: Asymmetric
microarray data produces gene lists highly predictive of research
literature on multiple cancer types. BMC Bioinformatics.
11:4832010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dawany NB, Dampier WN and Tozeren A:
Large-scale integration of microarray data reveals genes and
pathways common to multiple cancer types. Int J Cancer.
128:2881–2891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin J, Marquardt G, Mullapudi N, Wang T,
Han W, Shi M, Keller S, Zhu C, Locker J and Spivack SD: Lung cancer
transcriptomes refined with laser capture microdissection. Am J
Pathol. 184:2868–2884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rohrbeck A, Neukirchen J, Rosskopf M,
Pardillos GG, Geddert H, Schwalen A, Gabbert HE, von Haeseler A,
Pitschke G, Schott M, et al: Gene expression profiling for
molecular distinction and characterization of laser captured
primary lung cancers. J Transl Med. 6:692008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boelens MC, van den Berg A, Fehrmann RS,
Geerlings M, de Jong WK, te Meerman GJ, Sietsma H, Timens W, Postma
DS and Groen HJ: Current smoking-specific gene expression signature
in normal bronchial epithelium is enhanced in squamous cell lung
cancer. J Pathol. 218:182–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID Bioinformatics Resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35:W169–W175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scardoni G, Tosadori G, Faizan M, Spoto F,
Fabbri F and Laudanna C: Biological network analysis with
CentiScaPe: Centralities and experimental dataset integration.
F1000Res. 3:1392014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mangan ME, Williams JM, Lathe SM,
Karolchik D and Lathe WC III: UCSC genome browser: Deep support for
molecular biomedical research. Biotechnol Annu Rev. 14:63–108.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou J, Aerts J, den Hamer B, van Ijcken W,
den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens
JA, Hoogsteden HC, et al: Gene expression-based classification of
non-small cell lung carcinomas and survival prediction. PLoS One.
5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garber ME, Troyanskaya OG, Schluens K,
Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen
GD, Perou CM, Whyte RI, et al: Diversity of gene expression in
adenocarcinoma of the lung. Proc Natl Acad Sci USA. 98:13784–13789.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wachi S, Yoneda K and Wu R:
Interactome-transcriptome analysis reveals the high centrality of
genes differentially expressed in lung cancer tissues.
Bioinformatics. 21:4205–4208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou J, Aerts J, den Hamer B, van Ijcken W,
den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens
JA, Hoogsteden HC, et al: Gene expression-based classification of
non-small cell lung carcinomas and survival prediction. PLoS One.
5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou GX, Liu P, Yang J and Wen S: Mining
expression and progQixing M, Gaochao D, Wenjie X, Anpeng W, Bing C,
Weidong M, Lin X and Feng J: Microarray analyses reveal genes
related to progression and prognosis of esophageal squamous cell
carcinoma. Oncotarget. 8:78838–78850. 2017.PubMed/NCBI
|
|
29
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: a cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sholl LM, Aisner DL, Varella-Garcia M,
Berry LD, Dias-Santagata D, Wistuba II, Chen H, Fujimoto J, Kugler
K, Franklin WA, et al: Multi-institutional Oncogenic Driver
Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation
Consortium ExperienceJ Thorac Oncol; 10. pp. 768–777. 2015,
PubMed/NCBI
|
|
32
|
Mantripragada K and Khurshid H: Targeting
genomic alterations in squamous cell lung cancer. Front Oncol.
3:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heist RS, Sequist LV and Engelman JA:
Genetic changes in squamous cell lung cancer: A review. J Thorac
Oncol. 7:924–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brambilla C, Laffaire J, Lantuejoul S,
Moro-Sibilot D, Mignotte H, Arbib F, Toffart AC, Petel F, Hainaut
P, Rousseaux S, et al: Lung squamous cell carcinomas with basaloid
histology represent a specific molecular entity. Clin Cancer Res.
20:5777–5786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao W, Bai Y, Li Y, Guo L, Zeng P, Wang Y,
Qi B, Liu S, Qin X, Li Y and Zhao B: Upregulation of MALAT-1 and
its association with survival rate and the effect on cell cycle and
migration in patients with esophageal squamous cell carcinoma.
Tumour Biol. 37:4305–4312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee CH, Lee MK, Kang CD, Kim YD, Park DY,
Kim JY, Sol MY and Suh KS: Differential expression of hypoxia
inducible factor-1 alpha and tumor cell proliferation between
squamous cell carcinomas and adenocarcinomas among operable
non-small cell lung carcinomas. J Korean Med Sci. 18:196–203. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirano T, Franzén B, Kato H, Ebihara Y and
Auer G: Genesis of squamous cell lung carcinoma. Sequential changes
of proliferation, DNA ploidy, and p53 expression. Am J Pathol.
144:296–302. 1994.PubMed/NCBI
|
|
39
|
Liu GM and Zhang YM: Targeting FBPase is
an emerging novel approach for cancer therapy. Cancer Cell Int.
18:362018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Keku T, Millikan R, Worley K, Winkel S,
Eaton A, Biscocho L, Martin C and Sandler R:
5,10-methylenetetrahydrofolate reductase codon 677 and 1298
polymorphisms and colon cancer in African Americans and whites.
Cancer Epidemiol Biomarkers Prev. 11:1611–1621. 2002.PubMed/NCBI
|
|
42
|
Ferlazzo N, Currò M, Zinellu A, Caccamo D,
Isola G, Ventura V, Carru C, Matarese G and Ientile R: Influence of
MTHFR genetic background on p16 and MGMT methylation in oral
squamous cell cancer. Int J Mol Sci. 18:E7242017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fujii T, Toyooka S, Ichimura K, Fujiwara
Y, Hotta K, Soh J, Suehisa H, Kobayashi N, Aoe M, Yoshino T, et al:
ERCC1 protein expression predicts the response of cisplatin-based
neoadjuvant chemotherapy in non-small-cell lung cancer. Lung
Cancer. 59:377–384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kasai D, Ozasa H, Oguri T, Miyazaki M,
Uemura T, Takakuwa O, Kunii E, Ohkubo H, Maeno K and Niimi A:
Thymidylate synthase gene copy number as a predictive marker for
response to pemetrexed treatment of lung adenocarcinoma. Anticancer
Res. 33:1935–1940. 2013.PubMed/NCBI
|
|
45
|
Lu Y, Zhuo C, Cui B, Liu Z, Zhou P, Lu Y
and Wang B: TYMS serves as a prognostic indicator to predict the
lymph node metastasis in Chinese patients with colorectal cancer.
Clin Biochem. 46:1478–1483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
da Silva Nogueira J Jr, de Lima Marson FA
and Sílvia Bertuzzo C: Thymidylate synthase gene (TYMS)
polymorphisms in sporadic and hereditary breast cancer. BMC Res
Notes. 5:6762012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shichijo S, Azuma K, Komatsu N, Ito M,
Maeda Y, Ishihara Y and Itoh K: Two proliferation-related proteins,
TYMS and PGK1, could be new cytotoxic T lymphocyte-directed
tumor-associated antigens of HLA-A2+ colon cancer. Clin Cancer Res.
10:5828–5836. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chao YL and Anders CK: TYMS gene
polymorphisms in breast cancer patients receiving
5-fluorouracil-based chemotherapy. Clin Breast Cancer.
18:e301–e304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He Y, Penney ME, Negandhi AA, Parfrey PS,
Savas S and Yilmaz YE: XRCC3 Thr241Met and TYMS variable number
tandem repeat polymorphisms are associated with time-to-metastasis
in colorectal cancer. PLoS One. 13:e01923162018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Knizhnik AV, Kovaleva OB, Laktionov KK,
Mochal'nikova VV, Komel'kov AV, Chevkina EM and Zborovskaia IB:
Arf6, RalA and BIRC5 protein expression in non small cell lung
cancer. Mol Biol (Mosk). 45:307–315. 2011.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu X, Zhang Y, Cavazos D, Ma X, Zhao Z, Du
L and Pertsemlidis A: miR-195 targets cyclin D3 and survivin to
modulate the tumorigenesis of non-small cell lung cancer. Cell
Death Dis. 9:1932018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Phiboonchaiyanan PP, Petpiroon N,
Sritularak B and Chanvorachote P: Phoyunnanin E induces apoptosis
of non-small cell lung cancer cells via p53 activation and
down-regulation of survivin. Anticancer Res. 38:6281–6290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ganapathy-Kanniappan S: Evolution of GAPDH
as a druggable target of tumor glycolysis? Expert Opin Ther
Targets. 22:295–298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hao L, Zhou X, Liu S, Sun M, Song Y, Du S,
Sun B, Guo C, Gong L, Hu J, et al: Elevated GAPDH expression is
associated with the proliferation and invasion of lung and
esophageal squamous cell carcinomas. Proteomics. 15:3087–3100.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mo ML, Chen Z, Li J, Li HL, Sheng Q, Ma
HY, Zhang FX, Hua YW, Zhang X, Sun DQ, et al: Use of serum
circulating CCNB2 in cancer surveillance. Int J Biol Markers.
25:2010. View Article : Google Scholar
|
|
56
|
Takashima S, Saito H, Takahashi N, Imai K,
Kudo S, Atari M, Saito Y, Motoyama S and Minamiya Y: Strong
expression of cyclin B2 mRNA correlates with a poor prognosis in
patients with non-small cell lung cancer. Tumour Biol.
35:4257–4265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lei CY, Wang W, Zhu YT, Fang WY and Tan
WL: The decrease of cyclin B2 expression inhibits invasion and
metastasis of bladder cancer. Urol Oncol. 34:237.e1–e10. 2016.
View Article : Google Scholar
|
|
58
|
Wang XC, Yue X, Zhang RX, Liu TY, Pan ZZ,
Yang MJ, Lu ZH, Wang ZY, Peng JH, Le LY, et al: Genome-wide RNAi
screening identifies RFC4 as a factor that mediates radioresistance
in colorectal cancer by facilitating nonhomologous end joining
repair. Clin Cancer Res. 25:4567–4579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xiang J, Fang L, Luo Y, Yang Z, Liao Y,
Cui J, Huang M, Yang Z, Huang Y, Fan X, et al: Levels of human
replication factor C4, a clamp loader, correlate with tumor
progression and predict the prognosis for colorectal cancer. J
Transl Med. 12:3202014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu YZ, Wang BS, Jiang YY, Cao J, Hao JJ,
Zhang Y, Xu X, Cai Y and Wang MR: MCMs expression in lung cancer:
Implication of prognostic significance. J Cancer. 8:3641–3647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Behrens C, Solis LM, Lin H, Yuan P, Tang
X, Kadara H, Riquelme E, Galindo H, Moran CA, Kalhor N, et al: EZH2
protein expression associates with the early pathogenesis, tumor
progression, and prognosis of non-small cell lung carcinoma. Clin
Cancer Res. 19:6556–6565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi R, Li M, Raghavan V, Tam S, Cabanero
M, Pham NA, Shepherd FA, Moghal N and Tsao MS: Targeting the
CDK4/6-Rb pathway enhances response to PI3K inhibition in
PIK3CA-mutant lung squamous cell carcinoma. Clin Cancer Res.
24:5990–6000. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen L and Pan J: Dual cyclin-dependent
kinase 4/6 inhibition by PD-0332991 induces apoptosis and
senescence in oesophageal squamous cell carcinoma cells. Br J
Pharmacol. 174:2427–2443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ma Y, Lin D, Sun W, Xiao T, Yuan J, Han N,
Guo S, Feng X, Su K, Mao Y, et al: Expression of targeting protein
for xklp2 associated with both malignant transformation of
respiratory epithelium and progression of squamous cell lung
cancer. Clin Cancer Res. 12:1121–1127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mollinari C, Kleman JP, Jiang W, Schoehn
G, Hunter T and Margolis RL: PRC1 is a microtubule binding and
bundling protein essential to maintain the mitotic spindle midzone.
J Cell Biol. 157:1175–1186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhan P, Xi GM, Liu HB, Liu YF, Xu WJ, Zhu
Q, Zhou ZJ, Miao YY, Wang XX, Jin JJ, et al: Protein regulator of
cytokinesis-1 expression: Prognostic value in lung squamous cell
carcinoma patients. J Thorac Dis. 9:2054–2060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang J, Zhou Y, Thomas GS, Gu Z, Yang Y,
Xu H, Tricot G and Zhan F: NEDD8 inhibition overcomes CKS1B-induced
drug resistance by upregulation of p21 in multiple myeloma. Clin
Cancer Res. 21:5532–5542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhan F, Colla S, Wu X, Chen B, Stewart JP,
Kuehl WM, Barlogie B and Shaughnessy JD Jr: CKS1B, overexpressed in
aggressive disease, regulates multiple myeloma growth and survival
through SKP2- and p27Kip1-dependent and -independent mechanisms.
Blood. 109:4995–5001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Romero Arenas MA, Whitsett TG, Aronova A,
Henderson SA, LoBello J, Habra MA, Grubbs EG, Lee JE, Sircar K,
Zarnegar R, et al: Protein expression of PTTG1 as a diagnostic
biomarker in adrenocortical carcinoma. Ann Surg Oncol. 25:801–807.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Guo XC, Li L, Gao ZH, Zhou HW, Li J and
Wang QQ: The long non-coding RNA PTTG3P promotes growth and
metastasis of cervical cancer through PTTG1. Aging (Albany NY).
11:1333–1341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang JL, Cao SW, Ou QS, Yang B, Zheng SH,
Tang J, Chen J, Hu YW, Zheng L and Wang Q: The long non-coding RNA
PTTG3P promotes cell growth and metastasis via up-regulating PTTG1
and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol
Cancer. 17:932018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
You H, Lin H and Zhang Z: CKS2 in human
cancers: Clinical roles and current perspectives (Review). Mol Clin
Oncol. 3:459–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hauge S, Naucke C, Hasvold G, Joel M,
Rødland GE, Juzenas P, Stokke T and Syljuåsen RG: Combined
inhibition of Wee1 and Chk1 gives synergistic DNA damage in S-phase
due to distinct regulation of CDK activity and CDC45 loading.
Oncotarget. 8:10966–10979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jensen JB, Munksgaard PP, Sørensen CM,
Fristrup N, Birkenkamp-Demtroder K, Ulhøi BP, Jensen KM, Ørntoft TF
and Dyrskjøt L: High expression of karyopherin-α2 defines poor
prognosis in non-muscle-invasive bladder cancer and in patients
with invasive bladder cancer undergoing radical cystectomy. Eur
Urol. 59:841–848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Goto Y, Kurozumi A, Arai T, Nohata N,
Kojima S, Okato A, Kato M, Yamazaki K, Ishida Y, Naya Y, et al:
Impact of novel miR-145-3p regulatory networks on survival in
patients with castration-resistant prostate cancer. Br J Cancer.
117:409–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Garnett MJ, Mansfeld J, Godwin C,
Matsusaka T, Wu J, Russell P, Pines J and Venkitaraman AR: UBE2S
elongates ubiquitin chains on APC/C substrates to promote mitotic
exit. Nat Cell Biol. 11:1363–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Akter KA, Hyodo T, Asano E, Sato N,
Mansour MA, Ito S, Hamaguchi M and Senga T: Erratum to: UBE2S is
associated with malignant characteristics of breast cancer cells.
Tumor Biol. 37:69992016. View Article : Google Scholar
|
|
78
|
Ben-Eliezer I, Pomerantz Y, Galiani D,
Nevo N and Dekel N: Appropriate expression of Ube2C and Ube2S
controls the progression of the first meiotic division. FASEB J.
29:4670–4681. 2015. View Article : Google Scholar : PubMed/NCBI
|