Introduction
Liver cancer is one of the most common types of
cancers in the world, with ~841,000 newly diagnosed cases and
782,000 deaths globally per year, according to the global burden
data in 2018. Hepatocellular carcinoma (HCC) constitutes 75–85% of
liver cancer cases globally (1). In
spite of the rapid development of clinical treatment methods in
recent years, the prognosis of HCC patients remains dismal
(2). Overall, the choice of surgical
approach significantly affects prognosis and therefore, should be
carefully considered (3).
In current clinical practice, the Barcelona Clinic
Liver Cancer staging system (4) is
recommended as the standard for surgical approach selection by both
the European Association for the Study of the Liver and the
American Association for the Study of Liver Disease (5,6).
According to this standard, curative treatments are recommended as
the optimal choice only for patients with very-early-stage and
early-stage tumors (Barcelona Clinic Liver Cancer staging 0-A;
solitary tumors or multinodular tumors with ≤3 nodules and size ≤3
cm with no vascular invasion or extrahepatic spread,
Child-Turcotte-Pugh A or B, performance status 0) (7). Kutlu et al (8) demonstrated that radiofrequency ablation
(RFA) is an appropriate method of treatment for patients with
tumors measuring ≤30 mm, but that overall and cancer-specific
survival (CSS) are worse for RFA compared with surgical resection
or transplantation for tumors >30 mm. For patients with
unresecTable tumors, Shimose et al (9) suggested that transcatheter arterial
chemoembolization (TACE) combined with RFA may prolong survival
compared with TACE alone. However, some guidelines recommend
surgical treatment for a broader spectrum of patients with HCC,
such as the Asian Pacific Association for the Study of the Liver
(10), the American
Hepato-Pancreato-Biliary Association (11), the Korean Liver Cancer Study Group
(12) and the Japan Society of
Hepatology (13). In addition, Hyun
et al (7) in 2019 performed a
systematic review and reported that surgical treatment provides
survival benefits in advanced-stage patients with HCC compared with
chemoembolization. Due to these inconsistencies, further
exploration is needed to establish the best surgical method for
patients with HCC.
Previous studies have identified various factors
that influence the prognosis of patients with HCC, such as age
(14), tumor size (15,16),
marital status (17), α-fetoprotein
level (18), lymph node involvement,
metastasis and co-infection with hepatitis B and C viruses
(19), Epstein-Barr virus-induced
gene 3 (20), serum interleukin-34
(IL-34) (21), however their
relative importance remains unclear.
Random forest (RF) is a widely used classification
machine learning method that does not require a prior hypothesis
(22,23) and may provide another statistical
option for researchers evaluating large datasets. RF has become a
promising computational approach for determining patterns and
associations based on high-dimensional datasets (24–26). The
variable importance measure, a byproduct of the RF algorithm is
calculated according to the predictive power of a variable and is
often used to order the importance of variables, especially in
genetics (27–29).
The Surveillance, Epidemiology and End Results
(SEER) program collects data on cancer cases from various locations
and sources throughout the USA, covering ~28% of the population
(30). This dataset has been
indicated to be valuable for predicting the prognosis of numerous
types of malignant tumors, such as spinal ependymoma (31), breast carcinoma (32) and lung cancer (33).
In the present study, the RF model was used to
identify the most important variables influencing the survival
outcomes of patients with HCC. A longitudinal analysis was
subsequently performed to determine the overall survival (OS) time
and CSS outcomes of patients from SEER program with HCC (www.seer.cancer.gov) treated with different surgical
methods, including no surgery, local tumor destruction, wedge or
segmental resection, lobectomy and liver transplantation. The
findings of the present study may provide clinicians and
researchers with new evidence regarding HCC treatment
selection.
Patients and methods
Population selection and data
pre-processing
This cohort study was based on the newly released
(1975–2016) Surveillance, Epidemiology and End Results (SEER)
national database (www.seer.cancer.gov). Patients with an International
Classification of Diseases Oncology, 3rd Edition
(34) code C220 and those with
histology codes 8170–8175 were identified as cases with HCC. In
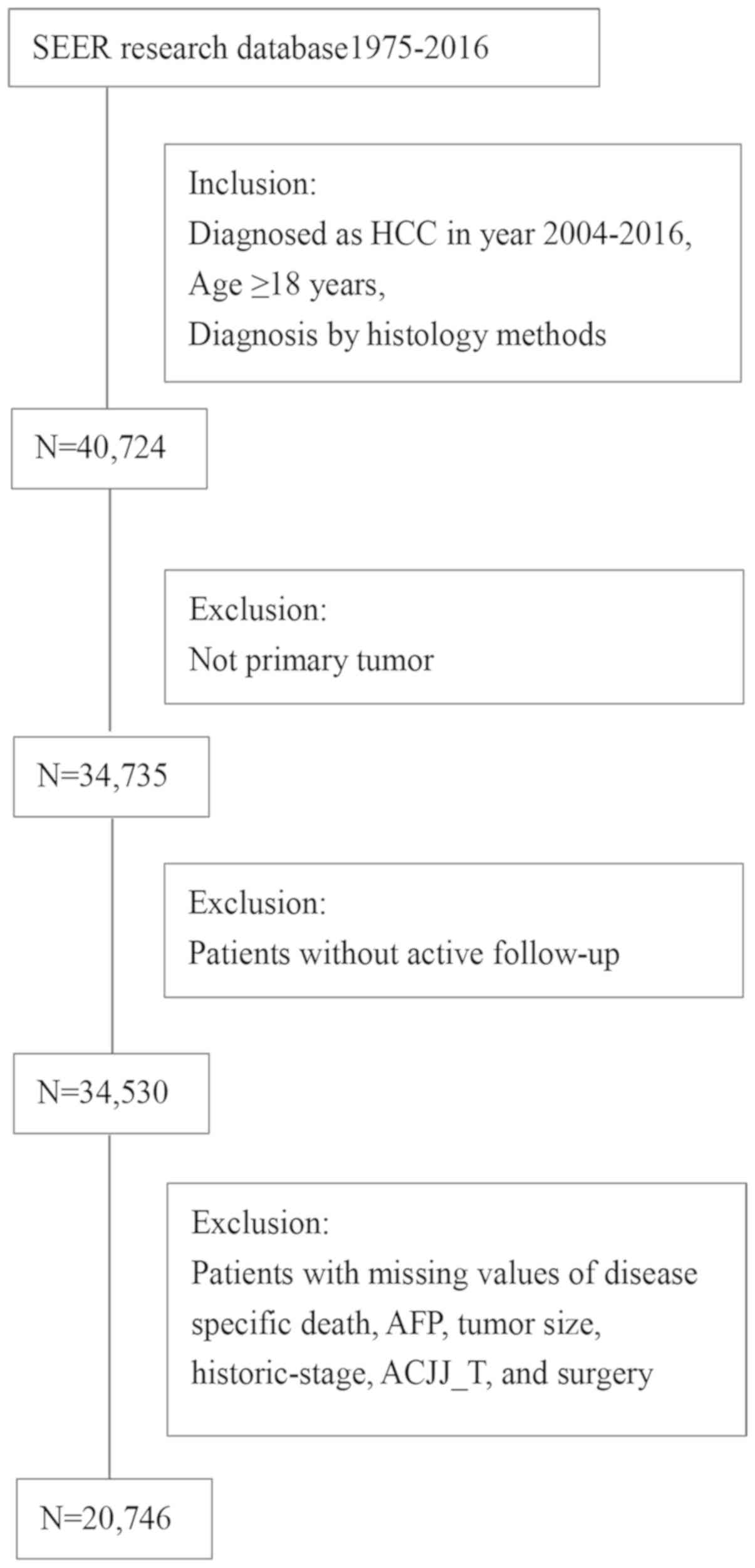
total, 20,746 patients with HCC were identified who met the
following inclusion criteria: (1)
Diagnosed in 2004–2016, (2)
diagnosed by histology methods and (3) aged ≥18 years. The exclusion criteria
were no active follow-up data and missing values for surgery
method, cancer-specific death, AFP, tumor size, historic stage and
the T category according to the American Joint Committee on Cancer
6th edition (AJCC6_T) (https://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/).
The detailed selection process is summarized in Fig. 1.
The data for surgery methods was categorized into no
surgery (no surgery of the primary site), local tumor destruction
(including photodynamic therapy, electrocautery, cryosurgery,
laser, percutaneous ethanol injection, heat RFA, ultrasound and use
of acetic acid), wedge or segmental resection, lobectomy (including
right lobectomy, left lobectomy, lobectomy and local tumor
destruction and extended lobectomy), and liver transplantation
groups. Surgery of other sites (distant lymph nodes or other
tissue(s)/organ(s) beyond the primary site) was divided into a
surgery group and a non-surgery group. The year of diagnosis was
divided into three time periods: 2004–2008, 2009–2012 and
2013–2016. The data for scope of regional lymph node surgery were
divided into a non-surgical group and a surgical group. Data for
marital status were dichotomously divided into a married group and
an unmarried group, which included those who were divorced,
separated, single, unmarried, had a domestic partner or were
widowed. In the present study, data regarding insurance as
uninsured or insured were recoded. The insured group included those
with Medicaid, Indian or a public health service or private
insurance, and those >65 years (as they were eligible for
Medicare) (35).
Presentation of data and
statistics
Missing values consisted of 2.38% of the total data
extracted; missing continuous variables were replaced with the mean
and missing categorical variables were recorded as unknown. In
Table I, continuous data are
presented as mean ± SD and categorical data are presented as
frequencies (%). One-way ANOVA, χ2 test and the
Kruskal-Wallis H test were used to compare differences among the
treatment groups for continuous, categorical and ordinal variables,
respectively. All the post hoc tests were adjusted using
Bonferroni's method. All the statistical analyses were performed
using the R version 3.4.3 (https://cran.r-project.org/). Two-sided P-values
<0.05 were considered to indicate a statistically significant
difference.
 | Table I.Demographic and clinical
characteristics of patients with hepatocellular carcinoma by
categories of surgery methods. |
Table I.
Demographic and clinical
characteristics of patients with hepatocellular carcinoma by
categories of surgery methods.
|
Characteristics | No surgery | Local tumor
destruction | Wedge or segmental
resection | Lobectomy | Liver
transplantation | Statistics | P-value |
|---|
| Diagnosis year, n
(%) |
|
|
|
|
| Kruskal-Wallis | <0.001 |
|
|
|
|
|
|
| χ2=188.0 |
|
|
2004-2008 | 3,531 (29.34) | 923 (37.43) | 575
(27.34) | 519
(32.97) | 1008
(39.25) |
|
|
|
2009-2012 | 3,929 (32.65) | 722 (29.28) | 663
(31.53) | 517
(32.85) | 855
(33.29) |
|
|
|
2013-2015 | 4,575 (38.01) | 821 (33.29) | 865
(41.13) | 538
(34.18) | 705
(27.45) |
|
|
| Age, mean ±
SDa | 64.17±11.32 | 63.19±10.00 | 62.06±11.08 | 61.52±12.64 | 57.64±7.63 | F=201.2 | <0.001 |
| Education
levelb, mean ± SD | 14.89±5.94 | 13.81±5.50 | 14.34±5.54 | 14.21±5.73 | 14.08±5.83 | F=26.3 | <0.001 |
| Family
incomec, mean ± SD | 75.76±19.27 | 80.89±19.43 | 80.80±20.60 | 82.27±20.85 | 77.49±20.20 | F=79.8 | <0.001 |
| Tumor size, mm,
mean ± SD | 72.09±46.08 | 41.23±27.78 | 53.85±41.50 | 82.78±54.40 | 33.36±24.49 | F=724.4 | <0.001 |
| Histological
stage, |
|
|
|
|
| Kruskal-Wallis | <0.001 |
| n (%) |
|
|
|
|
| χ2=3,269.7 |
|
|
Localized | 4,899 (40.71) | 1,917 (77.74) | 1,720 (81.79) | 1,142 (72.55) | 2,022 (78.74) |
|
|
|
Regional | 4,250 (35.31) | 482
(19.55) | 333
(15.83) | 356
(22.62) | 528
(20.56) |
|
|
|
Distant | 2,886 (23.98) | 67
(2.72) | 50
(2.38) | 76
(4.83) | 18
(0.70) |
|
|
| AJCC_T n
(%)e |
|
|
|
|
| χ2=2,639.3 | <0.001 |
| T1 | 3,764 (31.28) | 1,372 (55.64) | 1,130 (53.73) | 664
(42.19) | 1,216 (47.35) |
|
|
| T2 | 2,182 (18.13) | 632
(25.63) | 494
(23.49) | 350
(22.24) | 1,077 (41.94) |
|
|
| T3 | 4,097 (34.04) | 227 (9.21) | 202
(9.61) | 339
(21.54) | 129
(5.02) |
|
|
| T4 | 603
(5.01) | 24
(0.97) | 59
(2.81) | 87
(5.23) | 17
(0.66) |
|
|
| TX | 1,389 (11.54) | 211 (8.56) | 218
(10.37) | 134
(8.51) | 129
(5.02) |
|
|
| AJCC_N n
(%)f |
|
|
|
|
|
χ2=1,082.1 | <0.001 |
| N0 | 8,566 (71.18) | 2,134 (86.54) | 1831 (87.07) | 1,373 (87.23) | 2,384 (92.83) |
|
|
| N1 | 1,225 (10.18) | 48
(1.95) | 22
(1.05) | 45
(2.86) | 20
(0.78) |
|
|
| NX | 2,244 (18.65) | 284
(11.52) | 250
(11.89) | 156
(9.91) | 164
(6.39) |
|
|
| AJCC_M n
(%)g |
|
|
|
|
| χ2=1,897.8 | <0.001 |
| M0 | 7,946 (66.02) | 2,179 (88.36) | 1,834 (87.21) | 1,379 (87.61) | 2,414 (94.00) |
|
|
| M1 | 2,425 (20.15) | 57
(2.31) | 39
(1.85) | 50
(3.18) | 16
(0.62) |
|
|
| MX | 1,664 (13.83) | 230 (9.33) | 230
(10.94) | 145
(9.21) | 138
(5.37) |
|
|
| Grade, n
(%)d |
|
|
|
|
|
χ2=2,058.9 | <0.001 |
| I | 2,299 (19.10) | 624
(25.30) | 396
(18.83) | 246
(15.63) | 557
(21.69) |
|
|
| II | 2,785 (23.14) | 736
(29.85) | 1,019 (48.45) | 773
(49.11) | 1,008 (39.25) |
|
|
| II | 1,598 (13.28) | 199 (8.07) | 446
(21.21) | 370
(23.51) | 220
(8.57) |
|
|
| IV | 136
(1.13) | 9
(0.36) | 31
(1.47) | 46
(2.92) | 14
(0.55) |
|
|
|
Unknown | 5,217 (43.35) | 898
(36.42) | 211
(10.03) | 139
(8.83) | 769
(29.95) |
|
|
Variable selection and importance
ranking using RF analysis
For the generation of the RF model, the data
included in the present study was first trained using general
demographic information [(age, insurance, education level (36), family income (36), race (37), marital status (17), sex (38), region)] and factors that may be
associated with survival outcomes according to previous studies and
clinical practice as follows: Surgery method (39–41),
tumor size (15), diagnosis year
(42), historic stage, grade
(43), AJCC_Tumor (T), AJCC_Node
(N), AJCC_Metastasis (M) (44),
scope of regional lymph node surgery, AFP (18), surgery of other sites, number of
benign or borderline tumors and number of malignant tumors
(45). Fibrosis score, radiation and
chemotherapy were initially considered in the model, but were later
excluded to preserve the power of the test as missing values
accounted for over half of the population.
The RF model was employed to rank the importance of
the predictors of HCC survival outcomes. This version of the RF
model was performed in the R package ‘randomForest’ (v. 4.6–14)
(https://www.stat.berkeley.edu/~breiman/RandomForests/).
All data were randomly split into a training set and a validation
set by a ratio of 6:4. The importance rankings of predictors of
2-year survival were obtained as a by-product of the RF model. The
association between the top 10 most important predictors and 2-year
survival outcome was analyzed using the logistic regression
method.
Comparison of surgical approaches
using Cox regression analysis
Univariate Cox regression analysis was used to
compare the overall survival (OS) time and CSS of patients with HCC
treated with different surgical methods. To explore whether the
association between surgical method and survival outcome is
modified by other predictors, multivariate models were constructed
using the 10 variables with the highest Gini index, which is a tool
describing the relative contribution of each feature in predicting
the outcome (46). In addition,
analyses stratified by tumor size (patients were divided into two
groups by median tumor size, cut-off value 54 mm), age (patients
were divided into two groups by median age, cut-off value 62
years), histological stage and grade were conducted to minimize the
effect of confounding factors.
Results
Predictive factors and comparison of
surgical methods
A total of 20,746 patients diagnosed with HCC from
2004–2016 were included in the present study. The 10 most important
factors influencing survival outcomes were surgery method, tumor
size, age, AJCC_T, family income, education level, historic stage,
grade, AJCC_M, and diagnosis year (Fig.
2). Overall, local tumor destruction [(hazard ratio (HR)=0.48;
95% confidence interval (CI), 0.45–0.51; P<0.001)], wedge or
segmental resection (HR, 0.31; 95% CI, 0.29–0.33; P<0.001),
lobectomy (HR=0.29; 95% CI, 0.27–0.31; P<0.001) and liver
transplantation (HR=0.16; 95% CI; 0.14–0.17; P<0.001)
demonstrated improved OS outcomes compared with no surgery, except
for undifferentiated tumors and those with distant metastasis, with
the HRs demonstrating a decreasing trend (Tables III, SI and SIV).
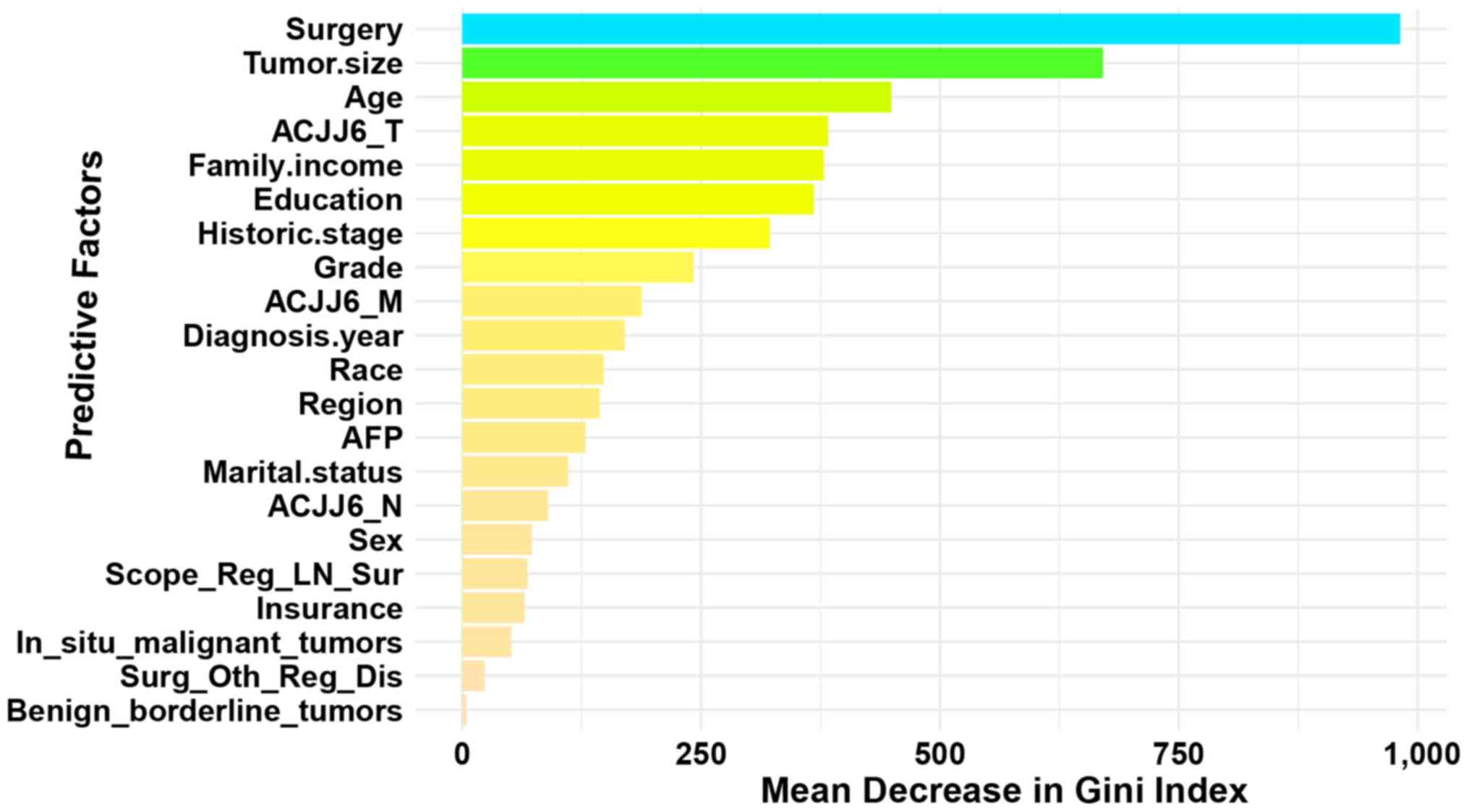 | Figure 2.Order of importance of predictive
factors associated with the survival outcome of patients with HCC.
The relative importance was ranked according to Gini index, which
is a tool describing the relative contribution of each feature in
predicting the outcome. Surgery, surgery of the primary site; tumor
size, the largest dimension or diameter of the primary tumor; age,
patient age at diagnosis; marital status, patients' marital status
at diagnosis; insurance, if patients had medical insurance at
diagnosis; family income, median family income values by county;
education, percentages of county populations (≥25 years) with less
than a high school education between 2011–2015; historic stage,
localized, regional and distant; grade, the degree of cell
differentiation; diagnosis year, year of diagnosis; region, groups
of countries at diagnosis; AFP, alpha fetoprotein; AJCC, the
American Joint Committee on Cancer; T, tumor; N, node; M,
metastasis; AJCC6_T, the T category according to the American Joint
Committee on Cancer 6th edition; AJCC6_M, the M category according
to the AJCC 6th edition; AJCC6_N, the N category according to the
AJCC 6th edition; scope_reg_ln_sur, scope of regional lymph node
surgery; in situ_malignant_tumor, total number of malignant tumors
in patients; surg_oth_reg_dis, the surgical removal of distant
lymph nodes or other tissues or organs beyond the primary site;
benign_borderline_tumor, total number of benign or borderline
tumors; HCC, hepatocellular carcinoma. |
 | Table III.Cox regression and competing risk
analysis for association of survival outcomes with surgery types in
patients with HCC. |
Table III.
Cox regression and competing risk
analysis for association of survival outcomes with surgery types in
patients with HCC.
| A, Univariate
analysis |
|---|
|
|---|
|
| Overall
survival | Cancer-specific
survival |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| No surgery | Reference |
|
|
|
|
|
| Local tumor
destruction | 0.34 | 0.32–0.36 | <0.001 | 0.31 | 0.29–0.33 | <0.001 |
| Wedge or segmental
resection | 0.23 | 0.22–0.25 | <0.001 | 0.22 | 0.20–0.24 | <0.001 |
| Lobectomy | 0.27 | 0.25–0.29 | <0.001 | 0.26 | 0.24–0.28 | <0.001 |
| Liver
transplantation | 0.11 | 0.10–0.12 | <0.001 | 0.07 | 0.07–0.08 | <0.001 |
|
| B, Multivariate
analysisa |
|
|
| Overall
survival | Cancer-specific
survival |
|
|
|
|
|
Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| No surgery | Reference |
|
|
|
|
|
| Local tumor
destruction | 0.48 | 0.45–0.51 | <0.001 | 0.46 | 0.43–0.49 | <0.001 |
| Wedge or segmental
resection | 0.31 | 0.29–0.33 | <0.001 | 0.30 | 0.28–0.32 | <0.001 |
| Lobectomy | 0.29 | 0.27–0.31 | <0.001 | 0.28 | 0.26–0.31 | <0.001 |
| Liver
transplantation | 0.16 | 0.14–0.17 | <0.001 | 0.11 | 0.10–0.12 | <0.001 |
Demographic and clinical
characteristics of patients with HCC
Of the total patients with HCC, 2,568 had undergone
liver transplantation, 1,574 lobectomy, 2,103 wedge or segmental
resection and 2,466 local tumor destruction. A total of 12,035
patients had no surgery. All the 10 most important predictors were
distributed unevenly among the 5 treatment groups (all P-values
<0.001). Post hoc analyses using Bonferroni's method
demonstrated significant difference between the no surgery group
and all 4 surgery groups (all P-values <0.05). These detailed
demographic and clinical characteristics of the patients are
presented in Table I.
Ranking of important predictors
association with survival outcomes of patients with HCC based on
the RF model
To compare the relative importance of predictive
factors, an RF model using all predictors was first trained. This
21-predictor RF model achieved an accuracy of 78.05%, with
ntree=1,400 (Number of trees to grow) and mtry=3 (Number of
variables randomly sampled as candidates at each split). Based on
the Gini index, the 10 most important predictors associated with
the survival outcomes of patients with HCC were obtained. These
were surgery method, tumor size, age, AJCC_T, family income,
education level, historic stage, grade, AJCC_M and diagnosis year.
The importance of the ranking results are presented in Fig. 2. Next, 10 factors with the highest
Gini were included in the subsequent analysis. The association
between predictors and 2-year OS were further analyzed using the
logistic regression method. The results indicated that more radical
surgery methods, for example liver transplantation and lobectomy,
higher family income and more recent diagnosis time, were
protective predictive factors and that larger tumor size, older
age, lower education level, metastasis, higher stage of AJCC_T and
AJCC_M, less differentiated tissue were negative predictive factors
(Table II).
 | Table II.Logistic regression analysis of
2-year survival and predictors in patients with hepatocellular
carcinoma. |
Table II.
Logistic regression analysis of
2-year survival and predictors in patients with hepatocellular
carcinoma.
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|---|
| Predictive
factor | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Surgery method |
|
|
|
|
|
|
| No
surgery | Reference |
|
| Reference |
| <0.001 |
| Local
tumor destruction | 0.18 | 0.16–0.20 | <0.001 | 0.29 | 0.26–0.32 | <0.001 |
| Wedge
or segmental resection | 0.10 | 0.09–0.12 | <0.001 | 0.15 | 0.13–0.16 | <0.001 |
|
Lobectomy | 0.15 | 0.13–0.16 | <0.001 | 0.13 | 0.11–0.15 | <0.001 |
| Liver
transplantation | 0.05 | 0.05–0.06 | <0.001 | 0.08 | 0.07–0.10 | <0.001 |
| Tumor size, mm | 1.02 | 1.02–1.02 | <0.001 | 1.01 | 1.01–1.01 | <0.001 |
| Age,
yearsa | 1.02 | 1.02–1.02 | <0.001 | 1.01 | 1.01–1.02 | <0.001 |
| Education
level | 1.01 | 1.01–1.02 | <0.001 | 0.98 | 0.98–0.99 | <0.001 |
| Family income | 0.99 | 0.99–0.99 | <0.001 | 0.99 | 0.99–0.99 | <0.001 |
| Historic stage |
|
|
|
|
|
|
|
Localized | Reference |
|
| Reference |
|
|
|
Regional | 5.78 | 5.34–6.28 | <0.001 | 2.32 | 1.97–2.73 | <0.001 |
|
Distant | 1.04 | 0.98–1.11 | 0.202 | 1.09 | 0.98–1.22 | 0.105 |
| AJCC_T |
|
|
|
|
|
|
| T1 | Reference |
|
| Reference |
|
|
| T2 | 1.17 | 1.09–1.26 | <0.001 | 1.21 | 1.10–1.33 | <0.001 |
| T3 | 5.50 | 5.06–5.97 | <0.001 | 1.77 | 1.58–1.98 | <0.001 |
| T4 | 7.00 | 5.78–8.54 | <0.001 | 1.66 | 1.31–2.12 | <0.001 |
| Diagnosis year |
|
|
|
|
|
|
|
2004-2008 | Reference |
|
| Reference |
|
|
|
2009-2012 | 0.88 | 0.82–0.94 | <0.001 | 0.72 | 0.66–0.79 | <0.001 |
|
2013-2015 | 0.57 | 0.54–0.61 | <0.001 | 0.48 | 0.44–0.53 | <0.001 |
| Grade |
|
|
|
|
|
|
| I | Reference |
|
| Reference |
|
|
| II | 0.97 | 0.90–1.06 | <0.001 | 1.30 | 1.18–1.43 | <0.001 |
|
III | 2.22 | 2.01–2.45 | <0.001 | 2.44 | 2.15–2.76 | <0.001 |
| IV | 3.34 | 2.50–4.51 | <0.001 | 3.68 | 2.59–5.29 | <0.001 |
| AJCC_M | 2.04 | 1.89–2.21 | <0.001 |
|
|
|
| M0 | Reference |
|
| Reference |
|
|
| M1 | 11.69 | 10.14–13.55 | <0.001 | 1.74 | 1.33–2.27 | <0.001 |
Cox regression analysis of survival
outcomes for surgery methods among patients with HCC
In univariate Cox regression analysis, patients
undergoing local tumor destruction (HR, 0.34; 95% CI, 0.32–0.36;
P<0.001), wedge or segmental resection (HR, 0.23; 95% CI,
0.22–0.25; P<0.001), lobectomy (HR, 0.27; 95% CI, 0.25–0.29;
P<0.001) and liver transplantation (HR, 0.11; 95% CI, 0.10–0.12;
P<0.001) had improved overall survival time compared with
patients not undergoing surgery (Table
III). Following adjustment for confounding factors in the
multivariate analysis, this trend was weakened but remained
significant. In multivariate analyses, patients undergoing local
tumor destruction (HR, 0.48; 95% CI, 0.45–0.51; P<0.001), wedge
or segmental resection (HR, 0.31; 95% CI, 0.29–0.33; P<0.001),
lobectomy (HR, 0.29; 95% CI, 0.27–0.31; P<0.001), and liver
transplantation (HR, 0.16; 95% CI, 0.14–0.17; P<0.001) had
improved OS outcomes compared with patients not undergoing surgery
(Table III). Following adjustment,
the HRs of local tumor destruction, wedge or segmental resection,
lobectomy and liver transplantation demonstrated a decreasing
trend, but there was no significant difference observed for wedge
or segmental resection and lobectomy (Table III). CSS analysis demonstrated a
similar trend to OS for both univariate and multivariate analyses.
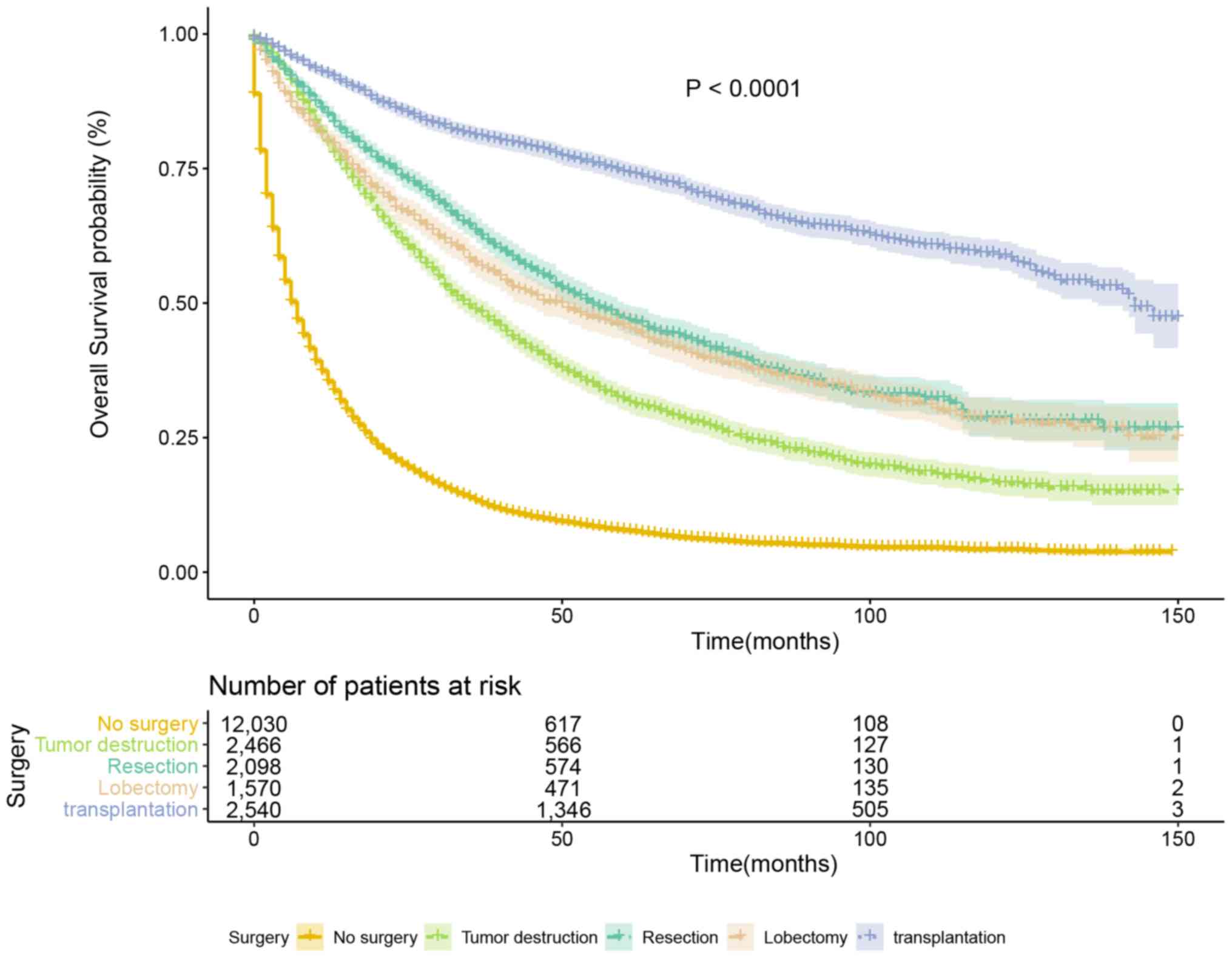
Survival curves showed that patients undergoing liver
transplantation had the longest survival time, and patients who did
not undergo surgery had the shortest survival time compared with
surgical methods (P<0.001, Fig.
3).
Stratified analysis
To minimize the effect of confounding factors,
stratified analyses were conducted based on tumor size (median
tumor size, <54 mm or ≥54 mm), age (median age, <62 years or
≥62 years), historic stage (localized, regional or distant), and
grade (grades I–IV). The stratified survival curves are presented
in Figs. S1–S4 and detailed information is provided in
Tables SI-SIV. Altogether, the
results demonstrated that the protective trend remained following
stratification. However, there were two exceptions: In patients
with distant metastasis and patients with undifferentiated tumors,
no significant difference was observed for the four surgery types
following stratification.
Discussion
The present longitudinal study included 20,746
patients with HCC from the SEER database and a RF model was used to
predict 2-year OS and CSS outcomes. Firstly, the relative
importance of predictive factors was evaluated. Subsequently, the
factor that was ranked most important, surgery method was further
analyzed by Cox regression analysis. The the no surgery group,
local tumor destruction group, wedge or segmental resection group,
and lobectomy group demonstrated improved OS and CSS outcomes
compared with liver transplantation group, with the HRs exhibiting
a gradually decreasing trend overall. This result remained stable
following stratification by tumor size, age, historic stage and
grade, except for undifferentiated tumors and those with distant
metastasis.
RF models are popular as they have high prediction
accuracy and provide information on the relative importance of
variables for classification (47).
In recent years, the RF model has been commonly applied for
investigating the quantitative importance of predictors for
different cancers like pancreatic neuroendocrine tumors (25) and colorectal carcinoma (48); however, studies using this model on
patients with HCC are very limited. Choi et al (23) constructed a prognostic model
estimating the outcomes of 480 advanced-stage patients with HCC,
all of whom were treated with sorafenib. Similarly, Kawaguchi et
al (49) evaluated the prognosis
of 247 patients with non-alcoholic fatty liver disease-HCC in
Japan, indicating treatment and serum albumin level to be the two
most important distinguishing factors. Our results suggested
treatment was the most important prognostic factor, which was
consistent with these studies. The present study had a large sample
size and therefore provided a sTable result.
The 10 most important predictors found in the
present study were surgery method (top of the list), tumor size,
age, AJCC_T, family income, education level, historic stage, grade,
AJCC_M and diagnosis year. Previous studies have suggested that RFA
along with TACE prolongs the survival of patients with HCC
(9) and that chemotherapy may
achieve favorable results in patients with advanced HCC (50). However, in the present study, data on
RFA therapy and chemotherapy was lacking for the majority of the
study population; therefore, these methods were not investigated.
No surgery and four surgery methods were discussed in the present
study, including tumor destruction, wedge or segmental resection,
lobectomy and transplantation. The findings of the present study
demonstrated that liver transplantation was the first choice for
all patients with HCC, except for those with undifferentiated
tumors or distant metastasis. This result is consistent with
previous studies, which have suggested that surgical resection
offers a significant survival benefit over thermal ablation
(41) and transarterial
chemoembolization (2). Another study
based on the SEER database (2013.5–2014.1) reported similar trends;
local tumor destruction, partial surgery and total surgery compared
with no surgery were all significant positive prognostic factors
(3). The present study included more
recently released data for participants, analyzed more treatment
methods and applied more reliable variable selection criteria
compared with the aforementioned study providing clinicians and
researchers with more evidence. Other important outcome predictors,
such as tumor size, age, AJCC_T, family income, education level,
historic stage, grade, AJCC_M and diagnosis year, were consistent
with previous studies. The present study is novel as it identified
predictors according to their relative importance. Due to the
limitations of the cohort studied, some important factors could not
be included in the present study, such as genetic factors and
cytokines, which are reported to be significantly associated with
survival outcomes. For instance, EBI3 is suggested to be a cancer
suppressor (20) and IL-34 may be
associated with survival outcomes by regulating tumor growth and
hepatic fibrosis in patients with HCC (21). Future prospective studies are
required in order to investigate the relative importance of these
factors.
The present study had several strengths. The RF
model was used to rank the importance of variables associated with
the survival outcomes of patients with HCC, providing new evidence
to currently limited research. To date, studies utilizing the RF
method to rank the importance of predictive clinical variables for
patients with HCC are very limited (23,49). The
surgical methods included in the present study are broader and more
detailed compared with those in previous studies. Besides, SEER
collects data with a wide temporal and spatial range and the time
of diagnosis was adjusted by multifactoral regression analysis
considering that the treatment modality varies year by year. In
total, 20,746 patients with HCC were included in the analysis,
making the models very reliable. However, there are several
limitations in the present study. First, as with any observational
study, the effect of residual confounding or unmeasured factors
cannot be completely ruled out inspite of the attempts to account
for major potential confounders. Secondly, although, prognostic
models for patients with HCC were generated with a large sample
size, the present study lacked external validation. There is still
a need to verify the results of the present study with external
results for consistency.
The three most important predictors of survival
outcomes of patients with HCC were surgery method, tumor size and
education level. Liver transplantation had the best prognosis for
patients with HCC, except for those with undifferentiated tumors or
distant metastasis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are
available in the SEER repository (www.seer.cancer.gov).
Authors' contributions
HT and SC designed the study, analyzed and
interpreted the data and drafted the manuscript. MH, YW and QF
designed the study, acquired the data and revised the manuscript.
YP and TQ interpreted the data and revised the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Labgaa I, Demartines N and Melloul E:
Surgical resection versus transarterial chemoembolization for
intermediate stage hepatocellular carcinoma (BCLC-B): An unsolved
question. Hepatology. 69:9232019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baddour HM Jr, Fedewa SA and Chen AY:
Five- and 10-year cause-specific survival rates in carcinoma of the
minor salivary gland. JAMA Otolaryngol Head Neck Surg. 142:67–73.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lexe G, Monaco J, Doyle S, Basavanhally A,
Reddy A, Seiler M, Ganesan S, Bhanot G and Madabhushi A: Towards
improved cancer diagnosis and prognosis using analysis of gene
expression data and computer aided imaging. Exp Biol Med (Maywood).
234:860–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
European Association For The Study of The
Liver and European Organisation For Research and Treatment of
Cancer, . EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK,
Seo YS, Yim HJ, Yeon JE and Byun KS: Hepatic resection compared to
chemoembolization in intermediate- to advanced-stage hepatocellular
carcinoma: A meta-analysis of high-quality studies. Hepatology.
68:977–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kutlu OC, Chan JA, Aloia TA, Chun YS,
Kaseb AO, Passot G, Yamashita S, Vauthey JN and Conrad C:
Comparative effectiveness of first-line radiofrequency ablation
versus surgical resection and transplantation for patients with
early hepatocellular carcinoma. Cancer. 123:1817–1827. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimose S, Tanaka M, Iwamoto H, Niizeki T,
Shirono T, Aino H, Noda Y, Kamachi N, Okamura S, Nakano M, et al:
Prognostic impact of transcatheter arterial chemoembolization
(TACE) combined with radiofrequency ablation in patients with
unresectable hepatocellular carcinoma: Comparison with TACE alone
using decision-tree analysis after propensity score matching.
Hepatol Res. 49:919–928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Omata M, Lesmana LA, Tateishi R, Chen PJ,
Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al: Asian
pacific association for the study of the liver consensus
recommendations on hepatocellular carcinoma. Hepatology Int.
4:439–474. 2010. View Article : Google Scholar
|
|
11
|
Jarnagin W, Chapman WC, Curley S,
D'Angelica M, Rosen C, Dixon E and Nagorney D; American
Hepato-Pancreato-Biliary Association, Society of Surgical Oncology
and Society for Surgery of the Alimentary Tract, : Surgical
treatment of hepatocellular carcinoma: Expert consensus statement.
HPB (Oxford). 12:302–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korean Liver Cancer Study Group (KLCSG)
and National Cancer Center, Korea (NCC), . 2014 KLCSG-NCC Korea
practice guideline for the management of hepatocellular carcinoma.
Gut Liver. 9:267–317. 2015.PubMed/NCBI
|
|
13
|
Kudo M, Matsui O, Izumi N, Iijima H,
Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et
al: JSH consensus-based clinical practice guidelines for the
management of hepatocellular carcinoma: 2014 update by the liver
cancer study group of Japan. Liver Cancer. 3:458–468. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaibori M, Yoshii K, Yokota I, Hasegawa K,
Nagashima F, Kubo S, Kon M, Izumi N, Kadoya M, Kudo M, et al:
Impact of advanced age on survival in patients undergoing resection
of hepatocellular carcinoma: Report of a Japanese nationwide
survey. Annals Surg. 269:692–699. 2019. View Article : Google Scholar
|
|
15
|
Yang A, Xiao W, Chen D, Wei X, Huang S,
Lin Y, Zhang C, Lin J, Deng F, Wu C and He X: The power of tumor
sizes in predicting the survival of solitary hepatocellular
carcinoma patients. Cancer Med. 7:6040–6050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinkawa H, Tanaka S, Takemura S, Ishihara
T, Yamamoto K and Kubo S: Tumor size drives the prognosis after
hepatic resection of solitary hepatocellular carcinoma without
vascular invasion. J Gastrointest Surg. June 13–2019.(Epub ahead of
print). View Article : Google Scholar
|
|
17
|
Wu W, Fang D, Shi D, Bian X and Li L:
Effects of marital status on survival of hepatocellular carcinoma
by race/ethnicity and gender. Cancer Manag Res. 10:23–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai DS, Zhang C, Chen P, Jin SJ and Jiang
GQ: The prognostic correlation of AFP level at diagnosis with
pathological grade, progression, and survival of patients with
hepatocellular carcinoma. Sci Rep. 7:128702017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sarveazad A, Agah S, Babahajian A, Amini N
and Bahardoust M: Predictors of 5 year survival rate in
hepatocellular carcinoma patients. J Res Med Sci. 24:862019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song Q, Chen X, Hu W, Mei G, Yang X and Wu
H: Downregulation of Epstein-Barr virus-induced gene 3 is
associated with poor prognosis of hepatocellular carcinoma after
curative resection. Oncol Lett. 15:7751–7759. 2018.PubMed/NCBI
|
|
21
|
Noda Y, Kawaguchi T, Korenaga M, Yoshio S,
Komukai S, Nakano M, Niizeki T, Koga H, Kawaguchi A, Kanto T and
Torimura T: High serum interleukin-34 level is a predictor of poor
prognosis in patients with non-viral hepatocellular carcinoma.
Hepatol Res. 49:1046–1053. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pang W, Jiang H and Li S: Sparse
contribution feature selection and classifiers optimized by
concave-convex variation for HCC image recognition. Biomed Res Int.
2017:97183862017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi GH, Han S, Shim JH, Ryu MH, Ryoo BY,
Kang YK, Kim KM, Lim YS and Lee HC: Prognostic scoring models for
patients undergoing sorafenib treatment for advanced stage
hepatocellular carcinoma in real-life practice. Am J Clin Oncol.
40:167–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Y, Wu W, Zheng HM, Li P, McDonald D,
Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, et al: Regional
variation limits applications of healthy gut microbiome reference
ranges and disease models. Nat Med. 24:1532–1535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song Y, Gao S, Tan W, Qiu Z, Zhou H and
Zhao Y: Multiple machine learnings revealed similar predictive
accuracy for prognosis of PNETs from the surveillance,
epidemiology, and end result database. J Cancer. 9:3971–3978. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X and Ishwaran H: Random forests for
genomic data analysis. Genomics. 99:323–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Ackerman HH and Carulli JP: A
genome-wide screen of gene-gene interactions for rheumatoid
arthritis susceptibility. Hum Genet. 129:473–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Briggs FB, Ramsay PP, Madden E, Norris JM,
Holers VM, Mikuls TR, Sokka T, Seldin MF, Gregersen PK, Criswell LA
and Barcellos LF: Supervised machine learning and logistic
regression identifies novel epistatic risk factors with PTPN22 for
rheumatoid arthritis. Genes Immun. 11:199–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Degenhardt F, Seifert S and Szymczak S:
Evaluation of variable selection methods for random forests and
omics data sets. Brief Bioinform. 20:492–503. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu C, Ren X and Zhang Q: Incidence, risk
factors, and prognosis in patients with primary hepatocellular
carcinoma and lung metastasis: A population-based study. Cancer
Manag Res. 11:2759–2768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryu SM, Lee SH, Kim ES and Eoh W:
Predicting survival of spinal ependymoma patients using machine
learning algorithms with SEER database. World Neurosurg. Dec
28–2018.(Epub ahead of print).
|
|
32
|
Shukla N, Hagenbuchner M, Win KT and Yang
J: Breast cancer data analysis for survivability studies and
prediction. Comput Methods Programs Biomed. 155:199–208. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lynch CM, Abdollahi B, Fuqua JD, de Carlo
AR, Bartholomai JA, Balgemann RN, van Berkel VH and Frieboes HB:
Prediction of lung cancer patient survival via supervised machine
learning classification techniques. Int J Med Inform. 108:1–8.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adamo MB, Johnson CH, Ruhl JL and Dickie
LA: SEER Program Coding and Staging Manual. National Cancer
Institute, NIH Publication no. 12-5581. (Bethesda, MD). 2012.
|
|
35
|
Raghu G, Chen SY, Yeh WS, Maroni B, Li Q,
Lee YC and Collard HR: Idiopathic pulmonary fibrosis in US medicare
beneficiaries aged 65 years and older: Incidence, prevalence, and
survival, 2001-11. Lancet Respir Med. 2:566–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen Y, Guo H, Wu T, Lu Q, Nan KJ, Lv Y
and Zhang XF: Lower education and household income contribute to
advanced disease, less treatment received and poorer prognosis in
patients with hepatocellular carcinoma. J Cancer. 8:3070–3077.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jones PD, Diaz C, Wang D, Gonzalez-Diaz J,
Martin P and Kobetz E: The impact of race on survival after
hepatocellular carcinoma in a diverse American population.
digestive diseases and sciences. Dig Dis Sci. 63:515–528. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai MW, Chu YD, Lin CL, Chien RN, Yeh TS,
Pan TL, Ke PY, Lin KH and Yeh CT: Is there a sex difference in
postoperative prognosis of hepatocellular carcinoma? BMC Cancer.
19:2502019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee GC, Ferrone CR, Vagefi PA, Uppot RN,
Tanabe KK, Lillemoe KD, Blaszkowsky LS and Qadan M: Surgical
resection versus ablation for early-stage hepatocellular carcinoma:
A retrospective cohort analysis. Am J Surg. 218:157–163. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu B, Ding Y, Liao X, Wang C, Wang B and
Chen X: Radiofrequency ablation versus surgical resection in
elderly patients with early-stage hepatocellular carcinoma in the
era of organ shortage. Saudi J Gastroenterol. 24:317–325. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mills A, Thayer D, Noda C, Salter A, Tao
Y, Xing M, Martin R, Ramaswamy R and Akinwande O: Thermal ablation
versus surgical resection for localized hepatocellular carcinoma: A
population study using the SEER database. Future Oncol. 14:631–645.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yim SY, Seo YS, Jung CH, Kim TH, Lee JM,
Kim ES, Keum B, Jong YK, An H, Kim JH, et al: The management and
prognosis of patients with hepatocellular carcinoma: What has
changed in 20 years? Liver Int. 36:445–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen J, Liu J, Li C, Wen T, Yan L and Yang
J: The impact of tumor differentiation on the prognosis of
HBV-associated solitary hepatocellular carcinoma following
hepatectomy: A propensity score matching analysis. Dig Dis Sci.
63:1962–1969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang JF, Shu ZJ, Xie CY, Li Q, Jin XH, Gu
W, Jiang FJ and Ling CQ: Prognosis of unresectable hepatocellular
carcinoma: Comparison of seven staging systems (TNM, Okuda, BCLC,
CLIP, CUPI, JIS, CIS) in a Chinese cohort. PLoS One. 9:e881822014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoshizumi T, Ikegami T, Yoshiya S,
Motomura T, Mano Y, Muto J, Ikeda T, Soejima Y, Shirabe K and
Maehara Y: Impact of tumor size, number of tumors and
neutrophil-to-lymphocyte ratio in liver transplantation for
recurrent hepatocellular carcinoma. Hepatol Res. 43:709–716. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jain SS, Sarkar IN, Stey PC, Anand RS,
Biron DR and Chen ES: Using demographic factors and comorbidities
to develop a predictive model for ICU mortality in patients with
acute exacerbation COPD. AMIA Annu Symp Proc. 2018:1319–1328.
2018.PubMed/NCBI
|
|
47
|
Touw WG, Bayjanov JR, Overmars L, Backus
L, Boekhorst J, Wels M and van Hijum SA: Data mining in the life
sciences with random forest: A walk in the park or lost in the
jungle? Brief Bioinform. 14:315–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu G, Dong C, Wang X, Hou G, Zheng Y, Xu
H, Zhan X and Liu L: Regulatory activity based risk model
identifies survival of stage II and III colorectal carcinoma.
Oncotarget. 8:98360–98370. 2017.PubMed/NCBI
|
|
49
|
Kawaguchi T, Tokushige K, Hyogo H, Aikata
H, Nakajima T, Ono M, Kawanaka M, Sawada K, Imajo K, Honda K, et
al: A data mining-based prognostic algorithm for NAFLD-related
hepatoma patients: A nationwide study by the Japan study group of
NAFLD. Sci Rep. 8:104342018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheong JY, Lee KM, Cho SW, Won JH, Kim JK,
Wang HJ, Hahm KB and Kim JH: Survival benefits of intra-arterial
infusion chemotherapy in patients with advanced hepatocellular
carcinoma with portal vein tumor thrombosis. Hepatol Res.
32:127–133. 2005. View Article : Google Scholar : PubMed/NCBI
|