Hepatocellular carcinoma (HCC) was the sixth most
common cancer and fourth most common cause of cancer-related death
globally in 2018 (1). The World
Health Organization estimates that >1 million patients will die
from liver cancers by 2030 (2). HCC
occurs in patients with underlying liver diseases, mostly as a
result of hepatitis B (HBV) or C virus infection or alcohol abuse
(3). Recently, nonalcoholic fatty
liver disease (NAFLD) and metabolic syndrome becoming hot topics of
research as both are important risk factors for HCC (4). At present, HCC is treated mainly by
resection and occasionally in combination with other therapies,
including ablation, transarterial embolization and radiotherapy,
transplantation and targeting therapies, however, the efficacy of
treatments for patients with advanced HCC are limited (4). Immunotherapies aimed at reactivating
the activity of antitumoral T cells have attracted wide attention
in recent years (5). However, the
efficacy of nivolumab (a programmed death-1 inhibitor) was
unsatisfactory in patients with advanced HCC (6). This maybe in part due to the
immunosuppressive microenvironment (7).
The liver is a pivotal immunological organ which
serves a crucial role in host defense via numerous innate, such as
dendritic cells, kupffer cells and natural killer T (NKT) cells and
adaptive immune cells, such as CD4+ and CD8+
T cells (8). Dysregulation of the
liver's immune system leads to the occurrence of necroinflammation,
which is characterized by the dysregulation of the immune
regulatory network with upregulation of pro-inflammatory signals
and the breakdown of immune tolerance in chronic liver disease
(9). The necroinflammatory response
promotes the development of HCC via the infiltration of various
innate and adaptive immune cells, such as dendritic cells, natural
killer (NK) cells, monocytes, neutrophils, T cells and B cells
(9). It is widely accepted that the
T cells serve important roles in the development of HCC (10). There is always a large number of
exhaustive CD8+ T cells in the tumor microenvironment,
which makes the treatment of HCC more difficult (11). In fact, innate immune cells also
contribute to tumor immunosurveillance and immune escape by
assisting T cells or by secreting cytokines (9). For example, macrophages assist
CD4+ helper T cells to remove senescent hepatocytes
(12). Regulatory dendritic cells
(DCs) produce indoleamine-2,3-dioxygenase (IDO) to promote tumor
immune escape in HCC (13). Hence,
it is important to understand the underlying interplay between
immune cells and HCC in the tumor microenvironment. Interestingly,
innate immune cells can themselves affect tumor progression, but
also regulate T-cell responses to affect tumor progression
(7). Considering the regulatory
properties of innate immune cells, understanding the roles of
innate immune cells in HCC and interaction with T cells in the
tumor microenvironment are necessary.
In the present study several kinds of innate immune
cells were reviewed that have been widely reported in HCC,
including DCs, macrophages, neutrophils and innate lymphoid cells
(ILCs), and the underlying mechanisms by which they regulate T-cell
responses in the occurrence and development of HCC was discussed.
The present review will improve the understanding of innate immune
cells in HCC and pave the way for developing new immunotherapies
for patients with HCC.
Macrophages, which are differentiated cells of the
mononuclear phagocytic lineage and are activated in response to
microbe-associated molecular patterns, such as bacterial
lipopolysaccharide or cytokines, such as interleukin (IL)-4, IL-5
and IL-10, have long been recognized as M1 and M2 macrophages
(27,28). M1 macrophages possess proinflammatory
and antitumor properties, whereas M2 macrophages possess regulatory
properties for tumor growth and metastasis (29,30). In
addition, co-inhibitory molecules, such as B7-H3; signaling
pathways, such as the Wnt/β-catenin and STAT3 pathway and long
non-coding RNAs (lncRNAs), such as cyclooxygenase 2 serve important
roles in regulating the polarization of macrophages in the HCC
microenvironment (31–34). The antitumoral role of M1 macrophages
has been documented in HCC (28).
However, a recent study found that M1 macrophages promoted the
expression of programmed death ligand 1 (PD-L1) on HCC cells via
IL-1β, which supports the protumor role of M1 macrophages (35).
Tumor-associated macrophages (TAMs), mainly M2 type,
can be recruited by various cytokines, such as colony stimulating
factor (CFS)-1, vascular endothelial growth factor (VEGF) and
chemokines (CCL2) and serve a protumor role in HCC (29). For example, TAMs can secrete IL-6 and
IL-8 to promote the proliferation of HCC stem cells and
epithelial-mesenchymal transition (EMT) in HCC cells (36,37). In
addition, NF-κB, STAT-3 and hypoxia inducible factor-1 (HIF-1)
signaling pathways serve key roles in the interaction between TAMs
and HCC cells (38). Recently, Zhang
et al (36) demonstrated that
TAMs promoted the metastasis and EMT of HCC cells through
HIF-1α/IL-1β signaling under a hypoxic microenvironment.
Oxaliplatin has been widely used to treat patients with HCC, and a
recent study indicated that TAMs contributed to oxaliplatin
resistance through autophagy in HCC (39).
On the other hand, Kupffer cells (KCs), as the
sessile resident live macrophages in the liver can sense injury of
the liver and activate inflammation and promote tumor growth by
releasing proinflammatory or proangiogenic factors, such as IL-6,
IL-1β, VEGF and platelet-derived growth factor and recruit large
numbers of inflammatory monocytes (40,41). A
previous study indicated that KCs can promote the occurrence and
development of HCC by increasing the production of IL-6 in a manner
dependent on the Toll-like receptor adaptor protein myeloid
differentiation primary response 88 (42). Recent studies also demonstrated that
KCs can promote the occurrence and development of HCC in the
context of inflammation and fibrosis (43,44).
Similarly, the M1/M2 polarization of KCs regulated by lncRNA FTX
can influence the progression from NAFLD to HCC (45).
In conclusion, M2 macrophages and kupffer cells
promote invasion and metastasis of HCC cells and the HCC
microenvironment further promotes the protumor effect of M2
macrophages and kupffer cells.
Neutrophils are the first line of defense against
microbial pathogens, are the predominant leukocyte subset (50–70%)
in human peripheral blood and have protumoral functions in HCC
(46). Previous studies focused more
on the association between neutrophil-to-lymphocyte ratio and the
prognosis of HCC (47).
Subsequently, Kuang et al (46) found that the accumulation of
neutrophils was associated with poor prognosis in patients with HCC
and promoted angiogenesis at the invading tumor edge via MMP-9.
These findings were consistent with those of Li et al
(48). Meanwhile, chemokines, such
as chemokine (C-X-C) ligand (CXCL)1 and CXCL5 in HCC promote the
infiltration of neutrophils and predict poor prognosis of patients
with HCC (49,50). As understanding about neutrophils has
increased, the close relationship between neutrophils and HCC cells
has been discovered (51). HCC cells
promote the production of hepatocyte growth factor (HGF) in
neutrophils via granulocyte-macrophage colony stimulating factor
and in turn neutrophils promote the metastasis of hepatoma cells
via the HGF/c-mesenchymal-epithelial transition factor (c-Met) axis
(52). In addition,
neutrophil-mediated reactive oxygen species production and telomere
DNA damage contributed to the development of HCC and NF-κB1
weakened the protumoral effect of neutrophils in diethylnitrosamine
induced murine models (53). On the
other hand, neutrophils can release extensive extracellular
web-like structure called neutrophil extracellular traps (NETs)
that are composed of cytosolic protein, then NETs can entrap and
kill bacteria and serve important roles in cancers, such as small
bowel cancer, lung cancer and HCC (54). Recent studies indicated that NETs
promoted the progression from nonalcoholic steatohepatitis to HCC
and metastasis potential of HCC by promoting inflammation (55,56).
Myeloid-derived suppressor cells (MDSCs) are divided
into polymorphonuclear MDSCs and monocytic MDSCs, characterized by
their immunosuppressive properties, maturity status and pathology
(62). The association between
frequency of MDSCs and clinical prognosis in patients with HCC has
been observed (63). Several studies
have demonstrated that MDSCs have a protumoral effect by
suppressing the functions of DCs and NK cells as well as by
regulating T-cell responses in HCC (64–66).
Meanwhile, HCC cells themselves can promote the accumulation or
activation of MDSCs by hypoxia, receptor-interacting protein kinase
3 deficiency or IL-6 produced by HCC cells (67–69). In
short, MDSCs serve an important role in shaping the
immunosuppressive tumor microenvironment in HCC.
Innate lymphoid cells (ILCs), including group 1 ILCs
(ILC1s), group 2 ILCs (ILC2s) and group 3 ILCs (ILC3s) mainly
reside in tissues and have similar cytokine-secreting profiles as
helper T cell subsets in response to infections or tissue damage
(70). ILCs play protumoral or
antitumoral roles in immune regulation in different tumor
microenvironments (71). However,
the exact roles of ILCs in HCC are still unknown. A recent study
found that ILCs secreted IFN-γ to promote hepatocellular
tumorigenesis in HBV transgenic mice (72). The aforementioned study suggested
that the ILCs play protumoral role in HCC, however, further
molecular mechanisms remain to be explored.
NK cells, which account for about a third of the
lymphocytes in the liver belong to the ILC1s and are one of the
main antitumor cells found in the liver (70). Functional impairment, such as
decreased production of IFN-γ from NK cells in patients with HCC
has been observed (73). In
addition, the infiltration of functional NK cells in HCC tissues
can suppress disease progression and have a favorable effect on
rates of overall survival and disease free survival in patients
with HCC (74). It is widely
accepted that the exhaustion of NK cells may contribute to the
development of HCC and natural killer group 2D (NKG2D) an
activatory receptor on NK cells serves a crucial role in regulating
the functions of NK cells during the development of HCC (75). For instance, suppressing enhancer of
zeste homolog 2 (EZH2), a transcriptional repressor of NKG2D
ligands, promoted HCC cell eradication by NK cells in a NKG2D
ligand-dependent manner (76).
Notably, NK cell-derived IFN-γ promotes HCC through the epithelial
cell adhesion molecule-epithelial-to-mesenchymal transition (EMT)
axis in hepatitis B virus transgenic (HBs-Tg) mice (77), which suggests that the roles of NK
cells in the development of HCC are complicated. In addition,
tumor-derived soluble MHC class I-chain related protein A (MICA),
which is a ligand of NKG2D or AFP inhibit the functions of NK cells
in vitro (78,79). Hence, NK cells can fight against
tumors, however, the tumor itself inhibits the activity of NK cells
to create conditions for HCC cell growth.
In addition, NKT cells, which are closely related to
NK cells, have also been implicated in HCC (80). NKT cells are characterized by the
expression of surface markers of NK cells together with a single
invariant T cell receptor in humans (81). NKT cells can directly kill tumor
cells by recognizing CD1d antigen or indirectly by activating NK
cells (82). Studies have
demonstrated that the number of NKT cells in HCC is positively
associated with rates of overall survival and recurrence-free
survival, which may be related to the involvement of NKT cells in
angiogenesis in the tumor microenvironment (82,83).
Transforming growth factor beta (TGF-β) derived from HCC cells is a
crucial factor in the development of HCC and can inhibit the
antitumoral functions of NKT cells, NK cells and T cells (84). The relationship between all of the
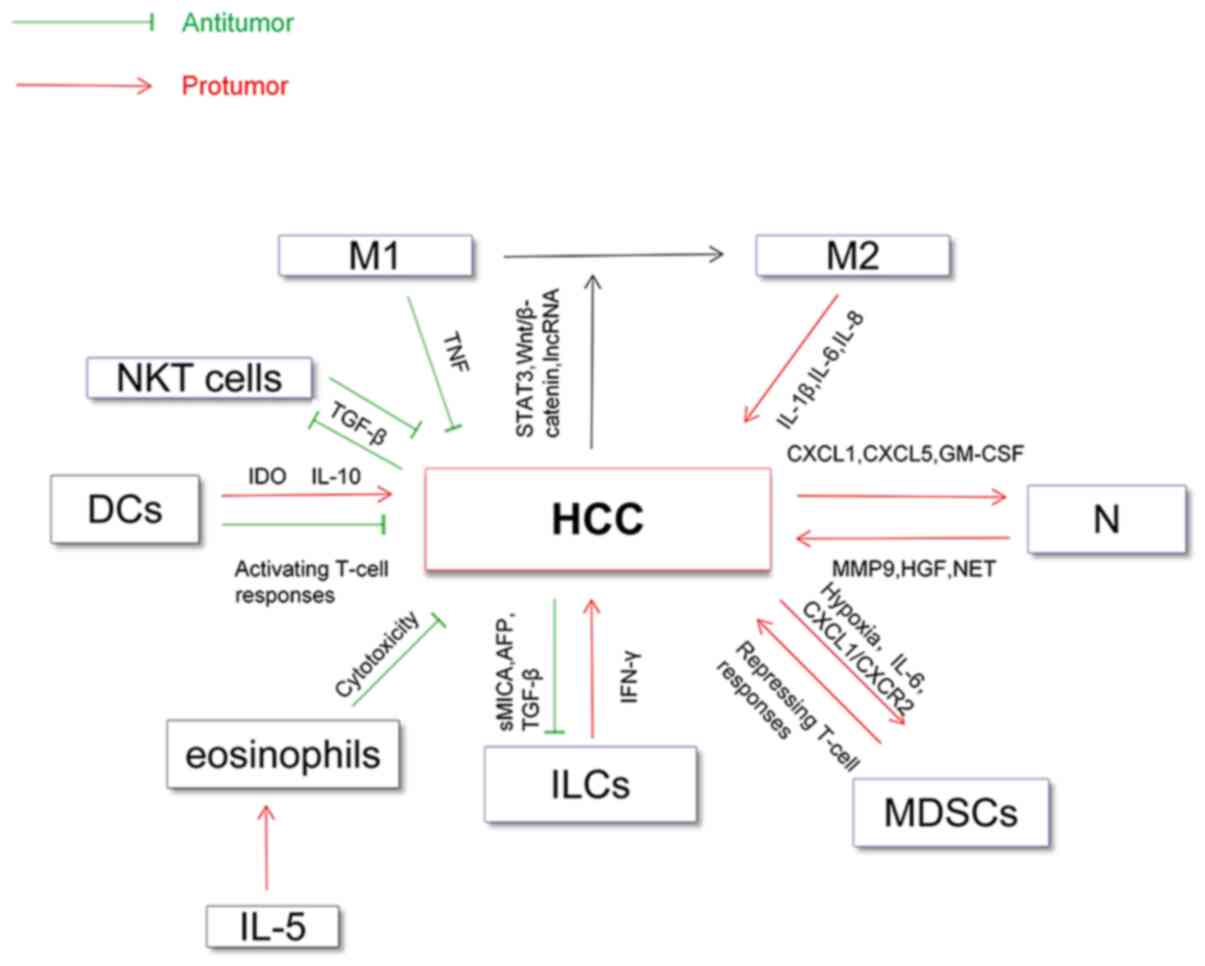
aforementioned innate immune cells (DCs, macrophages and
neutrophils) and HCC has been summarized in Fig. 1.
In addition to adaptive immune cells, innate immune
cells are also important cells for tumor surveillance (88). Meanwhile, innate and adaptive immune
cells do not act independently (9).
Innate immune cells can perform protumoral or antitumoral functions
by regulating the CD8+ T-cell response in HCC. For
example, DCs which are professional APCs can enhance the
antitumoral functions of CD8+ T cells by
tumor-associated antigen in HCC (20). A previous study demonstrated that
dendritic cell-derived exosomes (DEXs), as a cell-free vaccine, can
improve the antitumor activation of CD8+ T cells and
reshapes the tumor immune microenvironment in HCC mice (89). MDSCs can promote the exhaustion of
CD8+ T cells via the PD-1/PD-L1 pathway and arginase-I
(62). Although the relationship
between NK cells and CD8+ T cells has also been observed
in malignant tumors (90), the
molecular mechanisms and the relationship between NK cells and
CD8+ T cells remains unclear.
Tregs play a crucial role in exhaustion of T cells
and immune escape of HCC cells (7,100). So
far, increasing studies have shown the relationship between innate
immune cells and Tregs, as well as underlying molecular mechanisms
in HCC. For example, the increase in intratumoral pDCs was
associated with increased intratumoral infiltration of forkhead
box-3 (Foxp3)+ regulatory T cells (24). In terms of molecular mechanisms,
increased regulatory DCs induced by carcinoma-associated
fibroblasts contributed to T-cell proliferation impairment and
promotion of Treg expansion via IDO (13). Triggering receptor expressed on
myeloid cells-1-positive tumor-associated macrophages
(TREM-1+TAMs) can secrete CCL20 to promote the
accumulation of CCR6+Foxp3+ Tregs and the
upregulated expression of PD-L1 in TREM-1+ TAMs
contributed to depletion of CD8+ T cells in HCC both
in vitro and in vivo (101). Similarly, tumor-associated
neutrophils can recruit macrophages and Tregs by the expression of
CCL2 and CCL17 to promote the growth of HCC and resistance to
sorafenib (102) (Fig. 2).
In addition, helper T cells can mediate the immune
response by secreting various cytokines, such as interferon-γ,
IL-4, IL-10 (103). Th1
(CXCR3+) mainly secret interferon-γ phenotypic, while
Th2 (CRTH2+) mainly secret IL-4, IL-5 and IL-13, which
can resist the harm of intracellular pathogens and extracellular
parasites respectively (104).
Furthermore, ILC2s promoted Th2 differentiation while inhibiting
Th1 differentiation in a contact-dependent manner in a co-culture
system (105). Membrane-coated
microvesicles derived from neutrophils suppressed a subset of Th
cells by downregulating IL-2 and IL-2R expression and signaling
(106). The aforementioned studies
demonstrated the relationship between innate immune cells and
helper T cells in a non-hepatocellular environment. However, the
exact relationship and underlying molecular mechanisms between
innate immune cells and helper T cells in HCC need further
exploration. The roles of CD4+ and CD8+ T
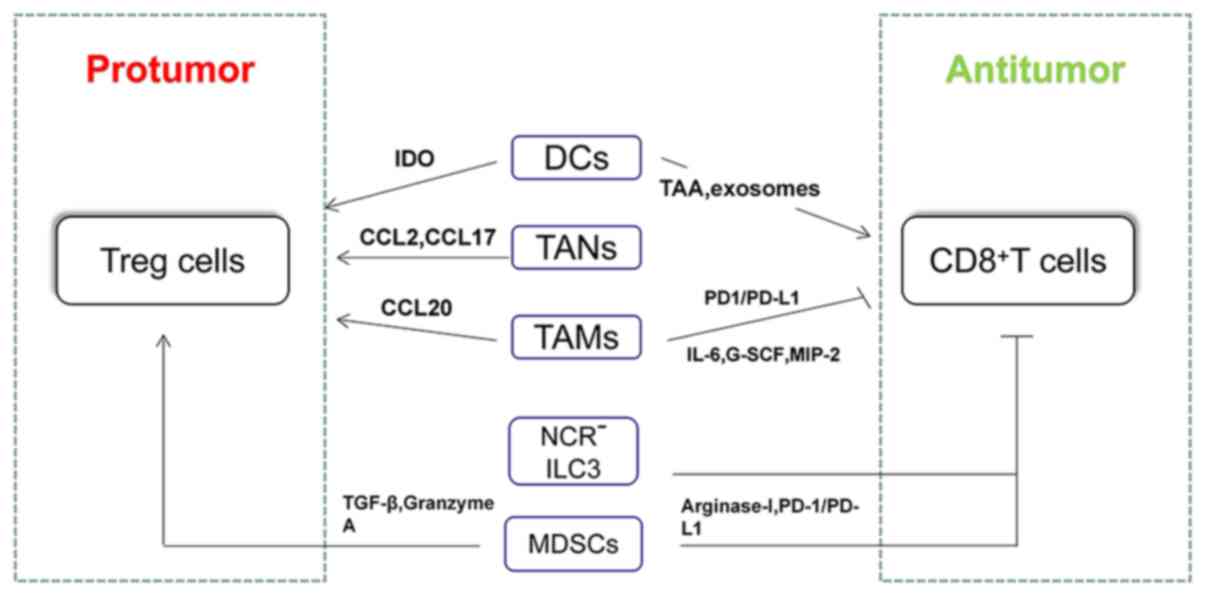
cells in HCC and their relationship with various innate immune
cells have been summarized in Fig.
2.
Immunotherapy has been a hotspot of research since
cytotoxic T lymphocyte-associated protein 4 and PD-1 inhibitors
were approved to treat melanoma (51). Similarly, immunotherapy based on T
cells has become an important therapy for patients with HCC,
especially for advanced HCC, but the efficacy of treatment is still
not satisfactory (107–109). Hence, a novel treatment strategy is
necessary to complement immunotherapy in patients with HCC. In
addition to T cells, innate immune cells which are important parts
of the tumor microenvironment can also serve crucial roles and
regulate T-cell responses in HCC (9). Hence, innate immune cells will be
promising candidates for the treatment of patients with HCC. So
far, the clinical value of DCs, macrophages and NK cells have been
noticed. For example, several animal experiments have confirmed the
efficacy of innate immune cells in HCC. For example, the dendritic
cell-DEXs, being a cell-free vaccine can elicit tumor regression in
autochthonous hepatocellular carcinoma mouse models (89). Injection of M1 hydrogels (a poly
ethylene glycol diacrylate and thiolated gelatin poly ethylene
glycol cross-linked hydrogels capsulated with M1 macrophages) can
reduce metastasis and induce tumor regression in ectopic nude mice
liver cancer models and subcutaneous HCC models (110). Human IL-15 gene-modified NK cells
exhibited strong growth inhibition of transplanted human HCC tumors
in xenograft nude mouse models (111). In terms of clinical trials, an
early phase II study has confirmed the safety of autologous DC
vaccination in patients with HCC (112). A randomized phase II study
suggested that adjuvant immunotherapy with DC vaccine reduces the
risk of tumor recurrence in patients with HCC who underwent
standard treatment modalities other than radiofrequency ablation
(113). Some ongoing clinical
trials based on DCs or NK cells that have been registered are shown
in Table I (https://clinicaltrials.gov/).
So far, immunotherapy based on T cells, such as
PD-1/PD-L1 and chimeric antigen receptor-T cell therapy have
demonstrated efficacy in partial malignant tumors, such as
malignant tumors of the blood system (114–116).
However, the efficacy of T-cell based immunotherapy is not
satisfactory in solid tumor (liver cancer), which is partly due to
the suppression of T-cell responses induced by innate immune cells
in the tumor microenvironment (7).
With the development of precision medicine, more attention will be
paid to improving immunotherapy (117). Considering the regulation of T-cell
responses by innate immune cells and complex protumoral mechanisms
in HCC, therapies combining innate immune cells with cytotoxic T
cells may be a promising choice.
HCC remains a threat for human health due to its
high degree of malignancy, poor clinical outcome and therapeutic
effect (2). Increasing HCC induced
by non-infectious diseases, such as NAFLD and alcoholic hepatitis
occurs with the improvement of living standards and sanitary
conditions (4). The occurrence and
development of these HCC are associated with a chronic inflammatory
microenvironment and infiltration of immune cells (109). T cells as important
tumor-associated immune cells have become a hotspot of research in
HCC (5). In fact, innate immune
cells, as the other ‘weapon’ of defense in humans also serve
important roles in the development of HCC (117). In fact, in addition to immune
cells, hypoxia and glycolysis further promote the formation of the
immunosuppressive tumor microenvironment and ultimately promote the
metastasis and immune escape of HCC (118,119).
However, how these metabolic factors exactly regulate various types
of immune cells and whether they are regulated by immune cells in
turn remains to be explored.
With the emergence of single-cell RNA sequencing
technology, people have realized the importance of the
heterogeneity of innate immune cell populations in the HCC
microenvironment (120,121). A number of studies have indicated
that the innate immune cells play opposite roles in different
stages of HCC (122,123). In future, studies of innate immune
cells in the HCC microenvironment will become more precise and this
will help in improved understanding the functions and mechanisms of
immune cell subsets. In addition, the revelation of heterogeneity
in the tumor microenvironment in HCC is helpful for overcoming the
drug resistance and for designing targets for immunotherapy for HCC
in the future (124).
In conclusion, both innate immune cells and T cells
can directly mediate the development of HCC. In addition, innate
immune cells can also regulate specific T-cell responses to mediate
the development of HCC. This may be the key to the immune
surveillance and escape in HCC. The present review will help
understand the importance of innate immune cells in HCC and
facilitate improved immunotherapy for patients with HCC.
Not applicable.
No funding was received.
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
GQH conceived this review, searched, analyzed and
drafted the manuscript. DC and JPG participated in discussing,
collecting literature and revising the manuscript for important
intellectual details. XL conceived and revised the manuscript for
important intellectual details. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulik L and El-Serag H: Epidemiology and
management of hepatocellular carcinoma. Gastroenterology.
156:477–491.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macek Jilkova Z, Aspord C and Decaens T:
Predictive factors for response to PD-1/PD-L1 checkpoint inhibition
in the field of hepatocellular carcinoma: Current status and
challenges. Cancers (Basel). 11:15542019. View Article : Google Scholar
|
|
6
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Syn NL, Teng MWL, Mok TSK and Soo RA:
De-novo and acquired resistance to immune checkpoint targeting.
Lancet Oncol. 18:e731–e741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jenne CN and Kubes P: Immune surveillance
by the liver. Nat Immunol. 14:996–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ringelhan M, Pfister D, O'Connor T,
Pikarsky E and Heikenwalder M: The immunology of hepatocellular
carcinoma. Nat Immunol. 19:222–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greten T, Wang X and Korangy F: Current
concepts of immune based treatments for patients with HCC: From
basic science to novel treatment approaches. Gut. 64:842–848. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang TW, Yevsa T, Woller N, Hoenicke L,
Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova
A, et al: Senescence surveillance of pre-malignant hepatocytes
limits liver cancer development. Nature. 479:547–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS,
Chen WJ, Liu W, Tai Y, Peng YW and Zhang Q: Hepatic
carcinoma-associated fibroblasts induce IDO-producing regulatory
dendritic cells through IL-6-mediated STAT3 activation.
Oncogenesis. 5:e1982016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinman R and Cohn Z: Identification of a
novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benites BD, Alvarez MC and Saad STO: Small
particles, Big effects: The interplay between exosomes and
dendritic cells in antitumor immunity and immunotherapy. Cells.
8:16482019. View Article : Google Scholar
|
|
16
|
Osada T, Clay T, Hobeika A, Lyerly HK and
Morse MA: NK cell activation by dendritic cell vaccine: A mechanism
of action for clinical activity. Cancer Immunol Immunother.
55:1122–1131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shang N, Figini M, Shangguan J, Wang B,
Sun C, Pan L, Ma Q and Zhang Z: Dendritic cells based
immunotherapy. Am J Cancer Res. 7:2091–2102. 2017.PubMed/NCBI
|
|
18
|
Chen S: Absence of CD83-positive mature
and activated dendritic cells at cancer nodules from patients with
hepatocellular carcinoma: Relevance to hepatocarcinogenesis. Cancer
Lett. 148:49–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai XY, Gao Q, Qiu SJ, Ye SL, Wu ZQ, Fan J
and Tang ZY: Dendritic cell infiltration and prognosis of human
hepatocellular carcinoma. J Cancer Res Clin Oncol. 132:293–301.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shibolet O, Alper R, Zlotogarov L,
Thalenfeld B, Engelhardt D, Rabbani E and Ilan Y: NKT and CD8
lymphocytes mediate suppression of hepatocellular carcinoma growth
via tumor antigen-pulsed dendritic cells. Int J Cancer.
106:236–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YX, Man K, Ling GS, Chen Y, Sun BS,
Cheng Q, Wong OH, Lo CK, Ng IO, Chan LC, et al: A crucial role for
dendritic cell (DC) IL-10 in inhibiting successful DC-based
immunotherapy: Superior antitumor immunity against hepatocellular
carcinoma evoked by DC devoid of IL-10. J Immunol. 179:6009–6015.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tatsumi T, Takehara T, Kanto T, Miyagi T,
Kuzushita N, Sugimoto Y, Jinushi M, Kasahara A, Sasaki Y, Hori M
and Hayashi N: Administration of interleukin-12 enhances the
therapeutic efficacy of dendritic cell-based tumor vaccines in
mouse hepatocellular carcinoma. Cancer Res. 61:7563–7567.
2001.PubMed/NCBI
|
|
23
|
Rai V, Abdo J, Alsuwaidan AN, Agrawal S,
Sharma P and Agrawal DK: Cellular and molecular targets for the
immunotherapy of hepatocellular carcinoma. Mol Cell Biochem.
437:13–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Santos PM, Menk AV, Shi J, Tsung A,
Delgoffe GM and Butterfield LH: Tumor-derived alpha-fetoprotein
suppresses fatty acid metabolism and oxidative phosphorylation in
dendritic cells. Cancer Immunol Res. 7:1001–1012. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu
Y, Lin C, Pan Z, Yu Y, Jiang M, et al: Human CD14+ CTLA-4+
regulatory dendritic cells suppress T-cell response by cytotoxic
T-lymphocyte antigen-4-dependent IL-10 and
indoleamine-2,3-dioxygenase production in hepatocellular carcinoma.
Hepatology. 59:567–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng Z, Yao WJ, Xu X, Xu GQ, Long JH, Wang
X, Wen ZY and Chien S: Hepatocellular carcinoma cells deteriorate
the biophysical properties of dendritic cells. Cell Biochem
Biophys. 55:33–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vitale I, Manic G, Coussens L, Kroemer G
and Galluzzi L: Macrophages and metabolism in the tumor
microenvironment. Cell Metab. 30:36–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li
CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively
activated (M2) macrophages promote tumour growth and invasiveness
in hepatocellular carcinoma. J Hepatol. 62:607–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang FB, Wang L, Li D, Zhang YG and Sun
DX: Hepatocellular carcinomas promote tumor-associated macrophage
M2-polarization via increased B7-H3 expression. Oncol Rep.
33:274–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC,
Han H, Liu WC and Qin HY: Crosstalk between hepatic tumor cells and
macrophages via Wnt/β-catenin signaling promotes M2-like macrophage
polarization and reinforces tumor malignant behaviors. Cell Death
Dis. 9:7932018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S
and Jian Z: IL-6/STAT3 pathway intermediates M1/M2 macrophage
polarization during the development of hepatocellular carcinoma. J
Cell Biochem. 119:9419–9432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, Luo
X, Chen R and Chen T: Long non-coding RNA cox-2 prevents immune
evasion and metastasis of hepatocellular carcinoma by altering
M1/M2 macrophage polarization. J Cell Biochem. 119:2951–2963. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zong Z, Zou J, Mao R, Ma C, Li N, Wang J,
Wang X, Zhou H, Zhang L and Shi Y: M1 macrophages induce PD-L1
expression in hepatocellular carcinoma cells through IL-1β
signaling. Front Immunol. 10:16432019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, Wei
T, Yang J, Tang J, Wang J, Chen Y, et al: Hypoxia-inducible
factor-1alpha/interleukin-1beta signaling enhances hepatoma
epithelial-mesenchymal transition through macrophages in a
hypoxic-inflammatory microenvironment. Hepatology. 67:1872–1889.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ,
Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB and Fan J:
Macrophage-secreted IL-8 induces epithelial-mesenchymal transition
in hepatocellular carcinoma cells by activating the
JAK2/STAT3/Snail pathway. Int J Oncol. 46:587–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Capece D, Fischietti M, Verzella D,
Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F and Alesse E:
The inflammatory microenvironment in hepatocellular carcinoma: A
pivotal role for tumor-associated macrophages. Biomed Res Int.
2013:1872042013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu XT, Song K, Zhou J, Shi YH, Liu WR, Shi
GM, Gao Q, Wang XY, Ding ZB and Fan J: Tumor-associated macrophages
modulate resistance to oxaliplatin via inducing autophagy in
hepatocellular carcinoma. Cancer Cell Int. 19:712019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tacke F: Targeting hepatic macrophages to
treat liver diseases. J Hepatol. 66:1300–1312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dou L, Shi X, He X and Gao Y: Macrophage
phenotype and function in liver disorder. Front Immunol.
10:31122019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Naugler W, Sakurai T, Kim S, Maeda S, Kim
K, Elsharkawy A and Karin M: Gender disparity in liver cancer due
to sex differences in MyD88-dependent IL-6 production. Science.
317:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martínez-Cardona C, Lozano-Ruiz B,
Bachiller V, Peiró G, Algaba-Chueca F, Gómez-Hurtado I, Such J,
Zapater P, Francés R and González-Navajas J: AIM2 deficiency
reduces the development of hepatocellular carcinoma in mice. Int J
Cancer. 143:2997–3007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miura K, Ohnishi H, Morimoto N, Minami S,
Ishioka M, Watanabe S, Tsukui M, Takaoka Y, Nomoto H, Isoda N and
Yamamoto H: Ezetimibe suppresses development of liver tumors by
inhibiting angiogenesis in mice fed a high-fat diet. Cancer Sci.
110:771–783. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu H, Zhong Z, Wang A, Yuan C, Ning K, Hu
H, Wang C and Yin X: LncRNA FTX represses the progression of
non-alcoholic fatty liver disease to hepatocellular carcinoma via
regulating the M1/M2 polarization of Kupffer cells. Cancer Cell
Int. 20:2662020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu
Z, Yin XY and Zheng L: Peritumoral neutrophils link inflammatory
response to disease progression by fostering angiogenesis in
hepatocellular carcinoma. J Hepatol. 54:948–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gomez D, Farid S, Malik HZ, Young AL,
Toogood GJ, Lodge JPA and Prasad KR: Preoperative
neutrophil-to-lymphocyte ratio as a prognostic predictor after
curative resection for hepatocellular carcinoma. World J Surg.
32:1757–1762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao
YS and Xu YF: Intratumoral neutrophils: A poor prognostic factor
for hepatocellular carcinoma following resection. J Hepatol.
54:497–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li L, Xu L, Yan J, Zhen ZJ, Ji Y, Liu CQ,
Lau WY, Zheng L and Xu J: CXCR2-CXCL1 axis is correlated with
neutrophil infiltration and predicts a poor prognosis in
hepatocellular carcinoma. J Exp Clin Cancer Res. 34:1292015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH,
Wang Z, Huang XW, Fan J and Zhou J: Overexpression of CXCL5
mediates neutrophil infiltration and indicates poor prognosis for
hepatocellular carcinoma. Hepatology. 56:2242–2254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu C, Rong D, Zhang B, Zheng W, Wang X,
Chen Z and Tang W: Current perspectives on the immunosuppressive
tumor microenvironment in hepatocellular carcinoma: Challenges and
opportunities. Mol Cancer. 18:1302019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He M, Peng A, Huang XZ, Shi DC, Wang JC,
Zhao Q, Lin H, Kuang DM, Ke PF and Lao XM: Peritumoral stromal
neutrophils are essential for c-Met-elicited metastasis in human
hepatocellular carcinoma. Oncoimmunology. 5:e12198282016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wilson CL, Jurk D, Fullard N, Banks P,
Page A, Luli S, Elsharkawy AM, Gieling RG, Chakraborty JB, Fox C,
et al: NFκB1 is a suppressor of neutrophil-driven hepatocellular
carcinoma. Nat Commun. 6:68182015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jorch SK and Kubes P: An emerging role for
neutrophil extracellular traps in noninfectious disease. Nat Med.
23:279–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
van der Windt DJ, Sud V, Zhang H, Varley
PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O'Doherty RM,
Minervini MI, et al: Neutrophil extracellular traps promote
inflammation and development of hepatocellular carcinoma in
nonalcoholic steatohepatitis. Hepatology. 68:1347–1360. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei
R, Lin ZF, Wang XY, Wang CQ, Lu M, et al: Increased neutrophil
extracellular traps promote metastasis potential of hepatocellular
carcinoma via provoking tumorous inflammatory response. J Hematol
Oncol. 13:32020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fulkerson P: Transcription factors in
eosinophil development and as therapeutic targets. Front Med
(Lausanne). 4:1152017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bernsmeier C, van der Merwe S and Périanin
A: Innate immune cells in cirrhosis. J Hepatol. 73:186–201. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Grisaru-Tal S, Itan M, Klion A and Munitz
A: A new dawn for eosinophils in the tumour microenvironment. Nat
Rev Cancer. 10:594–607. 2020. View Article : Google Scholar
|
|
60
|
Kataoka S, Konishi Y, Nishio Y,
Fujikawa-Adachi K and Tominaga A: Antitumor activity of eosinophils
activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA
Cell Biol. 23:549–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Steel JL, Kim KH, Dew MA, Unruh ML, Antoni
MH, Olek MC, Geller DA, Carr BI, Butterfield LH and Gamblin TC:
Cancer-related symptom clusters, eosinophils, and survival in
hepatobiliary cancer: An exploratory study. J Pain Symptom Manage.
39:859–871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gabrilovich D, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Arihara F, Mizukoshi E, Kitahara M, Takata
Y, Arai K, Yamashita T, Nakamoto Y and Kaneko S: Increase in
CD14+HLA-DR-/low myeloid-derived suppressor cells in hepatocellular
carcinoma patients and its impact on prognosis. Cancer Immunol
Immunother. 62:1421–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hoechst B, Voigtlaender T, Ormandy L,
Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns M, Greten T
and Korangy F: Myeloid derived suppressor cells inhibit natural
killer cells in patients with hepatocellular carcinoma via the
NKp30 receptor. Hepatology. 50:799–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hu C, Gan J, Zhang R, Cheng Y and Huang G:
Up-regulated myeloid-derived suppressor cell contributes to
hepatocellular carcinoma development by impairing dendritic cell
function. Scand J Gastroenterol. 46:156–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lu L, Chang C and Hsu C: Targeting
myeloid-derived suppressor cells in the treatment of hepatocellular
carcinoma: Current state and future perspectives. J Hepatocell
Carcinoma. 6:71–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK,
Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, et al: Hypoxia inducible
factor HIF-1 promotes myeloid-derived suppressor cells accumulation
through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun.
8:5172017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li Y, Liu Z, Wang J, Yu J, Li Z, Yang H,
Tang J and Chen Z: Receptor-interacting protein kinase 3 deficiency
recruits myeloid-derived suppressor cells to hepatocellular
carcinoma through the chemokine (C-X-C Motif) ligand 1-chemokine
(C-X-C Motif) receptor 2 axis. Hepatology. 70:1564–1581. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu M, Zhao Z, Song J, Lan X, Lu S, Chen M,
Wang Z, Chen W, Fan X, Wu F, et al: Interactions between
interleukin-6 and myeloid-derived suppressor cells drive the
chemoresistant phenotype of hepatocellular cancer. Exp Cell Res.
351:142–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sonnenberg GF and Hepworth MR: Functional
interactions between innate lymphoid cells and adaptive immunity.
Nat Rev Immunol. 19:599–613. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Warner K and Ohashi PS: ILC regulation of
T cell responses in inflammatory diseases and cancer. Semin
Immunol. 41:1012842019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Han X, Huang T and Han J: Cytokines
derived from innate lymphoid cells assist Helicobacter hepaticus to
aggravate hepatocellular tumorigenesis in viral transgenic mice.
Gut Pathog. 11:232019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cai L, Zhang Z, Zhou L, Wang H, Fu J,
Zhang S, Shi M, Zhang H, Yang Y, Wu H, et al: Functional impairment
in circulating and intrahepatic NK cells and relative mechanism in
hepatocellular carcinoma patients. Clin Immunol. 129:428–437. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM,
Wang D, Li XF and Zheng L: Monocyte/macrophage-elicited natural
killer cell dysfunction in hepatocellular carcinoma is mediated by
CD48/2B4 interactions. Hepatology. 57:1107–1116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tatsumi T and Takehara T: Impact of
natural killer cells on chronic hepatitis C and hepatocellular
carcinoma. Hepatol Res. 46:416–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bugide S, Green MR and Wajapeyee N:
Inhibition of Enhancer of zeste homolog 2 (EZH2) induces natural
killer cell-mediated eradication of hepatocellular carcinoma cells.
Proc Natl Acad Sci USA. 115:E3509–E3518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen Y, Hao X, Sun R, Wei H and Tian Z:
Natural killer cell-derived interferon-gamma promotes
hepatocellular carcinoma through the epithelial cell adhesion
molecule-epithelial-to-mesenchymal transition axis in hepatitis B
virus transgenic mice. Hepatology. 69:1735–1750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Luo Q, Luo W, Zhu Q, Huang H, Peng H, Liu
R, Xie M, Li S, Li M, Hu X and Zou Y: Tumor-derived soluble MICA
obstructs the NKG2D pathway to restrain NK cytotoxicity. Aging Dis.
11:118–128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Vujanovic L, Stahl EC, Pardee AD, Geller
DA, Tsung A, Watkins SC, Gibson GA, Storkus WJ and Butterfield LH:
Tumor-derived α-fetoprotein directly drives human natural
killer-cell activation and subsequent cell death. Cancer Immunol
Res. 5:493–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mossanen J, Kohlhepp M, Wehr A, Krenkel O,
Liepelt A, Roeth A, Möckel D, Heymann F, Lammers T, Gassler N, et
al: CXCR6 inhibits hepatocarcinogenesis by promoting natural killer
T- and CD4 T-cell-dependent control of senescence.
Gastroenterology. 156:1877–1889.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Miyagi T, Takehara T, Tatsumi T, Kanto T,
Suzuki T, Jinushi M, Sugimoto Y, Sasaki Y, Hori M and Hayashi N:
CD1d-mediated stimulation of natural killer T cells selectively
activates hepatic natural killer cells to eliminate experimentally
disseminated hepatoma cells in murine liver. Int J Cancer.
106:81–89. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vivier E, Ugolini S, Blaise D, Chabannon C
and Brossay L: Targeting natural killer cells and natural killer T
cells in cancer. Nat Rev Immunol. 12:239–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xiao Y, Gao Q, Xu X, Li Y, Ju M, Cai M,
Dai C, Hu J, Qiu S, Zhou J and Fan J: Combination of intratumoral
invariant natural killer T cells and interferon-gamma is associated
with prognosis of hepatocellular carcinoma after curative
resection. PLoS One. 8:e703452013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen J, Gingold J and Su X:
Immunomodulatory TGF-β signaling in hepatocellular carcinoma.
Trends Mol Med. 25:1010–1023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li J, Lee Y, Li Y, Jiang Y, Lu H, Zang W,
Zhao X, Liu L, Chen Y, Tan H, et al: Co-inhibitory molecule B7
superfamily member 1 expressed by tumor-infiltrating myeloid cells
induces dysfunction of anti-tumor CD8+ T cells.
Immunity. 48:773–786.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hiroishi K, Eguchi J, Baba T, Shimazaki T,
Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, et al:
Strong CD8(+) T-cell responses against tumor-associated antigens
prolong the recurrence-free interval after tumor treatment in
patients with hepatocellular carcinoma. J Gastroenterol.
45:451–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zong L, Peng H, Sun C, Li F, Zheng M, Chen
Y, Wei H, Sun R and Tian Z: Breakdown of adaptive immunotolerance
induces hepatocellular carcinoma in HBsAg-tg mice. Nat Commun.
10:2212019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sachdeva M: Immunology of hepatocellular
carcinoma. World J Hepatol. 7:2080–2090. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z,
Qi H, Guo H and Yin H: Dendritic cell-derived exosomes elicit tumor
regression in autochthonous hepatocellular carcinoma mouse models.
J Hepatol. 67:739–748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sconocchia G, Eppenberger S, Spagnoli GC,
Tornillo L, Droeser R, Caratelli S, Ferrelli F, Coppola A, Arriga
R, Lauro D, et al: NK cells and T cells cooperate during the
clinical course of colorectal cancer. Oncoimmunology.
3:e9521972014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li X, Yao W, Yuan Y, Chen P, Li B, Li J,
Chu R, Song H, Xie D, Jiang X and Wang H: Targeting of
tumour-infiltrating macrophages via CCL2/CCR2 signalling as a
therapeutic strategy against hepatocellular carcinoma. Gut.
66:157–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liu Y, Song Y, Lin D, Lei L, Mei Y, Jin Z,
Gong H, Zhu Y, Hu B, Zhang Y, et al: NCR− group 3 innate
lymphoid cells orchestrate IL-23/IL-17 axis to promote
hepatocellular carcinoma development. EBioMedicine. 41:333–344.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu CQ, Xu J, Zhou ZG, Jin LL, Yu XJ, Xiao
G, Lin J, Zhuang SM, Zhang YJ and Zheng L: Expression patterns of
programmed death ligand 1 correlate with different
microenvironments and patient prognosis in hepatocellular
carcinoma. Br J Cancer. 119:80–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lim T, Chew V, Sieow J, Goh S, Yeong J,
Soon A and Ricciardi-Castagnoli P: PD-1 expression on dendritic
cells suppresses CD8 T cell function and antitumor immunity.
Oncoimmunology. 5:e10851462016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liu J, Fan L, Yu H, Zhang J, He Y, Feng D,
Wang F, Li X, Liu Q, Li Y, et al: Endoplasmic reticulum stress
causes liver cancer cells to release exosomal miR-23a-3p and
up-regulate programmed death ligand 1 expression in macrophages.
Hepatology. 70:241–258. 2019.PubMed/NCBI
|
|
96
|
Mossanen JC, Kohlhepp M, Wehr A, Krenkel
O, Liepelt A, Roeth AA, Mockel D, Heymann F, Lammers T, Gassler N,
et al: CXCR6 inhibits hepatocarcinogenesis by promoting natural
killer T- and CD4+ T-cell-dependent control of
senescence. Gastroenterology. 156:1877–1889.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shigeta K, Datta M, Hato T, Kitahara S,
Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, et
al: Dual programmed death receptor-1 and vascular endothelial
growth factor receptor-2 blockade promotes vascular normalization
and enhances antitumor immune responses in hepatocellular
carcinoma. Hepatology. 71:1247–1261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou Y, Xu X, Ding J, Jing X, Wang F, Wang
Y and Wang P: Dynamic changes of T-cell subsets and their relation
with tumor recurrence after microwave ablation in patients with
hepatocellular carcinoma. J Cancer Res Ther. 14:40–45. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang H, Jiang Z and Zhang L: Dual effect
of T helper cell 17 (Th17) and regulatory T cell (Treg) in liver
pathological process: From occurrence to end stage of disease. Int
Immunopharmacol. 69:50–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kondo Y and Shimosegawa T: Significant
roles of regulatory T cells and myeloid derived suppressor cells in
hepatitis B virus persistent infection and hepatitis B
virus-related HCCs. Int J Mol Sci. 16:3307–3322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wu Q, Zhou W, Yin S, Zhou Y, Chen T, Qian
J, Su R, Hong L, Lu H, Zhang F, et al: Blocking triggering receptor
expressed on myeloid cells-1-positive tumor-associated macrophages
induced by hypoxia reverses immunosuppression and anti-programmed
cell death ligand 1 resistance in liver cancer. Hepatology.
70:198–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z,
Chen EB, Fan J, Cao Y, Dai Z and Zhou J: Tumor-associated
neutrophils recruit macrophages and T-regulatory cells to promote
progression of hepatocellular carcinoma and resistance to
sorafenib. Gastroenterology. 150:1646–1658.e7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Murphy K and Reiner S: The lineage
decisions of helper T cells. Nat Rev Immunol. 2:933–944. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Geginat J, Paroni M, Maglie S, Alfen J,
Kastirr I, Gruarin P, De Simone M, Pagani M and Abrignani S:
Plasticity of human CD4 T cell subsets. Front Immunol. 5:6302014.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mirchandani AS, Besnard AG, Yip E, Scott
C, Bain CC, Cerovic V, Salmond RJ and Liew FY: Type 2 innate
lymphoid cells drive CD4+ Th2 cell responses. J Immunol.
192:2442–2448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shen G, Krienke S, Schiller P, Nießen A,
Neu S, Eckstein V, Schiller M, Lorenz H-M and Tykocinski LO:
Microvesicles released by apoptotic human neutrophils suppress
proliferation and IL-2/IL-2 receptor expression of resting T helper
cells. Eur J Immunol. 47:900–910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Duffy A, Ulahannan S, Makorova-Rusher O,
Rahma O, Wedemeyer H, Pratt D, Davis J, Hughes M, Heller T, ElGindi
M, et al: Tremelimumab in combination with ablation in patients
with advanced hepatocellular carcinoma. J Hepatol. 66:545–551.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ,
Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW and Yoon JH: Adjuvant
immunotherapy with autologous cytokine-induced killer cells for
hepatocellular carcinoma. Gastroenterology. 148:1383–1391.e6. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Okusaka T and Ikeda M: Immunotherapy for
hepatocellular carcinoma: Current status and future perspectives.
ESMO Open. 3:e0004552018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Guerra AD, Yeung OWH, Qi X, Kao WJ and Man
K: The anti-tumor effects of M1 macrophage-loaded poly (ethylene
glycol) and gelatin-based hydrogels on hepatocellular carcinoma.
Theranostics. 7:3732–3744. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jiang W, Zhang C, Tian Z and Zhang J:
hIL-15 gene-modified human natural killer cells (NKL-IL15) augments
the anti-human hepatocellular carcinoma effect in vivo.
Immunobiology. 219:547–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Palmer DH, Midgley RS, Mirza N, Torr EE,
Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS and Adams DH: A
phase II study of adoptive immunotherapy using dendritic cells
pulsed with tumor lysate in patients with hepatocellular carcinoma.
Hepatology. 49:124–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lee JH, Tak WY, Lee Y, Heo MK, Song JS,
Kim HY, Park SY, Bae SH, Lee JH, Heo J, et al: Adjuvant
immunotherapy with autologous dendritic cells for hepatocellular
carcinoma, randomized phase II study. Oncoimmunology.
6:e13283352017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sawada Y, Yoshikawa T, Shimomura M, Iwama
T, Endo I and Nakatsura T: Programmed death-1 blockade enhances the
antitumor effects of peptide vaccine-induced peptide-specific
cytotoxic T lymphocytes. Int J Oncol. 46:28–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gao H, Li K, Tu H, Pan X, Jiang H, Shi B,
Kong J, Wang H, Yang S, Gu J and Li Z: Development of T cells
redirected to glypican-3 for the treatment of hepatocellular
carcinoma. Clin Cancer Res. 20:6418–6428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Majzner R and Mackall C: Clinical lessons
learned from the first leg of the CAR T cell journey. Nat Med.
25:1341–1355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kole C, Charalampakis N, Tsakatikas S,
Vailas M, Moris D, Gkotsis E, Kykalos S, Karamouzis M and Schizas
D: Immunotherapy for hepatocellular carcinoma: A 2021 update.
Cancers (Basel). 12:E28592020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chang C, Dinh T, Lee Y, Wang F, Sung Y, Yu
P, Chiu S, Shih Y, Wu C, Huang Y, et al: Nanoparticle delivery of
MnO2 and anti-angiogenic therapy to overcome
hypoxia-driven tumor escape and suppress hepatocellular carcinoma.
ACS Appl Mater Interfaces. 12:44407–44419. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tian H, Zhu X, Lv Y, Jiao Y and Wang G:
Glucometabolic reprogramming in the hepatocellular carcinoma
microenvironment: Cause and effect. Cancer Manag Res. 12:5957–5974.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang Q, Lou Y, Bai X and Liang T:
Intratumoral heterogeneity of hepatocellular carcinoma: From
single-cell to population-based studies. World J Gastroenterol.
26:3720–3736. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liu F, Qin L, Liao Z, Song J, Yuan C, Liu
Y, Wang Y, Xu H, Zhang Q, Pei Y, et al: Microenvironment
characterization and multi-omics signatures related to prognosis
and immunotherapy response of hepatocellular carcinoma. Exp Hematol
Oncol. 9:102020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Xiong X, Kuang H, Ansari S, Liu T, Gong J,
Wang S, Zhao XY, Ji Y, Li C, Guo L, et al: Landscape of
intercellular crosstalk in healthy and NASH liver revealed by
single-cell secretome gene analysis. Mol Cell. 75:644–660.e5. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao
R, Modak M, Carotta S, Haslinger C, Kind D, et al: Landscape and
dynamics of single immune cells in hepatocellular carcinoma. Cell.
179:829–845.e20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Caruso S, O'Brien D, Cleary S, Roberts L
and Zucman-Rossi J: Genetics of HCC: Novel approaches to explore
molecular diversity. Hepatology. May 28–2020.(Epub ahead of print).
View Article : Google Scholar
|