Introduction
Lung cancer is one of the most frequently diagnosed
malignant tumors and the leading cause of cancer-associated death
worldwide. For example, in the United States lung cancer caused
more deaths than breast, prostate, colorectal and brain cancer
combined in 2017, and is projected to account for one-quarter of
all cancer-associated deaths in 2020 (1). Histologically, lung cancer can be
classified as small cell lung cancer (SCLC) and non-SCLC. SCLC is
considered to have the poorest prognosis among all types of lung
cancer due to its rapid growth and early distant metastases;
clinically, around two-thirds of patients with SCLC present with
multiple organ metastases, particularly to the bone (2). Bone metastases are often associated
with skeletal-associated events, including pain, hypercalcemia,
fracture and nerve compression syndromes, all of which may decrease
the quality of life of patients (3,4). The
occurrence of bone metastasis directly affects the choice of
treatment options (5). Therefore,
timely diagnosis of bone metastasis is important to improve the
treatment of SCLC. At present, the diagnosis of bone metastasis
mainly depends on imaging examination, such as bone scans and
computed tomography (CT), which are harmful to patients and not
economical; however, due to the rapid progression of SCLC, frequent
monitoring of the status of bone metastases is clinically required
(6). Therefore, finding biomarkers
associated with bone metastasis is important to improve the
diagnosis and treatment of patients with SCLC.
Annexin A1 (ANXA1), a member of calcium-dependent
phospholipid-binding proteins, serves a role in regulating cell
proliferation, apoptosis, phagocytosis and carcinogenesis (7,8). Studies
have demonstrated that ANXA1 expression is different in various
types of tumor tissue (8,9). For example, ANXA1 expression is
upregulated in liver, colorectal and pancreatic cancer, but
downregulated in prostate, esophageal and cervical cancer (9). Abnormal ANXA1 expression and the
changes of its location may be associated with the differentiation
and metastases of malignant tumors (10,11).
ANXA1 may be a potential new biomarker for the diagnosis and
treatment of tumors; however, the role of ANXA1 in the pathogenesis
of SCLC remains unclear. In addition, ANXA1 is involved in
regulating bone development and the bone marrow microenvironment
(12,13). However, whether this indicates that
ANXA1 participates in the bone metastases of tumors requires
further confirmation.
The present study analyzed the association between
the expression levels of ANXA1 in serum and the clinical
characteristics of patients with SCLC, and discussed the diagnostic
value of ANXA1 expression in bone metastasis. To further confirm
the association between ANXA1 and bone metastasis, ANXA1 was
overexpressed in SBC-3 cells and inhibited in SBC-5 cells, and the
effects of ANXA1 on the biological behavior of SCLC cells and bone
metastasis-associated signaling pathways were investigated.
Materials and methods
Patients and specimens
Serum samples were collected from 82 patients with
SCLC who were admitted to Tangdu Hospital of the Air Force Military
Medical University (Xi'an, China) between March 2016 and May 2017,
and ANXA1 1 expression was detected using a Human Annexin A1 ELISA
kit (cat. no. ab222868; Abcam). The present study was based on a
cohort of consecutive patients with histopathological diagnosis of
SCLC, without inflammatory disease and who had never received
chemotherapy or radiotherapy. Detailed information was obtained
from the medical records of the enrolled patients in a computerized
registry database, including patient age, sex and smoking history.
Tumor assessment was performed using CT, radionuclide bone scanning
or integrated positron emission tomography-CT. Magnetic resonance
imaging or enhanced CT was performed is symptomatic patients. At
least one radiologist and one physician confirmed the diagnosis.
All tumors were staged according to the pathological
tumor/node/metastasis (pTNM) classification (8th edition) of the
Union for International Cancer Control (UICC) (14). The study protocol was approved by the
ethical review board of Tangdu Hospital. All patients provided
written informed consent for use of their medical records and
samples for research purposes.
Cell lines and culture
The human SCLC SBC-3 and SBC-5 cell lines were
provided by Professor Saburo Sone and Professor Seiji Yano
(University of Tokushima School of Medicine, Tokushima, Japan).
Both cell lines were cultured in RPMI-1640 medium supplemented with
10% heat-inactivated FBS (both Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in an atmosphere of 5% CO2.
Transfection
For ANXA1-knockdown in SBC-5 cells, lentiviral
vectors for human ANXA1 short hairpin RNAs (shRNAs) were designed
according to the human ANXA1 mRNA sequence (GenBank accession no.
NM-000700): shANXA1-1, 5′-AACCATCATTGACATTCTA-3′; shANXA1-2,
5′-CTTGTATGAAGCAGGAGAA-3′; shANXA1-3, 5′-AGCGCAATTTGATGCTGAT-3′;
shANXA1-4, 5′-ATTCTATCAGAAGATGTAT-3′; and negative control
(non-targeting), 5′-TTCTCCGAACGTGTCACGT-3′. ANXA1 and negative
control shRNAs were constructed, packed and purified by Shanghai
GeneChem Co., Ltd.. The second generation of self-inactivated
lentivirus packaging system was used for virus packaging, which
involved three plasmids (Shanghai GeneChem Co., Ltd.): Tool vector
plasmid GV248 carrying the target sequence, pHelper 1.0 (carrying
gag, pol and rev genes) and pHelper 2.0 (carrying the VSV-G gene).
Viral vector generation was obtained by co-transfection of
5×106 293T cells (Shanghai GeneChem Co., Ltd.)/15 ml
medium (DMEM with 10% FBS; Gibco; Thermo Fisher Scientific, Inc.)
with 20 µg GV248, 15 µg pHelper1.0 and 10 µg pHelper2.0 on 10-cm
plates. To overexpress ANXA1 in SBC-3 cells, the cDNA of ANXA1 was
transfected into the GV166 virus to build the ANXA1/GV166-LV
plasmid, which was then packed into lentiviral vectors. SBC-3 cells
and SBC-5 cells were seeded in 6-well plates before transfection.
When the cells reached ~80% confluence, SBC-5 cells were either
transfected with ANXA1 shRNAs (SBC-5-shANXA1) or the negative
control shRNA (SBC-5-NC), and SBC-3 cells were transfected with
pcDNA-ANXA1 (SBC-3-ANXA1) or the negative control (empty) vector by
co-incubating with Enhance Infection Solution (Shanghai GeneChem
Co., Ltd.) for 12 h at 37°C at a multiplicity of infection of 20,
after which the medium was replaced by fresh RPMI-1640 with 10%
FBS. Three days after transfection, cells were selected using 0.5
µg/ml puromycin and harvested for subsequent experiments.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT was performed to obtain cDNA using the SuperScript III
First-Strand Synthesis kit (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. mRNA expression in
each cell line was standardized to β-actin. The following primer
pairs were synthesized by Shanghai GeneChem Co., Ltd., for qPCR:
ANXA1 forward, 5′-TTTTGGCATCAAGAACTAAC-3′ and reverse,
5′-CCTCAGATCGGTCACCCT-3′; parathyroid hormone-related protein
(PTHrP) forward, 5′-GGAGACTGGTTCAGCAGTGG-3′ and reverse,
5′-TTGTCATGGAGGAGCTGATG-3′; β-actin forward,
5′-ATCGTGCGTGACATTAAGGACAAG-3′ and reverse,
5′-AGGAAGGAAGGGCTGGAAGAGTG-3′. qPCR was preformed using SYBR Premix
Ex Taq™ II (Takara Bio, Inc.). The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 3
min; 35 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 1
min; extension at 72°C for 7 min. Each sample was detected in
triplicate, and a melting curve was analyzed to confirm
amplification specificity. Relative mRNA expression was calculated
using the 2−ΔΔCq method (15).
Western blotting
Harvested cells were washed once with PBS and lysed
to extract total cellular protein using RIPA buffer (Beyotime
Institute of Biotechnology). The proteins were boiled for 5 min and
protein concentration was quantified using a Micro-BCA protein
assay. Subsequently, 20 µg/lane protein was subjected to 10%
SDS-PAGE and transferred to PVDF membranes. The membranes were then
blocked in 5% skimmed milk for 1 h at room temperature and
incubated overnight at 4°C with the following primary antibodies:
Anti-ANXA1 (1:500; cat. no. AMAB90558; Sigma-Aldrich; Merck KGaA),
anti-PTHrP (1:200; cat. no. sc-53936; Santa Cruz Biotechnology,
Inc.), anti-Smad2 (1:500, cat. no. 5339; Cell Signaling Technology,
Inc.), anti-phosphorylated (p)-Smad2 (1:500; cat. no. 18338; Cell
Signaling Technology, Inc.) and anti-β-actin (1:1,000; cat. no.
A1978; Sigma-Aldrich; Merck KGaA). Subsequently, the membranes were
incubated with peroxidase-conjugated goat anti-rabbit IgG (1:5,000;
cat. no. ZB-2301; OriGene Technologies, Inc.) or goat anti-mouse
IgG (1:5,000; cat. no. ZB-2305; OriGene Technologies, Inc.)
secondary antibodies for 1 h at room temperature. Finally, the
target proteins were visualized by chemiluminescence (Pierce;
Thermo Fisher Scientific, Inc.) and measured using ImageJ software
(version 1.52a; National Institutes of Health).
MTT assay
SBC-3 and SBC-5 cells in the logarithmic phase were
harvested and seeded into 96-well plates (5×103
cells/well). To assess the relative cell number, 20 µl of 5 mg/ml
MTT solution was added to the wells and cells were incubated for 3
h at 37°C. The supernatant was removed and 150 µl DMSO was added to
each well to dissolve the blue formazan crystals. The absorbance
was determined at a wavelength of 490 nm using a Model 680
microplate reader (Bio-Rad Laboratories, Inc.). To draw the growth
curve, the cell number was measured at 24, 48, 72, 96 and 120 h.
For accuracy, each assay contained six replicates and was repeated
three times.
Colony formation assay
Single-cell suspensions were plated in a 35-mm
diameter plate at a density of 1×103 cells/plate and
incubated at 37°C with 5% CO2 for 7 days. Subsequently,
at room temperature, the colonies were fixed with 4% formaldehyde
for 15 min, stained with 0.5% crystal violet for 15 min and counted
manually using a light microscope (one colony contained ≥50 cells).
Three independent plates were set up for accuracy.
Cell cycle assay
The cells were resuspended at 1×106
cells/ml, fixed and permeabilized with 75% ethanol at 4°C overnight
and washed with cool PBS. Subsequently, the cells were stained with
staining solution (50 µg/ml propidium iodide and 20 µg/ml RNase in
PBS; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min in the dark. The
analysis of cellular DNA content was performed using a flow
cytometer (FACSCalibur; BD Biosciences) at an excitation wavelength
of 488 nm. The distribution of these cells was analyzed using
CellQuest v3.3 and ModFit v3.1 softwares (BD Biosciences).
In vitro migration and invasion
assay
Both the migratory and invasive abilities of cells
were assessed using Transwell inserts (8-µm pore size) in a 24-well
plate (Corning, Inc.). The cells were starved for 24 h prior to the
migration assay using serum-free RPMI-1640. A total of
3×104 cells in 100 µl RPMI-1640 with 0.1% BSA
(Sigma-Aldrich; Merck KGaA) were seeded in triplicates into the
upper chamber of the Transwell insert. RPMI-1640 (500 µl) with 10%
FBS was added into the lower chamber as a chemoattractant.
Following 48 h of incubation at 37°C, cells that did not migrate
through the membranes were gently removed using a cotton swab. At
room temperature, cells adhering to the lower surface of the
membranes were fixed with 4% formaldehyde for 15 min and stained
with 0.5% crystal violet for 10 min. Finally, migrated cells were
imaged at ×400 magnification using a light microscope and counted
manually in five randomly selected fields per well. For the
invasion assay, the inserts were coated with 70 µl
Matrigel® (1:8 dilution with serum-free RPMI-1640; BD
Biosciences) at 37°C for 30 min before the cells were seeded into
the upper chamber. Subsequent steps were performed as
aforementioned for the migration assay.
In vitro bone adhesion ability
assay
A total of 30 female NOD-SCID mice (age, 5 days old;
weight, 4.4–5.8 g) were obtained from the Laboratory Animal
Research Center of the Air Force Military Medical University
(Xi'an, China) and sacrificed by inhaling carbon dioxide
(CO2 flow rate, 20% of the container volume per min). As
previously described (16), skulls
of mice were dissected, swabbed with alcohol wipes and soaked in
PBS containing 1×104 U/ml penicillin for 24 h at room
temperature. Once the supernatant was removed following
centrifugation at 1,000 × g for 15 min at 20°C, collagenase I (5
mg/ml; 400 µl; Sigma-Aldrich; Merck KGaA), collagenase II (5 mg/ml;
400 µl; Sigma-Aldrich; Merck KGaA) and 0.05% trypsin (200 µl) were
added to digest the skulls at 37°C for 2 h. The skulls were then
washed with PBS twice, and were cut into pieces of 3×3
mm2 for subsequent experimentation. Agar (1%; 0.5
ml/well) was added into a 24-well plate, and the bone pieces were
fixed to the solidified agar surface. Subsequently, each well was
washed with RPMI-1640 without FBS and incubated in 1 ml RPMI-1640
with 10% FBS in an incubator for 30 min at 37°C. A total of
1×104 cells were planted onto the skulls and incubated
for 24 h at 37°C. Subsequently, the bone pieces were taken out,
washed with PBS three times, fixed with 95% alcohol for 10 min and
stained with 0.5% crystal violet for 15 min, all at room
temperature. Finally, cells adhering to the bone surface were
visualized under a light microscope (magnification, ×100). Cells
adhering to the bone were digested using trypsin and resuspended in
PBS to measure the optical density at a wavelength of 600 nm using
a spectrophotometer.
In vivo xenograft bone metastasis
assay
A total of 10 female NOD/SCID mice (age, 3–5 weeks
old; weight, 19–24 g) were obtained from the Laboratory Animal
Center of the Air Force Military Medical University and raised
under germ-free conditions (temperature, 22°C; ventilation rate,
15/h; light/dark cycle, 12/12 h; food was sterilized with Cobalt-60
irradiation and water was autoclaved; access to food and water was
ad libitum). SBC-5-NC/SBC-5-shANXA1 cells were harvested
from 80–90% confluent conditions, washed twice and resuspended in
PBS. Subsequently, 2×106 cells in 200 µl PBS were
implanted into the NOD/SCID mice (4–6 weeks old) via intravenous
injection. The mice were divided into two groups according to the
expression levels of ANXA1. On the 35th day post-inoculation, mice
were anesthetized by intraperitoneal injection of 1% pentobarbital
(40 mg/kg). Resultant bone metastases were visualized using X-ray
irradiation. At the end of the experiment, the mice were sacrificed
by inhaling carbon dioxide (CO2 flow rate, 20% of the
container volume per min). All animal experimental procedures,
including the aforementioned bone adhesion ability assays, were
approved by the Animal Ethics Committee of the Air Force Military
Medical University and were in accordance with the ‘Animal
Research: Reporting In Vivo Experiments’ guidelines
(17).
ELISA and TGF-β treatment
The cells at 80% confluence were harvested, plated
into 6-well tissue culture plates (1×105 cells/2
ml/well) and incubated for 24 h at 37°C with 5% CO2.
Culture supernatants were collected and the concentrations of PTHrP
were determined using the PTHrP ELISA kit (cat. no. CSB-E08649h;
Cusabio Technology LLC). For experiments involving TGF-β treatment,
0.5 ng/ml recombinant human TGF-β1 (cat. no. rcyc-htgfb1;
InvivoGen) was added to the medium and incubated for 12 h at 37°C
with 5% CO2.
Statistical analysis
The data are presented as the mean ± standard
deviation. The Kruskal-Wallis test followed by Bonferroni
correction for multiple comparisons was used to compare ANXA1
expression in patients with SCLC. Fisher's exact test was used for
the association between ANXA1 expression and clinicopathological
characteristics. When the variables were normally distributed,
unpaired Student's t-test was used to compare two groups and
one-way ANOVA was used to compare three groups, followed by Tukey's
post hoc test. Receiver operating characteristic (ROC) curve
analysis was used to evaluate the diagnostic value of ANXA1 for
bone metastasis in SCLC. Data analysis was performed using SPSS
software (version 25.0; IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
ANXA1 expression is associated with
bone metastases in patients with SCLC
Initially, ANXA1 expression was determined in the
serum of 82 patients with SCLC (28 females and 54 males; median
age, 64 years; age range, 33–83 years). UICC staging evaluation
demonstrated stage I disease in 23 patients, stage II disease in 13
patients, stage III disease in 15 patients and stage IV disease in
31 patients. Among the 82 patients with SCLC, distant organ
metastasis occurred in 31 cases at initial diagnosis, including 6
cases of brain metastasis, 17 cases of bone metastasis, 13 cases of
liver metastasis and 5 cases of adrenal metastasis. ELISA results
revealed that ANXA1 expression in the serum ranged between 0.6–6.6
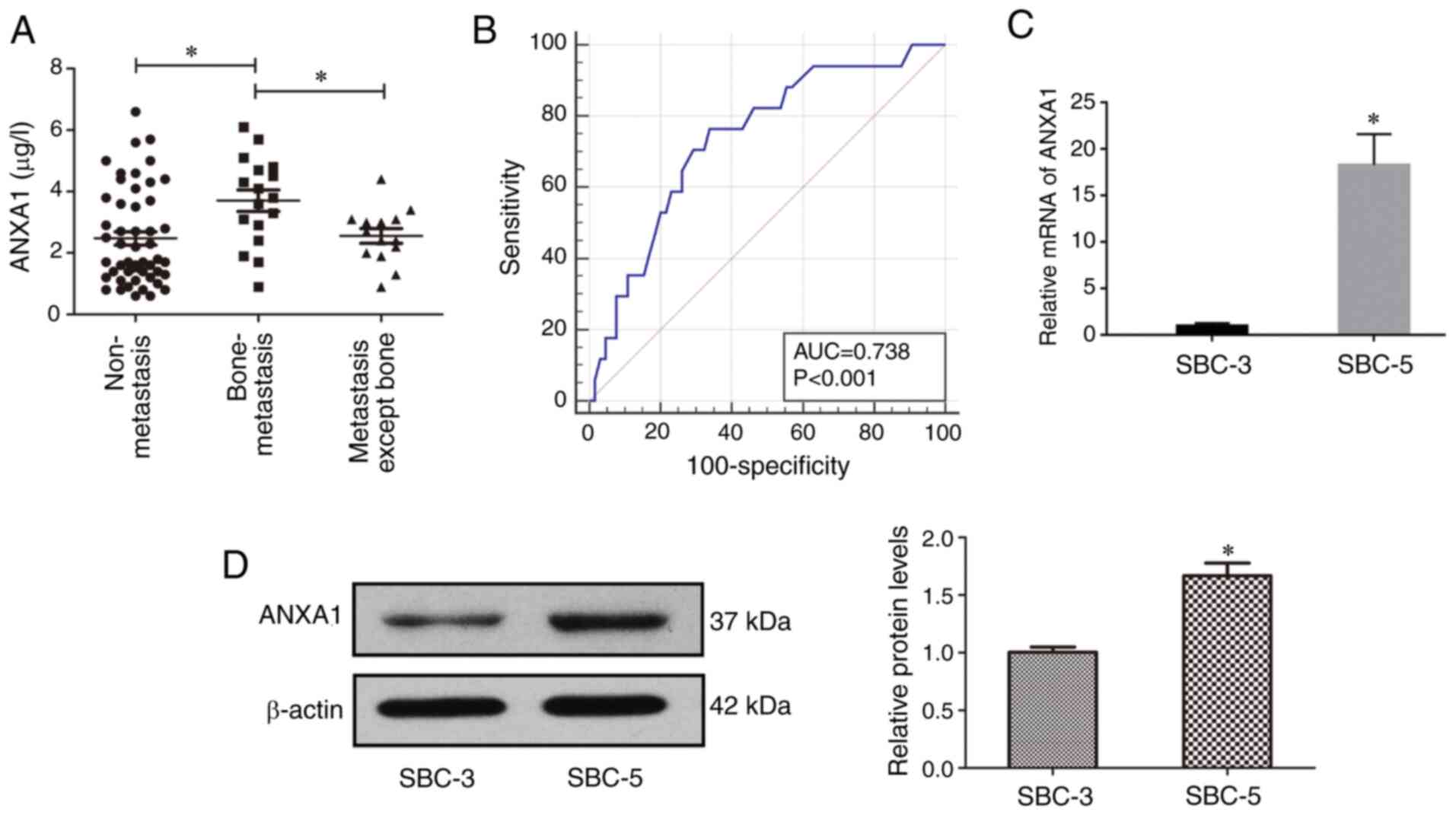
µg/l (Fig. 1A; median, 2.45 µg/l;
mean, 2.74 µg/l).
Subsequently, the associations between ANXA1
expression and clinicopathological characteristics of the patients
were analyzed (Table I). The results
revealed that ANXA1 expression was significantly associated with
lymphatic invasion (P<0.01), bone metastasis (P=0.03) and TNM
stage (P<0.01). No significant association was observed between
ANXA1 expression and age (P=0.62), sex (P=0.82), smoking history
(P=0.48), tumor invasion (P=0.44), metastasis (P=0.07), liver
metastasis (P>0.99) and brain metastasis (P=0.68).
 | Table I.Clinicopathological associations of
ANXA1 expression in 82 patients with small cell lung cancer. |
Table I.
Clinicopathological associations of
ANXA1 expression in 82 patients with small cell lung cancer.
|
| ANXA1
expressiona |
|
|---|
|
|
|
|
|---|
| Category | N | High | Low | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 23 | 13 | 10 | 0.62 |
|
≥60 | 59 | 28 | 31 |
|
| Sex |
|
|
|
|
|
Male | 54 | 26 | 28 | 0.82 |
|
Female | 28 | 15 | 13 |
|
| Smoking
history |
|
|
|
|
|
Yes | 73 | 35 | 38 | 0.48 |
| No | 9 | 6 | 3 |
|
| Tumor invasion |
|
|
|
|
|
T1-2 | 62 | 29 | 33 | 0.44 |
|
T3-4 | 20 | 12 | 8 |
|
| Lymphatic
invasion |
|
|
|
|
|
N0-1 | 40 | 13 | 27 | <0.01 |
|
N2-3 | 42 | 28 | 14 |
|
| Metastasis |
|
|
|
|
|
Yes | 31 | 20 | 11 | 0.07 |
| No | 51 | 21 | 30 |
|
| Bone
metastasis |
|
|
|
|
|
Yes | 17 | 13 | 4 | 0.03 |
| No | 65 | 28 | 37 |
|
| Liver
metastasis |
|
|
|
|
|
Yes | 13 | 6 | 7 | >0.99 |
| No | 69 | 35 | 34 |
|
| Brain
metastasis |
|
|
|
|
|
Yes | 6 | 2 | 4 | 0.68 |
| No | 76 | 39 | 37 |
|
| TNM stage |
|
|
|
|
|
I–II | 36 | 11 | 25 | <0.01 |
|
III–IV | 46 | 30 | 16 |
|
To further clarify the specific association between
ANXA1 expression and bone metastasis, patients with SCLC were
divided into three groups: ‘No metastasis’ group (n=51); ‘bone
metastasis’ group (n=17) and ‘organ metastasis except bone
metastasis’ group (n=14). ANXA1 expression in patients with SCLC
with bone metastasis was significantly higher compared with the ‘no
metastasis’ and ‘organ metastasis except bone metastasis’ groups
(3.70±1.44 vs. 2.47±1.56 and 2.56±0.89, respectively; P<0.05),
but there was no significant difference between the latter two
groups (Fig. 1A). Furthermore, the
results of the ROC curve analysis revealed that ANXA1 expression
was of diagnostic value for SCLC bone metastasis (area under the
curve, 0.74; 95% CI, 0.63–0.83; P<0.05). When the cut-off value
was 2.80, the sensitivity was 76.47% and the specificity was 66.15%
(Fig. 1B).
SBC-3 and SBC-5 cell lines have similar genetic
backgrounds; however, SBC-5 cells are more prone to bone metastasis
(18). Total RNA and protein of
SBC-3 and SBC-5 cells were extracted to examine ANXA1 mRNA and
protein expression using RT-qPCR (Fig.
1C) and western blotting (Fig.
1D), respectively. As expected, the mRNA and protein expression
levels of ANXA1 in SBC-5 cells were significantly higher compared
with those in SBC-3 cells (Fig. 1C and
D).
ANXA1 overexpression enhances the
proliferation, migration and invasion of SCLC cell lines
RT-qPCR and western blotting revealed that shRNA-2
was the most efficient shRNA, and this was therefore used for
subsequent experiments (Fig. S1A and
C). ANXA1 cDNA was transfected to overexpress ANXA1 in SBC-3
cells (Fig. S1B and D). The effects
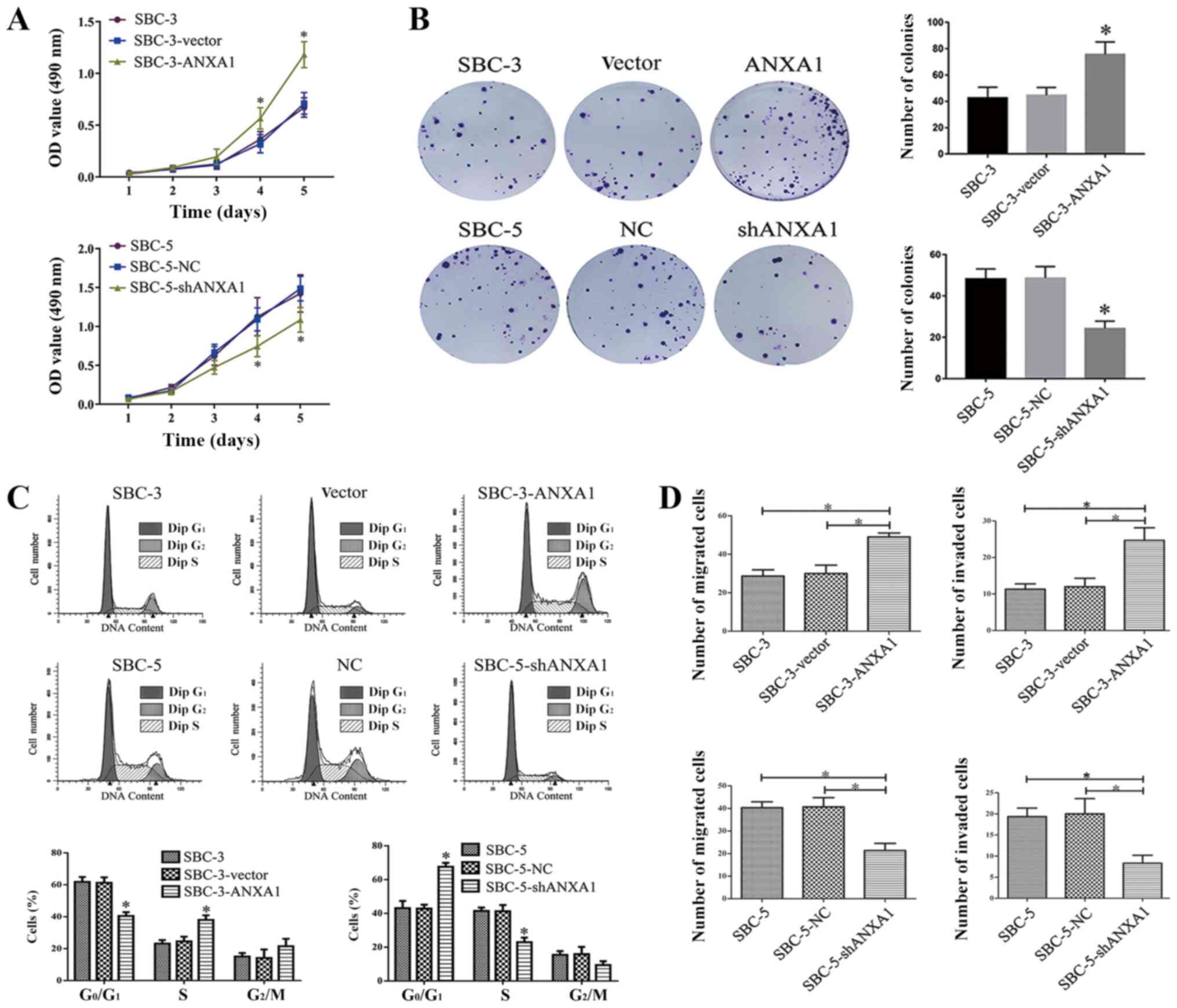
of ANXA1 on cell proliferation were detected using MTT and colony
formation assays. In the MTT assay, SBC-3-ANXA1 cells grew at a
faster rate compared with SBC-3-vector or parental SBC-3 cells
(Fig. 2A). On the other hand, the
proliferative rate of SBC-5-shANXA1 cells was lower compared with
SBC-5-NC and SBC-5 cells, with a significant difference in the cell
proliferation curve observed from day 4 (Fig. 2A). In the planar cloning experiment,
colony formation was counted at 1 week after inoculation. ANXA1
overexpression significantly increased cell colony formation
ability, while inhibition of ANXA1 expression significantly
decreased cell colony formation ability (Fig. 2B).
Cell cycle analysis was further applied to assess
the change in cell proliferation following overexpression or
inhibition of ANXA1. As shown in Fig.
2C, the percentage of cells in the G1 phase was
higher in SBC-3 and SBC-3-vector cells compared with in SBC-3-ANXA1
cells (61.83±3.06 and 61.32±3.37 vs. 40.47.29±2.44, respectively;
P<0.05), while the percentages of cells in S phase were
23.14±2.21, 24.55±2.97 and 38.04±2.88 (P<0.05), respectively.
Additionally, SBC-5-shANXA1 cells had a higher proportion of cells
in the G1 phase compared with SBC-5 and SBC-5-NC cells
(67.59±2.38 vs. 43.09±4.25 and 42.82±2.33, respectively; P<0.05)
and a lower proportion in S phase (22.95±2.76 vs. 41.42±2.05 and
41.27±3.71, respectively; P< 0.05) (Fig. 2C).
Transwell assays were performed to determine the
effects of ANXA1 on the invasion and migration of SCLC cells.
ANXA1-transfected SBC-3 cells exhibited significantly increased
migratory and invasive abilities compared with controls (Figs. 2D and S2). Consistently, functional inhibition of
ANXA1 significantly impaired the migration and invasion of SBC-5
cells (Figs. 2D and S2).
ANXA1 facilitates the adhesion of SCLC
cells to bone in vitro and bone metastases in NOD/SCID mice
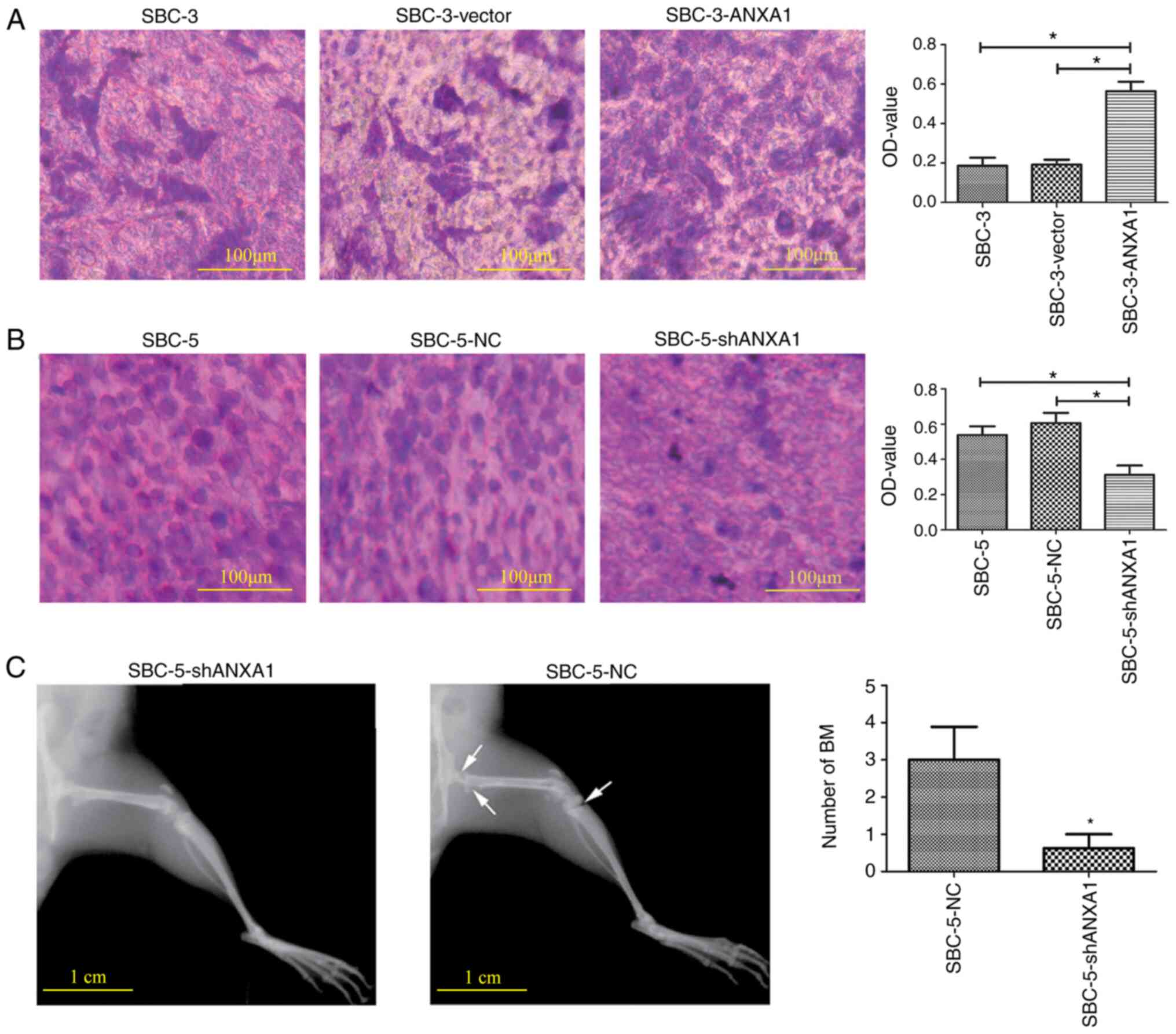
The effects of ANXA1 on the bone adhesion ability of
SCLC cells were detected via a bone adhesion model in vitro.
In the attachment assay, the number of SBC-3-ANXA1 cells that
adhered to bone was significantly higher compared with controls
(Fig. 3A). On the other hand,
functional inhibition of ANXA1 significantly impaired the adhesive
ability of SBC-5 cells compared with that of controls (Fig. 3B).
Furthermore, an in vivo xenograft bone
metastasis model in NOD/SCID mice was established. Five weeks after
SBC-5-NC and SBC-5-shANXA1 cells were injected into the tail vein,
X-ray images displayed significantly decreased lesions and damage
on bones in the ANXA1-inhibition group (Fig. 3C). The bone metastasis ability of
cells with high or low ANXA1 expression was quantified by the
number of bone metastases (3.00±2.51 vs. 0.63±1.06, respectively;
P<0.05). Lung and liver metastases were also observed in mice
(data not shown). These results indicated that inhibition of ANXA1
significantly decreased bone metastasis in animal models.
Synthesis and secretion of PTHrP in
SCLC cells is mediated by ANXA1
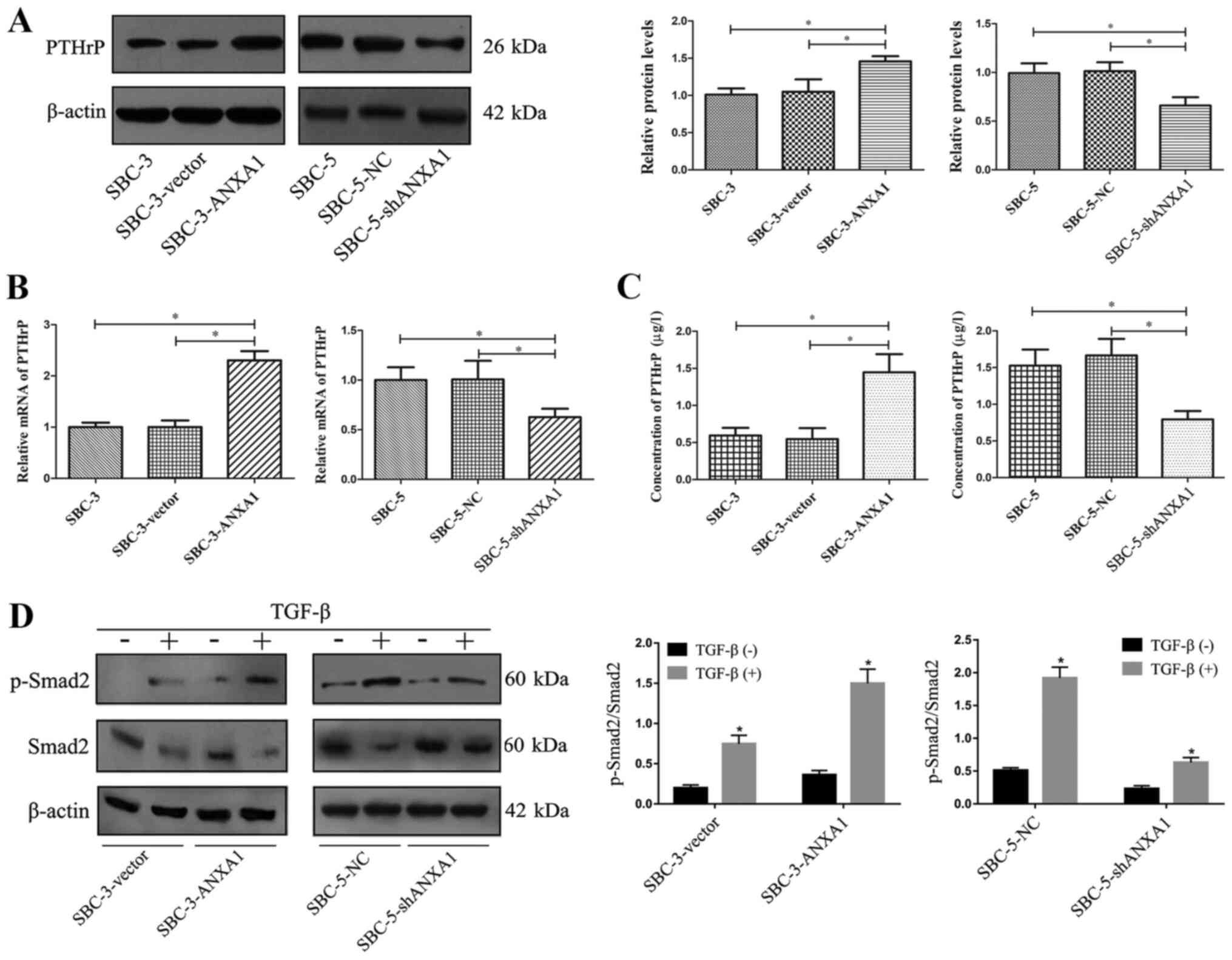
To confirm the association between ANXA1 and bone
metastasis at the mechanistic level, the effects of ANXA1 on PTHrP
expression, which serves an important role in bone metastasis of
SCLC (19,20), were investigated. To elucidate
whether modulation of ANXA1 affected PTHrP synthesis, western
blotting, RT-qPCR and ELISA were applied to quantify PTHrP levels
secreted by SCLC cells. SBC-3-ANXA1 cells secreted significantly
higher PTHrP levels compared with parental and control SBC-3 cells,
while PTHrP synthesis in SBC-5-shANXA1 cells was significantly
decreased compared with parental and control SBC-5 cells (Fig. 4A-C).
A previous study has demonstrated that TGF-β/Smad
signaling participates in regulating PTHrP secretion and serves an
important role in bone metastasis (21). Therefore, the expression levels of
Smad2 and p-Smad2 after exposure to TGF-β were detected. As shown
in Fig. 4D, TGF-β treatment
significantly increased Smad2 phosphorylation in the cells. ANXA1
transfection into SBC-3 cells enhanced the function of TGF-β to
induce Smad2 phosphorylation, while ANXA1-knockdown in SBC-5 cells
impaired Smad2 phosphorylation in response to TGF-β exposure.
Discussion
Bone metastasis hinders the treatment of SCLC, and
timely diagnosis of bone metastasis is a prerequisite to improve
treatment effects. Therefore, it is of scientific importance and
clinical value to identify biomarkers associated with bone
metastasis in SCLC.
Compared with other subtypes of lung cancer, SCLC
treatment mainly depends on radiotherapy and chemotherapy; hence,
access to tumor tissue samples is limited (22). By contrast, blood samples are easy to
obtain, and several biomarkers in the blood, such as lactate
dehydrogenase, can be effective in reflecting bone metastases
(23). Therefore, serum samples of
patients with SCLC were used in the present study to study
biomarkers for bone metastasis. By detecting the expression levels
of ANXA1 in the serum of 82 patients with SCLC, a strong
association was identified between ANXA1 expression and bone
metastasis. First, compared with patients with SCLC without
metastasis, ANXA1 expression was significantly increased in
patients with bone metastasis, while no significant increase in
ANXA1 expression was detected in patients with other organ
metastasis. Second, ROC curve analysis revealed that ANXA1 had
diagnostic significance for bone metastasis of SCLC. Additionally,
in SBC-3/SBC-5 cell lines with similar genetic background and
significantly different bone metastasis ability, ANXA1 expression
levels were also associated with bone metastasis abilities.
Previous studies have confirmed that ANXA1 is a potential tumor
biomarker with abnormal expression in a variety of tumors and is
associated with multiple clinical factors, such as metastasis and
prognosis (6,11,24).
However, the expression and function of ANXA1 are not consistent
among different tumors. For example, ANXA1 expression is increased
in lung adenocarcinoma, gastric cancer and hepatocellular
carcinoma, which is associated with a poor prognosis and decreased
disease-free survival and metastasis-free survival (25–27). By
contrast, several studies reported that ANXA1 expression is
downregulated in head and neck squamous cell carcinoma,
nasopharyngeal carcinoma and esophageal carcinoma, and is
associated with the differentiation grades (28–30). The
aforementioned studies suggest that the use of ANXA1 as a
therapeutic or prognostic marker for cancer should be carefully
evaluated depending on cancer type, grade and stage.
Next, a series of in vivo and in vitro
experiments were conducted to further confirm the role of ANXA1 in
bone metastasis. By regulating the expression levels of ANXA1 in
SCLC cell lines, the present study confirmed that ANXA1 exerted
promoting effects on the proliferation, migration and invasion of
SCLC cells. Similar results have been reported in previous studies
(9,31). For example, autophagy induced by
ANXA1 inhibition promoted nasopharyngeal carcinoma cell invasion
and metastasis (32). Bone adhesion
experiments in vitro and xenograft bone metastasis assay
in vivo further confirmed the role of ANXA1 in promoting
bone metastasis in SCLC, consistent with the present results of
serological testing on patients.
Bone tissue has a distinct structure and
composition; hence, the mechanism involved in bone metastasis is
different from that of other organs. PTHrP stimulates osteoclastic
bone resorption, resulting in bone destruction and TGF-β release,
which is stored in the bone matrix (33,34).
TGF-β binds to receptors on the surface of tumor cells and induces
the phosphorylation of Smad family members (such as the production
of p-Smad2), which then activates downstream signaling pathways and
regulates the expression of bone metastasis-associated target genes
(21,35). The expression levels of PTHrP and
p-Smad2 are closely associated with the degree of bone metastasis
(21). To confirm the association
between ANXA1 and bone metastasis at the mechanistic levels, the
effects of ANXA1 on PTHrP and p-Smad2 expression in SCLC cells were
assessed. Using western blotting, RT-qPCR and ELISA, the present
study confirmed that ANXA1-overexpressing SCLC cells secreted
higher levels of PTHrP, while PTHrP synthesis in ANXA1-knockdown
SCLC cells was significantly suppressed. In addition, following
exposure to TGF-β, ANXA1-overexpressing SCLC cells exhibited higher
levels of p-Smad2 compared with in ANXA1-knockdown SCLC cells.
The present study has some limitations. First, the
current study was a single-center study with a limited clinical
sample size. Second, due to the limitations of specimen collection,
the present study failed to assess the expression levels of ANXA1,
PTHrP and pSMAD in tumor tissues, nor the association between ANXA1
and other markers, such as PTHrP in serum. Post-mortem studies of
tissue samples may be useful to overcome these problems. Third, the
expression level of ANXA1 in patients was not continuously
monitored, and methods such as Kaplan-Meier analysis were not used
to reflect the influence of ANXA1 on the incidence of bone
metastasis. Finally, ANXA1 secretion has not been studied
thoroughly. Detection of ANXA1 secretion under different conditions
using neutralizing antibodies may help to further clarify the
function of ANXA1. In addition, the downstream signaling pathways
of ANXA1 in promoting bone metastasis, such as the specific role of
PTHrP/Smad2, lack of in-depth study. Overcoming these limitations
is important to further confirm the role of ANXA1 in bone
metastasis and should be further investigated in future
studies.
In conclusion, the present study confirmed the
strong association between ANXA1 expression and bone metastasis in
SCLC from different perspectives, suggesting that ANXA1 may be a
potential marker of bone metastasis and providing new potential for
the diagnosis and treatment of SCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81702246) and the
Key Research and Development Program in Shaanxi Province (grant no.
2019SF-069).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PC, JM and FZ conceived and designed the
experiments. PC, JM, CW, GT and FZ performed the experiments and
collected the data. HZ and HW analyzed and interpreted the data.
PC, HW and FZ drafted the initial manuscript and revised it for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The research involving human samples was approved by
the ethical review board of Tangdu Hospital (approval no.
TDLL-2017024) and was performed according to the Declaration of
Helsinki. All participants signed informed consent. All animal
experimental procedures were approved by the Animal Ethics
Committee of the Air Force Military Medical University and were in
accordance with the ‘Animal Research: Reporting In Vivo
Experiments’ guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang S, Zimmermann S, Parikh K, Mansfield
AS and Adjei AA: Current diagnosis and management of small-cell
lung cancer. Mayo Clin Proc. 94:1599–1622. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conen K, Hagmann R, Hess V, Zippelius A
and Rothschild SI: Incidence and predictors of bone metastases (BM)
and skeletal-related events (SREs) in small cell lung cancer
(SCLC): A swiss patient cohort. J Cancer. 7:2110–2116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti GV, Hirsh V, Siena S, Henry DH,
Woll PJ, Manegold C, Solal-Celigny P, Rodriguez G, Krzakowski M,
Mehta ND, et al: Overall survival improvement in patients with lung
cancer and bone metastases treated with denosumab versus zoledronic
acid: Subgroup analysis from a randomized phase 3 study. J Thorac
Oncol. 7:1823–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L and Gong Z: Clinical
characteristics and prognostic factors in bone metastases from lung
cancer. Med Sci Monit. 23:4087–4094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugimoto MA, Ribeiro ALC, Costa BRC, Vago
JP, Lima KM, Carneiro FS, Ortiz MMO, Lima GLN, Carmo AAF, Rocha RM,
et al: Plasmin and Plasminogen induce macrophage reprogramming and
regulate key steps of inflammation resolution via annexin A1.
Blood. 129:2896–2907. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foo SL, Yap G, Cui J and Lim LHK:
Annexin-A1-A blessing or a curse in cancer? Trends Mol Med.
25:315–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo C, Liu S and Sun MZ: Potential role of
Anxa1 in cancer. Future Oncol. 9:1773–1793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mussunoor S and Murray GI: The role of
annexins in tumour development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boudhraa Z, Bouchon B, Viallard C, D'Incan
M and Degoul F: Annexin A1 localization and its relevance to
cancer. Clin Sci (Lond). 130:205–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Damazo AS, Moradi-Bidhendi N, Oliani SM
and Flower RJ: Role of annexin 1 gene expression in mouse
craniofacial bone development. Birth Defects Res A Clin Mol
Teratol. 79:524–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan X, Liu P and Yin G: Downregulation of
annexin A1 by short hairpin RNA inhibits the osteogenic
differentiation of rat bone marrow-derived mesenchymal stem cells.
Int J Mol Med. 36:406–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma N, Shen W, Pang H, Zhang N, Shi H, Wang
J and Zhang H: The effect of RCAN1 on the biological behaviors of
small cell lung cancer. Tumour Biol. 39:10104283177004052017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG; NC3Rs Reporting Guidelines Working Group, : Animal
research: Reporting in vivo experiments: The ARRIVE guidelines. Br
J Pharmacol. 160:1577–1579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miki T, Yano S, Hanibuchi M and Sone S:
Bone metastasis model with multiorgan dissemination of human
small-cell lung cancer (SBC-5) cells in natural killer
cell-depleted SCID mice. Oncol Res. 12:209–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mak IW, Turcotte RE and Ghert M:
Parathyroid hormone-related protein (PTHrP) modulates adhesion,
migration and invasion in bone tumor cells. Bone. 55:198–207. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frieling JS, Shay G, Izumi V, Aherne ST,
Saul RG, Budzevich M, Koomen J and Lynch CC: Matrix
metalloproteinase processing of PTHrP yields a selective regulator
of osteogenesis, PTHrP1-17. Oncogene. 36:4498–4507.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kakonen SM, Selander KS, Chirgwin JM, Yin
JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y and Guise TA:
Transforming growth factor-beta stimulates parathyroid
hormone-related protein and osteolytic metastases via Smad and
mitogen-activated protein kinase signaling pathways. J Biol Chem.
277:24571–24578. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao X, Kallakury B, Chahine JJ, Hartmann
D, Zhang Y, Chen Y, Zhang H, Zhang B, Wang C and Giaccone G:
Surgical resection of SCLC: Prognostic factors and the tumor
microenvironment. J Thorac Oncol. 14:914–923. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katakami N, Kunikane H, Takeda K, Takayama
K, Sawa T, Saito H, Harada M, Yokota S, Ando K, Saito Y, et al:
Prospective study on the incidence of bone metastasis (BM) and
skeletal-related events (SREs) in patients (pts) with stage IIIB
and IV lung cancer-CSP-HOR 13. J Thorac Oncol. 9:231–238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sobral-Leite M, Wesseling J, Smit VT,
Nevanlinna H, van Miltenburg MH, Sanders J, Hofland I, Blows FM,
Coulson P, Patrycja G, et al: Annexin A1 expression in a pooled
breast cancer series: Association with tumor subtypes and
prognosis. BMC Med. 13:1562015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biaoxue R, Xiling J, Shuanying Y, Wei Z,
Xiguang C, Jinsui W and Min Z: Upregulation of Hsp90-beta and
annexin A1 correlates with poor survival and lymphatic metastasis
in lung cancer patients. J Exp Clin Cancer Res. 31:702012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin Y, Lin G, Fang W, Zhu H and Chu K:
Increased expression of annexin A1 predicts poor prognosis in human
hepatocellular carcinoma and enhances cell malignant phenotype. Med
Oncol. 31:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato Y, Kumamoto K, Saito K, Okayama H,
Hayase S, Kofunato Y, Miyamoto K, Nakamura I, Ohki S, Koyama Y and
Takenoshita S: Up-regulated annexin A1 expression in
gastrointestinal cancer is associated with cancer invasion and
lymph node metastasis. Exp Ther Med. 2:239–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garcia Pedrero JM, Fernandez MP, Morgan
RO, Herrero Zapatero A, Gonzalez MV, Suarez Nieto C and Rodrigo JP:
Annexin A1 down-regulation in head and neck cancer is associated
with epithelial differentiation status. Am J Pathol. 164:73–79.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodrigo JP, Garcia-Pedrero JM, Fernandez
MP, Morgan RO, Suarez C and Herrero A: Annexin A1 expression in
nasopharyngeal carcinoma correlates with squamous differentiation.
Am J Rhinol. 19:483–487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia SH, Hu LP, Hu H, Ying WT, Xu X, Cai Y,
Han YL, Chen BS, Wei F, Qian XH, et al: Three isoforms of annexin I
are preferentially expressed in normal esophageal epithelia but
down-regulated in esophageal squamous cell carcinomas. Oncogene.
21:6641–6648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bizzarro V, Belvedere R, Migliaro V,
Romano E, Parente L and Petrella A: Hypoxia regulates ANXA1
expression to support prostate cancer cell invasion and
aggressiveness. Cell Adh Migr. 11:247–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu JF, Huang W, Yi HM, Xiao T, Li JY,
Feng J, Yi H, Lu SS, Li XH, Lu RH, et al: Annexin A1-suppressed
autophagy promotes nasopharyngeal carcinoma cell invasion and
metastasis by PI3K/AKT signaling activation. Cell Death Dis.
9:11542018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guise TA, Mohammad KS, Clines G, Stebbins
EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L
and Chirgwin JM: Basic mechanisms responsible for osteolytic and
osteoblastic bone metastases. Clin Cancer Res. 12:6213s–6216s.
2016. View Article : Google Scholar
|
|
34
|
Roodman GD: Biology of osteoclast
activation in cancer. J Clin Oncol. 19:3562–3571. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kunihiro AG, Brickey JA, Frye JB, Luis PB,
Schneider C and Funk JL: Curcumin, but not curcumin-glucuronide,
inhibits Smad signaling in TGFβ-dependent bone metastatic breast
cancer cells and is enriched in bone compared to other tissues. J
Nutr Biochem. 63:150–156. 2019. View Article : Google Scholar : PubMed/NCBI
|