Hepatocellular carcinoma (HCC) accounts for 85–90%
of primary liver cancer cases. HCC is an aggressive cancer, which
has a marked clinical and epidemiological impact, with 600,000
mortalities and ~1,000,000 new cases of HCC being reported
annually, worldwide (1,2). Following a diagnosis of HCC, patients
survive <1 year. In addition, the mortality rates are high, with
an overall survival rate of <12% (3). HCC arises from the aggregation of
normal cells following accumulation of several genetic changes that
activates oncogenes and deactivates tumor suppressor genes, nuclear
factors, growth factors and cytokines (4). The functions of the liver, as well as
the liver reserve, are altered and damaged during the course of
disease. Despite advancements in the treatment strategies of HCC,
the prognosis of patients remains poor due to metastasis and
development of drug resistance. Currently, hepatic resection or
transplantation are considered the only curative therapies
(5). Even with surgery, ~30% of
patients with HCC undergo hepatectomy as they receive a diagnosis
at an advanced tumor stage. Furthermore, radiofrequency ablation is
used to treat patients with early-stage HCC. Several factors lead
to the poor prognosis of patients with HCC, including history of
cirrhosis, poverty and limited medical resources. The rapid onset
and fast-growing characteristics of HCC results in limited
treatment options for patients. In addition, HCC is characterized
by a high angioinvasive capacity due to portal vein obstruction
(6). Thus, there is an urgent
requirement to identify and develop novel therapeutic drugs for the
treatment of HCC.
HSPGs are highly anionic carbohydrate compounds.
HSPGs are composed of a limited quantity of a core protein that is
covalently linked to ≥1 sugar chain, representing HS side chains.
These chains are considered linear polysaccharides, which are built
of ≤200 units of a repeated disaccharide formed by N-acetyl
glucosamine with uronic acid, glucuronic or iduronic acids
(7).
The structure of HSPGs is modified in the Golgi
apparatus following the addition of sulfate groups instead of
acetyl groups, or by sulfation of the hydroxyl groups at C-6 and
C-3 in the N-acetylglucosamine moiety, or by sulfation of the
hydroxyl group at C-2 in the uronic acid moiety (8). There are several families of cell
surface HSPGs, such as syndecan and glypican. The presence of
several carboxyl and sulfate groups in HS is similar to the
polyanionic nature of mammalian cells compared with the neighboring
cells and extracellular matrix (ECM) (9). Thus, cell surface HSPGs produce
multiple structural and signaling functions due to their capability
to interact with several protein ligands, including growth factors
and their receptors, proteases, cytokines, chemokines, adhesion
molecules and ECM proteins, including fibronectin, collagen and
fibrin (10).
HSPGs are one of the most important elements of the
ECM, and are located on the surface of the cell membrane of most
animal cells, such as hepatocytes and leukocytes (11). HSPGs are involved in several
interactions between adjacent cells or between cells and the ECM.
HSPGs regulate several signaling pathways and receptor trafficking,
and control ligand secretion. The variability of HS generated by
its modifying enzymes led to the hypothesis of ‘sugar code’, which
is characterized by specific HS alterations observed in the embryo
to orchestrate development through modification of certain
signaling pathways. It depends on the regulation of special areas
of HS-modifying enzymes to regulate their activity or even change
their functions. The sugar code is considered a dynamic process as
HS chains can be hydrolyzed by heparanase or sulfatase enzymes
(12). HSPGs participate in an
extensive range of biological processes, such as development
(13), homoeostasis control
(11) and enhancement of
inflammatory and malignant diseases (14). In addition, HSPGs control cell
adhesion, motility, proliferation, differentiation and apoptosis
(8).
HSPGs act as anchors for the lipoprotein lipase
located on the outer surface of capillary endothelial cells. They
protect against invasion of tumor cells by preventing both cell
infiltration and intercellular adhesion (15). The activities of HSPG-degrading
enzymes are elevated in highly invasive cancer cells compared with
less invasive cells (16). When the
basal membrane is ruptured by hematogenous metastatic cancer cells,
HSPGs located inside the tumor microenvironment are attacked by
several enzymes, such as heparanase, matrix metalloproteinase-9,
sulfatase-2, which are capable of modifying the proteoglycan
structure, which alters transportation of inflammatory cells from
vessels into the surrounding tissues (17). Consequently, cytokines, proteases,
growth factors and angiogenic factors, which bind to HSPGs, are
released and promote the infiltration and metastasis of cancer
cells (15).
MMPs constitute a family of transmembrane
zinc-dependent endopeptidases that have the ability to digest the
ECM and basement membrane. The MMP family consists of 25 members in
vertebrates and 22 in humans (18).
Previously, MMP-9 was called type IV collagenase or gelatinase B.
MMP-9 is capable of degrading type IV collagen, a major constituent
of the basement membrane (19,20). The
active zone of MMP-9 consists of two zinc ions and five calcium
ions. The proteolytic activity of MMP-9 is maintained by the two
zinc ions and cysteine switch motif of the pro-domain (21). MMP-9 also contains a fibronectin-like
domain, which is strongly O-glycosylated and is important for
binding to collagen or gelatin (18).
MMPs play an important role in proliferation,
invasion and metastasis of tumor cells (22). Deryugina and Quigley (23) demonstrated an association between ECM
degradation by MMPs and the invasion of cancer cells. MMP-9
releases fibroblast growth factor (FGF)-1 and FGF-2 from their
stores, producing potent angiogenic effects (24). In addition, MMP-9 attacks HSPGs
inside the tumor microenvironment to enhance the proteolytic
release of syndecan-1 and to potentiate tumor growth and metastasis
(17,25). Thus, tumors that express MMP-9 at
high levels are more likely to exhibit relapse or metastasis
compared with tumors that express low levels of MMP-9.
Previous studies have demonstrated the role of
certain MMP-9 inhibitors in the treatment of HCC both in
vivo and in vitro (Table
I). Although, several synthetic MMP inhibitors have been
developed, none of them have reached phase III clinical trials due
to either lack of efficacy or serious side effects.
Heparanase is an endo-β-glucuronidase that belongs
to the glycoside hydrolase 79 family. Heparanase hydrolyses HS at
specific intrachain positions with low sulfation and participates
in the degradation and remodeling of the ECM (26).
Heparanase is upregulated in several types of human
tumor, such as HCC, myeloma and breast cancer, and it strongly
enhances the invasiveness of tumors in experimental animals
(27). Heparanase releases HS
fragments associated with angiogenic factors from the tumor
microenvironment to produce an angiogenic response. In addition,
heparanase facilitates vascularization, accelerates primary tumor
growth and provides a gate for invading metastatic cells, thus
leading to cancer progression (28).
Heparanase inhibitors notably decrease the incidence
of metastasis in experimental animals (29). Suramin was subsequently assessed in
rats with HCC, where it was demonstrated to elevate the percentage
of survival rate of rats with HCC, and decrease the level of serum
α-fetoprotein. Furthermore, suramin has been demonstrated to
ameliorate fibrosis, thus producing an hepatoprotective effect
(15). Table II summarizes several studies that
assessed heparanase inhibitors in the treatment of HCC.
Sulfatase-2 is an extracellular enzyme that enhances
the removal of 6-O-sulfate from HS disaccharides, and controls the
interactions between HSPGs and extracellular factors. Sulfatase-1
and −2 are expressed in malignant tumors, including highly invasive
brain cancer (30). Sulfatase-1 acts
as a tumor suppressor gene, which downregulates the phosphorylation
and activation of tyrosine kinase receptors (31). Conversely, sulfatase-2 decreases the
affinity of HSPGs for several signaling molecules, such as
glypican-3 and syndean-1, detaching them from HSPGs and preparing
the transition of different signaling pathways, particularly the
insulin-like growth factor (IGF) pathway (32).
Upregulation of sulfatase-2 is considered oncogenic,
and is associated with HCC in human, animal and tissue culture
models (33,34). Sulfatase-2 enhances the expression of
growth factors available to cell surface receptors, thus promoting
the proliferation and migration of tumor cells. In addition, it
enhances the activity of glypican-3, activates FGF signaling,
potentiates the phosphorylation of both Erk and Akt, and induces
Wnt/β-catenin signaling (35).
Some studies have focused on the use of sulfatase-2
inhibitors for treating HCC. Adiponectin, a suppressor of the
synthesis of sulfatase-2 protein, has been reported to exhibit
antitumor activity both in vivo and in vitro
(35). In addition, OKN-007, an
inhibitor of sulfatase-2, significantly decreases solid tumor
growth (36). Table III summarizes the results of
previous studies that used sulfatase-2 inhibitors for the treatment
of HCC.
Syndecan-1 is a transmembrane HSPG that is located
on epithelial cells. The syndecan family consists of four members,
syndecan-1, syndecan-2, syndecan-3 and syndecan-4. Among these four
members, syndecan-1 has been extensively studied. Its name is
derived from the Latin syndein, which means binding
together, since syndecans are involved in the binding of cells to
the ECM (37). Syndecans are
composed of three domains forming highly conserved intracellular
and transmembrane domains, as well as an extracellular domain,
which is uniquely characteristic to each member (38).
Syndecan-1 controls cell-cell and cell-ECM adhesion
interactions, as well as their activities through its HS chains. It
modulates certain proteolytic enzymes and chemokines in
vivo, and controls the recruitment of leukocytes and the
remodeling of tissues during inflammation (39). In addition, syndecan-1 modulates
proteolytic v, thus leading to the regulation of leucocyte
recruitment with subsequent remodeling of tissues (40).
The release of syndecan-1 from its membrane-bound
form by MMP-9 (syndecan-1 sheddase) to the soluble molecule inside
the circulation represents the transition of the tumor from a
proliferative stage to an invasive stage (25). Syndecan-1 binds to both the ECM and
FGF family. Overexpression of the MMP-9/syndecan-1/FGF-2 axis
potentiates the apoptosis pathway in several tumor models (41,42).
A previous study demonstrated the role of inhibiting
syndecan-1 by synstatin, which exhibits promising antitumor
activity against rats with HCC (43).
Glypican-3 is the most commonly studied member of
the glypican family of glycosyl-phosphatidylinositol-(GPI)
cell-surface HSPGs (44). It
consists of six medium-sized HSPGs that are attached to the cell
surface via a GPI anchor, with an insertion of 2–4 HS chains.
Glypican-3 regulates Wnt, Hedgehog and FGF signaling (38).
Glypican-3 is considered an attractive therapeutic
target in HCC. Antibodies against glypican-3 exhibit strong
antitumor activities in several models of HCC (33,34).
Recently, several mouse monoclonal antibodies targeting glypican-3
have been produced (48). One of
these antibodies is the humanized GC33 (hGC33), which has been
assessed in a phase I clinical trial. hGC33 acts against the
carboxyl-terminal region of glypican-3 and is effective in HepG2
×enografts (49). In addition,
another human heavy chain variable domain antibody, NH3,
inhibits the proliferation of glypican-3-positive cells and blocks
HCC xenograft growth in nude mice by modulating the TGF-β/SMAD
pathway (50). Zaghloul et al
(34) demonstrated that treatment of
rats with HCC with monoclonal anti-glypican-3 increased survival
rate up to 90% and decreased the level of serum AFP. In addition,
anti-glypican-3 was demonstrated to affect the sulafatase-2/IFG-II
pathway. Glypican-3 has also been reported to act as a predictive
marker of HCC recurrence following radial surgery (51). Table
IV represents a summary of studies that have assessed the role
of glypican-3 inhibitors in treating HCC.
Fascin is an actin-binding protein that controls
cell movement under physiological or pathological conditions
(52). It regulates cell motility
and is considered one of the cytoskeleton-regulatory proteins
(53).
Fascin expression has been associated with tumor
invasion and metastasis, and its expression is low in normal
tissues (52). Overexpression of
fascin elevates cell membrane processes, such as broken
intercellular junctions, and enforces cell movement associated with
changes to the cytoskeleton and ECM, thus facilitating tumor
metastasis (54). It has been
reported that upregulation of fascin in several tumors, including
HCC, is associated with tumor invasion and metastasis (55). In addition, fascin is unable to
control cell migration alone, unless it is supported by other
factors, such as MMP-9. This can be explained by the fact that the
ability of fascin to act as a migration factor is only associated
with epithelial-to-mesenchymal transition or MMP-9, which
facilitate their invasiveness (56).
Glucosaminoglycans are linear polysaccharides
composed of repeat units, with areas of glucuronic acid and
N-acetyl glucosamine. They contain regions of 2-O-sulfated iduronic
acid and N-sulfoglucosamine. Between these regions, there are
transition zones with both sulfo-glucosamine and
acetyl-glucosamine, which are associated with polypeptide
core-forming HSPGs (57).
Glucosamine is an amino saccharide that is present
in almost all tissues, and abundant in liver, kidney and cartilage
(58). It is the predominant
building unit in the synthesis of glycolipids, glycoproteins,
glycosaminoglycans and proteoglycans (59). Glucosamine induces autocrine TGF-β
activity (60) and helps in the
O-linked glycosylation of proteins. As an alteration of the
structure of proteins with O-linked N-acetylglucosamine,
glucosamine has evolved as an important regulator of cellular
physiology. This alteration is associated with several diseases,
such as cancer, neurodegenerative disorders and cardiovascular
diseases (61). Notable elevation in
the serum levels of glucosamine has been observed in patients and
animals with HCC (15,62,63).
Glucuronic acid is synthesized from UDP-glucose
inside the liver via UDP-glucosedehydrogenase. It participates in
several detoxification pathways, such as xenobiotic and bilirubin
(64). Elevated levels of hyaluronic
acid in liver diseases are the main cause for increased levels of
serum glucosamine and glucuronic acid (65). Degradation of hyaluronic acid, which
is initiated by its binding to CD44, notably enhances the
activation of cell migration molecules, thus leading to tumor
motility (66).
Sialic acid is part of the plasma membrane of
mammalian cells. It binds to N-acetyl galactosamine via an
O-glycosidic linkage, which is associated with the proteins that
form glycoproteins (58). Sialic
acid is a major player in several physiological and pathological
processes, such as progression and spread of multiple malignancies,
such as neuroblastoma, oral cancer and breast cancer (67). A variation in the sialic acid levels
in patients with cirrhosis and HCC is an important diagnostic tool.
Elevated sialic acid levels in HCC may be explained by endothelial
cell dysfunction or macrovascular disease (68).
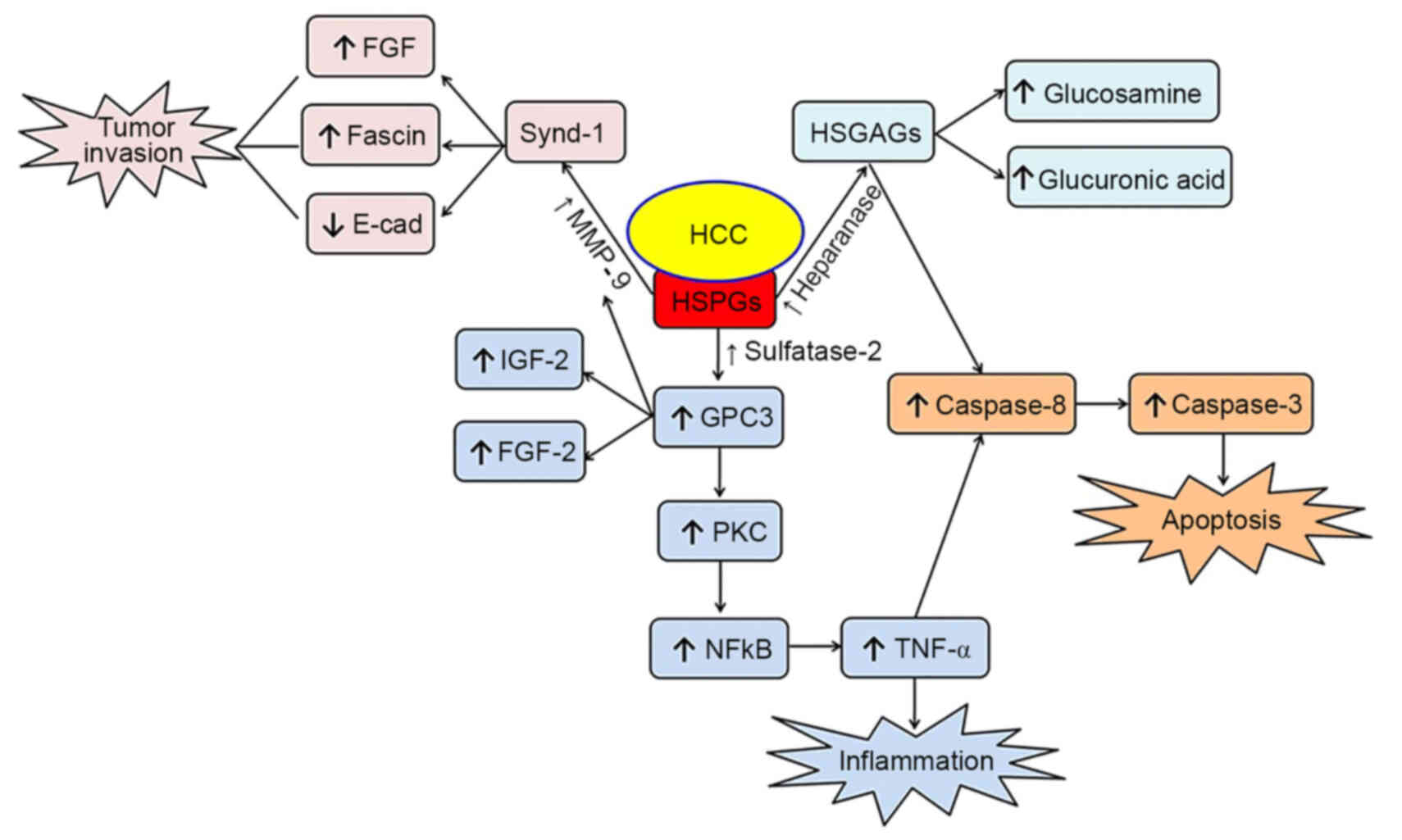
HCC triggers metabolic and dynamic modifications
that lead to the activation of certain enzymes, such as MMP-9,
sulfatase-2 and heparanase, resulting in the degradation of HSPGs.
Increasing evidence suggests that some of the HSPG degradation
products, such as syndecan-1 and glypican-3, are associated with
the activation, migration and apoptosis of tumor cells (Fig. 1). Thus, an improved understanding of
the role of HSPGs and their degradation products will aid the
identification of novel effective therapeutic targets and
strategies for preventing and treating HCC.
Cancer treatment has shifted from single target
treatment to multiple target therapies. HSPGs represent a goal for
a new trend in multiple target therapies, since they comprise
several enzymes and important compounds located in the tumor
microenvironment that control multiple biological and pathological
processes. Prospective studies will focus on the specific
post-translational modifications of these compounds in the HSPG
pathway, along with further assessment of the inhibitors and
modulators of cell signaling.
Not applicable.
Not applicable.
All data and materials are included in the present
review.
MAA and NNA contributed to the study design. MMA and
MAAG acquired the data. MMHAG contributed to the study concept and
design. All authors helped draft the initial manuscript, and read
and approved the final version.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Balkan E, Bilici M, Gundogdu B, Aksungur
N, Kara A, Yasar E, Dogan H and Ozturk G: ERCC2 Lys751Gln rs13181
and XRCC2 Arg188His rs3218536 gene polymorphisms contribute to
subsceptibility of colon, gastric, HCC, lung and prostate cancer. J
BUON. 25:574–581. 2020.PubMed/NCBI
|
|
2
|
Lv Y, Xu A, Wang N, Mu K, Wang Z, Zhao L,
Huang Y, Peng L, Xiang K, Hu D and Qi J: Retrospective study of
TACE in the treatment of lobaplatin-induced thrombocytopenia in
primary hepatocellular carcinoma. J BUON. 24:2385–2393.
2019.PubMed/NCBI
|
|
3
|
Nazmy EA, El-Khouly OA, Zaki MMA, MM A,
Elsherbiny NM, Said E, Al-Gayyar MMH and Salem HA: Targeting
p53/TRAIL/caspase-8 signaling by adiponectin reverses
thioacetamide-induced hepatocellular carcinoma in rats. Environ
Toxicol Pharmacol. 72:1032402019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia Y, Zhang J and Ni X: Diagnsosis,
treatment and prognosis of hepatocellualr carcinoma with inferior
vena cava/right atrium tumor thrombus. Oncol Lett. 20:1012020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng Z, Yang B and Liao ZY: Current
progress and prospect of immune checkpoint inhipitros in
hepatocellualr carcinoma. Oncol Lett. 2:452020.
|
|
7
|
Iozzo RV: Heparan sulfate proteoglycans:
Intricate molecules with intriguing functions. J Clin Invest.
108:165–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar AV, Katakam SK, Urbanowitz AK and
Gotte M: Heparan sulphate as a regulator of leukocyte recruitment
in inflammation. Curr Protein Pept Sci. 16:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Christianson HC and Belting M: Heparan
sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix
Biol. 35:51–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosen SD and Lemjabbar-Alaoui H: Sulf-2:
An extracellular modulator of cell signaling and a cancer target
candidate. Expert Opin Ther Targets. 14:935–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bishop JR, Schuksz M and Esko JD: Heparan
sulphate proteoglycans fine-tune mammalian physiology. Nature.
446:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poulain FE and Yost HJ: Heparan sulfate
proteoglycans: A sugar code for vertebrate development?
Development. 142:3456–3467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Häcker U, Nybakken K and Perrimon N:
Heparan sulphate proteoglycans: The sweet side of development. Nat
Rev Mol Cell Biol. 6:530–541. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lindahl U and Kjellén L: Pathophysiology
of heparan sulphate: Many diseases, few drugs. J Intern Med.
273:555–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tayel A, Abd El Galil KH, Ebrahim MA,
Ibrahim AS, El-Gayar AM and Al-Gayyar MM: Suramin inhibits hepatic
tissue damage in hepatocellular carcinoma through deactivation of
heparanase enzyme. Eur J Pharmacol. 728:151–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toyoshima M and Nakajima M: Human
heparanase. Purification, characterization, cloning, and
expression. J Biol Chem. 274:24153–24160. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong S and Wu XZ: Heparanase and
hepatocellular carcinoma: Promoter or inhibitor? World J
Gastroenterol. 16:306–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yabluchanskiy A, Ma Y, Iyer RP, Hall ME
and Lindsey ML: Matrix metalloproteinase-9: Many shades of function
in cardiovascular disease. Physiology (Bethesda). 28:391–403.
2013.PubMed/NCBI
|
|
19
|
Powell WC and Matrisian LM: Complex roles
of matrix metalloproteinases in tumor progression. Curr Top
Microbiol Immunol. 213:1–21. 1996.PubMed/NCBI
|
|
20
|
Gonçalves JL, Roma EH, Gomes-Santos AC,
Aguilar EC, Cisalpino D, Fernandes LR, Vieira AT, Oliveira DR,
Cardoso VN, Teixeira MM and Alvarez-Leite JI: Pro-inflammatory
effects of the mushroom Agaricus blazei and its consequences on
atherosclerosis development. Eur J Nutr. 51:927–937. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rowsell S, Hawtin P, Minshull CA, Jepson
H, Brockbank SM, Barratt DG, Slater AM, McPheat WL, Waterson D,
Henney AM and Pauptit RA: Crystal structure of human MMP9 in
complex with a reverse hydroxamate inhibitor. J Mol Biol.
319:173–181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tassi E, McDonnell K, Gibby KA, Tilan JU,
Kim SE, Kodack DP, Schmidt MO, Sharif GM, Wilcox CS, Welch WJ, et
al: Impact of fibroblast growth factor-binding protein-1 expression
on angiogenesis and wound healing. Am J Pathol. 179:2220–2232.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lambaerts K, Wilcox-Adelman SA and
Zimmermann P: The signaling mechanisms of syndecan heparan sulfate
proteoglycans. Curr Opin Cell Biol. 21:662–669. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vlodavsky I, Ilan N, Naggi A and Casu B:
Heparanase: Structure, biological functions, and inhibition by
heparin-derived mimetics of heparan sulfate. Curr Pharm Des.
13:2057–2073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKenzie EA: Heparanase: A target for drug
discovery in cancer and inflammation. Br J Pharmacol. 151:1–14.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ilan N, Elkin M and Vlodavsky I:
Regulation, function and clinical significance of heparanase in
cancer metastasis and angiogenesis. Int J Biochem Cell Biol.
38:2018–2039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferro V, Hammond E and Fairweather JK: The
development of inhibitors of heparanase, a key enzyme involved in
tumour metastasis, angiogenesis and inflammation. Mini Rev Med
Chem. 4:693–702. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morimoto-Tomita M, Uchimura K, Werb Z,
Hemmerich S and Rosen SD: Cloning and characterization of two
extracellular heparin-degrading endosulfatases in mice and humans.
J Biol Chem. 277:49175–49185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang XP, Liu L, Wang P and Ma SL: Human
sulfatase-1 improves the effectiveness of cytosine deaminase
suicide gene therapy with 5-fluorocytosine treatment on
hepatocellular carcinoma cell line HepG2 in vitro and in vivo. Chin
Med J (Engl). 128:1384–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bret C, Moreaux J, Schved JF, Hose D and
Klein B: SULFs in human neoplasia: Implication as progression and
prognosis factors. J Transl Med. 9:722011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zaghloul RA, Al-Gayyar MM, El-Shishtawy MM
and Ebrahim MA: Cytotoxic effects of antiglypican-3 against HepG2
cell lines. J App Pharm Sci. 3:31–35. 2013.
|
|
34
|
Zaghloul RA, El-Shishtawy MM, El Galil KH,
Ebrahim MA, Metwaly AA and Al-Gayyar MM: Evaluation of
antiglypican-3 therapy as a promising target for amelioration of
hepatic tissue damage in hepatocellular carcinoma. Eur J Pharmacol.
746:353–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Gayyar MM, Abbas A and Hamdan AM:
Chemopreventive and hepatoprotective roles of adiponectin (SULF2
inhibitor) in hepatocelluar carcinoma. Biol Chem. 397:257–267.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng X, Gai X, Han S, Moser CD, Hu C,
Shire AM, Floyd RA and Roberts LR: The human sulfatase 2 inhibitor
2,4-disulfonylphenyl-tert-butylnitrone (OKN-007) has an antitumor
effect in hepatocellular carcinoma mediated via suppression of
TGFB1/SMAD2 and Hedgehog/GLI1 signaling. Genes Chromosomes Cancer.
52:225–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stepp MA, Pal-Ghosh S, Tadvalkar G and
Pajoohesh-Ganji A: Syndecan-1 and its expanding list of contacts.
Adv Wound Care (New Rochelle). 4:235–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baghy K, Tátrai P, Regős E and Kovalszky
I: Proteoglycans in liver cancer. World J Gastroenterol.
22:379–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Götte M, Kersting C, Radke I, Kiesel L and
Wülfing P: An expression signature of syndecan-1 (CD138),
E-cadherin and c-met is associated with factors of angiogenesis and
lymphangiogenesis in ductal breast carcinoma in situ. Breast Cancer
Res. 9:R82007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Metwaly HA, Al-Gayyar MM, Eletreby S,
Ebrahim MA and El-Shishtawy MM: Relevance of serum levels of
interleukin-6 and syndecan-1 in patients with hepatocellular
carcinoma. Sci Pharm. 80:179–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schulze D, Plohmann P, Höbel S and Aigner
A: Anti-tumor effects of fibroblast growth factor-binding protein
(FGF-BP) knockdown in colon carcinoma. Mol Cancer. 10:1442011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao B, Li S, Tan Z, Ma L and Liu J: ACTG1
and TLR3 are biomarkers for alcohol-associated hepatocellular
carcinoma. Oncol Lett. 17:1714–1722. 2019.PubMed/NCBI
|
|
43
|
Metwaly HA, El-Gayar AM and El-Shishtawy
MM: Inhibition of the signaling pathway of syndecan-1 by synstatin:
A promising anti-integrin inhibitor of angiogenesis and
proliferation in HCC in rats. Arch Biochem Biophys. 652:50–58.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aggio A, Grassi D, Onori E, D'Alessandro
A, Masedu F, Valenti M and Ferri C: Endothelium/nitric oxide
mechanism mediates vasorelaxation and counteracts vasoconstriction
induced by low concentration of flavanols. Eur J Nutr. 52:263–272.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jeon Y, Jang ES, Choi YS, Kim JW and Jeong
SH: Glypican-3 level assessed by the enzyme-linked immunosorbent
assay is inferior to alpha-fetoprotein level for hepatocellular
carcinoma diagnosis. Clin Mol Hepatol. 22:359–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stigliano I, Puricelli L, Filmus J,
Sogayar MC, Bal de Kier Joffé E and Peters MG: Glypican-3 regulates
migration, adhesion and actin cytoskeleton organization in mammary
tumor cells through Wnt signaling modulation. Breast Cancer Res
Treat. 114:251–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng W, Tseng CJ, Lin TT, Cheng I, Pan
HW, Hsu HC and Lee YM: Glypican-3-mediated oncogenesis involves the
Insulin-like growth factor-signaling pathway. Carcinogenesis.
29:1319–1326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ho M: Advances in liver cancer antibody
therapies: A focus on glypican-3 and mesothelin. BioDrugs.
25:275–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakano K, Ishiguro T, Konishi H, Tanaka M,
Sugimoto M, Sugo I, Igawa T, Tsunoda H, Kinoshita Y, Habu K, et al:
Generation of a humanized anti-glypican 3 antibody by CDR grafting
and stability optimization. Anticancer Drugs. 21:907–916. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun CK, Chua MS, He J and So SK:
Suppression of glypican 3 inhibits growth of hepatocellular
carcinoma cells through up-regulation of TGF-β2. Neoplasia.
13:735–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miura M, Fujinami N, Shimizu Y, Mizuno S,
Saito K, Suzuki T, Konishi M, Takahashi S, Gotohda N, Suto K, et
al: Usefulness of plasma full-length glypican-3 as a predictive
marker of hepatocellular carcinoma recurrence after radial surgery.
Oncol Lett. 19:2657–2666. 2020.PubMed/NCBI
|
|
52
|
Elewa MA, Al-Gayyar MM, Schaalan MF, Abd
El Galil KH, Ebrahim MA and El-Shishtawy MM: Hepatoprotective and
anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma
and its relation to vascular invasion markers. Clin Exp Metastasis.
32:479–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jayo A and Parsons M: Fascin: A key
regulator of cytoskeletal dynamics. Int J Biochem Cell Biol.
42:1614–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang X, Ji J, Xue H, Zhang F, Han X, Cai
Y, Zhang J and Ji G: Fascin and cortactin expression is correlated
with a poor prognosis in hepatocellular carcinoma. Eur J
Gastroenterol Hepatol. 24:633–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oh SY, Kim YB, Suh KW, Paek OJ and Moon
HY: Prognostic impact of fascin-1 expression is more significant in
advanced colorectal cancer. J Surg Res. 172:102–108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lai JP, Thompson JR, Sandhu DS and Roberts
LR: Heparin-degrading sulfatases in hepatocellular carcinoma: Roles
in pathogenesis and therapy targets. Future Oncol. 4:803–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Abdel-Hamid NM: Premalignant variations in
extracellular matrix composition in chemically induced
hepatocellular carcinoma in rats. J Membr Biol. 230:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
de los Reyes GC, Koda RT and Lien EJ:
Glucosamine and chondroitin sulfates in the treatment of
osteoarthritis: A survey. Prog Drug Res. 55:81–103. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang L, Liu WS, Han BQ, Peng YF and Wang
DF: Antitumor activities of D-glucosamine and its derivatives. J
Zhejiang Univ Sci B. 7:608–614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tan HY, Eskandari R, Shen D, Zhu Y, Liu
TW, Willems LI, Alteen MG, Madden Z and Vocadlo DJ: Direct one-step
fluorescent labeling of O-GlcNAc-modified proteins in live cells
using metabolic intermediates. J Am Chem Soc. 140:15300–15308.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tayel A, Ebrahim MA, Ibrahim AS, El-Gayar
AM and Al-Gayyar MM: Cytotoxic effects of suramin against HepG2
cells through activation of intrinsic apoptotic pathway. J BUON.
19:1048–1054. 2014.PubMed/NCBI
|
|
63
|
Al-Gayyar MMH, Ebrahim MA and Shams MEE:
Measuring serum levels of glycosaminoglycans for prediction and
using viscum fraxini-2 for treatment of patients with
hepatocellular carcinoma. J Pharm Res. 7:571–575. 2013.
|
|
64
|
Bezabeh T, Ijare OB, Albiin N, Arnelo U,
Lindberg B and Smith IC: Detection and quantification of
D-glucuronic acid in human bile using 1H NMR spectroscopy:
Relevance to the diagnosis of pancreatic cancer. MAGMA. 22:267–275.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Attallah AM, Toson el-SA, El-Waseef AM,
Abo-Seif MA, Omran MM and Shiha GE: Discriminant function based on
hyaluronic acid and its degrading enzymes and degradation products
for differentiating cirrhotic from non-cirrhotic liver diseased
patients in chronic HCV infection. Clin Chim Acta. 369:66–72. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Aruffo A, Stamenkovic I, Melnick M,
Underhill CB and Seed B: CD44 is the principal cell surface
receptor for hyaluronate. Cell. 61:1303–1313. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Varki NM and Varki A: Diversity in cell
surface sialic acid presentations: Implications for biology and
disease. Lab Invest. 87:851–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Arif S, Najeeb-ul-Haq, Hanif R, Khan AS,
Jamil-ur-Rehman and Mufti TA: Variations of serum sialic acid level
in liver cirrhosis. J Ayub Med Coll Abbottabad. 17:54–57. 2005.
|
|
69
|
Liu J, Wen X, Liu B, Zhang Q, Zhang J,
Miao H and Zhu R: Diosmetin inhibits the metastasis of
hepatocellular carcinoma cells by downregulating the expression
levels of MMP-2 and MMP-9. Mol Med Rep. 13:2401–2408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Niclosamide suppresses
migration of hepatocellular carcinoma cells and downregulates
matrix metalloproteinase-9 expression. Oncol Lett. 10:3515–3518.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu Z, Dou C, Jia Y, Li Q, Zheng X, Yao Y,
Liu Q and Song T: RIG-I suppresses the migration and invasion of
hepatocellular carcinoma cells by regulating MMP9. Int J Oncol.
46:1710–1720. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen X, Bo L, Zhao X and Chen Q:
MicroRNA-133a inhibits cell proliferation, colony formation
ability, migration and invasion by targeting matrix
metallopeptidase 9 in hepatocellular carcinoma. Mol Med Rep.
11:3900–3907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu L, Wang T, Meng WY, Wei J, Ma JL, Shi M
and Wang YG: Salinomycin inhibits hepatocellular carcinoma cell
invasion and migration through JNK/JunD pathway-mediated MMP9
expression. Oncol Rep. 33:1057–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hsieh MJ, Lin CW, Yang SF, Chen MK and
Chiou HL: Glabridin inhibits migration and invasion by
transcriptional inhibition of matrix metalloproteinase 9 through
modulation of NF-κB and AP-1 activity in human liver cancer cells.
Br J Pharmacol. 171:3037–3050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yuxian X, Feng T, Ren L and Zhengcai L:
Tanshinone II-A inhibits invasion and metastasis of human
hepatocellular carcinoma cells in vitro and in vivo. Tumori.
95:789–795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Darweish MM, Abbas A, Ebrahim MA and
Al-Gayyar MM: Chemopreventive and hepatoprotective effects of
Epigallocatechin-gallate against hepatocellular carcinoma: Role of
heparan sulfate proteoglycans pathway. J Pharm Pharmacol.
66:1032–1045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen XP, Luo JS, Tian Y, Nie CL, Cui W and
Zhang WD: Downregulation of heparanase expression results in
suppression of invasion, migration, and adhesion abilities of
hepatocellular carcinoma cells. Biomed Res Int. 2015:2419832015.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen Z, Zhu L, Li X, Tian H, Fang Y, Liu
H, Li S, Li L, Yue W and Li W: Down-regulation of heparanase leads
to the inhibition of invasion and proliferation of A549 cells in
vitro and in vivo. Acta Biochim Biophys Sin (Shanghai). 45:188–193.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu CJ, Chang J, Lee PH, Lin DY, Wu CC,
Jeng LB, Lin YJ, Mok KT, Lee WC, Yeh HZ, et al: Adjuvant heparanase
inhibitor PI-88 therapy for hepatocellular carcinoma recurrence.
World J Gastroenterol. 20:11384–11393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB,
Lin PW, Mok KT, Lee WC, Yeh HZ, Ho MC, et al: Heparanase inhibitor
PI-88 as adjuvant therapy for hepatocellular carcinoma after
curative resection: A randomized phase II trial for safety and
optimal dosage. J Hepatol. 50:958–968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Alyoussef A and Al-Gayyar MMH: Cytotoxic
and partial hepatoprotective activity of sodium ascorbate against
hepatocellular carcinoma through inhibition of sulfatase-2 in vivo
and in vitro. Biomed Pharmacother. 103:362–372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen G, Nakamura I, Dhanasekaran R, Iguchi
E, Tolosa EJ, Romecin PA, Vera RE, Almada LL, Miamen AG,
Chaiteerakij R, et al: Transcriptional induction of periostin by a
sulfatase 2-TGFβ1-SMAD signaling axis mediates tumor angiogenesis
in hepatocellular carcinoma. Cancer Res. 77:632–645. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gao W, Kim H and Ho M: Human monoclonal
antibody targeting the heparan sulfate chains of glypican-3
inhibits HGF-mediated migration and motility of hepatocellular
carcinoma cells. PLoS One. 10:e01376642015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gao H, Li K, Tu H, Pan X, Jiang H, Shi B,
Kong J, Wang H, Yang S, Gu J and Li Z: Development of T cells
redirected to glypican-3 for the treatment of hepatocellular
carcinoma. Clin Cancer Res. 20:6418–6428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu S, Li Y, Chen W, Zheng P, Liu T, He W,
Zhang J and Zeng X: Silencing glypican-3 expression induces
apoptosis in human hepatocellular carcinoma cells. Biochem Biophys
Res Commun. 419:656–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qi XH, Wu D, Cui HX, Ma N, Su J, Wang YT
and Jiang YH: Silencing of the glypican-3 gene affects the
biological behavior of human hepatocellular carcinoma cells. Mol
Med Rep. 10:3177–3184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yu D, Dong Z, Yao M, Wu W, Yan M, Yan X,
Qiu L, Chen J, Sai W and Yao D: Targeted glypican-3 gene
transcription inhibited the proliferation of human hepatoma cells
by specific short hairpin RNA. Tumour Biol. 34:661–668. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yao M, Wang L, Dong Z, Qian Q, Shi Y, Yu
D, Wang S, Zheng W and Yao D: Glypican-3 as an emerging molecular
target for hepatocellular carcinoma gene therapy. Tumour Biol.
35:5857–5868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ikeda M, Ohkawa S, Okusaka T, Mitsunaga S,
Kobayashi S, Morizane C, Suzuki I, Yamamoto S and Furuse J:
Japanese phase I study of GC33, a humanized antibody against
glypican-3 for advanced hepatocellular carcinoma. Cancer Sci.
105:455–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sawada Y, Sakai M, Yoshikawa T, Ofuji K
and Nakatsura T: A glypican-3-derived peptide vaccine against
hepatocellular carcinoma. Oncoimmunology. 1:1448–1450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhu AX, Gold PJ, El-Khoueiry AB, Abrams
TA, Morikawa H, Ohishi N, Ohtomo T and Philip PA: First-in-man
phase I study of GC33, a novel recombinant humanized antibody
against glypican-3, in patients with advanced hepatocellular
carcinoma. Clin Cancer Res. 19:920–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang N, Lin J, Ruan J, Su N, Qing R, Liu
F, He B, Lv C, Zheng D and abd Luo R: MiR-219-5p inhibits
hepatocellular carcinoma cell proliferation by targeting
glypican-3. FEBS Lett. 586:884–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang K, Kievit FM, Sham JG, Jeon M,
Stephen ZR, Bakthavatsalam A, Park JO and Zhang M: Iron-oxide-based
nanovector for tumor targeted siRNA delivery in an orthotopic
hepatocellular carcinoma xenograft mouse model. Small. 12:477–487.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hanaoka H, Nakajima T, Sato K, Watanabe R,
Phung Y, Gao W, Harada T, Kim I, Paik CH, Choyke PL, et al:
Photoimmunotherapy of hepatocellular carcinoma-targeting glypican-3
combined with nanosized albumin-bound paclitaxel. Nanomedicine
(Lond). 10:1139–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li SQ, Lin J, Qi CY, Fu SJ, Xiao WK, Peng
BG and Liang LJ: GPC3 DNA vaccine elicits potent cellular antitumor
immunity against HCC in mice. Hepatogastroenterology. 61:278–284.
2014.PubMed/NCBI
|