Introduction
The prostate gland is the major accessory organ of
the male reproductive system. Prostate cancer is the most
frequently diagnosed non-skin cancer in men in developed countries,
and is one of the leading causes of cancer-related death (1). In 2020, 191,930 men in the United
States were expected to be diagnosed with prostate cancer, with an
estimated 33,330 deaths from the disease (2). Prostate cancer is a clinically
heterogeneous disease with a variable prognosis (3), and as such, early diagnosis, accurate
prognostic prediction and successful management are challenging and
controversial (4). The incidence of
prostate cancer is age-related and several signaling systems,
including the generation of reactive oxygen species (ROS), are
known to play an important role in the development and progression
of prostate cancer (5).
ROS consist of superoxide anion (O2·−)
and hydroxyl radicals (OH·), as well as non-radical
molecules such as hydrogen peroxide (H2O2)
(6). ROS are primarily produced by
mitochondrial respiratory chain enzymes, including NADPH oxidase
(NOX), xanthine oxidase and nitric oxide synthase, as well as
arachidonic acid oxidation and metabolic enzymes such as cytochrome
P450s, lipoxygenase and cyclooxygenase (7). Low or moderate levels of ROS act as
signaling molecules for cellular proliferation, differentiation and
stress-responsive survival pathways (8). However, high ROS levels can induce cell
cycle arrest, apoptosis and necrosis (9).
Benzimidazole derivatives (BZMs) are used as
anthelmintic drugs, but BZMs such as albendazole, flubendazole and
mebendazole have also been repurposed for their anticancer effects
(10–12). Albendazole is a broad-spectrum
anti-parasitic drug (13) with low
toxicity, which inhibits glycolytic metabolism in the parasite,
resulting in death (14,15). Albendazole has also been reported to
inhibit cancer cell glycolysis (16), induce cell cycle arrest (17), and downregulate vascular endothelial
growth factor receptor and hypoxia inducible factor 1A expression
(18,19). A recent study demonstrated that
albendazole induces leukemia cell apoptosis by increasing ROS
production in a non-mitotic manner (20). Albendazole has also been reported to
inhibit the development of hepatocellular carcinoma (21), as well as that of colorectal
(22), ovarian (23), lung (16), breast (24), gastric (25) and head and neck (26) cancer. Although albendazole inhibits
the development of prostate cancer when used in combination with
other anticancer agents, the mechanism of these anticancer effects
have not been evaluated in detail (27).
In the present study, the anticancer effects of
albendazole on prostate cancer cells were investigated, and the
results confirmed that ROS play an important role in promoting its
anticancer effects. Oxidative stress-related and Wnt signaling
genes are downregulated in the presence of ROS. Therefore,
albendazole may be used as a novel antitumor agent for prostate
cancer.
Materials and methods
Cell lines and reagents
The PC-3 and DU145 human prostate cancer cell lines
were acquired from the American Type Culture Collection, and AT-2
rat prostate cancer cells was obtained from the Korean Cell Line
Bank. The non-tumorigenic human prostate epithelial cell line
(RWPE-1) was acquired from Dr Won-Woo Lee and the human prostate
cancer cell line (LNCaP) was acquired from Dr So Yeong Lee (both of
the College of Veterinary Medicine, Seoul National University,
Seoul, South Korea). PC-3, DU145, LNCaP and AT-2 cells were
cultured in RPMI-1640 medium (Welgene, Inc.) supplemented with 10%
fetal bovine serum and 1% penicillin/streptomycin (both Gibco;
Thermo Fisher Scientific, Inc.). RWPE-1 cells were cultured in
keratinocyte serum-free medium supplemented with 50 mg/l bovine
pituitary extract and 5 µg/l epidermal growth factor (Gibco; Thermo
Fisher Scientific, Inc.). All cell lines were maintained at 37°C
(95% air, 5% CO2). Albendazole (cat. no. A1943; Tokyo
Chemical Industry Co., Ltd.) and diphenyleneiodonium chloride (DPI;
Sigma-Aldrich; Merck KGaA) were dissolved in dimethyl sulfoxide
(DMSO) to a concentration of 10 mM each. The final DMSO
concentration in the culture media was 0.1%, and the same final
concentration of DMSO was used for the control. Glutathione (GSH;
Sigma-Aldrich; Merck KGaA) and N-acetylcysteine (NAC;
Sigma-Aldrich; Merck KGaA) were dissolved in distilled water to a
concentration of 100 and 75 mM, respectively, with the same
distilled water as their respective controls.
Cellular viability assay
Cellular proliferative capacity was analyzed using
MTT (Sigma-Aldrich; Merck KGaA) based on the ability of living
cells to convert tetrazolium salts to formazan. Briefly, cells were
seeded into 96-well culture plates at a density of
1.6×104 per well in 200 µl media. After culturing for 24
h at 37°C, the media were replaced with FBS-free media for 24 h.
The cells were then treated with 0.1, 0.5, 2.5, 5 and 10 µM
albendazole or the vehicle control (DMSO), and then cultured for a
further 24 h. The treatment concentration of albendazole was
determined by referring to previous reports (12,20). The
media were then replaced with fresh media containing 100 µl MTT
(diluted to 0.5 mg/ml in FBS-free medium from a 5 mg/ml stock
solution) and incubated at 37°C for 3 h. The supernatant was
removed and 100 µl DMSO was added to each well to dissolve the
formazan crystals. The absorbance was read at 470 nm using a
microplate reader (BioTek Instruments, Inc.), and all treatments
were performed in triplicate.
Clonogenic assay
To determine the longer-term effects of albendazole,
a clonogenic assay was performed using PC-3 and DU145 cells in the
logarithmic growth phase. Briefly, ~1,000 cells obtained from a
sub-confluent cell culture flask were seeded into 6-well culture
plates in 2 ml media per well. After 24 h, 0.1 and 0.5 µM
albendazole, or the vehicle (DMSO), were added to the culture
medium. The cells were allowed to form colonies for 7 days at 37°C,
and were rinsed with fresh medium every 3 days. When discrete and
well-defined colonies had formed, the plates were washed with
phosphate-buffered saline (PBS), fixed with 100% methanol for 10
min at room temperature and stained with hematoxylin for 30 min at
room temperature. The colonies with >50 cells were counted using
an inverted microscope (IX70; Olympus Corporation). Plating
efficiency (PE) is the ratio of the number of colonies to the
number of cells seeded. The number of colonies that arise after
treatment, expressed in terms of PE, is the surviving fraction.
However, PC3 cells exhibited a more scattered pattern, which made
it hard to determine the colony number. Therefore, their colony
area was measured instead using the ‘colony area’ plugin feature of
ImageJ software (28). The relative
colony area was calculated by multiplying the colony area by the
colony intensity.
Wound-healing assay
Cell motility was analyzed using an in vitro
wound-healing assay. PC-3 and DU145 cells were seeded into a 6-well
plate and cultured in RPMI-1640 medium (supplemented with 10% FBS
and 1% penicillin/streptomycin) at 37°C (95% air and 5%
CO2) until ≥90% confluent. Prior to the assay, a
preliminary experiment was conducted to determine the lowest FBS
concentration required for survival and migration in the control
group, and 10% FBS was deemed necessary for survival (29). A wound was then created in the
prostate cell monolayers using a sterile pipette tip. Wound closure
was monitored using an inverted microscope (IX70; Olympus
Corporation) following 24-h exposure to albendazole at
concentrations of 0.1 and 0.5 µM, or the vehicle (DMSO). All
treatments were performed in triplicate, and the wound areas were
measured using ImageJ software version 1.51k (National Institutes
of Health).
ROS measurement
The generation of intracellular ROS was determined
using 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Sigma-Aldrich;
Merck KGaA), which is converted to fluorescent
2′,7′-dichlorofluorescin (DCF) in the presence of peroxides. After
exposure to different concentrations of albendazole, 200 µM GSH,
300 µM NAC and 10 µM DPI simultaneously for 24 h, PC-3 and DU145
cells were treated with 10 µM DCFH-DA for 1 h at 37°C, and then
washed with PBS. To confirm the association between ROS production
by albendazole and NOX, the cells were treated with 10 µM DPI, an
inhibitor of NOX, in accordance with a previous report (20). The cells were detached using
trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc.) and
intracellular ROS was detected using a fluorescence spectrometer
(Victor 3; PerkinElmer, Inc.) at 485 nm exposure and 535 nm
emission.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the cells using a
Hybrid-R RNA extraction kit (GeneAll Biotechnology Co., Ltd.), and
cDNA was subsequently synthesized using the M-MLV cDNA Synthesis
kit (Enzynomics Co., Ltd.) according to the suppliers'
instructions. qPCR was performed with the TOPreal™ qPCR 2X PreMIX
(Enzynomics Co., Ltd.) using a CFX Connect Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc.). qPCR was performed with
initial denaturation at 95°C for 10 min, followed by 35 cycles of
denaturation at 95°C for 10 sec, annealing at 56–66°C (depending on
the primers) for 15 sec and elongation at 72°C for 30 sec. The
following human gene primers were used for qPCR: Catalase
(CAT) forward, 5′-ACAGCAAACCGCACGCTATG-3′ and reverse,
5′-CAGTGGTCAGGACATCAGCTTTC-3′; glutathione peroxidase 1
(GPX1) forward, 5′-CGCTTCCAGACCATTGACATC-3′ and reverse,
5′-CGAGGTGGTATTTTCTGTAAGATCA-3′; GPX3 forward,
5′-ACATGCCTACAGGTATGCGT-3’ and reverse, 5′-GAGCAGAACAATTGGACCTA-3′;
CDGSH iron sulfur domain 2 (CISD2) forward,
TTGGCTACCTTGCAGTTCGT-3′ and reverse, 5′-ATGTGAACCATCGCAGGCA-3′;
hypoxia-inducible factor 1α (HIF1A) forward,
5′-GCCAGACGATCATGCAGCTA-3′ and reverse, 5′-ATCCATTGATTGCCCCAGCA-3′;
catenin β1 (CTNNB1) forward, 5′-ATGACTCGAGCTCAGAGGGT-3′ and
reverse, 5′-ATTGCACGTGTGGCAAGTTC-3′; twist family BHLH
transcription factor 1 (TWIST1) forward,
5′-CTCGGACAAGCTGAGCAAGA-3′ and reverse, 5′-GCTCTGGAGGACCTGGTAGA-3′;
transcription factor 4 (TCF4) forward,
5′-CCTGGCACCGTAGGACAAAT-3′ and reverse, 5′-TGGGACCATATGGGGAGGG-3′;
BCL2 forward, 5′-CTTTGAGTTCGGTGGGGTCA-3′ and reverse,
5′-GGGCCGTACAGTTCCACAAA-3′; and ACTB forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The ratio of target gene fold-change
was normalized to that of ACTB expression using the
2−ΔΔCq method (30).
Western blot analysis
Cells were lysed using buffer containing 25 mM
Tris-HCl (pH 7.4), 120 mM NaCl, 0.5% NP-40, 4 mM NaF, 100 µM
Na3VO4 and protease inhibitor cocktail
(GenDEPOT). The protein concentration in the cell lysates was
determined using Bradford protein assay (Bio-Rad Laboratories,
Inc.). The cell lysates (20 µg/lane) were resolved by 15% SDS-PAGE
before transferring the proteins to nitrocellulose membranes. After
blocking with 5% skimmed milk (BD Biosciences) and 1% sodium azide
(PanReac AppliChem; ITW Reagents Division) diluted in PBS-Tween
(0.1% Tween-20) for 1 h at room temperature, the membranes were
incubated with anti-TCF4 (1:1,000; cat. no. 2569s; Cell Signaling
Technology, Inc.), anti-BCL2 (1:1,000; cat. no. 15071t; Cell
Signaling Technology, Inc.) and anti β-actin (1:1,000; cat. no.
A5441; Sigma-Aldrich; Merck KGaA) primary antibodies overnight at
4°C. The blots were then incubated with horseradish
peroxidase-conjugated IgG secondary antibodies (1:5,000; cat. nos.
31430 and 31460; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature, and developed using Clarity Western ECL Substrate
(Bio-Rad Laboratories, Inc.). The density of each band was
quantified with ImageJ software v1.51k (National Institutes of
Health) and expressed as fold-change relative to that of the
control treated with DMSO.
Statistical analyses
All data are presented as the mean ± standard error.
Experiments were repeated three times. Statistical significance was
determined using GraphPad Prism 5 software (GraphPad Software,
Inc.). The data were analyzed by one-way ANOVA followed by
Dunnett's or Tukey's post hoc test, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Albendazole decreases the
proliferative potential and colony formation capacity of prostate
cancer cells
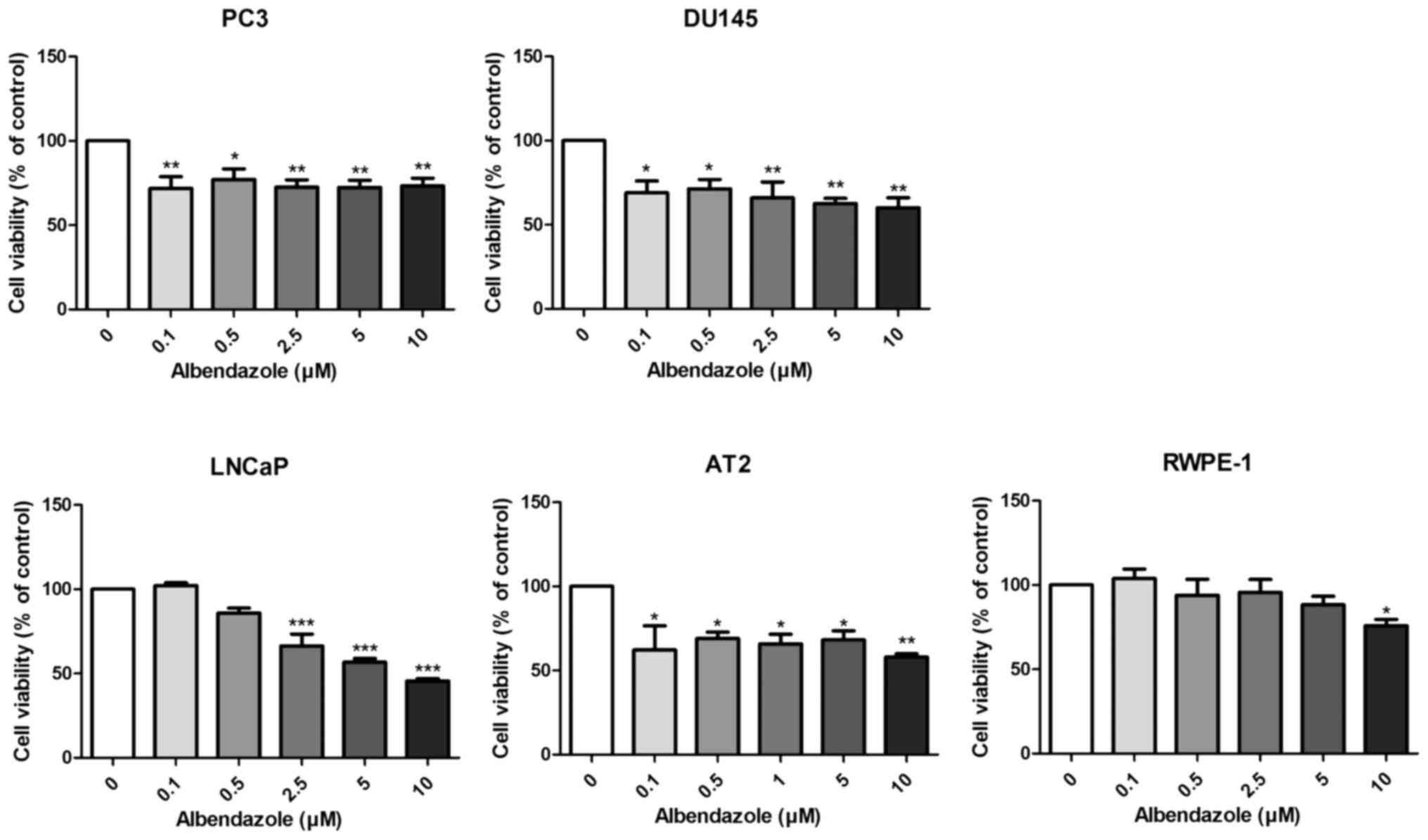
The effects of albendazole on the proliferative and
colony formation capacities of prostate cancer cells were
investigated. Albendazole reduced the proliferative potential of
PC3, DU145 and LNCaP human prostate cancer cells, as well as that
of the AT-2 rat prostate cancer cells (Fig. 1). Normal prostate RWPE-1 cells were
treated with albendazole as a negative control, which did not
affect the proliferative potential at concentrations <10 µM. As
0.1 and 0.5 µM albendazole reduced the proliferative potential of
PC3 and DU145 cells, these concentrations were used for subsequent
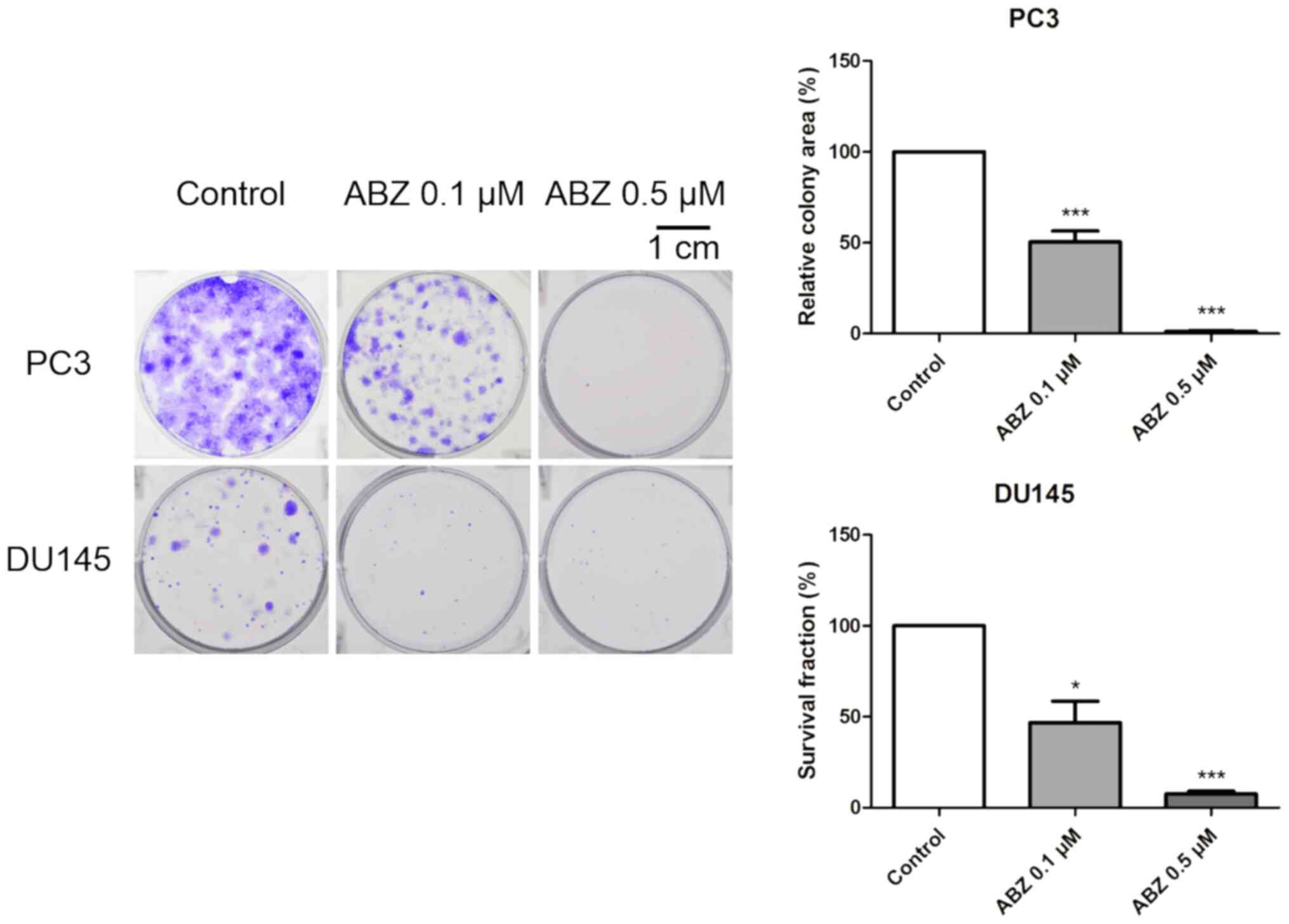
experimentation. To determine the longer-term effects of
albendazole, PC3 and DU145 cells were treated with albendazole for
7 days. Albendazole inhibited the colony formation capacity of both
cell lines, compared with that of the vehicle-treated control cells
(Fig. 2).
Albendazole treatment decreases the
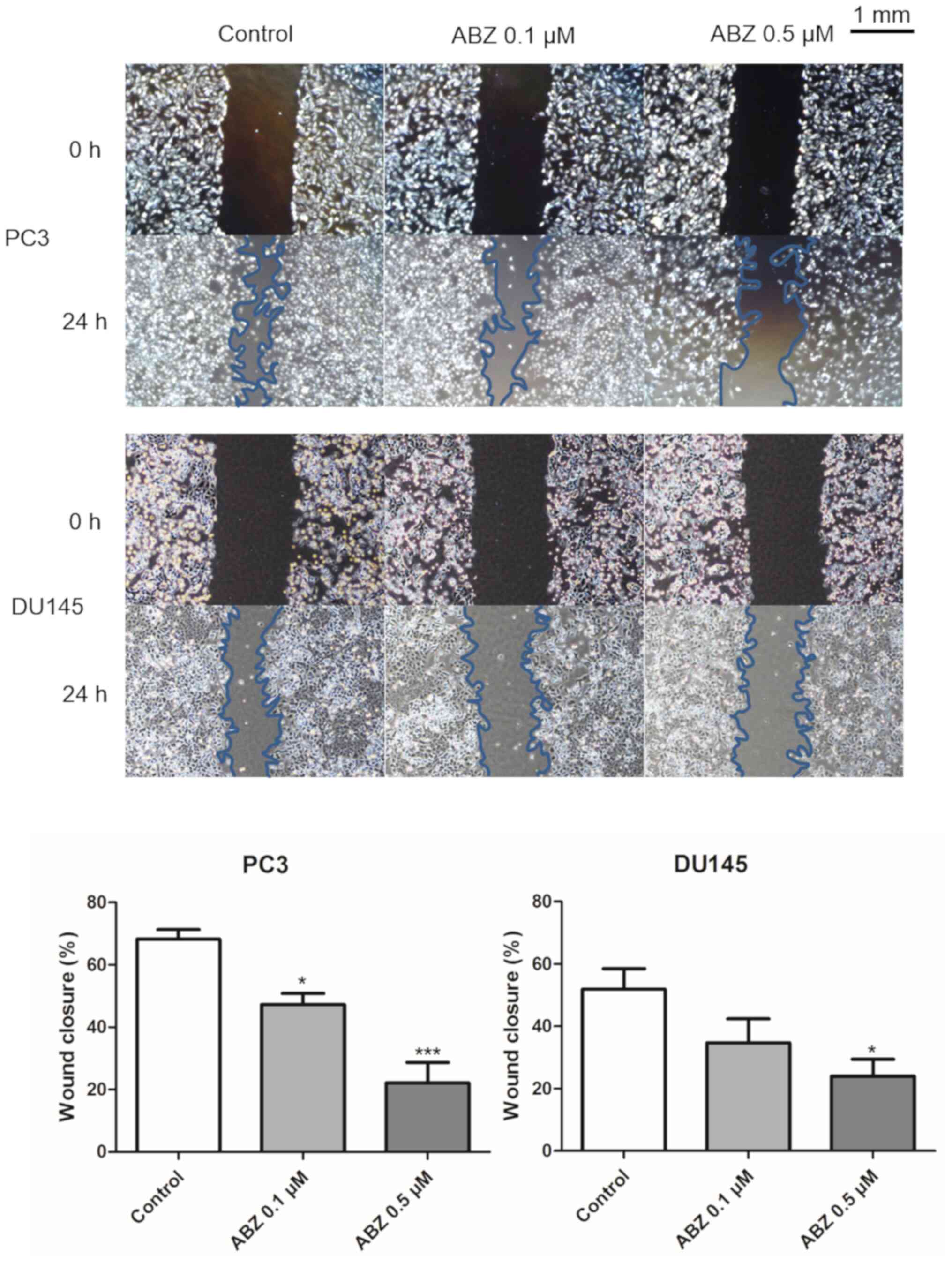
migration ability of PC3 and DU145 cells
A wound-healing assay was performed to determine the
effects of albendazole treatment on the migration of PC3 and DU145
cells. After 24 h of treatment with 0.1 and 0.5 µM albendazole, the
potential for migration of both cell lines was decreased in a
concentration-dependent manner compared with that of the control
(Fig. 3).
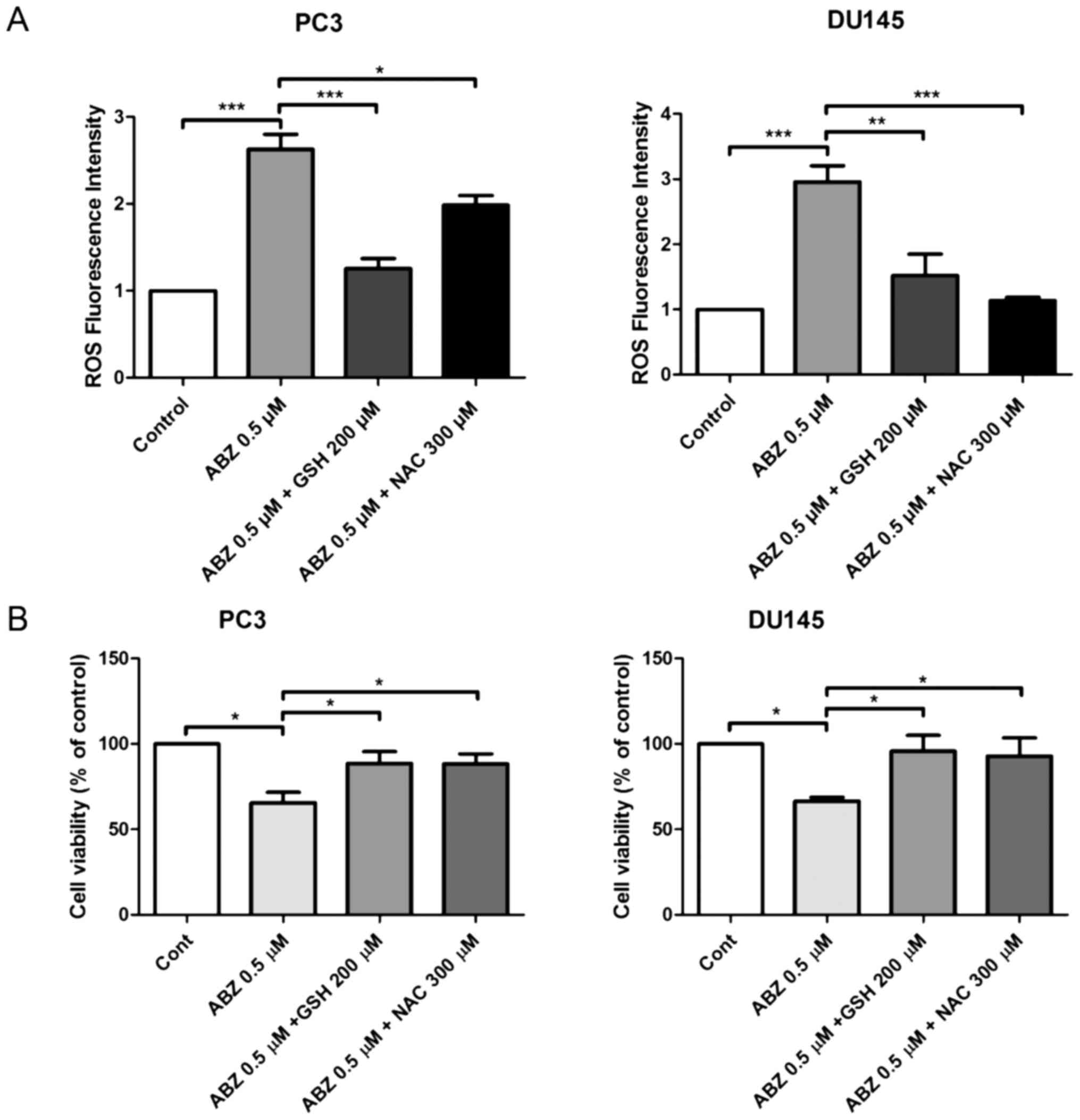
Albendazole promotes ROS production in
PC3 and DU145 cells
ROS production was measured in both PC3 and DU145
cells 24 h after albendazole treatment, using the fluorescent dye,
DCFH-DA. Compared with the control, albendazole increased
intracellular ROS levels in a concentration-dependent manner
(Fig. 4A). NOX is the primary source
of ROS generation (31). Compared
with the control groups, treatment with DPI, a NOX inhibitor,
reduced the basal levels of ROS in PC3 and DU145 cells, but only
PC3 cells were significantly impacted. However, treatment with DPI
did not alter the effects of albendazole on ROS generation in
either PC3 or DU145 cells (Fig. 4B).
Subsequently, PC3 and DU145 cells were treated with a combination
of albendazole and the antioxidants GSH and NAC, to determine
whether albendazole-induced ROS levels were associated with its
anticancer effects. Indeed, treatment with GSH and NAC decreased
albendazole-induced ROS levels (Fig.
5A). Moreover, treatment with GSH and NAC inhibited the
antiproliferative effects of albendazole (Fig. 5B).
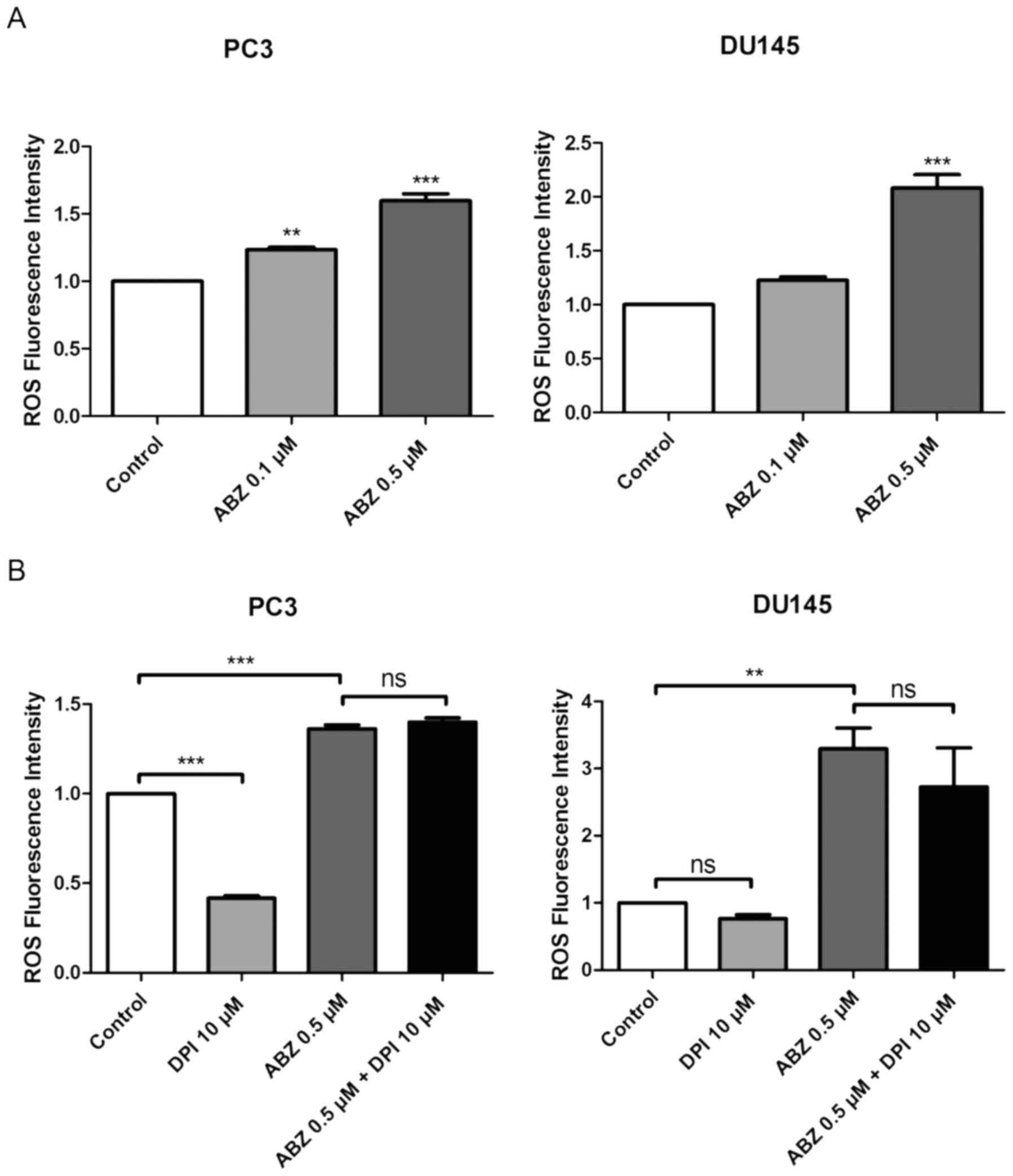 | Figure 4.Albendazole treatment increases ROS
production in PC3 and DU145 cells. (A) After treating PC3 and DU145
cells with albendazole and incubating with DCFH-DA, ROS levels were
measured using a fluorescence spectrometer (n=4). 0.1 and 0.5 µM
albendazole increased ROS production in PC3 and DU145 cells in a
concentration-dependent manner. (B) To determine whether NOX
influences ROS production, cells were treated with a combination of
DPI, a NOX inhibitor, and albendazole. ROS production was assessed
by adding DCFH-DA and measuring fluorescence (n=4). DPI alone
decreased the basal ROS levels of PC3 and DU145 cells. However,
co-treatment with albendazole did not decrease albendazole-induced
ROS production. **P<0.01 and ***P<0.001 vs. control, or as
indicated. Results are presented as the mean ± SEM. ROS, reactive
oxygen species; NOX, NADPH oxidase; DPI, diphenyleneiodonium
chloride; ns, not significant; ABZ, albendazole. |
Albendazole downregulates oxidative
stress-related and Wnt/β-catenin signaling genes
Treatment with albendazole increased ROS levels in
PC3 and DU145 cells. CAT, GPX1 and GPX3 encode
antioxidant enzymes that reduce oxidative stress by catalyzing
H2O2 produced by superoxide dismutase into
H2O (32). In addition,
CISD2 is a redox-sensitive gene that is responsible for
increasing the antioxidant capacity of cancer cells against ROS
(33). HIF1A is an important
mediator of the hypoxic response, which is also involved in tumor
initiation and progression (34). As
indicated in Fig. 6A, the mRNA
expression levels of CAT, GPX1, GPX3, CISD2 and HIF1A
were reduced following albendazole treatment. Furthermore,
oxidative stress regulates Wnt/β-catenin signaling (35), and albendazole was shown to decrease
the mRNA expression of CTNNB and TCF4, genes that
regulate Wnt/β-catenin signaling (36,37)
(Fig. 6B). In DU145 cells, both 0.1
and 0.5 µM albendazole decreased the mRNA expression of
CTNNB1 and TCF4, whereas in PC3 cells only 0.5 µM
albendazole decreased the expression of TCF4. The mRNA
expression of CTNNB1 decreased at 0.1 and 0.5 µM albendazole
in PC3 cells. The mRNA expression of BCL2 and TWIST1,
targets of Wnt/β-catenin signaling (38,39) were
also decreased following albendazole treatment in the present study
(Fig. 6B). In DU145 cells, 0.1 and
0.5 µM albendazole decreased the mRNA expression of BCL2 and
TWIST1, whereas in PC3 cells only 0.5 µM albendazole
decreased the expression of BCL2, and 0.1 µM albendazole
decreased the expression of TWIST1. Furthermore, albendazole
reduced TCF4 and BCL2 protein expression in PC3 cells (Fig. 6C).
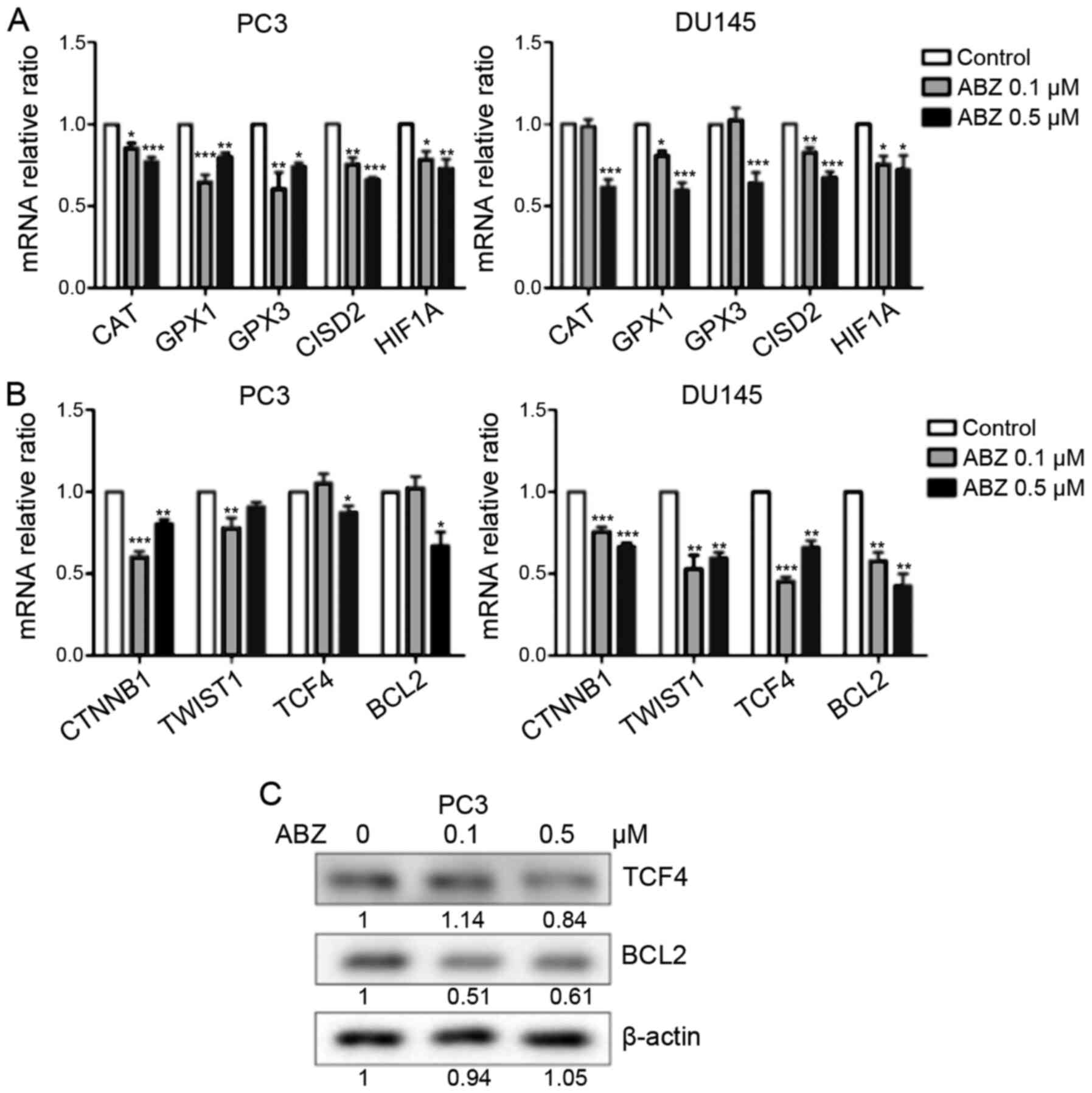 | Figure 6.Effects of albendazole treatment on
oxidative stress-related and Wnt/β-catenin signaling genes in PC3
and DU145 cells. Following treatment with albendazole, gene
expression in PC3 and DU145 cells was analyzed by reverse
transcription-quantitative PCR and western blotting. (A) mRNA
expression levels of the antioxidant enzymes CAT, GPX1, GPX3, CISD
and HIF1A were downregulated by albendazole treatment (n=6). (B)
The mRNA expression levels of CTNNB1, TWIST1, TCF4 and BCL2 were
also downregulated by albendazole treatment (n=6). (C) Albendazole
decreased TCF4 and BCL2 protein expression in PC3 cells.
*P<0.05, **P<0.01 and ***P<0.001 vs. control. Results are
presented as the mean ± SEM. CAT, catalase; GPX, glutathione
peroxidase; CISD2, CDGSH iron sulfur domain 2; HIF1A,
hypoxia-inducible factor 1α; CTNNB1, catenin β1; TWIST1, Twist
family BHLH transcription factor 1; TCF4, transcription factor 4;
ABZ, albendazole. |
Discussion
Due to the need for effective therapeutic options,
the demand for novel anticancer drugs against prostate cancer is
increasing. Drug repurposing is emerging as a new strategy to
replace existing drug development paradigms, and has been used to
discover new anticancer drugs by reusing existing or abandoned
compounds approved by the United States Food and Drug
Administration (40,41). Albendazole is a safe,
well-established antiparasitic agent (13). In the current study, albendazole was
demonstrated to suppress the proliferation of PC3, DU145, LNCaP and
AT2 prostate cancer cells. Notably, as albendazole did not affect
the proliferative potential of normal prostate RWPE-1 cells at
concentrations <10 µM, the anticancer effect of albendazole
appeared to be targeted towards cancer cells.
Furthermore, the anticancer effects of albendazole
were also confirmed to be associated with ROS production, as
previously reported (20,42). Treating prostate cancer cells with a
combination of DPI and albendazole did not affect ROS levels. ROS
generation by albendazole-treated prostate cancer cells was thus
unrelated to the presence of NOX, a finding which is also
consistent with previous reports (20). Antioxidants have been reported to
inhibit ROS-induced anticancer effects (43). Thus, in the present study, cells were
treated with a combination of albendazole and the antioxidants GSH
and NAC. Both of these antioxidants decreased the effects of
albendazole on prostate cancer cells, suggesting that ROS levels
are important for its anticancer effects.
ROS is known to activate and stabilize HIF1A
(34), and ROS-induced HIF1A
activation is thought to interfere with the anticancer effects of
albendazole. However, albendazole-induced HIF1A inhibition has been
reported in ovarian and lung cancer (16,18). In
the present study, the mRNA expression levels of HIF1A were
analyzed, and were significantly reduced by albendazole treatment
in PC3 and DU145 cells. As in previous reports (16,18),
albendazole was confirmed to decrease the expression of
HIF1A in prostate cancer. In addition, HIF1A overexpression
has been reported to reduce ROS levels (44). Thus, in the present study, it is
highly likely that the decreased expression of HIF1A was
involved in albendazole-associated ROS production.
CAT, GPX1 and GPX3 encode antioxidant
enzymes that reduce oxidative stress in cancer cells (32). CISD2 expression reduces ROS
levels in lung cancer cells and is associated with a poor prognosis
in lung adenocarcinoma (33). An
increased antioxidant capacity through several mechanisms improves
cancer cell survival by increasing the resistance to oxidative
stress (45). Albendazole is thought
to enhance the anticancer effects of ROS by reducing the expression
of these antioxidant enzymes, as well as that of CISD2.
Albendazole treatment has also been found to suppress Wnt/β-catenin
signaling. Tang et al (46)
showed that Wnt/β-catenin signaling inhibited ROS production in
melanocytes. The results of the present study suggested that
decreased Wnt/β-catenin signaling resulting from albendazole
treatment may be associated with increased ROS levels in prostate
cancer cells. Albendazole treatment was also found to suppress
Wnt/β-catenin signaling and decrease the mRNA expression of its
target gene, BCL2. An association between the anticancer
effects of albendazole and BCL2 has been reported in breast
cancer, leukemia and gastric cancer (25,42,47).
Therefore, the anticancer effects of albendazole may be associated
with the downregulation of BCL2 through the inhibition of
Wnt/β-catenin signaling.
As primary cultures of malignant prostatic cells
have more in vivo physiological characteristics than
established cell lines (48), they
are considered an appropriate model to verify the anticancer
effects of albendazole. However, in the present study, the use of
primary cell cultures was considered to be excessively challenging.
Therefore, various prostate cancer cell lines were used to verify
the anticancer effects of albendazole, including human cell lines
(PC3, DU145 and LNCaP) and a rat cell line (AT2). Nonetheless,
based on the current data, it is difficult to determine how
albendazole may affect prostate cancer metastasis in vivo,
or to predict its affects in clinical trials. Prostate cancer has
been shown to metastasize to the lungs and lymph nodes in TRAMP
mice (49,50), the study of which is a future
research consideration, which aims to confirm how albendazole
affects the metastasis of prostate cancer in mice. These findings
may then be used to highlight the physiological and clinical
relevance of albendazole in patients with prostate cancer.
In summary, the results of the present study
indicate that albendazole selectively reduced the proliferative
potential of prostate cancer cells. The anticancer effect of
albendazole was associated with increased ROS levels, which were
associated with the downregulation of antioxidant enzymes and the
redox-sensitive gene, CISD2. Albendazole also suppressed
Wnt/β-catenin signaling. In conclusion, these findings suggest that
albendazole may be used as a potential novel anticancer agent for
prostate cancer.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the
Brain Korea 21 PLUS Program for Creative Veterinary Science
Research, Research Institute for Veterinary Science, College of
Veterinary Medicine of Seoul National University.
Availability of data and materials
The datasets used and/or during the current study
are available from the corresponding author upon reasonable
request
Author's contributions
JHP and CS conceived and designed the experiments.
UK, CYK, BR, JK and JB performed the experiments. UK analyzed the
data and wrote the manuscript. JHP and UK confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barbieri CE, Bangma CH, Bjartell A, Catto
JW, Culig Z, Grönberg H, Luo J, Visakorpi T and Rubin MA: The
mutational landscape of prostate cancer. Eur Urol. 64:567–576.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cuzick J, Thorat MA, Andriole G, Brawley
OW, Brown PH, Culig Z, Eeles RA, Ford LG, Hamdy FC, Holmberg L, et
al: Prevention and early detection of prostate cancer. Lancet
Oncol. 15:e484–e492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khandrika L, Kumar B, Koul S, Maroni P and
Koul HK: Oxidative stress in prostate cancer. Cancer Lett.
282:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiseman H and Halliwell B: Damage to DNA
by reactive oxygen and nitrogen species: Role in inflammatory
disease and progression to cancer. Biochem J. 313:17–29. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finkel T: Signal transduction by
mitochondrial oxidants. J Biol Chem. 287:4434–4440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janssen-Heininger YM, Mossman BT, Heintz
NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG and
van der Vliet A: Redox-based regulation of signal transduction:
Principles, pitfalls, and promises. Free Radic Biol Med. 45:1–17.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pantziarka P, Bouche G, Meheus L, Sukhatme
V and Sukhatme VP: Repurposing Drugs in Oncology (ReDO)-mebendazole
as an anti-cancer agent. ecancermedicalscience. 8:2014. View Article : Google Scholar
|
|
11
|
Armando RG, Mengual Gómez DL and Gomez DE:
New drugs are not enough drug repositioning in oncology: An update.
Int J Oncol. 56:651–684. 2020.PubMed/NCBI
|
|
12
|
Rushworth LK, Hewit K, Munnings-Tomes S,
Somani S, James D, Shanks E, Dufès C, Straube A, Patel R and Leung
HY: Repurposing screen identifies mebendazole as a clinical
candidate to synergise with docetaxel for prostate cancer
treatment. Br J Cancer. 122:517–527. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horton J: Albendazole: A broad spectrum
anthelminthic for treatment of individuals and populations. Curr
Opin Infect Dis. 15:599–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li QZ, Hao YH, Gao XJ, Gao WX and Bing Z:
The target of benzimidazole carbamate against cysticerci
cellulosae. Agric Sci China. 6:1009–1017. 2007. View Article : Google Scholar
|
|
15
|
Xiao SH, Feng JJ, Guo HF, Jiao PY, Yao MY
and Jiao W: Effects of mebendazole, albendazole, and praziquantel
on fumarate hydratase, pyruvate kinase, and phosphoenolpyruvate
carboxykinase of Echinococcus granulosus cyst wall harbored in
mice. Zhongguo Yao Li Xue Bao. 15:69–72. 1994.PubMed/NCBI
|
|
16
|
Zhou F, Du J and Wang J: Albendazole
inhibits HIF-1α-dependent glycolysis and VEGF expression in
non-small cell lung cancer cells. Mol Cell Biochem. 428:171–178.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pourgholami MH, Cai ZY, Wang L, Badar S,
Links M and Morris DL: Inhibition of cell proliferation, vascular
endothelial growth factor and tumor growth by albendazole. Cancer
Invest. 27:171–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pourgholami MH, Cai ZY, Badar S, Wangoo K,
Poruchynsky MS and Morris DL: Potent inhibition of tumoral
hypoxia-inducible factor 1α by albendazole. BMC Cancer. 10:1432010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pourgholami MH, Khachigian LM, Fahmy RG,
Badar S, Wang L, Chu SW and Morris DL: Albendazole inhibits
endothelial cell migration, tube formation, vasopermeability, VEGF
receptor-2 expression and suppresses retinal neovascularization in
ROP model of angiogenesis. Biochem Biophys Res Commun. 397:729–734.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang LJ, Lee YC, Huang CH, Shi YJ, Chen
YJ, Pei SN, Chou YW and Chang LS: Non-mitotic effect of albendazole
triggers apoptosis of human leukemia cells via SIRT3/ROS/p38
MAPK/TTP axis-mediated TNF-α upregulation. Biochem Pharmacol.
162:154–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pourgholami MH, Woon L, Almajd R, Akhter
J, Bowery P and Morris DL: In vitro and in vivo suppression of
growth of hepatocellular carcinoma cells by albendazole. Cancer
Lett. 165:43–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang BS, Choi JS, Lee SE, Lee JK, Kim TH,
Jang WS, Tunsirikongkon A, Kim JK and Park JS: Enhancing the in
vitro anticancer activity of albendazole incorporated into
chitosan-coated PLGA nanoparticles. Carbohydr Polym. 159:39–47.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi EK, Kim SW, Nam EJ, Paek J, Yim GW,
Kang MH and Kim YT: Differential effect of intraperitoneal
albendazole and paclitaxel on ascites formation and expression of
vascular endothelial growth factor in ovarian cancer cell-bearing
athymic nude mice. Reprod Sci. 18:763–771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang J, Li R, He Y, Ling C, Wang Q, Huang
Y, Qin J, Lu W and Wang J: A novel tumor-targeting treatment
strategy uses energy restriction via co-delivery of albendazole and
nanosilver. Nano Res. 11:4507–4523. 2018. View Article : Google Scholar
|
|
25
|
Zhang X, Zhao J, Gao X, Pei D and Gao C:
Anthelmintic drug albendazole arrests human gastric cancer cells at
the mitotic phase and induces apoptosis. Exp Ther Med. 13:595–603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghasemi F, Black M, Vizeacoumar F, Pinto
N, Ruicci KM, Le CCSH, Lowerison MR, Leong HS, Yoo J, Fung K, et
al: Repurposing Albendazole: New potential as a chemotherapeutic
agent with preferential activity against HPV-negative head and neck
squamous cell cancer. Oncotarget. 8:71512–71519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ehteda A, Galettis P, Pillai K and Morris
DL: Combination of albendazole and 2-methoxyestradiol significantly
improves the survival of HCT-116 tumor-bearing nude mice. BMC
Cancer. 13:862013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guzmán C, Bagga M, Kaur A, Westermarck J
and Abankwa D: ColonyArea: An ImageJ plugin to automatically
quantify colony formation in clonogenic assays. PLoS One.
9:e924442014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
D'Autréaux B and Toledano MB: ROS as
signalling molecules: Mechanisms that generate specificity in ROS
homeostasis. Nat Rev Mol Cell Biol. 8:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marengo B, Nitti M, Furfaro AL, Colla R,
Ciucis CD, Marinari UM, Pronzato MA, Traverso N and Domenicotti C:
Redox homeostasis and cellular antioxidant systems: Crucial players
in cancer growth and therapy. Oxid Med Cell Longev.
2016:62356412016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li SM, Chen CH, Chen YW, Yen YC, Fang WT,
Tsai FY, Chang JL, Shen YY, Huang SF, Chuu CP, et al: Upregulation
of CISD2 augments ROS homeostasis and contributes to tumorigenesis
and poor prognosis of lung adenocarcinoma. Sci Rep. 7:118932017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galanis A, Pappa A, Giannakakis A, Lanitis
E, Dangaj D and Sandaltzopoulos R: Reactive oxygen species and
HIF-1 signalling in cancer. Cancer Lett. 266:12–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Korswagen HC: Regulation of the
Wnt/β-catenin pathway by redox signaling. Dev Cell. 10:687–688.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang G, Huang Y-X, Zhang R, Hou LD, Liu H,
Chen XY, Zhu JS and Zhang J: Toosendanin suppresses oncogenic
phenotypes of human gastric carcinoma SGC 7901 cells partly via miR
200a mediated downregulation of β-catenin pathway. Int J Oncol.
51:1563–1573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ravindranath A, Yuen HF, Chan KK, Grills
C, Fennell DA, Lappin TR and El-Tanani M: Wnt-β-catenin-Tcf-4
signalling-modulated invasiveness is dependent on osteopontin
expression in breast cancer. Br J Cancer. 105:542–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kypta RM and Waxman J: Wnt/β-catenin
signalling in prostate cancer. Nat Rev Urol. 9:418–428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng H, Jia L, Liu C-C, Rong Z, Zhong L,
Yang L, Chen XF, Fryer JD, Wang X, Zhang YW, et al: TREM2 promotes
microglial survival by activating Wnt/β-catenin pathway. J
Neurosci. 37:1772–1784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carley DW: Drug repurposing: identify,
develop and commercialize new uses for existing or abandoned drugs.
Part II. IDrugs. 8:3102005.PubMed/NCBI
|
|
41
|
Carley DW: Drug repurposing: identify,
develop and commercialize new uses for existing or abandoned drugs.
Part I. IDrugs. 8:3062005.PubMed/NCBI
|
|
42
|
Castro LS, Kviecinski MR, Ourique F,
Parisotto EB, Grinevicius VM, Correia JF, Wilhelm Filho D and
Pedrosa RC: Albendazole as a promising molecule for tumor control.
Redox Biol. 10:90–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiao D, Powolny AA, Moura MB, Kelley EE,
Bommareddy A, Kim SH, Hahm ER, Normolle D, Van Houten B and Singh
SV: Phenethyl isothiocyanate inhibits oxidative phosphorylation to
trigger reactive oxygen species-mediated death of human prostate
cancer cells. J Biol Chem. 285:26558–26569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun R, Meng X and Pu Y, Sun F, Man Z,
Zhang J, Yin L and Pu Y: Overexpression of HIF-1a could partially
protect K562 cells from 1,4-benzoquinone induced toxicity by
inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol In
Vitro. 55:18–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Traverso N, Ricciarelli R, Nitti M,
Marengo B, Furfaro AL, Pronzato MA, Marinari UM and Domenicotti C:
Role of glutathione in cancer progression and chemoresistance.
Oxidative medicine and cellular longevity. 2013:9729132013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang L, Fang W, Lin J, Li J, Wu W and Xu
J: Vitamin D protects human melanocytes against oxidative damage by
activation of Wnt/β-catenin signaling. Lab Invest. 98:1527–1537.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Khalilzadeh A, Wangoo KT, Morris DL and
Pourgholami MH: Epothilone-paclitaxel resistant leukemic cells
CEM/dEpoB300 are sensitive to albendazole: Involvement of apoptotic
pathways. Biochem Pharmacol. 74:407–414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peehl DM: Primary cell cultures as models
of prostate cancer development. Endocr Relat Cancer. 12:19–47.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Greenberg NM, DeMayo F, Finegold MJ,
Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik
RJ and Rosen JM: Prostate cancer in a transgenic mouse. Proc Natl
Acad Sci USA. 92:3439–3443. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gingrich JR, Barrios RJ, Morton RA, Boyce
BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM and Greenberg
NM: Metastatic prostate cancer in a transgenic mouse. Cancer Res.
56:4096–4102. 1996.PubMed/NCBI
|