Introduction
A gastrointestinal stromal tumor (GIST) is the most
common mesenchymal tumor of the human gastrointestinal tract, with
an estimated incidence of 10–15 per 1 million per year (1–5).
Approximately 90% of GISTs are located in the stomach and small
intestine, with gastric lesions being the most prevalent (~60%)
(1,3,6,7). GIST occurs with similar frequency in
males and females (6,8); nevertheless, some studies claim slight
predominance in males (1). Patients
might be diagnosed with GISTs at any age, yet they rarely occur
(0.5%) in individuals younger than 20 years. The median age of
detection is estimated to be 65 years of age (1,2,6,8).
Generally, patients presenting with GIST are
asymptomatic; nevertheless, some demonstrate non-specific symptoms
such as abdominal distension, pain, nausea or vomiting (1,8). The
median tumor size at diagnosis is ~6 cm; however, it may reach 20
cm (8). Although nodal metastases
rarely follow a primary tumor, distant metastasis encompassing the
abdominal cavity or the liver concern ~20% of patients at diagnosis
(8,9). The leading treatment for GIST cases
remains surgical resection (10).
Standard first-line therapy for inoperable, metastatic or recurrent
issues is the tyrosine-kinase inhibitor imatinib (11).
Some of the most critical GIST carcinogenesis
mutations occur in the tyrosine kinase family (KIT) or
platelet-derived growth factor receptor A (PDGFRA) gene. Only a
small proportion of GISTs seems to be associated with neither KIT
nor PDGFR sporadic mutations and is assigned to the wild-type (WT)
group (5). KIT and PDGFRA mutation
types might predict advanced or metastatic GIST response to
imatinib (12). GISTs with KIT exon
11 mutations are the most sensitive to imatinib treatment, whereas
KIT exon 9 mutations require a higher dosage of this inhibitor. On
the other hand, cases with PDGRA D842V mutation are
imatinib-resistant (7,12). Despite the significant improvement in
disease control and overall survival (OS) in advanced GIST cases
associated with imatinib usage, patients frequently suffer from
acquired or secondary resistance. Therefore, it appears essential
to identify the mechanism underlying the resistance to develop an
intervention that might be applied in this group of patients
(13,14).
Risk stratification of GISTs attempts to evaluate
the risk of an unfavorable outcome and select patients who may
benefit from adjuvant therapy (15).
Various risk stratification systems have evolved over the years,
but none is proved superior to the other (6). The first risk stratification was
proposed by Schaefer et al (12), predicting GIST malignant behavior by
classification into very low, low, intermediate and high-risk
categories based on tumor size and mitotic rate. One of the widely
used classification, Armed Force Institute of Pathology (AFIP) risk
classification, is based on primary tumor site (extra-gastric
location has worse predicted outcome), mitotic count and primary
tumor size (16). Notwithstanding
this, all risk assessments present one common drawback concerning
the non-linear continuous character of variables such as tumor size
and mitotic count (17). Moreover,
behaviors of specific GIST subgroups [for example, succinate
dehydrogenase (SDH) deficiency] are less well predicted by all
systems (18).
All things considered, while preparing holistic care
for patients with GIST diagnosis, scientists might face several
difficulties: Insufficient risk stratification, acquired or
secondary resistance to imatinib or the need for an exceptional
therapy method associated with wild-type tumors. The present review
summarizes recent advances associated with GIST biology that might
enhance diagnostic and therapeutic strategies. The described area
embraces angiogenesis, immunology, epithelial-to-mesenchymal
transition, origin from Cajal cells, microRNAs, and Raf kinase
inhibitory proteins. According to the authors' best knowledge,
similar reviews encompassing the described area have not been
published yet.
Angiogenic markers
Angiogenesis is proved to be one of the principal
processes in tumor growth and metastasis promotion. It is regulated
by a balance of angiogenic and anti-angiogenic cytokines (19). Anti-angiogenic strategies are an area
of great interest throughout the scientific world. They might be
categorized into three groups: i) Protein-based immunotherapeutics
directly neutralizing vascular endothelial growth factor (VEGF)
(Bevacizumab); ii) receptor tyrosine kinase inhibitors (Sunitinib);
and iii) antagonists of the mammalian target of rapamycin
(Everolimus) (20).
Anti-angiogenic therapies eradicate the existing
tumor vessels and obstruct the formation of new ones and
consequently avert the tumor cell's nutrition. Moreover, such
strategies decrease the degree of malignancy and increase the
efficiency of conventional treatment. Notwithstanding this, single
inhibition of VEGF receptors or tyrosine kinase receptors is
insufficient for hindering the entire angiogenesis process and
might develop an adaptive resistance towards treatment, partly due
to changes in the immune microenvironment of the tumor (21). Despite their defects, anti-angiogenic
therapies present high potential and need further research,
encompassing specific molecules that may contribute to establishing
highly effective personalized oncological treatment.
As far as GIST is concerned, the enormous role of
angiogenesis in tumor progression is verified by the high
efficiency of second-line Sunitinib. The primary mechanism of
Sunitinib targets multiple receptor tyrosine kinases, including
these for VEGF-critical mediators of angiogenesis (13).
VEGF expression is frequently increased in
GIST-accounted positively for 60–80% of all studied cases (22–24).
Null or weak expression of VEGF is associated with better prognosis
[higher progression-free survival (PFS) and OS], independently of
the tumor genotype. Moreover, low VEGF expression is associated
with a high therapeutic response to imatinib mesylate (13,24). In
general, GIST cells do not express VEGF-C, playing a critical role
in node metastasis via lymphangiogenesis (25). The lack of VEGF-C expression could be
one of the pivotal mechanisms explaining the rare occurrence of
lymph node metastases among patients with GIST diagnosis (26).
Among other angiogenic factors, endoglin (CD105) and
platelet endothelial cell adhesion molecule (PECAM-1) are
considered to be associated with patients outcomes (24). In GISTs, the association between the
strong immunohistochemical staining of CD105 with various
morphological criteria is associated with a worse prognosis,
encompassing a mitotic index above 5 mitoses per 50 high-power
fields and a high degree of risk (27). Moreover, the average value for the
CD105 and PECAM-1 expression is significantly higher in patients
with poor prognosis compared with the group of patients who are
presented without recurrence (24,28).
Fibroblast growth factors (FGFs) and their receptors
(FGFRs) are known as the potent regulators of angiogenesis
(29). Massive secretion of the
multiple chemokines, including FGF-2, was discovered to be induced
by the imatinib c-KIT inhibition. Increased production of FGF-2 by
GIST cells treated with imatinib activates the FGF-2/FGFR autocrine
loop that presents a negative impact on the disease progression and
might become one of the most important mechanisms underlying
imatinib-resistance among some patients with GIST (30). Inhibition of FGF-signaling in
imatinib-resistant patients was proved to restore their sensitivity
to the applied treatment (31).
Moreover, the combined inhibition of KIT and FGFR signaling
increases growth inhibition in GIST cells both in vitro and
in vivo (32).
Collagen and calcium-binding EGF domain-containing
protein 1 (CCBE1) is suggested to function as an independent
regulator of budding and migration of lymphangioblasts, and as a
result, to promote lymphangiogenesis (33). Notwithstanding this, in the study
conducted on GIST tissues, CCBE1 was proved to be specifically
located in the vessel wall with co-localization of a marker of
vascular endothelial cells - CD31 (34). Higher levels of CCBE1 were associated
with higher risk groups of GIST, lower survival and were suspected
of counteracting the anti-tumor effects of imatinib (34). However, studies conducted on ovarian
and breast cancers presented contradictory findings - higher CCBE1
expression was associated with better survival rates (35). In conclusion, CCBE1 action seems to
be contextually based upon tumor origin - epithelial or
mesenchymal.
Origin from Cajal cells
Hirota et al (36) found in 1998 that GISTs originate from
interstitial cells of Cajal (ICCs) in the myenteric plexus of the
alimentary tract. Pacemaker potentials of ICCs suggest that
mutations in genes required for synapse and neural development
might underlie some GIST behaviors (37). Moreover, the neuroendocrine phenotype
of GIST was proved by the presence of synaptic-like micro-vesicle
proteins, ghrelin, and peptide hormone receptors in the analyzed
GIST specimens (38).
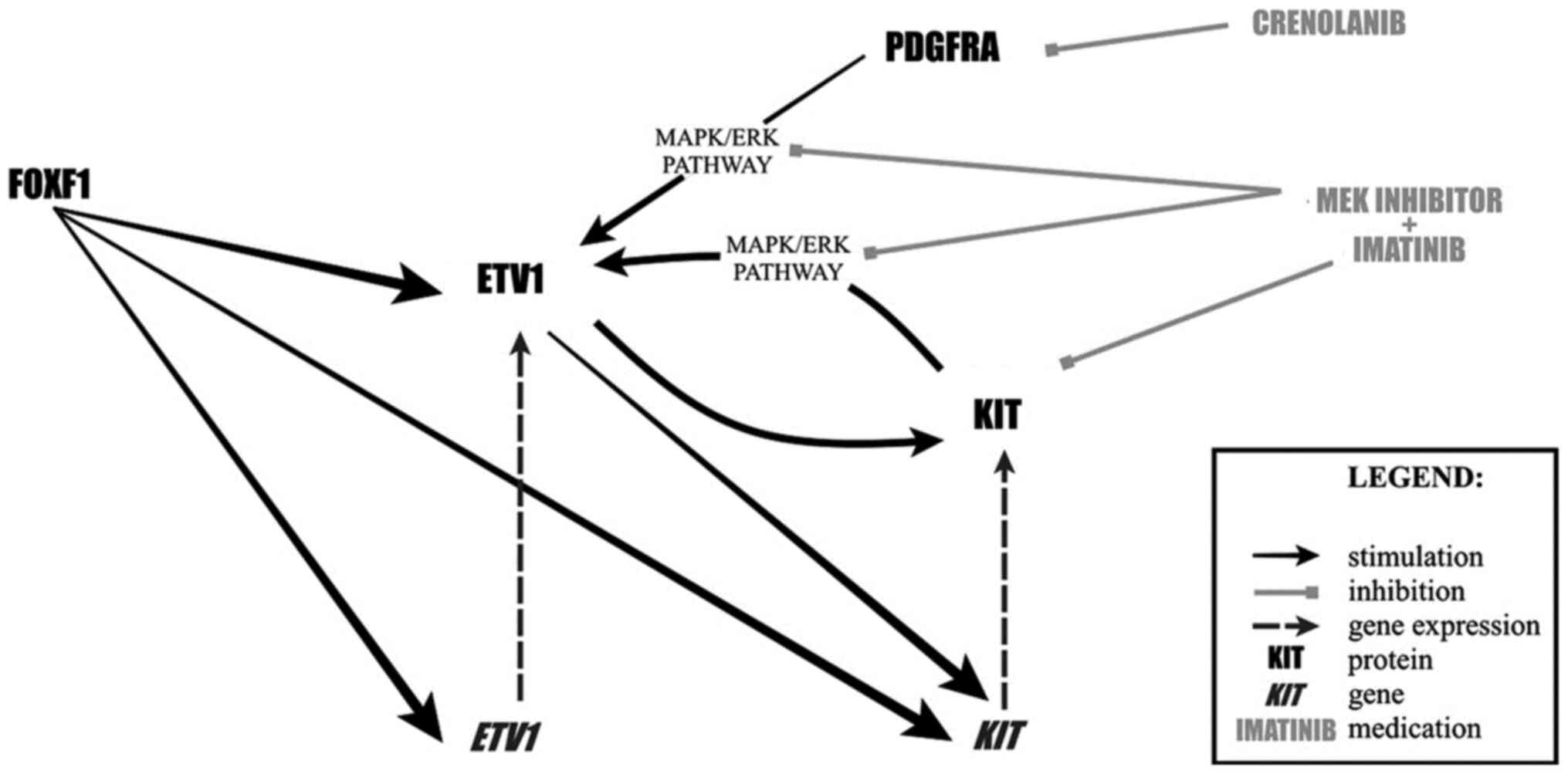
ICCs requires the principal signaling
regulator-KIT, and a lineage-specific master transcription
factor-ETS translocation variant 1 (ETV1), for lineage
specification and survival (39).
ETV1 is a master regulator of an ICC-GIST-specific transcription
network predominantly through enhancer-binding (40). The transcription of KIT and
ETV1 is directly regulated by the forkhead family member,
FOXF1, that co-localizes with ETV1 at enhancers (39). Mutant KIT and ETV1 form a positive
feedback loop, in which KIT excessively activates downstream
mitogen-activated protein kinase (MAPK) signaling that stabilizes
ETV1. In turn, ETV1 consolidates mutant KIT overexpression
(39,40). In GIST xenografts, the treatment
combination of imatinib and MEK162 (a MEK inhibitor) resulted in a
practically complete response with rapid inhibition of the MAPK
activity, loss of ETV1 protein, and downregulation of ETV1 target
genes. This therapy represents a significantly more effective
treatment strategy than imatinib alone and might prevent the
development of imatinib-resistance (41). Another therapeutic option includes
the inhibition of PDGFRA by crenolanib, a novel potent
pharmacological inhibitor of wild-type and oncogenic type of
receptor tyrosine kinase III with high selectivity for PDGFRA
relative to KIT. The inhibition of PDGFRA disturbs a
KIT-EKR-ETV1-KIT signaling loop and promotes proteasomal
degradation of ETV1 through decreased ERK-MAPK phosphorylation
(42). The protein level not
significantly affected by the KIT or MARP pathways perturbations is
FOXF1. Notably, FOXF1 loss results in decreased ETV1 protein
expression and global loss of ETV1 chromatin binding. It creates a
unique therapeutic opportunity to target the cellular context for
all GIST cases, including those that do not pose drug-sensitive
mutations, such as SDH-deficient ones (Fig. 1) (39).
Contrary to all the aforementioned findings
concerning ETV1, in the study by Sakamaki et al (43), ETV1 mRNA expression was negatively
associated with malignancy, with detected attenuation in aggressive
and malignant cases of GIST. Patients with low ETV1 expression
experienced shorter relapse-free survival (RFS) compared with
patients with a higher one. Notwithstanding this, the findings
aforementioned concerned only ETV1 mRNA, being different from the
protein, and a negative feedback system regulating mRNA by the
level of ETV1 protein might try to explain the results (43).
Cell adhesion molecules, including Slitrk3 (ST3),
are essential for establishing and regulating the synaptic
connections (44). The function of
ST3 in carcinogenesis remains unclear; however, its expression was
detected in GIST tissues in accordance with clinicopathological
features (37). ST3 expression was
correlated with decreased OS and disease-free survival (DFS) and
was proposed as a new enhancement for widely applied AFIP risk
stratification classification (37,45).
Cell adhesion molecule L1-like protein (CHL1) is a
multidomain type 1 membrane glycoprotein of the immunoglobulin
superfamily playing various functions in developing the neuronal
system. Its role in cancer cell growth, invasion and migration was
demonstrated in numerous studies encompassing different types of
malignancies (46). GIST expresses
CHL1 on mRNA as well as on protein level. Moreover, systemic CHL1
levels are increased in patients with GIST and is associated with a
shortened RFS, regardless of other clinicopathological parameters
(47).
Phosphodiesterase 3A (PDE3A) is identified as an
ICCs marker playing an influential role in their development;
however, not essential for their occurrence (48). PDE3A is found in most GIST samples,
regardless of their histological type, and thus, might be suggested
as a prospective novel marker for the patient prognosis (49).
MicroRNAs
MicroRNAs (miRNAs) are small endogenous RNAs that
regulate post-transcriptional silencing of target genes (50). Deregulated expression of various
miRNAs confers the malignant cells tumorigenic potential.
Considering the complexity of miRNAs connections involved in
carcinogenesis, focusing on a single miRNA molecule represents a
limited clinical approach (51).
Emerging evidence highlights that miRNA dysregulation is an
essential component in GIST expansion; nevertheless, the entire
mechanism remains unclear (52).
Some miRNAs (miR-148b-3p, miR-494, miR-218) negatively regulate KIT
protein expression and inhibit GIST cell proliferation and invasion
(53–55).
On the other hand, miR-218 might improve GIST cells'
sensitivity to imatinib through PI3K/AKT signaling pathway
(56). As far as prognosis is
concerned, overexpression of miR-196a and low expression of miR-186
are associated with poorer prognosis in patients with GIST
(57,58). As previously mentioned, focusing on a
single miRNA brings limited evaluation; however, undoubtedly,
miRNAs present an enormous impact on GIST biology. miRNA is
suspected of building regulatory networks controlling various
cellular functions (59). Generating
miRNA networks that would predict miRNAs' regulatory functions is a
promising approach that might help select potential biomarkers and
therapeutical targets in cancer, including GISTs (51).
Due to the involvement of miRNA in carcinogenesis,
therapeutics based on these molecules represent one of the
significant areas of scientists' interest. Various candidates have
been identified as potential therapeutic applications;
nevertheless, there is still much to learn about transforming them
into effective, targeted drug delivery systems (60). Commonly, miRNA-based therapeutics are
tolerated well in humans in various ongoing cancer-associated
clinical trials. The treatment strategy is based mainly on the
anti-miRNAs that inhibit the mature miRNAs from binding to their
targets and consequently block the participation of these miRNAs in
cancer development (61). Leading
barriers associated with this therapy are specific delivery
platforms to reach the targeted cell or miRNA. Suggested areas that
can be used to formulate miRNAs delivery effectively are
virus-based carriers (lentiviruses, adenoviruses or
adeno-associated viruses), biocompatible and biodegradable
liposomes, or nanoparticles (62).
Extensive research on the mechanism of pharmacological miRNA
targeting and the optimization of application methods might enable
the future implementation of miRNA-based therapeutics into the
oncological schemes, including those for patients with GIST
diagnosis.
Immune system
Imatinib prolongs patients' survival not only by its
direct effect on tumor cells but also by indirect immunostimulatory
effects on T and NK cells; thus, appropriate complementary
immunotherapy might further improve the patients' outcomes
(63). GIST microenvironment
presents a suppressed immune system, due to the high infiltration
of tumor-associated macrophages (TAMs) that promote tumor
development by suppressing Th1-mediated inflammation and
stimulating angiogenesis (64).
In a GIST animal model, imatinib increases the
activation, proliferation and frequency of intratumoral
CD8+T cells and, on the other hand, results in the
apoptosis of regulatory T cells (Tregs) (65). The mechanism underlying this
phenomenon might be associated with the overexpression of the
enzyme indoleamine 2,3-dioxygenase (IDO) in malignant cells. IDO
functions as one of the primary regulators in the biological
progression of malignancies by suppressing T and natural killer
(NK) cells, generating and activating Treg cells. Imatinib was
proved to decrease IDO expression, leading to CD8+T
cells activation and Tregs apoptosis (66). Moreover, IDO inhibition by imatinib
partially accounts for the anti-tumor efficacy of complementary
programmed death receptor 1 (PD-1)/programmed cell death 1 ligand 1
(PD-L1) blockade. PD-L1 expression, triggered by interferon-γ
(INF-γ) has been proven to be an independent factor of poor
prognosis in GIST (67,68). Both in vivo and in
vitro, anti-PD-1 and anti-PD-L1 had no efficacy when used
alone. Still, they enhanced the effectiveness of imatinib by
increasing T cell effector function in the presence of KIT and IDO
inhibition (67,69). The primary resistance to PD-1 might
be associated with the residence of GIST-associated macrophages
expressing IDO1 leading to the immune-suppressive phenotype of
malignancy cells (70).
Chemokines are a class of chemotactic cytokines with
low molecular mass involved in cancer progression (71). CC chemokine receptor type 8 (CCR8) is
one of the most critical chemokine receptors, mainly expressed in
Tregs. Its ligand, CCL1, enhances Treg immunosuppressive activity
through CCR8 recruitment, with a positive feedback loop (72). In GIST specimens, low expression of
CCR8 was positively associated with the patients' survival
(10). CCR8 recruits
FOXP3+ Treg cells to reveal an immunosuppressive
function, which results in a decreased proportion of
CD8+ T cells/Tregs and leads to a poor prognosis in
solid malignancies (73,74). Interaction between another chemokine
receptor, CXC chemokine receptor (CXCR) 4 and its ligand CXC
chemokine ligand (CXCL) 12, is one of the postulated mechanisms
leading to the increased organ-specific metastasis development
(75). One of the most repeated GIST
mutations, KIT exon 11 557–558 deletion, enhances ETV1 and
increases CXCR4 expression in GIST cells. Typically, GIST
metastases occur in the liver. CXCL12 expressed by hepatic cells
attracts GIST cells harboring upregulated expression of CXCR4
(8,71).
Raf kinase inhibitory protein (RKIP)
Raf kinase inhibitory protein (RKIP) is a highly
conserved kinase inhibitor functioning as a metastasis suppressor
in various malignancies; thus, its downregulation is proved to be a
frequent occurrence in metastatic tumors (76). The high possibility of negative RKIP
expression in GISTs is correlated with larger tumor size, despite
no association with the number of mitotic figures (77). The lack of RKIP expression restrains
the MAPK signaling pathway regulating the cell cycle, resulting in
increased proliferation of tumor cells (78). Patients with higher RKIP expression
are suspected of having an improved prognosis with higher survival
rates; however, it cannot be an independent prognostic factor in
GIST (77,79).
Epithelial-to-mesenchymal transition
(EMT)
EMT is a reversible cellular program that
transiently changes epithelial cells into a mesenchymal phenotype
characterized by loss of apical-basal polarity, reorganization of
their cytoskeleton and increased cellular motility. EMT enables
cancer cells to fulfill the invasion-metastasis cascade,
encompassing local invasion, intravasation and extravasation. On
the other hand, to efficiently form macroscopic metastases,
carcinoma cells need to revert to a more epithelial phenotype by
undergoing mesenchymal-epithelial transition (MET). Pathologists
might use the detection of many of the EMT-associated protein
markers as highly specific indicators of high-grade malignancy
(80,81).
Osteopontin (OPN) plays a predominant regulatory
role in expressing many well-known EMT activators, thus being
recognized as a critical regulator of the entire process. Moreover,
OPN can modify the tissue and tumor microenvironment to support EMT
by generating cancer-associated fibroblasts (82,83). The
clinical significance of OPN as a biomarker for poor prognosis has
been reported in GISTs; increased OPN expression was significantly
associated with higher mitosis rate, poor recurrence prognosis,
high-risk status and worse DFS (84). OPN, upon its interaction and
upregulating effect on CD44 surface expression, was proved to
contribute to tumor cell proliferation. CD44 is an OPN receptor
highly expressed in the vast majority of malignancies and promotes
processes involved in metastases via interaction with appropriate
extracellular matrix ligands (85).
High OPN expression and its interaction with CD44 is correlated
with elevated mitosis rate in GIST tissues, possibly through
subsequent downstream signaling contributing to the enhanced
proliferation (84). Moreover, OPN
elicits an anti-apoptotic effect through β-catenin-mediated
upregulation of anti-apoptotic protein-induced myeloid leukemia
cell differentiation protein (Mcl-1), and as a result, attenuates
imatinib-induced apoptosis in GIST in vitro. The discussed
mechanism might underly drug resistance to imatinib among some
patients with GIST (14).
Another molecule affecting EMT and being studied in
GIST is long non-coding RNA (lncRNA) AOC4P. AOC4P regulates EMT by
affecting the production of vimentin, one of the EMT and metastasis
markers. The expression of various EMT markers, including vimentin,
transforming growth factor-β1, ZEB1 and Snail, was significantly
higher in GIST tissues compared with normal ones. In contrast, the
expression of E-cadherin was found to be lower. This association
was particularly significant in high-risk GIST cases. The
downregulation of E-cadherin is the hallmark of the EMT in cancer.
The decrease in E-cadherin leads to the exacerbation of GIST
development, conversion of cytokeratin into vimentin, and
consequently, EMT acceleration. The EMT process might be inhibited
by AOC4P silencing that induces the increase in E-cadherin and the
decrease in vimentin in carcinoma cells (86–88).
Slug, a member of the SNAIL family, is the most
thoroughly investigated EMT regulator. Overexpression of Slug
suppresses the expression of E-cadherin and increases the cancer
cells' invasiveness (87).
Approximately 90% of GIST cases might display SLUG overexpression.
Nuclear positivity for SLUG is observed in GIST cases with distant
metastasis, especially strong in extra-gastrointestinal ones
(89). SLUG acts as a nuclear
transcription factor and is more commonly expressed by large GISTs
with pleomorphic nuclei and a high mitotic index. SLUG is described
as an indicator of patients' unfavorable RFS. Downregulation of
this member of the SNAIL family inhibits cell proliferation,
induces cell death, and sensitizes GIST cells to the lower
concentrations of imatinib (90).
The molecules involved in the EMT are summarized in the Table I.
 | Table I.Molecules involved in the EMT in
GIST. |
Table I.
Molecules involved in the EMT in
GIST.
| Molecule | Expression rate in
GIST | Mechanism | Clinical
significance | Refs. |
|---|
| E-cadherin | Decreased | Adhesive ability,
chromosome elimination, and gene mutation downregulation;
cytokeratin into vimentin conversion | Lower expression in
high-risk compared with low/medium-risk GIST | (86) |
| Vimentin | Increased | The functions of
cell signal transduction, adhesion, migration and apoptosis | Higher expression
in high-risk compared with low/medium-risk GIST | (86) |
| lncRNA AOC4P | Increased | E-cadherin
downregulation; vimentin and Snail upregulation | Higher expression
in high-risk compared with low- and intermediate-risk GISTs | (86) |
| Slug | Increased | E-cadherin
downregulation | Nuclear positivity
in GIST cases with distant metastasis, especially strong in
extra-gastrointestinal; the indicator of unfavorable RFS | (89,90) |
| Snail | Increased | E-cadherin
downregulation through E-box recognition and integration | Higher expression
in high-risk compared with low/medium-risk GIST | (86) |
| TGF-β1 | Increased | E-cadherin
transcriptional repressors (Snail, ZEB and TWIST) upregulation |
| (86,88) |
| ZEB1 | Increased | TGF-β pathway
activation |
| (86,88) |
| Osteopontin | Increased | Mitosis rate
upregulation in GIST tissues, possibly through subsequent
downstream signaling; the anti-apoptotic effect through
β-catenin-mediated upregulation of anti-apoptotic protein Mcl-1.
EMT initiation through activating an autocrine MAPK intracellular
signaling pathway resulting in Twist activation and Bmi1
expression | Higher expression
significantly associated with poor recurrence prognosis, high-risk
status, and worse DFS | (14,83,84) |
Conclusion
Broadened knowledge considering GIST biology is a
promising window for developing improved diagnostic and therapeutic
strategies. Mutations in genes required for synapse and neural
development may underlie some GIST behaviors, while miRNA
dysregulation is an essential component in GIST expansion. On the
other hand, resistance to imatinib may be associated with
epithelial-mesenchymal transition. New molecules might be
incorporated into risk stratification schemes due to their proven
association with outcomes; however, further research is required.
Therapies based on the significant role of angiogenesis, immunology
and neural origin in the GIST biology could become a valuable
enhancement of currently implemented treatment schemes. Although
multiple obstacles must be defeated, developing an understanding of
GIST carcinogenesis presents a promising future.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors' contributions
MMF and AMBK designed the study. MMF collected the
data and researched the literature. MMF and AMBK drafted the
manuscript. MMF and AMBK confirmed the authenticity of all the raw
data. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed M: Recent advances in the management
of gastrointestinal stromal tumor. World J Clin Cases. 8:3142–3155.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamamoto H and Oda Y: Gastrointestinal
stromal tumor: Recent advances in pathology and genetics. Pathol
Int. 65:9–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirota S: Differential diagnosis of
gastrointestinal stromal tumor by histopathology and
immunohistochemistry. Transl Gastroenterol Hepatol. 3:272018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khoshnood A: Gastrointestinal stromal
tumor-A review of clinical studies. J Oncol Pharm Pract.
25:1473–1485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kupcinskas J: Small molecules in rare
tumors: Emerging role of MicroRNAs in GIST. Int J Mol Sci.
19:3972018. View Article : Google Scholar
|
|
6
|
Parab TM, DeRogatis MJ, Boaz AM, Grasso
SA, Issack PS, Duarte DA, Urayeneza O, Vahdat S, Qiao JH and Hinika
GS: Gastrointestinal stromal tumors: A comprehensive review. J
Gastrointest Oncol. 10:144–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H and Liu Q: Prognostic indicators
for gastrointestinal stromal tumors: A review. Transl Oncol.
13:1008122020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Mehren M and Joensuu H:
Gastrointestinal stromal tumors. J Clin Oncol. 36:136–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi YN, Li Y, Wang LP, Wang ZH, Liang XB,
Liang H, Zhang L, Li B, Fan LQ, Zhao Q, et al: Gastrointestinal
stromal tumor (GIST) with liver metastases: An 18-year experience
from the GIST cooperation group in North China. Medicine
(Baltimore). 96:e82402017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li HL, Wang LH, Hu YL, Feng Y, Li XH, Liu
YF, Li P, Mao QS and Xue WJ: Clinical and prognostic significance
of CC chemokine receptor type 8 protein expression in
gastrointestinal stromal tumors. World J Gastroenterol.
26:4656–4668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishida T, Blay JY, Hirota S, Kitagawa Y
and Kang YK: The standard diagnosis, treatment, and follow-up of
gastrointestinal stromal tumors based on guidelines. Gastric
Cancer. 19:3–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaefer IM, Marino-Enriquez A and
Fletcher JA: What is new in gastrointestinal stromal tumor? Adv
Anat Pathol. 24:259–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Den Hollander D, Van der Graaf WTA, Desar
IME and Le Cesne A: Predictive factors for toxicity and survival of
second-line sunitinib in advanced gastrointestinal stromal tumours
(GIST). Acta Oncol. 58:1648–1654. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu KH, Tsai HW, Lin PW, Hsu YS, Lu PJ and
Shan YS: Anti-apoptotic effects of osteopontin through the
up-regulation of Mcl-1 in gastrointestinal stromal tumors. World J
Surg Oncol. 12:1892014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei SC, Xu L, Li WH, Li Y, Guo SF, Sun XR
and Li WW: Risk stratification in GIST: Shape quantification with
CT is a predictive factor. Eur Radiol. 30:1856–1865. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Review on morphology, molecular pathology,
prognosis, and differential diagnosis. Arch Pathol Lab Med.
130:1466–1478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Vita M, Zanghi A, Cavallaro A, Cardì F,
Uhlig M, Ursi P, Lo Menzo E, Panebianco V and Cappellani A: Gastric
GIST and prognostic models. Which is the best to predict survival
after surgery? Ann Ital Chir. 90:31–40. 2019.PubMed/NCBI
|
|
18
|
Wong NA: Gastrointestinal stromal
tumours--an update for histopathologists. Histopathology.
59:807–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Imamura M, Yamamoto H, Nakamura N, Oda Y,
Yao T, Kakeji Y, Baba H, Maehara Y and Tsuneyoshi M: Prognostic
significance of angiogenesis in gastrointestinal stromal tumor. Mod
Pathol. 20:529–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rey S, Schito L, Wouters BG, Eliasof S and
Kerbel RS: Targeting Hypoxia-Inducible factors for antiangiogenic
cancer therapy. Trends Cancer. 3:529–541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teleanu RI, Chircov C, Grumezescu AM and
Teleanu DM: Tumor angiogenesis and anti-angiogenic strategies for
cancer treatment. J Clin Med. 9:842019. View Article : Google Scholar
|
|
22
|
Liu N, Huang J, Sun S, Zhou Z, Zhang J,
Gao F and Sun Q: Expression of matrix metalloproteinase-9,
cyclooxygenase-2 and vascular endothelial growth factor are
increased in gastrointestinal stromal tumors. Int J Clin Exp Med.
8:6495–6501. 2015.PubMed/NCBI
|
|
23
|
Tasdemir A, Soyuer I, Unal D and Artis T:
Prognostic value of NF-κB, CD9, and VEGF in gastrointestinal
stromal tumors. Contemp Oncol (Pozn). 17:493–498. 2013.PubMed/NCBI
|
|
24
|
Basilio-de-Oliveira RP and Pannain VL:
Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell
proliferation (Ki67) for gastrointestinal stromal tumors. World J
Gastroenterol. 21:6924–6930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kigure W, Fujii T, Sutoh T, Morita H,
Katoh T, Yajima RN, Yamaguchi S, Tsutsumi S, Asao T and Kuwano H:
The association of VEGF-C expression with tumor lymphatic vessel
density and lymph node metastasis in patients with gastric cancer
and gastrointestinal stromal tumor. Hepatogastroenterology.
60:277–280. 2013.PubMed/NCBI
|
|
26
|
Yang Z, Wang F, Liu S and Guan W:
Comparative clinical features and short-term outcomes of gastric
and small intestinal gastrointestinal stromal tumours: A
retrospective study. Sci Rep. 9:100332019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gromova P, Rubin BP, Thys A, Cullus P,
Erneux C and Vanderwinden JM: ENDOGLIN/CD105 is expressed in KIT
positive cells in the gut and in gastrointestinal stromal tumours.
J Cell Mol Med. 16:306–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Oliveira AT, Reis RM, Afonso J,
Martinho O, Matos D, Carvalho AL, Vazquez VL, Silva TB,
Scapulatempo C, Saad SS and Longatto-Filho A: Lymphangiogenic
VEGF-C and VEGFR-3 expression in genetically characterised
gastrointestinal stromal tumours. Histol Histopathol. 26:1499–1507.
2011.PubMed/NCBI
|
|
29
|
Hui Q, Jin Z, Li X, Liu C and Wang X: FGF
family: From drug development to clinical application. Int J Mol
Sci. 19:18752018. View Article : Google Scholar
|
|
30
|
Boichuk S, Galembikova A, Mikheeva E,
Bikinieva F, Aukhadieva A, Dunaev P, Khalikov D, Petrov S,
Kurtasanov R, Valeeva E, et al: Inhibition of FGF2-Mediated
Signaling in GIST-promising approach for overcoming resistance to
imatinib. Cancers (Basel). 12:16742020. View Article : Google Scholar
|
|
31
|
Sergei B, Pavel D, Aigul G, Firyuza B,
Ilmira N, Ilshat M, Aida A, Refat K, Natalia A, Elena S and Vera G:
Inhibition of FGFR2-Signaling Attenuates a Homology-Mediated DNA
Repair in GIST and Sensitizes Them to DNA-Topoisomerase II
Inhibitors. Int J Mol Sci. 21:3522020. View Article : Google Scholar
|
|
32
|
Li F, Huynh H, Li X, Ruddy DA, Wang Y, Ong
R, Chow P, Qiu S, Tam A, Rakiec DP, et al: FGFR-Mediated
Reactivation of MAPK signaling attenuates antitumor effects of
imatinib in gastrointestinal stromal tumors. Cancer Discov.
5:438–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li P, Cong Z, Qiang Y, Xiong L, Tang L,
Zhang Y, Wu H, Yi J, Jing H, Li D and Shen Y: Clinical significance
of CCBE1 expression in lung cancer. Mol Med Rep. 17:2107–2112.
2018.PubMed/NCBI
|
|
34
|
Tian GA, Zhu CC, Zhang XX, Zhu L, Yang XM,
Jiang SH, Li RK, Tu L, Wang Y, Zhuang C, et al: CCBE1 promotes GIST
development through enhancing angiogenesis and mediating resistance
to imatinib. Sci Rep. 6:310712016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mesci A, Huang X, Taeb S, Jahangiri S, Kim
Y, Fokas E, Bruce J, Leong HS and Liu SK: Targeting of CCBE1 by
miR-330-3p in human breast cancer promotes metastasis. Br J Cancer.
116:1350–1357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang CJ, Zhang ZZ, Xu J, Wang M, Zhao WY,
Tu L, Zhuang C, Liu Q, Shen YY, Cao H and Zhang ZG: SLITRK3
expression correlation to gastrointestinal stromal tumor risk
rating and prognosis. World J Gastroenterol. 21:8398–8407. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bumming P, Nilsson O, Ahlman H, Welbencer
A, Andersson MK, Sjölund K and Nilsson B: Gastrointestinal stromal
tumors regularly express synaptic vesicle proteins: Evidence of a
neuroendocrine phenotype. Endocr Relat Cancer. 14:853–863. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ran L, Chen Y, Sher J, Wong EWP, Murphy D,
Zhang JQ, Li D, Deniz K, Sirota I, Cao Z, et al: FOXF1 defines the
core-regulatory circuitry in gastrointestinal stromal tumor. Cancer
Discov. 8:234–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chi P, Chen Y, Zhang L, Guo X, Wongvipat
J, Shamu T, Fletcher JA, Dewell S, Maki RG and Zheng D: ETV1 is a
lineage survival factor that cooperates with KIT in
gastrointestinal stromal tumours. Nature. 467:849–853. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ran L, Sirota I, Cao Z, Murphy D, Chen Y,
Shukla S, Xie Y, Kaufmann MC, Gao D, Zhu S, et al: Combined
inhibition of MAP kinase and KIT signaling synergistically
destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov.
5:304–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hayashi Y, Bardsley MR, Toyomasu Y,
Milosavljevic S, Gajdos GB, Choi KM, Reid-Lombardo KM, Kendrick ML,
Bingener-Casey J, Tang CM, et al: Platelet-Derived Growth Factor
Receptor-α Regulates proliferation of gastrointestinal stromal
tumor cells with mutations in KIT by Stabilizing ETV1.
Gastroenterology. 149:420–432.e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sakamaki K, Funasaka K, Miyahara R,
Furukawa K, Yamamura T, Ohno E, Nakamura M, Kawashima H, Hirooka Y,
Fujishiro M and Goto H: Low ETV1 mRNA expression is associated with
recurrence in gastrointestinal stromal tumors. Sci Rep.
10:147672020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Han W, Pelkey KA, Duan J, Mao X,
Wang YX, Craig MT, Dong L, Petralia RS, McBain CJ and Lu W:
Molecular Dissection of Neuroligin 2 and Slitrk3 reveals an
essential framework for GABAergic Synapse Development. Neuron.
96:808–826.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Agaimy A: Gastrointestinal stromal tumors
(GIST) from risk stratification systems to the new TNM proposal:
More questions than answers? A review emphasizing the need for a
standardized GIST reporting. Int J Clin Exp Pathol. 3:461–471.
2010.PubMed/NCBI
|
|
46
|
Hotzel J, Melling N, Muller J, Polonski A,
Wolters-Eisfeld G, Izbicki JR, Karstens KF and Tachezy M: Protein
expression of close homologue of L1 (CHL1) is a marker for overall
survival in non-small cell lung cancer (NSCLC). J Cancer Res Clin
Oncol. 145:2285–2292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Karstens KF, Bellon E, Polonski A,
Wolters-Eisfeld G, Melling N, Reeh M, Izbicki JR and Tachezy M:
Expression and serum levels of the neural cell adhesion molecule
L1-like protein (CHL1) in gastrointestinal stroma tumors (GIST) and
its prognostic power. Oncotarget. 11:1131–1140. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Thys A, Vandenberghe P, Hague P, Klein OD,
Erneux C and Vanderwinden JM: Hyperplasia of interstitial cells of
cajal in sprouty homolog 4 deficient mice. PLoS One.
10:e01248612015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vandenberghe P, Hague P, Hockman SC,
Manganiello VC, Demetter P, Erneux C and Vanderwinden JM:
Phosphodiesterase 3A: A new player in development of interstitial
cells of Cajal and a prospective target in gastrointestinal stromal
tumors (GIST). Oncotarget. 8:41026–41043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dragomir M, Mafra ACP, Dias SMG, Vasilescu
C and Calin GA: Using microRNA networks to understand cancer. Int J
Mol Sci. 19:18712018. View Article : Google Scholar
|
|
52
|
Isosaka M, Niinuma T, Nojima M, Kai M,
Yamamoto E, Maruyama R, Nobuoka T, Nishida T, Kanda T, Taguchi T,
et al: A screen for epigenetically silenced microRNA genes in
gastrointestinal stromal tumors. PLoS One. 10:e01337542015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Li J, Kuang D, Wang X, Zhu Y, Xu
S, Chen Y, Cheng H, Zhao Q, Duan Y and Wang G: MiR-148b-3p
functions as a tumor suppressor in GISTs by directly targeting KIT.
Cell Commun Signal. 16:162018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fan R, Zhong J, Zheng S, Wang Z, Xu Y, Li
S, Zhou J and Yuan F: MicroRNA-218 inhibits gastrointestinal
stromal tumor cell and invasion by targeting KIT. Tumour Biol.
35:4209–4217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yun S, Kim WK, Kwon Y, Jang M, Bauer S and
Kim H: Survivin is a novel transcription regulator of KIT and is
downregulated by miRNA-494 in gastrointestinal stromal tumors. Int
J Cancer. 142:2080–2093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fan R, Zhong J, Zheng S, Wang Z, Xu Y, Li
S, Zhou J and Yuan F: MicroRNA-218 increase the sensitivity of
gastrointestinal stromal tumor to imatinib through PI3K/AKT
pathway. Clin Exp Med. 15:137–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Niinuma T, Suzuki H, Nojima M, Nosho K,
Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki
Y, et al: Upregulation of miR-196a and HOTAIR drive malignant
character in gastrointestinal stromal tumors. Cancer Res.
72:1126–1136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Niinuma T, Kai M, Kitajima H, Yamamoto E,
Harada T, Maruyama R, Nobuoka T, Nishida T, Kanda T, Hasegawa T, et
al: Downregulation of miR-186 is associated with metastatic
recurrence of gastrointestinal stromal tumors. Oncol Lett.
14:5703–5710. 2017.PubMed/NCBI
|
|
59
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Awasthi R, Rathbone MJ, Hansbro PM, Bebawy
M and Dua K: Therapeutic prospects of microRNAs in cancer treatment
through nanotechnology. Drug Deliv Transl Res. 8:97–110. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mansoori B, Mohammadi A, Shirjang S and
Baradaran B: MicroRNAs in the diagnosis and treatment of cancer.
Immunol Invest. 46:880–897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Catela Ivkovic T, Voss G, Cornella H and
Ceder Y: MicroRNAs as cancer therapeutics: A step closer to
clinical application. Cancer Lett. 407:113–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rusakiewicz S, Semeraro M, Sarabi M,
Desbois M, Locher C, Mendez R, Vimond N, Concha A, Garrido F,
Isambert N, et al: Immune infiltrates are prognostic factors in
localized gastrointestinal stromal tumors. Cancer Res.
73:3499–3510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tan Y, Garcia-Buitrago MT, Trent JC and
Rosenberg AE: The immune system and gastrointestinal stromal tumor:
A wealth of opportunities. Curr Opin Oncol. 27:338–342. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tan Y, Trent JC, Wilky BA, Kerr DA and
Rosenberg AE: Current status of immunotherapy for gastrointestinal
stromal tumor. Cancer Gene Ther. 24:130–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Balachandran VP, Cavnar MJ, Zeng S,
Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C,
Rossi F, et al: Imatinib potentiates antitumor T cell responses in
gastrointestinal stromal tumor through the inhibition of Ido. Nat
Med. 17:1094–1100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Seifert AM, Zeng S, Zhang JQ, Kim TS,
Cohen NA, Beckman MJ, Medina BD, Maltbaek JH, Loo JK, Crawley MH,
et al: PD-1/PD-L1 Blockade Enhances T-cell activity and antitumor
efficacy of imatinib in gastrointestinal stromal tumors. Clin
Cancer Res. 23:454–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bertucci F, Finetti P, Mamessier E,
Pantaleo MA, Astolfi A, Ostrowski J and Birnbaum D: PDL1 expression
is an independent prognostic factor in localized GIST.
Oncoimmunology. 4:e10027292015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui
Y, Yi M, Xia L, Zhuang W, Wu X and Zhou Y: PD-1/PD-L1 blockade
rescue exhausted CD8+ T cells in gastrointestinal stromal tumours
via the PI3K/Akt/mTOR signalling pathway. Cell Prolif.
52:e125712019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Toulmonde M, Penel N, Adam J, Chevreau C,
Blay JY, Le Cesne A, Bompas E, Piperno-Neumann S, Cousin S,
Grellety T, et al: Use of PD-1 Targeting, Macrophage Infiltration,
and IDO pathway activation in sarcomas: A Phase 2 clinical trial.
JAMA Oncol. 4:93–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang HC, Li TY, Chao YJ, Hou YC, Hsueh YS,
Hsu KH and Shan YS: KIT Exon 11 Codons 557–558 deletion mutation
promotes liver metastasis through the CXCL12/CXCR4 axis in
gastrointestinal stromal tumors. Clin Cancer Res. 22:3477–3487.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
De Simone M, Arrigoni A, Rossetti G,
Gruarin P, Ranzani V, Politano C, Bonnal RJP, Provasi E, Sarnicola
ML, Panzeri I, et al: Transcriptional landscape of human tissue
lymphocytes unveils uniqueness of Tumor-Infiltrating T regulatory
cells. Immunity. 45:1135–1147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tanaka A and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Cell Res. 27:109–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Soler D, Chapman TR, Poisson LR, Wang L,
Cote-Sierra J, Ryan M, McDonald A, Badola S, Fedyk E, Coyle AJ, et
al: CCR8 expression identifies CD4 memory T cells enriched for
FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol.
177:6940–6951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12 / CXCR4 / CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yesilkanal AE and Rosner MR: Targeting Raf
kinase inhibitory protein regulation and function. Cancers (Basel).
10:3062018. View Article : Google Scholar
|
|
77
|
Wang Y, Chen JJ, Wang XF and Wang Q:
Clinical and prognostic significance of Raf kinase inhibitory
protein expression in gastrointestinal stromal tumors. World J
Gastroenterol. 24:2508–2517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yesilkanal AE and Rosner MR: Raf kinase
inhibitory protein (RKIP) as a metastasis suppressor: Regulation of
signaling networks in cancer. Crit Rev Oncog. 19:447–454. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Valadao M, Braggio D, Santos AF,
Pimenta-Inada HK, Linhares E, Gonçalves R, Romano S, Vilhena B,
Small I, Cubero D, et al: Involvement of signaling molecules in the
prediction of response to imatinib treatment in metastatic GIST
patients. J Surg Res. 178:288–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang Y and Weinberg RA:
Epithelial-to-mesenchymal transition in cancer: Complexity and
opportunities. Front Med. 12:361–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kothari AN, Arffa ML, Chang V, Blackwell
RH, Syn WK, Zhang J, Mi Z and Kuo PC: Osteopontin-A master
regulator of Epithelial-Mesenchymal transition. J Clin Med.
5:392016. View Article : Google Scholar
|
|
83
|
Shevde LA and Samant RS: Role of
osteopontin in the pathophysiology of cancer. Matrix Biol.
37:131–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hsu KH, Tsai HW, Lin PW, Hsu YS, Shan YS
and Lu PJ: Osteopontin expression is an independent adverse
prognostic factor in resectable gastrointestinal stromal tumor and
its interaction with CD44 promotes tumor proliferation. Ann Surg
Oncol. 17:3043–3052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Senbanjo LT and Chellaiah MA: CD44: A
multifunctional cell surface adhesion receptor is a regulator of
progression and metastasis of cancer cells. Front Cell Dev Biol.
5:182017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hu JC, Wang Q, Jiang LX, Cai L, Zhai HY,
Yao ZW, Zhang ML and Feng Y: Effect of long non-coding RNA AOC4P on
gastrointestinal stromal tumor cells. Onco Targets Ther.
11:6259–6269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang K, Lu C, Huang X, Cui J, Li J, Gao
Y, Liang W, Liu Y, Sun Y, Liu H, et al: Long noncoding RNA AOC4P
regulates tumor cell proliferation and invasion by
epithelial-mesenchymal transition in gastric cancer. Therap Adv
Gastroenterol. 12:17562848198276972019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hao Y, Baker D and Ten Dijke P:
TGF-beta-Mediated Epithelial-Mesenchymal transition and cancer
metastasis. Int J Mol Sci. 20:27672019. View Article : Google Scholar
|
|
89
|
Kovecsi A, Gurzu S, Szentirmay Z, Kovacs
Z, Bara TJ and Jung I: Paradoxical expression pattern of the
epithelial mesenchymal transition-related biomarkers CD44, SLUG,
N-cadherin and VSIG1/Glycoprotein A34 in gastrointestinal stromal
tumors. World J Gastrointest Oncol. 9:436–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pulkka OP, Nilsson B, Sarlomo-Rikala M,
Reichardt P, Eriksson M, Hall KS, Wardelmann E, Vehtari A, Joensuu
H and Sihto H: SLUG transcription factor: A pro-survival and
prognostic factor in gastrointestinal stromal tumour. Br J Cancer.
116:1195–1202. 2017. View Article : Google Scholar : PubMed/NCBI
|