Introduction
Due to the aging population and numerous
environmental factors, the incidence of malignant tumors is
increasing, which poses a major threat to human health (1,2).
Patients with malignant tumors often experience very subtle signs
and symptoms. This, combined with a lack of early diagnostic
methods, lead to diagnosis at advanced stages and missed
opportunity for early and effective treatments. In addition, due to
the heterogeneity of tumor cells with respect to gene expression,
metabolic activity, proliferation and metastatic potential, actual
treatment methods often fail to achieve satisfactory therapeutic
outcomes. Personalized diagnostic methods and therapeutic options
are therefore needed to improve the diagnosis and treatment of
malignant tumors (3).
Previous studies have reported that fat metabolism
is related to the development and progression of cancer (4,5). Fatty
acid metabolism plays a key role in maintaining the
microenvironment of malignant tumors, and lipid droplet metabolism
has been demonstrated to be highly involved in the development and
progression of multiple types of tumor (4). Adiponectin, an adipocytokine secreted
by fat cells, exhibits some insulin-sensitizing, anti-diabetic,
anti-atherosclerosis and anti-inflammatory properties on all cells
(4). Furthermore, adiponectin was
reported to be strongly associated with the development of several
types of tumor, such as prostate cancer and breast tumor,
suggesting its potential role as a therapeutic target and marker
(6–8). Subsequently, it is hypothesized that
the effects of fat metabolism on tumors may be associated with
adiponectin and its homologs.
Complement Cq1/tumor necrosis factor-related protein
(CTRP), a member of the adipokine superfamily expressed in and
excreted by adipose tissue, has a high degree of sequence homology
with adiponectin (9). Previous
studies have demonstrated that CTRP family members are involved in
the regulation of multiple physiological and pathological
processes, including glucose and lipid metabolism, inflammation,
cartilage formation and development, myocardial protection and
vasodilation (10,11). In addition, CTRPs might also be
involved in the pathogenesis of multiple sclerosis and may be
considered as biomarkers or therapeutic targets (12). The role of the CRTP family in cancer
has attracted great interest and is being extensively studies. At
present, several CTRP family members are considered as molecular
mediators that can regulate tumorigenesis and tumor cell invasion
and metastasis (13,14). The present review aimed to determine
the pathophysiological roles of CTRP family members in the
development and progression of different types of tumor. CTRPs may
therefore be considered as potential new targets for future
research and clinical treatment of cancers.
CTRP family: General characteristics
Previous studies have demonstrated that adipose
tissue serves as the primary energy storage organ of the body and
has potent endocrine functions, since it secretes a variety of
adipokines. CTRP is an adipokine superfamily of proteins discovered
by Wong et al (15), which
are structurally similar to adiponectin (15) and exhibit numerous functions,
including blood lipid regulation (16,17),
insulin sensitization (18) and
anti-inflammatory effects (19).
Structurally, CTRPs are predominantly homotrimers; however, some
CTRPs exist as heterohexamers (20).
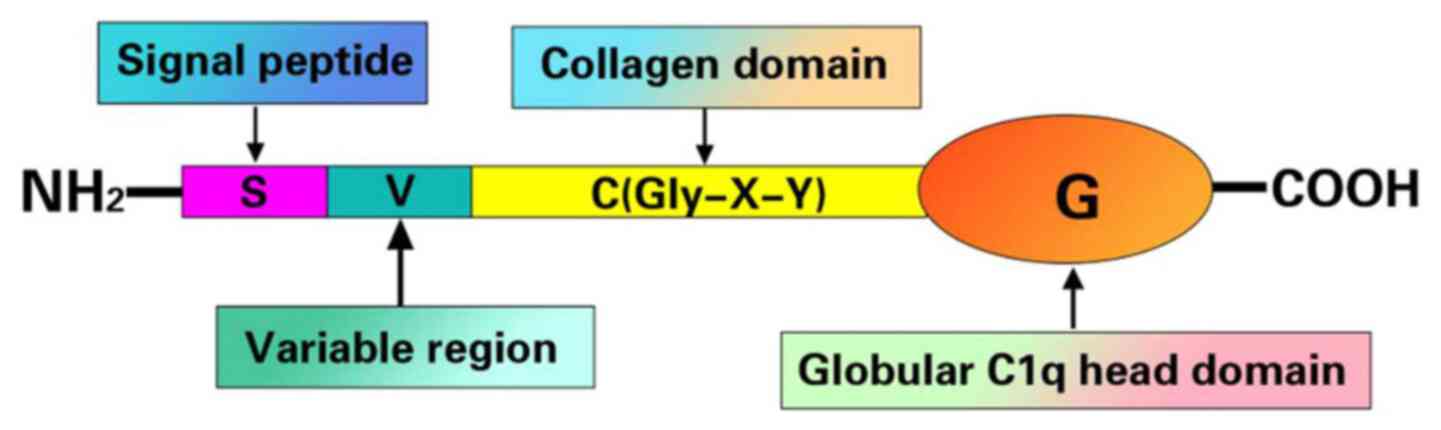
Apart from CTRP4, all CTRPs consist of an amino-terminal signal
peptide, a short variable region, a collagen-like domain and most
importantly, a globular carboxy-terminal domain homologous to the
complement protein C1q, which is crucial for their biological
functions (9,13) (Fig.
1).
Four CTRP family members, CTRP3, CTRP4, CTRP6 and
CTRP8, have been reported to be associated with tumor promotion. In
humans, CTRP3 mRNA is expressed in multiple tissues and organs,
including adipose tissue, cartilage and the kidney, and it is
highly abundant in the lung and spleen (21,22). It
was reported that CTRP3 is the closest functional homolog of
adiponectin, whereas CTRP4 is a classic secreted protein. Unlike
other CTRPs, CTRP4 contains two globular C1q domains in series and
lacks a collagen-like domain (23).
In mice, increased CTRP4 level has been observed in the heart,
liver, brain and kidneys (10). In
humans, CTRP4 is expressed in the testes, kidneys, fat and brain.
In the brain, it is primarily expressed and secreted by neurons
(23). A recent study demonstrated
that CTRP4 is not only associated with metabolism but is also
involved in the regulation of bone metabolism and the promotion of
osteoblast differentiation (24).
CTRP6 is extensively expressed in the uterus, placenta, skin, lungs
and fat in humans. CTRP6 functions as a novel
metabolic/immunomodulator by binding to a variety of microorganisms
and endogenous ligands (25) and
acts as an intermediate between obesity and the inflammation of
adipose tissue or insulin resistance (26). The last tumor-associated CTRP, CTRP8,
is the least studied protein. This is partly attributed to the fact
that CTRP8 is not expressed in mice, which makes its study more
difficult. In humans, CTRP8 is expressed in the lungs and testes
(27). It is currently known that
CTRP3 promotes the proliferation of osteosarcoma cells (28). CTRP4 and CTRP6 are involved in the
survival of human liver cancer cells and tumor tissue angiogenesis
(14). CTRP8 enhances the
invasiveness of glioblastoma (GBM) (29). The role and underlying mechanisms of
other CTRP family members in tumors require further
investigation.
Osteosarcoma (OS) and CTRP3
Previous studies have reported that CTRP3 is
strongly associated with OS (29,30),
which is the most common malignant bone tumor in adolescents, with
an incidence of ~0.0004% worldwide. CTRP3 is a growth factor which
presents two subtypes in humans, CTRP3A and CTRP3B. CTRP3 protein
expression was demonstrated to be increased in the mouse OS cell
lines NHOS and LM8, and it was shown to promote cell proliferation,
and the effect of promoting cell proliferation is increased with
the increase in CTRP3 level (30).
In cartilage progenitor and endothelial cells, CTRP3 can activate
the MAPK and PI3K/AKT pathways (27,31),
which are crucial for cell proliferation, in response to growth
factors and other control signals (32,33). The
progression of OS is induced by a complex intracellular signaling
network, and both the MAPK and PI3K/AKT pathways are key regulators
of the proliferation and survival of OS cells (34,35).
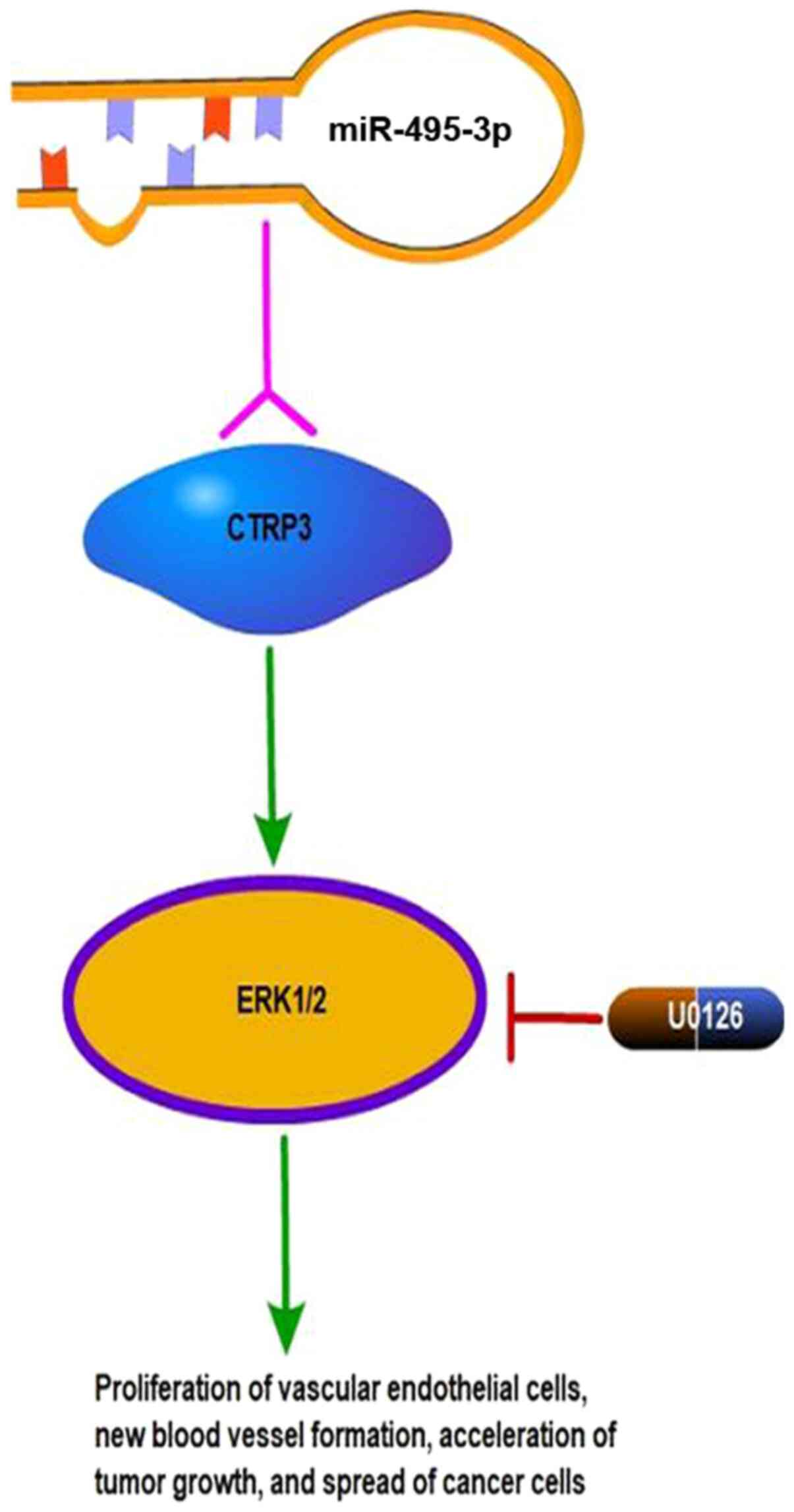
Exogenous CTRP3 can promote the proliferation of OS
cells via the ERK1/2 pathway, and activation of the ERK1/2 pathway
is associated with tumor angiogenesis. U0126, which is an inhibitor
of the MAPK/ERK1/2 (MEK1/2) pathway, can block CTRP3-mediated cell
proliferation (36), suggesting that
CTRP3 may play an important role in the development of OS. One
possible mechanism may involve the promotion of vascular
endothelial cell proliferation and new blood vessel formation via
the MAPK/ERK1/2 signal transduction pathway, which would accelerate
tumor growth and facilitate the spread of tumor cells (Fig. 2). MEK5 also plays an important role
in the occurrence and development of tumors and can be inhibited by
U0126. Subsequently, MEK5 is likely to play a role in the
development of OS via one of the pathways regulated by CTRP3.
However, there are currently no supporting evidence. Further
investigation is therefore needed to explore this possibility. A
previous study demonstrated that overexpression of the micro-RNA
(miR)-495-3p in human OS cell lines inhibits the proliferation,
migration and invasion of OS cells by downregulating the expression
of CTRP3, which is a direct target of miR-495-3p (36). CTRP3 could therefore be considered as
a therapeutic target for OS. In addition, Kim et al
(37) demonstrated that CTRP3 has a
negative effect on osteoclast bone resorption activity both in
vitro and in vivo through inhibition of osteoclast
formation by acting directly on osteoclast precursors. Based on
these data, the authors proposed that CTRP3 could be a potential
treatment target for bone diseases associated with excessive
osteoclast differentiation and bone destruction. At present, the
underlying mechanism of CTRP3 effect in OS progression is unknown,
and identification of the CTRP3 receptor would facilitate the
investigation of its function and help determining its potential
use in clinical application. These findings should be further
verified in additional cell lines.
The role of CTRP4 and CTRP6 in liver
cancer
Chronic infection and inflammation are primary
predisposing factors for several types of tumor. For example,
simultaneous infection with hepatitis B and hepatitis C viruses
increases the risk of developing hepatocellular carcinoma (38). In addition, inflammatory signals play
key roles in tumor development and progression, such as JAK/signal
transducer and activator of transcription 3 (STAT3) in multiple
myeloma (39) and PPARγ/nuclear
factor-κB (NF-κB) in lung cancer (40). CTRP4, which is highly expressed in
hepatocytes and bile duct epithelial cells, is a novel
tumor-promoting factor involved in the regulation of inflammatory
signaling pathways in tumor cells. Minimal expression of CTRP4 mRNA
has been observed in the hepatoma cell line HepG2 and the human
colon cancer cell line HT29 (38).
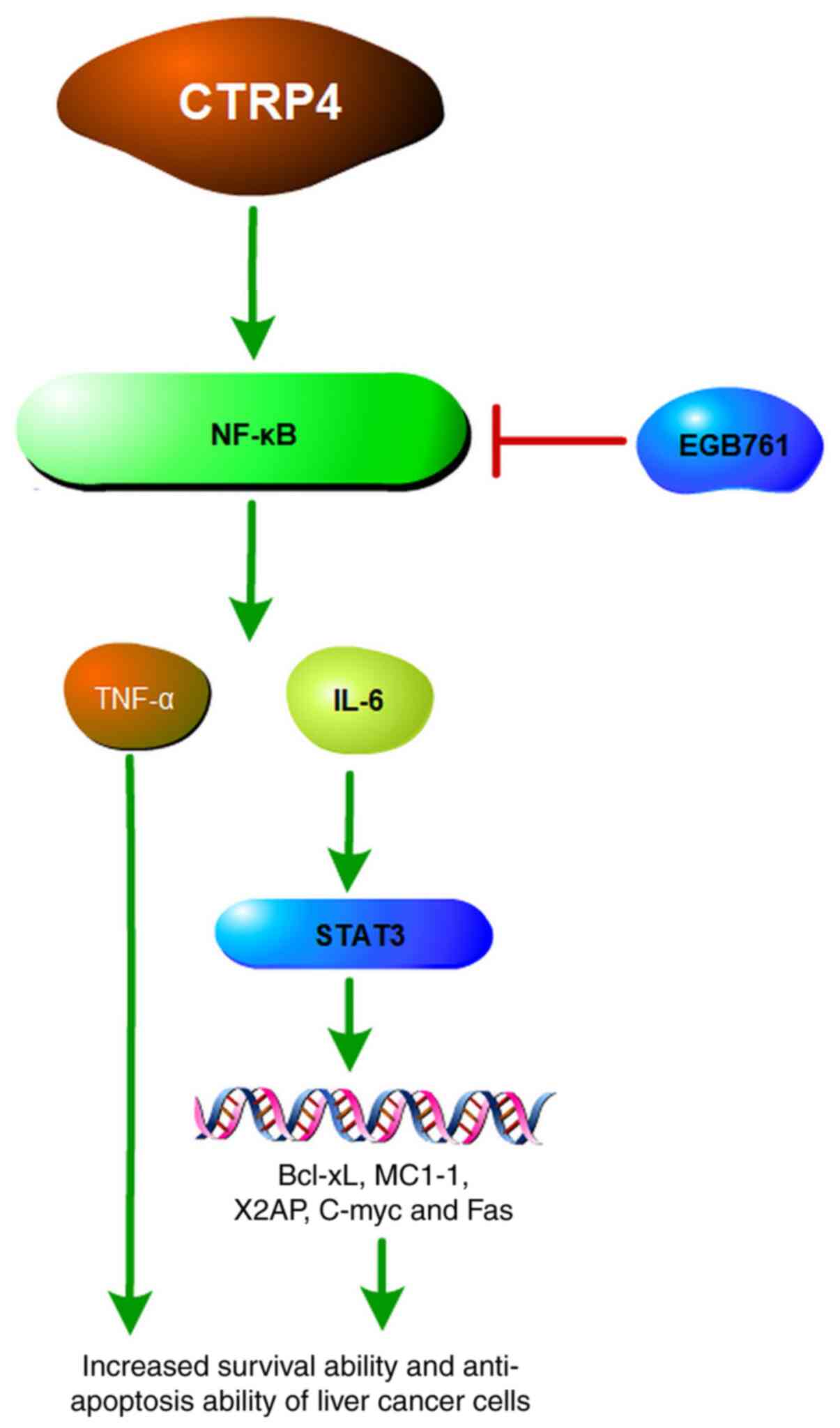
Furthermore, overexpression of CTRP4 activates the NF-κB signaling
pathway, which in turn induces the production of multiple
tumor-related cytokines, such as interleukin (IL-)6 and tumor
necrosis factor-α (TNF-α) (41).
The NF-κB signaling pathway induces and maintains a
chronic inflammation microenvironment, which is the most important
mechanism of tumorigenesis (42).
The Ginkgo biloba extract EGB761 has been shown to induce
apoptosis in hepatoma cells by inhibiting the NF-κB/p53 signaling
pathway (43). IL-6 is related to
liver cancer and IL-6 signaling upregulates androgen receptor
expression. Androgen receptors decrease the expression of tumor
suppressor p53 and enhances the production of reactive oxygen
species (ROS) and subsequent DNA damage and mutation, eventually
contributing to malignant transformation of liver cells (44). A previous study demonstrated that
inhibition of IL-6 by estrogen can reduce the risk of liver cancer
in women (45). IL-6 is also the
primary cytokine that regulates the STAT3 pathway. A previous study
reported that CTRP4 can activate STAT3 signaling pathway (46), which regulates the transcription of
various genes, including apoptosis suppressor genes Bcl-xL, MC1-1,
X2AP and two-way regulatory gene C-myc. Through STAT3 pathway,
CTRP4 regulates the survival, proliferation, differentiation and
invasion of tumor cells as well as inflammation and
immunosuppression (47). A previous
study in animal models have demonstrated that colorectal carcinoma
cell-derived TNF-α can facilitate tumor growth and metastasis
(48). Studies examining the effects
of chemotherapy on the hepatoma cell line HepG2 have reported that
activation of inflammatory factors by CTRP4 can promote tumor cell
survival through the activation of anti-apoptosis pathways
(14,49). Although there is no direct evidence
that CTRP4 could induce liver cancer, it is reasonable to propose
CTRP4 as a potential hepatocarcinogen that may upregulate the
expression of inflammatory factors by activating the NF-κB/STAT3
signaling pathway, regulating therefore the pathological processes
of liver cancer (Fig. 3).
Another member of the CTRP family, CTRP6, is also
overexpressed in liver cancer. A previous study reported that CTRP6
mediates a dose-dependent increase in IL-10 synthesis by
macrophages following activation of ERK1/2 (24), suggesting that CTRP6 might have some
anti-inflammatory effects. In addition, CTRP6 inhibits the
IL-8/VEGF pathway to inhibit the proliferation and metastasis of
ovarian cancer cells (50). However,
in Hep3B hepatoma cells, CTRP6 knockout triggers apoptosis and
inhibits cell migration and invasion (51), suggesting that CTRP6 could have
either carcinogenic or anti-tumor effects depending on the type of
cancer. Overexpression of CTRP6 in hepatoma cells not only
facilitates the phosphorylation of AKT in sinusoidal endothelial
cells but also promotes tumor angiogenesis and inhibits the
apoptosis of hepatocellular carcinoma cells (52). Furthermore, inhibition of CTRP6
blocks the migration and survival of hepatocellular carcinoma cells
by inactivating the AKT signaling pathway (49). In xenotransplants of hepatoma cells,
CTRP6 reduces the area of central intravascular necrosis by
accelerating tumor neovascularization (53). In a study on clear cell renal cell
carcinoma (ccRCC), Lin et al demonstrated that CTRP6
mediates tumorigenesis by modulating the cell cycle at the G2M
checkpoint, epithelial-mesenchymal transition and angiogenesis.
Furthermore, CTRP6 may also participate in tumor progression by
activating the AKT and ERK1/2 signaling pathways (54). According to these findings, the
underlying mechanism of CTRP6 in hepatoma cells is likely to be
similar to that in ccRCC; however, further investigation is
required. Modulating the levels of secretory CTRP6 may therefore be
considered as a new treatment approach for liver cancer.
Roles of CTRP4 and CTRP6 in colon
cancer
Colon cancer is a primary malignant tumor of the
digestive tract. The risk factors for colon cancer are complex and
diverse, and the mechanisms underlying its development and
progression remain unclear. However, it is well known that some
inflammatory factors are implicated in the development of colon
cancer, and a variety of inflammatory mediators, including IL-6,
TNF-α and IL-10, have been reported to be involved in regulating
the progression and metastasis of colon cancer (55,56).
Previous studies have reported an increased expression of CTRP6 in
colon cancer tissues, regardless of patient sex and age, tumor
size, degree of differentiation and depth of invasion (57). In addition, CTRP6 overexpression was
demonstrated to decrease the expression levels of pro-inflammatory
factors, such as IL-1β, IL-6 and TNF-α, and increase the expression
of the anti-inflammatory factor IL-10 (58). A decrease in CTRP6 expression
increases the aggressiveness of ovarian cancer cells, while CTRP6
overexpression inhibits the proliferation of cancer cells (51). Conversely, CTRP6 knockout in
glomerular mesangial cells inhibits the generation of intracellular
ROS and inflammation (59). The
expression level of CTRP6 is significantly increased in high
glucose-induced glomerular mesangial cells, and CTRP6 knockdown
causes significant decrease in TNF-α, IL-1β and IL-6 production
levels in these cells. Treatment with LY294002, an inhibitor of
Akt, reverses the induction effects of CTRP6 overexpression on ROS
production, inflammation and extracellular matrix accumulation in
mesangial cells. In addition, activation of the AKT/NF-κB pathway
promotes the growth, migration and invasion of colon cancer cells
(60). Because of these
discrepancies, the specific underlying mechanisms of CTRP6 in colon
cancer remain to be determined. However, CTRP6 is closely related
to the occurrence and development of colon cancer.
CTRP4 expression is increased in the human colon
cancer cell line HT29 where it functions as a dual activator of
NF-κB and IL-6 (41), which are
important regulators of cancer development and progression.
However, a previous study on mice has shown that overexpression of
CTRP4 not only inhibits the production of IL-6 but also inhibits
the initiation of colon cancer induced by DSS/AOM, which is a
method used to extablish colon cancer model through drugs (47). These findings were further supported
by in vitro experiments. For example, the human recombinant
CTRP4 can inhibit the lipopolysaccharide-induced overexpression of
the inflammatory factor TNF-α in macrophages, suggesting that CTRP4
may have a potential protective factor in inflammatory bowel
disease (61), which is an
independent risk factor for colon cancer (62). The role of CTRP4 in colon cancer
remains therefore controversial. Although the therapeutic
significance of CTRP4 in colon cancer has yet to be determined, it
is clear that CTRP4 is involved in the regulation of the
inflammatory network and that it serves a crucial role in the
development of colon cancer.
GBM and CTRP8
GBM is a malignant brain cancer with the highest
malignancy grade among astrocytic tumors (63). CTRP8 was recently discovered as a
novel ligand of the G protein-coupled receptor relaxin family
peptide receptor-1 (RXFP1) and was detected in fatal GBM cases
(64). CTRP8 can activate the PI3K,
PKC and JAK2/STAT3 signaling cascade in brain tumor cells (65) Owing to its important molecular role
in cancer, RXFP1 has been extensively investigated in breast,
endometrial, prostate and thyroid cancers. The effects of RXFP1
activation in GBM cells include increased cell motility, tumor
expansion, tissue invasion and metastasis (66), tumor growth and angiogenesis
(67). The mechanism underlying
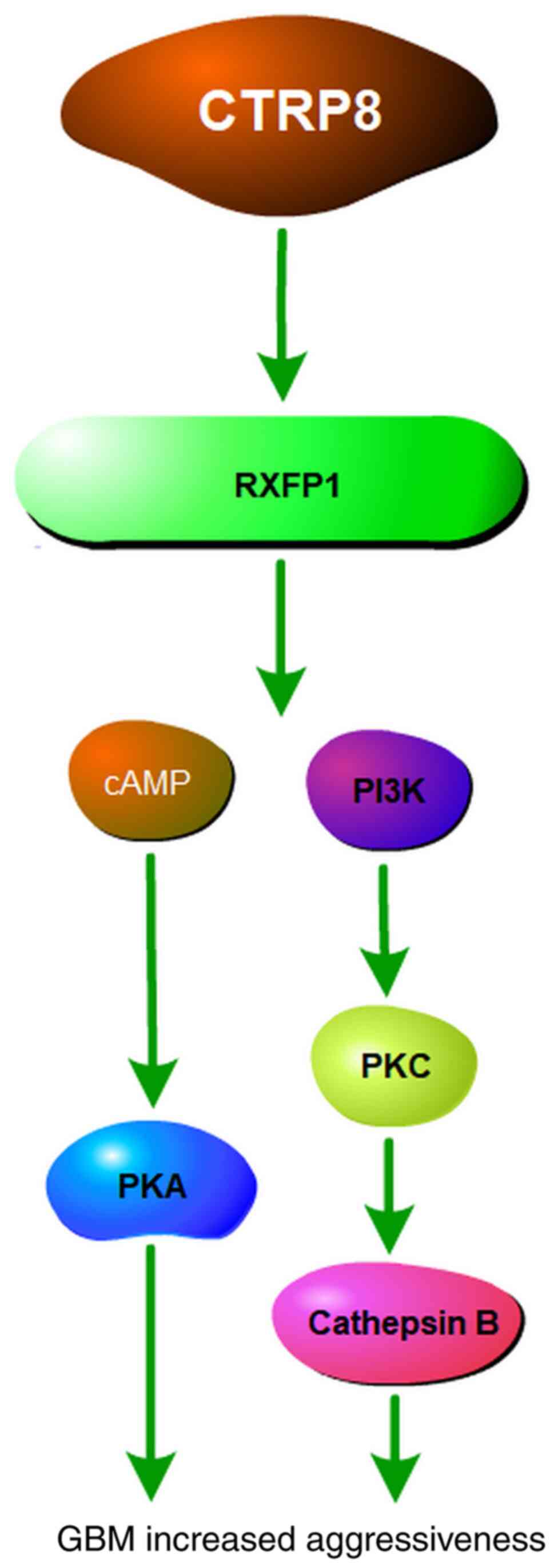
CTRP8/RXFP1-mediated aggressiveness of GBM tumors involves the
RXFP1/PI3K/PKC/cathepsin B signaling cascade. In GBM cells, two
biologically active peptides with homologous sequences in the
N-terminal region of the C1q globular domain of CTRP8 activate
RXFP1 via cAMP and PI3K-PKC/PKC-ERK1/2 signaling. In addition,
activation of RXFP1 by CTRP8 increases the production and secretion
of cathepsin-B protein and increases the invasiveness of GBM cells
via the laminin matrix (68). The
U251 GMB cell line and HEK293 cells, which do not express RXFP1 but
have exogenous expression of RXFP2, do not respond to elevated
cAMP, indicating that the RXFP1-mediated signal activation is
cell-type specific (27). In
addition, the inhibition of cathepsin B secretion and invasion by
CTRP6 suggests that CTRP6 may act as a competitor of CTRP8 for
binding to RXFP1 and may subsequently block CTRP8 signaling as the
concentration of CTRP6 increases (66). We therefore hypothesized that CTRP6
may affect the development and progression of GBM. However, further
clinical verification is required. A recent study by Thanasupawat
et al (64) reported a novel
role for CTRP8 in the protection of GBM cells from DNA alkylation
damage induced by the chemotherapeutic drug temozolomide (TMZ)
(69). This study showed that the
mechanism underlying CTRP8-induced chemotherapy resistance involves
activation of the newly discovered RXFP1/STAT3 signaling pathway
and upregulation of a new target of this pathway, N-methylpurine
DNA glycosylase. This mechanism boosts the DNA repair system and
consequently promotes activation of the anti-apoptotic pathway
involving Bcl-2 and Bcl-XL in GBM cells. Treatment with TMZ
increases cell resistance to DNA alkylation via
CTRP8/-RXFP1/-STAT3, thereby improving the survival rates of
patients with GBM. The effects of CTRP8 in GBM are summarized in
Fig. 4.
Tumor-related roles of the other CTRP family
members
A recent study demonstrated that CTRP1 mRNA
expression is significantly higher in GBM tissues compared with
normal tissues. Knockout of the CTRP1 gene significantly
inhibits the proliferation and migration of human GBM cells,
suggesting that CTRP1 may promote the progression of human GBM,
thereby leading to a poor prognosis in patients (70). Regarding the development and
progression of chondroblastoma, CTRP3 induces the activation of
liver AKT signaling pathway and inhibits the expression of liver
gluconeogenesis enzymes, thus reducing liver gluconeogenesis and
lowering glucose levels, which promote the proliferation of
chondrogenic cells (27,71). Qu et al (72) reported CTRP8 overexpression in
gastric cancer as well as its involvement in the proliferation and
migration of gastric cancer cells. CTRP6 downregulation induces
cell cycle arrest at the G2M checkpoint and apoptosis in gastric
cancer cells. This finding indicates that CTRP6 increases the
sensitivity of gastric cancer cells to apoptosis. In addition, as a
direct target of miR-29b, CTRP6 regulates tumor progression and may
function as either a tumor promoter or a tumor suppressor,
depending on the type of cell or tissue. Downregulation of CTRP6 in
MCF-7 breast cancer cells increases tumor aggressiveness (73), while CTRP6 overexpression in SKOV3,
3AO and HO8910 epithelial ovarian cancer cells inhibits
proliferation and migration by blocking the IL-8/VEGF pathway.
Furthermore, a previous study reported that CTRP6 expression is
increased in ccRCC and is positively correlated with disease stage
(51). A very recent study
demonstrated that inhibition of CTRP6 can attenuate cell
proliferation, migration, invasion and promote apoptosis in
vitro and in vivo in non-small cell lung cancer
(74). However, in a previous study,
aberrant C1QTNF6 expression is implicated in terrible prognosis
accompanied with damage of cell potential in lung adenocarcinoma
(75). CRTP6 seems therefore to play
opposite roles in different studies. All these studies are still at
early stages and the underlying mechanisms of numerous CTRPs remain
unknown.
Conclusions
By activating numerous signaling pathways, the CTRP
family plays important roles in human cancers in various tissues
and organs, such as the digestive tract, brain and bone. CTRP3
promotes angiogenesis in bone tumors by activating the ERK1/2
pathway, thereby accelerating tumor growth and promoting the spread
of cancer cells (27). CTRP4
modulates tumor development and progression by activating the
NF-κB/STAT3 signaling pathway in hepatocytes and colon cancer cells
and inducing the expression of multiple tumor-associated cytokines
(10). CTRP6 exhibits both
carcinogenic and anti-tumor effects, which are cancer-type
dependent. CTRP6 is expressed in colon cancer and ovarian cancer
cells where it exerts different functions. In colon cancer, CTRP6
inhibits tumor development and progression by decreasing
pro-inflammatory factors and increasing anti-inflammatory factors
(26). In ovarian cancer, CTRP6
inhibits the proliferation and migration of cancer cells by
blocking the expression of IL-8 and vascular endothelial growth
factor pathways (52). Conversely,
overexpression of CTRP6 in hepatocellular carcinoma increases tumor
angiogenesis, whereas CTRP6 silencing can activate the AKT
signaling pathway. Subsequently, CTRP6 promotes apoptosis in
hepatoma cells and prevents cell invasion. The effects of CTRP8
have been reported in brain tumors. CTRP8 binds to RXFP1 to
activate the RXFP1/PI3K/PKC/cathepsin B signaling pathway and
consequently increases the invasiveness of GBM cells (29). The roles of these CTRP family members
have also been investigated in other cancers, such as gastric
cancer, ovarian cancer and renal cell carcinoma, and are summarized
in Table I. CTRP family members are
widely expressed, which provides the basis for their function in
multi-system tumors. However, studies examining CTRPs are still
insufficient, and further investigation is crucial to expand our
understanding on CTRP biological functions and CTRP-related
diseases. At present, studies demonstrated that CTRPs have the
potential to be considered as therapeutic targets in numerous types
of cancer, which offers glimmer of hope for potential treatment
strategies.
 | Table I.Roles and underlying mechanism of
CTRP family members in various types of tumor. |
Table I.
Roles and underlying mechanism of
CTRP family members in various types of tumor.
|
| Location | Related
pathways | Relationship with
tumor | Function | (Refs.) |
|---|
| CTRP3 | Osteosarcoma,
chondroblastoma, giant cell tumor, fat cells, chondrocytes,
monocytes, fibroblasts, placenta, small intestine, pancreas, brain,
kidneys, thymus, ovaries | PI3K/AKT/eNos
pathway, NF-ĸB pathway, MAPK/ERK1/2 pathway | Promotes the growth
of osteosarcoma; is associated with the development and progression
of chondroblastoma | Improves
neovascularization; accelerates tumor growth; promotes the spread
of cancer cells; simulates cell proliferation | (21,22,28–37) |
| CTRP4 | Testes, kidneys,
fat, brain, hepatoma cells, colon cancer cells | NF-ĸB/STAT3
pathway | Participates in the
development of hepatoma cells; relationship with colon cancer is
controversial | Upregulates the
expression of inflammatory factors to promote the survival of
cancer cells and apoptosis resistance in tumors | (14,23,38,41,46,47,49,61,62) |
| CTRP6 | Uterus, skin,
placenta, lungs, fat | ERK1/2 pathway,
IL-8/VEGF pathway, AKT pathway | Promotes tumor
angiogenesis and inhibits the apoptosis of hepatocellular carcinoma
cells; inhibits the development of colon cancer; antagonizes the
effect of CTRP8 in glioblastoma; increases the aggressiveness of
breast cancer cells; participates in the proliferation and
migration of gastric cancer cells; inhibits the proliferation and
migration of ovarian cancer cells; is associated with advanced
clear cell renal cell carcinoma and lung cancer | Upregulates the
expression of IL-10; downregulates the expression of IL-1β, IL-6
and TNF-α; exerts anti-inflammatory effects; has carcinogenic and
anti-tumor effects depending on the type of cancer | (14,24–26,50–60,64,74,75) |
| CTRP8 | Lungs, testes | cAMP, PI3K/PKC and
PKC/ERK1/2 pathways | Is involved in the
aggressiveness of glioblastoma | Promotes cell
movement, tumor expansion, tissue invasion and metastasis by
activating the RXFP1 receptor | (27,28,63–68,72) |
Acknowledgements
Not applicable.
Funding
This study was supported by the Hebei Province
Talent Training Project (grant no. A201802018).
Availability of data and materials
Not applicable.
Authors' contributions
MK wrote the manuscript. YG, XG, YX and YY reviewed
and revised the manuscript. All authors have read and approved the
final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Archambault AN, Su YR, Jeon J, Thomas M,
Lin Y, Conti DV, Win AK, Sakoda LC, Lansdorp-Vogelaar I, Peterse
EFP, et al: Cumulative burden of colorectal cancer-associated
genetic variants is more strongly associated with early-onset vs.
late-onset cancer. Gastroenterology. 158:1274–1286.e12. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sagnelli E, Macera M, Russo A, Coppola N
and Sagnelli C: Epidemiological and etiological variations in
hepatocellular carcinoma. Infection. 48:7–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Işçi Bostanci E, Durmuş Y, Duru Çöteli SA,
Kayikçioğlu F and Boran N: Outcomes of the conservative management
of the patients with endometrial intraepithelial
neoplasia/endometrial cancer: Wait or treat! Turk J Med Sci. May
20–2021.(Epub ahead of print).
|
|
4
|
Heyn GS, Corrêa LH and Magalhães KG: The
impact of adipose tissue-derived miRNAs in metabolic syndrome,
obesity, and cancer. Front Endocrinol (Lausanne). 11:5638162020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marino N, German R, Rao X, Simpson E, Liu
S, Wan J, Liu Y, Sandusky G, Jacobsen M, Stoval M, et al:
Upregulation of lipid metabolism genes in the breast prior to
cancer diagnosis. NPJ Breast Cancer. 6:502020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Philp LK, Rockstroh A, Lehman M, Sadowski
MC, Bartonicek N, Wade JD, Otvos L and Nelson CC: Adiponectin
receptor activation inhibits prostate cancer xenograft growth.
Endocr Relat Cancer. 27:711–729. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llanos AAM, Yao S, Singh A, Aremu JB,
Khiabanian H, Lin Y, Omene C, Omilian AR, Khoury T, Hong CC, et al:
Gene expression of adipokines and adipokine receptors in the tumor
microenvironment: Associations of lower expression with more
aggressive breast tumor features. Breast Cancer Res Treat.
185:785–798. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Long X, Yang C, Chen H, Sharkey C,
Rashid K, Hu M, Liu Y, Huang Q, Chen Q, et al: Icaritin reduces
prostate cancer progression via inhibiting high-fat diet-induced
serum adipokine in TRAMP mice model. J Cancer. 11:6556–6564. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong GW, Krawczyk SA, Kitidis-Mitrokostas
C, Revett T, Gimeno R and Lodish HF: Molecular, biochemical and
functional characterizations of C1q/TNF family members:
Adipose-tissue-selective expression patterns, regulation by
PPAR-gamma agonist, cysteine-mediated oligomerizations,
combinatorial associations and metabolic functions. Biochem J.
416:161–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarver DC, Stewart AN, Rodriguez S, Little
HC, Aja S and Wong GW: Loss of CTRP4 alters adiposity and food
intake behaviors in obese mice. Am J Physiol Endocrinol Metab.
319:E1084–E1100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shanaki M, Shabani P, Goudarzi A, Omidifar
A, Bashash D and Emamgholipour S: The C1q/TNF-related proteins
(CTRPs) in pathogenesis of obesity-related metabolic disorders:
Focus on type 2 diabetes and cardiovascular diseases. Life Sci.
256:1179132020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rasooli Tehrani A, Gholipour S, Sharifi R,
Yadegari S, Abbasi-Kolli M and Masoudian N: Plasma levels of
CTRP-3, CTRP-9 and apelin in women with multiple sclerosis. J
Neuroimmunol. 333:5769682019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kishore U, Gaboriaud C, Waters P, Shrive
AK, Greenhough TJ, Reid KB, Sim RB and Arlaud GJ: C1q and tumor
necrosis factor superfamily: Modularity and versatility. Trends
Immunol. 25:551–561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schäffler A and Buechler C: CTRP family:
Linking immunity to metabolism. Trends Endocrinol Metab.
23:194–204. 2012. View Article : Google Scholar
|
|
15
|
Wong GW, Wang J, Hug C, Tsao TS and Lodish
HF: A family of Acrp30/adiponectin structural and functional
paralogs. Proc Natl Acad Sci USA. 101:10302–10307. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan SY, Little HC, Sarver DC, Watkins PA
and Wong GW: CTRP12 inhibits triglyceride synthesis and export in
hepatocytes by suppressing HNF-4α and DGAT2 expression. FEBS Lett.
594:3227–3239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei X and Wong GW: C1q/TNF-related protein
2 (CTRP2) deletion promotes adipose tissue lipolysis and hepatic
triglyceride secretion. J Biol Chem. 294:15638–15649. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moradi N, Najafi M, Sharma T, Fallah S,
Koushki M, Peterson JM, Meyre D and Fadaei R: Circulating levels of
CTRP3 in patients with type 2 diabetes mellitus compared to
controls: A systematic review and meta-analysis. Diabetes Res Clin
Pract. 169:1084532020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian M, Yang Q, Li J, Zhao B, Zhang Y and
Zhao Y: C1q/TNF-related protein-9 alleviates airway inflammation in
asthma. Int Immunopharmacol. 81:1062382020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peterson JM, Wei Z and Wong GW: CTRP8 and
CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF
family members. Biochem Biophys Res Commun. 388:360–365. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schäffler A, Ehling A, Neumann E, Herfarth
H, Paul G, Tarner I, Gay S, Schölmerich J and Müller-Ladner U:
Genomic organization, promoter, amino acid sequence, chromosomal
localization, and expression of the human gene for CORS-26
(collagenous repeat-containing sequence of 26-kDa protein). Biochim
Biophys Acta. 1630:123–129. 2003. View Article : Google Scholar
|
|
22
|
Maeda T, Abe M, Kurisu K, Jikko A and
Furukawa S: Molecular cloning and characterization of a novel gene,
CORS26, encoding a putative secretory protein and its possible
involvement in skeletal development. J Biol Chem. 276:3628–3634.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Byerly MS, Petersen PS, Ramamurthy S,
Seldin MM, Lei X, Provost E, Wei Z, Ronnett GV and Wong GW:
C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with
two tandem C1q domains that functions in the hypothalamus to
modulate food intake and body weight. J Biol Chem. 289:4055–4069.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Q, Wu J, Xi W, Chen X, Wang W, Zhang T,
Yang A and Wang T: Ctrp4, a new adipokine, promotes the
differentiation of osteoblasts. Biochem Biophys Res Commun.
512:224–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kirketerp-Møller N, Bayarri-Olmos R,
Krogfelt KA and Garred P: C1q/TNF-related protein 6 is a pattern
recognition molecule that recruits collectin-11 from the complement
system to ligands. J Immunol. 204:1598–1606. 2020. View Article : Google Scholar
|
|
26
|
Lei X, Seldin MM, Little HC, Choy N,
Klonisch T and Wong GW: C1q/TNF-related protein 6 (CTRP6) links
obesity to adipose tissue inflammation and insulin resistance. J
Biol Chem. 292:14836–14850. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akiyama H, Furukawa S, Wakisaka S and
Maeda T: CTRP3/cartducin promotes proliferation and migration of
endothelial cells. Mol Cell Biochem. 304:243–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao G, Zhang L, Qian D, Sun Y and Liu W:
miR-495-3p inhibits the cell proliferation, invasion and migration
of osteosarcoma by targeting C1q/TNF-related protein 3. Onco
Targets Ther. 12:6133–6143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glogowska A, Thanasupawat T, Beiko J, Pitz
M, Hombach-Klonisch S and Klonisch T: Novel CTRP8-RXFP1-JAK3-STAT3
axis promotes Cdc42-dependent actin remodeling for enhanced
filopodia formation and motility in human glioblastoma cells. Mol
Oncol. May 7–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akiyama H, Furukawa S, Wakisaka S and
Maeda T and Maeda T: Elevated expression of CTRP3/cartducin
contributes to promotion of osteosarcoma cell proliferation. Oncol
Rep. 21:1477–1481. 2009.PubMed/NCBI
|
|
31
|
Akiyama H, Furukawa S, Wakisaka S and
Maeda T: Cartducin stimulates mesenchymal chondroprogenitor cell
proliferation through both extracellular signal-regulated kinase
and phosphatidylinositol 3-kinase/Akt pathways. FEBS J.
273:2257–2263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li JM, Zhang X, Nelson PR, Odgren PR,
Nelson JD, Vasiliu C, Park J, Morris M, Lian J, Cutler BS and
Newburger PE: Temporal evolution of gene expression in rat carotid
artery following balloon angioplasty. J Cell Biochem. 101:399–410.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hill CS and Treisman R: Transcriptional
regulation by extracellular signals: Mechanisms and specificity.
Cell. 80:199–211. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coltella N, Manara MC, Cerisano V,
Trusolino L, Di Renzo MF, Scotlandi K and Ferracini R: Role of the
MET/HGF receptor in proliferation and invasive behavior of
osteosarcoma. FASEB J. 17:1162–1164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bentov I, LeRoith D and Werner H: The WT1
Wilms' tumor suppressor gene: A novel target for insulin-like
growth factor-I action. Endocrinology. 144:4276–4279. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JY, Min JY, Baek JM, Ahn SJ, Jun HY,
Yoon KH, Choi MK, Lee MS and Oh J: CTRP3 acts as a negative
regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling
in vitro and RANKL-induced calvarial bone destruction in vivo.
Bone. 79:242–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127 (5 Suppl 1):S35–S50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung YY, Um JY, Nasif O, Alharbi SA, Sethi
G and Ahn KS: Blockage of the JAK/STAT3 signaling pathway in
multiple myeloma by leelamine. Phytomedicine. Apr 15–2021.(Epub
ahead of print). View Article : Google Scholar
|
|
40
|
Hang T, Yang L, Zhang X, Li J, Long F, Zhu
N, Li Y, Xia J, Zhang Y, Zhang P, et al: Peroxisome
proliferator-activated receptor γ improves pemetrexed therapeutic
efficacy in non-squamous non-small cell lung cancer. Am J Transl
Res. 13:2296–2307. 2021.PubMed/NCBI
|
|
41
|
Li Q, Wang L, Tan W, Peng Z, Luo Y, Zhang
Y, Zhang G, Na D, Jin P, Shi T, et al: Identification of
C1qTNF-related protein 4 as a potential cytokine that stimulates
the STAT3 and NF-κB pathways and promotes cell survival in human
cancer cells. Cancer Lett. 308:203–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xia Y, Shen S and Verma IM: NF-kappaB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang R, Shao X, Yang J, Liu Z, Chew L and
Shao Y: Ginkgo biloba extract mechanism inhibits
hepatocellular carcinoma through the nuclear factor-κB/p53
signaling pathway. J Environ Pathol Toxicol Oncol. 39:179–189.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang YM, Kim SY and Seki E: Inflammation
and liver cancer: Molecular mechanisms and therapeutic targets.
Semin Liver Dis. 39:26–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Naugler WE, Sakurai T, Kim S, Maeda S, Kim
K, Elsharkawy AM and Karin M: Gender disparity in liver cancer due
to sex differences in MyD88-dependent IL-6 production. Science.
317:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Y, Ye L, Jia G, Chen H, Yu L and Wu D:
C1q/TNF-related protein 4 induces signal transducer and activator
of transcription 3 pathway and modulates food intake. Neuroscience.
429:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Balkwill F: TNF-alpha in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim MJ, Lee W, Park EJ and Park SY:
C1qTNF-related protein-6 increases the expression of interleukin-10
in macrophages. Mol Cells. 30:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang L, Liu Z, Duan L, Ma B and Sun Z: C1q
tumor necrosis factor-related protein 6 (CTRP6) inhibits the
proliferation and migration of ovarian cancer cells. Xi Bao Yu Fen
Zi Mian Yi Xue Za Zhi. 31:1664–1668. 2015.(In Chinese). PubMed/NCBI
|
|
52
|
Wan X, Zheng C and Dong L: Inhibition of
CTRP6 prevented survival and migration in hepatocellular carcinoma
through inactivating the AKT signaling pathway. J Cell Biochem.
120:17059–17066. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Takeuchi T, Adachi Y and Nagayama T:
Expression of a secretory protein CTRP6, a C1qTNF family member, in
hepatocellular carcinoma. Anal Cell Pathol (Amst). 34:113–121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lin W, Chen X, Chen T, Liu J, Ye Y, Chen
L, Qiu X, Chia-Hsien Cheng J, Zhang L, Wu J and Qiu S: C1QTNF6 as a
novel diagnostic and prognostic biomarker for clear cell renal cell
carcinoma. DNA Cell Biol. 39:1000–1011. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Milone M, Desiderio A, Velotti N,
Manigrasso M, Vertaldi S, Bracale U, D'Ambra M, Servillo G, De
Simone G, De Palma FDE, et al: Surgical stress and metabolic
response after totally laparoscopic right colectomy. Sci Rep.
11:96522021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
James S, Aparna JS, Babu A, Paul AM,
Lankadasari MB, Athira SR, Kumar SS, Vijayan Y, Namitha NN,
Mohammed S, et al: Cardamonin attenuates experimental colitis and
associated colorectal cancer. Biomolecules. 11:6612021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gou JX, Jiang XJ, Qu HX and Cui YX:
Expression of Complement C1q/Tumor Necrosis Factor Related Protein
6 in Colon Cancer. J Gastroenterol Hepatol. 28:313–316. 2019.
|
|
58
|
Lei H, Wu D, Wang JY, Li L, Zhang CL, Feng
H, Fu FY and Wu LL: C1q/tumor necrosis factor-related protein-6
attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A
pathway and inhibiting myofibroblast differentiation. Basic Res
Cardiol. 110:352015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xu E, Yin C, Yi X and Liu Y: Knockdown of
CTRP6 inhibits high glucose-induced oxidative stress, inflammation
and extracellular matrix accumulation in mesangial cells through
regulating the Akt/NF-κB pathway. Clin Exp Pharmacol Physiol.
47:1203–1211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang YP, Zhao YJ and Kong XL: A
metalloproteinase of the disintegrin and metalloproteinases and the
ThromboSpondin Motifs 6 as a novel marker for colon cancer:
Functional experiments. Genet Mol Biol. 43:e201902662020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cao L, Tan W, Chen W, Huang H, He M, Li Q,
Zhu X and Wang L: CTRP4 acts as an anti-inflammatory factor in
macrophages and protects against endotoxic shock. Eur J Immunol.
51:380–392. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gillen CD, Walmsley RS, Prior P, Andrews
HA and Allan RN: Ulcerative colitis and Crohn's disease: A
comparison of the colorectal cancer risk in extensive colitis. Gut.
35:1590–1592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lv W, Li Q, Jia B, He Y, Ru Y, Guo Q, Li X
and Lin W: Differentiated embryonic chondrocyte-expressed gene 1
promotes temozolomide resistance by modulating the SP1-MGMT axis in
glioblastoma. Am J Transl Res. 13:2331–2349. 2021.PubMed/NCBI
|
|
64
|
Thanasupawat T, Glogowska A, Burg M, Wong
GW, Hoang-Vu C, Hombach-Klonisch S and Klonisch T: RXFP1 is
targeted by complement C1q tumor necrosis factor-related factor 8
in brain cancer. Front Endocrinol (Lausanne). 6:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Guo X, Liu Y, Huang X, Wang Y, Qu J and Lv
Y: Serum relaxin as a diagnostic and prognostic marker in patients
with epithelial ovarian cancer. Cancer Biomark. 21:81–87. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Klonisch T, Bialek J, Radestock Y,
Hoang-Vu C and Hombach-Klonisch S: Relaxin-like ligand-receptor
systems are autocrine/paracrine effectors in tumor cells and
modulate cancer progression and tissue invasiveness. Adv Exp Med
Biol. 612:104–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Silvertown JD, Symes JC, Neschadim A,
Nonaka T, Kao JC, Summerlee AJ and Medin JA: Analog of H2 relaxin
exhibits antagonistic properties and impairs prostate tumor growth.
FASEB J. 21:754–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Klonisch T, Glogowska A, Thanasupawat T,
Burg M, Krcek J, Pitz M, Jaggupilli A, Chelikani P, Wong GW and
Hombach-Klonisch S: Structural commonality of C1q TNF-related
proteins and their potential to activate relaxin/insulin-like
family peptide receptor 1 signalling pathways in cancer cells. Br J
Pharmacol. 174:1025–1033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Thanasupawat T, Glogowska A, Burg M, Krcek
J, Beiko J, Pitz M, Zhang GJ, Hombach-Klonisch S and Klonisch T:
C1q/TNF-related peptide 8 (CTRP8) promotes temozolomide resistance
in human glioblastoma. Mol Oncol. 12:1464–1479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen L and Su G: Identification of CTRP1
as a prognostic biomarker and oncogene in human glioblastoma.
Biomed Res Int. 2019:25824162019.PubMed/NCBI
|
|
71
|
Maeda T, Jikko A, Abe M, Yokohama-Tamaki
T, Akiyama H, Furukawa S, Takigawa M and Wakisaka S: Cartducin, a
paralog of Acrp30/adiponectin, is induced during chondrogenic
differentiation and promotes proliferation of chondrogenic
precursors and chondrocytes. J Cell Physiol. 206:537–544. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qu HX, Cui L, Meng XY, Wang ZJ, Cui YX, Yu
YP, Wang D and Jiang XJ: C1QTNF6 is overexpressed in gastric
carcinoma and contributes to the proliferation and migration of
gastric carcinoma cells. Int J Mol Med. 43:621–629. 2019.PubMed/NCBI
|
|
73
|
Wang C, Gao C, Zhuang JL, Ding C and Wang
Y: A combined approach identifies three mRNAs that are
down-regulated by micro RNA-29b and promote invasion ability in the
breast cancer cell line MCF-7. J Cancer Res Clin Oncol.
138:2127–2136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang W and Feng G: C1QTNF6 regulates cell
proliferation and apoptosis of NSCLC in vitro and in vivo. Biosci
Rep. 41:BSR202015412021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Han M, Wang B, Zhu M and Zhang Y: C1QTNF6
as a novel biomarker regulates cellular behaviors in A549 cells and
exacerbates the outcome of lung adenocarcinoma patients. In Vitro
Cell Dev Biol Anim. 55:614–621. 2019. View Article : Google Scholar : PubMed/NCBI
|