Introduction
The majority of thyroid cancers have an excellent
prognosis, although the presence of locoregional or distant
metastases has a considerable negative effect on patient survival
and morbidity (1). At present,
thyroglobulin and calcitonin are the only two established
biomarkers associated with thyroid cancer management and are the
subject of active surveillance within patient serum following
thyroidectomy to indicate disease recurrence (2,3).
However, no markers associated with thyroid cancer aggressiveness
have been incorporated into clinical practice.
In recent years various molecular markers have been
extensively studied as predictors of patient outcome. For example,
the BRAFV600E mutation has been associated with aggressive clinical
behaviours such as metastatic spread or recurrent tumour burden
(4). In addition, multiple microRNAs
(miRs), such as miR-221, miR-222 and miR-146b have been reported as
overexpressed in papillary thyroid cancer (PTC) specimens and may
also be associated with disease aggressiveness (5–7). A wide
range of both soluble and tissue-retained biomarkers for thyroid
cancer have been studied previously (Table I). The expression of biomarkers
within a tissue, although interesting from a biological
perspective, commonly do not reflect the level within circulation
(8), and it is clearly impractical
to sample routinely making them less attractive for clinical
utilisation. On the other hand, patient sera, potentially offer a
higher degree of translatability for the identification of
progression and disease-specific biomarkers (9–11); thus
far both serum VEGF-C and MMP-2 have been correlated with thyroid
tumour metastases (11,12).
 | Table I.Seminal findings pertaining to the
elucidation of thyroid cancer biomarkers. |
Table I.
Seminal findings pertaining to the
elucidation of thyroid cancer biomarkers.
| Author (year) | Key findings | Sample | Techniques | Formats | (Refs.) |
|---|
| Xie et al
(2019) | TGF-β1 may promote
the invasion and migration of PTC cells by inhibiting the
expression of lncRNA-NEF. | n=62 | RT-qPCR, western
blotting | Fresh patient
tissue samples and sera | (48) |
| Zhang et al
(2019) | Detecting the
levels of MMP-2, MMP-9, TIMP-1 and TIMP-2 in peripheral blood is
helpful in the diagnosis of benign and malignant thyroid
lesions. | n=57 | ELISA | Peripheral blood of
patients with DTC | (49) |
| Jang et al
(2019) | Serum VEGF-C might
be a clinically relevant biomarker of lateral neck metastasis in
patients with PTC. | n=150 | ELISA | Peripheral blood of
patients with PTC | (11) |
| Liu et al
(2019) | MMP-9 expression in
thyroid carcinoma samples may represent a potential and
supplementary tool for the diagnosis and prognostication of
PTC. | n=112 | IHC | Fresh patient
tissue samples | (50) |
| Selemetjev et
al (2018) | High expression of
survivin and VEGF-C closely associated with lymph node metastasis
in patients with PTC. | n=75 | IHC, western
blotting | Fresh patient
tissue samples | (51) |
| Guan et al
(2018) | INAVA expression
was significantly upregulated in PTC compared with adjacent benign
tissue and was significantly associated with lymph node
metastasis. | n=112 | RT-qPCR, IHC | Banked FFPE tissue,
fresh frozen tissue. | (52) |
| Cui et al
(2018) | Upregulation of
lncRNA-ATB by TGF-β1 promotes migration and invasion of PTC
cells. | n=76 | RT-qPCR | Peripheral blood
and tissue-patients with PTC | (53) |
| Lu et al
(2018) | Both fibrinogen A
and complement C4A/B were identified as potential markers for
diagnosis of PTC. | n=88 | MADLI-TOF | Fresh sera of
patients with PTC | (54) |
| Shi et al
(2018) | Preoperative serum
MMP-2 may serve as a biomarker for diagnosing PTC and a predictive
indicator for LNM and SPRD in male patients with PTC. | n=193 | ELISA | Fresh sera of
patients with PTC | (12) |
| Selemetjev et
al (2016) | High levels of
VEGF-C and active MMP-9 correlated with lymphatic spreading and
local invasiveness of PTC. | n=60 | IHC, western | PTC tissue samples
blotting | (10) |
| Wang et al
(2014) | Immunohistochemical
and real-time RT-PCR evaluation of TGF-β1, SNAI1 and MMP-9
expression in PTC may be useful to predict the risk of LNM in
patients | n=83 | RT-qPCR, western
blotting | PTC tissue samples
with PTC. | (55) |
| Makki et al
(2013) | TIMP-1 and GAL-3
serum levels were elevated in patients with thyroid masses relative
to normal values. | n=99 | ELISA | Bio-banked patient
sera | (56) |
| Zhou et al
(2012) | Serum VEGF
increased in patients with recurrent thyroid cancer following
surgical therapies. The predictive value of serum VEGF requires
further investigation. | n=79 | ELISA | Fresh patient
sera | (57) |
| Liang et al
(2011) | MMP2, PTTG, VEGF-C,
CXCR4 and bFGF are potential markers for identifying thyroid cancer
with greater risk for metastasis. | n=113 | Microarray IHC | Mixed metastatic
and non-metastatic tissue samples | (9) |
| Liang et al
(2011) | VEGF-C and bFGF
were most useful for the differential diagnosis between metastatic
and non-metastatic PTC. | n=58 | IHC | PTC tissue
samples | (58) |
| Wang et al
(2009) | Immunohistochemical
detection of MMP-9, TIMP-1, VEGF and TGFβ-1 expression may carry
clinical significance in evaluating thyroid cancer
aggressiveness. | n=85 | IHC | PTC and FTC tissue
samples | (59) |
| Wang et al
(2009) | Apolipoprotein C-I,
apolipoprotein C-III, a-globin and b-globin may have utility for
diagnosis of papillary thyroid carcinoma. | n=35 | MALDI-TOF | Fresh sera of
patients with PTC | (60) |
The use of a precision cut tumour slice microfluidic
device to maintain ex vivo thyroid cancer tissue has been
demonstrated previously, and offers a novel means of assessing the
levels of soluble markers released specifically from the malignant
tissue (13). Proteins involved in
the process of blood vessel formation are ideal candidates as
markers of cancer progression and metastasis, with angiogenesis
being fundamental to these processes (14). The current research aimed to
elucidate the levels of common angiogenic biomarkers within
effluent produced in the described system and correlating the
findings with the clinical features of the patients.
Materials and methods
Patient sample acquisition
Human thyroid tissue samples were collected during
thyroidectomy following written informed consent under ethical
approval from Northeast-Newcastle and North Tyneside Research
Ethics Committee (15/NE/0412) and Hull University Teaching
Hospitals NHS Trust R&D (R1925). Where possible, tissue was
excised from the contralateral lobe alongside the malignant sample
(aggressive n=9; non-aggressive n=8; pathologically benign (n=3;
Table II). Tissues were classed as
‘aggressive’ if they presented clinically with a minimum of N1b
level metastases [tumour spread beyond the central compartment,
including unilateral (on 1 side of the neck), bilateral cervical
(on both sides of the neck), contralateral cervical (the opposite
side to the tumour), or mediastinal (chest) lymph nodes].
‘Non-aggressive’ samples were T3 or lower (localised to the thyroid
gland) without evidence of multifocality.
 | Table II.Clinicopathological features of
thyroid patients, ordered by descending tumour-node-metastasis
stage. |
Table II.
Clinicopathological features of
thyroid patients, ordered by descending tumour-node-metastasis
stage.
| Patient | Staging | Age, years | Sex | Tissue type | Survival,
months |
|---|
| 1 | T4aN1b | 70–80 | F | A | 16a |
| 2 | T4aN1b | 70–80 | F | A | 23a |
| 3 | T4N0M1 | 50–60 | F | A | 29a |
| 4 | T4aN1bMX | 70–80 | M | A | 28 |
| 5 | T3N1bMX | 20–30 | F | A | 43 |
| 6 | T3bN1aMX | 40–50 | M | A/BC | 35 |
| 7 | T3aN1bM0 | 20–30 | F | A | 31 |
| 8 | T3aN1bMX | 30–40 | F | A | 32 |
| 9 | T3N1bMX | 40–50 | F | A | 40a |
| 10 | T3aNXMX | 60–70 | F | L | 28 |
| 11 | T3aNXMX | 50–60 | F | L | 26 |
| 12 | T2NXMX | 40–50 | M | L | 47 |
| 13 | T1aNXMX | 50–60 | F | L | 23 |
| 14 | T1NXMX | 60–70 | F | L | 33 |
| 15 | T1aNXMX | 10–20 | M | L | 33 |
| 16 | T1aNXMX | 40–50 | F | L | 30 |
| 17 | T1bNXMX | 40–50 | M | L | 35 |
| 18 | – | 50–60 | F | BC | 25 |
| 19 | – | 60–70 | F | BC | 23 |
Microculture device set up. Tissue samples were
‘live’ sliced (350–500 µm) in ice-cold PBS using a vibratome (Leica
VT1200S, Milton Keynes) with a blade speed of 0.1 mms−1
and amplitude of 2.5 mm. A skin biopsy punch (Stiefel) was used to
generate a precision cut tumour slice (PCTS), 5 mm in diameter.
Each PCTS was weighed before insertion into the microculture
device. Average PCTS wet weight was 15.92±2.41 mg. A schematic
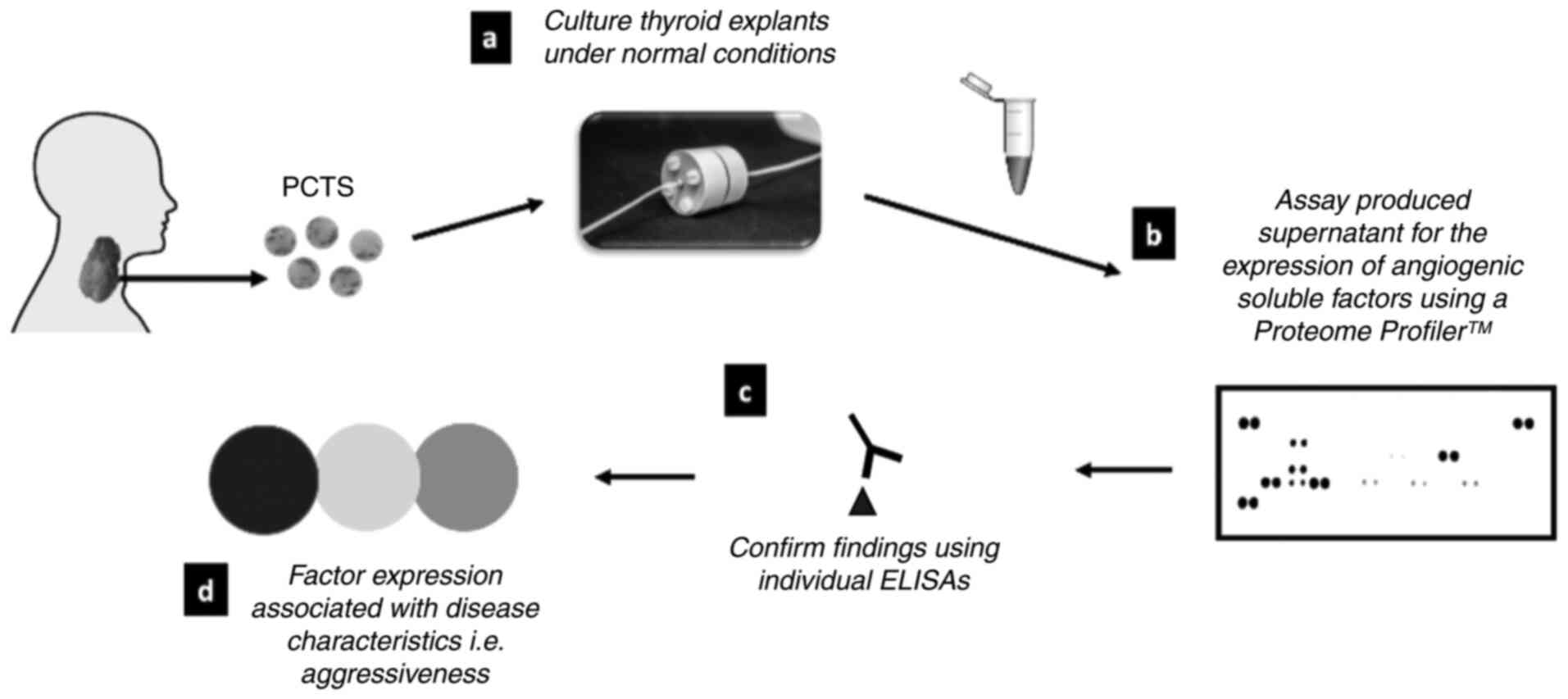
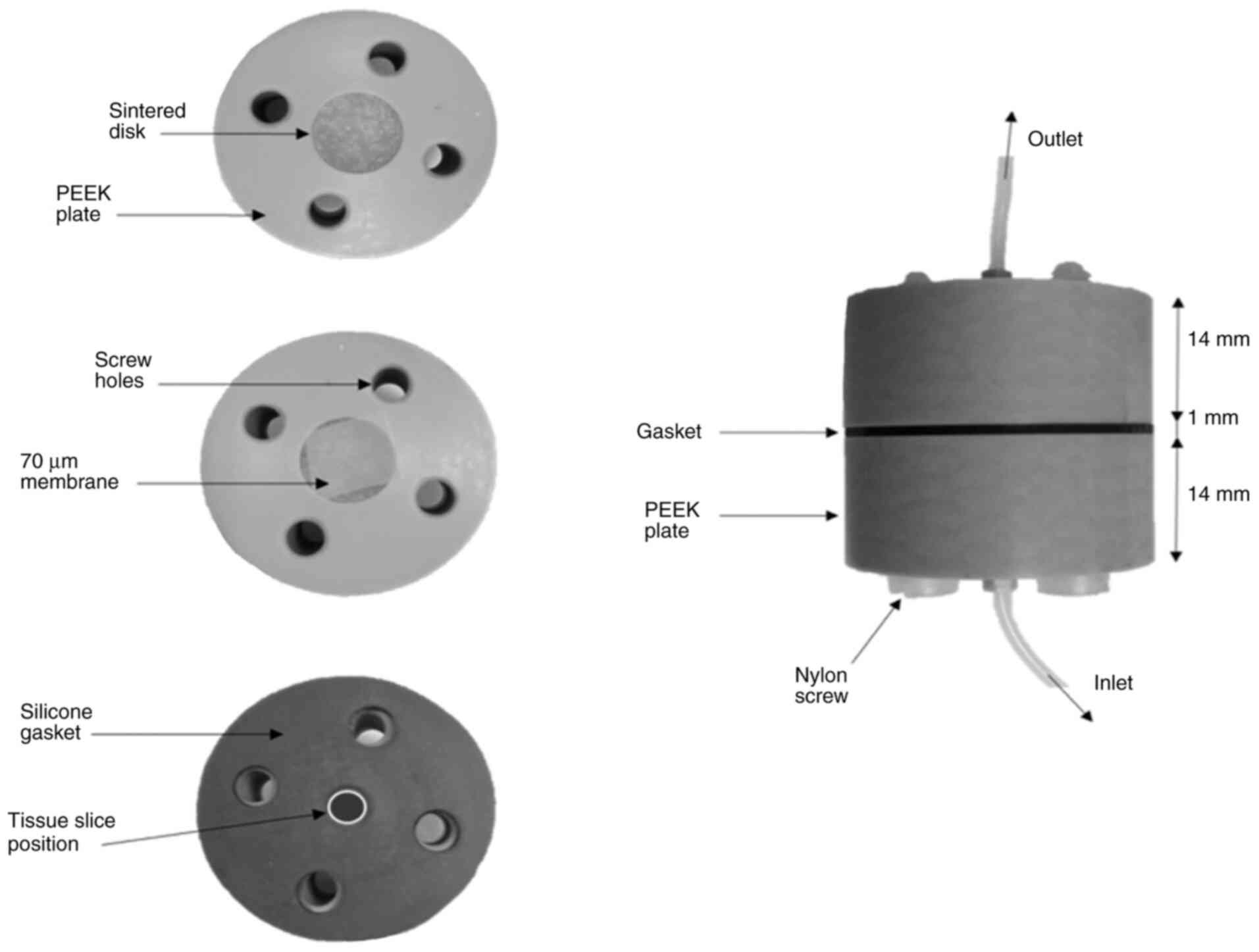
demonstrating the study design is shown in Fig. 1. A previously described PCTS device
(13) was used to house the thyroid
tissue whilst being perfused at a rate of 2 µl min−1
with Dulbecco's modified eagles medium (DMEM; GE Healthcare)
containing 10% (v/v) heat inactivated foetal bovine serum (FBS;
Biosera), penicillin/streptomycin (0.1 U/ml and 0.1 mg/ml
respectively; GE Healthcare), 0.4 mM glutamine (GE Healthcare), 2.5
µg/ml Amphotericin B (Life Technologies, Paisley, UK), thyrotropin
(TSH; 2 mIU/l) and sodium iodide (0.1 µg/ml). The culture device
(shown in Fig. 2) was maintained at
37°C for 72 h; medium coming off the device was collected after 2 h
culture, then once per day thereafter and frozen (−80°C) prior to
use in the assays. Effluent was collected from at least 2
patient-derived tissue slices from each patient, which had been
cultured in parallel, was used in this study in order to minimise
the impact of tumour tissue heterogeneity.
Proteome profiler™ angiogenesis
array
An Array Kit (Proteome Profiler™; R&D Systems)
was used, as directed, to detect the presence of 55
angiogenesis-related proteins released from the thyroid tissue
whilst maintained on the microfluidic device. Following a 1 h
incubation of the array membranes with blocking buffer, detection
antibody cocktail, reconstituted in dH20 (15 µl), was
added to 700 µl of thawed and centrifuged (300 × g; to remove
cellular debris) culture effluent collected following a 72 h
incubation on the microfluidic device. The 72 h timepoint was
chosen as data produced previously demonstrated a stabilisation of
cellular death (such as tissue LDH leakage) within 72 h incubation
(13). Effluent from a minimum of 2
explants from each patient, cultured in parallel, was utilised. The
sample volume was made up to 1.5 ml using buffer provided and
incubated for 1 h at room temperature, before adding to the
membranes in separate wells of a 4-well multi-dish (provided). The
membranes and samples were incubated overnight at 4°C on a rocking
platform before being washed in 20 ml wash buffer (provided) for
3×10 min. Streptavidin-HRP (1:2,000) was added to each membrane (2
ml) and incubated for 30 min with gentle agitation. The membranes
were washed a further three times before antibody binding was
detected with chemiluminescence (Thermo Fisher Scientific) and
autoradiography. Dot densitometry was then analysed using the
‘analyse gels’ function within ImageJ Fiji software (open source;
http://imagej.net/Fiji). The mean densitometry of
each protein duplicate was calculated and expressed as a percentage
of the average density of the 6 positive-control dots within the
membrane. The resultant expression intensities were correlated to
thyroid disease aggressiveness, by comparing their levels in three
tissue groups: benign, non-aggressive and aggressive.
Quantification of factor expression
using ELISA
Following initial detection of biomarkers of
interest using a Proteome Profiler™ angiogenesis array, marker
specific ELISAs were utilised, allowing fully quantitative
evaluation of their levels within microculture effluent. ELISAs
[CCL2 (DY279); serpin-F1 (DY1177-05); R&D, Oxford, UK] were
carried out according to the manufacturer's instruction. Effluent
samples were combined from a minimum of two replicate culture
devices set-up from each resected patient sample (aggressive n=9;
non-aggressive n=8; benign n=3). Due to limitations of effluent
availability, effluent collected after 96 h culture was tested by
ELISA (as opposed to the 72 h timepoint used for Proteome Profiler
experiments). Following removal of the capture antibody and washing
of the plate with the wash buffer provided, effluent (100 µl/well)
samples were added to the plate in duplicate alongside prepared
standards, covered with an adhesive strip, and incubated at room
temperature for two hours. Following three washes with TBS,
incubation with matched biotinylated detection antibodies was
carried out for a further two hours before addition of the
streptavidin-biotin complex for 20 min. Colour development was
induced by the addition of substrate reagent containing stabilised
tetramethylbenzidine (TMB; R&D Systems). The optical density of
each well was measured at 450 nm with wavelength correction at 570
nm. The mean of duplicate readings was taken for each sample. A
4-parameter logistic curve fit was used to produce a standard curve
from which protein concentration in the unknown samples was
determined.
Statistical analysis
Data were grouped into results for benign,
non-aggressive and aggressive tumours and analysed using one-way
ANOVA and post-hoc Tukey/Sidak tests on GraphPad Prism 8.0. All
data is reported as mean ± SEM unless otherwise stated.
Results
Semi-quantitative measurement of
angiogenic factors release from thyroid tissue maintained on a
microfluidic device using a Proteome Profiler™ array
The analysis of microfluidic culture effluent for
the presence of 55 soluble angiogenesis-related protein factors,
using the proteome profiler™ array, demonstrated that 22 of those
factors were detectable (Fig. 3).
While the majority of detectable factors showed relatively little
variance between the three disease sub-groups, inter-group variance
existed in the cases of chemokine (C-C motif) ligand 2 (CCL2),
Serpin-F1, vascular endothelial growth factor (VEGF) and
Thrombospondin-1 (TSP-1; Fig. 3).
The effluent of aggressive thyroid cancers contained a
significantly higher level (72.85±21.38 a.u.; P=0.0002 and
P=0.04) of CCL2 when compared to the effluent of
non-aggressive (32.06±13.57 a.u.) and benign (39.72±32.96 a.u.)
thyroid tissue, respectively. No significance was detected between
the levels of CCL2 released by benign and non-aggressive tumour
tissue.
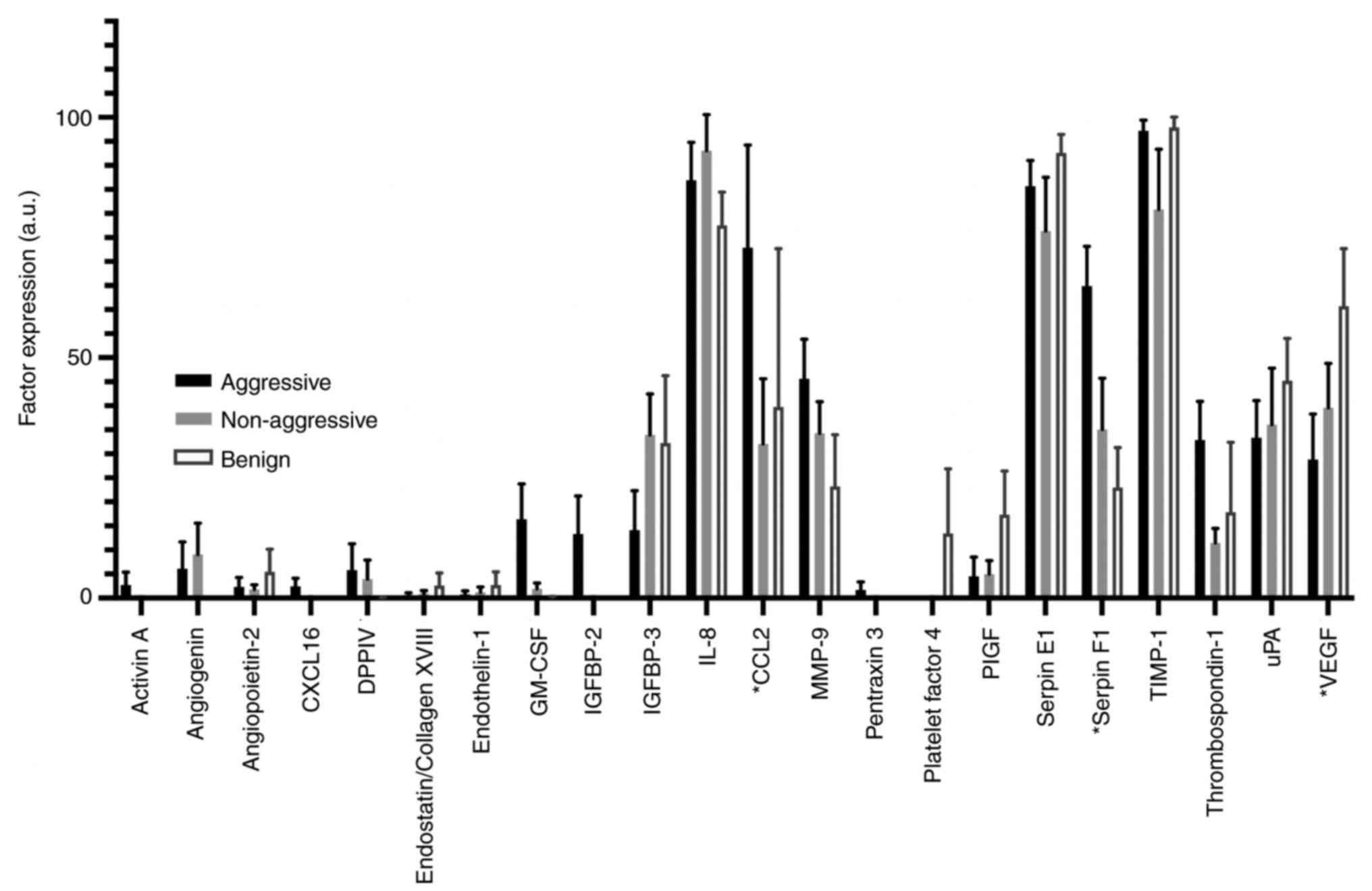 | Figure 3.Bar chart displaying the relative
expression of 22 detectable angiogenic factors released by human
thyroid explants (aggressive n=7; non-aggressive n=8; benign n=3)
cultured within the novel microfluidic device. Of those 22 factors
detected, CCL2, Serpin-F1 and VEGF demonstrated significant
variation between the three tissue subgroups to some degree when
significance was tested using a one-way ANOVA. Data produced were
used to guide marker selection for subsequent fully quantitative,
ELISA analysis. *P<0.05 between the three tissue subgroups. a.u,
absorbance units; CXCL16, chemokine (C-X-C motif) ligand 16; DPPIV,
dipeptidyl peptidase-4; GM-CSF, granulocyte macrophage
colony-stimulating factor; IGFBP, insulin-like growth
factor-binding protein; CCL2, chemokine (C-C motif) ligand; MMP,
matrix metalloproteinase; PIGF, phosphatidylinositol-glycan
biosynthesis class F; TIMP, Tissue inhibitor of metalloproteinases;
uPA, urokinase-type plasminogen activator; VEGF, vascular
endothelial growth factor. |
In addition, the level of Serpin-F1 increased with
disease severity; benign thyroid tissue released 22.99±8.28 a.u.,
compared with 35.06±10.64 a.u. in non-aggressive cancer tissue and
64.87±8.25 a.u. in the effluent of aggressive thyroid cancer tissue
explants. Significance was detected when comparing the levels of
Serpin-F1 in aggressive with both non-aggressive (P=0.008)
and benign thyroid tissue (P=0.005). In contrast, VEGF
release decreased with disease severity and was found to be
significantly lower in concentration in the effluent collected from
aggressive thyroid tumours compared to that collected from benign
thyroid tissue (28.79±9.46 vs. 60.69±12.02 au.; P=0.044).
Finally, although not significant, thrombospondin-1 was markedly
higher in effluent from the aggressive tissue sub-group, when
compared to the non-aggressive group (32.86±8.06 vs. 11.46±2.99
a.u.; P=0.08). Further, benign tissue appeared to release a
higher level of thrombospondin-1 than the counterpart
non-aggressive tissue, although the difference was minimal and
there was large variation in the levels.
Quantification of factor expression
using ELISA
Following initial, semi-quantitative biomarker,
analysis within the culture supernatant, and due to a limited
volume of available supernatants for subsequent analyses, Serpin F1
and CCL2 were chosen to be studied by ELISA as they represented the
most interesting potential biomarkers of thyroid disease
aggressiveness due to the clear differences in levels between
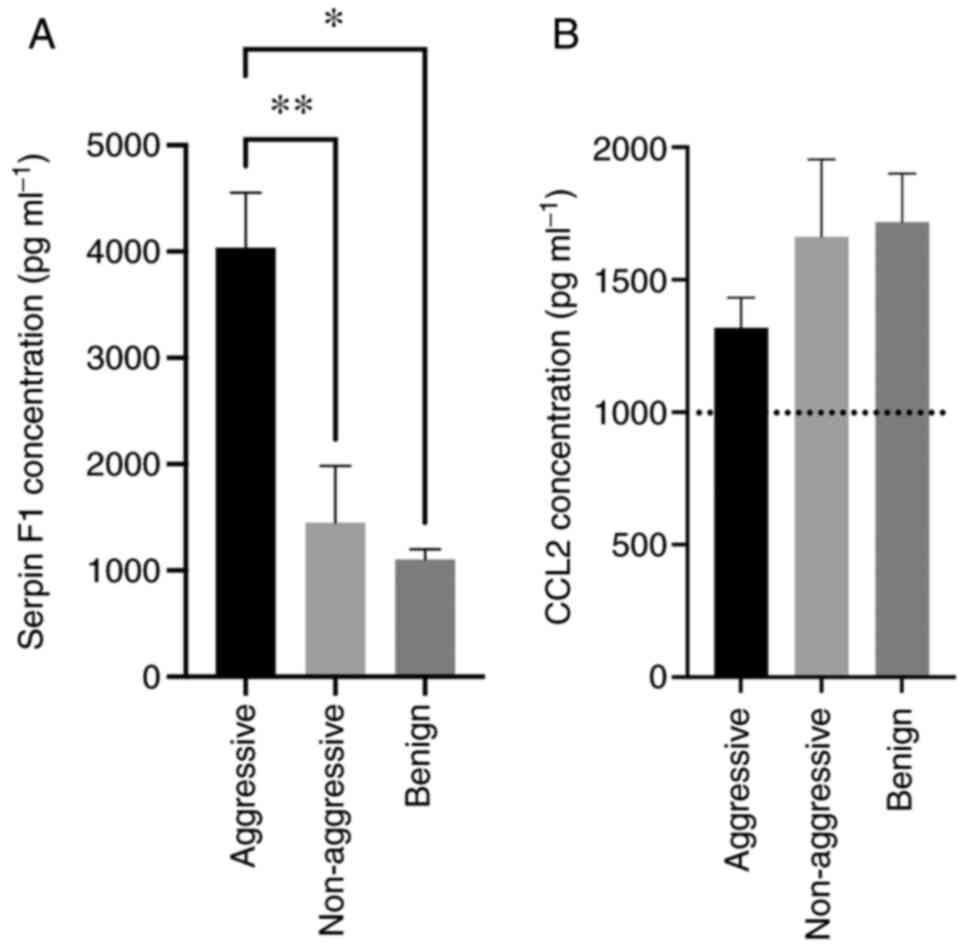
tissue types. The concentration of Serpin-F1 within the effluent of
aggressive thyroid cancer tissue (4,038±518.01 pg•ml−1)
was significantly higher (P=0.005) than that from non-aggressive
cancer tissue (1,450.48±532.34 pg•ml−1) and benign
thyroid tissue (P=0.01; 1,104.28±95.24 pg•ml−1; Fig. 4A). The overall pattern of Serpin F1
release detected by ELISA directly mirrored the pattern detected by
the aforementioned array experiments. Four of the eight patients
within the ‘aggressive’ group (1,3,4,9)
experienced disease progression; furthermore, three of those four
patients (1,3,9) passed
away due to complications with progression of their thyroid disease
750±236 days following their initial thyroid cancer surgery. No
significant difference in factor secretion existed between those
patients who experienced a fatal progression of their disease and
those who did not. All patients within the ‘non-aggressive’
subcategory are currently alive.
The concentration of CCL2 detectable by ELISA across
the three tissue types was above the top standard supplied (1,000
pg•ml−1). Due to the limited volumes of culture
supernatant available, it was not possible to dilute and repeat the
analysis, thus the results were extrapolated and therefore should
be regarded with caution and may be why the pattern observed
(decreasing with disease severity) was not the same as detected in
the array experiments (Fig. 4B). No
significant differences between the tissue cohorts were
observed.
Discussion
A number of previous studies have investigated
whether quantifiable changes in protein expression can be used
diagnostically or prognostically for thyroid malignancies,
especially as biomarkers for disease characteristics such as
lateral neck metastasis in thyroid cancer (11). As is the case for most cancers
(>90% cancer-related deaths are due to tumour invasion),
metastasis remains one of the main reasons for patient mortality in
PTC (15,16). Currently no clinical utilised
biomarkers of thyroid cancer invasiveness or metastasis exist.
Effluent samples from thyroid patient tumour tissue
were grouped into three categories: Aggressive, non-aggressive and
benign thyroid tissue. Culture effluent was initially analysed
semi-quantitatively; of the 55 proteins investigated, a total of 22
proteins were detectable following their release by ex vivo
thyroid tissue. Four of the 22 detectable proteins were
differentially expressed depending on the thyroid tissue group from
which they were derived. Levels of Serpin-F1, CCL2, and
Thrombospondin-1 were all significantly higher in the effluent
derived from aggressive thyroid cancers than those which had not
metastasised. VEGF, on the other hand, was inversely correlated
with aggressiveness; appearing to be released in lesser quantities
by aggressive thyroid tissue, comparatively. Due to logistical
difficulties regarding the quantity of culture effluent produced by
on-chip maintenance of thyroid tissue explants, fully quantitative
analysis was carried out on Serpin-F1 and CCL2 by ELISA only, as
those factors appeared most profoundly modulated between the
subgroups.
Serpin-F1, also known as pigment epithelial-derived
factor, is a 50-kDa glycoprotein with numerous biological functions
including antiangiogenic and anti-tumorigenesis, and was initially
purified from conditioned medium from human retinal pigment
epithelial cells (17). The protein
is commonly expressed in normal tissues (18) and to a lesser extent, in malignant
tissues (19), in contrast to the
findings in the current study. Approximately a decade after the
proteins' initial discovery it was observed to be a potent
inhibitor of angiogenesis, acting as a major antagonist to a range
of pro-angiogenic factors such as VEGF; multiple studies have shown
an inverse relationship in the expression of both serpin-F1 and
VEGF (20,21). In addition to its anti-angiogenic
activity, serpin-F1 has been demonstrated as able to induce tumour
cell re-differentiation and block tumour cell invasion and
metastasis (22,23). These anti-tumour effects of serpin-F1
have been demonstrated in a range of human cancers such as prostate
(23), ovarian (24) and glioma (25). A previous study carried out by Lv
et al utilising immunohistochemistry (IHC) and RT-qPCR
established a correlation between the reduced expression of
serpin-F1 in the thyroid and lymph node metastasis, extrathyroidal
invasion and BRAFV600E mutation in cases of papillary
thyroid cancer (26). Although these
findings apparently contradict the results obtained for serpin-F1
release in the current study, the two sets of data should be
compared with caution; as Lv et al studied tissue-specific
levels of the molecule, the current study investigated the levels
released directly from the tissue.
CCL2 is a chemokine produced by a wide range of cell
types including fibroblasts, endothelial and tumour cells (27). It has previously been demonstrated
that CCL2 is overexpressed by a range of cancer types such as
melanoma, ovarian, breast, and oesophageal (28–31).
Furthermore, increased CCL2 expression has been correlated with
poor prognosis and advanced stage in cancer types such as prostate
and oesophageal (32,33) as well as predicting recurrence and
vascular invasion in breast cancer (30,34).
Importantly, increased CCL2 expression, was significantly
correlated with lymph node involvement and poor prognosis in
thyroid cancer tissue (35,36). These scant findings in the current
literature appear to agree with the current results regarding CCL2
release by thyroid cancer tissue. However, as was the case with Lv
et al who investigated Serpin-F1 release, Tanaka's study was
carried out using IHC to assess tissue-specific marker expression,
whereby the current study tested the level of released, soluble,
CCL2.
Thrombospondin-1 is a 450 kDa homotrimeric
glycoprotein with diverse functionality such as the modulation of
endothelial cell adhesion, motility, and growth, mediated through
interaction between its structural domains and multiple
cell-surface molecules and is produced by a range of cell types
including platelets, endothelial cells, cancer cells and
circulating immune cells (37,38). In
thyroid cancer, TSP-1 has been shown to be upregulated and mediate
invasiveness and aggressiveness in B-RAFV600E mutant
tumours; murine implantation of TSP-1 knockdown cancer cells
yielded significantly smaller tumours by volume, and fewer
metastases compared with control (39). Further, Soula-rothhut and colleagues
proposed that TSP-1 is a positive effector in thyroid
tumorigenesis; they used an in vitro model to demonstrate
the proteins' stimulation of cell proliferation, migration and
invasion in FTC133 and FTC-238 thyroid cancer cells (40). The results found in the current study
would appear to agree with the evidence for the role of TSP-1 in
mitigating tumour invasion since a significantly higher
concentration of TSP-1 was detected in the effluent of aggressive
thyroid cancer tissue compared to that of non-aggressive thyroid
cancer tissue. However, in contrast, TSP-1 has been shown to
inhibit kidney cancer cell migration in vitro (41). In addition, oesophageal cancer
patients with low tumour expression of TSP-1 were associated with
worse progression free survival (42). This incongruence between studies
investigating the role of TSP-1 in cancer tissues reveals a
potentially multifaceted, pleiotropic role which depends on both
the tumour microenvironment and downstream receptor presence
(43). VEGF is a pro-angiogenic
growth factor which binds one of two receptors (VEGFR-1 and
VEGFR-2), both of which are expressed on the surface of endothelial
cells and transduce pro-angiogenic signalling (44). VEGF is a key mediator of angiogenesis
in tumour tissue, where it is up regulated due to oncogene
activation and hypoxia within the tumour microenvironment (45). Interestingly, a previous study
carried out by Soh and colleagues found an increased level of VEGF
secretion by thyroid cancer cells when compared to their benign
counterparts. The discrepancy is perhaps as the group employed
fully quantitative ELISA, which was not possible in the current
study (46). A different group
discovered that serum VEGF was more likely to be elevated in
patients with differentiated thyroid cancer, as opposed to those
suffering poorly differentiated thyroid cancer (47). It would be possible in future studies
to investigate the effect of hypoxia on the relative levels of VEGF
release by ex vivo thyroid tissue.
The logical next stage of the work would involve the
collection of data concerning tissue-specific levels of the
proteins investigated, by separate means such as IHC, in order to
correlate the tumour microenvironment with secreted products.
Furthermore, maximising the quantity of tissue collected at the
point of surgical resection would increase the number of tissue
slices able to be cultured in parallel. Thus, an increased volume
of effluent could be collected and combined, strengthening the
statistical analyses, and improving the clinical applicability of
the system. However, the quantity of human tissue provided will
always be a limiting factor, and thus focussing on a smaller subset
of targets is an equally valuable approach. In addition, a
potentially interesting avenue of work would be the investigation
of serpin-F1 and CCL2 in vitro and in vivo, assessing
how inhibition of these proteins affects thyroid cancer development
and aggressiveness.
The data presented within this manuscript
demonstrates first and foremost the application of microfluidic
technology for the detection of soluble tissue-derived factors.
This is, to the best of the authors knowledge, the first time
effluent derived from the microfluidic culture of ex vivo
human thyroid tissue has been tested for the expression of a panel
of angiogenic markers. Initial testing of effluent identified four
potential markers of thyroid cancer progression/aggressiveness
(Serpin-F1, CCL2, VEGF, and TSP-1). Further testing by ELISA
demonstrated the significant modulation of serpin-F1 release
according to tissue severity, and therefore its' potential as a
marker of thyroid cancer aggressiveness. An interesting facet of
the data presented herein is the prediction of disease progression
by elevated Serpin-F1 and CCL2 in culture effluent at the point of
testing. These data represent a pilot study, demonstrating the
correlation between a number of identified markers and thyroid
cancer aggressiveness.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Committee of The
British Association of Endocrine and Thyroid Research (grant no.
256168).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AR and VG undertook the experimental work. AR, JE,
VG and JG designed the project. JE collected the thyroid specimens
and relevant clinical data. JG and AR confirm the authenticity of
all the raw data. AR and HJ undertook the writing of the manuscript
and data analysis. DK undertook microfluidic device design and
manufacture. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present project received ethical approval from
Northeast-Newcastle and North Tyneside Research Ethics Committee
(approval no. 15/NE/0412) and from Hull University Teaching
Hospital NHS Trust R&D (approval no. R1925). Patient tissue
samples were taken after obtaining written, informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang LY and Ganly I: Nodal metastases in
thyroid cancer: Prognostic implications and management. Future
Oncol. 12:981–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM and
Schlumberger M: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Indrasena BSH: Use of thyroglobulin as a
tumour marker. World J Biol Chem. 8:81–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SJ, Lee KE, Myong JP, Park JH, Jeon
YK, Min HS, Park SY, Jung KC, Koo DH and Youn YK: BRAF V600E
mutation is associated with tumor aggressiveness in papillary
thyroid cancer. World J Surg. 36:310–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato MA and Fahey TJ 3rd: Molecular
markers in thyroid cancer diagnostics. Surg Clin North Am.
89:1139–1155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yip L, Kelly L, Shuai Y, Armstrong MJ,
Nikiforov YE, Carty SE and Nikiforova MN: MicroRNA signature
distinguishes the degree of aggressiveness of papillary thyroid
carcinoma. Ann Surg Oncol. 18:2035–2041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Acibucu F, Dökmetaş HS, Tutar Y, Elagoz S
and Kilicli F: Correlations between the expression levels of
micro-RNA146b, 221, 222 and p27Kip1 protein mRNA and the
clinicopathologic parameters in papillary thyroid cancers. Exp Clin
Endocrinol Diabetes. 122:137–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cookson VJ, Bentley MA, Hogan BV, Horgan
K, Hayward BE, Hazelwood LD and Hughes TA: Circulating microRNA
profiles reflect the presence of breast tumours but not the
profiles of microRNAs within the tumours. Cell Oncol (Dordr).
35:301–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang H, Zhong Y, Luo Z, Huang Y, Lin H,
Zhan S, Xie K and Li QQ: Diagnostic value of 16 cellular tumor
markers for metastatic thyroid cancer: An immunohistochemical
study. Anticancer Res. 31:3433–3440. 2011.PubMed/NCBI
|
|
10
|
Šelemetjev S, Ðoric I, Paunovic I, Tatic S
and Cvejic D: Coexpressed high levels of VEGF-C and active MMP-9
are associated with lymphatic spreading and local invasiveness of
papillary thyroid carcinoma. Am J Clin Pathol. 146:594–602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang JY, Kim DS, Park HY, Shin SC, Cha W,
Lee JC, Wang SG and Lee BJ: Preoperative serum VEGF-C but not
VEGF-A level is correlated with lateral neck metastasis in
papillary thyroid carcinoma. Head Neck. 41:2602–2609. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi Y, Su C, Hu H, Yan H, Li W, Chen G, Xu
D, Du X and Zhang P: Serum MMP-2 as a potential predictive marker
for papillary thyroid carcinoma. PLoS One. 13:e01988962018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riley A, Green V, Cheah R, McKenzie G,
Karsai L, England J and Greenman J: A novel microfluidic device
capable of maintaining functional thyroid carcinoma specimens ex
vivo provides a new drug screening platform. BMC Cancer.
22:2592019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 (Suppl 16):S15–S18. 2002.
View Article : Google Scholar
|
|
15
|
Kohn EC and Liotta LA: Molecular insights
into cancer invasion: Strategies for prevention and intervention.
Cancer Res. 55:1856–1862. 1995.PubMed/NCBI
|
|
16
|
Ito Y, Miyauchi A, Kihara M, Fukushima M,
Higashiyama T and Miya A: Overall survival of papillary thyroid
carcinoma patients: A single-institution long-term follow-up of
5897 patients. World J Surg. 42:615–622. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tombran-Tink J, Chader GG and Johnson LV:
PEDF: A pigment epithelium-derived factor with potent neuronal
differentiative activity. Exp Eye Res. 53:411–414. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bilak MM, Corse AM, Bilak SR, Lehar M,
Tombran-Tink J and Kuncl RW: Pigment epithelium-derived factor
(PEDF) protects motor neurons from chronic glutamate-mediated
neurodegeneration. J Neuropathol Exp Neurol. 58:719–728. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Chen J, Ke Y, Mansel RE and Jiang
WG: Expression of pigment epithelial derived factor is reduced in
non-small cell lung cancer and is linked to clinical outcome. Int J
Mol Med. 17:937–944. 2006.PubMed/NCBI
|
|
20
|
Dawson DW, Volpert OV, Gillis P, Crawford
SE, Xu H, Benedict W and Bouck NP: Pigment epithelium-derived
factor: A potent inhibitor of angiogenesis. Science. 285:245–248.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Han J, Yang X, Shao C, Xu Z,
Cheng R, Cai W, Ma J, Yang Z and Gao G: Pigment epithelium-derived
factor inhibits angiogenesis and growth of gastric carcinoma by
down-regulation of VEGF. Oncol Rep. 26:681–686. 2011.PubMed/NCBI
|
|
22
|
Crawford SE, Stellmach V, Ranalli M, Huang
X, Huang L, Volpert O, De Vries GH, Abramson LP and Bouck N:
Pigment epithelium-derived factor (PEDF) in neuroblastoma: A
multifunctional mediator of schwann cell antitumor activity. J Cell
Sci. 114((Pt 24)): 4421–4428. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Filleur S, Volz K, Nelius T, Mirochnik Y,
Huang H, Zaichuk TA, Aymerich MS, Becerra SP, Yap R, Veliceasa D,
et al: Two functional epitopes of pigment epithelial-derived factor
block angiogenesis and induce differentiation in prostate cancer.
Cancer Res. 65:5144–5152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheung LW, Au SC, Cheung AN, Ngan HY,
Tombran-Tink J, Auersperg N and Wong AST: Pigment
epithelium-derived factor is estrogen sensitive and inhibits the
growth of human ovarian cancer and ovarian surface epithelial
cells. Endocrinology. 147:4179–4191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan M, Pang CP, Yam HF, Cheung KF, Liu WW
and Lu Y: Inhibition of glioma invasion by overexpression of
pigment epithelium-derived factor. Cancer Gene Ther. 11:325–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv Y, Sun Y, Shi T, Shi C, Qin H and Li Z:
Pigment epithelium-derived factor has a role in the progression of
papillary thyroid carcinoma by affecting the HIF1α-VEGF signaling
pathway. Oncol Lett. 12:5217–5222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graves DT, Barnhill R, Galanopoulos T and
Antoniades HN: Expression of monocyte chemotactic protein-1 in
human melanoma in vivo. Am J Pathol. 140:9–14. 1992.PubMed/NCBI
|
|
29
|
Negus RP, Stamp GW, Relf MG, Burke F,
Malik ST, Bernasconi S, Allavena P, Sozzani S, Mantovani A and
Balkwill FR: The detection and localization of monocyte
chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin
Invest. 95:2391–2396. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saji H, Koike M, Yamori T, Saji S, Seiki
M, Matsushima K and Toi M: Significant correlation of monocyte
chemoattractant protein-1 expression with neovascularization and
progression of breast carcinoma. Cancer. 92:1085–1091. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohta M, Kitadai Y, Tanaka S, Yoshihara M,
Yasui W, Mukaida N, Haruma K and Chayama K: Monocyte
chemoattractant protein-1 expression correlates with macrophage
infiltration and tumor vascularity in human esophageal squamous
cell carcinomas. Int J Cancer. 102:220–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koide N, Nishio A, Sato T, Sugiyama A and
Miyagawa S: Significance of macrophage chemoattractant protein-1
expression and macrophage infiltration in squamous cell carcinoma
of the esophagus. Am J Gastroenterol. 99:1667–1674. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu Y, Cai Z, Galson DL, Xiao G, Liu Y,
George DE, Melhem MF, Yao Z and Zhang J: Monocyte chemotactic
protein-1 (MCP-1) acts as a paracrine and autocrine factor for
prostate cancer growth and invasion. Prostate. 66:1311–1318. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ueno T, Toi M, Saji H, Muta M, Bando H,
Kuroi K, Koike M, Inadera H and Matsushima K: Significance of
macrophage chemoattractant protein-1 in macrophage recruitment,
angiogenesis, and survival in human breast cancer. Clin Cancer Res.
6:3282–3289. 2000.PubMed/NCBI
|
|
35
|
Tanaka K, Kurebayashi J, Sohda M, Nomura
T, Prabhakar U, Yan L and Sonoo H: The expression of monocyte
chemotactic protein-1 in papillary thyroid carcinoma is correlated
with lymph node metastasis and tumor recurrence. Thyroid. 19:21–25.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryder M, Gild M, Hohl TM, Pamer E, Knauf
J, Ghossein R, Joyce JA and Fagin JA: Genetic and pharmacological
targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and
impairs BRAF-induced thyroid cancer progression. PLoS One.
8:e543022013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dawes J, Pratt DA, Dewar MS and Preston
FE: Do extra-platelet sources contribute to the plasma level of
thrombospondin? Thromb Haemost. 59:273–276. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen H, Herndon ME and Lawler J: The cell
biology of thrombospondin-1. Matrix Biol. 19:597–614. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nucera C, Porrello A, Antonello ZA, Mekel
M, Nehs MA, Giordano TJ, Gerald D, Benjamin LE, Priolo C, Puxeddu
E, et al: B-Raf(V600E) and thrombospondin-1 promote thyroid cancer
progression. Proc Natl Acad Sci USA. 107:10649–10654. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soula-Rothhut M, Coissard C, Sartelet H,
Boudot C, Bellon G, Martiny L and Rothhut B: The tumor suppressor
PTEN inhibits EGF-induced TSP-1 and TIMP-1 expression in FTC-133
thyroid carcinoma cells. Exp Cell Res. 304:187–201. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bienes-Martínez R, Ordóñez A,
Feijoo-Cuaresma M, Corral-Escariz M, Mateo G, Stenina O, Jiménez B
and Calzada MJ: Autocrine stimulation of clear-cell renal carcinoma
cell migration in hypoxia via HIF-independent suppression of
thrombospondin-1. Sci Rep. 2:7882012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tzeng HT, Tsai CH, Yen YT, Cheng HC, Chen
YC, Pu SW, Wang YS, Shan YS, Tseng YL, Su WC, et al: Dysregulation
of Rab37-mediated cross-talk between cancer cells and endothelial
cells via thrombospondin-1 promotes tumor neovasculature and
metastasis. Clin Cancer Res. 23:2335–2345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang T, Sun L, Yuan X and Qiu H:
Thrombospondin-1 is a multifaceted player in tumor progression.
Oncotarget. 8:84546–84558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schlaeppi JM and Wood JM: Targeting
vascular endothelial growth factor (VEGF) for anti-tumor therapy,
by anti-VEGF neutralizing monoclonal antibodies or by VEGF receptor
tyrosine-kinase inhibitors. Cancer Metastasis Rev. 18:473–481.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shweiki D, Itin A, Soffer D and Keshet E:
Vascular endothelial growth factor induced by hypoxia may mediate
hypoxia-initiated angiogenesis. Nature. 359:843–845. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Soh EY, Duh QY, Sobhi SA, Young DM,
Epstein HD, Wong MG, Garcia YK, Min YD, Grossman RF, Siperstein AE
and Clark OH: Vascular endothelial growth factor expression is
higher in differentiated thyroid cancer than in normal or benign
thyroid. J Clin Endocrinol Metab. 82:3741–3747. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tuttle RM, Fleisher M, Francis GL and
Robbins RJ: Serum vascular endothelial growth factor levels are
elevated in metastatic differentiated thyroid cancer but not
increased by short-term TSH stimulation. J Clin Endocrinol Metab.
87:1737–1742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie J, Liu Y, Du X and Wu Y: TGF-β1
promotes the invasion and migration of papillary thyroid carcinoma
cells by inhibiting the expression of lncRNA-NEF. Oncol Lett.
17:3125–3132. 2019.PubMed/NCBI
|
|
49
|
Zhang W, van Weerden WM, de Ridder CMA,
Erkens-Schulze S, Schönfeld E, Meijer TG, Kanaar R, van Gent DC and
Nonnekens J: Ex vivo treatment of prostate tumor tissue
recapitulates in vivo therapy response. Prostate. 79:390–402. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu X, Su C, Xu J, Zhou D, Yan H, Li W,
Chen G, Zhang N, Xu D and Hu H: Immunohistochemical analysis of
matrix metalloproteinase-9 predicts papillary thyroid carcinoma
prognosis. Oncol Lett. 17:2308–2316. 2019.PubMed/NCBI
|
|
51
|
Selemetjev S, Savin S, Paunovic I, Tatic S
and Cvejic D: Concomitant high expression of survivin and vascular
endothelial growth factor-C is strongly associated with metastatic
status of lymph nodes in papillary thyroid carcinoma. J Cancer Res
Ther. 14 (Suppl):S114–S119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guan H, Guo Y, Liu L, Ye R, Liang W, Li H,
Xiao H and Li Y: INAVA promotes aggressiveness of papillary thyroid
cancer by upregulating MMP9 expression. Cell Biosci. 8:262018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cui M, Chang Y, Du W, Liu S, Qi J, Luo R
and Luo S: Upregulation of lncRNA-ATB by transforming growth factor
β1 (TGF-β1) promotes migration and invasion of papillary thyroid
carcinoma cells. Med Sci Monit. 24:5152–5158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lu ZL, Chen YJ, Jing XY, Wang NN, Zhang T
and Hu CJ: Detection and identification of serum peptides biomarker
in papillary thyroid cancer. Med Sci Monit. 24:1581–1587. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang N, Jiang R, Yang JY, Tang C, Yang L,
Xu M, Jiang QF and Liu ZM: Expression of TGF-β1, SNAI1 and MMP-9 is
associated with lymph node metastasis in papillary thyroid
carcinoma. J Mol Histol. 45:391–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Makki FM, Taylor SM, Shahnavaz A, Leslie
A, Gallant J, Douglas S, Teh E, Trites J, Bullock M, Inglis K, et
al: Serum biomarkers of papillary thyroid cancer. J Otolaryngol
Head Neck Surg. 42:162013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou ZH, Cui XN, Xing HG, Yan RH, Yao DK
and Wang LX: Changes and prognostic value of serum vascular
endothelial growth factor in patients with differentiated thyroid
cancer. Med Princ Pract. 22:24–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liang H, Zhong Y, Luo Z, Huang Y, Lin H,
Luo M, Zhan S, Xie K, Ma Y and Li QQ: Assessment of biomarkers for
clinical diagnosis of papillary thyroid carcinoma with distant
metastasis. Int J Biol Markers. 25:38–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang T, Jiang CX, Li Y and Liu X:
Pathologic study of expression and significance of matrix
metalloproteinases-9, tissue inhibitor of metalloproteinase-1,
vascular endothelial growth factor and transforming growth factor
beta-1 in papillary carcinoma and follicular carcinoma of thyroid.
Zhonghua Bing Li Xue Za Zhi. 38:824–828. 2009.(In Chinese).
PubMed/NCBI
|
|
60
|
Wang JX, Dong R, Liu QL, Yang SB, Fan YX,
Zhang Q, Yang FQ, Wu P, Yu JK and Zheng S: Detection and
identification of specific serum biomarkers in papillary thyroid
cancer. Zhonghua Zhong Liu Za Zhi. 31:265–268. 2009.(In Chinese).
PubMed/NCBI
|