Introduction
According to the National Comprehensive Cancer
Network (NCCN) guidelines, treatment options for patients with
locoregional recurrence of head and neck squamous cell carcinoma
(HNSCC) without prior radiotherapy (RT) includes salvage surgery,
RT combined with chemotherapy, or combination chemotherapy followed
by RT (1). The classical
chemotherapy treatment options include high-dose platinum plus
5-fluorouracil or cetuximab (Cmab) (1). Moreover, immune checkpoint inhibitors
(ICIs), such as pembrolizumab with or without the platinum or
EXTREME regimens, are recommended as category 1 therapies because
of their effectiveness in very advanced HNSCC (2,3).
Among the treatment options, salvage surgery with curative intent
has shown a survival benefit (4–6).
However, salvage surgery often results in a poorer quality of life
(QOL) because of dysphagia or speech problems. Therefore,
participating in multidisciplinary discussions regarding treatment
options is recommended to maximize survival while preserving form
and function (1). At present, no
clear criteria exist for salvage surgery treatment. Since salvage
surgery may result in a significantly lower QOL, then a shift to
chemoradiotherapy as the treatment strategy might be appropriate.
In our previous report regarding Cmab-containing chemotherapy, we
suggested that high expression of the 110-kDa catalytic subunit of
class IA phosphatidylinositol 3-kinase (PI3Kp110α) may play an
important role in Cmab resistance, and PI3Kp110α is a predictor for
Cmab therapy in recurrent and metastatic oral squamous cell
carcinoma (OSCC) (7).
Based on the treatment strategy in our department,
patients commonly undergo surgery with or without adjuvant RT or
concurrent chemoradiotherapy against primary OSCC according to the
NCCN guidelines (1). We describe
our experiences of three patients who received Cmab in combination
with RT (Cmab + RT) for loco-regional recurrent OSCC and achieved
long-term survival of 5 years or more while maintaining the QOL. We
also reported PI3Kp110α findings from an immunohistochemical study
to clarify the validity of our previous report (7).
Case report
Case 1
A 63-year-old man who was complaining of a painful
mass at the right side of his tongue was referred to Nagasaki
University Hospital (Nagasaki, Japan) in March 2017. Intraoral
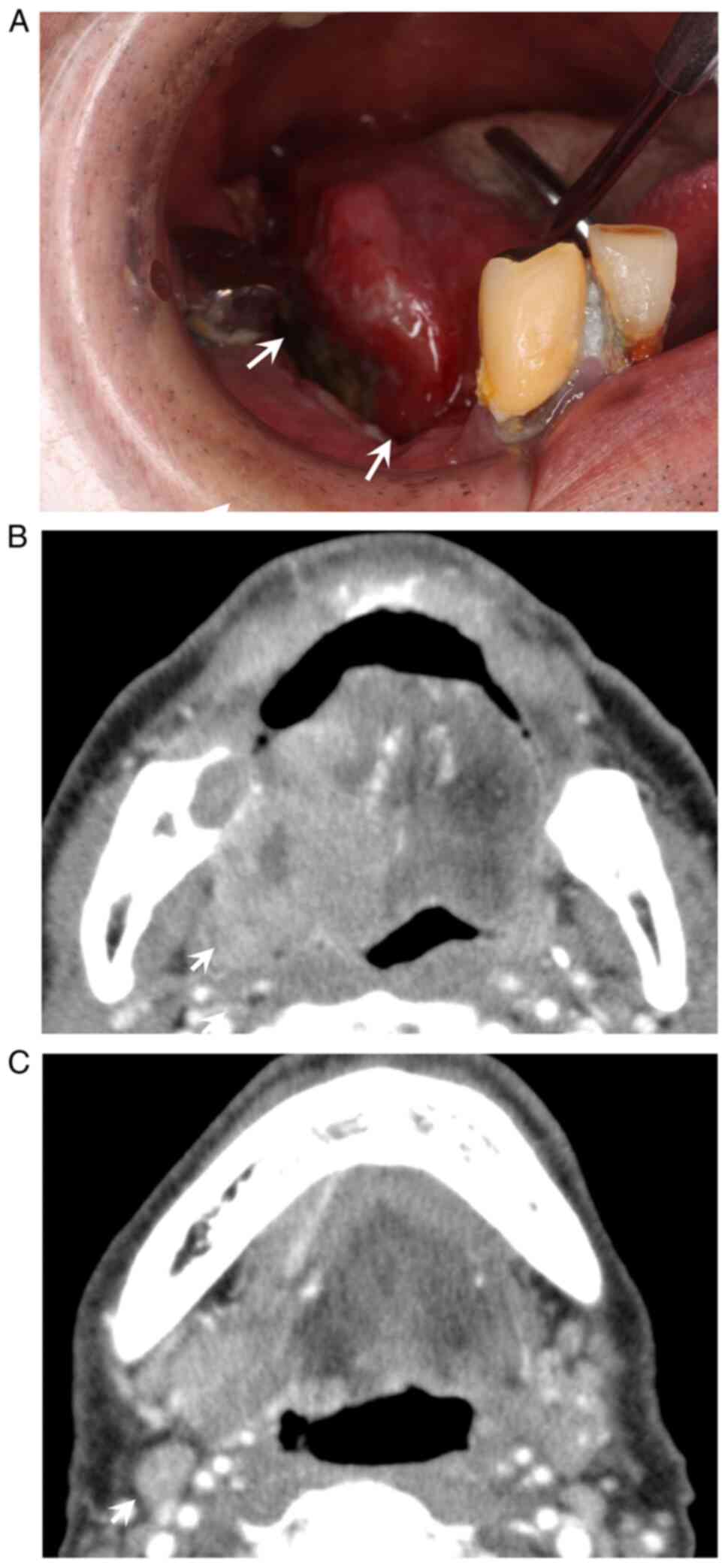
examination revealed a 48×35 mm elastic and hard mass with a
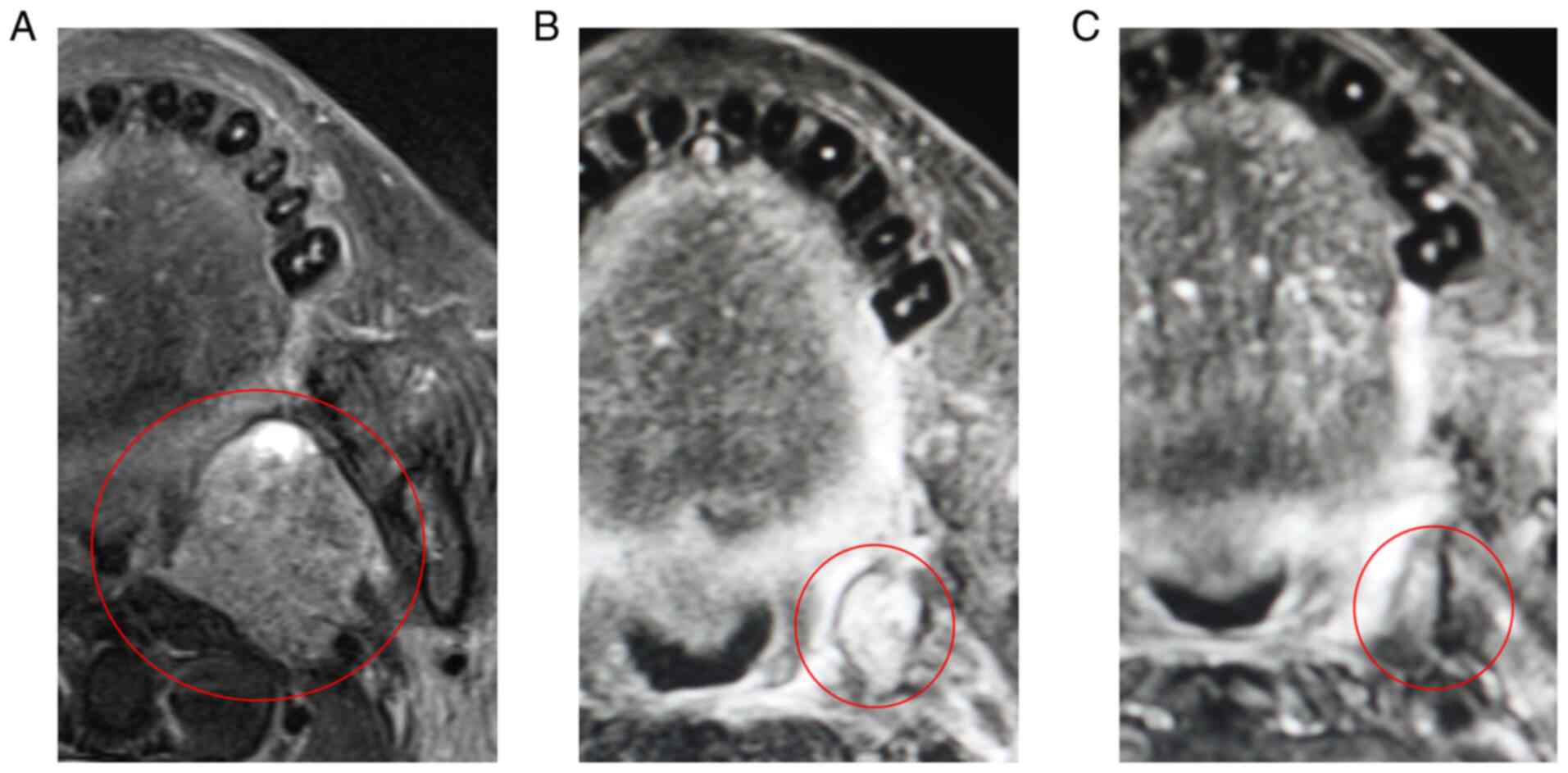
central ulceration involving the right tongue (Fig. 1A). Contrast-enhanced axial computed
tomography (CT) revealed a poorly marginated lesion and right
regional lymph node involvement (Fig.
1B and C). The depth of clinical invasion was 27 mm. He was
diagnosed with SCC of the tongue (cT4aN1M0 stage IVA) based on the
clinical and biopsy findings. Under general anesthesia, the patient
underwent subtotal glossectomy, modified radical neck dissection,
and reconstruction with a pectoral major musculocutaneous flap.
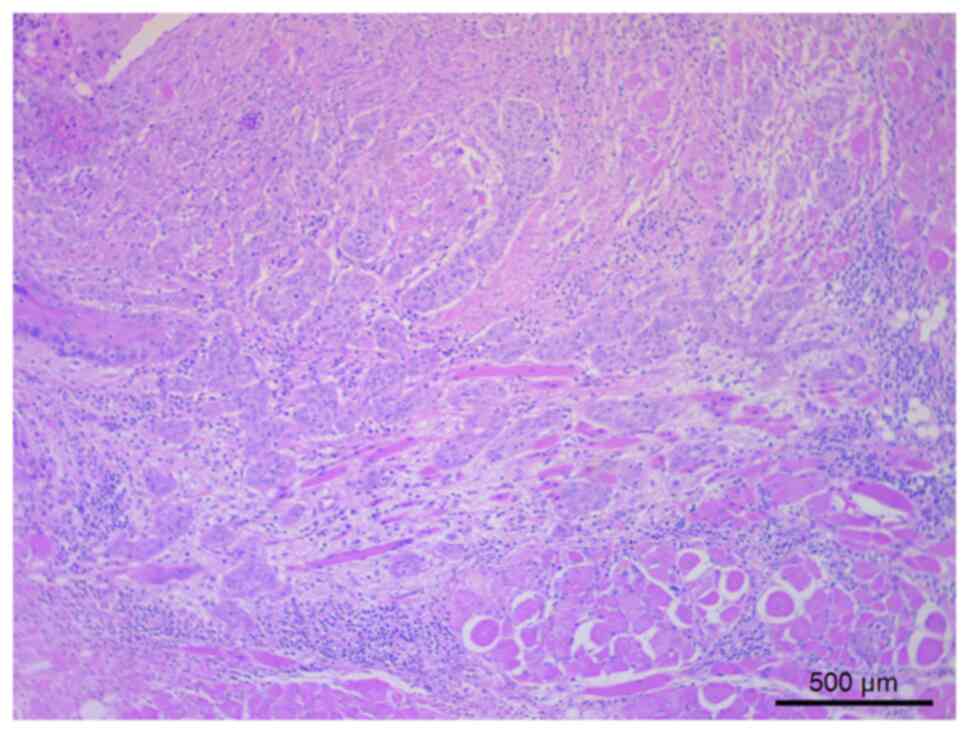
Pathological findings of the primary tumor showed keratinizing
tumor cells that had relatively round or cord-like tumor nests with
deeply stained irregular nuclei, which infiltrated the submucosa
and surrounded the deep muscle layer of the tongue (Fig. 2). However, 4 months after surgery,
regional recurrence was noted at the lower right mastoid region
contacting the cervical vertebrae processus transversus. Although
salvage surgery was considered, the patient underwent Cmab + RT
because extensive resection would result in dysphagia and lead to a
poorer QOL. RT was administered at a total dose of 66 Gy.
Concomitant RT was administered at 2 Gy/day for 6 days/week. Cmab
was administered for 6 courses at a dose of 400 mg/m2 of
body surface area (BSA) for the first injection followed by 250
mg/m2 BSA/week thereafter. Among the adverse events,
grade 2 radiation dermatitis, oral mucositis, and acne like rash
were observed. All the adverse events were manageable. Disease-free
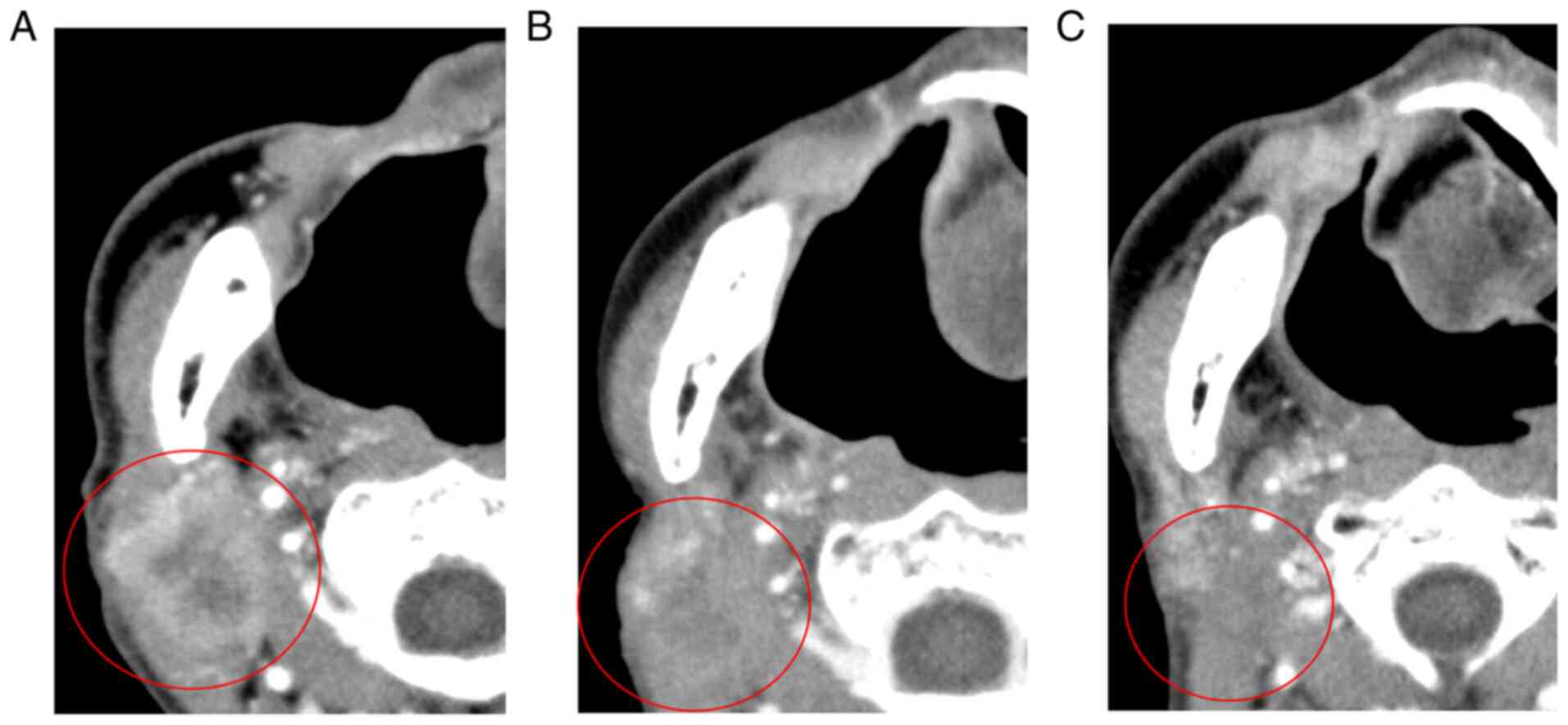
status was confirmed by enhanced CT 4 months after Cmab + RT
(Fig. 3A-C), and there were no
signs of recurrence or progression 5 years after Cmab + RT.
Case 2
A 77-year-old woman who was complaining of a painful
mass at the right buccal mucosa was referred to Nagasaki University
Hospital in May 2015. History revealed that she had undergone
neoadjuvant chemotherapy [cisplatin (117.75
mg)/tegafur/gimeracil/oteracil potassium (2,640 mg)], marginal
mandibulotomy, and radical neck dissection for SCC of the right
mandible (cT4N1M0 stage IVA) 6 years ago in our department.
Intraoral examination revealed a 27×18 mm elastic and hard mass
with a central ulceration at the right buccal mucosa.
Contrast-enhanced axial magnetic resonance imaging (MRI) revealed a
poorly marginated lesion and left regional lymph node involvement.
The clinical invasion depth was 14 mm. She was diagnosed with SCC
of the buccal mucosa (cT2N2bM0 stage IVA) based on the clinical and
biopsy findings. Under general anesthesia, the patient underwent
tumorectomy of the buccal mucosa, modified radical neck dissection,
and reconstruction with a forearm flap. SCC of the buccal mucosa
was diagnosed pathologically. However, regional recurrence was
noted at the parotid lymph node 7 months after surgery. Although
salvage surgery was considered, she refused this treatment as it
may result in facial nerve paralysis and xerostomia, which may lead
to a poorer QOL; she instead underwent Cmab + RT. RT was
administered at a total dose of 66 Gy. Concomitant RT was
administered at 2 Gy/day for 6 day/week. Cmab was administered for
6 courses at a dose of 400 mg/m2 of BSA for the first
injection followed by 250 mg/m2 BSA/week thereafter. In
the adverse events, grade 2 radiation dermatitis, grade 3 oral
mucositis and grade 2 acne like rash were observed. All the adverse
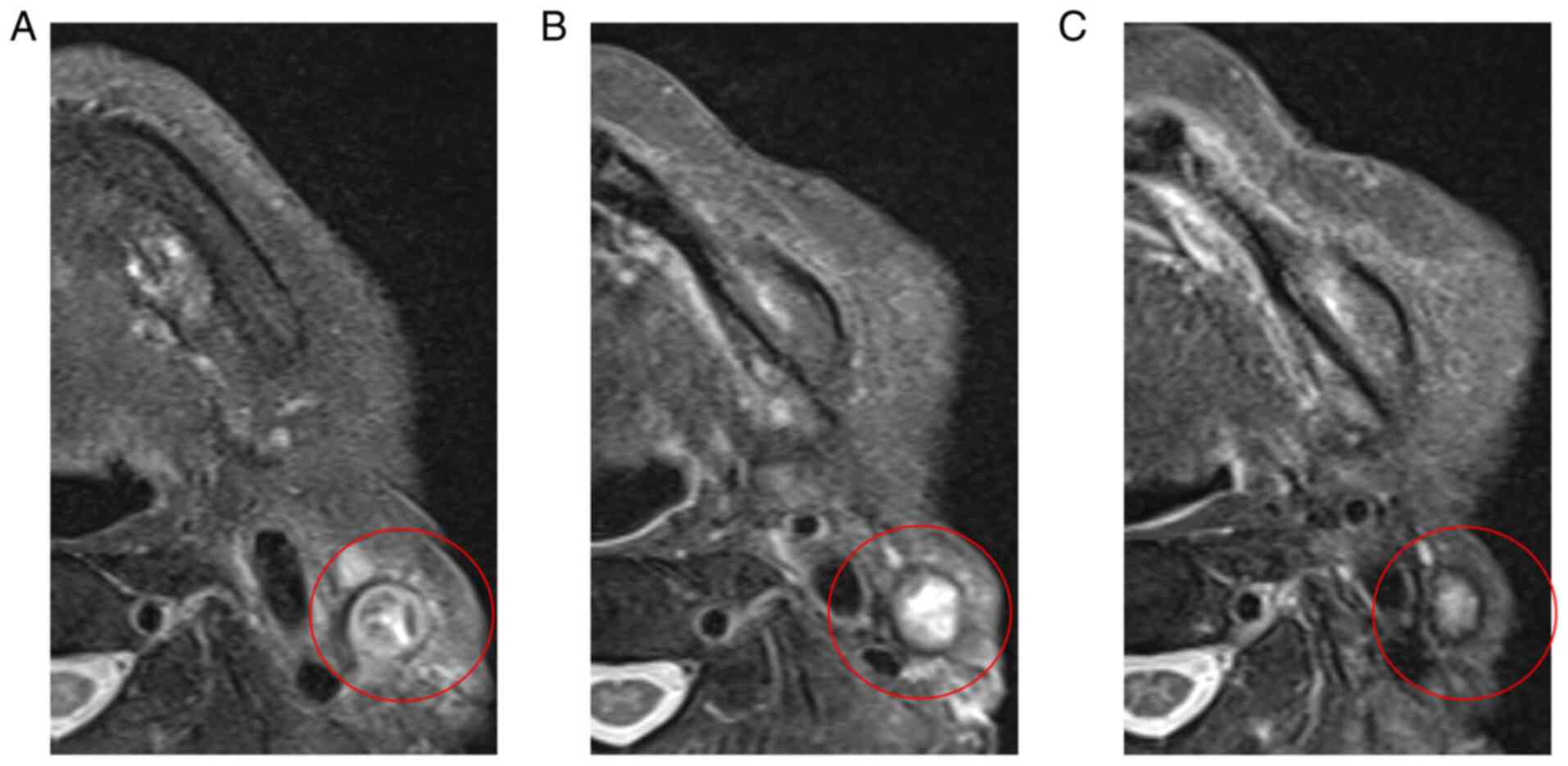
events were manageable. Disease-free status was confirmed using
enhanced MRI 2 years and 6 months after Cmab + RT (Fig. 4A-C), and there were no signs of
recurrence or progression 6 years after Cmab + RT.
Case 3
A 71-year-old man who was complaining of a painful
mass at the left mandible was referred to Nagasaki University
Hospital in March 2015. Intraoral examination revealed a 15×15 mm
elastic and hard mass with a central ulceration at the lingual side
of left retromolar region. Contrast-enhanced axial MRI revealed a
poorly marginated lesion and left regional lymph node involvement.
The clinical invasion depth was 8 mm. The patient was diagnosed
with SCC of the mandibular gingiva (cT2N2bM0 stage IVA) based on
the clinical and biopsy findings. Under general anesthesia, the
patient underwent marginal mandibulotomy and modified radical neck
dissection. SCC of the mandibular gingiva was pathologically
diagnosed. However, local recurrence was noted at the masticator
space and regional recurrence was noted at the Rouviere's lymph
nodes 1 and 3 months after surgery, respectively. Although salvage
surgery was considered, the patient underwent Cmab + RT because the
lesion was unresectable. RT was administered at a total dose of 66
Gy. Concomitant RT was administered at 2 Gy/day for 6 day/week.
Cmab was administered at a dose of 400 mg/m2 of BSA for
the first injection followed by 250 mg/m2 BSA/week
thereafter. After RT, Cmab was maintained for approximately 2 years
because of a residual, but localized tumor in the masticatory
muscle. In the adverse events, grade 1 radiation dermatitis, grade
3 oral mucositis, and grade 1 acne like rash were observed. All the
adverse events were manageable. Disease-free status was confirmed
using enhanced MRI 10 months after RT (Fig. 5A-C), and there were no signs of
recurrence or progression 5 years and 9 months after RT.
Immunohistochemical staining and
evaluation
Immunohistochemical staining was performed by using
the EnVision method (EnVision+; Dako), as previously described
(7). For the assay, specimens were
obtained immediately before Cmab + RT. Because neoadjuvant and
adjuvant chemotherapy were not administered to all the patients
before Cmab + RT, paraffin-embedded sections of the resected
primary tumor specimens were selected. PI3Kp110α (dilution 1:400)
rabbit polyclonal primary antibody from Abcam was used. Negative
controls were prepared by replacing the primary antibody with
phosphate-buffered saline. Normal oral mucosal specimens from three
healthy individuals were used as positive controls.
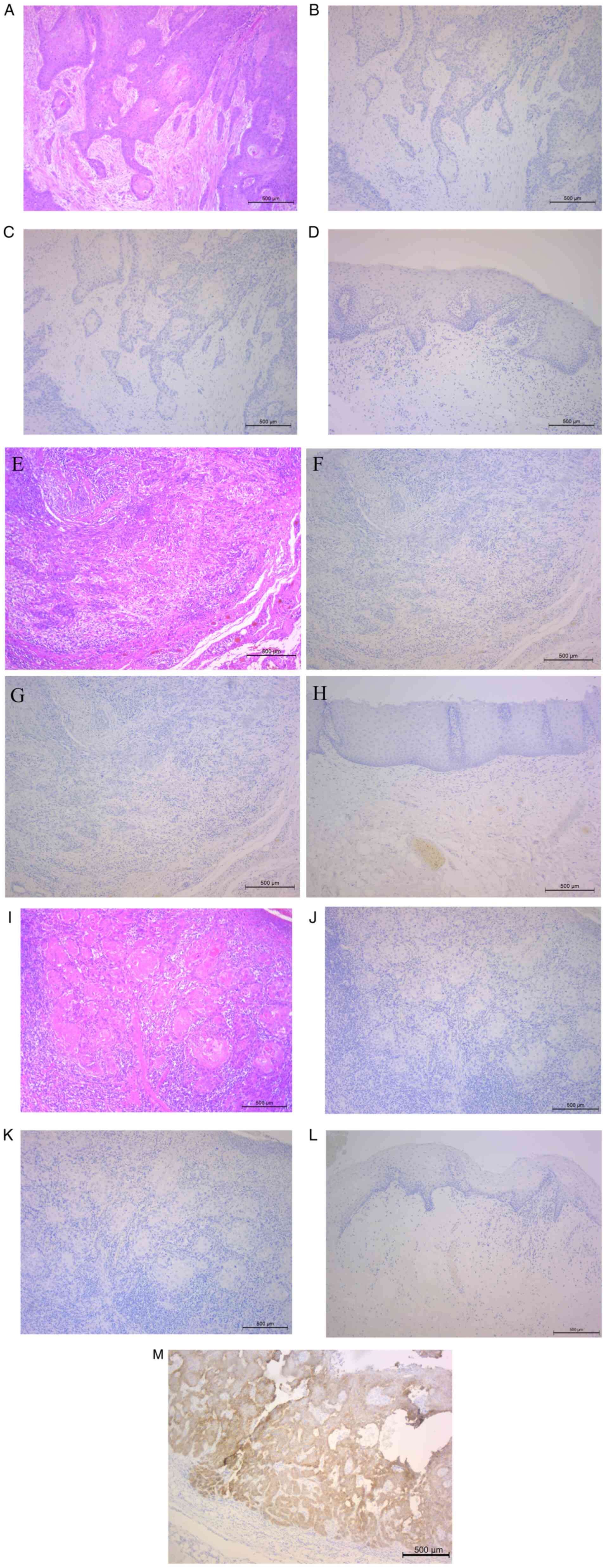
Among the three patients, no PI3Kp110α expression
was detected in all patients (Fig.
6A-L). High PI3Kp110α expression in a case of OSCC described in
our previous study (7) was shown
in Fig. 6M. Our previous study
revealed that high PI3Kp110α expression was significantly
associated with cetuximab resistance (7). In these results, weak PI3Kp110α
expression showed Cmab sensitivity.
Discussion
The goal of this article was to describe the
usefulness of Cmab + RT and assess the predictors of PI3Kp110α for
Cmab therapy in locoregional recurrence of OSCC. Treatment options
recommended for patients with locoregional recurrence of HNSCC
without prior RT include salvage surgery, RT combined with
chemotherapy, or combination chemotherapy followed by RT (1). Salvage surgery with curative intent
has shown survival benefit (4–6).
However, salvage surgery often lowers the patient's QOL. Horn et
al reported that impaired swallowing and speech lowered the
overall QOL 3 months after salvage surgery, although the patients
recovered within 5 years (8).
Recently, the development of molecular targeted drugs and ICIs has
resulted in improved patient survival and QOL, which have been
turning points in the treatment strategy for locoregional HNSCC.
Therefore, if salvage surgery will lower a patient's QOL,
chemoradiotherapy should be performed instead.
We experienced three cases of favorable locoregional
control against locoregional recurrence of OSCC with Cmab + RT. In
a prospective study of locoregional recurrence of HNSCC, Hecht
et al reported that Cmab + RT had superior progression-free
survival and overall survival (OS) compared to Cmab alone, with a
1-year OS of 53% (9). Other two
studies have reported that the 1-year OS of Cmab + RT were 44 and
47.5%, respectively (10,11). The overall response rate (ORR) in
these reports was 16–58%, which was different from previous study
(9–11). In contrast, the 1-year OS of
salvage surgery was approximately 50% (5,6) and
1-year OS of chemotherapy with Cmab (EXTREME regimen and Cmab plus
paclitaxel) was approximately 40% in recurrent or metastatic (R/M)
OSCC (12,13). Meanwhile, the ORRs for the EXTREME
regimen and Cmab plus paclitaxel were 46.2 and 48.4%, respectively.
More recently, pembrolizumab was approved as first-line treatment
for patients with R/M HNSCC (1);
its 1-year-OS with immunotherapy (pembrolizumab alone or with
chemotherapy) is 49–57% (2).
However, in a subgroup analysis of patients with locoregional
recurrence, the efficacy of pembrolizumab alone did not differ from
the EXTREME regimen in selected patients (programmed death ligand-1
combined positive score ≥1) (2).
Although the comparison of different treatment modalities was
challenging because of differences, such as regions of recurrent
OSCC and previous therapy, Cmab + RT was not inferior to other
treatments for locoregional recurrence of OSCC.
Regarding lowered QOL, late adverse events after RT,
such as impairments in swallowing and eating, and salvage surgery
result in a lower QOL. Osteoradionecrosis (ORN) of the jaw is a
severe late adverse event of RT that occurs in the head and neck
region. ORN interferes with the patient's daily activities due to
persistent pain, drainage from the exposed bone, trismus, and
eating disorders, resulting in a poor QOL. Kojima et al
reported that ORN developed in 30 of 392 patients (7.7%)
administered with 50 Gy of RT in the head and neck region (14). The standard therapy for ORN has not
yet been established; however, it sometimes requires extensive
jawbone resection. Although ORN did not develop in our patients,
periapical periodontitis and tooth extraction after radiotherapy
are independent risk factors for ORN, which should be monitored in
patients receiving RT in the head and neck region (14,15).
As a predictor of response to Cmab therapy, Argiris
et al (16) has reported
that vascular endothelial growth factor (VEGF) and interleukin
(IL)-6 were identified as potentially useful serum biomarker
predictors. In addition, Oliveras-Ferraros et al (17) reported that interferon/STAT1 and
neuregulin signaling pathways are predictors of Cmab efficacy in
KRAS wild-type squamous carcinomas. Cmab promotes
epithelial-to-mesenchymal transition (EMT), which Cmab resistance
is associated with. In previous reports, the association between
increased expression of EMT markers and Cmab resistance have been
reported (18,19). We have previously reported that
based on the immunohistochemical staining of recurrent tumors, as
in this case, before and after long-term Cmab administration,
increased expression of EMT markers was observed (20). In addition, we have also previously
suggested that inhibition of PI3Kp110α was not only a good
predictor for Cmab therapy, but that it also inhibits the potential
of Cmab-resistant cells to undergo EMT in OSCC (7). Therefore, we believe that PI3Kp110α
is one of the best useful predictors for Cmab therapy in OSCC. In
our three cases, either no or weak expression of PI3Kp110α was
observed, resulting in better clinical outcomes. Therefore, this
case series provides strong evidence of the association between
PI3Kp110α expression and Cmab resistance, as was reported in our
previous study. Bonner et al (21) reported that Cmab-induced moderate
or severe skin rash is associated with better survival. In our case
series, two patients with severe oral mucositis were observed, but
in all the patients with adverse events (radiation dermatitis and
acne like rash), these were mild or moderate, and there was no
relationship between adverse events and PI3Kp110α expression.
A potential limitation of our experience is that
only three cases were reported. Additionally, patient backgrounds,
such as the regions of recurrent OSCC, previous therapies, and
tumor heterogeneity, differed among the patients. Moreover, we did
not compare the efficacy of Cmab + RT with that of other therapies
(salvage surgery or chemotherapy). Therefore, our findings should
be interpreted with caution.
In conclusion, our experience suggests that if
salvage surgery for recurrent OSCC will result in a significantly
low QOL, then shifting to chemoradiotherapy is appropriate as one
of the treatment strategies. Furthermore, our experience provides
strong evidence that PI3Kp110α expression is associated with Cmab
therapy efficacy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TN, KF, TM, KM, MO and MU contributed to the
conception and design of the study. Clinical data were collected by
TN, TM and KM. Data analysis for the immunohistochemical study was
performed by TN, KF and MU. TN and MU confirm the authenticity of
all the raw data. The first draft of the manuscript was written by
TN, and all authors commented on the previous versions of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This immunochemical study was approved by the
independent ethics committee of Nagasaki University Hospital
(approval no. 20042012). Patients were given the opportunity to opt
out of participation in the research involving the
immunohistochemical study of tissues.
Patient consent for publication
Written informed consent for publication was
obtained from the three patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
RT
|
radiotherapy
|
|
Cmab
|
cetuximab
|
|
ICI
|
immune checkpoint inhibitor
|
|
QOL
|
quality of life
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PI3Kp110α
|
110-kDa catalytic subunit of class IA
phosphatidylinositol 3-kinase
|
|
Cmab + RT
|
Cmab in combination with RT
|
|
CT
|
computed tomography
|
|
BSA
|
body surface area
|
|
MRI
|
magnetic resonance imaging
|
|
OS
|
overall survival
|
|
ORR
|
overall response rate
|
|
R/M
|
recurrent or metastatic
|
|
ORN
|
osteoradionecrosis
|
References
|
1
|
National Comprehensive Cancer Network, .
Clinical practice guidelines in oncology head and neck cancers,
version 2. National Comprehensive Cancer Network. 2021.
|
|
2
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akali NR, Buggaveeti R, Sukumaran SV,
Balasubramanian D, Iyer S and Thankappan K: Prior chemoradiotherapy
and pathological perineural invasion predict the survival outcomes
of salvage surgery in head and neck squamous cell carcinoma. Head
Neck. 43:874–883. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao CT, Chang JT, Wang HM, Ng SH, Hsueh
C, Lee LY, Lin CH, Chen IH, Huang SF, Cheng AJ and Yen TC: Salvage
therapy in relapsed squamous cell carcinoma of the oral cavity: How
and when? Cancer. 112:94–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nandy K, Rai S, Bhatt S, Puj K, Rathod P
and Gangopadhyay A: Salvage surgery for recurrent carcinoma of the
oral cavity: Assessment of prognostic factors. Int J Oral
Maxillofac Surg. 51:602–611. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuchihashi H, Naruse T, Yanamoto S,
Okuyama K, Furukawa K, Omori K and Umeda M: Selective inhibition of
PI3K110α as a novel therapeutic strategy for cetuximab-resistant
oral squamous cell carcinoma. Oncol Rep. 44:863–872. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horn D, Zittel S, Moratin J, Metzger K,
Ristow O, Krisam J, Bodem J, Engel M, Freudlsperger C, Hoffmann J
and Freier K: Prospective feasibility analysis of salvage surgery
in recurrent oral cancer in terms of quality of life. Oral Oncol.
102:1045802020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hecht M, Hahn D, Wolber P, Hautmann MG,
Reichert D, Weniger S, Belka C, Bergmann T, Göhler T, Welslau M, et
al: A prospective real-world multi-center study to evaluate
progression-free and overall survival of radiotherapy with
cetuximab and platinum-based chemotherapy with cetuximab in locally
recurrent head and neck cancer. Cancers (Basel). 13:34132021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balermpas P, Keller C, Hambek M,
Wagenblast J, Seitz O, Rödel C and Weiss C: Reirradiation with
cetuximab in locoregional recurrent and inoperable squamous cell
carcinoma of the head and neck: Feasibility and first efficacy
results. Int J Radiat Oncol Biol Phys. 83:e377–e383. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lartigau EF, Tresch E, Thariat J, Graff P,
Coche-Dequeant B, Benezery K, Schiappacasse L, Degardin M, Bondiau
PY, Peiffert D, et al: Multi institutional phase II study of
concomitant stereotactic reirradiation and cetuximab for recurrent
head and neck cancer. Radiother Oncol. 109:281–285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naruse T, Yanamoto S, Otsuru M, Yamakawa
N, Kirita T, Shintani Y, Matsumura T, Okura M, Sasaki M, Ota Y, et
al: Multicenter retrospective study of weekly cetuximab plus
paclitaxel for recurrent or metastatic oral squamous cell
carcinoma. Anticancer Res. 41:5785–5791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yanamoto S, Umeda M, Kioi M, Kirita T,
Yamashita T, Hiratsuka H, Yokoo S, Tanzawa H, Uzawa N, Shibahara T,
et al: Multicenter retrospective study of cetuximab plus
platinum-based chemotherapy for recurrent or metastatic oral
squamous cell carcinoma. Cancer Chemother Pharmacol. 81:549–554.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kojima Y, Yanamoto S, Umeda M, Kawashita
Y, Saito I, Hasegawa T, Komori T, Ueda N, Kirita T, Yamada SI, et
al: Relationship between dental status and development of
osteoradionecrosis of the jaw: A multicenter retrospective study.
Oral Surg Oral Med Oral Pathol Oral Radiol. 124:139–145. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito I, Hasegawa T, Kawashita Y, Kato S,
Yamada SI, Kojima Y, Ueda N, Umeda M, Shibuya Y, Kurita H, et al:
Association between dental extraction after radiotherapy and
osteoradionecrosis: A multi-centre retrospective study. Oral Dis.
28:1181–1187. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Argiris A, Lee SC, Feinstein T, Thomas S,
Branstetter BF IV, Seethala R, Wang L, Gooding W, Grandis JR and
Ferris RL: Serum biomarkers as potential predictors of antitumor
activity of cetuximab-containing therapy for locally advanced head
and neck cancer. Oral Oncol. 47:961–966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oliveras-Ferraros C, Vazquez-Martin A,
Queralt B, Adrados M, Ortiz R, Cufí S, Hernández-Yagüe X, Guardeño
R, Báez L, Martin-Castillo B, et al: Interferon/STAT1 and
neuregulin signaling pathways are exploratory biomarkers of
cetuximab (Erbitux®) efficacy in KRAS wild-type squamous
carcinomas: A pathway-based analysis of whole human-genome
microarray data from cetuximab-adapted tumor cell-line models. Int
J Oncol. 39:1455–1479. 2011.PubMed/NCBI
|
|
18
|
Schmitz S, Bindea G, Albu RI, Mlecnik B
and Machiels JP: Cetuximab promotes epithelial to mesenchymal
transition and cancer associated fibroblasts in patients with head
and neck cancer. Oncotarget. 6:34288–34299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimura I, Kitahara H, Ooi K, Kato K,
Noguchi N, Yoshizawa K, Nakamura H and Kawashiri S: Loss of
epidermal growth factor receptor expression in oral squamous cell
carcinoma is associated with invasiveness and
epithelial-mesenchymal transition. Oncol Lett. 11:201–207. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naruse T, Tokuhisa M, Yanamoto S, Sakamoto
Y, Okuyama K, Tsuchihashi H and Umeda M: Lower gingival squamous
cell carcinoma with brain metastasis during long-term cetuximab
treatment: A case report. Oncol Lett. 15:7158–7162. 2018.PubMed/NCBI
|
|
21
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|