Introduction
Breast cancer (BC), one of the most common tumor
types, is the second leading cause of cancer-related mortality
among females (1–3). In China, BC cases account for 12.2%
of all new BC diagnoses worldwide and 9.6% of all BC-related deaths
(4). In previous years, a number
of treatments have been developed for improving the survival rate
of patients with BC, including surgery, radiotherapy, chemotherapy,
endocrine therapy and antibody therapy (5,6). The
early diagnosis and treatment of the disease usually leads to a
good prognosis with a high survival rate (7). However, patients diagnosed with
advanced BC usually have a low survival rate and poor prognosis,
and recurrence often occurs (8).
The majority of deaths from BC are mainly due to drug resistance
and metastasis to distant organs, such as the bone, liver, lungs
and brain (9,10). Therefore, the development of novel
biomarkers for the early diagnosis and treatment of BC is
mandatory.
Recently, heat shock protein (HSP) 20 has become a
hotspot for tumor research due to its role in regulating
proliferation and apoptosis (11).
As a molecular chaperone, HSP20 belongs to the small HSP family and
is expressed in various organs (12). In 2007, HSP20 was found to be
downregulated in hepatocellular carcinoma (HCC) tissues and was
associated with tumor progression (13). Later, the detailed pathological
mechanisms of HSP20 in HCC were reported by the same group
(14–16). In addition, another research group
reported decreased expression of HSP20 in colorectal cancer tissues
(17). However, whether HSP20
serves a functional role in BC remains unclear.
MAPKs and AKT are responsible for intracellular
signal transduction and are usually activated by phosphorylation in
response to various stimuli (18,19).
It is well known that MAPK-related factors, ERK, JNK and p38, serve
a vital regulatory role in cell proliferation, invasion and
metastasis (20–22). Notably, accumulating evidence
suggests that the inhibition of the MAPK and AKT signaling pathways
can alleviate BC cell migration, invasion and proliferation
(23,24). The present study aimed to explore
whether HSP20 serves a regulatory role in BC through the MAPK and
AKT signaling pathways.
In the present study, data from Gene Expression
Omnibus (GEO) datasets and the Kaplan-Meier Plotter database were
employed to investigate the expression of HSP20 in BC tissues and
determine the association between HSP20 expression and the
prognosis of patients with BC, respectively. The association of
HSP20 with patient clinicopathological features was first
identified and the effects of HSP20 on cell proliferation,
migration and invasion were also examined. Mechanistically, the
MAPK and AKT signaling pathways may be involved in the
HSP20-mediated suppression of BC progression.
Materials and methods
Clinical samples
A total of 53 tumor samples were collected from
female patients (age range, 33–82 years; mean age, 55 years)
diagnosed with BC at Wuxi 9th Affiliated Hospital of Soochow
University (Wuxi, China) between March 2021 and February 2022. The
inclusion criteria were as follows: i) ≥18 years old; and ii) had
never undergone any type of anticancer therapy, such as
chemotherapy and radiotherapy prior to surgery. Patients diagnosed
with other cancers were excluded. The use of human tissues was
approved by the Ethics Committee of Wuxi 9th Affiliated Hospital of
Soochow University (approval no. LW2021008; Wuxi, China), and
written informed consent was provided by each participant. The
expression of HSP20 was examined using immunohistochemical (IHC)
staining of BC tissues, and the scoring system method was performed
manually according to the literature (25). Briefly, the tumor samples were
fixed in 4% paraformaldehyde for 24 h at room temperature and
embedded in paraffin. The paraffin-embedded samples were cut into
5-µm thick sections. After dewaxing with xylene for 15 min, the
sections were rehydrated with gradient alcohol solution (95, 85 and
75% for 2 min each) at room temperature. Following antigen
retrieval by boiling in citrate buffer for 10 min in a microwave
oven, H2O2 (3%) was added to the sections for
15 min. Subsequently, the sections were blocked with 1% goat serum
(SL038; Beijing Solarbio Science & Technology Co., Ltd.) for 15
min at room temperature. Subsequently, the sections were incubated
with anti-HSP20 (diluted 1:100; AF6003; Affinity Biosciences) at
4°C overnight. The horseradish peroxidase-conjugated
goat-anti-rabbit secondary antibody (diluted 1:500; #31460; Thermo
Fisher Scientific, Inc.) was added for 1 h at 37°C. The staining
was visualized using 100 µl DAB chromogenic fluid (DA1010; Beijing
Solarbio Science & Technology Co., Ltd.). Following hematoxylin
counterstaining at room temperature for 3 min, sections were
observed under a light microscope (magnification, ×400; Olympus
Corporation). The IHC scores of HSP20 expression are shown in
Fig. S1A. The median value of
HSP20 was a score of 2. If the HSP20 measurement was a score >2,
it was considered high expression; if the HSP20 was a score ≤2, it
was considered low expression. Representative IHC images are
provided in Fig. S1B. The results
of IHC scoring were used to divide patients into two groups [HSP20
high expression group (n=18) and HSP20 low expression group
(n=35)]. Subsequently, the association between HSP20 and patient
clinicopathological features was analyzed (Table I). Data from the GEO database
[https://www.ncbi.nlm.nih.gov/geo/; GSE139038, n=18; GSE115144,
n=21; GSE109169, n=25 (26,27)]
were applied to evaluate the expression of HSP20 in paired BC
tissues and adjacent non-cancerous tissues. The original data from
GEO are shown in Table SI. The
Kaplan-Meier Plotter database (http://kmplot.com/analysis/; survival curve, BC mRNA;
gene symbol, 214767_s_at; split patients by median; follow up
threshold, 120 months; cut-off value used in analysis, 172) was
used to assess the association between HSP20 expression and the
recurrence-free survival of 4,929 patients with BC.
 | Table I.Association between clinicopathologic
parameters and HSP20 expression in 53 patients with breast
cancer. |
Table I.
Association between clinicopathologic
parameters and HSP20 expression in 53 patients with breast
cancer.
|
| HSP20
expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameters | High, n (n=18) | Low, n (n=35) | P-value |
|---|
| Age, years |
|
| 0.504 |
|
<50 | 3 | 10 |
|
|
≥50 | 15 | 25 |
|
| ER |
|
| 0.144 |
|
Positive | 17 | 27 |
|
|
Negative | 1 | 8 |
|
| PR |
|
| 0.539 |
|
Positive | 14 | 24 |
|
|
Negative | 4 | 11 |
|
| HER2 |
|
| 0.174 |
|
Positive | 10 | 11 |
|
|
Negative | 8 | 23 |
|
|
Unknown | 0 | 1 |
|
| Ki-67, % |
|
| 0.901 |
|
<14 | 7 | 13 |
|
|
≥14 | 11 | 22 |
|
| pT |
|
| 0.015a |
| T1 | 6 | 20 |
|
| T2 | 5 | 10 |
|
| T3 | 0 | 3 |
|
|
Tis | 7 | 2 |
|
| pN |
|
| 0.179 |
| N0 | 13 | 23 |
|
| N1 | 3 | 5 |
|
| N2 | 2 | 1 |
|
| N3 | 0 | 6 |
|
| pStage |
|
| 0.031a |
| 0 | 7 | 2 |
|
| I | 6 | 15 |
|
| II | 3 | 10 |
|
|
III | 2 | 8 |
|
Cells and cell culture
The MDA-MB-231, MDA-MB-453 and MDA-MB-468 human BC
cell lines were incubated in L15 medium (LA9510; Beijing Solarbio
Science & Technology Co., Ltd.) containing 10% FBS (SH30084.03;
HyClone; Cytiva), 1% penicillin (C8251; Beijing Solarbio Science
& Technology Co., Ltd.) and 1% streptomycin (S8290; Beijing
Solarbio Science & Technology Co., Ltd.) in a 37°C, 5%
CO2 incubator. The MCF-7 and ZR-75-1 cells were cultured
with Minimum Essential Medium (MEM; 41500; Beijing Solarbio Science
& Technology Co., Ltd.) containing 10% FBS, 1% penicillin
(C8251; Beijing Solarbio Science & Technology Co., Ltd.) and 1%
streptomycin (S8290; Beijing Solarbio Science & Technology Co.,
Ltd.) and RPMI-1640 (31800; Beijing Solarbio Science &
Technology Co., Ltd.) medium containing 10% FBS, 1% penicillin
(C8251; Beijing Solarbio Science & Technology Co., Ltd.) and 1%
streptomycin (S8290; Beijing Solarbio Science & Technology Co.,
Ltd.) in a 37°C, 5% CO2 incubator, respectively. The
MCF-10A cells were cultured in Mammary Epithelium Basal Medium
(CC-3150; iCell Bioscience, Inc.) in a 37°C, 5% CO2
incubator. The MDA-MB-231, MDA-MB-453, MDA-MB-468, MCF-7 and
MCF-10A cells were purchased from iCell Bioscience, Inc. The
ZR-75-1 cells were purchased from Procell Life Science &
Technology Co., Ltd.
Cell transfection
The plasmid containing pcDNA3.1-HSP20 (exHSP20) and
HSP20 small interference RNA sequences (si-HSP20-1/2) were
synthesized by GenScript and JTSBio, respectively. The
corresponding vector or non-targeting control (si-NC) served as a
negative control. The control group consisted of untransfected
cells, and the vector group consisted of cells transfected with an
empty vector. The sequences of si-HSP20-1/2 and si-NC were as
follows: si-HSP20-1 sense, 5′-CGGUGCUGCUAGACGUGAATT-3′ and
antisense, 5′-UUCACGUCUAGCAGCACCGTT-3′; si-HSP20-2 sense,
5′-CGGAGGAAAUUGCUGUCAATT-3′ and antisense,
5′-UUGACAGCAAUUUCCUCCGTT-3′; and si-NC sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. The MDA-MB-231 and MDA-MB-468 cells
were transfected with 2.5 µg exHSP20 plasmid or vector using
Lipofectamine 3000® (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 24 h. G418 (300 µg/ml; 11811023;
Invitrogen; Thermo Fisher Scientific, Inc.) was then added to the
cells for further selection, and stable HSP20-overexpressing cells
were obtained in the presence of G418 (300 µg/ml) 2 weeks later.
G418 (150 µg/ml) was added to stable HSP20-overexpressing cells for
maintenance. The MCF-7 cells were transfected with 75 pmol
si-HSP20-1/2 or si-NC at 37°C for 48 h using Lipofectamine
3000® to obtain HSP20-silenced cells. Directly after
transfection for 48 h, cells were harvested for the subsequent
experiments.
RT-qPCR
Total RNA was extracted from the cells using TRIpure
lysis buffer (BioTeke Corporation). The extracted RNA was treated
with the BeyoRT II m-MLV reverse transcriptase kit (Beyotime
Institute of Biotechnology) to obtain cDNA according to the
manufacturer's instructions. The qPCR reaction system was then
constructed according to the SYBR-Green (Beijing Solarbio Science
& Technology Co., Ltd.) kit instructions. The thermocycling
conditions were as follows: 94°C for 5 min, followed by 40 cycles
of 94°C for 15 sec, 60°C for 25 sec and 72°C for 30 sec. Relative
gene expression was calculated using the 2−ΔΔCq method
(28) and β-actin was used as an
internal control. The details of primers used were as follows:
HSP20 forward, 5′-CGGACGCCTCTTTGACCAG-3′ and reverse,
5′-CGGTAGCGACGGTGGAACT-3′; and β-actin forward,
5′-CACTGTGCCCATCTACGAGG-3′ and reverse,
5′-TAATGTCACGCACGATTTCC-3′.
Western blot analysis
Protein was extracted from the cells using IP cell
lysate buffer (Beyotime Institute of Biotechnology) and the
concentration was quantified using a BCA kit (Beyotime Institute of
Biotechnology). Quantitative protein samples (~20 µg/lane) were
separated on an 10% SDS-PAGE gel and transferred to PVDF
(MilliporeSigma) membranes. The membranes were then blocked with 5%
(w/v) skimmed milk at room temperature for 1 h. Subsequently, the
membranes were incubated with the corresponding primary antibodies
at 4°C overnight. The membranes were then covered with
HRP-conjugated goat-anti-rabbit/mouse secondary antibody (A0208 and
A0216; diluted 1:5,000; Beyotime Institute of Biotechnology) at
37°C for 45 min. After the bands were visualized using ECL (P0018;
Beyotime Institute of Biotechnology), the optical density of each
band was analyzed using Gel-Pro-Analyzer software (version 4.0;
Beijing Liuyi Biotechnology Co., Ltd.). The primary antibodies used
were as follows: Anti-HSP20 (AF6003; diluted 1:500),
anti-phosphorylated (p-)ERK (AF1015; diluted 1:500), anti-ERK
(AF0155; diluted 1:500), anti-p-JNK (AF3318; diluted 1:1,000),
anti-JNK (AF6318; diluted 1:500), anti-p-AKT (#4060; diluted
1:1,000), anti-AKT (#4691; diluted 1:2,000), anti-Bax (A19684;
diluted 1:500), anti-Bcl-2 (A0208; diluted 1:1,000), anti-caspase-3
(#14220; diluted 1:1,000), anti-poly (ADP-ribose) polymerase (PARP;
#9542; diluted 1:1,000), anti-p-p38 (bs-0636R; diluted 1:400),
anti-p38 (bs-0637R; diluted 1:500). HSP20, p-ERK, ERK, p-JNK and
JNK antibodies were purchased from Affinity Biosciences. The p-AKT,
AKT, caspase-3 and PARP antibodies were purchased from Cell
Signaling Technology, Inc. The Bax and Bcl-2 antibodies were
purchased from ABclonal Biotech Co., Ltd. The p-p38 and p38
antibodies were purchased from BIOSS. The internal reference
β-actin antibody (sc-47778; diluted 1:1,000) was purchased from
Santa Cruz Biotechnology, Inc.
Cell viability assay
The cells in each group were seeded in 96-well
plates (4×103 cells/well) and incubated at 37°C for 0,
24, 48 and 72 h, respectively. Following incubation, cell viability
was detected using a Cell Counting Kit-8 (CCK-8; MilliporeSigma).
Briefly, the cells were covered with the CCK-8 reagent (10 µl/well)
for 2 h. Subsequently, the optical density values were measured at
450 nm using a microplate reader.
In rescue experiments, HSP20-overexpressing stable
MDA-MB-231 cells were treated with 10 µM SC79 (S80614; Yuanye
Biology) or 50 µM LM22B-10 (L879472; Macklin, Inc.) at 37°C for 48
h. Subsequently, a CCK-8 assay was performed as aforementioned.
Colony formation assay
The MDA-MB-231 and MDA-MB-468 cells
(3×102 cells/plate) were plated into a 35-mm cell
culture plastic and incubated at 37°C with 5% CO2 for ~2
weeks. Colonies were fixed in 4% paraformaldehyde at room
temperature for 20 min and stained with Ray-Giemsa dye (Nanjing
KeyGen Biotech Co., Ltd.) at room temperature for 5 min, then
visualized and counted manually. Colonies consisted of >50
cells. The colony formation rate=colony number/300×100%.
Apoptosis detection
The apoptosis of the MDA-MB-231 and MDA-MB-468 cells
was analyzed using an Annexin V-FITC Apoptosis Detection Kit
(C1062; Beyotime Institute of Biotechnology). All reagents
mentioned in this subsection were included in this kit. In brief,
the cells were washed twice with PBS and mixed with 195 µl Annexin
V-FITC binding buffer. The cells were then incubated with 5 µl
Annexin V-FITC and 10 µl PI in the dark at room temperature for 15
min. Subsequently, a NovoCyte flow cytometer (ACEA Bioscience,
Inc.) was used to evaluate cell apoptosis. The apoptotic cells were
analyzed using NovoCyte software (version 1.5.6, ACEA Bioscience,
Inc.).
Wound healing assay
After the MDA-MB-231, MDA-MB-468 and MCF-7 cells
(reached 100% confluency) were incubated in serum-free medium
supplemented with 1 µg/ml mitomycin C (MilliporeSigma) for 1 h, a
200-µl pipette tip was used to create a scratch. The cells treated
with or without SC79 (10 µM)/LM22B-10 (50 µM) were cultured at 37°C
for 24 h. Cell fragments were removed by washing with PBS.
Subsequently, cells were observed under a light microscope at ×100
magnification and images were captured. The distance of the wound
was measured using ipwin32 (version 6.0; National Institutes of
Health). Migration rate=distance from edge at 24 h/distance from
edge at 0 h ×100%.
Cell invasion assay
For the cell invasion assay, 24-well Transwell
chambers (Corning, Inc.) were applied. After the Matrigel was added
to the upper chambers at 37°C for 2 h, cells (3×104)
were suspended in serum-free L15 medium or MEM (200 µl) and seeded
on it. L15 medium or MEM supplemented with 10% FBS (800 µl) was
added to the lower chamber. After incubation at 37°C for 24 h,
paraformaldehyde (4%) was used to fix the invasive cells. The cells
were then stained with 0.4% crystal violet solution at room
temperature for 5 min and observed under a light IX53 microscope at
a magnification of ×200 (Olympus Corporation).
Statistical analysis
The statistical analysis was carried out using
GraphPad Prism (V8.0; GraphPad Software, Inc.). A paired Student's
t-test was used for comparisons between two groups. One-way ANOVA
with Tukey's post hoc test was used for multiple group comparisons.
Pearson's χ2 test (Ki-67) or Fisher's exact test (age,
ER, PR, HER2, pT, pN and pStage) was performed to examine the
association between the HSP20 expression and the patient
clinicopathologic characteristics. Experiments were performed in
triplicate. All experimental data are presented as the mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Decreased HSP20 expression is
associated with disease progression and prognosis of patients with
BC
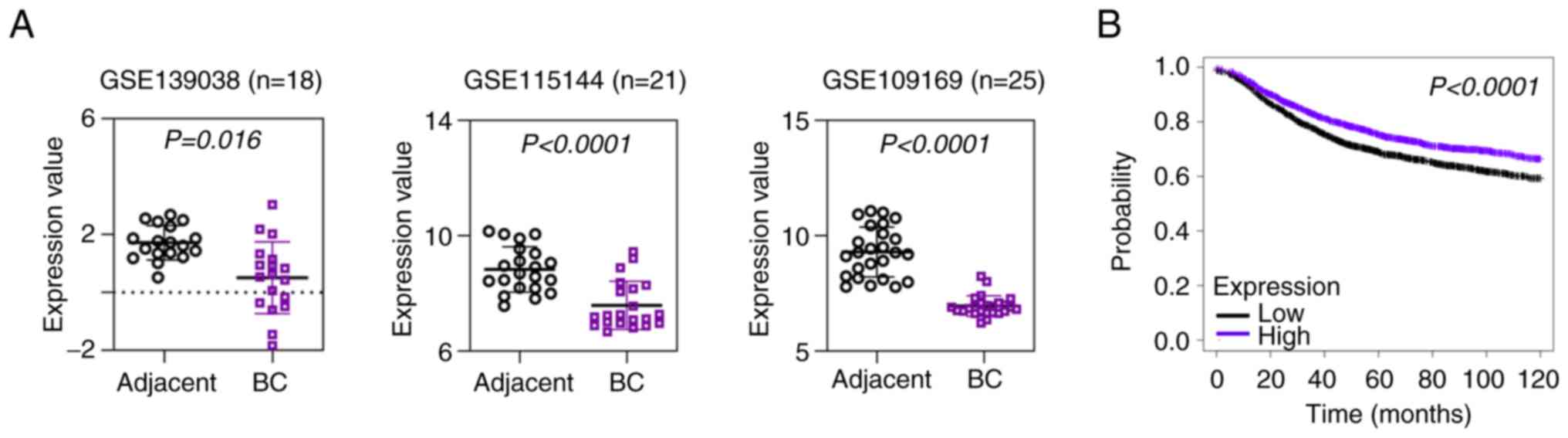
Data from three published GEO datasets (GSE139038,
GSE115144 and GSE109169) revealed that HSP20 expression was
downregulated in BC tissues compared with in paired non-cancer
tissues (Fig. 1A). In addition,
the results from the Kaplan-Meier Plotter database indicated that
low HSP20 expression was significantly associated with the poor
prognosis of patients with BC (Fig.
1B). Furthermore, the present study analyzed the association
between HSP20 expression and the clinicopathological parameters of
53 patients diagnosed with BC. The clinicopathological
characteristics are presented in Table
I. The results illustrated that HSP20 expression was markedly
associated with the pathological tumor stage [pT: T1, T2, T3 and
Tis; according to the staging system of the International Union
Against Cancer (29)] and
pathological tumor node metastasis [pStage: N0, N1, N2 and N3;
according to the staging system of the International Union Against
Cancer (29)] of BC.
HSP20 inhibits BC cell
proliferation
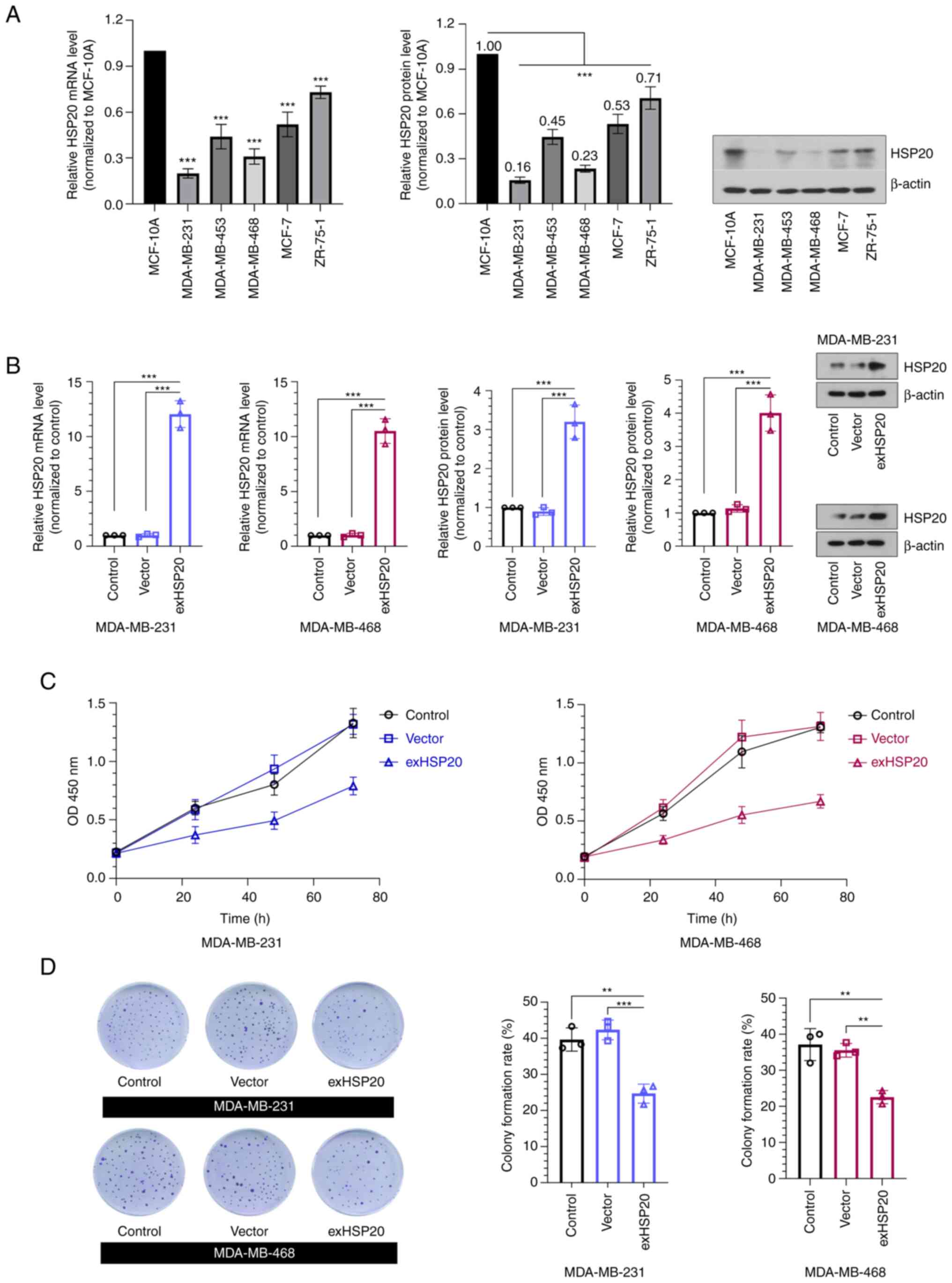
The results of RT-qPCR and western blot analysis
revealed that HSP20 expression was significantly decreased in five
BC cell lines compared with the MCF-10A normal mammary epithelial
cell line (Fig. 2A). Among these
cell lines, the two cell lines with the lowest expression of HSP20,
MDA-MB-231 (relative protein expression of HSP20, 0.16) and
MDA-MB-468 (relative protein expression of HSP20, 0.23), were
selected to establish stable HSP20-overexpressing cell lines.
Additionally, the MCF-7 cell line is hormone receptor-positive and
is the most common subtype of BC. The MCF-7 cell line with
relatively high HSP20 protein expression (0.53) was selected to
establish the HSP20-silenced cell line. The analysis of the
transfection efficiency demonstrated that the mRNA and protein
expression levels of HSP20 in the exHSP20 group were significantly
enhanced compared with those of the empty vector group (Fig. 2B). Additionally, HSP20 expression
was successfully silenced in MCF-7 cells (Fig. S2A).
In order to investigate the association between
HSP20 and cell proliferation, CCK-8 and colony formation assays
were performed. The results revealed that overexpression of HSP20
markedly decreased cell viability (Fig. 2C), while knockdown of HSP20 exerted
opposite effects (Fig. S2B).
Consistently, the colony formation assay confirmed that the
overexpression of HSP20 inhibited BC cell colony formation
(Fig. 2D). These data suggested
that HSP20 suppressed BC cell proliferation.
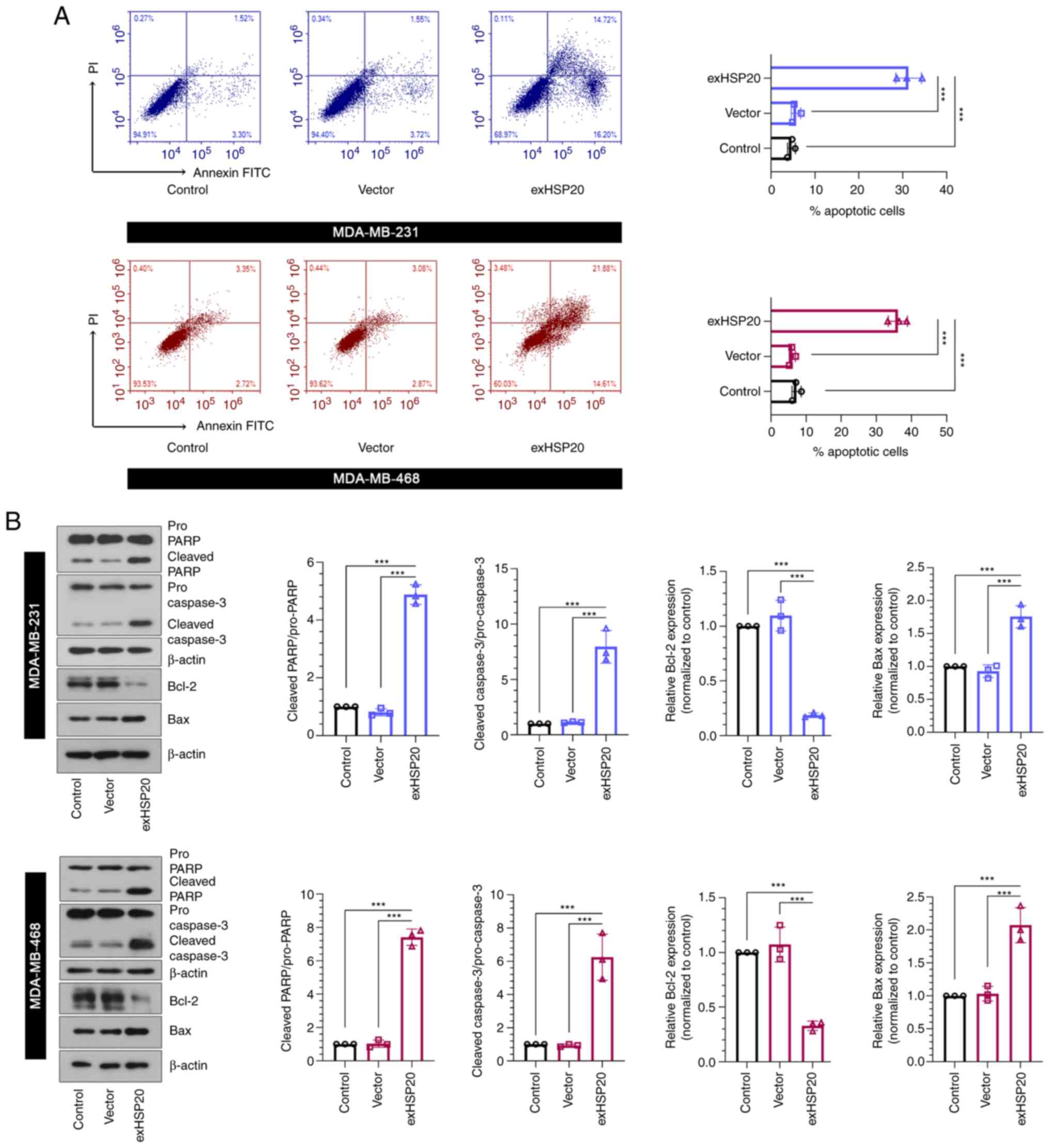
HSP20 promotes BC cell apoptosis
To clarify the association between HSP20 and cell
apoptosis, flow cytometric analysis was performed to evaluate cell
apoptosis. The apoptotic rate of the exHSP20 group was observably
enhanced compared with that of the empty vector group (Fig. 3A). In addition, the protein levels
of pro-PARP, cleaved (active) PARP, pro-caspase-3, cleaved (active)
caspase-3, Bcl2 and Bax were examined using western blot analysis.
The results indicated that the levels of cleaved PARP/pro-PARP,
cleaved caspase-3/pro-caspase-3 and Bax were significantly
upregulated, whereas the level of Bcl-2 was significantly
downregulated in HSP20-overexpressing BC cells (Fig. 3B). These findings indicated that
overexpression of HSP20 facilitated BC cell apoptosis.
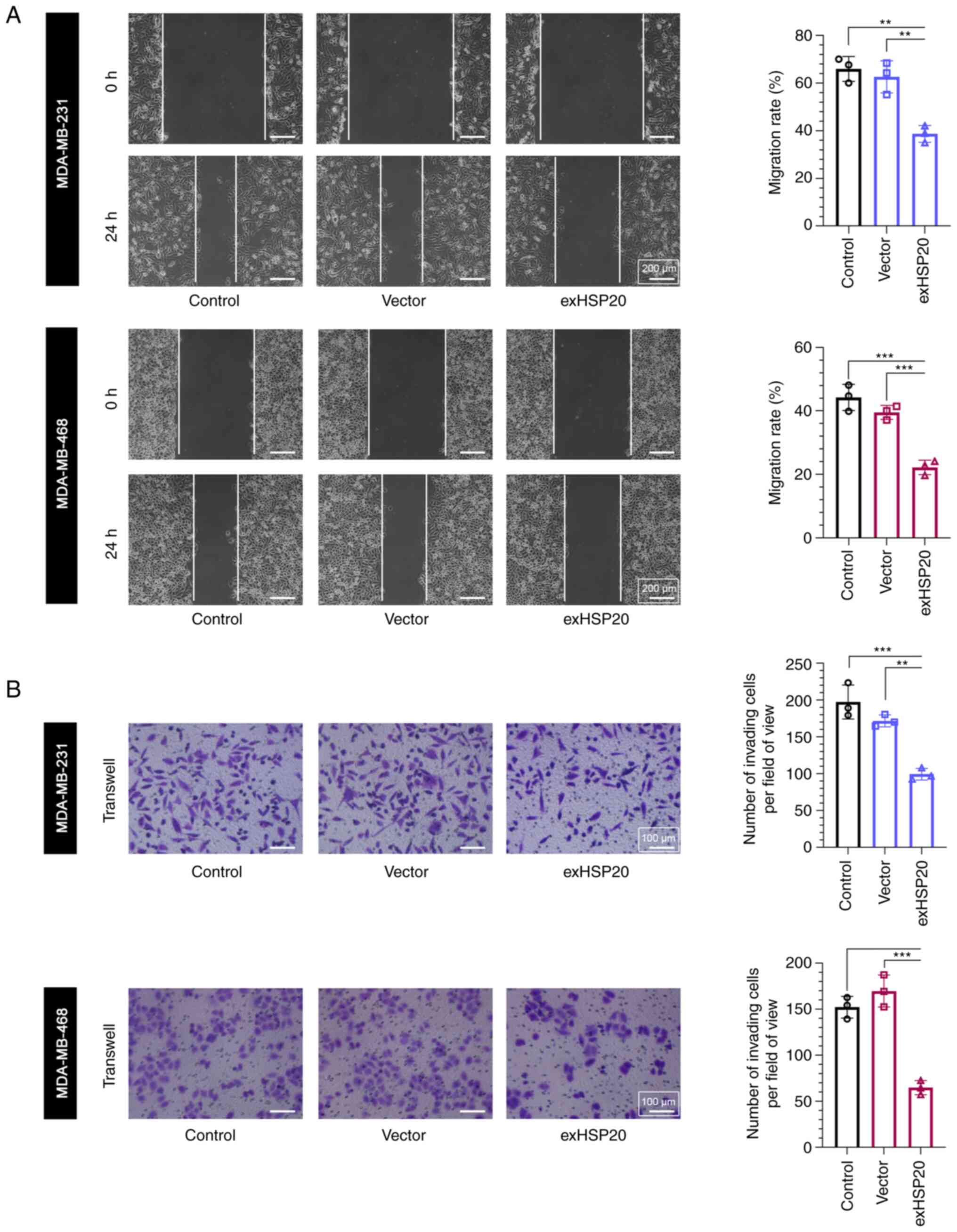
HSP20 suppresses BC cell migration and
invasion
Subsequently, the present study verified the effects
of HSP20 on BC cell migration and invasion. As shown in Fig. 4A, the results of the wound healing
assay revealed that overexpression of HSP20 decreased migration,
while knockdown of HSP20 increased the BC cell migration rate
(Fig. S2C). In addition, the
results of the Transwell assay demonstrated that the number of
invaded cells in the exHSP20 group was significantly reduced
(Fig. 4B). These results revealed
that HSP20 inhibited the migration and invasion of BC cells.
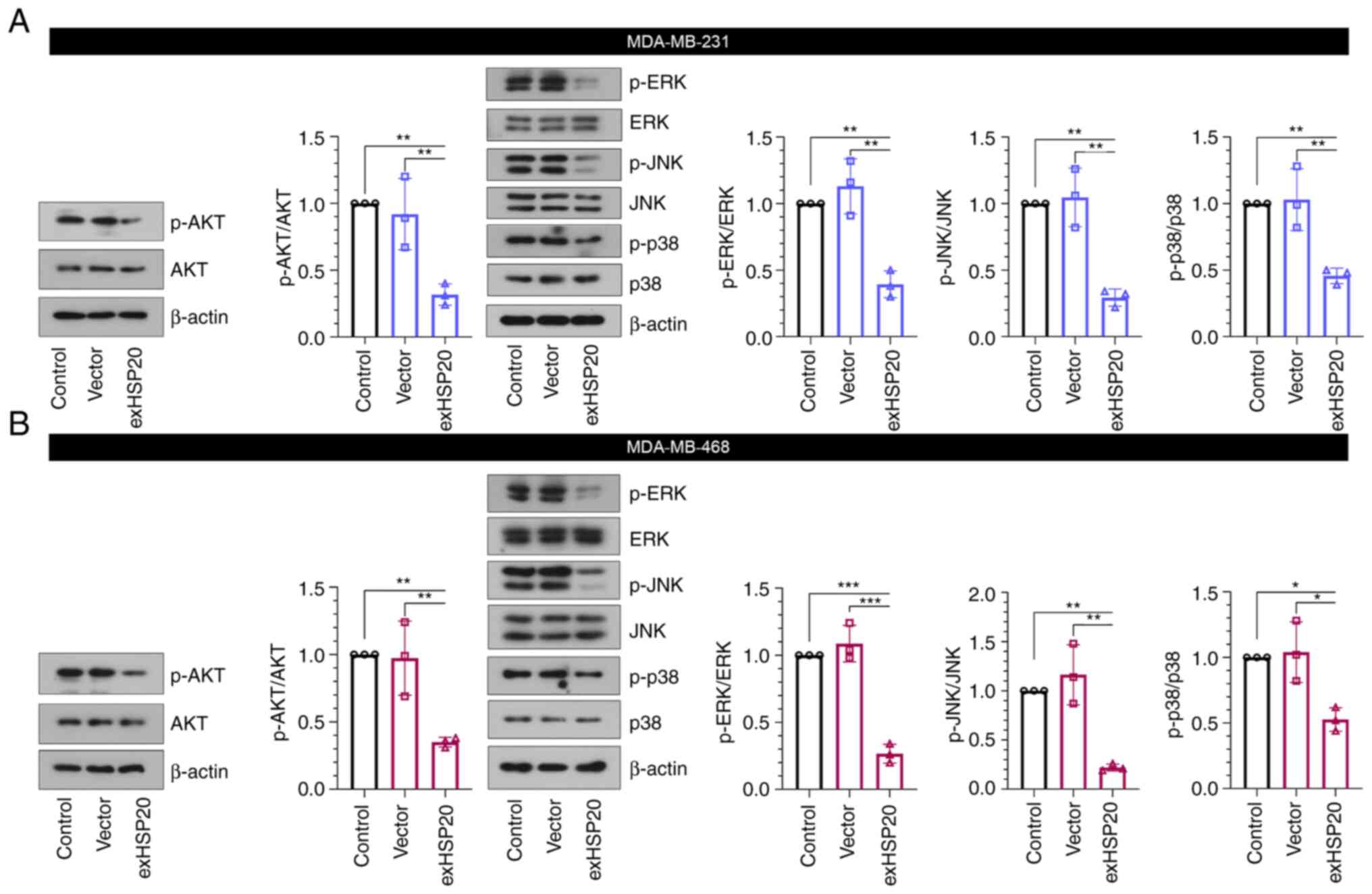
HSP20 inhibits the AKT and MAPK
signaling pathways
The AKT and MAPK signaling pathways are two crucial
signaling pathways in cancer (22,23).
In the present study, the results of western blot analysis
indicated that HSP20 overexpression suppressed the AKT and MAPK
signaling pathways, as evidenced by the reduced phosphorylation
levels of AKT, as well as ERK, JNK and p38 in BC cells (Fig. 5A and B). However, HSP20 silencing
exerted the opposite effects (Fig.
S2D). Thus, the regulatory effects of HSP20 on the malignant
phenotype of BC cells may be associated with the inhibition of
these two signaling pathways.
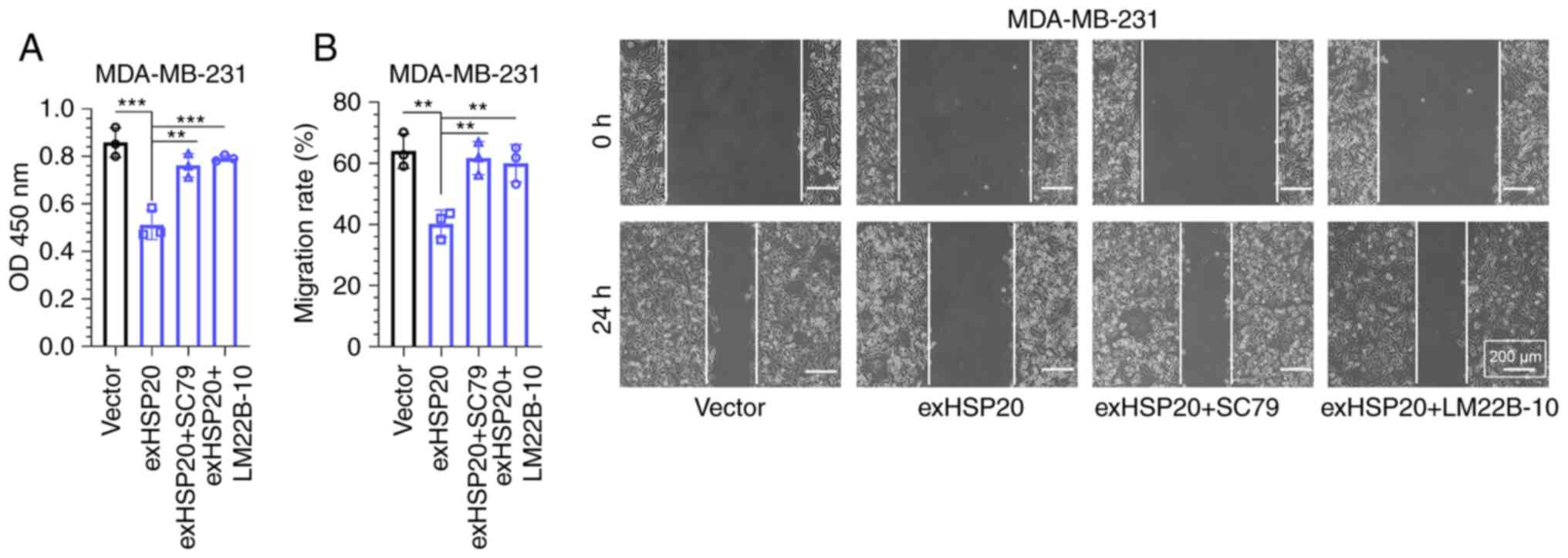
SC79 or LM22B-10 treatment reverses
the inhibitory effects of HSP20 on cell proliferation and
migration
The AKT and ERK agonists, SC79 and LM22B-10, were
applied in rescue experiments. MDA-MB-231 cells were randomly
selected for these experiments. As demonstrated by the CCK-8 assay,
the decreased cell viability induced by HSP20 overexpression was
reversed by SC79 or LM22B-10 treatment (Fig. 6A). Consistently, SC79 or LM22B-10
treatment also reversed the suppressive effects of HSP20
overexpression on the migration of MDA-MB-231 cells (Fig. 6B).
Discussion
The mortality of patients with BC is usually
ascribed to cell metastasis (30,31).
HSP20 participates in a number of pathological processes, including
the prevention of vasospasms, insulin resistance and
cardioprotection (32,33). Furthermore, HSP20 has become a
research hotspot in the field of cancer; however, the function of
HSP20 in BC is not yet fully understood. The present study
demonstrated that HSP20 expression was downregulated in BC tissues
and BC cell lines, which was associated with clinicopathological pT
and pStage parameters in patients with BC. It was further
demonstrated that HSP20 suppressed cell proliferation, migration
and invasion by inhibiting the MAPK and AKT signaling pathways.
One hallmark of cancer cells is the loss of
apoptotic control. The purpose of apoptosis is to kill abnormal
cells and prevent tumor growth (34). The Bcl-2 family, consisting of the
anti-apoptotic protein (Bcl-2) and pro-apoptotic proteins (Bax and
Bcl-2 antagonist/killer 1), are coupled to the activation of
caspase-3 and caspase-7 in regulating mitochondria-mediated
apoptosis (35,36). Bax expression could also directly
induce apoptosis, which has therapeutic relevance (37). Caspases, a family of cysteine
proteases, are central regulators of apoptosis (16). Caspase-3 is an important key
protease that is activated during cell apoptosis (38). The cleavage of PARP occurs by
upstream caspase-3 molecules, which are key drivers of apoptosis in
tumors (39). In the present
study, overexpression of HSP20 downregulated the anti-apoptotic
protein, Bcl-2, and upregulated pro-apoptotic Bax, cleaved PARP and
cleaved caspase-3 levels, inducing BC cell apoptosis. Therefore,
the overexpression of HSP20 in BC may induce the apoptosis of BC
cells.
Cancer invasion and metastasis are multistep and
complex processes, which have become a huge obstacle in the
clinical treatment of various tumors (40,41).
Furthermore, metastasis is one the major reasons for therapeutic
failure in BC (42,43). Therefore, reducing cell migration
and invasion effectively controls the metastasis of cancer cells.
In the present study, it was found that the overexpression of HSP20
significantly inhibited the metastasis of BC cells, as identified
using migration and invasion assays.
Additionally, the activation of the MAPK and AKT
signaling pathways predicts a poor prognosis and the early
recurrence of BC in patients (24). The MAPK signaling pathway is an
important pathway in cell migration, and the inhibition of the MAPK
signaling pathway can inhibit the migration of BC cells (44). The MAPK pathway consists of ERK,
JNK and p38; ERK is an important signal transducer for cell
survival, and JNK and p38 contribute to acquiring invasion and
migration capabilities (45).
Accumulating evidence suggests that MAPK has the potential to
prevent invasion and metastasis of various tumors (20,46).
Furthermore, the AKT signaling pathway participates in numerous
cellular events, such as the cell cycle and glucose metabolism,
particularly in cancer cells (47). In BC, the AKT signaling pathway is
frequently activated, promoting BC cell invasion and metastasis
(24,48). The present study demonstrated that
overexpression of HSP20 inhibited MAPK and AKT signaling in BC.
In conclusion, the findings of the present study
suggest that the downregulation of HSP20 in BC tissues may be
associated with the progression of BC. In addition, HSP20
overexpression suppressed the malignant phenotype of BC cells. This
effect may be associated with the inhibition of the AKT and MAPK
signaling pathways. HSP20 may thus prove to be a potential
prognostic marker or a candidate therapeutic target for BC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, YW, LH, XG and GS performed the experiments. YY
and HJ confirmed the authenticity of all the raw data. YY and YW
wrote the manuscript. HJ designed this study and polished the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Wuxi 9th Affiliated Hospital of Soochow University
(approval no. LW2021008; Wuxi, China), and written informed consent
was provided by each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmad A: Breast cancer statistics: Recent
trends. Adv Exp Med Biol. 1152:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh SK, Singh S, Lillard JW Jr and Singh
R: Drug delivery approaches for breast cancer. Int J Nanomedicine.
12:6205–6218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sultan AS, Marie MA and Sheweita SA: Novel
mechanism of cannabidiol-induced apoptosis in breast cancer cell
lines. Breast. 41:34–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McDonald ES, Clark AS, Tchou J, Zhang P
and Freedman GM: Clinical diagnosis and management of breast
cancer. J Nucl Med. 57 (Suppl 1):9S–16S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsen CB and Neumayer LA: Breast cancer:
A review for the general surgeon. JAMA Surg. 148:971–979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He X, Wang J, Yu H, Lv W, Wang Y, Zhang Q,
Liu Z and Wu Y: Clinical significance for diagnosis and prognosis
of POP1 and its potential role in breast cancer: A comprehensive
analysis based on multiple databases. Aging (Albany NY).
14:6936–6956. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dyan B, Seele PP, Skepu A, Mdluli PS,
Mosebi S and Sibuyi NRS: A review of the nucleic acid-based lateral
flow assay for detection of breast cancer from circulating
biomarkers at a point-of-care in low income countries. Diagnostics
(Basel). 12:19732022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu
ZY, Shi W, Jiang J, Yao PP and Zhu HP: Risk factors and preventions
of breast cancer. Int J Biol Sci. 13:1387–1397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang Y, Zhang H, Song X and Yang Q:
Metastatic heterogeneity of breast cancer: Molecular mechanism and
potential therapeutic targets. Semin Cancer Biol. 60:14–27. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Xie Y, Wu Y, He M, Yang M, Fan Y, Li
X, Qiao F and Deng D: HSP20 exerts a protective effect on
preeclampsia by regulating function of trophoblast cells via Akt
pathways. Reprod Sci. 26:961–971. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan GC, Chu G and Kranias EG: Hsp20 and
its cardioprotection. Trends Cardiovasc Med. 15:138–141. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noda T, Kumada T, Takai S,
Matsushima-Nishiwaki R, Yoshimi N, Yasuda E, Kato K, Toyoda H,
Kaneoka Y, Yamaguchi A and Kozawa O: Expression levels of heat
shock protein 20 decrease in parallel with tumor progression in
patients with hepatocellular carcinoma. Oncol Rep. 17:1309–1314.
2007.PubMed/NCBI
|
|
14
|
Matsushima-Nishiwaki R, Adachi S, Yoshioka
T, Yasuda E, Yamagishi Y, Matsuura J, Muko M, Iwamura R, Noda T,
Toyoda H, et al: Suppression by heat shock protein 20 of
hepatocellular carcinoma cell proliferation via inhibition of the
mitogen-activated protein kinases and AKT pathways. J Cell Biochem.
112:3430–3439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagasawa T, Matsushima-Nishiwaki R, Yasuda
E, Matsuura J, Toyoda H, Kaneoka Y, Kumada T and Kozawa O: Heat
shock protein 20 (HSPB6) regulates TNF-α-induced intracellular
signaling pathway in human hepatocellular carcinoma cells. Arch
Biochem Biophys. 565:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagasawa T, Matsushima-Nishiwaki R, Toyoda
H, Matsuura J, Kumada T and Kozawa O: Heat shock protein 20 (HSPB6)
regulates apoptosis in human hepatocellular carcinoma cells: Direct
association with Bax. Oncol Rep. 32:1291–1295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ju YT, Kwag SJ, Park HJ, Jung EJ, Jeong
CY, Jeong SH, Lee YJ, Choi SK, Kang KR, Hah YS and Hong SC:
Decreased expression of heat shock protein 20 in colorectal cancer
and its implication in tumorigenesis. J Cell Biochem. 116:277–286.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pritchard AL and Hayward NK: Molecular
pathways: Mitogen-activated protein kinase pathway mutations and
drug resistance. Clin Cancer Res. 19:2301–2309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haddadi N, Lin Y, Travis G, Simpson AM,
Nassif NT and McGowan EM: PTEN/PTENP1: ‘Regulating the regulator of
RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy.
Mol Cancer. 17:372018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anjum J, Mitra S, Das R, Alam R, Mojumder
A, Emran TB, Islam F, Rauf A, Hossain MJ, Aljohani ASM, et al: A
renewed concept on the MAPK signaling pathway in cancers:
Polyphenols as a choice of therapeutics. Pharmacol Res.
184:1063982022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asl ER, Amini M, Najafi S, Mansoori B,
Mokhtarzadeh A, Mohammadi A, Lotfinejad P, Bagheri M, Shirjang S,
Lotfi Z, et al: Interplay between MAPK/ERK signaling pathway and
MicroRNAs: A crucial mechanism regulating cancer cell metabolism
and tumor progression. Life Sci. 278:1194992021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang S, Wang D, Zhang S, Huang X, Wang D,
Ijaz M and Shi Y: Tunicamycin potentiates paclitaxel-induced
apoptosis through inhibition of PI3K/AKT and MAPK pathways in
breast cancer. Cancer Chemother Pharmacol. 80:685–696. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chun J and Kim YS: Platycodin D inhibits
migration, invasion, and growth of MDA-MB-231 human breast cancer
cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem
Biol Interact. 205:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y, Yang X, Xu X, Zhang J, Zhang L, Xu
H, Miao Z, Li D and Wang S: Deubiquitinase PSMD7 regulates cell
fate and is associated with disease progression in breast cancer.
Am J Transl Res. 12:5433–5448. 2020.PubMed/NCBI
|
|
26
|
Alam MS, Rahaman MM, Sultana A, Wang G and
Mollah MNH: Statistics and network-based approaches to identify
molecular mechanisms that drive the progression of breast cancer.
Comput Biol Med. 145:1055082022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xuan Z, Zhang Y, Jiang J, Zheng X, Hu X,
Yang X, Shao Y, Zhang G and Huang P: Integrative genomic analysis
of N6-methyladenosine-single nucleotide polymorphisms
(m6A-SNPs) associated with breast cancer. Bioengineered.
12:2389–2397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cserni G, Chmielik E, Cserni B and Tot T:
The new TNM-based staging of breast cancer. Virchows Arch.
472:697–703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brook N, Brook E, Dharmarajan A, Dass CR
and Chan A: Breast cancer bone metastases: Pathogenesis and
therapeutic targets. Int J Biochem Cell Biol. 96:63–78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Winters S, Martin C, Murphy D and Shokar
NK: Breast cancer epidemiology, prevention, and screening. Prog Mol
Biol Transl Sci. 151:1–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li R, Shi Y, Zhao S, Shi T and Zhang G:
NF-κB signaling and integrin-β1 inhibition attenuates osteosarcoma
metastasis via increased cell apoptosis. Int J Biol Macromol.
123:1035–1043. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin TP, Currie S and Baillie GS: The
cardioprotective role of small heat-shock protein 20. Biochem Soc
Trans. 42:270–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tunjung WAS and Sayekti PR: Apoptosis
induction on human breast cancer T47D cell line by extracts of
Ancorina sp. F1000Res. 8:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li XX, Wang DQ, Sui CG, Meng FD, Sun SL,
Zheng J and Jiang YH: Oleandrin induces apoptosis via activating
endoplasmic reticulum stress in breast cancer cells. Biomed
Pharmacother. 124:1098522020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pluta P, Smolewski P, Pluta A,
Cebula-Obrzut B, Wierzbowska A, Nejc D, Robak T, Kordek R, Gottwald
L, Piekarski J and Jeziorski A: Significance of Bax expression in
breast cancer patients. Pol Przegl Chir. 83:549–553. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bumbat M, Wang M, Liang W, Ye P, Sun W and
Liu B: Effects of Me2SO and trehalose on the cell
viability, proliferation, and Bcl-2 family gene (BCL-2, BAX, and
BAD) expression in cryopreserved human breast cancer cells.
Biopreserv Biobank. 18:33–40. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Affar EB, Germain M, Winstall E,
Vodenicharov M, Shah RG, Salvesen GS and Poirier GG:
Caspase-3-mediated processing of poly(ADP-ribose) glycohydrolase
during apoptosis. J Biol Chem. 276:2935–2942. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noble P, Vyas M, Al-Attar A, Durrant S,
Scholefield J and Durrant L: High levels of cleaved caspase-3 in
colorectal tumour stroma predict good survival. Br J Cancer.
108:2097–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uekita T and Sakai R: Roles of CUB
domain-containing protein 1 signaling in cancer invasion and
metastasis. Cancer Sci. 102:1943–1948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Friedl P, Locker J, Sahai E and Segall JE:
Classifying collective cancer cell invasion. Nat Cell Biol.
14:777–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dattachoudhury S, Sharma R, Kumar A and
Jaganathan BG: Sorafenib inhibits proliferation, migration and
invasion of breast cancer cells. Oncology. 98:478–486. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun B, Zhang S, Zhang D, Li Y, Zhao X, Luo
Y and Guo Y: Identification of metastasis-related proteins and
their clinical relevance to triple-negative human breast cancer.
Clin Cancer Res. 14:7050–7059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsushima-Nishiwaki R, Toyoda H, Nagasawa
T, Yasuda E, Chiba N, Okuda S, Maeda A, Kaneoka Y, Kumada T and
Kozawa O: Phosphorylated heat shock protein 20 (HSPB6) regulates
transforming growth factor-α-induced migration and invasion of
hepatocellular carcinoma cells. PLoS One. 11:e01519072016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Igaki T, Pagliarini RA and Xu T: Loss of
cell polarity drives tumor growth and invasion through JNK
activation in Drosophila. Curr Biol. 16:1139–1146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen PN, Hsieh YS, Chiang CL, Chiou HL,
Yang SF and Chu SC: Silibinin inhibits invasion of oral cancer
cells by suppressing the MAPK pathway. J Dent Res. 85:220–225.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsushima-Nishiwaki R, Toyoda H,
Takamatsu R, Yasuda E, Okuda S, Maeda A, Kaneoka Y, Yoshimi N,
Kumada T and Kozawa O: Heat shock protein 22 (HSPB8) reduces the
migration of hepatocellular carcinoma cells through the suppression
of the phosphoinositide 3-kinase (PI3K)/AKT pathway. Biochim
Biophys Acta Mol Basis Dis. 1863:1629–1639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kulawiec M, Owens KM and Singh KK: Cancer
cell mitochondria confer apoptosis resistance and promote
metastasis. Cancer Biol Ther. 8:1378–1385. 2009. View Article : Google Scholar : PubMed/NCBI
|