Introduction
A yolk sac tumor (YST), also known as an endodermal
sinus tumor, is a rare and highly malignant germ cell tumor (MGCT)
(1), which usually occurs in
children. Most of the tumors originate from the gonads (testis and
ovaries), with only 20% originating from other locations, including
the mediastinum, vagina, cervix, vulva, pelvic cavity, liver,
prostate and diaphragm, among others (2–4). In
post-pubertal women, the most common location of extragonadal YSTs
(EGYST) is the mediastinum (5).
Primary pelvic EGYSTs are extremely rare in post-pubertal women
(6). A YST was first described in
rats by Teilum in 1959 (7). YST is
the most observed histology for vaginal GCTs. Primary YSTs of the
vagina are very rare and only constitute 3–8% of all GCTs (1,8); they
have been reported to occur in young girls, typically under the age
of 3 years (1,8), with a presentation of painless vagina
bleeding. Assays of serum α-fetoprotein (AFP) can potentially aid
in the diagnosis, monitor the effectiveness of treatment and detect
recurrences (9). Ultrasonography,
computed tomography (CT) and magnetic resonance imaging (MRI) are
common inspection methods. The final diagnosis is based on
pathological results (9,10). Over time, the treatment strategy has
changed from radical surgical treatment to conservative surgery
with adjuvant chemotherapy (11,12).
Due to the rarity of the tumors, most of the literature regarding
vaginal YSTs consists of case reports, and there is no published
standardized clinical practice guideline for vaginal YSTs in
children. The reported cases each provide a limited view of the
diagnosis and treatment of the tumor. The present study reports the
case of a 13-month-old girl with a vaginal YST that was treated
with minimally invasive surgery and adjuvant chemotherapy, and
reviews the relevant literature to outline the clinical management
of diagnosis, treatment and survival.
Case report
A 13-month-old girl was referred to The Third
Affiliated Hospital of Zhengzhou University (Zhengzhou, China) due
to vaginal bleeding that had persisted for >20 days. The patient
was born at full term with a birth weight of 3,700 g, without
gestational or neonatal complications. The recorded Apgar score was
10 for both 1 and 5 min after delivery. On presentation, the
patient was 80 cm tall (84% for age) and weighed 13 kg (99% for
age), without a history of weight loss. The familial history was
unremarkable. On physical examination, the patient's abdomen was
soft and there was no hepatosplenomegaly. There were no obvious
abnormalities in the appearance of the vulva. A small amount of
blood was found in the vaginal orifices, and a small amount of
bloody discharge and a partial tumor were visible from the vaginal
opening. No more obvious abnormalities were discovered in the
results. The blood routine examination, coagulation function and
biochemical indicators were normal. The serum carcinoma embryonic
antigen (CEA) level [chemiluminescence immunoassay; CEA assay kit;
cat. no. 00937450(110761); Siemens Centaur xp] was 0.17 µg/l
(normal range, 0–5 µg/l) and the carbohydrate antigen 125 (CA125)
level [chemiluminescence immunoassay; CA125 assay kit; no.
01678114(128533); Siemens Centaur xp] (Siemens Healthineers) was
11.7 U/ml (normal range, 0–35 U/ml). The serum AFP level
(chemiluminescence immunoassay; AFP determination kit; cat. no.
03305838(110764); Siemens Centaur xp; Siemens Healthineers) reached
a markedly high value of 2,695 IU/ml (normal range, 0–6.723 IU/ml).
Ultrasonography results showed a solid tumor with a diameter of ~3
cm and with high vascularity in the vagina. The vascularization of
the tumor on color Doppler displayed abundant blood flow, scoring 3
according to the International Ovarian Tumor Analysis (IOTA) color
score (13). The results of plain
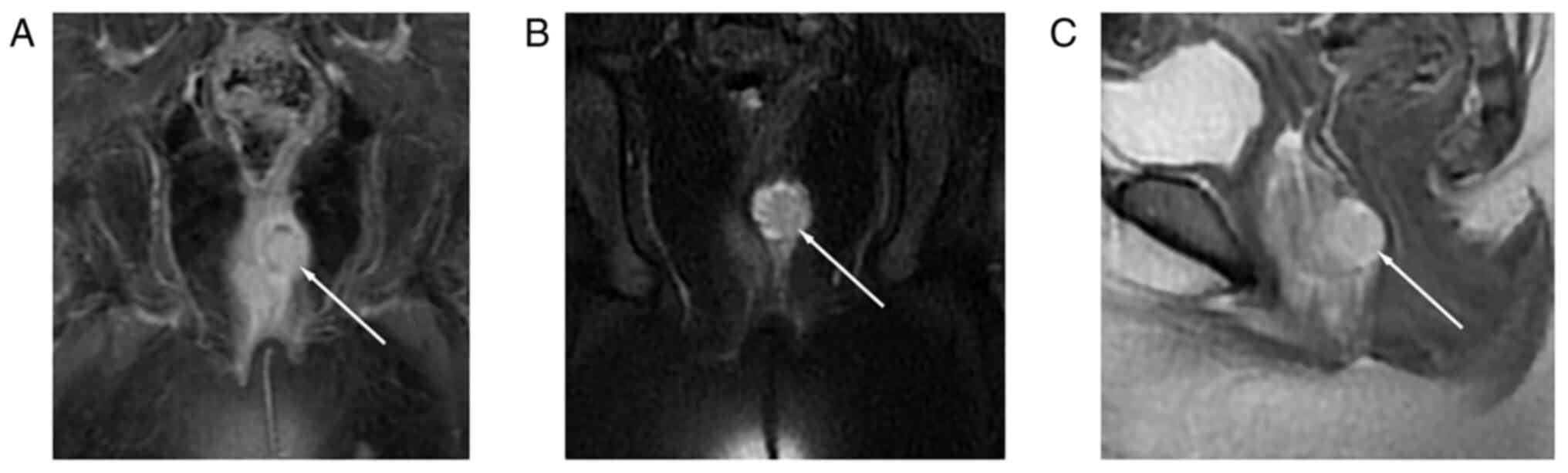
and contrast-enhanced MRI showed a heterogeneously enhanced tumor
(28.8×16.5 mm) in the vagina, without vulva or bladder invasion
(Fig. 1). There were no ascites in
the peritoneal and pelvic cavities. No abnormality was observed in
other parts of the pelvic and abdominal cavities. Imaging findings
also showed no distant metastasis. Under general anesthesia,
hysteroscopy results showed a tumor with a wide pedicle on the left
side of the vagina (Video S1).
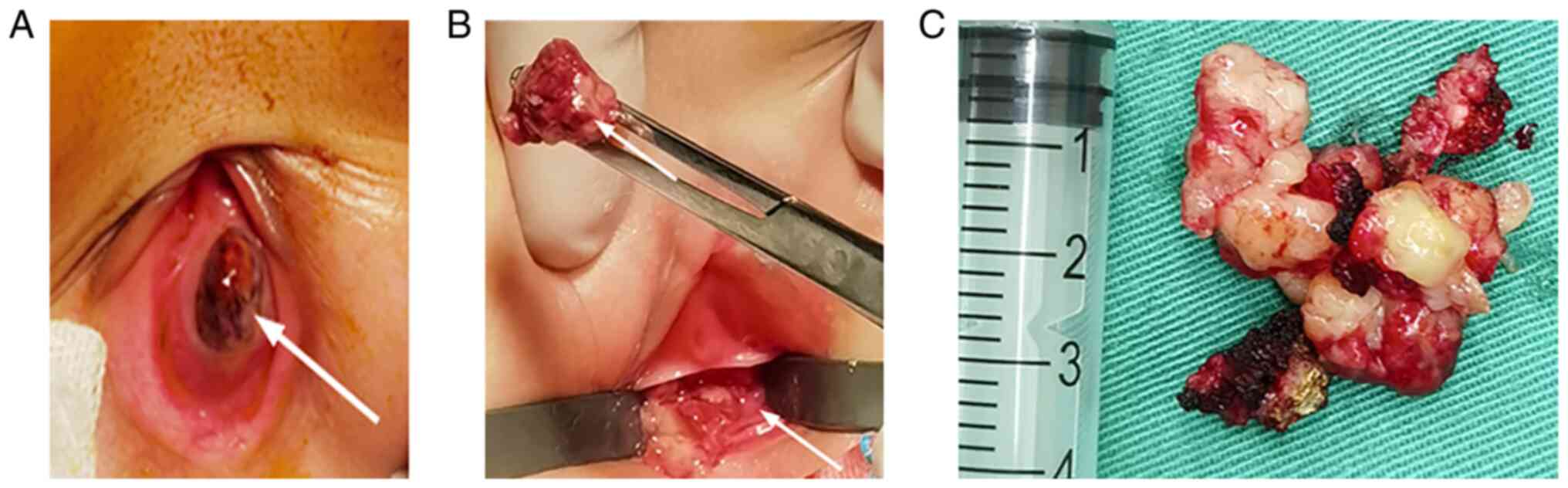
Transvaginal resections of the tumor and 0.5 cm of the vaginal
mucosa around the tumor were performed with no local tissue
adhesion and little blood loss. During the operation, it was
confirmed that the tumor was confined to the left vaginal mucosa
and did not invade the cervix or rectum. The tumor had a dark red
surface and was friable. The cross-section of the excised tumor was
grayish-white and grayish-yellow (Fig.
2). Sections (4-µm thick) from 10% formalin-fixed (12 h at room
temperature), paraffin-embedded blocks were cut for routine
hematoxylin and eosin staining. The pathologist used a light
microscope for observation (magnification, ×40 or ×15).
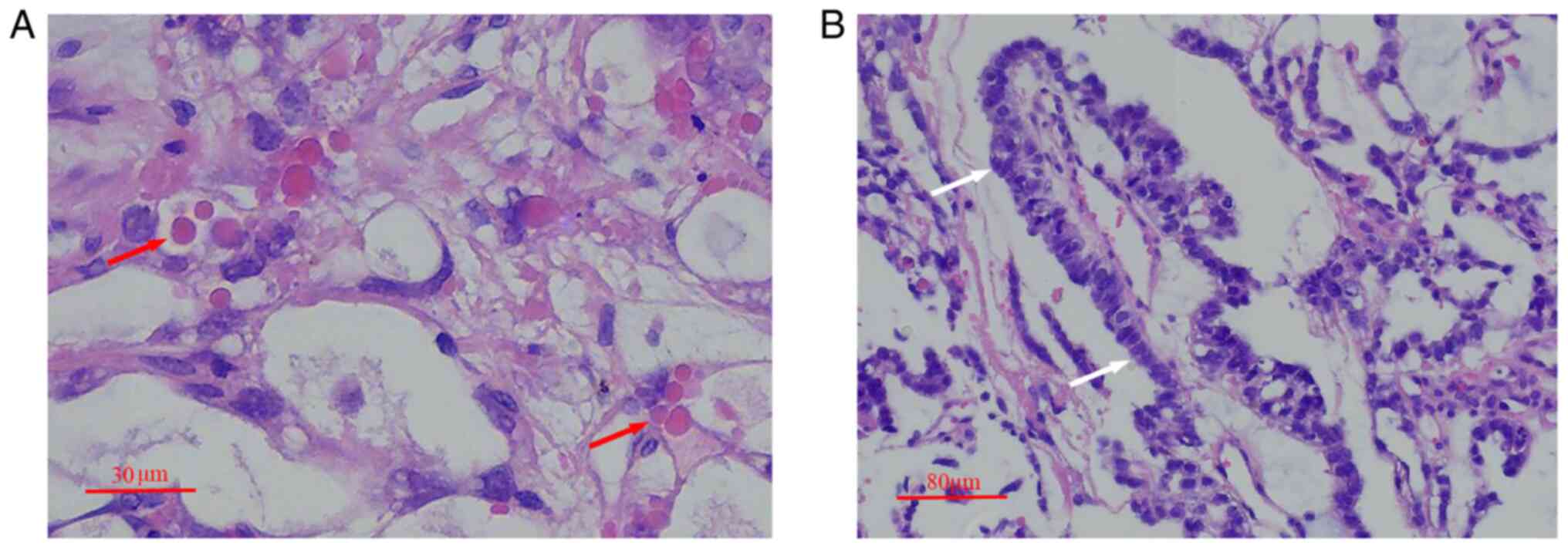
Histopathological examination results showed numerous intracellular
and extracellular periodic acid-Schiff-positive diastases and
papillary elements with a Schiller-Duval body cellular structure
(Fig. 3). The cells had large,
vesicular nuclei and prominent nucleoli. The results of
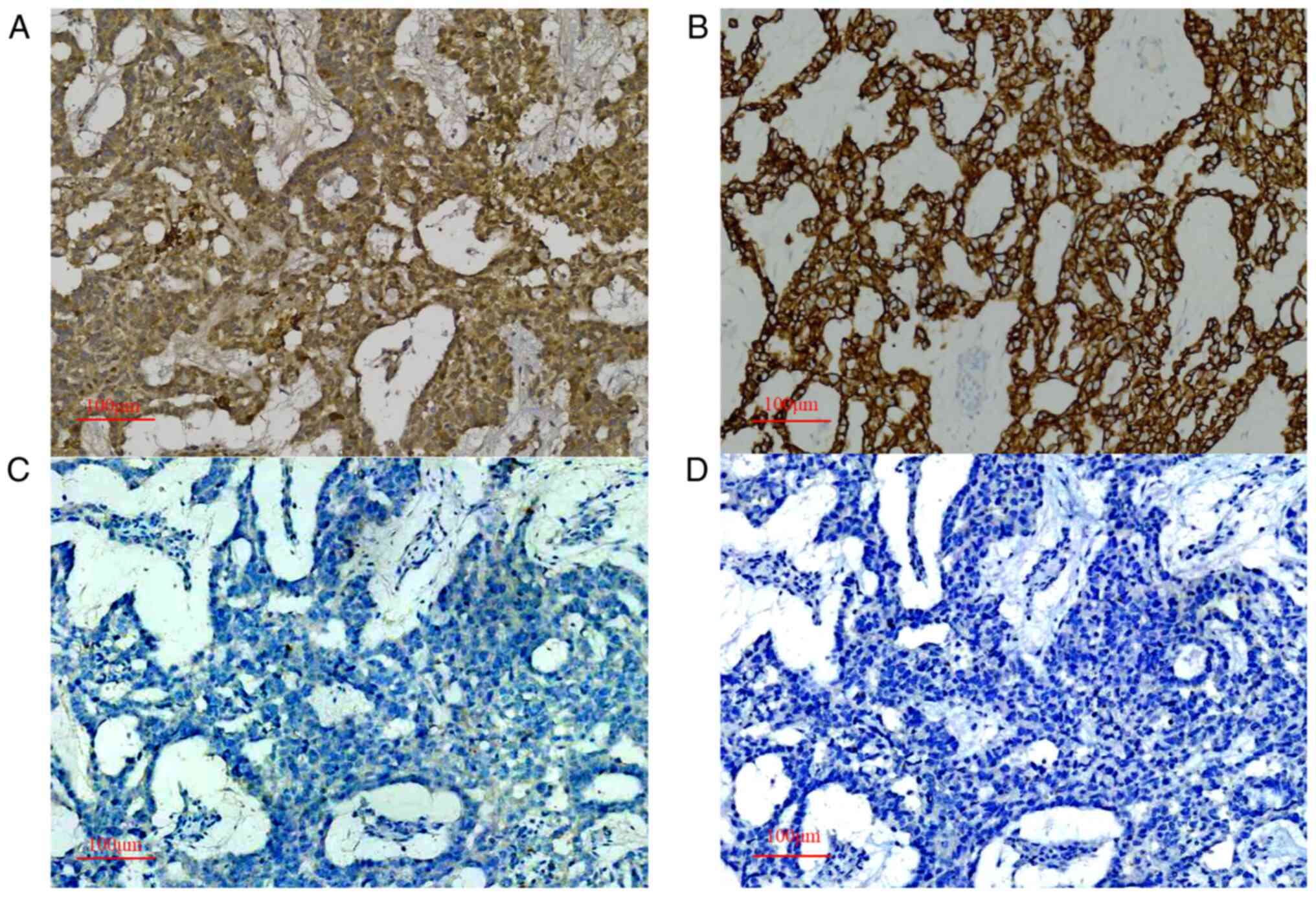
immunohistochemical staining (14)
showed positive results for AFP (Fig.
4A), cytokeratin (CK; Fig. 4B),
glypican-3 (Fig. S1), β-human
chorionic gonadotropin (β-hCG; Fig.
S2) and Sal-like protein 4 (SALL-4; Fig. S3), and negative results for of
CD-30 (Fig. 4C) and organic
cation/carnitine transporter 4 (OCT-4; Fig. 4D). The differential diagnosis
included clear cell carcinoma, rhabdomyosarcoma and dysgerminoma.
Based on the pathological and immunohistochemical findings, these
diagnoses were excluded. The vaginal wall margin was negative.
Preoperative imaging assessments showed no abnormalities in other
sites, so the tumor was considered to originate in the vagina. As a
result, the patient was diagnosed with a primary vaginal YST.
Four cycles of vincristine (1 mg/m2 from
days 1 to 5), cyclophosphamide (60 mg/m2 from days 1 to
5) and actinomycin D (0.2 mg/m2 from days 1 to 5) were
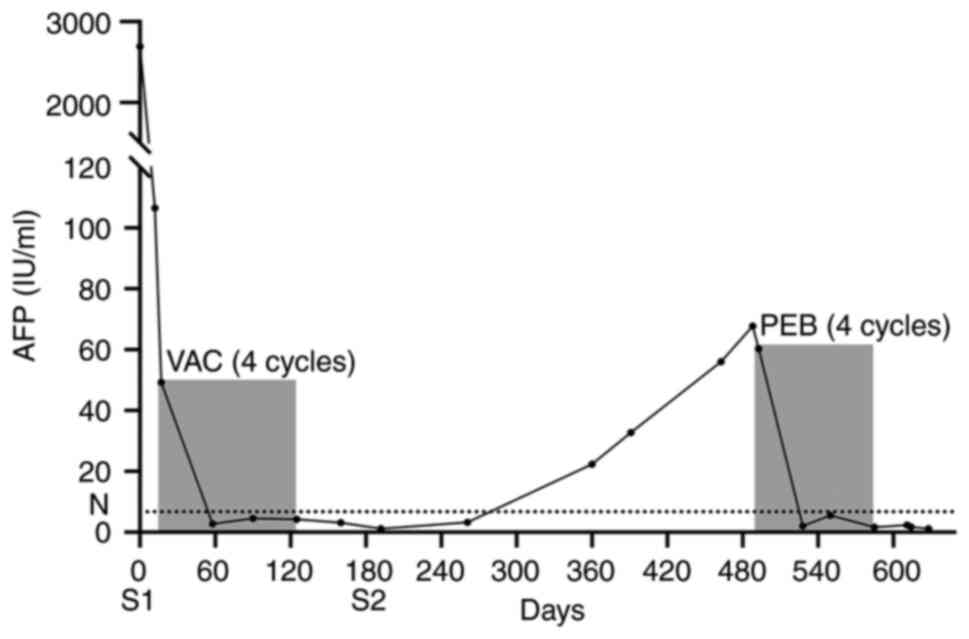
administered every 4 weeks from day 13 after surgery. On day 12
after surgery, the serum AFP level dropped to 106.5 IU/ml before
the first cycle of chemotherapy, and then decreased to 2.70 IU/ml
(within the normal range) after the second cycle of chemotherapy.
The serum AFP level remained normal during the third and fourth
cycles of chemotherapy. Pelvic ultrasound examination and a
second-look hysteroscopic examination were performed and no
positive results were found 6 months after the first surgery
(Video S2). Pelvic CT and MRI
scans were not suggested, as the results of the CT examination
performed recently in a local hospital were negative. The surgical
scar was almost invisible, with little influence on sexual and
reproductive functions. The serum AFP level increased to 22.4 IU/ml
within nearly 1 year of the surgery. The imaging result was
negative, and no abnormal vaginal bleeding was found. Due to the
COVID-19 situation in the area where the patient lived, the patient
received chemotherapy treatment 4 months after the blood AFP level
was found to be elevated. Combined chemotherapy was administered
for the second time using a belomycin, etoposide and carboplatin
(BEP) regimen, which consisted of 80 mg/m2 etoposide
from days 1 to 3, 200 mg/m2 carboplatin on day 2 and 8
mg/m2 bleomycin on day 3, every 4 weeks for 4 cycles.
The serum AFP level decreased to 2.04 IU/ml after the second cycle
of chemotherapy (Fig. 5). The side
effects of the chemotherapy were tolerable. To date, the patient
has been followed up for 2 years, and the AFP is at a normal level,
without any sign of recurrence. A perfect score was recorded for
the Activities of Daily Living scale (15). Regular medical check-ups, including
abdominal and rectal examinations, serum AFP analysis, and
ultrasonography of the pelvis and abdomen, were performed as
follow-up. Future follow-up examinations will occur once every 1
month for the first year, once every 2 months for the second year,
once every 3 months for the third and fourth years, and then once
every 6 months for the fifth year and afterwards. A complete blood
analysis, and renal and liver function tests will be performed
every year (16).
Literature review
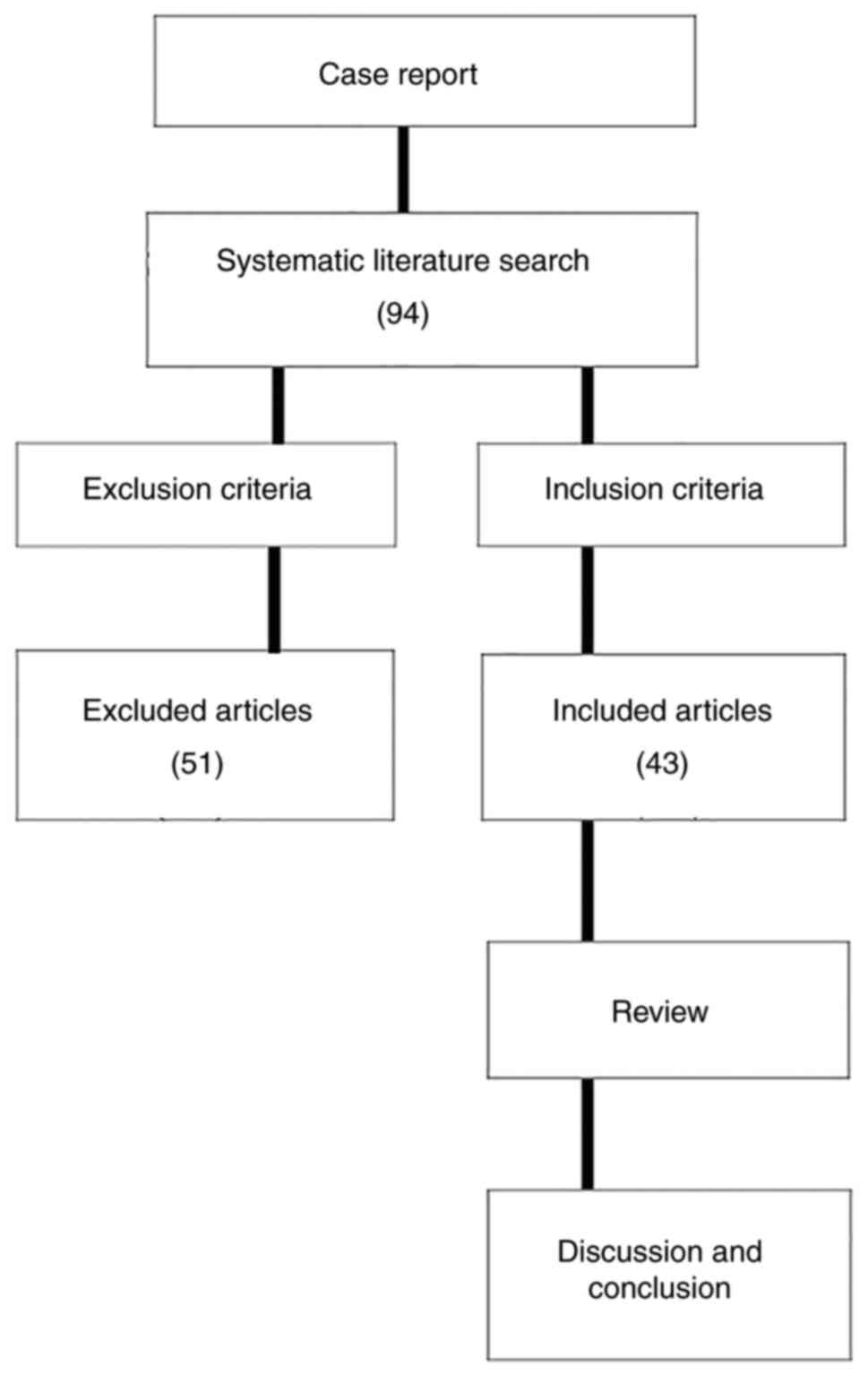
In November 2022, the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Medline (https://www.medline.com/) and Embase (https://www.embase.com/) databases were searched for
relevant studies published since January 2000. The keywords
‘vaginal’ and ‘yolk sac tumor’ or ‘endodermal sinus tumor’ were
used in the search. Studies were included if they met the following
criteria: i) Referring to vaginal YST or endodermal sinus tumor;
ii) concerning patients with a diagnosis of histologically
confirmed YST or endodermal sinus tumor; iii) containing relevant
information on clinical course, treatments, outcomes and follow-up;
iv) written in English; v) containing one or multiple clinical case
reports or letters; and vi) patients were <12 years old. The
exclusion criteria were as follows: i) An origin outside of the
vagina and extension or metastases to the vagina; ii) mixed tumors;
iii) incomplete data on diagnosis, treatment or follow-up; and iv)
repeated case reports (Fig. 6).
Reviewers resolved all discrepancies by discussion during the study
identification process. All included cases were reviewed to
consider the age at the time of diagnosis, symptoms, tumor size,
serum AFP level, treatment and follow-up. Table I summarizes 12 typical and
representative studies on vaginal YSTs, and describes the
therapeutic strategies used in the literature.
 | Table I.Review of primary vaginal yolk sac
tumors in 12 representative cases from the literature. |
Table I.
Review of primary vaginal yolk sac
tumors in 12 representative cases from the literature.
| First author | Year of
publication | Reported cases,
n | Age at diagnosis,
months | AFP, ng/ml | Tumor size (max
diameter), cm | Biopsy | Surgery | Brachytherapy | Chemotherapy (no.
of cycles) | Follow-up result
(months) | (Refs.) |
|---|
| Neels et
al | 2004 | 1 | 10 | 39324 | 6 | Yes | No | No | PEI (5) | CR (36) | (43) |
| Kumar et
al | 2005 | 1 | 12 | 9331 | 4 | No | Sv | No | PEB (5) | CR (12) | (30) |
| Lacy et
al | 2006 | 1 | 7 | 2364 | 4.7 | Yes | No | No | PEB (5) | CR (21) | (41) |
| Terenziani et
al | 2007 | Case 1/2 | 6/24 | 3006/104340 | 3.7/10 | Yes/Yes | No | No | PEB (6)/PEB (4) | CR (168)/CR
(35) | (42) |
| Watanabe et
al | 2010 | Case 1/2 | 12/46 | 34247/4.4 | 5/2 | Yes/No | Sel/Str | No | PEB (3) + JEB (3)/JEB (4) | CR (19)/CR (14) | (24) |
| Tang et
al | 2014 | Case 1 | 13 | 8127 | 5 | No | Spv | No | PEB (8) | CR (27) | (47) |
|
|
| Case 2 | 12 | 2255 | 2 | No (DT) | No | No | BIP (1) + IP (4) | CR (57) |
|
|
|
| Case 3 | 7 | 23650 | 4.5 | Yes | No | No | PEB (6) + VIP (6) | AWR (27) |
|
|
|
| Case 4 | 7 | 20592 | 4 | No (DT) | No | No | JEB + A (6) + PEB (6) | PR (29) |
|
| Rajagopal et
al | 2015 | Cases 1/2/6 | 17/18/23 | 80892/2139/ | 6.7/3.3/4.8 | Yes | No | No | PEB (6/4/12) | CR (60)/CR | (11) |
|
|
|
|
|
>10000a |
|
|
|
|
| (156)/CR (300) |
|
|
|
| Case 3 | 23 | 87680a | 7 | Yes | No | No | PEB (6) + JEB (6) + VAC (2) | CR (240) |
|
|
|
| Case 4 | 16 | 10580a | 3 | Yes | No | No | PVB (8) + PEB (3) + VAC (4) | Lost to follow-up
(17) |
|
|
|
| Case 5 | 3 | 2180a | 3 | Yes | Str | Yes | PEB (10) + JEB (7) + VAC (2) | CR (288) |
|
| Bhatt et
al | 2015 | 1 | 6 | 5565 | 3.7 | No | Str | No | PEB (6) | CR (24) | (9) |
| Lightfoot et
al | 2018 | 1 | 14 | 1386 | 3.6 | Yes | No | No | PEB (4) | CR (12) | (18) |
| Mayhew et
al | 2021 | 1 | 11 | 11636 | 5 | Yes | No | No | PEB (4) | CR (12) | (46) |
| Meng et
al | 2022 | 14 | 14b | 6002c | 3.9c | Yes | No | No | PEB
(6.4c) | CR
(98.2c) | (19) |
|
|
|
|
|
|
|
|
|
|
| (1 case DOD) |
|
|
|
| 5 | 21b | 12605c | 3.7c | No | Str | No | PEB
(6.4c) | CR
(107c) |
|
|
|
|
|
|
|
|
|
|
|
| (1 case local
relapse after 6 months) |
|
|
|
| 1 | 13 | 2100 | 3.4 | Yes | No | No | PV (4) + PVB (8) | CR (95) |
|
| Yin et
al | 2022 | 13 | 11b | 7840 | 3.6c | Yes | No | No | PEB (4.5) | CR (94c) | (12) |
|
|
|
|
|
|
|
|
|
|
| (1 case DOD) |
|
|
|
| Cases 1/2/8 | 20/44/10 | 15100/646/8754 | 3.7c | Yes | No | No | PEB
(3.7c) + PEV
(2.3c) + VAC (1) | CR (281)/CR
(278)/CR (96) |
|
|
|
| Cases 13/20 | 9/32 | 19766/33147 | 6/9 | Yes | No | No | PEB (5) + PE (1)/CEB (6)
+ PEV (2) + PEB (2) | CR (69)/CR
(10) |
|
|
|
| Cases 18/19/21 | 36/15/10 |
54000/1000/6977 | 7/5/4 | No | Stvr | No | PEB (10)/NVEB (1) + PEB (2)/VPEB(2)
+ CEB (1) + PEB (2) | CR (122)/CR (62)/CR
(51) |
|
Discussion
MGCTs account for 3% of all childhood malignant
tumors (17). Vaginal MGCTs can
cause vaginal bleeding. YSTs originating in the vagina account for
3–8% of all reported GCT cases, and patients in most GCT cases are
under the age of 3 years (1,8).
Rhabdomyosarcoma (RMS) is considered the most common pathological
type of vaginal tumor in prepubertal females, followed by YST
(18). However, Meng et al
(19) found that YST may be the
most common pathological type of vaginal tumor in Chinese children,
followed by RMS. Vaginal YST should be considered for a young girl
with vaginal bleeding. Any vaginal bleeding in children >10 days
should be considered abnormal, and further investigation is
necessary to eliminate vaginal malignant tumors in prepubertal
females (20).
The clinical presentation of vaginal YST includes
vaginal bleeding, blood-tinged vaginal discharge or a friable mass.
A rectal examination helps to assess the extent of the tumor and
rule out the possibility of invasion. Ultrasonography is usually
used as the first choice for diagnosis and follow-up. CT and MRI
are non-invasive methods for estimating the primary site,
extravaginal invasion and possible distant metastasis (10,21).
According to previous studies, vaginal YST is uniformly isointense
on T1-weighted imaging (T1WI), heterogeneously hyperintense on T2WI
and heterogeneously enhanced after administrating contrast agents,
which is different from vaginal RMS. Diffusion-weighted MRI may
also be useful for differentiating vaginal YST and RMS (10). Serum AFP is a sensitive and reliable
YST marker and plays an important role in evaluating the treatment
efficacy, remission status or disease progression (22). It is useful to examine the lectin
affinity fractionation of AFP, which can improve the specificity
and sensitivity of the final diagnosis (23). However, not all vaginal YSTs are
AFP-positive (24). A biopsy or
complete pathological examination of the tumor and an elevated
serum AFP level confirm the final diagnosis (18). Pathological examination typically
shows a loose reticular pattern of cells. Papillary structures with
a vascular core lined by a single layer of cells (Schiller-Duval
bodies) may be seen in yolk sac tumors from any site and are
pathognomonic when present (17).
Further histological patterns have been proposed by some studies
(8,25), including festoon, reticular, solid
and polyvesicular. Most tumors exhibit more than one of these
patterns. Immunohistochemically, CK AE1/AE3, CD117, AFP,
glypican-3, SALL-4, OCT-4, CD30 and β-hCG are usually assessed to
provide an accurate diagnosis. YSTs are characterized by the
positive expression of AFP (variable, focal), glypican-3 (patchy),
CK AE1/AE3, SALL4 and LIN28, and the lack of expression of OCT-4,
CD-30 and phospholipase A-2-activating protein, and have
variability in the expression of CK7, CK20 and β-hCG (2).
Due to the rarity of such tumors, there are no
clinical practice guidelines, expert consensus or standard
treatment principles or protocols for treating vaginal YSTs. Before
1965, radical surgery and radiotherapy were the main methods to
treat vaginal YSTs (26). Radical
surgery guaranteed complete tumor removal, but the prognosis was
very poor with a survival rate of 56.3% (27). Meanwhile, the sexual and
reproductive functions of patients were severely damaged, and even
the functions of the bladder and rectum were adversely affected
(28–30). Therefore, treatment effects on
sexual and reproductive functions should be considered in the
long-term treatment process, especially when deciding the scope of
surgical resection.
Improvements to surgery and the application of
adjuvant chemotherapy have since allowed young patients to receive
relatively conservative surgical regimens that can preserve their
reproductive and sexual functions (31,32).
Moreover, diversified treatment options contribute to a higher cure
rate (32). Numerous published
reports on a combination of chemotherapy and less invasive surgery
have confirmed the usefulness of this combined method (24,33,34),
including the report by Mauz-Korholz et al (35) about nationwide multicenter clinical
trials in Germany. Therefore, it is necessary and valuable to
perform early surgical resection of vaginal YSTs for patients,
without tumor residue or organ invasion or metastasis. Complete
resection of the tumor can provide a complete tissue specimen for
pathological examination to rule out mixed germ cell tumors, which
is critical to treatment plans. However, this regimen should still
be carefully evaluated, as there are risks and complications
associated with any type of surgery. Furthermore, it is not clear
whether the best time to operate is before or after chemotherapy.
After reviewing treatment options published over the past 20 years,
it was found that conservative treatment is showing a growing trend
(2,11,18,19,36,37). A
number of studies tended to adopt a biopsy combined with
chemotherapy or chemotherapy combined with conservative surgery
(38,39). Local tumor resection followed by
chemotherapy can also be an effective option (9,39,40).
For infants and young children with vaginal YSTs,
chemotherapy alone can also eliminate tumor tissue and restore
serum AFP to a normal level according to some reports (9,18,19,36,41,42),
which may avoid damaging vaginal or pelvic structures. Neels et
al (43) suggested that vaginal
YST with distant metastases and good response to chemotherapy could
be cured with intensive chemotherapy without surgery after their
study. By analyzing the reported cases, Bhatt et al
(9) found that the efficacy of
platinum chemotherapy alone was equivalent to that of surgery
combined with platinum chemotherapy, and the recurrence rates of
the two methods were 14 and 13%, respectively. Cisplatin-based
multiagent chemotherapy has been playing an important role in
improving outcomes in children with malignant GCTs since the 1970s
(26). The most frequently used
chemotherapeutic drugs nowadays include etoposide, cisplatin,
cyclophosphamide and bleomycin. Despite their effectiveness for
inhibiting tumor growth, they have some significant and long-term
toxic effects, such as hearing impairment, secondary malignancy,
pulmonary fibrosis and infertility in and after the whole course of
treatment. The longest period of follow-up for a patient receiving
chemotherapy was 25 years, and the patient had two spontaneous
vaginal deliveries (11). However,
it is hard to fully assess the long-term consequences of these
toxic effects, as such a case is still in the minority. The PEB
chemotherapy regimen is currently considered to be the most
effective regimen with minimal side effects (19,44).
Yuan et al (15) suggested
that PEB chemotherapy should be adopted for patients who
experienced early stage disease and received non-surgical
treatment. Based on the summary and analysis of 12 patients with
vaginal YST, Han et al (45)
suggested that standardized PEB chemotherapy could be used for
patients diagnosed with vaginal YSTs by pathology before surgery.
The study found that most patients could achieve complete responses
with a good prognosis after chemotherapy. According to existing
reports, all the patients receiving chemotherapy alone have been
treated with over 4 cycles of chemotherapy (18,19,36,44,46).
However, due to the scarcity of case reports and serial reports,
there is limited evidence of the effectiveness of chemotherapy
alone and a lack of critical information on the dosage and duration
of chemotherapy used for patients.
In the present case, based on the systemic
preoperative evaluation and hysteroscopy, the tumor was considered
to be localized at the vaginal wall. Therefore, a complete tumor
resection was performed without affecting the vaginal structure,
instead of just a biopsy. This procedure was advisable according to
the treatment plan published by the Children's Oncology Group
(18,19). Moreover, since mixed germ cell
tumors could not be ruled out, a complete resection would help to
give a more accurate diagnosis, which is important as the
subsequent treatment may vary based on the final pathological
results. Mixed component YSTs may exhibit recurrence even after
effective chemotherapy (19). Tang
et al (47) indicated that
chemotherapy alone was more suitable for simple YSTs and that
chemotherapy combined with local surgery should be used for tumors
with mixed components.
In conclusion, more attention should be paid to
vaginal bleeding in infants, and a complete evaluation is
essential. Imaging findings from ultrasonography or MRI benefit the
diagnosis. The final diagnosis is determined by histological
examination and AFP levels, which are also helpful during
follow-up. Prospective studies on a larger scale are needed to
determine the long-term effectiveness and safety of chemotherapy
alone among patients with vaginal YSTs. Thus far, conservative
surgery combined with adjuvant chemotherapy has been considered
effective, and it may be less toxic than chemotherapy alone
potentially in the long term due to the lower number of
chemotherapy cycles.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZM contributed to the study conception, reviewed the
literature and drafted the manuscript. CL collected medical images
(MRI scans and pathological section scans) and analyzed
patient-related data. Both authors read and approved the final
manuscript. ZM and CL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This case report has been approved by the Ethics
Committee of the Third Affiliated Hospital of Zhengzhou University
(Zhengzhou, China; approval no. 2023-031-01).
Patient's consent to publication
The patient's family provided oral consent for the
publication of the article. The Ethics Committee of the Third
Affiliated Hospital of Zhengzhou University (Zhengzhou, China)
approved that the oral consent was sufficient for this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davidoff AM, Hebra A, Bunin N, Shochat SJ
and Schnaufer L: Endodermal sinus tumor in children. J Pediatr
Surg. 31:1075–1078; discussion 1078–1079. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong YP, Yahaya A, Che Abdul Aziz R, Chia
PY, Loh CK and Tan GC: Primary extragonadal vaginal yolk sac
tumour: A case report. Malays J Pathol. 42:301–305. 2020.PubMed/NCBI
|
|
3
|
Euscher ED: Germ cell tumors of the female
genital tract. Surg Pathol Clin. 12:621–649. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ben Nsir A, Darmoul M, Arous SB and Hattab
N: Metastatic sacrococcygeal yolk sac tumor: A misleading
diagnosis. J Neurosci Rural Pract. 6:395–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ravishankar S, Malpica A, Ramalingam P and
Euscher ED: Yolk Sac tumor in extragonadal pelvic sites: Still a
diagnostic challenge. Am J Surg Pathol. 41:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rudaitis V, Mickys U, Katinaite J and
Dulko J: Successful treatment of advanced stage yolk sac tumour of
extragonadal origin: A case report and review of literature. Acta
Med Litu. 23:110–116. 2016.PubMed/NCBI
|
|
7
|
Teilum G: Endodermal sinus tumors of the
ovary and testis. Comparative morphogenesis of the so-called
mesoephroma ovarii (Schiller) and extraembryonic (yolk
sac-allantoic) structures of the rat's placenta. Cancer.
12:1092–1105. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopes LF, Chazan R, Sredni ST and de
Camargo B: Endodermal sinus tumor of the vagina in children. Med
Pediatr Oncol. 32:377–381. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatt MD, Braga LH, Stein N, Terry J and
Portwine C: Vaginal Yolk Sac tumor in an infant: A case report and
literature review of the last 30 years. J Pediatr Hematol Oncol.
37:e336–e340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun F, Zhao SH, Li HM, Bao L, Xu L and
Wang DB: Computed tomography and magnetic resonance imaging
appearances of malignant vaginal tumors in children: Endodermal
sinus tumor and rhabdomyosarcoma. J Comput Assist Tomogr.
44:193–196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajagopal R, Ariffin H, Krishnan S,
Abdullah WA and Lin HP: Pediatric vaginal yolk sac tumor:
Reappraisal of treatment strategy in a rare tumor at a unique
location. J Pediatr Hematol Oncol. 37:391–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin M, Yang J, Wang T, Li S and Zhang X:
Primary vaginal endodermal sinus tumor in infants and children:
Experience from a tertiary center. BMC Pediatr. 22:5792022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anfelter P, Testa A, Chiappa V, Froyman W,
Fruscio R, Guerriero S, Alcazar JL, Mascillini F, Pascual MA, Sibal
M, et al: Imaging in gynecological disease (17): Ultrasound
features of malignant ovarian yolk sac tumors (endodermal sinus
tumors). Ultrasound Obstet Gynecol. 56:276–284. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shojaei H, Hong H and Redline RW:
High-level expression of divergent endodermal lineage markers in
gonadal and extra-gonadal yolk sac tumors. Mod Pathol.
29:1278–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan Z, Cao D, Yang J, Keng S and Huang H:
Vaginal yolk sac tumors: Our experiences and results. Int J Gynecol
Cancer. 27:1489–1493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gantschnig BE, Fisher AG, Page J, Meichtry
A and Nilsson I: Differences in activities of daily living (ADL)
abilities of children across world regions: A validity study of the
assessment of motor and process skills. Child Care Health Dev.
41:230–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saltzman AF, Gills JRR, LeBlanc DM, Velez
MC, Craver RD and Roth CC: Multimodal management of a pediatric
cervical yolk sac tumor. Urology. 85:1186–1189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lightfoot MA, Bilgutay AN and Kirsch AJ: A
rare case of pediatric vaginal yolk sac tumor. Urology.
119:137–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng Z, Lin D, Liu C, Wang G and Sun N:
Vaginal tumours in childhood: A descriptive analysis from a large
paediatric medical centre. Pediatr Surg Int. 38:927–934. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Striegel AM, Myers JB, Sorensen MD,
Furness PD and Koyle MA: Vaginal discharge and bleeding in girls
younger than 6 years. J Urol. 176:2632–2635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wani NA, Robbani I, Andrabi AH, Iqbal A
and Qayum A: Vaginal yolk sac tumor causing infantile hydrometra:
Use of multidetector-row computed tomography. J Pediatr Adolesc
Gynecol. 23:e115–e118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tao T, Yang J, Cao D, Guo L, Chen J, Lang
J and Shen K: Conservative treatment and long-term follow up of
endodermal sinus tumor of the vagina. Gynecol Oncol. 125:358–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aihara M, Gotoh K, Maruyama K, Yamanaka M,
Sakemoto M, Suzuki H, Kato M, Hotta T and Kang D: An abnormal
alpha-fetoprotein fractionation provides additional information: A
case of retroperitoneal germ cell tumor accompanied by liver
cirrhosis Type C. Rinsho Byori. 64:1353–1356. 2016.(In Japanese).
PubMed/NCBI
|
|
24
|
Watanabe N, Okita H, Matsuoka K, Kiyotani
C, Fujii E, Kumagai M and Nakagawa A: Vaginal yolk sac (endodermal
sinus) tumors in infancy presenting persistent vaginal bleeding. J
Obstet Gynaecol Res. 36:213–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harms D and Janig U: Germ cell tumours of
childhood. Report of 170 cases including 59 pure and partial
yolk-sac tumours. Virchows Arch A Pathol Anat Histopathol.
409:223–239. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beller FK, Nienhaus H, Gizycki BS,
Schellong G, Bunte H and Schmandt W: Endodermal germ cell carcinoma
(endodermal sinus tumor) of the vagina in infant girls. J Cancer
Res Clin Oncol. 94:295–306. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Young RH and Scully RE: Endodermal sinus
tumor of the vagina: A report of nine cases and review of the
literature. Gynecol Oncol. 18:380–392. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goyal S, Puri A, Mishra K, Aggarwal SK,
Kumar M and Sonaker P: Endodermal sinus tumor of vagina posing a
diagnostic challenge and managed by chemotherapy and novel
posterior sagittal surgical approach: Lessons learned. J Obstet
Gynaecol Res. 40:632–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kano M, Furugane R, Hogetsu K, Yamada Y,
Maniwa J, Kobayashi T, Hashizume N, Mori T, Watanabe E, Takahashi
M, et al: Vaginal yolk sac tumor resected by a novel
laparo/endoscope-assisted posterior sagittal approach: A case
report. Surg Case Rep. 8:1622022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar V, Kini P, Vepakomma D and Basant M:
Vaginal endodermal sinus tumor. Indian J Pediatr. 72:797–798. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rescorla F, Billmire D, Vinocur C,
Colombani P, London W, Giller R, Cushing B, Lauer S, Cullen J,
Davis M and Hawkins E: The effect of neoadjuvant chemotherapy and
surgery in children with malignant germ cell tumors of the genital
region: A pediatric intergroup trial. J Pediatr Surg. 38:910–912.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou JY, Liu HC, Yeh TC, Sheu JC, Chen KH,
Chang CY and Liang DC: Treatment results of extracranial malignant
germ cell tumor with regimens of cisplatin, vinblastine, bleomycin
or carboplatin, etoposide, and bleomycin with special emphasis on
the sites of vagina and testis. Pediatr Neonatol. 56:301–306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arafah M and Zaidi SN: A case of yolk sac
tumor of the vagina in an infant. Arch Gynecol Obstet.
285:1403–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alhumidi A, Al Shaikh S and Alhammadi A:
Yolk sac tumor of vagina: A case report. Int J Clin Exp Pathol.
8:2183–2185. 2015.PubMed/NCBI
|
|
35
|
Mauz-Korholz C, Harms D, Calaminus G and
Gobel U: Primary chemotherapy and conservative surgery for vaginal
yolk-sac tumour. Maligne Keimzelltumoren Study Group. Lancet.
355:6252000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong W, Yang Q, Feng Y and Zhang J: A rare
case of vaginal yolk sac tumor in an infant. Asian J Surg.
45:580–581. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yin M, Wang T and Yang JX: Yolk sac tumor
of the uterus in a 2-year-old girl: A case report and literature
review. J Pediatr Adolesc Gynecol. 35:177–181. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elbaz M, Qadiry RE, Fouraiji K, Jalal H
and Elhoudzi J: Yolk sac tumor of vagina: A rare cause of vaginal
bleeding in adolescents-a case report. Pan Afr Med J. 37:1692020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang X, Du W, Wang Q and Zhao X:
Endoscopic surgery combining chemotherapy for vaginal yolk-sac
tumor: A case report. Eur J Gynaecol Oncol. 36:335–338.
2015.PubMed/NCBI
|
|
40
|
Chauhan S, Nigam JS, Singh P, Misra V and
Thakur B: Endodermal sinus tumor of vagina in infants. Rare Tumors.
5:83–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lacy J, Capra M and Allen L: Endodermal
sinus tumor of the infant vagina treated exclusively with
chemotherapy. J Pediatr Hematol Oncol. 28:768–771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Terenziani M, Spreafico F, Collini P,
Meazza C, Massimino M and Piva L: Endodermal sinus tumor of the
vagina. Pediatr Blood Cancer. 48:577–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neels NJ, Tissing WJ, Pieters R,
Oosterhuis JW, van de Ven CP and Devos AS: Treatment of an infant
with a vaginal yolk sac tumour and distant metastases with
chemotherapy only. Pediatr Blood Cancer. 43:296–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie W, Shen K, Yang J, Cao D, Yu M and
Wang Y: Conservative management of primary vaginal endodermal sinus
tumor and rhabdomyosarcoma. Oncotarget. 8:63453–63460. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han J, Chang X, Hong Q, Yang W, Han W,
Cheng H, He L and Wang H: Analysis of the effect of BEP regimen in
the treatment of children with vaginal endodermal sinus tumors. J
Clin Pediat Surg. 557–575. 2021.(In Chinese).
|
|
46
|
Mayhew AC, Rytting H, Olson TA, Smith E
and Childress KJ: Vaginal yolk sac tumor: A case series and review
of the literature. J Pediatr Adolesc Gynecol. 34:54–60. e42021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tang QL, Jiang XF, Yuan XP, Liu Y, Zhang
L, Tang XF, Zhou JJ, Li HG, Fang JP and Xue L: Prognosis of eight
Chinese cases of primary vaginal yolk sac tumor with a review of
the literature. Asian Pac J Cancer Prev. 15:9395–9404. 2014.
View Article : Google Scholar : PubMed/NCBI
|