Introduction
Venous thromboembolism (VTE) is one of the most
common non-tumor related causes of death in patients with cancer
(1), so its early diagnosis and
prevention are important for prolonging survival. Patients with
cancer have a 4.1-fold higher risk of VTE than other patients, and
the risk increases up to 6.5-fold with chemotherapy (2,3).
Studies on risk factors for cancer-associated thrombosis (CAT) have
been conducted mainly in Europe and North America, and risk
prediction models (RPM) based on that evidence have been developed
for stratifying patients with cancer according to their risk of VTE
(4–8). CAT risk studies can be broadly divided
into studies on occurrence risk and recurrence risk. The most
well-known RPM for CAT occurrence is the Khorana score, which is
based on site of cancer, white blood cell (WBC) count, platelet
(Plt) count, hemoglobin (Hb) and/or use of
erythropoiesis-stimulating agents, and body mass index (BMI)
(4). Since publication of the
original Khorana score, many studies have been conducted to improve
it (9–11). Ay et al (7) developed an RPM incorporating the
hemostasis biomarkers D-dimer and soluble P-selectin (sP-selectin)
into the Khorana score.
In Asian patients with cancer, there are few
prospective studies on risk factors for, and an RPM of, CAT
occurrence (12–14). Although the risk of CAT is lower in
Asian people than in other ethnic groups (15–17),
VTE risk is much higher than usual in patients with metastatic
cancer who are receiving chemotherapy, and VTE is one of the major
causes of death among them (14,18).
The occurrence of VTE can also interrupt or delay essential
treatment, worsen quality of life, and increase the use of health
care resources, including hospitalization (19). Therefore, predicting the risk of VTE
is an important issue in Asian cancer patients. In addition,
measurement of blood biomarkers for prediction of CAT has been
limited to a single point in time (often before treatment
initiation) in most previous reports, and to our knowledge there is
only one study with data measured repeatedly over time in cancer
patients (20). Examining the
relationship between longitudinal changes in blood biomarkers and
the occurrence of CAT would allow us to understand the
characteristics of the biomarkers and to consider the optimal
timing for measuring them in clinical practice.
We therefore conducted a prospective observational
study in Japanese patients undergoing anticancer drug therapy for
advanced cancer. The purpose was to identify clinical
characteristics and blood biomarkers that are risk factors for
predicting CAT, and to propose an RPM based on these risk factors.
The blood biomarkers included soluble fibrin (SF) and tissue
plasminogen activator/plasminogen activator inhibitor type 1
antigens complex (tPA/PAI-1), for which there is still no evidence
of an association with CAT. In addition, we investigated the
association between longitudinal data on blood biomarkers and the
occurrence of CAT.
Materials and methods
Patients and study design
This was a single-center, prospective, observational
study (UMIN000026826) conducted at Saga University Hospital in
accordance with the Declaration of Helsinki and with the approval
of the Saga University Hospital Institutional Review Board
(2016-12-05). From all study participants the informed written
consent was obtained. The patient enrollment period was from March
2017 to March 2020, and the study population consisted of patients
with unresectable cancer for whom anticancer drug therapy was
planned. Inclusion criteria were as follows: patients with cancers
of the pancreas, biliary tract, stomach, esophagus, colorectum, or
lung; UICC classification stage III–IV or postoperative recurrence
of cancer, and cancer not curatively resectable; scheduled by the
attending physician to begin anticancer drug therapies, including
chemotherapy, molecular targeted agents and immune checkpoint
inhibitors; expected survival at least 3 months; written consent
obtained; 20 years of age or older; and contrast-enhanced
whole-body CT scan planned before and after the start of anticancer
drug therapy (to ensure that the conditions for finding
asymptomatic VTE, which is included among the current study
endpoints, are as similar as possible). Exclusion criteria were as
follows: history of VTE; treatment within 4 weeks prior to
enrollment, where treatment could be any of radiation therapy (with
the exception of palliative radiation therapies), anticancer drug
therapy including adjuvant anticancer therapy, and surgery (with
the exception of minor surgeries); ongoing anticoagulant use;
active infection; pregnancy; history of lower extremity amputation;
inability to perform contrast-enhanced CT due to impaired renal
function or allergy to contrast media; or judged by the principal
investigator to be unsuitable for this study.
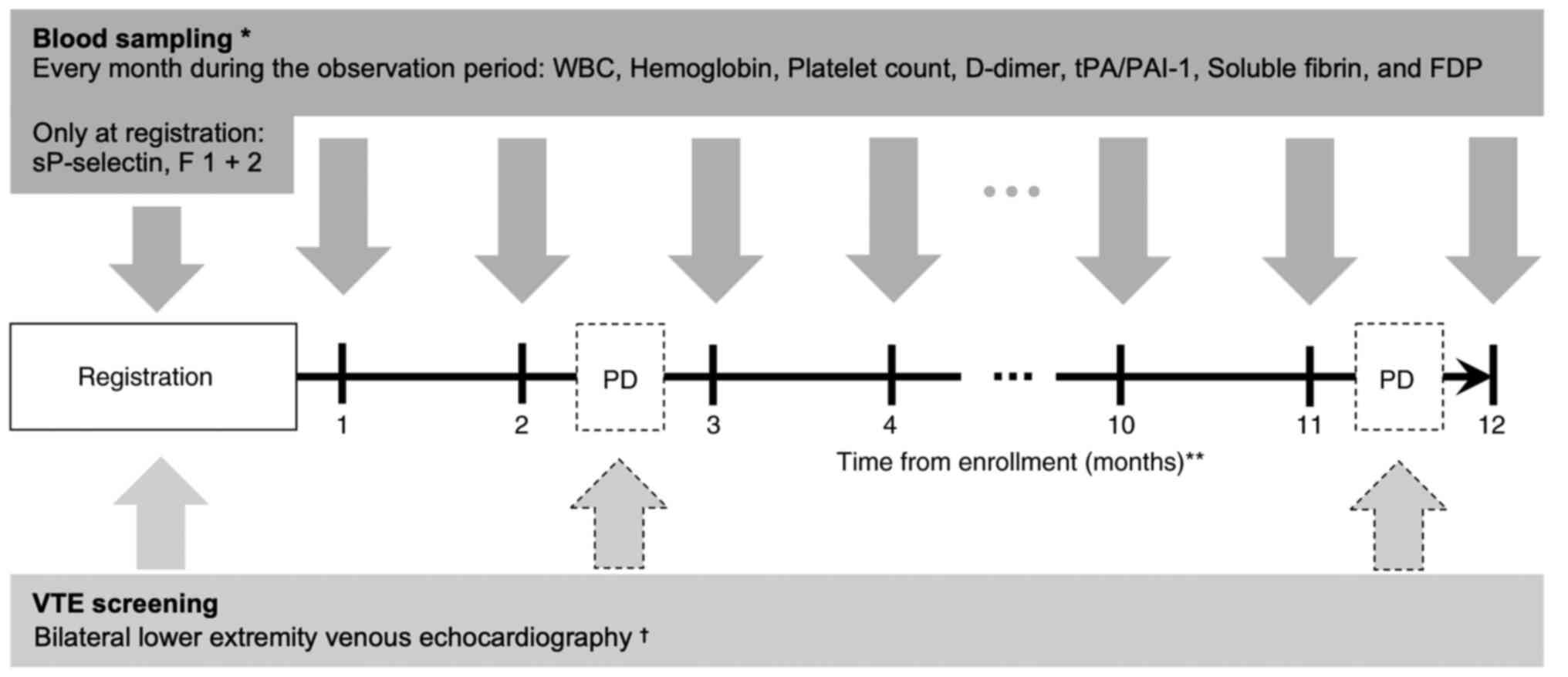
At the time of enrollment, bilateral lower extremity
venous vascular echocardiography was performed to assess the
presence of pre-existing deep vein thrombosis (Fig. 1). If the D-dimer value measured at
the time of registration was less than 1.2 µg/ml, the echo
examination could be omitted (21).
The presence of deep venous thrombus in the trunk was also assessed
by contrast-enhanced CT for the evaluation of cancer disease at the
time of registration.
As for blood biomarkers, WBC, Hb, and Plt were
measured and included in the Khorana score, and sP-selectin and
D-dimer were measured and included in the Vienna CATS score
(Fig. 1). In addition to the above,
we measured prothrombin fragment 1 + 2 (F 1 + 2) and SF as
coagulation system markers, and fibrin/fibrinogen degradation
products (FDP) and tPA/PAI-1 as fibrinolytic system markers. All
biomarkers were measured at enrollment, and WBC, Hb, Plt, D-dimer,
FDP, SF, and tPA/PAI-1 were measured additionally every month
during the observation period.
For detecting asymptomatic VTE during the
observation period, bilateral lower extremity venous
echocardiography was performed to evaluate the presence of deep
vein thrombosis when the physician judged that there was clinical
progression of cancer. The presence of VTE in the trunk, including
asymptomatic PE, was confirmed by using the results of whole body
CT, which was performed to assess the state of the cancer. If the
physician suspected VTE, imaging studies could be performed at any
time. The occurrence of VTE was defined as an objective diagnosis
using either contrast-enhanced CT or lower extremity venous
echocardiography.
Considering the prognosis of patients with advanced
cancer and the fact that VTE occurs most frequently during the
first year after diagnosis (22),
patients were followed until the end of a 1-year observation
period, the occurrence of VTE, death, or difficulties in follow-up
associated with best supportive care, whichever occurred first.
Outcome measures
The primary endpoint was the occurrence of VTE. The
definition of VTE occurrence in this study was the objective
confirmation of the diagnosis of symptomatic or asymptomatic VTE
using the detection methods described above from the time of
enrollment to the end of the observation period. WBC, Hb, and Plt
values were taken from the earliest day of the month in which they
were clinically examined. Plasma levels of D-dimer, FDP, SF, and
tPA/PAI-1 were measured by latex immunoagglutination with the LPIA
GENESIS D-dimer (cat. no. 756818; LSI Medience Corporation, Tokyo,
Japan), LPIA FDP-P (cat. no. 753725; LSI Medience Corporation,
Tokyo, Japan), IATRO SF II (cat. no. 757419; LSI Medience
Corporation, Tokyo, Japan), and LPIA tPAI test (cat. no. 757211;
LSI Medience Corporation, Tokyo, Japan). F 1 + 2 levels were
measured by enzyme-linked immunosorbent assay using
Enzygnost® F 1 + 2 (cat. no. 10445978; Siemens
Healthcare Diagnostics, Marburg, Germany), according to the
manufacturer's protocol. sP-Selectin levels were measured with the
human sP-selectin Immunoassay (cat. no. DPSE00; R&D Systems,
Minneapolis, MN) according to previous reports (23).
Data gathering and monitoring
To ensure data quality, data collection and regular
monitoring for protocol compliance were performed at an independent
clinical research center at Saga University Hospital.
Proposed RPM
Risk factors that were statistically significant in
multivariable Cox regression analysis and in covariate analysis
adjusted for the propensity score were used for our proposed RPM.
Numerical values of the elements constituting the RPM were assigned
by considering the hazard ratios in the above analyses. The RPMs
were evaluated by generating receiver operating characteristic
(ROC) curves and calculating sensitivity, specificity, positive
predictive value (PPV), and negative predictive value (NPV) for the
internal cohort only.
Sample size
Since the difference in Khorana score between the
high risk and low risk groups is smaller than the difference
between groups defined by Vienna CATS score ≥3 and <3, detecting
a significant difference in Khorana score between the high risk and
low risk groups requires a larger sample size (7). To estimate the necessary sample size,
we assumed that the occurrence proportion of VTE in the high risk
and low risk groups based on the Khorana score is 17.7 and 1.5%,
respectively [on the basis of previous studies (7)]. The number of cases required to detect
this difference by a chi-square test or a Fisher's exact test with
80% power and 5% two-sided significance level is 220. Further
allowing for dropouts, we considered that 240 patients would need
to be enrolled.
Data analysis and statistical
methods
As the principal analyses in this study, assessment
of the relationship between the occurrence of VTE and the two risk
scores, Khorana and Vienna cancer and thrombosis study (CATS), as
well as the relationships of individual risk factors to the
occurrence of VTE, were planned. For the Khorana score, occurrence
proportion of VTE in the high risk group (score ≥3) was compared to
that in the low risk group (score 0) with a Fisher's exact test. A
secondary comparison was made between the high risk group (score
≥3) and the low + intermediate risk group (score 0–2) as well. For
the Vienna CATS score, occurrence proportion of VTE in the group
with a score ≥3 was compared to that in the group with a score
<3 with a Fisher's exact test. In addition, to take into account
censoring during the observation period, we constructed
Kaplan-Meier plots that express the cumulative probability of VTE
and preformed log-rank tests to compare the same groups as above.
Single-variable and multivariable analyses by Cox proportional
hazards model were used for calculating the VTE risk of individual
risk factors as follows: the factors used in Cox proportional
hazards model included clinical characteristics and multiple blood
biomarkers, and some clinical factors that might have been
confounders at the time of study enrollment: age, gender, BMI, ECOG
performance status (PS), and Charlson comorbidity index (24). If previous studies had established a
cutoff value, that value was used; otherwise, the 75th percentile
in the current population was used. In the previous literature, the
cutoff value of D-dimer is ≥1.44 µg/ml, which is reported in
fibrinogen equivalent units (FEU) (6,7), and
the D-dimer measurement method used in Japan is reported in D-dimer
units (DDU). Therefore, because of the approximate relationship 2 ×
FEU=DDU (25,26), we used ≥2.88 µg/ml as the cutoff
value for D-dimer.
As secondary analyses, considering that the number
of variables that can be included in a Cox proportional hazards
model in a conventional multivariable analysis is limited by the
expected number of events, covariate analysis using propensity
scores was performed to adjust for confounding factors and to
calculate the VTE risk for individual factors. Moreover,
longitudinal data analyses were performed by using the enrollment
point values, final time-point values and average rate of change.
To address missing values in longitudinal data, we used the average
rate of change reflecting the values at all time points during the
observation period, which was calculated as the slope of a linear
trend with a mixed effects model for repeated measures. These
parameters were compared between the VTE and non-VTE groups using
Wilcoxon's rank-sum test. The level of statistical significance for
all analyses was defined as P<0.05. Statistical analyses were
performed with JMP Pro 15.2.0 software.
Results
Patient characteristics and VTE
events
In total, 200 patients were enrolled during the
recruitment period. Among these patients, 10 were excluded from the
analysis: two were judged unsuitable for assessing the risk of
developing VTE due to loss of baseline blood samples (n=2); seven
had VTE detected by the screening test at enrollment; and one had a
change in pathological diagnosis from lung cancer to mesothelioma,
a type of cancer that was not included in this study. Baseline
characteristics and blood biomarker values at enrollment (n=190)
are shown in Table I. The
proportion of males was high (73%), as is typical with lung or
esophagus cancer. Charlson comorbidity index was very high, ≥6 in
all patients. With this index, metastatic solid cancer is counted
as 6, and 1 or 2 represents complications (27% of patients in this
study had Charlson index 7 or 8). Distributions of WBC, Hb, and Plt
values were similar to those in the Vienna CATS score cohort in
Austria (7), but D-dimer and
sP-selectin tended to be higher and lower, respectively. BMI was
much lower in our cohort. Ninety percent of the patients received
chemotherapy as anti-cancer therapies, and 50% were treated with
other anti-cancer drugs in addition to chemotherapy. During the
observation period, 31% (n=58) and 9% (n=17) of patients were
censored because active treatment was discontinued due to
deterioration of the patient's general condition or death,
respectively. Two patients were censored because anticoagulants
were administered due to portal vein thrombosis. The maximum
observation period was one year of planned observation; mean
observation period was 265 days with median 365 days and 25th
percentile 165 days.
 | Table I.Baseline characteristics of patients
(n=190). |
Table I.
Baseline characteristics of patients
(n=190).
|
Characteristics | Value |
|---|
| Median age, years
(IQR) | 69 (62–74) |
| Sex, n (%) |
|
|
Male | 139 (73) |
|
Female | 51 (27) |
| Primary site of
cancer, n (%) |
|
|
Lung | 61 (32) |
|
Stomach | 44 (23) |
|
Colorectal | 34 (18) |
|
Pancreas | 26 (14) |
|
Esophagus | 15 (8) |
| Biliary
tract | 10 (5) |
| CCI, n (%) |
|
| 6 | 140 (74) |
| ≥7 | 50 (27) |
| ECOG PS, n (%) |
|
| 0 | 105 (55) |
| 1 | 71 (37) |
| Median BMI,
kg/m2 (IQR) | 21.6
(18.7–23.7) |
| Anti-cancer
therapy, n (%) |
|
|
Chemotherapy | 170 (89) |
|
Platinum-based
Chx | 122 (64) |
|
MTA | 94 (49) |
|
Anti-VEGF mAb | 69 (36) |
|
ICI | 33 (17) |
| Median laboratory
values (IQR) |
|
| WBC,
×109/l | 6.8 (5.3-8.6) |
|
Hemoglobin, g/l | 127 (110–138) |
|
Platelet count,
×109/l | 254 (197–334) |
|
D-Dimer, µg/ml | 1.46
(0.79–3.14) |
| Soluble
P-selectin, ng/ml | 33.5
(26.9–43.4) |
| FDP,
µg/ml | 1.95
(1.20–3.63) |
| Soluble
fibrin, µg/ml | 3.2 (1.6–6.2) |
|
tPA/PAI-1, ng/ml | 18.0
(12.0–25.3) |
| F 1 +
2, pmol/l | 261 (190–371) |
| Khorana score, n
(%) |
|
| Low:
0 | 18 (10) |
|
Intermediate: 1–2 | 137 (72) |
| High:
≥3 | 35 (18) |
| Vienna CATS score,
n (%) |
|
| 0 | 15 (8) |
| 1 | 58 (31) |
| 2 | 60 (32) |
| 3 | 33 (17) |
| 4 | 14 (7) |
| ≥5 | 10 (5) |
During the follow-up period, 17 (9%) of the 190
patients eligible for analysis developed VTE. Site of VTE and
detailed information are shown in Table II. PE was complicated in 2 of these
patients (12% of VTE events). Among the VTE events, the most common
site of deep vein thrombosis was a distal lower extremity vein in
10 patients, and symptomatic VTE was observed in 5 patients.
Anticoagulant therapy was administered to 13 of these patients.
 | Table II.Overall occurrence of venous
thromboembolism events (n=17). |
Table II.
Overall occurrence of venous
thromboembolism events (n=17).
| Classification | No. of patients
(%) |
|---|
| Types |
|
| DVT
alone | 15 (88) |
| DVT +
PE | 2 (12) |
| Subtypes |
|
| Distal
lower extremity | 10 (59) |
|
Proximal lower and distal
lower extremity | 2 (12) |
|
Internal jugular vein | 2 (12) |
|
Superior vena cava | 1 (6) |
|
Inferior vena cava | 1 (6) |
|
Superior mesenteric vein | 1 (6) |
| Symptomatic or
asymptomatic |
|
|
Symptomatic | 5 (29) |
|
Asymptomatic | 12 (71) |
| Anticoagulant
therapy |
|
|
Received | 13 (77) |
|
Edoxaban | 11 (65) |
|
Apixaban | 1 (1) |
|
Unfractionated
heparin | 1 (1) |
| Not
Received | 4 (24) |
Assessment of VTE risk using Khorana
and Vienna CATS risk scores
At first, we evaluated whether the Khorana and
Vienna CATS risk scores could predict VTE in our cohort (Fig. 2). The higher the Khorana score, the
more frequently VTE occurred; occurrence proportion of VTE was
about twice in the High risk group what it was in the Low risk
group. However, a Fisher's exact test using a 2×2 contingency table
with Low risk vs. High risk and with VTE vs. without VTE showed no
statistically significant differences (P=0.44) (Table SI). A secondary analysis of low +
intermediate risk (score 0–2) vs. High risk (score ≥3) was
performed similarly but did not show a significant difference
(P=0.38) (Table SI). A log-rank
test (based on the Kaplan-Meier curve to account for data
censoring) showed HR 1.8 (95% CI, 0.3-11.9; P=0.60) for High vs Low
(Fig. 2B), and HR 1.6 (95% CI,
0.5-6.0; P=0.38) for High vs. Low + Int (Fig. 2C). Because the number of patients in
the analysis did not reach the target number, it is possible that
power was insufficient. With the Vienna CATS risk score, there was
a trend toward higher occurrence proportion of VTE in patients with
a score of ≥3 than in those with a score of <3, but the Fisher's
exact test using a 2×2 contingency table with score ≥3 vs. score
<3 and with VTE vs. without VTE was not significant (P=0.09)
(Table SI). On the other hand, the
log-rank test showed a statistically significant difference in
cumulative probability of VTE between patients with a score of ≥3
and those with a score of <3, with an HR of 2.8 (95% CI,
0.9-8.7; P=0.045) (Fig. 2F).
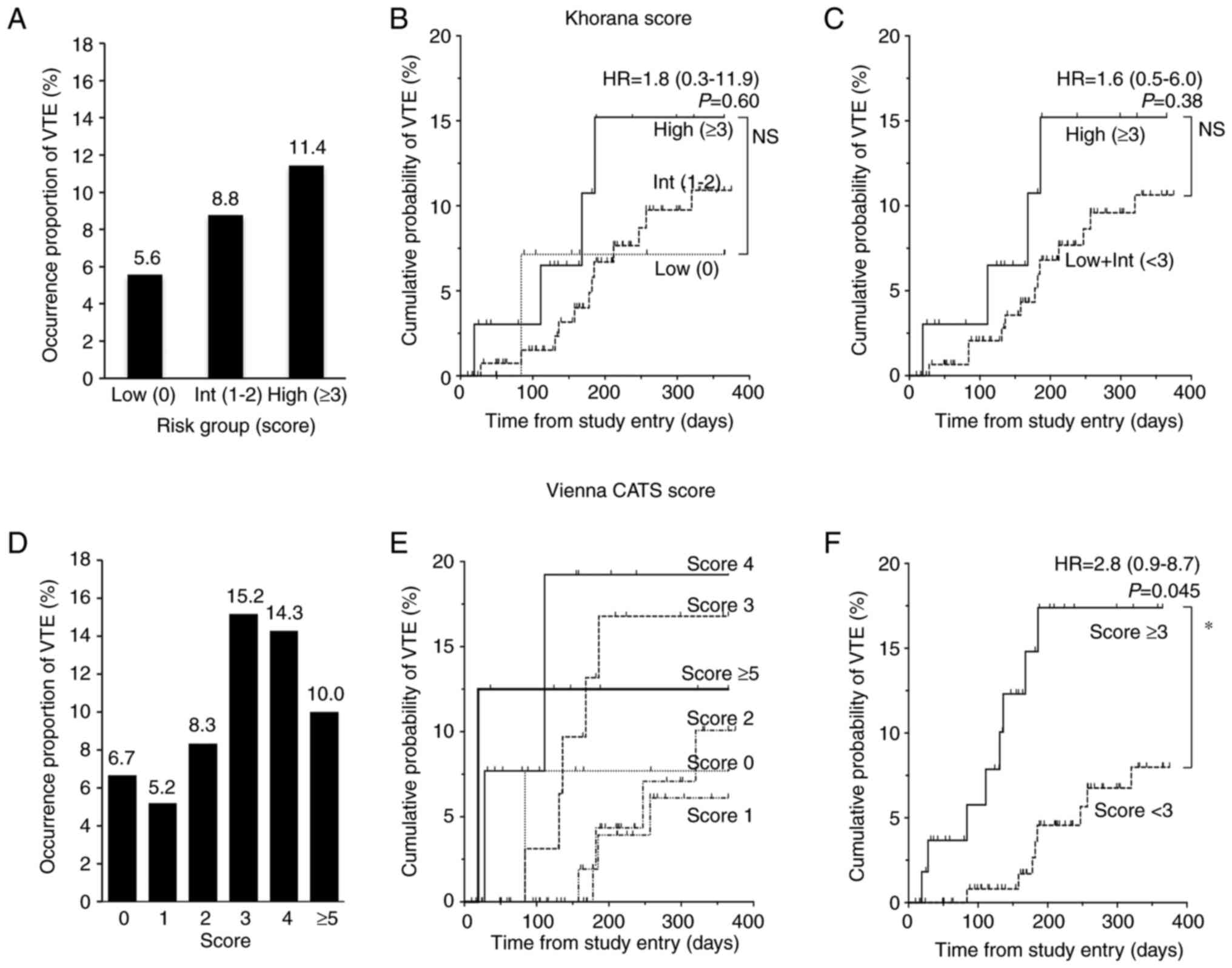 | Figure 2.Occurrence proportion and cumulative
probabilities of VTE for Khorana and Vienna CATS scores.
Comparisons among risk groups based on the Khorana score are shown
for (A) occurrence proportion of VTE, and (B and C) cumulative
probability of VTE. Comparisons in (A and B) are among all three
risk groups: Low, Int and High; the comparison in (C) is between
High and Low + Int groups. (D-F) Analogous comparisons based on the
Vienna CATS score. Comparisons in (D and E) are among all score
levels and the comparison in (F) is between the group with a score
<3 and the group with a score ≥3, the intermediate point of the
scores. Data in (B, C and F) were analyzed using the log-rank test.
No statistical comparisons were performed for (E). The Khorana
score assigns 2 points for very high-risk cancer sites (pancreas,
stomach) and 1 point for high-risk cancer sites (lung, ovary,
bladder). One point is also assigned for each of the following:
White blood cell count ≥11×109/l, hemoglobin <100 g/l
or use of erythropoiesis-stimulating agents, platelet count
≥350×109/l, and body mass index 35 kg/m2. The
Vienna CATS score adds one point to the Khorana score if D-dimer
≥2.88 µg/ml (when using the fibrinogen equivalent unit test, the
cutoff value is ≥1.44 µg/ml) or soluble P-selectin ≥53.1 ng/ml,
respectively. *P<0.05. CATS, cancer and thrombosis study; HR,
hazard ratio; Int, intermediate; Low + Int, combined low plus
intermediate groups; NS, not significant; VTE, venous
thromboembolism. |
Association between VTE occurrence and
each clinical factor at baseline using single-variable and
multivariable analyses
Next, we examined the relationship between risk
factors at the time of enrollment, including blood biomarkers, and
subsequent VTE occurrence. Single-variable Cox regression analysis
showed that pancreatic cancer (HR, 4.0; 95% CI 1.5-10.8; P=0.007),
F 1 + 2 (HR, 2.8; 95% CI 1.0-7.2; P=0.041), and SF (HR, 3.6; 95% CI
1.4-9.4; P=0.008) were associated with increased risk of VTE during
chemotherapy (Tables III and
IV). With multivariable Cox
regression analysis using the above three risk factors,
statistically significant differences remained with pancreatic
cancer (HR, 3.2; 95% CI, 1.1-8.8; P=0.028) and SF (HR, 3.7; 95% CI,
1.1-7.8; P=0.036) (Tables III and
IV). To confirm the above results
in analyses adjusted for confounding factors, covariate analyses
were performed as secondary analyses using a propensity score
calculated from six patients' background factors: age, gender, BMI,
PS, Charlson comorbidity index, and site of primary lesion. The
analysis with propensity score showed that pancreatic cancer (HR,
4.4; 95% CI 1.5-12.3; P=0.006) and SF (HR, 3.9; 95% CI 1.4-10.5;
P=0.008) were associated with an increased risk of VTE during
chemotherapy, with statistical significance (Tables III and IV). This analysis also showed a
statistically significant increase in VTE risk with a binary
indicator of Hb <100 g/l (HR, 3.9; 95% CI 1.1-14.0;
P=0.034).
 | Table III.Association between VTE occurrence
and baseline characteristics of patients. |
Table III.
Association between VTE occurrence
and baseline characteristics of patients.
|
|
|
|
Single-variable |
Multivariableb | Propensity score
adjustment |
|---|
|
|
|
|
|
|
|
|---|
| Variable | No. | VTE%a | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age ≥65 years |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 130 | 10 | 1.5 | 0.5-4.7 | 0.448 |
|
|
| 1.4 | 0.4-4.3 | 0.597 |
| No | 60 | 7 |
|
|
|
|
|
|
|
|
|
| Sex, female |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 51 | 12 | 1.5 | 0.6-4.2 | 0.400 |
|
|
| 1.5 | 0.5-4.1 | 0.492 |
| No | 139 | 8 |
|
|
|
|
|
|
|
|
|
| BMI
≥25c
kg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 31 | 13 | 1.4 | 0.4-4.2 | 0.593 |
|
|
| 1.7 | 0.5-5.9 | 0.416 |
| No | 159 | 8 |
|
|
|
|
|
|
|
|
|
| ECOG PS ≥1 |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 85 | 7 | 0.8 | 0.3-2.1 | 0.634 |
|
|
| 0.9 | 0.3-2.6 | 0.834 |
| No | 105 | 11 |
|
|
|
|
|
|
|
|
|
| CCI ≥7 |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 50 | 10 | 1.2 | 0.4-3.4 | 0.742 |
|
|
| 1.0 | 0.3-3.1 | 0.966 |
| No | 140 | 9 |
|
|
|
|
|
|
|
|
|
| Primary site:
Lung |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 61 | 5 | 0.4 | 0.1-1.3 | 0.118 |
|
|
| 0.4 | 0.1-1.3 | 0.117 |
| No | 129 | 11 |
|
|
|
|
|
|
|
|
|
| Primary site:
Stomach |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 44 | 7 | 0.8 | 0.2-2.6 | 0.647 |
|
|
| 0.7 | 0.2-2.6 | 0.634 |
| No | 146 | 10 |
|
|
|
|
|
|
|
|
|
| Primary site:
Colorectal |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 34 | 12 | 1.4 | 0.5-4.4 | 0.530 |
|
|
| 1.3 | 0.4-4.2 | 0.630 |
| No | 156 | 8 |
|
|
|
|
|
|
|
|
|
| Primary site:
Pancreas |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 26 | 23 | 4.0 | 1.5-10.8 | 0.007 | 3.2 | 1.1-8.8 | 0.028 | 4.4 | 1.5-12.3 | 0.006 |
| No | 164 | 7 |
|
|
|
|
|
|
|
|
|
| Primary site:
Esophagus |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 15 | 0 | NE |
|
|
|
|
| NE |
| 0.999 |
| No | 175 | 10 |
|
|
|
|
|
|
|
|
|
| Primary site:
Biliary tract |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 10 | 10 | 1.3 | 0.2-6.0 | 0.818 |
|
|
| 1.2 | 0.2-9.1 | 0.865 |
| No | 180 | 9 |
|
|
|
|
|
|
|
|
|
 | Table IV.Association between VTE occurrence
and laboratory values at baseline. |
Table IV.
Association between VTE occurrence
and laboratory values at baseline.
|
|
|
|
Single-variable |
Multivariableb | Propensity score
adjustment |
|---|
|
|
|
|
|
|
|
|---|
| Variable | No. | VTE%a | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| WBC
≥11×109/l |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 19 | 11 | 1.7 | 0.4-7.5 | 0.471 |
|
|
| 2.5 | 0.5-11.4 | 0.247 |
| No | 171 | 9 |
|
|
|
|
|
|
|
|
|
| Hemoglobin <100
g/l |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 23 | 17 | 2.8 | 0.9-8.6 | 0.071 |
|
|
| 3.9 | 1.1-14.0 | 0.034 |
| No | 167 | 8 |
|
|
|
|
|
|
|
|
|
| Platelet count
≥350×109/l |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 38 | 5 | 0.6 | 0.1-2.7 | 0.506 |
|
|
| 0.7 | 0.2-3.5 | 0.689 |
| No | 152 | 10 |
|
|
|
|
|
|
|
|
|
| D-dimer
≥2.88c µg/ml |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 52 | 14 | 2.4 | 0.9-6.4 | 0.071 |
|
|
| 2.4 | 0.9-6.6 | 0.085 |
| No | 138 | 7 |
|
|
|
|
|
|
|
|
|
| sP-selectin ≥53.1
ng/ml |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 20 | 10 | 1.9 | 0.4-8.2 | 0.402 |
|
|
| 1.9 | 0.4-8.6 | 0.433 |
| No | 170 | 9 |
|
|
|
|
|
|
|
|
|
| F 1 + 2 ≥358
pmol/l |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 49 | 14 | 2.8 | 1.0-7.2 | 0.041 | 1.8 | 0.7-5.1 | 0.255 | 2.3 | 0.8-6.6 | 0.110 |
| No | 141 | 7 |
|
|
|
|
|
|
|
|
|
| Soluble fibrin
≥6.3d µg/ml |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 47 | 17 | 3.6 | 1.4-9.4 | 0.008 | 3.7 | 1.1-7.8 | 0.036 | 3.9 | 1.4-10.5 | 0.008 |
| No | 143 | 6 |
|
|
|
|
|
|
|
|
|
| tPA/PAI-1
≥26d ng/ml |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 47 | 9 | 1.1 | 0.4-3.4 | 0.841 |
|
|
| 1.2 | 0.4-3.7 | 0.793 |
| No | 143 | 9 |
|
|
|
|
|
|
|
|
|
| FDP
≥3.7d µg/ml |
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 47 | 13 | 2.3 | 0.8-6.1 | 0.109 |
|
|
| 2.3 | 0.8-6.6 | 0.111 |
| No | 143 | 8 |
|
|
|
|
|
|
|
|
|
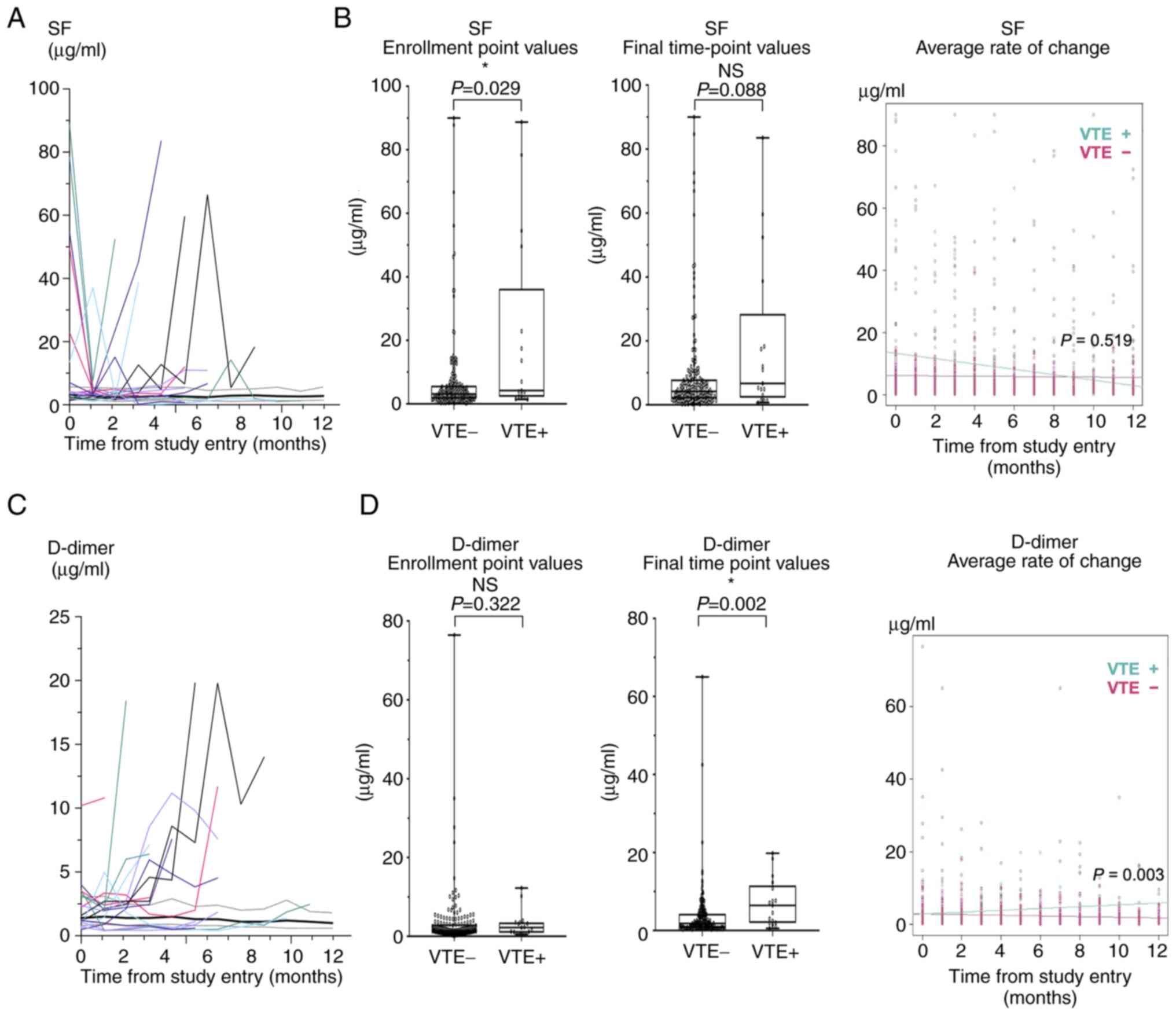
Longitudinal changes of biomarkers and
VTE occurrence
In addition, we investigated the relationship
between VTE occurrence and changes in blood biomarkers during the
observation period. Among the seven biomarkers examined in this
study, longitudinal patterns of SF, D-dimer, and FDP differed
between the VTE and non-VTE groups (Figs. 3A and C, and S1). FDP and D-dimer showed similar
patterns in the two groups. Therefore, SF and D-dimer values were
compared between the two groups by using three parameters: value at
the time of enrollment, value at the final time point, and average
rate of change during the observation period (Fig. 3). SF showed a significant difference
only at the time of enrollment (Fig.
3B). D-dimer showed significant differences at the final
time-point and in average rate of change, but was not different at
the time of enrollment (Fig.
3D).
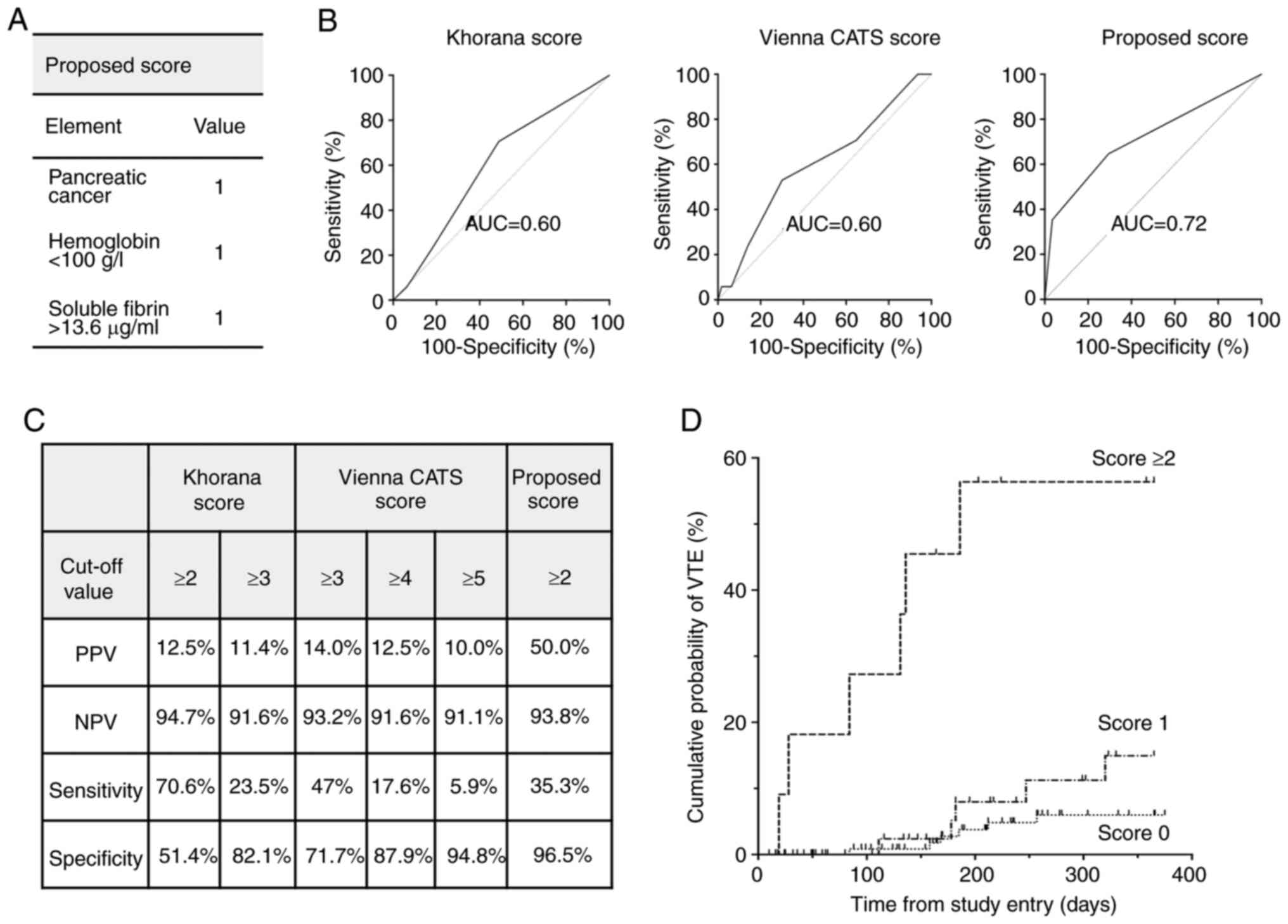
Proposed RPM of VTE
On the basis of the results with individual risk
factors, we propose an RPM including existence of pancreatic
cancer, SF (>13.6 ng/ml), and Hb (<100 g/l) (Fig. 4A), with SF and Hb dichotomized at
their respective cutoff values. We calculated the optimal cutoff
value of SF for predicting development of VTE by using the ROC
curve, and found that the sum of specificity and sensitivity was
greatest when SF >13.6 ng/ml. In our RPM, each of the elements
is assigned the same dichotomous value-‘1’ if present, ‘0’ if
absent, on the basis of their estimated hazard ratios being
similar. The area under the curve (AUC) of the ROC curve for
assessing performance of our RPM was 0.72 (95% CI 0.57-0.86),
better for predicting VTE than the Khorana score (AUC=0.60; 95% CI
0.47-0.73) and Vienna CATS score (AUC=0.60; 95% CI 0.46-0.75)
(Fig. 4B). When a score ≥2 was used
as the cutoff value, the sensitivity was 35%, specificity was 97%,
PPV was 50%, and NPV was 94% (Fig.
4C). A Kaplan-Meier plot showed that the group with score ≥2
had a much higher incidence of VTE than the other groups (Fig. 4D).
Discussion
There are few prospective studies in Asian patients
on the risk factors for CAT, especially on blood biomarkers of CAT
occurrence and RPM. In this prospective study with examination of
seven blood biomarkers, we showed that Vienna CATS risk score ≥3
was associated with VTE occurrence in Japanese patients receiving
chemotherapy for treatment of advanced cancer. Furthermore,
pancreatic cancer, low Hb, and high SF were associated with CAT
risk, suggesting that the inclusion of blood biomarkers in RPM may
help improve the accuracy of predicting VTE occurrence even in
persons of Asian ethnicity.
Asians have a lower risk of developing VTE than
persons of Caucasian or Black ethnicity (15,16)
and several studies have been conducted on the biological
mechanisms that may be involved in this difference. Studies on
coagulation factors related to VTE risk across races have reported
that factor VIII levels are significantly higher in persons of
Black ethnicity than in other ethnic groups (27) and that the factor V Leiden
polymorphism and the factor II G20210A variant, which both increase
VTE risk, are more common in Caucasians from northern and southern
Europe (28). Recently, an
increasing trend in VTE occurrence even in Asians has been reported
(18,29), and cancer is the most critical risk
factor for VTE, as in other races (30,31).
However, studies of risk factors for CAT are particularly limited
in Asians, with few prospective studies, apart from a study in
China on the risk of CAT occurrence in the perioperative period
among patients with cancer (n=262) (12) and a study in Korea on the risk of
CAT occurrence in hospitalized patients with cancer (n=140)
(13). There was also a
retrospective study in Taiwan on CAT occurrence risk and RPM in
patients with cancer, which used Taiwan's National Health Insurance
Research Database (29), but only
clinical parameters were used in those studies, not blood
biomarkers. In prospective studies performed in Europe and North
America, blood biomarkers including WBC count, Hb level, and Plt
count (4,32), as well as hemostasis biomarkers such
as D-dimer (6,7), F 1 + 2 (6), and sP-selectin (23,33),
were evaluated. In the present study, having pancreatic cancer, low
Hb levels, and high SF levels were associated with risk of CAT
occurrence. The Khorana score incorporates Hb <100 g/l or the
use of erythropoiesis-stimulating agents as risk factors. However,
in Japan, erythropoiesis-stimulating agents are not used to treat
anemia in patients with cancer, and there were no instances of
their use in the present study. Therefore, our results indicate
that Hb <100 g/l alone is a risk factor for CAT occurrence. SF,
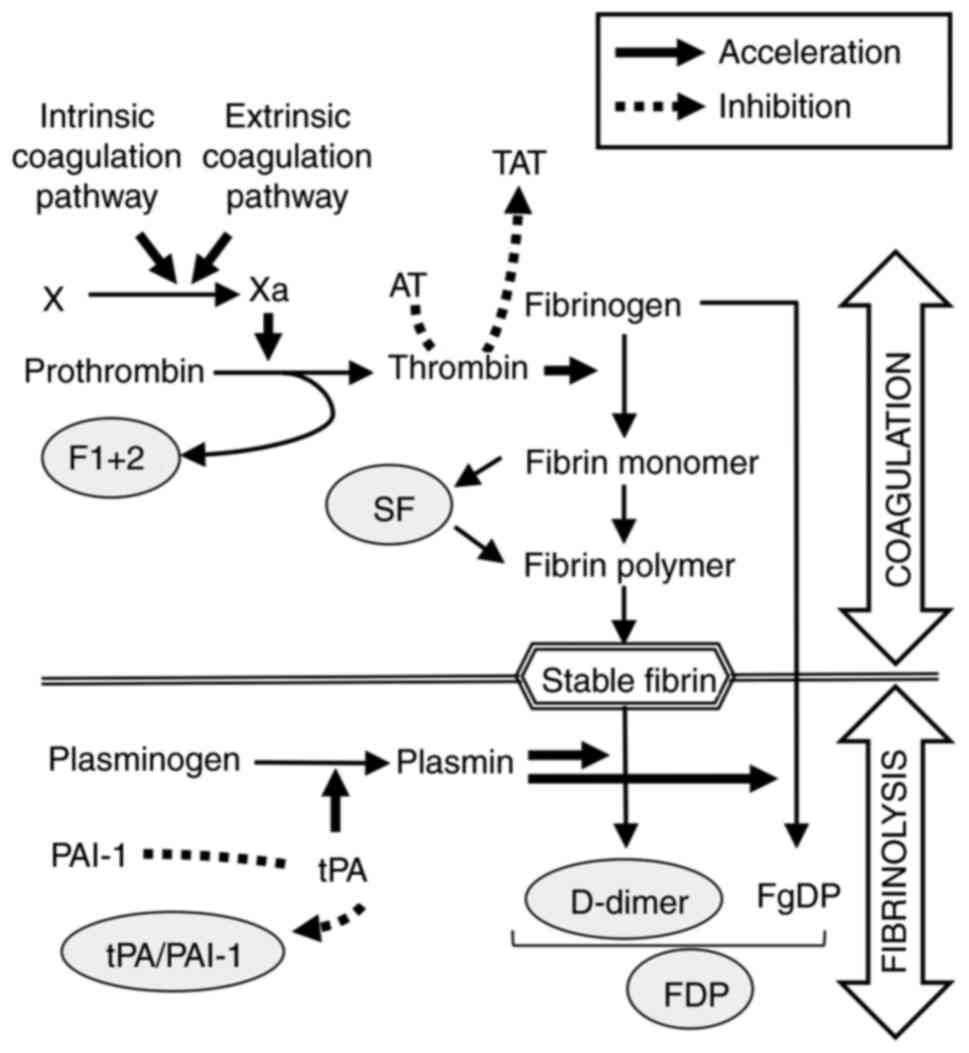
which is a hemostasis biomarker reflecting thrombin activation, as
well as F 1 + 2 (Fig. 5), have been
reported to be useful in diagnosis of VTE (34,35)
and disseminated intravascular coagulation (36,37),
as well as in predicting VTE after orthopedic surgery (38,39).
The present study is the first to demonstrate the usefulness of SF
in predicting CAT occurrence. In this study, patients received a
variety of anticancer drug therapies (Table SII). Among these treatments,
platinum-based chemotherapy and anti-angiogenesis agents in
particular have been reported to confer a risk of VTE (40). We have not been able to eliminate
the influence of all confounding factors due to our sample size.
However, at least there was no significant relationship between
these drug types and either SF or Hb (Table SIII), the biomarkers that we
identified as useful in the present study. On the other hand, the
present study did not show a significant association of CAT
occurrence with D-dimer, F 1 + 2, or sP-selectin. However, the
hazard ratios for those markers were relatively large, and the
lower bounds of their confidence intervals with both
single-variable and multivariable analyses, shown in Tables III and IV, were close to, if not greater than,
1.0. That they are not statistically significant is considered to
be due to the small number of cases. Moreover, the present study
showed that the Vienna CATS score, which includes hemostasis
biomarkers, was better at predicting CAT than the Khorana score,
although the small number of events precluded sufficient
validation. These results support a conclusion that including
hemostasis biomarkers improves the accuracy of RPM in patients of
Asian ethnicity who have cancer.
Data on longitudinal changes in biomarkers of
hemostasis in patients with cancer is only available from an
Austrian study that examined 112 patients with any of four cancer
types (20). In that study, D-dimer
values at the last blood-sampling time point before VTE onset in 14
patients who developed VTE tended to be higher than D-dimer values
in patients without VTE. Similarly, in the present study we found
that D-dimer was significantly higher at the final time point
before VTE onset in patients who developed VTE than in patients
without VTE. In addition, an increase in D-dimer during the
observation period was associated with occurrence of VTE. In
contrast, with SF the value of the point at enrollment, not at the
time point before the onset of VTE, was significantly associated
with occurrence of VTE. One reason for the difference between SF
and D-dimer is that SF reflects the early phase of VTE whereas
D-dimer reflects secondary fibrinolysis after thrombus formation
(Fig. 5) (34,41).
Another reason could be different lengths of times that these two
biomarkers persist: SF decreases relatively quickly after thrombus
formation, whereas D-dimer values remain high even for 7 days
(35). In fact, SF quickly
decreased after the point of estimated VTE occurrence in some
patients (Fig. 3A). Therefore, we
speculate that SF is more advantageous for revealing the
hypercoagulable state before VTE formation, whereas D-dimer is more
suitable for confirming the VTE state after thrombus formation has
started. From this perspective, SF may be a better biomarker than
D-dimer for predicting VTE. Based on these data, we proposed an RPM
to estimate the risk of VTE among Japanese patients undergoing
anticancer drug therapy. In some countries, prophylactic
anticoagulation therapy is being considered for populations at high
risk of VTE (42–44). However, cancer patients are known to
be at higher risk of bleeding than the general population (45), so prophylactic use of anticoagulants
should be made very cautiously. RPM should be easy to calculate in
clinical practice and so should contribute effectively to clinical
decision making. The present RPM is a simple score consisting of
only three factors with high NPV and specificity, and may be
clinically useful in identifying populations that do not require
prophylactic anticoagulation, but it needs to be validated in other
cohorts in the future. In post-hoc analyses, median overall
survival (OS) of all patients was 747 days (Fig. S2A), and there was no statistical
difference in prognosis between patients with vs. without VTE
(Fig. S2B). This may be because we
performed detection of asymptomatic VTE and therefore treatment for
VTE was initiated early. Similar to previous reports that
predictive scores for VTE are associated with OS in cancer patients
(46,47), our proposed score was also
associated with OS (Fig. S2C).
We acknowledge several limitations of this study.
First, it was conducted at a single center with a relatively small
number of patients, resulting in insufficient power to assess the
Khorana scores and the Vienna CATS score adequately. However, a
main objective of this study was to examine multiple blood
biomarkers to predict the risk of VTE, and we obtained longitudinal
data of blood biomarkers in each individual patient. We restricted
our study to a single center to maintain quality control over the
measurements of hemostasis biomarkers, as the time and conditions
between blood collection and plasma separation can affect the
results. A second limitation is that only six cancer types (lung,
colorectum, pancreas, stomach, biliary tract, and esophagus) were
included in the study; patients with other types of cancer that are
considered to have a high risk of thrombosis, such as gynecological
cancer and brain tumors, were not enrolled. In addition, it was
difficult to adequately assess differences in usefulness of the
biomarkers for each individual type of tumor given our sample size.
Therefore, the usefulness of SF should be validated in further
studies with more patients, including those with other types of
cancer. The strengths of the present study are that we
prospectively evaluated multiple blood biomarkers of risk for VTE,
which is still scarce in Asian cancer patients, and we collected
longitudinal data.
In conclusion, we showed that having pancreatic
cancer, high SF, and Hb <100 g/l were significantly associated
with VTE occurrence among Japanese patients undergoing anticancer
drug therapy for cancer. Our study supports a conclusion that blood
biomarkers can improve RPM performance in Asian cancer patients. In
particular, our study suggests that SF could be a promising
predictive factor for VTE in cancer patients, and further
evaluation in other cohorts of Asians and other ethnic groups is
expected in the future. Also, other biomarkers reflecting
inflammation, such as CRP, may help predict thrombosis, and we
would like to consider them in further studies.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, NSA, AS, AN, SO, MM, ES and SK were responsible
for the conception and design of the study. YH, AS, AN and SO
collected the data. YH, AK and NSA analyzed the data. YH and NSA
drafted the manuscript. YH and NSA confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Institutional
Review Board of the Saga University Hospital (Saga, Japan), and all
patients provided written informed consent. This observational
study was registered at UMIN Clinical Trials Registry System, using
identifier 000026826.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VTE
|
venous thromboembolism
|
|
CAT
|
cancer-associated thrombosis
|
|
RPM
|
risk prediction models
|
|
WBC
|
white blood cell count
|
|
Plt
|
platelet
|
|
Hb
|
hemoglobin
|
|
BMI
|
body mass index
|
|
sP-selectin
|
soluble P-selectin
|
|
SF
|
soluble fibrin
|
|
tPA/PAI-1
|
tissue plasminogen
activator/plasminogen activator inhibitor type 1 antigens
complex
|
|
F 1 + 2
|
prothrombin fragment 1 + 2
|
|
FDP
|
fibrin/fibrinogen degradation
products
|
|
CATS
|
cancer and thrombosis study
|
|
PS
|
performance status
|
|
FEU
|
fibrinogen equivalent units
|
|
DDU
|
D-dimer units
|
|
ROC
|
receiver operating characteristic
|
|
PPV
|
positive predictive value
|
|
NPV
|
negative predictive value
|
|
OS
|
overall survival
|
References
|
1
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Thromboembolism is a leading cause of
death in cancer patients receiving outpatient chemotherapy. J
Thromb Haemost. 5:632–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heit JA, Silverstein MD, Mohr DN,
Petterson TM, O'Fallon WM and Melton LJ III: Risk factors for deep
vein thrombosis and pulmonary embolism: A population-based
case-control study. Arch Intern Med. 160:809–815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silverstein MD, Heit JA, Mohr DN,
Petterson TM, O'Fallon WM and Melton LJ III: Trends in the
incidence of deep vein thrombosis and pulmonary embolism: A 25-year
population-based study. Arch Intern Med. 158:585–593. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khorana AA, Kuderer NM, Culakova E, Lyman
GH and Francis CW: Development and validation of a predictive model
for chemotherapy-associated thrombosis. Blood. 111:4902–4907. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khorana AA and Connolly GC: Assessing risk
of venous thromboembolism in the patient with cancer. J Clin Oncol.
27:4839–4847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ay C, Vormittag R, Dunkler D, Simanek R,
Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C and
Pabinger I: D-dimer and prothrombin fragment 1 + 2 predict venous
thromboembolism in patients with cancer: Results from the Vienna
cancer and thrombosis study. J Clin Oncol. 27:4124–4129. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ay C, Dunkler D, Marosi C, Chiriac AL,
Vormittag R, Simanek R, Quehenberger P, Zielinski C and Pabinger I:
Prediction of venous thromboembolism in cancer patients. Blood.
116:5377–5382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Louzada ML, Carrier M, Lazo-Langner A, Dao
V, Kovacs MJ, Ramsay TO, Rodger MA, Zhang J, Lee AY, Meyer G and
Wells PS: Development of a clinical prediction rule for risk
stratification of recurrent venous thromboembolism in patients with
cancer-associated venous thromboembolism. Circulation. 126:448–454.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verso M, Agnelli G, Barni S, Gasparini G
and LaBianca R: A modified Khorana risk assessment score for venous
thromboembolism in cancer patients receiving chemotherapy: The
protecht score. Intern Emerg Med. 7:291–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muñoz Martín AJ, Ortega I, Font C, Pachón
V, Castellón V, Martínez-Marín V, Salgado M, Martínez E, Calzas J,
Rupérez A, et al: Multivariable clinical-genetic risk model for
predicting venous thromboembolic events in patients with cancer. Br
J Cancer. 118:1056–1061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pelzer U, Sinn M, Stieler J and Riess H:
Primary pharmacological prevention of thromboembolic events in
ambulatory patients with advanced pancreatic cancer treated with
chemotherapy? Dtsch Med Wochenschr. 138:2084–2088. 2013.(In
German). PubMed/NCBI
|
|
12
|
Song C, Shargall Y, Li H, Tian B, Chen S,
Miao J, Fu Y, You B and Hu B: Prevalence of venous thromboembolism
after lung surgery in China: A single-centre, prospective cohort
study involving patients undergoing lung resections without
perioperative venous thromboembolism prophylaxis†. Eur J
Cardiothorac Surg. 55:455–460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JO, Lee JY, Chun EJ, Choi SI, Kim JW,
Kim SH, Kim YJ, Lee KW, Kim JH, Lee JS and Bang SM: Incidence and
predictors of venous thromboembolism in medically ill hospitalized
elderly cancer patients: A prospective observational study. Support
Care Cancer. 27:2507–2515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitayama H, Kondo T, Sugiyama J, Kurimoto
K, Nishino Y, Hirayama M and Tsuji Y: Venous thromboembolism in
hospitalized patients receiving chemotherapy for malignancies at
Japanese community hospital: Prospective observational study. BMC
Cancer. 17:3512017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Frequency, risk factors, and trends for
venous thromboembolism among hospitalized cancer patients. Cancer.
110:2339–2346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh SY, Kim JH, Lee KW, Bang SM, Hwang JH,
Oh D and Lee JS: Venous thromboembolism in patients with pancreatic
adenocarcinoma: Lower incidence in Asian ethnicity. Thromb Res.
122:485–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiredu C, Haynes N, Guerra C and Ky B:
Racial and ethnic disparities in cancer-associated thrombosis.
Thromb Haemost. 122:662–665. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee LH, Gallus A, Jindal R, Wang C and Wu
CC: Incidence of venous thromboembolism in Asian populations: A
systematic review. Thromb Haemost. 117:2243–2260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashrani AA, Silverstein MD, Rooke TW, Lahr
BD, Petterson TM, Bailey KR, Melton LJ III and Heit JA: Impact of
venous thromboembolism, venous stasis syndrome, venous outflow
obstruction and venous valvular incompetence on quality of life and
activities of daily living: A nested case-control study. Vasc Med.
15:387–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reitter EM, Kaider A, Ay C, Quehenberger
P, Marosi C, Zielinski C and Pabinger I: Longitudinal analysis of
hemostasis biomarkers in cancer patients during antitumor
treatment. J Thromb Haemost. 14:294–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka S; Members of The Subcommittee for
Preparing Guidelines for Ultrasound Diagnosis of Venous Thrombosis
of Lower Extremities of The Terminology and Diagnostic Criteria
Committee, Japan Society of Ultrasonics in Medicine, . Nishigami K,
Taniguchi N, Matsuo H, Hirai T, Kaneda S, Ogasawara M, Satoh H and
Tobe H: Criteria for ultrasound diagnosis of deep venous thrombosis
of lower extremities. J Med Ultrason (2001). 35:33–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sørensen HT, Mellemkjaer L, Steffensen FH,
Olsen JH and Nielsen GL: The risk of a diagnosis of cancer after
primary deep venous thrombosis or pulmonary embolism. N Engl J Med.
338:1169–1173. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ay C, Simanek R, Vormittag R, Dunkler D,
Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C and
Pabinger I: High plasma levels of soluble P-selectin are predictive
of venous thromboembolism in cancer patients: Results from the
Vienna cancer and thrombosis study (CATS). Blood. 112:2703–2708.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dempfle CE, Zips S, Ergül H and Heene DL;
Fibrin Assay Comparative Trial study group, : The fibrin assay
comparison trial (FACT): Evaluation of 23 quantitative D-dimer
assays as basis for the development of D-dimer calibrators. FACT
study group. Thromb Haemost. 85:671–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Favaloro EJ and Thachil J: Reporting of
D-dimer data in COVID-19: Some confusion and potential for
misinformation. Clin Chem Lab Med. 58:1191–1199. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lutsey PL, Cushman M, Steffen LM, Green D,
Barr RG, Herrington D, Ouyang P and Folsom AR: Plasma hemostatic
factors and endothelial markers in four racial/ethnic groups: The
MESA study. J Thromb Haemost. 4:2629–2635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang L and Hu Y: Ethnic diversity in the
genetics of venous thromboembolism. Thromb Haemost. 114:901–909.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu YB, Gau JP, Liu CY, Yang MH, Chiang SC,
Hsu HC, Hong YC, Hsiao LT, Liu JH, Chiou TJ, et al: A nation-wide
analysis of venous thromboembolism in 497,180 cancer patients with
the development and validation of a risk-stratification scoring
system. Thromb Haemost. 108:225–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee CH, Lin LJ, Cheng CL, Kao Yang YH,
Chen JY and Tsai LM: Incidence and cumulative recurrence rates of
venous thromboembolism in the Taiwanese population. J Thromb
Haemost. 8:1515–1523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura M, Miyata T, Ozeki Y, Takayama M,
Komori K, Yamada N, Origasa H, Satokawa H, Maeda H, Tanabe N, et
al: Current venous thromboembolism management and outcomes in
Japan. Circ J. 78:708–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khorana AA, Francis CW, Culakova E and
Lyman GH: Risk factors for chemotherapy-associated venous
thromboembolism in a prospective observational study. Cancer.
104:2822–2829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ay C, Jungbauer LV, Sailer T, Tengler T,
Koder S, Kaider A, Panzer S, Quehenberger P, Pabinger I and
Mannhalter C: High concentrations of soluble P-selectin are
associated with risk of venous thromboembolism and the P-selectin
Thr715 variant. Clin Chem. 53:1235–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuji A, Wada H, Matsumoto T, Abe Y, Ota
S, Yamada N, Sugiyama T, Sudo A, Onishi K, Nakatani K, et al:
Elevated levels of soluble fibrin in patients with venous
thromboembolism. Int J Hematol. 88:448–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wada H, Kobayashi T, Abe Y, Hatada T,
Yamada N, Sudo A, Uchida A and Nobori T: Elevated levels of soluble
fibrin or D-dimer indicate high risk of thrombosis. J Thromb
Haemost. 4:1253–1258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dempfle CE, Wurst M, Smolinski M, Lorenz
S, Osika A, Olenik D, Fiedler F and Borggrefe M: Use of soluble
fibrin antigen instead of D-dimer as fibrin-related marker may
enhance the prognostic power of the ISTH overt DIC score. Thromb
Haemost. 91:812–818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aota T, Wada H, Yamashita Y, Matsumoto T,
Ohishi K, Suzuki K, Imai H, Usui M, Isaji S, Asakura H, et al: An
evaluation of the modified diagnostic criteria for DIC established
by the Japanese society of thrombosis and hemostasis. Clin Appl
Thromb Hemost. 23:579–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yukizawa Y, Inaba Y, Watanabe S, Yajima S,
Kobayashi N, Ishida T, Iwamoto N, Choe H and Saito T: Association
between venous thromboembolism and plasma levels of both soluble
fibrin and plasminogen-activator inhibitor 1 in 170 patients
undergoing total hip arthroplasty. Acta Orthop. 83:14–21. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yukizawa Y, Inaba Y, Kobayashi N, Ike H,
Kubota S and Saito T: Selective pharmacological prophylaxis based
on individual risk assessment using plasma levels of soluble fibrin
and plasminogen-activator inhibitor-1 following total hip
arthroplasty. Mod Rheumatol. 24:835–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ay C, Pabinger I and Cohen AT:
Cancer-associated venous thromboembolism: Burden, mechanisms, and
management. Thromb Haemost. 117:219–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bockenstedt P: D-dimer in venous
thromboembolism. N Engl J Med. 349:1203–1204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khorana AA, Carrier M, Garcia DA and Lee
AYY: Guidance for the prevention and treatment of cancer-associated
venous thromboembolism. J Thromb Thrombolysis. 41:81–91. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carrier M, Abou-Nassar K, Mallick R,
Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D,
Spadafora S, Marquis K, et al: Apixaban to prevent venous
thromboembolism in patients with cancer. N Engl J Med. 380:711–719.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj
S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, et
al: Rivaroxaban for thromboprophylaxis in high-risk ambulatory
patients with cancer. N Engl J Med. 380:720–728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Prandoni P, Lensing AW, Piccioli A,
Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins
MH, Noventa F and Girolami A: Recurrent venous thromboembolism and
bleeding complications during anticoagulant treatment in patients
with cancer and venous thrombosis. Blood. 100:3484–3488. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mansfield A, Tafur AJ, Wang CE, Kourelis
TV, Wysokinska EM and Yang P: Predictors of active cancer
thromboembolic outcomes: Validation of the Khorana score among
patients with lung cancer. J Thromb Haemost. 14:1773–1778. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vathiotis I, Dimakakos EP, Boura P,
Ntineri A, Charpidou A, Gerotziafas G and Syrigos K: Khorana score:
Νew predictor of early mortality in patients with lung
adenocarcinoma. Clin Appl Thromb Hemost. 24:1347–1351. 2018.
View Article : Google Scholar : PubMed/NCBI
|