Introduction
Magnetic Resonance Imaging (MRI) has become a common
diagnostic method in hospitals, and the intensity of MRI is 1.5–3 T
(1). For 1.5 T MRI, which is
applied in most hospitals, the maximum exposure of patients and MRI
operators is ~1.2 T. Most of the time, patients are exposed to
150–200 mT static magnetic field (SMF), or less (2). For diagnostic accuracy, 7 T MRI has
applied in clinic (3). Due to the
increasing demand in clinical diagnostics, 9.4 T MRI in healthy
human volunteers has also been applied successfully (4–6). The
application of MRI is mainly for patients in the clinic, and the
limits of MRI are constantly being explored for patients with
severe conditions. Regarding high magnetic field strength, 21.1 T
MRI has provided high resolution brain images in normal and
tumor-bearing rats (7,8). The application of high or ultra-high
MRI not only depends on the magnetic field and imaging technology,
but also on the biosafety, since this is the main concern of the
public regarding the development of magnetic technologies (9–15).
With the development of magnetic biology, several
studies have shown that novel of moderate or ultra-high SMF had
antitumor potential. For example, Tian et al (16) revealed that 0.2–1 T SMFs long-term
exposure inhibits the growth of gastrointestinal stromal tumor T1
in mice, and our previous study indicated that upward 9.4 T SMF
exposure can inhibit lung cancer growth by 44.7% (12). These studies suggest that MRI may be
used in a wide variety of clinical tumor treatments and adjuvant
therapies in the future. On the other hand, previous studies on
strong magnetic fields in mice have all demonstrated good biosafety
effect. For example, Wang et al (17) found that 4 weeks of exposure to
2.0–12.0 T SMF exerts no obvious damage on healthy mice, and Tian
et al (15) reported that 33
T exposure for 1 h does not have detrimental effects on normal
adult mice. Notably, Lv et al (18) found that 7–33 T SMF has potential as
an anti-depressant and previous studies have reported that magnetic
fields can reduce the side effects of chemotherapy drugs; for
example, Tian at al (19)
found that 9.4 T static magnetic field ameliorates imatinib
mesylate-induced toxicity in mice and Yu et al (20) found that SMFs protect against
cisplatin-induced kidney toxicity.
According to the World Health Organization, 17.5
million individuals are expected to succumb to cancer by 2050
globally (21). The current
treatments for cancer in the clinic include surgery, chemotherapy
and radiotherapy, but the effect of chemotherapy is limited due to
its high toxicity and price (22).
Platycodin D (PD) is one of the major bioactive components of the
Traditional Chinese Medicinal herb Platycodon grandiflorum,
and previous toxicological studies have shown that a single oral
dose of PD <2,000 mg/kg can be safely administered without
causing treatment-related abnormal signs in mice (23–25).
PD is a potential anticancer compound, an in vitro study has
demonstrated that PD inhibits the proliferation of cancer cells via
several mechanisms (26). PD also
has antitumor ability in vivo, as reported by Chun et
al (27), who found that PD
inhibits the growth of MDA-MB-231 ×enograft tumors in BALB/c nude
mice. Moreover, Zhou et al (28) found that 1 mg/kg PD reduces PC3
tumor size, although not significantly, while intraperitoneal
administration of 2.5 mg/kg PD markedly inhibits tumor growth
[tumor growth inhibition (TGI)=56%]. Due to its high safety and
antitumor effect, PD has been investigated in combination with
chemotherapy drugs for the treatment of cancer (29,30).
Therefore, the present study evaluated antitumor
effect of PD on A549 ×enograft tumor-bearing mice in an ultra-high
or moderate intensity SMFs environment, and compared the potential
antitumor efficacy and biosafety of PD with or without SMFs. The
present findings provides data to support preclinical studies of
ultra-high MRI, and suggest the use of PD as a potential
alternative drug alone or combination with physical therapy for the
treatment of patients with lung cancer.
Materials and methods
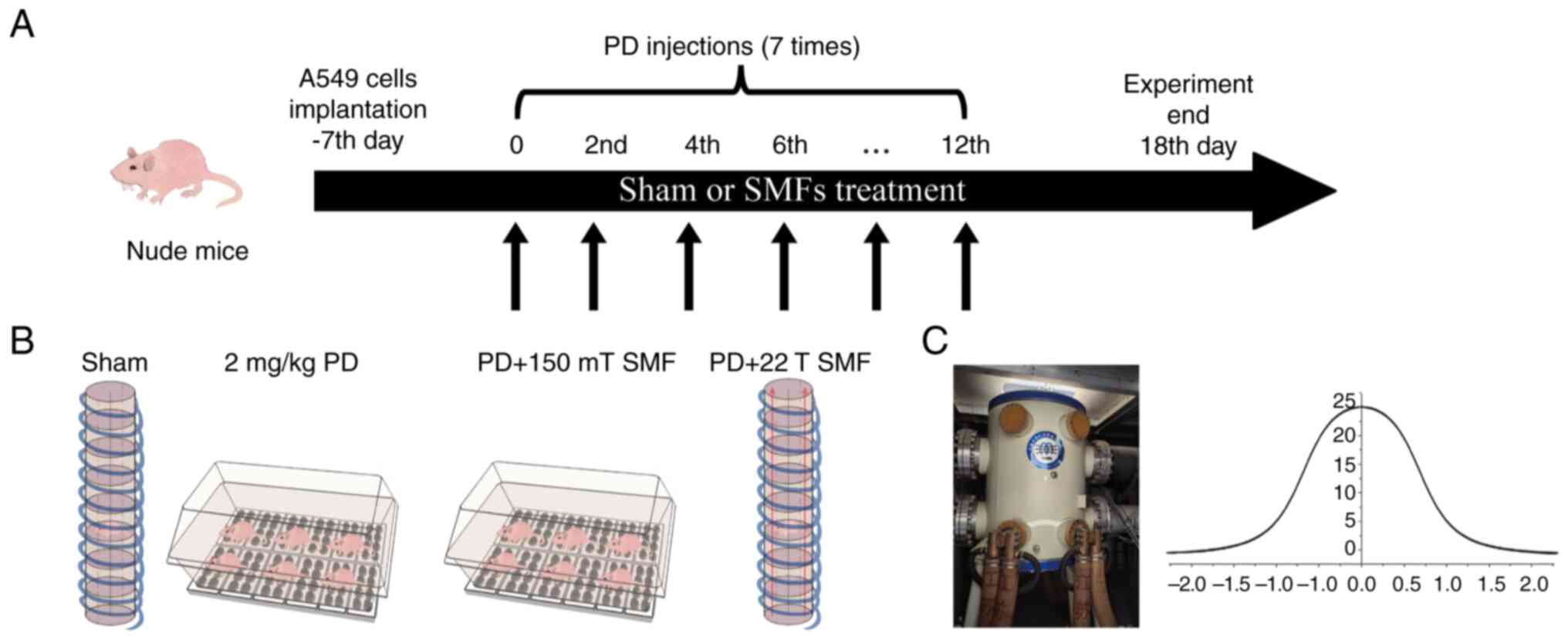
Magnetic fields exposure for mice
The magnetic field strength of MRI used in most
hospitals at present is 1.5 T, the maximum exposure of patients and
MRI operators is ~1.2 T, but the exposure time is very short. Most
of the time, they are exposed to 150–200 mT SMF, or even less
(2). Therefore, the present study
selected 150 mT SMF for the experiment and the exposure system is
the same as our previous reports (31). Briefly, the magnetic plate used in
mice experiment was composed of 10 small magnetic plates, each
small magnetic plate contained eight north polar magnets, which
strength were 500 mT, and formed the upward 150 mT magnetic plate
to the surface of mice. Subsequently, 22 T SMF was generated by a
water-cooled magnet (WM2) in Chinese High Magnetic Field Laboratory
(Hefei, China), and the parameters are the same as in our previous
study (10). Since the intensity is
22 T only at the center of the magnet, the others are gradient
(Fig. 1), the inner diameter of the
device is only 41 mm and only one mouse was exposed by 22 T SMF at
one time. The water-cooled magnet only can run for 4 h at a time,
so the present study treated each marked mouse in 22 T groups for 4
h. During the experiment, pre-cooled air was continuously pumped
into the magnet, thus ensuring that the mice had sufficient oxygen
and suitable temperature. The sham group had the same temperature
and humidity, but the device of the sham had no magnetic field. At
1 week after 549 cells were subcutaneously injected, mice were
exposed to 22 T SMF at day 2 and continuous exposure for 6
days.
Xenograft tumor model in nude
mice
A total of 24 25-day-old (~16 g) male SPF BALB/c
(Nu/Nu) mice were purchased from Gempharmatech (Nanjing, China).
The mice were housed in an air-conditioned room maintained at
22–24°C and 50–60% humidity with a 12 h of light and dark cycle,
with access to water and food ad libitum and housed in
plastic cages. All protocols were approved by the Ethics and Humane
Committee of Hefei Institute of Physical Sciences, Chinese Academy
of Sciences (approval no. DWLL-2019-25), and strictly followed the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publication no. 8023, revised 1978). Mice
were randomly divided into four groups with six mice in each group.
To adapt the magnetic field environment, 150 mT and 22 T SMFs mice
were maintained in the 150 mT magnetic environment for 2 weeks.
Then, 2 weeks later, 5×105 A549 cells were
subcutaneously injected into the right upper flank of sham, PD and
SMF treated mice.
In vivo chemotherapy
The dose of PD and the solution were prepared
according to a previous report (28). Briefly, PD was dissolved in saline
and prepared to 2.0 mg/kg solution. At 1 week after injection of
A549 cells, 2.0 mg/kg PD was injected into mice at 1-day intervals
for 2 weeks, and sham group received saline only. Water and food
intake, body weight and tumor volume of mice were recorded every
two days. When the animals had a low body weight (<20% of the
initial body weight), poor condition (mice do not eat or drink for
a long period of time, mental depression (elevated plus-maze test
and open field test), mice are in a stationary state for a long
period of time), as well as when the volume of a single tumor in
the mice exceeded 1,500 cm3, sacrifice of the mice was
performed using carbon dioxide. On day 18, all mice were sacrificed
by CO2 inhalation.
Behavioral tests
The present study mainly tested the behaviors
related to mouse emotion and vital activity, including open field,
elevated plus maze test and non-invasive pulse oximetry test,
considering the impact of environment on the behavior of mice, all
behavioral tests were performed from 9:00 am to 5:00 pm.
Elevated plus-maze test
The elevated plus maze test which can evaluate the
anxiety-like behavior of mice. Firstly, the device was wiped with
75% alcohol, then mice were placed in the center where the
intersection of two horizontally closed arms and two vertically
open arms were crossed. The mouse was allowed to explore for 5 min
freely, and the entries and time in the open arms were recorded by
ANY-Maze video tracking system (version 4.72; Stoelting).
Open field test (OFT)
The OFT can evaluate the locomotion and exploration
abilities of mice. On the board, the nine spaces in the middle were
defined as the central area, and the others were defined as the
peripheral area. The mice were placed in the center area and
allowed to explore for 6 min freely. The distance, entries and time
of mice spend in central and around area were recorded and analyzed
by ANY-Maze video tracking system automatically. The device was
cleaned with 75% ethanol after each test to avoid being affected by
previous mice.
Non-invasive pulse oximetry test
The non-invasive pulse oximetry test can evaluate
the physical condition of mice. In order for the experiment to be
carried out successfully, the mice need to be acclimated to the
test container and collar for 10 min before the test. In the
experiment, each awake mouse with the recording collar was placed
in the test container and allowed to move for 6 min freely. During
the test, the MouseOx system (Starr Life Sciences Corp.) recorded
the heart rate, breathing rate, arterial O2 and pulse
distension. The device was cleaned with 75% ethanol after each test
to avoid being affected by previous mice.
Complete blood count and blood
biochemistry test
The mice were anesthetized using 1% pentobarbital
sodium anesthesia, then blood samples were collected through the
orbital venous plexus before mice were sacrificed by 100%
CO2, 30% volume displacement rate per min according to
the 2013 edition of the AVMA Guidelines for the Euthanasia of
Animals (32). A total of 200 µl
blood was collected and placed in the 1.5 ml EP tube with or
without 1.5% EDTA-K2, and the serum was collected using 4,000 × g
centrifugation after blood was placed at 4°C centrifuge for 20 min.
The blood samples were sent to Wuhan Servicebio Technology Co.,
Ltd. in dry ice for blood biochemistry tests immediately. Blood
biochemistry mainly detected the level of serum creatinine (CREA),
urea (UREA), uric acid (UA), alanine aminotransferase (ALT),
aspartic transaminase (AST), triglyceride (TG), cholesterol (CHO),
high-density lipoprotein cholesterol (HDL-c) and low-density
lipoprotein cholesterol (LDL-c).
Hematoxylin and eosin (H&E)
staining
Heart, liver, spleen, lung, kidney and tumor tissues
were fixed in 4% paraformaldehyde (Wuhan Servicebio Technology Co.,
Ltd.) at room temperature for 24 h after mice were sacrificed.
Tissue was put into an embedding box for paraffin embedding and
sectioning (5-µm); tissue was treated using Xylene I and Xylene II
at room temperature for 15 min, rehydrated by 100–50% ethanol for
5–10 min. The tissue sections were put into hematoxylin staining
solution for 5 min at room temperature, rinsed gently with running
water, and then washed with 1% HCl and PBS buffer, then put the
tissue sections into Eosin staining solution for 5 min at room
temperature. The tissue sections were sequentially dehydrated in
50–100% ethanol, and then subjected to xylene I and xylene II,
dried at 65°C, and sealed with neutral gum. Microscopic images were
taken using a Nikon Eclipse E600 light microscope equipped with a
Nikon Digital Sight DS-U1 unit (Nikon Corporation).
RNA extraction and RNA-sequencing
(RNA-seq)
Total RNA was isolated using the TRIzol™ LS reagent
(cat. No. 10296010; Invitrogen™; Thermo Fisher Scientific, Inc.),
after which the concentration, quality and integrity were
determined using a NanoDrop spectrophotometer (Thermo Scientific).
A total of 3 µg RNA were used as input material for the RNA sample
preparations. The library was constructed with the NEBNext Ultra
RNA Library Prep Kit (New England BioLabs, Inc.), following the
manufacturer's recommendations. Sequencing libraries were generated
according to the following steps. Firstly, mRNA was purified from
total RNA using poly-T oligo-attached magnetic beads. Fragmentation
was carried out using divalent cations under elevated temperature
in an Illumina proprietary fragmentation buffer. First strand cDNA
was synthesized using random oligonucleotides and Super Script II.
Second strand cDNA synthesis was subsequently performed using DNA
Polymerase I and RNase H. Remaining overhangs were converted into
blunt ends via exonuclease/polymerase activities and the enzymes
were removed. After adenylation of the 3′ ends of the DNA
fragments, Illumina PE adapter oligonucleotides were ligated to
prepare for hybridization. To select cDNA fragments of the
preferred 400–500 bp paired end in length, the library fragments
were purified using the AMPure XP system (Beckman Coulter, Inc.).
DNA fragments with ligated adaptor molecules on both ends were
selectively enriched using Illumina PCR Primer Cocktail in a 15
cycle PCR reaction. Products were purified (AMPure XP system) using
the Agilent high sensitivity DNA assay on a Bioanalyzer 2100 system
(Agilent Technologies, Inc.) Agilent High Sensitivity DNA kit (cat.
no. 5067-4626; Agilent Technologies, Inc.). Total library
concentration was measured using Pico green (cat. no. E6090,
Quantifluor-ST fluorometer, Promega Corporation; Quant-iT PicoGreen
dsDNA Assay kit, cat. no. P7589, Invitrogen, Thermo Fisher
Scientific, Inc.). QPCR quantitative detection of effective library
concentration (StepOnePlus Real Time PCR Systems; Thermo Fisher
Scientific, Inc.). The sequencing library was then sequenced with
the concentration of 150 pm on NovaSeq 6000 platform (Illumina,
Inc.) by Shanghai Personal Biotechnology Co., Ltd. using NovaSeq
6000 S4 Reagent Kit v1.5 (cat. no. 20028312; 300 cycles; Illumina,
Inc.). The sequencing data were submitted and deposited at the
National Center for Biotechnology Information (NCBI) Sequence Read
Archive (accession no. PRJNA991961). Metabolic pathway analysis of
the assembled unigenes was performed according to the KEGG
(http://www.genome.jp/kegg/) database and
GO database (http://www.geneontology.org/) using BLASTX.
Statistical analysis
There were 6 mice in each group, and all data were
expressed as mean ± SEM and analyzed using Shapiro-Wilk test, and
they all conformed to normal distribution. If the data were
distributed normally, one-way ANOVA was used to evaluate the
difference between groups using GraphPad Prism 9.4.1 (GraphPad
Software, Inc.). Otherwise, the Mann-Whitney U test was used.
P<0.05 was considered to indicate a statistically significant
difference. For the accuracy of the experimental results, the
analysis was performed in a blind way and the person who analyzed
the data did not know the exposure conditions of the mice.
Results
Moderate and ultra-high SMFs enhance
the antitumor efficacy of PD against lung cancer
The present study used the center of a WM2
water-cooled magnet which can generate ~22 T SMF. As previously
reported (10), the current study
used a separate device of identical mice exposure system as the
sham which has the same gas and temperature as 22 T SMF (Fig. 1). A549 cells were injected into mice
to construct mouse lung cancer-bearing model. The mice were treated
separately and subjected to a series of tests before they were
sacrificed.
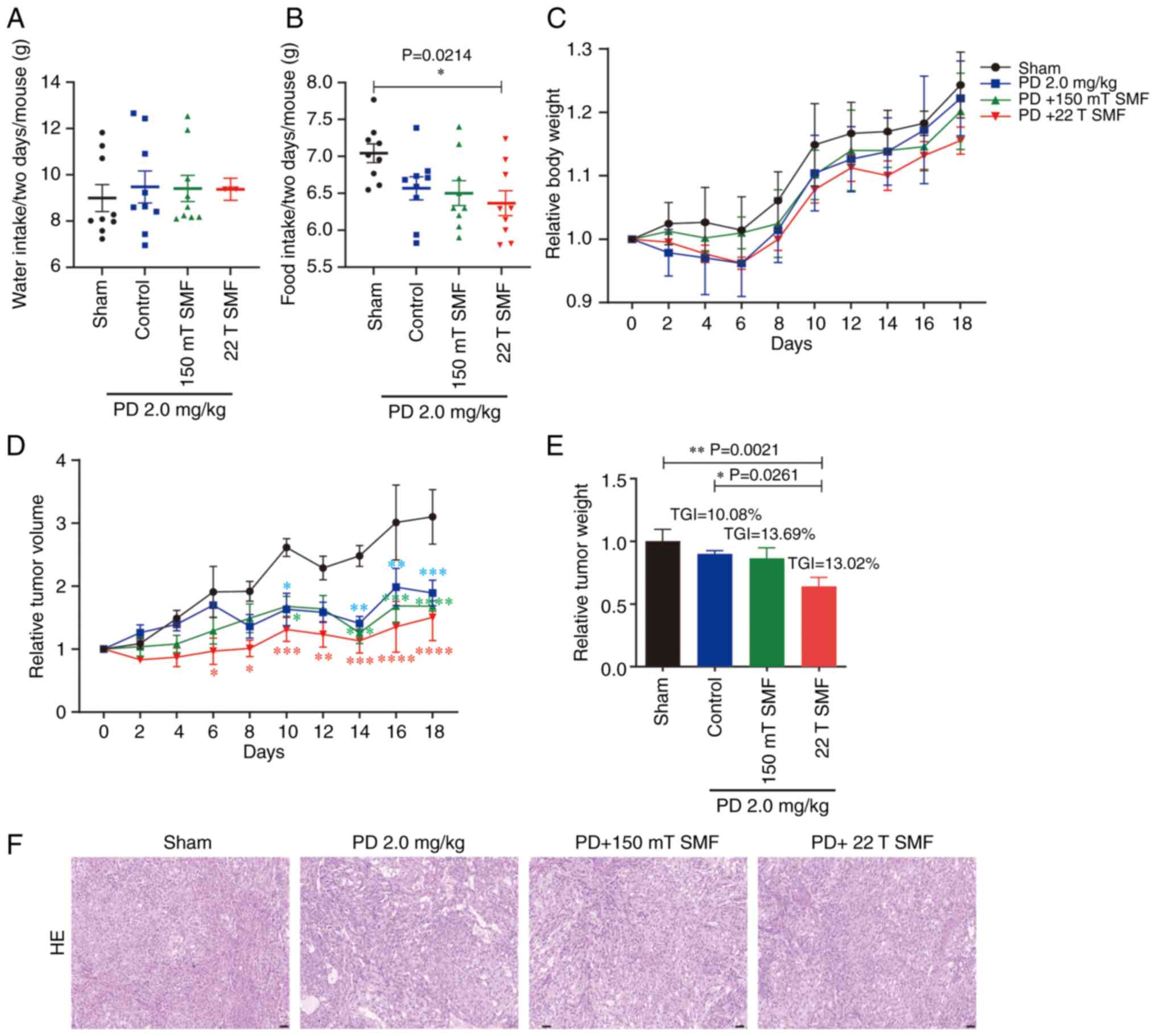
To evaluate the health status of the mice, their
body weight and food and water consumptions were measured every 2
days. The data showed that the water intake of the sham group was
lower compared with that of other groups, albeit this difference
was not statistically significant (Fig.
2A). By contrast, a significant reduction in food consumption
was observed in the PD treatment groups compared with that of the
sham group (7.0430±0.1274 g), while there was no obvious difference
between the upward 22 T SMF (6.3660±0.1696 g) and PD (6.568±0.1560
g) groups (Fig. 2B). Lower food
consumption directly led to a lower body weight, and it was found
that the weights of the PD-treated groups were all lower compared
with that of the sham group, although the results were not
statistically significant (Fig.
2C). Compared with the sham group, the 2 mg/kg PD group not
only reduced the size of the tumor (Fig. 2D), but also reduced tumor mass;
moreover, the combined antitumor effect of 150 mT SMF and 2 mg/kg
PD was 1.36-fold higher compared with that of the 2 mg/kg PD alone
(P>0.05); however, 22 T SMF had the most effective antitumor
effect, namely, 3.6-fold higher compared with that of the 2 mg/kg
PD group (P>0.05; Fig. 2E).
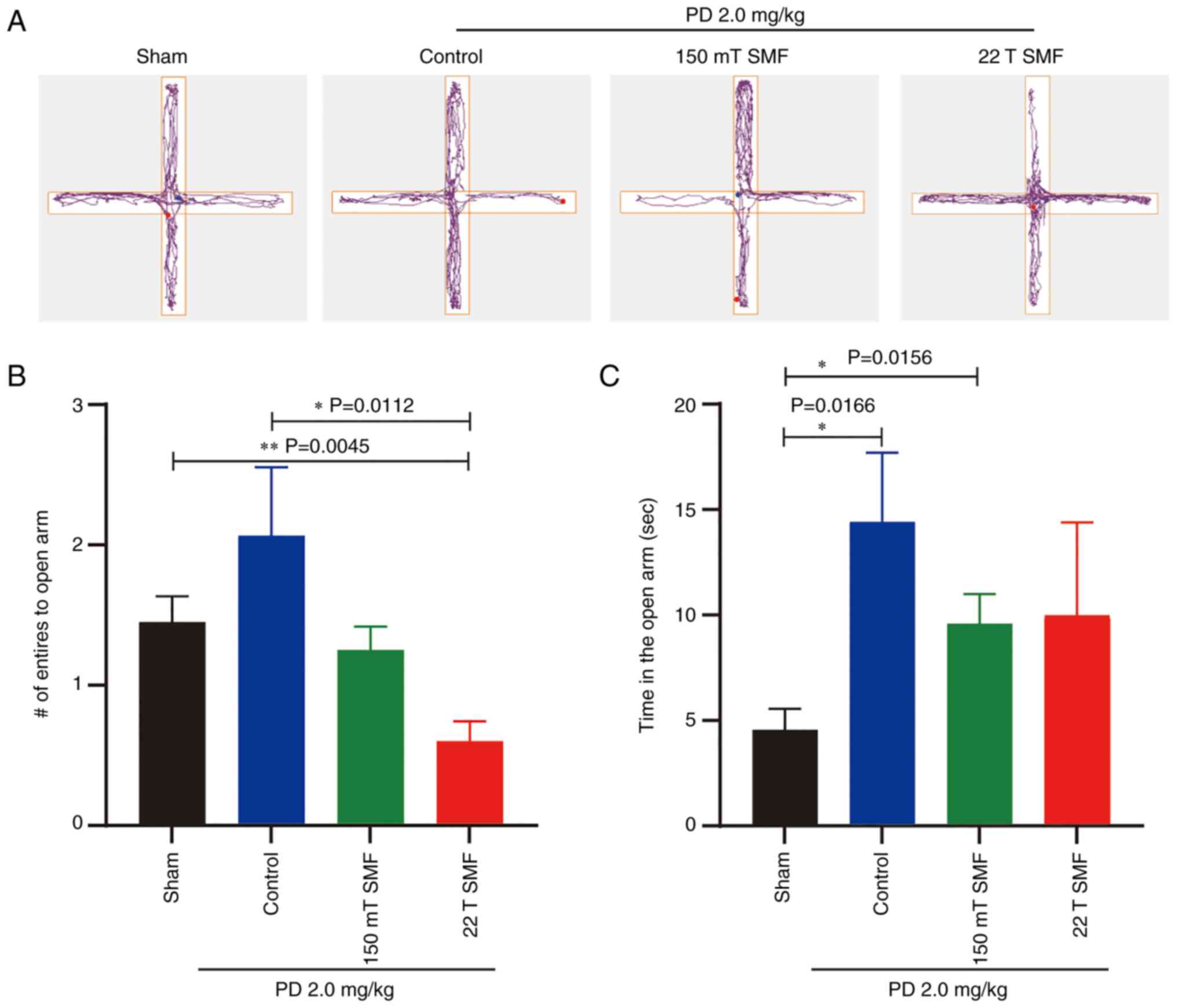
PD ameliorates anxiety-like emotion in
tumor-bearing mice, while SMFs reduces the ameliorative effect
To fully assess disease status, as well as the
efficacy and side effects of PD combined with SMFs, the elevated
plus maze and OFTs were performed. Firstly, the elevated plus maze
test, which reflects anxiety in mice, was performed (Fig. 3A). In this experiment, mice tended
to stay within the two closed arms and move to the open arms
occasionally; thus, the number of entries into the open arms and
the duration of the stay in the open arms are indicators of
anti-anxiety levels. The results showed that 2.0 mg/kg PD increased
the number of entries to the open arm (2.0670±0.4863; P>0.05)
compared with the sham group (1.4500±0.1839), although the results
had no statistical significance (Fig.
3B). Additionally, the time of that tumor-bearing mice spent in
the open arm increased from 4.558±1.001 to 14.400±13.281 (P=0.0166)
sec after 2 weeks of treatment with 2.0 mg/kg PD (Fig. 3C). However, this ameliorative effect
was reduced by exposure to 150 mT or 22 T SMFs. It was found that
the number of times that mice entered the open arm was reduced from
2.0670±0.4863 to 0.600±0.1438 (P=0.0112), and the time spent in the
open arm was reduced from 14.400±13.281 to 9.968±4.415 sec
(P>0.05) after 22 T SMF exposure (Fig. 3B and C).
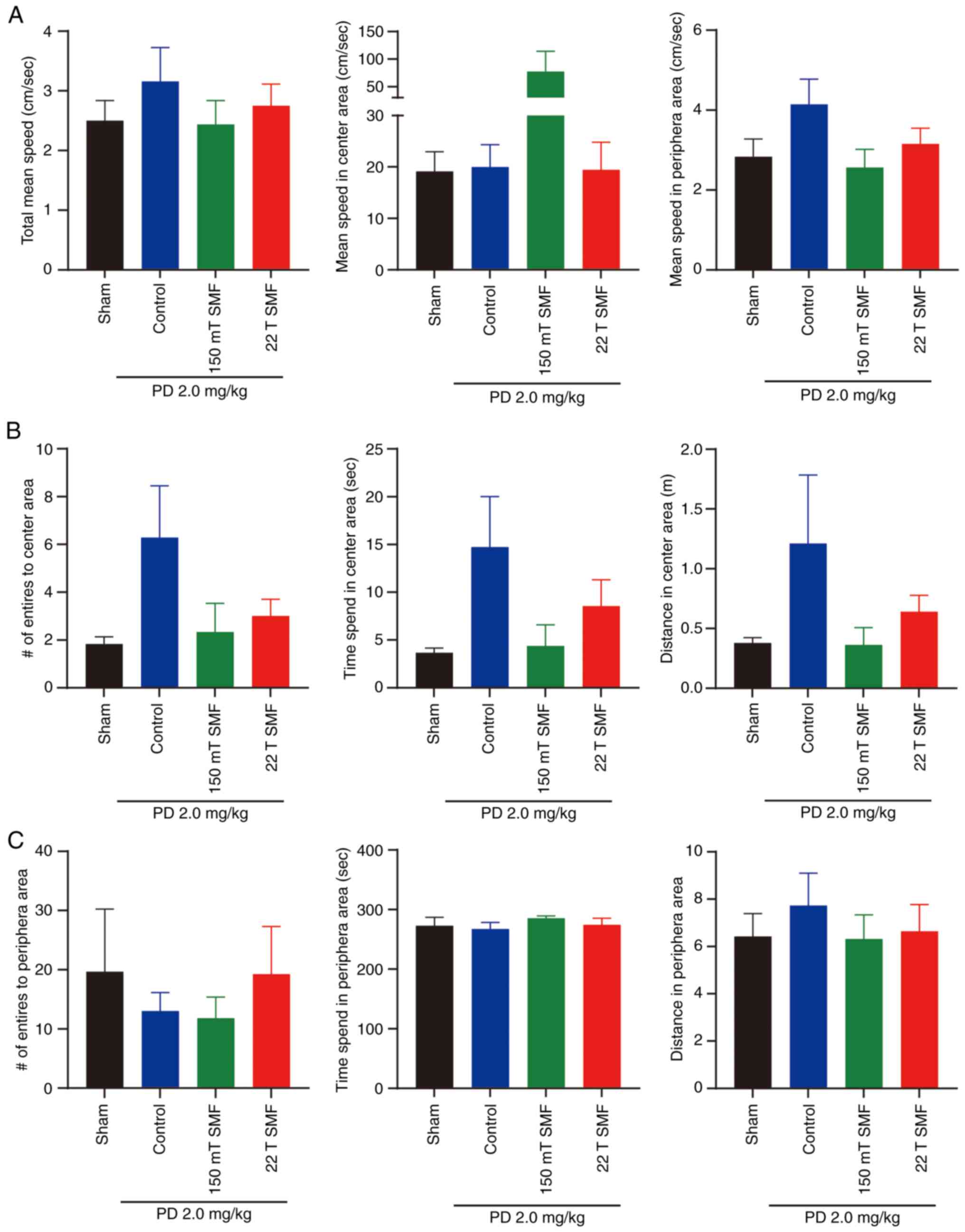
Besides the elevated plus maze test, the OFT also
can reflect the anxiety and depression of mice. In the OFT, there
was no significant difference in mean speed between the four groups
(Fig. 4A), and the number of
entries and time spent in the center area reflected the anxiety of
mice, since mice are likely to stay in the peripheral area when
they are depressed. The present data showed that the number of
entries into the center area increased from 1.8330±0.3073 to
6.286±2.168 (P>0.05), and the time spent in the center area
increased from 3.683±0.4750 to 14.730±5.282 sec (P>0.05) after
2.0 mg/kg PD treatment, although there was no statistical
significance. Of note, the indicators in the peripheral area had no
obvious change. Consistent with the results of the elevated plus
maze test, in the 150 mT and 22 T SMF exposure groups, the entries
and time spent in the center area were all reduced (Fig. 4B and C). Besides emotions, OFT also
can reflect the locomotive activity of mice. In the present study,
PD, 150 mT or 22 T SMFs exposure had no significant effect on mouse
locomotive activity (Figs. 4 and
S1).
To further examine the physical condition of the
tumor-bearing mice, the present study also measured their heart
rate, breathing rate, arterial O2 saturation and pulse
distension. In the 2 mg/kg PD group, which exhibited an increased
heart (P=0.0003) and breathing rate (P>0.05), 150 mT and 22 T
SMFs exposure could reduce the PD-induced increase in heart and
breathing rates (Fig. S2A and B).
Compared with those of the sham group, there were no obvious
difference in arterial O2 saturation or pulse distension
in the PD, 150 mT or 22 T SMFs exposure groups (Fig. S2C and D).
Upward of 22 T SMF improves the
physiological status of PD treated tumor-bearing mice
Terminal stage of lung cancer is often accompanied
by infections which lead to a high level of white blood cells
(WBC), and patients with high WBC have poorer prognosis and lower
survival rates; thus, reducing the number of WBCs is crucial in
lung cancer treatment (33). The
present study found that the level of WBCs was increased after PD
treatment (7.7600±0.3957×109/l) which was higher
compared with the normal range (0.8–6.8×109/l). However,
after 150 mT (P=0.0005) and 22 T (P=0.0008) SMFs treatment, the
level of WBC was reduced by 50%, which was within the normal range
(Fig. 5A). Additionally, the
present results showed that 22 T SMF
(4.9220±0.6488×1012/l) could increase the low level of
red blood cells (RBC) associated with sham
(2.9470±0.3681×1012/l; P=0.0363) or PD therapy
(2.8200±0.5737×1012/l; P=0.0331) (Fig. 5B). 22 T SMF exposure not only
increased the hematocrit (HCT) of tumor-bearing mice to the normal
range (Fig. 5C), but also
maintained the other indicators within their normal range (Fig. 5D-F).
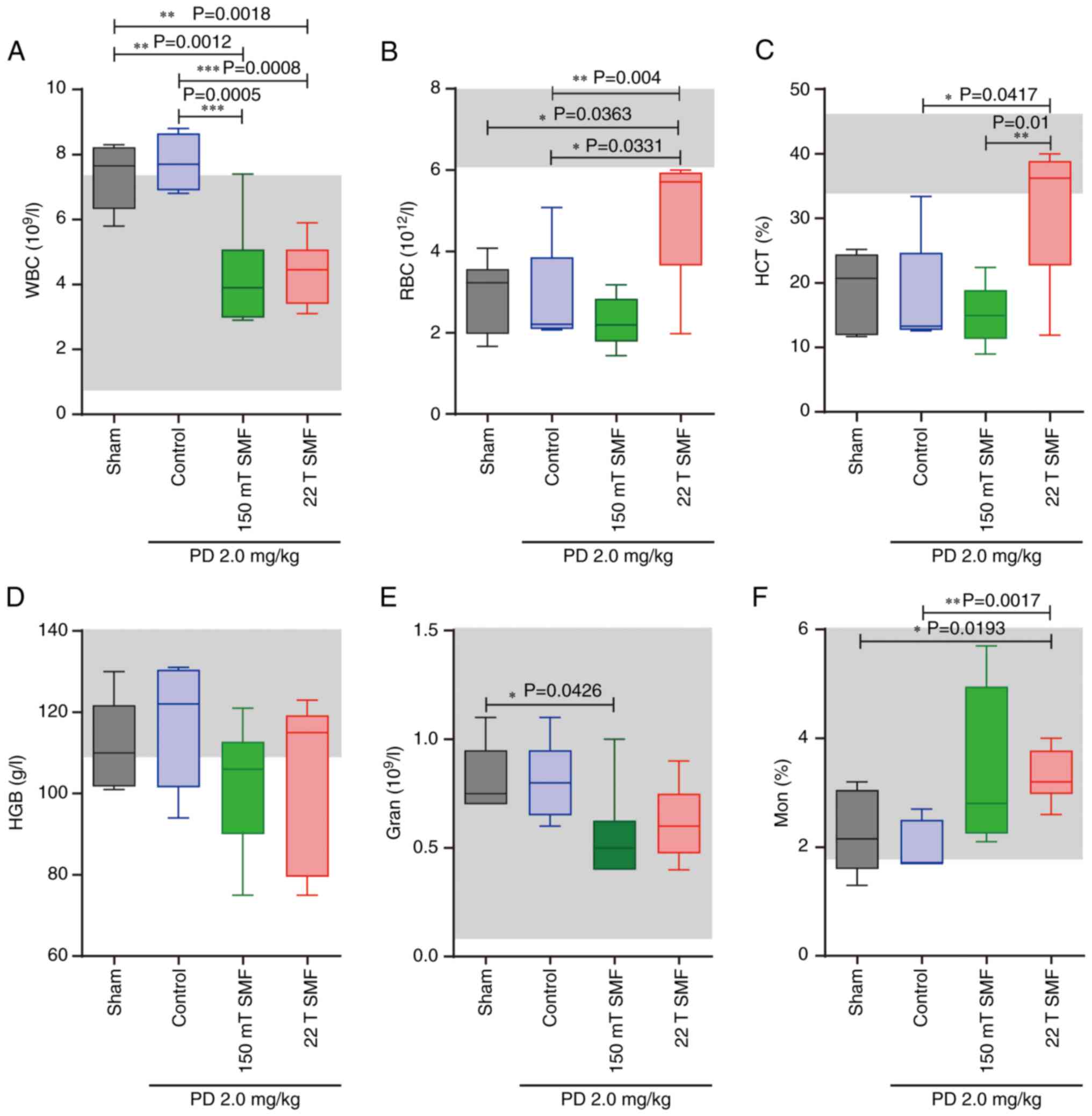 | Figure 5.Complete blood count of mice in each
group. After mice were sacrificed, complete blood count was
measured, including (A) WBC, (B) RBC, (C) HCT, (D) HGB, (E) Gran
and (F) percentage of mon. (n=6 mice/group). *P<0.05,
**P<0.01 and ***P<0.001. WBC, white blood cell; RBC, red
blood cell; HCT, hematocrit; HGB, hemoglobin; gran, granulocytes;
mon, monocytes; PD, Platycodin D; SMF, static magnetic field. |
Moderate or ultra-high SMF does not
cause side effects during PD against lung cancer
To confirm the biosafety, the current study measured
the organ index which is the ratio of organ weight to animal body
weight. This study measured the organ index of heart, kidney, lung,
liver and spleen and found that combined treatment of magnetic
fields and PD did not cause significant damage to major organs in
mice (Fig. 6A and C-E), with the
exception of the kidneys. Notably, the data showed that 2 mg/kg PD
treatment reduced the renal index significantly (P=0.0004), while
SMFs had no effect on this decrease (Fig. 6B). However, HE staining did not
reveal any clear damage to the liver in the PD or combined
treatment groups (Fig. 6F).
Furthermore, the serum of mice was subjected to blood biochemistry
for analysis of renal function, and it was revealed that there were
no significant difference in creatinine, urea nitrogen or uric acid
among any of the groups (Fig.
S3).
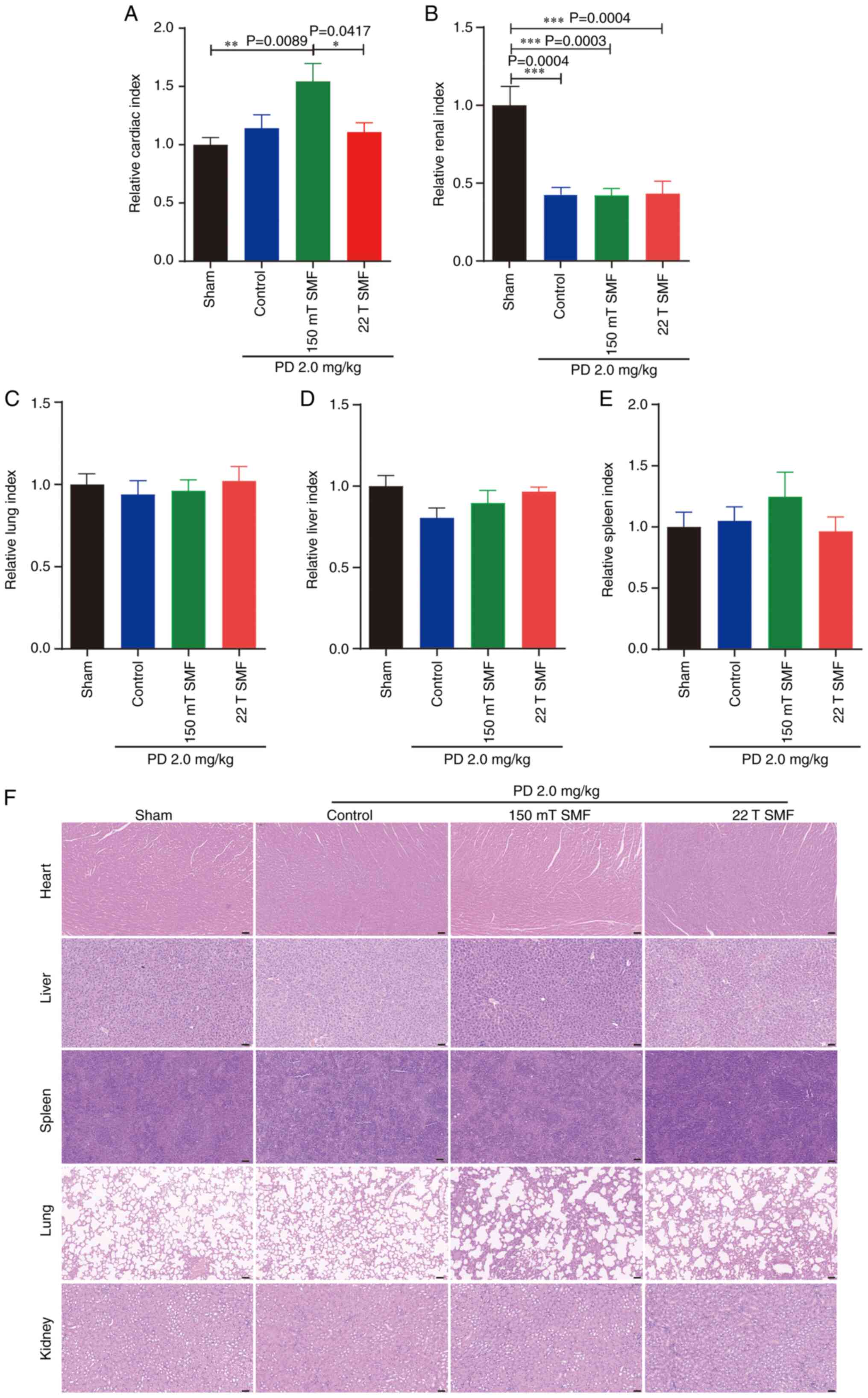 | Figure 6.Exposure to 22 T SMF did not cause
significant damage to major organs during PD therapy in
tumor-bearing mice. The (A) heart, (B) kidney, (C) lung, (D) liver
and (E) spleen weight index were analyzed. The ratio of organ
weight to the corresponding animal body weight is displayed. (F) HE
staining of heart, kidney, lung, liver and spleen. Scale bar, 50
µm. n=6. *P<0.05, **P<0.01 and ***P<0.001. PD, Platycodin
D; HE, hematoxylin and eosin; SMF, static magnetic field. |
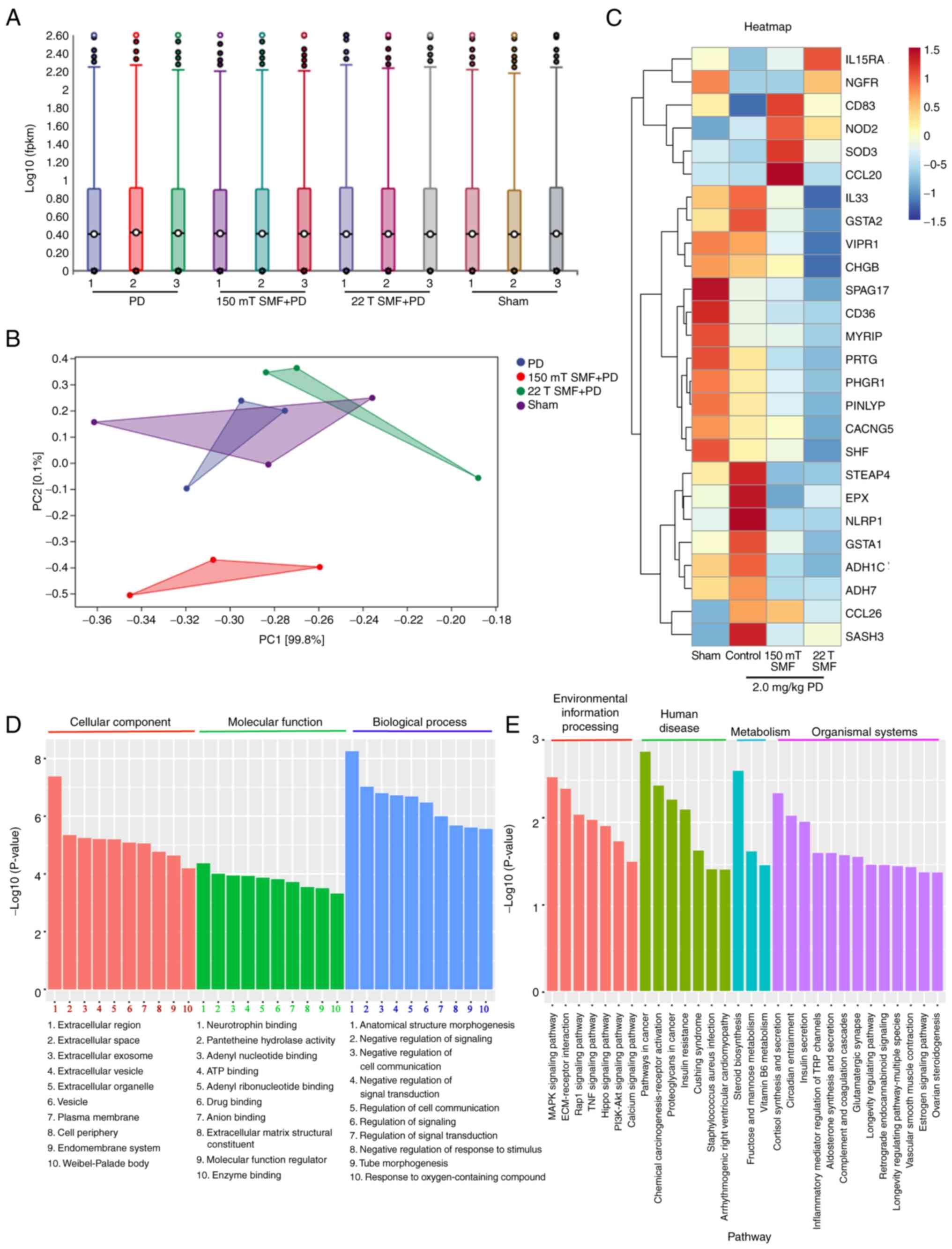
Moderate or ultra-high SMF and PD
together reduce the expression of related target genes
To investigate the mechanisms of the combined
biological effect of moderate or ultra-high magnetic fields, the
present study sequenced tumor tissues and obtained Gene Ontology
(GO) terms from tumor tissue transcriptome RNA-seq analysis
(Fig. 7). Notably, the results
showed that SMFs downregulated the expression of genes such as
vasoactive intestinal peptide receptor 1 (VIPR1), CD36, protogenin,
chromogranin B and calcium voltage-gated channel auxiliary subunit
γ 5. The gene heat map further confirmed that SMFs acted with PD on
multiple targets, including ‘tumor growth’, as well as
‘inflammatory’ and ‘psych-regulatory pathways’ (Fig. 7C).
Discussion
The safety of ultra-high SMFs was previously studied
on healthy mice, and no detrimental effects were reported (14,18).
However, the purpose of exploring high or ultra-high MRI is for
clinical application of patients; thus, it is logical to explore
the biosafety of pathological conditions. For example, Yu et
al (34) found that prolonged
treatment of gradient high SMFs (1.0–8.6 T) had negative effects on
mice with type 1 and type 2 diabetes. Our previous study also
showed that, as a non-invasive physical therapy, high SMFs have
antitumor effects (12), and
previous reports have shown that moderate and high SMFs can
ameliorate the adverse reactions and toxic side effects of
chemotherapy drugs (19,20). Compared with chemotherapy, as a
medicinal food, Platycodon grandiflorum has a high safety,
but it is only used as a complementary treatment due to the fact
that its antitumor effect is not as strong as chemotherapy. The
present study used physiological and pathological tests, and found
that short-term exposure to 150 mT moderate or 22 T ultra-high SMFs
could achieve a greater anti-lung cancer effect combined with 2
mg/kg PD compared with that of PD on its own, and the enhancement
effect was associated with the strength of SMF. This is not only
consistent with the previous report by Tian et al (19), who found that 9. 4 T SMF enhances
the antitumor efficacy of imatinib mesylate, but also provides a
novel therapeutic idea and theoretical base for the treatment of
lung cancer in the clinic. Since magnetic fields are non-invasive
and highly penetrating physical fields with tumor-killing effects,
it can be hypothesized that magnetic fields may be widely used in
the future as an adjuvant in the clinical treatment of numerous
diseases, including cancer.
In addition to the tumor inhibition effects, Tian
et al (19) found that 200 h
exposure to 9.4 T SMF can reduce the toxicity of low-dose imatinib
mesylate, and Yu et al (20)
found that 24 days (24 h/day) of exposure to moderate SMF can also
reduce cisplatin side effects in kidney. In addition, our previous
study showed that 150 mT exposure for 18 weeks continuously reduces
the anxiety-like emotion (31).
Compared with the sham group, the present behavioral experiments
showed that PD reduced the anxiety-like emotion in tumor-bearing
mice, but neither 150 mT or 22 T ultra-high SMFs ameliorated this
phenomenon, suggesting that SMFs disturbed this effect. This was
inconsistent with previous reports, which may be due to the
exposure time and intensity of SMF. Although the present study used
150 mT, the duration of SMF treatment was only 5 weeks, and
co-treatment with PD only lasted 3 weeks, thus the exposure time
was <18 months. The exposure time of 22 T SMF was even shorter
at only 4 h due to the limitations of the magnet itself. It can be
hypothesized that, if the SMFs exposure time was longer, the
results would be improved. Lv et al (18) found that 1 h exposure to 11.1–33.0 T
increases anxiety, and reduces locomotion and exploration in heathy
mice in the short time, but these phenomena are reversed after 2
weeks. The current study evaluated the behavior of tumor-bearing
mice at 1 week after 22 T SMF exposure, rather than immediately,
and used tumor-bearing rather than healthy mice; thus, the response
to SMFs may be different in a disease state with the physiological
state. It could be hypothesized that magnetic fields only not could
exert antitumor effects by reducing the dose of various antitumor
drugs, including PD, but could reduce the toxic side effects of a
large number of drugs, thus alleviating patient pain and prolonging
their survival in the future.
The terminal stage of lung cancer is often
accompanied by infection, which leads to a high level of WBCs, and
is associated with poorer prognosis and low survival rates; thus,
reducing the WBC number is crucial in lung cancer treatment
(33). Compared with the sham
group, PD treatment alone increased the WBC levels in tumor-bearing
mice, and the level of WBCs were reduced to the normal range by
combination with 150 mT or 22 T SMF. Besides WBC, numerous blood
count indicators returned to normal levels gradually after SMF
exposure, particularly 22 T SMF. All these changes were consistent
with a previous report by Tian et al (10), who also found that SMFs could bring
the levels of complete blood count indicators to their normal range
in healthy mice. Moreover, the organ index indicated that PD
treatment with or without SMFs had good biosafety in tumor-bearing
mice, with exception of renal index, which was reduced by PD with
or without SMFs. The kidneys are an essential organ for humans, and
are responsible for excretion. Thus, the present study performed
kidney H&E staining and blood biochemistry analysis; however,
no abnormalities were detected, which indicated that PD did not
affect the function but may affect the weight of the kidneys in
tumor-bearing mice. It has also been reported PD ameliorates
cisplatin-induced nephrotoxicity in mice (35). The present data showed that neither
150 mT moderate or 22 T ultra-high SMF caused no additional damage
to the mice, which indicated that ultra-high or moderate SMF
combined with 2 mg/kg PD had good biosafety against lung
cancer.
Magnetic fields could be combined with drugs to
inhibit tumor growth. For example, Luo et al (36) found that SMFs enhance the antitumor
effects of 5-FU and Taxol through inducing spindle abnormalities.
The present tumor tissue transcriptome RNA-seq demonstrated that
the anti-lung cancer effect and biosafety of SMFs and PD was due to
their targeted expression of multiple genes that regulate tumor
growth, inflammation and mental health, such as vasoactive
intestinal peptide receptor-1 (VIPR1) which plays an important role
on the growth of tumors and controls lung physiology. Zhao et
al (37) found that
overexpression of VIPR1 inhibits proliferation, migration and
invasion of H1299 cells, while another study reported that a VIPR1
antagonist can strengthen the ability of chemotherapy to kill
breast cancer cells (38). In the
present study, both PD and SMFs acted as an antagonist of VIPR1,
since they inhibited tumor growth. With the exception of VIPR1, a
scavenger receptor, CD36, which is involved in angiogenesis,
inflammatory and atherothrombotic diseases (39) was also downregulated by PD with or
without SMFs, and reduced the inflammatory response in
tumor-bearing mice. Additionally, RNA-seq indicated that PD and
SMFs targeted the CACNG5 gene. It has been reported that CACNG5 is
associated with schizophrenia, which is a complex psychiatric
disorder characterized by delusions, hallucinations, altered
cognition, emotional reactivity and disorganized behavior (40). Therefore, it can be suggested that
PD and SMFs may regulate the expression of the CACNG5 gene to
modulate the behavior of tumor-bearing mice.
Overall, the present results showed that the
combination of 2 mg/kg PD and 150 mT or 22 T SMF not only can
improve the antitumor effect of PD, but also have a good biosafety
on A549 tumor-bearing mice. These results suggest that SMFs may be
used in combination with traditional Chinese herbal medicines to
increase its antitumor effect and to improve the quality of life of
patients in clinic. The majority of hospitals use MRI with 1.5 T
intensity, and most of the time the patients are exposed to 150–200
mT SMF (2); thus, 1.5 T MRI is safe
for patients with lung cancer treated with PD. Although >20 T
SMFs are not currently applied in clinic and do not have public
acceptance at present, the current findings provide support for the
clinical application of >20 T SMFs in the future.
The current study has certain limitations. Firstly,
only one cell line, A549 was used to construct the lung cancer
model, as A549 cells have been widely used as an in vitro
model and their high success rate of tumor formation. Secondly,
since there is only one water-cooled WM2 magnet, the 150 mT or 22 T
SMF was not set up separately for consistency. Thirdly, the WM2
magnet is a uniform magnetic field with no gradient in the middle,
and the magnet cartridge only can hold 1 mouse; thus, the 6 mice in
the 22 T SMF and PD combined treatment group were not evaluated at
the same time. Moreover, since the WM2 magnet is water-cooled and
can only operate for 4 h at a time twice a day, these 6 mice were
not exposed to the SMF on the same day. The size of the tumor
varies considerably from day to day in the late stages of tumor
growth, which may lead to a large variability in the results.
Additionally, due to the short duration of study, behavioral assays
before and after the 22 T SMF treatment were not immediately
performed to avoid the mice becoming familiar with the environment
of the behavioral apparatus, which could affect the reliability of
the results. Fourthly, the PD used in this study was not extracted
by the present authors, and its purity may be high; thus, the PD
dose was downregulated according to the literature and a
concentration gradient of PD was not evaluated. If the PD used in
the current study had been extracted by the present authors, a
higher concentration (<2,000 mg/kg) could have been used and
improved results could have been obtained. Lastly, RNA-seq results
only showed genes that PD and SMFs may target together, and
validation of the genes, proteins and signaling pathways were
performed by RT-qPCR and western blotting. However, no conclusions
were obtained which may be due to individual differences in tissue
cells, and external factors such as magnetic field environment and
temperature changes. The regulatory mechanism of magnet biology is
complex; thus, further studies are required to fully clarify
it.
In conclusion, the present study demonstrated that
150 mT and 22 T SMFs not only improved the antitumor effect of 2
mg/kg PD, but also reduced PD-induced abnormalities of blood
parameters in mice with lung cancer. Although it appeared that SMFs
had a negative effect on PD-induced behavioral improvement in
tumor-bearing mice in the short term, the results was not
statistically significant and did not cause significant damage to
the mice. Overall, the combination of moderate or ultra-high SMFs
and PD for lung cancer has a good biosafety, which suggests that
SMFs have potential for application in clinical treatment and
diagnosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was funded by Anhui Provincial Natural Science
Research Project of Higher Education (grant nos. KJ2021A0925 and
KJ2020A0095), Anhui Provincial Natural Science Foundation (grant
no. 2008085MC105), Research Foundation for Talented Scholars of
Hefei Normal University (grant nos. 2020rcjj46 and 2022rcjj21) and
Hefei Institutes of Physical Science Director's Fund (grant no.
YZJJ2023QN43).
Availability of data and materials
The sequencing datasets generated and/or analyzed
during the current study are available in the NCBI BioProject
repository, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA991961/.
The other datasets used and/or analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
XXY and BY performed the experiments, and drafted
the manuscript. CYX operated the magnet. CS, CLF, XYW and GFC were
responsible for the methodology. RY, WW, XW and XHZ acquired and
analyzed the data. YSC and YZ conceived and designed the study. XXY
and BY confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Ethics and Humane
Committee of Hefei Institute of Physical Sciences, Chinese Academy
of Sciences (approval no. DWLL-2019-25), and strictly followed the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publication No. 8023, revised 1978).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sarracanie M, LaPierre CD, Salameh N,
Waddington DEJ, Witzel T and Rosen MS: Low-cost high-performance
MRI. Sci Rep. 5:151772015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karpowicz J and Gryz K: The pattern of
exposure to static magnetic field of nurses involved in activities
related to contrast administration into patients diagnosed in 1.5 T
MRI scanners. Electromagn Biol Med. 32:182–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoff MN, McKinney A IV, Shellock FG,
Rassner U, Gilk T, Watson RE Jr, Greenberg TD, Froelich J and Kanal
E: Safety considerations of 7-T MRI in clinical practice.
Radiology. 292:509–518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thulborn K, Lui E, Guntin J, Jamil S, Sun
Z, Claiborne TC and Atkinson IC: Quantitative sodium MRI of the
human brain at 9.4 T provides assessment of tissue sodium
concentration and cell volume fraction during normal aging. NMR
Biomed. 29:137–143. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruhm L, Avdievich N, Ziegs T, Nagel AM, De
Feyter HM, de Graaf RA and Henning A: Deuterium metabolic imaging
in the human brain at 9.4 Tesla with high spatial and temporal
resolution. Neuroimage. 244:1186392021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaughan T, DelaBarre L, Snyder C, Tian J,
Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, et al:
9.4T human MRI: Preliminary results. Magn Reson Med. 56:1274–1282.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagel AM, Umathum R, Rösler MB, Ladd ME,
Litvak I, Gor'kov PL, Brey WW and Schepkin VD: (39) K and (23) Na
relaxation times and MRI of rat head at 21.1 T. NMR Biomed.
29:759–766. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schepkin VD, Bejarano FC, Morgan T,
Gower-Winter S, Ozambela M Jr and Levenson CW: In vivo magnetic
resonance imaging of sodium and diffusion in rat glioma at 21.1 T.
Magn Reson Med. 67:1159–1166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yilmaz S: The safety of high-field MRI?
Int J Occup Environ Med. 5:63–64. 2014.PubMed/NCBI
|
|
10
|
Tian X, Wang D, Feng S, Zhang L, Ji X,
Wang Z, Lu Q, Xi C, Pi L and Zhang X: Effects of 3.5–23.0 T static
magnetic fields on mice: A safety study. Neuroimage. 199:273–280.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franco J: Magnetic resonance imaging
safety. Radiol Technol. 91:343–356. 2020.PubMed/NCBI
|
|
12
|
Yang X, Song C, Zhang L, Wang J, Yu X, Yu
B, Zablotskii V and Zhang X: An upward 9.4 T static magnetic field
inhibits DNA synthesis and increases ROS-P53 to suppress lung
cancer growth. Transl Oncol. 14:1011032021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tkáč I, Benneyworth MA, Nichols-Meade T,
Steuer EL, Larson SN, Metzger GJ and Uğurbil K: Long-term
behavioral effects observed in mice chronically exposed to static
ultra-high magnetic fields. Magn Reson Med. 86:1544–1559. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan MH, Huang X, Tian X, Ouyang C, Wang
D, Feng S, Chen J, Xue T, Bao J and Zhang X: Short- and long-term
effects of 3.5–23.0 Tesla ultra-high magnetic fields on mice
behaviour. Eur Radiol. 32:5596–5605. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian X, Lv Y, Fan Y, Wang Z, Yu B, Song C,
Lu Q, Xi C, Pi L and Zhang X: Safety evaluation of mice exposed to
7.0–33.0 T high-static magnetic fields. J Magn Reson Imaging.
53:1872–1884. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian X, Wang D, Zha M, Yang X, Ji X, Zhang
L and zhang X: Magnetic field direction differentially impacts the
growth of different cell types. Electromagn Biol Med. 37:114–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Luo J, Lv H, Zhang Z, Yang J, Dong
D, Fang Y, Hu L, Liu M, Liao Z, et al: Safety of exposure to high
static magnetic fields (2 T-12 T): A study on mice. Eur Radiol.
29:6029–6037. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv Y, Fan Y, Tian X, Yu B, Song C, Feng C,
Zhang L, Ji X, Zablotski V and Zhang X: The anti-depressive effects
of ultra-high static magnetic field. J Magn Reson Imaging.
56:354–365. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian X, Wang C, Yu B, Fan Y, Zhang L and
Zhang X: 9.4 T static magnetic field ameliorates imatinib
mesylate-induced toxicity and depression in mice. Eur J Nucl Med
Mol Imaging. 50:314–327. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu X, Ji X, Fan Y, Yu B, Wang X, Feng C,
Zhang L, Song C and Zhang X: Static magnetic fields protect against
cisplatin-induced kidney toxicity. Antioxidants (Basel). 12:732022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Begnini KR, Moura de Leon PM, Thurow H,
Schultze E, Campos VF, Martins Rodrigues F, Borsuk S, Dellagostin
OA, Savegnago L, Roesch-Ely M, et al: Brazilian red propolis
induces apoptosis-like cell death and decreases migration potential
in bladder cancer cells. Evid Based Complement Alternat Med.
2014:6398562014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rahmani AH, Alzohairy MA, Khan MA and Aly
SM: Therapeutic implications of black seed and its constituent
thymoquinone in the prevention of cancer through inactivation and
activation of molecular pathways. Evid Based Complement Alternat
Med. 2014:7246582014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tada A, Kaneiwa Y, Shoji J and Shibata S:
Studies on the saponins of the root of Platycodon grandiflorum A.
De Candolle. I. Isolation and the structure of platycodin-D. Chem
Pharm Bull (Tokyo). 23:2965–2972. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Liu Y, Wang Z, Han Y, Tian YH, Zhang
GS, Sun YS and Wang YP: Platycodin D isolated from the aerial parts
of Platycodon grandiflorum protects alcohol-induced liver injury in
mice. Food Funct. 6:1418–1427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee WH, Gam CO, Ku SK and Choi SH: Single
oral dose toxicity test of platycodin d, a saponin from platycodin
radix in mice. Toxicol Res. 27:217–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan M, Maryam A, Zhang H, Mehmood T and
Ma T: Killing cancer with platycodin D through multiple mechanisms.
J Cell Mol Med. 20:389–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chun J and Kim YS: Platycodin D inhibits
migration, invasion, and growth of MDA-MB-231 human breast cancer
cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem
Biol Interact. 205:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou R, Lu Z, Liu K, Guo J, Liu J, Zhou Y,
Yang J, Mi M and Xu H: Platycodin D induces tumor growth arrest by
activating FOXO3a expression in prostate cancer in vitro and in
vivo. Curr Cancer Drug Targets. 14:860–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye Y, Han X, Guo B, Sun Z and Liu S:
Combination treatment with platycodin D and osthole inhibits cell
proliferation and invasion in mammary carcinoma cell lines. Environ
Toxicol Pharmacol. 36:115–124. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang ZH, Li T, Gao HW, Sun W, Chen XP,
Wang YT and Lu JJ: Platycodin D from Platycodonis Radix enhances
the anti-proliferative effects of doxorubicin on breast cancer
MCF-7 and MDA-MB-231 cells. Chin Med. 9:162014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang X, Yu B, Song C, Feng C, Zhang J,
Wang X, Cheng G, Yang R, Wang W and Zhu Y: The effect of long-term
moderate static magnetic field exposure on adult female mice.
Biology (Basel). 11:15852022.PubMed/NCBI
|
|
32
|
Schwartz DJ, Conover MS, Hannan TJ and
Hultgren SJ: Uropathogenic Escherichia coli superinfection enhances
the severity of mouse bladder infection. PLoS Pathog.
11:e10045992015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kasuga I, Makino S, Kiyokawa H, Katoh H,
Ebihara Y and Ohyashiki K: Tumor-related leukocytosis is linked
with poor prognosis in patients with lung carcinoma. Cancer.
92:2399–2405. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu B, Song C, Feng CL, Zhang J, Wang Y,
Zhu YM, Zhang L, Ji XM, Tian XF, Cheng GF, et al: Effects of
gradient high-field static magnetic fields on diabetic mice. Zool
Res. 44:249–258. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim TW, Song IB, Lee HK, Lim JH, Cho ES,
Son HY, Park SJ, Kim JW and Yun HI: Platycodin D, a triterpenoid
sapoinin from Platycodon grandiflorum, ameliorates
cisplatin-induced nephrotoxicity in mice. Food Chem Toxicol.
50:4254–4259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo Y, Ji X, Liu J, Li Z, Wang W, Chen W,
Wang J, Liu Q and Zhang X: Moderate intensity static magnetic
fields affect mitotic spindles and increase the antitumor efficacy
of 5-FU and Taxol. Bioelectrochem. 109:31–40. 2016. View Article : Google Scholar
|
|
37
|
Zhao L, Yu Z and Zhao B: Mechanism of
VIPR1 gene regulating human lung adenocarcinoma H1299 cells. Med
Oncol. 36:912019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moody TW, Leyton J, Chan D, Brenneman DC,
Fridkin M, Gelber E, Levy A and Gozes I: VIP receptor antagonists
and chemotherapeutic drugs inhibit the growth of breast cancer
cells. Breast Cancer Res Treat. 68:55–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Silverstein RL and Febbraio M: CD36, a
scavenger receptor involved in immunity, metabolism, angiogenesis,
and behavior. Sci Signal. 2:re32009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guan F, Zhang T, Liu X, Han W, Lin H, Li
L, Chen G and Li T: Evaluation of voltage-dependent calcium channel
γ gene families identified several novel potential susceptible
genes to schizophrenia. Sci Rep. 6:249142016. View Article : Google Scholar : PubMed/NCBI
|