Introduction
HIV attacks the human immune system and targets
CD4+T lymphocytes, which are important immune cells. The
introduction of highly active antiretroviral therapy (HAART) has
markedly improved outcomes in patients with HIV infection and
long-term survival can be expected even following the onset of
acquired immune deficiency syndrome (1). However, patients with HIV are at
increased risk of cancer due to oncogenic factors, including the
immune dysregulated state, direct pathogenicity of the virus,
chronic stimulation and prolonged drug exposure (2). Acute promyelocytic leukemia (APL), the
French-American-Britain classification of acute myeloid leukemia
(AML)-M3 (3), is distinct among AML
subtypes due to its unique prognosis and pathogenesis. The
malignant clone is characterized by a specific translocation
t(15;17), which results in rearrangement the retinoic acid receptor
α (RARα) genes and promyelocytic leukemia (PML) (4). The resulting protein product
interferes with maturation of the immature myeloid morphology
(5). APL is now a highly curable
disease with an overall response rate of 95% and current cure rate
is >80% (5). The incidence of
HIV with APL is extremely rare, with only 12 cases reported to
date, and it is even rarer in the chromosomally complex APL
population. Due to the small number of cases, there is no uniform
standard of treatment for APL complicated with HIV, and the
prognosis remains unclear (2,4,6–14).
The status of patients infected with HIV and initiation and course
of chemotherapy are increasingly a cause of concern due to the
highly effective HAART and its success in controlling viral load
(6,7). The present report describes a case of
high-risk APL with additional chromosomal abnormalities and HIV
infection and discusses the existing literature on this unique
population.
Case report
A 49-year-old previously healthy female patient
presented in July 2022 following abdominal pain for 1 week and
intermittent mild fever combined with fatigue for 3 days. Before
admission to The First Affiliated Hospital of Jishou University
(Jishou, China), the patient was admitted to Fenghuang County
People's Hospital (Fenghuang, China) at which the initial
hematological assessment determined the following: An elevated
white blood cell (WBC) count of 40.97×109/l (normal
range, 4.0–10.0×109/l); neutrophil count,
4.30×109/l (normal range, 1.8–6.3×109/l);
platelet count of 14×109/l (normal range,
100–400×109/l) and a hemoglobin, 75 g/l (normal range,
110–150 g/l) (15). Whole abdominal
computed tomography (CT) scan (KVP:120, MA:177, SL-573.5MM,
TITLE:0, Head 5.0, Hr40 3) and abdominal standing films showed no
abnormalities. Intravenous cephalosporin antibiotic administration
(2 g ceftazidim twice/day for 4 days) and fluid replacement were
used to control the fever but the efficacy was limited.
Subsequently, the patient was admitted to the Emergency Department
of The First Affiliated Hospital of Jishou University for further
diagnosis and treatment in July 2022.
Physical examination revealed an afebrile case with
conjunctival pallor and abdominal tenderness without tonsillar
exudates. Old ecchymosis was visible on the skin without fresh
petechiae and ecchymoses. There was no hepatosplenomegaly or
lymphadenopathy. Physical examination of the heart and lungs showed
no positive signs. The patient had no history of tobacco, alcohol
or illicit drug use.
Laboratory assessment demonstrated the following:
Hemoglobin, 66 g/l; total leukocyte count, 41.86×109/l;
platelet count of 9×109/l and reticulocyte count of
32.3×109/l. Blood tests showed differential leukocyte
counts were as follows: 19.8% neutrophils, 12.9% lymphocytes and
66.5% monocytes (Table I). Immature
cells and rod-shaped bodies were visible but no schistocytes were
observed in the peripheral blood smear. Prothrombin time and
activated partial thromboplastin time were 17.4 and 41.6 sec,
respectively. Fibrinogen levels were 1.294 g/l, D-dimer levels were
23.97 µg/ml and fibrin monomer concentration was 40.05 µg/ml. Serum
potassium levels were 2.79 mmol/l and lactate dehydrogenase
concentration was 808 U/l. Serum electrolytes, calcium, magnesium,
urea and creatinine were within normal range. Liver function tests
were normal. Hepatitis B surface antigen index was 2362.00 and the
HIV antibody index was 1303.00. The hepatitis B virus (HBV) titer
was 2.96×103 IU/ml, as evidenced by HBV-DNA virus
nucleic acid quantitative detection-internal standard
quantification (High Pure Viral Nucleic Acid kit, Roche
Diagnostics, Mannhein, Germany). The lymphocyte subsets [analyzed
by flow cytometry (MoFlo® Astrios; Beckman Coulter)
(16)] were as follows:
Lymphocytes, 4.01%; B lymphocytes, 22.99%; helper/induced T
lymphocytes, 21.30%; CD4/CD8, 0.55% and absolute helper/induced T
lymphocytes count, 335.00/µl.
 | Table I.Initial laboratory test data. |
Table I.
Initial laboratory test data.
| Laboratory
measure | On admission | Normal value |
|---|
| White blood cell
count, ×109/l | 41.86 | 4.00–10.00 |
| Differential count,
% |
|
|
|
Neutrophils | 19.80 | 40.00–75.00 |
|
Lymphocytes | 12.60 | 20.00–50.00 |
|
Monocytes | 66.50 | 3.00–10.00 |
|
Eosinophils | 0.00 | 0.40–8.00 |
|
Basophilic granulocyte | 0.80 | 0.00–1.00 |
| Hemoglobin,
g/l | 66.00 | 110.00–150.00 |
| Hematocrit, % | 19.90 | 35.00–45.00 |
| Platelet count,
×109/l | 9.00 | 100.00–400.00 |
| Mean corpuscular
volume, fl | 92.50 | 82.00–100.00 |
| Activated partial
thromboplastin time, sec | 41.60 | 27.00–45.00 |
| Prothrombin time,
sec | 17.40 | 11.00–16.00 |
| International
normalized ratio, sec | 1.48 | 0.80–1.30 |
| Fibrinogen,
g/l | 1.29 | 2.00–4.00 |
| Fibrin-split
products, µg/ml | 40.50 | 0.00–5.00 |
| D-dimer, µg/ml | 23.97 | 0.00–0.50 |
| Lactate
dehydrogenase, U/l | 808.00 | 125.00–274.00 |
| Sodium, mmol/l | 138.00 | 135.00–145.00 |
| Potassium,
mmol/l | 2.79 | 3.50–5.50 |
| Chloride,
mmol/l | 106.00 | 96.00–108.00 |
| Urea nitrogen,
mmol/l | 5.40 | 2.50–7.10 |
| Creatinine,
µmol/l | 77.50 | 40.00–120.00 |
| Glucose,
mmol/l | 9.12 | 3.89–6.11 |
| Total protein,
g/l | 69.80 | 60.00–85.00 |
| Total bilirubin,
µmol/l | 16.20 | 3.40–20.50 |
| Aspartate
aminotransferase, U/l | 24.00 | 0.00–40.00 |
| Alanine
aminotransferase, U/l | 16.00 | 0.00–40.00 |
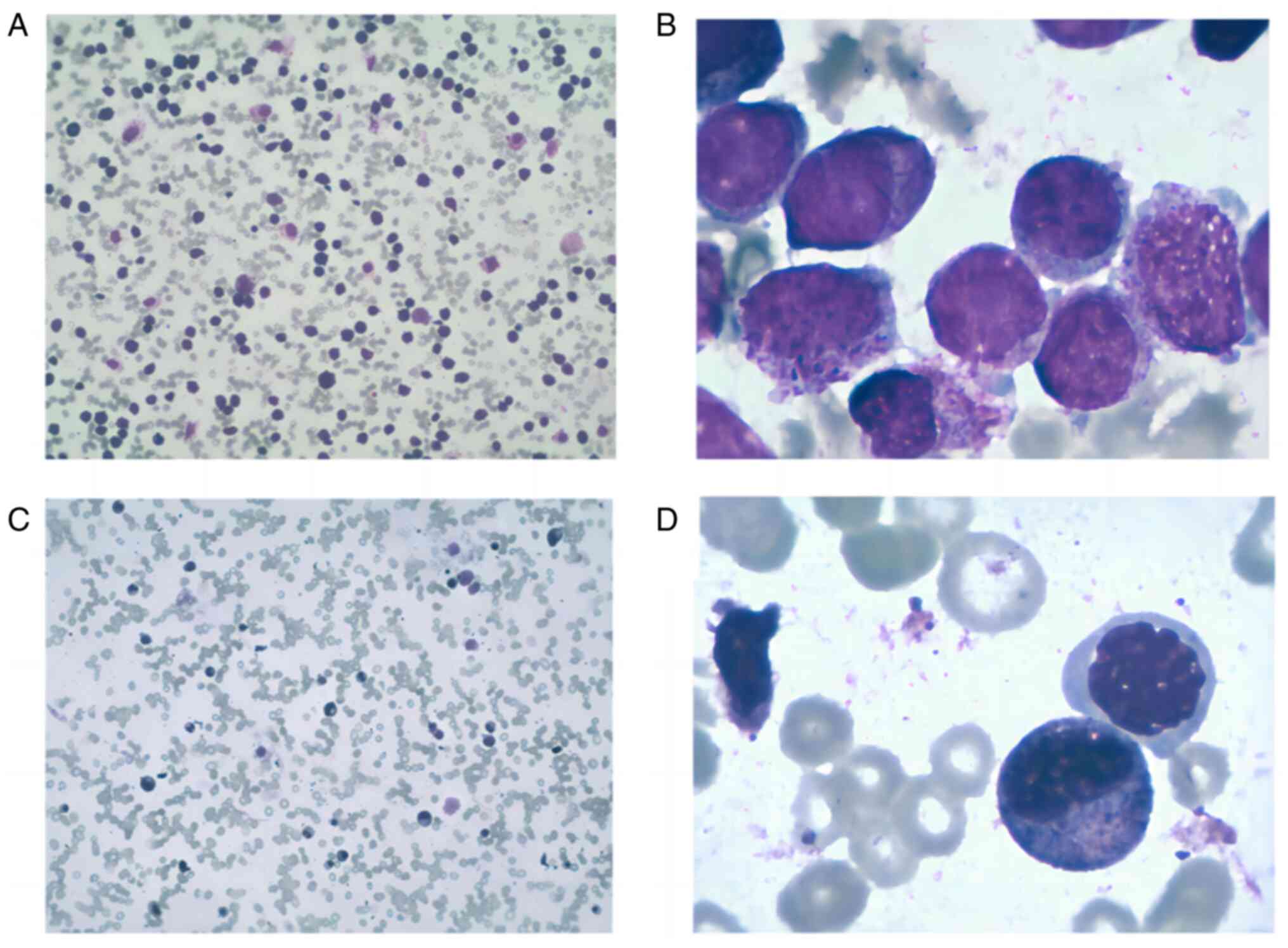
Based on morphology (cells from the bone marrow
aspirate smears stained with Wright's stain for 10–15 min and
myeloperoxidase respectively. For myeloperoxidase staining: 10–15
drops of 0.3% benzidine ethanol solution in the slices, after 1
min, 10–15 drops of hydrogen peroxide solution were added for 5
min. Slides were rinsed and Wright's stain was added for 30 min,
followed by another rinse. Images were captured using an optical
microscope at magnifications, ×10 and ×100) (Fig. 1), 92% of marrow cells were
promyelocytes [myeloperoxidase (MPO+++)]. These variant
(monocytoid) promyelocytes expressed CD13, CD33, cMPO and human
leukocyte antigen-DR, whilst CD34 and CD19 were absent. Monocytic
markers were either absent (CD14) or scarcely expressed (CD64).
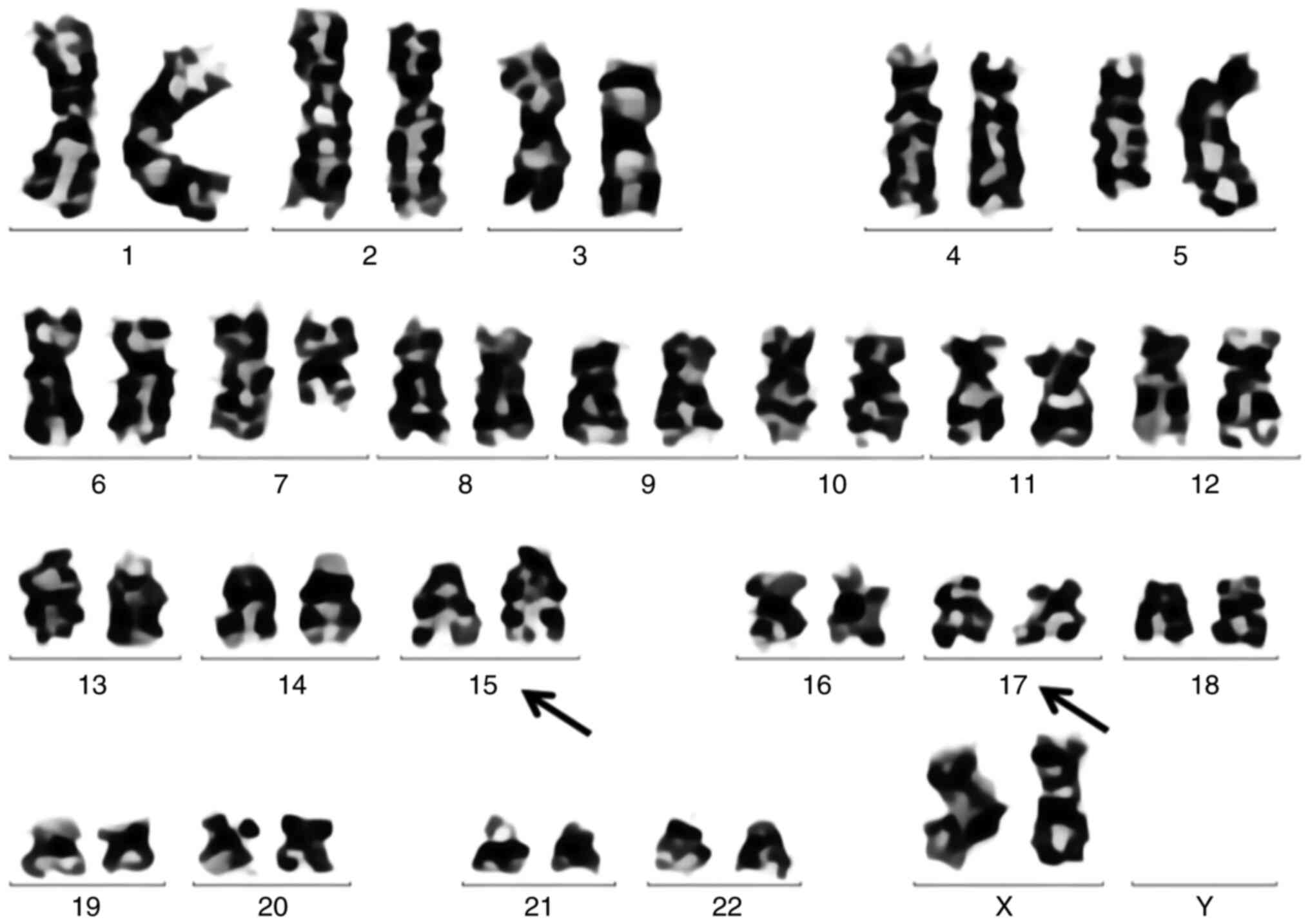
Cytogenetic analysis revealed an abnormal female karyotype [46, XX,
add(5)(q15), add(5)(q31), add(7)(q11.2), add(12)(p13),
t(15;17)(q24; q21)[19]/46, XX; Fig.
2], whilst myeloid leukemia fusion gene results were negative
for AML1/ETO, mixed-lineage leukemia and core-binding factor
subunit β rearrangements. For fusion gene detection,
leukemia-associated fusion gene analysis detected positive
expression of PML-RARα, and negative expression of BCR/ABL1,
MLL/ELL, MLL/SEPT6, SET/CAN, TEL/PDGFRB, TLS/ERG, MLL/AF6,
NPM1/RARα, TEL/ABL1, AML1/ETO, CBFβ/MYH11, PLZF/RARα,
FIP1L1/PDGFRA, DEK/CAN, AML1-MDS1/EVI1, AML1/MTG16, AML1/EAP,
NPM1/MLF, MLL/AF9, MLL/AF10, MLL/ENL, MLL/AF17, MLL/AF1q, MLL/AF1p
(Total RNA was extracted from the patient's bone marrow mononuclear
cells by TRIzol method. The reaction solution was prepared
according to the instructions of leukemia fusion gene detection
kit, and the amplification reaction was performed by ABI7500
amplification instrument). For the mutated gene fraction, sample
transfer/genomic DNA levels were detected by deep target sequencing
(Next-generation sequencing; NGS). Gene mutations in AML suggested
the following: Calreticulin (CALR) p.E371fs, variant abundance,
2.40%) and lysine methyltransferase 2C (KMT2C) p.H1826R, variant
abundance, 49.30%. NGS was performed by Golddomain
Medicine/Guangzhou Jinyu Inspection using DNA extracted from bone
marrow mononuclear cells of the patient Using illumina platform
NextSeq550 sequencing platform (Thermo Fisher Scientific, Inc.), 72
genes (ANKRD26, ABCB1, ARID1A, ARID1B, ARID2, ASXL1, ASXL2, ATG2B,
BCOR, BCORL1, BRAF, CALR, CBL, CEBPA, CREBBP, CSF3R, CTCF, DDX41,
DIS3, DNMT3A, ETNK1, ETV6, EZH2, FLT3, GATA1, GATA2, GFI1, GNB1,
GSKIP, HRAS, IDH1 IDH2 IKZF1 JAK1 JAK2 JAK3 KDM6A KIT KMT2A KMT2C
KRAS MPL MYC NBN NF1 NPM1 NRAS NTRK1 PHF6 PML PPM1D PTPN11 RAD21
RARA RUNX1 SBDS SETBP1 SETD2 SF3B1 SMC1A SMC3 SRSF2 STAG2 STAT5A
TERC TERT TET1 TET2 TP53 U2AF1 WT1 ZRSR2) of patients were deeply
sequenced by targeted amplicon method (NGS amplicon sequencing
primers were designed and synthesized by Thermo Fisher company).
Sequencing depth of 170-fold.] Reverse transcription (RT)-qPCR
testing was positive for the PML/RARα translocation [Type L;
PML-retinoic acid receptor α (RARα) gene copy number, 15,006
copies; ABL1 gene copy number, 138499 copies;
PML-RARα/ABL1:10.835%; The relative quantitation of
PML/RARa=(copiesPML/RARa/copiesABL) ×100% (17)]. PML-RARα mRNA expression was
measured using RT-qPCR [The patient's bone marrow RNA was
reverse-transcribed onto the cDNA and tested for PML/RAR α
transcripts with primers: forward, 5′-GCAATTTAGGTATGAAAGCCAGC-3′,
and reverse, 5′-CTTTCAGCATTTTGACGGCAACC-3′; and fluorescein
amidite-labeled probe (Boshang Biotechnology Co., Ltd.):
5′-CTGCTCTGGGTCTCAATGGCTGCCTCC-3′; ABL was used as the reference
gene and detected with the primers: forward,
5′-TCCATCTCGCTGAGATACGAAG-3′, and reverse,
5′-ATGATGAACCAACTCGGCCA-3′; and VIC-labeled probe
5′CAACACTGCTTCTGATGGCAAGCTCTACG3′. RT-qPCR was tested for 2 min at
50°C, pre-denatured for 3 min at 95°C, and then 40 cycles of
denatured for 5 sec at 95°C, annealed and extended for 30 sec at
58°C were performed using the ABI 7500 Real-time PCR system. Data
was collected and analyzed using ABI 7500 software v2.3 (Thermo
Fisher Scientific, Inc.)].
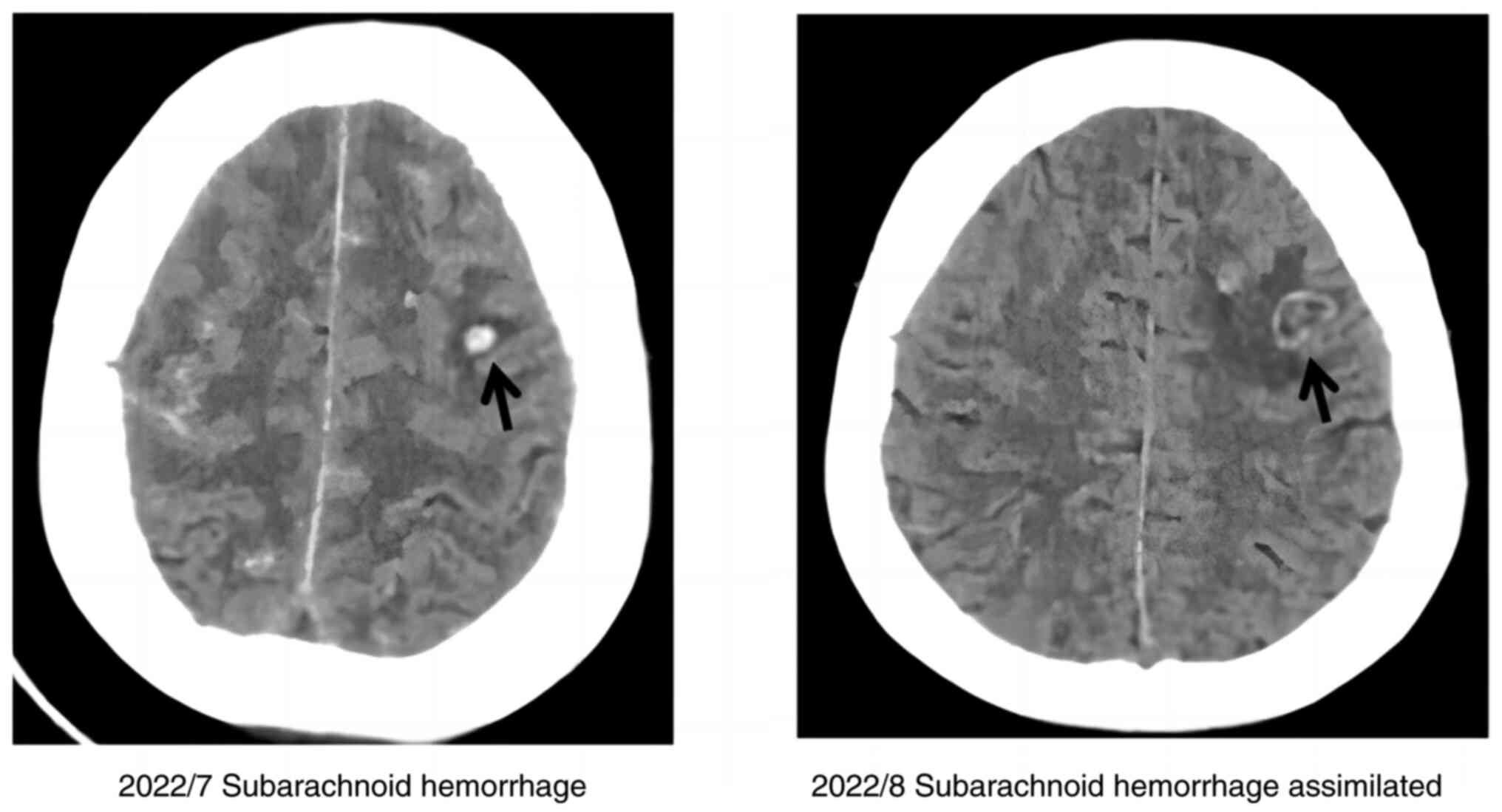
Chest CT and electrocardiography were normal.
Multigated equilibrium radionucleotide cineangiography revealed
normal cardiac wall motion and ejection fraction of 58%. However,
cranial CT suggested a subarachnoid hemorrhage (Fig. 3).
Remission was induced using all-trans-retinoic acid
(ATRA; 25 mg/m2). The patient was administered
Pirarubicin hydrochloride (45 mg/m2) via a continuous
intravenous infusion over 24 h (20 mg for 3 day, 10 mg × 2 day) and
arsenic trioxide (ATO; 0.16 mg/m2) as an intravenous
bolus once daily for three days. As the corrected QT interval of
the patient increased from normal to 492 msec, ATO was
intermittently used during induction. The patient developed
differentiation syndrome during chemotherapy but it did not recur
following ATRA dose reduction (20 mg twice/day). In the absence of
disseminated intravascular coagulation, heparin was not
administered. Red cells, platelets, fibrinogen and cryoprecipitate
were transfused as required.
Following discussion with the Department of
Infection, HAART was initiated during the induction. HAART regimen
comprised efavirenz (600 mg daily) and lamivudine (300 mg daily).
Simultaneously, the patient received anti-HBV therapy (tenofovir
disoproxil fumarate, 300 mg daily) due to infection with hepatitis
B. A total of 4 weeks later, the CD4+ T cell count was decreased,
whilst the HIV-1 titer was below the normal range of detection
values.
Following one cycle of chemotherapy, the patient was
in complete morphological remission (CMR; Fig. 1C and D). Subsequently, the patient
was administered one cycle of consolidation chemotherapy with
idarubicin (IDA; 8 mg/sqm/d; days 1–3; bolus intravenous injection)
and cytosine arabinoside (1 g/sqm/12 h; days 1–3, continuous
intravenous infusion), and HAART was administered throughout. The
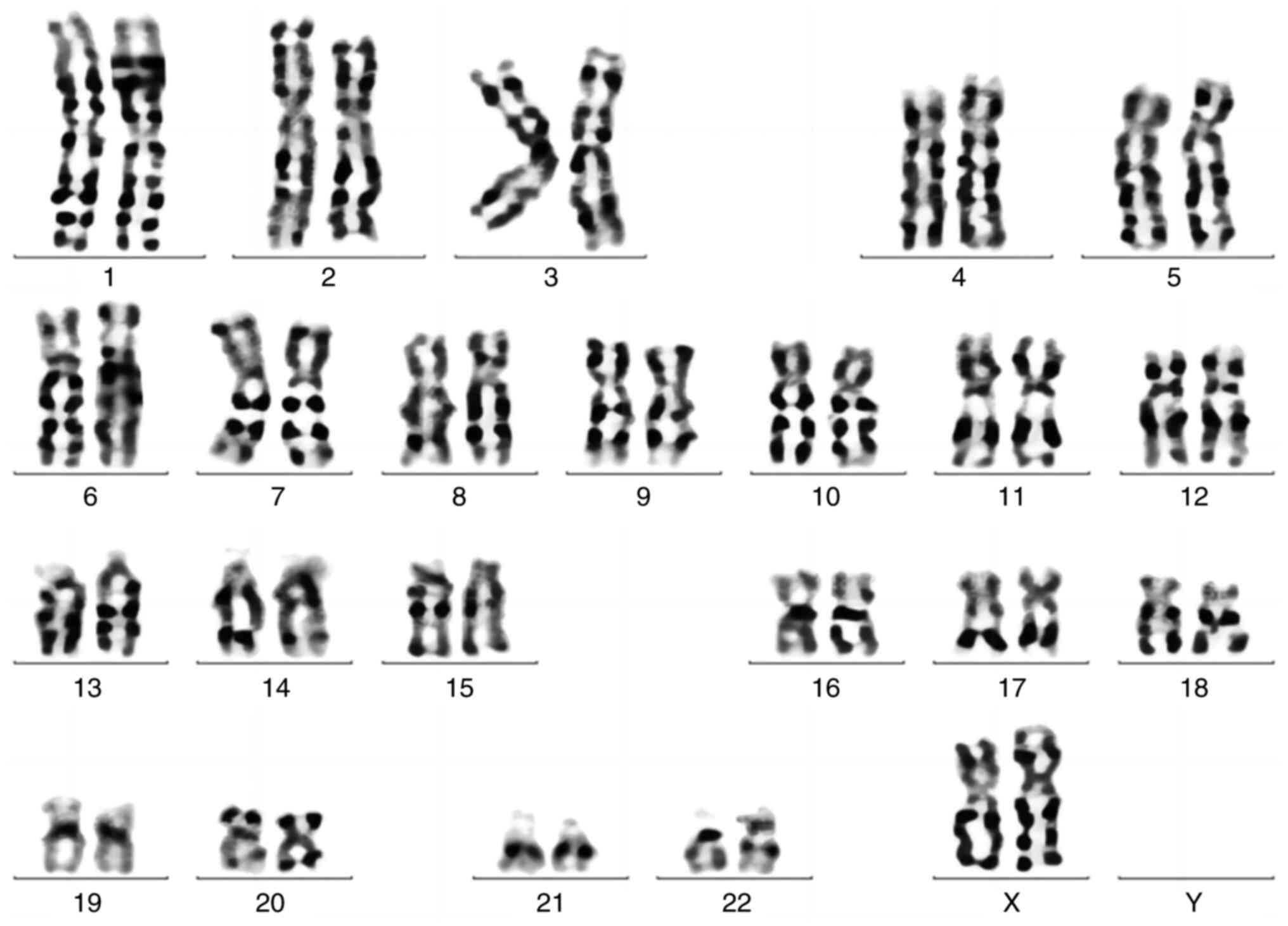
chromosome karyotype of the patients was normalized after
chemotherapy. As of January 2024 (Fig.
4), the patient is receiving regular chemotherapy, but is
asymptomatic and has tolerated both chemotherapy and HAART well;
however, there is risk of recurrence and need further
observation
Discussion
HIV attacks the human immune system and may increase
risk of malignant tumors, which may be associated with oncogenic
factors, including immune dysregulation status, direct viral
pathogenicity, chronic stimulation and long-term medication
exposure. An association between HIV infection and several types of
malignancy has been reported (1).
Although the most frequent neoplasms are non-Hodgkin lymphoma,
Kaposi sarcoma and invasive cervical carcinoma, other cancers are
increasingly reported (2,18). A few cases of AML have been
described in patients with AML and HIV infection (9,10). As
the incidence of APL in patients infected with HIV is sporadic, the
therapeutic approach is individualized and often challenging
(2,4,8).
Recently, with the advent of novel therapies, survival of patients
with HIV and APL has improved but there are no relevant guidelines
for treatment of the concomitant HIV and APL (6,7,9–14).
Therefore, evaluating the mechanism and clinical characteristics of
these cases is important. The present report described a case of an
HIV-positive patient with a high-risk AML M3L presenting with
hyperleukocytosis complicated by hematencephalon and prolonged QT
interval during induction therapy. HIV may also infect monocytes
and macrophages in addition to functioning as a tropic retrovirus
and neurotropic virus for helper inducer (CD4) lymphocytes
(19). This increases DNA-binding
activity of the NF-κB transcription factor, which can further
activate genes that may be involved in leukemogenesis [for example,
IL-6, granulocyte-colony stimulating factor (CSF) or
granulocyte-macrophage-CSF] through paracrine or autocrine loops
(8). Immunodeficiency may also
explain. The high incidence of APL in two disorders associated with
chronic T cell abnormality, severe combined immunodeficiency and
Wiskott Aldrich syndrome, suggests that an immunodeficient state is
associated with APL (2,20). Furthermore, during HIV infection,
tumor cells evade immune surveillance via lost or decreased immune
response. The development of APL in patients infected with HIV may
involve the potent transactivator protein Tat, which serves a
crucial role in angiogenesis and can replace the preformed basic
fibroblast growth factor (bFGF). bFGF increases myelopoiesis
directly via FGF receptors on myeloid progenitors (21). HIV may also alter the bone marrow
microenvironment to make it more favorable for proliferation of
leukemic cells (19,21). Lastly, in the era of ART, the
improvement in survival rate following HIV infection has led to an
increase in long-term morbidity, including APL. Exposure to drugs,
including cell inhibitors, HIV nucleoside analogs, benzene, alkanes
and cytotoxic molecules, can increase the risk of leukemia
complications in patients with HIV. In addition to the
aforementioned factors, ionizing radiation can cause chromosome
breakage and recombination, along with alterations and mutations in
the c-myc and ras genes, which serve an important role in inducing
leukemia. The greater the radiation dose, the higher the risk of
leukemia. It is hypothesized that the occurrence of APL in HIV may
be coincidental but certain authors suggest that incidence of APL
is higher in HIV-infected patients (22). Cytopenia of patients with HIV
infection is usually attributed to action of viruses and antiviral
drugs, and the accompanying malignant tumors of the hematopoietic
system are often ignored (23).
Therefore, further assessment and monitoring of potential
associations is needed to determine the cause of concomitant HIV
and APL. Although prognostic variables have been assessed to
stratify patients, the data concerning the prognostic relevance of
CK are conflicting (24–29). Most patients with APL who have
t(15;17) chromosome heterotopia are considered to have a good
prognosis, but certain factors affect prognosis including high WBC
count, the male sex, elevated serum creatinine levels, advanced age
and fibrinogen levels (30). The
prognosis of complex chromosome karyotype in patients with AML but
no HIV is poor but whether CK affects the prognosis of patients
with APL is debatable and, to the best of our knowledge, few
studies have assessed this (25,31).
The additional chromosome abnormality does not affect overall
survival (OS). Moreover, the additional chromosome abnormality
population has advantages in duration of complete remission (CR)
and event-free survival rate (EFS) (25–26).
Wiernik et al (25), through
uni- and multivariate survival analysis, reported that treatment
regimen with arsenic acid could prolong the disease-free survival
of patients with APL and improve prognoses. Arsenic acid and
retinoic acid may have a synergistic effect on clearing
promyelocytic leukemia clones, thus improving the curative effect
(32). Wan et al (27) reported that the additional
chromosome does not affect the OS rate but patients with APL
carrying additional chromosome abnormalities have delayed
recurrence, which may be related to the lack of a serine proline
enrichment region in PML-RARα fusion gene S (33). However, Vu et al (28) reported that patients with additional
chromosomal abnormalities have aggressive disease, and additional
chromosomal abnormalities are independent adverse prognostic
factors for these patients. Another study (29) demonstrated inferior EFS for patients
harboring complex karyotypes but not for patients harboring
additional cytogenetic abnormalities. In conclusion, prognosis of
patients with APL with additional chromosomes remains controversial
and needs more evidence. At the molecular level, mutations were
detected in CALR (p.E371fs) and KMT2C genes in the present patient.
CALR is a multifunctional protein with 417 amino acids and is
mainly localized in the luminal of the endoplasmic reticulum
(34). A study reported that gene
expression of CALR is downregulated in patients with APL (35). Another study reported that CALR may
participate in clearance of tumor cells by reducing angiogenesis
and immune system activation (36).
Moreover, increased CALR expression may cause tumor metastasis,
which may be associated with lack of matrix attachment or
regulation of Ca2+ signaling (37). In the nucleus, CALR inhibits the
interaction between the retinoic acid receptor and its DNA response
elements and CALR silencing causes a significant decrease in both
erythroid and MK differentiation of human HSPC (38). KMT2C is an epigenetic modifier gene
that participates in histone methylation and affects
transcriptional coactivation of gene expression. KMT2C is expressed
in several types of tumor tissues, including leukemia, and is among
the most frequently mutated genes in human cancer (39). KMT2C is a haploinsufficient tumor
suppressor (40) and its inhibition
impairs the differentiation of hematopoietic stem cells and
progenitor cells. In a study on AML with fms-related receptor
tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD)
mutations, RFS and OS were markedly decreased in patients with
recurrent KMT2C mutations and deletions compared with patients with
FLT3-ITD mutations without KMT2C (41). To the best of our knowledge,
however, there are no reports of CALR or KMT2C mutations or CK with
HIV and more studies are needed to reveal their relevance.
APL is among the highly curable hematological
neoplastic diseases with a 10-year OS rate of 93.9% owing to the
use of ATRA and ATO (42). A total
of 13 cases of APL with HIV have been reported (Tables II and III) (2,4,6–14).
Of these, treatment and survival details are unavailable for one
case (9). ATRA was used in 12
patients and 10/12 (83.3%) evaluable patients remained in CR at the
time of reporting, which is similar to the experience with classic
APL (32). For the 13 patients, the
median age was 37 years (range, 22–67 years); 9 were male (69.2%)
and 4 (30.8%) were female. A total of four cases were in the
high-risk and 9 in the low-median group (based on NCCN guidelines)
(43). The patients in the
high-risk and the low-median group who were still in the CR status
at the time of reporting accounted for 50.0 and 88.9%,
respectively. The treatment failed for one patient as ATRA was used
inappropriately (CR was not maintained for a long period after
chemotherapy). A total of 6/12 (50.0%) patients who received
chemotherapy-alone were alive during CR at a median follow-up of 10
months, which is consistent with classical APL treated with
chemotherapy-alone (44). Thus,
even from a small number of cases, it appears that there is no
difference in survival between individuals with HIV. However,
extensive data are needed to confirm this observation. Therefore,
chemotherapy should not be rejected even for patients with HIV.
Despite therapeutic advances, early mortality of APL is 32.6–34.6%
(45). Hemorrhage remains one of
the most common causes of early mortality (46) and mainly occurs in the brain and
lungs. High WBC count and prolonged Prothrombin time predict severe
bleeding in patients with high-risk APL (47,48).
Strategies to reduce early death are key for improving the survival
of patients with APL (42). To
prevent early death, patients with high risk of early death and
hemorrhage should be identified. The patient in the present report
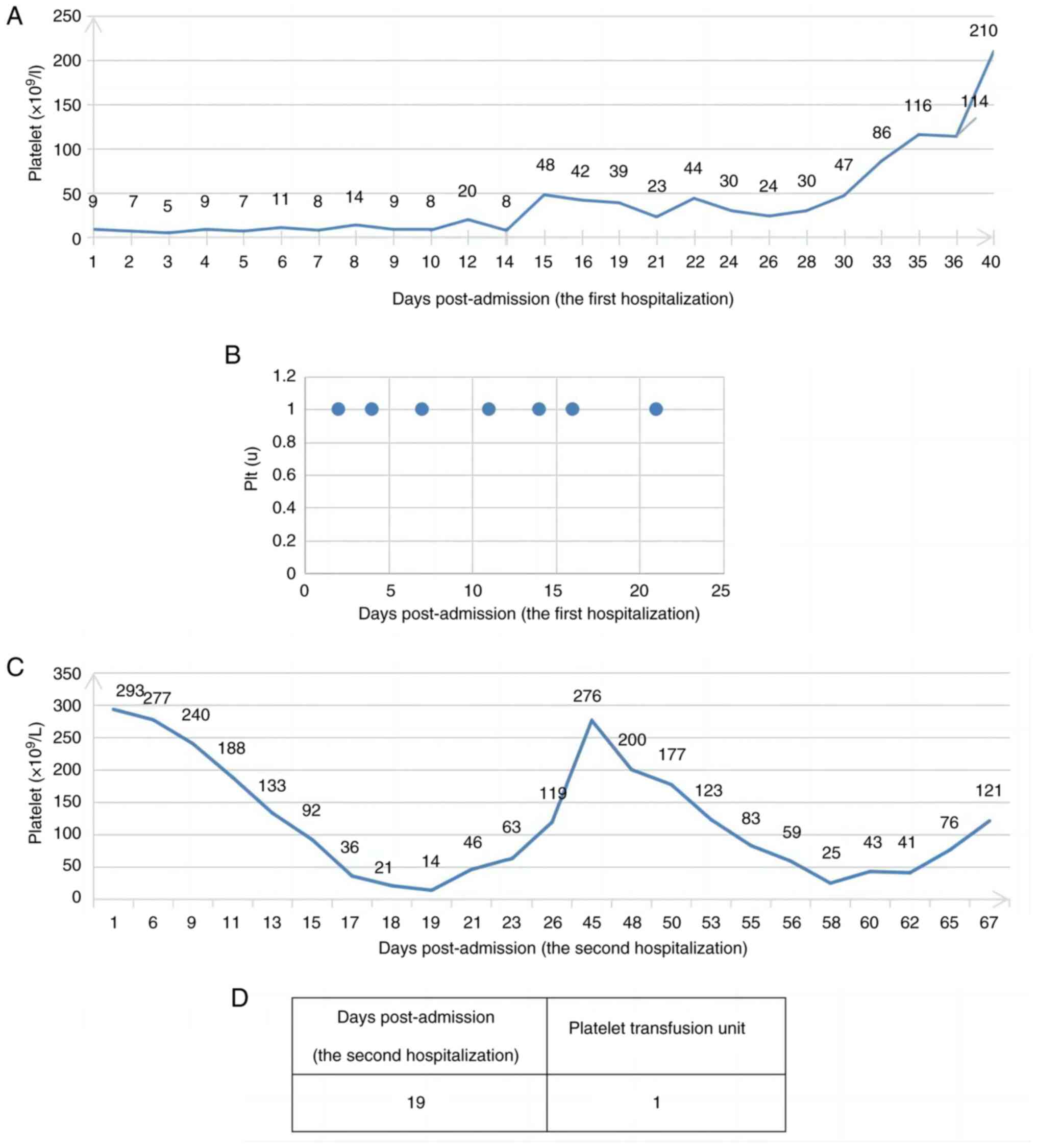
suffered from a subarachnoid hemorrhage after diagnosis. Platelet
transfusion was administered during induction chemotherapy but the
efficacy was not obvious, which may be associated with heavy tumor
burden and the immune dysfunction caused by HIV. Platelet
transfusion resistance (PTR) refers to persistently inadequate
increments in post-transfusion platelet count. It is commonly
defined as a corrected count increment of the platelet count
<7.5×109/l or a % platelet recovery of <30% within
60 min post-transfusion. PTR can result from non-immune and immune
factors; non-immune causes are more common. These factors include
infection, disseminated intravascular coagulation, fever (body
temperature ≥38°C), bleeding, heparin administration, splenomegaly
and intravenous antibiotic use. Immune factors include
incompatibility of non-specific antigens, such as human leukocyte
antigen class I, ABO, CD36 and human platelet antigen (48,49).
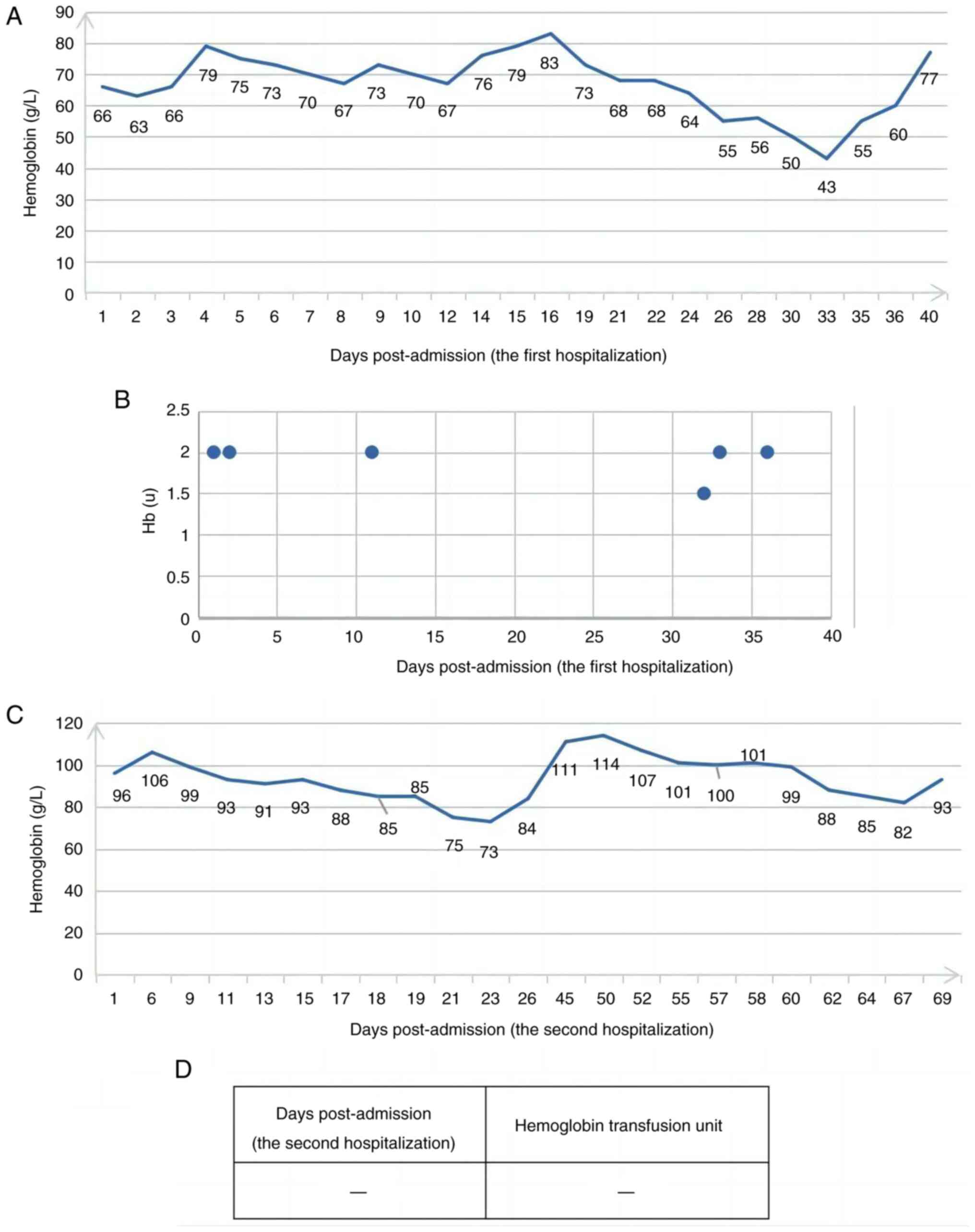
Following one cycle of induction chemotherapy and HAART,
re-examination of the bone marrow morphology of the patient in the
present report revealed CR, effective platelet transfusion and a
notably decreased transfusion volume of platelets (Fig. 5), which further confirms that the
poor efficacy of platelet transfusion may be related to the high
tumor burden. Simultaneously, red blood cell infusion was markedly
decreased compared with before (Fig.
6). However, prolonging QT interval is common, especially in
frail patients, and attention should be paid to the risk to their
heart (50). The patient in the
present report experienced repeated prolonging of QT interval
during the induction of chemotherapy, during which potassium
supplementation and electrocardiogram monitoring were performed,
while arsenic treatment was intermittent. The patient in the
present report had HIV and APL, and received treatment that is
considered to be ‘standard of care’. Standard treatment for APL
together with HAART should be used in patients with HIV infection
when possible. Attempting to prolong the maintenance treatment
cycle may overcome the adverse effects of complex karyotype on
relapse-free survival but needs further clinical research. The
disease state of patients with APL with complex chromosome
karyotype needs to be monitored to identify early recurrence and
ultimately improve their prognoses. It is difficult to establish a
definite association between HIV and APL due to the scarcity of
cases. Multicenter clinical studies are needed to define
epidemiology, standardize cytogenetic/molecular features and
improve therapeutic management.
 | Table II.Previous cases of acute promyelocytic
leukemia with human immunodeficiency virus infection. |
Table II.
Previous cases of acute promyelocytic
leukemia with human immunodeficiency virus infection.
| First author/s,
year | HIV case | Age, years | Sex | HIV detection time,
months | ART | Risk group | CD4+ cell count,
/µl HIV RNA | Induction | Consolidation | Maintenance | Treatment
outcome | Survival
status | Observati on
period | (Refs.) |
|---|
| Kunitomi et
al, 2019 | 1 | 46 | Male | 5 | RAL, FTC, TDF | Intermediate | 264, 325 | ATRA, IDA | ATRA, IDA, MTZ | Not possible due to
liver dysfunction | CCR at 30
months | Alive | 30 months | (2) |
|
| 2 | 32 | Male | 5 | ABC/3T C, DRV,
RTV | Intermediate | 38, 75.4 | ATRA, IDA, MTZ | ATRA, IDA, MTZ | ATRA, MTX 6-MP | CCR at 38
months | Alive | 38 months | (2) |
| Mahmoud et
al, 2023 | 3 | 67 | Male | 0 | Biktarvy | Low | 491/548.74 | ATRA, ATO | ATRA, ATO | ATRA | CR at 4 weeks | Alive | 8 months | (4) |
| Drilon et
al, 2010 | 4 | 43 | Female | 0 | ATV, TVD, RAL | High | 118,
>500,000 | ATRA, IDA | ATRA, IDA, MTZ | ATRA, MTX,
6-MP | CR at day 29; CCR
at 8 months | Alive | 8 months | (6) |
| Mendes-de-Almeida
et al, 2022 | 5 | 49 | Male | 18 | ATV, 3TC, TDF | M3v/high | 673 | ATRA, Ara-C,
Dauno | Nil | Nil | Died on day 10 | Deceased | 10 days | (7) |
| Calvo et al,
1997 | 6 | 30 | Male | 24 | ND | Intermediate | 240, ND | ATRA | DNR, Ara-C,
MTZ | ND | CCR at 8
months | Alive | 8 months | (8) |
| Gatphoh et
al, 2001 | 7 | 22 | Female | ND | ND | High | ND, ND | ND | ND | ND | CR not reached | ND | ND | (9) |
| Sutton et
al, 2001 | 8 | 36 | Male | 0 | ND |
Low-intermediate | 400, ND | ATRA | ND | MTX, 6-MP | CR/relaps e day
305 | Deceased | 350 days | (10) |
| Kudva et al,
2004 | 9 | 27 | Male | 72 | IDV, 3TC, ZDV | Intermediate | 356,
undetectable | ATRA, IDA,
Ara-C | High dose
Ara-C | ATRA, MTX, 6-MP;
maintenance therapy interrupted due to liver dysfunction | Molecular CR at 9
weeks; CCR at 40 months | Alive | 40 months | (11) |
| De Vita et
al, 2006 | 10 | 46 | Female | 24 | EFV, TDF, 3TC | Intermediate | >500,
<50 | ATRA, IDA | ATRA, IDA, MTZ | 6-ATRA, 7-MTX,
8-MP | CCR at 21
months | Alive | 21 months | (12) |
| Boban et al,
2009 | 11 | 35 | Male | 120 | D4T, LPV | Intermediate | 184, <50 | ATRA, IDA | ATRA | ND | CCR at 14
months | Alive | 14 months | (13) |
| Malik et al,
2009 | 12 | 37 | Male | 7 | LPV/RTV 3TC, NVP,
DDI |
M3/intermediate | >800 | ATRA, IDA | NA | NA | CR at day 77;
relapse at 1 year and retreated with ATO; CR at 3 months and CCR at
17 months | Alive | 17 months | (14) |
| Present case | 13 | 49 | Female | 0 | ATV, TVD, RAL | High | ND | ATRA, ATO, THP | IDA, Ara-C | NS | CR at 4 weeks; CCR
at 2 months | Alive | 3 months | - |
 | Table III.
|
Table III.
| HIV case | Induction | Consolidation | Maintenance | (Refs.) |
|---|
| 1 | ATRA, IDA | ATRA, IDA, MTZ | Not possible due to
liver | (2) |
|
| Oral ATRA (45
mg/m2/d) divided into 2 | Three monthly
risk-adapted | dysfunction |
|
|
| daily doses, which
was maintained until | consolidation
cycles with ATRA | Because of liver
dysfunction |
|
|
| complete
hematologic remission and | (45
mg/m2/day for 15 days) and | due to fatty liver
(AST: |
|
|
| idarubicin (12
mg/m2/d) given as an | received a
reinforced dose of | 50-230 IU/L,
ALT:50-270 |
|
|
| intravenous bolus
on days 2, 4, 6, and 8 | idarubicin in the
first cycle | IU/L), he did not
receive |
|
|
| (ATRA and
idarubicin [AIDA] regimen) | (7
mg/m2/day) and third cycle | maintenance
therapy |
|
|
|
| (12
mg/m2/day for 2 days) |
|
|
| 2 | ATRA, IDA, MTZ | ATRA, IDA, MTZ | ATRA, MTX 6-MP | (2) |
|
| ATRA (45
mg/m2, po) D1-12 | ATRA (45
mg/m2/d) was given on | Details are not
described |
|
|
| Idarubicin (12
mg/m2, ivgtt) D13-14 | days 1 through 15
in combination | in the original
text |
|
|
| Cytarabine (100
mg/m2, ivgtt) D13-17 | with the 3
single-agent |
|
|
|
|
| chemotherapy
courses |
|
|
|
|
| Reinforcement of
chemotherapy |
|
|
|
|
| consolidation
consisted of |
|
|
|
|
| increasing the
idarubicin dose in the |
|
|
|
|
| first course to 7
mg/m2/d and of |
|
|
|
|
| administering
idarubicin for 2 |
|
|
|
|
| consecutive
days |
|
|
| 3 | ATRA, ATO | ATRA, ATO | ATRA | (4) |
|
| The patient was
started on all-trans | The patient
received a total of 4 | Details are not
described in |
|
|
| retinoic acid
(ATRA) and arsenic trioxide | cycles of ATO, with
plans to receive | the original
text |
|
|
| (ATO) for APL | ATRA, for a total
of 7 cycles as an |
|
|
|
|
| outpatient |
|
|
|
| Details are not
described in the original | Details are not
described in the |
|
|
|
| text | original text |
|
|
| 4 | ATRA, IDA | ATRA, IDA, MTZ | ATRA, MTX,
6-MP | (6) |
|
| Idarubicin 12
mg/m2 on days 2, 4, 6, and | A first course of
consolidation | ATRA,
methotrexate, |
|
|
| 8; ATRA 45
mg/m2 orally daily | chemotherapy with
ATRA and | mercaptopurine |
|
|
|
| idarubicin; a
second course | Details are not
described in |
|
|
|
| of ATRA and
mitoxantrone; | the original
text |
|
|
|
| a final course of
ATRA and |
|
|
|
|
| idarubicin |
|
|
|
|
| Details are not
described in the |
|
|
|
|
| original text |
|
|
| 5 | ATRA, Ara-C,
Dauno | Nil | Nil | (7) |
|
| Cytarabine and
daunorubicin protocol |
|
|
|
|
| (7+3) (idarubicin
was unavailable |
|
|
|
|
| nationally) |
|
|
|
|
| Details are not
described in the original |
|
|
|
|
| text |
|
|
|
| 6 | ATRA | DNR, Ara-C,
MTZ | ND | (9) |
|
| ATRA (45 mg/sqm/d
oral) | Daunorubicin (60
mg/sqm/d days |
|
|
|
|
| 1-3, bolus
intravenous injection) |
|
|
|
|
| Cytosine
arabinoside (200 mg/sqm/d |
|
|
|
|
| days 1–7 in
continuous intravenous |
|
|
|
|
| infusion) |
|
|
|
|
| Mitoxantrone |
|
|
|
|
| Details are not
described in the |
|
|
|
|
| original text |
|
|
| 7 | ND | ND | ND | (11) |
| 8 | ATRA | ND | MTX, 6-MP | (12) |
|
| ATRA (25 mg/d for 2
months, oral) |
| Mp (90 mg/d
oral) |
|
|
|
|
| Mtx (15 mg/w
oral) |
|
| 9 | ATRA, IDA,
Ara-C | High dose
Ara-C | ATRA, MTX,
6-MP; | (13) |
|
| ATRA (45
mg/m2) administered orally | High-dose
cytarabine | maintenance
therapy |
|
|
| Cytarabine (200
mg/m2) as a | (3 g/m2
q 12 hr ivgtt) for 6 days | interrupted due to
liver |
|
|
| continuous
intravenous infusion over | Daily ATRA
orally | dysfunction |
|
|
| 24 hr for 7 days
(days 3–9) | was continued
through consolidation | This was followed
by oral |
|
|
| Daunorubicin (50
mg/m2) as an intra | with high-dose
cytarabine | maintenance
therapy |
|
|
| venous bolus once
daily for 3 days |
| with ATRA (45
mg/m2) |
|
|
| (days 3–5) |
| daily for 15 days
every |
|
|
|
|
| 3 months |
|
|
|
|
| MTX 15
mg/m2 weekly; |
|
|
|
|
| 6-MP 50
mg/m2 |
|
|
|
|
| daily until 2
years |
|
|
|
|
| after
diagnosis |
|
| 10 | ATRA, IDA | ATRA, IDA, MTZ | 6-ATRA, 7-MTX,
8-MP | (14) |
|
| ATRA 45
mg/m2/d until CR | [ATRA 45
mg/m2 for 15 days; | Oral maintenance
with ATRA |
|
|
| Idarubicin 12
mg/m2/d (day 2,4,6,8) | IDA 5
mg/m2 (days 1,2,3,4)] | for 15 days every 3
months, |
|
|
|
| [ATRA 45
mg/m2 for 15 days; | methotrexate once
weekly, |
|
|
|
| Mitoxantrone 10
mg/m2 (days | 6-mercaptopurine
daily |
|
|
|
| 1,2,3,4,5)] | More details are
not described |
|
|
|
| [Idarubicine 12
mg/m2 (day 1); | in the original
text |
|
|
|
| ATRA 45
mg/m2 for 15 days)] |
|
|
| 11 | ATRA, IDA | ATRA | ND | (15) |
|
| ATRA at 45
mg/m2/day taken orally | ATRA was given
during March and |
|
|
|
| for 34 days | April 2007 as two
cycles of 45 mg/ |
|
|
|
| Idarubicine in dose
12 mg/m2/day | m2/day
for 42 days |
|
|
|
| intravenously
through 4 days |
|
|
|
| 12 | ATRA, IDA | NA | NA | (16) |
|
| Details are not
described in the original |
|
|
|
|
| text |
|
|
|
| 13 | ATRA, ATO, THP ATRA
(25 mg/m2 | IDA, Ara-C | NS | Our |
|
| oral daily) | Idarubicin (8
mg/sqm/d; days 1–3; |
| case |
|
| Pirarubicin
hydrochloride (45 mg/m2) | bolus intravenous
injection) |
|
|
|
| via a continuous
intravenous infusion | Cytosine
arabinoside (1 g/sqm/12 h; |
|
|
|
| over 24 h for
several days (Total of | days 1–3,
continuous intravenous |
|
|
|
| 80 mg; 20 mg*3 day,
10 mg*2 day) | infusion) |
|
|
|
| Arsenic trioxide
(0.16 mg/m2) as an |
|
|
|
|
| intravenous bolus
once daily for three |
|
|
|
|
| days |
|
|
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation Platform and
talent program of Hunan Province (grant no. 2021SK4050) and the
Natural Science Foundation of Hunan Province (grant no.
2023JJ30609).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
KS and XL conceived and designed the study. XL and
ML collected all relevant data of patients and drafted the
manuscript. LW coordinated the clinical management. JT and ZS
analyzed the data. KS revised the manuscript. KS and XL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent for publication of the case report
and any accompanying images, without any potentially identifying
information, was provided by the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palella FJ Jr, Delaney KM, Moorman AC,
Loveless MO, Fuhrer J, Satten GA, Aschman DJ and Holmberg SD:
Declining morbidity and mortality among patients with advanced
human immunodeficiency virus infection. HIV Outpatient Study
Investigators. N Engl J Med. 338:853–860. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kunitomi A, Hasegawa Y, Lmamura J,
Yokomaku Y, Tokunaga T, Miyata Y, Iida H and Nagai H: Acute
promyelocytic leukemia and HIV: Case reports and a review of the
literature. Intern Med. 58:2387–2391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta V, Shariff M, Bajwa R, Patel I,
Ayyad HA, Levitt MJ, Mencel PJ and Hossain MA: Acute myeloid
leukemia acquiring promyelocytic leukemia-retinoic acid receptor
alpha at relapse. World J Oncol. 10:153–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahmoud A, Ghrewati M, Kania B, Naseer M,
Kapoor A and Michael P: Aleukemic acute promyelocytic leukemia: How
Concomitant HIV, Hepatitis C, and chronic alcohol use disorder may
have hidden an underlying malignancy. Am J Case Rep.
24:e9380862023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Korsos V and Miller WH Jr: How retinoic
acid and arsenic transformed acute promyelocytic leukemia therapy.
J Mol Endocrinol. 69:T69–T83. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drilon AD, Gamboa EO, Koolaee R and Goel
A: Acute promyelocytic leukemia in HIV-infected adults: A case
report and review of therapeutic considerations. Clin Lymphoma
Myeloma Leuk. 10:E47–E52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendes-de-Almeida DP, Fernandez TS,
Lovatel VL, da Rocha MM, Gomes BE, Monte-Mór BCR, Vianna DT,
Alcoforado MTG, Kronemberg JMPB, Cardoso JPSC, et al: Acute
promyelocytic leukemia in a long-standing HIV-positive patient:
Case report and literature review. Leuk Res Rep.
18:1003392022.PubMed/NCBI
|
|
8
|
Calvo R, Ribera JM, Battle M, Sancho JM,
Granada I, Flores A, Millá F and Feliu E: Acute promyelocytic
leukemia in a HIV seropositive patient. Leuk Lymphoma. 26:621–624.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gatphoh ED, Zamzachin G, Devi SB and
Punyabati P: AIDS related malignant disease at regional institute
of medical sciences. Indian J Pathol Microbiol. 44:1–4.
2001.PubMed/NCBI
|
|
10
|
Sutton L, Guénel P, Tanguy ML, Rio B,
Dhedin N, Casassus P and Lortholary O; French Study Group on Acute
Myeloid Leukaemia in HIV–Infected Patients, : Acute myeloid
leukaemia in human immunodeficiency virus-infected adults:
Epidemiology, treatment feasibility and outcome. Br J Haematol.
112:900–908. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudva GC, Maliekel K, Richart JM, Batanian
JR, Grosso LE, Sokol-Anderson M and Petruska PJ: Acute
promyelocytic leukemia and HIV-1 infection: Case report and review
of the literature. Am J Hematol. 77:287–290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Vita S, De Matteis S, Laurenti L, Sorà
F, Tarnani M, Cingolani A and Sica S: Acute promyelocytic leukemia
in an HIV-infected patient: A case report. Am J Hematol.
81:3002006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boban A, Radman I, Zadro R, Dubravcic K,
Maretic T, Civljak R, Lisic M and Begovac J: Acute promyelocytic
leukemia after whole brain irradiation of primary brain lymphoma in
an HIV-infected patient. Eur J Med Res. 14:42–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malik A and Levine RL: The First Case
Report of APL (Acute Promyelocytic Leukemia) in An HIV Positive
Patient On (Highly Active Antiretroviral Therapy) Treated with
Arsenic Trioxide. Blood. 114:41662009. View Article : Google Scholar
|
|
15
|
Wu P, Sun W and Li J: Rheumatoid arthritis
patients with peripheral blood cell reduction should be evaluated
for latent Felty syndrome: A case report. Medicine (Baltimore).
99:e236082020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Liao Q, Hu Y and Zhong D: T
lymphocyte subset imbalances in patients contribute to ankylosing
spondylitis. Exp Ther Med. 9:250–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caprodossi S, Pedinotti M, Amantini C,
Santoni G, Minucci S, Pelicci PG and Fanelli M: Differentiation
response of acute promyelocytic leukemia cells and PML/RARa
leukemogenic activity studies by real-time RT-PCR. Mol Biotechnol.
30:231–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bolduc P, Roder N, Colgate E and Cheeseman
SH: Care of patients With HIV infection: Medical complications and
comorbidities. FP Essent. 443:16–22. 2016.PubMed/NCBI
|
|
19
|
Monroe J and Godwin JH: HIV/AIDS case
histories: Acute leukemia in an AIDS patient. AIDS Patient Care
STDS. 14:221–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forghieri F, Nasillo V, Bettelli F, Pioli
V, Giusti D, Gilioli A, Mussini C, Tagliafico E, Trenti T,
Cossarizza A, et al: Acute myeloid leukemia in patients living with
HIV Infection: Several questions, fewer answers. Int J Mol Sci.
21:10812020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aboulafia DM, Meneses M, Ginsberg S,
Siegel MS, Howard WW and Dezube BJ: Acute myeloid leukemia in
patients infected with HIV-1. AIDS. 16:865–876. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kane D, Keating S, McCann S and Mulcahy F:
The management of acute myeloid leukaemia (AML) in human
immunodeficiency virus (HIV) infection: A case report and review.
Int J STD AIDS. 8:272–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katsura M, Okuhama A, Koizumi Y, Ando N,
Yanagawa Y, Mizushima D, Aoki T, Tsukada K, Teruya K, Kikuchi Y, et
al: Progressive cytopenia developing during treatment of
cryptococcosis in a patient with HIV infection and bone marrow
cryptococcal infection. Intern Med. 61:257–261. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Labrador J, Luño E, Vellenga E, Brunet S,
González-Campos J, Chillón MC, Holowiecka A, Esteve J, Bergua J,
González-Sanmiguel JD, et al: Clinical significance of complex
karyotype at diagnosis in pediatric and adult patients with de novo
acute promyelocytic leukemia treated with ATRA and chemotherapy.
Leuk Lymphoma. 60:1146–1155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wiernik PH, Sun Z, Gundacker H, Dewald G,
Slovak ML, Paietta E, Kim HT, Appelbaum FR, Cassileth PA and
Tallman MS: Prognostic implications of additional chromosome
abnormalities among patients with de novo acute promyelocytic
leukemia with t(15;17). Med Oncol. 29:2095–2101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai BB, Mu QT, Zhang YL, Chen Y and Ouyang
GF: Effect of Chromosomal Karyotype on the Prognosis of Patients
with Acute Promyelocytic Leukemia in Condition of the Maintenance
Treatment Based on Arsenic Trioxide. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 27:1380–1386. 2019.(In Chinese). PubMed/NCBI
|
|
27
|
Wan TS, Ma SK, Au WY, Liu HS, Chan JC and
Chan LC: Trisomy 21 and other chromosomal abnormalities in acute
promyelocytic leukemia. Cancer Genet Cytogenet. 140:170–173. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vu MP, Nguyen CN and Vu H: Cytogenetic
influence on prognosis in acute promyelocytic leukaemia: A cohort
study in vietnam. Hematol Oncol Stem Cell Ther. 15:151–153.
2022.PubMed/NCBI
|
|
29
|
Epstein-Peterson ZD, Derkach A, Geyer S,
Mrózek K, Kohlschmidt J, Park JH, Rajeeve S, Stein EM, Zhang Y,
Iland H, et al: Effect of additional cytogenetic abnormalities on
survival in arsenic trioxide-treated acute promyelocytic leukemia.
Blood Adv. 6:3433–3439. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cingam SR and Koshy NV: Acute
Promyelocytic Leukemia. 2022 Jun 27. StatPearls [Internet].
StatPearls Publishing; Treasure Island, FL: 2023
|
|
31
|
Rosenbaum MW, Pozdnyakova O, Geyer JT, Dal
Cin P and Hasserjian RP: Ring chromosome in myeloid neoplasms is
associated with complex karyotype and disease progression. Hum
Pathol. 68:40–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stahl M and Tallman MS: Acute
promyelocytic leukemia (APL): Remaining challenges towards a cure
for all. Leuk Lymphoma. 60:3107–3115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Avvisati G, Lo-Coco F, Paoloni FP, Petti
MC, Diverio D, Vignetti M, Latagliata R, Specchia G, Baccarani M,
Di Bona E, et al: GIMEMA, AIEOP, and EORTC Cooperative Groups. AIDA
0493 protocol for newly diagnosed acute promyelocytic leukemia:
Very long-term results and role of maintenance. Blood.
117:4716–4725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Diep R and Metjian A: A rare CALR variant
mutation and a review of CALR in essential thrombocythemia. J
Thromb Thrombolysis. 45:457–462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Liu X, Chen H, Xie P, Ma R, He J and
Zhang H: Bioinformatics analysis for the role of CALR in human
cancers. PLoS One. 16:e02612542021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martins I, Kepp O, Galluzzi L, Senovilla
L, Schlemmer F, Adjemian S, Menger L, Michaud M, Zitvogel L and
Kroemer G: Surface-exposed calreticulin in the interaction between
dying cells and phagocytes. Ann N Y Acad Sci. 1209:77–82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Papp S, Fadel MP, Kim H, McCulloch CA and
Opas M: Calreticulin affects fibronectin-based cell-substratum
adhesion via the regulation of c-Src activity. J Biol Chem.
282:16585–16598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Merlinsky TR, Levine RL and Pronier E:
Unfolding the role of calreticulin in myeloproliferative neoplasm
pathogenesis. Clin Cancer Res. 25:2956–2962. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao Z, Chen CC, Rillahan CD, Shen R,
Kitzing T, McNerney ME, Diaz-Flores E, Zuber J, Shannon K, Le Beau
MM, et al: Cooperative loss of RAS feedback regulation drives
myeloid leukemogenesis. Nat Genet. 47:539–543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kayser S, Feszler M, Krzykalla J, Schick
M, Kramer M, Benner A, Thol F, Platzbecker U, Müller-Tidow C, Ho
AD, et al: Clinical impact of KMT2C and SPRY4 expression levels in
intensively treated younger adult acute myeloid leukemia patients.
Eur J Haematol. 99:544–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garg M, Nagata Y, Kanojia D, Mayakonda A,
Yoshida K, Haridas Keloth S, Zang ZJ, Okuno Y, Shiraishi Y, Chiba
K, et al: Profiling of somatic mutations in acute myeloid leukemia
with FLT3-ITD at diagnosis and relapse. Blood. 126:2491–2501. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ciftciler R, Haznedaroglu IC, Aksu S,
Ozcebe O, Sayınalp N, Malkan UY and Buyukasık Y: The factors
affecting early death in newly diagnosed apl patients. Open Med
(Wars). 14:647–652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
O'Donnell MR, Tallman MS, Abboud CN,
Altman JK, Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE,
Damon LE, et al: National Comprehensive Cancer Network. Acute
myeloid leukemia, version 2.2013. J Natl Compr Canc Netw.
11:1047–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li X, Wang C, Chen G, Ji B and Xu Y:
Combined chemotherapy for acute promyelocytic leukemia: A
meta-analysis. Hematology. 22:450–459. 2017.PubMed/NCBI
|
|
45
|
Xiao M, Zhou P, Liu Y, Wei S, Li D, Li W,
Niu X, Niu J, Zhang Y, Cao W, et al: Predictive factors for
differentiating thrombohemorrhagic disorders in high-risk acute
promyelocytic leukemia. Thromb Res. 210:33–41. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jillella AP and Kota VK: The global
problem of early deaths in acute promyelocytic leukemia: A strategy
to decrease induction mortality in the most curable leukemia. Blood
Rev. 32:89–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pei Y, Shi M, Song J, Niu X, Wei S, Dou L,
Xiao M, Li D, Xu F, Bai Y and Sun K: Absolute circulating leukemic
cells as a risk factor for early bleeding events in patients with
non-high-risk acute promyelocytic leukemia. Cancer Manag Res.
13:4135–4146. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saris A and Pavenski K: Human leukocyte
antigen alloimmunization and alloimmune platelet refractoriness.
Transfus Med Rev. 34:250–257. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Prodger CF, Rampotas A, Estcourt LJ,
Stanworth SJ and Murphy MF: Platelet transfusion: Alloimmunization
and refractoriness. Semin Hematol. 57:92–99. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Trinkley KE, Page RL II, Lien H, Yamanouye
K and Tisdale JE: QT interval prolongation and the risk of torsades
de pointes: Essentials for clinicians. Curr Med Res Opin.
29:1719–1726. 2013. View Article : Google Scholar : PubMed/NCBI
|