Introduction
The incidence of esophageal adenocarcinoma (EAC) and
adenocarcinoma of the gastroesophageal junction has gradually
increased over the last three decades (Iincidence rate ratio, 2.45)
(1). Despite stable incidence rates
since then, the survival of these patients remains poor, which
highlights the need for biomarkers for early disease detection and
novel therapeutic strategies (1). A
hallmark of cancer is altered cellular metabolism (2). The energy production in cancer cells
is characterized by increased levels of the less efficient
oxygen-independent glycolysis, followed by lactate production.
While these pathways are also present in healthy cells, they are
overly activated in cancer cells (3). The expression levels of distinct
enzymes of the glycolysis pathway [hexokinase (HK)1 and pyruvate
kinase isozyme M2] have been shown to correlate with disease
progression, cancer cell invasion and poor patient survival in
esophageal squamous cell cancer (ESCC) (4). The first step of glycolysis is the
phosphorylation of glucose to glucose-6-phosphate by the HK enzyme;
five different isozymes of HK have been reported, with, for
instance, type I facilitating catabolic functions through
mitochondrial interaction and utilizing intramitochondrial ATP,
type II potentially serving anabolic roles, type III primarily
localized perinuclearly with less understood functions, type IV
involved in carbohydrate metabolism as a glucose sensor, and type V
involved in the gestational glucose regulation (5–8). These
isozymes show tissue-specific expression patterns and varying
affinities towards glucose. The expression of HK2, one of the five
isozymes, in human adults is limited, as HK2 is only expressed in
skeletal muscle and adipose tissues under physiological conditions
(6). However, HK2 becomes
upregulated in cancer cells (9).
Additionally, HK2 expression levels are increased in esophageal
cancer compared with those in normal esophageal tissues (10). Therefore, HK2 is not only of
interest in metabolic pathway studies or as a sole biomarker, but
also as a potential target for novel therapeutic options for
patients with esophageal cancer.
The increased activation of glycolysis and the
subsequent increased demand for glucose in cancer cells have become
integral elements in cancer diagnostics, notably in
fluorodeoxyglucose-positron emission tomography (FDG-PET) (11). Previous reports on the correlation
between FDG uptake and HK2 expression vary. In colorectal cancer
and cholangiocarcinoma, no correlation between FDG uptake and HK2
expression was detected. However, increased HK2 expression levels
were positively correlated with increased FDG uptake in
hepatocellular carcinoma (11,12).
This suggests that different enzymes of glycolysis are activated
depending on different tumor entities and therefore different tumor
microenvironments.
In a previous expression pattern study, high HK2
expression levels were associated with poor patient outcomes, as
well as higher tumor stages, the occurrence of lymph node
metastases and increased tumor size in colorectal cancer, gastric
cancer and hepatocellular cancer (13).
HK2 expression levels have been reported as
significantly increased in ESCC compared with those in EAC
(14). However, the role of HK2 and
its prognostic implications in EAC are currently unclear.
Therefore, the present study aimed to elucidate the impact of HK2
expression levels on the oncological outcome of patients with
EAC.
Materials and methods
Patients and tumor samples
The study present was approved by the Ethics
Committee of the University Hospital of Cologne (approval no.
21-1146; Cologne, Germany) and was conducted in accordance with the
Declaration of Helsinki. The study was reported in line with the
Strengthening the Reporting of Observational Studies in
Epidemiology guidelines (15).
Patients from 1998 until 2019 at the University Hospital of Cologne
(Cologne, Germany) were screened for the present retrospective,
single-center cohort study. Inclusion criteria were as follows: A
diagnosis of EAC, patients underwent an Ivor-Lewis esophagectomy,
curative treatment intention, and sufficient tumor tissue was
available for the tissue microarray. All patient data were
collected prospectively and analyzed retrospectively for the
present study. Written informed consent for inclusion in the
database and tissue bank was obtained from each patient. The median
age of all included patients was 63.1 years (range, 27.8–91.6
years). Overall survival (OS) was defined as the time from the date
of surgery until death or being censored in case of loss of
follow-up and was updated yearly. Patients who had experienced
survival periods of <90 days postoperatively or lacked
sufficient tissue for the subsequent analysis were excluded from
the present study. The pathological assessment of tumor samples was
conducted according to the 7th edition of the Union for
International Cancer Control (16).
The tumor borders of the primary tumor tissue samples were
demarcated by an experienced pathologist and 1.2-mm tissue
cylinders were punched out using a semi-automated precision
instrument. The tissue cylinders were then transferred to a
paraffin-embedded tissue microarray and were cut into 4-µm slices.
Tissues were fixed in a 4% formaldehyde solution at room
temperature for 24 h, followed by embedding in paraffin.
Fluorescence in situ hybridization
(FISH)
FISH was conducted as described in a previous study
(17). Briefly, analysis was
performed for the long (green) and short (red) arm of the Y
chromosome [cat. no. (long), 05J10-024; cat. no. (short),
05J27-079; Abbott]. The ready-to-use FISH pretreatment kit was
utilized (Vysis IntelliFISH Universal FFPE Tissue Pretreatment
Protease; cat. no. 08N85-005; Abbott), all in accordance with the
manufacturer's instructions. A fully automated upright fluorescence
microscope Leica DM5500 B (Leica Microsystems) was used. The
imaging was performed with a JVC KY-F75 digital camera (JVCKenwood)
(Fig. S1A and B). FISH data were
analyzed by two experienced pathologists. The absence of green and
red staining was defined as Y chromosome loss. Internal controls
were performed by screening normal epithelial tissue, fibroblasts
or lymphocytes on the tumor sample slide. Samples were excluded if
no clear control could be obtained.
Immunohistochemistry (IHC)
HK2 staining was conducted using the automatic
staining system Leica BOND-MAX (Leica Biosystems). Dilutions,
reagents and control tissues were used according to the
manufacturer. Here, the polymer refine detection kit BOND Epitope
retrieval Solution 1 (cat. no AR9961; Leica Biosystems) was used
(100°C for 5 min) to perform the automated staining according to
the manufacturer's instructions. IHC staining was performed using
primary antibodies for HER2 [cat. no. 4B5; Roche Diagnostics; with
EDTA (BOND Epitope retrieval Solution 1; Leica Biosystems) as a
buffer for the epitope retrieval; with positive control breast
carcinoma cells previously confirmed to be HER2-positive] and HK2
[1:500; cat. no. ab104836; Abcam; with EDTA (BOND Epitope retrieval
Solution 1, Leica Biosystems) as a buffer for the antigen
retrieval; with negative control normal human esophagus epithelium
cells]. HER2 and HK2 staining was analyzed by two experienced
pathologists. HER2 staining was defined as either negative or
positive. The staining intensity and the percentage of positive
cancer cells of the HK2 staining were assessed to calculate a
H-score, which was computed using these parameters as previously
described (18). The patient cohort
was divided into two groups using the median H-score: Low
expression of HK2 (H-score <100) and high expression of HK2
(H-score ≥100).
RNAScope™ for HK2
The RNAScope™ assay was performed as
described previously, following the manufacturer's instructions
(19). According to the user manual
of the kit, 5-µm thick tissue microarray sections were
deparaffinized, pretreated, digested, hybridized and counterstained
using hematoxylin before developing the signal, using the provided
RNAScope 2.5 HD Assay-RED (cat. no. 322360; Bio-Techne) and mRNA
probe RNAscope Probe-Hs-HK2 (cat. no. 487731; Advanced Cell
Diagnostics; Bio-Techne). Analyses of the RNAScope assay results
were performed independently by two experienced pathologists using
a Leica DM2500 light microscope (Leica Microsystems). For imaging,
the slides were scanned with the Aperio GT 450 DX (Leica
Biosystems). Signal scoring followed the manufacturer's guidelines
(score 0, <1 dots/cell; score 1, 1-3 dots/cell; score 2, 4-9
dots/cell; score 3, 10-15 dots/cell; and score 4, >15
dots/cell). Positivity was defined as a score >0, which
reflected the presence of detectable signals according to the
specified scoring criteria.
Validation of the IHC HK2
antibody
HK2 IHC results were compared with results using the
aforementioned RNAscope mRNA in situ hybridization probes
targeting HK2 in 10 early-stage esophageal adenocarcinoma cases,
which were included in the total study cohort, to validate use of
the HK2 antibody. In all cases, the expression levels detected by
the IHC HK2 antibody corresponded with those identified by
RNAScope, demonstrating agreement between the HK2 expression levels
detected using both methods. Of these, the four HK2 positive cases
exhibited high HK2 expression levels using both assays, which
confirmed the accuracy and reliability of the immunohistochemical
HK2 antibody.
Statistical analysis
All statistical analyses were conducted using SPSS
(version 29.0.1.1; IBM Corp.). Survival data are presented as
Kaplan-Meier curves and were analyzed using the log-rank test.
Associations between clinicopathological values and survival data
were assessed using univariate and multivariate Cox regression
analyses. Qualitative values were compared using the χ2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Correlation between HK2 expression and
clinicopathological values
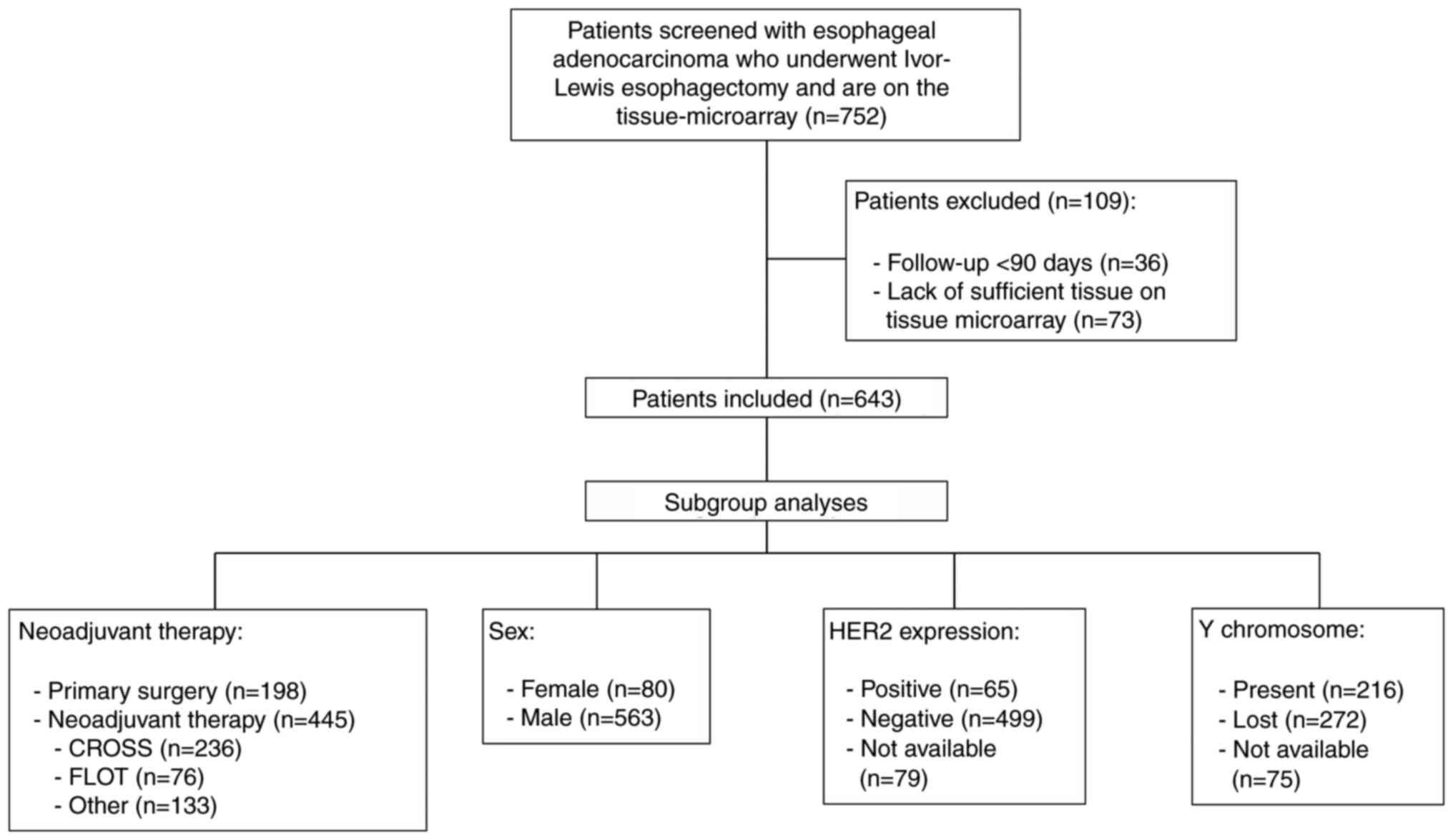
In the present study, 643 patients with EAC who
underwent Ivor-Lewis esophagectomy at the University Hospital of
Cologne were included (Fig. 1). A
large proportion of the included patients were male (87.6%). The
median OS time of the total patient cohort was 24.0 months.
Neoadjuvant therapy was administered to 69.2% of patients (n=445).
Lymph node metastases were diagnosed in 59.6% of the included
patients (n=383) (Table I). The
study cohort was stratified into two groups using the median
H-score for IHC staining: i) Tumors with low HK2 expression
(n=307); and ii) tumors with high HK2 expression (n=336) (Fig. 2A). IHC staining was verified through
comparison with RNAScope HK2 staining (Fig. S1C and D). In all cases evaluated,
the RNAScope and IHC results corresponded, which confirmed the
association between HK2 mRNA and protein expression levels. The
clinicopathological characteristics between these two groups were
compared (Table I). High HK2
expression levels were significantly associated with patients who
underwent perioperative therapy with chemoradiotherapy for
oesophageal cancer followed by surgery (CROSS; P=0.033). No other
significant differences between patients with low and high HK2
expression in all other variables mentioned in Table I could be detected.
 | Figure 2.Representative immunohistochemical
staining and survival analyses of patients with EAC. (A)
Representative immunohistochemical images of tumor samples with
high (left) and low (right) HK2 expression levels. Kaplan-Meier
curves for overall survival classed by HK2 expression levels of (B)
the total patient cohort (low, n=307; high, n=336; P=0.027) and the
following subgroups: (C) Primarily resected patients (low, n=101;
high, n=97; P=0.013), (D) patients following neoadjuvant therapy
(low, n=206, high, n=239; P=0.391), (E) patients who received CROSS
(low, n=95; high, n=141; P=0.400) and (F) patients who received
FLOT as perioperative treatment (low, n=41; high, n=35; P=0.712).
Scalebar, 50 µm. CROSS, Chemoradiotherapy for Oesophageal Cancer
Followed by Surgery Study; FLOT, fluorouracil, leucovorin,
oxaliplatin, docetaxel. |
 | Table I.General clinicopathological values of
the total study population (n=643) and patients with low (n=307) or
high (n=336) HK2 expression levels. |
Table I.
General clinicopathological values of
the total study population (n=643) and patients with low (n=307) or
high (n=336) HK2 expression levels.
| Characteristic | Patients | Low HK2
expression | High HK2
expression | P-value |
|---|
| Total patients, n
(%) | 643 (100.0) | 307 (100.0) | 336 (100.0) |
|
| Sex, n (%) |
|
|
| 0.250 |
|
Male | 563 (87.6) | 264 (86.0) | 299 (89.0) |
|
|
Female | 80 (12.4) | 43 (14.0) | 37 (11.0) |
|
| Age, n (%) |
|
|
| 0.401 |
| <65
years | 365 (56.8) | 169 (55.0) | 196 (58.3) |
|
| ≥65
years | 278 (43.2) | 138 (45.0) | 140 (41.7) |
|
| Alcohol, n (%) |
|
|
| 0.666 |
| No | 181 (28.1) | 91 (29.6) | 90 (26.8) |
|
|
Yes | 56 (8.7) | 30 (9.8) | 26 (7.8) |
|
|
Unknown | 406 (63.1) | 186 (60.6) | 220 (65.5) |
|
| Nicotine, n
(%) |
|
|
| 0.467 |
| No | 118 (18.4) | 65 (21.2) | 53 (15.8) |
|
| Active
smoker | 78 (12.1) | 37 (12.1) | 41 (12.2) |
|
| Former
smoker | 106 (16.5) | 51 (16.6) | 55 (16.4) |
|
|
Unknown | 341 (53.0) | 154 (50.2) | 187 (55.7) |
|
| Median overall
survival (range), months | 24.0
(3.0–233.6) | 25.4
(3.0–214.8) | 22.9
(3.0–233.6) |
|
| Neoadjuvant
therapy, n (%) |
|
|
| 0.269 |
| No | 198 (30.8) | 101 (32.9) | 97 (28.9) |
|
|
Yes | 445 (69.2) | 206 (67.1) | 239 (71.1) |
|
| Neoadjuvant therapy
regime, n (%) |
|
|
| 0.033 |
|
CROSS | 236 (53.0) | 95 (46.1) | 141 (59.0) |
|
|
FLOT | 76 (17.1) | 41 (19.9) | 35 (14.6) |
|
|
Other | 133 (29.9) | 70 (34.0) | 63 (26.4) |
|
| (y)pT, n (%) |
|
|
| 0.236 |
| 1 | 122 (19.0) | 68 (22.1) | 54 (16.1) |
|
| 2 | 106 (16.5) | 49 (16.0) | 57 (17.0) |
|
| 3 | 394 (61.3) | 179 (58.3) | 215 (64.0) |
|
| 4 | 21 (3.3) | 11 (3.6) | 10 (3.0) |
|
| (y)pN, n (%) |
|
|
| 0.483 |
| 0 | 260 (40.4) | 131 (42.7) | 129 (38.4) |
|
| 1 | 182 (28.3) | 86 (28.0) | 96 (28.6) |
|
| 2 | 106 (16.5) | 51 (16.6) | 55 (16.4) |
|
| 3 | 95 (14.8) | 39 (12.7) | 56 (16.7) |
|
| L, n (%) |
|
|
| 0.682 |
| 0 | 292 (45.4) | 142 (46.3) | 150 (44.6) |
|
| 1 | 351 (54.6) | 165 (53.7) | 186 (55.4) |
|
| V, n (%) |
|
|
| 0.603 |
| 0 | 478 (74.3) | 229 (74.6) | 249 (74.1) |
|
| 1 | 68 (10.6) | 29 (9.4) | 39 (11.6) |
|
| 2 | 97 (15.1) | 49 (16.0) | 48 (14.3) |
|
| Pn, n (%) |
|
|
| 0.586 |
| 0 | 416 (64.7) | 200 (65.1) | 216 (64.3) |
|
| 1 | 131 (20.4) | 58 (18.9) | 73 (21.7) |
|
| 2 | 96 (14.9) | 49 (16.0) | 47 (14.0) |
|
| G, n (%) |
|
|
| 0.285 |
| 1 | 1 (0.2) | 1 (0.3) | 0 (0.0) |
|
| 2 | 103 (16.0) | 56 (18.2) | 47 (14.0) |
|
|
3/4 | 86 (13.4) | 39 (12.7) | 47 (14.0) |
|
| Not
applicable or unknown | 453 (70.5) | 211 (68.7) | 242 (72.0) |
|
High HK2 expression is associated with
worse patient survival in the total cohort
Survival analyses was performed to assess the impact
of HK2 expression on patient survival. High HK2 expression levels
were significantly associated with reduced patient times survival
in the total cohort (median OS, 29.6 vs. 39.9 months, respectively;
P=0.027; Fig. 2B).
HK2 expression levels are correlated
with patient survival in primarily resected patients, but not in
neoadjuvant-treated patients
Due to the standard use of multimodal therapy for a
large portion of patients with EAC, the patient cohort was divided
into two subsets: i) Patients who had received neoadjuvant
treatment; and ii) patients who had undergone primary surgery
without preceding adjuvant interventions. The aforementioned impact
on patient survival was substantiated within the subgroup of
primarily resected patients, as high HK2 expression was
significantly associated with reduced patient survival time in the
primary surgery cohort (median OS, 33.9 vs. 140.9 months,
respectively; P=0.013; Fig. 2C).
However, no significant difference was demonstrated with regard to
the impact of HK2 expression levels on patient survival for those
who underwent neoadjuvant therapy (median OS, 28.1 vs. 31.6 months,
respectively; P=0.391; Fig. 2D).
Furthermore, survival analyses were conducted for two perioperative
therapy regimes, namely, CROSS and fluorouracil, leucovorin,
oxaliplatin and docetaxel (FLOT). No significant survival
differences associated with HK2 expression levels were demonstrated
in the CROSS (median OS, 27.2 vs. 26.8 months, respectively;
P=0.400; Fig. 2E) or the FLOT
(median OS, 24.1 vs. 22.8 months, respectively; P=0.712; Fig. 2F) subgroups.
High HK2 expression is an independent
risk factor for worse patient survival in multivariate Cox
regression analysis
Cox regression analysis was performed to assess the
association between clinicopathological values and patient
survival. In univariate analyses, high pathological tumor status
(y)pT-status, high pathological lymph node status (y)pN-status,
high grading (G) and high HK2 expression were associated with
reduced patient survival in the total patient cohort [(y)pT,
P<0.001; (y)pN, P<0.001; G, P=0.001; HK2 expression, P=0.028;
Table SI]. In multivariate
analyses, high HK2 expression levels were demonstrated to be an
independent risk factor, as a significant association with reduced
patient OS was demonstrated (HR, 1.629; 95% CI, 1.077–2.465;
P=0.021; Table II). Additionally,
(y)pT-, (y)pN- and lymphatic vessel invasion (L)-status were
associated with significantly reduced patient survival, thus
representing independent risk factors for patients with EAC [(y)pT,
P=0.009; (y)pN, P<0.001; L-status, P=0.009; Table II]. Similar findings could be found
in the primarily resected subgroup. Here, higher age, higher
(y)pT-, (y)pN- and G-status, and high HK2 expression were
correlated with worse patient survival in the univariate analyses
[age, P<0.001; (y)pT, P<0.001; (y)pN, P<0.001; G, P=0.002;
HK2 expression, P=0.014; Table
SI]. In the multivariate analyses, (y)pT-, (y)pN-stage and high
HK2 expression were independent risk factors for reduced patient
survival [(y)pT, P=0.004; (y)pN, P<0.001; HK2 expression,
P=0.040; Table II].
 | Table II.Multivariate Cox regression analyses
of the total cohort, patients following primary surgery and
patients with negative HER2 expression. |
Table II.
Multivariate Cox regression analyses
of the total cohort, patients following primary surgery and
patients with negative HER2 expression.
|
|
| Total cohort | Primary
surgery | Negative HER2
expression |
|---|
|
|
|
|
|
|
|---|
| Characteristic | Borders | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, years | ≥65 vs. <65 | 1.394 | 0.863–2.252 | 0.174 | 1.445 | 0.895–2.331 | 0.132 | 1.590 | 0.909–2.779 | 0.104 |
| (y)pT | ≥2 vs. 1 | 1.512 | 1.107–2.066 | 0.009 | 1.568 | 1.153–2.133 | 0.004 | 1.462 | 1.036–2.064 | 0.031 |
| (y)pN | ≥1 vs. 0 | 1.497 | 1.185–1.891 | <0.001 | 1.582 | 1.264–1.980 | <0.001 | 1.449 | 1.112–1.887 | 0.006 |
| L, stage | 1 vs. 0 | 2.121 | 1.208–3.724 | 0.009 | - | - | - | 1.582 | 0.824–3.035 | 0.168 |
| V, stage | ≥1 vs. 0 | 0.692 | 0.501–0.954 | 0.025 | 0.882 | 0.671–1.161 | 0.371 | 0.763 | 0.519–1.122 | 0.170 |
| G, stage | ≥2 vs. 1 | 1.055 | 0.670–1.661 | 0.817 | 1.216 | 0.784–1.887 | 0.382 | 1.102 | 0.639–1.899 | 0.727 |
| Hexokinase 2
expression | high vs. low | 1.629 | 1.077–2.465 | 0.021 | 1.541 | 1.021–2.327 | 0.040 | 1.687 | 1.029–2.764 | 0.038 |
High HK2 expression was associated with worse
patient survival in male patients, HER2-negative tumors and tumors
with Y chromosome loss.
Since patient sex, HER2 expression levels and Y
chromosome loss are reported factors that influence patient
survival in patients with EAC (20–22),
further analysis of the impact of HK2 expression on patient
survival in these subgroups was performed. The patient cohort was
divided into subgroups based on sex (female or male), HER2
expression (negative or positive) and the state of the Y chromosome
in the tumor cells [present (positive) or lost (negative)].
Patients with high HK2 expression showed a significantly reduced OS
in subgroups of male patients, HER2-negative tumors and tumors with
Y chromosome loss (sex, P=0.038; HER2 status, P=0.020; Y chromosome
loss, P=0.018; Fig. 3A-F).
 | Figure 3.Survival analyses of subgroups.
Kaplan-Meier curves for overall survival classed by HK2 expression
levels of (A) male (low, n=264; high, n=299; P=0.038) and (B)
female patients (low, n=43; high, n=37; P=0.512). The impact of HK2
expression levels on patient survival in subgroups of patients with
(C) negative (low, n=234; high, n=265; P=0.020) and (D) positive
(low, n=31; high, n=34; P=0.445) HER2 expression, and (E) negative
(low, n=124; high, n=148; P=0.018) and (F) positive (low, n=99;
high, n=117; P=0.925) Y chromosome expression. |
Multivariate analyses confirm high HK2
expression as an independent risk factor for reduced patient
survival in the subgroup of HER2-negative tumors
Univariate and multivariate Cox regression analyses
were performed on the sex, HER2 expression level and Y chromosome
patient subgroups. In univariate analyses, high HK2 expression
levels were associated with worse patient survival in the subgroup
of patients with tumors with Y chromosome loss (HR, 1.465; 95% CI,
1.066–2.014; P=0.018; Table SII)
and male patients (HR, 1.262; 95% CI, 1.012–1.572; P=0.039;
Table SII). However, high HK2
expression was not significantly associated with patient survival
in the subgroup of patients with tumors with Y chromosome loss (HR,
1.505; 95% CI, 0.830–2.727; P=0.178; Table SIII) or male patients in
multivariate analyses (HR, 1.395; 95% CI, 0.889–2.189; P=0.147;
Table SIII). In HER2-negative
tumors, the expression level of HK2 was significantly associated
with reduced patient survival in univariate and multivariate
analyses (univariate: HR, 1.332; 95% CI, 1.045–1.697, P=0.021;
Table SI; Multivariate: HR, 1.687;
95% CI, 1.029–2.764, P=0.038; Table
II). In addition, (y)pT- and (y)pN-status were significantly
associated with reduced patient survival and thus represented risk
factors for reduced patient survival [(y)pT, P=0.031; (y)pN,
P=0.006; Table II].
No survival difference is observed in
subgroups with specific risk behavior, such as smoking, in regard
to different HK2 expression levels
The impact of reported high-risk behaviors such as
nicotine and alcohol consumption on the association between HK2
expression levels and patient survival was investigated. No
significant differences in the HK2 expression levels were
demonstrated in these subgroups (Table
I). From survival analyses, HK2 expression levels showed no
significant survival differences associated with smoking and
alcohol consumption status (non-smoker, P=0.239; smoker, P=0.252;
no alcohol consumption, P=0.504; alcohol consumption, P=0.889;
Fig. S2).
In summary, the present study demonstrated that high
HK2 expression levels may potentially represent a risk factor
associated with the reduced OS of patients with EAC and in patients
with EAC who underwent primary resection. Moreover, high HK2
expression levels additionally represent a potential risk factor
for the specific high-risk subgroups of male patients and tumors
with Y chromosome loss.
Discussion
The present study aimed to evaluate the prognostic
significance of HK2 in patients diagnosed with EAC. The expression
levels of HK2 were analyzed in the tumor samples from 643 patients
with EAC and survival and subgroup analyses were performed. High
HK2 expression levels were significantly associated with reduced
patient OS time. Furthermore, high HK2 expression levels were
identified as an independent risk factor for reduced OS time of
patients with EAC. To the best of our knowledge, this is the first
report of the prognostic value of HK2 expression levels in EAC;
however, similar effects for high HK2 expression have been reported
in gastric adenocarcinoma and breast cancer (23,24).
Patients with high HK2 expression levels could potentially benefit
from more frequent follow-up exams but also from more aggressive
neoadjuvant therapy options. In the future, HK2 expression levels
could be assessed in biopsy material obtained during endoscopic
evaluation before the initiation of neoadjuvant therapy, as these
biopsies are routinely performed as part of the primary staging
process (25). Further subgroup
analyses would be necessary to confirm this hypothesis. The present
study demonstrated HK2 expression levels to be an independent risk
factor for patients with EAC following primary surgery. HK2
expression levels did not show prognostic value in patients who
underwent esophagectomy after neoadjuvant therapy. Consistent with
this observation, a previous study reported that neoadjuvant
radiochemotherapy or chemotherapy induces general alterations in
gene expression in EAC (26).
Furthermore, HK2 expression levels differ significantly between
pretreated patients and therapy-naive patients with EAC (27). Future studies could assess HK2
expression levels in pre- and post-neoadjuvant biopsies to further
evaluate the prognostic value of varying HK2 expression during
therapy. FDG uptake, a marker for glucose demand in cells used in
staging exams, negatively correlates with HK2 expression in
patients with esophageal carcinoma, and its increase is associated
with worse patient survival and more frequent detection in patients
with EAC (14), which could further
suggest prognostic value for the assessment of HK2 expression
levels. Patients with high HK2 expression levels could potentially
represent a high-risk patient group, which may have otherwise been
classified as low-risk based on the FDG-PET result. However, a
previous clinical trial reported a correlation between HK2
expression and the accumulation of FDG (28). Therefore, further clinical studies
are needed to assess the prognostic implications of the alteration
in HK2 expression levels during tumor progression and therapy.
The prognostic value of HK2 in subgroups defined by
the expression or loss of previously described biomarkers (for
instance, Y chromosome loss and HER2-negative tumors) for patients
with EAC were assessed in the present study. High HK2 expression
was associated with worse patient survival in the subgroups of male
patients and tumors with Y chromosome loss, which have previously
described as high-risk groups (20,21).
Furthermore, high HK2 expression levels were identified as an
independent risk factor for reduced survival in patients with
HER2-negative tumors, which is a subgroup that has previously been
associated with improved patient survival (22).
HK2 could also be targeted as a potential
therapeutic option for patients with EAC and hypothetically other
cancer types. For instance, in head and neck squamous cell
carcinoma, HK2 knockdown showed decreased cell growth in
vitro and inhibited tumor progression in vivo (29). Increased HK2 expression levels are
associated with decreased patient survival in nasopharyngeal
carcinoma, and inhibition of HK2 using the glycolysis inhibitor
3-bromo-2-oxopropionate-1-propyl-ester reduces cell proliferation
and invasion while increasing cell apoptosis in vitro
(30). Capsaicin, which naturally
occurs in red hot peppers, is recognized for its anticancer
properties (31). Mao et al
(32) reported that use of
capsaicin resulted in reduced HK2 expression levels in ESCC cells.
Glucose consumption and lactate production of these cells were
reduced by capsaicin in the study. High lactate levels are reported
to correlate with poorer patient survival (33). Diclofenac, a widely used analgesic,
is also described as an anticancer drug due to its ability to
reduce lactate levels in in vivo glioma models, which alters
the immune microenvironment and could help to overcome immune
escape mechanisms (34,35). HK2 expression levels were also
related to altered immune cell infiltrates (36). Therefore, in the future,
manipulation of HK2 expression levels via inhibitors could
potentially help to overcome tumor resistance caused by immune
escape. Nonetheless, the targeting of HK2 expression in patients
with cancer should be further investigated, as HK2 also
participates for in a number of physiological mechanisms of the
glucose metabolism, including phosphorylation of glucose, anabolic
functions, and subcellular localization and mitochondrial binding
(6).
HK2 expression levels have been reported to impact
the therapeutic response of a number of types of tumors. Patients
with ovarian cancer who exhibited high HK2 expression levels
demonstrated a significantly increased frequency of chemoresistance
(tumor recurrence within 6 months after the termination of
first-line chemotherapy) (37). In
addition, HK2 knockdown in head and neck squamous cell carcinoma
cells showed an increased sensitivity to cisplatin and
5-fluorouracil, which may be of significant importance for patients
with esophageal carcinoma (29).
According to the ESMO Clinical Practice Guideline for diagnosis,
treatment and follow-up, patients with locally advanced esophageal
carcinoma are eligible for perioperative radio-chemotherapy
(25). Here, patients receive
either chemotherapy with 5-fluorouracil, oxaliplatin and docetaxel
(FLOT protocol) or radiochemotherapy with carboplatin and
paclitaxel (CROSS protocol) (38,39).
From the aforementioned results of the present study and previous
studies discussed, it could be suggested that patients
characterized by low HK2 expression levels may theoretically have
improved benefits from perioperative therapy.
The present study had a number of limitations. The
inclusion of disease-free survival data could add more clinically
significant information, although it was not evaluated in the
present study. However, as a number of patients at the University
Hospital of Cologne were referred nationally and internationally,
reliable disease-free survival data was not available.
Additionally, as the present study was conducted retrospectively,
future prospective studies to assess the value of HK2 as a
biomarker are warranted. Finally, as a single-center cohort study
was conducted, potential selection biases may have been introduced,
such as the ethnicity of patients, as predominantly Caucasian
patients are treated at the University Hospital of Cologne.
Therefore, further multi-center studies are required to confirm the
generalizability of the present study.
In spite of the aforementioned limitations, the
present study demonstrated that HK2 expression levels represent an
independent risk factor for the reduced survival of patients with
EAC. Through future research, HK2 expression status may potentially
be used in daily clinical decision-making. The evaluation of
pre-neoadjuvant HK2 has potential to streamline perioperative
therapy selection. Moreover, the investigation of HK2 and
glycolysis as potential therapeutic targets could encompass
mechanisms relating to the induction of apoptosis, the inhibition
of cell proliferation and addressing immune escape pathways.
The present study assessed the prognostic
significance of the IHC expression levels of HK2 in 643 patients
diagnosed with EAC. An association between HK2 expression and
unfavorable patient outcomes, particularly in primary resections,
male patients, cases with Y chromosome loss and tumors negative for
HER2 was demonstrated. This suggested that the incorporation of HK2
as a biomarker has potential to identify a high-risk subgroup of
patients in the future. This high-risk subgroup may benefit from
more frequent follow-up examinations or even alternate therapeutic
interventions. Moreover, patients with low HK2 expression levels
could potentially derive benefits from multimodal treatment
protocols, such as perioperative FLOT therapy. In the future, HK2
may be considered as a therapeutic target to improve treatment
outcomes of patients with EAC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SIL, AQ, KK, WS, CJB and TS contributed to the
study's conception and design. Material preparation and data
collection were performed by SIL, JOJ, CF and KK. WS and AQ confirm
the authenticity of all the raw data. Analysis was performed by KK.
SIL, AGS and AQ analyzed pathology data. The first draft of the
manuscript was written by SL and KK. All authors commented on
previous versions of the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the principles of the Declaration of Helsinki. Approval was granted
by the Ethics Committee of the University of Cologne (Cologne,
Germany; approval no. 21-1146). Informed consent for inclusion in
the database and tissue bank was obtained from all individual
participants included in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Agarwal S, Bell MG, Dhaliwal L, Codipilly
DC, Dierkhising RA, Lansing R, Gibbons EE, Leggett CL, Kisiel JB
and Iyer PG: Population based time trends in the epidemiology and
mortality of gastroesophageal junction and esophageal
adenocarcinoma. Dig Dis Sci. 69:246–253. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Xu Z, Hong J and Xu Y: Expression
patterns of three regulation enzymes in glycolysis in esophageal
squamous cell carcinoma: association with survival. Med Oncol.
31:1182014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Irwin DM and Tan H: Molecular evolution of
the vertebrate hexokinase gene family: Identification of a
conserved fifth vertebrate hexokinase gene. Comp Biochem Physiol
Part D Genomics Proteomics. 3:96–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson JE: Isozymes of mammalian
hexokinase: Structure, subcellular localization and metabolic
function. J Exp Biol. 206:2049–2057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Schaftingen E: Hexokinase/glucokinase.
Encyclopedia of Biological Chemistry. Academic Press; London: pp.
543–547. 2013, View Article : Google Scholar
|
|
8
|
Guo C, Ludvik AE, Arlotto ME, Hayes MG,
Armstrong LL, Scholtens DM, Brown CD, Newgard CB, Becker TC, Layden
BT, et al: Coordinated regulatory variation associated with
gestational hyperglycaemia regulates expression of the novel
hexokinase HKDC1. Nat Commun. 6:60692015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinohara Y, Yamamoto K, Kogure K,
Ichihara J and Terada H: Steady state transcript levels of the type
II hexokinase and type 1 glucose transporter in human tumor cell
lines. Cancer Lett. 82:27–32. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li R, Mei S, Ding Q, Wang Q, Yu L and Zi
F: A pan-cancer analysis of the role of hexokinase II (HK2) in
human tumors. Sci Rep. 12:188072022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Izuishi K, Yamamoto Y, Sano T, Takebayashi
R, Nishiyama Y, Mori H, Masaki T, Morishita A and Suzuki Y:
Molecular mechanism underlying the detection of colorectal cancer
by 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography. J
Gastrointest Surg. 16:394–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paudyal B, Oriuchi N, Paudyal P, Tsushima
Y, Higuchi T, Miyakubo M, Ishikita T, Nakajima T and Endo K:
Clinicopathological presentation of varying 18F-FDG uptake and
expression of glucose transporter 1 and hexokinase II in cases of
hepatocellular carcinoma and cholangiocellular carcinoma. Ann Nucl
Med. 22:83–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Hu L, Wu F, Zou L and He T: Poor
prognosis of hexokinase 2 overexpression in solid tumors of
digestive system: A meta-analysis. Oncotarget. 8:32332–32344. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schreurs LMA, Smit JK, Pavlov K, Pultrum
BB, Pruim J, Groen H, Hollema H and Plukker JT: Prognostic impact
of clinicopathological features and expression of biomarkers
related to (18)F-FDG uptake in esophageal cancer. Ann Surg Oncol.
21:3751–3757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gotzsche PC and Vandenbroucke JP: The strengthening the reporting
of observational studies in epidemiology (STROBE) statement:
Guidelines for reporting observational studies. Lancet.
370:1453–1457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rous B, Asamura H, Van Eycken E and
Brierley JD: TNM atlas. 7th edition. Wiley-Blackwell; Hoboken, NJ:
2021
|
|
17
|
Loeser H, Waldschmidt D, Kuetting F, Heydt
C, Zander T, Plum P, Alakus H, Buettner R and Quaas A: Copy-number
variation and protein expression of DOT1L in pancreatic
adenocarcinoma as a potential drug target. Mol Clin Oncol.
6:639–642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mazières J, Brugger W, Cappuzzo F, Middel
P, Frosch A, Bara I, Klingelschmitt G and Klughammer B: Evaluation
of EGFR protein expression by immunohistochemistry using H-score
and the magnification rule: Re-analysis of the SATURN study. Lung
Cancer. 82:231–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schiffmann LM, Loeser H, Jacob AS, Maus M,
Fuchs H, Zhao Y, Tharun L, Essakly A, Iannos Damanakis A, Zander T,
et al: Dickkopf-2 (DKK2) as context dependent factor in patients
with esophageal adenocarcinoma. Cancers (Basel). 12:4512020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knipper K, Damanakis AI, Lyu SI, Simon AG,
Wahler I, Bruns CJ, Schröder W, Schmidt T and Quaas A: High NANOG
expression correlates with worse patients' survival in esophageal
adenocarcinoma. BMC Cancer. 23:6692023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loeser H, Wölwer CB, Alakus H, Chon SH,
Zander T, Buettner R, Hillmer AM, Bruns CJ, Schroeder W, Gebauer F
and Quaas A: Y chromosome loss is a frequent event in Barrett's
adenocarcinoma and associated with poor outcome. Cancers (Basel).
12:17432020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prins MJD, Ruurda JP, van Diest PJ, van
Hillegersberg R and Ten Kate FJW: The significance of the HER-2
status in esophageal adenocarcinoma for survival: An
immunohistochemical and an in situ hybridization study. Ann Oncol.
24:1290–1297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu MZ, Han B, Luo HY, Zhou ZW, Wang ZQ,
Wang FH, Li YH and Xu RH: Expressions of hypoxia-inducible
factor-1α and hexokinase-II in gastric adenocarcinoma: The impact
on prognosis and correlation to clinicopathologic features. Tumour
Biol. 32:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato-Tadano A, Suzuki T, Amari M, Takagi
K, Miki Y, Tamaki K, Watanabe M, Ishida T, Sasano H and Ohuchi N:
Hexokinase II in breast carcinoma: A potent prognostic factor
associated with hypoxia-inducible factor-1α and Ki-67. Cancer Sci.
104:1380–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Obermannová R, Alsina M, Cervantes A,
Leong T, Lordick F, Nilsson M, van Grieken NCT, Vogel A and Smyth
EC; ESMO Guidelines Committee, : Electronic address: simpleclinicalguidelines@esmo.org:
Oesophageal cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:992–1004. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wagener-Ryczek S, Schoemmel M, Kraemer M,
Bruns C, Schroeder W, Zander T, Gebauer F, Alakus H,
Merkelbach-Bruse S, Buettner R, et al: Immune profile and
immunosurveillance in treatment-naive and neoadjuvantly treated
esophageal adenocarcinoma. Cancer Immunol Immunother. 69:523–533.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fonteyne P, Casneuf V, Pauwels P, Van
Damme N, Peeters M, Dierckx R and Van de Wiele C: Expression of
hexokinases and glucose transporters in treated and untreated
oesophageal adenocarcinoma. Histol Histopathol. 24:971–977.
2009.PubMed/NCBI
|
|
28
|
Tohma T, Okazumi S, Makino H, Cho A,
Mochiduki R, Shuto K, Kudo H, Matsubara K, Gunji H and Ochiai T:
Relationship between glucose transporter, hexokinase and FDG-PET in
esophageal cancer. Hepatogastroenterology. 52:486–490.
2005.PubMed/NCBI
|
|
29
|
Li WC, Huang CH, Hsieh YT, Chen TY, Cheng
LH, Chen CY, Liu CJ, Chen HM, Huang CL, Lo JF and Chang KW:
Regulatory role of hexokinase 2 in modulating head and neck
tumorigenesis. Front Oncol. 10:1762020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang MX, Hua YJ, Wang HY, Zhou L, Mai HQ,
Guo X, Zhao C, Huang WL, Hong MH and Chen MY: Long-term prognostic
implications and therapeutic target role of hexokinase II in
patients with nasopharyngeal carcinoma. Oncotarget. 7:21287–21297.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clark R and Lee SH: Anticancer properties
of capsaicin against human cancer. Anticancer Res. 36:837–843.
2016.PubMed/NCBI
|
|
32
|
Mao X, Zhu H, Luo D, Ye L, Yin H, Zhang J
and Zhang Y and Zhang Y: Capsaicin inhibits glycolysis in
esophageal squamous cell carcinoma by regulating hexokinase-2
expression. Mol Med Rep. 17:6116–6121. 2018.PubMed/NCBI
|
|
33
|
Walenta S, Wetterling M, Lehrke M,
Schwickert G, Sundfør K, Rofstad EK and Mueller-Klieser W: High
lactate levels predict likelihood of metastases, tumor recurrence,
and restricted patient survival in human cervical cancers. Cancer
Res. 60:916–921. 2000.PubMed/NCBI
|
|
34
|
Pantziarka P, Sukhatme V, Bouche G, Meheus
L and Sukhatme VP: Repurposing drugs in oncology (ReDO)-diclofenac
as an anti-cancer agent. Ecancermedicalscience. 10:6102016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chirasani SR, Leukel P, Gottfried E,
Hochrein J, Stadler K, Neumann B, Oefner PJ, Gronwald W, Bogdahn U,
Hau P, et al: Diclofenac inhibits lactate formation and efficiently
counteracts local immune suppression in a murine glioma model. Int
J Cancer. 132:843–853. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu XS, Liu JM, Chen YJ, Li FY, Wu RM, Tan
F, Zeng DB, Li W, Zhou H, Gao Y and Pei ZJ: Comprehensive analysis
of hexokinase 2 immune infiltrates and m6A related genes in human
esophageal carcinoma. Front Cell Dev Biol. 9:7158832021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suh DH, Kim MA, Kim H, Kim MK, Kim HS,
Chung HH, Kim YB and Song YS: Association of overexpression of
hexokinase II with chemoresistance in epithelial ovarian cancer.
Clin Exp Med. 14:345–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Batran SE, Hartmann JT, Hofheinz R,
Homann N, Rethwisch V, Probst S, Stoehlmacher J, Clemens MR,
Mahlberg R, Fritz M, et al: Biweekly fluorouracil, leucovorin,
oxaliplatin, and docetaxel (FLOT) for patients with metastatic
adenocarcinoma of the stomach or esophagogastric junction: A phase
II trial of the arbeitsgemeinschaft internistische onkologie. Ann
Oncol. 19:1882–1887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eyck BM, van Lanschot JJB, Hulshof MCCM,
van der Wilk BJ, Shapiro J, van Hagen P, van Berge Henegouwen MI,
Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, et al:
Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for
esophageal cancer: The randomized controlled CROSS trial. J Clin
Oncol. 39:1995–2004. 2021. View Article : Google Scholar : PubMed/NCBI
|