Introduction
Breast cancer carries a considerable global burden
due to its high morbidity and mortality rates (1). Acknowledged as the most common cancer
affecting the female population worldwide, its incidence reached an
estimated 300,590 new cases, with 43,700 associated deaths (in both
sexes), in the United States by the year 2023 (2). In 2019, the Jordanian Cancer Registry
revealed that breast cancer represented 38.5% of all cancer cases
among female Jordanian patients (3). Histologically, invasive ductal
carcinoma of no special type constitutes the predominant subtype,
comprising 40–75% of all invasive breast carcinoma cases (4). Otherwise, there are other special
subtypes, each characterized by unique architectural,
immunohistochemical and prognostic features. One such rare subtype,
adenoid cystic carcinoma (AdCC), mimics the histology of salivary
gland tumors and is typically found in the salivary glands of the
head and neck region (5). Breast
cancer was shown to be the second most common site of AdCC
following the head and neck in a recent Surveillance, Epidemiology
and End Results database report (6). This variant represents <0.1% of all
breast cancer subtypes (7). AdCC
comprises three distinct subtypes: Classic, solid basaloid and
high-grade transformation; each exhibiting unique clinical
behaviors and prognostic outcomes (8). Although AdCC of the breast shares
features with its more aggressive salivary gland counterpart, and
typically lacks expression of estrogen receptors (ERs),
progesterone receptors (PRs) and human epidermal growth factor
receptor 2 (HER2/neu), AdCC of the breast is nonetheless
characterized by an indolent clinical course and a favorable
prognosis (6,9). AdCC typically remains localized within
the breast region, with extremely rare lymph node involvement and
sparse occurrence of hematogenous metastases, primarily to the
lungs (10,11). In such cases, recurrence often
occurs after an extended period, commonly several years
post-resection (12). The present
study reports the case of a patient diagnosed with cT3N0M0
triple-negative AdCC of the breast. Notably, the clinical course
and tumor spread possessed a unique pattern, with rapid progression
over a span of 6 months, with the eventual clinical deterioration
and mortality of the patient.
Case report
In March 2022, a 77-year-old female patient
(Gravida, 22; Para, 12+10), was referred to the King Hussein Cancer
Center (Amman, Jordan) complaining of a painless, gradually
enlarging right breast mass associated with left nipple inversion
and tenderness for a 6-month duration. Apart from this, the
systemic review of symptoms did not yield any significant findings.
The patient was known to have a history of hypertension, diabetes
mellitus type II, osteoarthritis and chronic kidney disease. The
surgical history included an open cholecystectomy and hysterectomy
with bilateral salpingo-oopherectomy performed at the age of 57
years related to post-menopausal bleeding due to non-malignant
causes. Menarche occurred at the age of 13 years, and the patient
had been in menopause for the past 22 years. There was no family
history of malignancies. The patient was a second-hand smoker with
no hormonal replacement therapy usage. During physical examination,
a mass measuring 4×5 cm was identified in the mid-upper quadrant
area of the right breast, situated 5 cm away from the nipple
areolar complex. The mass was mobile, without any concurrent skin
alterations or retraction of the right nipple. Notably, palpable
lymph nodes were observed in the ipsilateral axilla. Otherwise,
examination of the left breast, left axilla and bilateral
supraclavicular region revealed no notable findings.
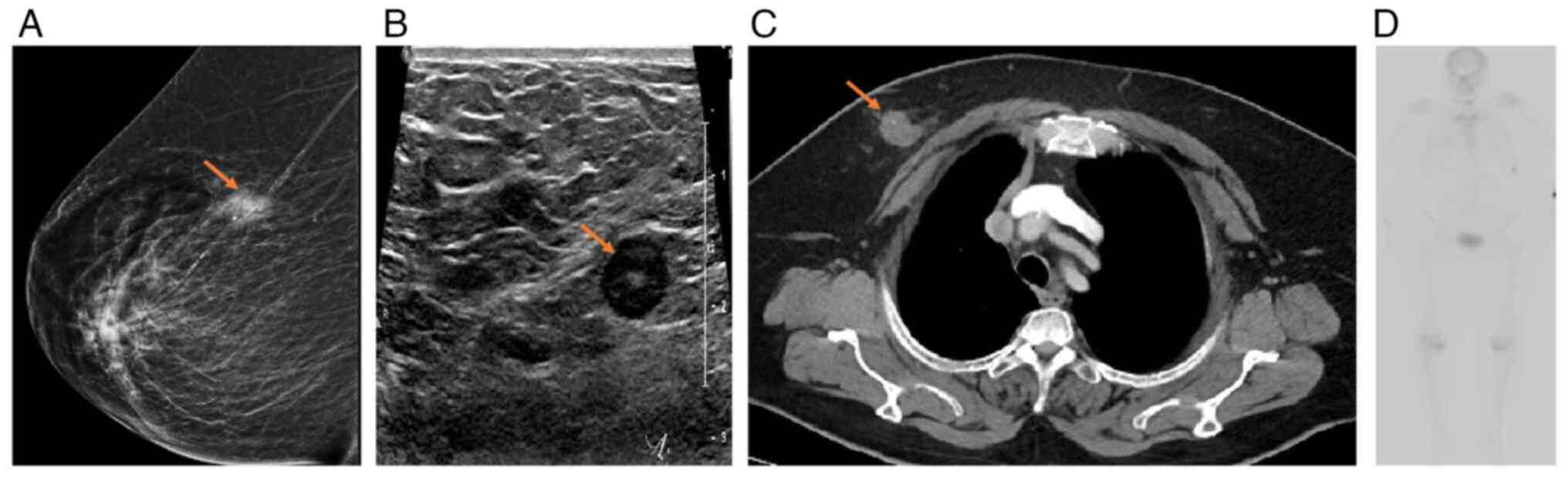
A mammogram demonstrated an irregular spiculated
hyperdense mass at the mid upper right breast ~9.5 cm away from the
nipple (Fig. 1A). Subsequent breast
ultrasound (U/S) imaging revealed an irregular, heterogeneous
hypoechoic mass with indistinct margins at the mid upper right
breast measuring 1.7×1.3×2 cm, with no concerning findings in the
left breast (Fig. 1B). Lymph node
involvement was notably absent in both axillae. Further staging
computed tomography (CT) scans revealed an ill-defined soft-tissue
lesion consistent with the previously identified right-sided breast
mass (Fig. 1C). Bone scans were
free of any active lesions, and there was no evidence of metastases
to nearby or distant organs (Fig.
1D). Additionally, CT scans revealed prominent subpectoral and
mediastinal lymph nodes on the ipsilateral side, measuring 0.8 cm
and 1.2 cm, respectively (Fig.
S1). A prior tru-cut biopsy performed outside our center
revealed Nottingham histological grade I invasive ductal carcinoma
and a ductal carcinoma in situ, characterized by a
cribriform pattern with intermediate nuclear grade (13). Repeated diagnostic testing was
performed to confirm the histological subtype and staging, to guide
the multidisciplinary tumor board management plan.
In May 2022, the patient underwent a wide local
excision along with additional excision of deep margins and a
biopsy of the sentinel lymph nodes. The procedure was carried out
successfully and there were no observed immediate or delayed
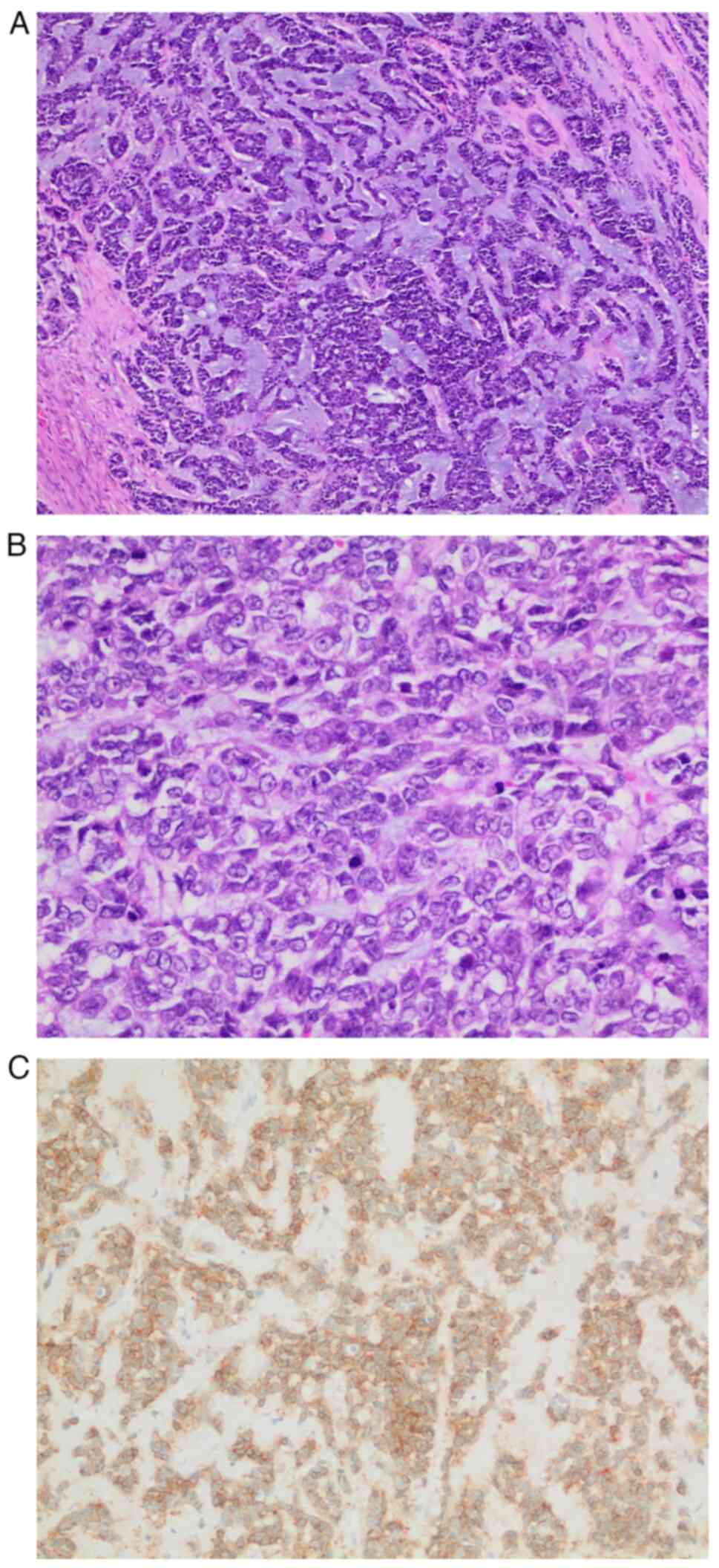
post-operative complications. Subsequent surgical pathology reports
revealed a 6.4-cm-diameter tumor composed of basaloid epithelial
cells arranged in solid sheets, nests and tubules. Formalin-fixed,
paraffin-embedded (FFPE) tissue specimens were prepared by fixing
in 10% neutral buffered formalin for 24 h at room temperature,
followed by paraffin embedding. Sections of 4-µm thickness were
cut, placed in an oven at 60°C for 15 min, stained with H&E
(hematoxylin for 8 min and eosin for 2 min at room temperature),
and examined using a light microscope. In some areas, the tumor
showed a lower grade component with evident myxoid stroma (Fig. 2A), whereas in other areas, the tumor
showed a higher nuclear grade with numerous mitotic figures
(Fig. 2B). The surgical margins
were free of tumor with the closest margin being the superior
margin at 0.3 cm. Perineural invasion was not observed. The tumor
was positive for c-Kit (Fig. 2C),
negative for S100 (Fig. S2) and
tumor protein p63 (p63) (Fig. S3),
and negative for chromogranin (Fig.
S4), synaptophysin (Fig. S5),
ER (Fig. S6) and HER2/neu
(Fig. S7), immunostaining
(procedure details described in Table
SI). The solid areas represented >30% of the tumor. The
diagnosis was consistent with solid-basaloid AdCC of the breast,
Nottingham grade II (13). Ductal
carcinoma in situ cribriform, intermediate grade was also
observed. The tumor was staged as pT3N0, per the American Joint
Committee on Cancer 8th edition staging system (14), indicating a large primary tumor size
with no extension to adjacent viscera or nearby lymph nodes.
The case was discussed at the multidisciplinary
tumor board meeting, and adjuvant radiation therapy was planned. At
1.5 months post-resection, upon evaluation at the Radiation
Oncology Clinic at King Hussein Cancer Center for a CT simulation
scan, a suspected lesion was detected in the axillary region, with
a slightly atypical appearance (Fig.
3A). A U/S scan was requested, which revealed the presence of a
new complex mass at the right axillary tail. Subsequently, a
U/S-guided biopsy was performed, which confirmed the recurrence of
known primary AdCC. Immunohistochemistry (IHC) analysis, carried
out as aforementioned, of the axillary lesion revealed negative ER,
weakly positive (2%) PR and negative HER2/neu expression, a
proliferation index (Ki-67) of 50%, a limited number of cells
staining positive for p63 and focal positivity for cytokeratin 8/18
(data not shown).
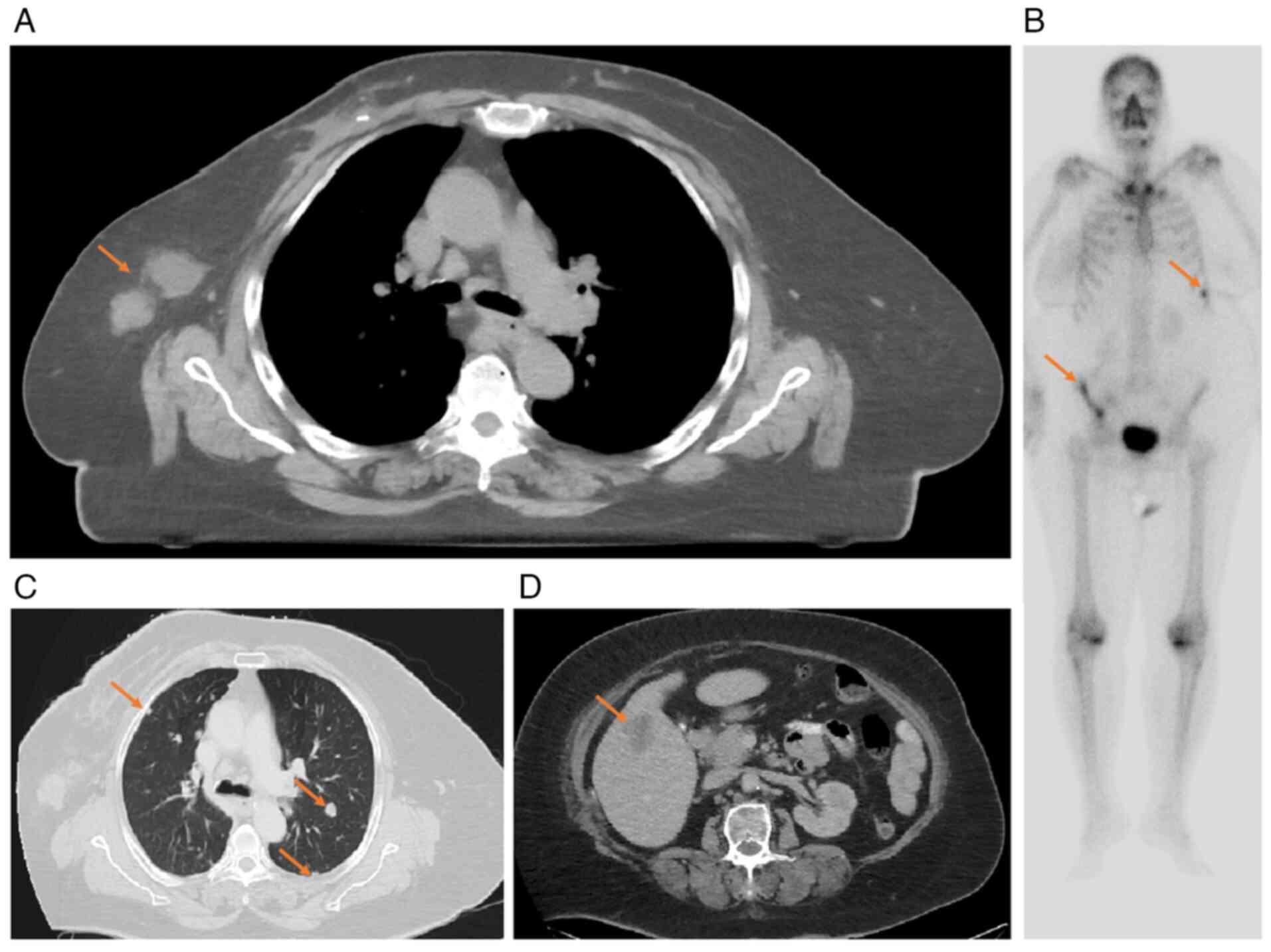
Given the unexpected findings, new staging work up
was performed including bone scan revealed new multiple active
lesions in the right iliac bone and ribs (Fig. 3B) and CT scan which shown
significant disease progression manifested by new innumerable
metastatic pulmonary nodules in both lungs, new liver metastases,
new right axillary metastatic lymph nodes, new minimal right
pleural effusion and thickening suggestive of metastatic deposits
(Fig. 3C and D).
Prompted by the discovery of the new findings, the
treatment plan included the initiation of chemotherapy sessions
using weekly paclitaxel (144 mg) with a 20% dose reduction from the
first cycle due to the age of the patient. The patient completed a
total of three cycles. However, a multitude of complications
occurred due to factors relating to the advanced age of the
patient, comorbid conditions, side effects of chemotherapeutic
agents and tumor infiltration. The patient presented to the
Emergency Department on six occasions complaining of severe lower
back pain, an episode of falling down and recurrent urinary tract
infections. A CT scan revealed a non-displaced fracture in the
right superior iliac crest and an inferior iliac crest fracture.
Despite being on oral Tramal 100 mg twice daily, Tramal 50 mg every
8 h as needed, paracetamol 1,000 mg every 6 h, and gabapentin 300
mg three times daily, the patient continued to experience
significant pain. Consequently, the patient required multiple
admissions and palliative pain interventions, including haloperidol
1 mg IV every 4 h as needed, a continuous morphine sulfate infusion
at 0.2 mg/h, and morphine 2 mg/2 ml IV every 2 h as needed for
breakthrough pain. She also received multiple courses of
intravenous antibiotics, with the most recent being meropenem 1 g
diluted in 100 ml of 0.9% sodium chloride, infused over 30 min
every 8 h. Unfortunately, chemotherapy sessions were postponed and
subsequently discontinued due to a worsening clinical condition
until stabilization was achieved. Over a span of 2 months, the
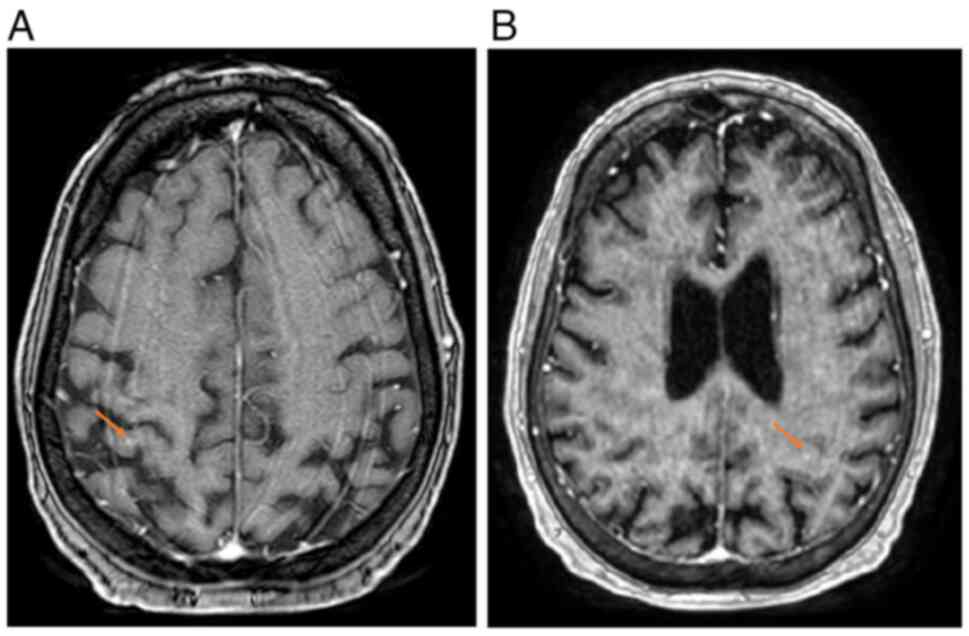
patient suffered from neurological symptoms, manifesting as a
decreased level of consciousness and seizures. Subsequent brain
magnetic resonance imaging findings confirmed the presence of small
bilateral hemispheric lesions suggestive of metastatic deposits
(Fig. 4A and B). Concurrently, the
urinary tract infections of the patient exhibited resistance to
treatment. A final CT scan performed before clinical deterioration
revealed no local recurrence of the right-sided breast cancer, with
a partial response of the right axillary lymph node, a mild
increase in right-sided pleural effusion and a new large lytic bone
metastasis in the right iliac bone, with a non-displaced fracture
of the right pubic bone (Fig.
S8).
At 7 months after the initial diagnosis, the patient
presented with a decreased level of consciousness. Clinical and
diagnostic evaluation detected urosepsis, concomitant aspiration
pneumonia and an acute-on-chronic kidney disease, with resultant
metabolic acidosis and poor Glasgow Coma Scale (6/15) (15). After discussion with the family, the
code status of the patient was changed to ‘do not resuscitate’ and
the patient was administered comfort measures until they passed
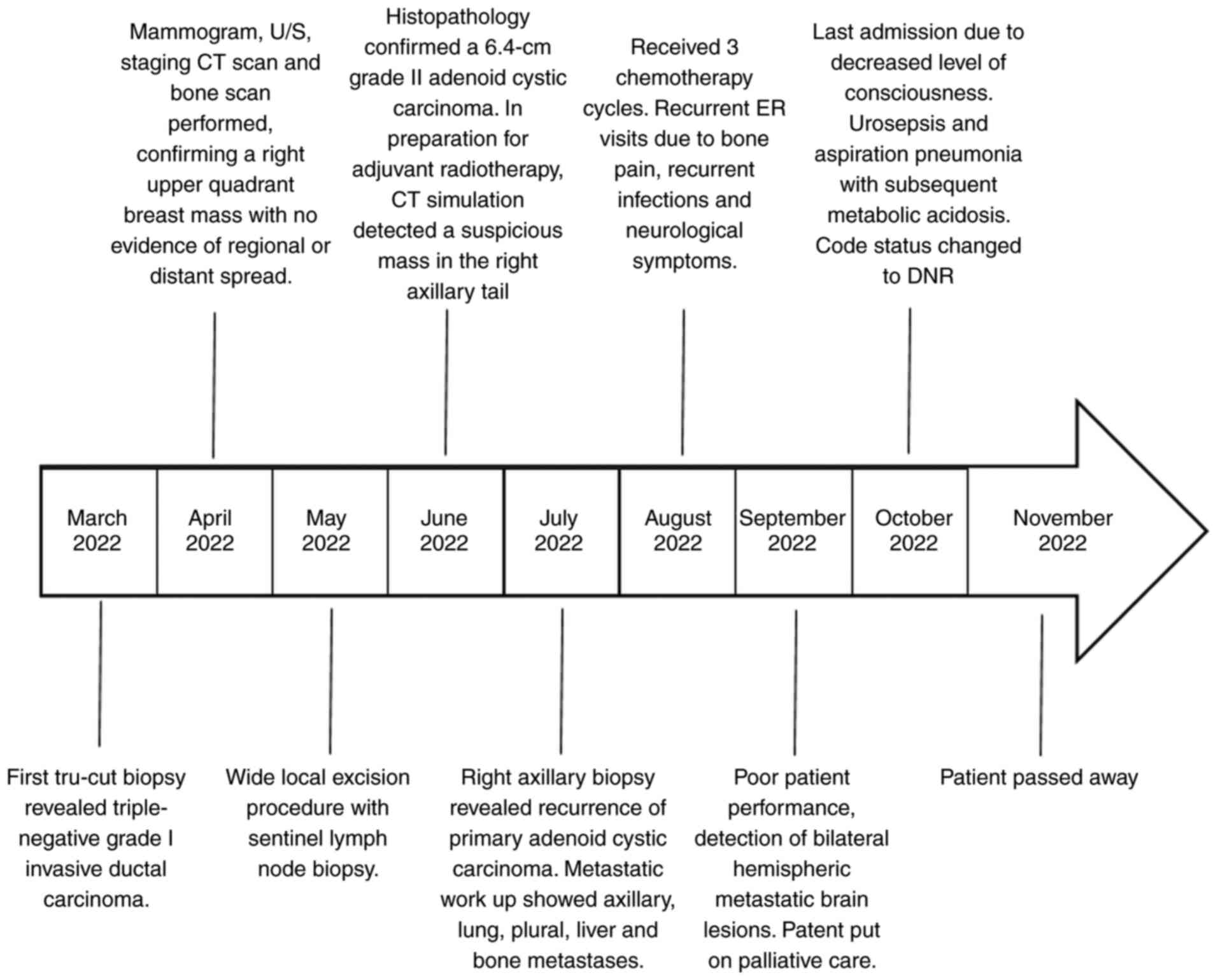
away 2 weeks later. The patient timeline of events is summarized in
Fig. 5.
Discussion
It is considered that AdCC was first described as a
‘tumeur heteradenique’ by Robin and Laboulbene in 1853 (16). Subsequently, it was referred to as a
‘cylindroma’ due to the presence of cylindrical-shaped cells in a
hyaline stroma (17). In 1953, the
term AdCC was introduced by Foote and Frazell (18) to describe a distinct category
primarily arising in the minor salivary, parotid and submandibular
glands, comprising 3–5% of head and neck malignancies (18,19).
AdCC also manifests as a rare subtype of primary breast carcinomas,
which was first described by Geschickter and Copeland (20) in 1945. AdCC accounts for <0.1% of
all breast cancer cases and 0.058% of total AdCC cases (7,9,21).
In a comprehensive cohort covering a 30-year
interval, the age-adjusted incidence ratio was calculated to be
0.92 per 1 million person-years, predominantly affecting the female
population, with a median age of onset falling between 50 and 60
years (11). Jang et al
(22) reported lower overall
survival (OS) rates in head and neck AdCC cases in age groups
>70 years old compared with those in groups <45 years old.
Zhang et al (23) supported
the aforementioned association; however, conflicting opinions
exist, with studies indicating lower OS in both age extremes
(<45 and >60 years) (24,25).
Notably, the age of the patient in the present study at symptom
onset exceeded the average age documented in the aforementioned
reports (24,25).
The clinical presentation and imaging findings of
breast AdCC are non-specific, typically manifesting as a
well-circumscribed, palpable mass, with no predilection for the
left or right breast or specific quadrants (23). However, some studies have
demonstrated a preference for the submastoid and upper outer
quadrant region, while others have shown a 50% tendency for the
subareolar region (10,23,26,27).
Pain and tenderness upon palpation may be present, possibly due to
the perineural invasion of the lesion (28). In the present case, the latter
findings were not observed, as the histological assessment of the
resected mass revealed no evidence of perineural invasion. The
tumor usually ranges in size between 2 and 3 cm and exhibits a slow
growth pattern (29,30).
Imaging modalities are able to detect AdCC; however,
the efficacy of mammography in accurately distinguishing AdCC from
its benign differentials remains uncertain. Typically, AdCC
manifests as irregular masses with spiculated margins (7,31,32).
In the present case, mammography revealed an irregular spiculated
hyperdense mass, with a prior biopsy-proven confirmation of
malignancy. Breast U/S and staging CT scans supported these
findings; however, it is important to note the role that the
post-resection CT simulation scan played in the early detection of
recurrence. In the present case, CT simulation imaging was able to
detect a suspected atypical lesion in the axillary tail at 1.5
months post-resection, aligning with existing studies of the
paramount diagnostic value of the imaging technique in detecting
new lesions and planning therapeutic strategies (33–35).
Histologically, AdCC diagnosis relies on identifying
a dual cell population of myoepithelial and epithelial cells,
presenting true duct-like structures with microvilli-projecting
epithelial cells and pseudo-cyst structures lined by myoepithelial
cells (11,36,37).
AdCC is categorized according to the predominant growth structure
into one or a combination of three groups, namely, cribriform,
tubular and solid, with histological subdivisions holding
prognostic value (38). Ro et
al (39) proposed a
classification for breast AdCC. Grade I is characterized by the
absence of solid components, grade II exhibits <30% solid
components and grade III exhibits >30% solid components. The
2019 World Health Organization classification of Tumors of the
Breast highlights that the classic form of AdCC with predominant
cribriform and tubular differentiation is more prognostically
favorable, and such cases are recommended to undergo conservative
treatment, in contrast to solid-basaloid AdCC and high-grade AdCC,
which exhibit elevated rates of both local and distant recurrence
(40,41). A comprehensive analysis by
Slodkowska et al (42)
involving 108 AdCC cases revealed a significantly higher incidence
of distant metastasis in basaloid AdCC compared with that in
classic AdCC (40 vs. 8%; P<0.0004). In a study by Marco et
al (43), high-grade
transformation of AdCC presented with skin and lung involvement in
one patient, and lung, colon and brain involvement in a second
patient, both of whom eventually died of disease. Examination of
previously published case reports with distant metastases (Table I) (9,24,27,39,44–59),
reveals a prevailing incidence of solid-basaloid AdCC and
high-grade AdCC.
 | Table I.Literature review of cases of breast
adenoid cystic carcinoma with aggressive behavior. |
Table I.
Literature review of cases of breast
adenoid cystic carcinoma with aggressive behavior.
| First author/s,
year | Age, years | Sex | Size
(grade/stage) | Genetic
alternations | Site of
metastasis | Lymph node
involvement | Surgical
intervention | Adjuvant
therapies | Time until
recurrence from primary surgery | Outcome | (Refs.) |
|---|
| Vasudevan et
al, 2023 | ~60 | Female | 4.5×4×4 cm, 3×2.5×2
cm (high grade cribriform-solid); Ki-67, 70–80% | NR | Lung | None | Mastectomy | R | 11 Y | NR | (44) |
| Ji et al,
2022 | 50 | Female | 1.5 cm
(solid-basaloid) | (+) MYB
rearrangement | Lung | None | Lumpectomy | C + R | 2.5 Y | Alive | (24) |
|
| 59 | Female | 4.0 cm
(solid-basaloid) | (−) MYB
rearrangement | Liver | None | Mastectomy | C | 1.5 Y | Alive |
|
| Collins et
al, 2020 | 49 | Female | (High grade,
>30% solid components) | NR | Skin (multiple
sites) | NR | Lumpectomy | NR | 1.3 Y post initial
diagnosis | NR | (45) |
| Sołek et al,
2020 | 52 | Female | Stage IIA (T2N0Mx)
(high grade solid-basaloid) | NR | Lung | None | Mastectomy | R | 1 M | Alive | (46) |
|
| 41 | Female | Stage IA (T1N0Mx)
(high grade solid-basaloid) | NR | Lung, liver,
brain | NR | Lumpectomy | C + R | 1 Y | Alive |
|
| Mhamdi et
al, 2017 | 65 | Female | 8.0 cm | (−) MYB
rearrangement | Lung, liver, brain,
kidney | None | Mastectomy | R | 4 Y | Alive | (47) |
| Miyai et al,
2014 | 83 | Female | 3.0 cm (grade I)
(stage IV) | NR | Lung | None | Lumpectomy | None | 7 Y | Alive | (9) |
| Kim et
al, | 33 | Female | (pT2N0M0) | NR | Lung | None | Quadrantectomy | C + R | 2.6 Y | Alive | (27) |
| 2014 | 58 | Female | (pT2N0M0) | NR | Lung, liver,
bone | None | Mastectomy | C | 6.25 Y | Alive |
|
| D'Alfonso et
al, 2014 | 25 | Female | 6.5 cm (grade II,
cribriform-solid) | (−) MYB
rearrangement | Lung, liver,
brain | NR | Mastectomy | None | 5 Y | Dead | (48) |
|
| 55 | Female | 2.0 cm (grade III,
solid-basaloid) | (−) MYB
rearrangement | Lung | None | Mastectomy | None | 4.58 Y post initial
diagnosis | Alive |
|
| Silva et al,
2011 | 37 | Female | 3 cm
(solid-basaloid) (T2N1Mx) | NR | Lung, liver, bone,
brain | Yes | Mastectomy | None | 2 Y | Dead | (49) |
| Vranić et
al, 2007 | 76 | Female | 1.8 cm (pT1cN0MxR0,
cribriform-solid) | (+) PIK-3CA and
PTEN mutations | Kidney | None | Mastectomy | None | 5 Y | NR | (50) |
| Millar et
al, 2004 | 53 | Male | 4.0 cm | NR | Bone, brain | Yes | Mastectomy | R | 10 Y | Dead | (51) |
| Herzberg et
al, 1991 | 57 | Female | 1.5 cm
(cribriform) | NR | Lung, kidney | None | Mastectomy | None | 6 Y | Alive | (52) |
| Ro et al,
1987 | 53 | Female | 2.5 cm (grade
II) | NR | Lung, thigh,
skull | None | Mastectomy | None | 9 Y | Dead | (39) |
|
| 68 | Female | 0.7 cm (grade
II) | NR | Lung | None | Mastectomy | R | 5 Y | Alive |
|
|
| 64 | Female | 6.0 cm (grade
III) | NR | Liver, brain | Yes | Mastectomy | None | 1 Y | Dead |
|
| Koller et
al, 1986 | 49 | Female | NR | NR | Lung, brain | None | Mastectomy | None | 1 Y local
recurrence; 8 Y distant metastasis | Alive | (53) |
| Peters and Wolff,
1983 | 73 | Female | 1.0 cm | NR | Lung, liver | None | Mastectomy | None | 10 Y | Alive | (54) |
| Lim et al,
1979 | 48 | Female | NR | NR | Lung | None | Mastectomy | None | 9 Y | NR | (55) |
| Verani and Van
der Bel-Kahn, 1973 | 63 | Female | 2.0 cm | NR | Lung,
vertebrae | Yes | Mastectomy | None | 14 Y | Dead | (56) |
|
| 78 | Male | 3.5×3×2 cm | NR | Lung, liver | None | Mastectomy | None | 6 M | Dead |
|
| Elsner, 1970 | 44 | Female | NR | NR | Lung | NR | Mastectomy | None | 7 Y | NR | (57) |
| Wilson and Spell,
1967 | 54 | Female | 2.8 cm | NR | Ribs | Yes | Mastectomy | None | 6 Y | NR | (76) |
| O'Kell, 1964 | 73 | Female | NR | NR | Lung, inferior vena
cava | NR | Mastectomy | None | 3 Y | Dead | (58) |
| Nayer, 1957 | 39 | Female | NR | NR | Lung | None | Mastectomy | None | 8 Y | Dead | (59) |
| Present study | 77 | Female | 6.4 cm (grade II)
(Nottingham 6/9) (pT3N0) | NR | Lung, liver, bone,
brain | Yes | Lumpectomy | R planned | 1.5 M | Dead | - |
Following the American Joint Committee on Cancer
guidelines (60), the utilization
of the Nottingham score was endorsed in the present study, where
scores exceeding 5 represent a higher grade and poorer outcome. The
present case had a total score of 6/9 (tubular differentiation,
2/3; nuclear pleomorphism, 2/3; and mitotic activity, 2/3)
(13). Despite the favorable score,
the observed tumor size was 6.4 cm, surpassing the median size
reported in prior studies (7,61).
This notable size difference may have contributed as a factor to
the subsequent aggressive behavior of the tumor in the patient,
despite absence of initial extension to adjacent structures or
lymph nodes (30). Factors
affecting prognosis in AdCC of the breast include a solid growth
pattern (>30%), basaloid morphology, lymphovascular invasion,
perineural invasion, necrosis, lymph node involvement, positive
margins and high Nottingham grade (12,40).
In the present case, a few factors may have contributed to the
worse prognosis, including the presence of significant solid and
basaloid components, the large tumor size and the higher tumor
grade, along with the increased mitotic activity and Ki-67 value,
which eventually resulted in distant generalized metastasis.
IHC, genomic investigations and the distinct
polarity of cellular structures are pivotal in achieving an
accurate diagnosis of AdCC. This arises from the inherent challenge
in distinguishing the solid-basaloid variant of AdCC from the more
aggressive triple-negative breast cancer (TNBC) with basal-like
features, where both entities share characteristics in terms of
negativity for ER, PR and HER2/neu receptors. Additionally, they
exhibit reactivity for basal high molecular weight CKs, including
CK5/6, CK14, CK17 and luminal progenitors CK8/18 (62). Specifically, the luminal cells in
AdCC exhibit positivity for CK7, CK8/18, epithelial membrane
antigen and c-KIT, while myoepithelial cells exhibit positivity for
CK5, CK5/6, CK14, CK17, p63, actin, calponin, S100, vimentin and
epidermal growth factor receptor (63,64). A
key difference between TNBC and AdCC lies in the loss of
myoepithelium, and the loss of reactivity to p63 and calponin in
TNBC (24). In the present case,
IHC analysis of the recurrent lesion revealed triple-negative
expression for ER, PR and HER2/neu, limited positivity for p63 and
focal positivity for CK8/18. Notably, the Ki-67 value was markedly
elevated at 50%, which is uncharacteristic of the proliferative
activity associated with low-grade AdCC. Wetterskog et al
(65) reported a low Ki-67 index in
69% of AdCC cases, with a moderate index in the remaining cases
(66). This observation is
particularly important, as Ki-67 has been established as a valuable
marker of cellular proliferation, with higher levels being
associated with increased tumor aggressiveness and poor clinical
outcomes (67). High Ki-67
expression was associated with advanced histological grade and
recurrence in a retrospective study (68). Another study found that Ki-67
immunoreactivity increased proportionally with tumor grade,
averaging 27.12% in grade I, 34.43% in grade II and 38.45% in grade
III tumors (69).
The majority of cases of AdCC of the breast harbor
the transcriptional activator Myb:: nuclear factor IB (MYB::NFIB)
fusion gene [t(6;9)(q22-23;p23-24)], which is considered a
molecular hallmark of the diagnosis (48). The gene fusion can be detected by
fluorescence in situ hybridization (FISH) or PCR (65,70).
In the absence of molecular testing, diffuse and strong MYB protein
expression detected by IHC is often used as a surrogate,
particularly in conventional AdCC. However, this surrogate is less
reliable for solid variants. Massé et al (71) reported that only 19% of solid-type
AdCCs exhibited MYB gene rearrangements based on FISH, suggesting
that this variant may be a distinct molecular entity enriched for
CREB-binding protein and neurogenic locus notch homolog protein
pathway mutations. We acknowledge the absence of MYB::NFIB testing
in the present case due to institutional limitations. This
represents a diagnostic limitation, as molecular confirmation would
have further strengthened the diagnosis in the present study,
particularly given the aggressive clinical course and high-grade
histological features.
Breast AdCC is renowned for its indolent nature and
favorable prognosis, distinguishing itself from its salivary gland
counterpart and other forms of TNBC (54). Axillary lymph node involvement is
rare, with Arpino et al (72) documenting lymph node metastasis in
only 1 of 23 cases studied. Distant metastasis is also uncommon,
following a hematogenous route and silent progression, and often
emerging several years after the initial presentation. The lung is
the most frequently affected organ, along with the occasional
involvement of the liver, bones and brain (73). Consequently, various reports have
argued against routine axillary lymph node dissection during
surgery and underscored the importance of prolonged follow-up
during remission (47,74,75).
In the present study, to the best of our knowledge, all the
previously reported cases of breast AdCC with confirmed recurrence
and distant metastasis have been compiled and presented in Table I (9,24,27,39,44–59).
The present case, alongside the case reported by Sołek et al
(46) represent, to the best of our
knowledge, the only two reported instances in the literature where
the interval between resection and the detection of the first
metastatic lesion was <3 months. While Sołek et al
(46) exclusively reported lung
involvement, the present case reported a broad spectrum of
metastases, including those of the lungs, pleura, liver, bones and
brain, culminating in a fatal outcome. Additionally, the discussed
case is among the limited reports revealing axillary lymph node
involvement, joining the case reports by Silva et al
(49) Verani and Van der Bel-Kahn
(56), Wilson and Spell (76), and Ro et al (39) in mentioning this uncommon
manifestation.
Management strategies for AdCC of the breast vary
across different clinical recommendations, particularly concerning
the adoption of an aggressive surgical approach despite the
recognized indolent nature of the tumor (77). Ro et al (39) proposed a treatment strategy
involving local excision for grade I tumors, simple mastectomy for
grade II tumors and mastectomy with axillary lymph node dissection
for grade III tumors. Hodgson et al (78) suggested a more comprehensive
approach due to the reported instances of positive margins
post-excision, irrespective of the year of surgery or local
practice patterns, but perhaps due to the silent and microscopic
infiltration of the tumor into breast tissue. This mechanism may
have contributed to the outcome of the present patient, as the
treatment plan involved a wide local excision surgery coupled with
radiotherapy sessions, which were halted by the discovery of local
and distant recurrence of the disease, and then the commencing of
chemotherapy. There is a consensus on the efficacy of lumpectomy or
mastectomy with adjuvant radiotherapy in achieving remission and
preventing recurrence, with no significant impact of adjuvant
chemotherapy on OS (11,51,79,80).
This was supported by a retrospective study of 488 AdCC cases
conducted by Gomez-Seoane et al (81), which demonstrated an increased OS
rate in the post-resection adjuvant radiation therapy group
compared with that in those subjected to surgery alone.
National Comprehensive Cancer Network guidelines
consider AdCC a favorable histology and only recommend adjuvant
chemotherapy for pathological node positive disease (82). Furthermore, limited responses to
neoadjuvant chemotherapy have been documented in solid-basaloid
variants, with a complete pathological response remaining rare
(83). However, evolving evidence
has raised the need to reconsider whether adjuvant chemotherapy
should be added for high-risk subtypes, particularly solid-basaloid
and high-grade AdCC. Such variants have shown to be associated with
more aggressive clinical behavior, higher rates of nodal
involvement and early distant metastasis (42).
There are several potential limitations to the
present study. First, its retrospective design may introduce bias
and limit the ability to establish causal relationships. Second,
the advanced age of the patient and their comorbidities presented
significant challenges in administering various therapeutic
options. Additionally, despite early surgical intervention, the
aggressive nature of the solid-basaloid subtype of AdCC resulted in
rapid metastasis and disease progression, underscoring the
limitations of current treatment strategies in managing high-risk
cases. In addition, the suitability of this tumor to represent a
high-grade metaplastic, triple-negative, matrix-producing carcinoma
is high. The case was subject to intradepartmental discussion and a
consensus diagnosis was made based on the available histology,
hormonal studies, immunohistochemical stains and clinical
presentation at the time of first diagnosis. The gold standard for
diagnosis is detecting MYB::NFIB gene fusion by molecular methods
or detecting upregulation of MYB expression by IHC; however,
neither tests were available at King Hussein Cancer Center. In
summary, despite its indolent nature, certain subtypes of AdCC of
the breast carry aggressive behavior manifested by short-term
locoregional and systemic spread. The present report highlights the
importance of a further understanding of AdCC of the breast, and
the challenges that can arise in achieving control of this
pathology.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL, FA and HAR designed the overall concept and
outline of the manuscript. HL and FA confirm the authenticity of
all the raw data. HL, FA, LW, RA, WA, AA, OJ, IM and HAR have
contributed to methodology, data collection and the review of the
literature. HL, FA, LW, RA, WA, AA, OJ, IM and HAR contributed to
writing and editing of the manuscript. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The requirement for consent for participation was
waived by the Institutional Review Board at King Hussein Cancer
Center (Amman, Jordan) due to the study nature and no added risk to
the participant.
Patient consent for publication
Consent for publication could not be obtained as the
patient passed away prior to the time of writing this report.
However, the Institutional Review Board at King Hussein Cancer
Center (Amman, Jordan) issued a determination waiving the
requirement for consent, as this case qualifies as non-human
subject research. All patient data have been fully anonymized in
compliance with ethical standards and publication guidelines.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI
|
|
3
|
Jordan Ministry of Health,
Non-Communicable Diseases Directorate, Jordan Cancer Registry,
Cancer Incidence in Jordan, . 2019.
|
|
4
|
Masood S: Breast cancer subtypes:
Morphologic and biologic characterization. Womens Health (Lond).
12:1032016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bradley PJ: Adenoid cystic carcinoma of
the head and neck: A review. Curr Opin Otolaryngol Head Neck Surg.
12:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahouma M, Khairallah S, Baudo M, Al-Thani
S, Dabsha A, Shenouda D, Mohamed A, Dimagli A, El Sherbiny M, Kamal
M, et al: Epidemiological study of adenoid cystic carcinoma and its
outcomes: insights from the surveillance, epidemiology, and end
results (SEER) database. Cancers (Basel). 16:33832024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glazebrook KN, Reynolds C, Smith RL,
Gimenez EI and Boughey JC: Adenoid cystic carcinoma of the breast.
AJR Am J Roentgenol. 194:1391–1396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seethala RR: An update on grading of
salivary gland carcinomas. Head Neck Pathol. 3:69–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyai K, Schwartz MR, Divatia MK, Anton
RC, Park YW, Ayala AG and Ro JY: Adenoid cystic carcinoma of
breast: Recent advances. World J Clin Cases. 2:732–741. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kulkarni N, Pezzi CM, Greif JM, Suzanne
Klimberg V, Bailey L, Korourian S and Zuraek M: Rare breast cancer:
933 Adenoid cystic carcinomas from the national cancer data base.
Ann Surg Oncol. 20:2236–2241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghabach B, Anderson WF, Curtis RE, Huycke
MM, Lavigne JA and Dores GM: Adenoid cystic carcinoma of the breast
in the United States (1977 to 2006): A population-based cohort
study. Breast Cancer Res. 12:R542010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khoury T, Rosa M, Nayak A, Karabakhtsian
R, Fadare O, Li Z, Turner B, Fang Y, Kumarapeli A, Li X, et al:
Clinicopathologic predictors of clinical outcomes in mammary
adenoid cystic carcinoma: A multi-institutional study. Mod Pathol.
36:1000062023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rakha EA, El-Sayed ME, Lee AHS, Elston CW,
Grainge MJ, Hodi Z, Blamey RW and Ellis IO: Prognostic significance
of nottingham histologic grade in invasive breast carcinoma. J Clin
Oncol. 26:3153–3158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teasdale G and Jennett B: Assessment of
coma and impaired consciousness. A practical scale. Lancet.
2:81–84. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Otto RA: Tumours of the upper jaw. By
Donald Harrison and Valerie J. Lund, Churchill Livingstone, New
York, 1993, 351 pp, $150.00. Head Neck,. 17:275. 1995. View Article : Google Scholar
|
|
17
|
Andreasen S: Molecular features of adenoid
cystic carcinoma with an emphasis on microRNA expression. APMIS.
126 (Suppl 140):S7–S57. 2018. View Article : Google Scholar
|
|
18
|
Foote FW Jr and Frazell EL: Tumors of the
major salivary glands. Cancer. 6:1065–1133. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ellington CL, Goodman M, Kono SA, Grist W,
Wadsworth T, Chen AY, Owonikoko T, Ramalingam S, Shin DM, Khuri FR,
et al: Adenoid cystic carcinoma of the head and neck: Incidence and
survival trends based on 1973–2007 surveillance, epidemiology, and
end results data. Cancer. 118:4444–4451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geschickter CF and Copeland MM: Diseases
of the breast: Diagnosis, pathology, treatment. J B Lippincott;
Philadelphia: 1945, PubMed/NCBI
|
|
21
|
Rosen PP: Adenoid cystic carcinoma of the
breast. A morphologically heterogeneous neoplasm. Pathol Annu.
24:237–254. 1989.PubMed/NCBI
|
|
22
|
Jang S, Patel PN, Kimple RJ and McCulloch
TM: Clinical outcomes and prognostic factors of adenoid cystic
carcinoma of the head and neck. Anticancer Res. 37:3045–3052.
2017.PubMed/NCBI
|
|
23
|
Zhang W, Fang Y, Zhang Z and Wang J:
Management of adenoid cystic carcinoma of the breast: A
single-institution study. Front Oncol. 11:6210122021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji J, Zhang F, Duan F, Yang H, Hou J, Liu
Y, Dai J, Liao Q, Chen X and Liu Q: Distinct clinicopathological
and genomic features in solid and basaloid adenoid cystic carcinoma
of the breast. Sci Rep. 12:85042022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi Y, Kim SB, Yoon DH, Kim JY, Lee SW
and Cho KJ: Clinical characteristics and prognostic factors of
adenoid cystic carcinoma of the head and neck. Laryngoscope.
123:1430–1438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas DN, Asarian A and Xiao P: Adenoid
cystic carcinoma of the breast. J Surg Case Rep. 2019:rjy3552019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim M, Lee DW, Im J, Suh KJ, Keam B, Moon
HG, Im SA, Han W, Park IA and Noh DY: Adenoid cystic carcinoma of
the breast: A case series of six patients and literature review.
Cancer Res Treat. 46:93–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McClenathan JH and de la Roza G: Adenoid
cystic breast cancer. Am J Surg. 183:646–649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boujelbene N, Khabir A, Boujelbene N,
Jeanneret Sozzi W, Mirimanoff RO and Khanfir K: Clinical
review-breast adenoid cystic carcinoma. Breast. 21:124–127. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Wang M, Wang Y, Shen X and Li C:
Diagnosis of adenoid cystic carcinoma in the breast: A case report
and literature review. Arch Med Sci. 18:279–283. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sheen-Chen SM, Eng HL, Chen WJ, Cheng YF
and Ko SF: Adenoid cystic carcinoma of the breast: Truly uncommon
or easily overlooked? Anticancer Res. 25:455–458. 2005.PubMed/NCBI
|
|
32
|
Santamaría G, Velasco M, Zanón G, Farrús
B, Molina R, Solé M and Fernández PL: Adenoid cystic carcinoma of
the breast: Mammographic appearance and pathologic correlation. AJR
Am J Roentgenol. 171:1679–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murakami R, Kumita SI, Yoshida T, Ishihara
K, Kiriyama T, Hakozaki K, Yanagihara K, Iida S and Tsuchiya S:
FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta
Radiol. 53:12–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu Y, Schöder H, Zakeri K, Chen L, Kang
JJ, McBride SM, Tsai CJ, Gelblum DY, Boyle JO, Cracchiolo JR, et
al: Post-operative PET/CT improves the detection of early
recurrence of squamous cell carcinomas of the oral cavity. Oral
Oncol. 141:1064002023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim B, Kim YC, Noh OK, Heo J, Lee HW, Kim
JH, Lee JH, Kim JK, Cho O, Oh YT and Chun M: Diagnostic evaluation
of simulation CT images for adjuvant radiotherapy in pancreatic
adenocarcinoma. Br J Radiol. 90:201702252017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koss LG, Brannan CD and Ashikari R:
Histologic and ultrastructural features of adenoid cystic carcinoma
of the breast. Cancer. 26:1271–1279. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anthony PP and James PD: Adenoid cystic
carcinoma of the breast: Prevalence, diagnostic criteria, and
histogenesis. J Clin Pathol. 28:647–655. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
da Cruz Perez DE, de Abreu Alves F, Nobuko
Nishimoto I, de Almeida OP and Kowalski LP: Prognostic factors in
head and neck adenoid cystic carcinoma. Oral Oncol. 42:139–146.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ro JY, Silva EG and Stephen Gallager H:
Adenoid cystic carcinoma of the breast. Hum Pathol. 18:1276–1281.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shin SJ and Rosen PP: Solid variant of
mammary adenoid cystic carcinoma with basaloid features: A study of
nine cases. Am J Surg Pathol. 26:413–420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 World Health Organization classification of tumours of the
breast. Histopathology. 77:181–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slodkowska E, Xu B, Kos Z, Bane A, Barnard
M, Zubovits J, Iyengar P, Faragalla H, Turbin D, Williams P, et al:
Predictors of outcome in mammary adenoid cystic carcinoma: A
multi-institutional study. Am J Surg Pathol. 44:214–223. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marco V, Garcia F, Rubio IT, Soler T,
Ferrazza L, Roig I, Mendez I, Andreu X, Mínguez CG and Tresserra F:
Adenoid cystic carcinoma and basaloid carcinoma of the breast: A
clinicopathological study. Rev Esp Patol. 54:242–249.
2021.PubMed/NCBI
|
|
44
|
Vasudevan G, John AM, D K V and
Vallonthaiel AG: Adenoid cystic carcinoma of the breast with late
recurrence and high-grade transformation. BMJ Case Rep.
16:e2523362023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Collins K, Prieto VG and Aung PP: Unusual
presentations of primary and metastatic adenoid cystic carcinoma
involving the skin. Am J Dermatopathol. 42:967–971. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sołek JM, Braun M, Kalwas M,
Jesionek-Kupnicka D and Romańska HM: Adenoid cystic carcinoma of
the breast-an uncommon malignancy with unpredictable clinical
behaviour. A case series of three patients. Contemp Oncol (Pozn).
24:263–265. 2020.PubMed/NCBI
|
|
47
|
Mhamdi HA, Kourie HR, Jungels C, Aftimos
P, Belbaraka R and Piccart-Gebhart M: Adenoid cystic carcinoma of
the breast-an aggressive presentation with pulmonary, kidney, and
brain metastases: A case report. J Med Case Rep. 11:3032017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
D'Alfonso TM, Mosquera JM, Macdonald TY,
Padilla J, Liu YF, Rubin MA and Shin SJ: MYB-NFIB gene fusion in
adenoid cystic carcinoma of the breast with special focus paid to
the solid variant with basaloid features. Hum Pathol. 45:2270–2280.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Silva I, Tome V and Oliveira J: Adenoid
cystic carcinoma of the breast with cerebral metastisation: A
clinical novelty. BMJ Case Rep. 2011:bcr08201146922011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vranić S, Bilalović N, Lee LMJ, Krušlin B,
Lilleberg SL and Gatalica Z: PIK3CA and PTEN mutations in adenoid
cystic carcinoma of the breast metastatic to kidney. Hum Pathol.
38:1425–1431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Millar BAM, Kerba M, Youngson B, Lockwood
GA and Liu FF: The potential role of breast conservation surgery
and adjuvant breast radiation for adenoid cystic carcinoma of the
breast. Breast Cancer Res Treat. 87:225–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Herzberg AJ, Bossen EH and Walther PJ:
Adenoid cystic carcinoma of the breast metastatic to the kidney. A
clinically symptomatic lesion requiring surgical management.
Cancer. 68:1015–1020. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koller M, Ram Z, Findler G and Lipshitz M:
Brain metastasis: A rare manifestation of adenoid cystic carcinoma
of the breast. Surg Neurol. 26:470–472. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peters GN and Wolff M: Adenoid cystic
carcinoma of the breast report of 11 new cases: Review of the
literature and discussion of biological behavior. Cancer.
52:680–686. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lim SK, Kovi JK and Warner OG: Adenoid
cystic carcinoma of breast with metastasis: A case report and
review of the literature. J Natl Med Assoc. 71:329–330.
1979.PubMed/NCBI
|
|
56
|
Verani RR and Van der Bel-Kahn J: Mammary
adenoid cystic carcinoma with unusual features. Am J Clin Pathol.
59:653–658. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Elsner B: Adenoid cystic carcinoma of the
breast. Review of the literature and clinico-pathologic study of
seven patients. Pathol Eur. 5:357–364. 1970.PubMed/NCBI
|
|
58
|
O'Kell RT: Adenoid cystic carcinoma of the
breast. Mo Med. 61:855–858. 1964.PubMed/NCBI
|
|
59
|
Nayer HR: Case report section; cylindroma
of the breast with pulmonary metastases. Dis Chest. 31:324–327.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu H and Doğan BE: American joint
committee on cancer's staging system for breast cancer, eighth
edition: Summary for clinicians. Eur J Breast Health. 17:234–238.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Canyilmaz E, Uslu GH, Memiş Y, Bahat Z,
Yildiz K and Yoney A: Adenoid cystic carcinoma of the breast: A
case report and literature review. Oncol Lett. 7:1599–1601. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vranic S, Bender R, Palazzo J and Gatalica
Z: A review of adenoid cystic carcinoma of the breast with emphasis
on its molecular and genetic characteristics. Hum Pathol.
44:301–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Badve S, Dabbs DJ, Schnitt SJ, Baehner FL,
Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR,
et al: Basal-like and triple-negative breast cancers: A critical
review with an emphasis on the implications for pathologists and
oncologists. Mod Pathol. 24:157–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wetterskog D, Lopez-Garcia MA, Lambros MB,
A'Hern R, Geyer FC, Milanezi F, Cabral MC, Natrajan R, Gauthier A,
Shiu KK, et al: Adenoid cystic carcinomas constitute a genomically
distinct subgroup of triple-negative and basal-like breast cancers.
J Pathol. 226:84–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kilickap S, Kaya Y, Yucel B, Tuncer E,
Babacan NA and Elagoz S: Higher Ki67 expression is associates with
unfavorable prognostic factors and shorter survival in breast
cancer. Asian Pac J Cancer Prev. 15:1381–1385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Davey MG, Hynes SO, Kerin MJ, Miller N and
Lowery AJ: Ki-67 as a prognostic biomarker in invasive breast
cancer. Cancers (Basel). 13:44552021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Al Zubidi M, Abdulhussain MM and Mohsin
AS: Assessment of the relationship between Ki-67 expression and
different clinicopathological factors of adenoid cystic carcinoma:
A retrospective immunohistochemical study. Int J Biomed.
15:141–145. 2025.
|
|
69
|
Bussari S, Jeergal PA, Sarode M, Namazi
NA, Kulkarni PG, Deshmukh A and Kulkarni D: Evaluation of
proliferative marker Ki-67 in adenoid cystic carcinoma: A
retrospective study. J Contemp Dent Pract. 20:211–215. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Persson M, Andrén Y, Mark J, Horlings HM,
Persson F and Stenman G: Recurrent fusion of MYB and NFIB
transcription factor genes in carcinomas of the breast and head and
neck. Proc Natl Acad Sci USA. 106:18740–18744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Massé J, Truntzer C, Boidot R, Khalifa E,
Pérot G, Velasco V, Mayeur L, Billerey-Larmonier C, Blanchard L,
Charitansky H, et al: Solid-type adenoid cystic carcinoma of the
breast, a distinct molecular entity enriched in NOTCH and CREBBP
mutations. Mod Pathol. 33:1041–1055. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Arpino G, Clark GM, Mohsin S, Bardou VJ
and Elledge RM: Adenoid cystic carcinoma of the breast: Molecular
markers, treatment, and clinical outcome. Cancer. 94:2119–2127.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Marchiò C, Weigelt B and Reis-Filho JS:
Adenoid cystic carcinomas of the breast and salivary glands (or
‘The strange case of Dr Jekyll and Mr Hyde’ of exocrine gland
carcinomas). J Clin Pathol. 63:220–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cantù G: Adenoid cystic carcinoma. An
indolent but aggressive tumour. Part A: From aetiopathogenesis to
diagnosis. Acta Otorhinolaryngol Ital. 41:206–214. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Page DL: Adenoid cystic carcinoma of
breast, a special histopathologic type with excellent prognosis.
Breast Cancer Res Treat. 93:189–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wilson WB and Spell JP: Adenoid cystic
carcinoma of breast: A case with recurrence and regional
metastasis. Ann Surg. 166:861–864. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang S, Ji X, Wei Y, Yu Z and Li N:
Adenoid cystic carcinoma of the breast: Review of the literature
and report of two cases. Oncol Lett. 4:701–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hodgson NC, Lytwyn A, Bacopulos S and
Elavathil L: Adenoid cystic breast carcinoma: High rates of margin
positivity after breast conserving surgery. Am J Clin Oncol.
33:28–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Khanfir K, Kallel A, Villette S, Belkacémi
Y, Vautravers C, Nguyen T, Miller R, Li YX, Taghian AG, Boersma L,
et al: Management of adenoid cystic carcinoma of the breast: A rare
cancer network study. Int J Radiat Oncol Biol Phys. 82:2118–2124.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li L, Zhang D and Ma F: Adenoid cystic
carcinoma of the breast may be exempt from adjuvant chemotherapy. J
Clin Med. 11:44772022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gomez-Seoane A, Davis A and Oyasiji T:
Treatment of adenoid cystic carcinoma of the breast: Is
postoperative radiation getting its due credit? Breast. 59:358–366.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Bailey J,
Burstein HJ, et al: Breast Cancer, Version 3.2024, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
22:331–357. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Schulz-Costello K, Fan F, Schmolze D,
Arias-Stella JA III, Taylor L, Tseng J, Afkhami M, Rand JG, Jones
V, Farmah P and Han M: Solid basaloid adenoid cystic carcinoma of
the breast: A high-grade triple negative breast carcinoma which
rarely responds to neoadjuvant chemotherapy. Hum Pathol.
157:1057602025. View Article : Google Scholar : PubMed/NCBI
|