Introduction
Due to the high prevalence of hepatitis B and C
virus infections in many countries, the incidence of hepatocellular
carcinoma (HCC) is increasing and the mortality rate of HCC is
extremely high in some countries (1). According to the data published by the
International Agency for Research on Cancer, there were 564,000 HCC
patients in 2000 and 55% of which were in China (2). Curative treatments for HCC include
surgery, local destruction techniques (radiofrequency ablation or
percutaneous ethanol injection) and liver transplantation.
Unfortunately, only ~40% of patients can benefit from curative
treatments and only 1/3 of patients are typically resectable
(3). Hence, for most patients with
unresectable HCC, palliative treatments, which include
transarterial chemoembolization, systemic therapy and radiotherapy
are their only choices, and the survival rate and quality of life
for them remain dismal (4,5). Low efficacy and severe adverse effects
still exist for chemotherapeutics. Development of more effective
and less toxic antineoplastic agent remains an urgent task.
A meta-analysis has shown that patients
simultaneously receiving chemotherapy and Chinese medicines may
have higher 1-, 2- and 3-year survival rates (6). Indeed, many traditional Chinese
medicines have long been used to fight various cancers and manage
the side effects of chemotherapy for several decades. For instance,
astragalus induced cancer cell apoptosis. It reversed the
immunosuppressive effects of chemotherapy drugs by stimulating the
production of interleukin-6 and TNF (7,8).
Hedyotis Diffusa Willd (HDW) is an ancient
Chinese medicine belonging to the Rubiaceae family, which is
capable of heat-clearing, detoxification and activating blood
according to the Chinese medicinal theory (9). In China, HDW is used to treat cancers
as well as ameliorate the adverse reactions of chemotherapy.
Pharmacological studies showed that it contains compounds with
anticancer activities, including anthraquinones, hemiterpenes,
flavones, polyphenols, organic acids and polysaccharides (10–12).
Our previous study demonstrated that HDW extract inhibited
angiogenesis and induced tumor cell apoptosis via the
mitochondrion-dependent pathway (13,14).
By performing molecular docking simulation, we found that
components in HDW (such as quercetin, asperuloside) could bind
cyclin-dependent kinase 2 (CDK2) (15). CDK2 and downstream transcription
factor E2F1 regulate the transition from G1 to S phase during cell
proliferation (16). Inhibition of
CDK2 activity can inhibit cell proliferation.
In this study, we showed that water extract of HDW
was able to suppress the expression of CDK2 and E2F1 mRNA in HepG2
cells, which is consistent with molecular docking simulation. More
importantly, our findings suggested that water extract of HDW
effectively inhibited HepG2 cell growth in vivo and promote
the anticancer efficiency of low-dose (5-fluorouracil) 5-FU via the
inhibition of the CDK2-E2F1 pathway.
Materials and methods
Reagents
5-FU was purchased from Tianjin King York
Aminophenol, Inc. (Tianjin, China). HDW was purchased from
Purapharm Co., Ltd. (Hong Kong, China). Specimens were
authenticated by the Department of Pharmacy of Fujian University of
Traditional Chinese Medicine (Fuzhou, China). To prepare the HDW
water extract, HDW was steeped in 10-fold volume of distilled water
and decocted two times, 20 min each time. The aqueous extract was
then filtered and concentrated to make a decoction, and
subsequently stored at 4°C until use. Each milliliter of water
extract was equivalent to 1.8 g crude HDW.
Animals
Forty female BALB/c nu/nu mice (6-weeks-old,
18–22 g) were purchased from Shanghai Slac Experimental Animal Co.,
Ltd. (Shanghai, China). The animals were maintained in a
pathogen-free facility (23±2°C, 55±5% humidity). Animal care and
experiment procedures were approved by the Ethics Committee of
Fujian University of Traditional Chinese Medicine.
Cell line and culture
The HepG2 cell line was obtained from the Shanghai
Institute of Life Science, Chinese Academy of Sciences (Shanghai,
China), and was grown in high glucose DMEM (Gibco, Carlsbad, CA)
supplemented with 10% fetal calf serum (Gibco).
MTT assay
To evaluate the effect of HDW on tumor cell
proliferation, 1×104 HepG2 cells were seeded in 96-well
plates for 24 h and treated with HDW at the final concentrations of
0, 1.25, 2.5, 5 and 10 mg/ml for 24 h. Then, 20 mu;l of 5 mg/ml
methyl thiazolyl tetrazolium (MTT) was added and incubated for
another 4 h before it was discarded. The purple-blue MTT formazan
precipitate was dissolved in 100 mu;l dimethyl sulfoxide (DMSO).
The absorbance was measured at 570 nm. Cell viability was
calculated according to the following formula: cell viability (%) =
average ODtreatment group/average ODblank
group ×100%.
Cell cycle assay
Cells were incubated in 6-well plates with either
4.62 mg/ml HDW (IC50) or vehicle. They were referred to
as HDW group and control group, respectively. After 24 h, cells
were digested and washed. Progression through the cell cycle was
measured with flow cytometery (BD Biosciences, Franklin, NJ)
according to the instructions of the Cycle-test Plus DNA assay kit
(BD Biosciences). The percentages of cells in G0/G1, S and G2/M
phase were evaluated by the ModFit software (BD Biosciences). The
proliferating index (PI) was calculated according to the following
formula: PI=(S+G2/M)/(G0/G1+S+G2/M) ×100%.
Mouse xenograft experiments
BALB/c nu/nu mice were inoculated with
1×104 HepG2 cells at the right flank and treatment was
initiated when the estimated tumor volumes uniformly reached ~50
mm3. The mice were randomly divided into 4 groups
(n=10/group): low-dose 5-FU group, HDW group, combination group
(HDW plus low-dose 5-FU) and vehicle control group, which received
5-FU [10 mg/kg/day, intraperitoneally (i.p.)], HDW [6 g/kg/day,
intragingivally (i.g.)], 5-FU (10 mg/kg/day, i.p.) plus HDW (6
g/kg/day, i.g.) and normal saline (NS, i.g.), respectively. The
mouse body weight as well as tumor width (d) and length (D) were
measured once every 3 days. Tumor volume (TV) was calculated by
formula: TV (mm3)=d2×D×0.52. After 4 weeks of
treatment, blood from each mouse was collected, and the tumors were
harvested and weighed. The levels of alanine aminotransferase
(ALT), aspartate transaminase (AST), blood urea nitrogen (BUN) and
creatinine (CRE) (Kehua, Shanghai, China) were measured by
Biochemical Analyzer (Toshiba, Kawasaki, Japan). The tumor
inhibitory rate (TIR) was calculated as follows: TIR (%) =
[1−average tumor weight(treatment group)/average tumor
weight(vehicle group)] ×100%.
Quantitative real-time polymerase chain
reaction (PCR) examination
Total cellular RNA and tissular RNA were isolated
using TRIzol one-step method as described in the manufacturer’s
recommendations (Invitrogen, Carlsbad, CA). Single-stranded cDNA
was synthesized using oligo(dt) primer (Promega, Madison, WI) in a
20 mu;l reaction mixture. The primer pairs of CDK2, E2F1, cyclin E
and GAPDH were designed and synthesized as follows: E2F1,
5′-TGATACCCCAACTCCCTCTACC-3′ and 5′-TGT CTCCCTCCCTCACTTTCC-3′;
CDK2, 5′-CGCAAATG CTGCACTACGACC-3′ and 5′-GCCCACCTGAGTCCAAATA
GCC-3′; Cyclin E, 5′-TGACTGCCTTGAATTTCCTTATG-3′ and
5′-GCACCACTGATACCCTGAAACC-3′; GAPDH, 5′-AC GGATTTGGTCGTATTGGGC-3′
and 5′-CTCGCTCCTGGA AGATGGTGAT-3′. Real-time PCR was performed with
power SYBR-Green I PCR Master mix kit (Applied Biosystems, Foster
City, CA) by a 7500 real-time PCR system (Applied Biosystems). The
relative quantitative PCR conditions for 40 cycles were performed
as follow: denaturation at 95°C for 15 sec, anneal and extension at
60°C for 1 min. After the amplification phase was completed, the
melting curve examination was performed in order to eliminate
non-specific products. The fold change in gene expression of each
sample was analyzed by SDS software (Applied Biosystems) using the
equation 2−ΔΔCt method to calculate the relative level
of each mRNA and expressed as a ratio relative to GAPDH housekeeper
genes, where Ct value is the fractional cycle number at which the
fluorescence exceeds that of background, and
ΔΔCt=(Cttarget−CtGAPDH)sample−
(Cttarget−CtGAPDH)control(17).
Western blot analysis
Tumor cells were put on dry ice for 10 min in lysis
buffer. After centrifugation at 11,000 rpm at 4°C for 20 min, the
supernatant was collected and the protein concentration determined
using the Bradford assay. Equal amounts of denatured protein were
separated on SDS-PAGE gels and transferred onto nitrocellulose (NC)
membranes. The NC membranes were then put into blocking solution
(1% bovine serum albumin) for 1 h and incubated in monoclonal
anti-mouse CDK2, E2F1 or cyclin E primary antibody solutions (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA) or anti-β-actin antibody
solution (Beyotime, Shanghai, China) overnight at 4°C with shaking,
and then in horseradish peroxidase (HRP)-conjugated secondary
antibody (Beyotime) for at least 1 h. Chemiluminescence was
detected using a Chemiluminescence imaging system (Bio-Rad,
Hercules, CA).
Statistical analysis
The data were expressed as mean ± SD of at least
triplicate experiments. Double and multiple comparisons were
performed using independent-samples t-test and one-way ANOVA,
respectively. P-value <0.05 was considered significant. All
results were analyzed using the SPSS 16.0 statistical software.
Results
HDW inhibits proliferation of HepG2 cells
in vitro
The effect of HDW on the proliferation of HepG2
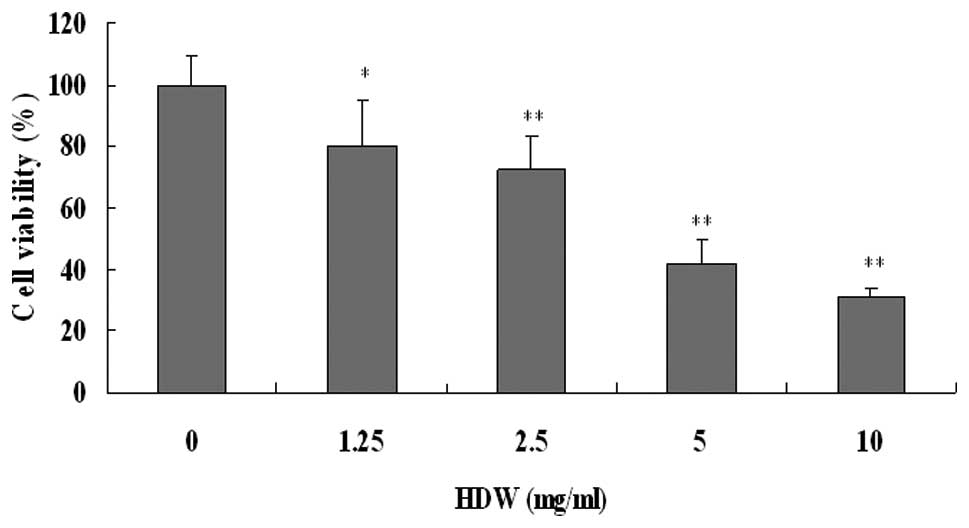
cells was evaluated by MTT method. As shown in Fig. 1, in the presence of various
concentrations of HDW, the proliferation of HepG2 was remarkably
inhibited in a dose-dependent manner. The concentration of HDW to
inhibit 50% cell growth (IC50) was estimated to be 4.62
mg/ml. The results demonstrated that water extract of HDW could
markedly inhibit the proliferation of HepG2 cells.
HDW arrests HepG2 cells at G0/G1 phase
and induces S phase delay
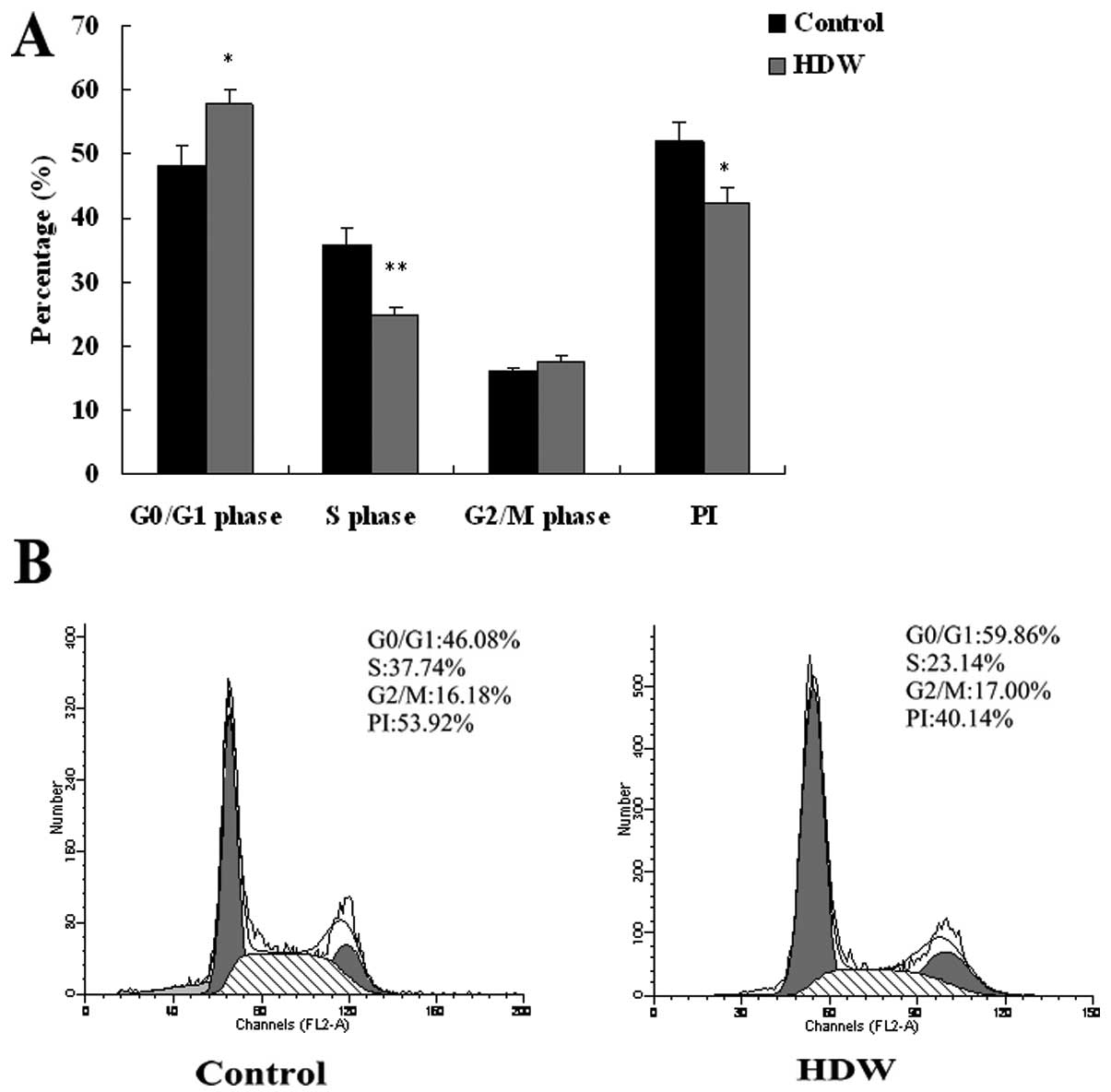
Progression through the cell cycle was examined
using flow cytometry and the results are shown in Fig. 2. When HepG2 cells were treated with
4.62 mg/ml HDW (IC50), the percentage of cells in G0/G1
phase increased from 48.16±3.11–57.71±2.29% (P<0.05). On the
contrary, the percentage of cells in S phase decreased from
35.73±2.56–24.71±1.43% (P<0.01). As expected, PI in the HDW
group was lower than that of the control group (42.29±2.30% vs.
51.85±3.11%, P<0.05). These results demonstrated that HDW
inhibited the proliferation of HepG2 cells by inducing G0/G1 phase
arrest and S phase delay.
HDW downregulates the expression of CDK2
and E2F1 mRNA of HepG2 cells
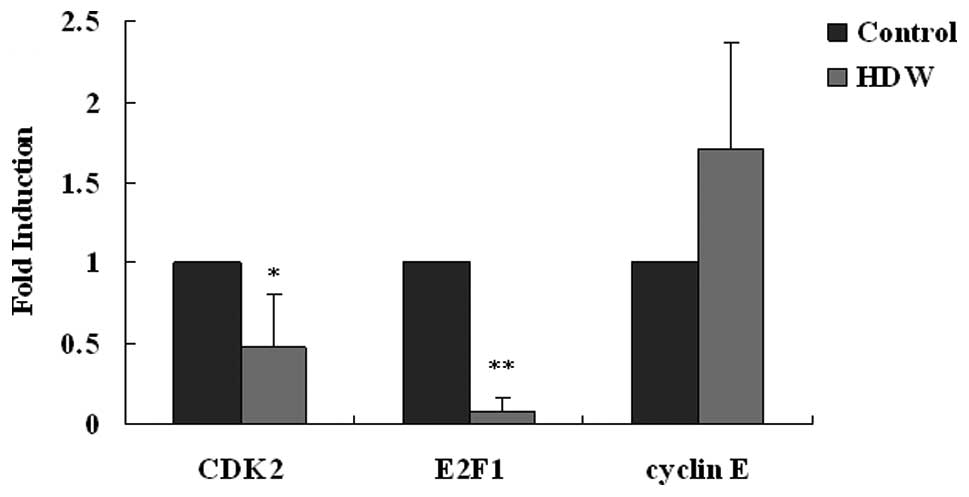
CDK2, cyclin E and E2F1 transcription factor play
critical roles in cell cycle progression from G0/G1 to S phase. To
investigate how HDW interferred G1/S transition of HepG2 cell
cycle, expression of CDK2, E2F1 and cyclin E mRNA was evaluated
with relative quantitative real-time PCR. As shown in Fig. 3, the levels of CDK2 and E2F1 mRNA
decreased to 48 and 0.08%, respectively by HDW. However, HDW did
not affect the transcription of cyclin E mRNA. These results
suggested that HDW induced G0/G1 phase arrest and S phase delay of
HepG2 cells at least partly through the CDK2-E2F1 pathway in
vitro.
Assessment of anticancer effects of HDW
alone or in combination with low-dose 5-FU in vivo
The reason of testing HDW for treatment of HCC is to
see if HDW can be used as an adjuvant therapeutic agent in
combination with low-dose chemotherapy, which is not as effective
as standard-dose therapy. To evaluate the effects of HDW on tumor
growth and if it could enhance the effect of low-dose 5-FU, the
leading chemotherapeutic drug for advanced HCC, we examined tumor
xenograft volume once every 3 days after initial treatment and
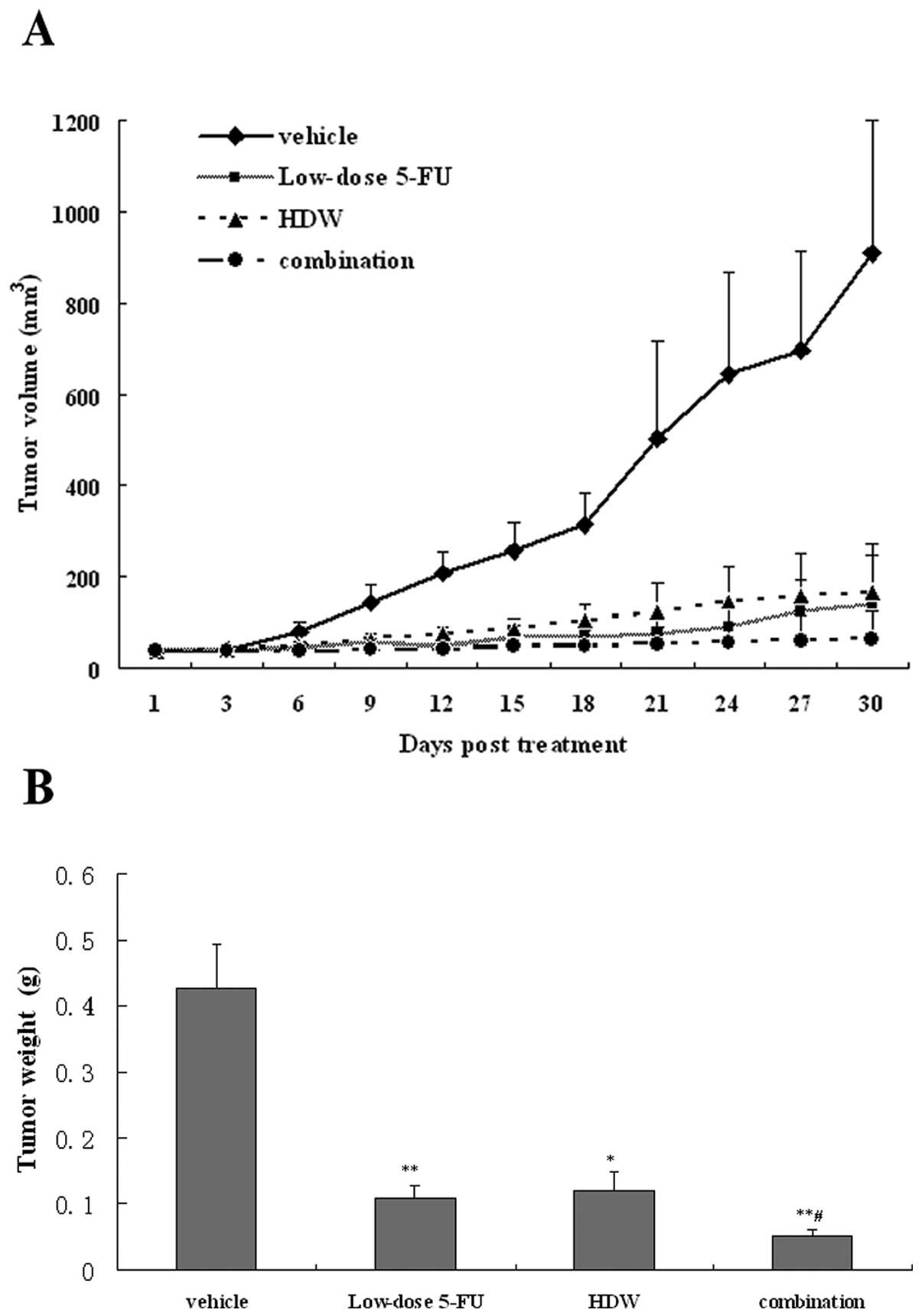
tumor weight at sacrifice. As shown in Fig. 4A, all treatment groups showed tumor
growth delay as assessed by tumor volume changes. At sacrifice, the
tumor volume was 909.92±289.99 mm3 in the vehicle group,
139.73±104.46 mm3 in the low-dose 5-FU group,
165.35±106.17 mm3 in the HDW group and 65.92±59.34
mm3 in the combination group. Thus, either HDW or
low-dose 5-FU could significantly inhibit the tumor growth. When
they were simultaneously administered, a higher inhibition was
achieved (P<0.01). A similar tendency was observed when the
tumor weights were compared among the 4 groups (Fig. 4B). The TIR was 74.51% in the
low-dose 5-Fu group, 71.83% in the HDW group and 87.87% in the
combination group, respectively. The anticancer efficacy of HDW was
comparable to low-dose 5-FU. Furthermore, HDW potentiated the
effect of low-dose 5-FU.
Evaluation of toxicity of HDW and
combined therapy
We also investigated the gross toxicity of HDW alone
or HDW combined with low-dose 5-FU in tumor-bearing mice. As tumor
growth progressed, mice in all groups gradually showed signs of
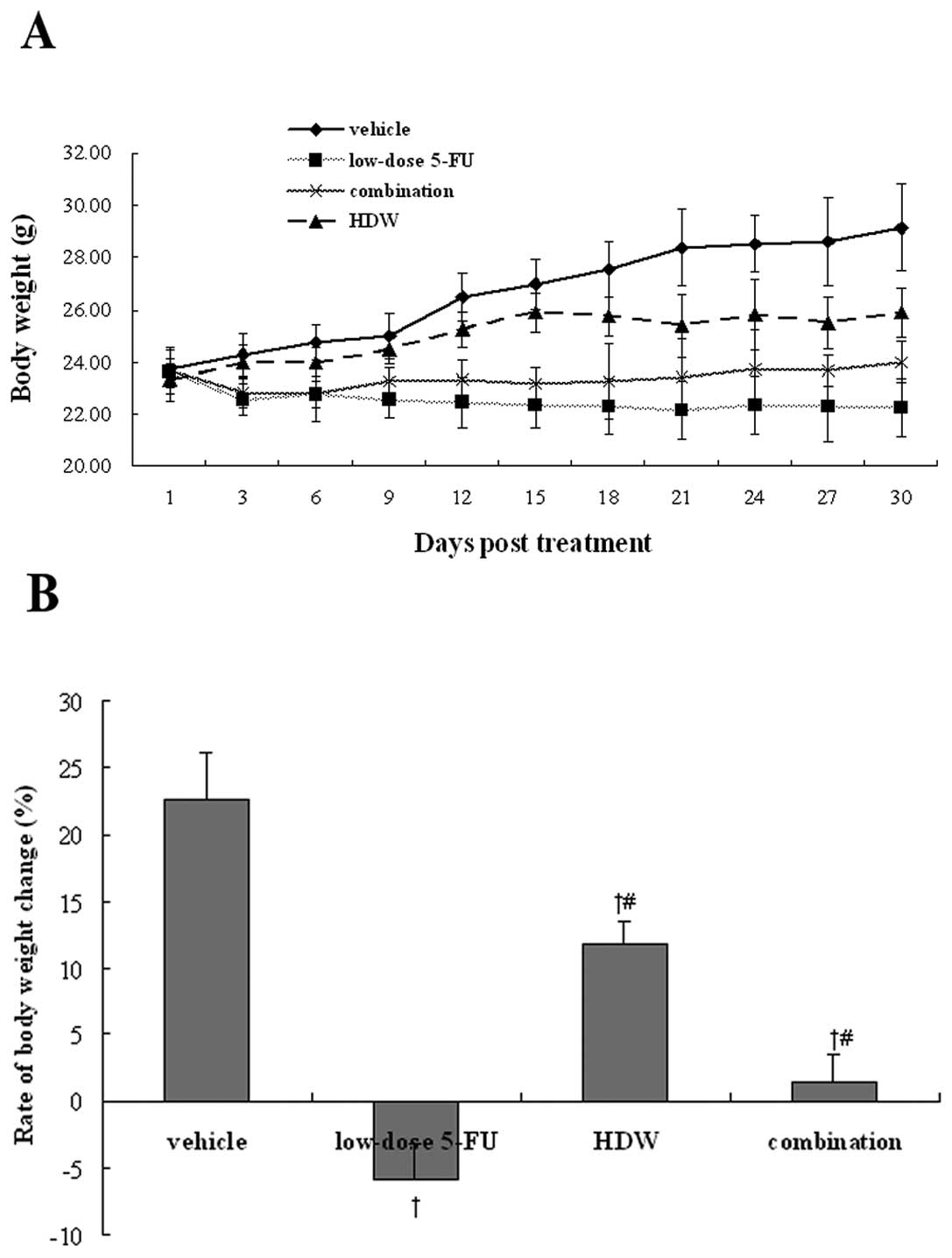
dullness, inertia, dim skin color, etc. The body weights of
tumor-bearing mice in each group are shown in Fig. 5. At sacrifice, body weight decreased
by 5.92±2.79% in the low-dose 5-FU group. On the contrary, the body
weight increased by 22.65±3.42% in the vehicle group, 11.79±1.81%
in the HDW group and 1.39±2.15% in the combination group. No overt
toxicity in liver and kidney function was observed in each group
(Table I). These results indicate
that addition of HDW to low-dose 5-FU does not increase the
toxicity of the latter.
 | Table IHepatorenal toxicity of low-dose
5-FU, HDW and a combination of low-dose 5-FU and HDW. |
Table I
Hepatorenal toxicity of low-dose
5-FU, HDW and a combination of low-dose 5-FU and HDW.
| Groups | ALT (IU/l) | AST (IU/l) | BUN (mmol/l) | CRE (mu;mol/l) |
|---|
| Vehicle | 58.44±15.00 | 217.88±53.20 | 8.07±1.05 | 31.36±6.50 |
| Low-dose 5-FU | 58.70±17.70 | 206.10±55.40 | 7.89±1.70 | 35.96±8.30 |
| HDW | 72.00±15.19 | 216.50±54.87 | 7.13±0.76 | 31.48±5.17 |
| Combination | 62.00±12.65 | 202.00±26.44 | 9.78±2.20 | 36.08±4.16 |
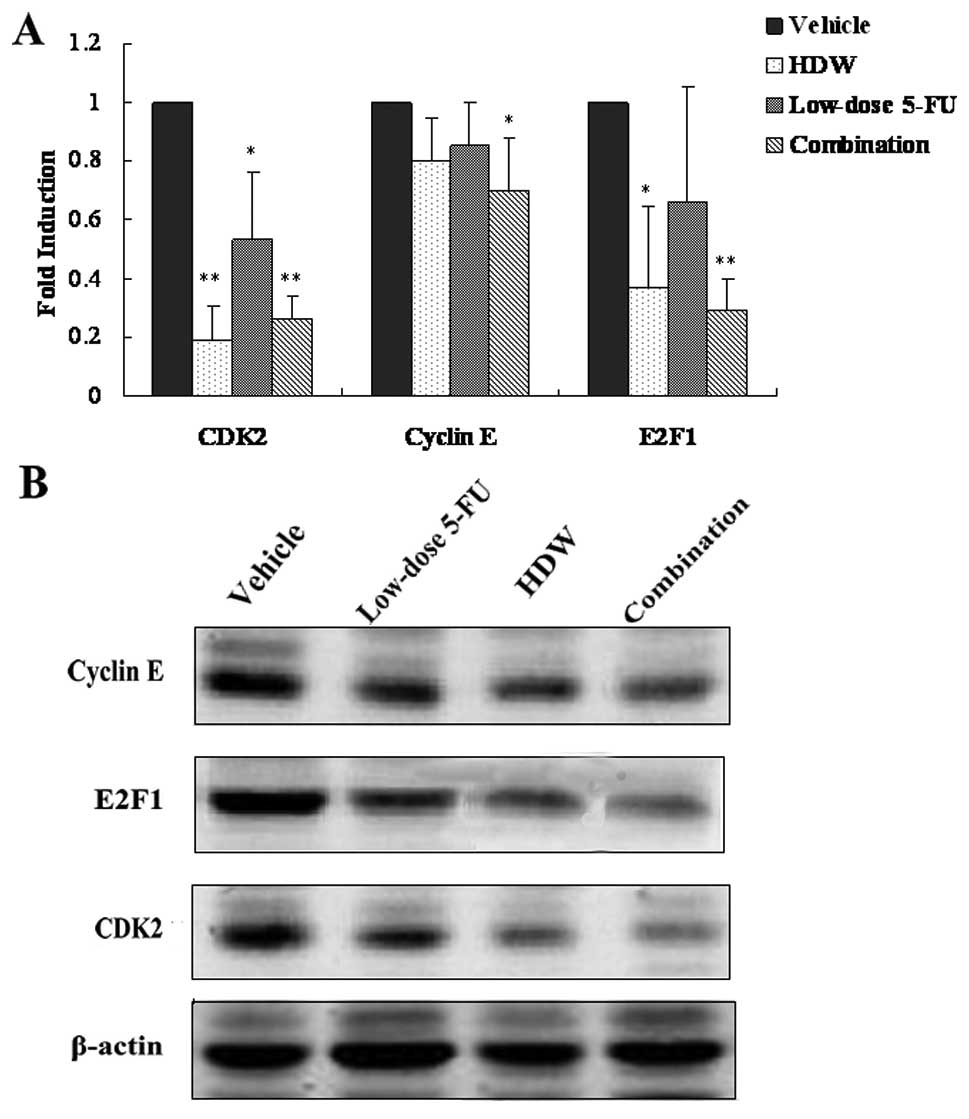
HDW and combination therapy downregulate
the expressions of cell cycle regulators in vivo
As shown above, HDW treatment downregulated the
expressions of CDK2 and E2F1 mRNA of HepG2 cells in vitro.
Here we further investigated HDW alone and HDW combined with
low-dose 5-FU on the expression of CDK2, E2F1 and cyclin E in
vivo using both western blot analysis and real-time PCR. The
results are shown in Fig. 6.
Compared with the vehicle group, HDW markedly downregulated the
expression levels of CDK2 and E2F1 mRNA. Low-dose 5-FU
significantly decreased the expression level of CDK2 mRNA but
showed no effects on E2F1 and cyclin E mRNA expression. As
expected, a combination of HDW and low-dose 5-FU caused a greater
decrease in the expression levels of CDK2 and E2F1 mRNA (Fig. 6A). Expression of cyclin E was also
decreased in the combination group. These results were further
supported by western blot analysis (Fig. 6B), indicating that HDW enhanced the
efficacy of low-dose 5-fluorouracil by further inhibiting the
CDK2-E2F1 pathway.
Discussion
5-FU-based regimens as standard treatment have been
widely used clinically in the treatment of various solid tumors,
such as colorectal cancer (18),
HCC (19), breast cancer (20) and glioma (21). Clinical trials have proven that
5-FU-based chemotherapy significantly improves overall and
disease-free survival of such patients (22). Despite these favorable results, low
response rate (13%) (23),
resistance (24), short half-life
(10 min) (25), severe cytotoxic
and immunosuppressive adverse reactions (26–29)
limit its clinical application. Recently, the regimens of
low-dose-based chemotherapy have been used against cancers with
relatively mild toxicities (30–37).
For example, regimen with low-dose 5-fluorouracil and cisplatin has
been reported to improve the median survival time of patients with
advanced HCC (36). Patients with
unresectable squamous cell carcinoma of the esophagus also had
favorable overall survival when treated with low-dose cisplatin and
continuous infusion of 5-FU (37).
Unfortunately, low-dose chemotherapy regimens are still undoubtedly
less effective than standard-dose therapy regimens.
The use of Chinese medicine as adjuvant treatment of
chemotherapy has many advantages over chemotherapy alone, such as
enhancement of the anticancer effects and reduction of the side
effects of chemotherapy and improvement of patients’ quality of
life (38). HDW, an important
component in many anticancer formulas of traditional Chinese
medicine, has been verified by many researchers (7,39).
However, its anticancer mechanism still remains largely unknown.
Our findings showed that HDW could inhibit the growth of HepG2
cells both in vitro and in vivo. It can arrest HepG2
cells at G0/G1 phase and induce S phase delay at least partly
through the CDK2-E2F1 pathway. In addition, we found in animal
experiments that although its antineoplastic effect was lower than
low-dose 5-FU, it might enhance the antitumor effect of the latter
in the absence of overt toxicity, indicating that HDW might be a
promising adjuvant therapy for chemotherapy.
The CDK2-E2F1 pathway is critical in regulating the
transition of G1 to S phase of cell cycle. CDK2 is one of the
members of cyclin-dependent kinases (CDKs). When CDK2 associates
with cyclin E, its serine-threonine kinase activity is activated
(40,41) and the conjunction facilitates
phosphorylation of retinoblastoma protein with the release of the
E2F1 transcription factor (42).
The release of the E2F1 transcription factor drives cells through
G1 into S phase and promotes cell cycle progression (43). The control of the pathway is
disrupted in virtually all human cancers (44), including HCC (45). Several studies have demonstrated
that the growth of cancer cells could be inhibited via
downregulating the mRNA or protein levels of CDK2, cyclin E or E2F1
transcription factor. For example, Tin et al(46) reported that artemisinin inhibited
the proliferation of breast cancer cells via selectively
downregulating the levels of the CDK2 and CDK4, cyclin E, cyclin D1
and the E2F1 transcription factor. Fang et al(47) reported that acetylbritannilactone
inhibited the growth of HT-29 human colon cancer cells by inducing
cell cycle arrest in G0/G1 phase and this suppression was
accompanied by a marked decrease of cyclin E and CDK4 protein
levels.
In this study, we observed the effect of HDW on the
transcription and protein levels of CDK2, cyclin E and E2F1
involved in CDK2/Rb/E2F signaling. Our results showed that HDW
interfered the G1/S transition of HepG2 cells at least via
downregulation of the transcript and protein levels of CDK2 and
E2F1 transcription factor. The results are consistent with our data
of molecular docking simulation (15). Furthermore, we also found in
vivo low-dose 5-FU could inhibit the expression of CDK2, as
reported by others (18). However,
it had no effect on the levels of cyclin E and E2F1 transcription
factor. Combined with HDW, low-dose 5-FU more remarkably
downregulated not only CDK2 but also E2F1 transcription factor and
cyclin E. This may partially explain why HDW can enhance the
antiproliferative effect of low-dose 5-FU.
In the present study, we also found that
administration of low-dose 5-FU alone caused diarrhea in the mice
(data not shown) and suppressed body weight gain. Indeed, studies
showed that the use of low-dose 5-FU was still associated with many
adverse effects, such as mucositis (18%) and diarrhea (39%)
(48), which may be the possible
reason of weight loss. However, when the mice were treated with the
combination of HDW and low-dose 5-FU, the effect was reversed. Wang
et al(49) reported that HDW
could protect the gastrointestinal mucosa. We thus hypothesize that
HDW could protect against low-dose 5-FU-induced weight loss by
preventing injury to the gastrointestinal mucosa that otherwise
would cause diarrhea.
In conclusion, HDW remarkably enhanced the antitumor
effect of low-dose 5-FU by inhibiting the CDK2-E2F1 pathway in the
absence of overt toxicity. These findings provide important data
for further testing the possibility of using HDW as adjuvant
therapy for clinical HCC patients. Further studies are necessary to
elucidate the compounds in HDW which are responsible for such
synergism.
Acknowledgements
The study was supported by the International Science
Joint Project, the Ministry of Science and Technology of the
People’s Republic of China (no. 2008DFA32200), the National Natural
Science Foundation of China (no. 81102582), the Fujian Province
Natural Science Foundation (no. 2009J01170), the Chen Keji
Integrative Medicine Developmental Foundation (no. CKJ2008002) and
the Open Fund of Fujian Key Laboratory of Integrative Medicine on
Geriatrics (2008J1004-15ckj2008052).
Abbreviations:
|
HDW
|
Hedyotis Diffusa Willd
|
|
HCC
|
hepatocellular carcinoma
|
|
5-FU
|
5-fluorouracil
|
|
CDK2
|
cyclin-dependent kinase 2
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
PCR
|
polymerase chain reaction
|
|
HRP
|
horseradish peroxidase
|
|
PI
|
proliferating index
|
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
2
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
3
|
Gish RG and Baron A: Hepatocellular
carcinoma (HCC): current and evolving therapies. IDrugs.
11:198–203. 2008.PubMed/NCBI
|
|
4
|
Llovet J, Di Bisceglie A, Bruix J, Kramer
B, Lencioni R, Zhu A, Sherman M, Schwartz M, Lotze M, Talwalkar J
and Gores GJ: Design and endpoints of clinical trials in
hepatocellular carcinoma. J Natl Cancer Inst. 100:698–711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DuBray BJ Jr, Chapman WC and Anderson CD:
Hepatocellular carcinoma: a review of the surgical approaches to
management. Mo Med. 108:195–198. 2011.PubMed/NCBI
|
|
6
|
Shu X, McCulloch M, Xiao H, Broffman M and
Gao J: Chinese herbal medicine and chemotherapy in the treatment of
hepatocellular carcinoma:a meta-analysis of randomized controlled
trials. Integr Cancer Ther. 4:219–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshida Y, Wang MQ, Liu JN, Shan BE and
Yamashita U: Immunomodulating activity of Chinese medicinal herbs
and Oldenlandia diffusa in particular. Int J Immunopharmacol.
19:359–370. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song Y, Yang J, Bai WL and Ji WY:
Antitumor and immunoregulatory effects of astragalus on
nasopharyngeal carcinoma in vivo and in vitro. Phytother Res.
25:909–915. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YG and Song LR: Zhong hua ben cao.
Shanghai Sciece and Technology Press; Shanghai: pp. 1530–1533.
1998
|
|
10
|
Zhang YY and Luo JB: Analysis of the
chemical constituents of Hedyotis diffusa. Nan Fang Yi Ke Da Xue
Xue Bao. 28:127–128. 2008.(In Chinese).
|
|
11
|
Lee HZ, Bau DT, Kuo CL, Tsai RY, Chen YC
and Chang YH: Clarification of the phenotypic characteristics and
anti-tumor activity of Hedyotis diffusa. Am J Chin Med. 39:201–213.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang W, Li Y and Jiang J: Chemical
constituents from Hedyotis diffusa. Zhongguo Zhong Yao Za Zhi.
34:712–714. 2009.(In Chinese).
|
|
13
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.
|
|
14
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis Diffusa Willd extract on tumor
angiogenesis. Mol Med Rep. 4:1283–1288. 2011.
|
|
15
|
Chen L, Zheng C and Du J: Study on
antitumor mechanism of Qingre Xiaozheng drink by molecular docking
method. Chin J Clin Pharmacol Ther. 12:324–328. 2007.
|
|
16
|
Wadler S: Perspectives for cancer
therapies with cdk2 inhibitors. Drug Resist Updat. 4:347–367. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
18
|
Sasaki K, Tsuno NH, Sunami E, Tsurita G,
Kawai K, Okaji Y, Nishikawa T, Shuno Y, Hongo K, Hiyoshi M, et al:
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on
colon cancer cells. BMC Cancer. 10:3702010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uka K, Aikata H, Takaki S, Kawaoka T,
Saneto H, Miki D, Takahashi S, Toyota N, Ito K and Chayama K:
Systemic gemcitabine combined with intra-arterial low-dose
cisplatin and 5-fluorouracil for advanced hepatocellular carcinoma:
Seven cases. World J Gastroenterol. 14:2602–2608. 2008. View Article : Google Scholar
|
|
20
|
Hutchins LF, Green SJ, Ravdin PM, Lew D,
Martino S, Abeloff M, Lyss AP, Allred C, Rivkin SE and Osborne CK:
Randomized, controlled trial of cyclophosphamide, methotrexate, and
fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil
with and without tamoxifen for high-risk, node-negative breast
cancer: treatment results of Intergroup Protocol INT-0102. J Clin
Oncol. 23:8313–8321. 2005. View Article : Google Scholar
|
|
21
|
Shapiro WR, Green SB, Burger PC, Selker
RG, VanGilder JC, Robertson JT, Mealey J Jr, Ransohff J and Mahaley
MS Jr: A randomized comparison of intra-arterial versus intravenous
BCNU, with or without intravenous 5-fluorouracil, for newly
diagnosed patients with malignant glioma. J Neurosurg. 76:772–781.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai WS, Hsieh PS, Yeh CY, Chiang JM, Tang
R, Chen JS, Changchien CR and Wang JY: Impact of
chemotherapy-related prognostic factors on long-term survival in
patients with stage III colorectal cancer after curative resection.
Int J Clin Oncol. Jan 20–2012.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Stehlin JS Jr, de Ipolyi PD, Greeff PJ,
McGaff CJ Jr, Davis BR and McNary L: Treatment of cancer of the
liver. Twenty years’ experience with infusion and resection in 414
patients. Ann Surg. 208:23–35. 1988.
|
|
24
|
Wang W, Cassidy J, O’Brien V, Ryan KM and
Collie-Duguid E: Mechanistic and predictive profiling of
5-fluorouracil resistance in human cancer cells. Cancer Res.
64:8167–8176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Regazzoni S, Pesce G, Marini G, Cavalli F
and Goldhirsch A: Low-dose continuous intravenous infusion of
5-fluorouracil for metastatic breast cancer. Ann Oncol. 7:807–813.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cameron DA, Gabra H and Leonard RC:
Continuous 5-fluorouracil in the treatment of breast cancer. Br J
Cancer. 70:120–124. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lokich J, Bothe A, Fine N and Perri J:
Phase I study of protracted venous infusion of 5-fluorouracil.
Cancer. 48:2565–2568. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemon HM: Reduction of 5-fluorouracil
toxicity in man with retention of anticancer effects by prolonged
intravenous administration in 5% dextrose. Cancer Chemother Rep.
8:97–101. 1960.PubMed/NCBI
|
|
29
|
Ng JS, Cameron DA and Leonard RC:
Infusional 5-fluorouracil in breast cancer. Cancer Treat Rev.
20:357–364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huan S, Pazdur R, Singhakowinta A, Samal B
and Vaitkevicius VK: Low-dose continuous infusion 5-fluorouracil
evaluation in advanced breast carcinoma. Cancer. 63:419–422. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Munoz R, Man S, Shaked Y, Lee CR, Wong J,
Francia G and Kerbel RS: Highly efficacious nontoxic preclinical
treatment for advanced metastatic breast cancer using combination
oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res.
66:3386–3391. 2006. View Article : Google Scholar
|
|
32
|
Iwamoto H, Torimura T, Nakamura T,
Hashimoto O, Inoue K, Kurogi J, Niizeki T, Kuwahara R, Abe M, Koga
H, et al: Metronomic S-1 chemotherapy and vandetanib: An
efficacious and nontoxic treatment for hepatocellular carcinoma.
Neoplasia. 13:187–197. 2011.PubMed/NCBI
|
|
33
|
Tanioka H, Tsuji A, Morita S, Horimi T,
Takamatsu M, Shirasaka T, Mizushima T, Ochi K, Kiura K and Tanimoto
M: Combination chemotherapy with continuous 5-fluorouracil and
low-dose cisplatin infusion for advanced hepatocellular carcinoma.
Anticancer Res. 23:1891–1897. 2003.PubMed/NCBI
|
|
34
|
Ueshima K, Kudo M, Takita M, Nagai T,
Tatsumi C, Ueda T, Kitai S, Ishikawa E, Yada N, Inoue T, et al:
Hepatic arterial infusion chemotherapy using low-dose
5-fluorouracil and cisplatin for advanced hepatocellular carcinoma.
Oncology. 78:148–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lai YC, Shih CY, Jeng CM, Yang SS, Hu JT,
Sung YC, Liu HT, Hou SM, Wu CH and Chen TK: Hepatic arterial
infusion chemotherapy for hepatocellular carcinoma with tumor
thrombosis of the portal vein. World J Gastroenterol. 9:2666–2670.
2003.PubMed/NCBI
|
|
36
|
Tang TC, Man S, Xu P, Francia G, Hashimoto
K, Emmenegger U and Kerbel RS: Development of a resistance-like
phenotype to sorafenib by human hepatocellular carcinoma cells is
reversible and can be delayed by metronomic UFT chemotherapy.
Neoplasia. 12:928–940. 2010.PubMed/NCBI
|
|
37
|
Takagawa R, Kunisaki C, Makino H, Kosaka
T, Ono HA, Akiyama H and Shimada H: Efficacy of chemoradiotherapy
with low-dose cisplatin and continuous infusion of 5-fluorouracil
for unresectable squamous cell carcinoma of the esophagus. Dis
Esophagus. 22:482–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Konkimalla VB and Efferth T:
Evidence-based Chinese medicine for cancer therapy. J
Ethnopharmacol. 116:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gupta S, Zhang D, Yi J and Shao J:
Anticancer activities of Oldenlandia diffusa. J Herb Pharmacother.
4:21–33. 2004. View Article : Google Scholar
|
|
40
|
Koff A, Giordano A, Desai D, Yamashita K,
Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR and Roberts
JM: Formation and activation of a cyclin E-cdk2 complex during the
G1 phase of the human cell cycle. Science. 257:1689–1694. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
D’Urso G, Marraccino RL, Marshak DR and
Roberts JM: Cell cycle control of DNA replication by a homologue
from human cells of the p34cdc2 protein kinase. Science.
250:786–791. 1990.PubMed/NCBI
|
|
42
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weinberg RA: E2F and cell proliferation: a
world turned upside down. Cell. 85:457–459. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Matsuda Y: Molecular mechanism underlying
the functional loss of cyclindependent kinase inhibitors p16 and
p27 in hepatocellular carcinoma. World J Gastroenterol.
14:1734–1740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tin AS, Sundar SN, Tran KQ, Park AH,
Poindexter KM and Firestone GL: Antiproliferative effects of
artemisinin on human breast cancer cells requires the downregulated
expression of the E2F1 transcription factor and loss of E2F1-target
cell cycle genes. Anticancer Drugs. 23:370–379. 2012. View Article : Google Scholar
|
|
47
|
Fang XM, Liu B, Liu YB, Wang JJ, Wen JK,
Li BH and Han M: Acetylbritannilactone suppresses growth via
upregulation of krüppel-like transcription factor 4 expression in
HT-29 colorectal cancer cells. Oncol Rep. 26:1181–1187.
2011.PubMed/NCBI
|
|
48
|
Smith IE, Johnston SR, O’Brien ME, Hickish
TF, de Boer RH, Norton A, Cirkel DT and Barton CM: Low-dose oral
fluorouracil with eniluracil as first-line chemotherapy against
advanced breast cancer: a phase II study. J Clin Oncol.
18:2378–2384. 2000.PubMed/NCBI
|
|
49
|
Wang GY, Li ZB, Shi JX, Wang H, Gao QF,
Gen LF, Zhu JJ and Zhao QS: Oldenlandia diffusa protects against
indomethacin-induced injury of the gastrointestinal mucosa in rats.
Hebei J Tradit Chin Med. 23:70–71. 2001.(In Chinese).
|