Introduction
The incidence of metastatic melanoma has increased
dramatically over the past two decades (1), while the prognosis remained poor with
an estimated 8600 persons died from melanoma in 2009 only in the
United States (2). In 2010, the
median survival of patients with melanoma who have distant
metastases is still less than one year (3). Treatment with standard
chemotherapeutic agents such dacarbazine, vinblastine or cisplatin
yields low response rates (4).
Cytokine therapy with high-dose IL-2 achieves similar response
rates but it may induce, although with low frequency, durable
complete response rates that lead to years of benefit (5). However, this treatment can be
accompanied by severe toxicities that require the patient to be
hospitalized for support during treatment (6). Cancer vaccines have also been largely
studied with some promising results (7). Adoptive cell transfer (ACT) of tumor
infiltrating lymphocytes in combination with IL-2 treatment after
host preconditioning by lympho-depletion represents one of the most
effective treatments for metastatic melanoma, but its application
is limited by several disadvantages such as the high technical
expertise required (8).
We have recently shown that covalent attachment of
the nitric oxide (NO) moiety to the HIV protease inhibitor
Saquinavir (Saq) produced a qualitatively new chemical entity
(NCE), named Saquinavir-NO (Saq-NO) (Fig. 1), with enhanced anticancer
properties and reduced toxicity. In particular, Saq-NO inhibited at
significantly lower IC50 values compared to the parental
compound the in vitro growth of rodent and human melanoma
cell lines B16 and A375 (9,10) showing potent anticancer effects also
against multidrug-resistant cancer cells (11). Neither p53 mutation nor depletion
nor expression of P-glycoprotein (P-gp), multidrug
resistance-associated protein 1 (MRP1), or breast cancer resistance
protein 1 (BRCP1) affected the anticancer activity of Saq-NO or
Saq. Moreover, Saq-NO sensitized P-gp-, MRP1-, or BCRP1-expressing
cancer cells to chemotherapy. Saq-NO enhanced sensitivity of P-gp-
or MRP1-expressing cancer cells to chemotherapy to a greater extent
than Saq (11). In agreement with
these in vitro effects, Saq-NO was more effective than its
parental compound Saq in reducing the in vivo growth of the
B16 and A375 xenotransplants (9,10) as
well as of the hormone resistant human prostate cancer PC3 cells
both in vitro and in vivo(12).
In vitro studies on the B16 and A375 melanoma
cell lines Saq-NO have shown a cell-dependent pharmacological
profile of Saq-NO. While Saq-NO efficiently exerted Akt-independent
cytostatic effects promoting terminal differentiation into
Schwann-like cells in B16 cells (9), it exerted a strong cytotoxic effect on
the inducible nitric oxide synthase (iNOS) positive A375 cells
(10). In these cells,
Saq-NO-triggered apoptosis was dependent on transient Akt
up-regulation and reduced pERK and iNOS expression that were
observed within the first 12 hours of exposure to the drug
(10). Thereafter, however, Saq-NO
up-regulated both iNOS transcription and NO endogenous synthesis
and subsequently sensitized A375 cells to TRAIL (10). In all the settings considered,
NO-release was modest thus qualifying Saq-NO as a soft NO-releaser
drug.
The impact of Saq-NO on the in vitro and
in vivo growth of A375 cells was of particular interest as
these cells constitutively produce large amounts of NO that appears
essential for exertion of its malignant potential (13). This is consistent with the emerging
pathogenic role of endogenous NO in the development and clinical
course of melanoma. In fact, constitutive expression of inducible
form of iNOS correlates with poor survival (14–16).
NO seems to initiate progression of human melanoma via a feedback
loop mediated by apurinic/apyrimidinic endonuclease-1/redox
factor-1 (17).
Therefore, the evidence that a soft NO-releaser such
as Saq-NO strongly influenced the in vitro and in
vivo growth of the two melanoma cell lines B16 and A375
requires to be carefully weighed on other experimental studies
before being further considered for translation to the clinical
setting. This prompted us to carry out this phenomenological study
where we evaluate the impact of single and combined effects of
Saq-NO, Saq, the NO-donor DETA NONOate and the iNOS inhibitor
L-NAME on the in vitro and in vivo growth of A375
cells xenotransplanted into nude mice.
The data confirm clear-cut evidence for a strong and
powerful anti-melanoma action of Saq-NO and add important insights
into its pharmacological profile. Surprisingly but fitting in with
the complex and multifaceted role of endogenous NO in A375 cells,
both DETA NONOate and L-NAME significantly suppressed the in
vivo growth of these cells.
Materials and methods
Reagents and cells
Fetal calf serum (FCS), RPMI-1640,
phosphate-buffered saline (PBS), were obtained from Sigma (Milan,
Italy). Matrigel was obtained from BD Bioscience (San Jose, CA).
Human melanoma A375 cell line was obtained from the American Type
Culture Collection (Rockville, MD, USA). Cells were routinely
maintained in RPMI-1640 medium supplemented with 10% FCS, 2 mM
L-glutamine and antibiotics (culture medium) at 37°C in a
humidified atmosphere with 5% CO2.
Test compounds
Nitro-L-arginine methyl ester (L-NAME) and DETA
NONOate were obtained from Sigma. Saq was obtained from Hoffmann La
Roche. Saq-NO, synthesized as described elsewhere (9), was provided from Onconox Aps
(Copenhagen, Denmark). Both Saq and Saq-NO were dissolved in DMSO
20%. L-NAME and DETA NONOate were dissolved in water for
injection.
In vitro studies and isobologram
analysis
To classify the type of interaction between Saq-NO
or Saq with L-NAME or DetaNONOate isobologram analysis was carried
out as described elsewhere (10).
Isobolograms were drawn from treatments with following
concentration of Saq-NO (4.7, 9.4, 18.8 μM) or the same
concentrations of Saq with different concentrations of L-NAME (5–20
μM) or DETA NONOate (125–500 μM). Combinations reaching 30–50% of
cytotoxicity were expressed as concentration of single agent alone
provoking this toxicity. Analysis was carried out on the basis of
dose-response curves of cell viability treated with mentioned
reagents for 24 h. Cell viability was measured by crystal violet
test as indicated previously (12).
Animals
Six- to eight-week-old male nude mice, weighing
25–28 g were purchased from Harlan-Nossan (San Pietro al Natisone,
Udine, Italy). The mice were kept under standard laboratory
conditions (non-specific pathogen-free) with free access to food
and water. The animal studies were carried out in accordance to
local guidelines and approved by local Institutional Animal Care
and Use Committee.
Induction of melanoma and experimental
treatment
On day 1 of the experiment, 5×106 A375
melanoma cells were subcutaneously injected between the shoulder
blades of each mouse using a 0.6-mm needle. Cells were injected in
a 200-μl suspension consisting of 100 μl of sterile Phosphate
Buffered Saline (PBS) and 100 μl of Matrigel, as previously
described (18). Tumor growth was
evaluated by measurement with calipers (2 perpendicular diameters)
twice a week. Tumor volume was calculated using the formula 0.52 ×
a × b2, where a is the longest and b is the shortest
diameter.
Several experimental groups of mice (n=8 to 11 mice
for each group) were created that were treated by single and
combined treatment with Saq, Saq-NO, DETA NONOate and L-NAME as
described in Figs. 2 and 3. The control group was treated with DMSO
20% that is the vehicle of Saq and Saq-NO. When the tumors were
already palpable, 8 to 10 days after tumor induction, animals were
randomly allocated to the different groups and were treated for 16
consecutive days. Post-randomization analysis revealed no
significant differences in tumor volumes at the beginning of the
treatment among the different groups. The study shown represents
two independent experiments. Since the data were highly
reproducible the results are merged and shown as a single
experiment.
Statistical analysis
Students t-test was used to determine statistical
significance. Values of p<0.05 were considered to be
statistically significant.
Results
Saq-NO down regulated A375 growth in
vitro and in vivo
A375 cells were exposed to both Saq or Saq-NO in
parallel with L-NAME or DETA NONOate. Cell viability was measured
by crystal violet assay and isobologram curve was drawn. Saq-NO
synergized with L-NAME while the effects with DETA NONOate was
antagonistic (Fig. 2). Combined
treatment of Saq with either L-NAME or DETA NONOate resulted in
less toxicity then in monotreatment (Fig. 2).
To confirm the data obtained in vitro, we
performed an in vivo xenograft model with cultured A375
cells. In both in vivo studies with the A375 cells, tumor
size in the vehicle-treated mice was 60–70 mm3 at the
beginning of the treatment. Drugs were administered for a period of
16 consecutive days starting from 8 to 10 days after xenograft.
Thereafter, the treatment was interrupted and the mice were
followed up for additional 11 days to observe the growth of the
tumor in the absence of the treatment. The readouts of the clinical
results are summarized in Table I
indicating the fold increase and Area Under the drug
concentration-time Curve (AUC) of the tumors both at the end of the
treatment period as well as at the end of the follow-up period. The
statistical significance between the groups is summarized in
Table II that indicates the days
of observation during the treatment of significant inhibition of
tumor growth. The kinetics of tumor development is shown in
Figs. 3 and 4.
 | Table ISummary of clinical readouts. |
Table I
Summary of clinical readouts.
| Treatment (16
days) | Fold increase (end
of treatment) | Fold increase (end
of study) | AUC (end of
treatment) | AUC (end of
study) |
|---|
| Saq-NO | 4.4 | 19.0 | 1243 | 13759 |
| Saq-NO + DETA
NONOate | 7.8 | 26.1 | 3358 | 24295 |
| Saq-NO +
L-NAME | 3.5 | 12.3 | 733.3 | 8561 |
| Saq | 6.4 | 21.3 | 2551 | 17311 |
| Saq + DETA
NONOate | 6.5 | 18.2 | 2340 | 16265 |
| Saq + L-NAME | 5.6 | 18.0 | 1974 | 14114 |
| DETA NONOate | 6.6 | 22.2 | 2283 | 18373 |
| L-NAME | 6.1 | 22.7 | 1778 | 16509 |
| Vehicle | 11.8 | 36.4 | 3671 | 26511 |
 | Table IISummary of statistical
significance. |
Table II
Summary of statistical
significance.
| Inhibition of tumor
growth during treatment (days) |
|---|
|
|
|---|
| T-test vs. | Vehicle | Saq-NO | Saq | DETA NONOate | L-NAME |
|---|
| Saq-NO | 6–16 ↑ | - | 6, 14 ↑ | 16 ↑ | 6 ↑ |
| Saq-NO + DETA
NONOate | NS | 6, 14–16 ↓ | - | NS | - |
| Saq-NO +
L-NAME | 6–16 ↑ | 9–11 ↑ | - | - | 6–11↑ |
| Saq | 11, 16 ↑ | 6, 14 ↓ | - | NS | NS |
| Saq + DETA
NONOate | 11, 16 ↑ | - | NS | NS | - |
| Saq + L-NAME | 11, 16 ↑ | - | NS | - | NS |
| DETA NONOate | 11, 16 ↑ | 16 ↓ | NS | - | NS |
| L-NAME | 11–16 ↑ | 6 ↓ | NS | NS | - |
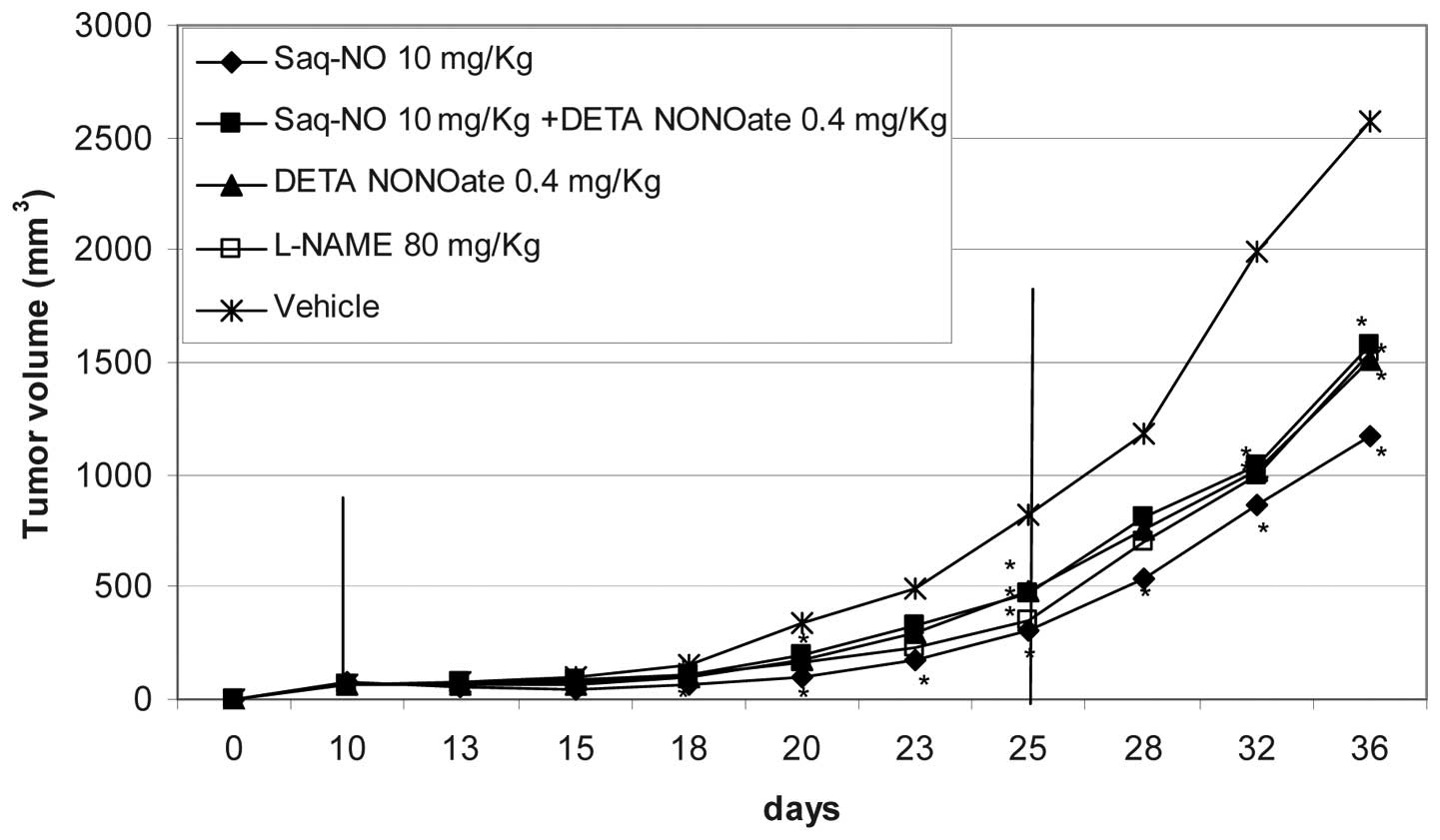
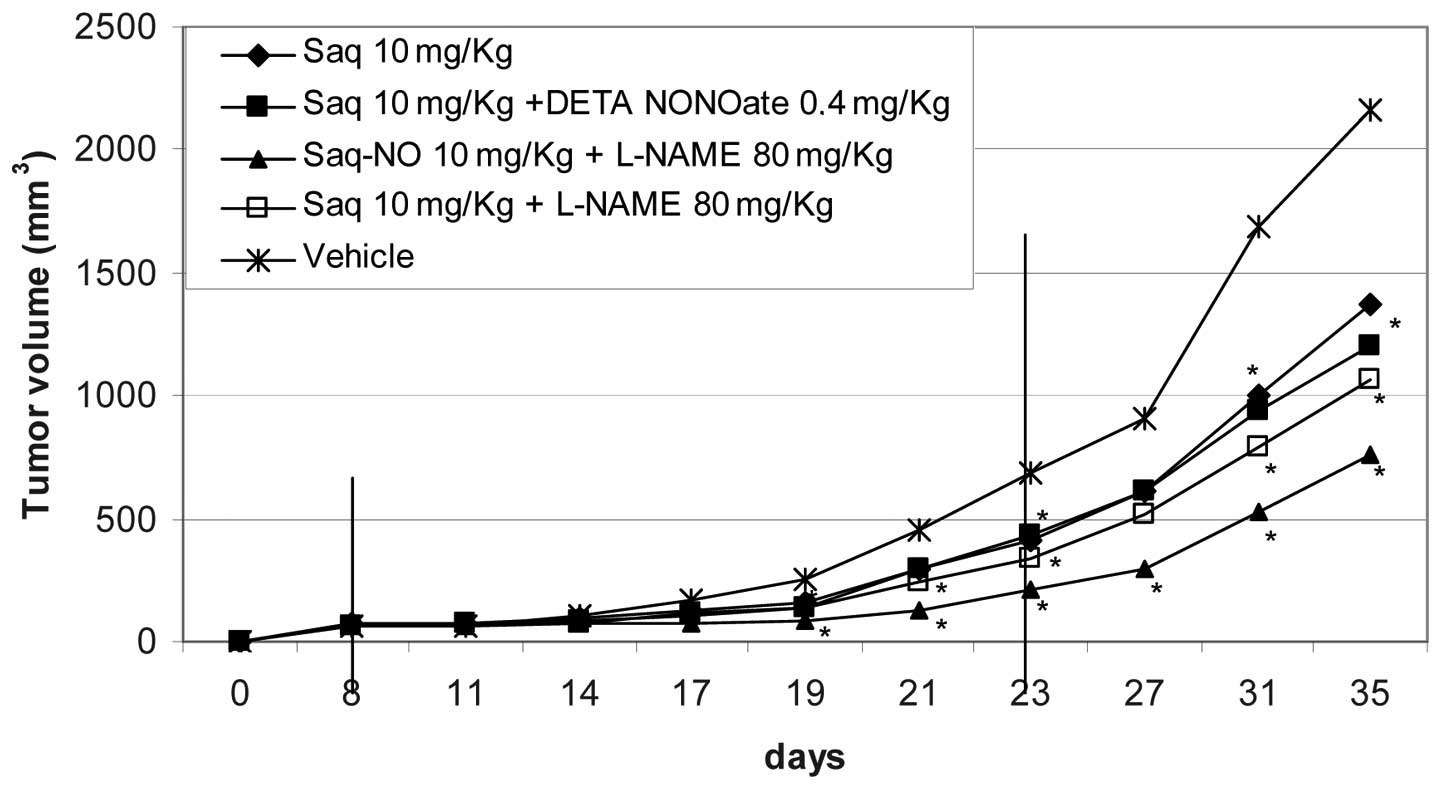
As seen from the observations of Table I and Figs. 3 and 4, Saq-NO appeared the most powerful of the
treatments given either alone or in combination. This treatment was
the most rapid of all those considered to achieve significant
inhibition of tumor volume, with an effect prolonged for the entire
period of treatment. This was also witnessed from the significant
reduction of fold tumor increase (4 vs. 12) and AUC (1243 vs. 3671)
observed at the end of the treatment as compared to vehicle-treated
mice (Table I). A substantial
increase of the tumor volumes was observed upon treatment
withdrawal that led to a 19-fold increase of the tumors in the
Saq-NO treated mice with an AUC of 13759. These values were however
still significantly lower than those observed at the same period of
time in the vehicle-treated mice that exhibited a 36-fold increase
of the tumor volumes with an AUC of 26511 (Table I).
As we reported previously (10), the effects of Saq-NO were markedly
stronger than those of its parental compound Saq. The mice treated
with Saq exhibited a significant reduction of their tumor volumes
on day 11 and 16 of the treatment. Although, relative to
vehicle-treated mice, Saq favourably influenced the in vivo
growth of A375 cells, its effects appeared of a significant lower
magnitude than those of Saq-NO (Table
II). Accordingly, both during the treatment period and at the
end of the follow-up, the fold increase of the tumor volumes and
the AUC were considerably higher than those observed in Saq-NO
treated mice (see Table I and
Figs. 3 and 4).
An additional group of mice was considered where the
NO donor DETA NONOate was given alone or in combination to Saq or
Saq-NO to mice xenotransplanted with A375 cells. On the one hand,
these studies aimed at understanding whether the powerful
anti-tumor effects of Saq-NO on the growth of A375 cells could have
also been achieved by NO donation monotherapy, and on the other at
evaluating whether the stronger in vivo anti-melanoma action
of Saq-NO than Saq could be reproduced by the combined treatment of
the latter with this prototypical NO donor. The data show that DETA
NONOate monotherapy favourably influenced the growth of A375 cells
at different time points during the study (days 11 and 16). The
effects were however of considerable lower magnitude than those of
Saq-NO and of comparable extent to those observed in mice treated
with Saq (Table I and Fig. 3). The combined treatment with Saq
and DETA NONOate failed to improve the chemotherapeutic efficacy of
the drugs given alone (Table I and
Fig. 4). Interestingly, and in
agreement with the in vitro data, combined treatment with
DETA NONOate reduced the anti-melanoma effects of Saq-NO (Table I and Fig. 3).
Finally, to evaluate the effects of NO inhibition in
this experimental setting, we included groups of nude mice
xenotransplanted with A375 cells treated with iNOS inhibitor L-NAME
either alone or in combination with Saq or Saq-NO. In apparent
contradiction with the beneficial effects of DETA NONOate, relative
to treatment with vehicle, L-NAME monotherapy also significantly
reduced at different time points during the study period the
clinical readouts considered (Tables
I and II; and Fig. 3). The effects were almost
superimposable to those achieved with DETA NONOate treatment
(Tables I and II; and Fig.
3). Interestingly, and extending to the in vivo setting
the synergistic effect observed between Saq-NO and L-NAME in
vitro, the combined treatment of L-NAME with Saq-NO (Tables I and II; and Fig.
4) appears to exert an additive effect so that AUC of the
tumors of mice treated with Saq-NO + L-NAME was ~70% lower than
those observed with either of the two drugs in monotherapy
(Table I and Fig. 4). However final reduction of tumor
growth in combined treatment with Saq and L-NAME was not
significantly potentiated in comparison to that found in
monotherapy (Fig. 4).
In summary, in vitro evaluation of
co-treatment of experimental drugs with NO donating compound or
iNOS inhibitor showed a strong correlation and high reproducibility
with results obtained in vivo.
Discussion
This study further characterizes the powerful
antimelanoma action of Saq-NO, revealing important pharmacological
insights of this compound. The results confirm that Saq-NO exerted
a much stronger protective effect on the in vitro and in
vivo cell growth of A375 cells than the parental compound Saq.
Saq-NO also inhibited the in vivo growth of A375 cells to a
much greater extent than the strong NO-releaser DETA NONOate and
its effects were not superimposable to those achieved by the
combined treatment of Saq and DETA NONOate. These data demonstrate
that Saq-NO represents a NCE with a unique chemotherapeutic profile
that can not be reproduced by the combined administration of two
related but different molecules, sharing only some features of
Saq-NO. The chemicophysical changes conferred by the NO-releasing
moiety to the Saq scaffold have clearly induced fundamental changes
of the pharmacological actions of the parental Saq molecule such as
Akt inhibition. Vice versa, the presence of Saq has plausibly
changed the chemico-physical properties of the NO-releasing moiety
that might have led to a substantial change in the kinetic profile
and/or compartment of NO release resulting in a different
pharmacological action.
Both in the in vitro and in vivo
studies we have shown that the growth of A375 cells in mice was
inhibited by the treatment with either the NO donor DETA NONOate or
the NOS inhibitor L-NAME. The effects of the two compounds were of
similar magnitude but lower than that achieved with either Saq or
Saq-NO.
By using isobologram analysis we have also shown
that Saq-NO exerted a synergistic action with the iNOS inhibitor
L-NAME and an antagonistic action with the NO donor DETA NONOate on
the in vitro growth of A375 cells. These effects were
confirmed in nude mice xenotransplanted with A375 cells. On the
other hand, Saq did not exert additive or synergistic effects with
either L-NAME or DETA NONOate neither in vitro and parallel
treatment with Saq and L-NAME just slightly potentiated the
reduction of tumor growth in comparison to that observed in
monotherapeutic regime and rate of inhibition was less than
additive.
The equal efficacy with which the NO donor DETA
NONOate and L-NAME prevent the growth of A375 cells in xenografted
mice with A375 clearly witnesses how the pleiotropism of NO in the
pathogenesis of melanoma may translate into apparently paradoxical
therapeutic effects when NO agonists and NO antagonists are used.
This latter observation may mirror the known dichotomic role of NO
in the regulation of oncogenesis that may result in pro- or
anti-tumor effects depending on concentration, the microenvironment
and local or peripheral localization within the tumor. This bimodal
action of NO has led this molecule to be considered both as a
target for cancer therapy and an anti-cancer agent (19–21).
The different contribution of endogenously produced and
exogenously-administered NO on the dysregulated growth of A375
cells, and possibly other cancer cells, should also be considered.
For example, a study from Chin and Deen (22) anticipates that because of the high
rates of cellular consumption, the elevation in NO concentration
may be localized, ~90% of the concentration decay occurring within
30 μm of the tumor edge. High concentrations of endogenous NO at
the periphery of a melanoma may contribute to metastasis by
stimulating cell proliferation, inhibiting apoptosis, or acting as
a lymphangiogenic factor.
In contrast, exogenously-administered NO may also
act at more distant sites of the tumor microenvironment and this
may results in the activation of anti-oncogenic pathways inhibiting
tumor formation. Antitumor action of NO is also manifested through
sensitization to apoptosis triggered by mediators of natural immune
response. This is very important especially in the light of the
fact that most of diagnosed malignancies are already resistant to
death signals delivered from surrounding tissues and immune cells.
In addition, the different NO concentrations ensuing from low
physiological amounts of endogenously produced NO or larger amounts
of NO released from NO donors may generate different concentrations
of other closely related reactive nitrogen species (RNS) such as
ONOO−. Different NO concentrations, and/or different
RNS, on different cell/tissue compartments can reach totally
different molecular targets triggering completely different, if not
opposite, pharmacological reactions. This may, for example, explain
the dichotomic effects DETA NONOate in the in vitro vs. the
in vivo setting. Whilst DETA NONOate treatment was presently
effective in reducing A375 growth in vivo, Yang et al
have demonstrated that it may promote melanoma progression in
vitro by induction of Apurinic/Apyrimidinic Endonuclease
endonuclease-1/redox factor-1 (APE/Ref-1) and related downstream
targets such as activator protein-1/JunD, matrix
metalloproteinase-1, Bcl-2, and iNOS (17). Similar paradoxes have been also
observed in other cancer diseases. For example, despite strong
correlation between eNOS expression/activity and poor prostate
cancer prognosis (23), the
moderate multi-functional NO-donor such as Saq-NO effectively
inhibited the in vitro and in vivo growth of human
hormone refractory prostate cancer (12). In the same manner, whilst increased
NOS2 predicts poor survival in estrogen receptor-negative breast
cancer patients (24) and has been
thought to play a major pathogenetic role in disease development
(25), McMurtry et
al(26) have demonstrated that
JS-K, a NO-releasing prodrug, induces breast cancer cell death
while sparing normal mammary epithelial cells.
The presently observed inhibitory activity of L-NAME
in the growth of melanoma is consistent with recent data from
Sikora et al(27) who found
that oral treatment with the specific iNOS-selective small molecule
antagonist N(6)-(1-iminoethyl)-l-lysine-dihydrochloride
(L-nil) significantly inhibited the growth of two human melanoma
cell lines mel624 and mel528 xenotransplanted into severe combined
immunodeficient (SCID) mice and extended the survival of
tumor-bearing animals. In their experiments, iNOS inhibition was
associated with tumor growth suppression, decrease in tumor
microvessels, down-regulation of the anti-apoptotic gene Bcl-2,
increased number of intratumoral apoptotic cells and enhanced
efficacy when L-nil treatment was combined with cisplatin in
vivo. This synergism closely resembles that observed in our
study between L-NAME and both Saq and Saq-NO. Although dismantling
the molecular and pharmacological mechanisms underlying this
synergism was outside the phenomenological scope of our work and
has not been studied, it is possible that in a similar manner to
that described by Sikora et al(27) for N(6)-(1-iminoethyl)-l-lysine-dihydrochloride
(L-nil) and cisplatin it may be due to combined and higher
effective influence on key oncogenic pathways of A375 cells. Taken
together these data support the hypothesis that targeting NOS may
represent a valuable novel target of melanoma therapies that may be
combined with other chemotherapeutic agents including soft NO donor
such as Saq-NO.
Acknowledgements
This work was partly supported by the Ministry of
Education and Science of the Republic of Serbia, grant no.
173013.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
3
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar
|
|
4
|
Bedikian AY, Johnson MM, Warneke CL, et
al: Does complete response to systemic therapy in patients with
stage IV melanoma translate into long-term survival? Melanoma Res.
21:84–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrella T, Quirt I, Verma S, Haynes AE,
Charette M and Bak K; Members of the Melanoma Disease Site Group of
Cancer Care Ontario’s Program in Evidence-Based Care. Single-agent
interleukin-2 in the treatment of metastatic melanoma. Curr Oncol.
14:21–26. 2007. View Article : Google Scholar
|
|
6
|
Garbe C, Eigentler TK, Keilholz U,
Hauschild A and Kirkwood JM: Systematic review of medical treatment
in melanoma: current status and future prospects. Oncologist.
16:5–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andersen MH, Junker N, Ellebaek E, Svane
IM and Thor Straten P: Therapeutic cancer vaccines in combination
with conventional therapy. J Biomed Biotechnol. 2010:2376232010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hershkovitz L, Schachter J, Treves AJ and
Besser MJ: Focus on adoptive T cell transfer trials in melanoma.
Clin Dev Immunol. 2010:2602672010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maksimovic-Ivanic D, Mijatovic S,
Miljkovic D, et al: The antitumor properties of a nontoxic, nitric
oxide-modified version of saquinavir are independent of Akt. Mol
Cancer Ther. 8:1169–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mijatovic S, Maksimovic-Ivanic D, Mojic M,
et al: Cytotoxic and immune-sensitizing properties of nitric
oxide-modified saquinavir in iNOS-positive human melanoma cells. J
Cell Physiol. 226:1803–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rothweiler F, Michaelis M, Brauer P, et
al: Anticancer effects of the nitric oxide-modified saquinavir
derivative saquinavir-NO against multidrug-resistant cancer cells.
Neoplasia. 12:1023–1030. 2010.
|
|
12
|
Donia M, Maksimovic-Ivanic D, Mijatovic S,
Mojic M, Miljkovic D, Timotijevic G, Fagone P, Caponnetto S,
Al-Abed Y, McCubrey J, Stosic-Grujicic S and Nicoletti F: In vitro
and in vivo anticancer action of Saquinavir-NO, a novel nitric
oxide-derivative of the protease inhibitor saquinavir, on hormone
resistant prostate cancer cells. Cell Cycle. 10:492–499. 2011.
View Article : Google Scholar
|
|
13
|
Tang CH and Grimm EA: Depletion of
endogenous nitric oxide enhances cisplatin-induced apoptosis in a
p53-dependent manner in melanoma cell lines. J Biol Chem.
279:288–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ekmekcioglu S, Ellerhorst J, Smid CM, et
al: Inducible nitric oxide synthase and nitrotyrosine in human
metastatic melanoma tumors correlate with poor survival. Clin
Cancer Res. 6:4768–4775. 2000.PubMed/NCBI
|
|
15
|
Ekmekcioglu S, Ellerhorst JA and Prieto
VG: Tumor iNOS predicts poor survival for stage III melanoma
patients. Int J Cancer. 119:861–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johansson CC, Egyházi S, Masucci G, et al:
Prognostic significance of tumor iNOS and COX-2 in stage III
malignant cutaneous melanoma. Cancer Immunol Immunother.
58:1085–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Yang S, Misner BJ, Chiu R, Liu F
and Meyskens FL Jr: Nitric oxide initiates progression of human
melanoma via a feedback loop mediated by apurinic/apyrimidinic
endonuclease-1/redox factor-1, which is inhibited by resveratrol.
Mol Cancer Ther. 7:3751–3760. 2008. View Article : Google Scholar
|
|
18
|
White N, Knight GE, Butler PE and
Burnstock G: An in vivo model of melanoma: treatment with ATP.
Purinergic Signal. 5:327–333. 2009.PubMed/NCBI
|
|
19
|
Huerta S, Chilka S and Bonavida B: Nitric
oxide donors: novel cancer therapeutics (Review). Int J Oncol.
33:909–927. 2008.PubMed/NCBI
|
|
20
|
Hickok JR and Thomas DD: Nitric oxide and
cancer therapy: the emperor has NO clothes. Curr Pharm Des.
16:381–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirst D and Robson T: Nitric oxide in
cancer therapeutics: interaction with cytotoxic chemotherapy. Curr
Pharm Des. 16:411–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chin MP and Deen WM: Prediction of nitric
oxide concentrations in melanomas. Nitric Oxide. 23:319–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nanni S, Benvenuti V, Grasselli A, et al:
Endothelial NOS, estrogen receptor beta, and HIFs cooperate in the
activation of a prognostic transcriptional pattern in aggressive
human prostate cancer. J Clin Invest. 119:1093–1108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glynn SA, Boersma BJ, Dorsey TH, et al:
Increased NOS2 predicts poor survival in estrogen receptor-negative
breast cancer patients. J Clin Invest. 120:3843–3854. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ambs S and Glynn SA: Candidate pathways
linking inducible nitric oxide synthase to a basal-like
transcription pattern and tumor progression in human breast cancer.
Cell Cycle. 10:619–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McMurtry V, Saavedra JE, Nieves-Alicea R,
Simeone AM, Keefer LK and Tari AM: JS-K, a nitric oxide-releasing
prodrug, induces breast cancer cell death while sparing normal
mammary epithelial cells. Int J Oncol. 38:963–971. 2011.
|
|
27
|
Sikora AG, Gelbard A, Davies MA, et al:
Targeted inhibition of inducible nitric oxide synthase inhibits
growth of human melanoma in vivo and synergizes with chemotherapy.
Clin Cancer Res. 16:1834–1844. 2010. View Article : Google Scholar : PubMed/NCBI
|