Introduction
Ovarian cancer is one of the most common and lethal
gynecologic neoplasm, and presently ranks fifth in causing female
cancer-related mortality (1). The
overall 5-year relative survival rate is no more than 50% (2). Due to its few and imperceptible early
symptoms, ovarian cancer is mostly at advanced stage during the
initial diagnosis. Although patients may initially respond to the
combination treatment of surgical and chemical therapies, and
various new chemotherapeutics have been developed for the treatment
of ovarian cancer, most of the patients will inevitably die from
relapse and the development of chemotherapy-resistant recurrence
(3). Drug resistance and severe
side effects remain unavoidable obstacles for chemotherapeutic
approaches. Development of new approaches to overcome the
therapeutic dilemma is of great urgency.
Interferon γ-inducible protein of 10 kDa
(IP-10/CXCL10), a member of the non-ELR-CXC chemokine superfamily,
is known to a highly inducible chemoattractant binding to the CXCR3
chemokine receptor (4,5). According to present studies, IP-10 has
been demonstrated to be a pleiotropic molecule eliciting potent
biological effects, including stimulation of monocytes, natural
killer (NK) cells and T-cell, regulation of T-cell and bone marrow
progenitor maturation, modulation of adhesion molecule expression
as well as inhibition of angiogenesis (6). It has also been reported that IP-10
may inhibit human non-small cell lung cancer (NSCLC) tumorigenesis
and spontaneous metastases (7).
Currently, much interest is focused on the anti-neoplastic effects
of IP-10 (8). IP-10 has been found
to possess anti-tumor and anti-metastatic properties by
immunological, antiangiogenic and anti-neoplastic mechanisms
(9,10). In the present study, we constructed
this recombinant plasmid expressing IP-10 (pVITRO-IP-10) to test
its antitumor effect on ovarian cancer.
In addition, the delivery system is a crucial factor
affecting the effectiveness of therapy for ovarian cancer.
Polyethyleneimine (PEI) has emerged as one of the most effective
nonviral gene carrier agent. Currently, commercial PEI25K (25
kg/mol) is widely considered to be the gold standard for assessing
the transfection efficiency of new polymer-based gene carriers
(11,12). However, PEI has the shortcoming of
inducing marked aggregation of erythrocytes and hemolysis (13,14).
Moreover, PEI whose transfection efficiency comes with relatively
high cytotoxicity is not biodegradable. In our previous study,
PEI2K was chemically conjugated with heparin to form a novel
biodegradable cationic nanogel, named HPEI. The novel biodegradable
cationic HPEI nanogels were then used to effectively deliver a
plasmid expressing vesicular stomatitis virus matrix protein
(pVSVMP) into C-26 cells in vitro and in vivo, where
the complex efficiently suppressed the growth of abdominal and
pulmonary metastases in C-26 colon carcinomas in BALB/c mice
(15). HPEI nanogels have also been
used to transfect the novel FILIP1LDC103-p gene into SKOV3 cells
efficiently (16). In the present
study, biodegradable cationic HPEI nanogels were used as gene
carriers to deliver a recombinant plasmid encoding IP-10 to treat a
SKOV3 intraperitoneal ovarian carcinomatosis in nude mice,
potential enhanced effect of IP-10 on ovarian cancer was
expected.
Materials and methods
Cell culture
Human epithelial serous cystadenocarcinoma SKOV3
cells were obtained from American Type Culture Collection
(Rockville, MD, USA) and cultured in DMEM (Gibco, Carlsbad, CA)
culture medium supplemented with 10% FCS, 2 mM L-glutamine, 100
U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated
in a humidified atmosphere containing 5% CO2 at 37°C and
passaged every 4 days at a split ratio of 1:3.
HUVECs were isolated from human umbilical vein
vascular wall as described previously (17). Briefly, the human umbilical vein
vascular wall was digested with collagenase IV at 37°C for 10 min,
and the homogenate was centrifuged at 750 g for 10 min. Cells were
suspended and seeded on fibronectin-coated plates and cultured in
Earle's salts medium supplemented with 10% fetal calf serum
(FCS).
Preparation of plasmids
A dual promoter plasmid, pVITRO2-neo-mcs
(Invitrogen, San Diego, CA, USA) was utilized to express IP-10
genes, which is a common expression vector in gene therapy
(18). The recombinant plasmid was
named pVITRO-IP-10. The pVITRO2-neo-mcs plasmid cDNA without IP-10
was used as the empty-vector control (hereafter called E-p). The
plasmids were purified using Qiagen Endo-free Giga kit (Qiagen,
Hilden, Germany) following the manufacturer's instructions
(19).
HPEI preparation and plasmid
transfection
Biodegradable HPEI nanogels were synthesized as
previously described (15). In
brief, 0.05 g of heparin was first dissolved in 2-(N-morpholino)
ethanesulfonic acid (MES) buffering agent (100 ml, 50 mM); 0.02 g
of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and 0.03 g
of N-hydroxysuccinimide (NHS) were subsequently added to the
solution to activate heparin's carboxylic acid groups. After 20 ml
of PEI2K solution (7.5 mg/ml) was dropped into the solution, the
reaction was carried out at room temperature overnight. The
resulting HPEI nanogels were then dialyzed in double-distilled
water for 3 days. Later, HPEI nanogels were filtered using a
syringe filter, then adjusted to a final concentration of 1.0 mg/ml
and stored at 4°C.
SKOV3 ovarian cancer cells (2.0×105) were
grown on 6-well plates in DMEM medium and cultured for 24 h. Twenty
micrograms of HPEI and 2 μg of pVITRO-IP-10 was diluted in 1 ml of
DMEM medium without antibiotic or serum, and then combined at a
ratio of 10:1. The combinations were transfected to cells in
triplicate and incubated overnight to 80% confluence. Meanwhile,
the medium alone and HPEI nanogels alone were used as control
agents. After 4–6 h, the medium was replaced by 2 ml of DMEM medium
with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml
streptomycin. After 48 h of incubation, the cells and supernatants
were collected for further assay.
Detection of transfection efficiency
In order to evaluate the transfection efficiency of
HPEI in vitro, 2 μg of green fluorescent protein (GFP)
plasmid was encapsulated with HPEI to transfect SKOV3 cells. The
GFP expression profile was detected as described in our previous
studies (20). In brief, SKOV3
cells (1×105 cells/well) were seeded on 6-well plates.
Before transfection, the DMEM medium in each well was replaced with
0.8 ml of fresh serum-free medium, and then the HPEI:GFP complex
was added and left to mix in serum-free medium at 37°C overnight.
Then the complete DMEM medium was added, and the plate was
maintained at 37°C for 48 h, after which we observed green
fluorescence expression with a fluorescence inverted microscope
(Nikon Eclipse 80i, Japan). The transfection efficiency was
determined based on the percentage of cells expressing GFP. The
number of GFP-expressing cells divided by the total cell quantity
under the microscope was defined as the transfection efficiency.
Cell counting was performed randomly in the microscopic observation
scope under magnifications ×10 with 3 repeats. All data are
presented as the mean ± standard deviation (SD).
RT-PCR
The IP-10 primers were designed based on their cDNA
sequences, upstream IP-10 primer: 5′-ATGAATCAAACTGCC ATTCTG-3′; and
downstream IP-10 primer: 5′-TTAAGGAGA TCTTTTAGACCTTTCC-3′.
Total RNA was purified using the TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), according to the manufacturer's
instructions. RT-PCR was performed as follows: incubation at 50°C
for 40 min and denaturation at 94°C for 2 min, followed by a
standard PCR regime of 94°C for 30 sec 53°C (IP-10) for 30 sec, and
72°C for 40 sec (30 cycles). Each RT-PCR product (5 μl) was
analyzed by agarose gel (1.5%) electrophoresis. The housekeeping
gene GAPDH served as the internal control. The density of PCR bands
was quantified by Quantity One software.
Western blot analysis
Western blot analysis was employed to determine the
expression level of IP-10 in vitro and in vivo. Cells
or tumor tissue were lysed in RIPA lysis buffer containing 50 mM
Tris-HCl (pH 7.4), 0.25% sodium deoxycholate, 150 mM NaCl, 0.1%
SDS, 1% NP-40, 1 mM EDTA, 1 mM NaF, and 1 mM
Na3VO4, supplemented with proteinase
inhibitor (1 mM cocktail plus 1 mM PMSF, Sigma, St. Louis, MO). The
lysed product was then centrifuged at 15,000 rpm, 4°C for 30 min.
Protein concentrations were measured with Bio-Rad protein assay
kits (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein
(40 μg) were loaded onto a 12% SDS-PAGE gel for electrophoresis and
transferred onto polyvinylidene difluoride (PVDF) membrane
(Millipore, Bedford, MA). After being blocked in 5% skim milk for 1
h, the membranes were incubated with anti-IP-10 antibody (1:100
dilution, Abcam 9807) at 4°C overnight. The membranes were then
incubated with goat anti-rabbit secondary antibody at 1:5,000
dilution (Abcam, Cambridge, MA) in PBS with Tween-20 for 1 h.
Targeted protein bands were detected by the enhanced
chemiluminescence detection system (Pierce Biotech Inc., Rockford,
IL) according to the manufacturer's instructions. GAPDH served as
the protein loading control. Bands were analyzed using Quantity One
4.52 software (Bio-Rad).
Assessment of proliferation in vitro
The effect of pVITRO-IP-10 on cell proliferation was
measured by MTT assay (21). SKOV3
transfection procedure was as mentioned above, then 10 ml of 5
mg/ml MTT (pH 4.7) was added to each well, and the cells were
incubated for another 4 h. The supernatant was then removed; 100 ml
DMSO/well was added followed by shaking for 15 min. Absorbance at
490 nm was measured with a micro-plate reader. Independent
experiments were performed in triplicate. Inhibition of cell
proliferation (%) was assessed using the formula: cell inhibition
(%) = (1-A490 of treated cells/A490 of control cells) × 100%.
Assessment of apoptosis in vitro
Cell apoptosis was analyzed by Hoechst 33258
staining. Briefly, 48 h after transfection, cells were immersed in
0.5 ml of methanol for 15 min, followed by two PBS rinses. Then the
cells were stained with 1 μg/ml Hoechst 33258 compounds in a dark
chamber at room temperature for 10 min and again rinsed two times
with PBS, then stained with 1× PI 500 μl for 10 min and rinsed two
times with PBS again. Cells were analyzed by fluorescence
microscopy using excitation and emission wavelengths of 350 and 460
nm, respectively (22).
Quantitative evaluation of cellular apoptosis was
performed by flow cytometric analysis. Briefly, after processing in
6-well plates as described above, the floated cells were discarded
while the attached cells were trypsinized and thereafter washed
twice with cold PBS. Cells were resuspended in prediluted binding
buffer and stained with propidium iodide (10 μg/ml, BD Pharmingen,
San Jose, CA, USA) for 10 min in the dark at room temperature, and
the mixtures were immediately analyzed by flow cytometry (23).
Assessment of antiangiogenesis in
vitro
Antiangiogenesis effect of pVITRO-IP-10 was
evaluated by tube formation of human umbilical vein endothelial
cells (HUVEC) (24,25). HUVEC were cultured as described
above. Tube formation assay is reported as an effectively model for
assessment of antiangiogenesis in vitro. Briefly, Matrigel
was incubate on ice at 4°C overnight. Pre-cooled matrigel (150 μl)
was transfered to a 48-well plate on ice and allowed to solidify at
37°C for 1 h. HUVEC were counted and diluted to 4×105
cells/ml in cell culture media. The molecules were diluted to
required concentrations in cell culture media. The diluted HUVEC
were transfer to wells of a 96-well plate. Respective diluted
molecules were added to protein solution by pipetting them up and
down three times, then ensuring the final concentration as 1× mix.
The mixture (200 μl) was transfered to a 48-well plate and
incubated at 37°C, 5% CO2 for 3 to 30 h. Endothelial
tube size and numbers were recorded under a microscope and
photograph. The increase or decrease in the formation of tubes in
the test wells as compared to control wells indicated whether the
molecule was angiogenic or anti-angiogenic.
Tumor xenograft model and animal
treatment
All animal research procedures were approved by the
Institutional Animal Care and Use Committee of Sichuan University
(Chengdu, China). Female athymic BALB/c nude mice, 6–8 weeks old,
were used to establish an intraperitoneal carcinomatosis model
using a previously-established method with some modifications
(26). In brief, 5×106
SKOV3 cells in 100 μl DMEM medium (free of serum and antibiotics)
were injected subcutaneously into the dorsal sides of 6 mice. When
the diameter of the resulting subcutaneous tumors reached ~10 mm,
the tumor nodes were collected and minced into tiny particles
(<1 mm3) by sterile stainless screen cloth. The tumor
particles were then resuspended by serum-free medium to a final
volume of 15 ml, and 30 mice were inoculated intraperitoneally with
0.5 ml of the mixture. Mice were assigned randomly to one of the
following groups (five per group): a) untreated, 100 ml of 5%
glucose solution; b) HPEI, 50 ml of HPEI in 50 ml of 5% glucose
solution; c) HPEI±E-p, 5 μg of pVITRO2-neo-mcs of HPEI in 50 ml of
5% glucose solution; and (d) HPEI+pVITRO-IP-10, 5 μg of
pVITRO-IP-10 and 50 ml of HPEI in 50 ml of 5% glucose solution;
intraperitoneal administration was initiated 5 days after
inoculation. The HPEI/DNA complexes were prepared at room
temperature before administration. The mice received therapy every
3 days and were sacrificed after 12 treatments. Intraperitoneal
tumors were resected and weighed immediately to assess antitumoral
efficacy.
Histological analysis
Each dissected tumor node group was divided into two
parts. One was fixed in 10% formalin for 48 h and then embedded in
paraffin. The other was stored at −80°C for frozen section and
protein extraction. Sections (3–5 μm) were made of the
paraffin-embedded tumors of each group. Then the paraffin sections
were stained with hematoxylin and eosin (HE) and examined by an
experienced pathologist using light microscopy to confirm
histology. Immunohistochemistry staining of IP-10, and Ki67 was
performed as described previously (27). Briefly, deparaffinized tumor
sections were immersed in 10 mM citrate buffer (pH 6.0) and then
heated in an autoclave for 5 min in saturated steam for antigen
retrieval. Endogenous peroxidase activity was quenched in 3%
H2O2 for 10 min, and the non-specific binding
sites of reagents were subsequently blocked with homeotypic
nonimmunoglobulin of the secondary antibody at 37°C for 20 min.
Then tumor sections were incubated with the primary antibody
(R&D Systems, Minneapolis, MN) at 4°C overnight. Tumor sections
were incubated with the biotinylated secondary antibody at 37°C for
40 min, followed by sequential incubation with a
streptavidin-biotin-horseradish peroxidase complex for 40 min at
37°C. Colorimetric detection was performed with
diaminobenzidine.
TUNEL assay for apoptotic cells
TUNEL staining was performed to analyze apoptotic
cells in tumor tissues using apoptotic cell kits according to the
manufacturer's protocol (Promega, Madison, WI) (16). The apoptosis index was calculated as
the average percentage of green fluorescence-positive cells in 10
random fields from different sections at magnification ×400.
Alginate-encapsulated tumor cell
assay
To explore inhibition of angiogenesis, we performed
an alginate-encapsulation assay. Briefly, SKOV3 cells were
resuspended in a 1.5% solution of alginate (Sigma) and added
dropwise into a solution of 250 mM CaCl2; an alginate
bead was formed containing 1×105 cells. Two beads were
then implanted subcutaneously in the backs of every nude mouse.
Eighteen mice were then grouped and treated as described earlier.
Treatment was initiated on the same day the beads were implanted.
After two weeks, mice were injected intravenously with 0.2 ml of a
50 mg/kg fluorescein isothiocyanate (FITC)-dextran (Sigma)
solution. Alginate beads were photographed after being exposed
surgically and then rapidly removed 20 min after FITC-dextran
injection. The uptake of FITC-dextran was measured as described by
Hoffmann et al(28).
Microvessel density assay
The frozen tumor masses stored in liquid nitrogen
were sliced into 5-μm sections for quantification of microvessel
density (MVD) with CD31 staining by the immunohistochemical ABC
method, following the protocol of the manufacturer with a few
modifications. Briefly, after fixing frozen sections in cold
acetone for 20 min, rabbit serum was used in the blocking steps.
Subsequently, endogenous peroxidase was blocked with 0.3%
H2O2. Sections were then incubated in
monoclonal CD31 primary antibody of rat anti-mouse PECAM-1 diluted
1:400 overnight at 4°C. After incubating and washing in PBS,
sections were incubated in biotinylated rabbit anti-rat IgG
(secondary antibody, 1:100 dilution) at 37°C for 40 min. The
sections were then treated with streptavidin-biotin reagents,
resulting in the brown staining of microvessel endothelial cells.
Then MVD was determined by examining vascular hot spots as
described previously (29). Three
vascular tumor areas without necrosis were selected at high
magnification (x200), and the three values were averaged for each
sample.
Evaluation of possible adverse
effects
For mice treated with pVITRO-IP-10 DNA complex,
gross measures such as weight loss, diarrhea, toxic death, life
span, behavior, and feeding were investigated in particular for
potential signs of toxicity. Heart, liver, spleen, lung, and kidney
tissues were fixed in 10% buffered formalin solution and embedded
in paraffin. Sections of 3–5 μm were stained with hematoxylin and
eosin (H&E) and observed by two different experienced
pathologists in a blinded manner.
Statistical analysis
All experimental data are expressed as mean ± SD.
One-way ANOVA, followed by Tukey's test for multiple comparisons,
was performed with SPSS software (version 15.0 for Windows).
Dunnett's t-test (2-sided) was employed as needed. Statistical
significance was determined by the log-rank test. Significance was
defined as p<0.05.
Results
Transfection efficiency and expression of
IP-10 in vitro and in vivo
The transfection efficiency of HPEI in vitro
was tested by green fluorescent protein (GFP). The transfection
efficiency, based on the percentage of cells expressing GFP, is
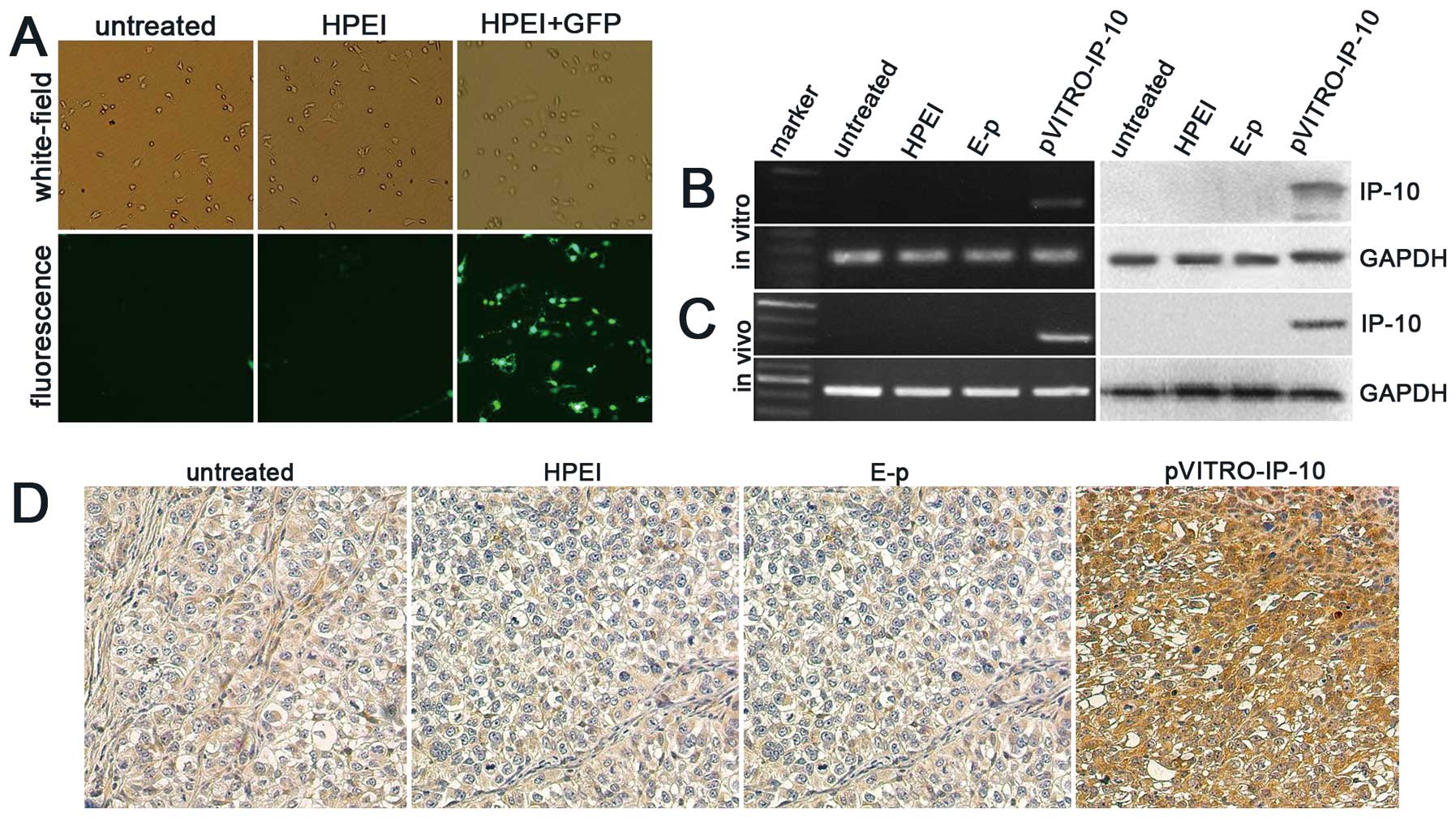
~48.8%±5.15, shown in Fig. 1A.
The in vitro expression of the IP-10 gene in
transfected SKOV3 cells was confirmed using RT-PCR and western blot
analysis. As shown in Fig. 1B, the
expression of IP-10 in SKOV3 cells transfected with
HPEI+pVITRO-IP-10 could be detected, whereas there was no
expression of IP-10 in SKOV3 cells from the untreated, HPEI, or
HPEI+E-p groups
To examine the expression of IP-10 in vivo,
an intraperitoneal ovarian carcinoma model was established in nude
mice, which were then treated with HPEI+pVITRO-IP-10, HPEI+E-p,
HPEI, or 5% glucose. Tumors were collected for RT-PCR and western
blotting on the third day after the final treatment. The level of
IP-10 mRNA and expression of IP-10 protein were detected in tumor
tissues from the HPEI+pVITRO-IP-10 group, whereas there was no
expression in the HPEI, E-p, and 5% glucose groups (Fig. 1C). In addition, immunochemical
staining of IP-10 in tumor tissues from the HPEI+pVITRO-IP-10 group
also suggested positive expression, as shown in Fig. 1D.
Inhibition of proliferation and increased
apoptosis induced by pVITRO-IP-10 in vitro
The effect of pVITRO-IP-10 on cell proliferation was
measured by MTT assay. The inhibition rate of SKOV3 cell
proliferation 72 h after transfection with HPEI reached 45.3% in
pVITRO-IP-10 treated samples, while it is 21.7% in E-p treated
samples, and 9.6% in samples treated only with HPEI, in comparison
with untreated samples. Cell viability was significantly lower in
the groups treated with pVITRO-IP-10 than in the other three
control groups (untreated, HPEI alone, and E-p), as shown in
Fig. 2A (p<0.05).
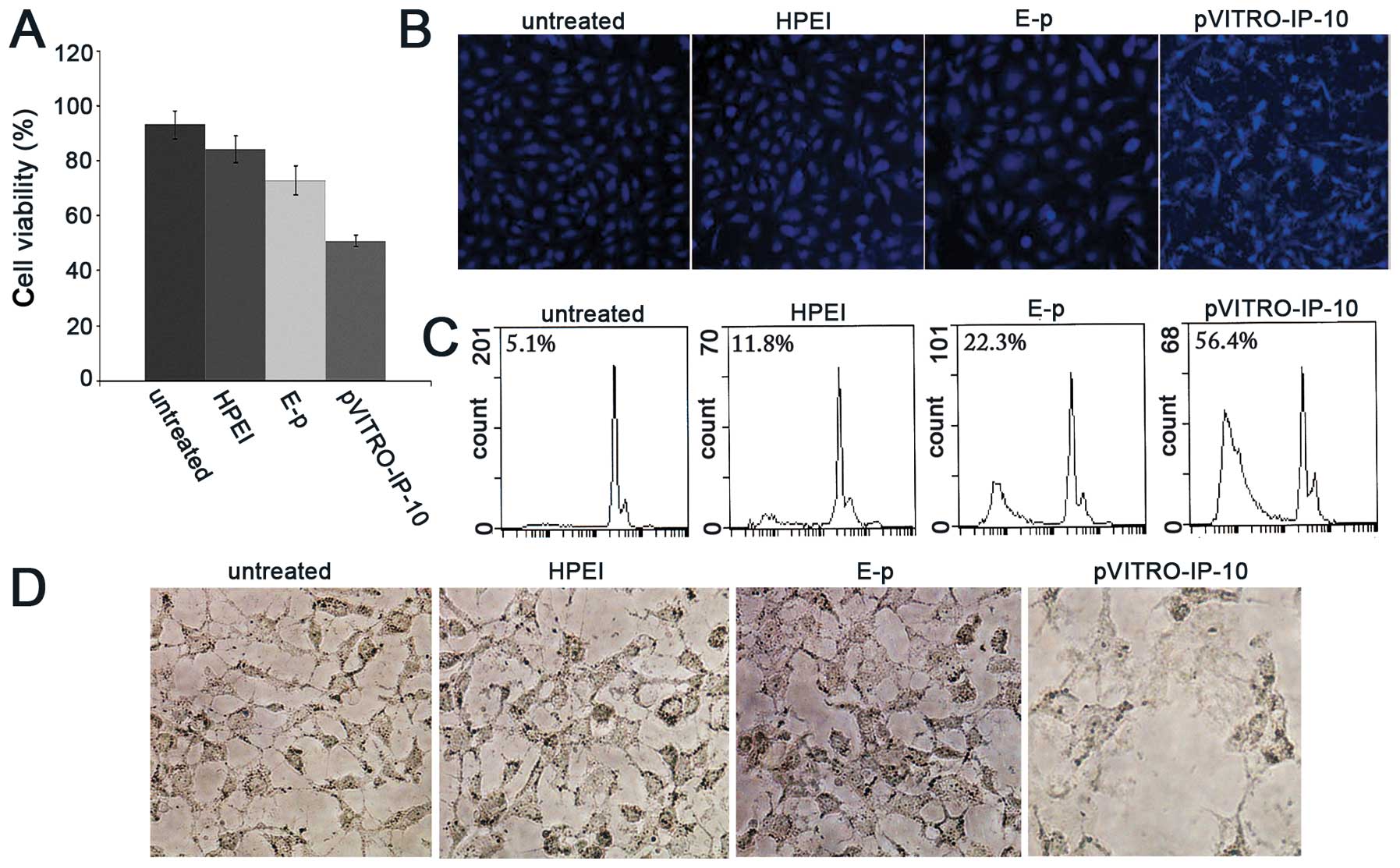 | Figure 2Inhibition of proliferation,
apoptosis and antiangiogenesis induced by pVITRO-IP-10 in
vitro. (A) SKOV3 cell proliferation was determined by MTT
assay. Seventy-two hours after transfection cell viability was
significantly lower in the groups treated with HPEI+pVITRO-IP-10
than in the other three control groups (untreated, HPEI alone, and
E-p). (B) Bright blue-fluorescent condensed nuclei (intact or
fragmented), decreased cell volume, blebbing, condensation of
nuclear chromatin, nuclear fragmentation, and apoptotic bodies were
common in the pVITRO-IP-10 treated cells, and less prominent in the
untreated, HPEI or E-p groups. (C) The percentage of apoptotic
cells was confirmed by flow cytometric analysis as significantly
higher in the pVITRO-IP-10 samples (56.4%) compared with samples
treated with E-p (22.3%), HPEI (11.8%), or untreated (5.1%). (D)
Obvious inhibition of HUVECs tubular network formation was found in
pVITRO-IP-10 treated group, while they display healthy tubular
network formation in untreated, HPEI or HPEI+E-p groups. |
Hoechst 33258/PI staining was used to test the
apoptosis. The pVITRO-IP-10 treated cells resulted in more
morphological changes compared with controls. As shown in Fig. 2B, bright blue-fluorescent condensed
nuclei (intact or fragmented), decreased cell volume, blebbing,
condensation of nuclear chromatin, nuclear fragmentation, and
apoptotic bodies were common in the pVITRO-IP-10 treated cells.
Those cells also displayed prominent red fluorescence. However,
these changes were less prominent, and slight bright blue or red
fluorescence appeared in the untreated, HPEI alone, and E-p
groups.
In addition, the number of apoptotic and
non-apoptotic cells were quantitated by flow cytometric analysis.
The results correlated strongly with those in the staining patterns
observed with fluorescence microscopy. The percentage of apoptotic
cells was significantly higher in the pVITRO-IP-10 samples (56.4%)
compared with samples treated with E-p (22.3%), HPEI (11.8%), or
untreated (5.1%) as shown in Fig.
2C.
Inhibition of angiogenesis in vitro
We tested the ability of IP-10 to inhibit
endothelial cell activity in vitro using tube formation
assay. Untreated HUVECs display a healthy tubular network
formation. In the case of HUVECs treated by HPEI alone or HPEI+E-p
supernatant, there was tubular network formation almost similar to
that of the control. In the case of HUVECs treated with
pVITRO-IP-10 supernatant, we found obvious inhibition of tubular
network formation (Fig. 2D).
Inhibition of intraperitoneal ovarian
cancer xenograft growth in nude mice
To evaluate the effect of HPEI+pVITRO-IP-10 on
suppressing ovarian cancer growth in vivo, we established an
intraperitoneal xenograft model of human ovarian cancer. All
intraperitoneal tumor nodules were collected carefully; each group
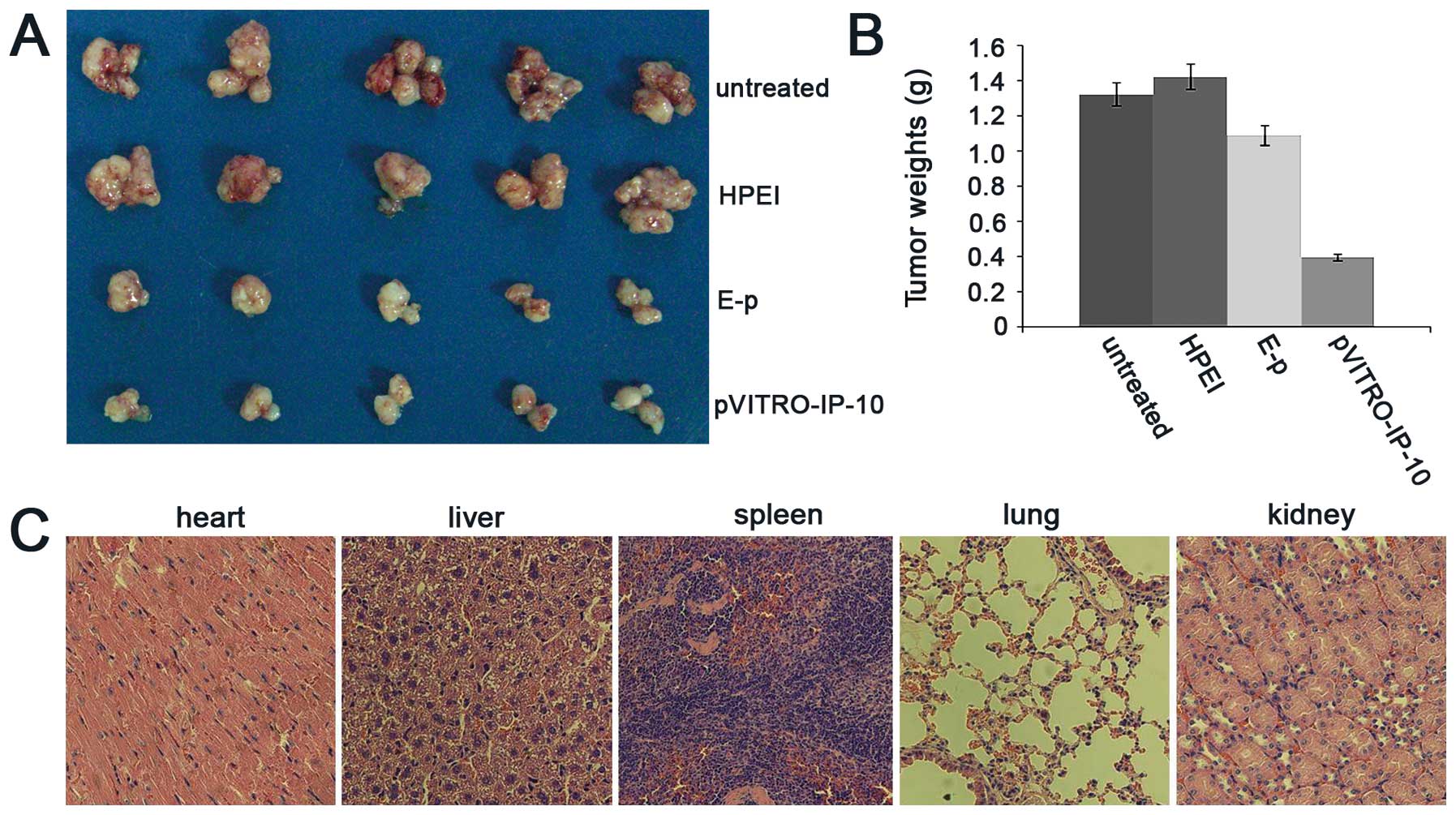
with 5 mice. As shown in Fig. 3A,
the average tumor weights were 1.33±0.41 (untreated), 1.43±0.29
(HPEI), 1.10±0.20 (HPEI+E-p), and 0.40±0.04 g (HPEI+pVITRO-IP-10),
(p<0.01). It suggested that HPEI+pVITRO-IP-10 reduced tumor
weight by 69.92% compared with the controls (Fig. 3B).
Observation of toxicity
To determine potential side effects during
treatment, we looked for signs of possible toxicity. No gross
abnormalities were found regarding weight loss, feeding, or
behavior in the treated groups. Furthermore, histologically, no
apparent pathological differences were noted between the treated
and control groups in the HE-stained organs, including heart,
liver, spleen, lung, and kidney (Fig.
3C).
Inhibition of cell proliferation in
vivo
To explore potential mechanisms underlying the
antitumor effect of HPEI+ pVITRO-IP-10 in vivo, we performed
Ki-67 immunohistochemistry to investigate whether the antitumor
effect of HPEI+pVITRO-IP-10 correlated with cell proliferation
inhibition. The reduction of Ki-67 expression relative to the three
control groups was obvious in the tumors of the HPEI+pVITRO-IP-10
treated groups. The Ki67-positive cells were 75.3±6.67 (untreated)
vs. 71.34±6.49 (HPEI) vs. 68.5±4.50 (HPEI+E-p) vs. 20.94±3.51
(HPEI+pVITRO-IP-10) (p<0.05; Fig.
4A).
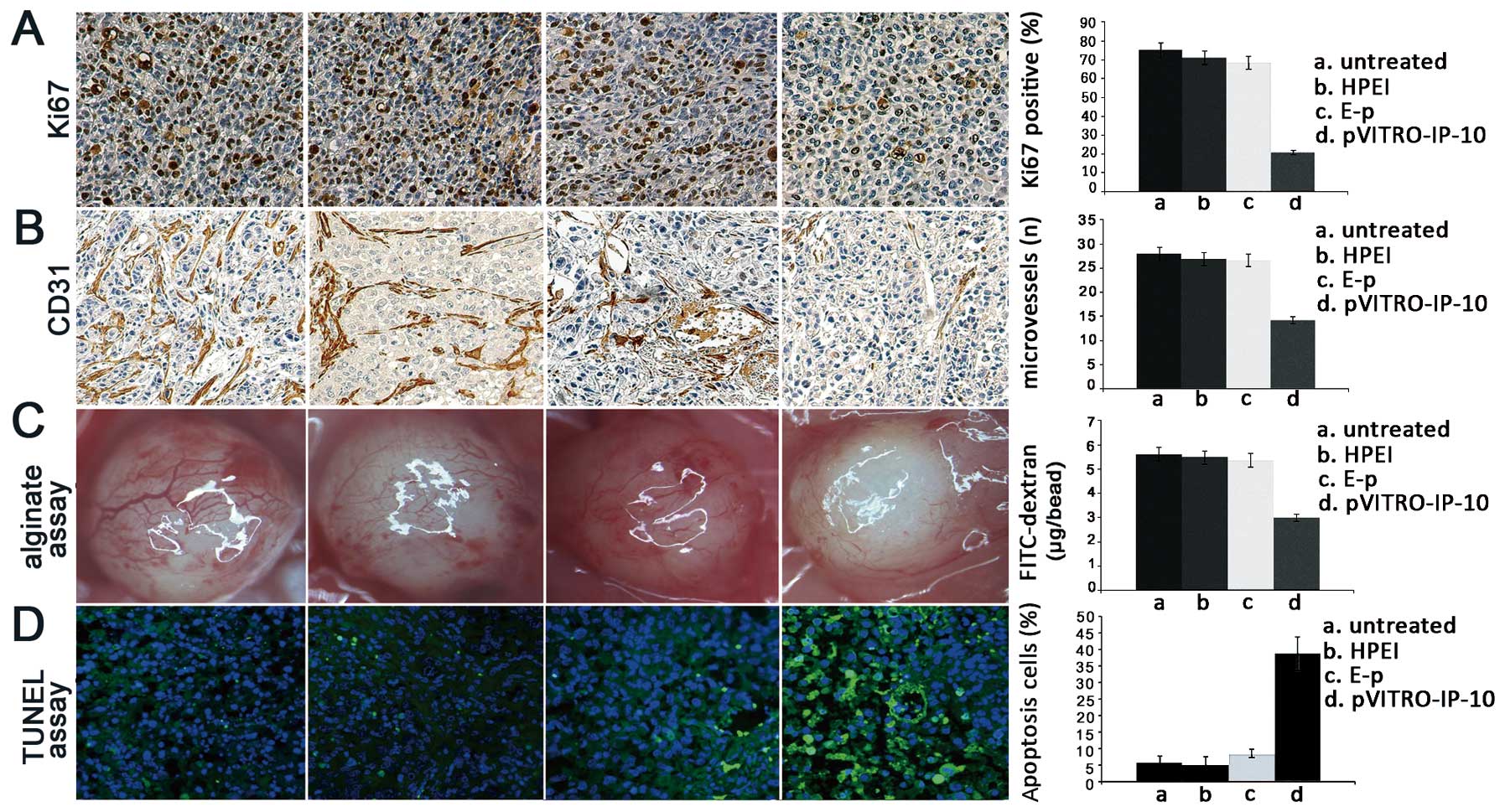 | Figure 4Inhibition of cell proliferation,
suppression of angiogenesis and induction of cell apoptosis by
pVITRO-IP-10 in vivo. (A) Ki-67 immunochemical staining was
performed to detect the cell proliferation inhibition. The
Ki67-positive cells were 75.3±6.67 (untreated) vs. 71.34±6.49
(HPEI) vs. 68.5±4.50 (HPEI + E-p) vs. 20.94±3.51
(HPEI+pVITRO-IP-10) vs. respectively. (B) The antiangiogenic effect
of HPEI+pVITRO-IP-10 was detected by CD31 immunochemical staining.
This staining revealed a significant reduction of MVD in tumor
tissues from the HPEI+pVITRO-IP-10 treated groups compared with the
untreated, HPEI, and HPEI + E-p groups. The MVD were 27.91±1.67
(untreated) vs. 26.97±1.23 (HPEI) vs. 26.74±0.85 (HPEI + E-p) vs.
14.32±1.36 (HPEI+pVITRO-IP-10) vs. respectively. (C)
Antiangiogenesis activity was also detected by the
alginate-encapsulated tumor cell assay. There were fewer newborn
blood vessels in the alginate bead nude mice treated with
HPEI+pVITRO-IP-10 than in those from the control groups. The
FITC-dextran uptake were 5.62±0.46 μg/bead (untreated) vs.
5.49±0.45 μg/bead (HPEI) vs. 5.37±0.37 μg/bead (HPEI+E-p) vs.
2.99±0.16 μg/bead (HPEI+pVITRO-IP-10), respectively. Compared with
the three control groups, the levels of FITC-dextran uptake were
significantly lower in mice treated with HPEI+pVITRO-IP-10. (D)
Apoptosis was detected by TUNEL assay. In tumor tissues, apoptotic
cells were stained with bright green fluorescence, while normal
live cells were stained by DAPI in bland blue. The TUNEL assay
showed many apoptotic cells in the tumor sections of the
HPEI+pVITRO-IP-10 groups, however, there were few such cells in the
three control groups. The apoptotic index, the ratio of bright
green-fluorescence cells to total cells, revealed that tumors in
the HPEI+pVITRO-IP-10 groups had significantly more TUNEL-positive
nuclei than tumors in the untreated, HPEI, or HPEI + E-p groups,
apoptosis ratio was 5.79±1.67 (untreated) vs. 5.11±1.69 (HPEI) vs.
8.38±2.16 (HPEI+E-p) vs. 39.92±4.84 (HPEI+pVITRO-IP-10), which
revealed that tumors in the HPEI+pVITRO-IP-10 groups had
significantly more TUNEL-positive nuclei than tumors in the
untreated, HPEI, or HPEI + E-p groups. |
Suppression of angiogenesis in vivo
Frozen sections were stained by CD31 antibody to
investigate the antiangiogenic effect of HPEI+pVITRO-IP-10.
Angiogenesis in tumor tissues was evaluated by microvessel density
(MVD) in different sections stained with an antibody reactive to
CD31, which has high specific affinity for vascular endothelial
cells. This staining revealed a significant reduction of MVD in
tumor tissues from the HPEI+pVITRO-IP-10 treated groups compared
with the untreated, HPEI, and HPEI+E-p groups. The MVD were
27.91±1.67 (untreated) vs. 26.97±1.23 (HPEI) vs. 26.74±0.85
(HPEI+E-p) vs. 14.32±1.36 (HPEI+pVITRO-IP-10) (p<0.01; Fig. 4B).
Antiangiogenesis activity was detected using the
alginate-encapsulated tumor cell assay; there were fewer newborn
blood vessels in alginate beads from nude mice treated with
HPEI+pVITRO-IP-10 than in those from the control groups. Moreover,
compared with the three control groups, the levels of FITC-dextran
uptake were significantly lower in mice treated with
HPEI+pVITRO-IP-10. The FITC-dextran uptake were 5.62±0.46
(untreated) vs. 5.49±0.45 (HPEI) vs. 5.37±0.37 (HPEI+E-p) vs.
2.99±0.16 μg/bead (HPEI+pVITRO-IP-10) (p<0.01; Fig. 4C).
Induction of apoptosis in vivo
TUNEL assay was applied to test apoptotic cells
in vivo. As described above, apoptotic cells were stained
with bright green fluorescence, while normal live cells were
stained by DAPI in bland blue. The TUNEL assay showed more
apoptotic cells in the tumor sections of the HPEI+pVITRO-IP-10
groups, while there were few such cells in the three control
groups. The apoptotic index, the ratio of bright green-fluorescence
cells to total cells, revealed that tumors in the HPEI+pVITRO-IP-10
groups had significantly more TUNEL-positive nuclei than tumors in
the untreated, HPEI, or HPEI+E-p groups. Apoptosis ratio was
5.79±1.67 (untreated) vs. 5.11±1.69 (HPEI) vs. 8.38±2.16 (HPEI+E-p)
vs. 39.92±4.84 (HPEI+pVITRO-IP-10) as shown in Fig. 4D.
Discussion
A previous study showed that growth of xenografted
human non-small cell lung cancer (NSCLC) can be suppressed by
introducing IP-10 (7). IP-10 has
been demonstrated to be a pleiotropic molecule eliciting potent
biological effects, including stimulation of monocytes, natural
killer (NK) cells and T-cell, regulation of T-cell and bone marrow
progenitor maturation, modulation of adhesion molecule expression
as well as inhibition of angiogenesis (6). However, the antitumoral efficacy of
the IP-10 gene in human ovarian cancer is largely unknown. In the
present study, we constructed a recombinant plasmid expressing
IP-10 which was then used to effectively inhibit intraperitoneal
xenograft growth of human ovarian cancer in nude mice, with
biodegradable cationic HPEI nanogels as a novel delivery system.
Treatment with HPEI+pVITRO-IP-10 complexes in nude mice was found
to have no detectable toxicity.
Although the exact mechanism of pVITRO-IP-10
antitumor activity remains to be determined, part of the answer may
lie in increased induction of antiangiogenesis and apoptosis. This
assumption is strongly supported by the result of our experiments.
Firstly, inhibition of angiogenesis was found in the tumors treated
with HPEI+pVITRO-IP-10 compared with the control group. In this
study, tube formation assay in vitro and CD31 staining,
alginate-encapsulated tumor cell assay in vivo showed
obvious inhibition of angiogenesis in HPEI+pVITRO-IP-10 treatment
compared with controls. Secondly, more apoptotic cells were found
in the tumors treated with HPEI+pVITRO-IP-10 than in control
groups. In our study, the Hoechst/PI staining in vitro and
the TUNEL assay in vivo suggested that pVITRO-IP-10
transfected by HPEI resulted in significant inhibition of cell
proliferation and induction of cell apoptosis. Other agents,
including soluble vascular endothelial growth factor receptor
(30), angiostatin (31), and endostatin (32,33)
have also been reported to inhibit tumor angiogenesis, induce
apoptosis, thus suppress tumor growth. The molecular mechanisms
underlying the inhibition of angiogenesis, and thus the induction
of apoptosis in tumor cells, have yet to be elucidated fully
(34). Taken together, these
previous findings support our hypothesis that the antitumor
efficacy in the present study may result in part from the
inhibition of angiogenesis and induction of apoptosis. These
proposed mechanisms are persuasive, but further experiments in
vitro and in vivo are required to resolve the relative
contribution of the hypothesized mechanism.
In ovarian cancer, which is mainly limited in the
abdominal cavity, systemic administration usually could not attain
desired drug concentration at the tumor site. Compared with
systemic administration, intraperitoneal administration of
HPEI+pVITRO-IP-10 avoids the systemic toxicity and first pass
effect (35), refrains from
clearance through bonding with plasma protein or phagocytized by
lymphocyte that was inevitable in cationic lipid-DNA complexes, and
maintains high focal concentration of the drug in the area where it
was topically applied.
Consequently, we may wonder whether transferring the
IP-10 gene into ovarian cancer cells by a suitable delivery system
could be an approach for treating ovarian cancer. Over the last
decade, many other functional genes have been reported to
effectively inhibit ovarian cancer in vitro and in
vivo(36,37). However, the lack of safe and
efficient gene delivery approach is a major obstacle to the
applications of gene treatments in clinical practice. Currently,
delivery systems for gene transfer are generally classified as
viral and non-viral vectors. Non-viral vectors have many advantages
over viral vectors, such as calcium phosphate, liposome, and
emerging chitosan (38–43). In our study, a novel nonviral gene
delivery system was developed based on PEI. In addition to the
advantages of PEI, the delivery system resolved the transfection
efficiency-dependent cytotoxicity and problems of
non-biodegradability. The heparin conjugated PEI into biodegradable
cationic nanogels were relatively stable in vitro and easy
to degrade through enzymolysis and hydrolysis in vivo. As a
natural polysaccharide, heparin is bio-compatible and non-toxic.
Furthermore, it improves the biocompatibility of the nanogels. In
our previous study, the transfection efficiency of HPEI was found
to be comparable to that of PEI25k, but with less toxicity.
Analyses of the erythrocyte aggregation and hemolysis showed that
HPEI had better blood compatibility than PEI25K. Moreover, HPEI
nanogels were stable in vitro and could be quickly degraded
into the low-molecular weight chemical compound PEI and excreted in
urine (15). We previously found
that HPEI could effectively transfect plasmids into SKOV3 cells.
Based on these advantages, HPEI nanogels were chosen to deliver a
recombinant plasmid to evaluate the antitumor ability of IP-10, as
well as the efficacy and safety of this delivery system.
In conclusion our study showed for the first time
that the antitumor efficacy of a novel strategy of HPEI
nanogel-delivered pVITRO-IP-10 could efficiently inhibit the growth
of ovarian cancer both in vitro and in vivo. IP-10 as
a potential angiogenesis inhibitor could efficiently inhibit the
growth of xenografted ovarian cancer through inhibition of
angiogenesis, inhibition of tumor cell proliferation and induction
of tumor-cell apoptosis. Biodegradable cationic HPEI nanogels might
also be used as a safe and effective delivery media. Hence, HPEI
nanogel-delivered pVITRO-IP-10 may have a great potential in
clinical practice against ovarian cancer in the future. However,
more studies are required to further demonstrate the mechanisms
involved.
Acknowledgements
This study was supported by the National Key Basic
Research Program (973 Program) of China (2010CB529905,
2011CB910700), National Natural Science Foundation of China
(0040215401068), and NIH81071861.
References
|
1
|
Jermal A, Siegel R, Xu J and Ward E:
Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. 2010.
View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
3
|
DiSaia PJ and Creasman WT: Epithelial
ovarian cancer. Clinical Gynecologic Oncology. 5th edition.
1997
|
|
4
|
Farber J and Mig M: IP-10: CXC chemokines
that target lymphocytes. J Leukoc Biol. 61:246–247. 1997.PubMed/NCBI
|
|
5
|
Loetscher M, Loetscher P, Brass N, Meese E
and Moser B: Lymphocyte-specific chemokine receptor CXCR3:
regulation, chemokine binding and gene localization. Eur J Immunol.
28:3696–3705. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neville LF, Mathiak G and Bagasra O: The
immunobiology of interferon-gamma inducible protein 10 kD (IP-10):
a novel, pleiotropic member of the CXC chemokine superfamily.
Cytokine Growth Factor Rev. 8:207–219. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douglas A, Arenberg A, Steven LK and
Unkelfl PJ: Cisplatin-based adjuvant chemotherapy in patients with
completely resected non-small-cell lung cancer. N Engl J Med.
350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maru SV, Holloway KA, Flynn G, Lancashire
CL, Loughlin AJ, Male DK and Romero IA: Chemokine production and
chemokine receptor expression by human glioma cells: role of CXCL10
in tumour cell proliferation. J Neuroimmunol. 199:35–45. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujita M, Zhu X, Ueda R, Sasaki K,
Kohanbash G, Kastenhuber ER, McDonald HA, Gibson GA, Watkins SC and
Muthuswamy R: Effective immunotherapy against murine gliomas using
type 1 polarizing dendritic cells - significant roles of CXCL10.
Cancer Res. 69:1587–1595. 2009. View Article : Google Scholar
|
|
10
|
Strieter RM, Polverini PJ, Kunkel SL, et
al: The functional role of the ELR motif in CXC chemokine mediated
angiogenesis. J Biol Chem. 270:348–357. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boussif O, Lezoualc'h F, Zanta MA, et al:
A versatile vector for gene and oligonucleotide transfer into cells
in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA.
92:7297–7301. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lungwitz U, Breunig M, Blunk T and
Gopferich A: Polyethylenimine-based non-viral gene delivery
systems. Eur J Pharm Biopharm. 60:247–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neu M, Fischer D and Kissel T: Recent
advances in rational gene transfer vector design based on poly
(ethyleneimine) and its derivatives. J Gene Med. 7:992–1009. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kunath K, Von HA, Fischer D, et al:
Low-molecular weight polyethyleneimine as a non-viral vector for
DNA delivery: comparison of physicochemical properties,
transfection efficiency and in vivo distribution with
high-molecular-weight polyethyleneimine. J Control Release.
89:113–125. 2003. View Article : Google Scholar
|
|
15
|
Gou M, Men K, Zhang J, et al: Efficient
inhibition of C-26 colon carcinoma by VSVMP gene delivered by
biodegradable cationic nanogel derived from polyethyleneimine. ACS
Nano. 4:5573–5584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuan X, Ma LG, Tao Y, et al: Efficient
inhibition of ovarian cancer by truncation mutant of FILIP1L gene
delivered by novel biodegradable cationic heparin-polyethyleneimine
nanogels. Hum Gene Ther. 22:1413–1422. 2011. View Article : Google Scholar
|
|
17
|
Baudin B, Bruneel A, Bosselut N and
Vaubourdolle M: A protocol for isolation and culture of human
umbilical vein endothelial cells. Nat Protoc. 2:481–485. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen Y, Wang C, Ma T, Li Z, Zhou L, Mu B,
Leng F, Shi H, Li Y and Wei Y: Immunotherapy targeting fibroblast
activation protein inhibits tumor growth and increases survival in
a murine colon cancer model. Cancer Sci. 101:2325–2332. 2010.
View Article : Google Scholar
|
|
19
|
Qiaoyun S, Andrew T, Guyen N, Angell Y, et
al: A combinatorial approach for targeted delivery using small
molecules and reversible masking to bypass non-specific uptake in
vivo. Gene Ther. 17:1085–1097. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jen-Tsan C, Howard YC, Nancy NW, Dustin
SC, Nina D and Patrick OB: Genome wide view of gene silencing by
small interfering RNAs. Proc Natl Acad Sci USA. 11:6343–6346.
2003.PubMed/NCBI
|
|
21
|
Selvakumaran M, Pisarcik DA, Bao R, Yeung
AT and Hamilton TC: Enhanced cisplatin cytotoxicity by disturbing
the nucleotide excision repair pathway in ovarian cancer cell
lines. Cancer Res. 63:1311–1316. 2003.PubMed/NCBI
|
|
22
|
Xu GX, Zhong Y, Munir S, Yang BB, Tsang BK
and Peng C: Nodal induces apoptosis and inhibits proliferation in
human epithelial ovarian cancer cells via activin receptor-like
kinase 7. J Clin Endocrinol Metab. 89:5523–5534. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin XJ, Chen XC, Wei YQ, et al: Efficient
inhibition of intraperitoneal human ovarian cancer growth and
prolonged survival by gene transfer of vesicular stomatitis virus
matrix protein in nude mice. Gynecol Oncol. 104:540–546. 2007.
View Article : Google Scholar
|
|
24
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery. Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hillen F and Griffioen AW: Tumour
vascularization: sprouting angiogenesis and beyond. Cancer
Metastasis Rev. 26:489–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin XJ, Chen XC, Wang L, et al: Dynamic
progression of an intraperitoneal xenograft model of human ovarian
cancer and its potential for preclinical trials. J Exp Clin Cancer
Res. 26:467–474. 2007.PubMed/NCBI
|
|
27
|
Sun C, Yi T, Song X, Li S, Qi X, Chen X,
Lin H, He X, Li Z, Wei Y and Zhao X: Efficient inhibition of
ovarian cancer by short hairpin RNA targeting claudin-3. Oncol Rep.
26:193–200. 2011.PubMed/NCBI
|
|
28
|
Hoffmann J, Schirner M, Menrad A and
Schneider MR: A highly sensitive model for quantification of in
vivo tumor angiogenesis induced by alginate-encapsulated tumor
cells. Cancer Res. 57:3847–3851. 1997.
|
|
29
|
Fan Y, Li Z, Hong XD, et al: Efficient
inhibition of ovarian cancer growth and prolonged survival by
transfection with a novel pro-apoptotic gene, hPNAS-4, in a mouse
model. Oncology. 75:137–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takayama K, Ueno H, Nakanishi Y, et al:
Suppression of tumor angiogenesis and growth by gene transfer of a
soluble form of vascular endothelial growth factor receptor into a
remote organ. Cancer Res. 60:2169–2177. 2000.PubMed/NCBI
|
|
31
|
Yokoyama Y, Dhanabal M, Griffioen AW,
Sukhatme VP and Ramakrishnan S: Synergy between angiostatin and
endostatin: inhibition of ovarian cancer growth. Cancer Res.
60:2190–2196. 2000.PubMed/NCBI
|
|
32
|
Herbst RS, Mullani NA, Davis DW, et al:
Development of biologic markers of response and assessment of
antiangiogenic activity in a clinical trial of human recombinant
endostatin. J Clin Oncol. 20:3804–3814. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmidt NO, Ziu M, Carrabba G, et al:
Antiangiogenic therapy by local intracerebral microinfusion
improves treatment efficiency and survival in an orthotopic human
glioblastoma model. Clin Cancer Res. 10:1255–1262. 2004. View Article : Google Scholar
|
|
34
|
Folkman J: Angiogenesis and apoptosis.
Semin Cancer Biol. 13:159–167. 2003. View Article : Google Scholar
|
|
35
|
Alberts DS, Clouser MC, Hess LM, Springer
V, Heidel B and Howell SB: Selection of drugs for intraperitoneal
chemotherapy for ovarian cancer. Intraperitoneal Ther Ovarian
Cancer. 1:77–88. 2010.
|
|
36
|
Malek A, Bakhidze E, Noske A, et al: HMGA2
gene is a promising target for ovarian cancer silencing therapy.
Int J Cancer. 123:348–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nguyen TMB, Subramanian IV, Xiao X, et al:
Adenoassociated virus-mediated delivery of kringle 5 of human
plasminogen inhibits orthotopic growth of ovarian cancer. Gene
Ther. 17:606–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Young LS and Mautner V: The promise and
potential hazards of adenovirus gene therapy. Gut. 48:733–736.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhdanov RI, Podobed OV and Vlassov V:
Cationic lipid-DNA complexes - lipoplexes - for gene transfer and
therapy. Bioelectrochemistry. 58:53–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao Y, Xu Z, Chen S, et al:
Arginine-chitosan/DNA self-assemble nanoparticles for gene
delivery: in vitro characteristics and transfection efficiency. Int
J Pharm. 359:241–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Montier T, Benvegnu T, Jaffre's PA, et al:
Progress in cationic lipid-mediated gene transfection: a series of
bioinspired lipids as an example. Curr Gene Ther. 8:296–312. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morille M, Passirani C, Vonarbourg A, et
al: Progress in developing cationic vectors for non-viral systemic
gene therapy against cancer. Biomaterials. 29:3477–3496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suzuki R, Namai E, Oda Y, et al: Cancer
gene therapy by IL-12 gene delivery using liposomal bubbles and
tumoral ultrasound exposure. J Control Release. 142:245–250. 2010.
View Article : Google Scholar : PubMed/NCBI
|